
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 May 2020
Sec. Plant Development and EvoDevo
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00516
This article is part of the Research Topic The Role of Flower Color in Angiosperm Evolution View all 30 articles
In social bees, the choice of food sources is based on several factors, including scent marks, color, and location of flowers. Here, we used similar setups, in which two stingless bee species, Melipona subnitida and Plebeia flavocincta, and the Western honeybee, Apis mellifera, were tested regarding the importance of chemical cues, color cues, and location-dependent cues for foraging behavior. It was determined whether workers chose food sources according to (1) scent marks deposited by conspecifics, (2) the color hue of a food source, (3) the trained location or the proximity of a food source to the hive. All three species preferred the scent-marked over an unmarked feeder that was presented simultaneously, but M. subnitida showed a weaker preference compared to the other species. When trained to blue feeders all three bee species preferred blue, but A. mellifera showed the strongest fidelity. The training to yellow feeders led to less distinct color choices. Only workers of M. subnitida mostly orientated at the training position and the close proximity to the nest. Whether the distance of a feeding site influenced the choice was dependent on the tested parameter (color or scent marks) and the species. Workers of M. subnitida preferably visited the feeder closer to the nest during the scent mark trials, but choose randomly when tested for color learning. Worker honeybees preferred the closer feeding site if trained to yellow, but not if trained to blue, and preferred the more distant feeder during the scent mark trials. Workers of P. flavocincta preferred the closer feeder if trained to blue or yellow, and preferred the more distant feeder during the scent mark trials. The disparity among the species corresponds to differences in body size. Smaller bees are known for reduced visual capabilities and might rely less on visual parameters of the target such as color hue, saturation, or brightness but use scent cues instead. Moreover, the dim-light conditions in forest habitats might reduce the reliability of visual orientation as compared to olfactory orientation. Honeybees showed the most pronounced orientation at floral color cues.
Foraging bees use visual and olfactory cues to find and select food sources and deploy innate or learned preferences to detect flowers (Lunau and Maier, 1995; Dyer et al., 2016). Primarily, a forager’s choice is biased by innate preferences for particular colors, shapes, and odors (Menzel, 1967; Giurfa et al., 1995; Lehrer et al., 1995; Lunau et al., 1996; Gumbert, 2000; Biesmeijer and Slaa, 2004; Raine and Chittka, 2007; Howard et al., 2019). These innate preferences differ among species. In several experiments, preferences for specific hues and saturation of colors could be found for honeybees and bumble bees (Lunau, 1990; Giurfa et al., 1995; Lunau et al., 1996; Papiorek et al., 2013; Rohde et al., 2013), while stingless bees sparsely show preferences for color hue or saturation (Spaethe et al., 2014; Dyer et al., 2016; Koethe et al., 2016, 2018).
With increasing foraging experience, initial individual preferences may be either consolidated or modified through associative learning (Gumbert, 2000; Sánchez et al., 2008; Roselino et al., 2016). For instance, species-specific chemical footprints deposited by bees while landing on and manipulating flowers indicate the recent presence of a forager to subsequent visitors (Hrncir et al., 2004; Jarau et al., 2004; Eltz, 2006; Saleh and Chittka, 2006; Witjes et al., 2011). An initial attraction toward the familiar scent of conspecifics (Schmidt et al., 2005) may be reinforced when individuals learn to associate the footprints with high reward levels or reversed when scent marks indicate depleted flowers (Saleh and Chittka, 2006; Roselino et al., 2016).
Learning and memory play a major role in bee foraging, enabling the repeated visit to sustainable food sources (Breed et al., 2002; Reinhard et al., 2004, 2006; Jesus et al., 2014), flower constancy (Free, 1963; Biesmeijer and Toth, 1998; Slaa et al., 1998, 2003), and the discovery of new patches of known food plants (Biesmeijer and Slaa, 2004). In addition to memorizing scent and location of resources (Reinhard et al., 2004, 2006), bees learn both color and position of landmarks, which facilitates the orientation toward food sources and the nest (Cartwright and Collett, 1983; Cheng et al., 1986, 1987; Chittka et al., 1995; Menzel et al., 2005). However, species differ concerning their learning ability (Pessotti and Lé’Sénéchal, 1981; Mc Cabe et al., 2007), which might be associated with differences in life-history and ecological traits among bee species, such as longevity of individuals (Ackerman and Montalvo, 1985), the degree of floral specialization (Cane and Snipes, 2006), and food niche-breath (Biesmeijer and Slaa, 2006).
In eusocial bees, including the stingless bees (Meliponini), bumble bees (Bombini), and honeybees (Apini), food source selection is not only based on individual foraging preferences, but relies to a large extent on social information. On their return to the nest, foragers transmit olfactory and gustatory information about the exploited food source to nestmates, which biases the subsequent food choice of the receivers (Farina et al., 2005, 2007; Mc Cabe and Farina, 2009). Moreover, returning foragers of many species announce the existence of lucrative food sources through thoracic vibrations (stingless bees: Lindauer and Kerr, 1958; Esch et al., 1965; Barth et al., 2008; Hrncir and Barth, 2014; honeybees: Esch, 1961; Waddington and Kirchner, 1992; Hrncir et al., 2011). Inactive individuals may use these mechanical signals for their decision of whether to engage in foraging or to remain in the nest. In addition, foragers of some eusocial bee species guide the recruits to the location of the exploited food patch. Honeybees (all species) use an elaborated dance language (waggle dance) communicating information about distance, direction, and quality of foraging sites (Von Frisch, 1967; Dyer, 2002). Stingless bees (few species), in contrast, lay polarized trails of species-specific pheromone marks that guide recruits with high precision toward the goal (Lindauer and Kerr, 1958; Schmidt et al., 2003; Nieh et al., 2004; Barth et al., 2008; Jarau, 2009). At the food patch, foraging choices are influenced by field-based social information, like olfactory footprints and the visual presence of con- or heterospecific foragers (Slaa et al., 2003). Depending on the composition of the foraging community at the food patch, these passively provided cues may cause local enhancement or local inhibition (Slaa and Hughes, 2009). Thus, food source selection in eusocial species is based on a complex interplay between individual preferences and social information.
Differences among social bee species regarding ecological (habitat, food niche), physiological (learning ability, visual capacity, color vision), and behavioral features (innate preferences, foraging strategy, recruitment mechanism) may result in differences concerning the parameters used in foraging decisions. With more than 500 described species, stingless bees (Meliponini) are the most speciose group of eusocial bees with very diverse characteristics regarding body size, colony size, nesting biology, brood cell arrangement, queen production, foraging strategies, and recruitment mechanisms (Michener, 1974, 2013; Johnson, 1983; Wille, 1983; Engels and Imperatriz-Fonseca, 1990; Roubik, 2006; Barth et al., 2008). Given this biological diversity, we can expect differences concerning the mechanisms of food source selection among species. In the present study, we investigated the food source selection by two stingless bee species, Melipona subnitida and Plebeia flavocincta, and the Western honeybee, Apis mellifera. Since stingless bees show only weak preferences for colors compared to other bee species (Dyer et al., 2016; Koethe et al., 2016, 2018), alternative parameters could be of importance for foraging choices. Of interest were the roles of scent marks (olfactory footprints), the color, and the location of a food source. Melipona species are known to mark food sources with olfactory footprints (Jarau, 2009; Roselino et al., 2016). For P. flavocincta, no specific information concerning scent communication is available so far (Aguilar et al., 2005). However, given that all bee species studied to this moment deposit chemical footprints at food sources (Goulson et al., 1998; Eltz, 2006; Yokoi et al., 2007; Jarau, 2009; Witjes et al., 2011), scent cues can also be postulated for this meliponine species. A. mellifera is known for marking food sources directly (Giurfa and Nunez, 1992).
The aim was to analyze how the three investigated social bee species use the parameters color, scent marks, or location differently during the colony foraging processes. We test the hypothesis that these bees possess a hierarchy in the use of the parameters color, scent marks, and location of flowers. We expect honeybees to rely more on color cues than the two stingless bee species. For the two stingless bee species, we assume that they follow scent markings of conspecifics more reliable than honeybees. Since small stingless bee might exploit nectarrich flowers by repeated visits to the same individual flower, we assume that the location of the flower is of higher importance in the smaller bees.
This study is part of a research project on color preferences in stingless bees conducted in Australia and Brazil (Köthe, 2019).
The foraging behavior of the stingless bee species was investigated at the Brazilian Federal University at Mossoró (Universidade Federal Rural do Semi-Árido), located in the Brazilian tropical dry forest, the Caatinga at 5°12′13.3″S 37°19′44.8″W. For our experiments, we used two stingless bee species native to the study region, M. subnitida (six colonies) and P. flavocincta (one colony) (Zanella, 2000; Imperatriz-Fonseca et al., 2017). P. flavocincta is the smallest bee with less than 5 mm body length (Cockerell, 1912), M. subnitida is intermediate with 7.5–8.5 mm (Schwarz, 1932), and A. mellifera is the largest with more than 11 mm (Amiet and Krebs, 2012). Colonies of the stingless bee species were kept in wooden nest-boxes at the university’s meliponary (Meliponário Imperatriz) and were freely foraging. The foraging behavior of the Western honeybee, A. mellifera, was studied at the botanical garden of the Heinrich Heine University Düsseldorf, Germany at 51°11′10.7″N 6°48′14.1″E. Foragers of five nests were trained to participate in the experiment. The colony size of the three tested species differs and ranges from several thousand individuals (20.000–80.000) in a single colony of A. mellifera to several hundred (up to 1000) in M. subnitida (Wilson, 1971; Michener, 1974). For P. flavocincta, colony size has not been determined yet, but in other Plebeia species, colony size has been shown to range from 2.000 to 3.000 individuals (Roldão-Sbordoni et al., 2018).
The reasons for conducting the study on stingless bees and honeybees at different study sites were as follows: Most experimental research in Western honeybees has been done in Europe and Australia, excluding the Africanized honeybees available in Mossoro. Moreover, A. mellifera is not native in South America. Thus, direct comparison with literature data is easier when working with European Western honeybees, although direct comparison of foraging strategies in stingless bees and honeybees in the same habitat might also yield interesting results (Roubik and Buchmann, 1984). The origin of the Western honeybee is in the Middle East or Africa (Han et al., 2012) and Western honeybees have developed adaptations to get along with temperate climates (Han et al., 2012).
For all tests and bee species, the training was identical. Workers of all three species were trained to mass feeders offering sugar solution (50%) affixed to tripods. The training to the mass feeders started at the respective nest’s entrance. After more than 10 workers regularly foraged at the feeder, it was moved in short steps (∼1 m) away from the nest until a distance of 15 m (site 1) or 17 m (site 2) was reached. Once at the final feeding site, the mass feeder was replaced by a colored gravity feeder (10 cm diameter, 5 cm height) that was used during the experiment. The gravity feeders were either blue (edding permanent spray RAL5010 enzianblau, edding International GmbH, Ahrensburg, Germany) or yellow (only for the color test; edding permanent spray RAL 1037 sonnengelb, edding International GmbH, Ahrensburg, Germany). The colors were measured using spectrometer analysis (USB4000 miniature fiber optic spectrometer, Ocean Optics GmbH, Ostfildern, Germany) at an angle of 45° using a UV-NIR deuterium halogen lamp (DH-2000-BAL, Ocean Optics GmbH), which was connected to the spectrometer by a UV–VIS fiber optic cable (Ø 600 μm, QR600-7-UV 125 BX, Ocean Optics GmbH). To calibrate the spectrometer, a black standard (black PTFE powder, Spectralon diffuse reflectance standard SRS-02-010, reflectance factor of 2.00%, Labsphere, Inc., North Sutton, NH, United States) and a white standard (white PTFE powder, Spectralon diffuse reflectance standard SRS99-010, reflectance factor of 99.00%, Labsphere, Inc., North Sutton, NH, United States) were used (Supplementary Figure S1). After the workers accepted the colored gravity feeder (henceforth “feeder”), a training period of 30 min started, in which the bees were allowed to forage ad libitum (approximate number of foragers during training phase: M. subnitida ≈ 10 individuals; P. flavocincta ≈ 30–50 individuals, A. mellifera ≈ 30–50 individuals). Workers were not marked during the training to keep the disturbance at the feeder to a minimum. Hence, no discrimination between experienced and inexperienced workers was possible.
We conducted experiments investigating the influence of scent marks deposited at the training feeder on the choice behavior of foragers. For this experimental series, we used only blue-colored feeders. In total, we performed three trials with each bee species. In preliminary studies, this approach turned out to be most reasonable for comparative studies between these bee species. Each trial consisted of three sets of a 30-min training phase and a subsequent 5-min test phase, switching the feeder positions in pseudo-randomized order (SM1–SM3; Supplementary Table S1). After the training phase, we offered the incoming bees both the training feeder (scent-marked) and a clean blue-colored feeder (unmarked), one at each feeding site (Supplementary Table S1). During this test phase, both feeders contained sugar solution (50%). In total, we performed three trials of this experimental series with each bee species. A trial consisted of three pairs of a 30-min training phase and a 5-min test phase intermitted by 30-min training phases (SM1–SM32; Supplementary Table S1), switching the feeder positions in pseudo-randomized order. The three different bee species (A. mellifera, M. subnitida, and P. flavocincta) were tested separately. Workers that visited the feeder were either marked with nail polish on their first visit (A. mellifera and M. subnitida) or caught after landing (P. flavocincta) and released at the end of the respective 5-min test phase. Workers were allowed to participate in all three trials. To avoid pseudo-replication (A. mellifera, M. subnitida), only the first landing of an individual in each test phase was considered for the analysis. During the third test, all foragers were captured and killed by freezing to avoid pseudo-replication.
In the second experimental series, we investigated the impact of color on the choice of food sites by workers. In this experimental series, we performed two different trial series with each bee species. Each trial consisted of two sets of a 30-min training phase and a subsequent 5-min test phase, switching the feeder positions in pseudo-randomized order (Supplementary Table S1). After the training phase (training feeder either blue or yellow; Supplementary Table S1), the training feeder was removed, and we offered the incoming bees a blue- and a yellow-colored feeder during the test phase, one at each feeding site (Supplementary Table S1). In trial series 1 (C1–C4; Supplementary Table S1), bees were trained to blue feeders in the first three training phases and a yellow feeder in the fourth (training to blue, retraining to yellow). In trial series 2 (C5–C8; Supplementary Table S1), foragers were trained to yellow feeders during three training phases and a blue feeder in the last training phase (training to yellow, retraining to blue). For the test phases, we used alcohol-cleaned feeders to eliminate the influence by any potential scent marks. During the test phase, both feeders offered sugar solution (50% weight on weight). Each trial series was repeated three to five times with different individuals. The bee species (A. mellifera, M. subnitida, and P. flavocincta) were tested separately and workers that visited the feeder were either marked with nail polish (A. mellifera and M. subnitida) or caught after landing on a feeder (P. flavocincta) and released at the end of the respective 5-min test phase. To avoid pseudo-replication (A. mellifera, M. subnitida), only the first landing of an individual in each test was considered for the analysis. During the fourth test, all workers were captured and killed by freezing.
To test whether bees visited the feeding site closer to the nest (site 1, 15 m) more often than the farther feeding site (site 2, 17 m) the results of all above described tests (scent marks and color) were analyzed concerning the influence of distance.
The statistical program R was used to analyze the data (R Development Core Team, 2019). The data were analyzed by testing the bees’ choices (the first decision of each test) for the different parameters (scent marks, color, distance) using a generalized linear mixed model (GLMM). We used the “lme4” package of R to analyze choices of the bees, which were assessed using GLMM with Poisson distribution of data (Bates et al., 2009; R Development Core Team, 2019). We analyzed the number of choices for each test as fixed effect and the position of the stimuli were used as random effect of the model when testing the influence of color and scent marks, while these parameters were used as random effect when testing the impact of distance on the bees’ choice behavior.
In the first experimental series (influence of scent marks), foragers of all three bee species significantly preferred the previously visited training feeder over the clean feeder (Figure 1; M. subnitida: n = 239, z-value = −8.346, p < 0.001; P. flavocincta: n = 355, z-value = −12.15, p < 0.001; A. mellifera: n = 303, z-value = −10.46, p < 0.001).
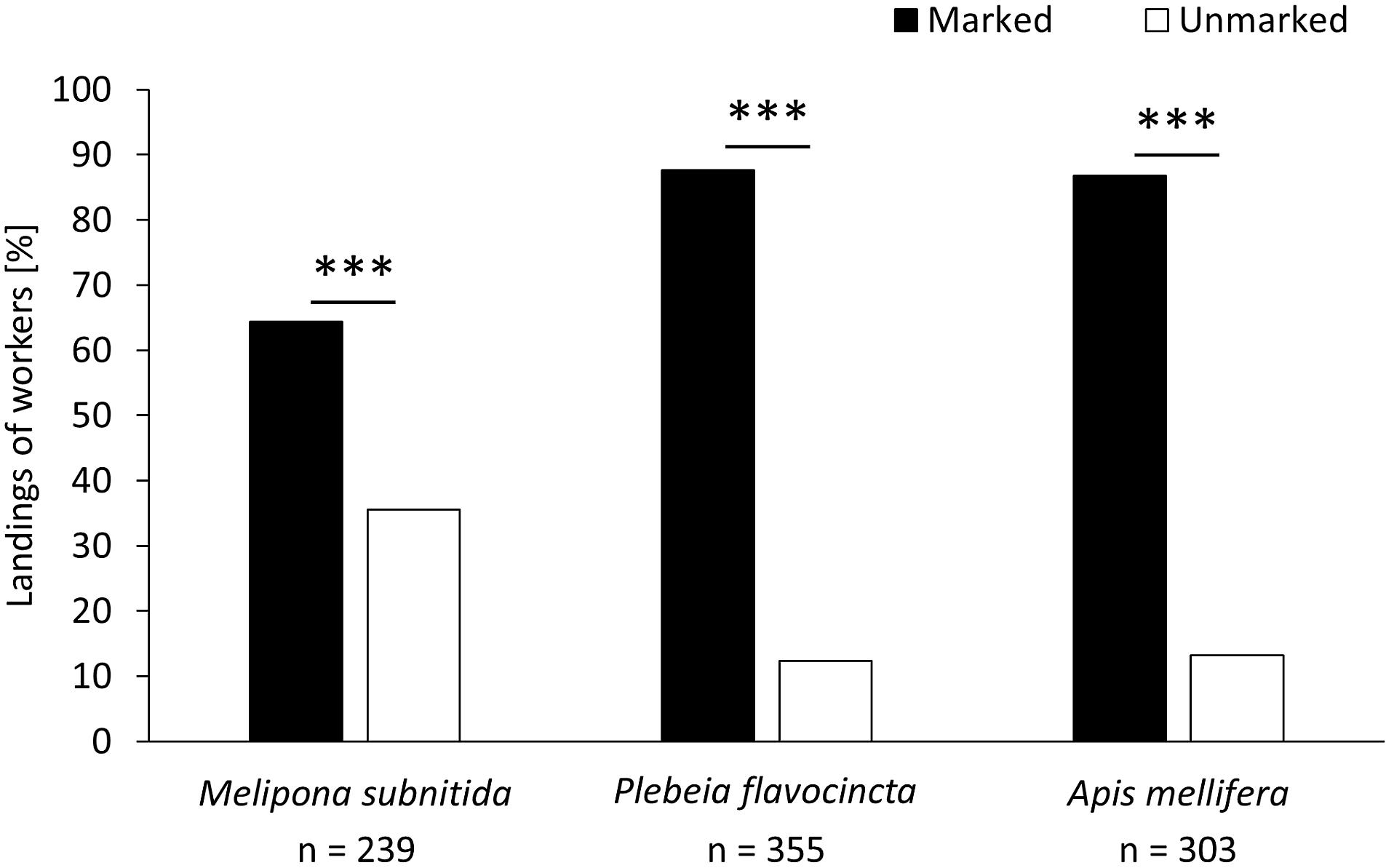
Figure 1. Landings of workers on a scent-marked and an unmarked feeder. A generalized linear mixed model was used for statistical analysis (∗∗∗p < 0.001).
In the second experimental series, we investigated the influence of color on the feeder choice by the three bee species. After training to a blue-colored feeder, all three species significantly preferred the blue feeder over the yellow feeder (Figure 2A; M. subnitida: n = 250, z-value = −10.24, p < 0.001; P. flavocincta: n = 230, z-value = −8.821, p < 0.001; A. mellifera: n = 538, z-value = −10.85, p < 0.001). When these workers were retrained to forage on a yellow feeder during the last training phase, the two stingless bee species significantly preferred the yellow feeder while honeybee workers visited both colors equally (Figure 2B; M. subnitida: n = 124, z-value = 2.667, p = 0.007; P. flavocincta: n = 71, z-value = 3.756, p < 0.001; A. mellifera: n = 278, z-value = 0.6, p = 0.549). When workers were initially trained to a yellow-colored feeder, both stingless bee species preferred the yellow feeder significantly over the blue feeder during the test, while A. mellifera preferred the blue feeder (Figure 2C; M. subnitida: n = 199, z-value = 3.318 p < 0.001; P. flavocincta: n = 303, z-value = 3.141, p = 0.002; A. mellifera: n = 556, z-value = 5.863, p < 0.001). Retraining to a blue feeder in the last training phase lead to a significant preference of the blue colored feeder in all three species (Figure 2D; M. subnitida: n = 52, z-value = −2.95, p = 0.003; P. flavocincta: n = 61, z-value = −2.632, p = 0.008; A. mellifera: n = 213, z-value = −6.74, p < 0.001).
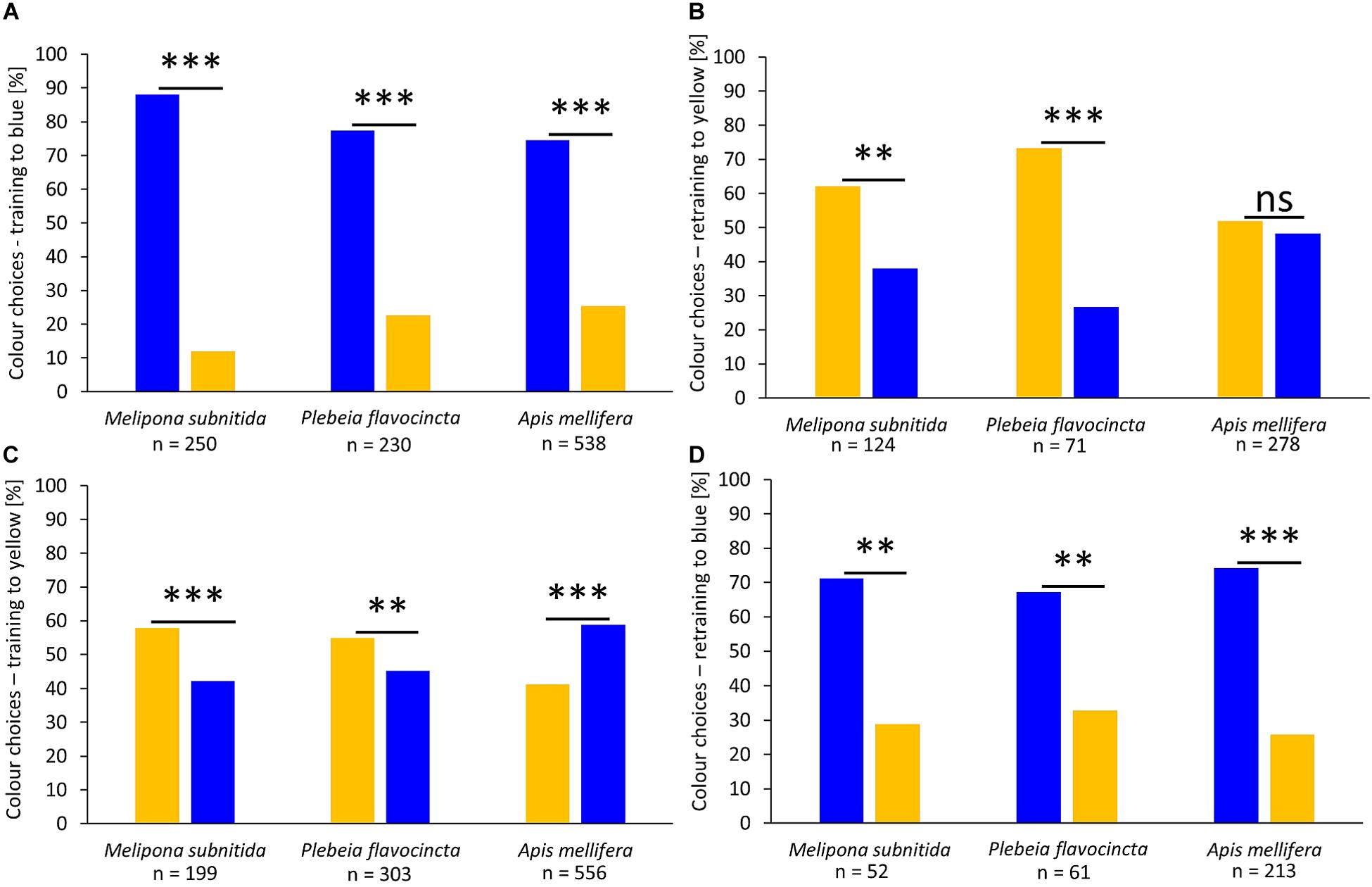
Figure 2. Color choices after training sessions. The three tested bee species were trained to forage on either a blue feeder (A) or a yellow feeder (C). Furthermore, the workers were retrained to the opposite color (B,D). A generalized linear mixed model was used for statistical analysis (∗∗p < 0.01; ∗∗∗p < 0.001; ns = not significant p > 0.05).
When analyzing the influence of the feeders’ positions on the food source choice, we observed that M. subnitida visited the feeding site closer to the nest during the scent mark trials (site 1, 15 m) significantly more often than the farther site (site 2, 17 m) (Figure 3; n = 239, z-value = −8.467, p < 0.001), while choosing randomly when tested for color differences (Figure 3; blue: n = 250, z-value = −0.045, p = 0.964; yellow: n = 199, z-value = 1.502, p = 0.133). Workers of A. mellifera significantly preferred the closer feeding site when they were trained to yellow (Figure 3; n = 556, z-value = −6.147, p < 0.001), but did not distinguish between the two sites when trained for blue (Figure 3; blue: n = 538, z-value = −0.951, p = 0.342; scent marks: n = 199, z-value = −1.502, p = 0.133). In the trial concerning scent marks workers of A. mellifera significantly preferred the farther away feeding site (Figure 3; n = 303, z-value = −10.46, p < 0.001). Workers of P. flavocincta preferred the closer feeding site when trained to blue or yellow (Figure 3; blue: n = 230, z-value = −5.036, p < 0.001; yellow: n = 71, z-value = −4.856, p < 0.001) and significantly visited the farther site when tested concerning scent marks (Figure 3; n = 355, z-value = −2.548, p = 0.011).

Figure 3. Landings of workers depending on the feeding site. The number of landings at the feeding sites with 15 m (site 1, black) and 17 m (site 2, white) distance to the hive were compared for A. mellifera, M. subnitida, and P. flavocincta for the two color trials, blue and yellow, and the scent mark trial (generalized linear mixed model *p < 0.05; ***p < 0.001; ns = not significant p > 0.05).
The results of this study show that the response to the color of feeder, scent marks, and locations differs among the tested species P. flavocincta, M. subnitida, and A. mellifera. Our results confirm previous findings about the important role of color for food plant detection in honeybees and add further findings to the diverse and sometimes less important role of color for food plant detection in stingless bees. In previous studies of color preferences in stingless bees, the results varied among species. While three species of the genus Melipona chose colors poorly, Tetragonula carbonaria chose colors according to their hue and Partamona helleri showed similar color choices as A. mellifera preferring spectrally purer colors and bluish color hues (Rohde et al., 2013; Dyer et al., 2016; Koethe et al., 2016, 2018). Particularly for the honeybee, it has been shown that besides innate preferences, absolute or differential conditioning and behavioral plasticity play important roles in how they exploit color information (Reser et al., 2012), and that strong color preferences impede learning of other features (Morawetz et al., 2013).
Workers of A. mellifera orientated most strongly according to colors. The blue-colored feeder was preferred in all tests with exception of the retraining to yellow, where A. mellifera showed no depicted choice for one of the two colors and the two stingless bee species preferred the yellow feeder. This is in accordance to previous studies showing that A. mellifera prefers blue colors over other color hues (Giurfa et al., 1995; Horridge, 2007). The two stingless bee species chose feeders according to their colors but rather preferred the feeder color of the previous training. Only when initially trained to yellow they showed weak (M. subnitida) or no preferences for the trained color (P. flavocincta). This preference for blue is in accordance with previous results of stingless bees, but also suggests that it is weaker in stingless bees than in honeybees (Dyer et al., 2016; Koethe et al., 2016). An explanation for less visually driven behavior in stingless bees could be the size differences compared to honeybees. P. flavocincta reaches a body size of 3.6–4.1 mm, M. subnitida of 7.5 mm, and A. mellifera is the largest of the three species with 13–16 mm (Hrncir and Maia-Silva, 2013; Maia-Silva et al., 2015; Imperatriz-Fonseca et al., 2017). Especially the size of the eyes, which is associated with body size, can impact the visual capacities of bees (Streinzer et al., 2016). Workers of P. flavocincta are rather small; consequently, their eyes are also small leading to poorer visual capabilities.
Both stingless bees and honeybees use scent cues to evaluate reward availability of food resources (Nunez, 1967; Butler et al., 1969; Ferguson and Free, 1979; Free and Williams, 1983; Corbet et al., 1984; Giurfa and Nunez, 1992; Giurfa, 1993; Stout et al., 1998; Williams, 1998; Stout and Goulson, 2001). In this study, all three species showed preferences for the marked feeder over the unmarked one. P. flavocincta and A. mellifera chose the marked feeder consistently (∼88% of choices), while M. subnitida preferred the marked feeder, but visited it less frequently (∼64% of choices).
Plebeia flavocincta was the only species that significantly preferred the closer feeding site when tested concerning colors and the farther feeding site when tested regarding scent marks. One interpretation is that P. flavocincta does not differentiate between colors and choses the closer feeding site, while the preference during the scent mark trial could be based on the fact that the scent marked feeder was positioned twice at the farther site and only once at the closer site. In contrast, M. subnitida was the only species in the scent mark trials that visited the food site with shorter distance to the hive more frequently. It seems likely that M. subnitida orientates on location rather than on scent marks. Previous studies showed that species of the genus Melipona mark food sites directly and do not lay scent trails (Hrncir et al., 2004). In order to recruit new foragers, it seems possible that M. subnitida relies strongly on piloting—leading new foragers from hive to food site during flight (Nieh et al., 2003). Foragers of M. subnitida could be observed to frequently arrive in small groups, while A. mellifera and P. flavocincta workers seemed more independent from each other. Scent marks play an important role for the communication of reward availability, but their impact on recruitment seems dependent on the specific strategy used by species (Free and Williams, 1983; Corbet et al., 1984; Giurfa and Nunez, 1992; Giurfa, 1993; Stout et al., 1998; Stout and Goulson, 2001; Schmidt et al., 2003). The attractiveness of scent marks, whether or not they were used for recruitment purposes, appears to be strong because scent-marked feeders were preferred by all three tested bee species. During the experiments workers foraged in groups and could be influenced by the presence of other individuals. An influence by social facilitation (Wilson, 1971) could not be excluded during the experiments, but when comparing the results for choices of blue and yellow feeders, after the respective training, an influence solely by the presence of conspecifics seems unlikely.
Another aspect that can explain the diverse results for the three tested bee species could be their natural habitat. M. subnitida originates from the Caatinga, which is an open habitat, while P. flavocincta inhabits a spacious habitat that extends from the Caatinga to the Atlantic Rainforest, which is a densely vegetated forest (Imperatriz-Fonseca et al., 2017). Because of its domestication, the honeybee is widespread all over the world. It originates from diverse habitats of Europe, the Middle East, and Africa. Open habitats are brightly illuminated, while forest habitats are characterized by dim-light conditions (Endler, 1992). Based on the light conditions of their respective habitat, it appears to be possible that M. subnitida and A. mellifera could rely to a greater extent on visual signals than P. flavocincta that encounters dim-light conditions and a less visually structured vegetation. In a densely vegetated habitat, scent marks could be a more reliable signal to guide foragers to a food source. Furthermore, temperate and sub-tropical regions experience more distinct seasons concerning weather conditions and the rhythm of flowering plants is directly influenced, while tropical and semi-arid regions have more steady weather conditions but are challenging for their inhabitants because of high temperatures (Prado, 2003; Zanella and Martins, 2003; Machado and Lopes, 2004; Maia-Silva et al., 2012, 2015; Hrncir et al., 2019).
Social bee species that face seasonal variations mass-collect floral resources for provision of the hive (Ramalho, 2004). These variations in floral resource availability could be another explanation for more distinct preferences for visual signals in honeybees when compared to tropical species, like M. subnitida and P. flavocincta, because only honeybees face strong seasonal variations (Michener, 1974; Kleinert-Giovannini, 1982; Roubik, 1982a; Seeley, 1985). Nonetheless, this would not explain the differences between M. subnitida and P. flavocincta.
The three tested bee species reacted vaguely similar to color, scent marks, and location of food sources, but their main focus varies: While A. mellifera choose food sites according to both color and scent marks, M. subnitida orientates on location and color of food sites, and P. flavocincta relies mainly on scent marks. These variations are possibly based on different recruitment mechanisms (e.g., waggle dance of honeybees vs. piloting, excited movements, vibration, and scent mark deposition by stingless bees) or they could be the result of adaptations to the bees’ respective habitat and obliged morphological constraints. Although highly eusocial stingless and honeybees do not communicate the color of flowers to nestmates (Michelsen, 2014), flower color has a large impact on foraging decisions. This impact is demonstrated by the results of this study, that bees exhibit a spontaneous response to color cues and that they memorize color cues following experience; spontaneous response of bees and discrimination after conditioning might rely on different color parameters, such as color saturation and color hue (Rohde et al., 2013). Flower color has also been identified as a floral filter excluding bees from visiting the less preferred flower colors, i.e., red, UV-absorbing and white, UV-reflecting hummingbird-pollinated flowers (Lunau et al., 2011). Stingless bees are known as nectar robbers of hummingbird-pollinated flowers (Roubik, 1982b); it remains to be tested if the less pronounced color preferences in stingless bees are helpful for finding food on flowers displaying colors that are not adapted to bee-color vision and color preferences.
All datasets generated for this study are included in the article/Supplementary Material.
KL conceived and developed the research concept and supervised the study. SK designed the experiments. MH and KL supported the design of the experiments. SK, VF, LR, and SB conducted the experiments and collected and analyzed the data. SK wrote the manuscript. SB, VF, LR, SB, MH, and KL supported the writing of the manuscript.
This study was supported by the Deutsche Forschungsgemeinschaft and by CGIAR Research Program on Roots, Tubers, and Bananas and CGIAR Fund Donors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the financial contribution of the CGIAR Research Program on Roots, Tubers, and Bananas and the CGIAR Fund Donors. The study was part of research project about “Color preferences in stingless bees—features of an outstanding visual ecology” (Köthe, 2019) funded by the Deutsche Forschungsgemeinschaft.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00516/full#supplementary-material
FIGURE S1 | Spectral reflectance curves of colored feeders.
TABLE S1 | Position of feeders in training and test phase. The order of tests was pseudo-randomized to ensure no influence of test order on the decisions of workers. Two experimental trials were conducted comprising four choice experiments each. C1–C4 are the tests which focused on blue color, while the tests C5–C8 focused on yellow color. SM1–3 = are tests which analyzed the impact of scent marks; m = marked feeder; u = unmarked feeder; C1–8 = are the tests analyzing the impact of color; b = blue; y = yellow; site 1 = 15 m distance to the hive; site 2 = 17 m distance to the hive.
Aguilar, I., Fonseca, A., and Biesmeijer, J. C. (2005). Recruitment and communication of food source location in three species of stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 36, 313–324. doi: 10.1051/apido:2005005
Amiet, F., and Krebs, A. (2012). Bienen Mitteleuropas: Gattungen, Lebensweise, Beobachtung. Bern: Haupt.
Barth, F. G., Hrncir, M., and Jarau, S. (2008). Signals and cues in the recruitment behavior of stingless bees (Meliponini). J. Comp. Physiol. A 194, 313–327. doi: 10.1007/s00359-008-0321-7
Bates, D., Maechler, M., and Dai, B. (2009). lme4: Linear Mixed-Effects Models Using S4 Classes. R package version 0.999375–999331.
Biesmeijer, J. C., and Slaa, E. J. (2004). Information flow and organization of stingless bee foraging. Apidologie 35, 143–157. doi: 10.1051/apido:2004003
Biesmeijer, J. C., and Slaa, J. E. (2006). The structure of eusocial bee assemblages in Brazil. Apidologie 37, 240–258. doi: 10.1051/apido:2006014
Biesmeijer, J. C., and Toth, E. (1998). Individual foraging, activity level and longevity in the stingless bee Melipona beecheii in Costa Rica (Hymenoptera, Apidae, Meliponinae). Insectes Soc. 45, 427–443. doi: 10.1007/s000400050099
Breed, M. D., Stocker, E. M., Baumgartner, L. K., and Vargas, S. A. (2002). Time-place learning and the ecology of recruitment in a stingless bee, Trigona amalthea (Hymenoptera, Apidae). Apidologie 33, 251–258. doi: 10.1051/apido:2002018
Butler, C. G., Fletcher, D. J. C., and Watler, D. (1969). Nest-entrance marking with pheromones by the honeybee, Apis mellifera L., and by the wasp, Vespula vulgaris L. Anim. Behav. 17, 142–147. doi: 10.1016/0003-3472(69)90122-5
Cane, J. H., and Snipes, S. (2006). “Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty,” in Plant-Pollinator Interactions, eds N. M. Waser and J. Ollerton (Chicago, IL: University Press of Chicago), 99–122.
Cartwright, B. A., and Collett, T. S. (1983). Landmark learning in bees. J. Comp. Physiol. A 151, 521–543. doi: 10.1007/bf00605469
Cheng, K., Collett, T. S., Pickhard, A., and Wehner, R. (1987). The use of visual landmarks by honeybees: bees weight landmarks according to their distance from the goal. J. Comp. Physiol. A 161, 469–475. doi: 10.1007/bf00603972
Cheng, K., Collett, T. S., and Wehner, R. (1986). Honeybees learn the colours of landmarks. J. Comp. Physiol. A 159, 69–73. doi: 10.1007/bf00612497
Chittka, L., Geiger, K., and Kunze, J. (1995). The influences of landmarks on distance estimation of honey bees. Anim. Behav. 50, 23–31. doi: 10.1006/anbe.1995.0217
Corbet, S. A., Kerslake, C. J. C., Brown, D., and Morland, N. E. (1984). Can bees select nectar-rich flowers in a patch? J. Apic. Res. 23, 234–242. doi: 10.1080/00218839.1984.11100638
Dyer, A. G., Boyd-Gerny, S., Shrestha, M., Lunau, K., Garcia, J. E., Koethe, S., et al. (2016). Innate colour preferences of the Australian native stingless bee Tetragonula carbonaria Sm. J. Comp. Physiol. A 202, 603–613. doi: 10.1007/s00359-016-1101-4
Dyer, F. C. (2002). The biology of the dance language. Annu. Rev. Entomol. 47, 917–949. doi: 10.1146/annurev.ento.47.091201.145306
Eltz, T. (2006). Tracing pollinator footprints on natural flowers. J. Chem. Ecol. 32, 907–915. doi: 10.1007/s10886-006-9055-6
Endler, J. A. (1992). The colour of light in forests and its implications. Ecol. Monogr. 63, 1–27. doi: 10.2307/2937121
Engels, W., and Imperatriz-Fonseca, V. L. (1990). “Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees,” in Social Insects: An Evolutionary Approach to Castes and Reproduction, ed. W. Engels (Berlin: Springer), 167–230. doi: 10.1007/978-3-642-74490-7_9
Esch, H. (1961). Über die Schallerzeugung beim Werbetanz der Honigbiene. Z. Vgl. Physiol. 45, 1–11. doi: 10.1007/bf00297754
Esch, H., Esch, I., and Kerr, W. E. (1965). Sound: an element common to communication of stingless bees and honey bees. Science 149, 320–321. doi: 10.1126/science.149.3681.320
Farina, W. M., Grüter, C., Acosta, L., and Mc Cabe, S. (2007). Honeybees learn floral odors while receiving nectar from foragers within the hive. Naturwissenschaften 94, 55–60. doi: 10.1007/s00114-006-0157-3
Farina, W. M., Grüter, C., and Díaz, P. C. (2005). Social learning of floral odours inside the honeybee hive. Proc. Biol. Sci. 272, 1923–1928. doi: 10.1098/rspb.2005.3172
Ferguson, A. W., and Free, J. B. (1979). Production of a forage-marking pheromone by the honeybee. J. Apic. Res. 18, 128–135. doi: 10.1080/00218839.1979.11099956
Free, J. B., and Williams, I. H. (1983). Foraging behaviour of honeybees and bumble bees on Brussels sprout grown to produce hybrid seed. J. Apic. Res. 22, 94–97. doi: 10.1080/00218839.1983.11100566
Giurfa, M. (1993). The repellent scent-mark of the honeybee Apis mellifera ligustica and its role as communication cue during foraging. Insectes Soc. 40, 59–67. doi: 10.1007/bf01338832
Giurfa, M., Nunez, J., Chittka, L., and Menzel, R. (1995). Colour preferences of flower-naive honeybees. J. Comp. Physiol. A 177, 247–259.
Giurfa, M., and Nunez, J. A. (1992). Honeybees mark with scent and reject recently visited flowers. Oecologia 89, 113–117. doi: 10.1007/BF00319022
Goulson, D., Hawson, S. A., and Stout, J. C. (1998). Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim. Behav. 55, 199–206. doi: 10.1006/anbe.1997.0570
Gumbert, A. (2000). Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 48, 36–43. doi: 10.1007/s002650000213
Han, F., Wallberg, A., and Webster, M. T. (2012). From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2, 1949–1957. doi: 10.1002/ece3.312
Horridge, A. (2007). The preferences of the honeybee (Apis mellifera) for different visual cues during the learning process. J. Insect Physiol. 53, 877–889. doi: 10.1016/j.jinsphys.2006.12.002
Howard, S. R., Shrestha, M., Schramme, J., Garcia, J. E., Avarguès-Weber, A., Greentree, A. D., et al. (2019). Honeybees prefer novel insect-pollinated flower shapes over bird-pollinated flower shapes. Curr. Zool. 65, 457–465. doi: 10.1093/cz/zoy095
Hrncir, M., and Barth, F. G. (2014). “Vibratory communication in stingless bees (Meliponini): the challenge of interpreting the signals,” in Studying Vibrational Communication, eds R. B. Cocroft, M. Gogala, P. S. M. Hill, and A. Wessel (Berlin: Springer), 349–374. doi: 10.1007/978-3-662-43607-3_18
Hrncir, M., Jarau, S., Zucchi, R., and Barth, F. G. (2004). On the origin and properties of scent marks deposited at the food source by a stingless bee, Melipona seminigra. Apidologie 35, 3–13. doi: 10.1051/apido:2003069
Hrncir, M., and Maia-Silva, C. (2013). “On the diversity of foraging-related traits in stingless bees,” in Pot-Honey: A Legacy of Stingless Bees, eds P. Vit, S. R. M. Pedro, and D. Roubik (New York, NY: Springer), 201–215. doi: 10.1007/978-1-4614-4960-7_13
Hrncir, M., Maia-Silva, C., Mc Cabe, S. I., and Farina, W. M. (2011). The recruiter’s excitement – features of thoracic vibrations during the honey bee’s waggle dance related to food source profitability. J. Exp. Biol. 214, 4055–4064. doi: 10.1242/jeb.063149
Hrncir, M., Maia-Silva, C., Teixeira-Souza, V. H. S., and Imperatriz-Fonseca, V. L. (2019). Stingless bees and their adaptations to extreme environments. J. Comp. Physiol. A 205, 415–426. doi: 10.1007/s00359-019-01327-3
Imperatriz-Fonseca, V. L., Koedam, D., and Hrncir, M. (2017). A Abelha Jandaíra no Passado, No Presente e No Futuro, EdUFERSA. Mossóro: Editora Universitária.
Jarau, S. (2009). “Chemical communication during food exploitation in stingless bees,” in Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches, eds S. Jarau and M. Hrncir (Boca Raton, FL: CRC Press), 223–249. doi: 10.1201/9781420075618.ch12
Jarau, S., Hrncir, M., Ayasse, M., Schulz, C., Francke, W., Zucchi, R., et al. (2004). A stingless bee (Melipona seminigra) marks food sources with a pheromone from its claw retractor tendons. J. Chem. Ecol. 30, 793–804. doi: 10.1023/b:joec.0000028432.29759.ed
Jesus, T. N. C. S., Venturieri, G. C., and Contrera, F. A. L. (2014). Time-place learning in the bee Melipona fasciculata (Apidae, Meliponini). Apidologie 45, 257–265. doi: 10.1007/s13592-013-0245-2
Johnson, L. K. (1983). “Foraging strategies and the structure of stingless bee communities in Costa Rica,” in Social Insects in the Tropics 2, ed. P. Jaisson (Paris: Universite Paris-Nord), 31–58.
Kleinert-Giovannini, A. (1982). The influence of climatic factors on flight activity of Plebeia emerina Friese (Hymenoptera, Apidae, Meliponinae) in winter. Rev. Bras. Entomol. 26, 1–13.
Koethe, S., Banysch, S., Alves-dos-Santos, I., and Lunau, K. (2018). Spectral purity, intensity and dominant wavelength: disparate colour preferences of two Brazilian stingless bee species. PLoS One 13:e0204663. doi: 10.1371/journal.pone.0204663
Koethe, S., Bossems, J., Dyer, A. G., and Lunau, K. (2016). Colour is more than hue: preferences for compiled colour traits in the stingless bees Melipona mondury and M. quadrifasciata. J. Comp. Physiol. A 202, 615–627. doi: 10.1007/s00359-016-1115-y
Köthe, S. (2019). Impact of Colour Preferences on the Foraging Behaviour of Tropical Stingless Bees. Doctoral dissertation, Heinrich-Heine-Universität Düsseldorf, Düsseldorf.
Lehrer, M., Horridge, G. A., Zhang, S. W., and Gadagkar, R. (1995). Shape and vision in bees: innate preference for flower-like patterns. Philos. Trans. R. Soc. B 347, 123–137. doi: 10.1098/rstb.1995.0017
Lindauer, M., and Kerr, W. E. (1958). Die gegenseitige Verständigung bei den stachellosen Bienen. Z. Vgl. Physiol. 41, 405–434. doi: 10.1007/bf00344263
Lunau, K. (1990). Colour saturation triggers innate reactions to flower signals: flower dummy experiments with bumblebees. J. Comp. Physiol. A 166, 827–834.
Lunau, K., and Maier, E. J. (1995). Innate colour preferences of flower visitors. J. Comp. Physiol. A 177, 1–19.
Lunau, K., Papiorek, S., Eltz, T., and Sazima, M. (2011). Avoidance of achromatic colours by bees provides a private niche for hummingbirds. J. Exp. Biol. 214, 1607–1612. doi: 10.1242/jeb.052688
Lunau, K., Wacht, S., and Chittka, L. (1996). Colour choices of naive bumble bees and their implications for colour perception. J. Comp. Physiol. A 178, 477–489.
Machado, I. C., and Lopes, A. V. (2004). Floral traits and pollination systems in the Caatinga, a Brazilian tropical dry forest. Ann. Bot. 94, 365–376. doi: 10.1093/aob/mch152
Maia-Silva, C., Da Silva, C. I., Hrncir, M., de Queiroz, R. T., and Imperatriz-Fonseca, V. L. (2012). Guia de Plantas Visitadas por Abelhas na Caatinga. Fortaleza: Editora Fundação Brasil Cidadão.
Maia-Silva, C., Hrncir, M., Da Silva, C. I., and Imperatriz-Fonseca, V. L. (2015). Survival strategies of stingless bees (Melipona subnitida) in an unpredictable environment, the Brazilian tropical dry forest. Apidologie 46, 631–643. doi: 10.1007/s13592-015-0354-1
Mc Cabe, S. I., and Farina, W. M. (2009). Odor information transfer in the stingless bee Melipona quadrifasciata: effect of in-hive experiences on classical conditioning of proboscis extension. J. Comp. Physiol. A 195, 113–122. doi: 10.1007/s00359-008-0391-6
Mc Cabe, S. I., Hartfelder, K., Santana, W. C., and Farina, W. M. (2007). Odor discrimination in classical conditioning of proboscis extension in two stingless bee species in comparison to Africanized honeybees. J. Comp. Physiol. A 193, 1089–1099. doi: 10.1007/s00359-007-0260-8
Menzel, R. (1967). Untersuchungen zum erlernen von spektralfarben durch die Honigbiene (Apis mellifica). Z. Vgl. Physiol. 56, 22–62. doi: 10.1007/bf00333562
Menzel, R., Greggers, U., Smith, A., Berger, S., Brandt, R., Brunke, S., et al. (2005). Honey bees navigate according to a map-like spatial memory. Proc. Natl. Acad. Sci. U.S.A. 102, 3040–3045. doi: 10.1073/pnas.0408550102
Michelsen, A. (2014). “Mechanical signals in honeybee communication,” in Studying Vibrational Communication. Animal Signals and Communication, Vol. 3, eds R. Cocroft, M. Gogala, P. Hill, and A. Wessel (Berlin: Springer), 333–347. doi: 10.1007/978-3-662-43607-3_17
Michener, C. D. (2013). “The Meliponini,” in Pot-Honey: A Legacy of Stingless Bees, eds P. Vit, S. R. M. Pedro, and D. Roubik (New York, NY: Springer), 3–17. doi: 10.1007/978-1-4614-4960-7_1
Morawetz, L., Svoboda, A., Spaethe, J., and Dyer, A. G. (2013). Blue colour preference in honeybees distracts visual attention for learning closed shapes. J. Comp. Physiol. A 199, 817–827. doi: 10.1007/s00359-013-0843-5
Nieh, J. C., Contrera, F. A., Ramírez, S., and Imperatriz-Fonseca, V. L. (2003). Variation in the ability to communicate three-dimensional resource location by stingless bees from different habitats. Anim. Behav. 66, 1129–1139. doi: 10.1006/anbe.2003.2289
Nieh, J. C., Contrera, F. A. L., Yoon, R. R., Barreto, L. S., and Imperatriz-Fonseca, V. L. (2004). Polarized short odor-trail recruitment communication by a stingless bee, Trigona spinipes. Behav. Ecol. Sociobiol. 56, 435–448.
Nunez, J. A. (1967). Sammelbienen markieren versiegende Futterquellen durch Duft. Naturwissenschaften 54, 322–323. doi: 10.1007/bf00640625
Papiorek, S., Rohde, K., and Lunau, K. (2013). Bees’ subtle colour preferences: how bees respond to small changes in pigment concentration. Naturwissenschaften 100, 633–643. doi: 10.1007/s00114-013-1060-3
Pessotti, I., and Lé’Sénéchal, A. M. (1981). Aprendizagem em abelhas. I. Discriminacao simples em onze especies. Acta Amazon 11, 653–658. doi: 10.1590/1809-43921981113653
Prado, D. (2003). “As caatingas da América do Sul,” in Ecologia e Conservação da Caatinga, eds I. R. Leal, M. Tabarelli, and J. M. C. Silva (Recife: Editora Universitária UFPE), 75–134.
R Development Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raine, N. E., and Chittka, L. (2007). The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. PLoS One 2:e556. doi: 10.1371/journal.pone.0000556
Ramalho, M. (2004). Stingless bees and mass flowering trees in the canopy of Atlantic Forest: a tight relationship. Acta Bot. Brasilica 18, 37–47. doi: 10.1590/s0102-33062004000100005
Reinhard, J., Srinivasan, M. V., Guez, D., and Zhang, S. W. (2004). Floral scents induce recall of navigational and visual memories in honeybees. J. Exp. Biol. 207, 4371–4381. doi: 10.1242/jeb.01306
Reinhard, J., Srinivasan, M. V., and Zhang, S. W. (2006). Complex memories in honeybees: can there be more than two? J. Comp. Physiol. A 192, 409–416. doi: 10.1007/s00359-005-0079-0
Reser, D. H., Wijesekara Witharanage, R., Rosa, M. G. P., and Dyer, A. G. (2012). Honeybees (Apis mellifera) learn color discriminations via differential conditioning independent of long wavelength (green) photoreceptor modulation. PLoS One 7:e48577. doi: 10.1371/journal.pone.0048577
Rohde, K., Papiorek, S., and Lunau, K. (2013). Bumblebees (Bombus terrestris) and honeybees (Apis mellifera) prefer similar colours of higher spectral purity over trained colours. J. Comp. Physiol. A 199, 197–210. doi: 10.1007/s00359-012-0783-5
Roldão-Sbordoni, Y. S., Nascimento, F. S., and Mateus, S. (2018). Estimating colonies of Plebeia droryana (Friese, 1900) (Hymenoptera, Apidae, Meliponini): adults, brood and nest structure. Sociobiology 65, 280–284.
Roselino, A. C., Rodrigues, A. V., and Hrncir, M. (2016). Stingless bees (Melipona scutellaris) learn to associate footprint cues at food sources with a specific reward context. J. Comp. Physiol. A 202, 657–666. doi: 10.1007/s00359-016-1104-1
Roubik, D. W. (1982a). Seasonality in colony food storage, brood production and adult survivorship: studies of Melipona in tropical forest (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 55, 789–800.
Roubik, D. W. (1982b). The ecological impact of nectar-robbing bees and pollinating hummingbirds on a Tropical Shrub. Ecology 63, 354–360. doi: 10.2307/1938953
Roubik, D. W. (2006). Stingless bee nesting biology. Apidologie 37, 124–143. doi: 10.1051/apido:2006026
Roubik, D. W., and Buchmann, S. L. (1984). Nectar selection by Melipona and Apis mellifera (Hymenoptera: Apidae) and the ecology of nectar intake by bee colonies in a tropical forest. Oecologia 61, 1–10. doi: 10.1007/BF00379082
Saleh, N., and Chittka, L. (2006). The importance of experience in the interpretation of conspecific chemical signals. Behav. Ecol. Sociobiol. 61, 215–220. doi: 10.1007/s00265-006-0252-7
Sánchez, D., Nieh, J. C., and Vandame, R. (2008). Experience-based interpretation of visual and chemical information at food sources in the stingless bee Scaptotrigona mexicana. Anim. Behav. 76, 407–414. doi: 10.1016/j.anbehav.2008.04.003
Schmidt, V. M., Zucchi, R., and Barth, F. G. (2003). A stingless bee marks the feeding site in addition to the scent path (Scaptotrigona depilis). Apidologie 32, 237–248. doi: 10.1051/apido:2003021
Schmidt, V. M., Zucchi, R., and Barth, F. G. (2005). Scent marks left by Nannotrigona testaceicornis at the feeding site: cues rather than signals. Apidologie 36, 285–291. doi: 10.1051/apido:2005002
Schwarz, H. F. (1932). The genus Melipona. The type genus of the Meliponidae or stingless bees. Bull. Am. Mus. Nat. Hist. 63, 231–460.
Seeley, T. D. (1985). Honeybee Ecology: A Study of Adaptation in Social Life. Princeton, NJ: Princeton University Press.
Slaa, E. J., and Hughes, W. H. O. (2009). “Local enhancement, local inhibition, eavesdropping, and the parasitism of social insect communication,” in Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches, eds S. Jarau and M. Hrncir (Boca Raton, FL: CRC Press), 147–164. doi: 10.1201/9781420075618.ch8
Slaa, J. E., Cevaal, A., and Sommeijer, M. J. (1998). Floral constancy in Trigona stingless bees foraging on artificial flower patches: a comparative study. J. Apic. Res. 37, 191–198. doi: 10.1080/00218839.1998.11100971
Slaa, J. E., Wassenberg, J., and Biesmeijer, J. C. (2003). The use of field-based social information on eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 28, 369–379. doi: 10.1046/j.1365-2311.2003.00512.x
Spaethe, J., Streinzer, M., Eckert, J., May, S., and Dyer, A. G. (2014). Behavioural evidence of colour vision in free flying stingless bees. J. Comp. Physiol. A 200, 485–496. doi: 10.1007/s00359-014-0886-2
Stout, J. C., and Goulson, D. (2001). The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim. Behav. 62, 183–189. doi: 10.1006/anbe.2001.1729
Stout, J. C., Goulson, D., and Allen, J. A. (1998). Repellent scent-marking of flowers by a guild of foraging bumblebees (Bombus spp.). Behav. Ecol. Sociobiol. 43, 317–326. doi: 10.1007/s002650050497
Streinzer, M., Huber, W., and Spaethe, J. (2016). Body size limits dim-light foraging activity in stingless bees (Apidae: Meliponini). J. Comp. Physiol. A 202, 643–655. doi: 10.1007/s00359-016-1118-8
Von Frisch, K. (1967). The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press.
Waddington, K. D., and Kirchner, W. H. (1992). Acoustical and behavioral correlates of profitability of food sources in honey bee round dances. Ethology 92, 1–6. doi: 10.1111/j.1439-0310.1992.tb00945.x
Williams, C. S. (1998). The identity of the previous visitor influences flower rejection by nectar collecting bees. Anim. Behav. 56, 673–681. doi: 10.1006/anbe.1998.0794
Witjes, S., Witsch, K., and Eltz, T. (2011). Reconstructing the pollinator community and predicting seed set from hydrocarbon footprints on flowers. Oecologia 165, 1017–1029. doi: 10.1007/s00442-010-1816-9
Yokoi, T., Goulson, D., and Fujisaki, K. (2007). The use of heterospecific scent marks by the sweat bee Halictus aerarius. Naturwissenschaften 94, 1021–1024. doi: 10.1007/s00114-007-0285-4
Zanella, F. C. (2000). The bees of the Caatinga (Hymenoptera, Apoidea, Apiformes): a species list and comparative notes regarding their distribution. Apidologie 31, 579–592. doi: 10.1051/apido:2000148
Keywords: eusocial bees, chemical cues, color cues, location-dependent cues, foraging behavior
Citation: Koethe S, Fischbach V, Banysch S, Reinartz L, Hrncir M and Lunau K (2020) A Comparative Study of Food Source Selection in Stingless Bees and Honeybees: Scent Marks, Location, or Color. Front. Plant Sci. 11:516. doi: 10.3389/fpls.2020.00516
Received: 05 February 2020; Accepted: 06 April 2020;
Published: 06 May 2020.
Edited by:
Eduardo Narbona, Universidad Pablo de Olavide, SpainReviewed by:
Islam S. Sobhy, Keele University, United KingdomCopyright © 2020 Koethe, Fischbach, Banysch, Reinartz, Hrncir and Lunau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Klaus Lunau, S2xhdXMuTHVuYXVAaGh1LmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.