- Institute of Basic Biological Problems (RAS), Pushchino, Russia
Recruitment of H2O as the final donor of electrons for light-governed reactions in photosynthesis has been an utmost breakthrough, bursting the evolution of life and leading to the accumulation of O2 molecules in the atmosphere. O2 molecule has a great potential to accept electrons from the components of the photosynthetic electron transfer chain (PETC) (so-called the Mehler reaction). Here we overview the Mehler reaction mechanisms, specifying the changes in the structure of the PETC of oxygenic phototrophs that probably had occurred as the result of evolutionary pressure to minimize the electron flow to O2. These changes are warranted by the fact that the efficient electron flow to O2 would decrease the quantum yield of photosynthesis. Moreover, the reduction of O2 leads to the formation of reactive oxygen species (ROS), namely, the superoxide anion radical and hydrogen peroxide, which cause oxidative stress to plant cells if they are accumulated at a significant amount. From another side, hydrogen peroxide acts as a signaling molecule. We particularly zoom in into the role of photosystem I (PSI) and the plastoquinone (PQ) pool in the Mehler reaction.
Introduction
Mehler reaction is the major source of reactive oxygen species (ROS), such as O2∙– and H2O2, in chloroplasts. During the Mehler reaction, O2 molecules serve as an alternative electron acceptor from the photosynthetic electron transfer chain (PETC), being a safety valve to release surplus electrons and thus alleviating the PETC over-reduction. This reaction also contributes to building up of ΔpH across the thylakoid membrane and produces a signaling messenger, H2O2, which is capable of initiating various signaling pathways (Ivanov et al., 2012). However, an efficient electron flow to O2 would decrease the photosynthetic quantum yield. Moreover, ROS, if not neutralized efficiently, lead to oxidative damage. Thus, the PETC evolution could have been guided toward minimizing and/or taking strong control over the Mehler reaction.
Most of the PETC components were proposed as sites of O2∙– photoproduction, the first step of the Mehler reaction. Among them, there are water-soluble and water-exposed components (Figure 1, open circles) and the components situated in hydrophobic zones (Figure 1, closed circles). The former produce O2∙– in water bulk phases, e.g., stroma, while the latter produce O2∙–, which can be detected outside the membrane when diffused there or can be detected within the thylakoid membranes (Kozuleva et al., 2011). The value of Em (O2/O2∙–) in water is −160 mV, while in hydrophobic zones of proteins and membranes it is more negative, approximately −550 mV (Wardman, 1990). Only few components in PETC possess enough negative Em for O2 reduction within a thylakoid membrane. Numerous experiments unambiguously demonstrated that photosystem I (PSI) is the major site of O2∙– photoproduction (Kozuleva and Ivanov, 2016). O2∙– generation by other components was shown under the disturbed PETC function. The second step of the Mehler reaction is H2O2 production via O2∙– dismutation in stroma as catalyzed by superoxide dismutase. Apart from O2∙– dismutation, another mechanism was shown to operate in the thylakoid membranes (Mubarakshina et al., 2006). It involves O2∙– reduction by the plastoquinone (PQ) pool, namely, by plastoquinol (PQH2) (Borisova-Mubarakshina et al., 2019). Thus, the Mehler reaction proceeds at a variety of sites, still leading to O2∙– and subsequent H2O2 production.
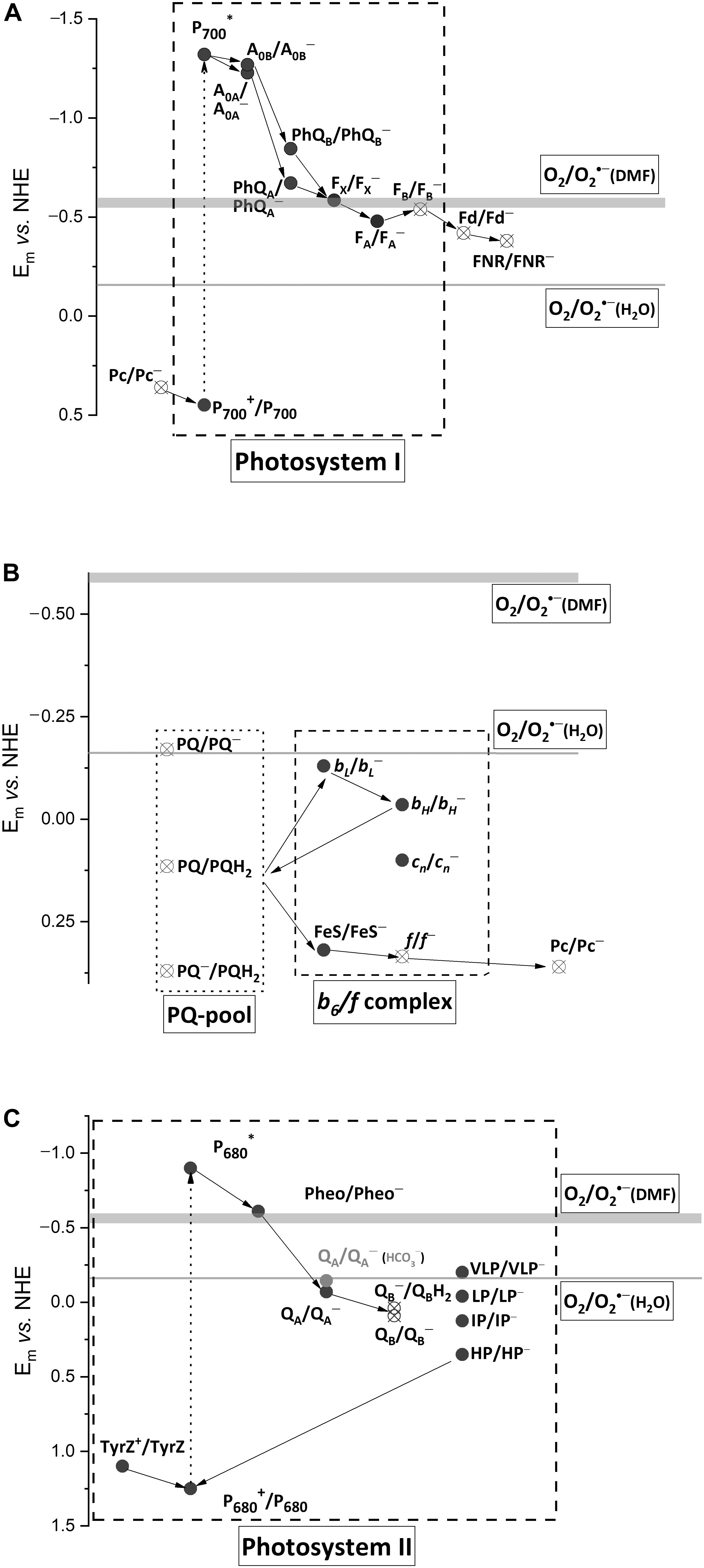
Figure 1. Schematic diagram of forward electron transfer in PSI (A), PQ-pool and cytochrome b6/f complex (B), and PSII (C), with Em values of the cofactors. The Em values of (O2/O2∙–) in water, –160 mV, and dimethylformamide (DMF), from –550 to –600 mV, are shown by thin and thick horizontal lines, respectively. Pc, plastocyanin; P700, the dimer of Chl a molecules in PSI; A0, the primary electron acceptor in PSI; PhQ, phylloquinone, a secondary electron acceptor in PSI; FX, a 4Fe–4S cluster, a secondary electron acceptor in PSI; FA and FB, 4Fe–4S clusters, the terminal electron acceptors in PSI; Fd, ferredoxin, the mobile electron acceptor; FNR, ferredoxin/NADP+ reductase; PQ, PQ∙–, and PQH2, plastoquinone, plastosemiquinone, and plastoquinol; FeS, a 2Fe-2S cluster of Rieske protein; f, cytochrome f; bL and bH, the low- and high-potential forms of cytochrome b6; cn, the heme covalently bound to cytochrome b6; TyrZ, the redox active tyrosine residue; P680, the dimer of Chl a molecules in PSII; Pheo, pheophytin, a primary electron acceptor in PSII; QA, the tightly bound plastoquinone in PSII, the secondary electron acceptor in PSII; QB, the loosely bound plastoquinone in PSII, the terminal electron acceptor in PSII; VLP, LP, IP, and HP, the very low-, low-, intermediate-, and high-potential forms of cytochrome b559 in PSII. The Em-values for PSI cofactors are according to Ptushenko et al. (2008), those for b6/f complex are according to Alric et al. (2005), those for PSII are according to Brinkert et al. (2016) and Causmaecker et al. (2019), except cytochrome b559 (Khorobrykh, 2019). Closed circles, components situated in hydrophobic zones; open circles, water bulk phases exposed components.
The evolution of various photosynthetic complexes has been a subject of several recent reviews (Jagannathan et al., 2012; Rutherford et al., 2012; Pierella Karlusich and Carrillo, 2017; Orf et al., 2018). Here, we briefly summarize the structural changes which could have happened in PETC to control and minimize an electron flow to O2. The general evolutionary trends could include: (i) kinetic control, making the forward reactions faster than the competing electron flow to O2, (ii) redox tuning of cofactors, disabling spontaneous exergonic reactions with O2, and (iii) shielding of cofactors with protein environment, restricting O2 accessibility (Rutherford et al., 2012).
Photosystem I
All secondary electron acceptor cofactors of PSI were proposed as the sites of O2 photoreduction. The terminal FeS clusters FA/FB are inevitably oxidized by O2 in the absence of ferredoxin (Fd). The role of intermediate cofactor FeS cluster FX was claimed in Takahashi and Asada (1988) based on experiments showing that the primary H2O2 photoproduction site was a PsaA/PsaB heterodimer, which harbors FX. However, the PsaA/PsaB heterodimer also binds two phylloquinone (PhQ) molecules at the A1 sites and they could also contribute to H2O2 photoproduction. For the first time, the role of PhQs was proposed by Kruk with coauthors (Kruk et al., 2003) since adding PhQ to quinone-depleted thylakoid membranes re-established the O2 uptake at a single light flash. This result still does not rule out that FeS clusters reduce O2 by electrons from P700 via PhQ re-incorporated to the A1 sites. The PhQ involvement in O2 photoreduction in intact PSI under steady-state illumination was proposed based on comparing O2 photoreduction as a function of irradiance in the wild-type PSI with that in the mutant PQ-containing PSI (Kozuleva et al., 2014). The authors concluded that the PhQs at the A1 sites are the major contributor to O2∙– generation.
From an evolutionary point of view, the terminal cofactor FB can be one of the sites where the Mehler reaction should have been taken under control. This cofactor possesses negative Em, allowing for the efficient reduction of both Fd and O2. However, Fd is a mobile protein, diffusing to and out of PSI and leaving FB– transiently open to O2. If FB– is oxidized by O2 efficiently, it would be insufficient in a steady-state reduction of Fd. However, the electron lives mostly on FA, not FB, because of a positive shift of Em (FA/FA–) relative to (FB/FB–) (Figure 1A; Fischer et al., 1997; Shinkarev et al., 2000). FA is embedded deeper in the protein, which shields it from O2. This feature allows keeping of electrons for Fd and avoiding any wasteful electron leakage to O2.
The PsaC protein carrying FA and FB is homologous to mobile ferredoxins in anoxigenic phototrophs (Jagannathan and Golbeck, 2009). It is widely accepted that, during evolution, the ancestral mobile Fd was tightly bound to the ancestral homodimeric reaction center (RC). This binding resulted in an elongation of the ET chain in the RC that could have aimed at stabilizing the charge separation state and minimizing the charge recombination, which could lead to 3P700 and, hence, 1O2 formation (Orf et al., 2018). However, that binding probably provided an additional protein shielding for FX, which was the terminal cofactor in the ancestral RC, and for FA, limiting O2 diffusion and preventing unproductive electron leakage (Jagannathan et al., 2012). The protein shielding of these FeS clusters, being potentially capable of catalyzing H2O2 decomposition into a highly reactive HO∙ (Šnyrychová et al., 2006), could have additionally protected the PSI acceptor side from HO∙ formation.
Binding of the ancestral Fd to the ancestral homodimeric RC resulted in RC asymmetry through locating the FA cluster closer to one of the quinones (PhQB), bringing about a negative shift in Em (PhQB/PhQB∙–) (Rutherford et al., 2012). The difference in Em between PhQA and PhQB is up to 170 mV (Ptushenko et al., 2008). Rutherford with coauthors presented an elegant hypothesis explaining the benefit of this asymmetry as it eliminates 3P700 (and hence 1O2) formation under the conditions of the Fd pool over-reduction (Rutherford et al., 2012). In line with this hypothesis, PhQ∙– oxidation by O2 sustains a forward ET and contributes to both alleviating PETC over-reduction and preventing charge recombination (Kozuleva and Ivanov, 2016). Both PhQs in PSI have one of the most negative Em in the PETC (−671 and −840 mV for PhQA and PhQB, respectively; Figure 1A), which allows phyllosemiquinones to reduce O2 even in the hydrophobic zones of the thylakoid membranes, where Em (O2/O2∙–) is close to −550 mV (see above). Due to a longer lifetime, PhQA∙– gets higher chances to react with O2, although the more negative Em of PhQB/PhQB∙– provides a larger −ΔG in reaction with O2. However, the particular impact of each PhQ as well as clarifying the FX role is still open questions.
Ferredoxin and FNR
In bacterial type Fd, two 4Fe-4S clusters are partially exposed to solvent and accessible for O2 attacks (Jagannathan et al., 2012). After binding the ancestral Fd to RC, the organisms recruited another Fd, where a single 2Fe–2S cluster is shielded by a protein.
A long-lasting controversy on the role of Fd in the Mehler reaction was solved nearly a decade ago. In the absence of NADP+, which is the major electron sink for Fd, O2 inevitably oxidizes the reduced Fd (Fd–). In the presence of NADP+, simultaneously with its photoreduction, the electron flow to O2 was shown to be significant in high light; however, the contribution of Fd was almost negligible relative to that of the membrane-bound PETC components (Kozuleva and Ivanov, 2010). These results reveal a low reactivity of Fd– toward O2, which enables Fd to fulfill the function of stromal hub-donating electrons to multiple enzymes and proteins, including ferredoxin-NADP+ reductase (FNR) (Hanke and Mulo, 2013).
The Fd affinity to its redox partners, i.e., PSI acceptor side, was also raised to ensure the competition with O2 for electrons. However, this is not entirely the case of FNR. Although a semiquinone form of FAD prosthetic group in FNR can react with O2 (Massey, 1994), so far there are no reliable experimental data demonstrating that FNR is involved in O2 photoreduction in the thylakoid membranes (Kozuleva and Ivanov, 2016). The FNR of oxygenic phototrophs possesses ∼10 times higher catalytic activity than the bacterial FNR (Pierella Karlusich and Carrillo, 2017), with affinity remaining roughly the same. The high catalytic activity is likely achieved through conformational changes caused by NADP+ binding to FNR, which greatly facilitate both the Fd– oxidation (Batie and Kamin, 1984) and the liberation of the oxidized Fd from the complex (Mulo and Medina, 2017). This enhancement in the FNR catalytic activity most possibly decreased the chances for both the FAD semiquinone (Q∙–) oxidation by O2 and the formation of Fd:FNR∙– complex in the absence of NADP+.
Plastoquinone Pool
O2∙– photoproduction by PQ∙– in the PQ pool was demonstrated (Khorobrykh and Ivanov, 2002; Vetoshkina et al., 2017). However, the maximal O2∙– production rates observed in the pool were 10 times lower than in the PSI.
While anoxygenic phototrophs use menaquinone (MQ) and ubiquinone (UQ), the oxygenic ones recruited PQ, a representative of a “more recent” group of quinones (Schoepp-Cothenet et al., 2009). MQ was probably the first quinone in ancient photosynthetic membranes. The rationale for replacing MQ with PQ is clear: the Em values of (Q/Q∙–) and (Q/QH2) are ∼100 mV (Kishi et al., 2017) and ∼180 mV (Bergdoll et al., 2016), more negative for MQ than for PQ (Figure 1B). This means that PQ∙– and the PQ pool itself in the reduced state are more stable in the presence of O2. Furthermore, pKa (Q∙–/QH) for PQ is higher than for MQ, providing an easier protonation and, hence, a higher stability of plastosemiquinone (Hasegawa et al., 2017).
A possible rationale for choosing PQ instead of UQ in the PETC of oxygenic phototrophs is still vague. Firstly, the O2∙– generation by free UQ∙– in the mitochondria was discovered as early as in 80-s (Turrens et al., 1985). This reaction has long been considered as an important source of O2∙– in animal cells. On the contrary, PQ∙– in photosynthetic cells has little impact on O2∙– production, as stated above. Secondly, PQH2 is more efficient as an antioxidant than UQH2 (Borisova-Mubarakshina et al., 2019), e.g., in lipid peroxidation prevention (Kruk et al., 1997). A consequence of higher antioxidant activity of PQH2 is its higher ability to reduce O2∙– to H2O2. It was shown that the PQ pool in the thylakoid membranes (presumably PQH2) is indeed oxidized by O2∙– (Borisova-Mubarakshina et al., 2018). Therefore, despite the low O2∙–-generating activity, the contribution of the PQ pool to the Mehler reaction can be essential due to the production of H2O2 from O2∙–. We hypothesize that ensuring the efficient transformation of O2∙–, which is generated by PSI, to H2O2 could be one of the evolutionary driving forces for the choice of PQ.
Replacing MQ with PQ as a mobile pool in the thylakoid membrane inevitably affected all of the complexes interacting with quinone. All cofactors in photosystem II (PSII) and cytochrome b6/f complexes have 110–150 mV more positive Em values than in their MQ-based analogs (Schoepp-Cothenet et al., 2009; Bergdoll et al., 2016).
Cytochrome b6/f Complex
The cytochrome b6/f complex is also considered to be an O2 photoreduction site (Taylor et al., 2018). The high Em values of the b6f complex cofactors are a consequence of MQ replacement with PQ (Bergdoll et al., 2016). Among its ET cofactors, the bL heme possesses one of the lowest Em, −130 mV (Alric et al., 2005). Thermodynamically, this heme can hardly reduce O2 since Em (O2/O2∙–) in the membrane is close to −550 mV (Figure 1B, see above). The fast ET from bL to bH decreases the possibility of a bL reaction with O2.
In several studies, PQ∙– at the quinol-oxidizing (Qo) site of the complex is considered as the electron donor to O2. However, the concerted oxidation of PQH2 diminishes the PQ∙– lifetime. If semiquinone is produced, it is either quickly oxidized by bL heme or reduced by it, if the heme is pre-reduced. The dimer organization of the b6/f complex was proposed to lower the chances of O2∙– generation at the Qo site (Rutherford et al., 2012). In the bc1 complex, a spin–spin complex state between the semiquinone and the Rieske cluster was shown to suppress O2∙– generation (Bujnowicz et al., 2019). This keeps up well with the experimental observations that PQ∙– can reduce O2 once it leaves the Qo pocket (Forquer et al., 2006), becoming a part of the pool (see above). It was demonstrated that O2∙– production by the isolated b6/f complexes was 10 times higher than the one by the isolated bc1 complexes (Baniulis et al., 2013). This can be explained by an easier liberation of semiquinone from the Qo site in the former case. It is important that, in vivo, such PQ∙– would appear at the luminal side of the thylakoid membrane. The lumen pH determines the protonation of PQ∙–. Since PQH∙ has a lower chance to reduce O2, the lumen pH can regulate the O2∙– production there.
The appearance of semiquinone at the quinone-reducing site (Qr) of the bc1 complex from purple bacteria was shown (Drachev et al., 1989). There are still no reliable data on semiquinone formation at the Qr site of the b6/f complex. The double reduction of PQ occurs there when the second electron is transferred to the bH heme (Ivanov, 1993). The residence of the first electron at the bH heme can be a result of the cn heme situated in close vicinity to the bH in the b6/f complex.
Photosystem II
Three major tasks could have been solved during the evolution of PSII: (i) the existence of highly oxidizing P680+, (ii) dealing with charge recombination leading to 1O2 production, and (iii) stabilization of QB– waiting for the second electron (Rutherford et al., 2012). O2∙– production in PSII was shown many times (Pospíšil, 2012). Pheophytin (Pheo), QA, QB, and cytochrome b559 were suggested as the sites of O2 reduction to O2∙–, based presumably on the experiments with PSII complexes with disrupted function, e.g., after modifications of the water-oxidizing complex.
Although Pheo– possesses Em, −610 mV (Rappaport et al., 2002), negative enough to reduce O2 even in hydrophobic media (Figure 1C), its lifetime is rather short (300 ps) such that it prevents the electron leakage to O2. This reaction with QA– (Ivanov and Khorobrykh, 2003; Pospíšil, 2012) is thermodynamically unfavorable due to a more positive Em (QA/QA–), −70 mV (Brinkert et al., 2016), than Em (O2/O2∙–). However, the binding of HCO3– to non-heme Fe situated between the QA and the QB shifts Em (QA/QA–) to −145 mV, making the electron leakage from QA– to O2 more probable. In contrast to QA, QB undergoes two sequential reduction steps, meaning that QB– lives for a longer time waiting for the second electron. However, QB– is thermodynamically stable due to the positive Em potentials (Causmaecker et al., 2019).
The role of a very low potential form of cytochrome b559 (Em is −150 to −200 mV) in O2 reduction was also proposed (Khorobrykh, 2019). However, the fraction of this form is extremely small under normal conditions and increases only when the PSII functioning is severely perturbed. The b559 heme is embedded in the hydrophobic zone of the membrane; therefore, O2 photoreduction by b559 heme is thermodynamically unfavorable.
Discussion
In this review, we briefly summarize some features of the modern PETC, which have evolved at the background of the Mehler reaction. The main site of O2∙– generation is PSI. Several experiments revealed that PhQ could be the major contributor to this process (Kruk et al., 2003; Kozuleva et al., 2011, 2014). The reactivity of the FeS components with O2, especially FB and Fd, was diminished by redox tuning and protein shielding. The recruitment of a high-potential PQ to the membrane quinone pool instead of a low-potential MQ was driven by the necessity to keep the pool in the reduced state under illumination in the presence of O2. Replacing MQ with PQ triggered a redox tuning of PSII and cytochrome b6/f complex cofactors, disabling, among other things, efficient O2∙– generation in these complexes. The only MQ-based cofactor preserved in the modern PETC is PhQ, which is likely to be the main site of O2∙– generation.
The stromal production of O2∙– via Fd greatly increases if the NADP+ recovering in the Calvin–Benson–Bassham cycle is retarded, e.g., due to closed stomata. In the stroma, H2O2 is produced from O2∙– under catalysis by superoxide dismutase. O2 reduction by PhQ∙– can account for O2∙– appearance within the thylakoid membrane (Kozuleva et al., 2011); however, a significant part of O2∙– formed by PhQ∙– still likely diffuses outside the membrane. Nevertheless, the increasing irradiance resulted in both a larger O2∙– production just within the thylakoid membrane and a larger H2O2 production via O2∙– reduction by PQH2, i.e., by the mechanism different from dismutation (Borisova-Mubarakshina et al., 2012).
Thus, in chloroplasts, H2O2 is produced via two distinct reactions in two distinct chloroplast compartments. We believe that this observation may be important for the understanding of H2O2-mediated signal transduction. The stromal H2O2, which might be considered as a messenger of NADP+/NADPH status, can oxidize thioredoxins (Hofmann, 2010; Netto and Antunes, 2016). Therefore, a temporary H2O2 accumulation in the stroma can affect the expression of chloroplast genes and/or the translation aimed at the fast adaptation of photosynthetic apparatus. H2O2 formed by the membrane PQ pool might be considered as a messenger of PETC status. It is important in terms of the PQ pool function as a central hub, of which the redox state represents a signal for both the chloroplast gene expression (Pfannschmidt et al., 2009) and the retrograde signaling pathways from the chloroplast to the nucleus (Pfannschmidt et al., 2003). For example, the PQ pool redox state initiates the changes in the PSII light-harvesting antenna size as a long-term acclimation to light conditions (Escoubas et al., 1995; Frigerio et al., 2007). We demonstrated that it is H2O2 rather than the PQ pool reduction state itself that is responsible for the antenna size reduction in high light (Borisova-Mubarakshina et al., 2015, 2019). Therefore, we suppose that a high potential of the PQ pool to form H2O2 in high light and under stress conditions could serve as evolutionarily set to signal about the PETC redox state to adjust to the ever-changing environmental conditions.
Author Contributions
MK and MB-M designed the concept of the article. All authors contributed to the writing of the first draft and manuscript revision, and approved the submitted version. MK incorporated all inputs from the coauthors, reviewers, and editor.
Funding
This work was funded by the Russian Science Foundation, project 17-14-01371 and by The Ministry of Science and Higher Education of the Russian Federation, State Scientific Program, theme no. AAAA-A17-117030110135-1.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alric, J., Pierre, Y., Picot, D., Lavergne, J., and Rappaport, F. (2005). Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc. Natl. Acad. Sci. U.S.A. 102, 15860–15865. doi: 10.1073/pnas.0508102102
Baniulis, D., Hasan, S. S., Stofleth, J. T., and Cramer, W. A. (2013). Mechanism of enhanced superoxide production in the cytochrome b6f complex of oxygenic photosynthesis. Biochemistry 52, 8975–8983. doi: 10.1021/bi4013534
Batie, C. J., and Kamin, H. (1984). Ferredoxin:NADP (oxidoreductase. Equilibria in binary and ternary complexes with NADP (and ferredoxin. J. Biol. Chem. 259, 8832–8839.
Bergdoll, L., ten Brink, F., Nitschke, W., Picot, D., and Baymann, F. (2016). From low- to high-potential bioenergetic chains: Thermodynamic constraints of Q-cycle function. Biochim. Biophys. Acta 1857, 1569–1579. doi: 10.1016/j.bbabio.2016.06.006
Borisova-Mubarakshina, M. M., Ivanov, B. N., Vetoshkina, D. V., Lubimov, V. Y., Fedorchuk, T. P., Naydov, I. A., et al. (2015). Long-term acclimatory response to excess excitation energy: evidence for a role of hydrogen peroxide in the regulation of photosystem II antenna size. J. Exp. Bot. 66, 7151–7164. doi: 10.1093/jxb/erv410
Borisova-Mubarakshina, M. M., Kozuleva, M. A., Rudenko, N. N., Naydov, I. A., Klenina, I. B., and Ivanov, B. N. (2012). Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta 1817, 1314–1321. doi: 10.1016/j.bbabio.2012.02.036
Borisova-Mubarakshina, M. M., Naydov, I. A., and Ivanov, B. N. (2018). Oxidation of the plastoquinone pool in chloroplast thylakoid membranes by superoxide anion radicals. FEBS Lett. 592, 3221–3228. doi: 10.1002/1873-3468.13237
Borisova-Mubarakshina, M. M., Vetoshkina, D. V., and Ivanov, B. N. (2019). Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant. 166, 181–198. doi: 10.1111/ppl.12936
Brinkert, K., Causmaecker, S. D., Krieger-Liszkay, A., Fantuzzi, A., and Rutherford, A. W. (2016). Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. PNAS 113, 12144–12149. doi: 10.1073/pnas.1608862113
Bujnowicz, Ł, Borek, A., Kuleta, P., Sarewicz, M., and Osyczka, A. (2019). Suppression of superoxide production by a spin-spin coupling between semiquinone and the Rieske cluster in cytochrome bc1. FEBS Lett. 593, 3–12. doi: 10.1002/1873-3468.13296
Causmaecker, S. D., Douglass, J. S., Fantuzzi, A., Nitschke, W., and Rutherford, A. W. (2019). Energetics of the exchangeable quinone, QB, in Photosystem II. PNAS 116, 19458–19463. doi: 10.1073/pnas.1910675116
Drachev, L. A., Kaurov, B. S., Mamedov, M. D., Mulkidjanian, A. Y., Semenov, A. Yu, Shinkarev, V. P., et al. (1989). Flash-induced electrogenic events in the photosynthetic reaction center and bc1 complexes of Rhodobacter sphaeroides chromatophores. Biochim. Biophys. Acta 973, 189–197. doi: 10.1016/S0005-2728(89)80421-9
Escoubas, J. M., Lomas, M., LaRoche, J., and Falkowski, P. G. (1995). Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. PNAS 92, 10237–10241. doi: 10.1073/pnas.92.22.10237
Fischer, N., Sétif, P., and Rochaix, J.-D. (1997). Targeted mutations in the psac gene of Chlamydomonas reinhardtii: preferential reduction of FB at low temperature is not accompanied by altered electron flow from Photosystem I to Ferredoxin. Biochemistry 36, 93–102. doi: 10.1021/bi962244v
Forquer, I., Covian, R., Bowman, M. K., Trumpower, B. L., and Kramer, D. M. (2006). Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 281, 38459–38465. doi: 10.1074/jbc.M605119200
Frigerio, S., Campoli, C., Zorzan, S., Fantoni, L. I., Crosatti, C., Drepper, F., et al. (2007). Photosynthetic antenna size in higher plants is controlled by the plastoquinone redox state at the post-transcriptional rather than transcriptional level. J. Biol. Chem. 282, 29457–29469. doi: 10.1074/jbc.M705132200
Hanke, G., and Mulo, P. (2013). Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ. 36, 1071–1084. doi: 10.1111/pce.12046
Hasegawa, R., Saito, K., Takaoka, T., and Ishikita, H. (2017). pKa of ubiquinone, menaquinone, phylloquinone, plastoquinone, and rhodoquinone in aqueous solution. Photosynth. Res. 133, 297–304. doi: 10.1007/s11120-017-0382-y
Hofmann, N. R. (2010). A new thioredoxin is involved in plastid gene expression. Plant Cell 22:1423. doi: 10.1105/tpc.110.220512
Ivanov, B., and Khorobrykh, S. (2003). Participation of photosynthetic electron transport in production and scavenging of reactive oxygen species. Antioxid. Redox Signal. 5, 43–53. doi: 10.1089/152308603321223531
Ivanov, B., Kozuleva, M., and Mubarakshina, M. (2012). “Oxygen metabolism in chloroplast,” in Cell Metabolism-Cell Homeostasis and Stress Response, ed. P. Bubulya (London: InTech).
Ivanov, B. N. (1993). “Stoichiometry of proton uptake by thylakoids during electron transport in chloroplasts,” in Photosynthesis: Photoreactions to Plant Productivity, eds Y. P. Abrol and P. Mohanty (Dordrecht: Springer Netherlands), 109–128. doi: 10.1007/978-94-011-2708-0_4
Jagannathan, B., and Golbeck, J. H. (2009). Understanding of the binding interface between PsaC and the PsaA/PsaB heterodimer in Photosystem I. Biochemistry 48, 5405–5416. doi: 10.1021/bi900243f
Jagannathan, B., Shen, G., and Golbeck, J. H. (2012). “The evolution of type i reaction centers: the response to oxygenic photosynthesis,” in Functional Genomics and Evolution of Photosynthetic Systems Advances in Photosynthesis and Respiration, (Dordrecht: Springer), 285–316. doi: 10.1007/978-94-007-1533-2_12
Khorobrykh, A. (2019). Hydrogen peroxide and superoxide anion radical photoproduction in PSII preparations at various modifications of the water-oxidizing complex. Plants 8:329. doi: 10.3390/plants8090329
Khorobrykh, S. A., and Ivanov, B. N. (2002). Oxygen reduction in a plastoquinone pool of isolated pea thylakoids. Photosynth. Res. 71, 209–219. doi: 10.1023/A:1015583502345
Kishi, S., Saito, K., Kato, Y., and Ishikita, H. (2017). Redox potentials of ubiquinone, menaquinone, phylloquinone, and plastoquinone in aqueous solution. Photosynth. Res. 134, 193–200. doi: 10.1007/s11120-017-0433-4
Kozuleva, M., Klenina, I., Proskuryakov, I., Kirilyuk, I., and Ivanov, B. (2011). Production of superoxide in chloroplast thylakoid membranes: ESR study with cyclic hydroxylamines of different lipophilicity. FEBS Lett. 585, 1067–1071. doi: 10.1016/j.febslet.2011.03.004
Kozuleva, M. A., and Ivanov, B. N. (2010). Evaluation of the participation of ferredoxin in oxygen reduction in the photosynthetic electron transport chain of isolated pea thylakoids. Photosynth. Res. 105, 51–61. doi: 10.1007/s11120-010-9565-5
Kozuleva, M. A., and Ivanov, B. N. (2016). The mechanisms of oxygen reduction in the terminal reducing segment of the chloroplast photosynthetic electron transport chain. Plant Cell Physiol. 57, 1397–1404. doi: 10.1093/pcp/pcw035
Kozuleva, M. A., Petrova, A. A., Mamedov, M. D., Semenov, A. Y., and Ivanov, B. N. (2014). O2 reduction by photosystem I involves phylloquinone under steady-state illumination. FEBS Lett. 588, 4364–4368. doi: 10.1016/j.febslet.2014.10.003
Kruk, J., Jemioła-Rzemiłska, M., Burda, K., Schmid, G. H., and Strzałka, K. (2003). scavenging of superoxide generated in Photosystem I by Plastoquinol and other Prenyllipids in Thylakoid membranes. Biochemistry 42, 8501–8505. doi: 10.1021/bi034036q
Kruk, J., Jemioła-Rzemińska, M., and Strzałka, K. (1997). Plastoquinol and (-tocopherol quinol are more active than ubiquinol and (-tocopherol in inhibition of lipid peroxidation. Chem. Phys. Lipids 87, 73–80. doi: 10.1016/S0009-3084(97)00027-3
Massey, V. (1994). Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 269, 22459–22462.
Mubarakshina, M., Khorobrykh, S., and Ivanov, B. (2006). Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane. Biochim. Biophys. Acta 1757, 1496–1503. doi: 10.1016/j.bbabio.2006.09.004
Mulo, P., and Medina, M. (2017). Interaction and electron transfer between ferredoxin–NADP (oxidoreductase and its partners: structural, functional, and physiological implications. Photosynth. Res. 134, 265–280. doi: 10.1007/s11120-017-0372-0
Netto, L. E. S., and Antunes, F. (2016). The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cells 39, 65–71. doi: 10.14348/molcells.2016.2349
Orf, G. S., Gisriel, C., and Redding, K. E. (2018). Evolution of photosynthetic reaction centers: insights from the structure of the heliobacterial reaction center. Photosynth. Res. 138, 11–37. doi: 10.1007/s11120-018-0503-2
Pfannschmidt, T., Bräutigam, K., Wagner, R., Dietzel, L., Schröter, Y., Steiner, S., et al. (2009). Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Ann. Bot. 103, 599–607. doi: 10.1093/aob/mcn081
Pfannschmidt, T., Schütze, K., Fey, V., Sherameti, I., and Oelmüller, R. (2003). Chloroplast redox control of nuclear gene expression—a new class of plastid signals in interorganellar communication. Antioxid. Redox Signal. 5, 95–101. doi: 10.1089/152308603321223586
Pierella Karlusich, J. J., and Carrillo, N. (2017). Evolution of the acceptor side of photosystem I: ferredoxin, flavodoxin, and ferredoxin-NADP (oxidoreductase. Photosyn. Res. 134, 235–250. doi: 10.1007/s11120-017-0338-2
Pospíšil, P. (2012). Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817, 218–231. doi: 10.1016/j.bbabio.2011.05.017
Ptushenko, V. V., Cherepanov, D. A., Krishtalik, L. I., and Semenov, A. Y. (2008). Semi-continuum electrostatic calculations of redox potentials in photosystem I. Photosynth. Res. 97:55. doi: 10.1007/s11120-008-9309-y
Rappaport, F., Guergova-Kuras, M., Nixon, P. J., Diner, B. A., and Lavergne, J. (2002). Kinetics and Pathways of Charge Recombination in Photosystem II. Biochemistry 41, 8518–8527. doi: 10.1021/bi025725p
Rutherford, A. W., Osyczka, A., and Rappaport, F. (2012). Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett. 586, 603–616. doi: 10.1016/j.febslet.2011.12.039
Schoepp-Cothenet, B., Lieutaud, C., Baymann, F., Verméglio, A., Friedrich, T., Kramer, D. M., et al. (2009). Menaquinone as pool quinone in a purple bacterium. PNAS 106, 8549–8554. doi: 10.1073/pnas.0813173106
Shinkarev, V. P., Vassiliev, I. R., and Golbeck, J. H. (2000). A kinetic assessment of the sequence of electron transfer from F(X) to F(A) and further to F(B) in photosystem I: the value of the equilibrium constant between F(X) and F(A). Biophys. J. 78, 363–372. doi: 10.1016/S0006-3495(00)76599-4
Šnyrychová, I., Pospíšil, P., and Nauš, J. (2006). Reaction pathways involved in the production of hydroxyl radicals in thylakoid membrane: EPR spin-trapping study. Photochem. Photobiol. Sci. 5, 472–476. doi: 10.1039/B514394B
Takahashi, M., and Asada, K. (1988). Superoxide production in aprotic interior of chloroplast thylakoids. Arch. Biochem. Biophys. 267, 714–722. doi: 10.1016/0003-9861(88)90080-X
Taylor, R. M., Sallans, L., Frankel, L. K., and Bricker, T. M. (2018). Natively oxidized amino acid residues in the spinach cytochrome b6f complex. Photosynth. Res. 137, 141–151. doi: 10.1007/s11120-018-0485-0
Turrens, J. F., Alexandre, A., and Lehninger, A. L. (1985). Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 237, 408–414. doi: 10.1016/0003-9861(85)90293-0
Vetoshkina, D. V., Ivanov, B. N., Khorobrykh, S. A., Proskuryakov, I. I, and Borisova-Mubarakshina, M. M. (2017). Involvement of the chloroplast plastoquinone pool in the Mehler reaction. Physiol. Plant. 161, 45–55. doi: 10.1111/ppl.12560
Keywords: photosystems, evolution, plastoquinone, phylloquinone, oxygen, reactive oxygen species
Citation: Kozuleva MA, Ivanov BN, Vetoshkina DV and Borisova-Mubarakshina MM (2020) Minimizing an Electron Flow to Molecular Oxygen in Photosynthetic Electron Transfer Chain: An Evolutionary View. Front. Plant Sci. 11:211. doi: 10.3389/fpls.2020.00211
Received: 30 September 2019; Accepted: 11 February 2020;
Published: 13 March 2020.
Edited by:
Chikahiro Miyake, Kobe University, JapanReviewed by:
Yoshitaka Nishiyama, Saitama University, JapanChristine Helen Foyer, University of Leeds, United Kingdom
Copyright © 2020 Kozuleva, Ivanov, Vetoshkina and Borisova-Mubarakshina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina A. Kozuleva, a296dWxldmFAZ21haWwuY29t; Maria M. Borisova-Mubarakshina, bXViYXJha3NoaW5hbW1AZ21haWwuY29t
 Marina A. Kozuleva
Marina A. Kozuleva Boris N. Ivanov
Boris N. Ivanov Maria M. Borisova-Mubarakshina
Maria M. Borisova-Mubarakshina