
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 17 March 2020
Sec. Plant Systematics and Evolution
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00263
This article is part of the Research Topic Origins and Domestication of the Grape View all 8 articles
 Jun Wen1*
Jun Wen1* Sterling A. Herron2
Sterling A. Herron2 Xue Yang3
Xue Yang3 Bin-Bin Liu1,4
Bin-Bin Liu1,4 Yun-Juan Zuo5
Yun-Juan Zuo5 AJ Harris6,7
AJ Harris6,7 Yash Kalburgi1
Yash Kalburgi1 Gabriel Johnson1
Gabriel Johnson1 Elizabeth A. Zimmer1
Elizabeth A. Zimmer1Despite the commercial importance of the Concord grape, its origin has remained unresolved for over 150 years without a comprehensive phylogenetic analysis. In this study we aimed to reconstruct the evolutionary history of the Concord grape using sequence data from four nuclear markers (AT103, GAI1, PHYA, and SQD1), six plastid markers (matK, psbA-trnH, petN-trnC, ycf1, trnL-F, and trnS-G), and the plastid genome. We sampled extensively the Vitis species native to northeastern North America as well as representative species from Europe and Asia, including the commercially important Vitis vinifera (wine grape), a native European species with hermaphroditic flowers, and its wild progenitor, V. vinifera subsp. sylvestris. We also sequenced the plastid genome of one accession of the Concord grape and compared the plastid genome data to the recently published data set of Vitis plastomes. Phylogenetic analyses of the plastid and nuclear data using maximum likelihood and Bayesian inference support the hybrid origin of the Concord grape. The results clearly pinpoint the wine grape, V. vinifera, as the maternal donor and the fox grape, Vitis labrusca, which is common in northeastern North America, as the paternal donor. Moreover, we infer that the breeding history of the Concord grape must have involved the backcrossing of the F1 hybrid with the paternal parent V. labrusca. This backcrossing also explains the higher morphological similarity of the Concord grape to V. labrusca than to V. vinifera. This study provides concrete genetic evidence for the hybrid origin of a widespread Vitis cultivar and is, therefore, promising for similar future studies focused on resolving ambiguous origins of major crops or to create successful hybrid fruit crops.
The Concord grape is an economically important cultivar in the United States and Canada as a source of juice, jelly, jam, table grape, candy, and sweet wine, as well as a popular garden plant. It is a hardy and productive vine that bears hermaphroditic flowers and large blue-black berries. However, the origin of the Concord grape has been ambiguous since its introduction by Ephraim Wales Bull in the town of Concord, MA, United States in the 1840’s. Bull labored for 6 years on his grape plants in order to develop the perfect, cold-hardy crop with hermaphroditic flowers (Schofield, 1988). Many early attempts to grow European Vitis vinifera cultivars failed in North America due to diseases and pests such as phylloxera as well as climatic reasons (Gerrath et al., 2015). The introduction of the Concord grape, which was well adapted to conditions in the eastern United States, was revolutionary to grape cultivation (Miller, 1954). Many workers believe that the Concord grape was derived from selection from the native local fox grape Vitis labrusca that Bull planted from seeds (Munson, 1909; Galet, 1979; Schofield, 1988). Schofield (1988) cited that Bull told Liberty Hyde Bailey decades later that “boys brought up from the Concord River some wild grapes and scattered them about the place.” Bull is then reported to have used the seeds of these grapes to produce the Concord variety (Schofield, 1988). However, a pure selection scenario may be less likely considering the relatively short time frame in which the Concord grape was developed. Others argue that it is a hybrid of two or more grape species (Munson, 1909; Bailey, 1934). Munson (1909) hypothesized that the Concord grape was primarily derived from V. labrusca L. but might contain a trace of V. vulpina L. (=Vitis riparia Michx. sensu Moore and Wen, 2016, not sensu Munson as V. riparia was misidentified as V. vulpina by Munson). V. riparia is a common native species near Concord and throughout the northern part of North America. However, the wine grape V. vinifera L. was also regarded as a potential parent due to the Concord grape having hermaphroditic flowers, a trait only found in V. vinifera (Munson, 1909; Schofield, 1988), even though the Concord grape is highly similar to the fox grape V. labrusca in many other morphological characters.
The grape genus, Vitis L., is one of 16 genera in the grape family Vitaceae (Wen, 2007; Wen et al., 2018b). Vitis includes about 70 species primarily distributed in Asia, and North to Central America, and has one species in Europe (Chen et al., 2007; Wen, 2007; Moore and Wen, 2016; Wen et al., 2018a). Vitis has been classified into two subgenera: subgenus Vitis (c. 68 spp.) and subgenus Muscadinia (Planch.) Rehder (2 spp.) (Brizicky, 1965; Moore, 1991; Wen, 2007; Wen et al., 2018b). The chromosome number for Vitis subgenus Vitis is 2n = 38, with no polyploidy having been reported for the genus (Wen, 2007). Several recent studies have shed new light on the phylogeny of Vitis (Aradhya et al., 2008, 2013; Tröndle et al., 2010; Péros et al., 2011; Zecca et al., 2012; Miller et al., 2013; Wan et al., 2013; Liu et al., 2016; Klein et al., 2018; Ma et al., 2018a, b; Wen et al., 2018a). Notably, there is incongruence among studies concerning the monophyly of the taxa of Vitis subgenus Vitis in North America (cf. Wan et al., 2013; Liu et al., 2016; Ma et al., 2018a; Wen et al., 2018a). Nevertheless, several studies have resolved Vitis subgenus Vitis into two main clades corresponding to their geographic distributional areas in Eurasia and North America (e.g., Zecca et al., 2012; Klein et al., 2018; Ma et al., 2018b; Wen et al., 2018a).
The goal of this study was to clarify the genetic donor(s) of the Concord grape using a broad sampling scheme of the putative relatives from North America, especially from northeastern North America. To achieve our goal, we performed phylogenetic analyses on plastid (matK, trnL-F, petN-trnC, trnH-psbA, trnS-G, and ycf1) and nuclear (GAI1, AT103, PHYA, and SQD1) sequence data from the sampled Vitis species. The markers were selected based on our success in using them in prior phylogenetic studies of plant species (Ren et al., 2011; Zimmer and Wen, 2012; Zhao et al., 2016, 2018; Hearn et al., 2018; Lu et al., 2018). Even though GAI1 (the grapevine derived GA INSENSITIVE or GAI-like gene), the phytochrome genes (e.g., PHYA), and the sulfoquinovosyldiacylglycerol 1 (or SQD1) gene may have significant functions (Li et al., 2008; Zimmer and Wen, 2012), our purpose of utilizing these markers in this study is as nuclear phylogenetic markers as discussed in detail in Zimmer and Wen (2012). We also sequenced the plastid genome of one accession of the Concord grape and analyzed it alongside the recently published data set of plastomes of Vitis (Wen et al., 2018a). Our work has implications not only for understanding the cultivation history of this species, but also for future breeding efforts of grape cultivars.
To achieve a comprehensive sampling of the species which Mr. Bull may have selected for his breeding of the Concord grape, we collected specimens of Vitis from North America emphasizing the northeastern United States, including the following species: Vitis acerifolia Raf., V. aestivalis Michx., V. arizonica Engelm., V. cinerea (Engelm.) Millardet [var. baileyana (Munson) Comeaux, var. cinerea and var. floridana Munson], V. labrusca L., V. mustangensis Buckley, V. riparia Michx., V. rotundifolia Michx., V. vulpina L. and the cultivated wine grape V. vinifera L. (Moore and Wen, 2016). Five cultivars of V. vinifera were included: Pinot Noir, Reichensteiner, Riesling, Syrian, and Thompson Seedless, to represent the gene pool of the wine grape as a potential parental species, not necessarily to genotype the exact cultivar that Mr. Bull might have used to breed the Concord grape. The original Concord grape V. labruscana L. H. Bailey grown by Ephraim Bull was sampled (Wen 12570) from the Grape Cottage in Concord, Massachusetts, as well as a cutting from the original Concord grape growing at Welch’s Foods Inc., in Concord, MA (Wen 12568). Furthermore, we included representative taxa of Vitis from Eurasia, V. flexuosa Thunb. (var. flexuosa and var. parvifolia (Roxb.) Gagnep.), V. lanata Roxb. ex Wall., V. thunbergii Siebold and Zucc. and V. vinifera subsp. sylvestris (Gmelin) Hegi (Supplementary Table S1). The overall sampling was designed to include all potential species of Vitis that Mr. Bull may have had access to in Concord, Massachusetts at that time. The most likely species that he may have possessed were the native species of Vitis from northeastern North America and the various cultivars of V. vinifera. Therefore, we included five cultivars of the wine grape, and all native species from northeastern North America, as well as a few other Eurasian taxa sampled to ensure that the phylogenetic diversity of the genus was also represented.
We also compared the plastid genome sequence of the Concord grape with a large published data set of North American Vitis plastid genome sequences with two accessions from Europe and West Asia (V. vinifera ssp. vinifera and V. vinifera ssp. sylvestris), and nine representative species from eastern Asia to cover the morphological diversity of subgenus Vitis (Wen et al., 2018a).
We selected ten DNA markers for resolving phylogenetic relationships and parentage in this study: six plastid (matK, psbA-trnH, petN-trnC, ycf1, trnL-F, and trnS-G) and four nuclear (AT103, SQD1, PHYA, and GAI1). Throughout, we follow the accepted nomenclature for these genes according to UniProt1 (The UniProt Consortium., 2017). AT103 (or CRD1) is involved in chlorophyll biosynthesis (e.g., Gene Ontogeny term, GO:0015995; Bang et al., 2008), while SQD1 has a role in synthesis of thylakoid membrane structures (e.g., GO:0046507; Sanda et al., 2001), and PHYA is a well-known member of the phytochrome family involved in photoperiodism and other photo-regulated pathways (e.g., GO:0010161, GO:0031516; Kim et al., 2002; Yang et al., 2009). GAI1 is known especially from Vitis and its close relatives, but is likley a transcription factor in the gibberellin (GA) signaling pathway related to RGA in Arabidopsis thaliana (L.) Heynh. (e.g., GO:0009740; Silverstone et al., 1998) according to a protein BLAST (Altschul et al., 1990) search using Uniprot accession Q8S4W7 (V. vinifera L.) performed on the NCBI webserver (Madden et al., 1996). Each of these genes are essential for plant growth and almost assuredly play roles in responses to environmental stimuli that merit investigation outside of the context of this molecular phylogenetic study, in which we use them to resolve evolutionary relationships. For the purposes here, it is noteworthy that all four nuclear genes are ubiquitous in vascular plants and yield phylogenies believed to be consistent with the vascular plant tree of life (Vandenbussche et al., 2007; Mathews, 2010; Chen et al., 2017).
We extracted DNA from leaf tissue samples, dried in silica gel, using the DNeasy Plant Mini Kit (QIAGEN, Valencia, CA, United States) following a modification of the manufacturer’s protocol. For each sample, six separate lysate solutions were prepared and processed through a single QIAShredder column and DNeasy column. We amplied the ten selected markers using standard polymerase chain reactions (PCR). Primer information for the six plastid markers can be found in Taberlet et al. (1991), Soejima and Wen (2006), Ren et al. (2011), and Lu et al. (2013, 2018); and that of the nuclear markers can be located in Wen et al. (2007), Li et al. (2008), Nie et al. (2012), Liu et al. (2016), and Lu et al. (2018). The PCRs were carried out in 25 μL that contained 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.4 mM of each primer, 1.0 U of Taq polymerase (Bioline, Aberdeen, United Kingdom), and 10–50 ng (2.5 μL) template DNAs. The amplification reactions for all ten genes were run with the following PCR program: (1) a denaturation step at 94°C for 5 min, (2) 35 cycles with a denaturing step at 94°C for 45 s, an annealing step at 50°C for 45 s, and an extension step at 72°C for 90 s, and (3) a final extension at 72°C for 10 min, using a BioRad T100 thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, United States). The PCR products were purified using the ExoSAP-IT enzyme (cat. #78201, USB Corporation, Cleveland, OH, United States) based on the manufacturer’s protocol. Sequencing primers were the same as amplification primers, and fluorescently labeled Sanger fragments were generated using BigDyeTM Terminator v3.1 cycle sequencing kit (cat. #4337455, Thermo Fisher Scientific, Inc., Waltham, MA, United States) at 1/4 of the manufacturer’s suggested concentration. The resulting products were read on an ABI 3730xl automated capillary sequencer (Applied Biosystems, Foster City, CA, United States), following the manufacturer’s protocols, at the Laboratories of Analytical Biology at the National Museum of Natural History, the Smithsonian Institution (Washington, DC, United States).
We sequenced the plastid genome for one accession of the Concord grape (Wen 12529) using the genome skimming approach (Zhang et al., 2015). The genomic library was constructed with the NEBNext Ultra II library prep kit for Illumina (New England Biolabs, Ipswich, MA, United States). Paired-end reads (2 × 150 bp) were produced using an Illumina NextSeq 500 Sequencing System at the Genomic Sequencing and Analysis Facility (GSAF) at the University of Texas, Austin. The raw reads were filtered and trimmed to remove adapters and lower quality bases at the end using Trimmomatic version 0.32 (Bolger et al., 2014) with default settings. The trimmed paired-end reads were used to assemble the plastid genome with NOVOPlasty 3.2 (Dierckxsens et al., 2016). The plastid genome sequence of the Concord grape was then analyzed phylogenetically with sequences of Vitis from Wen et al. (2018b).
We performed multiple sequence alignments of the datasets in MAFFT (Katoh et al., 2002; Katoh and Toh, 2008), followed by manual adjustment within Geneious 10.2.4 (Kearse et al., 2012).
The best fit partitioning schemes and nucleotide substitution models for the data sets (whole plastome, combined plastid regions, AT103, GAI1, PHYA, SQD1, and combined nuclear sequences) were estimated using PartitionFinder2 (Lanfear et al., 2016). Under the corrected Akaike information criterion (AICc) and linked branch lengths, PartitionFinder2 was performed with the greedy (Lanfear et al., 2012) and rcluster (Lanfear et al., 2014) algorithm options for these three datasets, with prior defined data blocks by codon positions of each protein-coding gene and all models. The partitioning schemes and evolutionary model for each subset were used for the downstream maximum likelihood (ML, Stamatakis, 2006, 2014) and Bayesian Inference (BI, Rannala and Yang, 1996; Mau et al., 1999) analyses. The ML trees were inferred by IQ-TREE v.1.6.9 (Nguyen et al., 2015) with 1000 bootstrap replicates using UFBoot2 (Hoang et al., 2017) and the collapsing near zero branches option. The BI was performed with MrBayes 3.2.7 (Ronquist et al., 2012). The Markov chain Monte Carlo (MCMC) analyses were run for 10,000,000 generations. Trees were sampled at every 1,000 generations with the first 25% discarded as burn-in. The remaining trees were used to build a 50% majority-rule consensus tree. The stationarity was considered to be reached when the average standard deviation of split frequencies remained below 0.01. The ML and BI trees were visualized with Tree View using Geneious Prime (Kearse et al., 2012).
The trees from the combined Sanger plastid data placed the samples of the Concord grapes nested within the Eurasian V. vinifera clade (Figure 1). The clade of Concord grapes plus the Eurasian V. vinifera (both subsp. vinifera and subsp. sylvestris) had bootstrap support of 63% and a Bayesian posterior probability of 1.00 (Figure 1). The maximum likelihood analysis and Bayesian inference of the plastid genome data of Vitis generated an identical topology, which placed the Concord grape (Wen 12529, chloroplast genome GenBank accession number MN577933) as nested within V. vinifera (sister to V. vinifera subsp. sylvestris) (Figure 2). Of great interest, there are several significant insertions and deletions in the plastid DNA, such as a 5-bp insertion in the trnL-F region and a 54-bp deletion in the petN-trnC intergenic spacer that were only shared between the Concord grape and V. vinifera.
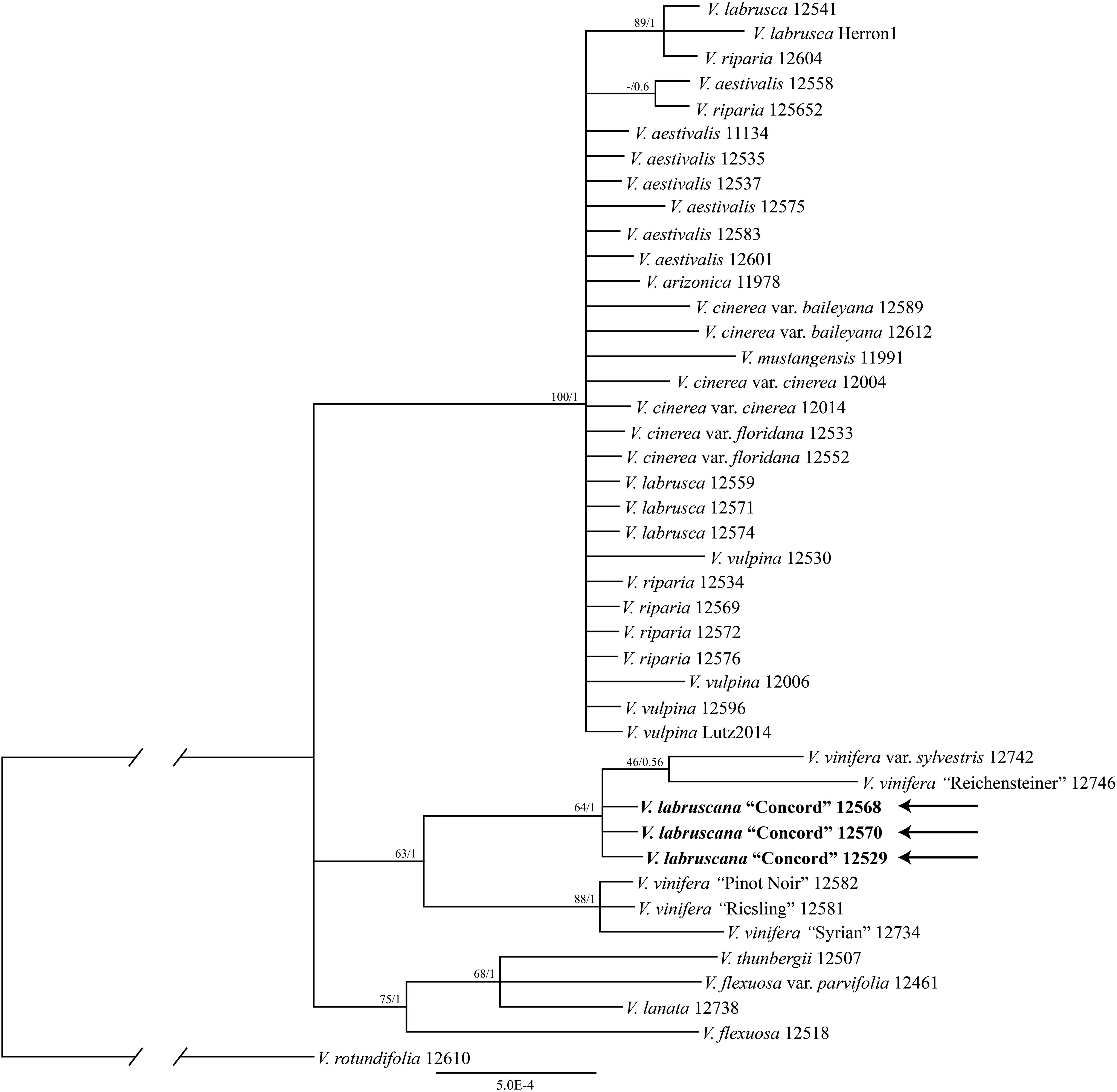
Figure 1. Phylogenetic relationships of the Concord grape and its close allies in Vitis using combined chloroplast DNA marker sequences and Bayesian inference. Numbers associated with the branches are ML bootstrap support and Bayesian posterior probability values.
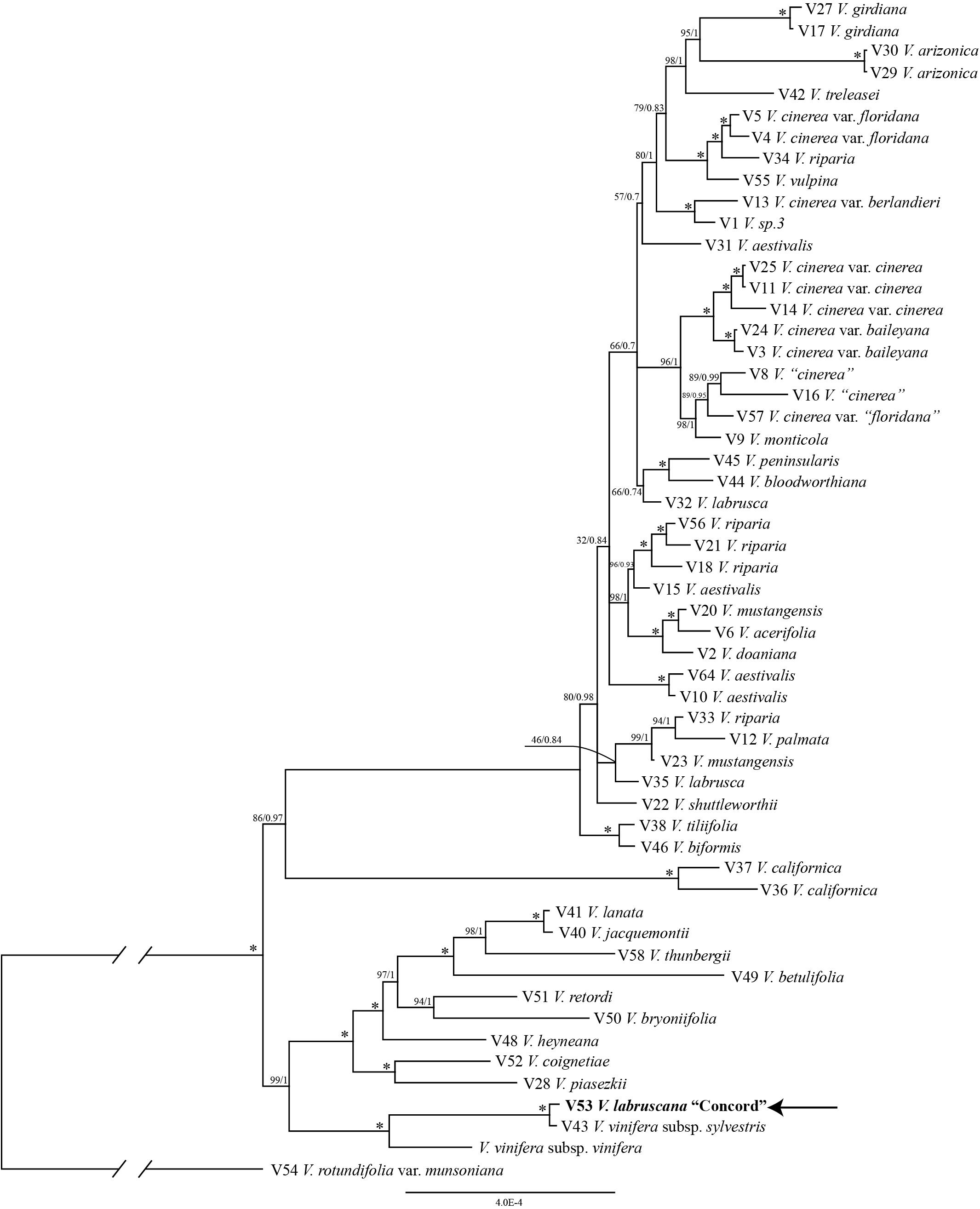
Figure 2. Phylogenetic relationships of the Concord grape and its close allies in Vitis using complete chloroplast DNA genome sequences and Bayesian inference. Numbers associated with the branches are ML bootstrap support and Bayesian posterior probability values, and asterisks (∗) indicate bootstrap support/posterior probability of 100/1.00.
Each of the nuclear gene regions had very few informative sites. Separate analyses of the four nuclear gene regions did not provide much phylogenetic resolution (Supplementary Figures S1–S4) except that the SQD1 tree supported a clade of V. labrusca and the Concord grape (Supplementary Figure S1). Furthermore, the AT103 data showed that the Concord grape had two recombinational sites between V. labrusca and V. vinifera, supporting the hybrid status of the Concord grape.
The tree of the combined nuclear data (AT103, GAI1, PHYA, and SQD1) strongly supported that the Concord grape samples formed a clade with multiple samples of the fox grape V. labrusca from eastern North America (bootstrap support 98%, PP 1.00; Figure 3).
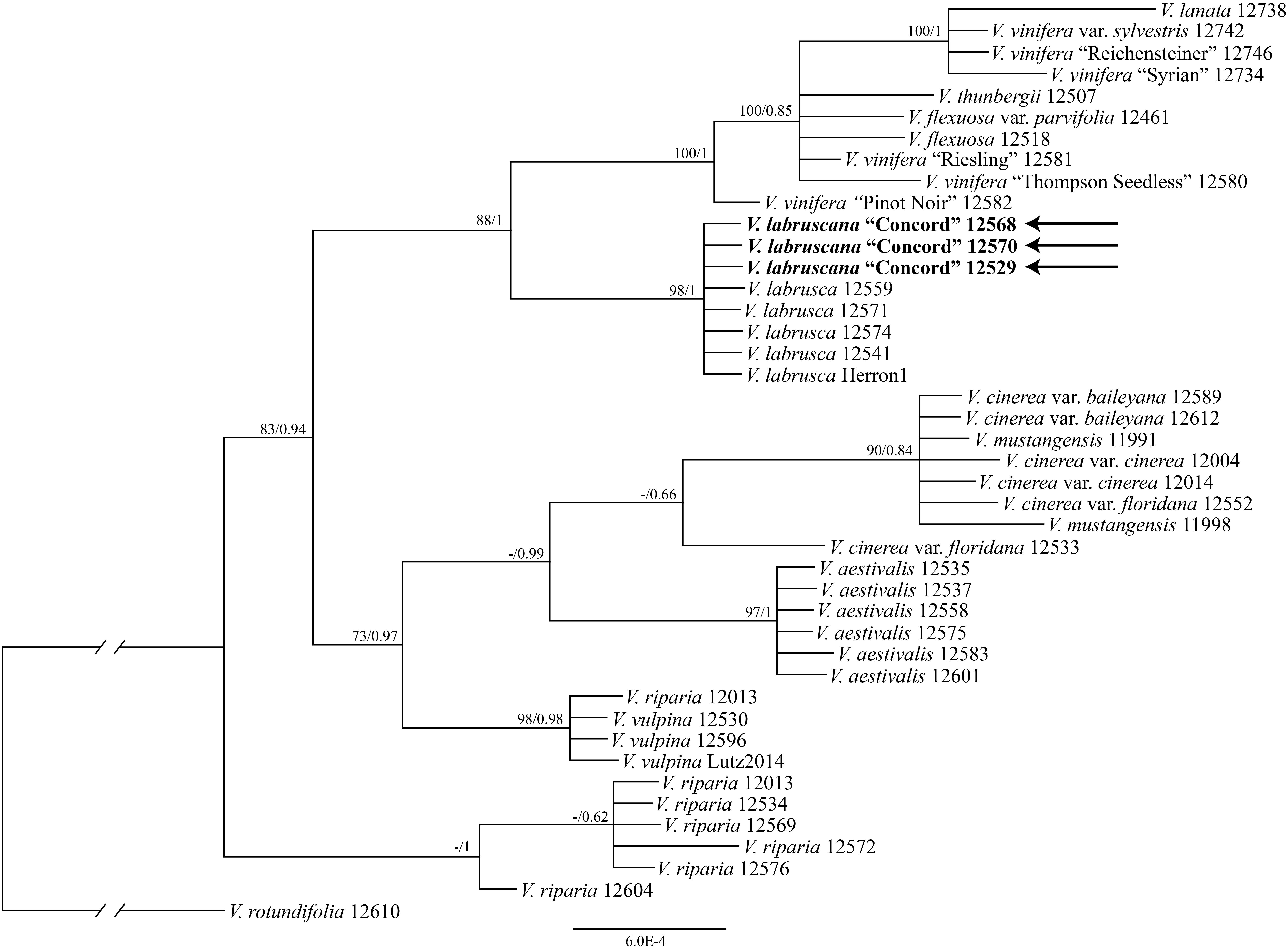
Figure 3. Phylogenetic relationships of the Concord grape and its close allies using combined nuclear sequences and Bayesian inference. Numbers associated with the branches are ML bootstrap support and Bayesian posterior probability values.
The plastid and nuclear trees clearly showed topological incongruence concerning the position of the Concord grape (cf. Figures 1–3). Furthermore, all samples of V. riparia formed a clade in the nuclear tree, but they did not constitute a monophyletic group in the plastid tree (Figure 2). A similar pattern of discordance is observed in V. aestivalis, although our sampling covered only V. aestivalis var. aestivalis in this study. A pattern of nuclear monophyly and plastid non-monophyly is also seen in V. vulpina (Figures 2, 3). Vitis cinerea showed a complex pattern such that the three varieties, var. baileyana, var. cinerea and var. floridana, did not form a clade.
The plastid results (the combined 6-marker data as well as the complete plastid genome data; Figures 1, 2) show that the Concord grape forms a clade with the Eurasian V. vinifera. As plastid DNA is maternally inherited, the close alliance of the Concord grape accessions with V. vinifera (including two subspecies) suggests that V. vinifera was the maternal parent from which the Concord grape was derived. With their shared hermaphroditic flowers, a relationship between the Concord grape and V. vinifera had long been suspected (Munson, 1909; Schofield, 1988). Within Vitis, hermaphroditic flowers are only predominantly found in V. vinifera ssp. vinifera (Chen et al., 2007; Moore and Wen, 2016; Gerrath et al., 2017). Nevertheless, it has also been proposed that the Concord grape may have been developed from the fox grape V. labrusca alone by repeated rounds of selection (Galet, 1979; Gerrath et al., 2015). The chloroplast topology clearly refutes the selection hypothesis, and instead shows the genetic relationship of the Concord grape with the wine grape V. vinifera. The Concord grape is best interpreted as a hybrid that involved V. vinifera as the maternal parent.
The strong similarity between the nuclear sequences of the Concord grape and the fox grape V. labrusca suggests the latter as the paternal parent of the Concord grape. The distinct nuclear sequence similarities between V. labrusca and the Concord grape, as well as their morphological similarities, indicate that the F1 hybrid was backcrossed with V. labrusca in the development of the Concord grape by Bull (Figure 4). Morphologically the Concord grape possesses continuous tendrils and a whitish to rusty tomentum on the adaxial surface of the leaf blade, similar to the fox grape V. labrusca.
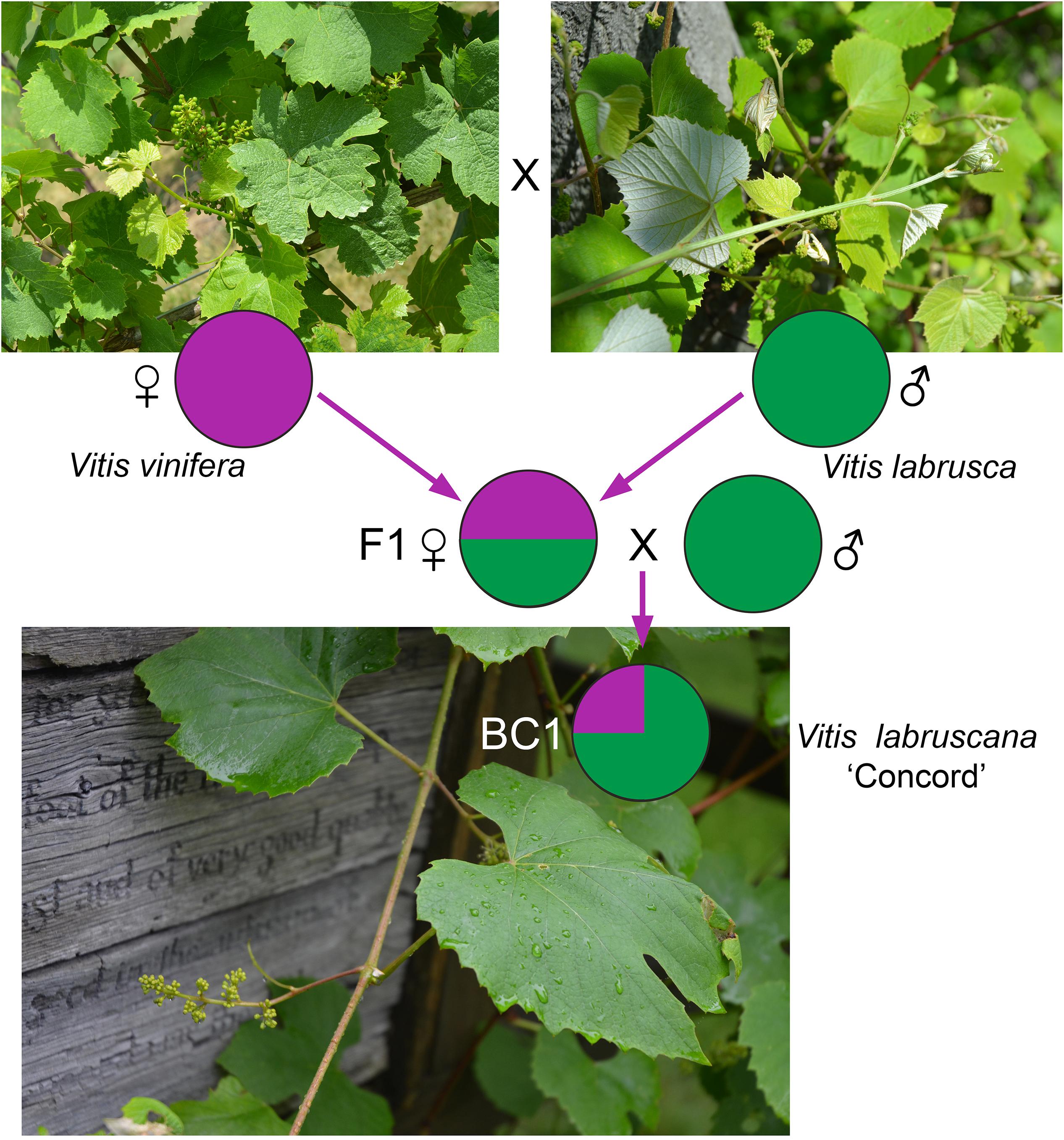
Figure 4. A model for the Concord grape origin based on chloroplast and nuclear DNA evidence. BC1 represents the backcrossed progeny of the F1 hybrid toward one of the parental species, Vitis labrusca.
The comparative genomics study by Sawler et al. (2013), using SNP data, reported that the Concord grape contains c. 30% of the V. vinifera genome. Based on this evidence, the backcrossing likely occurred just once (Figure 4); in this scenario, the Concord grape would contain c. 75% of the V. labrusca nuclear genome and c. 25% of the V. vinifera nuclear genome. V. labruscana L. H. Bailey has been commonly used to designate American grape cultivars that have a V. labrusca parentage (Bailey, 1934; Moore and Wen, 2016).
Ephraim Wales Bull passed away in 1895 without profiting financially from the great Concord grape that he cleverly created. The epitaph on his tombstone reads: “He Sowed, Others Reaped” (Schofield, 1988). We hope our deciphering of the enigmatic origin of the Concord grape will help bring due honor to such an ingenious plant breeder for his labor and legendary contribution to the American grape culture!
It is worth noting that our plastid and nuclear DNA trees for Vitis showed some topological discordances (c.f. Figures 1–3). Of particular interests, a pattern of nuclear monophyly and cpDNA non-monophyly was seen in V. aestivalis, V. riparia and V. vulpina (Figures 2, 3). Vitis cinerea seems to present a complex pattern both in the nuclear and plastid DNA trees. Currently five varieties are recognized within V. cinerea (Moore and Wen, 2016), and our sampling included only var. baileyana, var. cinerea and var. floridana. The three varieties did not form a clade, and their taxonomic status needs to be reassessed [also see section “Discussion” in Wen et al. (2018b)].
Many mechanisms may contribute to topological incongruence, especially lineage sorting, hybridization, and introgression (Soltis and Kuzoff, 1995; Hipp et al., 2004; Yi et al., 2015). Hybridization among North American Vitis species has long been discussed (Bailey, 1897, 1934; Munson, 1909; Comeaux et al., 1987; Moore, 1991; Aradhya et al., 2013; Wan et al., 2013; Moore and Wen, 2016; Wen et al., 2018a); and introgression has recently been proposed as an important driver for North American Vitis diversification (Nie et al., 2019). Thus, our preliminary data on the incongruence between the nuclear and plastid markers are consistent with the hypothesis of extensive reticulate evolution in North American Vitis. The nuclear gene tree (Figure 3) suggests that widespread species such as V. aestivalis, V. riparia and V. vulpina may have served as pollen donors in multiple hybridization events within Vitis (cf. Figures 1–3). Much work remains to be done concerning the patterns of hybridization and introgression and their potential impact on North American Vitis taxonomy, conservation and utilization (Moore and Wen, 2016; Wen et al., 2018a). We will explore incongruence among these data and its likely mechanisms using a broader taxon sampling scheme and additional genes from both the nuclear and plastid genomes in the near future using the target enrichment approach (Weitemier et al., 2014; Wanke et al., 2017; Kleinkopf et al., 2019; Li et al., 2019; Nie et al., 2019).
The sequencing data generated in this study has been deposited in the GenBank of NCBI and can be found using accession numbers MN702013–MN702384.
JW and EZ conceived and oversaw the study. SH, YK, AH, XY, B-BL, Y-JZ, and GJ performed experiments and implemented the data analyses. JW collected the specimens. JW, SH, and EZ wrote the manuscript. All authors read the manuscript and approved the final version.
The Washington Biologists Field Club provided partial funding for the study. SH was supported by the Natural History Research Experiences (NHRE) internship for undergraduates and funded by the National Science Foundation (REU Site, EAR-1062692) for the summer of 2014.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Ms. Linda Merwin, the owner of the Grape Cottage, for her permission to sample the original Concord grape vine, John Pacheco and Rich Tombeno for assistance with collecting the cultivated accessions at Welsh’s Food Inc., Concord, MA, United States, Ning Zhang for helpful discussions, Matt Kweskin for assistance with data analyses, and Alice Tangerini for making Figure 4. The lab work was conducted in the Laboratories of Analytical Biology in the National Museum of Natural History at the Smithsonian Institution. We also thank Bernie Prins and Malli Aradhya for providing accessions of Vitis vinifera subsp. sylvestris and V. vinifera subsp. vinifera through the National Clonal Germplasm Repository, United States Department of Agriculture-Agricultural Research Service, University of California, Davis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00263/full#supplementary-material
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410.
Aradhya, M., Koehmstedt, A., Prins, B. H., Dangl, G. S., and Stover, E. (2008). Genetic structure, differentiation, and phylogeny of the genus Vitis: implications for genetic conservation. Acta Hort. 799, 43–49. doi: 10.17660/actahortic.2008.799.4
Aradhya, M., Wang, Y., Walker, M. A., Prins, B. H., Koehmstedt, A. M., Velasco, D., et al. (2013). Genetic diversity, structure, and patterns of differentiation in the genus Vitis. Plant Syst. Evol. 299, 317–330. doi: 10.1007/s00606-012-0723-4
Bailey, L. H. (1897). “Vitaceae,” in Synoptical Flora of North America, Vol. 1, ed. A. Gray (New York, NY: American Book Company), 419–433.
Bang, W. Y., Jeong, I. S., Kim, D. W., Im, C. H., Ji, C., Hwang, S. M., et al. (2008). Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant Cell Physiol. 49, 1350–1363. doi: 10.1093/pcp/pcn111
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Brizicky, G. K. (1965). The genera of Vitaceae in the southeastern United States. J. Arnold Arbor. 46, 48–67.
Chen, G. E., Canniffe, D. P., and Hunter, C. N. (2017). Three classes of oxygen-dependent cyclase involved in chlorophyll and bacteriochlorophyll biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 114, 6280–6285. doi: 10.1073/pnas.1701687114
Chen, Z. D., Ren, H., and Wen, J. (2007). “Vitaceae,” in Flora of China, Vol. 12, eds C. Y. Wu, D.-Y. Hong, and P. H. Raven (Beijing: Science Press), 173–222.
Comeaux, B. L., Nesbitt, W. B., and Fantz, P. R. (1987). Taxonomy of the native grapes of North Carolina. Castanea 52, 197–215. doi: 10.1128/mBio.02527-14
Dierckxsens, N., Mardulyn, P., and Smits, G. (2016). NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45:e18. doi: 10.1093/nar/gkw955
Galet, P. (1979). A Practical Ampelography: Grapevine Identification, trans. Morton, L. T. (English translation). Ithaca, NY: Cornell University Press.
Gerrath, J., Posluszny, U., Ickert-Bond, S. M., and Wen, J. (2017). Inflorescence morphology and development in the basal rosid lineage Vitales. J. Syst. Evol. 55, 542–558. doi: 10.1111/jse.12261
Gerrath, J., Posluszny, U., and Melville, L. (2015). Taming the Wild Grape: Botany and Horticulture in the Vitaceae. Heidelberg: Springer.
Hearn, D. J., Evans, M., Wolf, B., McGinty, M., and Wen, J. (2018). Dispersal is associated with morphological innovation, but not increased diversification, in Cyphostemma (Vitaceae). J. Syst. Evol. 56, 340–359. doi: 10.1111/jse.12417
Hipp, A. L., Hall, J. C., and Sytsma, K. J. (2004). Congruence versus phylogenetic accuracy, revisiting the incongruence length difference test. Syst. Biol. 53, 81–89. doi: 10.1080/10635150490264752
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q., and Vinh, L. S. (2017). UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522. doi: 10.1093/molbev/msx281
Katoh, K., Misawa, K., Kuma, K., and Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. doi: 10.1093/nar/gkf436
Katoh, K., and Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinformatics 9, 286–298. doi: 10.1093/bib/bbn013
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kim, D.-H., Kang, J.-G., Yang, S.-S., Chung, K.-S., Song, P.-S., and Park, C.-M. (2002). A phytochrome-associated protein phosphatase 2A modulates light signals in flowering time control in Arabidopsis. Plant Cell 14, 3043–3056. doi: 10.1105/tpc.005306
Klein, L. L., Miller, A. J., Ciotir, C., Hyma, K., Uribe-Convers, S., and Londo, J. (2018). High-throughput sequencing data clarify evolutionary relationships among North American Vitis species and improve identification in USDA Vitis germplasm collections. Am. J. Bot. 105, 215–226. doi: 10.1002/ajb2.1033
Kleinkopf, J. A., Roberts, W. R., Wagner, W. L., and Roalson, E. H. (2019). Diversification of Hawaiian Cyrtandra (Gesneriaceae) under the influence of incomplete lineage sorting and hybridization. J. Syst. Evol. 57, 561–578. doi: 10.1111/jse.12519
Lanfear, R., Calcott, B., Ho, S. Y. W., and Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. doi: 10.1093/molbev/mss020
Lanfear, R., Calcott, B., Kainer, D., Mayer, C., and Stamatakis, A. (2014). Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol. Biol. 14:82. doi: 10.1186/1471-2148-14-82
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., and Calcott, B. (2016). PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773. doi: 10.1093/molbev/msw260
Li, J., Stukel, M., Bussies, P., Skinner, K., Lemmon, A. R., Lemmon, E. M., et al. (2019). Maple phylogeny and biogeography inferred from phylogenomic data. J. Syst. Evol. 57, 594–606. doi: 10.1111/jse.12535
Li, M., Wunder, J., Bissoli, G., Scarponi, E., Gazzani, S., Barbaro, E., et al. (2008). Development of COS genes as universally amplifiable markers for phylogenetic reconstructions of closely related plant species. Cladistics 24, 727–745. doi: 10.1111/j.1096-0031.2008.00207.x
Liu, X. Q., Ickert-Bond, S. M., Nie, Z. L., Zhou, Z., Chen, L. Q., and Wen, J. (2016). Phylogeny of the Ampelocissus–Vitis clade in Vitaceae supports the New World origin of the grape genus. Mol. Phylogenet. Evol. 95, 217–228. doi: 10.1016/j.ympev.2015.10.013
Lu, L., Cox, C. J., Mathews, S., Wang, W., Wen, J., and Chen, Z. D. (2018). Optimal data partitioning, multispecies coalescent and Bayesian concordance analyses resolve early divergences of the grape family (Vitaceae). Cladistics 34, 57–77. doi: 10.1111/cla.12191
Lu, L. M., Wang, W., Chen, Z. D., and Wen, J. (2013). Phylogeny of the non-monophyletic Cayratia Juss. (Vitaceae) and implications for character evolution and biogeography. Mol. Phylogenet. Evol. 68, 502–515. doi: 10.1016/j.ympev.2013.04.023
Ma, Z. Y., Wen, J., Ickert-Bond, S. M., Nie, Z. L., Chen, L. Q., and Liu, X. Q. (2018a). Phylogenomics, biogeography, and adaptive radiation of grapes. Mol. Phylogenet. Evol. 129, 258–267. doi: 10.1016/j.ympev.2018.08.021
Ma, Z. Y., Wen, J., Tian, J. P., Chen, L. Q., and Liu, X. Q. (2018b). Testing reticulate evolution of four Vitis species from East Asia using restriction-site associated DNA sequencing. J. Syst. Evol. 56, 331–339. doi: 10.1111/jse.12444
Madden, T. L., Tatusov, R. L., and Zhang, J. (1996). Applications of network BLAST server. Methods Enzymol. 266, 131–141. doi: 10.1016/s0076-6879(96)66011-x
Mathews, S. (2010). Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 22, 4–16. doi: 10.1105/tpc.109.072280
Mau, B., Newton, M., and Larget, B. (1999). Bayesian phylogenetic inference via Markov chain Monte Carlo methods. Biometrics 55, 1–12. doi: 10.1111/j.0006-341x.1999.00001.x
Miller, A. J., Matasci, N., Schwaninger, H., Aradhya, M. K., Prins, B., Zhong, G. Y., et al. (2013). Vitis phylogenomics: hybridization intensities from a SNP array outperform genotype calls. PLoS One 8:e78680. doi: 10.1371/journal.pone.0078680
Miller, E. V. (1954). The natural origins of some popular varieties of fruit. Econ. Bot. 8, 337–348. doi: 10.1038/srep16794
Moore, M. O. (1991). Classification and systematics of eastern North American Vitis L. (Vitaceae) north of Mexico. SIDA 14, 339–367.
Moore, M. O., and Wen, J. (2016). “Vitaceae,” in Flora of North America North of Mexico, Magnoliophyta: Vitaceae to Garryaceae, Vol. 12, (Oxford: Oxford University Press), 3–23.
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. doi: 10.1093/molbev/msu300
Nie, Z. L., Ma, Z. Y., Johnson, G., Ren, C., Ickert-Bond, S., Zimmer, E. A., et al. (2019). Phylogenomic Discordance in the New World Grapes Suggests Widespread Introgressions as a Driver for Species Diversifications. Oral Paper (PHYL2, Phylogenomics II). Tucson: Botanical Society of America.
Nie, Z. L., Sun, H., Manchester, S. R., Meng, Y., Luke, Q., and Wen, J. (2012). Evolution of the intercontinental disjunctions in six continents in the Ampelopsis clade of the grape family (Vitaceae). BMC Evol. Biol. 12:17. doi: 10.1186/1471-2148-12-17
Péros, J. P., Berger, G., Portemont, A., Boursiquot, J.-M., and Lacombe, T. (2011). Genetic variation and biogeography of the disjunct Vitis subg. Vitis (Vitaceae). J. Biogeogr. 38, 471–486. doi: 10.1111/j.1365-2699.2010.02410.x
Rannala, B., and Yang, Z. H. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. doi: 10.1007/pl00006090
Ren, H., Lu, L. M., Soejima, A., Luke, Q., Zhang, D. X., Chen, Z. D., et al. (2011). Phylogenetic analysis of the grape family (Vitaceae) based on the noncoding plastid trnC-petN, trnH-psbA, and trnL-F sequences. Taxon 60, 629–637. doi: 10.1002/tax.603001
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sanda, S., Leustek, T., Theisen, M. J., Garavito, R. M., and Benning, C. (2001). Recombinant Arabidopsis SQD1 converts UDP-glucose and sulfite to the sulfolipid head group precursor UDP-sulfoquinovose in vitro. J. Biol. Chem. 276, 3941–3946. doi: 10.1074/jbc.m008200200
Sawler, J., Reisch, B., Aradhya, M. K., Prins, B., Zhong, G.-Y., Schwaninger, H., et al. (2013). Genomics assisted ancestry deconvolution in grape. PLoS One 8:e80791. doi: 10.1371/journal.pone.0080791
Schofield, E. A. (1988). “He sowed; others reaped”: Ephraim Wales Bull and the origins of the ‘Concord’ grape. Arnoldia 48, 4–15.
Silverstone, A. L., Ciampaglio, C. N., and Sun, T.-P. (1998). The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10, 155–169. doi: 10.1105/tpc.10.2.155
Soejima, A., and Wen, J. (2006). Phylogenetic analysis of the grape family (Vitaceae) based on three chloroplast markers. Amer. J. Bot. 93, 278–287. doi: 10.3732/ajb.93.2.278
Soltis, D. E., and Kuzoff, R. K. (1995). Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution 49, 727–742. doi: 10.1111/j.1558-5646.1995.tb02309.x
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Taberlet, P., Gielly, L., Pautou, G., and Bouvet, J. (1991). Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17, 1105–1109. doi: 10.1007/bf00037152
The UniProt Consortium. (2017). UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169. doi: 10.1093/nar/gkw1099
Tröndle, D., Schröder, S., Kassemeyer, H. H., Kiefer, C., Koch, M. A., and Nick, P. (2010). Molecular phylogeny of the genus Vitis (Vitaceae) based on plastid markers. Amer. J. Bot. 97, 1168–1178. doi: 10.3732/ajb.0900218
Vandenbussche, F., Fierro, A. C., Wiedemann, G., Reski, R., and Van Der Straeten, D. (2007). Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol. 7:65. doi: 10.1186/1471-2229-7-65
Wan, Y., Schwaninger, H. R., Baldo, A. M., Labate, J. A., Zhong, G., and Simon, C. J. (2013). A phylogenetic analysis of the grape genus (Vitis L.) reveals broad reticulation and concurrent diversification during neogene and quaternary climate change. BMC Evol. Biol. 13:141. doi: 10.1186/1471-2148-13-141
Wanke, S., Mendoza, C. G., Mueller, S., Guillen, A. P., Neinhuis, C., Lemmon, A. R., et al. (2017). Recalcitrant deep and shallow nodes in Aristolochia (Aristolochiaceae) illuminated using anchored hybrid enrichment. Mol. Phylogenet. Evol. 117, 111–123. doi: 10.1016/j.ympev.2017.05.014
Weitemier, K., Straub, S. C. K., Cronn, R. C., Fishbein, M., Schmickl, R., McDonnell, A., et al. (2014). Hyb−Seq: combining target enrichment and genome skimming for plant phylogenomics. Appl. Plant Sci. 2:1400042. doi: 10.3732/apps.1400042
Wen, J. (2007). “Vitaceae,” in The Families and Genera of Vascular Plants, Vol. 9, ed. K. Kubitzki (Berlin: Springer-Verlag), 466–478.
Wen, J., Harris, A. J., Kalburgi, Y., Zhang, N., Xu, Y., Zheng, W., et al. (2018a). Chloroplast phylogenomics of the New World grape species (Vitis, Vitaceae). J. Syst. Evol. 56, 297–308. doi: 10.1016/j.ympev.2011.11.015
Wen, J., Lu, L. M., Nie, Z. L., Liu, X. Q., Zhang, N., Ickert-Bond, S., et al. (2018b). A new phylogenetic tribal classification of the grape family (Vitaceae). J. Syst. Evol. 56, 262–272. doi: 10.1111/jse.12427
Wen, J., Nie, Z. L., Soejima, A., and Meng, Y. (2007). Phylogeny of Vitaceae based on the nuclear GAI1 gene sequences. Can. J. Bot. 85, 731–745. doi: 10.1139/b07-071
Yang, S. W., Jang, I.-C., Henriques, R., and Chua, N.-H. (2009). FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE Associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell 21, 1341–1359. doi: 10.1105/tpc.109.067215
Yi, T. S., Jin, G. H., and Wen, J. (2015). Chloroplast capture and intra- and inter-continental biogeographic diversification in the Asian – New World disjunct plant genus Osmorhiza (Apiaceae). Mol. Phylogenet. Evol. 85, 10–21. doi: 10.1016/j.ympev.2014.09.028
Zecca, G., Abbott, J. R., Sun, W. B., Spada, A., Sala, F., and Grassi, F. (2012). The timing and the mode of evolution of wild grapes (Vitis). Mol. Phylogenet. Evol. 62, 736–747. doi: 10.1016/j.ympev.2011.11.015
Zhang, N., Wen, J., and Zimmer, E. A. (2015). Congruent deep relationships in the grape family (Vitaceae) based on sequences of chloroplast genomes and mitochondrial genes via genome skimming. PLoS One 10:e0144701. doi: 10.1371/journal.pone.0144701
Zhao, L., Jiang, X. W., Zuo, Y. J., Liu, X. L., Chin, S. W., Haberle, R., et al. (2016). Multiple events of allopolyploidy in the evolution of the racemose lineages in Prunus (Rosaceae) based on integrated evidence from nuclear and plastid data. PLoS One 11:e015712. doi: 10.1371/journal.pone.0157123
Zhao, L., Potter, D., Xu, Y., Liu, P. L., Johnson, G., Chang, Z. Y., et al. (2018). Phylogeny and spatio−temporal diversification of Prunus subgenus Laurocerasus section Mesopygeum (Rosaceae) in the Malesian region. J. Syst. Evol. 56, 637–651.
Keywords: Concord grape, grape, origin, Vitis, Vitaceae
Citation: Wen J, Herron SA, Yang X, Liu B-B, Zuo Y-J, Harris A, Kalburgi Y, Johnson G and Zimmer EA (2020) Nuclear and Chloroplast Sequences Resolve the Enigmatic Origin of the Concord Grape. Front. Plant Sci. 11:263. doi: 10.3389/fpls.2020.00263
Received: 11 October 2019; Accepted: 19 February 2020;
Published: 17 March 2020.
Edited by:
Fabrizio Grassi, University of Bari Aldo Moro, ItalyReviewed by:
Peter Nick, Karlsruhe Institute of Technology (KIT), GermanyCopyright © 2020 Wen, Herron, Yang, Liu, Zuo, Harris, Kalburgi, Johnson and Zimmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wen, d2VuakBzaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.