- College of Life and Environment Science, Shanghai Normal University, Shanghai, China
NaHSO3 addition greatly increases the yield of H2 photoproduction in a unicellular green alga Chlamydomonas reinhardtii through removing O2 and activating hydrogenase but significantly impairs the activity of PSII, an electron source for H2 photoproduction. Here, a stepwise addition mode of total 13 mM NaHSO3, an optimal concentration for H2 photoproduction of C. reinhardtii identified in a previous one step addition method, significantly improved H2 photoproduction. Such improvement was believed to be the result of increased residual PSII activity in an anaerobic background, but was at least independent of two alternative electron sinks for H2 photoproduction, cyclic electron transport around PSI and CO2 assimilation. Based on the above results, we propose that increased residual PSII activity in an anaerobic environment is an efficient strategy to enhance H2 photoproduction in C. reinhardtii, and the stepwise NaHSO3 addition mode is a case study in the strategy.
Introduction
With the increasing awareness of fossil fuel depletion and global warming, efforts have been undertaken to develop clean and sustainable energy sources (McKendry, 2002). Molecular hydrogen (H2) is one of the potential future energy sources (Hansel and Lindblad, 1998; Momirlan and Veziroglu, 2002). C. reinhardtii, a unicellular green alga, has been recognized as an ideal system for sustainable H2 photoproduction under anaerobic conditions; however, this alga cannot efficiently and continuously produce H2 in an aerobic environment because its H2ase is extremely sensitive to O2 (Ghirardi et al., 1997). To activate H2ase for sustainable and efficient H2 photoproduction in C. reinhardtii, therefore, numerous strategies have been extensively developed mainly through engineering O2 tolerance in H2ase (Flynn et al., 2002; Liebgott et al., 2011; Wu et al., 2011) or decreasing O2 content around H2ase (Melis et al., 2000; Kruse et al., 2005; Surzycki et al., 2007; Wu et al., 2010; Xu et al., 2011; Jurado-Oller et al., 2015; Xiong et al., 2015; Shu et al., 2018). Meanwhile, our studies demonstrate that NaHSO3 addition strategy is capable of decreased the O2 content around H2ase, thereby activating the enzyme activity and promoting H2 photoproduction (Wang et al., 2010; Ma et al., 2011; Wei et al., 2017). This strategy can result in an approximately 10-fold or 200-fold increase in H2 photoproduction in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 (Wang et al., 2010) or the unicellular green alga C. reinhardtii (Ma et al., 2011; Wei et al., 2017), respectively. Despite these increases, this yield is still not sufficient to meet the requirements of industrial applications. Thus, extensive optimization of this NaHSO3 addition strategy is necessary to increase H2 photoproduction in C. reinhardtii further.
The yield of H2 photoproduction caused by sulfur deprivation is also not sufficient to meet the requirements of industrial applications. Under sulfur deprivation conditions, therefore, many strategies have been developed to improve the yield of H2 photoproduction in C. reinhardtii via metabolic and genetic engineering (for review, see Esquível et al., 2011; Dubini and Ghirardi, 2015). Among them, increased residual photosystem II (PSII) activity was found to play a vital role in efficient H2 photoproduction (Zhang et al., 2002; Kosourov et al., 2005; Kim et al., 2010; Volgusheva et al., 2013; Grewe et al., 2014; Steinbeck et al., 2015; Chen et al., 2016), since the PSII activity is significantly impaired by sulfur deprivation (Melis et al., 2000). Similarly, in the NaHSO3 addition background, the PSII activity is also significantly impaired (Wang et al., 2010). To test whether increased residual PSII activity in the NaHSO3 addition background can also enhance H2 photoproduction, we monitored the accumulated H2 level and residual PSII activity in the stepwise mode of total 13 mM NaHSO3, an optimal concentration for H2 production of C. reinhardtii identified in a previous one step addition method (Ma et al., 2011). We also measured the content of dissolved O2 and activities of two alternative electron sinks for H2 photoproduction, CET and CO2 assimilation, in the stepwise NaHSO3 addition mode. Our results demonstrate that the stepwise NaHSO3 addition mode evidently enhances the yield of H2 photoproduction in C. reinhardtii; such enhancement is mostly the result of increased residual PSII activity in an anaerobic environment, but is at least independent of two alternative electron sinks for H2 photoproduction.
Materials and Methods
Culture Conditions
Cells of C. reinhardtii (CC-503 strain) were cultured at 25°C in TAP medium (Harris, 1989). The medium was buffered with Tris–HCl (20 mM; pH 7.3), bubbled with air under continuous illumination with cool-white fluorescent lamps (40 μmol photons m-2s-1), and inoculated with approximately 8.1 × 104 cells mL-1 of C. reinhardtii (inoculum size, 1%).
Sample Preparation and NaHSO3 Addition
Cells of C. reinhardtii were continuously illuminated by growth light of 40 μmol photons m-2s-1 and were cultured in 0.5 L of TAP medium for 2 days with bubble aeration (A750 = 0.8–1.0), after which a fixed volume of cells containing 300 μg of Chl was transferred to 60 mL serum bottles (30 mL head space and 30 mL cells) with rubber seals. After cells were statically pre-cultured under continuous illumination of 200 μmol photons m-2s-1 for 36 h, total 13 mM of NaHSO3 was directly or step by step added to the serum bottles, as indicated in Figures 1, 2, Supplementary Figure S1, and described in Table 1, with Lin of 5 mM (final concentration) or AA of 10 μM (final concentration) or GA of 2 mM (final concentration) or DCMU of 20 μM (final concentration) or not. Subsequently, the cells were still illuminated at 200 μmol photons m-2s-1 or were incubated in the dark to induce the production of H2.

FIGURE 1. A stepwise NaHSO3 addition mode significantly increases (A) H2 photoproduction, and (B) in vivo and (C) in vitro H2ase activity in C. reinhardtii. After cells were statically pre-cultured under continuous illumination of 200 μmol photons m-2s-1 for 36 h, total 13 mM of NaHSO3 was directly or step by step added to the serum bottles, as indicated by arrows 1–3 and described in Table 1. Values are means ± SD (n = 5).
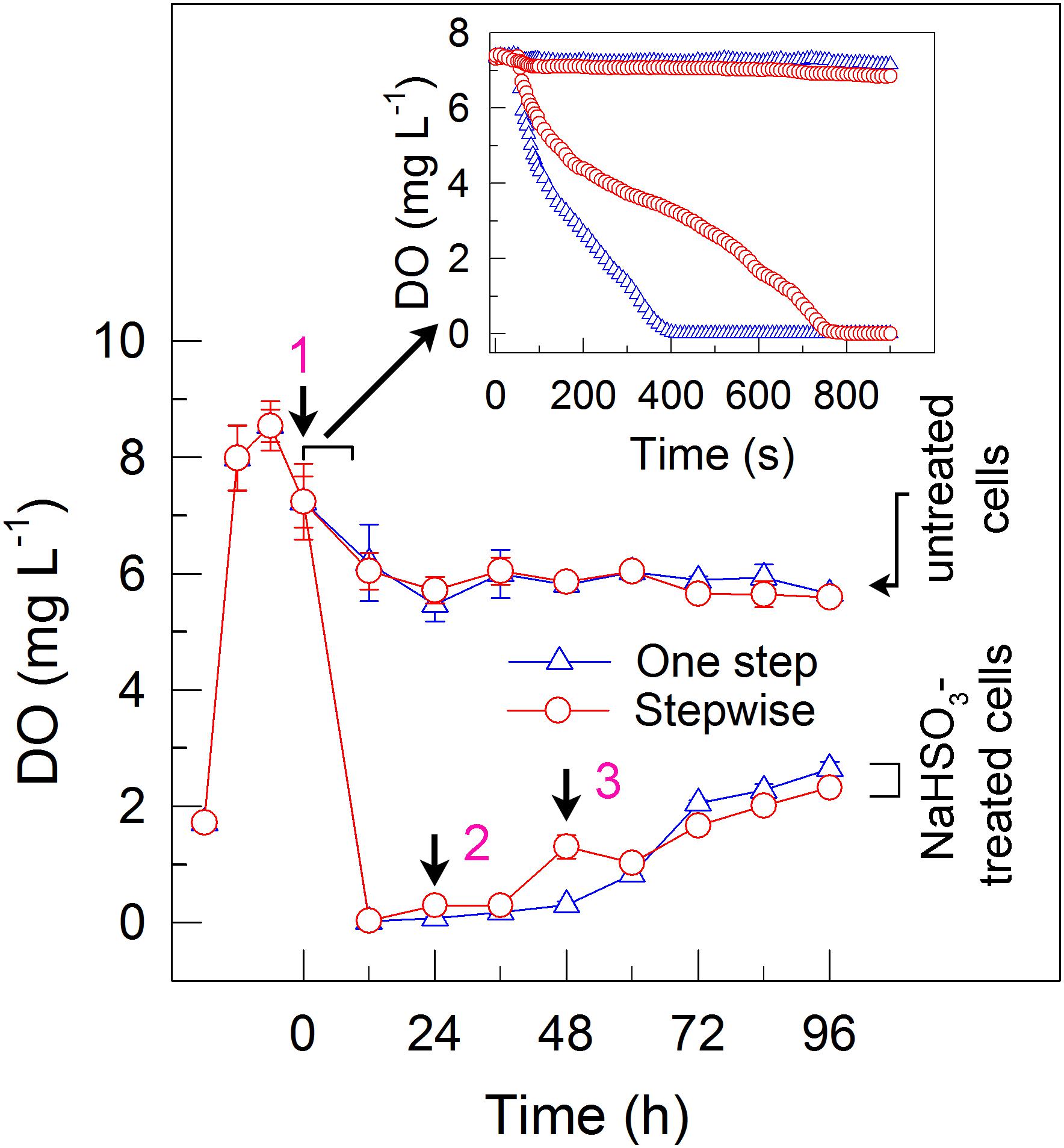
FIGURE 2. A stepwise NaHSO3 addition mode influences the content of dissolved oxygen (DO) in C. reinhardtii. Pre-culture conditions of cells and addition modes of NaHSO3 are shown in the legend of Figure 1. Values are means ± SD (n = 5).
Monitoring H2 Photoproduction
At predetermined time intervals, 200 μL of gas samples were withdrawn from the bottles using a gas-tight syringe and injected into a gas chromatograph (Agilent 7890A; Agilent Technologies Inc., United States) with a thermal conductivity detector for determining the concentrations of H2, O2, and N2 simultaneously. The column of the gas chromatograph was a molecular sieve column (type 5Å; 2 m × 1/8 mm), and argon was used as the carrier gas.
H2ase Activity Assay
In vivo and in vitro H2ase activity was monitored as described earlier (Ma et al., 2011; Wei et al., 2013, 2017) with some modifications. In brief, 1 mL cell suspension samples upon exposure to 200 μmol photons m-2s-1 were anaerobically withdrawn from the 60 mL serum bottles at designated time points (see Figures 1B,C) and then injected into 10 mL glass vials. To measure in vivo H2ase activity, the cell suspension samples were immediately purged with argon gas for 1 min to eliminate the inhibitory effect of O2 on the H2ase activity. The cell suspension samples were then placed in a 25°C water bath for 1 h and shaken continuously (150 rpm) whilst exposed to a constant light of 200 μmol photons m-2s-1. To measure in vitro H2ase activity, we used vials containing 1 mL of 10 mM oxidized MV prepared in O2-free 50 mM Tris buffer (for pH 7.1–9.0) and 0.2% (w/v) Triton X-100. The reaction was started when MV was reduced by the addition of 100 μL of 100 mM anaerobic sodium dithionite in 0.03 N NaOH. This assay was performed at 37°C in the dark for 20 min. We determined the amount of H2 produced in the headspace of the glass vial by gas chromatography, and the rate of H2 production was calculated on the basis of the total Chl content in the glass vial, unless otherwise indicated.
Dissolved Oxygen Measurement
Dissolved oxygen was monitored as described earlier (Wei et al., 2017). In brief, a DO meter (Orion Star A213, Thermo Scientific, Untied States) was used to monitor the DO attenuation process after the addition of NaHSO3 to the cell suspension cultures of C. reinhardtii. The DO meter was corrected before each measurement. The DO meter probe was placed in the middle of the cell suspension cultures and the data were recorded at several designated time points.
Chl Fluorescence and P700 Analysis
The yields of Chl fluorescence at a steady-state of electron transport were measured at room temperature using a Dual-PAM-100 monitoring system (Walz, Effeltrich, Germany) equipped with an ED-101US/MD unit (Schreiber et al., 1986; Ma et al., 2008; Wei et al., 2013, 2017). Minimal fluorescence at open PSII centers in the dark-adapted state (Fo) was excited by a weak measuring light (650 nm) at a photon flux density of 0.05–0.15 μmol photons m-2s-1. A saturating pulse of red light (600 ms, 10,000 μmol photons m-2s-1) was applied to determine the maximal fluorescence at closed PSII centers in the dark-adapted state (Fm). Fv/Fm was evaluated as (Fm-Fo)/Fm (Kitajima and Butler, 1975; Wei et al., 2013, 2017).
The reduction of P700+ in darkness was measured with the aforementioned Dual-PAM-100 fluorometer by monitoring absorbance changes at 830 nm and using 875 nm as a reference. Cells were kept in the dark for 2 min, and 10 μM of DCMU was added to the cell suspension cultures prior to the measurement. The P700 was oxidized by far-red light with a maximum at 720 nm from a light-emitting diode lamp for 30 s, and the subsequent re-reduction of P700+ in the dark was monitored and its half-time was calculated.
Oxygen Evolution Activity
Oxygen production in intact C. reinhardtii cells by photosynthesis was determined at 25°C by monitoring the changes in O2 levels with a Clark-type oxygen electrode (Hansatech Instruments, King’s Lynn, United Kingdom). Prior to the measurements, 10 mM of NaHCO3 was added to the cell suspension cultures. The intensity of light used for the measurements was 1,000 μmol photons m-2s-1.
Results
A Stepwise NaHSO3 Addition Mode Significantly Increases the Yield of H2 Photoproduction in C. reinhardtii
To test whether a stepwise NaHSO3 addition mode can enhance the yield of H2 photoproduction, we monitored accumulated H2 amounts in the one step addition method and stepwise mode of total 13 mM NaHSO3 (hereafter one step method and stepwise mode, respectively; see Table 1), an optimal concentration for H2 photoproduction of C. reinhardtii identified in a previous one step method (Ma et al., 2011). Compared to the one step method, stepwise mode evidently enhanced the yield of H2 photoproduction (Figure 1A and Table 2). The H2 level in stepwise mode was approximately 1.5 times greater than that in one step method and, approximately 350 times greater than that in untreated cells (Figure 1A and Table 2). This was confirmed by the results of in vivo (Figure 1B) and in vitro (Figure 1C) H2ase activity. We therefore conclude that the stepwise mode considerably improves H2 photoproduction in the green alga C. reinhardtii.
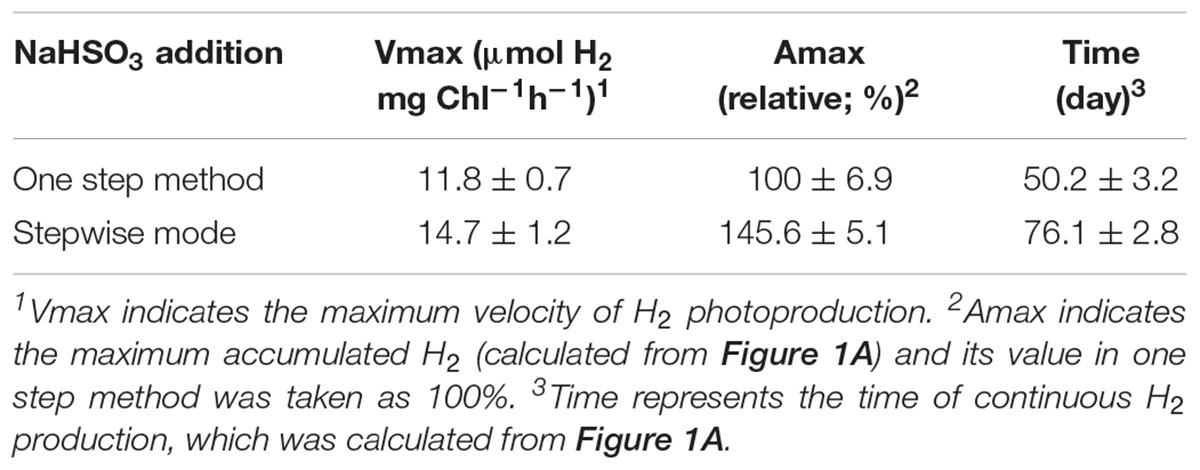
TABLE 2. Comparison of H2 photoproduction characteristics in C. reinhardtii between one step method and stepwise mode.
The Stepwise Mode Can Also Establish an Anaerobic Environment
To elucidate the mechanism underlying the increase in the H2 yield under stepwise mode, we monitored the dissolved O2 (DO) content in the cell suspension cultures of C. reinhardtii. The results indicated that addition of total 13 mM NaHSO3 to the cell suspension cultures in both the one step method and stepwise mode can similarly create an anaerobic environment (Figure 2), although the stepwise mode was slightly slow to generate an anaerobic environment when compared to the one step method (insert in Figure 2). It is worthy of note that, when an initial concentration of NaHSO3 in stepwise mode was less than or equal to 7 mM, the cell suspension cultures did not enter or maintain an anaerobic environment, which evidently suppressed the increase of H2 photoproduction in the stepwise mode (data not shown). Based on the above results, we propose that the stepwise mode is necessary to operate in an anaerobic environment as an efficient strategy for H2 photoproduction in C. reinhardtii.
The Stepwise Mode Maintains a Relatively High Residual Activity of Electron Source for H2 Photoproduction
To understand why the stepwise mode can increase the yield of H2 photoproduction, we measured the activity of PSII, an electron source for H2 photoproduction. After the addition of total 13 mM NaHSO3 to the cell suspension cultures, the residual activity of PSII was much higher in stepwise mode than that in one step method, as evaluated by the Fv/Fm values (Figure 3). This was supported by the results that the stepwise mode maintained a slightly high DO content at an efficient H2 production stage when compared to the one step method (Figure 2). This implies that the relatively high PSII activity under anaerobic conditions is an important reason for improved H2 photoproduction in the stepwise mode.
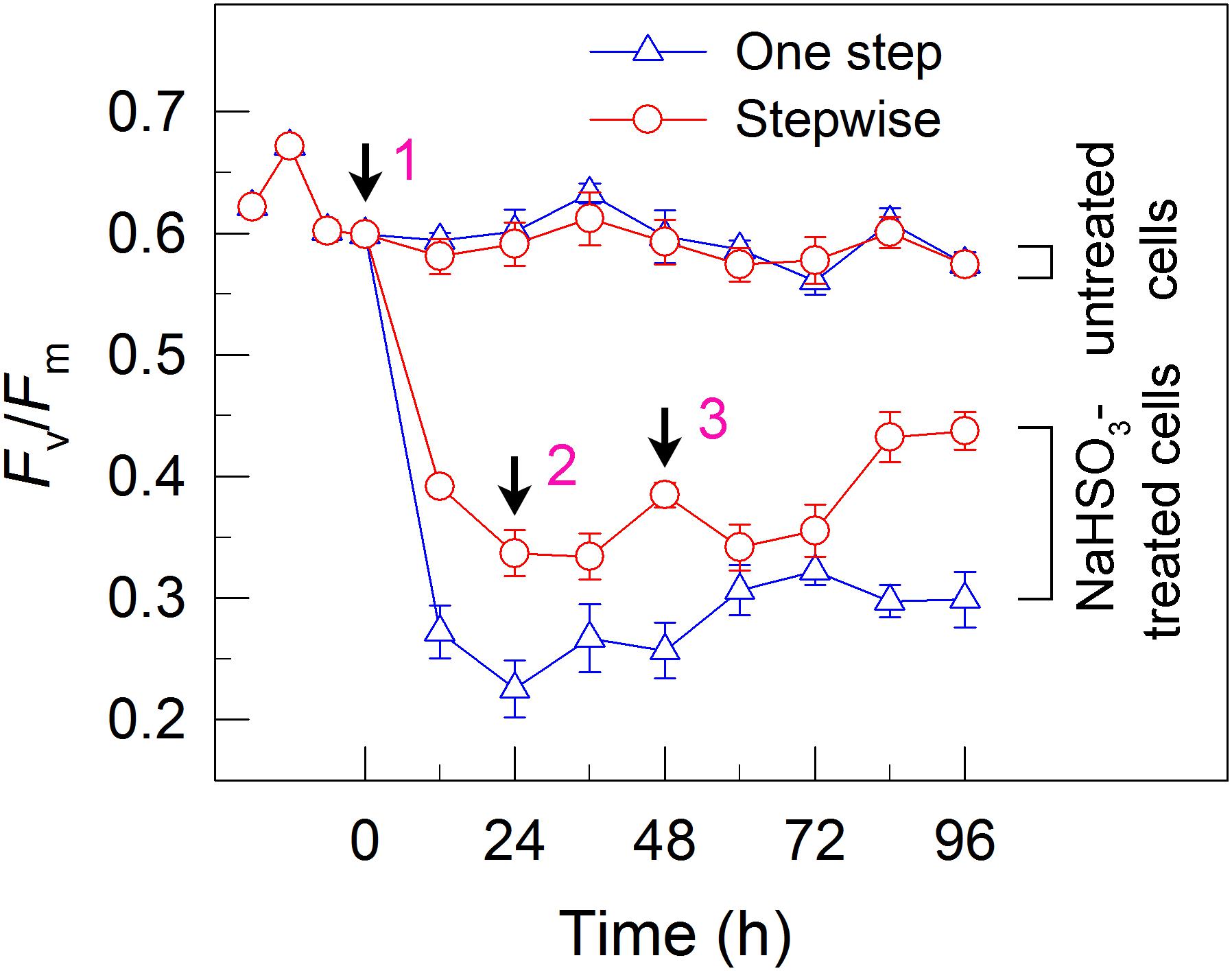
FIGURE 3. A stepwise addition mode alleviates the inhibitory effects of NaHSO3 on PSII activity in C. reinhardtii. The Chl concentration was adjusted to 10 μg mL-1 before measurement. PSII activity was evaluated by the Fv/Fm values. Values are means ± SD (n = 5).
The Stepwise Mode Slightly Enhances the Activities of Two Alternative Electron Sinks for H2 Photoproduction
We also measured the activities of CET and CO2 assimilation, two alternative electron sinks for H2 photoproduction. The rates of CET and CO2 assimilation were slightly faster in the stepwise mode than those in the one step method, as estimated by the rate of re-reduction of P700+ (Figure 4), and photosynthetic production of O2 with NaHCO3 as an artificial electron acceptor (Figure 5), respectively. It appears plausible that at least the two alternative electron sinks for H2 photoproduction do not contribute to enhance the photoproduction of H2 in the stepwise mode. If this possibility is true, an increase in H2 photoproduction caused by impaired the activity of either CET or CO2 assimilation will be higher in the stepwise mode than that in the one step method. The results shown in Figure 6 support our hypothesis that the increase in H2 photoproduction was slightly higher in the stepwise mode than that in the one step method in the presence of either AA that specifically inhibits the CET activity (Tagawa et al., 1963) or GA that disrupts the Calvin–Benson cycle activity via inhibiting the phosphoribulokinase (Rühle et al., 2008). Taking all these results together, we may conclude that in the anaerobic background, increased residual PSII activity can significantly enhance the yield of H2 photoproduction in C. reinhardtii.
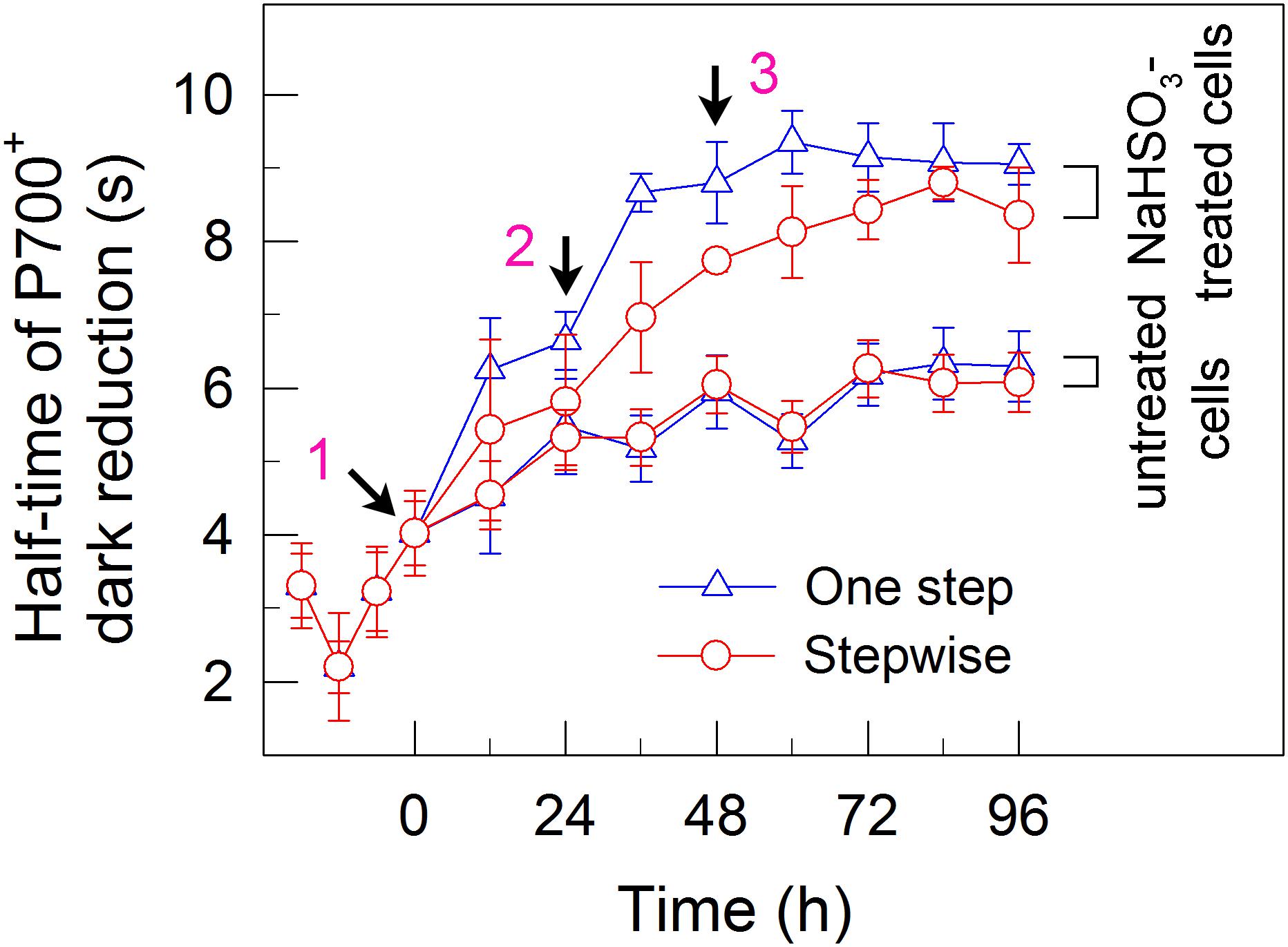
FIGURE 4. A stepwise addition mode slightly alleviates the inhibitory effects of NaHSO3 on cyclic electron transport around PSI in C. reinhardtii. The rate of cyclic electron transport around PSI was judged by half-time of P700+ dark reduction. Values are means ± SD (n = 5).
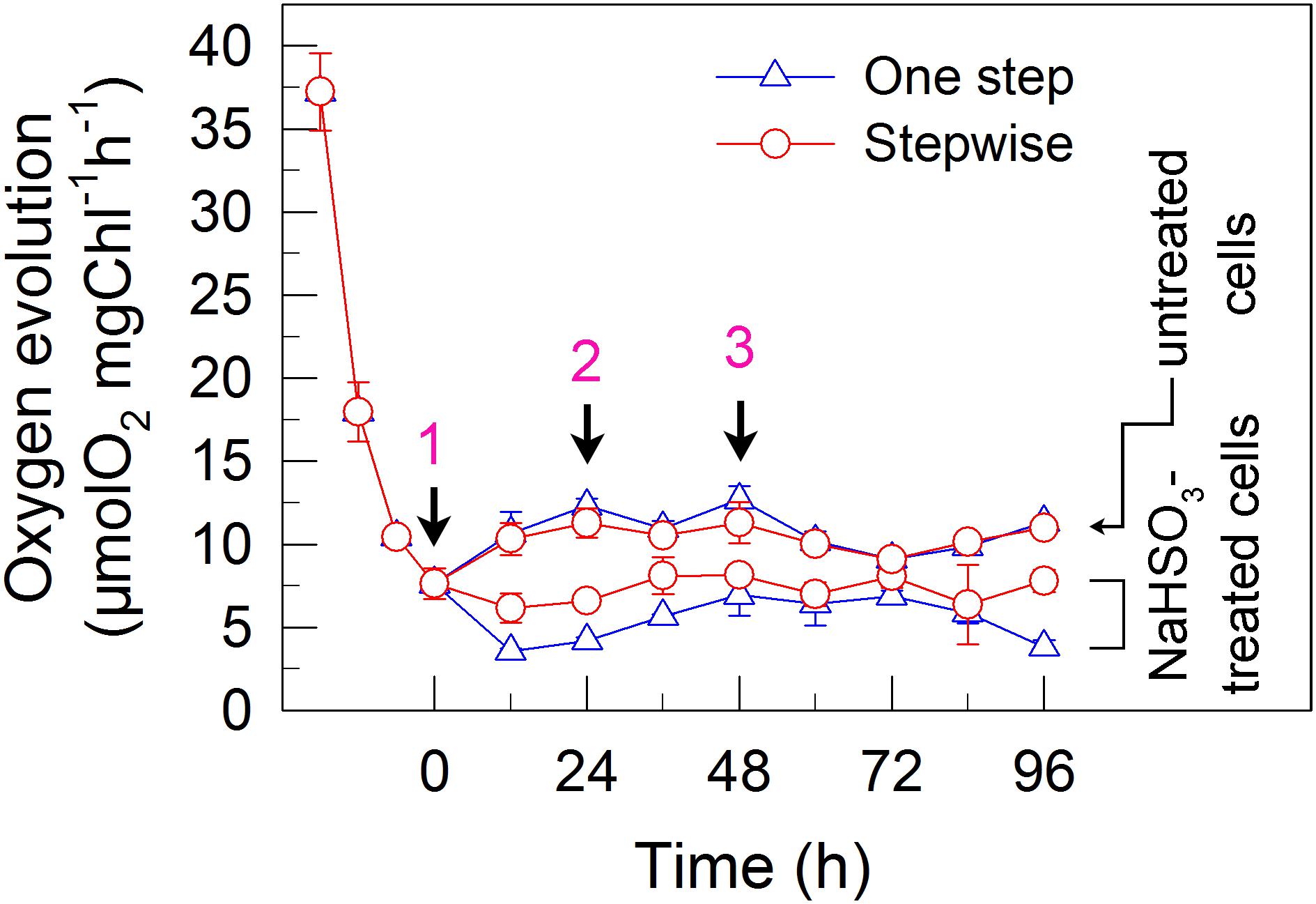
FIGURE 5. A stepwise addition mode slightly alleviates the inhibitory effects of NaHSO3 on CO2 assimilation in C. reinhardtii. Activity of CO2 assimilation was assessed by photosynthetic production of O2 with NaHCO3 as an artificial electron acceptor. Values are means ± SD (n = 5).
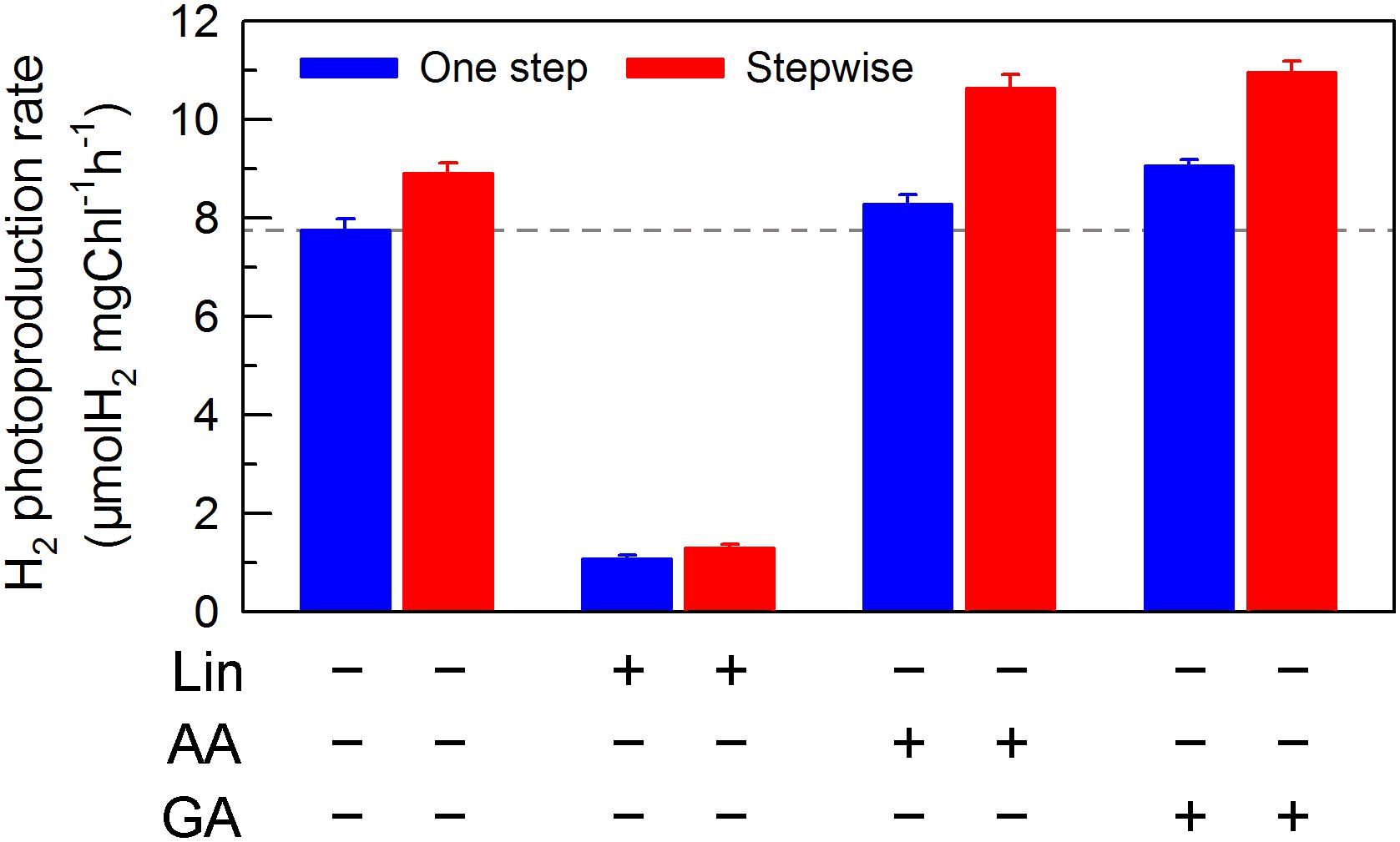
FIGURE 6. Comparison of the H2 photoproduction in C. reinhardtii between one step method and stepwise mode with multiple inhibitors. After cells were statically pre-cultured under continuous illumination of 200 μmol photons m-2s-1 for 36 h, NaHSO3 was added as described in the legend for Figure 1, as well as several inhibitors, lincomycin (Lin; 5 mM), antimycin A (AA; 10 μM), and glycolaldehyde (GA; 2 mM) were also added to the serum bottles, respectively. Values are means ± SD (n = 5).
If this conclusion is true, impaired PSII activity in an anaerobic environment created by NaHSO3 addition will inevitably decrease the yield of H2 photoproduction in C. reinhardtii at a significant level. As expected, the H2 photoproduction rate was significantly decreased in the presence of Lin, which impairs the PSII activity via inhibiting the D1 protein synthesis (Vavilin et al., 1995), regardless of either one step method or stepwise mode (Figure 6). This consolidates our conclusion that increased residual PSII activity in an anaerobic environment is an efficient strategy to improve H2 photoproduction in C. reinhardtii and the stepwise NaHSO3 addition mode is a case study in this strategy.
Discussion
Whether NaHSO3 addition promotes photosynthesis or H2 photoproduction depends on its concentrations: NaHSO3 in a low amount improves photosynthesis (Wang et al., 2003) but in a moderate amount can enhance H2 photoproduction (Wang et al., 2010; Ma et al., 2011). Wang et al. (2003) demonstrate that a low amount (100 μM) of NaHSO3 increases cyclic photophosphorylation and consequently improves photosynthesis via optimizing the ATP/NADPH ratio. By contrast, Wei et al. (2017) demonstrate that a moderate amount (13 mM) of NaHSO3 can remove O2 efficiently through the reaction of bisulfite with superoxide anion produced at the acceptor side of PSI, especially under sufficient light conditions, consequently activates H2ase and promotes H2 photoproduction. The results of this study indicate that a moderate amount of NaHSO3 under a stepwise addition mode can quickly establish an anaerobic environment (Figure 2) and significantly improves H2 photoproduction in a unicellular green alga C. reinhardtii (Table 1 and Figure 1). Such improvement is at least independent of two alternative electron sinks for H2 photoproduction, CET (Figures 4, 6), and CO2 assimilation (Figures 5, 6) and, most likely the result of maintained a relatively high electron source for H2 photoproduction, PSII activity (Figures 3, 6).
Under a photon flux density of 200 μmol photons m-2s-1, the Mehler reaction is usually considered to also be an important alternative electron sink for H2 photoproduction. However, we found that the Mehler reaction is almost absent in NaHSO3 addition strategy, regardless of either one step method or stepwise mode (data not shown), possibly because addition of NaHSO3 to the cell suspension cultures quickly results in entering of cells to an anaerobic environment (less than 800 s) (Figure 2). Therefore, the improvement of H2 photoproduction in C. reinhardtii by a moderate amount of NaHSO3 under a stepwise addition mode is also independent of a third alternative electron sink for H2 photoproduction, Mehler reaction, and consolidating the above mentioned possibility that such improvement is the result of increased residual PSII activity, an electron source for H2 photoproduction.
Based on different sources of electrons to H2ase, three pathways for H2 production have been identified in C. reinhardtii. Their sources of electrons to H2ase come from water photolysis via PSII (Melis et al., 2000; Kosourov et al., 2003), NADPH through type II NAD(P)H dehydrogenase (Baltz et al., 2014) and the fermentative degradation of endogenous compounds (Gfeller and Gibbs, 1985), respectively. We observed that production of H2 under photon flux densities of 200 μmol photons m-2s-1 by NaHSO3 addition was almost completely suppressed in cells incubated in the dark (Supplementary Figures S1A,B) or treated with DCMU (Supplementary Figures S1A,C). A quick establishment of anaerobic environment by NaHSO3 addition (Figure 2) suppresses the acetate uptake (Jurado-Oller et al., 2015) and impairs the mitochondrial respiratory electron transport chain function. Taking all these results together, we propose that in the NaHSO3 addition strategy, the source of electrons for H2 production predominantly, if not totally, comes from water photolysis via PSII, regardless of either the one step method or the stepwise mode.
The results of this study further indicate that the stepwise mode increased the maximum accumulated H2 levels, produced a higher maximum velocity of H2 photoproduction, and prolonged the time length of H2 photoproduction when compared to the one step method (Table 2). We thus propose that the stepwise mode developed in this study is an efficient and sustained strategy for improving H2 photoproduction in the green alga C. reinhardtii.
Although a moderate amount of NaHSO3 can remove efficiently O2, the activity of PSII, an electron source for H2 photoproduction, is also significantly impaired (Wang et al., 2010; Ma et al., 2011; Wei et al., 2017). The results of this study observe that in the anaerobic background, a stepwise mode maintains a relatively high PSII activity (Figure 3) and consequently promotes H2 photoproduction (Figure 1). The cause and effect of PSII activity and H2 photoproduction is also present in the sulfur-deprived strategy (Zhang et al., 2002; Kosourov et al., 2005; Kim et al., 2010; Volgusheva et al., 2013; Grewe et al., 2014; Steinbeck et al., 2015; Chen et al., 2016) but the reasons why H2 photoproduction is terminated in sulfur deprivation and NaHSO3 addition strategies are distinctly different. It is known that H2 photoproduction is terminated in the sulfur deprivation strategy because of cell death (Nguyen et al., 2008) and in the NaHSO3 addition strategy because of conversion of too much bisulfite to sulfate (Wei et al., 2017). It is worthy of note that the relationship between H2 production and biomass accumulation in sulfur deprivation and NaHSO3 addition strategies is also distinctly different. Regardless of either one step method or stepwise mode, the simultaneous production of H2 and biomass is present in NaHSO3 addition strategy, as observed in mixotrophic nutrient-replete cultures under low light conditions (Jurado-Oller et al., 2015), but is absent in sulfur deprivation strategy. Therefore, it appears reasonable that improved PSII activity in the NaHSO3 background is considered to be a better strategy to meet future application requirements in comparison with that in the sulfur-deprived background.
Author Contributions
WM designed and supervised the experiments. XL, BF, and ZR performed the experiments and analyzed the data. LW and WM analyzed and interpreted the data and wrote the article.
Funding
This work was supported by the Shanghai Science and Technology Committee (Grant No. 17070502900) and National Natural Science Foundation of China (Grant Nos. 31570235 and 31770259).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01532/full#supplementary-material
Abbreviations
AA, antimycin A; CET, cyclic electron transport around PSI; Chl, chlorophyll; C. reinhardtii, Chlamydomonas reinhardtii; DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea; DO, dissolved oxygen; Fv/Fm, maximal quantum yield of PSII; GA, glycolaldehyde; H2ase, hydrogenase; Lin, lincomycin; MV, methyl viologen; one step method, a one step addition method of NaHSO3; stepwise mode, a stepwise addition mode of NaHSO3; TAP, Tris-acetate-phosphate.
References
Baltz, A., Dang, K. V., Beyly, A., Auroy, P., Richaud, P., Cournac, L., et al. (2014). Plastidial expression of type II NAD(P)H dehydrogenase increases the reducing state of plastoquinones and hydrogen photoproduction rate by the indirect pathway in Chlamydomonas reinhardtii. Plant Physiol. 165, 1344–1352. doi: 10.1104/pp.114.240432
Chen, M., Zhang, J., Zhao, L., Xing, J., Peng, L., Kuang, T., et al. (2016). Loss of algal proton gradient regulation 5 increases reactive oxygen species scavenging and H2 evolution. J. Integr. Plant Biol. 58, 943–946. doi: 10.1111/jipb.12502
Dubini, A., and Ghirardi, M. L. (2015). Engineering photosynthetic organisms for the production of biohydrogen. Photosynth. Res. 123, 241–253. doi: 10.1007/s11120-014-9991-x
Esquível, M. G., Amaro, H. M., Pinto, T. S., Fevereiro, P. S., and Malcata, F. X. (2011). Efficient H2 production via Chlamydomonas reinhardtii. Trends Biotechnol. 29, 595–600. doi: 10.1016/j.tibtech.2011.06.008
Flynn, T., Ghirardi, M. L., and Seibert, M. (2002). Accumulation of O2-tolerant phenotypes in H2-producing strains of Chlamydomonas reinhardtii by sequential applications of chemical mutagenesis and selection. Int. J. Hydrogen Energy 27, 1421–1430. doi: 10.1016/S0360-3199(02)00117-9
Gfeller, R. P., and Gibbs, M. (1985). Fermentative metabolism of Chlamydomonas reinhardtii: II. Role of plastoquinone. Plant Physiol. 77, 509–511. doi: 10.1104/pp.77.2.509
Ghirardi, M. L., Togasaki, R. K., and Seibert, M. (1997). Oxygen sensitivity of algal H2 production. Appl. Microbiol. Biotechnol. 63, 141–151. doi: 10.1007/BF02920420
Grewe, S., Ballottari, M., Alcocer, M., D’Andrea, C., Blifernez-Klassen, O., Hankamer, B., et al. (2014). Light-harvesting complex protein LHCBM9 is critical for photosystem II activity and hydrogen production in Chlamydomonas reinhardtii. Plant Cell 26, 1598–1611. doi: 10.1105/tpc.114.124198
Hansel, A., and Lindblad, P. (1998). Towards optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. Appl. Microbiol. Biotechnol. 50, 153–160. doi: 10.1007/s002530051270
Harris, E. H. (1989). The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego, CA: Academic Press.
Jurado-Oller, J. L., Dubini, A., Galván, A., Fernández, E., and González-Ballester, D. (2015). Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol. Biofuels 8:149. doi: 10.1186/s13068-015-0341-9
Kim, J. P., Kim, K. R., Choi, S. P., Han, S. J., Kim, M. S., and Sim, S. J. (2010). Repeated production of hydrogen by sulfate re-addition in sulfur deprived culture of Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 35, 13387–13391. doi: 10.1016/j.ijhydene.2009.11.113
Kitajima, M., and Butler, W. (1975). Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 376, 105–115. doi: 10.1016/0005-2728(75)90209-1
Kosourov, S., Makarova, V., Fedorov, A. S., Tsygankov, A., Seibert, M., and Ghirardi, M. L. (2005). The effect of sulfur re-addition on H2 photoproduction by sulfur-deprived green algae. Photosynth. Res. 85, 295–305. doi: 10.1007/s11120-005-5105-0
Kosourov, S., Seibert, M., and Ghirardi, M. L. (2003). Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 44, 146–155. doi: 10.1093/pcp/pcg020
Kruse, O., Rupprecht, J., Bader, K. P., Thomas-Hall, S., Schenk, P. M., Finazzi, G., et al. (2005). Improved photobiological H2 production in engineered green algal cells. J. Biol. Chem. 280, 34170–34177. doi: 10.1074/jbc.M503840200
Liebgott, P. P., Dementin, S., Léger, C., and Rousset, M. (2011). Towards engineering O2-tolerance in [Ni–Fe] hydrogenases. Energy Environ. Sci. 4, 33–41. doi: 10.1039/C0EE00093K
Ma, W., Chen, M., Wang, L., Wei, L., and Wang, Q. (2011). Treatment with NaHSO3 greatly enhances photobiological H2 production in the green alga Chlamydomonas reinhardtii. Bioresour. Technol. 102, 8635–8638. doi: 10.1016/j.biortech.2011.03.052
Ma, W., Wei, L., and Wang, Q. (2008). The response of electron transport mediated by active NADPH dehydrogenase complexes to heat stress in the cyanobacterium Synechocystis 6803. Sci. China C Life Sci. 51, 1082–1087. doi: 10.1007/s11427-008-0139-0
McKendry, P. (2002). Energy production from biomass (Part 1): overview of biomass. Bioresour. Technol. 83, 37–46. doi: 10.1016/S0960-8524(01)00118-3
Melis, A., Zhang, L., Forestier, M., Ghirardi, M. L., and Seibert, M. (2000). Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 122, 127–136. doi: 10.1104/pp.122.1.127
Momirlan, M., and Veziroglu, T. N. (2002). Current status of hydrogen energy. Renew. Sustain. Energ. Rev. 6, 141–179. doi: 10.1016/S1364-0321(02)00004-7
Nguyen, A. V., Thomas-Hall, S. R., Malnoë, A., Timmins, M., Mussgnug, J. H., Rupprecht, J., et al. (2008). Transcriptome for photobiological hydrogen production induced by sulfur deprivation in the green alga Chlamydomonas reinhardtii. Eukaryot. Cell 7, 1965–1979. doi: 10.1128/EC.00418-07
Rühle, T., Hemschemeier, A., Melis, A., and Happe, T. (2008). A novel screening protocol for the isolation of hydrogen producing Chlamydomonas reinhardtii strains. BMC Plant Biol. 8:107. doi: 10.1186/1471-2229-8-107
Schreiber, U., Schliwa, U., and Bilger, W. (1986). Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62. doi: 10.1007/BF00024185
Shu, L., Xiong, W., Shao, C., Huang, T., Duan, P., Liu, K., et al. (2018). Improvement in the photobiological hydrogen production of aggregated Chlorella by dimethyl sulfoxide. Chembiochem 19, 669–673. doi: 10.1002/cbic.201700637
Steinbeck, J., Nikolova, D., Weingarten, R., Johnson, X., Richaud, P., Peltier, G., et al. (2015). Deletion of Proton Gradient Regulation 5 (PGR5) and PGR5-Like 1 (PGRL1) proteins promote sustainable light-driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front. Plant Sci. 6:892. doi: 10.3389/fpls.2015.00892
Surzycki, R., Cournac, L., Peltier, G., and Rochaix, J. D. (2007). Potential for hydrogen production with inducible chloroplast gene expression in Chlamydomonas. Proc. Natl. Acad. Sci. U.S.A. 104, 17548–17553. doi: 10.1073/pnas.0704205104
Tagawa, K., Tsujimoto, H. Y., and Arnon, D. I. (1963). Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 49, 567–572. doi: 10.1073/pnas.49.4.567
Vavilin, D. V., Tyystjärvi, E., and Aro, E. M. (1995). In search of a reversible stage of photoinhibition in a higher plant: no changes in the amount of functional photosystem II accompany relaxation of variable fluorescence after exposure of lincomycin-treated Cucurbita pepo leaves to high light. Photosynth. Res. 45, 239–247. doi: 10.1007/BF00015564
Volgusheva, A., Styring, S., and Mamedov, F. (2013). Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 110, 7223–7228. doi: 10.1073/pnas.1220645110
Wang, H., Mi, H., Ye, J., Deng, Y., and Shen, Y. (2003). Low concentrations of NaHSO3 increase cyclic photophosphorylation and photosynthesis in cyanobacterium Synechocystis PCC6803. Photosynth. Res. 75, 151–159. doi: 10.1023/A:1022813402265
Wang, L., Chen, M., Wei, L., Gao, F., Lv, Z., Wang, Q., et al. (2010). Treatment with moderate concentrations of NaHSO3 enhances photobiological H2 production in the cyanobacterium Anabaena sp. strain PCC 7120. Int. J. Hydrogen Energy 35, 12777–12783. doi: 10.1016/j.ijhydene.2010.08.115
Wei, L., Li, X., Yi, J., Yang, Z., Wang, Q., and Ma, W. (2013). A simple approach for the efficient production of hydrogen from Taihu Lake Microcystis spp. blooms. Bioresour. Technol. 139, 136–140. doi: 10.1016/j.biortech.2013.04.026
Wei, L., Yi, J., Wang, L., Huang, T., Gao, F., Wang, Q., et al. (2017). Light intensity is important for hydrogen production in NaHSO3-treated Chlamydomonas reinhardtii. Plant Cell Physiol. 58, 451–457. doi: 10.1093/pcp/pcw216
Wu, S., Huang, R., Xu, L., Yan, G., and Wang, Q. (2010). Improved hydrogen production with expression of hemH and lba genes in chloroplast of Chlamydomonas reinhardtii. J. Biotechnol. 146, 120–125. doi: 10.1016/j.jbiotec.2010.01.023
Wu, X., Liang, Y., Li, Q., Zhou, J., and Long, M. (2011). Characterization and cloning of oxygen-tolerant hydrogenase from Klebsiella oxytoca HP1. Res. Microbiol. 162, 330–336. doi: 10.1016/j.resmic.2010.12.003
Xiong, W., Zhao, X., Zhu, G., Shao, C., Li, Y., Ma, W., et al. (2015). Silicification-induced cell aggregation for the sustainable production of H2 under aerobic conditions. Angew. Chem. Int. Ed. Engl. 54, 11961–11965. doi: 10.1002/anie.201504634
Xu, F. Q., Ma, W. M., and Zhu, X. G. (2011). Introducing pyruvate oxidase into the chloroplast of Chlamydomonas reinhardtii increases oxygen consumption and promotes hydrogen production. Int. J. Hydrogen Energy 36, 10648–10654. doi: 10.1016/j.ijhydene.2011.05.130
Keywords: NaHSO3, stepwise mode, anaerobic environment, PSII activity, H2 photoproduction, Chlamydomonas reinhardtii
Citation: Wei L, Li X, Fan B, Ran Z and Ma W (2018) A Stepwise NaHSO3 Addition Mode Greatly Improves H2 Photoproduction in Chlamydomonas reinhardtii. Front. Plant Sci. 9:1532. doi: 10.3389/fpls.2018.01532
Received: 27 February 2018; Accepted: 28 September 2018;
Published: 31 October 2018.
Edited by:
Yandu Lu, Hainan University, ChinaReviewed by:
Emilio Fernandez, Universidad de Córdoba, SpainHongmin Qin, Texas A&M University, United States
Weichao Huang, Carnegie Institution for Science (CIS), United States
Copyright © 2018 Wei, Li, Fan, Ran and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weimin Ma, d21hQHNobnUuZWR1LmNu
†These authors have contributed equally to this work
 Lanzhen Wei
Lanzhen Wei Xin Li
Xin Li Baoqiang Fan
Baoqiang Fan Zhaoxing Ran
Zhaoxing Ran Weimin Ma
Weimin Ma