
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 19 June 2019
Sec. Plant Abiotic Stress
Volume 10 - 2019 | https://doi.org/10.3389/fpls.2019.00767
This article is part of the Research TopicThe Role of Light in Abiotic Stress AcclimationView all 9 articles
Mediterranean ecosystems harbor a very important part of Earth’s biodiversity, and they are a conservation priority due to the effects of global change. Here, we examined the performance of the semi-deciduous shrub Cistus albidus under Mediterranean conditions during winter, including changes in leaf angle governed by diurnal, seasonal, and positional effects and their relationship with winter photoinhibition and photoprotection. We found marked diurnal variations in leaf angle during the day in autumn, which disappeared as the photoperiod shortened during winter due to a progressive decrease in the predawn leaf angle from November to January. During this period, auxin contents decreased, while those of melatonin increased, and the Fv/Fm ratio, chlorophyll, and tocopherol contents kept unaltered, thus indicating the absence of photoinhibitory damage. A marked decrease in the leaf angle toward the shoot apex occurred during winter, which was associated with slightly higher Fv/Fm ratios. An analysis of the inter-individual variability and sun orientation effects on leaf movements in a population growing in the Montserrat mountains revealed a very marked variability of 46.8% in the leaf angle, while Fv/Fm ratio showed a variation of 7.5% only. West orientation, which was associated with reduced leaf temperatures, but with no differences in the photosynthetic photon flux density, led to enhanced tocopherol contents, while leaf angle, Fv/Fm ratio, chlorophyll, auxin, and melatonin contents kept unaltered. It is concluded that (1) C. albidus has very effective and fine-regulated photoprotection mechanisms, including an adequate orientation of decussate leaves as part of the developmental program, (2) leaf angle is modulated on a diurnal and seasonal basis, thus contributing to prevent photoinhibition as a first line of defense, and (3) enhanced tocopherol contents help withstand combined high light with low temperature stress in C. albidus growing at high elevation.
The Mediterranean basin is one of the most unique biomes that harbors an important portion of Earth’s plant biodiversity. Mediterranean ecosystems have been described as especially delicate upon the predicted extreme climatic events due to climate change (Peñuelas et al., 2017), and given that there is already a constant loss of biodiversity (Butchart et al., 2010), Mediterranean plant species are a conservation priority (Bellard et al., 2014). Light has been classically considered as one of the main stressors in Mediterranean-type ecosystems for plants (Joffre et al., 1999; Karavatas and Manetas, 1999), particularly excess of light, which together with drought (typically occurring during summer) or low temperatures (during winter) can cause considerable damage to the photosynthetic apparatus (Martínez-Ferri et al., 2004; Fernández-Marín et al., 2017).
Mediterranean plants have evolved different photoprotection mechanisms to avoid this excess of light, building a first line of defense. There are structural adaptations to avoid excess of light absorption such as tomentosus leaves that reflect light (Ehleringer and Björkman, 1978), chloroplast movements during the day (Haupt and Scheuerlein, 1990), changes in leaf orientation (Ehleringer and Comstock, 1987), paraheliotropism (Lambers et al., 2008), and a phyllotaxis with an opposite disposition of leaves (Brites and Valladares, 2005). In the case of leaf paraheliotropism, for instance, not all plants species exhibit it but it has developed in multiple lineages, including the well-known common bean (Phaseolus vulgaris L.), where this movement is triggered by an endogenous circadian oscillator and light-induced signals that lead to turgor-dependent changes in the pulvinus, which is located at the juncture of the leaf base and the petiole (Mayer et al., 1985). In several Mediterranean plants, such as the white-leaved rockrose (Cistus albidus L.), reduced leaf angles occur as part of the developmental program with opposite and decussate leaves (without petioles) increasing their leaf angle as they develop at the shoot apex and progressively occupy more distal positions (De Bolòs, 1993).
Once light has gone through the structural photoprotection mechanisms, it is absorbed by leaves and penetrates the chloroplasts, plants activate a second line of defense to counteract excess solar radiation. The first and one of the most flexible mechanisms to counteract the excess excitation energy and, consequently, photo-oxidative stress in chloroplasts is the xantophyll cycle (Demmig-Adams and Adams, 2006). However, if the excited triple chlorophyll (3Chl*) derived from excited chlorophyll a (1Chl*) is not quenched by the non-photochemical quenching (NPQ) in the photosystem II, this can lead to the production of singlet oxygen (1O2) and ultimately to the oxidation of lipids and other molecules in the chloroplast. At the same time, O2 can also be over-reduced by the electrons originated in the photosystem I to superoxide radicals (O2−) and then form hydrogen peroxide (H2O2) and finally hydroxyl radical (OH−), which is highly reactive (Asada, 2006). The joint effects of these ROS can cause photoinhibition, and if this is sustained, plants will experience photo-oxidative damage (Takahashi and Badger, 2011). This can be prevented by the concerted action of a myriad of antioxidant compounds. Among them, lipophilic compounds, such as tocopherols (vitamin E), have been shown to play a major role in photoprotection (Havaux et al., 2005) acting as a sentinel for stress sensing and signaling (Munné-Bosch, 2019). On the other hand, melatonin, a tryptophan-derived compound (sharing part of the biosynthesis pathway with the phytohormone indole-3-acetic acid, Pérez-Llorca et al., 2019) has been postulated as a putative antioxidant and photoprotective compound in plants (Ding et al., 2017). However, it is still not fully elucidated whether or not melatonin always acts directly as an antioxidant or indirectly through its modulatory effects on gene expression (Arnao and Hernández-Ruiz, 2019).
The aim of this study was to get new insights into the performance of the semi-deciduous shrub, C. albidus during high light stress and suboptimal (low) temperatures during the Mediterranean winter, with a particular emphasis on the causes and consequences of changes in leaf orientation as part of the developmental program. We hypothesized that leaf movements may be governed on a diurnal and seasonal basis and that they may significantly influence the extent of photoinhibition and photoprotective demand of leaves during winter. To test this hypothesis, we measured (1) leaf angles during predawn and midday from November to January, (2) leaf positional effects on leaf angles during winter (January), and (3) the inter-individual variability and sun orientation influence on leaf angles and the Fv/Fm ratio in a natural population growing at 1,100 m.a.s.l., which was exposed to a combination of high light and low temperatures. Furthermore, we studied the endogenous variations in melatonin in relation to a biosynthesis-related phytohormone (auxin) and a well-known chloroplastic antioxidant (α-tocopherol) to get new insights into the possible protective effects of this compound against environmental stress in plants.
Three independent, complementary experiments were performed using white-leaved rockrose (Cistus albidus L.). The first experiment was focused on the study of diurnal variations in leaf angle from autumn to winter, including measurements at predawn (1 h before sunrise) and midday (at maximum diurnal photosynthetic photon flux density; PPFD). The samplings were performed on November 6 and December 4, 2017 (autumn), and January 16, 2018 (winter) in a population growing in the experimental garden of the Faculty of Biology at the University of Barcelona (41.384 N, 2.119E, 59 m.a.s.l.). All measurements from this study were made using leaves situated on the second whorl of the plant (second leaf position) from the top of shoots. The second experiment was focused on the study of leaf positional effects on leaf angle during winter. Leaves situated on the first, second, third, fourth, and fifth whorl (designated here, arbitrarily, as positions 1–5 and corresponding to two different leaf dispositions on the stem) from the top of shoots of the same plants as those used for the first experiment were selected for measurements during January 16, 2018 at midday (at maximum diurnal PPFD). The third experiment focused on sun orientation and inter-individual driven variability in the leaf angle of a natural population in the Natural Park of the Montserrat mountains, Spain (41.586 N–1.830 E, 1100 m.a.s.l.) during March 22, 2018. A stressful cold and sunny day was selected for measurements, with maximum and minimum air temperatures during the day of 14.7°C and −0.2°C, respectively (mean relative humidity was 30% and maximum diurnal PPFD 1570 μmol m−2 s−1, while no precipitation during the day was recorded). Sixty individuals of the same population, but with different sun orientations, were sampled; 30 were East oriented and 30 were West oriented (with an average leaf temperature of 11°C and 19°C, respectively, with no difference in the average relative humidity and maximum diurnal PPFD). All measurements for this study were made using leaves situated on the second whorl (position 2) from the top of shoots in all individuals. Details of soil characteristics of both sites (experimental garden from the University of Barcelona and the Montserrat mountains) can be found in Müller et al. (2013).
For the three experiments, all samplings at each time point were performed using two leaves of the same whorl per shrub from same-aged individuals that were randomly selected at the beginning of the study. Aside from leaf angle, which was described with the zenith and the azimuth angles of the surface normal to a small flat plate (Itakura and Hosoi, 2018), leaf biomass, leaf mass per area ratio, leaf hydration, chlorophyll contents, Fv/Fm ratio, indole-3-acetic acid (IAA), melatonin, and α-tocopherol contents were measured as described below. For all biochemical analyses, samples were immediately snap frozen in liquid nitrogen and stored at −80°C until subsequent processing in the laboratory.
PPFD, leaf temperature, and the Fv/Fm ratio (after 1 h of darkness), the latter used as an indicator of photoinhibition (Bolhar-Nordenkampf et al., 1991), were measured with a Mini-PAM II (Photosynthesis Yield Analyzer, Walz, Germany) in situ. Leaves were immediately weighed to estimate leaf biomass and leaf area was measured using a flatbed scanner (model Officejet Pro 8610, HP, California, USA). Then, dry mass was estimated by weighing the sample after oven drying it at 65°C to constant weight. Leaf hydration (H) was calculated as (fresh mass-dry mass)/dry mass, and leaf mass per area (LMA) was calculated as dry mass/area.
The chlorophyll a + b content of leaves was determined spectrophotometrically in methanol extracts using the equations described by Lichtenthaler (1983). Melatonin and auxin contents were determined by ultrahigh-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS), and α-tocopherol was determined by high-performance liquid chromatography (HPLC). In short, 50 mg per sample was extracted with 250 μl of cold methanol using ultrasonication and vortexing (Branson 2510 ultrasonic cleaner, Bransonic, Danbury, CT, USA) for 30 min. Deuterium-labeled indole-3-acetic and melatonin were then added, and after centrifugation at 12,000 rpm for 10 min at 4°C, the pellet was re-extracted using the same procedure. Supernatants were pooled and filtered through a 0.22-μm PTFE filter (Waters, Milford, MA, USA) before UHPLC-MS/MS and HPLC analyses. Indole-3-acetic and melatonin contents were analyzed by using UHPLC-ESI-MS/MS as described in Müller and Munné-Bosch (2011). Quantification was made considering recovery rates for each sample by using deuterium-labeled internal standards. α-Tocopherol contents were determined by using a normal-phase HPLC system coupled to a fluorescent detector as described in Cela et al. (2011). α-Tocopherol was identified by co-elution with an authentic standard (Sigma-Aldrich, Steinheim, Germany) and quantified using a calibration curve.
To assess the combined effects of month (“November,” “December,” “January”) and daytime (“Predawn,” “Midday”) on leaf angle, leaf biomass, leaf mass per area, hydration, chlorophyll a + b, Fv/Fm ratio, IAA, melatonin, and α-tocopherol, we used a linear mixed model (LMM). Different combinations of “Month,” “Daytime,” and their interaction were tested as fixed terms using Akaike information criterion (AIC), i.e., the model that best fitted the data should have the lowest AIC value. “Plant” was included in all models as a random term to account for repeated measures (Zuur et al., 2009). Models were fitted with maximum likelihood (ML) for model comparison. Final models were fitted using restricted maximum likelihood (REML). Additionally, when “Daytime” had a significant effect, an LMM with “Daytime” as an explanatory variable was used to test for significant differences within each month. To assess significant differences within leaf position on the response variables above mentioned, we used a LMM where “Leaf position” was fitted as the fixed term and “Plant” as a random term. To assess the effect of sun orientation in the Montserrat natural-growing population, a linear regression was used with “Orientation” as explanatory variable. In all models, the p of the main fixed effects were estimated using conditional F-tests (generally, ANOVA type II or ANOVA type III when an interaction term was included), and when main effects were significant, multiple comparisons between “Month” and “Daytime,” or “Leaf position” levels, were tested with the Tukey HSD post hoc test. All models were validated by visually checking the distribution of residuals for normality and homoscedasticity (Zuur et al., 2009). Before analyses, leaf angle, chlorophyll a + b, IAA, and melatonin were log10-transformed; leaf mass per area and α-tocopherol were square root-transformed; and Fv/Fm ratio was logit-transformed. Finally, to explore possible relationships between all response variables, we used the Spearman’s rank correlation test. All analyses were performed using the computing environment R (R Core Team 2019).
Leaf angle measurements at predawn and midday during 6th November, 4th December, and 16th January revealed significant diurnal variations in leaf orientation. Leaves had a reduced angle during the day in autumn (November and December) but not in winter (January), when maximum orientation parallel to sun’s rays was attained already at predawn (Figure 1). It is interesting to note that despite diurnal variations in leaf orientation in autumn, maximum orientation parallel to the sunbeam was always attained at midday, with leaf angles around 35°, irrespective of the day of measurements (Figure 1). Diurnal PPFD during this period changed from non-detectable values at predawn to values ranging between 892 and 989 μmol m−2 s−1 at midday, while the photoperiod was shortened from 10.3 h on 6th November to 9.5 h on 16th January. Leaf biomass, leaf mass per area ratio, and leaf hydration kept unaltered throughout the study (Figure 1). Despite chlorophyll contents decreased slightly on 4th December at predawn, which might be associated with temperatures reaching minimum values of 3.9°C (Table 1), the Fv/Fm ratio, an indicator of photoinhibition, kept unaltered throughout the study (Figure 1).
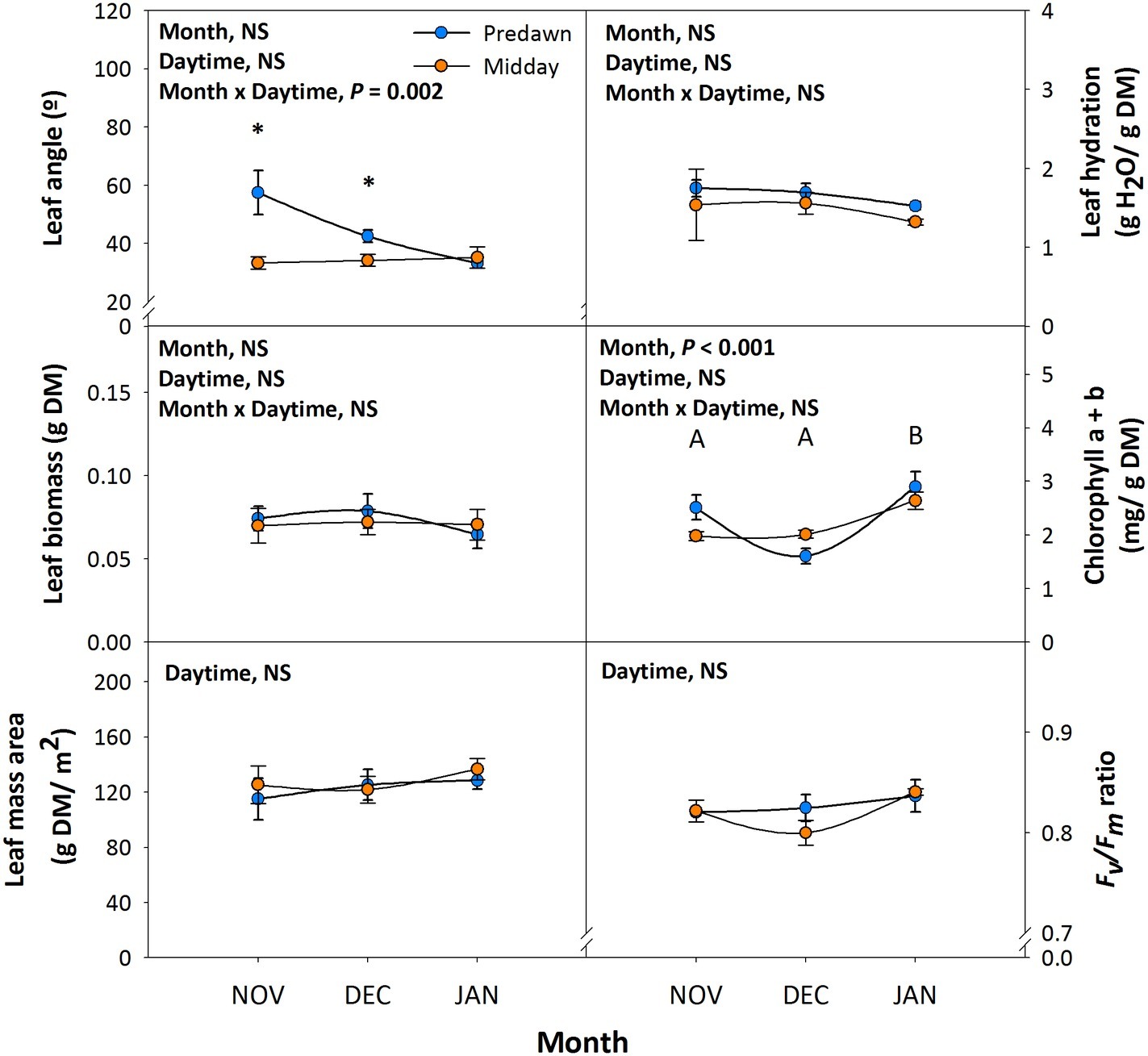
Figure 1. Diurnal and seasonal changes in leaf orientation in the Mediterranean shrub, C. albidus. A comparison of leaf angle, biomass, mass per area ratio, hydration, chlorophyll contents, and the Fv/Fm ratio between predawn and midday from autumn (November) to winter (January) in plants growing at the experimental garden of the University of Barcelona is shown. Data correspond to leaf position 2 from the apex (see photograph in Figure 3 for details) and represent the mean ± SE of n = 6 individuals. After model selection, significant differences in “Month” and “Daytime” were tested by conditional Fisher tests (p ≤ 0.05). Different capital letters indicate significant differences in Tukey’s HSD multiple comparison test (p ≤ 0.05) with all data. Asterisks indicate significant differences in Tukey’s HSD multiple comparison test in “Daytime” within “Month”. NS, not significant; DM, dry matter.
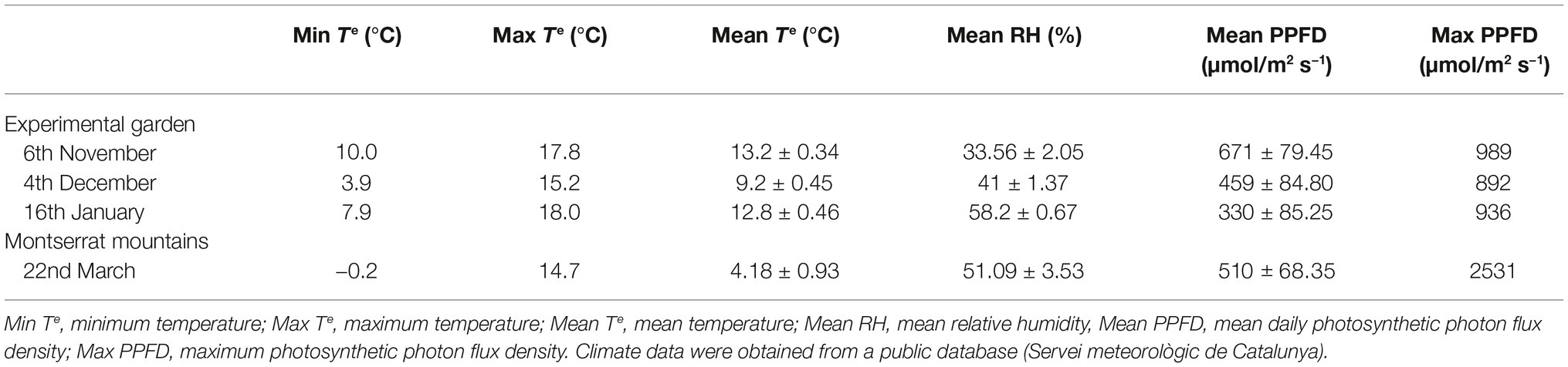
Table 1. Environmental conditions during the sampling days both in the Experimental garden at the Faculty of Biology of the University of Barcelona and in the Natural Park of the Montserrat mountains.
To investigate the possible protective effects of melatonin against environmental stress in plants, we studied the endogenous variations in melatonin in relation to its biosynthesis-related phytohormone auxin (indole-3-acetic acid, IAA) and a well-known chloroplastic antioxidant (α-tocopherol). Endogenous auxin contents decreased as the season progressed from autumn to winter, and values at midday were higher than those at predawn, whereas melatonin contents increased slightly during the same period with higher values at predawn than at midday (Figure 2). While melatonin contents were on the same order of magnitude than those of auxin, they were, however, four orders of magnitude smaller than those of α-tocopherol, the levels of which kept unaltered throughout the study (Figure 2).
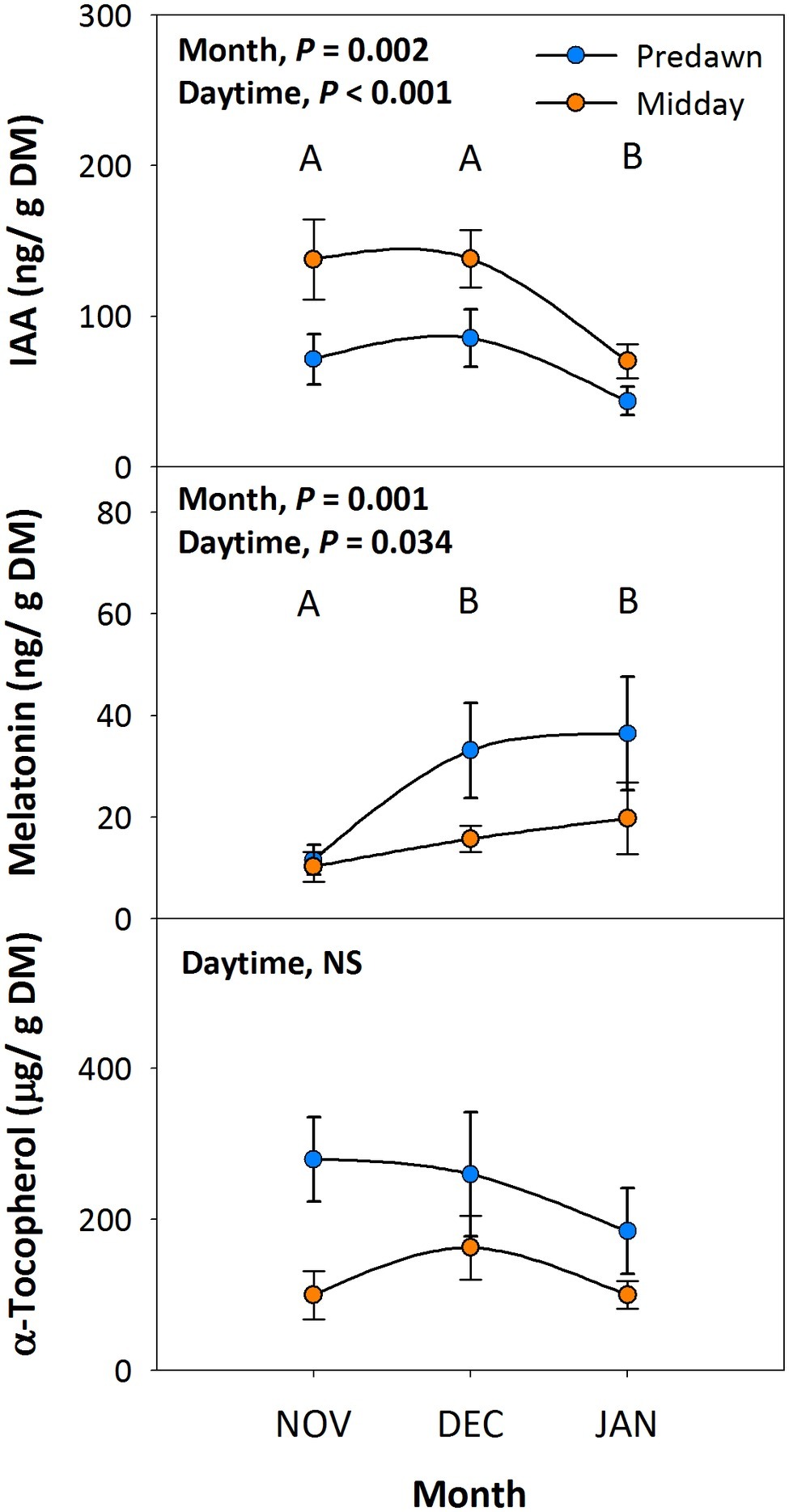
Figure 2. Diurnal and seasonal changes in endogenous melatonin contents in the Mediterranean shrub, C. albidus. A comparison between melatonin contents relative to those of the biosynthesis-related phytohormone auxin and the well-known chloroplastic antioxidant α-tocopherol between predawn and midday from autumn (November) to winter (January) in plants growing at the experimental garden of the University of Barcelona is shown. Data correspond to leaf position 2 from the apex (see photograph in Figure 3 for details) and represent the mean ± SE of n = 6 individuals. After model selection, significant differences in “Month” and “Daytime” were tested by conditional Fisher tests (p ≤ 0.05). Different capital letters indicate significant differences in Tukey’s HSD multiple comparison test (p ≤ 0.05) with all data. Asterisks indicate significant differences in Tukey’s HSD multiple comparison test in “Daytime” within “Month”. NS, not significant; DM, dry matter.
Leaf angle measurements at midday during 16th January revealed a marked positional effect on leaf orientation, with opposite, decussate leaves progressively showing an increased leaf angle from top to bottom of the shoot (Figure 3). Leaf biomass and leaf mass per area ratio kept unaltered with leaf position, while leaf hydration slightly increased by a 20% from leaf position 1 to 5 (Figure 3). Despite chlorophyll contents kept unaltered with leaf position, the Fv/Fm ratio decreased slightly with leaf opening. It is noteworthy, however, that this ratio kept always above 0.80 in all studied leaf positions (Figure 3).
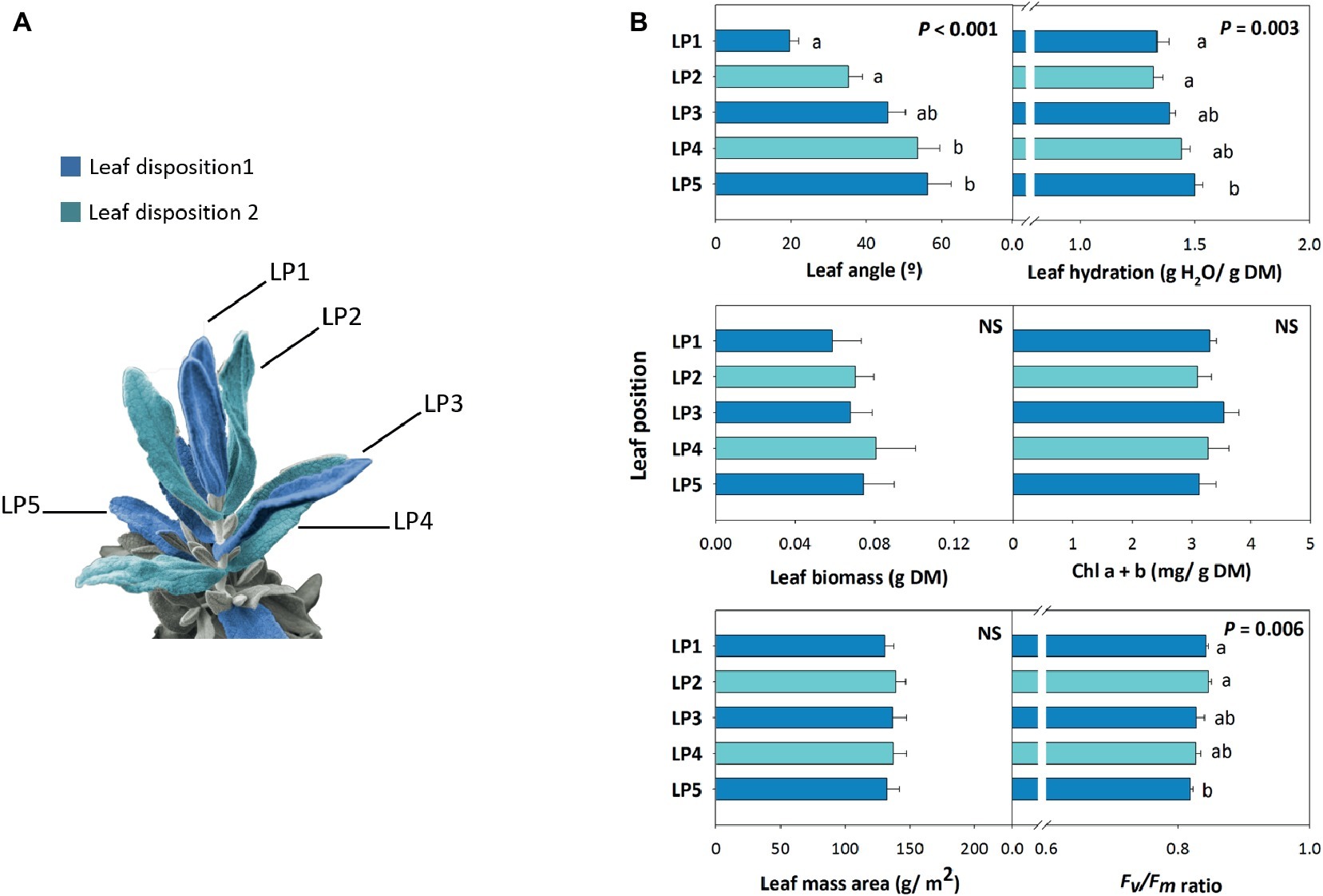
Figure 3. Positional effects in leaf orientation in the Mediterranean shrub, C. albidus. (A) Schematic representation of the disposition on the shoot (Leaf disposition 1 and 2 shown in dark blue and light blue, respectively) of the five leaf positions (LP1…LP5). (B) A comparison between leaf positions on leaf angle, biomass, mass per area ratio, hydration, chlorophyll contents, and the Fv/Fm ratio in plants growing at the experimental garden of the University of Barcelona in January at midday is shown. Data represent the mean ± SE of n = 6 individuals. Significant differences in “Leaf position” were tested by conditional Fisher tests (p ≤ 0.05), and different letters indicate significant differences in Tukey’s HSD multiple comparison test (p ≤ 0.05). NS, not significant; DM, dry matter.
Positional effects on the endogenous variations in melatonin in relation to auxin and α-tocopherol revealed that, although endogenous auxin contents were different between even and odd whorls, neither auxin nor melatonin contents were altered by increased leaf angle from top to bottom (Figure 4). In contrast, the contents of α-tocopherol increased concomitantly with less steep leaf angles from top to bottom (Figure 4).
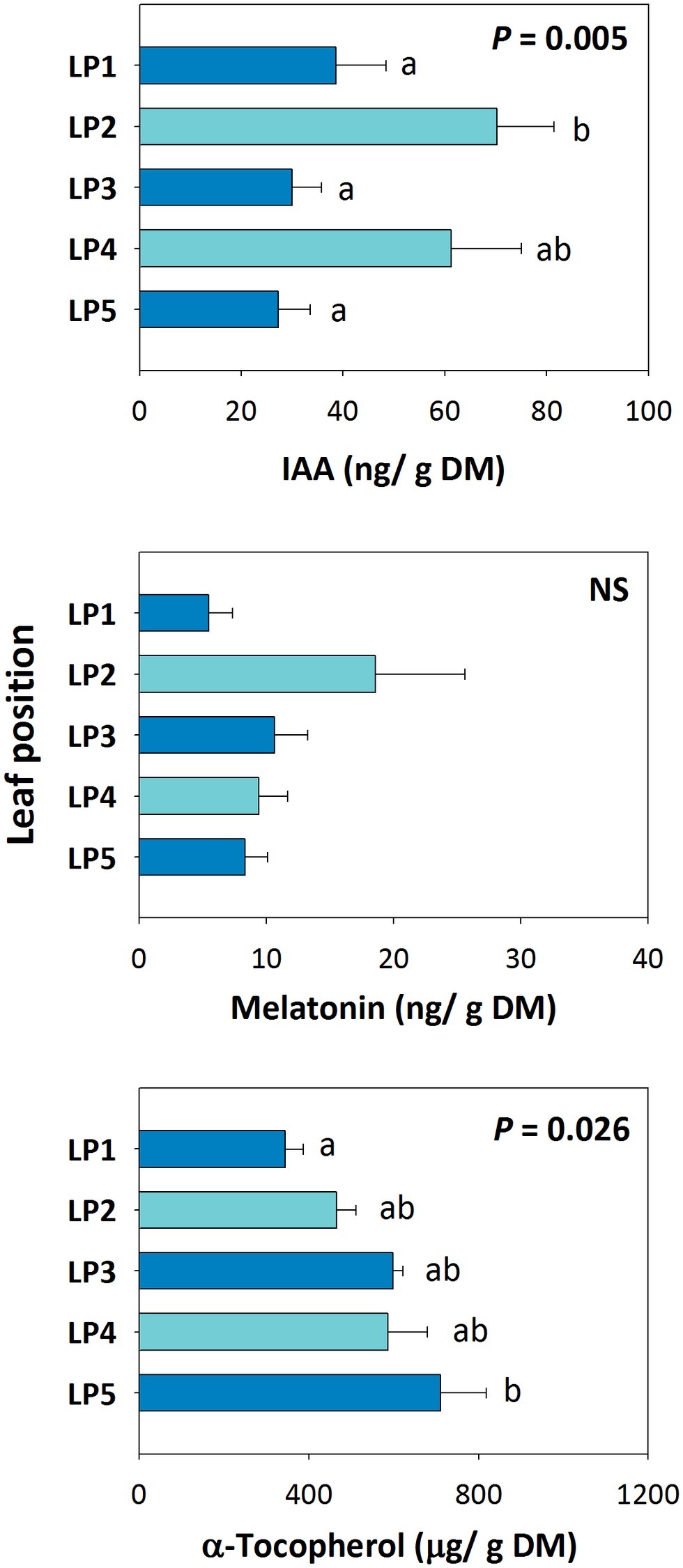
Figure 4. Positional effects in endogenous melatonin contents in the Mediterranean shrub, C. albidus. A comparison between melatonin contents relative to those of the biosynthesis-related phytohormone auxin and the well-known chloroplastic antioxidant α-tocopherol in plants growing at the experimental garden of the University of Barcelona during January at midday is shown. Data represent the mean ± SE of n = 6 individuals. Significant differences in “Leaf position” were tested by conditional Fisher tests (p ≤ 0.05), and different letters indicate significant differences in Tukey’s HSD multiple comparison test (p ≤ 0.05). NS, not significant; DM, dry matter.
Leaf angle measurements at midday during 22nd March in a natural population of C. albidus growing in the Montserrat mountains at 1,100 m.a.s.l. revealed a strong variability – quantified as percentage deviation (standard deviation/population mean) × 100 – on leaf orientation. Leaf at position 2 from top of the shoot from 60 individuals showed angles ranging from 10 to 80° (Figure 5). The variation in the leaf angle of both West-oriented and East-oriented individuals was much higher than that of leaf biomass, leaf mass per area ratio, leaf hydration, chlorophyll contents, and the Fv/Fm ratio (Figure 5). Indeed, variability in leaf angle was 46.8%, while that of the Fv/Fm ratio was 7.5% only. West orientation, which was associated with markedly reduced leaf temperatures and slightly lower leaf water contents compared to the East – but with no differences in the PPFD (Figure 5, see also materials and methods for details) –, led to enhanced tocopherol contents, while leaf angle, Fv/Fm ratios, chlorophyll, IAA, and melatonin contents kept unaltered (Figures 5, 6).
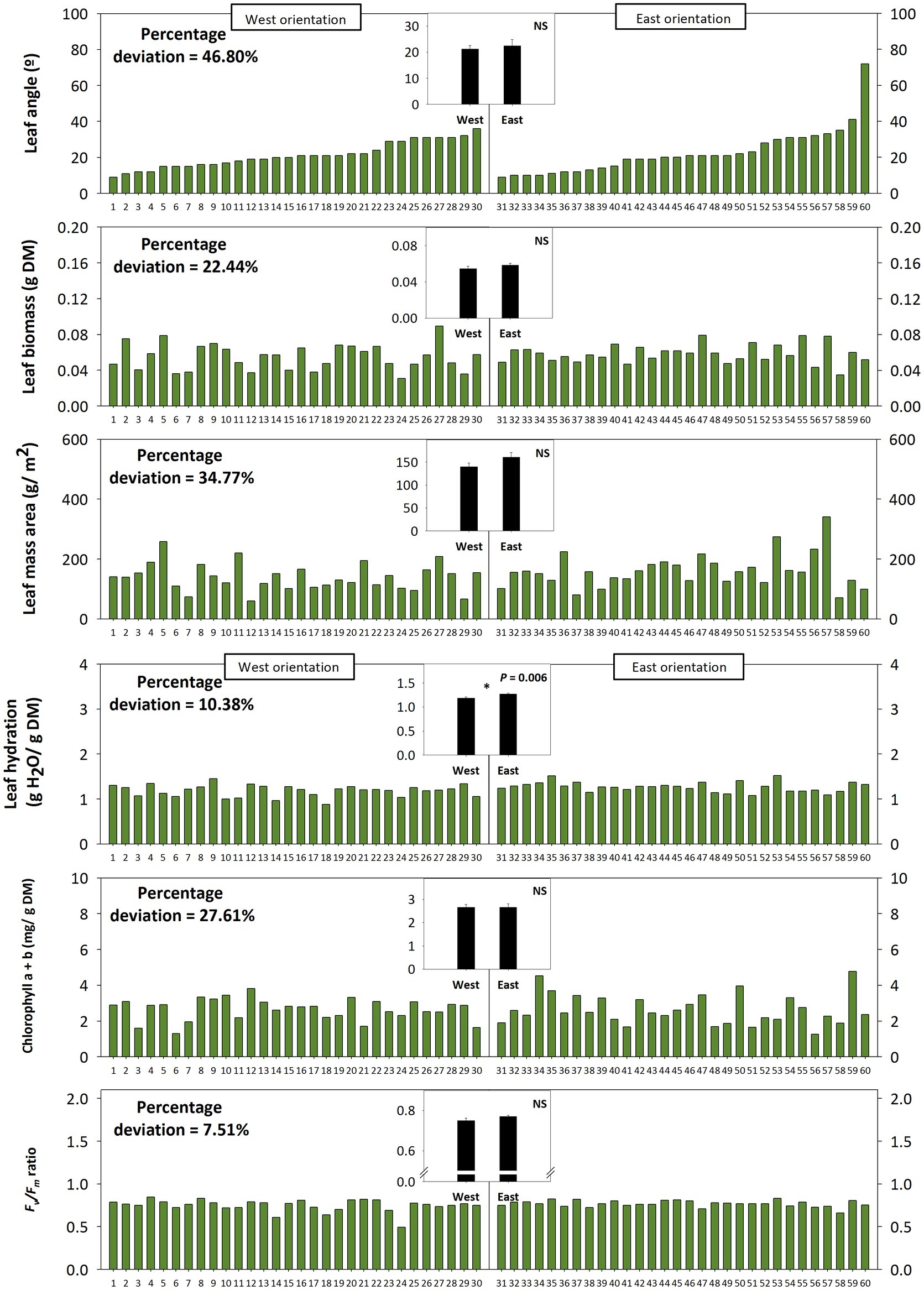
Figure 5. Effects of sun orientation and inter-individual variability on leaf orientation in the Mediterranean shrub, C. albidus. A comparison between leaf angle, biomass, mass per area ratio, hydration, chlorophyll contents, and the Fv/Fm ratio in a C. albidus population growing at 1,100 m.a.s.l. in the Natural Park of the Montserrat Mountains with different sun orientation (East orientation, n = 30 individuals; West orientation, n = 30 individuals) during winter (22nd March 2018) is shown. Data correspond to leaf position 2 from the apex (see photograph in Figure 3 for details) for all studied individuals. The number of the individual (ordered from lower to higher leaf angle) is indicated in the x-axis. Percent deviation for every variable is given as (SD/Population mean) × 100, where SD corresponds to standard deviation. The mean ± SE for each sun orientation is shown in the graphs located in the inlets. Significant differences between groups were tested by a one-way analysis of variance (ANOVA, p ≤ 0.05) and indicated with an asterisk. NS, not significant.
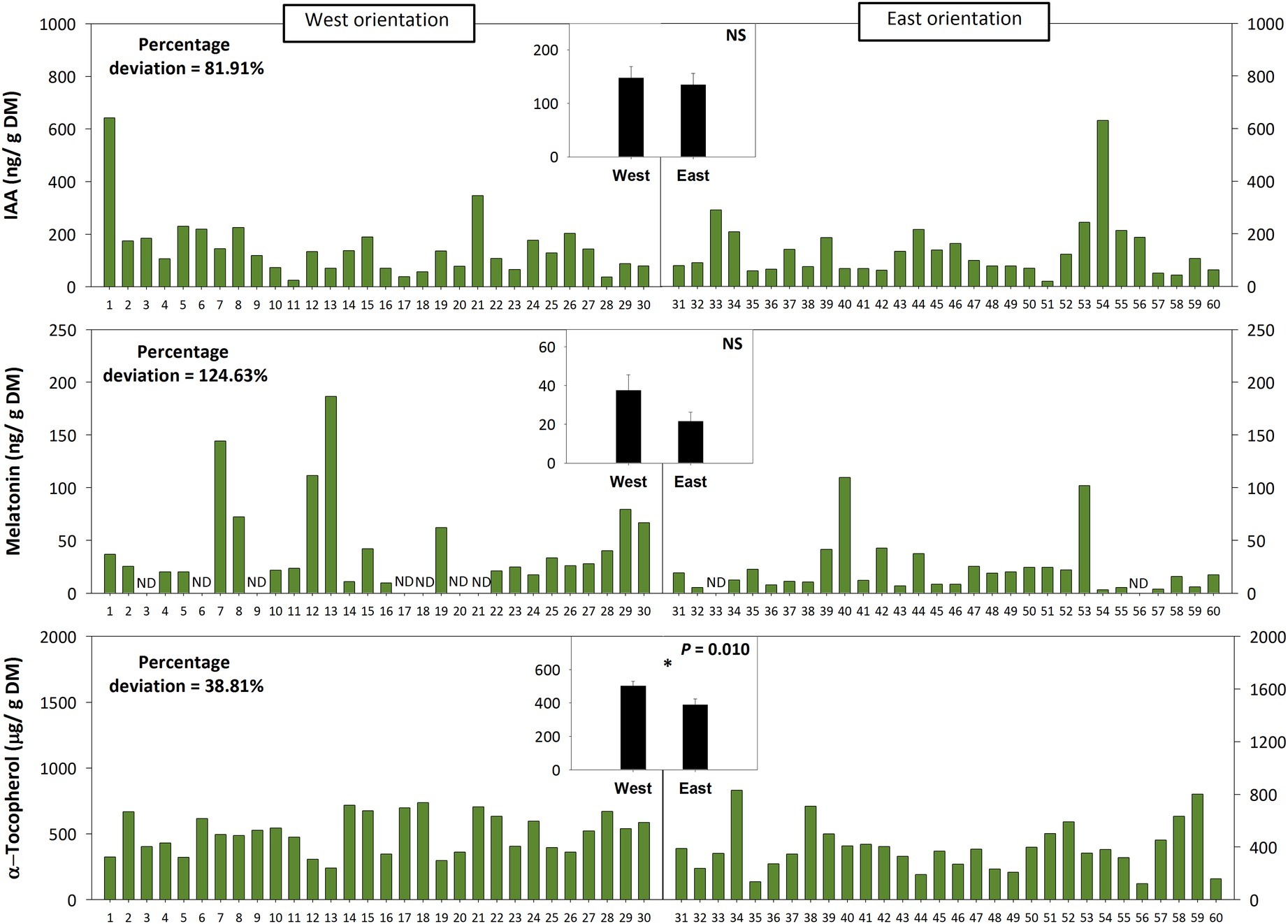
Figure 6. Effects of sun orientation and inter-individual variability on endogenous melatonin contents in the Mediterranean shrub, C. albidus. A comparison of melatonin contents relative to those of the biosynthesis-related phytohormone auxin and the well-known chloroplastic antioxidant α-tocopherol in a C. albidus population growing at 1,100 m.a.s.l. in the Natural Park of the Montserrat Mountains with different sun orientation (East orientation, n = 30 individuals, West orientation, n = 30 individuals) during winter (22nd March 2018) is shown. Data correspond to leaf position 2 from the apex (see photograph in Figure 3 for details) for all studied individuals. The number of the individual (ordered from lower to higher leaf angle) is indicated in the x-axis. Percent deviation for every variable is given as (SD/population mean) × 100, where SD corresponds to standard deviation. The mean ± SE for each sun orientation is shown in the graphs located in the inlets. Significant differences between groups were tested by a one-way analysis of variance (ANOVA, p ≤ 0.05) and indicated with an asterisk. NS, not significant; DM, dry matter.
Several semi-deciduous shrubs that represent an important hotspot of the Mediterranean flora, such as the white-leaved rockrose (Cistus albidus L.), show changes in leaf angle as part of their developmental program with opposite and decussate leaves without petioles increasing their leaf angle as they develop from top to bottom of the shoot apex. An enhanced leaf angle in more distal positions from the uppermost side of the shoot progressively leads to a leaf orientation more perpendicular to the sun’s rays, which results in an increased exposure to high light. Therefore, the youngest leaves, which develop later in time, are the ones with smaller leaf angles and, therefore, enhanced photoprotection due to reduced light incidence. There are several studies in Cistus spp. that report that higher leaf inclinations (i.e., steeper leaf angles) prevent photoinhibition and confer a higher stress tolerance during summer drought (C. incanus, Grattani and Bombelli, 2000; C. monspeliensis, Werner et al., 1999, 2001; C. ladanifer, Brites and Valladares, 2005; C. salviifolius and C. monspeliensis, Puglielli et al., 2017). However, the implications of these leaf movements to prevent winter photoinhibition have not been considered in detail thus far, with the exception of the study performed by Oliveira and Peñuelas (2002), which showed reduced cold-induced photoinhibition in leaves with smaller leaf angles and therefore less sun-exposed leaves in C. albidus. Here, we show that changes in leaf angle are governed by leaf position, as part of the leaf developmental program of shoots, and by environmental conditions both diurnally and seasonally, all providing a first line of defense to prevent photoinhibition in this plant species. At the same time, we also show that, in nature, the variability in leaf angles is so high that other mechanisms operating at the chloroplast level, such as an enhanced vitamin E biosynthesis, are required for counteracting the potential harmful effects of combined high light with low temperatures in populations growing at high elevation.
Leaf orientation was not only influenced by the intrinsic shoot developmental program in C. albidus, but it also changed on a diurnal and seasonal basis, both factors interacting between them. As it occurs with paraheliotropism in some legumes (Mayer et al., 1985; Raeini-Sarjaz, 2011), the leaf angle seemed to be governed to some extent, at least, by circadian rhythms in C. albidus since leaves had a steeper angle at midday compared to predawn, particularly in autumn. However, the absence of a petiole and most probably of a pulvinus in C. albidus leaves suggests that this movement is not as “active” as in some legumes such as common bean (Lambers et al., 2008), but the result of a more “passive” process associated with the plant developmental program. However, the fact that leaves move on a diurnal basis indicates that this leaf movement responds to light and can, therefore, be considered a type of paraheliotropism, as recently suggested by Puglielli et al. (2017) and sensu Häder and Lebert (2001).
Enhanced tocopherol contents were observed in the West-oriented individuals compared to the East-oriented ones, which might help withstand combined high light with low temperature stress in C. albidus growing at high elevation. Mean leaf temperature during samplings in the West orientation was 11°C, in contrast to the 19°C in East orientation, while the PPFD did not differ between both orientations. Reduced leaf temperature in the West led to a slightly reduced leaf hydration, which may be associated with dehydration caused by chilling (Hussain et al., 2018). In turn, low temperatures have been shown to increase α-tocopherol contents in other plant species as a mean to counteract ROS production and allow stabilization of membranes (Munné-Bosch, 2005; El Kayal et al., 2006). Indeed, tocopherols may not only protect the photosynthetic apparatus during plant exposure to extreme temperatures but also influence retrograde signaling, thus acting in stress sensing and signaling (Fang et al., 2019; Munné-Bosch, 2019). Interestingly, the Fv/Fm ratio did not differ between the West and East orientation, thus suggesting that enhanced tocopherol contents in the West orientation served as a photoprotective strategy, as it has been previously shown in model plant species (Havaux et al., 2005). By contrast, melatonin contents did not increase in response to low temperature stress in the West-oriented individuals, despite exogenous applications of this compound have been shown to protect the photosynthetic apparatus from excess light stress, either caused by drought (Fleta-Soriano et al., 2017) or low temperatures (Ding et al., 2017). Inter-individual variability in melatonin contents was very high, as it occurred with leaf angles, which might mask a possible protective effect of these two mechanisms in populations growing under natural conditions. Indeed, the endogenous contents of melatonin found (in the order of ng/g dry matter, even lower than those found for indole-3-acetic acid) in C. albidus leaves suggest a modulatory role of this compound in plants, rather than a direct function as an antioxidant, which is in agreement with previous studies (Fleta-Soriano et al., 2017, see also Arnao and Hernández-Ruiz, 2019).
Correlation analyses revealed interesting relationships between various parameters in the natural population, both when the analysis included all data and the East and West individuals separately (Figure 7). In the natural population from the Montserrat mountains, leaf angle did not correlate with any of the other studied parameters but the Fv/Fm ratio correlated very significantly, strongly, and positively with leaf hydration (ρ = 0.68, p < 0.001), which might indicate a prevalent occurrence of increased photoinhibition in the more water-stressed leaves. Small reductions in leaf hydration, which may be caused by chilling stress in the West-oriented individuals, could explain this result. This correlation was confirmed when all data were pooled together, although the relationship was more moderate (ρ = 0.56, p < 0.001). Furthermore, a moderate, but very significant, positive correlation was found between leaf hydration and total chlorophylls (ρ = 0.51, p < 0.001) pooling all data together that were consistent in both West and East-oriented individuals (ρ = 0.53, p < 0.001; ρ = 0.63, p < 0.001, respectively). Simultaneously, the relationship between Fv/Fm ratio and α-tocopherol was also significant, although not too strong, in both orientations but this time being negative (ρ = −0.32, p < 0.05; ρ = −0.46, p < 0.05, respectively). In this manner, given that leaf hydration was slightly lower and tocopherol contents were higher in the West-oriented individuals than in the East-oriented ones, α-tocopherol might be acting as an important photoprotection compound under abiotic stress conditions. Therefore, these results suggest that C. albidus plants activate two photoprotection mechanisms. First, reductions in leaf angle, which might serve as a first line of defense from winter photoinhibition in leaves, particularly occurring in the uppermost position of shoots; and second, increased accumulation of α-tocopherol, which might serve as a stress sensing and signaling mechanism to counteract combined high light and low temperature stress during winter. The increased accumulation of α-tocopherol appears to be particularly relevant when photoprotection by leaf orientation is minimal and leaves consequently require more sophisticated biochemical, photoprotection mechanisms. In this study, higher levels of α-tocopherol are observed under more stressful conditions (e.g., West-oriented individuals exposed to a combination of abiotic stresses) and when leaves occupy positions more distal to the top of the shoot, due to the developmental program, and they orient more perpendicular to sun’s rays.
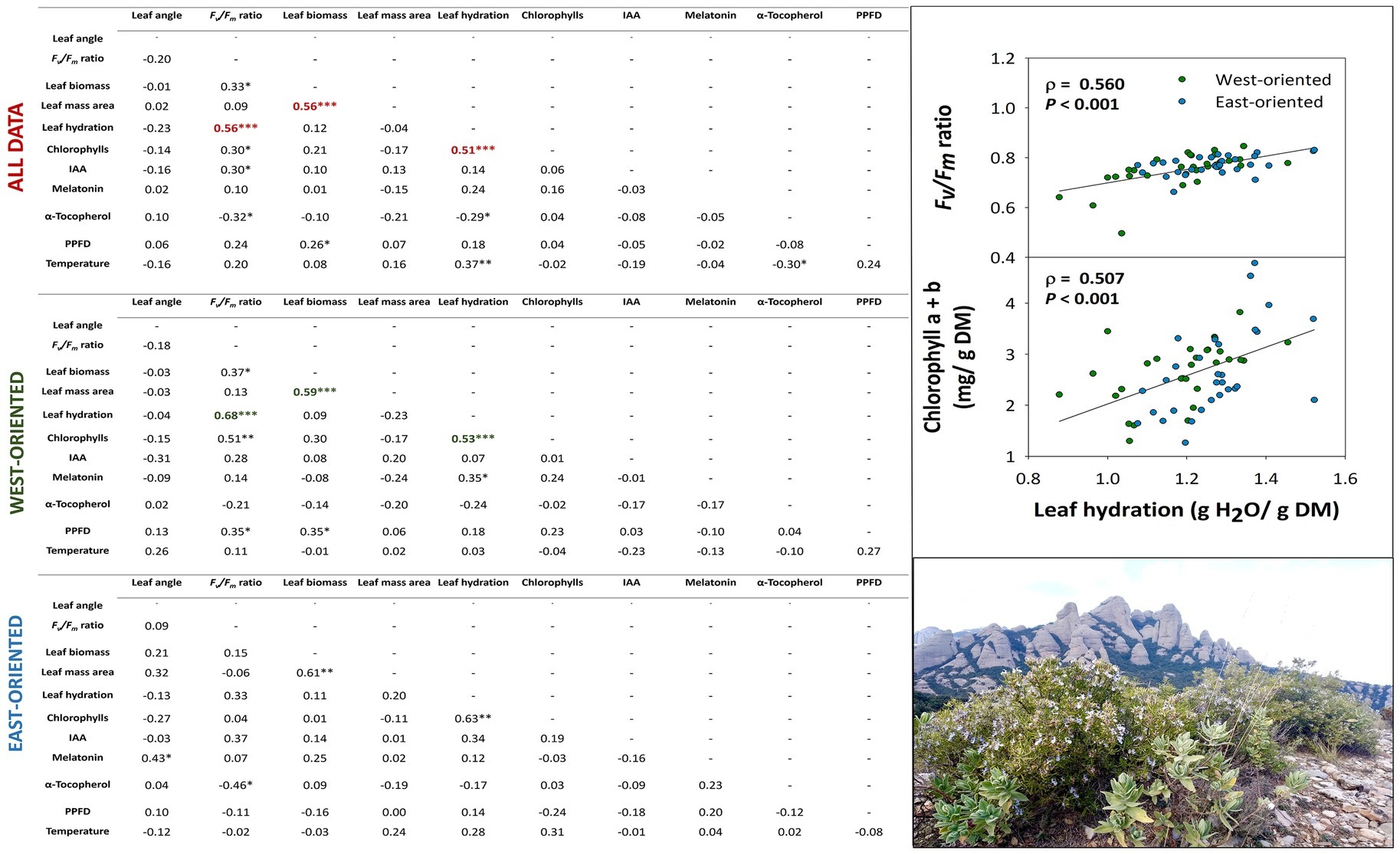
Figure 7. Relationship between all measured parameters in C. albidus from a natural population in the Natural Park of the Montserrat Mountains. Tables show the results of the Spearman’s rank correlation analyses (including the correlation coefficient, ρ, and p indicated with asterisks) for all data pooled together, for West-oriented individuals only and for East-oriented individuals only. All correlations were considered statistically significant at p ≤ 0.05, with one, two, and three asterisks indicating p below 0.05, 0.01, and 0.001, respectively. Correlations with a correlation coefficient, ρ ≥ 0.50, are shown in red, green, and blue for all data pooled together, West-oriented individuals, and East-oriented individuals, respectively. The plots in the right side of the figure show a graphical representation of the most biologically significant correlations. IAA, indole-3-acetic acid; PPFD, photosynthetic photon flux density.
It is concluded that C. albidus has very effective and fine-regulated photoprotection mechanisms, including (1) an adequate orientation of decussate leaves as part of the developmental program, in which the leaf angle is additionally modulated on a diurnal and seasonal basis, therefore contributing to prevent photoinhibition as a first line of defense and (2) enhanced tocopherol contents that may help withstand combined high light and low temperature stress in C. albidus growing under Mediterranean field conditions.
All datasets generated for this study are included in the manuscript and/or the supplementary files.
MP-L and SM-B conceived and designed the experiment. MP-L, AC, and MM performed the experiments. MP-L analyzed the data. SM-B wrote the manuscript with the help of MP-L. All authors revised and approved the final manuscript.
This research was supported by the Spanish Government through BFU2015-64001/FEDER and Generalitat de Catalunya through the ICREA Academia prize given to SM-B.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to Serveis Científico-tècnics (University of Barcelona) for their help with the biochemical analyses.
Arnao, M. B., and Hernández-Ruiz, J. (2019). Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci. 24, 38–48. doi: 10.1016/j.tplants.2018.10.010
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396. doi: 10.1104/pp.106.082040
Bellard, C., Leclerc, C., Leroy, B., Bakkenes, M., Veloz, S., Thuiller, W., et al. (2014). Vulnerability of biodiversity hotspots to global change. Glob. Ecol. Biogeogr. 23, 1376–1386. doi: 10.1111/geb.12228
Bolhar-Nordenkampf, H. R., Hofer, M., and Leclmer, E. G. (1991). Analysis of light-induced reduction of the photochemical capacity in field-grown plants. Evidence for photoinhibition? Photosynthesis Res. 27, 31–39. doi: 10.1007/BF00029974
Brites, D., and Valladares, F. (2005). Implications of opposite phyllotaxis for light interception efficiency of Mediterranean woody plants. Trees 19, 671–679. doi: 10.1007/s00468-005-0431-6
Butchart, S. H. M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P. W., Almond, R. E. A., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
Cela, J., Chang, C., and Munné-Bosch, S. (2011). Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 52, 1389–1400. doi: 10.1093/pcp/pcr085
Demmig-Adams, B., and Adams, W. W. (2006). Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 172, 11–21. doi: 10.1111/j.1469-8137.2006.01835.x
Ding, F., Wang, M., Liu, B., and Zhang, S. (2017). Exogenous melatonin mitigates photoinhibition by accelerating non-photochemical quenching in tomato seedlings exposed to moderate light during chilling. Front. Plant Sci. 8:244. doi: 10.3389/fpls.2017.00244
Ehleringer, J. R., and Björkman, O. (1978). Pubescence and leaf spectral characteristics in a desert shrub, Encelia farinosa. Oecologia 36, 151–162. doi: 10.1007/BF00349805
Ehleringer, J. R., and Comstock, J. (1987). “Leaf absorptance and leaf angle: mechanisms for stress avoidance” in Plant response to stress - functional analysis in Mediterranean ecosystems. eds. J. D. Tenhunen, F. M. Catarino, O. L. Lange, and W. C. Oechel (Berlin: Springer), 55–76.
El Kayal, W., Keller, G., Debayles, C., Kumar, R., Weier, D., Teulières, C., et al. (2006). Regulation of tocopherol biosynthesis through transcriptional control of tocopherol cyclase during cold hardening in Eucalyptus gunnii. Physiol. Plant. 126, 212–223. doi: 10.1111/j.1399-3054.2006.00614.x
Fang, X., Zhao, G., Zhang, S., Li, Y., Gu, H., Li, Y., et al. (2019). Chloroplast-to-nucleus signaling regulates microRNA biogenesis in Arabidopsis. Dev. Cell 48, 371–382. doi: 10.1016/j.devcel.2018.11.046
Fernández-Marín, B., Hernández, A., Garcia-Plazaola, J. I., Esteban, R., Míguez, F., Artetxe, U., et al. (2017). Photoprotective strategies of Mediterranean plants in relation to morphological traits and natural environmental pressure: a meta-analytical approach. Front. Plant Sci. 8:1051. doi: 10.3389/fpls.2017.01051
Fleta-Soriano, E., Díaz, L., Bonet, E., and Munné-Bosch, S. (2017). Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 203, 286–294. doi: 10.1111/jac.12201
Grattani, L., and Bombelli, A. (2000). Correlation between leaf age and other leaf traits in three Mediterranean maquis shrub species: Quercus ilex, Phillyrea latifolia and Cistus incanus. Environ. Exp. Bot. 43, 141–153. doi: 10.1016/S0098-8472(99)00052-0
Haupt, W., and Scheuerlein, R. (1990). Chloroplast movement. Plant Cell Environ. 13, 595–614. doi: 10.1111/j.1365-3040.1990.tb01078.x
Havaux, M., Eymery, F., Porfirova, S., Rey, P., and Dörmann, P. (2005). Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17, 3451–3469. doi: 10.1105/tpc.105.037036
Hussain, H. A., Hussain, S., Khaliq, A., Ashraf, U., Anjum, S. A., Men, S., et al. (2018). Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front. Plant Sci. 9:393. doi: 10.3389/fpls.2018.00393
Itakura, K., and Hosoi, F. (2018). Automatic leaf segmentation for estimating leaf area and leaf inclination angle in 3D plant images. Sensors 18:3576. doi: 10.3390/s18103576
Joffre, R., Rambal, S., and Damesin, C. (1999). “Functional attributes in Mediterranean-type ecosystems” in Handbook of functional plant ecology. eds. F. I. Pugnaire and F. Valladares (New York: Marcel Dekker), 347–380.
Karavatas, S., and Manetas, Y. (1999). Seasonal patterns of photosystem 2 photochemical efficiency in evergreen sclerophylls and drought semi-deciduous shrubs under Mediterranean field conditions. Photosynthetica 3, 41–49.
Lambers, H., Chapin, F. S., and Pons, T. L. (2008). Plant physiological ecology. 2nd edn. (New York: Springer).
Lichtenthaler, H. K., and Wellburn, A. R. (1983). Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11, 591–592. doi: 10.1042/bst0110591
Martínez-Ferri, E., Manrique, E., Valladares, F., and Balaguer, L. (2004). Winter photoinhibition in the field involves different processes in four co-occurring Mediterranean tree species. Tree Physiol. 24, 981–990. doi: 10.1093/treephys/24.9.981
Mayer, E.-W., Flach, D., Raju, M. V. S., Starrach, N., and Wiech, E. (1985). Mechanics of circadian pulvini movements in Phaseolus coccineus L. Planta 163, 381–390. doi: 10.1007/BF00395147
Müller, M., and Munné-Bosch, S. (2011). Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods 7:37. doi: 10.1186/1746-4811-7-37
Müller, M., Siles, L., Cela, J., and Munné-Bosch, S. (2013). Perennially young: seed production and quality in controlled and natural populations of Cistus albidus reveal compensatory mechanisms that prevent senescence in terms of seed yield and viability. J. Exp. Bot. 65, 287–297. doi: 10.1093/jxb/ert372
Munné-Bosch, S. (2005). The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 162, 743–748. doi: 10.1016/j.jplph.2005.04.022
Munné-Bosch, S. (2019). Vitamin E function in stress sensing and signaling in plants. Dev. Cell 48, 290–292. doi: 10.1016/j.devcel.2019.01.023
Oliveira, G., and Peñuelas, J. (2002). Comparative protective strategies of Cistus albidus and Quercus ilex facing photoinhibitory winter conditions. Environ. Exp. Bot. 47, 281–289. doi: 10.1016/S0098-8472(02)00003-5
Peñuelas, J., Sardans, J., Filella, I., Estiarte, M., Llusià, J., Ogaya, R., et al. (2017). Impacts of global change on Mediterranean forests and their services. Forests 8, 972–980. doi: 10.3390/f8120463
Pérez-Llorca, M., Muñoz, P., Müller, M., and Munné-Bosch, S. (2019). Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front. Plant Sci. 10:136. doi: 10.3389/fpls.2019.00136
Puglielli, G., Redondo-Gómez, S., Gratani, L., and Mateos-Naranjo, E. (2017). Highlighting the differential role of leaf paraheliotropism in two Mediterranean Cistus species under drought stress and well-watered conditions. J. Plant Physiol. 213, 199–208. doi: 10.1016/j.jplph.2017.02.015
Raeini-Sarjaz, M. (2011). Circadian rhythm leaf movement of Phaseolus vulgaris and the role of calcium ions. Plant Signal. Behav. 6, 962–967. doi: 10.4161/psb.6.7.15483
R Core Team (2019). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/
Takahashi, S., and Badger, M. R. (2011). Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 16, 53–60. doi: 10.1016/j.tplants.2010.10.001
Werner, C., Correia, O., and Beyschlag, W. (1999). Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecol. 20, 15–23. doi: 10.1016/S1146-609X(99)80011-3
Werner, C., Ryel, R. J., Correia, O., and Beyschlag, W. (2001). Structural and functional variability within the canopy and its relevance for carbon gain and stress avoidance. Acta Oecol. 22, 129–138. doi: 10.1016/S1146-609X(01)01106-7
Keywords: abiotic stress, Cistus albidus L., leaf orientation, leaf positional effects, low temperature stress, melatonin, photoinhibition, photoprotection
Citation: Pérez-Llorca M, Casadesús A, Müller M and Munné-Bosch S (2019) Leaf Orientation as Part of the Leaf Developmental Program in the Semi-Deciduous Shrub, Cistus albidus L.: Diurnal, Positional, and Photoprotective Effects During Winter. Front. Plant Sci. 10:767. doi: 10.3389/fpls.2019.00767
Received: 15 March 2019; Accepted: 27 May 2019;
Published: 19 June 2019.
Edited by:
Éva Hideg, University of Pécs, HungaryReviewed by:
Raquel Esteban, University of the Basque Country, SpainCopyright © 2019 Pérez-Llorca, Casadesús, Müller and Munné-Bosch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergi Munné-Bosch, c211bm5lQHViLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.