
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 27 December 2019
Sec. Technical Advances in Plant Science
Volume 10 - 2019 | https://doi.org/10.3389/fpls.2019.01732
Xylella fastidiosa (Xf) is an insect-borne bacterium confined to the xylem vessels of plants. This plant pathogen has a broad host range estimated to 560 plant species. Five subspecies of the pathogen with different but overlapping host ranges have been described, but only three subspecies are widely accepted, namely subspecies fastidiosa, multiplex, and pauca. Initially limited to the Americas, Xf has been detected in Europe since 2013. As management of X. fastidiosa outbreaks in Europe depends on the identification of the subspecies, accurate determination of the subspecies in infected plants as early as possible is of major interest. Thus, we developed various tetraplex and triplex quantitative PCR (qPCR) assays for X. fastidiosa detection and subspecies identification in planta in a single reaction. We designed primers and probes using SkIf, a bioinformatics tool based on k-mers, to detect specific signatures of the species and subspecies from a data set of 58 genome sequences representative of X. fastidiosa diversity. We tested the qPCR assays on 39 target and 30 non-target strains, as well as on 13 different plant species spiked with strains of the different subspecies of X. fastidiosa, and on samples from various environmental and inoculated host plants. Sensitivity of simplex assays was equal or slightly better than the reference protocol on purified DNA. Tetraplex qPCR assays had the same sensitivity than the reference protocol and allowed X. fastidiosa detection in all spiked matrices up to 103 cells.ml−1. Moreover, mix infections of two to three subspecies could be detected in the same sample with tetraplex assays. In environmental plant samples, the tetraplex qPCR assays allowed subspecies identification when the current method based on multilocus sequence typing failed. The qPCR assays described here are robust and modular tools that are efficient for differentiating X. fastidiosa subspecies directly in plant samples.
Xylella fastidiosa (Xf) is a worldwide insect-transmitted plant-pathogenic bacterium that presents a very large host range. Altogether, 563 plant species grouped into 82 botanical families have been reported as Xf hosts (EFSA, 2018a). Plants with a major socio-economic interest such as grapevine, citrus, coffee, and olive trees are hosts of Xf (EFSA, 2018a). Forest trees, shade trees, ornamentals, and landscape species are included in the host plant database making this pathogen a potential worldwide threat (EFSA, 2018a). Disease management of Xf is impeded by its asymptomatic period that can last several years (EFSA, 2018b).
This bacterial species is genetically diverse as five subspecies including fastidiosa, morus, multiplex, pauca, and sandyi are currently described (EFSA, 2018b). Although this subspecies delineation was initially associated to Xf host range and places of occurrence, more and more observations report infection of a given host by various subspecies (Denancé et al., 2017; EPPO, 2018b; Denancé et al., 2019; Nunney et al., 2019). Homologous recombination events were detected in Xf and were suspected to be associated with host-shift, as documented for the subspecies morus (Nunney et al., 2014). But intrasubspecific homologous recombination events could be more frequent than intersubspecific events (Potnis et al., 2019). Based on genome sequence analyses, it was proposed to merge the subspecies fastidiosa, morus, and sandyi in the subspecies fastidiosa [hereafter referred to Xff sensu lato (Xffsl) to avoid confusion with classical Xff], the subspecies multiplex and pauca remaining coherent groups and distantly related from Xff (Marcelletti and Scortichini, 2016; Denancé et al., 2019). The method generally used to identify strains at the subspecies level is based on the sequencing of seven housekeeping genes (cysG, gltT, holC, leuA, malF, nuoL, and petC) of the dedicated multilocus sequence typing (MLST) scheme (Yuan et al., 2010).
In Europe, Xf has been reported for the first time in the Apulia area, Italy, in olive trees (Saponari et al., 2013). Then, Xf was detected in 2015 in France, more precisely in Corsica and in the French Riviera region, mainly on Polygala myrtifolia and other ornamentals (Denancé et al., 2017). Two years later, Xf has been reported in the Balearic Islands mostly in olive tree, grapevine, and sweet cherry and in continental Spain in almond trees (Landa, 2017). More recently, in October 2018, the presence of X. fastidiosa subsp. multiplex was reported in Monte Argentario (Tuscany, Italy), and in January 2019, the subsp. multiplex was identified in Portugal (region of Porto), and both reports concerned ornamentals (EPPO, 2019). Since the first report, four subspecies, fastidiosa, multiplex, pauca, and sandyi, have been identified in Europe (Jacques et al., 2016; Denancé et al., 2017; Cruaud et al., 2018). A number of cases of imported plants being infected by Xf has also been reported in Europe since 2012 (EPPO, 2019). Being present in Europe, Xf was first listed as an A1 regulated pathogen. Xf is now reported in the Annex I/A2 of the directive 2000/29/CE and in the EPPO A2 list (C/2017/4883, 2017; EPPO, 2018a).
Apart the sympatry of several subspecies at the local, regional, or state level, cases of mix infection of plants have been described. In 2005 in California, an almond tree has been reported infected by two types of Xf strains, revealing the first case of mix infection by Xf (Chen et al., 2005). Recently, in coffee trees imported into Europe from Central America, the MLST revealed a mix infection with two different sequence types (STs) of Xf from two subspecies: pauca and fastidiosa (Bergsma-Vlami et al., 2017). In France, a P. myrtifolia plant was found mix infected with strains of two different STs (Denancé et al., 2017). Reported cases of undetermined sequences of housekeeping gene alleles were an indication of mix infections in plants (Denancé et al., 2017).
Because in Europe, the subspecies identification is necessary to set up outbreak management, it is of major interest to have access to reliable tools for the detection and identification of Xf. As Xf isolation is tedious, detection and identification of subspecies are performed directly on plant extracts (Denancé et al., 2017). To date, tests based on loop-mediated isothermal amplification (LAMP) (Harper et al., 2010), conventional PCR (Minsavage et al., 1994; Hernandez-Martinez et al., 2006), and quantitative PCR (qPCR) (Francis et al., 2006; Harper et al., 2010; Li et al., 2013; Ouyang et al., 2013) targeting specific regions at the species or subspecies level are available. Among these tests, the qPCR assay developed by Harper et al. (2010) has been identified as one of the most appropriate for the detection of Xf, as it has shown a high diagnostic sensitivity compared to other qPCR assays, detects all subspecies, has no cross-reactivity with any other bacterial species, and has been successfully used on a wide range of plants (Modesti et al., 2017; Reisenzein, 2017). Several tests have been proposed to identify one or more subspecies, but no test is currently available to identify all subspecies. The subspecies identification is then routinely performed by MLST, but this method, while accurate and portable, is time consuming, labor intensive, and expensive. From 2018, sequences of only two housekeeping genes (rpoD and cysG or rpoD and malF) are required for subspecies identification in France, while other sets of gene pairs are recommended by EPPO (EPPO, 2018b).
In recent years, multiplexed TaqMan qPCR has become a useful tool for the identification and quantification of pathogens in different areas such as food safety (Köppel et al., 2019; Wei et al., 2019), medical environment (Janse et al., 2013; Kamau et al., 2013), agronomics (Wei et al., 2008; Zitnick-Anderson et al., 2018), GMO detection (Choi et al., 2018; Wang et al., 2018), and the environment (Hulley et al., 2019). For plant pathogens, these methods have been tested on samples of naturally infected plants, spiked samples (Li et al., 2009; Willsey et al., 2018), and on mixtures of plant and pathogen DNAs (Abraham et al., 2018). Xf-specific multiplexed qPCR assays have already been developed based on the combination of primers designed by Harper et al. (2010) and Ouyang et al. (2013) (Bonants et al., 2018). Other tests were proposed to differentiate Xf from phytoplasmas sharing common host plants (Ito and Suzaki, 2017) and to differentiate the subspecies fastidiosa from the subspecies multiplex (Burbank and Ortega, 2018). However, none of them allows the differential identification of all Xf subspecies.
In this study, we described the development and evaluation of six multiplex qPCR assays for the detection and identification of Xf subspecies. These tests have been designed and tested in silico on a wide range of target and non-target genomic sequences, in vitro on target and non-target bacterial strains, on Xf-spiked plant extracts, and finally in planta on samples from environmental or inoculated plants. These assays allowed the detection of Xf subspecies up to 10 pg.ml−1 of DNA, 1×103 CFU.ml−1 in spiked samples and allow the identification of Xf subspecies in environmental plant samples that cannot be typed using MLST. These multiplex qPCR assays offer a new, faster, more reliable, more specific, more sensitive, and less expensive tool than MLST.
Collections of 39 strains representing the different Xf subspecies, 28 strains from other plant-pathogenic bacterium genera (Agrobacterium, Clavibacter, Dickeya, Erwinia, Pantoea, Pseudomonas, Stenotrophomonas, Xanthomonas, and Xylophilus), and two strains from plant endosymbionts (Ensifer and Rhizobium) were used (Table 1). A set of 12 Xf strains of the subsp. multiplex and one strain of the subsp. sandyi were kindly provided by Leonardo de la Fuente (Auburn University, AL, USA). The other 57 strains were provided by the French Collection of Plant-Associated Bacteria (CIRM-CFBP; https://www6.inra.fr/cirm_eng/CFBP-Plant-Associated-Bacteria). Xf strains were grown on BCYE (Wells et al., 1981) or modified PWG media [agar 12 g.L−1; soytone 4 g.L−1; bacto tryptone 1 g.L−1; MgSO4.7H2O 0.4 g.L−1; K2HPO4 1.2 g.L−1; KH2PO4 1 g.L−1; hemin chloride (0.1% in NaOH 0.05 M) 10 ml.L−1; BSA (7.5%) 24 ml.L−1 ; L-glutamine 4 g.L−1] at 28°C for 1 to 2 weeks. Other strains were grown at 25°C for 1 to 2 days on: MG media (Mougel et al., 2001) for Agrobacterium and Rhizobium; TSA (tryptone soy broth 30 g.L−1; agar 15 g.L−1) for Clavibacter, Ensifer, Stenotrophomonas, Xanthomonas, and Xylophilus; and King's B medium (KH2PO4 1.5 g.L−1; MgSO4, 7H2O 1.5 g.L−1; protease peptone 20 g.L−1, glycerol 10 ml.L−1; agar 15 g.L−1) for Dickeya, Erwinia, Pantoea, and Pseudomonas. For qPCR assays, bacterial suspensions were prepared from fresh cultures in sterile distilled water, adjusted at OD600 nm = 0.1. To evaluate assay specificity, bacterial suspensions were boiled for 20 min, followed by a thermal shock on ice and a centrifugation at 10,000 g during 10 min.
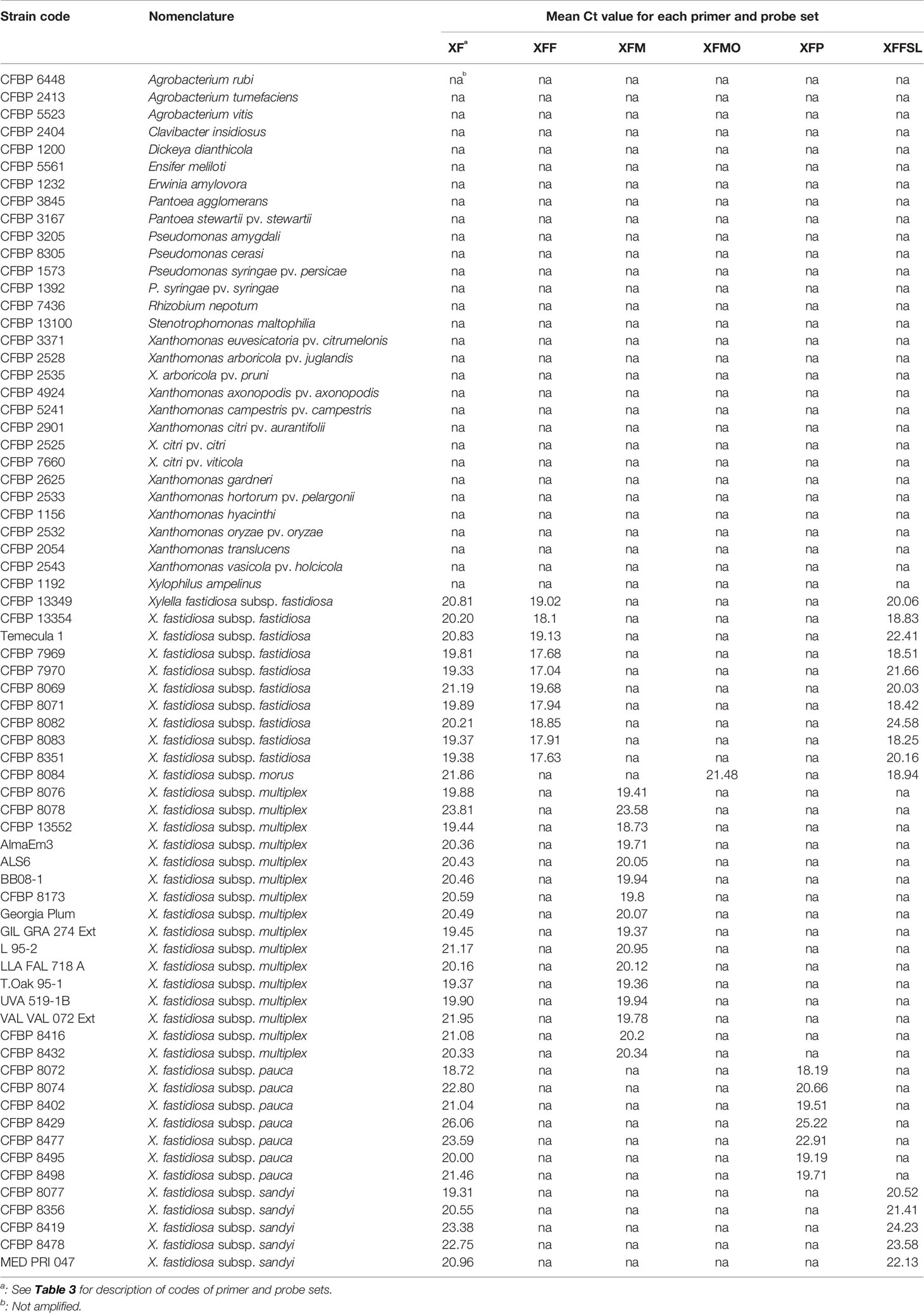
Table 1 List of strains used in this study and signals obtained with the primers and probe combinations in simplex quantitative PCR (qPCR) assays on DNA suspensions calibrated at OD600nm = 0.1.
Petioles or midribs were collected in 2018 from healthy plants of 13 species (Helichrysum italicum, Lavandula angustifolia, Nerium oleander, Olea europaea, Prunus cerasus, Prunus dulcis, Quercus ilex, Quercus robur, and Rosmarinus officinalis) growing in orchards adjacent to INRA center or purchased in nurseries (Vitis vinifera, Citrus clementina, and P. myrtifolia). These species are either not known to be host of Xf in France or were sampled in Xf-free areas. Symptomless Cistus monspeliensis plant material was collected in Corsica outside any recorded Xf-focus by the National Botanical Conservatory of Corsica (CNBC).
Plants were collected in June 2017 and in October 2018 in Corsica, France, based on symptoms and were pre-tested using a modified extraction procedure based on CTAB and/or QuickPickTM SML Plant DNA Kit (Bio-Nobile, Turku, Finland) as described in PM7/24 (EPPO, 2018b). Samples were first finely chopped and then sonicated (1 min, 42 KHz) in a Branson apparatus. A 15 min incubation step at room temperature was performed before DNA extraction. The frozen DNA solutions of 20 greenhouse inoculated plant materials were used to evaluate the multiplex qPCR assays.
X. fastidiosa strains CFBP 7970 (Xff), CFBP 8077 (Xfs), CFBP 8402 (Xfp), CFBP 8416 (Xfm), and CFBP 8418 (Xfm) were inoculated in 6-month-old grafted plants of V. vinifera cv Chardonnay, V. vinifera cv Cabernet Franc, in 1.5-year-old grafted plants of Prunus armeniaca var Bergeron, O. europaea cv Aglandau, O. europaea cv Capanaccia, and O. europaea cv Sabine. Plants were grown in a confined growth chamber at 24°C with 16 h of daylight and at 20°C during night, under 70% relative humidity. Plants were watered daily with water supplemented with 1.4 g.L−1 nitrogen:phosphorus:potassium fertilizer (16:8:32). Plants were inoculated by the needle puncture method. A 10 µl drop of inoculum calibrated at OD600nm = 0.5 was placed on the node of a growing young stem and punctured with a needle. After 6 months for vines and apricot trees, and 1 year for olive trees, samples at the inoculation point were tested by the Harper's qPCR test and typed using the classical Xf MLST scheme as described in Denancé et al. (2017). The samples were stored at −20°C before being analyzed. Plant inoculations were carried out under quarantine at IRHS, Centre INRA, Beaucouzé, France, under the agreement no. 2013119-0002 from the Prefecture de la Région Pays de la Loire, France.
Prior to DNA extraction, plant samples were inoculated by mixing 1 g of healthy plant material with 0.5 ml of a bacterial suspension, at a known concentration, and ground with 4.5 ml of sterile distilled water. Each matrix was spiked in order to end up with concentrations ranging from 1×106 to 10 CFU.ml−1. Spiking with more than one strain was done in equal amounts to end up with final concentrations ranging from 1×106 to 1×10 CFU.ml−1. Samples from P. myrtifolia were spiked with individual strains representing each subspecies of Xf (Xff: CFBP 7970, Xfmo: CFBP 8084, Xfp: CFBP 8402, Xfm: CFBP 8416). Other plant materials were spiked with the strain representing the only subspecies that infects them naturally. However, as several subspecies may co-occur in the same area and plant species may be hosts of several subspecies, samples of N. oleander, O. europaea, P. dulcis, and P. myrtifolia were also spiked with duos or trios of strains. A total of 29 plant species–Xf subspecies were combined. For negative controls, the samples were directly ground in sterile distilled water (5 ml). Samples were treated as above before DNA extraction. All DNA extractions were performed using the QuickPickTM SML Plant DNA Kit (Bio-Nobile, Turku, Finland) as in PM7/24 (EPPO, 2018b) with an automated system (Caliper Zephyr, PerkinElmer). A control composed of DNAs extracted from bacterial suspensions was systematically performed.
Fresh suspensions of CFBP 7970 strain calibrated at OD600 nm = 0.1 were plated on PWG medium and incubated at 28°C for 8 days before counting. They contained 1×108 CFU.ml−1. Genomic DNA from the same suspensions was extracted using QuickPickTM SML Plant DNA Kit (Bio-Nobile, Turku, Finland) as described in PM7/24 (EPPO, 2018b). DNA concentration was measured using Qubit fluorimeter and serial dilutions of Xf genomic DNA at concentrations ranging from 1 µg.ml−1 to 1 pg.ml−1 were prepared. The DNA was amplified using the Harper's et al. (2010) qPCR assay in a Bio-Rad CFX384 thermocycler. Results of the amplified serial dilutions were used to establish standard curves relating the amount of fluorescence to the amount of DNA. The bacterial concentration of the corresponding DNA solution was calculated based on DNA measures using an estimated genome size of 2,493,794 bp for the strain CFBP 7970 (Denancé et al., 2017) and knowing that 1 pg = 9.78×108 bp (Doležel et al., 2003). Using the following equation curve a Ct = 19.8 correlated to 1.04 × 108 genome equivalent.ml−1.
SkIf tool (Briand et al., 2016) was used on 58 Xylella genomic sequences to target specific sequences of the Xf species, each subspecies, and the fastidiosa sensu lato (Xffsl) subspecies, i.e. the group including the fastidiosa, morus, and sandyi subspecies (Denancé et al., 2019) (Table 2). Six primer and probe combinations were designed using Primer3 2.3.4 (Koressaar and Remm, 2007), on these specific sequences to target the whole Xf species (XF primers), and the various subspecies: fastidiosa (XFF primers), fastidiosa sensu lato (XFFSL primers), morus (XMO primers), multiplex (XFM primers), and pauca (XFP primers) (Table 3). The parameters were set up with an optimal size of 20 bp (sizing between 18 and 27 bp), an optimal product size of 85 to 150 bp, and a Tm of 60°C (± 3°C) and 70°C (± 3°C) for primers and probes, respectively. Then, the individual primer and probe combinations and the six sets of four combinations were tested using Amplify to check the absence of dimer and cross-amplification (Engels, 1993). The specificity of all primers and probes was tested in silico using PrimerSearch (Val Curwen, Human Genome Mapping Project, Cambridge, UK) on the initial set of 58 genomic sequences of Xylella and on the 154,478 bacterial Whole Genome Shotgun (WGS) sequences available in the NCBI database (as on August 22, 2018). BLASTn of the amplicons were run on the NCBI WGS database to evidence their specificity.
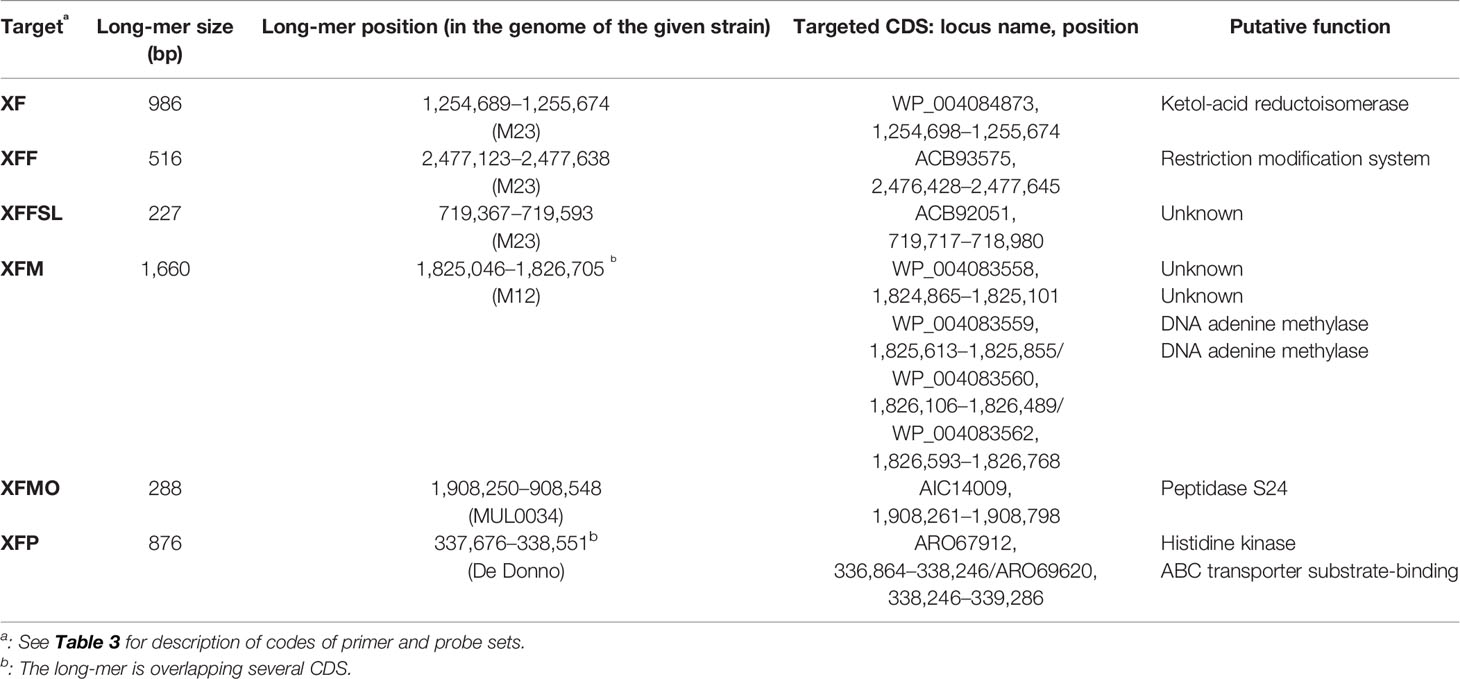
Table 2 Description and composition of the longest specific long-mers obtained using SkIf for the various targets.
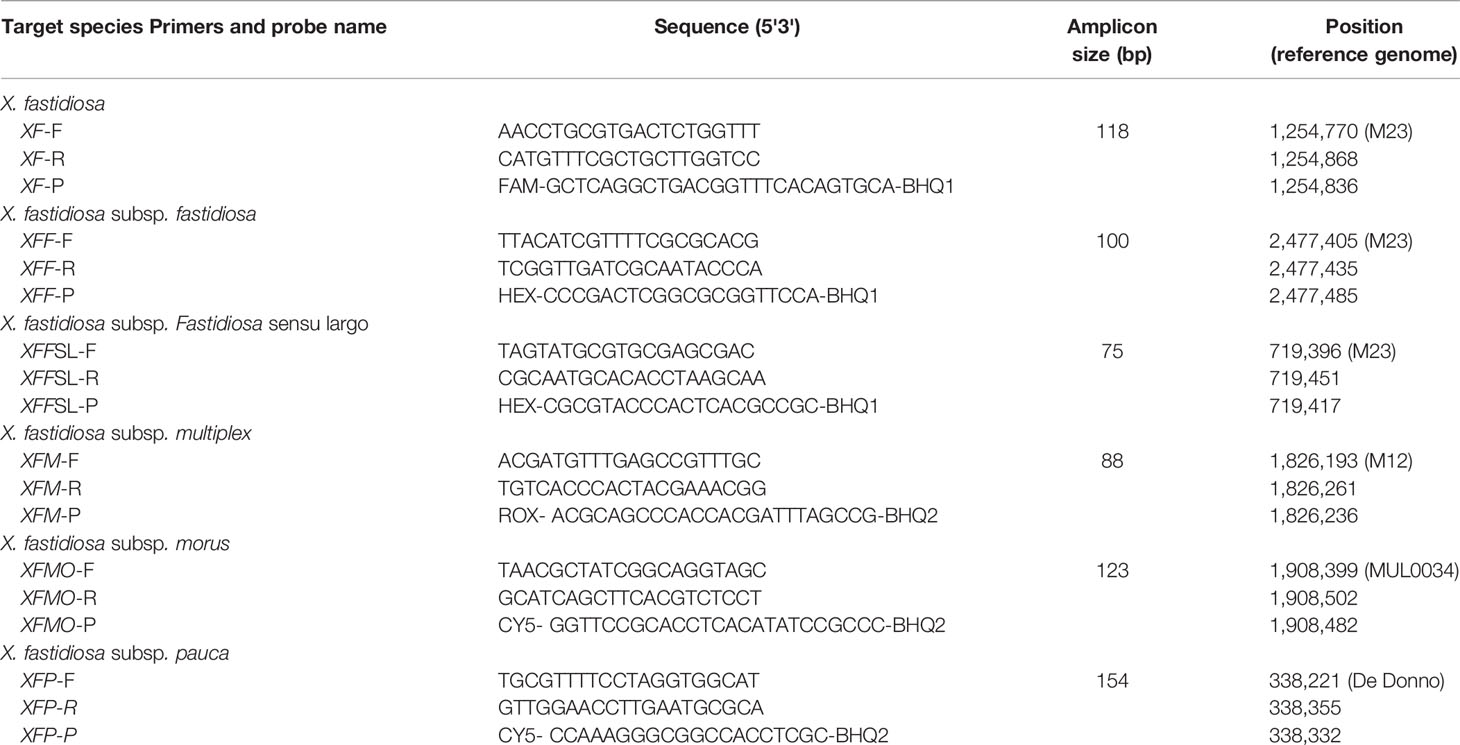
Table 3 Primers and probes designed in this study for Xf detection at the species and subspecies level.
Four other primer and probe combinations previously published were used in this study. The first targets the rimM gene of Xf (Harper et al., 2010) and was used as reference protocol. The second targets the eukaryotic rRNA18S gene (Ioos et al., 2012) and was used as internal control. The remaining two tests target fastidiosa or multiplex subspecies (Burbank and Ortega, 2018).
The tetraplex qPCR assays designed in this study were optimized for: i) primer and probe hybridization temperature that was checked individually by PCR using a gradient ranging from 57.5 to 61.4°C in intervals of 0.8°C (CFX96 Touch™ Bio-Rad), ii) concentrations of 250, 575, or 900 nM for primers combined with 150, 200 or 250 nM for probes according to PCR mix manufacturer instructions, and iii) addition of 600 ng.µl−1 of BSA. All the optimization analyses were performed in triplicates using SsoAdvanced™ Universal Probes Supermix (Bio-Rad) and performed in a Bio-Rad CFX thermocycler using the “all channels” reading mode. To allow simultaneous detection of Xf and identification at the subspecies level, primer and probe combinations were then declined in six different triplex and tetraplex qPCR sets, i.e. set n°1: XF–XFFSL–XFM–XFP, set n°2: XF–XFF–XFM–XFP, set n°3: XF–XFF–XFM–XMO, set n°4: XFFSL–XFM–XFP, set n°5: Harper–XFFSL–XFM–XFP, and set n°6: 18S–XFFSL–XFM–XFP.
The optimized final reaction conditions were performed in a final volume of 10 µl containing 1X of SsoAdvanced™ Universal Probes Supermix (Bio-Rad), 575 nM of primers, 200 nM of probes and 600 ng.µl−1 of BSA (ThermoFisher), and 1 µl of extracted DNA. The optimal thermocycling conditions selected were: 3 min at 95°C, followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. The qPCR assays results were analyzed, with expert verification, using Bio-Rad CFX Manager 3.1 software and its regression mode. The reaction efficiency was calculated using serial dilutions with the formula: E = 10(−1/slope).
The specificity of the newly designed primer and probe combinations was validated using the optimized protocol on the boiled bacterial suspensions of the 69 strains listed in the Table 1. The efficiency of each combination was evaluated on bacterial DNA solutions ranging from 1 µg.ml−1 to 1 pg.ml−1, in simplex or tetraplex assays (set n°1 to 3), on the strains CFBP 7970 (Xff) for the primers XF, XFF, and XFFSL, CFBP 8416 (Xfm) for the primers XF and XFM, CFBP 8084 (Xfmo) for the primers XF and XFMO, and CFBP 8402 (Xfp) for the primers XF and XFP. In addition, each set was also evaluated with spiked plant material. All analyses were performed in triplicate. Two independent experiments were carried out on O. europaea, P. myrtifolia, P. cerasus, P. dulcis, Q. ilex , and V. vinifera using the set n°1: XF–XFFSL–XFM–XFP, leading to the analysis of 46 combinations of plant/strain(s) for this set. The assays were also performed on environmental plant samples and inoculated plant samples. For plant samples, the lowest concentration with a positive result in at least two out of the three replicates was considered the limit of detection (LOD).
The LOD of the tetraplex qPCR assays sets n°1 to 3 was compared to the Harper's qPCR detection test (Harper et al., 2010) using the TaqMan™ Universal PCR Master Mix (Applied Biosystems) as in PM7/24 (EPPO, 2018b). The LOD of the tetraplex qPCR assay set n°1 was compared to the ones of sets n°4, 5, and 6. The specificity of the qPCR assay recently proposed by Burbank and Ortega (2018) was also evaluated on the Xf strain collection using the SsoAdvanced™ Universal Probes Supermix (Bio-Rad) master mix.
Species-specific and subspecies-specific long-mers were identified with SkIf (Briand et al., 2016; Denancé et al., 2019) on genomic sequences. For the Xf species and the subspecies fastidiosa, morus, multiplex, and pauca, one of the two longest long-mers identified by Denancé et al. (2019) was selected for this study (Table 2). For the subspecies fastidiosa sl specific long-mers were searched for on our 58 genome sequences of Xf, using the subspecies fastidiosa, morus, and sandyi genomes as ingroups and the multiplex and pauca genomes as outgroups. In total, 3,345 long-mers were identified, ranging from 22 to 235 bp (Supplemental Data 1).
Primers and probes were designed within specific long-mers (Table 3). Specific amplifications were obtained in silico on XF genome sequences and WGS bacterial sequences from NCBI at the expected amplification size, without any mismatch for the five primer and probe combinations (XFF, XFFSL, XFM, XFMO, and XFP). Only two mismatches were observed and concerned the XF primer and probe combination. One mismatch was on the eighth nucleotide on the XF probe for the Xfm Dixon, Griffin1, M12, Sycamore, CFBP 8416, CFBP 8417, CFBP 8418 strains, and the second one was on the sixth nucleotide of the forward XF primer of the Ann-1 Xfs strain. As there were not many possible combinations of primers and probes for the XF set, this combination was nevertheless retained, and subsequent in silico checks proved the specificity of all primer and probe combinations.
The specificity of each newly designed primer and probe combination was validated in simplex qPCR assays on 39 Xf strains and on 30 plant associated-bacterial strains (Table 1). These strains were selected as they potentially share the same niche than Xf or for being phylogenetically closely related. No amplification was detected on non-target strains or healthy host plant species and the primer and probe combinations allowed amplification of all strains or subspecies of Xf, for which they were designed (XF: 39/39, XFF: 10/10, XFM: 16/16, XFMO: 1/1, XFP: 7/7, XFFSL: 16/16).
In simplex qPCR assays, the LODs of the new primer and probe combinations designed in this study were as good as the LODs obtained with the Harper's qPCR assay or 10 times better for XFM primers (Table 4). The efficiency of each combination was evaluated on serial dilutions of calibrated DNA solutions. The XF, XFM, XFMO, XFP, and XFFSL primers and probes allowed detection of Xf up to 10 pg.ml−1 (4 copies/reaction). XFF primers were slightly less sensitive with a threshold up to 100 pg.ml−1 (40 copies/reaction). On the same DNA solutions, Harper et al. (2010) qPCR assay allowed the detection of strains CFBP 8402 (Xfp) and CFBP 8084 (Xfmo) up to 10 pg.ml−1, and CFBP 7970 (Xff) and CFBP 8416 (Xfm) strain up to 100 pg.ml−1. This makes our new primer qPCR assays good alternatives to Harper's qPCR assay.
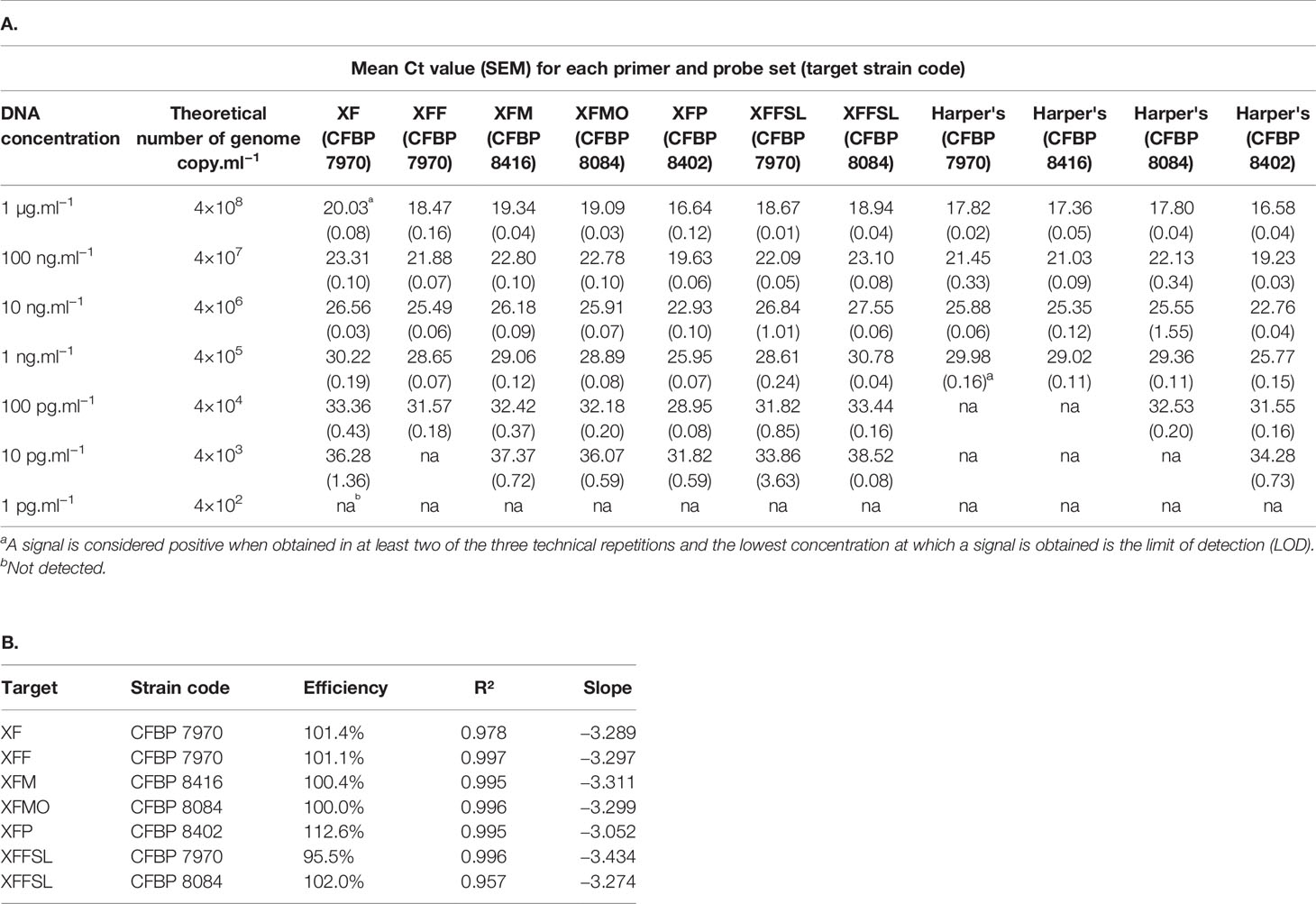
Table 4 Efficiency of the primer and probe sets in simplex qPCR assays on extracted DNA of bacterial strains in comparison with the Harper's test (Harper et al., 2010). A, Mean Ct value for each primer and probe set on target strains. B, Percentage of efficiency and standard curve parameters of each primer and probe set on target strains.
The three tetraplex qPCR assays (set n°1: XF–XFFSL–XFM–XFP, set n°2: XF–XFF–XFM–XFP, and set n°3: XF–XFF–XFM–XFMO) allowed both detection and identification of Xf and its subspecies (Supplemental Data 2). On calibrated DNA solutions, these assays were as good as Harper's test or had an LOD 10 times higher depending of the tetraplex assays. When used in tetraplex, the Ct values obtained were always lower than the Ct values obtained with Harper's test. Except for morus primers (XFMO), the LOD of tetraplex qPCR assays was usually 10 times higher than the LOD of the simplex test on DNA (Table 4 and Supplemental Data 2). In addition, it should be noted that the closer the Ct value was to the detection limit, the higher the SEM was. In tetraplex qPCR assays, set n°1, XF, XFM ,and XFP primers allowed a detection up to 100 pg.ml−1. The XFFSL primers allowed the detection of Xff up to 10 pg.ml−1 and of Xfmo up to 100 pg.ml−1. The set n°2 allowed detection up to 100 pg.ml−1 using XFF and XFM primers and up to 10 pg.ml−1with XFP primers. The XF primers allowed the detection of Xff and Xfm up to 100 pg.ml−1and of Xfp up to 10 pg.ml−1. The set n°3, allowed a detection up to 100 pg.ml−1 with XF, XFF, and XFM primers and up to 10 pg.ml−1 with XFMO primers.
A triplex qPCR assay for the simultaneous detection of subspecies fastidiosa and multiplex has recently been published (Burbank and Ortega, 2018). In order to analyze the potential of their targets and potentially introduce them into our sets to improve Xf detection, we tested their specificity in silico and in vitro on selected bacterial strains. According to BLASTn searches, Xff primers potentially amplified two of the three strains of the subsp. sandyi (CFBP 8073: ST75 and Co33: ST72) without mismatches and seven strains of the subsp. pauca (CoDiRo, COF0407, De Donno, OLS0478, OLS0479, Salento-1, and Salento-2) with one and two mismatches on the forward and reverse primers, respectively (Supplemental Data 3). In silico, Xfm primers potentially amplified eight strains of subsp. pauca (CFBP 8072, CoDiRo, COF0407, De Donno, OLS0478, OLS0479, Salento-1, Salento-2) with three mismatches on the forward primer, two mismatches on the reverse primer, and one mismatch on the probe, and amplicons had the expected size. We double-checked the specificity of these two sets in vitro on bacterial suspensions (Supplemental Data 4). Xff primers amplified the three tested strains of subsp. sandyi (CFBP 8356, CFBP 8419, and CFBP 8077) and six of the seven tested strains of subsp. pauca (CFBP 8074, CFBP 8402, CFBP 8429, CFBP 8477, CFBP 8495, and CFBP 8498). The sequencing of all amplicons confirmed the results of the qPCR assays. Xfm primers amplified five of the seven tested strains of Xf subsp. pauca (CFBP 8072, CFBP 8074, CFBP 8402, CFBP 8495, and CFBP 8498). Burbank and Ortega (2018) used a cutoff at Ct=35 for categorizing a result as positive. In that case only two pauca strains (CFBP 8072 and CFBP 8495) would have been identified as Xfm, the others having values ranging between 35.33 and 35.83. For Xfm, due to the high Ct values, no sequencing was feasible to confirm the identification.
After validation of the efficiency and specificity of the primers and probe, the three sets of tetraplex qPCR assays n°1, 2, and 3, were tested on spiked samples. As the three sets gave similar results, this section is focused on the tetraplex set n°1: XF–XFFSL–XFM–XFP, which covers the full known diversity of Xf (Table 5). The results of the other two tetraplex assays are provided in Supplemental Data 5 and Supplemental Data 6. This tetraplex qPCR assay (set n°1) was tested on 29 combinations of plant petioles and midribs spiked with one to three strains of the different subspecies. (The full results of the dilution ranges are available in Supplemental Data 7.) This tetraplex allowed the detection and correct identification of all subspecies in all combinations without false positive result. Although the detection limit was expected to be similar for all plants, since they were all enriched with the same bacterial suspensions, different LODs were observed ranging from 1×103 to 1×105 CFU.ml−1 (5 to 5×103 CFU/reaction) depending on the matrix for plants spiked with only one strain. An independent repetition of this test was performed 2 months after the first one. For O. europaea, P. myrtifolia, P. cerasus, P. dulcis, and Q. ilex the LOD was either identical between the two assays or 10 times higher. The LOD of Xf in V. vinifera was 100 times higher in the second assay highlighting a potential accumulation of qPCR inhibitors between the two experiments. Moreover, on 11 combinations out of 46, XF primers had an LOD 10 times higher in planta than the one obtained for the subspecies. Xf subspecies could be identified until a Ct value of 35.08 using Harper's qPCR assay in a spiked sample of P. dulcis. In other matrices, the LOD of the tetraplex qPCR assay corresponded usually to a Ct value ranging from 30 to 34 using Harper's qPCR.
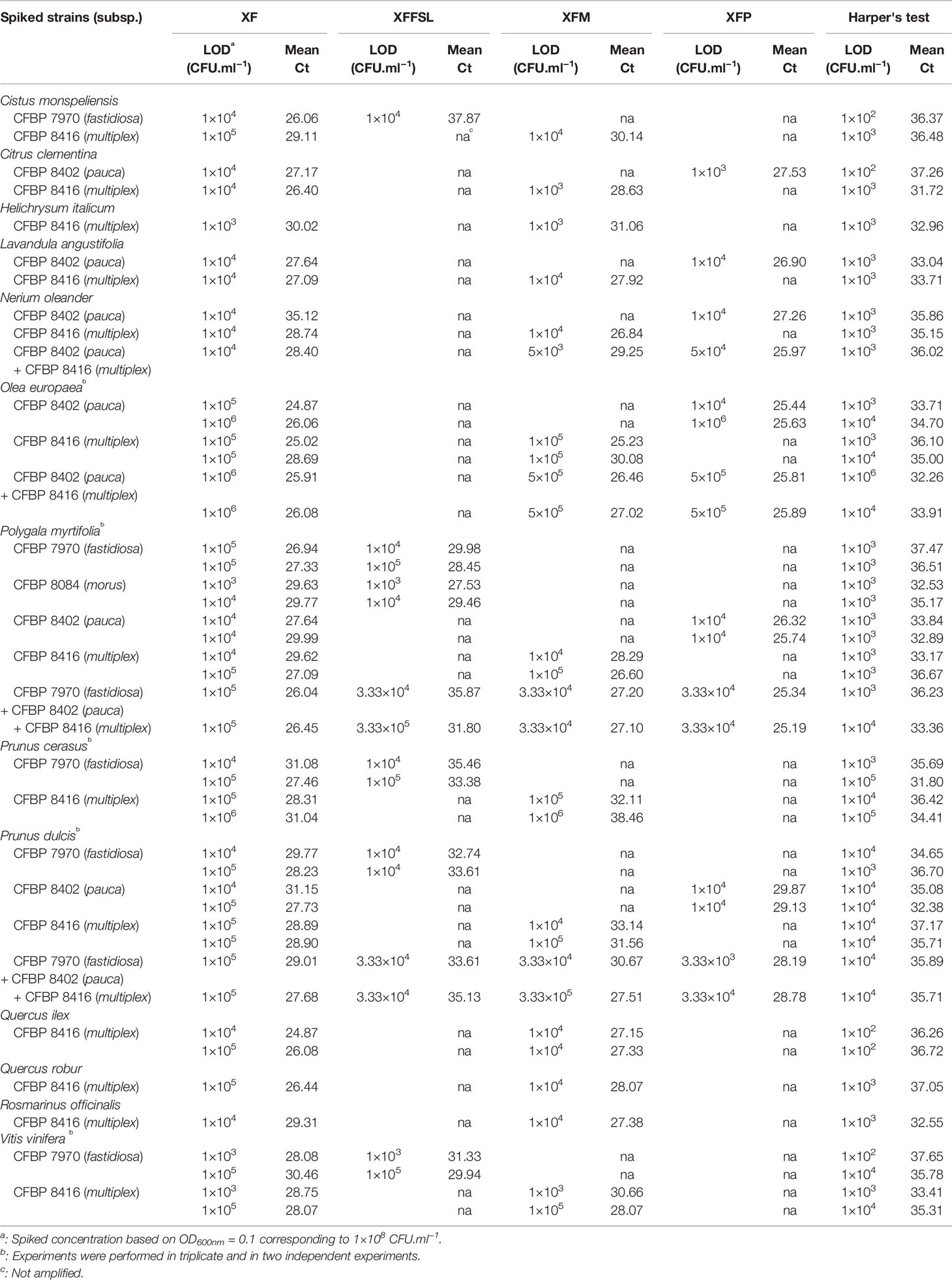
Table 5 Limit of detection (LOD) of X. fastidiosa strains in spiked matrices using the tetraplex qPCR assay XF–XFFSL–XFM–XFP (set n°1) in comparison with the reference test (Harper's test, Harper et al., 2010).
Moreover, the tetraplex qPCR assay set n°1 allowed the detection and identification of mix infections with two to three subspecies simultaneously. On N. oleander, O. europaea, P. myrtifolia, and P. dulcis, the LOD for the two or three inoculated subspecies is similar of the one obtained for single inoculations (Table 5).
To demonstrate that our multiplex qPCR assays are modular tools, which can be adapted to one's needs, three other primer and probe sets were evaluated. In one set, we removed the primers and probe targeting the species (set n°4: XFFSL–XFM–XFP). In a second one, we replaced it by the Harper's primers and probe as this test is known to be highly sensitive (set n°5: Harper–XFFSL–XFM–XFP), and we also tested the use of primers and probes targeting the 18S rRNA as an internal control (set n°6: 18S–XFFSL–XFM–XFP). Evaluation of these three sets on calibrated DNA suspensions of the Xff strain CFBP 7970 indicated that the LOD for the XFFSL primers was the same than the one found previously for the sets n°1, 4, 5, and 6 (10 pg.ml−1) (Supplemental Data 8). In Q. robur and C. monspeliensis samples spiked with the Xfm strain CFBP 8416, the LOD obtained for the primers detecting the multiplex subspecies (XFM) was the same for the three sets (1×105 CFU.ml−1) (Supplemental Data 9). The use of Harper's primers and probe in set n°5 allowed the detection of Xf strain at the same LOD than for XF primers and probe in spiked Q. robur samples, but the detection was slightly better (a gain of 1 log unit) in the spiked C. monspeliensis samples. A Ct value was obtained for all spiked samples with the 18s rRNA primers, highlighting that these primers and probe were reliable internal amplification controls.
Ten plant samples from Corsica, France (Table 6), and 10 samples from inoculated plants (Table 7) were tested using the tetraplex set n°1. Our tetraplex qPCR assay was able to detect the bacterium in samples declared contaminated with Harper's qPCR assay up to Ct =34.97. However, this LOD was variable depending on the matrices (Table 7). While the bacterium was detected at the subspecies level with one or the other primer and probe combinations in eight environmental plant samples, the XF primers and probe was less efficient and allowed the detection in only five samples (Table 6) indicating that primer and probe combinations designed for subspecies were more sensitive than the one designed to detect the species. The subspecies was hence identified in samples that were not successfully typed using the MLST protocol. Samples of Centranthus trinervis, O. europaea, and Phylirea angustifolia (n°1, 6, and 7) were infected by a Xffsl strain, and samples of H. italicum, Lavandula stoechas, P. myrtifolia, and Spartium junceum (n°2, 3, 8, 9, and 10) were detected infected by a multiplex strain. The partial MLST subspecies identification of the sample n°8 was hence validated. The assay also identified the subspecies in the 10 samples obtained from inoculated plants and confirmed the identity of the inoculated strain.
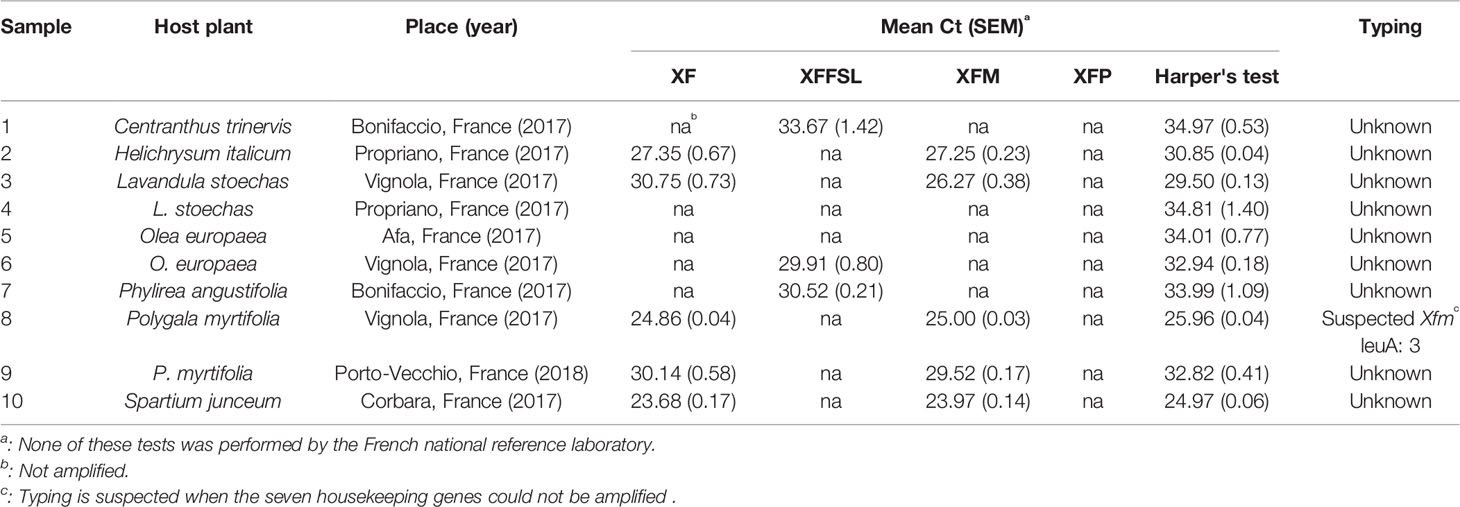
Table 6 Detection of X. fastidiosa in environmental plant samples with low population sizes using the tetraplex qPCR assay set n°1 in comparison with the reference test (Harper's test, Harper et al., 2010).
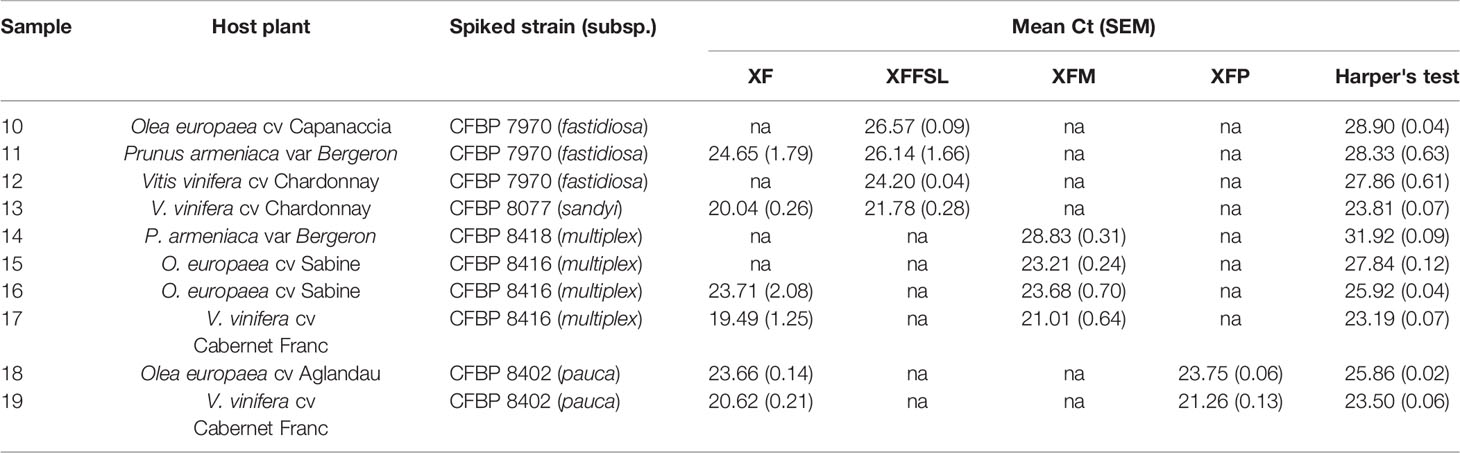
Table 7 Detection of X. fastidiosa in inoculated plants using the tetraplex qPCR assay (set n°1) in comparison with the reference test (Harper's test, Harper et al., 2010).
Since its first detection in Europe in 2013, Xf has been reported in various EU member states and on a wide host range (https://ec.europa.eu/food/sites/food/files/plant/docs/ph_biosec_legis_emergency_db-host-plants_update12.pdf). It is hence considered as an emergent plant bacterium in Europe, and it is regulated in the EU as a quarantine organism under Council Directive 2000/29/EC. Control measures to prevent the spread of this pathogen within the EU are limited to eradication and containment measures (EFSA, 2018b). Application of these outbreak management strategies requires the identification of Xf strains at the subspecies level. Indeed, the list of host plants is provided per Xf subspecies with only a limited number of plants (currently 15) being hosts of all subspecies currently detected in the EU. Identifying Xf at the subspecies level is thus highly important to limit the number of host plants to be eradicated once an outbreak is detected.
In this context, on the basis of a large data set of in-house and publicly available genome sequences of Xf and SkIf, a powerful bioinformatics tool (Briand et al., 2016; Denancé et al., 2019), we identified species and subspecies signatures. These long-mers were used as targets to designed primer and probe combinations with different levels of specificity. These combinations target single-copy genes encoding proteins involved in bacterial metabolism. This is the case for the XF primers and probe targeting a gene encoding a ketol-acid reductoisomerase, an enzyme essential in the biosynthesis pathway of the L-isoleucine and L-valine; XFF primers and probe target a gene encoding a restriction modification system DNA specificity, involved in defense against foreign DNA (Wilson and Murray, 1991); XFM primers and probe target a gene coding a DNA methyltransferase; XFMO primers and probe target a gene coding an S24 peptidase involved in a stress-response against DNA lesions and leading to the repair of single-stranded DNA (Erill et al., 2007); XFP primers and probe target a gene coding a histidine kinase and an ABC transporter substrate, two membrane proteins involved in signal transduction across the cellular membrane (Yoshida et al., 2007; Tanaka et al., 2018). The targets of our subspecific assays were selected to be exactly identical among all strains of a given subspecies and absent from any other bacteria; thus, these targets are not recombining elements.
Tested on a large collection of target and non-target strains, the primers and probes showed high specificity for Xf and its subspecies and no cross-reactions. In vitro, the specificity was tested in two steps. Inclusivity was evaluated on strains of Xf subspecies and exclusivity on a range of strains chosen to be present in the same plant and insect niches as Xf (Rogers, 2016) or to be genetically closely related to it. With the exception of a few studies (Boureau et al., 2013; Hulley et al., 2019), only 1 to 10 non-target strains are selected to test the specificity of novel molecular detection tools (Francis et al., 2006; Harper et al., 2010; Burbank and Ortega, 2018). Here a larger collection including 30 non-target strains and 39 Xf strains was analyzed to ensure the specificity of the primer and probe combinations based on the advice of the PM 7/98 of the EPPO (2014) and the MIQE of Bustin et al. (2009).
At the moment there are only few methods allowing the simultaneous detection and identification of different subspecies of Xf, and none of them is specific. The conventional PCR test of Hernandez-Martinez et al. (2006) was designed to differentiate the subspecies multiplex, fastidiosa, and sandyi. Nevertheless, the analysis of more than 300 samples collected in France and infected with subsp. multiplex revealed the amplification of additional bands leading to unclear patterns (Denancé et al., 2017). A triplex qPCR assay was recently developed to identify Xff and Xfm and was tested on grapevine, almond, and insects (Burbank and Ortega, 2018). Compared to this assay, our tetraplex qPCR assays gave similar results for the analysis of spiked almond and grapevine samples. However, we did not detect any cross-reaction with our primers and probes, while the test proposed by Burbank and Ortega in 2018 could lead to cross-reactions with strains from the subspecies pauca and sandyi. While pauca strains have not been so far detected in grapevine samples in any outbreaks, it was demonstrated that grapevine is susceptible to pauca strains (Li et al., 2013), and caution should be taken not to misidentify Xf strains infecting grapevine.
Primers and probes optimized for qPCR tetraplex assays allowed simultaneously the detection of Xf and its identification at the subspecies level, providing two complementary results as the targets of the tests are different. The use of one of these tetraplex assays hence corresponds to the first requirement for Xf detection as reported in PM 7/98 (EPPO, 2014). So far, subspecies identification is done by sequencing two to seven housekeeping genes (Yuan et al., 2010; EPPO, 2018b). If one of the gene amplifications fails, or if sequencing is not feasible (in case of a too-low amount of DNA), then the subspecies cannot be assigned. The average value of the LOD for every gene in the Xf MLST scheme is at the best at 105 CFU.ml−1 (Cesbron et al., in prep). As demonstrated with single strain suspensions and mix-suspensions, these assays display high efficiency (i.e. low LOD), even if, as Ito and Suzaki (2017) have shown, multiplexing increases the LOD by up to 1 log unit. With an LOD of 10 to 100 pg.ml−1 (i.e. 4×103 to 4×104 copies.ml−1), these multiplex qPCR assays still present a sensitivity that is similar to the one of the reference protocol, on single bacterial suspensions (Harper et al., 2010).
In spiked and environmental plant samples, the benefit from the use of our tetraplex assays is obvious. The tetraplex qPCR assays developed here are able to identify Xf subspecies up to 103 CFU.ml−1 in spiked samples. They allowed the identification of the Xf subspecies in environmental plant samples, as well, leading to subspecies identification when MLST failed and confirmed partial MLST identification. Subspecies was identified in samples detected infected but with high Ct values (determined at 35 with the Harper's qPCR assay), which corresponds to a bacterial load of only 103 CFU.ml−1. It should be mentioned here that to increase the chance of detecting Xf in low contaminated samples, a sonication step has been added before DNA extraction. Indeed, it has been known for a while that sonication allows bacterial recovery from plant samples (Morris et al., 1998), and this was recently demonstrated to improve Xf isolation from plant samples (Bergsma-Vlami et al., 2017). We hypothesize that a sonication step while disrupting biofilm will allow a better cell lysis through a better access of chemicals to the cells. Although analysis of more samples is necessary to confirm this LOD, the tetraplex qPCR assays allow the identification of Xf subspecies in samples for which it was not possible with the current MLST scheme, even considering only two genes.
In spiked plant samples the LOD of our tetraplex qPCR assays were 10 to 100 times higher than in bacterial suspensions. This could be linked to the presence of plant metabolites, mostly polyphenols, polysaccharides, but also pectin or xylan, that act as inhibitors of the polymerase. To avoid such a problem, we already included BSA in the PCR reaction mix to chelate polyphenols (Wei et al., 2008; Harper et al., 2010). Moreover, we used polymerases that are known to be less susceptible to inhibitors than regular ones. The TaqMan™ Universal PCR Master Mix (used in the qPCR Harper's test) contains an AmpliTaq Gold DNA polymerase, and the SsoAdvanced™ Universal Probes Supermix (Bio-Rad) (used in our tetraplex qPCR assays) contains an Sso7d fusion polymerase. Both Taq polymerases were highlighted to have good amplification performance in comparison to nine other Taq polymerases (Witte et al., 2018). The Sso7d fusion polymerase was optimized for multiplex qPCR and to amplify samples rich in inhibitors such as polysaccharides, cellulose, or pectin. Grapevine and olive tree are known to be rich in polyphenols (Ortega-Garcia et al., 2008; Schneider et al., 2008). These compounds are accumulated in the plant during stress or fruit ripening (Ortega-Garcia et al., 2008; Ennajeh et al., 2009). These variations could explain the 10- to 100-fold-higher LOD obtained for the second repetition that was performed with grapevine and olive tree sampled 2 months after the first sample set.
While we added a sonication step to improve DNA extraction, we did not test here other ways to improve per se the DNA extraction step and improve the LOD of our assays. Various options are available. A phenol–chloroform step could be added to the DNA extraction method to reduce the level of extracted proteins (Schrader et al., 2012). Reagents such as Tween 20, DMSO, polyethylene glycol, or active carbon could be used to precipitate the polysaccharides before DNA precipitation (Schrader et al., 2012). Phenol levels may be reduced with the use of polyvinyl-pyrrolidone or the addition of borate (Wilkins and Smart, 1996). Drying plant samples at 65°C for 2 days, prior to DNA extraction, could also help to cancel out the effect of phenolic inhibitors (Sipahioglu et al., 2006).
One of the great advantages of the multiplex qPCR assays we developed is that they are modular and reliable. Combinations of primers and probe can be adapted to include sets aiming at detecting infections at the species and/or only at the subspecies level, and having internal controls for each reaction. We showed here as proofs of concept, that replacing our XF primers and probe with the ones from Harper's test is feasible and leads to highly susceptible test, as using 18S rRNA primers and probe as internal control is efficient.
In addition, unlike with identification relying on MLST scheme, the qPCR tetraplex assays allow the simultaneous identification of several subspecies in one sample, as demonstrated with spiked samples. In fact, mix infections with two subspecies of Xf have already been observed in naturally infected plants (Chen et al., 2005; Bergsma-Vlami et al., 2017; Denancé et al., 2017). This leads to the observation of multiple peaks on the sequencing sequence of a housekeeping gene and is complex to analyze and differentiate from a sequencing error (Denancé et al., 2017). The simultaneous detection and identification of multiple subspecies brings the tetraplex qPCR assays powerful tools to easily and quickly detect mixed infection or to study Xf in areas such as Europe where several subspecies live in sympatry (Denancé et al., 2017).
When a new assay is developed, the time and cost difference with current protocols must be taken into account. The tetraplex qPCR assays are much faster and cheaper than using a test for detection and then a reduced MLST scheme for subspecies assignation. The current protocol costs are for Harper's qPCR detection at the writing time ~0.52€ for reagents (for a volume of 10 µl) ~1.62€ for the amplification of two housekeeping genes (~0.81€/gene for a volume of 20 µl), and ~10.2€ for their sequencing (~5.1€/gene in both directions), hence totalizing ~12.35€ per sample. In comparison, a single tetraplex qPCR assay costs ~0.37€ per sample (for a volume of 10 µl). None of these costs includes the cost of plastic materials or specialized equipment such as a qPCR thermocycler.
To conclude, we developed specific, effective, fast, cost-efficient, and easy-to-set-up tools allowing in one step to detect and identify at the subspecies level Xf infection directly in plant samples. Compared to current protocols, the LOD of our tetraplex assays allowed subspecies identification at levels where regular amplifications such as the one used for MLST failed. Tetraplex qPCR assays are also easy to perform in a routine lab and as such should be easily transferable to laboratories and are modular according to the user's needs.
All datasets generated for this study are included in the article/Supplementary Material.
ED performed the experiments. ED and SC conducted the study. MB designed the bioinformatics tool. ED, MB, M-AJ, and SC designed the in silico analysis and interpreted the data. M-AJ conceived the study and applied for funding. ED, M-AJ, and SC wrote the manuscript. All authors read and approved the final version of the manuscript.
ED’s salary was funded by INRA SPE division and Anses. This work received support from the European Union’s Horizon 2020 research and innovation program under grant agreement 727987 XF_ACTORS (Xylella Fastidiosa Active Containment Through a multidisciplinary-Oriented Research Strategy). The present work reflects only the authors’ view, and the EU funding agency is not responsible for any use that may be made of the information it contains.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The present work reflects only the authors' view, and no analysis has been made in the French Reference Lab; in particular, ED is not authorized to perform any official tests at Anses.
BLAST, Basic Local Alignment Search Tool; CNBC, National Botanical Conservatory of Corsica; INRA, French National Institute for Agricultural Research; IRHS, Research Institute of Horticulture and Seeds; LAMP, loop-mediated isothermal amplification; MIQE, Minimum Information for the Publication of Quantitative Real-Time PCR Experiments; MLST, multilocus sequence typing; NCBI, National Center for Biotechnology Information; ST, sequence type; Xf, Xylella fastidiosa; Xff, Xylella fastidiosa subsp. fastidiosa; Xffsl, Xylella fastidiosa subsp. fastidiosa sensu lato; Xfm, Xylella fastidiosa subsp. multiplex; Xfmo, Xylella fastidiosa subsp. morus; Xfp, Xylella fastidiosa subsp. pauca; Xfs, Xylella fastidiosa subsp. sandyi; WGS, Whole Genome Shotgun.
We thank Muriel Bahut (ANAN technical facility, SFR QUASAV, Angers, FR) for DNA extraction automatization, CIRM-CFBP (Beaucouzé, INRA, France; http://www6.inra.fr/cirm_eng/CFBP-Plant-Associated-Bacteria) for strain preservation and supply, Leonardo de la Fuente (Auburn University, AL, USA) and LSV-ANSES for sharing strains, and colleagues from CNBC for sampling plants in Corsica, France. We acknowledge Nicolas Denancé for preliminary experiments to design specific PCR tests. We thank Charles Manceau (Anses, Angers, FR) for his contribution while applying for funding, and Armelle Darrasse and Matthieu Barret for fruitful discussions and critical reading of the manuscript. This manuscript has been released as a pre-print at bioRxiv (Dupas et al., 2019).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.01732/full#supplementary-material
Abraham, N. D., Chitrampal, P., Keriö, S., LeBoldus, J. M. (2018). Multiplex qPCR for detection and quantification of Sphaerulina musiva in Populus stems. Plant Pathol. 67, 1874–1882. doi: 10.1111/ppa.12913
Bergsma-Vlami, M., van de Bilt, J. L. J., Tjou-Tam-Sin, N. N. A., Helderman, C. M., Gorkink-Smits, P. P. M. A., Landman, N. M., et al. (2017). Assessment of the genetic diversity of Xylella fastidiosa in imported ornamental Coffea arabica plants. Plant Pathol. 66, 1065–1074. doi: 10.1111/ppa.12696
Bonants, P., Griekspoor, Y., Houwers, I., Krijger, M., Zouwen van der, P., van der Lee, T., et al. (2018). The development and evaluation of a triplex TaqMan assay and next generation sequence analysis for improved detection of Xylella in plant material. Plant Dis. 103, 645–655. doi: 10.1094/PDIS-08-18-1433-RE
Boureau, T., Kerkoud, M., Chhel, F., Hunault, G., Darrasse, A., Brin, C., et al. (2013). A multiplex-PCR assay for identification of the quarantine plant pathogen Xanthomonas axonopodis pv. phaseoli. J. Microbiol. Methods 92, 42–50. doi: 10.1016/j.mimet.2012.10.012
Briand, M., Gaborieau, R., Jacques, M.-A., Barret, M., Boureau, T., Gaillard, S., et al. (2016). SkIf: a tool for rapid identification of genes or regulators of interest. FResearch 5. doi: 10.7490/f1000research.1112490.1
Burbank, L. P., Ortega, B. C. (2018). Novel amplification targets for rapid detection and differentiation of Xylella fastidiosa subspecies fastidiosa and multiplex in plant and insect tissues. J. Microbiol. Methods 155, 8–18. doi: 10.1016/j.mimet.2018.11.002
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi: 10.1373/clinchem.2008.112797
C/2017/4883 (2017). Commission implementing directive (EU) 2017/1279 of 14 July 2017 amending Annexes I to V to Council Directive 2000/29/EC on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community.Available at: http://data.europa.eu/eli/dir_impl/2017/1279/oj/fra [Accessed November 20, 2018].
Chen, J., Groves, R., Civerolo, E. L., Viveros, M., Freeman, M., Zheng, Y. (2005). Two xylella fastidiosa genotypes associated with almond leaf scorch disease on the same location in California. Phytopathology 95, 708–714. doi: 10.1094/PHYTO-95-0708
Choi, W., Seol, M.-A., Jo, B.-H., Kim, I. R., Lee, J. R. (2018). Development and application of a novel multiplex PCR method for four living modified soybeans. Appl. Biol. Chem. 61, 635–641. doi: 10.1007/s13765-018-0399-8
Cruaud, A., Gonzalez, A.-A., Godefroid, M., Nidelet, S., Streito, J.-C., Thuillier, J.-M., et al. (2018). Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: a case study in Corsica. Sci. Rep. 8, 15628. doi: 10.1038/s41598-018-33957-z
Denancé, N., Legendre, B., Briand, M., Olivier, V., de Boisseson, C., Poliakoff, F., et al. (2017). Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathol. 66, 1054–1064. doi: 10.1111/ppa.12695
Denancé, N., Briand, M., Gaborieau, R., Gaillard, S., Jacques, M.-A. (2019). Identification of genetic relationships and subspecies signatures in Xylella fastidiosa. BMC Genomics 20, 239. doi: 10.1186/s12864-019-5565-9
Doležel, J., Bartoš, J., Voglmayr, H., Greilhuber, J. (2003). Nuclear DNA content and genome size of trout and human. Cytometry A 51, 127–128. doi: 10.1002/cyto.a.10013
Dupas, E., Briand, M., Jacques, M.-A., Cesbron, S. (2019). Comparison of real-time PCR and droplet digital PCR for the detection of Xylella fastidiosa in plants. J. Microbiol. Methods 162, 86–95. doi: 10.1016/j.mimet.2019.05.010
EFSA. (2018a). Update of the Xylella spp. host plant database. EFSA J. 16, 1–87. doi: 10.2903/j.efsa.2018.5408
EFSA. (2018b). Updated pest categorisation of Xylella fastidiosa. Eur. Food Saf. Authority. 16, 1–61. doi: 10.2903/j.efsa.2018.5357
Engels, W. R. (1993). Contributing software to the internet: the amplify program. Trends Biochem. Sci. 18, 448–450. doi: 10.1016/0968-0004(93)90148-G
Ennajeh, M., Vadel, A. M., Khemira, H. (2009). Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol. Plant 31, 711–721. doi: 10.1007/s11738-009-0283-6
EPPO. (2014). PM 7/98 (2) Specific requirements for laboratories preparing accreditation for a plant pest diagnostic activity. EPPO Bull. 44, 117–147. doi: 10.1111/epp.12118
EPPO. (2018a). EPPO A2 List of pests recommended for regulation as quarantine pests - version 2018-09 -. Available at: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed April 12, 2018].
EPPO. (2019). Xylella fastidiosa - Reporting articles. EPPO Global Databse. Available at: https://gd.eppo.int/reporting/article-6447 [Accessed March 11, 2019].
Erill, I., Campoy, S., Barbé, J. (2007). Aeons of distress: an evolutionary perspective on the bacterial SOS response. FEMS Microbiol. Rev. 31, 637–656. doi: 10.1111/j.1574-6976.2007.00082.x
Francis, M., Lin, H., Cabrera-La Rosa, J., Doddapaneni, H., Civerolo, E. L. (2006). Genome-based PCR primers for specific and sensitive detection and quantification of Xylella fastidiosa. Eur. J. Plant Pathol. 115, 203–213. doi: 10.1007/s10658-006-9009-4
Harper, S. J., Ward, L. I., Clover, G. R. G. (2010). Development of LAMP and real-time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology 100, 1282–1288. doi: 10.1094/PHYTO-06-10-0168
Hernandez-Martinez, R., Costa, H. S., Dumenyo, C. K., Cooksey, D. A. (2006). Differentiation of strains of xylella fastidiosa infecting grape, almonds, and oleander using a multiprimer PCR assay. Plant Dis. 90, 1382–1388. doi: 10.1094/PD-90-1382
Hulley, E. N., Tharmalingam, S., Zarnke, A., Boreham, D. R. (2019). Development and validation of probe-based multiplex real-time PCR assays for the rapid and accurate detection of freshwater fish species. PloS One 14, e0210165. doi: 10.1371/journal.pone.0210165
Ioos, R., Fourrier, C., Wilson, V., Webb, K., Schereffer, J.-L., de Labrouhe, D. T. (2012). An optimized duplex real-time PCR tool for sensitive detection of the quarantine oomycete plasmopara halstedii in sunflower seeds. Phytopathology 102, 908–917. doi: 10.1094/PHYTO-04-12-0068-R
Ito, T., Suzaki, K. (2017). Universal detection of phytoplasmas and Xylella spp. by TaqMan singleplex and multiplex real-time PCR with dual priming oligonucleotides. PloS One 12, e0185427. doi: 10.1371/journal.pone.0185427
Jacques, M.-A., Denancé, N., Legendre, B., Morel, E., Briand, M., Mississipi, S., et al. (2016). New coffee plant-infecting xylella fastidiosa variants derived via homologous recombination. Appl. Environ. Microbiol. 82, 1556–1568. doi: 10.1128/AEM.03299-15
Janse, I., Hamidjaja, R. A., Hendriks, A. C., van Rotterdam, B. J. (2013). Multiplex qPCR for reliable detection and differentiation of Burkholderia mallei and Burkholderia pseudomallei. BMC Infect. Dis. 13, 86. doi: 10.1186/1471-2334-13-86
Köppel, R., Schum, R., Habermacher, M., Sester, C., Piller, L. E., Meissner, S., et al. (2019). Multiplex real-time PCR for the detection of insect DNA and determination of contents of Tenebrio molitor, Locusta migratoria and Achaeta domestica in food. Eur. Food Res. Technol. 245, 559–567. doi: 10.1007/s00217-018-03225-5
Kamau, E., Alemayehu, S., Feghali, K. C., Saunders, D., Ockenhouse, C. F. (2013). Multiplex qPCR for detection and absolute quantification of malaria. PloS One 8, e71539. doi: 10.1371/journal.pone.0071539
Koressaar, T., Remm, M. (2007). Enhancements and modifications of primer design program Primer3. Bioinformatics 23, 1289–1291. doi: 10.1093/bioinformatics/btm091
Landa, B. (2017). Emergence of Xylella fastidiosa in Spain: current situation. Presentation made at the European Conference on Xylella 2017. Available at: https://www.efsa.europa.eu/en/events/event/171113 [Accessed January 17, 2019].
Li, W., Abad, J. A., French-Monar, R. D., Rascoe, J., Wen, A., Gudmestad, N. C., et al. (2009). Multiplex real-time PCR for detection, identification and quantification of ‘Candidatus Liberibacter solanacearum' in potato plants with zebra chip. J. Microbiol. Methods 78, 59–65. doi: 10.1016/j.mimet.2009.04.009
Li, W., Teixeira, D. C., Hartung, J. S., Huang, Q., Duan, Y., Zhou, L., et al. (2013). Development and systematic validation of qPCR assays for rapid and reliable differentiation of Xylella fastidiosa strains causing citrus variegated chlorosis. J. Microbiol. Methods 92, 79–89. doi: 10.1016/j.mimet.2012.10.008
Marcelletti, S., Scortichini, M. (2016). Genome-wide comparison and taxonomic relatedness of multiple Xylella fastidiosa strains reveal the occurrence of three subspecies and a new Xylella species. Arch. Microbiol. 198, 803–812. doi: 10.1007/s00203-016-1245-1
Minsavage, G., Thompson, C., Hopkins, D., Leite, R., Stall, R. (1994). Development of a polymerase chain reaction protocol for detection of xylella fastidiosa in plant tissue. Phytopathology 84, 456. doi: 10.1094/Phyto-84-456
Modesti, V., Pucci, N., Lucchesi, S., Campus, L., Loreti, S. (2017). Experience of the Latium region (Central Italy) as a pest-free area for monitoring of Xylella fastidiosa: distinctive features of molecular diagnostic methods. Eur. J. Plant Pathol. 148, 557–566. doi: 10.1007/s10658-016-1111-7
Morris, C. E., Monier, J.-M., Jacques, M.-A. (1998). A technique to quantify the population size and composition of the biofilm component in communities of bacteria in the phyllosphere. Appl. Environ. Microbiol. 64, 4789–4795.
Mougel, C., Cournoyer, B., Nesme, X. (2001). Novel tellurite-amended media and specific chromosomal and Ti plasmid probes for direct analysis of soil populations of agrobacterium Biovars 1 and 2. Appl. Environ. Microbiol. 67, 65–74. doi: 10.1128/AEM.67.1.65-74.2001
Nunney, L., Schuenzel, E. L., Scally, M., Bromley, R. E., Stouthamer, R. (2014). Large-scale intersubspecific recombination in the plant-pathogenic bacterium xylella fastidiosa Is associated with the host shift to mulberry. Appl. Environ. Microbiol. 80, 3025–3033. doi: 10.1128/AEM.04112-13
Nunney, L., Azad, H., Stouthamer, R. (2019). An experimental test of the host-plant range of nonrecombinant strains of North American Xylella fastidiosa subsp. multiplex. Phytopathology 109, 294–300. doi: 10.1094/PHYTO-07-18-0252-FI
Ortega-Garcia, F., Blanco, S., Peinado, M. A., Peragon, J. (2008). Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. “Picual“ trees during fruit ripening. Tree Physiol. 28, 45–54. doi: 10.1093/treephys/28.1.45
Ouyang, P., Arif, M., Fletcher, J., Melcher, U., Corona, F. M. O. (2013). Enhanced reliability and accuracy for field deployable bioforensic detection and discrimination of Xylella fastidiosa subsp. pauca, Causal Agent of Citrus Variegated Chlorosis Using Razor Ex Technology and TaqMan Quantitative PCR. PloS One 8, e81647. doi: 10.1371/journal.pone.0081647
Potnis, N., Kandel, P. P., Merfa, M. V., Retchless, A. C., Parker, J. K., Stenger, D. C., et al. (2019). Patterns of inter- and intrasubspecific homologous recombination inform eco-evolutionary dynamics of Xylella fastidiosa. ISME J. 13, 2319–2333. doi: 10.1038/s41396-019-0423-y
Reisenzein, H. (2017). “PCR assays for the detection of Xylella fastidiosa. Review and comparison of published protocols,” in Xylella fastidiosa & the Olive Quick Decline Syndrome (OQDS). A serious worldwide challenge for the safeguard of olive trees Options Méditerranéennes: Série A. Séminaires Méditerranéens. Eds. D'Onghia, A. M., Valentini, F., Brunel, S. (Bari: CIHEAM), 57–60. Available at: http://om.ciheam.org/om/pdf/a121/00007213.pdf.
Rogers, E. E. (2016). Deep 16S rRNA gene sequencing of anterior foregut microbiota from the blue-green sharpshooter (Graphocephala atropunctata). J. Appl. Entomol. 140, 801–805. doi: 10.1111/jen.12303
Saponari, M., Boscia, D., Nigro, F., Martelli, G. P. (2013). Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 95, 659–668. doi: 10.4454/JPP.V95I3.035
Schneider, E., von der Heydt, H., Esperester, A. (2008). Evaluation of polyphenol composition in red leaves from different varieties of vitis vinifera. Planta Med. 74, 565–572. doi: 10.1055/s-2008-1034370
Schrader, C., Schielke, A., Ellerbroek, L., Johne, R. (2012). PCR inhibitors – occurrence, properties and removal. J. Appl. Microbiol. 113, 1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x
Sipahioglu, H. M., Usta, M., Ocak, M. (2006). Use of dried high-phenolic laden host leaves for virus and viroid preservation and detection by PCR methods. J. Virol. Methods 137, 120–124. doi: 10.1016/j.jviromet.2006.06.009
Tanaka, K. J., Song, S., Mason, K., Pinkett, H. W. (2018). Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim. Biophys. Acta 1860, 868–877. doi: 10.1016/j.bbamem.2017.08.011
Wang, F., Feng, J., Ye, S., Huang, H., Zhang, X. (2018). Development of a multiplex fluorescence quantitative PCR for detection of genetically modified organisms. Biologia 73, 21–29. doi: 10.2478/s11756-018-0004-y
Wei, T., Lu, G., Clover, G. (2008). Novel approaches to mitigate primer interaction and eliminate inhibitors in multiplex PCR, demonstrated using an assay for detection of three strawberry viruses. J. Virol. Methods 151, 132–139. doi: 10.1016/j.jviromet.2008.03.003
Wei, S., Daliri, E. B.-M., Chelliah, R., Park, B.-J., Lim, J.-S., Baek, M.-A., et al. (2019). Development of a multiplex real-time PCR for simultaneous detection of Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus in food samples. J. Food Saf. 39, e12558. doi: 10.1111/jfs.12558
Wells, J. M., Raju, B. C., Nyland, G., Lowe, S. K. (1981). Medium for isolation and growth of bacteria associated with plum leaf scald and phony peach diseases. Appl. Environ. Microbiol. 42, 357–363.
Wilkins, T. A., Smart, L. B. (1996). “Isolation of RNA from plant tissue,” in A laboratory guide to RNA: isolation, analysis, and synthesis. Eds. Krieg, P. A. (New York: Wiley-Liss), 21–42.
Willsey, T. L., Chatterton, S., Heynen, M., Erickson, A. (2018). Detection of interactions between the pea root rot pathogens Aphanomyces euteiches and Fusarium spp. using a multiplex qPCR assay. Plant Pathol. 67, 1912–1923. doi: 10.1111/ppa.12895
Wilson, G. G., Murray, N. E. (1991). Restriction and modification systems. Ann. Rev. Genet. 25, 585–627. doi: 10.1146/annurev.ge.25.120191.003101
Witte, A. K., Sickha, R., Mester, P., Fister, S., Schoder, D., Rossmanith, P. (2018). Essential role of polymerases for assay performance – impact of polymerase replacement in a well-established assay. Biomol. Detect. Quantif. 16, 12–20. doi: 10.1016/j.bdq.2018.10.002
Yoshida, T., Phadtare, S., Inouye, M. (2007). “The Design and Development of Tar-EnvZ Chimeric Receptors,” in Methods in Enzymology Two-Component Signaling Systems, Part B. Eds. Simon, M. I., Crane, B. R., Crane, A. (Elsvier: Academic Press), 166–183. doi: 10.1016/S0076-6879(07)23007-1
Yuan, X., Morano, L., Bromley, R., Spring-Pearson, S., Stouthamer, R., Nunney, L. (2010). Multilocus sequence typing of xylella fastidiosa causing pierce's disease and oleander leaf scorch in the United States. Phytopathology 100, 601–611. doi: 10.1094/PHYTO-100-6-0601
Keywords: real-time PCR, TaqMan, subspecies differentiation, diagnostic, SkIf, multiplexing
Citation: Dupas E, Briand M, Jacques M-A and Cesbron S (2019) Novel Tetraplex Quantitative PCR Assays for Simultaneous Detection and Identification of Xylella fastidiosa Subspecies in Plant Tissues. Front. Plant Sci. 10:1732. doi: 10.3389/fpls.2019.01732
Received: 19 July 2019; Accepted: 09 December 2019;
Published: 27 December 2019.
Edited by:
Roger Deal, Emory University, United StatesReviewed by:
Paul Rugman-Jones, University of California, Riverside, United StatesCopyright © 2019 Dupas, Briand, Jacques and Cesbron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Agnès Jacques, bWFyaWUtYWduZXMuamFjcXVlc0BpbnJhLmZy; Sophie Cesbron, PGEgaHJlZj0ibWFpbHRvOnNvcGhpZS5jZXNicm9uQGlucmEuZnIiPnNvcGhpZS5jZXNicm9uQGlucmEuZnI8L2E+
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.