
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 10 March 2020
Sec. Plant Breeding
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00034
This article is part of the Research Topic Wild Plants as Source of New Crops View all 23 articles
 Sterling A. Herron1,2*
Sterling A. Herron1,2* Matthew J. Rubin2
Matthew J. Rubin2 Claudia Ciotir1†
Claudia Ciotir1† Timothy E. Crews3
Timothy E. Crews3 David L. Van Tassel3
David L. Van Tassel3 Allison J. Miller1,2*
Allison J. Miller1,2*Herbaceous perennial species are receiving increased attention for their potential to provide both edible products and ecosystem services in agricultural systems. Many legumes (Fabaceae Lindl.) are of special interest due to nitrogen fixation carried out by bacteria in their roots and their production of protein-rich, edible seeds. However, herbaceous perennial legumes have yet to enter widespread use as pulse crops, and the response of wild, herbaceous perennial species to artificial selection for increased seed yield remains under investigation. Here we compare cultivated and wild accessions of congeneric annual and herbaceous perennial legume species to investigate associations of lifespan and cultivation with early life stage traits including seed size, germination, and first year vegetative growth patterns, and to assess variation and covariation in these traits. We use “cultivated” to describe accessions with a history of human planting and use, which encompasses a continuum of domestication. Analyses focused on three annual and four perennial species of the economically important genus Phaseolus. We found a significant association of both lifespan and cultivation status with seed size (weight, two-dimensional lateral area, length), node number, and most biomass traits (with cultivation alone showing additional significant associations). Wild annual and perennial accessions primarily showed only slight differences in trait values. Relative to wild forms, both cultivated annual and cultivated perennial accessions exhibited greater seed size and larger overall vegetative size, with cultivated perennials showing greater mean trait differences relative to wild accessions than cultivated annuals. Germination proportion was significantly lower in cultivated relative to wild annual accessions, while no significant difference was observed between cultivated and wild perennial germination. Regardless of lifespan and cultivation status, seed size traits were positively correlated with most vegetative traits, and all biomass traits examined here were positively correlated. This study highlights some fundamental similarities and differences between annual and herbaceous perennial legumes and provides insights into how perennial legumes might respond to artificial selection compared to annual species.
Life history theory traditionally categorizes plants as annuals, typified by fast growth and high reproductive effort, and perennials, typified by long-term survival and delayed reproduction (Cole, 1954; Harper, 1977). However, exceptions to this general trend occur; patterns of plant resource allocation exist along a gradient, with some perennial species showing fast growth and high reproductive output (e.g., Verboom et al., 2004; González-Paleo and Ravetta, 2015). Exploration of this life history diversity is important in plant breeding, as wild, herbaceous perennial species are increasingly considered as sources of novel genetic variation through crosses and as entirely new domesticates targeted for seed products. Many major crops have perennial wild relatives that remain uncharacterized despite their potential.
Nearly all grain and legume crops grown for human consumption are annual plants that complete their life cycle in a single year, or are perennial species cultivated as annuals (Van Tassel et al., 2010). Although thousands of herbaceous perennial species exist within major crop families (e.g., Ciotir et al., 2016; Ciotir et al., 2019), annual plant species were likely selected during the early stages of domestication due to pre-existing agriculturally favorable traits, e.g., high reproductive yield in a single season and accelerated germination and flowering (Van Tassel et al., 2010). Over time, artificial selection has led to exceptional gains in reproductive output in annual crops, particularly in the last century (Mann, 1997); however, cultivation intensity and other agronomic practices have resulted in widespread soil loss (FAO, 2019). Current research focuses in part on the development of crops to support the ecological intensification of agriculture, which aims to achieve both high yields and ecosystem services, such as soil and water retention (Ryan et al., 2018).
The use of herbaceous perennial species as seed crops has been proposed as one method of achieving ecological intensification (Jordan and Warner, 2010; Ryan et al., 2018). Longer-lived species have deep, persistent root systems that mitigate erosion and enhance nutrient uptake, they produce perennating shoots which reduce yearly planting costs, and they have a longer photosynthetically active growth period, allowing for high biomass production each year (Cox et al., 2006; Crews et al., 2018). However, only a few perennial seed crops (principally cereals, oilseeds, and pulses) have entered the domestication process, and we know relatively little about how artificial selection for increased seed production will impact the rest of the perennial plant.
Artificial selection is the human-mediated evolutionary process that leads to changes in plant traits over the course of generations. This process happens as a result of selective cultivation, the act of preferentially planting individuals with desired features. Over time cultivated populations evolve in response to artificial selection, leading to domestication: the evolution of morphological and genetic changes in cultivated populations relative to their wild progenitors (Harlan, 1995; Meyer et al., 2012). Because domestication is an ongoing, evolutionary process, a continuum of cultivated populations exist in species undergoing domestication, ranging from cultivated populations which display little or no differences relative to their wild (uncultivated) progenitors, to highly modified elite breeding lines, such contemporary maize, which differs dramatically from its closest wild relatives (Harris, 1989; Bharucha and Pretty, 2010; Breseghello and Coelho, 2013). To determine the extent to which domestication has occurred, the precise identity of the wild ancestor of a crop and the exact cultivated populations derived from it are required. Because these data are not available for all species examined here, we use the term “cultivated” to refer to any accession that has a history of cultivation, acknowledging that this encompasses a broad spectrum of phenotypic and genetic change under artificial selection.
The “domestication syndrome” describes a common suite of trait changes seen across different species in response to artificial selection for seed and/or fruit production (Hammer, 1984; Harlan, 1992). In annual species, the domestication syndrome typically includes loss of seed dormancy, higher germination, loss of shattering, greater seed size or number, and erect, determinate growth, among many others (Harlan et al., 1973; Olsen and Wendel, 2013; Abbo et al., 2014). In contrast to annuals, the domestication syndrome of woody perennials, such as fruit and nut trees, is characterized by an extended juvenile phase, outcrossing mating system, and often clonal propagation; consequently, woody perennials have typically undergone fewer cycles of sexual selection under domestication and retain a greater proportion of wild genetic diversity relative to annual domesticates (Zohary and Spiegel-Roy, 1975; Miller and Gross, 2011; Gaut et al., 2015). Herbaceous perennial plants cultivated for both edible seeds and sustained perennation were only recently targeted for selection (Suneson et al., 1963; DeHaan et al., 2016; Kane et al., 2016; Kantar et al., 2016; Crews and Cattani, 2018), and it is unclear if they will follow an evolutionary trajectory similar to domesticated annual and woody perennial species, or if they will show a unique domestication syndrome.
The agricultural context provides an entirely new adaptive landscape that may allow novel combinations of traits in herbaceous perennial species (e.g., high reproductive output and longevity), combinations that are often unfavorable in natural environments (Crews and DeHaan, 2015; Cox et al., 2018). Ongoing work seeks to understand how artificial selection for increased seed yield in herbaceous perennial species might impact vegetative traits and the capacity for perennation more generally. One hypothesis is that perennial seed crops are constrained by a vegetative-reproductive trade-off, where high reproductive allocation and sufficient storage allocation for perennation cannot coexist (Van Tassel et al., 2010). In other words, it may be possible for artificial selection to drive increases in seed yield in wild, herbaceous perennial species, but those increases may cause losses in allocation to vegetative and perennating structures, resulting in a shift from perenniality to annuality (Denison, 2012; Smaje, 2015). Some studies have supported such a trade-off (e.g., González-Paleo et al., 2016; Vico et al., 2016; Cattani, 2017; Pastor-Pastor et al., 2018). An alternative hypothesis is that reproductive yield and vegetative biomass may be selected for in concert, leading to sustained perennation. Concomitant perennation and high seed yield have been observed in some perennial cereals (Sacks et al., 2007; Jaikumar et al., 2012; Culman et al., 2013; Huang et al., 2018).
Herbaceous perennial seed crop studies can benefit from including comparisons with closely related annual domesticates, to clarify if domestication responses are dependent upon lifespan. Vico et al. (2016), in a meta-analysis of 67 annual-perennial pair studies across nine plant families, found greater reproductive allocation in annual crops and greater root allocation in perennial crops, which was the same pattern found in unselected wild groups. Since a majority of the studies in this meta-analysis were from the grass family, such annual-perennial meta-analyses may be augmented by empirical research on specific lineages of plants, to determine if phylogenetically focused trends are similar to the broader patterns observed, as well as to allow a more precise biological interpretation.
Here we focus on the legume family (Fabaceae), which includes 19,500+ species of which more than 30% are predominantly herbaceous perennials (Ciotir et al., 2019). Fabaceae is the second most economically important plant family after the grasses (Poaceae), with 41 domesticated species dating back to the first agricultural systems, and 1,000+ species cultivated for various purposes across the world (Harlan, 1992; Lewis et al., 2005; Hammer and Khoshbakht, 2015). To date, few herbaceous perennial legume species have been thoroughly assessed for agriculturally relevant traits such as seed size, germination rate, time to maturity, root/shoot allocation, and reproductive yield. Characterizing these and similar traits in herbaceous perennial crops and their wild relatives will be critical to assess what genetic variation is available for crop improvement through introgression and de novo domestication (Schlautman et al., 2018; Smýkal et al., 2018).
While there are known consistent differences in growth and resource allocation in some groups of annual and perennial species, gaps remain in our knowledge about how herbaceous perennials respond to artificial selection relative to their annual congeners in many plant lineages. Here we explore life history differences in members of the common bean genus Phaseolus by addressing the following questions: 1) How do wild annual and perennial Phaseolus species from multiple geographic origins allocate resources to seed production and vegetative growth? 2) What is the phenotypic signature of artificial selection in cultivated annual and perennial Phaseolus species? 3) What covariation exists between seed and adult vegetative growth traits, and is it consistent across lifespans and between cultivated and wild forms in Phaseolus? We address these questions by examining seed size, germination, and vegetative growth allocation among three annual and four perennial Phaseolus species (Figure 1). Through this work, we hope to contribute to ongoing efforts characterizing life history differences in closely related annuals and perennials within Fabaceae and shed light on how these lifespan groups may change with artificial selection.
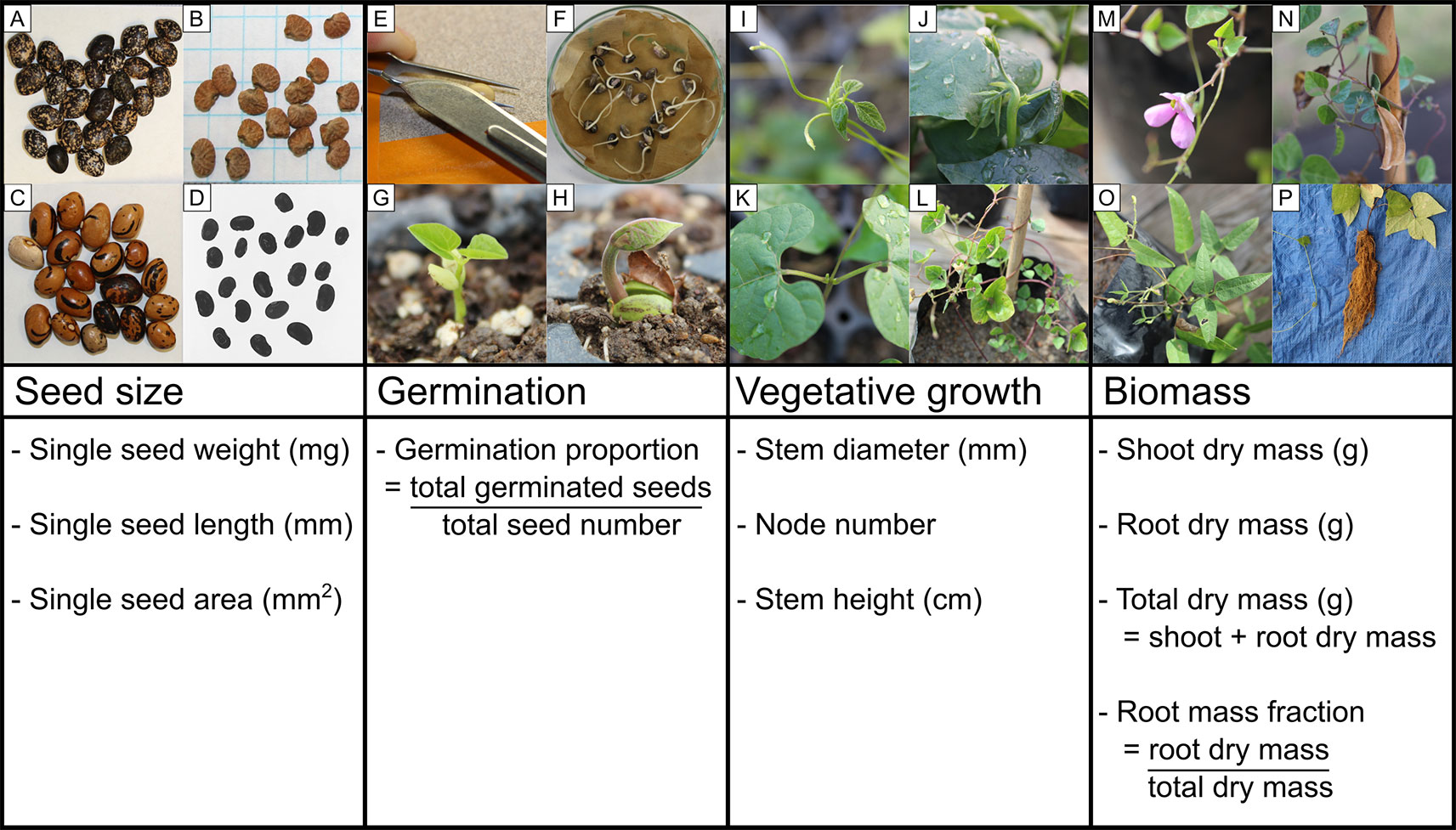
Figure 1 Schematic of the four developmental stages analyzed in this study (seed size, germination, early vegetative growth, and biomass harvest) and examples of phenotypic diversity across the Phaseolus species studied: seeds of (A) Phaseolus acutifolius, (B) P. angustissimus, and (C) P. coccineus; (D) P. coccineus seeds prepared for analysis in ImageJ; (E) scarification by nicking the seed coat; (F) P. vulgaris germinants; (G) epigeal germination of P. filiformis; (H) hypogeal germination of P. coccineus; (I, J) Phaseolus shoot apex at which stem height was measured to; (K) Phaseolus first node (unifoliate) below which stem diameter was measured; (L) fully grown P. filiformis shoot with developed nodes (unfolded leaves); (M, N) P. filiformis flower and ripe fruit; (O) P. acutifolius shoot biomass; (P) P. coccineus root biomass. Photo credit: SH.
Three annual Phaseolus species (P. acutifolius, P. filiformis, P. vulgaris; 58 accessions) and four perennial Phaseolus species (P. angustissimus, P. coccineus, P. dumosus, P. maculatus; 66 accessions) were included in this study (Tables 1 and 2). Species were chosen based on phylogenetic proximity and similar habitat types. The perennials P. coccineus and P. dumosus and annuals P. acutifolius and P. vulgaris are in the Vulgaris clade, and the perennial P. angustissimus and annual P. filiformis are in the Filiformis clade. Perennial P. maculatus is the only species in this study from the Polystachios clade (Delgado-Salinas et al., 2006). Geographically, our sampling of Phaseolus includes arid-adapted species of the Sonoran Desert (P. acutifolius, P. angustissimus, P. filiformis, and P. maculatus) and more tropically distributed species (primarily Mesoamerica: P. coccineus, P. dumosus, and P. vulgaris, with some South American accessions of the latter; Supplementary Table S4).
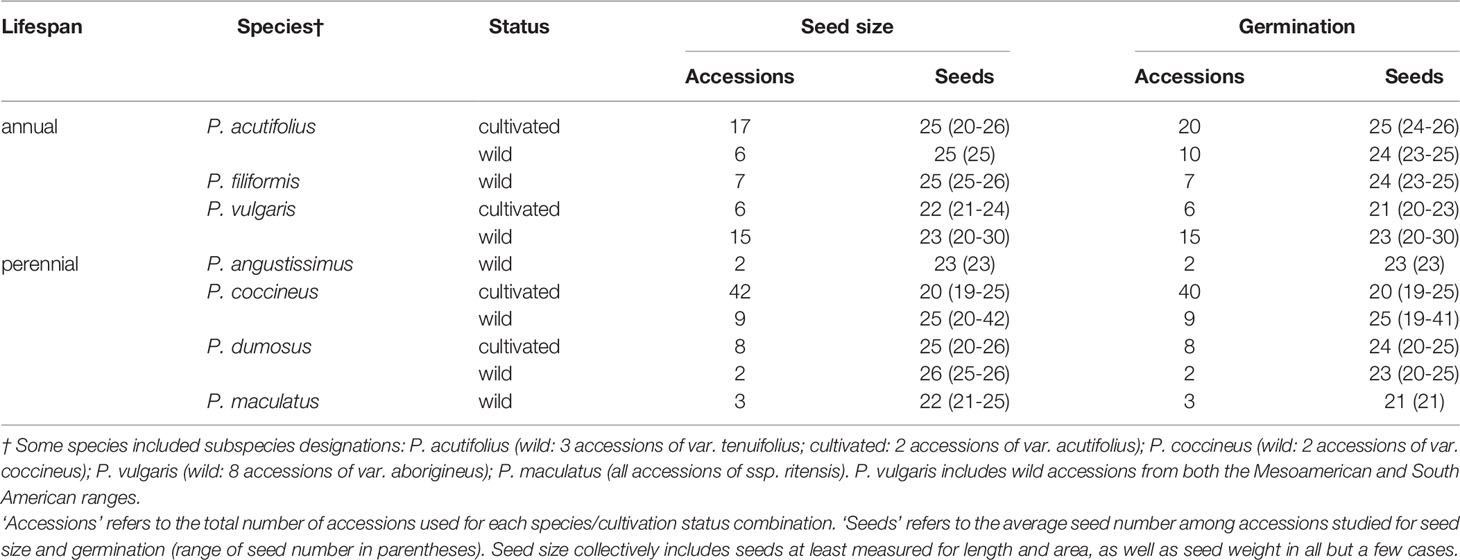
Table 1 Summary of sampling for seed size and germination traits for Phaseolus, by species and cultivation status.
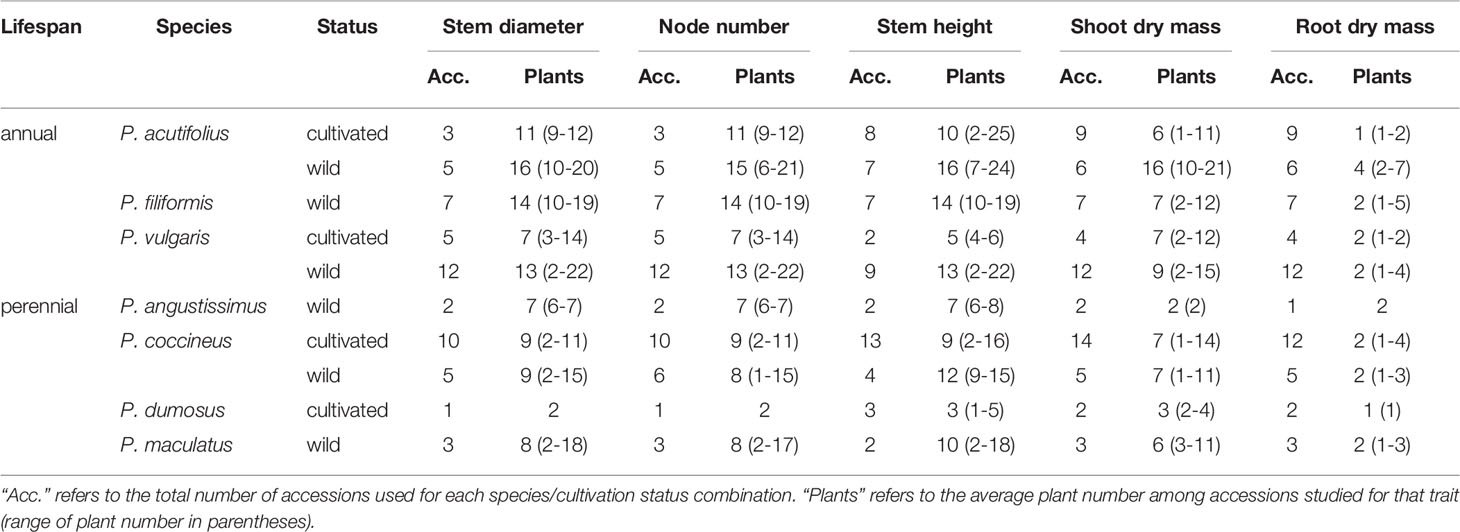
Table 2 Summary of sampling for early vegetative growth and biomass traits for Phaseolus, by species and cultivation status.
In total, we obtained seeds from 124 accessions from the United States Department of Agriculture's National Plant Germplasm System (Western Regional PI Station, Pullman, WA, stored at -18°C) in spring 2016, which were stored in a desiccator at 4°C, 33-50% relative humidity. All seeds were derived from plants regrown from the original collection material at the germplasm facility, but there were nevertheless possible genotype by environment and maternal effects that cannot be resolved here. 2,759 seeds were germinated and a subset of these grown from July to September 2016. The seed age for each accession, i.e., the length of time they were in frozen storage, ranged from one year to greater than 46 years (coded as 46).
We classified species in terms of the predominant lifespan observed in wild populations from their native range. In some cases, this differed from lifespan assignment in the USDA accession description, in which case it was confirmed by extensive literature review; this occurred for P. coccineus, P. dumosus, and P. filiformis. Wild P. coccineus is a vigorous, perennial, indeterminate vine with an extensive root system; perenniality is also maintained in many cultivated forms (Delgado-Salinas, 1988; Smartt, 1988; Debouck, 1992; Freytag and Debouck, 2002). P. dumosus, a hybrid of P. coccineus and P. vulgaris, is also perennial, although it is less frost tolerant than P. coccineus (Smartt, 1988; Schmit and Debouck, 1991; Debouck, 1992; Freytag and Debouck, 2002; Mina-Vargas et al., 2016). Lastly, P. filiformis is an ephemeral, annual vine primarily found in the Sonoran Desert, which can survive up to seven months in favorable conditions (Buhrow, 1983; Nabhan and Felger, 1985; Freytag and Debouck, 2002). In addition, one accession of P. maculatus (PI 494138) was labeled as annual, although the species in general and and other accessions of this species are classified as perennial (Freytag and Debouck, 2002).
Cultivation status was taken directly from the USDA's description, with the descriptors “cultivated,” “cultivar,” and “landrace” all categorized as “cultivated” for the purpose of this data set. We use the umbrella term “cultivated” rather than “domesticated,” since we do not have the data to determine the extent to which phenotypic and genetic change has occurred from the original wild population selected upon (see Introduction). All Phaseolus species are native to the Americas and were originally cultivated in either Mesoamerica or South America with some later selection occurring in Eurasia (Bitocchi et al., 2017). Cultivated Phaseolus species included here were first domesticated at least 1000 years before present, with most being domesticated much earlier (Kaplan and Lynch, 1999). Species-specific details on geographic origin and domestication are available in Supplementary Table S4.
A total of 51 annual accessions (1,227 seeds) and 66 perennial accessions (1,436 seeds) were analyzed for size traits (Figure 1; Table 1). Seeds from each accession were weighed in bulk to the nearest mg and mean single seed weight was estimated by dividing the bulk weight by the total number of seeds for that accession. We imaged all accessions on a light table with a fixed camera at a resolution of 640 × 360 or 1349 × 748 pixels (differences accounted for in linear models), with all seeds oriented on their lateral side. From these two-dimensional images, we used ImageJ (Rasband, 1997-2016) to measure mean single seed length and area.
58 annual accessions (1,390 seeds) and 64 perennial accessions (1,369 seeds) were monitored for germination (sampling differences from seed size are due to a few germinated accessions not being analyzed for seed size and vice versa, and some seed loss in the germination procedure). Number of germinated seeds was monitored for each accession and used to calculate germination proportion (Figure 1; Table 1). Seeds were germinated on RO-water dampened germination paper or with a dampened cotton ball in petri dishes, following 1) sterilization by soaking in 1% bleach for 2 minutes and rinsing, 2) scarification by nicking the seed coat with a scalpel (to break physical dormancy, i.e., a water-impermeable seed coat), and 3) soaking for an average of 23 hours (range: 13-32 hours) by submersion in RO water. The soaking start date ranged from June 26 to July 8, 2016 (one accession soaked on June 19); this was considered time point 0 (i.e., the sowing date) for germination counts, since all necessary resources were available for the seeds to germinate. The germination apparatus was placed onto a 24°C heat mat in 24-hour dark conditions (except for germinant counts and planting; López Herrera et al., 2001). Germination was defined as an extension of the radicle past the seed coat. In rare cases where the seed coat was lost or very hard, it was defined as a distinct vigorous movement of the radicle away from the seed or a distinct pushing of the seed coat away from the seed, respectively. Petri dishes were treated with Banrot 40WP fungicide solution (prepared according to the product label). Fungus-infected, potentially salvageable seeds were soaked in 1% to 2% bleach and rinsed with RO water. Germinated seeds were also scored for seed quality following any potential damage from pre-germination treatments (0-2, with 0 being no damage and 2 being the highest damage), and seeds which were compromised due to procedural damage or had prematurely germinated in storage were removed from the analysis. Germination was monitored prior to planting; accessions with remaining seeds were checked once more 7–10 days after planting for any new germinants. Any variation in germination protocol was noted and addressed in statistical models, as well as the covariates seed quality and seed age (years of frozen storage at the germplasm center since the last seed increase). Subsets of individuals from the original number germinated were chosen for further growth measurement based on the presence of ≥10 vigorous individuals, if the accession was from the native range of the species (preferred), and if the accession was from a duplicate geographic location (removed).
495 annual individuals (40 accessions) and 224 perennial individuals (29 accessions) of Phaseolus were transplanted to a greenhouse and measured for at least one vegetative trait (details below; Figure 1; Table 2). Seedlings were initially planted on July 13–14, 2016 in a mixture of unsterilized local riverine soil (Smoky Hill River, Salina, KS: 38.765948 N, -97.574213 W), sand, and potting soil (PRO-MIX) in small trays until they could be planted in 8” tall x 4” wide bag pots after 2-3 weeks. Initial planting date in small trays was used as the baseline for future measurements (days after planting, DAP), since despite different sowing dates, they were developmentally similar upon planting. Bag pots were filled with a mixture of the same riverine soil and coarse sand, to mimic field soil while also maximizing drainage. All Phaseolus were twining and were trained up four-foot bamboo poles. Plants did not receive any rhizobial inoculant treatment. Plants were initially bottom-watered twice daily (10AM, 6PM) for 20 minutes, and a shade cloth was incorporated in the greenhouse. After measurement of early vegetative growth (before biomass), some modifications were made. On August 18, 2016, watering was changed to once for 10 minutes every two days, and the shade cloth was removed on August 23, 2016. 90 of the most vigorous Phaseolus plants were moved from the greenhouse to the outdoors on August 25-26, 2016 to expose them to a more light-intense, natural environment. At this time, individual plants in both the greenhouse and outdoors were randomized to reduce spatial bias. All growth analyses were conducted at the research facilities of The Land Institute (Salina, KS).
At 19-23 DAP (25-40 days after sowing), plants were measured for early vegetative growth traits, which included stem diameter below the first node, total developed node number counted from the unifoliate node to the last node with an unfolded leaf, and stem height from ground to shoot apex on the tallest main stem, with twining stems being uncoiled from their poles and straightened as far as possible without damaging the plant. Plants were checked for reproductive status before biomass harvest. At 68-75 DAP (74-93 days after sowing), a random subset of plants was harvested for shoot and root (washed) biomass (Figure 1; Table 2), which was dried at a minimum of 37°C for at least 24 hours. Biomass was weighed on a precision or analytical scale depending on plant size. Root mass fraction was calculated on an individual plant basis from biomass measurements (root dry mass/total dry mass). Variation in growth conditions (greenhouse or outdoors), plant health (ordinal rating: 0,1,2; unhealthy, moderate health, healthy), and reproductive status (ordinal rating: 0,1,2,3; no reproduction, budding, flowering, fruiting) were recorded for individual plants and accounted for in statistical analyses (see below).
In order to assess associations of lifespan and cultivation status with trait variation, we used linear models and post hoc comparative analyses on a set of mean values for each trait from each accession (Table 3). Associations of lifespan, cultivation (nested within lifespan), and species (nested within lifespan and cultivation), in addition to any relevant covariates for the focal trait, were tested using linear models. All potentially confounding factors were included in the original model and were then dropped sequentially if found to be nonsignificant. The base model for all main analyses was: trait = lifespan + lifespan/cultivation + lifespan/cultivation/species. Analyses were calculated for accession-level means for all traits and covariates. Due to concerns about lifespan lability of P. dumosus, linear models for all traits were checked with this species included and then with the species removed from the dataset. Accessions with any uncertainty associated with their lifespan or cultivation status were also dropped from the model and checked in the same manner; these included PI 494138 (P. maculatus; called annual while usually perennial—see Lifespan Assignment) and PI 390770 (P. vulgaris, noted as “wild or naturalized” in the USDA description). Pairwise comparisons of lifespan and cultivation effects for each trait were evaluated with post-hoc Tukey HSD tests (Supplementary Table S1).
We ran separate linear models and Tukey HSD tests for Phaseolus wild accessions to detect any phenotypic signatures of geographic origin, divided broadly into desert-adapted and tropical-adapted species (Supplementary Tables S2 and S3). The base model for the geographic analyses was: trait = geography + lifespan + geography×lifespan + geography/lifespan/species, including any relevant covariates. Cultivated accessions were not included in this model due to potentially confounding effects of artificial selection. See Supplementary Table S4 for the assignment of geographic origin categories to species.
To assess trait covariation within the whole dataset, Pearson product-moment correlations were performed on all pairwise combinations of the 11 measured traits using mean accession-level data, to determine the magnitude and direction of their relationships. Pearson correlations were also run on the following subsets of the data to qualitatively assess any trait covariation differences: annual, perennial, cultivated, and wild (Supplementary Figure S1). Each subset of the data was only restrictive in regard to one criterion, e.g., the annual subset contained data for both cultivated and wild annual accessions. Statistical analyses and figure generation were performed in R v. 3.6.1 (R Core Team, 2019).
All accession-level and individual plant data can be found in Supplemental Table S5. All individual seed size data may be found in Supplemental Table S6.
We investigated annual and perennial Phaseolus species for potential differences and covariation in seed size, germination, and vegetative growth. We found that wild accessions of annual species showed nonsignificantly greater germination and vegetative trait values than wild perennial accessions, but did not show greater seed size traits (Figure 2). Cultivated accessions of both annual and perennial species had greater trait values compared to wild accessions for almost all traits measured, with greater mean increases observed in perennial species (Figure 2). Lastly, seed and vegetative traits were significantly positively correlated, with some variation in this trend within subsets of the data (Figure 3; Supplementary Figure S1).
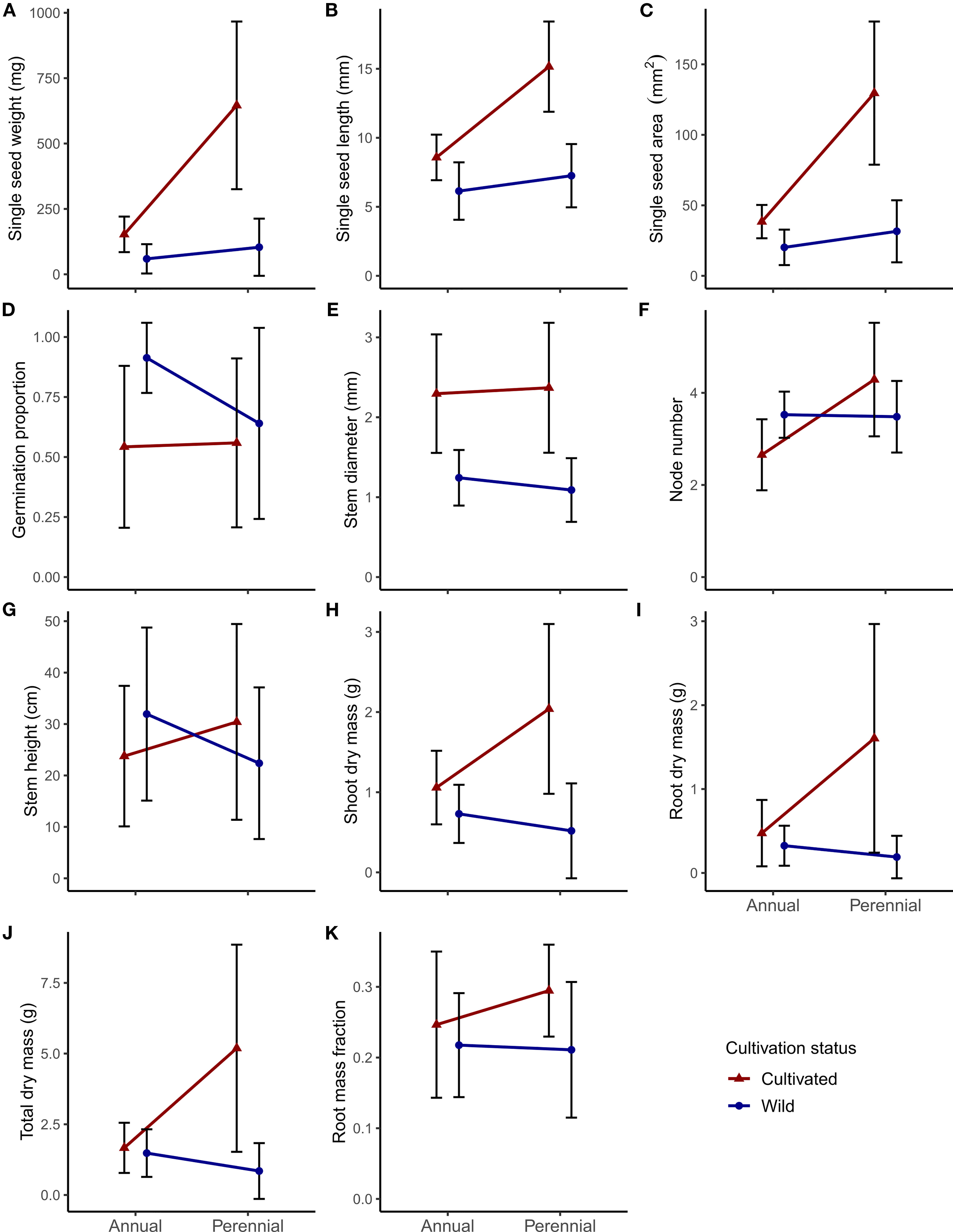
Figure 2 Panel of phenotypic differences between lifespan (annual or perennial) and cultivation status (cultivated or wild) for the 11 focal traits in Phaseolus: (A–C) seed size traits; (D) germination proportion; (E–G) early vegetative growth traits; (H–K) shoot and root biomass traits. Central points represent means of all accessions for that category; error bars represent one standard deviation.
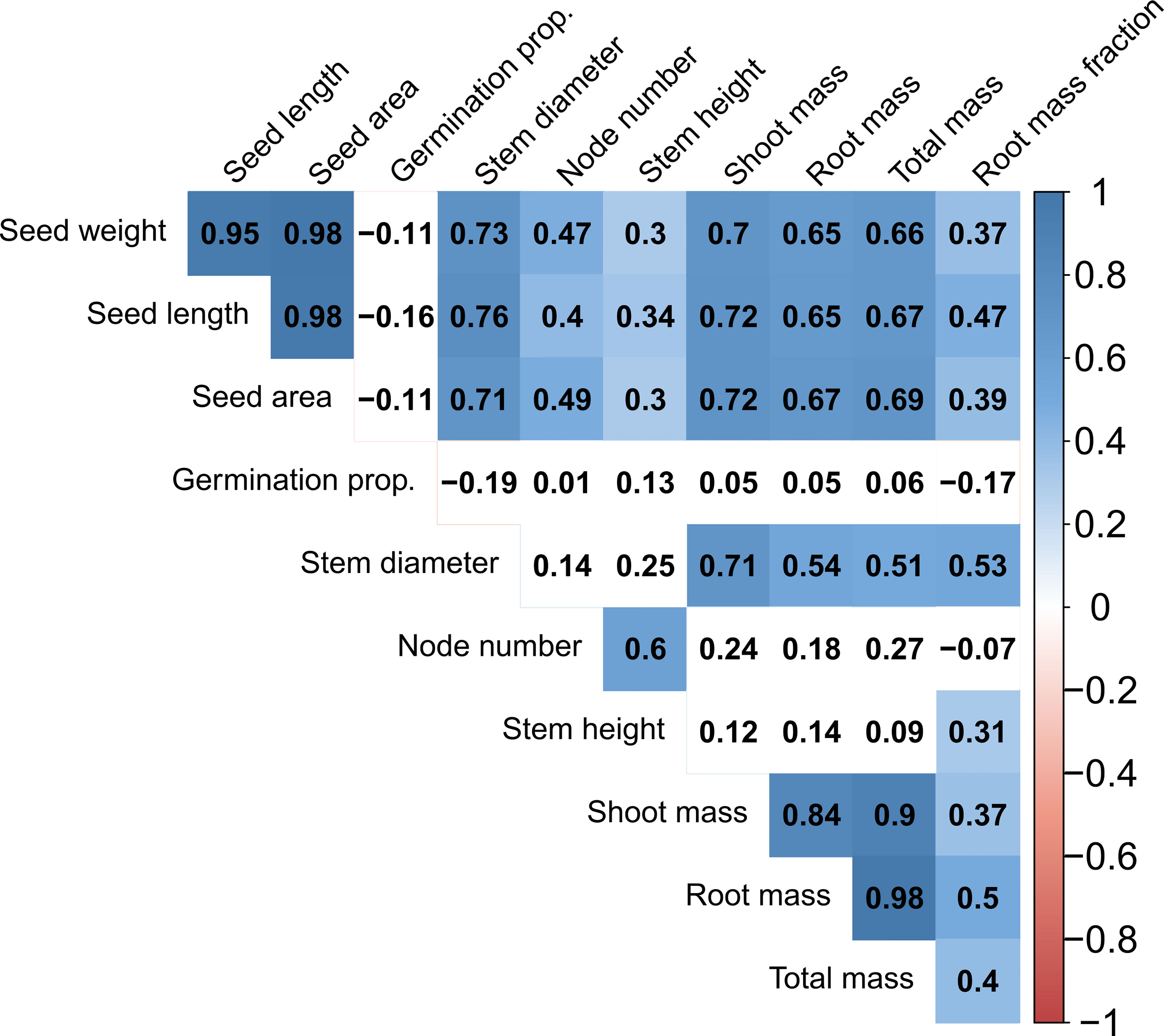
Figure 3 Correlation diagram of all traits for all accessions in the Phaseolus dataset. Numbers in boxes represent the Pearson correlation coefficient. Blue and red colors indicate significant positive and negative correlations (at P < 0.05), respectively; absence of color indicates lack of significance.
Although nonsignificant, wild perennial Phaseolus mean seed weight was nearly twice that of wild annuals (59 mg annual vs. 104 mg perennial; Figure 2A; see Supplementary Table S1 for all mean values, standard deviation, and Tukey test significance). Wild annual germination proportion was nonsignificantly higher than that of wild perennials (0.91 annual vs. 0.64 perennial; Figure 2D; Supplementary Table S1). Wild annual Phaseolus had similar to nonsignificantly larger vegetative trait values compared to wild perennials, with the largest relative differences seen in root dry mass (0.32 g annual vs. 0.19 g perennial) and total dry mass (1.48 g annual vs. 0.85 g perennial; Figures 2I, J; Supplementary Table S1). Mean root mass fraction was nearly equivalent for wild annuals (0.22) and wild perennials (0.21; Figure 2K; Supplementary Table S1).
In our main linear models, lifespan explained a significant amount of the variation seen in all seed size traits, node number, and most biomass traits (except root mass fraction; Table 3). Seed age and soak time were not significant predictors of germination; seed quality had significance at P < 0.001 (Table 3). Plant health was significant (at least at P < 0.01) in the linear models for all vegetative growth and biomass traits except stem diameter and shoot dry mass (Table 3). Reproductive status was not significant for any biomass trait; outdoor proportion was significant at P < 0.05 for shoot dry mass and root mass fraction (Table 3). The removal of P. dumosus and the accessions PI 494138 (P. maculatus) and PI 390770 (P. vulgaris; see Methods) changed some linear model results; here we note traits for which any variable's significance was lost or gained in the main model, which occurred for some vegetative traits. The exclusion of P. dumosus resulted in lifespan becoming significant for stem diameter (P < 0.05), in species becoming nonsignificant for shoot dry mass, and in outdoor proportion becoming nonsignificant for root mass fraction. Similarly, the exclusion of PI 494138 resulted in lifespan becoming significant for stem diameter (P < 0.05) but also for root mass fraction (P < 0.05). The exclusion of PI 390770 did not change significance in any of our models.
While our data do not allow precise interpretation of geographic effects, linear models including geographic origin as a main effect along with lifespan found that geographic origin explained a significant portion of the variation seen in all seed size traits and most vegetative traits for wild Phaseolus accessions (Supplementary Table S2). In general, tropically distributed Phaseolus accessions had larger seed and vegetative growth traits compared to desert species (Supplementary Table S3). Mean germination proportion was relatively high (> 0.85) for all Phaseolus groups except tropical perennials (0.48, although tropical perennials also had the greatest standard deviation; Supplementary Table S3).
Cultivated annual and perennial Phaseolus accessions showed generally larger seed and vegetative size characteristics compared to wild relatives (Figures 2). Cultivation differences in seed size were only significant for seed length in annual Phaseolus, although cultivated annual seed weight (153 mg) was nearly three times larger than wild annuals (59 mg) (Figure 2A; Supplementary Table S1). Cultivated perennial Phaseolus had significantly greater seed size in all traits, with seed weight over six times larger in cultivated perennials (646 mg) than wild perennials (104 mg) (Figure 2A; Supplementary Table S1). Germination proportion was significantly lower in cultivated annual Phaseolus (0.54) relative to wild annuals (0.91), while cultivated perennial germination proportion was only slightly lower (0.56) than wild perennials (0.64) (Figure 2D; Supplementary Table S1).
Cultivated perennial Phaseolus accessions tended to have significantly larger vegetative features compared to their wild relatives, whereas cultivated annual Phaseolus accessions usually displayed nonsignificantly larger vegetative features compared to their wild relatives (Figures 2E–K; Supplementary Table S1). Stem diameter was however significantly greater in both cultivated annual and perennial Phaseolus (Figure 2E; Supplementary Table S1). Cultivated annual Phaseolus showed nonsignificantly lower values in node number and stem height compared to wild annuals, while cultivated perennial Phaseolus showed nonsignificantly larger values in both traits compared to wild perennials (Figures 2F, G; Supplementary Table S1). Both cultivated annual and perennial Phaseolus had greater dry biomass trait values than their wild relatives, but for cultivated annual Phaseolus, the only significantly larger biomass value was shoot dry mass (1.06 g cultivated vs. 0.73 g wild; Figure 2H; Supplementary Table S1). In contrast, all biomass trait values were significantly greater in cultivated relative to wild perennial Phaseolus (except root mass fraction), with a greater than six-fold larger root dry mass value (1.60 g cultivated vs. 0.19 g wild; Figure 2I; Supplementary Table S1). In our linear models, cultivation explained a significant amount of variation for all traits except stem height (Table 3).
Trait covariation was predominantly positive within the total dataset (Figure 3) and each subset of the data (Supplementary Figures S1A-D). Considering the entire dataset, all seed dimensions (weight, length, area) were nearly perfectly correlated (R2 = 0.95-0.98; Figure 3). Seed traits were also significantly positively correlated with all vegetative growth traits, having the highest correlation with stem diameter and most biomass traits (R2 = 0.65-0.76; Figure 3). Stem diameter had positive albeit non-significant correlations with node number and stem height, and significant positive correlations with all biomass traits. Node number and stem height had nonsignificant positive correlations with most biomass traits (Figure 3). Biomass traits including shoot dry mass, root dry mass, total dry mass, and root mass fraction were significantly positively correlated with one another, and there was a tight relationship between shoot and root dry mass (R2 = 0.84; Figure 3). Germination proportion was the only trait in the total dataset to have no significant correlations and to have more than two negative correlations with other traits (Figure 3).
Subsets of our data also displayed similarly positive trait correlations with some exceptions. Due to sample size, each subset was restricted in regard to only one criterion (lifespan or cultivation status), e.g., the perennial subset contained both cultivated and wild perennial accessions. Notably, the annual subset showed negative correlations between node number and most traits (significant for seed size traits, stem diameter, and shoot dry mass), as well as a significant negative correlation of germination proportion with seed weight and stem diameter (Supplementary Figure S1A). In contrast, the perennial, cultivated, and wild subsets respectively tended to show positive correlations for the same trait pairings which were negatively correlated in annuals, although germination proportion and node number showed at least one negative correlation in all subsets (Supplementary Figure S1B-D). Significant positive seed size to vegetative trait correlations tended to be stronger and more common in the perennial and cultivated subsets than the annual and wild subsets (Supplementary Figures S1).
This study examined lifespan and cultivation effects among annual and herbaceous perennial legume species in the economically important genus Phaseolus, with the aim of identifying common trends and potential covariation among traits. Consistent trends included greater seed and plant size in cultivated relative to wild accessions for both annual and perennial species, and positive correlations among most seed and vegetative traits for annual and perennial species in the wild and under cultivation.
Wild perennial Phaseolus accessions in this study exhibited somewhat greater seed size trait values relative to wild annual accessions, which is consistent with the general life history expectation that later successional species produce larger seeds that are better able to compete for resources (Thompson and Hodkinson, 1998). This trend was consistent for Phaseolus species native to the desert and the tropics (Supplementary Table S3). Tropical Phaseolus species had higher mean seed size than desert Phaseolus, which is consistent with the broad pattern of increasing seed size at lower latitudes (Moles et al., 2007; Supplementary Table S3). The large range of phenotypic variation observed in annual and perennial Phaseolus (Figures 2A–K) is also consistent with the large distributions of several species, such as P. vulgaris and P. coccineus, which inhabit environments ranging from very arid to very humid (Dohle et al., 2019).
While few studies have compared germination in wild Phaseolus species, Bayuelo-Jiménez et al. (2002) reported a similarly high germination proportion (0.85+) for scarified wild annual and perennial Phaseolus accessions, including the desert species P. angustissimus and P. filiformis. Although wild annuals showed higher mean germination than wild perennials considering the whole dataset, desert perennial accessions all reached 100% germination, suggesting that the broader trend is more applicable to the tropical species studied here (Supplementary Table S3). Our lowest wild mean germination was observed in the tropical perennials (P. coccineus and P. dumosus; Supplementary Table S3), which may reflect the trend that seed dormancy is less common in tropical herbaceous species in regions of higher rainfall (Baskin and Baskin, 2014).
While germination showed consistent differences between wild annuals and perennials, we cannot make ecologically robust conclusions here, since we effectively removed the legumes' primary form of physical dormancy through scarification, and each accession was stored frozen for different lengths of time at USDA facilities, which can have diverse effects on species' germination biology (Walters et al., 2005). Our results may be more reflective of the viability of seeds after long-term storage. In this light, our findings are consistent with life history predictions that annual species will maintain a complex, long-lived seed bank in which dormancy breaking and germination is staggered over time to ensure offspring survival in variable environments (Venable and Lawlor, 1980; Gremer et al., 2016). Perennials may be less selectively constrained by this pressure due to the parent plant's persistent survival in more stable environments (Cohen, 1966; Thompson et al., 1998).
The lack of significant differences in vegetative traits between wild annual and perennial Phaseolus suggests that the traits studied here do not diverge at this growth stage among different Phaseolus life history strategies in nature. Wild perennials' slightly lower mean vegetative growth could be due to a slower growth rate and the early stage of growth at which the traits were measured (three to eleven weeks after planting), since some perennials have been shown to be able to achieve a higher total biomass than related annuals when the entire growing season is considered (Dohleman and Long, 2009). Previous studies have also found that vegetative growth, shoot biomass, and root biomass are similar between closely related annuals and perennials, up to 40 days of growth, before their resource allocation patterns diverge (De Souza and Vieira Da Silva, 1987; Garnier, 1992). The significance of plant health in vegetative linear models for stem height and node number may reflect a greater sensitivity of response in stem length traits to environmental stressors compared to stem diameter; the health effect on root dry mass and derived traits (total dry mass and root mass fraction) may be due to a lower sample size for these traits compared to shoot dry mass alone (Tables 2 and 3).
Phaseolus species showed larger seed and vegetative trait values in cultivated relative to wild accessions, consistent with domestication syndrome expectations; however, there were some notable differences in this trend between the annual and perennial groups. Consistent with previous studies in Phaseolus (Smartt, 1988; Koinange et al., 1996; Aragao et al., 2011), all cultivated annuals and perennials exhibited greater seed size relative to wild accessions. Perennial Phaseolus showed a particularly large difference in cultivated vs. wild seed size, as well as substantial variation in this trait, suggesting a large amount of genetic diversity. Much of the perennial seed size variation stemmed from P. coccineus, which is primarily outcrossing and more genetically diverse than other Phaseolus crops (Bitocchi et al., 2017; Guerra-García et al., 2017). Our seed size observations in USDA accessions are consistent with an analysis of wild and domesticated Phaseolus accessions from the International Center for Tropical Agriculture (CIAT), with the exception that they observed larger ranges of seed weight in cultivated annual species (P. acutifolius, P. vulgaris; Chacón-Sánchez, 2018). They similarly found that the perennial P. coccineus had the largest range in seed size in both cultivated and wild accessions, that P. coccineus showed the largest mean seed weight increase with cultivation, and that the perennial P. dumosus also showed some of the largest cultivated seed weights (Chacón-Sánchez, 2018).
Cultivation effects on Phaseolus germination may reflect different annual-perennial seed dormancy strategies. Domestication in annual common bean (P. vulgaris) and other seed crops has been known to reduce seed coat thickness and seed dormancy (Koinange et al., 1996; Fuller and Allaby, 2009), which could expose cultivated accessions to premature water imbibition and seed mortality in storage (López Herrera et al., 2001). Comparably low germination proportion in cultivated and wild perennial Phaseolus could be due to a less selective pressure for seed dormancy (and therefore lower seed longevity) in wild perennials relative to wild annuals, resulting in less change in germination proportion with domestication. Since our accessions were of various ages and geographic origin, more precise studies on fresh seedstock are necessary to confirm germination differences with cultivation for both annual and perennial species.
Vegetative size was generally larger in cultivated relative to wild annuals and perennials, although there were some notable exceptions. Mean node number and stem height were lower in cultivated annual Phaseolus and higher in cultivated perennial Phaseolus relative to wild accessions. Similarly, node number and height have been found to be lower in cultivated relative to wild annual P. vulgaris (Koinange et al., 1996; Berny Mier y Teran et al., 2018). This suggests that on average cultivated annual Phaseolus exhibit lower degrees of stem growth, which may be the result of direct selection for determinate growth or an indirect consequence of increased harvest index, i.e., biomass allocated to reproductive structures at the expense of vegetative growth. This is in spite of all of the cultivated accessions in this study retaining their twining habit. In contrast, cultivated perennial Phaseolus exhibited consistently higher values for vegetative growth relative to wild perennials, possibly indicating a persistence of indeterminate growth in cultivation (Smartt, 1988), allowing for simultaneous selection on increased vegetative growth and reproduction (but see Delgado-Salinas, 1988). Both cultivated annual and cultivated perennial accessions displayed higher shoot and root dry mass relative to wild accessions, in agreement with the findings of Berny Mier y Teran et al. (2018) in P. vulgaris; this coincided with higher values in stem diameter for cultivated accessions of both lifespans, in agreement with Smartt (1988). Under cultivation, higher shoot and root biomass relative to wild accessions have also been observed in 30 diverse annual crop species, attributed to greater seed size and leaf area allowing enhanced downstream effects on growth (Milla et al., 2014; Milla and Matesanz, 2017). Our study suggests that some cultivated perennial species in Phaseolus exhibit similar size increases.
Root mass fraction, a resource-conservative trait, exhibited higher values in cultivated relative to wild accessions for both lifespans; this contradicts the expectation that plants become more resource-acquisitive with domestication (McKey et al., 2012; Vilela and González-Paleo, 2015; Pastor-Pastor et al., 2018). Higher root mass fraction was also observed in the annuals Phaseolus vulgaris (Berny Mier y Teran et al., 2018) and Pisum sativum (Weeden, 2007) relative to wild forms. This difference could be a byproduct of a more general higher allocation to vegetative growth, allowing greater amounts of photosynthate to be allocated to roots (Weeden, 2007). In summary, while many vegetative traits exhibit higher values in cultivated relative to wild accessions for both annual and perennial Phaseolus species, some traits may exhibit divergent lifespan patterns. It remains to be determined if this is due to lifespan per se or each species' unique history of artificial selection.
Positive correlations among traits observed in our dataset suggest that some suites of traits are synergistic, including seed size, some early growth traits, and biomass allocation (Figure 3). Positive seed-vegetative size correlations have also been found in other herbaceous plant systems (Geber, 1990; Kleyer et al., 2019), as well as across vascular plants more generally (Díaz et al., 2015). The nearly perfect correlation among seed size parameters (weight, area, and length) suggests that both two-dimensional seed features and mass are jointly selected upon and can be used interchangeably for these species. Significant positive correlations between seed size and vegetative biomass are consistent with the fundamental constraint of plant size on seed size, i.e., small plants cannot produce very large seeds (Venable and Rees, 2009). Furthermore, positive correlations between stem diameter and seed size support the hypothesis that seed size is biomechanically limited by the size of the subtending branch (Aarssen, 2005; Venable and Rees, 2009). Seed size alone may be less reflective of whole-plant reproductive allocation (Vico et al., 2016) and more so of a tolerance-fecundity tradeoff, where larger seeds are produced in fewer numbers but have greater competitive ability in stressful conditions (Muller-Landau, 2010). Nevertheless, the perennial grain crop Thinopyrum intermedium showed a positive relationship of total biomass and single seed mass with total reproductive yield across three years of growth, suggesting that these traits are positively correlated in some perennial species (Cattani and Asselin, 2018; also see Kleyer et al., 2019). Lastly, significant positive associations between all dry biomass traits do not support aboveground-belowground tradeoffs; rather, the data suggest that larger plants require greater resource acquisition with proportionally larger roots. This is consistent with a previous study reporting positive correlations among shoot dry mass, root dry mass, and root mass fraction in Phaseolus vulgaris (Berny Mier y Teran et al., 2018).
There were a few notable exceptions to the general trend of positive correlations. There were unexpectedly no significant correlations between seed size and germination proportion considering the entire dataset, with only slight negative correlations detected. There is a theoretical expectation that germination dormancy and seed bank persistence are negatively related to seed size, since dormancy and seed size entail different strategies of surviving in different environmental conditions, i.e., small, dormant seeds dominate seasonal environments and large, non-dormant seeds are typical of aseasonal environments (Baskin and Baskin, 2014; Rubio de Casas et al., 2017). The annual and wild subsets showed greater negative seed size-germination correlations (significant for germination vs. seed weight for annuals), contrasting with positive correlations in the perennial and cultivated subsets, suggesting some association of lifespan and cultivation with this trait relationship (Supplementary Figure S1). The removal of the physical dormancy barrier through scarification must also be considered, although we may still expect differences in seed longevity in storage proportional to seed size.
There was also a general lack of significant covariation between early vegetative stem growth traits (stem height and node number) and most biomass traits (Figure 3). This is in contrast to a previous study reporting that stem height is a highly interconnected hub trait in herbaceous perennial species (Kleyer et al., 2019), although we find many of the same positive associations between vegetative and seed size traits. The significant negative correlations detected in annual species between seed size traits and node number may reflect tradeoffs between seed size and stem length allocation due to the biomechanical restraints of bearing larger seeds on a longer stem (Kleyer et al., 2019) or may be the result of reduced stem growth during artificial selection (see above discussion), although this correlation was positive in all other data subsets (Supplementary Figure S1).
Overall, positive correlations among traits measured here suggests that future breeding efforts targeting greater seed size in perennials may be possible without concomitant reductions in vegetative allocation. Consistently high correlations may also allow for measurement of simpler traits (e.g., stem diameter, shoot dry mass) as proxies for traits that are more difficult to assess (e.g., root dry mass, total yield).
For this set of Phaseolus species, we found that cultivation is associated with an increase in seed size and overall vegetative size in both annuals and perennials, and that seed size and most vegetative traits were positively correlated in both cultivated and wild annuals and perennials. Traits are shaped and predicted by more specific factors than lifespan alone, including habitat differences, evolutionary history, and which specific organs were targeted during artificial selection. Resolving these factors will require a focused sample of close sister species from the same native range, or intraspecific ecotypes of differing lifespan. Also, all perennials in our study were analyzed in their first year of growth, while their overall life history strategy is contingent upon their survival across multiple years. Thus, this study may also be augmented by multiyear assessments of reproductive and vegetative trait variation in perennial individuals. Such studies will advance our basic knowledge of life history evolution and inform plant breeding as we determine viable methods of ecological intensification. Conclusively, we highlight here common features in cultivated annual and perennial species compared to their wild relatives, and we observed few tradeoffs among seed and vegetative traits in the first year of growth. This offers insight into how perennial legume crops may respond to artificial selection relative to their annual relatives, and it suggests that many traits of interest may be selected for in concert.
All datasets generated for this study are included in the article/Supplementary Material.
AM and SH designed the project. SH implemented the project and wrote the manuscript, with significant input from AM and MR. MR assisted in statistical analysis and figure generation. CC, TC, and DT provided extensive technical assistance, helpful feedback on the basis and design of the study, and revisions to the manuscript. All authors read and approved the final version of the manuscript.
This research was funded by the Perennial Agriculture Project (Malone Family Land Preservation Foundation and The Land Institute). SH is supported by a graduate assistantship from Saint Louis University. MR is supported by the Donald Danforth Plant Science Center and the Perennial Agriculture Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Land Institute provided research tools and space for this project. Seeds were provided by the United States Department of Agriculture Western Regional PI Station (Pullman, WA). We are especially grateful to Daniel Debouck and Colin Khoury for their extensive feedback on Phaseolus species' biology and lifespan assignment. We thank the following Land Institute researchers and associates for valuable input in experimental design and intellectual contributions: Lee R. DeHaan, Matthew Newell, Damian A. Ravetta, Alejandra Vilela, and Shuwen Wang. We extend a special thanks to The Land Institute interns, technicians, and associates who assisted in experimental set-up, tending plants, and measuring traits: Mindelena Adams, Sheila Cox, Eliot Cusick, Tiffany Durr, Nick Feijen, Katherine Fortin, Maya Kathrineberg, Laura Kemp, Ron Kinkelar, Jordan Lowry, Maged Nosshi, Diego Sanchez, Codie Van de Meter, and Eline Van de Ven. Brandon Schlautman and REU student Dahlia Martinez also assisted in phenotyping and plant caretaking. We are grateful to the Miller Lab Group for valuable comments on previous versions of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00034/full#supplementary-material
Aarssen, L. W. (2005). Why don't bigger plants have proportionately bigger seeds? Oikos 111, 199–207. doi: 10.1111/j.0030-1299.2005.14206.x
Abbo, S., Pinhasi, R., Gopher, A., Saranga, Y., Ofner, I., Peleg, Z. (2014). Plant domestication versus crop evolution: a conceptual framework for cereals and grain legumes. Trends Plant Sci. 19, 351–360. doi: 10.1016/j.tplants.2013.12.002
Aragao, F. J., Brondani, R. P., Burle, M. L. (2011). “Phaseolus. ” in Wild Crop Relatives: Genomic and Breeding Resources. Legume Crops and Forages, 1st ed. Ed. Kole, C. (Springer-Verlag Berlin Heidelberg), 223–236. doi: 10.1007/978-3-642-14387-8
Baskin, C. C., Baskin, J. M. (2014). Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. (San Diego, CA: Academic Press), 1600.
Bayuelo-Jiménez, J. S., Craig, R., Lynch, J. P. (2002). Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci. 42, 1584–1594. doi: 10.2135/cropsci2002.1584
Berny Mier y Teran, J. C., Konzen, E. R., Medina, V., Palkovic, A., Ariani, A., Tsai, S. M., et al. (2018). Root and shoot variation in relation to potential intermittent drought adaptation of Mesoamerican wild common bean (Phaseolus vulgaris L.). Ann. Bot. 20, mcy221. doi: 10.1093/aob/mcy221
Bharucha, Z., Pretty, J. (2010). The roles and values of wild foods in agricultural systems. Philos. Trans. R. Soc. B.: Biol. Sci. 365, 2913–2926. doi: 10.1098/rstb.2010.0123
Bitocchi, E., Rau, D., Bellucci, E., Rodriguez, M., Murgia, M. L., Gioia, T., et al. (2017). Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front. Plant Sci. 8, 722. doi: 10.3389/fpls.2017.00722
Breseghello, F., Coelho, A. S. G. (2013). Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 61, 8277–8286. doi: 10.1021/jf305531j
Cattani, D. J., Asselin, S. R. (2018). Has selection for grain yield altered intermediate wheatgrass? Sustainability 10, 688. doi: 10.3390/su10030688
Cattani, D. J. (2017). Selection of a perennial grain for seed productivity across years: intermediate wheatgrass as a test species. Can. J. Plant Sci. 524, 516–524. doi: 10.1139/cjps-2016-0280
Chacón-Sánchez, M. I. (2018). “The domestication syndrome in Phaseolus crop plants: A review of two key domestication traits,” in Origin and Evolution of Biodiversity. Ed. Pontarotti, P. (Cham, Switzerland: Springer), 37–59. doi: 10.1007/978-3-319-95954-2
Ciotir, C., Townesmith, A., Applequist, W., Herron, S., Van Tassel, D., DeHaan, L., et al. (2016). Global Inventory and Systematic Evaluation of Perennial Grain, Legume, and Oilseed Species for Pre-breeding and Domestication. [http://www.tropicos.org/Project/PAPGI: month, 2016]. Missouri Botanical Garden, St. Louis, Missouri, USA.
Ciotir, C., Applequist, W., Crews, T. E., Cristea, N., DeHaan, L. R., Frawley, E., et al. (2019). Building a botanical foundation for perennial agriculture: Global inventory of wild, perennial herbaceous Fabaceae species. Plants People Planet 1, 375–386. doi: 10.1002/ppp3.37
Cohen, D. (1966). Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 12, 119–129. doi: 10.1016/0022-5193(66)90188-3
Cole, L. C. (1954). The population consequences of life history phenomena. Q. Rev. Biol. 29, 103–137. doi: 10.1086/400074
Cox, T. S., Glover, J. D., Van Tassel, D. L., Cox, C. M., DeHaan, L. R. (2006). Prospects for developing perennial grain crops. BioScience 56, 649–659. doi: 10.1641/0006-3568(2006)56
Cox, S., Nabukalu, P., Paterson, A. H., Kong, W., Nakasagga, S. (2018). Development of perennial grain sorghum. Sustainability 10, 172. doi: 10.3390/su10010172
Crews, T. E., Cattani, D. J. (2018). Strategies, advances, and challenges in breeding perennial grain crops. Sustainability 10, 2192. doi: 10.3390/su10072192
Crews, T. E., DeHaan, L. R. (2015). The strong perennial vision: a response. Agroecol. Sustain. Food Syst. 39, 500–515. doi: 10.1080/21683565.2015.1008777
Crews, T. E., Carton, W., Olsson, L. (2018). Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Global Sustainability 1, e11. doi: 10.1017/sus.2018.11
Culman, S. W., Snapp, S. S., Ollenburger, M., Basso, B., DeHaan, L. R. (2013). Soil and water quality rapidly responds to the perennial grain Kernza wheatgrass. Agron. J. 105, 735–744. doi: 10.2134/agronj2012.0273
Díaz, S., Kattge, J., Cornelissen, J. H. C., Wright, I. J., Lavorel, S., Dray, S., et al. (2015). The global spectrum of plant form and function. Nature 529, 167–171. doi: 10.1038/nature16489
De Souza, J. G., Vieira Da Silva, J. (1987). Partitioning of carbohydrates in annual and perennial cotton (Gossypium hirsutum L.). J. Exp. Bot. 38, 1211–1218. doi: 10.1093/jxb/38.7.1211
Debouck, D. G. (1992). “Frijoles, Phaseolus spp,” in Cultivos marginados: otra perspectiva de 1492. Eds. Hernández Bermejo, E., León, J. (Rome, Italy: Food and Agriculture Organization of the United Nations), 45–60.
DeHaan, L. R., Van Tassel, D. L., Anderson, J. A., Asselin, S. R., Barnes, R., Baute, G. J., et al. (2016). A pipeline strategy for grain crop domestication. Crop Sci. 56, 1–14. doi: 10.2135/cropsci2015.06.0356
Delgado-Salinas, A., Bibler, R., Lavin, M. (2006). Phylogeny of the genus Phaseolus (Leguminosae): A recent diversification in an ancient landscape. Syst. Bot. 31, 779–791. doi: 10.1600/036364406779695960
Delgado-Salinas, A. (1988). “Variation, taxonomy, domestication, and germplasm potentialities in Phaseolus coccineus.,” in Genetic Resources of Phaseolus Beans. Ed. Gepts, P. (Norwell, MA: Kluwer Academic Publishers), 441–463. doi: 10.1007/978-94-009-2786-5_18
Denison, R. F. (2012). “What won't work: Misguided mimicry of natural ecosystems,” in Darwinian Agriculture: How Understanding Evolution Can Improve Agriculture (Princeton, NJ: Princeton University Press), 95–119.
Dohle, S., Carlos, J., Mier, B., Egan, A., Kisha, T., Khoury, C. K. (2019). “Wild Beans (Phaseolus L.) of North America,” in North American Crop Wild Relatives, vol. 2 . Eds. Greene, S. L., Williams, K. A., Khoury, C. K., Kantar, M. B., Marek, L. F. (Cham, Switzerland: Springer), 99–127. doi: 10.1007/978-3-319-97121-6
Dohleman, F. G., Long, S. P. (2009). More productive than maize in the Midwest: How does Miscanthus do it? Plant Physiol. 150, 2104–2115. doi: 10.1104/pp.109.139162
FAO (Food and Agriculture Organization of the United Nations) (2019). Outcome document of the Global Symposium on Soil Erosion. Rome, Italy.
Freytag, G. F., Debouck, D. G. (2002). Taxonomy, distribution, and ecology of the genus Phaseolus (Leguminosae-Papilionoideae) in North America, Mexico, and Central America (Fort Worth, TX: Botanical Research Institute of Texas (BRIT)).
Fuller, D. Q., Allaby, R. (2009). Seed dispersal and crop domestication: Shattering, germination and seasonality in evolution under cultivation. Annu. Plant Rev. 38, 238–295. doi: 10.1002/9781444314557.ch7
Garnier, E. (1992). Growth analysis of congeneric annual and perennial grass species. J. Ecol. 80, 665–675. doi: 10.2307/2260858
Gaut, B. S., Díez, C. M., Morrell, P. L. (2015). Genomics and the contrasting dynamics of annual and perennial domestication. Trends Genet. 31, 709–719. doi: 10.1016/j.tig.2015.10.002
Geber, M. A. (1990). The cost of meristem limitation in Polygonum arenastrum: Negative genetic correlations between fecundity and growth. Evolution 44, 799–819. doi: 10.1111/j.1558-5646.1990.tb03806.x
González-Paleo, L., Ravetta, D. A. (2015). Carbon acquisition strategies uncoupled from predictions derived from species life-cycle. Flora 212, 1–9. doi: 10.1016/j.flora.2015.02.004
González-Paleo, L., Vilela, A. E., Ravetta, D. A. (2016). Back to perennials: Does selection enhance tradeoffs between yield and longevity? Ind. Crops Products 91, 272–278. doi: 10.1016/j.indcrop.2016.07.018
Gremer, J. R., Kimball, S., Venable, D. L. (2016). Within-and among-year germination in Sonoran Desert winter annuals: bet hedging and predictive germination in a variable environment. Ecol. Lett. 19, 1209–1218. doi: 10.1111/ele.12655
Guerra-García, A., Suárez-Atilano, M., Mastretta-Yanes, A., Delgado-Salinas, A., Piñero, D. (2017). Domestication genomics of the open-pollinated scarlet runner bean (Phaseolus coccineus L.). Front. Plant Sci. 8, 1891. doi: 10.3389/fpls.2017.01891
Hammer, K., Khoshbakht, K. (2015). A domestication assessment of the big five plant families. Genet. Resour. Crop Evol. 62, 665–689. doi: 10.1007/s10722-014-0186-2
Harlan, J. R., de Wet, J. M. J., Price, E. G. (1973). Comparative evolution of cereals. Evolution 27, 311–325. doi: 10.2307/2406971
Harlan, J. R. (1995). The Living Fields: Our Agricultural Heritage (Cambridge, UK: Cambridge University Press).
Harris, D. R. (1989). “An evolutionary continuum of people–plant interaction,” in Foraging and farming: the evolution of plant exploitation. Eds. Harris, D. R., Hillman, G. C. (London, UK: Unwin Hyman Ltd.), 11–26. doi: 10.4324/9781315746425
Huang, G., Qin, S., Zhang, S., Cai, X., Wu, S., Dao, J., et al. (2018). Performance, economics and potential impact of perennial rice PR23 relative to annual rice cultivars at multiple locations in Yunnan Province of China. Sustainability 10, 1086. doi: 10.3390/su10041086
Jaikumar, N. S., Snapp, S. S., Murphy, K., Jones, S. S. (2012). Agronomic assessment of perennial wheat and perennial rye as cereal crops. Agron. J. 104, 1716–1726. doi: 10.2134/agronj2012.0291
Jordan, N., Warner, K. D. (2010). Enhancing the multifunctionality of US agriculture. BioScience 60, 60–66. doi: 10.1525/bio.2009.60.1.10
Kane, D. A., Rogé, P., Snapp, S. S. (2016). A systematic review of perennial staple crops literature using topic modeling and bibliometric analysis. PloS One 11, e0155788. doi: 10.1371/journal.pone.0155788
Kantar, M., Tyl, C., Dorn, K., Zhang, X., Jungers, J., Kaser, J. M., et al. (2016). Perennial grain and oilseed crops. Annu. Rev. Plant Biol. 67, 1–27. doi: 10.1146/annurev-arplant-043015-112311
Kaplan, L., Lynch, T. F. (1999). Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-Colombian agriculture. Econ. Bot. 53 (3), 261–272. doi: 10.1007/BF02866636
Kleyer, M., Trinogga, J., Cebrián, M. A., Ejrnæs, R., Trenkamp, A., Fløjgaard, C., et al. (2019). Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 107, 829–842. doi: 10.1111/1365-2745.13066
Koinange, E. M. K., Singh, S. P., Gepts, P. (1996). Genetic control of the domestication syndrome in common bean. Crop Sci. 36, 1037–1045. doi: 10.2135/cropsci1996.0011183X003600040037x
López Herrera, M., Aguirre Rivera, J. R., Trejo, C., Peña-Valdivia, C. B. (2001). Differences in seed germination of wild and domesticated common bean (Phaseolus vulgaris L.) in response to storage. South Afr. J. Bot. 67, 620–628. doi: 10.1016/S0254-6299(15)31192-3
Lewis, G., Schrire, B., Mackinder, B., Lock, M. (2005). Legumes of the World. (Surrey, UK: Royal Botanic Garden, Kew).
Mann, C. (1997). Reseeding the green revolution. Science 277, 1038–1043. doi: 10.1126/science.277.5329.1038
McKey, D. B., Elias, M., Pujol, B., Duputié, A. (2012). “Ecological approaches to crop domestication,” in Biodiversity in Agriculture: Domestication, Evolution, and Sustainability. Eds. Gepts, P., Famula, T. R., Bettinger, R. L. (Cambridge, UK: Cambridge University Press), 377–406. doi: 10.1017/CBO9781139019514.023
Meyer, R. S., DuVal, A. E., Jensen, H. R. (2012). Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytol. 196, 29–48. doi: 10.1111/j.1469-8137.2012.04253.x
Milla, R., Matesanz, S. (2017). Growing larger with domestication: a matter of physiology, morphology or allocation? Plant Biol. 19, 475–483. doi: 10.1111/plb.12545
Milla, R., Morente-Lopez, J., Alonso-Rodrigo, J., Martin-Robles, N., Chapin, F. (2014). Shifts and disruptions in resource-use trait syndromes during the evolution of herbaceous crops. Proc. R. Soc B. 281, 20141429. doi: 10.1098/rspb.2014.1429
Miller, A. J., Gross, B. L. (2011). From forest to field: perennial fruit crop domestication. Am. J. Bot. 98, 1389–1414. doi: 10.3732/ajb.1000522
Mina-Vargas, A. M., McKeown, P. C., Flanagan, N. S., Debouck, D. G., Kilian, A., Hodkinson, T. R., et al. (2016). Origin of year-long bean (Phaseolus dumosus Macfady, Fabaceae) from reticulated hybridization events between multiple Phaseolus species. Ann. Bot. 118, 957–969. doi: 10.1093/aob/mcw138
Moles, A. T., Ackerly, D. D., Tweddle, J. C., Dickie, J. B., Smith, R., Leishman, M. R., et al. (2007). Global patterns in seed size. Global Ecol. Biogeogr. 16, 109–116. doi: 10.1111/j.1466-822x.2006.00259.x
Muller-Landau, H. C. (2010). The tolerance – fecundity trade-off and the maintenance of diversity in seed size. Proc. Natl. Acad. Sci. 107, 4242–4247. doi: 10.1073/pnas.0911637107
Nabhan, G. P., Felger, R. S. (1985).Wild desert relatives of crops: their direct uses as food, in: Plants for arid lands: proceedings of the Kew International Conference on Economic Plants for Arid Lands held in the Jodrell Laboratory, Royal Botanical Gardens, London/Boston. pp. 19–33, In Allen & Unwin (Eds.). doi: 10.1007/978-94-011-6830-4_3
Olsen, K. M., Wendel, J. F. (2013). Crop plants as models for understanding plant adaptation and diversification. Front. Plant Sci. 4, 290. doi: 10.3389/fpls.2013.00290
Pastor-Pastor, A., Vilela, A. E., González-Paleo, L. (2018). The root of the problem of perennials domestication: is selection for yield changing key root system traits required for ecological sustainability? Plant Soil 435, 161–174. doi: 10.1007/s11104-018-3885-1
R Core Team (2019). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). URL https://www.R-project.org/.
Rasband, W. S. (1997-2016). ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/.
Rubio de Casas, R., Willis, C. G., Pearse, W. D., Baskin, C. C., Baskin, J. M., Cavender-Bares, J. (2017). Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytol. 214, 1527–1536. doi: 10.1111/nph.14498
Ryan, M. R., Crews, T. E., Culman, S. W., Dehaan, L. R., Hayes, R. C., Jungers, J. M., et al. (2018). Managing for multifunctionality in perennial grain crops. BioScience 68, 294–304. doi: 10.1093/biosci/biy014
Sacks, E. J., Dhanapala, M. P., Sta. Cruz, M. T., Sallan, R. (2007). Clonal performance of perennial Oryza sativa/O. rufipogon selections and their combining ability with O. sativa cultivars for survival, stolon production and yield. Field Crops Res. 100, 155–167. doi: 10.1016/j.fcr.2006.06.003
Schlautman, B., Barriball, S., Ciotir, C., Herron, S. A., Miller, A. J. (2018). Perennial grain legume domestication phase I: Criteria for candidate species selection. Sustainability 10, 730. doi: 10.3390/su10030730
Schmit, V., Debouck, D. G. (1991). Observations on the origin of Phaseolus polyanthus Greenman. Econ. Bot. 45 (3), 345–364. doi: 10.1007/BF02887077
Smýkal, P., Nelson, M. N., Berger, J. D., von Wettberg, E. J. (2018). The impact of genetic changes during crop domestication. Agronomy 8, 119. doi: 10.3390/agronomy8070119
Smaje, C. (2015). The strong perennial vision: a critical review. Agroecol. Sustain. Food Syst. 39, 471–499. doi: 10.1080/21683565.2015.1007200
Smartt, J. (1988). “Morphological, physiological, and biochemical changes in Phaseolus beans under domestication,” in Genetic Resources of Phaseolus Beans. Ed. Gepts, P. (Norwell, MA: Kluwer Academic Publishers), 143–161. (Ed.),. doi: 10.1007/978-94-009-2786-5_8
Suneson, C. A., Sharkawy, E., Hall, W. E. (1963). Progress in 25 years of perennial wheat development. Crop Sci. 3, 437–439. doi: 10.2135/cropsci1963.0011183x000300050021x
Thompson, K., Hodkinson, D. J. (1998). Seed mass, habitat and life history: A re-analysis of Salisbury, (1942, 1974). New Phytol. 138 (1), 163–167. doi: 10.1046/j.1469-8137.1998.00886.x
Thompson, K., Bakker, J. P., Bekker, R. M., Hodgson, J. G. (1998). Ecological correlates of seed persistence in soil in the north-west European flora. J. Ecol. 86 (1), 163–169. doi: 10.1046/j.1365-2745.1998.00240.x
Van Tassel, D. L., Dehaan, L. R., Cox, T. S. (2010). Missing domesticated plant forms: can artificial selection fill the gap? Evol. Appl. 3, 434–452. doi: 10.1111/j.1752-4571.2010.00132.x
Venable, D. L., Lawlor, L. (1980). Delayed germination and dispersal in desert annuals: Escape in space and time. Oecologia 46, 272–282. doi: 10.1007/BF00540137
Venable, D. L., Rees, M. (2009). The scaling of seed size. J. Ecol. 97, 27–31. doi: 10.1111/j.1365-2745.2008.01461.x
Verboom, G. A., Linder, H. P., Stock, W. D. (2004). Testing the adaptive nature of radiation: Growth form and life history divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae). Am. J. Bot. 91, 1364–1370. doi: 10.3732/ajb.91.9.1364
Vico, G., Manzoni, S., Nkurunziza, L., Murphy, K., Weih, M. (2016). Trade-offs between seed output and life span - a quantitative comparison of traits between annual and perennial congeneric species. New Phytol. 209, 104–114.
Vilela, A. E., González-Paleo, L. (2015). Changes in resource-use strategy and phenotypic plasticity associated with selection for yield in wild species native to arid environments. J. Arid Environ. 113, 51–58. doi: 10.1111/nph.13574
Walters, C., Wheeler, L. M., Grotenhuis, J. M. (2005). Longevity of seeds stored in a genebank: species characteristics. Seed Sci. Res. 15, 1–20. doi: 10.1079/SSR2004195
Weeden, N. F. (2007). Genetic changes accompanying the domestication of Pisum sativum: Is there a common genetic basis to the ‘domestication syndrome' for legumes? Ann. Bot. 100, 1017–1025. doi: 10.1093/aob/mcm122
Keywords: perennial grain, Phaseolus, Fabaceae, legume, pulse, crop wild relative, domestication
Citation: Herron SA, Rubin MJ, Ciotir C, Crews TE, Van Tassel DL and Miller AJ (2020) Comparative Analysis of Early Life Stage Traits in Annual and Perennial Phaseolus Crops and Their Wild Relatives. Front. Plant Sci. 11:34. doi: 10.3389/fpls.2020.00034
Received: 25 October 2019; Accepted: 13 January 2020;
Published: 10 March 2020.
Edited by:
Petr Smýkal, Palacký University, CzechiaReviewed by:
Steven B. Cannon, United States Department of Agriculture, United StatesCopyright © 2020 Herron, Rubin, Ciotir, Crews, Van Tassel and Miller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sterling A. Herron, c3RlcmxpbmcuaGVycm9uQHNsdS5lZHU=; Allison J. Miller, YWxsaXNvbi5qLm1pbGxlckBzbHUuZWR1
†Present address: Claudia Ciotir, Department of Evolutionary and Environmental Biology and Institute of Evolution, University of Haifa, Haifa, Israel
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.