
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 08 January 2019
Sec. Plant Abiotic Stress
Volume 9 - 2018 | https://doi.org/10.3389/fpls.2018.01920
This article is part of the Research TopicChlorophyll Fluorescence Measurements and Plant Stress ResponsesView all 26 articles
The aim of experiments was to investigate a maximal efficiency of PSII, as a marker indicating growth, vigor, energetic value and physiological activity of sorghum fertilized with wastes from a biomass biodigestion to methane in a distillery integrated with a biogas plant using corn grains as substrate. The sorghum plants grown outdoor in different climate and in pots and in field were fertilized with different doses of the waste or Apol-humus – a soil improver and Stymjod – a nano-organic-mineral fertilizer. The maximal efficiency of PSII, in comparison with plant growth and health, chlorophyll content, gas exchange, activity of selected enzymes, element content in leaves and energetic value were studied. The wastes applied to soil resulted in increased maximal efficiency of PSII and the doses of 30 m3 ha-1 and 40–50 m3 ha-1 of the non-centrifuged and centrifuged ones, respectively, were most efficient. This enhancement was associated with the increased kinetics of plant growth, their health, fresh and dry biomass and physiological activity of plants as evidenced by activity of acid and alkaline phosphatase, RNase and dehydrogenase, as well as by gas exchange: net photosynthesis, transpiration, stomatal conductance, intercellular CO2 concentration and index of chlorophyll content in leaves. The fertilization with Apol-humus and Stymjod additionally increased maximal photochemical efficiency of PSII and plant development, biomass yield and physiological activity. The results indicate that waste from a biomass biodigestion to methane can be used as a natural fertilizer in sorghum crops and this ensures their recycling and environmental protection. The measurement values of maximal efficiency of PSII were proportionally to the vigor, growth and physiological activity of the plants. The obtained results indicate that the maximal efficiency of PSII in sorghum plants is a non-destructive method for defining the degree of growth and may be used as a marker of plant vigor and health, development and physiological activity expressed by gas exchange and activity of selected enzymes.
Management of waste from a biomass biodigestion to methane and its ecological use in plant production as fertilizers are among important issues in the world agriculture. This problem has become serious because the biogas plants are a prospective branch of energy production, as e.g., increase in electricity production from biogas in 2020 will amount to 125% as compared to 2010. Thus, the production of biogas is increasing and consequently the problem of economic management of the waste from a biomass biodigestion to methane has become very important from economic and environmental point of view. Given the environmental risks and benefits, it is most rational to use these wastes in agriculture (Alburquerque et al., 2012; Jasiulewicz and Janiszewska, 2013; Łagocka et al., 2016), probably to a greater extent than sewage sludge, which may contain toxic substances and should be refined before use in plant crops (Fang et al., 2017; Kołecka et al., 2017). World literature available on these issues, especially concerning agricultural management of waste from corn grains biodigestion to methane, is hard to find and in the majority of cases it refers to the waste produced by specific biogas plants and the raw materials used there (Lukehurst et al., 2010; Nakamura et al., 2014; Lalak et al., 2015).
The problem in the waste application in agriculture is a quick and reliable assessment of the effects of plant fertilization that would enable rational management of this treatment. For this reason, technologists are looking for physiological methods that immediately show the reaction of the plants to different treatments, which enables quick cultivation decisions to be made in the growing season without waiting for the completing plant growth in autumn. One of such checked tests is the evaluation of key for growth enzyme activity (acid and alkaline phosphatase, RNase, nitrate reductase, dehydrogenase), index of chlorophyll content and gas exchange in leaves (net photosynthesis, transpiration, stomatal conductance, intercellular CO2 concentration (Górnik et al., 2008; Grzesik and Romanowska-Duda, 2014, 2015; Piotrowski et al., 2016; Grzesik et al., 2017a,b). Kalaji M.H. et al. (2014), Kalaji et al. (2018) indicated that chlorophyll fluorescence parameters may be taken into account as an interesting new indicator which can measure most types of plant stress and perhaps their vigor and thus they published a practical information on the hardware, methodology and the hands on application of this technology. Chlorophyll fluorescence measurements allow the recognition of changes in the general bioenergetic state of the photosynthetic apparatus. Moreover, such measurements relate, directly or indirectly, to all stages of light-dependent photosynthetic reactions, including water splitting, electron transport, and pH gradient formation across the thylakoid membrane and ATP synthesis (Kalaji et al., 2012). The chosen fluorescence parameters can give insights into the ability of plants to tolerate environmental stresses and into the extent to which those stresses have damaged the photosynthetic apparatus. By measuring the intensity and nature of this fluorescence, plant ecophysiology can be investigated (Lichtenthaler et al., 1986; Żebrowska and Michałek, 2014). Strasser et al. (2000) suggested six parameters which can be used in screening tests: F0/Fm, (dV/dt)0, VJ, VI, tFmax and Sm. They describe that F0/Fm, (dV/dt)0 and VJ refer to the structure and function of PSII while VI, tFmax and Sm concern the activity of the electron transport chain (ETC) beyond QA. Among parameters of chlorophyll fluorescence, the maximum PSII efficiency, as given by Fv/Fm in dark-adapted leaves is more often used in research (Su et al., 2015) probably because it is more frequently used and commonly available from early pulse amplitude modulated (PAM) fluorimeters (Osinga et al., 2012). The ratio-metric normalization of Fv/Fm also facilitates ease of results interpretation. The Fv/Fm is calculated as (Fm - Fo)/Fm when Fo means a (minimal) fluorescence level of dark-adapted sample when all reaction centers of the photosystem II are open and Fm is a (maximal) fluorescence level of dark-adapted sample when a high intensity pulse has been applied and the all reaction centers of the photosystem II are closed (Kitajima and Butler, 1975).
Chlorophyll fluorescence has been found as the genetic marker of barley productivity (Planchon et al., 1989). Jalink et al. (1998) and Górnik et al. (2013) indicated that it can be used for assessment of maturation advancement of cabbage and coriander seeds, taking into consideration that their degreening process is directly related with the amount of chlorophyll in seed coats, which was monitored by measuring of maximal efficiency of PSII. Górnik and Lachuta (2017) demonstrated that the changes in maximal photochemical efficiency of PSII (Fv/Fm) in sunflower seedlings was related to their growth, which was reduced by previous chilling and increased by subsequent priming of seeds, sown then after these treatments to obtain seedlings subjected to assessments of physiological activity. Chlorophyll fluorescence was also proposed as a marker of the plant reactions to herbicides mechanism of action (Dayan et al., 2012), cellular damage by glyphosate herbicide in Raphanus sativus L. plants (Silva et al., 2014) and it was used in physiological evaluation of strawberry varieties (Żebrowska and Michałek, 2014). Available literature indicates fragmentarily on changes in maximal efficiency of PSII under the influence of selected plant treatments. Checking the applicability of maximal efficiency of PSII measurements as a marker of plant responses to different fertilization methods requires further extensive research using different cultivation conditions and fertilization methods, as well as comparison to the tests of vigor and plant reaction recognized in literature so far. To the best our knowledge, the use of maximal efficiency of PSII as the marker of energy plant vigor, including sorghum, fertilized with waste from a biomass biodigestion to methane and selected biostimulators, as well as its comparison to a wide range of previously used plant vigor evaluation tests, was not studied yet, despite the urgent need to develop a quick and non-invasive methods for assessing plant response to fertilization and making rapid decisions concerning their cultivation before completing growth.
The purpose of this work was to investigate the suitability of maximal efficiency of PSII, as the marker indicating growth, vigor, energetic value and physiological activity of sorghum fertilized with wastes from a corn grain biodigestion to methane, supplemented with new generation biostimulators, Apol-humus and Stymjod, and its usefulness in monitoring of waste applicability in sorghum crops.
The commercial seeds of sorghum (Sorghum bicolor L.) ‘Rona 1’ obtained from the breeding company “Kutnowska Hodowla Buraka Cukrowego” in Poland, were used in experiments. The chosen variety is cultivated for silage with a lower content of non-digestible fiber and as an energy plant. It gives high yields on weak soils of IV and V bonitation class and in conditions of shortage precipitation. Thus, it is an alternative to maize that requires more fertile and moistening soils, which assurance is difficult in a changing climate.
The liquid, non-centrifuged and centrifuged wastes from a corn grain biodigestion to methane, were supplied by the distillery integrated with the biogas plant in Piaszczyna (Gamawind Sp. z o.o., Poland), which produce alcohol and biogas. The tested waste were rich in most of macro- and microelements necessary for plant growth. The non-centrifuged waste contained more nutrients than the centrifuged one (Table 1).
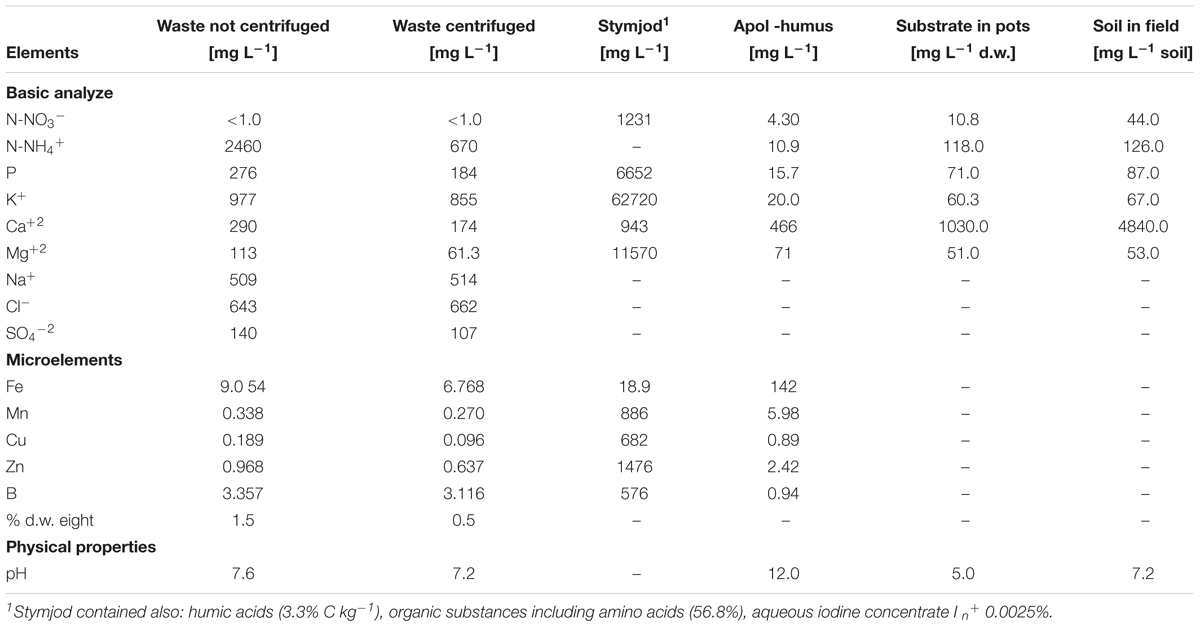
Table 1. Content of macro- and micronutrients and physical properties of liquid non-centrifuged and centrifuged wastes from a biomass biodigestion to methane, Stymjod, Apol-humus, and peat substrate in 5-l pots and in podzolic soil in field.
Apol-humus, the ecological soil improver was supplied by the producer, Poli-Farm Sp. z o.o., Poland, while Stymjod, the nano-organic-mineral fertilizer was obtained from manufacturer PHU Jeznach Sp. J., Poland (Jeznach, 2015) (Table 1).
The sphagnum peat substrate, used for filling of 5-l pots and containing nutrients listed in Table 1, were supplied by Alonet Substrate KS (Latvia).
The soil on the field plots (3 × 3 m each) has been classified as podzolic and contained nutrients listed in Table 1.
In order to check the usefulness of the maximum PSII efficiency, compared to other tests used so far, in various cultivation conditions, research was performed and plants were cultured outdoor in two places 400 km apart, slightly differing in climate, one in central Poland where pots (filled with peat substrate) were used and the other in northern Poland in the fields, in podzolic soil. In central Poland, temperature in July fluctuates from 8 to 32°C, annual average precipitation is 528.3 mm and dry air, while in the north these parameters are respectively from 11 to 21°C, 655 mm and humid air from the nearby Baltic Sea. In July, when physiological properties of plants were assessed, the temperature in central Poland was much over 30°C (nearby Baltic Sea less of 1–3°C) in sun and through the all month a more sunny and less windy days were noted than in the north. All cultivation treatments and plant measurements were carried out at similar times in experiments performed in pots and in the field.
In the middle of April, the peat substrate in each 10 pots forming a plot and the podzolic soil in field plots were fertilized with waste dosages and biostimulants, as follows:
(I) non-centrifuged liquid waste from a biomass biodigestion to methane (E) at dosages of 10, 20, 30, and 40 m3 ha-1,
(II) centrifuged liquid waste (C) at dosages of 20, 30, 40, 50, and 60 m3 ha-1,
(III) pot and field plots fertilized with the most favorable doses of waste (E 30 m3 ha-1 and C 40 m3 ha-1) were additionally fertilized with Apol-humus (AH, 10 L ha-1), and also with applied together Apol-humus (10 L ha-1) to soil and Stymjod 1.5% (5 L ha-1) used as twice leaf sprayings.
The applied waste and Apol-humus were mixed with soil directly after fertilization.
The fertilized differently pot plots and field plots were situated randomly and in three repetitions per treatment.
In the middle of May, seeds of sorghum were sown to (fertilized previously with waste and Apol-humus) peat substrate, one per pot, and to soil in field, 175 ones on plot, in rows 35 cm apart, and the distance between seeds in row 12 cm, according to the common practice.
Not fertilized plants and watered or sprayed with water at the same doses as waste or biostimulants, were served as control. Additional controls were the treatments of soil with Apol-humus and plants with Stymjod, which were used also to check whether they increase the plant’s response to used fertilization, as suggested by their producers. The applied dose range (allowing their optimization) was selected on the previous laboratory research in modernized Phytotoxkit plates, which showed that centrifuged waste should be used in a larger quantity than not subjected to centrifugation.
Non-destructive measurements of physiological activity (maximal efficiency of PSII, gas exchange, index of chlorophyll content, mycological analyzes) and destructive ones (enzymatic activity, nutrient content in leaves) were carried out on fully developed leaves located at the highest level on the plants cultivated in pots and field. In each treatment, one leaf from each of the five plants or their 100 g for nutrient content evaluation, was used for assessment at the end of July at temperature over 30°C in sun and air humidity of about 50%.
Chlorophyll fluorescence were assessed by the measurements of the maximum efficiency of photosystem II (PSII), as given by Fv/Fm in dark-adapted leaves, with a pulse modulated Fluorescence Monitoring System (FMS-1; Hansatech Instruments Ltd., Norfolk, United Kingdom) operated in the Fv/Fm mode, according to the instruction of producer. For measurement the fully developed leaves and placed in the highest part of plant were taken. Before assessment, the leaves were kept in dark for 20 min in order to initiate all reaction centers to open and minimize physiological processes associated with the energization of the thylakoid membrane. According to apparatus manufacturer, the fiber optic of the FMS-1 was situated by using the PPF/temperature leaf clip at a 60° angle from the leaf upper surface. This did not significantly interfere with PPF distribution at the leaf surface, yet it allowed delivery of a saturation pulse of actinic light and detection of fluorescence signals (Cheng et al., 2000). The space between the surface of leaves and fiber optic was constant for all evaluations. The maximum fluorescence (Fm), and variable fluorescence Fv, given as Fm – Fo, were assessed in the leaves adapted to darkness using leaf clips. The initial fluorescence (Fo) was evaluated at PPFD < 0.05 μmol m-2 s-1, following by a saturating pulse to determine the maximum fluorescence emission in the absence (Fm) of quenching was 3000 μmol m-2 s-1. The intensity of saturation pulses to evaluate a maximum fluorescence emission in the absence (Fm) of quenching was 1800 μmol m-2 s-1. Maximum photochemical efficiency of PSII was estimated by the calculation: Fv/Fm = (Fm - Fo)/Fm (Kalaji M.H. et al., 2014; Górnik and Lachuta, 2017).
Height of shoots was measured every month during vegetative season, from the soil up to the plant top (Grzesik et al., 2017b).
Activity of gas exchange, reflected by net photosynthesis rate (Pn; μm CO2 × m-2 × s-1), transpiration (E; mmol H2O × m-2 × s-1), stomatal conductance (Gs; mmol H2O-1 × m-2 × s-1), intercellular CO2 concentration (Ci; μmol CO2 air × mol-1), was measured using the portable photosynthesis measurement system TPS-2 (PP Systems, United States) (Grzesik et al., 2017a,b).
Index of chlorophyll content (in SPAD units) in leaves was evaluated using Minolta SPAD-502 chlorophyll meter (Konica Minolta, Japan) (Pacewicz and Gregorczyk, 2009; Grzesik and Romanowska-Duda, 2014; Grzesik et al., 2017a,b).
The infestation of plants by pathogenic fungi and their quality and quantity were evaluated after harvest of infected leaves (one from each of 10 plants) and subsequent their incubation for 7-day in humid cameras. The fungi were then transplanted from leaves into selective media, on which their species composition and quantity was identified using light microscopy (Leica) and available identification keys (Mehta et al., 2014). The percentage of plants infested with pathogenic fungi was assessed, taking into account 10 plants in pot experiment and 175 ones in the field.
Activities of alkaline (pH 7.5) (EC 3.1.3.1) and acid (pH 6) (EC 3.1.3.2) phosphatase [U g-1 (FM) min-1] and RNase (EC 3.1.27.5) [U g-1(FM) min-1] in leaves were examined using the methods elaborated by Knypl and Kabzińska (1977).
Total dehydrogenase activity (EC 1.1.1.-) [mg (formazan) g-1 (leaf fm] was evaluated, by the procedure described by Górnik and Grzesik (2002) and Grzesik et al. (2017a), using spectrophotometer UVmini-1240 (Shimadzu, Japan) for formazan determination at a wavelength of 480 nm. Enzyme activities were calculated on the basis of fresh mas (fm).
The weight of fresh (directly after harvest) and dry (after drying for 3 days at 130°C) biomass, of 5 plants per repetition and then calculated for 1 plant presented as average for treatment, was evaluated at the end of November (Grzesik et al., 2017b).
The content of elements in leaves, Stymjod, Apol-humus, peat substrate in pots and podzolic soil in field was evaluated in a certified laboratory at Research Institute of Horticulture in Skierniewice (Poland), using standard procedures. The material was concentrated by evaporation of excess water and then mineralized in a microwave oven “Etho-1” from Milestone, Italy. In the solution after mineralization, quantity and composition of elements were determined using a plasma spectrometer (ICP) model DV2000 from Perkin-Elmer, United States. Different wavelengths characteristic of a given element were used for the determination of elements. The total nitrogen content was determined by the Kjeldahl method after mineralization in concentrated sulfuric acid with the addition of a catalyst. The determination of the nitrogen content was carried out using a titration (Antweiler et al., 1996). Energetic value of plants was estimated by Carbochem, a certified laboratory (Poland) using Polish assessment standards listed in Table 8 (Grzesik et al., 2017b).
All experiments were performed in two series in central and northern Poland, in three replicates for each treatment. Within each series, each repetition was set up in a completely randomized block design. The obtained data, given as means from replicates, were processed applying analysis of variance (ANOVA I), by Statistica 12. The means of chosen parameters were grouped employing the Dunett’s test and the contrast between control sample and the remaining samples was used at α = 0.05 significance level. The confidence limit at 0.95 was shown in all drawings. The data in tables marked with the same letters within a column are not significantly different, according to Newman – Keuls multiple range test at an alpha level of 0.05. To predict sorghum yields based on chlorophyll fluorescence, linear regression was applied.
Maximum efficiency of photosystem II (PSII) measurements, as given by Fv/Fm in dark-adapted sorghum leaves in pot experiments, were closely related to the plant fertilization methods and showed that all used treatments with both, waste and biostimulants increased this parameter. However, the increase in the maximal efficiency of PSII depended on the fertilizers, their doses and method of application. The non-centrifuged waste applied to soil at the dose of 10–40 m3 ha-1 and the centrifuged one at 20–60 m3 ha-1 significantly increased the maximal efficiency of PSII, which showed also that the most profitable were their doses of 40 m3 and 40–50 m3, respectively. The positive impact of both forms of waste, shown by this parameter, was enhanced by the previous application of the soil improver Apol-humus at the dose of 10 L ha-1 and moreover, to higher degree, by additional twofold plant spraying with nano-organic-mineral fertilizer Stymjod, at 2-week interval, at the concentration of 1.5% (5 L ha-1) (Figure 1).
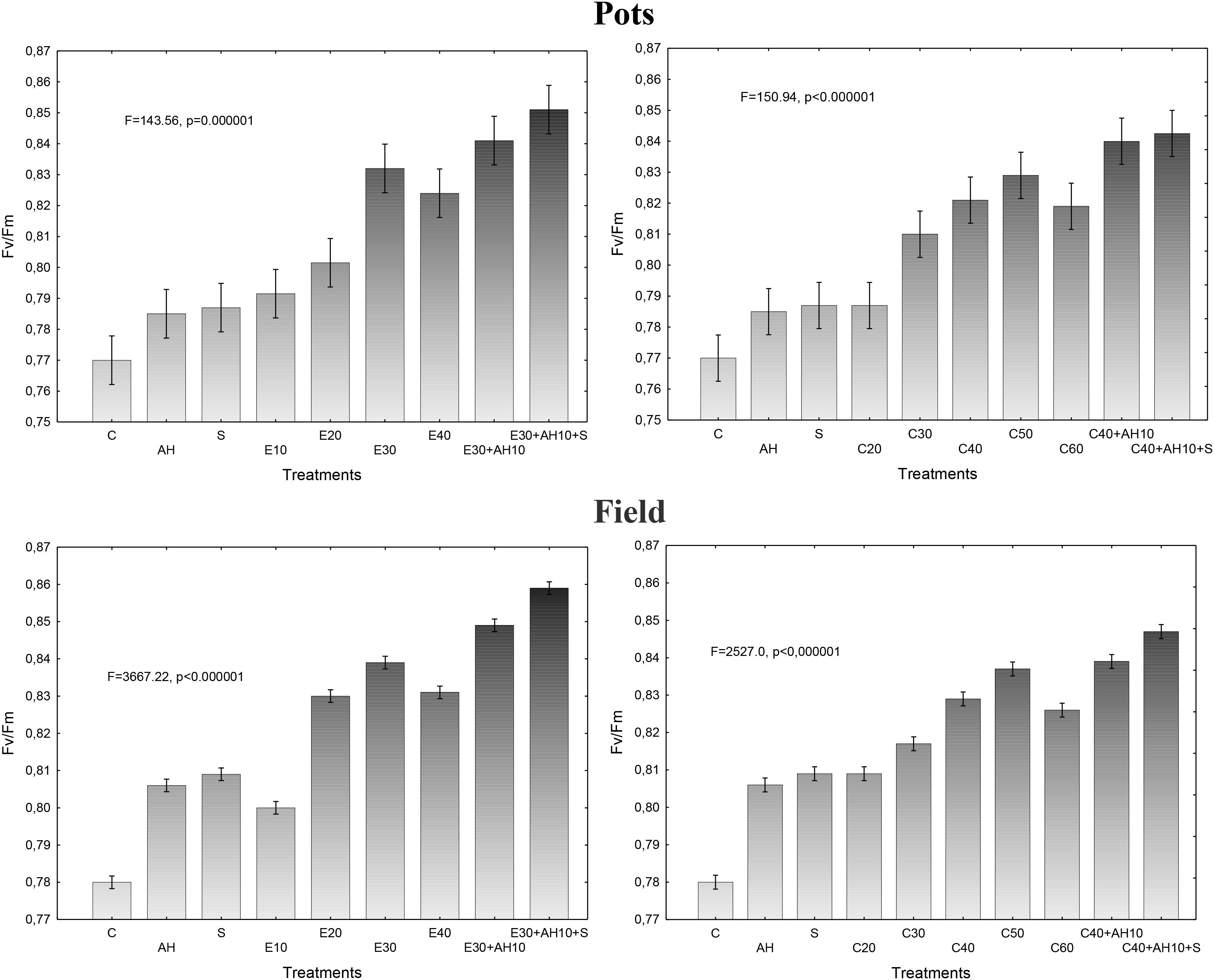
Figure 1. Maximum PSII efficiency in sorghum leaves cultivated in pots and field and fertilized with liquid waste from a biomass biodigestion to methane, non-centrifuged (E 10–40 m3 ha-1) and centrifuged (C 20–60 m3 ha-1), Apol-humus (AH; 10 L ha-1) and Stymjod (S; 5 L ha-1).
Established dependencies between the doses of non-centrifuged and centrifuged waste, supplemented with biostimulants and the maximum efficiency of PSII in the pot investigations were confirmed in field experiments conducted in more fertile podzolic soil and under slightly other climatic conditions of northern Poland nearby Baltic Sea than in center of the country. Due to more favorable soil conditions in field for plant development, the maximal efficiency of PSII was proportionally higher in plants cultivated in field, than in 5-l pots, as it was shown in all treatments (Figure 1).
Plant growth kinetics in 5-l pots, exhibited by results of the shoot height measurements every month during all vegetative season, were dependent on the waste dose and whether they were used alone or together with biostimulators, similarly as it was found in the study of maximal efficiency of PSII. Since the dependencies between effects of treatments were similar throughout the growing season, the presented results in Figure 2 show the results of final height measurement in November, as average of the measured plants in each repetition. Correspondingly, as the results of test described above showed, a non-centrifuged waste applied at the dose of 10–40 m3 ha-1 and the centrifuged one of 20–60 m3 ha-1 accelerated growth of plants. Similarly, the higher growth kinetics were observed when the doses were 30 m3 ha-1 and 40–50 m3 ha-1 for non-centrifuged and centrifuged wastes, respectively. Correspondingly, as maximal efficiency of PSII analyze showed also, application of Apol-humus to soil and Stymjod to plants increased their growth, as compare to control if they were used alone, or in comparison to optimal dosages of waste if these biostimulants were applied together with them (Figure 2).
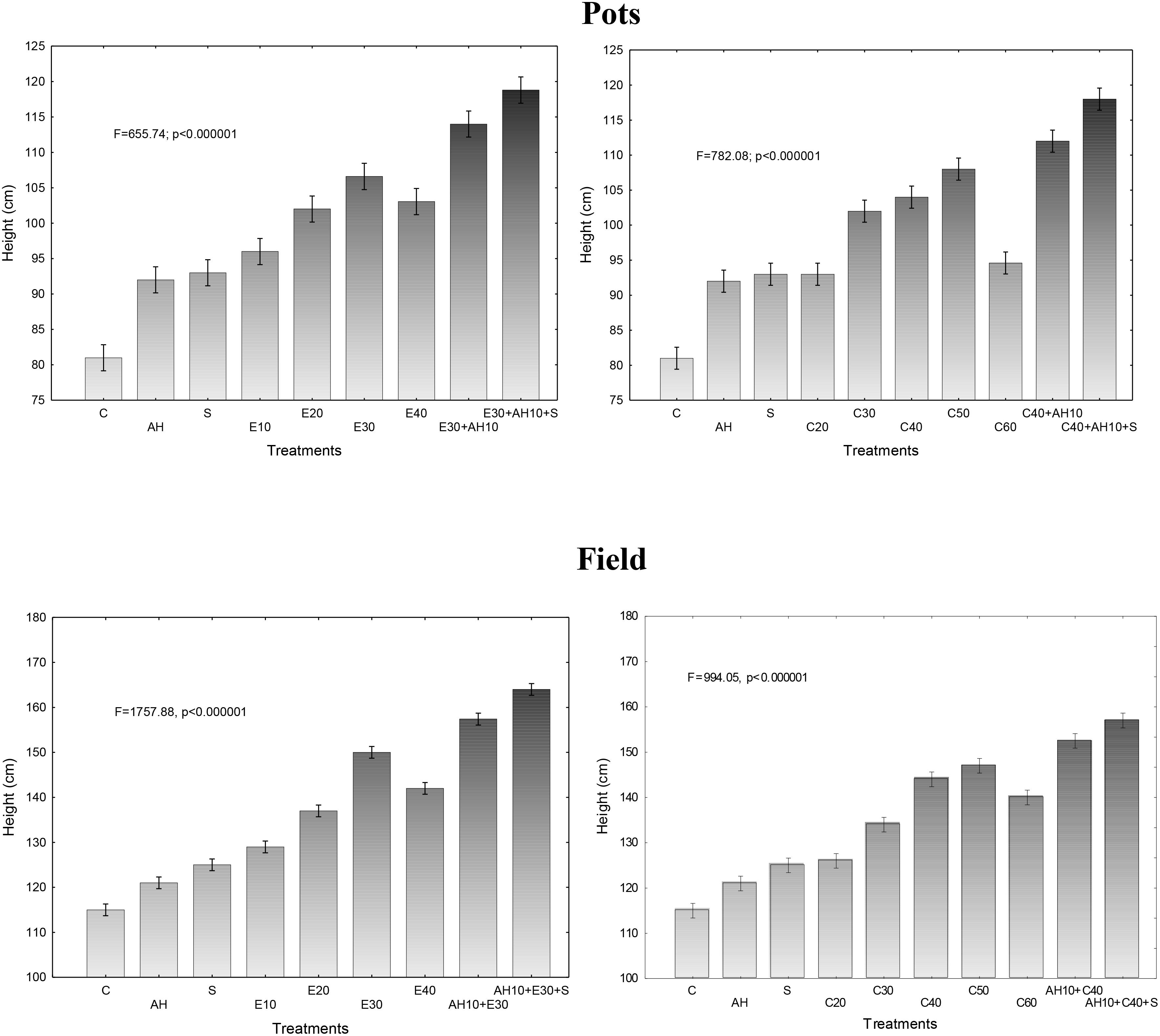
Figure 2. Final height of sorghum plants cultivated in pots and field and fertilized with liquid waste from a biomass biodigestion to methane, non-centrifuged (E 10–40 m3 ha-1) and centrifuged (C 20–60 m3 ha-1), Apol-humus (AH; 10 L ha-1) and Stymjod (S; 5 L ha-1).
The found relations between the doses of waste and biostimulators and the growth of plants in pot studies were confirmed in field experiments. Plants cultivated in the field in all experimental variants were proportionally higher than those grown in 5-liter pots. Similarly, they maintained the same relationship between the methods of fertilization with waste and biostimulators and the growth of plants that were found in the tests discussed above (Fv/Fm) (Figure 2).
The observed favorable changes in maximal efficiency of PSII comparable to changes in plant growth were confirmed by the enhanced proportionally activity of gas exchange and increased index of chlorophyll content in leaves, which were the consequence of the application of waste and stimulants in 5-l pots. Similarly, as the results of presented above two tests showed, the used waste and biostimulants increased activity of gas exchange processes and index of chlorophyll content in leaves, measured at high temperature, over 30°C in sun and in low air humidity about 50%. As shown in Table 2, the wastes increased the index of chlorophyll content, net photosynthesis, transpiration, stomatal conductance and decreased intercellular CO2 concentration, inversely proportional to mentioned gas exchange parameters. The dependencies between the impacts of the used waste doses and gas exchange features or index of chlorophyll content were similar to these observed between waste doses, plant height and maximal PSII efficiency. As they show, the non-centrifuged waste increased gas exchange parameters more than the centrifuged one used in the same doses and their application in doses of 30 m3 ha-1 and 40–50 m3 ha-1 respectively, together with biostimulants were most efficient (Figures 1, 2 and Table 2).
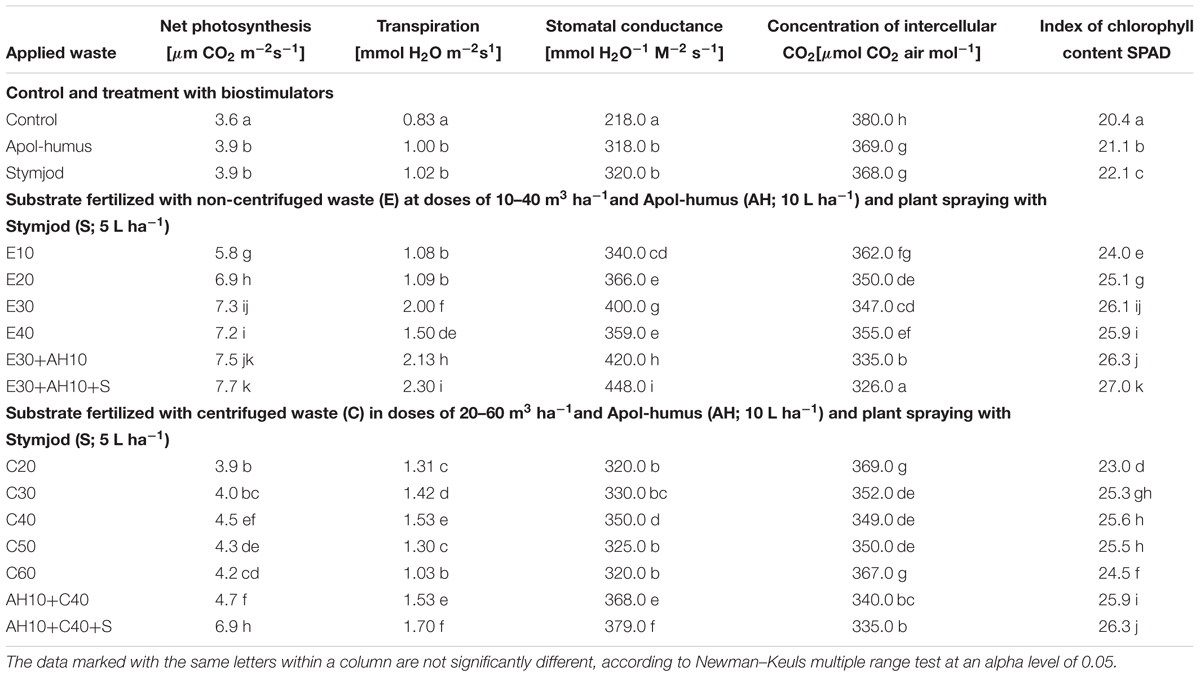
Table 2. Parameters of gas exchange and index of chlorophyll content in sorghum leaves of plant cultivated in pots and fertilized with liquid, non-centrifuged and centrifuged waste from a biomass biodigestion to methane, Apol-humus and Stymjod.
The examined dependences, found between all fertilization variants and the presented measurements in field conditions, closely coincided with the relationships found in the pot experiment. Plants growing in field, in more favorable soil conditions and at slightly lower temperature than in central Poland, showed slightly higher value of net photosynthesis, transpiration and stomatal conductance than in pots, however, the relationship between all these mentioned parameters and applied waste doses were similar (Tables 2, 3).
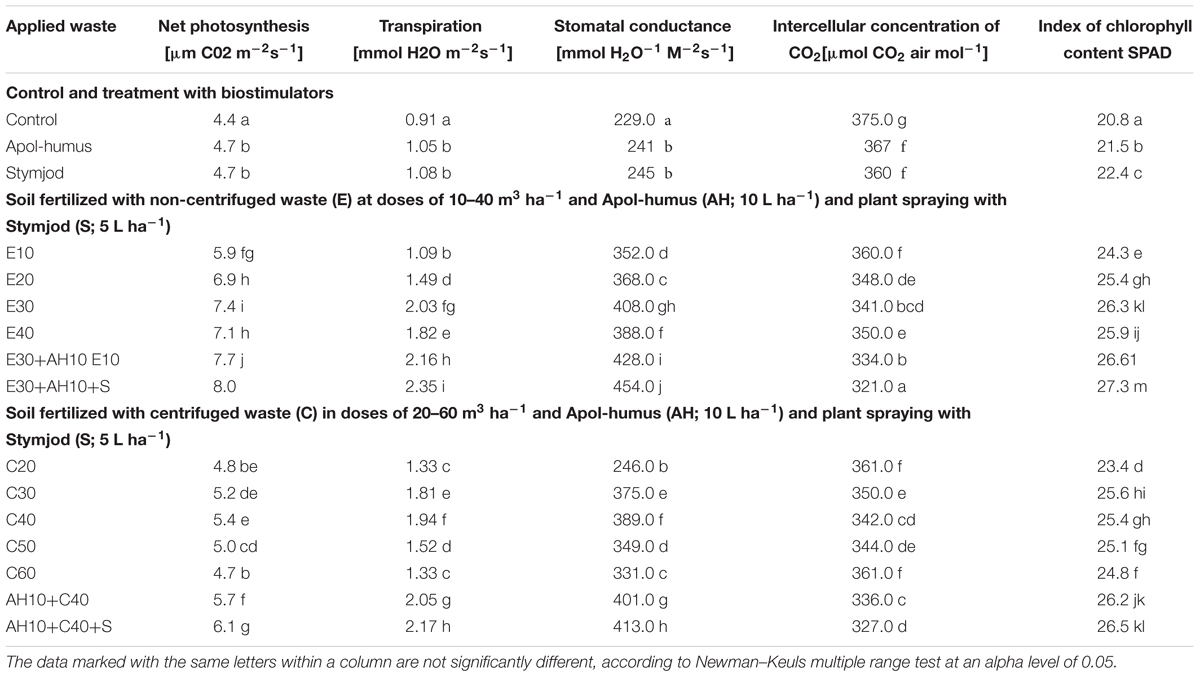
Table 3. Parameters of gas exchange and index of chlorophyll content in sorghum leaves of the plants cultivated in field and fertilized with liquid, non-centrifuged and centrifuged waste from a biomass biodigestion to methane, Apol-humus, and Stymjod.
Plant infestation by pathogenic mycoflora were similar in pot tests and field conditions. The infestation of plants grown in pots and field by pathogenic mycoflora was reduced by applied waste and biostimulants. As shown in Tables 4, 5, eight species of pathogenic fungi were isolated from the infected leaves. Percentage of all isolated pathogenic fungi in relation to total isolates, and the percent of infected plants in comparison to control, decreased under influence of all fertilization methods. The highest decrease in fungi infestation was observed after the use of waste doses of 30 m3 ha-1 (non-centrifuged) and 40 m3 ha-1 (centrifuged) and biostimulators (Tables 4, 5). The reduction of plant infestation by pathogenic fungi was inversely proportional to the increase in Fv/Fm (Figure 1 and Tables 4, 5).
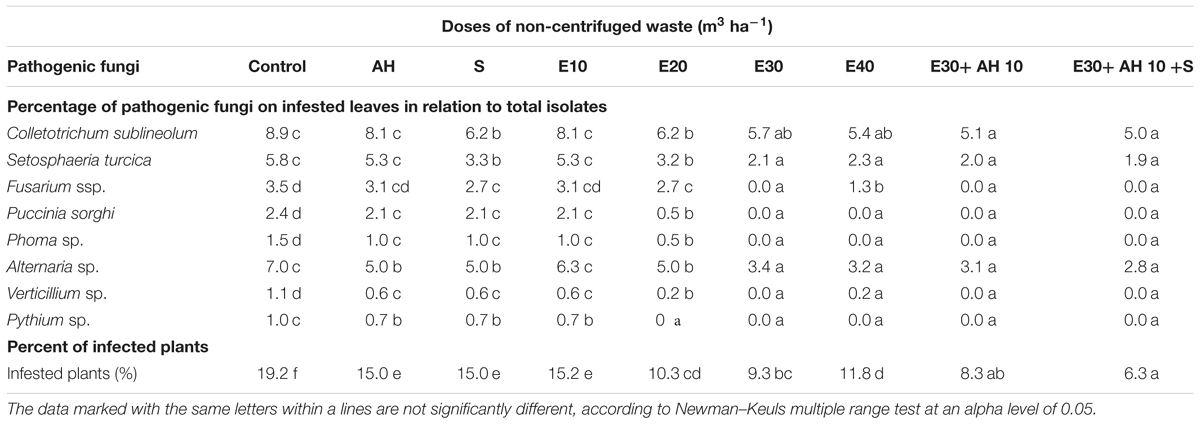
Table 4. Species composition and percentage of pathogenic fungi on infested leaves in relation to total isolates and the percent of infected plants, as effected by sorghum plant fertilization with waste from a biomass biodigestion to methane (E), Apol-humus (AH) and Stymjod (S) in pots.
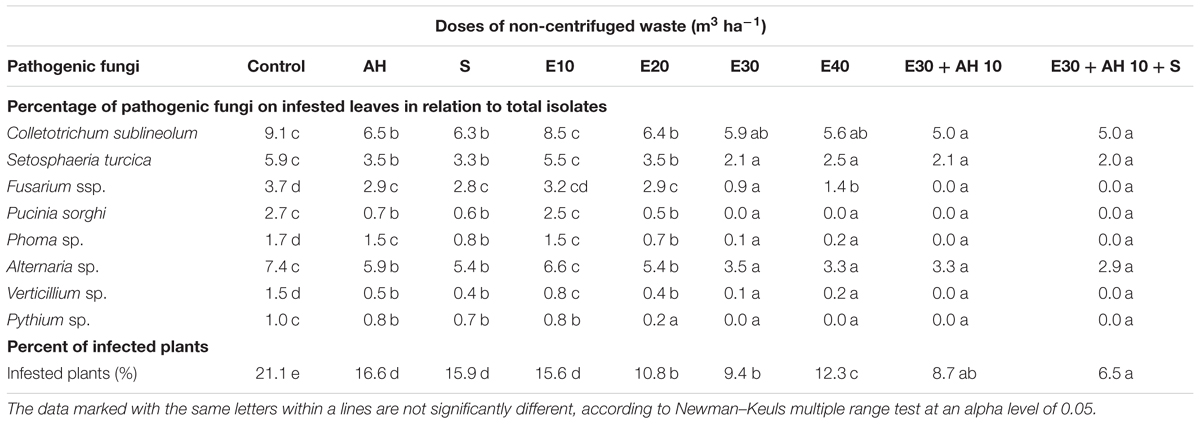
Table 5. Species composition and percentage of pathogenic fungi on infested leaves in relation to total isolates and the percent of infected plants, as effected by sorghum plant fertilization with waste from a biomass biodigestion to methane (E), Apol-humus (AH) and Stymjod (S) in field.
Applied doses of waste used separately or together with biostimulators caused a positive impact on the activity of selected enzymes (acid and alkaline phosphatase, RNase, dehydrogenases) that have a key impact on plant development. The changes in the activity of acid and alkaline phosphatase, RNase and dehydrogenase were closely related to the positive effect of fertilization with various doses of waste and biostimulants, and were proportional to the maximal efficiency of PSII and measurements of other studies. Here also, the more increased enzyme activity was affected by non-centrifuged and centrifuged waste application to the soil at a dose of 30–40 m3 ha-1 and 40–50 m3 ha-1, respectively. Their supplementation with Apol-humus Stymjod additionally improved this beneficial effect, similarly as it was found during maximal PSII efficiency measurements (Tables 6, 7).
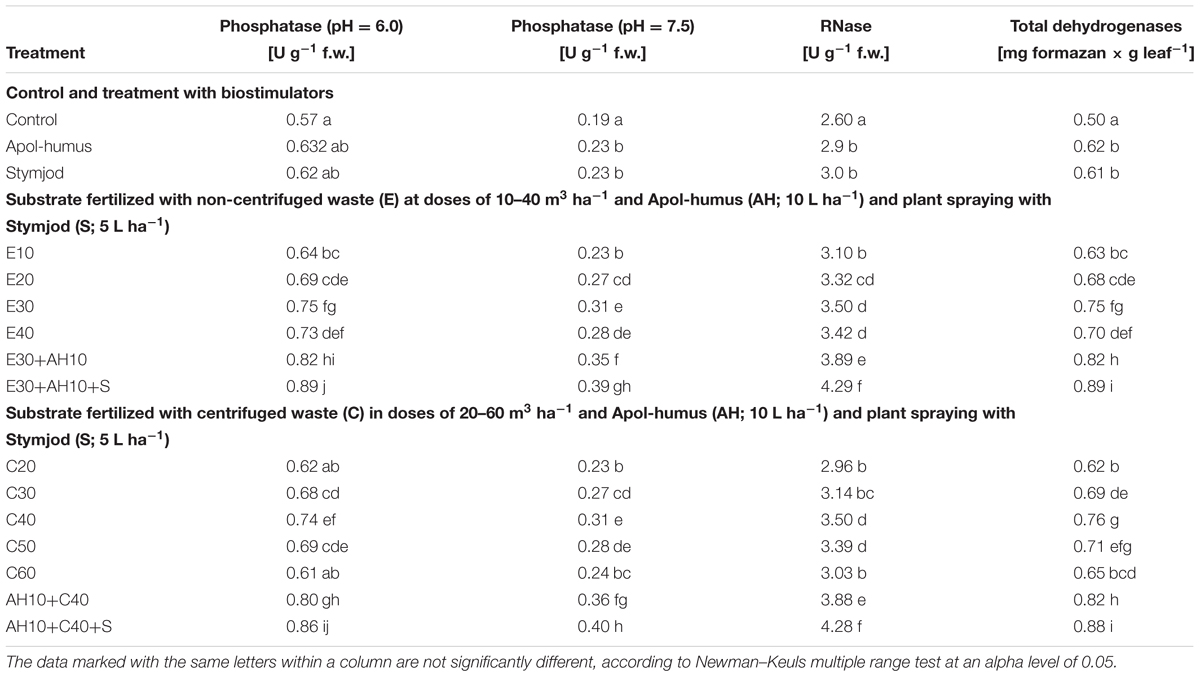
Table 6. Activity of selected enzymes in leaves of sorghum plants cultivated in pots and fertilized with liquid, non-centrifuged and centrifuged waste from a biomass biodigestion to methane, Apol-humus and Stymjod.
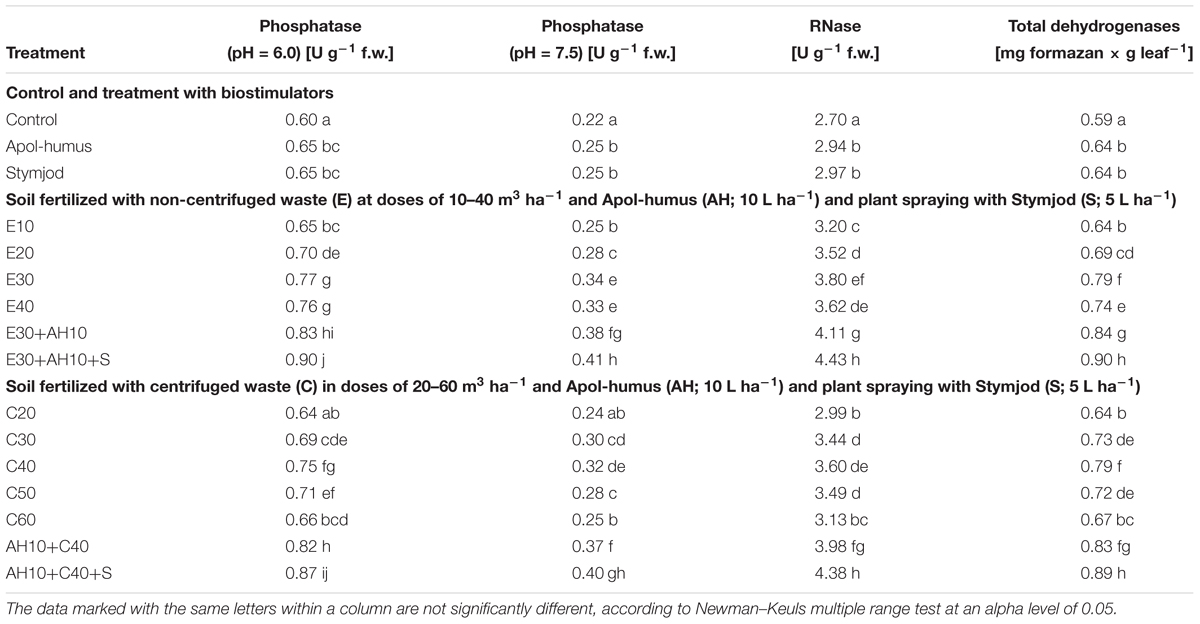
Table 7. Activity of selected enzymes in leaves of sorghum the plants cultivated in field and fertilized with liquid, non-centrifuged and centrifuged waste from a biomass biodigestion to methane, Apol-humus and Stymjod.
Enzymatic activity in plants grown in field were slightly higher in all experimental variants than in the pot experiments, however, the dependencies between doses of waste and results of enzyme activity were similar in pots and field, similarly as it was also found between waste doses and maximum PSII efficiency (Figure 1 and Tables 6, 7).
The applied fertilizers increased biomass yield in a degree depending on the dose of waste and the biostimulants used. Relationship between fresh or dry biomass yield and doses of waste and biostimulants were similar as shown in previous assessments. The applied non-centrifuged and centrifuged waste increased more fresh and dry biomass yield. Their doses of 30–40 m3 ha-1 and 40–50 m3 ha-1, respectively and supplemented with biostimulants were the most effective, similarly as it was shown by measurements of maximal PSII efficiency and other studies (Figures 1, 3, 4).
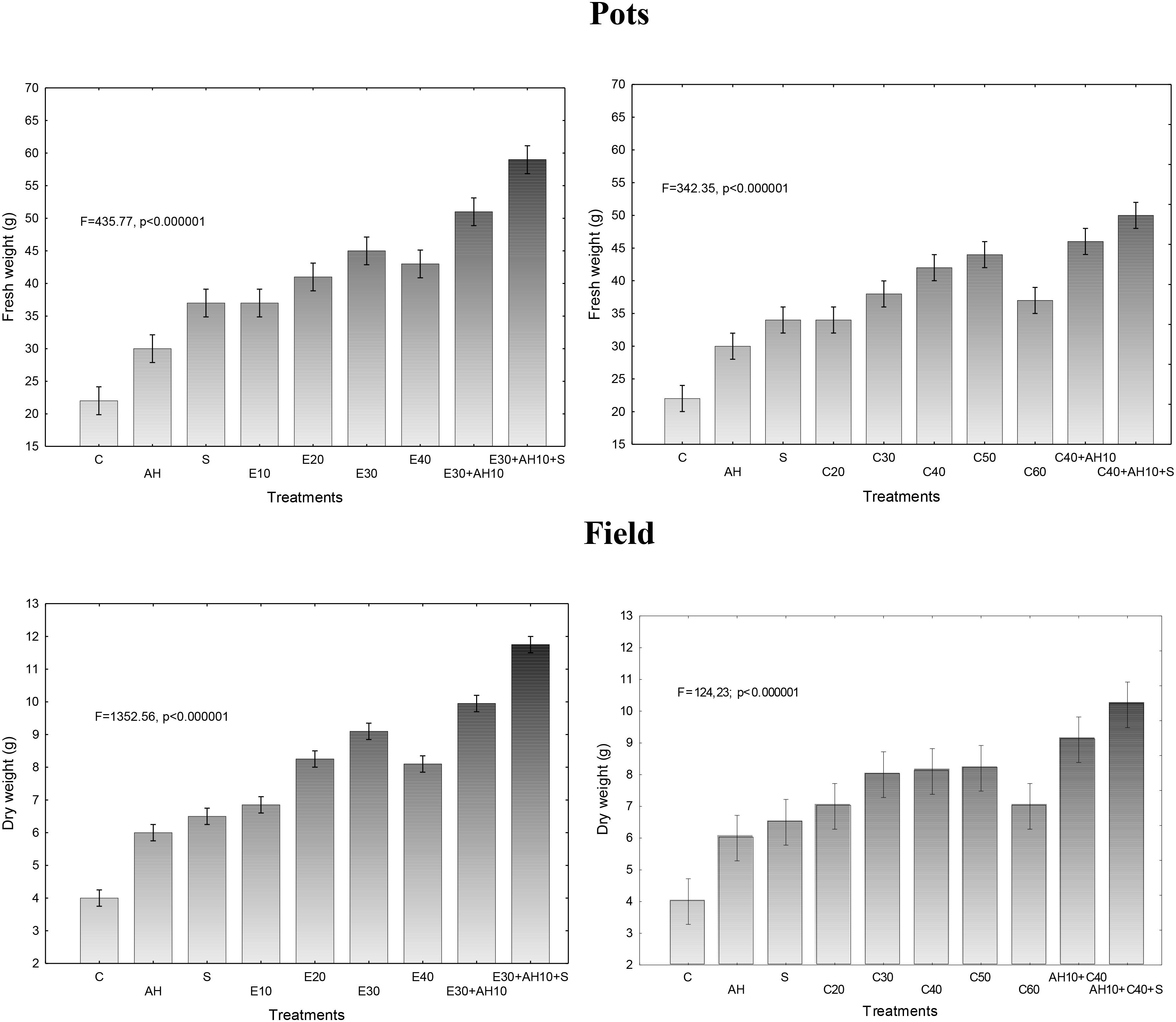
Figure 3. Final fresh and dry weight of one sorghum plant cultivated in pots fertilized with liquid waste from a biomass biodigestion to methane, non-centrifuged (E 10–40 m3 ha-1) and centrifuged (C 20–60 m3 ha-1), Apol-humus (AH; 10 L ha-1) and Stymjod (S; 5 L ha-1).
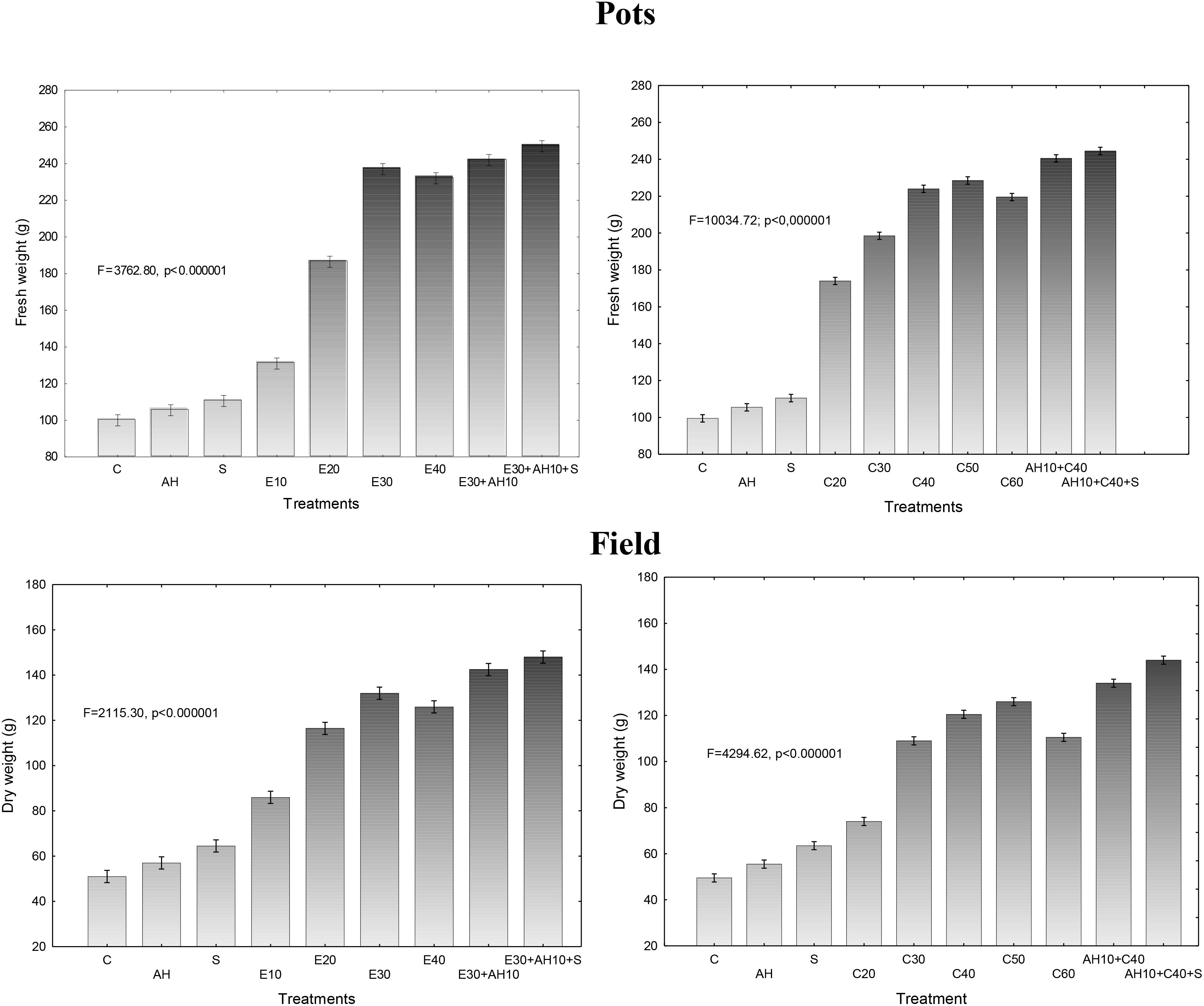
Figure 4. Final fresh and dry weight of one sorghum plant cultivated in field and fertilized with liquid waste from a biomass biodigestion to methane, non-centrifuged (E 10–40 m3 ha-1) and centrifuged (C 20–60 m3 ha-1), Apol-humus (AH; 10 L ha-1) and Stymjod (S; 5 L ha-1).
Changing Fv/Fm values depending on the doses of un-centrifuged and centrifuged waste from a biomass biodigestion to methane used were strictly correlated with the height and mass of Sorghum plants (Figures 5–7).
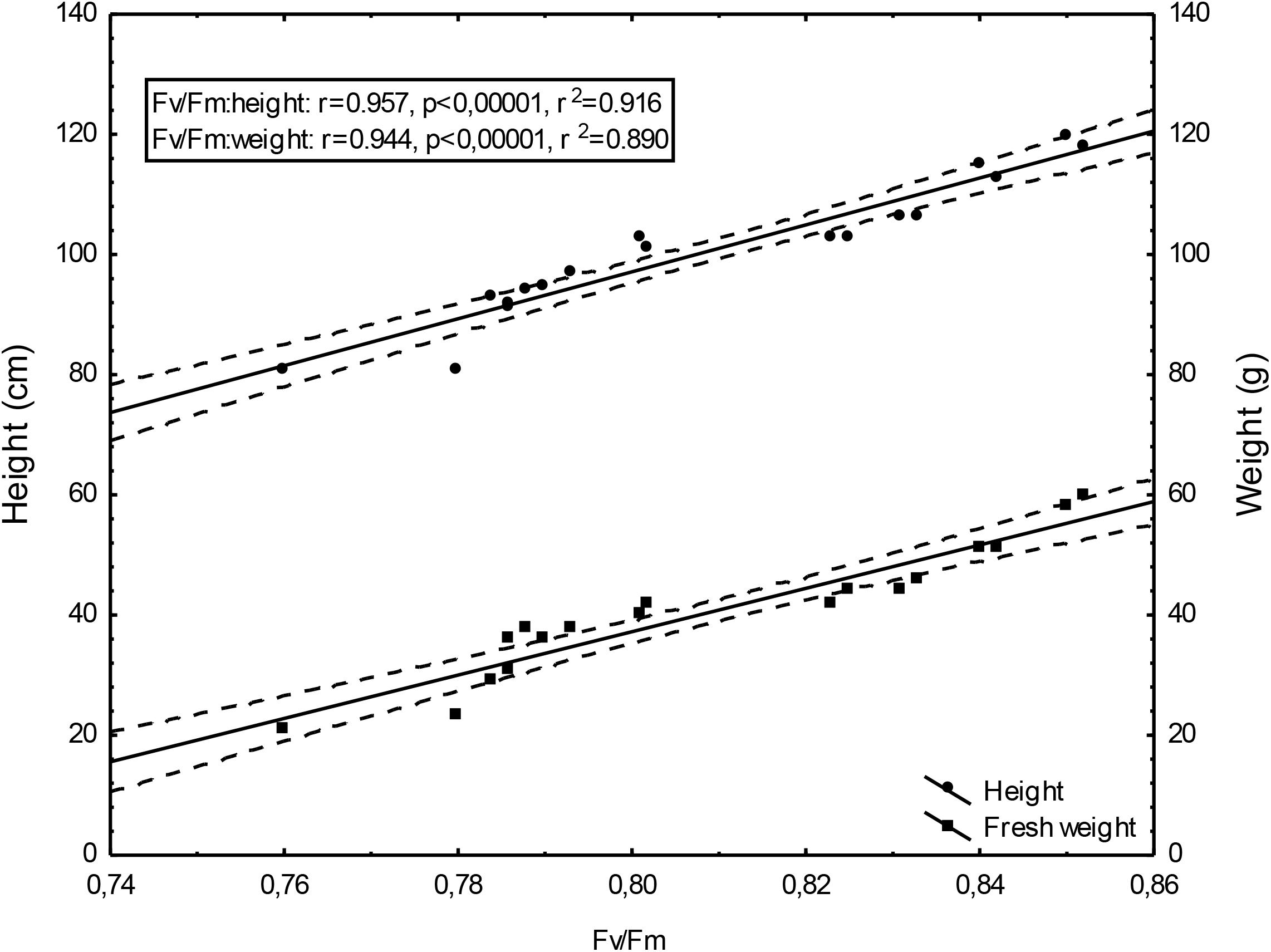
Figure 5. Height and fresh weight regressions against Fv/Fm: height = –215.27 + 390.48Fv/Fm and weight = –251.40 + 360.79Fv/Fm; 0,95 C.L. (Confidence limit).
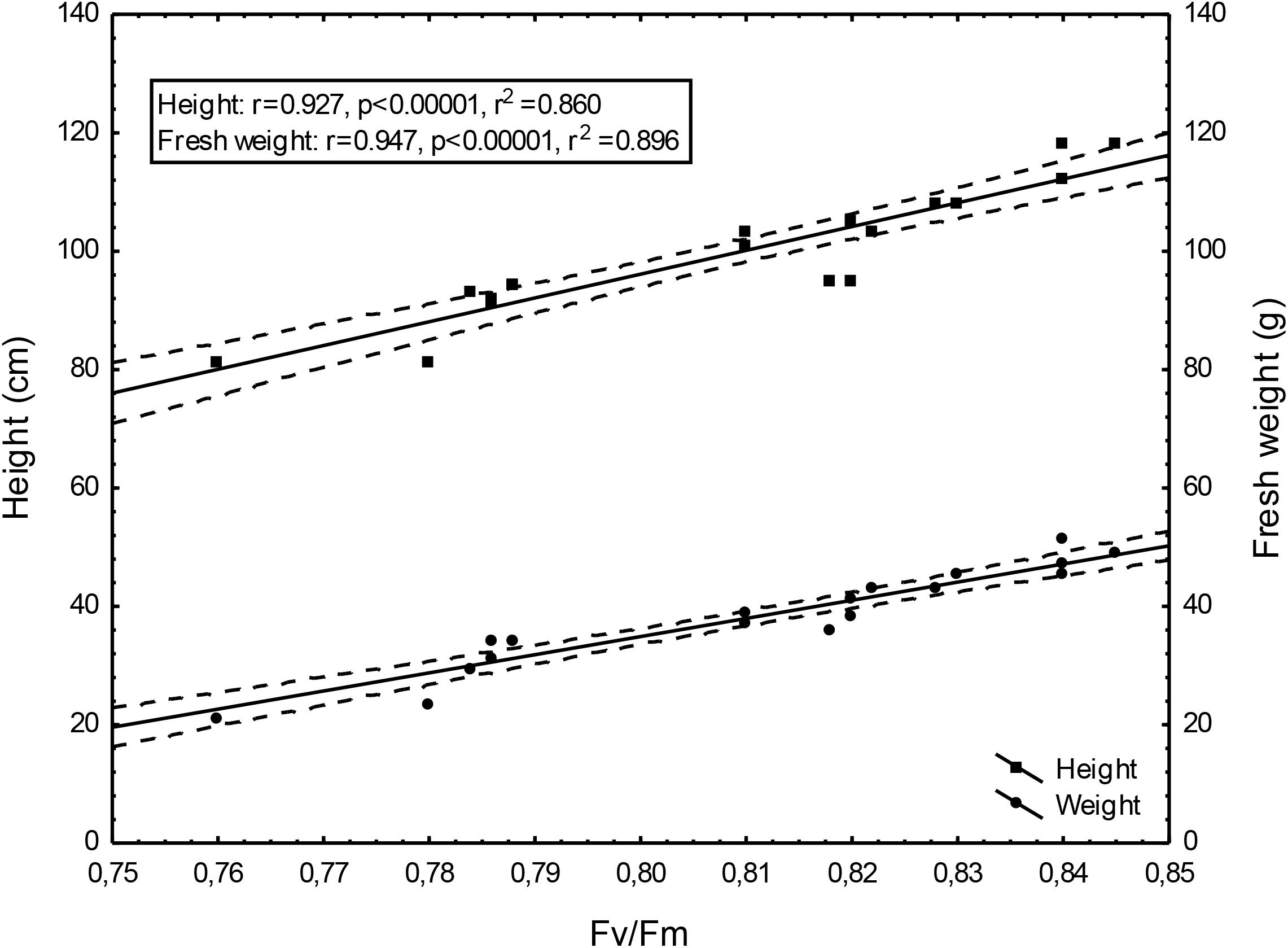
Figure 6. Height and fresh weight regressions against Fv/Fm: Height = –225.22 + 401.68Fv/Fm; fresh weight = –210.50 + 306.77Fv/Fm; 0,95 C.L.
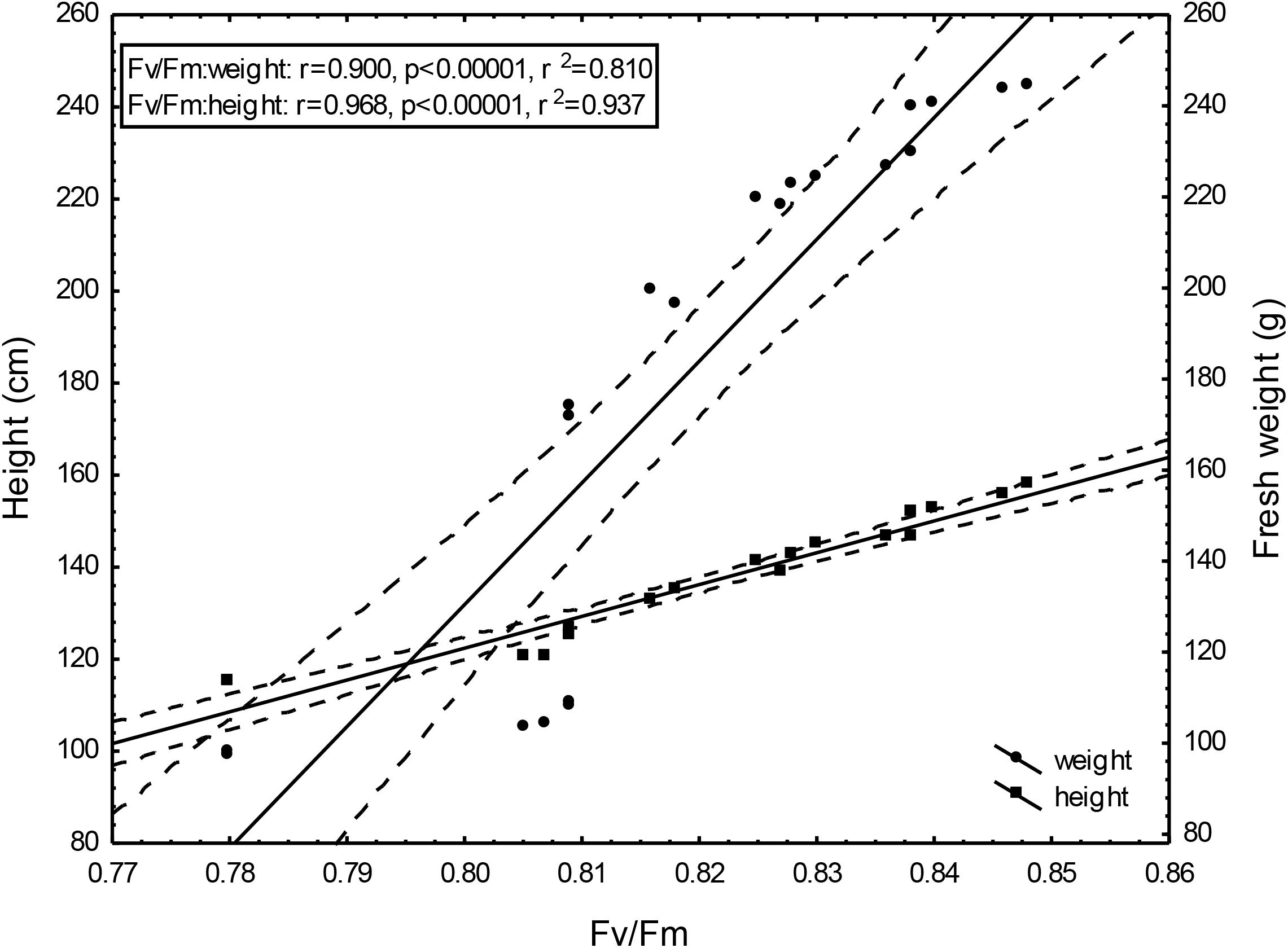
Figure 7. Height and fresh weight regressions against Fv/Fm: Fresh weight = –1984.95 + 2645.99Fv/Fm; 0.95 C.L. Height = –430.44 + 691,05x; 0.95 C.L.
Research assessing the content of macro- and micronutrients in leaves and the energy value of plants was carried out only in field conditions, due to the greater interest of energy biomass producers in them than in pot tests. The leaves of plants fertilized with the studied waste contained, in most cases, similar amounts of macro- and microelements as the non-fertilized ones, as shown in Table 8. The wastes applied to soil and supplemented with Apol-humus and Stymjod did not affected the energy value of plants and reduced only the ash content in them (Table 8).
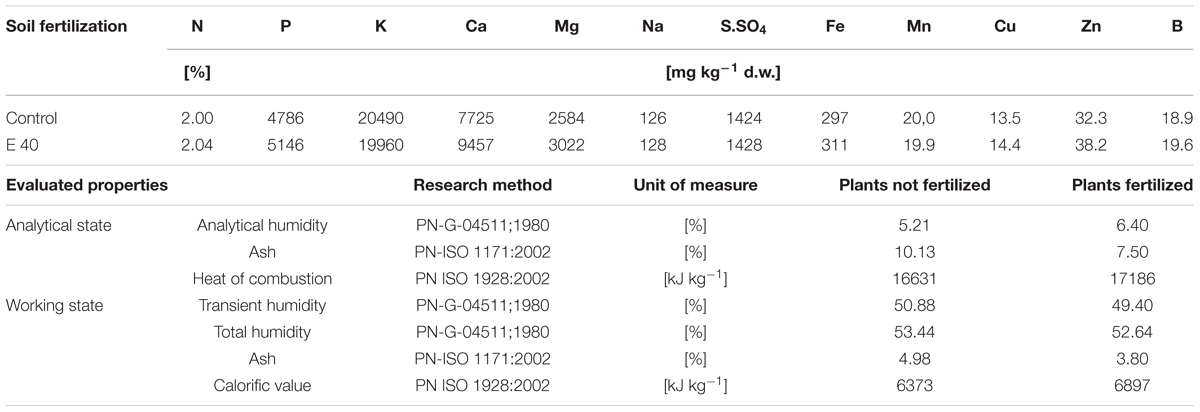
Table 8. Content of macro- and microelements in leaves of sorghum plants grown in field in non-fertilized soil (Control) and that fertilized with the non-centrifuged waste from a biomass biodigestion to methane at a dose of 40 m3 ha-1 (E40) and parameters of the energy value of sorghum plants grown in field in un-fertilized soil (Control) and that fertilized with the non-centrifuged waste from a biomass biodigestion to methane at a dose of 30 m3 ha-1, Apol-humus (15 L ha-1) and additionally foliar spraying with Stymjod 1.5% (5 L ha-1).
To the best of our knowledge, the investigation concerning the suitability of the maximum PSII efficiency, as the marker indicating vigor, growth, energetic value and physiological activity of sorghum fertilized with wastes from a biomass biodigestion to methane, in a distillery integrated with a biogas plant using corn grains as substrate, have not previously been reported. The performed studies demonstrate that the maximum PSII efficiency, as given by Fv/Fm in dark-adapted leaves of sorghum plants, can be used as a non-destructive marker for defining, the degree of plant development and similarly as other studied tests (gas exchange, index of chlorophyll content and activity of selected enzymes) can indicate the plant vigor, and physiological activity. Changing Fv/Fm values depending on the doses of un-centrifuged and centrifuged waste from a biomass biodigestion to methane used were strictly correlated with the height and mass of Sorghum plants. The linear regression equation makes it possible to predict the yields of sorghum plants based on Fv/Fm. This relationship is very tight. The study exhibit the usefulness of Fv/Fm in evaluation of impact of the waste from a corn grain biodigestion to methane on development and physiological activity of sorghum plants, thus showing their potential as an organic fertilizers. Obtained results indicate that Fv/Fm acts as a stable metric for measuring the impact of waste fertilization on the maximal quantum yield of PSII. Additionally, this parameter responded to the waste doses positively, allowing easy interpretation for practitioners. The same was stated in Banks (2018) studies in which the Fv/Fm responded to drought stress negatively. The found dependences in maximal efficiency of PSII, similarly as these in other laborious studies, closely reflected the effect of fertilization with various doses of waste, biostimulants, cultivation conditions and soil fertility on plant height, biomass yield, gas exchange and enzymatic activity and ash content in sorghum plants, which were determined in separate labor-intensive tests. These dependencies were observed independently these plants have been cultivated in peat substrate in pots or in podzolic soil in field, which showed other fertility, and under different climate in Central and North Poland. The changes in photochemical efficiency of PSII in relation to fertilization methods and sorghum growth, confirmed in other investigations, demonstrated positive impact of all studied waste doses on plant development and showed that the non-centrifuged waste is more effective in growth enhancement than the centrifuged one used in the same dose. Their application in doses of 30 m3 ha-1 and 40–50 m3 ha-1 respectively, were most efficient. These changes in Fv/Fm measurements were not in line with chemical analyze results showing that plant fertilization with the waste, did not increased macro- and microelement content in leaves but it decreased only ash content in burnt sorghum biomass, which is important when these plants are produced for energetic purposes. The obtained results confirm research of Shool and Shamshiri (2014) who demonstrated the enhanced Fv/Fm by improved nutritional status of the pistachio seedlings under four water regimes, especially by increasing content of the available phosphorus (P) element in soil. The close link between fluorescence emission and nitrogen fertilization was found also by Corp et al. (2003), Schächtl et al. (2005), Cendrero-Mateo et al. (2016), and Camino et al. (2018). This findings, that chlorophyll is the most widely used proxy for N content were also confirmed by research performed by Herrmann et al. (2010), Homolová et al. (2013), and Camino et al. (2018). In studies of Kalaji M.H. et al. (2014) on maize and tomato, the deficiencies of individual nutrients affected the photochemical processes of photosynthesis, as it was demonstrated by a complex of parameters derived from chlorophyll a fluorescence transient recorded in vivo. They stated that early detection of nutrient deficiency based on chlorophyll fluorescence data might be useful in particular species. Hamann et al. (2018) indicated that in young apple trees of two cultivars submitted to water restriction regimes fluorescence-based indices, related to chlorophyll content and nitrogen balance, promised to be a useful non-destructive tool to estimate physiological status of plants.
Studies suggest that the maximal PSII efficiency can be also used as indicator of plant health. In the conducted research, the value of this parameter grew, while when the percentage of all eight isolated pathogenic fungi in relation to the total number of isolates and the percentage of infected plants compared to controls decreased under the influence of all doses of fertilization. These found dependencies, demonstrating a lowest infection of plants showing simultaneously the highest maximal PSII efficiency, confirm usefulness of this chlorophyll fluorescence parameter in plant health status evaluation. The beneficial effect of fertilization on plant health is widely described in the literature. Possibility to use maximum PSII efficiency as indicator of plant health results from the fact that the increasing leaf colonization by pathogenic fungi damages progressively the photosynthetic system, whose activity can be assessed by Fv/Fm, chlorophyll content, gas exchange and other assessments. This is in line with research of Mandal et al. (2009) who demonstrated that downy mildew induced a reduction of chlorophyll content and PSII activity in Plantago ovata. According to Zhori et al. (2015), the non-invasive and rapid measurement of chlorophyll fluorescence induction allows characterizing the photosynthetic capacity of healthy and infected plants and of parts of them directly in the field. This test is highly sensitive not only concerning infection, but also toward other local environmental influences, as it was stated in Euphorbia cyparissias research. Chlorophyll a fluorescence has been satisfactorily used also for monitoring leaf health status in lamb’s lettuce (Ferrante and Maggiore, 2007).
The obtained results indicate that maximum PSII efficiency may be an rapid and not-destructive marker of sorghum plant vigor and can be profitable in fast evaluation of plant reactions to waste from a corn grain biodigestion to methane. It can be alternative or supplementary test in relation to labor-intensive and destructive studies of enzymatic activity or laboratory evaluation of chlorophyll content, which were useful in assessing the beneficial effects of various treatments on growth and physiological activity of plants. What’s more, this test is more useful in agriculture because it shows the total reaction of plants to specific treatments while others, such as chemical analysis, only results of some phenomena occurring in the tissues. In the performed research, the used maximal PSII efficiency measurements were comparable to activity of several enzymes (acid and alkaline phosphatase, RNase, dehydrogenase), index of chlorophyll content and gas exchange in leaves (net photosynthesis, transpiration, stomatal conductance and intercellular CO2 concentration), which were useful in assessment of physiological activity in plants and were recommended to be a markers of plant development. It was shown in research on China aster and tomato (Badek et al., 2014, 2016) and in studies showing stimulatory impact of algae on Virginia fanpetals, corn and willow growth and biostimulants on apple seed germination (Grzesik et al., 2009; Grzesik and Romanowska-Duda, 2014, 2015; Grzesik et al., 2015; Grzesik et al., 2017a,b) and sewage sludge on energy plants biomass yield (Romanowska-Duda et al., 2010). It is believed that maximum PSII efficiency measurement can be used to monitor parameters that depend on the content and activity of chlorophyll, and thus it can be an indicator of plant growth, gas exchange, activity of certain enzymes, biomass yield and the amounts of elements which depends on the functioning photosynthetic apparatus. The limiting factor of this parameter application for monitoring plant development can be a low temperature and insufficient nutrient supplementation that negatively affect the activity of the photosynthetic apparatus activity (Ostrem et al., 2018). Fv/Fm can be affected by numerous environmental factors, such as high-light, extreme temperature, CO2, drought, and can change diurnally. Too high light and temperature are considered to be the main factors responsible for the decrease of Fv/Fm at midday in terrestrial plants and in macrophytes. These changes are caused by either down-regulation or photodamage of photosystem II as a response to high irradiances (Jiang et al., 2018). In the conducted research too high temperature (well above 30°C in sun) and strong insolation and a too low air humidity (about 50%) could limit the activity of the photosynthetic system, which may cause the lower values of gas exchange parameters and photochemical efficiency of PSII (Banks, 2018). Drought is damaging when combined with other stressors, including high light intensities which cause an imbalance in the reaction centre leading to oxidative damage and photoinhibition (Goltsev et al., 2012).
Suggestions to use a maximal efficiency of PSII as a marker of sorghum growth, under influence of waste and biostimilants, are in line with research of Jalink et al. (1998) and Górnik et al. (2013) who proposed to use it in Brassica oleracea and coriander seeds as a non-destructive indicator for their maturity, taking into consideration that degreening process is directly related with the chlorophyll content in seed coats, which was monitored by the studied chlorophyll fluorescence parameter. Dayan et al. (2012) suggested chlorophyll fluorescence as a marker for herbicide mechanisms of action, while Silva et al. (2014) as an indicator of cellular damage by glyphosate herbicide in Raphanus sativus L. plants. Żebrowska and Michałek (2014) stated, that considerably differentiated phenotypic values of chlorophyll fluorescence parameters, including Fv/Fm, observed in the analyzed two cultivars of strawberry, were resulted from the specific response of photosynthetic apparatus, dependent on genotype to environmental conditions.
The found possibility to use maximal efficiency of PSII for rapid assessment of plant reactions to studied fertilizers become more important because amount of waste, produced by distilleries integrated with biogas plants, is rapidly growing around the world and thus its management and preferably ecological use as fertilizers for plant crops, have become one of important issues developed in the world. The tested non-centrifuged waste contained more macro- and microelements, essential for plant growth, than the centrifuged one in the unit of volume. The use of non-centrifuged waste in plant production is more justified since the costly centrifugation process is eliminated. Moreover, it contains more macro- and micronutrients thus slightly lower doses can be used to stimulate plant growth as compared to the centrifuged waste. All these measurements and findings under different climate conditions of central and northern Poland indicate, that the non-centrifuged waste from a corn grain biodigestion to methane can be used as a fertilizer in sorghum crops, solving the serious problem of its utilization and storage which is expensive and dangerous for environment. Moreover, the use of this waste in crop fertilization seems to be safer than of sewage sludge, that may contain toxic substances and thus should be refined before use in agriculture (Fang et al., 2017; Kołecka et al., 2017). The waste from corn grain biodigested to methane can be used in agriculture as fertilizers, similarly as waste from slurry digested to methane, which effectively reduce greenhouse gas emissions (Nakamura et al., 2014). However, the contents of nutrients in the studied waste was smaller (depending on the macronutrient) than in cattle manure and lower than in the waste from a maize residue biodigestion to methane in a biogas plant (Alburquerque et al., 2012; Łagocka et al., 2016), both recommended also as natural fertilizers in agriculture.
Demonstrated by maximal efficiency of PSII and other applied assessment tests, the stronger stimulating impact of the combined treatments on plant development could be caused by macro- and microelements contained in waste and by the presence of humic acids and chitosan polymers in Apol-humus, and by iodine, humic acids and nutrients in Stymjod, which additionally increased plant health, as their producers indicate. The chemical structure and stimulating impact of humic acids on plant development under normal and stress conditions was well described in literature (Calvo et al., 2014; Fahramand et al., 2014). The positive effect of chitosan on plant health, physiological activity and growth under different stress conditions was demonstrated in vines (Górnik et al., 2008) and in several other horticulture plants (Pichyangkura and Chandchawan, 2015; Bonilla and Sobral, 2016; Bistgani et al., 2017; Mukta et al., 2017; Pereira et al., 2017; Muxika et al., 2017). Kashyap et al. (2015) presented the positive impact of chitosan on production and protection of a wide number of crops and methods of its application in sustainable agriculture. The positive impact of Stymjod, applied alone and together with waste, on sorghum could be caused not only by, humic acids and elements but also by iodine. Iodine had the positive impact on the cyto-morphological changes in tomato and cabbage plants, as it was discovered by Jeznach (2011) after application of Biojodis, which was the preliminary form of Stymjod, contained similar elements, but was less absorbed by plants. Application of Biojodis to plants increased diameter of phloem and xylem tissues. Moreover, the well-formed single-layer epidermis bordered directly with one layer of collenchyma’s cells, and the parenchyma tissue greater than in control cells, was observed. Application of iodine resulted also in the predominance of open stomata which could increase gas exchange in leaves. Iodine stimulated also the physiological processes in cabbage, which were more resistant to stressed field conditions and increased the quantity of several macro- and microelements in leaves (Jeznach, 2011). In the stored carrot roots, not fertilized in field with N, treatment with iodine increased P, K, and Ca content and reduced Fe accumulation (Smoleń et al., 2011). Application of Biojodis also alleviated the negative influence of unfavorable temperature and drought on growth and physiological activity of corn plants (Piotrowski et al., 2016) and Virginia fanpetals (Grzesik et al., 2009). In lettuce, the winter and summer application of iodate (IO3-) or iodide (I-) increased quantity of these elements in leaves (Voogt et al., 2010). The mentioned processes found in vegetable crops, could stimulate also the growth of sorghum by increasing transportation of nutrients from reached by waste soil through the wider xylem cells, increased the plant growth and enhanced photosynthetic system activity, assessed by maximal efficiency of PSII, however, research in this area was not carried out.
The performed studies demonstrate that the maximum PSII efficiency, as given by Fv/Fm in dark-adapted leaves of sorghum plants, can be used as a non-destructive marker for defining, the degree of sorghum plant development. Similarly as other studied tests (gas exchange, index of chlorophyll content, activity of selected enzymes, mycological and chemical analyzes) it can be used to monitor the plant growth, physiological activity, infestation by pathogenic fungi, and ash content in burnt sorghum biomass. The presented maximum PSII efficiency assessments, confirming other physiological test measurements, shows prospects of ecological use of the waste from a corn grain biodigestion to methane in sorghum crops as fertilizers, together with the new generation soil improver Apol-humus and the nano-organic-mineral fertilizer, Stymjod, as the alternative to artificial fertilizers which pollute the environment. Thus, the maximum PSII efficiency can be seen as useful marker helping management of the studied waste as economically and environmentally friendly natural stimulants, if they are used in defined doses and in agreement with national, legal regulations on the safe application of fertilizers. Confirmed that the linear regression equation makes it possible to predict the yields of sorghum plants based on Fv/Fm. This relationship is very tight.
ZR-D carried out the experiments, processed the experimental data and wrote part of the research dealing with maximal efficiency of PSII, and enzyme activity. MG conducted the research, processed the experimental data, and wrote the part of article on plant development and the gas exchange. RJ conducted experiments on the health of plants, their infestation by pathogenic mycoflora and the quality and quantity of pathogenic microflora infesting plants. All authors provided critical feedback, made contributions to analysis and interpretation of data, discussed the results, contributed to the writing of the manuscript, and gave final approval of the version to be published.
This research was supported by National Centre for Research and Development Grant No. BIOSTRATEG2/296369/5/NCBR/2016.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thanks to Jolanta Zienkiewicz Expert-SITR Sp. z o.o. Poland for her participation in performance of the field experiments and plant height measurement.
Alburquerque, A. J., Fuente, C., Ferrer-Costa, A., Carrasco, L., Cegarra, J., Abad, M., et al. (2012). Assessment of the fertilizer potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 40, 181–189. doi: 10.1016/j.biombioe.2012.02.018
Antweiler, R. C., Patton, C. J., and Taylor, E. (1996). Automated Colorimetric Methods for Determination Nitrate Plus Nitrite, Nitrite, Ammonium, and Orthophosphate Ions un Natural Wather Samples. open-File Report No. 93-638. Denver, CO: United States Geological Survey, 1–28.
Badek, B., Romanowska-Duda, Z., Grzesik, M., and Kuras, A. (2016). Physiological markers for assessing germinability of Lycopersicon esculentum seeds primed by environment-friendly methods. Pol. J. Environ. Stud. 25, 1831–1838. doi: 10.15244/pjoes/63065
Badek, B., Romanowska-Duda, Z., Grzesik, M., and van Dujin, B. (2014). Rapid evaluation of germinability of primed china aster (Callistephus chinensis Ness.) seeds with physiological and biochemical markers. J. Hort. Res. 22, 5–18. doi: 10.2478/johr-2014-0017
Banks, J. M. (2018). Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Experim. Bot. 155, 118–127. doi: 10.1016/j.envexpbot.2018.06.022
Bistgani, Z. E., Siadad, S. A., Bakhshandeh, A., Pirbalouti, A. G., and Hashemi, M. (2017). Interactive effect of draught stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 5, 407–415. doi: 10.1016/j.cj.2017.04.003
Bonilla, J., and Sobral, P. J. A. (2016). Investigation of the physicochemical, antimicrobial and antioxidant properties gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 16, 17–25. doi: 10.1016/j.fbio.2016.07.003
Calvo, P., Nelson, L., and Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Camino, C., González-Dugo, V., Hernández, P., Sillero, J. C., and Zarco-Tejada, P. J. (2018). Improved nitrogen retrievals with airborne-derived fluorescence and plant traits quantified from VNIR-SWIR hyperspectral imagery in the context of precision agriculture. Int. J. Appl. Earth Obs. Geoinf. 70, 105–117. doi: 10.1016/j.jag.2018.04.013
Cendrero-Mateo, M. P., Moran, M. S., Papuga, S. A., Thorp, K. R., Alonso, L., Moreno, J., et al. (2016). Plant chlorophyll fluorescence: active and passive measurements at canopy and leaf scales with different nitrogen treatments. J. Exp. Bot. 67, 275–286. doi: 10.1093/jxb/erv456
Cheng, L., Leslie, H., Fuchigami, L. H., and Breen, P. J. (2000). Light absorption and partitioning in relation to nitrogen content in ‘Fuji’ apple leaves. J. Amer. Soc. Hortic. Sci. 125, 581–587.
Corp, L. A., McMurtrey, J. E., Middleton, E. M., Mulchi, C. L., Chappelle, E. W., and Daughtry, C. S. T. (2003). Fluorescence sensing systems: in vivo detection of biophysical variations in field corn due to nitrogen supply. Remote Sens. Environ. 86, 470–479. doi: 10.1016/S0034-4257(03)00125-1
Dayan, F. E., de, M., and Zaccaro, M. L. (2012). Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Phys. 102, 189–197. doi: 10.1016/j.pestbp.2012.01.005
Fahramand, M., Moradi, H., Noori, M., Sobhkhizi, A., Adibian, M., Abdollahi, S., et al. (2014). Influence of humic acid on increase yield of plants and soil properties. Inter. J. Farm. Allied Sci. 3, 339–341. doi: 10.1016/j.envres.2017.05.003
Fang, W., Delapp, R. C., Kosson, D. S., van der Sloot, H., and Liu, J. (2017). Release of heavy metals during long-term land application of sewage sludge compost: percolation leaching test with repeated additions of compost. Chemosphere 16, 271–280. doi: 10.1016/j.chemosphere.2016.11.086
Ferrante, A., and Maggiore, T. (2007). Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharv. Biol. Technol. 45, 73–80. doi: 10.1016/j.postbarvbio.2007.02.003
Goltsev, V., Zaharieva, I., Chernev, P., Kouzmanova, M., Kalaji, H. M., Yordanov, I., et al. (2012). Drought-induced modifications of photosynthetic electron transport in intact leaves: analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochim. Biophys. Acta 1817, 1490–1498. doi: 10.1016/j.bbabio.2012.04.018
Górnik, K., and Grzesik, M. (2002). Effect of Asahi SL on China aster ‘Aleksandra’ seed yield, germination and some metabolic events. Acta Physiol. Plant. 24, 379–383. doi: 10.1007/s11738-002-0033-5
Górnik, K., Grzesik, M., and Romanowska–Duda, B. (2008). The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornam. Plant Res. 16, 333–343.
Górnik, K., Janas, R., and Grzesik, M. (2013). Chlorophyll fluorescence of coriander seeds as maturation meter. Episteme 20, 317–322.
Górnik, K., and Lachuta, L. (2017). Application of phytohormones during seed hydropriming and heat shock treatment on sunflower (Helianthus annuus L.) chilling resistance and changes in soluble carbohydrates. Acta Physiol. Plant. 39:118. doi: 10.1007/s11738-017-2413-x
Grzesik, M., and Romanowska-Duda, Z. (2014). Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with cyanobacteria and microalgae. Pol. J. Environ. Stud. 23, 1147–1153.
Grzesik, M., Górnik, K., Janas, R., Lewandowki, M., Romanowska-Duda, Z., and van Duijn, B. (2017a). High efficiency stratification of apple cultivar Ligol seed dormancy by phytohormones, heat shock and pulsed radio frequency. J. Plant. Physiol. 21, 81–90. doi: 10.1016/j.jplph.2017.09.007
Grzesik, M., Romanowska-Duda, Z., and Kalaji, H. M. (2017b). Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 55, 510–521. doi: 10.1007/s11099-017-0716-1
Grzesik, M., Romanowska-Duda, Z., Piotrowski, K., and Janas, R. (2015). Diatoms (Bacillariophyceae) as an effective base of a new generation of organic fertilizers. Przem. Chem. 94, 391–396. doi: 10.15199/62.2015.3.27
Grzesik, M., and Romanowska-Duda, Z. B. (2015). Ability of Cyanobacteria and microalgae in improvement of metabolic activity and development of willow plants. Pol. J. Environ. Stud. 24, 1003–1012. doi: 10.15244/pjoes/34667
Grzesik, M., Romanowska-Duda, Z. B., and Piotrowski, K. (2009). The effect of potential climatic changes, Cyanobacteria, Biojodis and Asahi SL on development of the Virginia fanpetals (Sidahermaphrodita) plants. Pamiêtnik Puławski 151, 483–491.
Hamann, F. A., Czaja, S., Hunsche, M., Noga, G., and Fiebiga, A. (2018). Monitoring physiological and biochemical responses of two apple cultivars to water supply regimes with non-destructive fluorescence sensors. Sci. Hortic. 242, 51–61. doi: 10.1016/j.scienta.2018.07.008
Herrmann, I., Karnieli, A., Bonfil, D. J., Cohen, Y., and Alchanatis, V. (2010). SWIR-based spectral indices for assessing nitrogen content in potato fields. Int. J. Remote Sens. 31, 5127–5143. doi: 10.1080/01431160903283892
Homolová, L., Malenovský, Z., Clevers, J. G. P. W., García-Santos, G., and Schaepman, M. E. (2013). Review of optical-based remote sensing for plant trait mapping. Ecol. Complex 15, 1–16. doi: 10.1016/j.ecocom.2013.06.003
Jalink, H., van der Schoor, R., Frandas, A., van Pijlen, J. G., and Bino, R. J. (1998). Chlorophyll fluorescence of Brassica oleracea seeds as a non-destructive marker for seed maturity and seed performance. Seed Sci. Res. 8, 437–443. doi: 10.1017/S0960258500004402
Jasiulewicz, M., and Janiszewska, A. D. (2013). Possible opportunities for the development of biogas plants on the example of the Zachodniopomorskie province. Pol. Inżynieria Rolnicza 2, 91–102.
Jeznach, A. (2011). Effect of Iodine on the Cabbage Fruits Ontogenesis and Seed Quality. Master’s thesis, WSEH Skierniewice, Skierniewice.
Jeznach, A. (2015). General Description of Organic and Mineral Fertilizer “Stymjod” Intended for Foliar Fertilization of Plants. Available at: http://www.phu-jeznach.com.pl/doc/Stymjod/Opis_preparatu_STYMJOD_2.pdf [accessed July 28, 2018].
Jiang, H. S., Zhanga, Y., Yinb, L., Li, W., Jinb, Q., Fua, W., et al. (2018). Diurnal changes in photosynthesis by six submerged macrophytes measured using fluorescence. Aquatic Bot. 149, 33–39. doi: 10.1016/j.aquabot.2018.05.003
Kalaji, H. M., Oukarroum, A., Kouzmanova, M., Brestic, M., Zivcak, M., Samborska, I. A., et al. (2014). Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 81, 16–25. doi: 10.1016/j.plaphy.2014.03.029
Kalaji, H. M., Goltsev, V., Bosa, K., Allakhverdiev, S. I., Strasser, R. J., and Govindjee (2012). Experimental in vivo measurements of light emission in plants: a perspective dedicated to David Walker. Photosynth. Res. 114, 69–96. doi: 10.1007/s11120-012-9780-3
Kalaji, H. M., Račková, L., Paganová, V., Swoczyna, T., Rusinowski, S., and Sitko, K. (2018). Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill. Environ. Exp. Bot. 152, 149–157. doi: 10.1016/j.envexpbot.2017.11.001
Kalaji, M. H., Schanskser, G., Ladle, R. J., Goltsev, V., Bosak, K., Allakhverdiev, S. I., et al. (2014). Frequently asked questions about chlorophyll fluorescence: practical issues. Photosynth. Res. 122, 121–158. doi: 10.1007/s11120-014-0024-6
Kashyap, P. L., Xian, X., and Heiden, P. (2015). Chitosan nanoparticle based delivery system for sustainable agriculture. Internat. J. Biol. Macromol. 77, 36–51. doi: 10.1016/j.ijbiomac.2015.02.039
Kitajima, M., and Butler, W. L. (1975). Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 376, 105–115. doi: 10.1016/0005-2728(75)90209-1
Knypl, J. S., and Kabzińska, E. (1977). Growth, phosphatase and ribonuclease activity in phosphate deficient Spirodelaoligorrhiza cultures. Biochem. Physiol. 171, 279–287.
Kołecka, K., Gajewska, M., and Obarska-Pempkowiak, H. (2017). Integrated dewatering and stabilization system as the environmentally friendly technology in sewage sludge management in Poland. Ecol. Eng. 98, 346–353. doi: 10.1016/j.ecoleng.2016.08.011
Łagocka, A., Kamiński, M., Cholewiński, M., and Pospolita, W. (2016). Health and environmental benefits of utilization of post-fermentation pulp from agricultural biogas plants as a natural fertilizer Poland. Kosmos 65, 601–607.
Lalak, J., Kasprzycka, A., Paprota, E. M., Tys, J., and Murat, A. (2015). Development of optimum substrate compositions in the a methane fermentation proces. Int. Agrophys. 29, 313–321. doi: 10.1515/intag-2015-0037
Lichtenthaler, H., Buschmann, C., Rinderle, U., and Schmuck, G. (1986). Application of chlorophyll fluorescence in ecophysiology. Radiat. Environ. Biophys. 25, 297–308. doi: 10.1007/BF01214643
Lukehurst, C. T., Frost, P., and Al Seadi, T. (2010). Utilisation of Digestate from Biogas Plants as Biofertiliser. Available at: https://www.iea-biogas.net/_download/publitask37/Task37_Digestate_brochure9-2010 [accessed July 28, 2018].
Mandal, K., Saravanan, R., Maiti, S., and Kothari, I. L. (2009). Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J. Plant Dis. Protect. 116, 164–168. doi: 10.1007/BF03356305
Mehta, C. M., Palni, U., and Franke-Whittle, I. H. (2014). Compost its role mechanism and impact on reducing soil-borne plant diseases. Waste Manag. 34, 607–622. doi: 10.1016/j.wasman.2013.11.012
Mukta, J. A., Rahman, M., Sabir, A. A., Gupta, D. R., Surovy, M. Z., Rahman, M., et al. (2017). Chitosan and plant probiotic application enhance growth and yield of strawberry. Biocatal. Agric. Biotechnol. 11, 9–18. doi: 10.1016/j.bcab.2017.05.005
Muxika, A., Etxabide, A., Uranga, J., Guerrero, P., and de la Caba, K. (2017). Chitosan as the bioactive polymer: processing, properties and applications. Int. J. Biol. Macromol. 105, 1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087
Nakamura, M., Yuyama, Y., Yamaoka, M., and Shimizu, N. (2014). Global warming impacts of the process to utilize digested slurry from a methane fermentation as a fertilizer: case study of the Yamada Biomass Plant. Paddy Water Environ. 2(Suppl. 2), 295–299. doi: 10.1007/s10333-013-0375-1
Osinga, R., Iglesias-Prieto, R., and Enrquez, S. (2012). Measuring photosynthesis in symbiotic invertebrates: a review of methodologies, rates and processes. Appl. Photosynth. 11, 229–256. doi: 10.5772/29339
Ostrem, L., Rapacz, M., Larsen, A., Marum, P., Rognli, O., and Rognli, O. A. (2018). Chlorophyll a fluorescence and freezing tests as selection methods for growth cessation and increased winter survival in x Festulolium. Front. Plant Sci. 9:1200. doi: 10.3389/fpls.2018.01200
Pacewicz, K., and Gregorczyk, A. (2009). Comparison of chlorophyll content assesments with chlorophyll metter Spad-502 I N-Tester. Folia Pomer. Univ. Technol. Stetin Agric. Aliment. Pisc. Zootech. 269, 41–46.
Pereira, A. E. S., Silva, P. M., Oliveira, J. L., Oliveira, H. C., and Fraceto, L. F. (2017). Chitosan nanoparticles as carrier system for the plant hormone gibberellic acid. Colloids Surf. B Biointerfaces 150, 141–152. doi: 10.1016/j.colsurfb.2016.11.027
Pichyangkura, R., and Chandchawan, S. (2015). Biostimulant activity of chitosan in horticulture. Sci. Hortic. 196, 49–65. doi: 10.1016/j.scienta.2015.09.031
Piotrowski, K., Romanowska-Duda, Z., and Grzesik, M. (2016). How Biojodis and Cyanobacteria alleviate the negative influence of predicted environmental constraints on growth and physiological activity of corn plants. Pol. J. Environ. Stud. 25, 741–751. doi: 10.15244/pjoes/60894
Planchon, C., Sarrafi, A., and Ecochard, R. (1989). Chlorophyll fluorescence transient as a genetic marker of productivity in barley. Euphytica 42, 269–273. doi: 10.1007/BF00034464
Romanowska-Duda, Z., Grzesik, M., and Kalaji, H. M. (2010). Phytotoxkit test in growth assessment of corn as an energy plant fertilized with sewage sludge. Environ. Prot. Eng. 36, 73–81.
Schächtl, J., Huber, G., Maidl, F.-X., Sticksel, E., Schulz, J., and Haschberger, P. (2005). Laser induced chlorophyll fluorescence measurements for detecting the nitrogen status of wheat (Triticum aestivum L.) canopies. Precis. Agric. 6, 143–156. doi: 10.1007/s11119-004-1031-y
Shool, A., and Shamshiri, M. H. (2014). Effect of arbuscular mycorrhizal fungi and Pseudomonas fluorescence on chlorophyll fluorescence and photosynthetic pigments of pistachio seedlings (Pistacia vera cv. Qazvini) under four water regimes. Eur. J. Experiment. Biol. 4, 246–252.
Silva, F., Costa, A., Pereira Alves, R., and Megguer, C. (2014). Chlorophyll fluorescence as an indicator of cellular damage by glyphosate herbicide in Raphanussativus L. plants. Am. J. Plant Sci. 5, 2509–2519. doi: 10.4236/ajps.2014.516265
Smoleń, S., Sady, W., Rożek, S., Ledwożyw-Smoleń, I., and Strzetelski, P. (2011). Preliminary evaluation of the influence of iodine and nitro gen fertilization on the effectiveness of iodine biofortification and mineral composition of carrot storage roots. J. Elementol. 16, 275–285.
Strasser, R. J., Srivastava, A., and Tsimilli-Michael, M. (2000). “The fluorescence transient as a tool to characterize and screen photosynthetic samples,” in Probing Photosynthesis: Mechanism, Regulation and Adaptation, eds M. Yunus, U. Pather, and P. Mohanty (London: Taylor and Francis Ltd.), 445–480.
Su, L., Dai, Z., Li, S., and Xin, H. (2015). A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 15:82. doi: 10.1186/s12870-015-0459-8
Voogt, W., Holwerda, H. T., and Khodabaks, R. (2010). Biofortification of lettuce (Lactuca sativa L.) with iodine: the effect of iodine form and concentration in the nutrient solution on growth, development and iodine uptake of lettuce grown in water culture. J. Sci. Food Agric. 90, 906–913. doi: 10.1002/jsfa.3902
Żebrowska, J., and Michałek, W. (2014). Chlorophyll fluorescence in two strawberry (Fragaria x ananassa Duch.) cultivars. J. Central Europ. Agric. 15, 12–21. doi: 10.5513/JCEAO1/15.4.1501
Keywords: maximal efficiency of PSII, biomass biodigestion to methane, waste, fertilization, sorghum growth, physiological activity
Citation: Romanowska-Duda Z, Grzesik M and Janas R (2019) Maximal Efficiency of PSII as a Marker of Sorghum Development Fertilized With Waste From a Biomass Biodigestion to Methane. Front. Plant Sci. 9:1920. doi: 10.3389/fpls.2018.01920
Received: 07 August 2018; Accepted: 11 December 2018;
Published: 08 January 2019.
Edited by:
Marcin Rapacz, University of Agriculture in Kraków, PolandReviewed by:
Ruilian Jing, Institute of Crop Sciences (CAAS), ChinaCopyright © 2019 Romanowska-Duda, Grzesik and Janas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zdzisława Romanowska-Duda, cm9tYW5vQGJpb2wudW5pLmxvZHoucGw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.