- 1Key Laboratory of Biology and Genetic Improvement of Maize in Southwest Region, Maize Research Institute, Sichuan Agricultural University, Chengdu, China
- 2Research Center of Tumofous Stem Mustard, Chongqing Yudongnan Academy of Agricultural Sciences, Chongqing, China
- 3Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, China
Stalk lodging resistance, which is mainly measured by stem diameter (SD), stalk bending strength (SBS), and rind penetrometer resistance (RPR) in maize, seriously affects the yield and quality of maize (Zea mays L.). To dissect its genetic architecture, in this study multi-locus genome-wide association studies for stalk lodging resistance-related traits were conducted in a population of 257 inbred lines, with tropical, subtropical, and temperate backgrounds, genotyped with 48,193 high-quality single nucleotide polymorphisms. The analyses of phenotypic variations for the above traits in three environments showed high broad-sense heritability (0.679, 0.720, and 0.854, respectively). In total, 423 significant Quantitative Trait Nucleotides (QTNs) were identified by mrMLM, FASTmrEMMA, ISIS EM-BLASSO, and pLARmEB methods to be associated with the above traits. Among these QTNs, 29, 34, and 48 were commonly detected by multiple methods or across multiple environments to be related to SD, SBS, and RPR, respectively. The superior allele analyses in 30 elite lines showed that only eight lines contained more than 50% of the superior alleles, indicating that stalk lodging resistance can be improved by the integration of more superior alleles. Among sixty-three candidate genes of the consistently expressed QTNs, GRMZM5G856734 and GRMZM2G116885, encoding membrane steroid-binding protein 1 and cyclin-dependent kinase inhibitor 1, respectively, possibly inhibit cell elongation and division, which regulates lodging resistance. Our results provide the further understanding of the genetic foundation of maize lodging resistance.
Introduction
Lodging is one of the most important factors threatening grain yield in maize, and can result in reduced photosynthesis, nutrient transportation, and grain quality (Remison and Dele Akinleye, 1978). The annual yield losses caused by lodging are approximately 5–20% globally (Flintgarcia et al., 2003). In some areas where strong wind and heavy rain occur frequently, the risk of lodging will significantly increase (Adelana, 1980). Some properties of the stem itself are also strongly associated with lodging, such as the structure and mechanical strength of the stem, and the number of vascular bundles (Xu et al., 2017). In addition, Tesso and Ejeta (2011) showed that stalk rot disease can reduce stem strength, which further leads to lodging.
The most direct way to improve breeding populations for quantitative traits is phenotypic selection, where the frequency of favorable alleles is increased within a population over cycles of selection. Previous studies on crop morphological traits showed that plant stem diameter (SD), stalk bending strength (SBS), and rind penetrometer resistance (RPR) are closely associated with stalk lodging in the field (Kashiwagi et al., 2008; Hu et al., 2012, 2013). Furthermore, these three traits are significantly negatively correlated with stalk lodging rate in the field (Ling, 2008). The method for testing RPR involves the use of an electronic rind penetrometer to penetrate the rind of the maize stalk, and the maximum value of penetration is then indicated on the screen of the instrument (Sibale et al., 1992). This method does not affect maize seedling growth.
Genome-Wide Association Study (GWAS) is a very powerful tool for dissecting the genetic basis of complex traits (Korte and Farlow, 2013). To date, GWAS has been used to analyze many agronomic traits such as leaf architecture, maize kernel composition, plant height, oil biosynthesis in maize kernels (Tian et al., 2011; Weng et al., 2011; Cook et al., 2012; Li et al., 2013), and other traits, i.e., Some genetic research on crop lodging has also been conducted using GWAS. Hu et al. (2013) detected a complex polygenic inheritance for SBS-related traits, including the maximum load exerted to breaking (Fmax), the breaking moment (Mmax), and critical stress (σmax). A total of seven quantitative trait loci (QTLs) explaining 65.7% of the genotypic variance for these three traits. Ookawa et al. (2010) used chromosome segment substitution lines (CSSL) to identify an effective QTL, SCM2, for culm strength in rice, and the near-isogenic line (NIL) carrying SCM2 showed enhanced culm strength. Moreover, Lin et al. (2005) detected another six QTLs for stem strength, culm wall thickness, pith diameter, and stem diameter using a doubled-haploid (DH) population. Conversely, GWAS for maize lodging has rarely been reported, and the molecular mechanisms of variation for maize lodging-related traits remain poorly understood.
Currently, the Bonferroni correction is applied to control the false positive rate for single-marker GWAS, and some important loci with small effects could be excluded by this stringent correction. Multi-locus GWAS methodologies, such as FASTmrEMMA, ISIS EM-BLASSO, mrMLM, pLARmEB, and FarmCPU, have been shown to effectively resolve this issue. The first four methods have higher power and accuracy for quantitative trait nucleotide (QTN) detection and are more suitable for genetic models (Liu et al., 2016; Wang et al., 2016; Tamba et al., 2017; Wen et al., 2017; Zhang J. et al., 2017). Additionally, a combination of various methods for multi-locus GWAS has also been used to control the false positive rate (Wu et al., 2016; Misra et al., 2017).
Our objectives were to (i) estimate the genetic variance and heritability of SD, SBS, and RPR; (ii) estimate the correlations between these three traits; (iii) detect significant quantitative trait nucleotides (QTNS) for SD, RPR, and SBS in multiple environments; (iv) dissect the genetic basis of variation of lodging-related traits in maize, and (v) identify candidate genes controlling maize stalk lodging-related traits.
Materials and Methods
Phenotyping of Maize Lodging-Related Traits
The SD, SBS, and RPR tests were conducted in an association-mapping panel of 257 diverse inbred lines, which were collected from tropical or subtropical and temperate regions (Li et al., 2013). The names and pedigree information for this association panel are presented in Table S1. The 257 inbred lines were planted in three locations: Xishuangbanna (XSBN, N22°0, E100°79′, Yunnan province, China, 2014), Bijie at Guizhou (GZ, N27°32′, E105°29′, Guizhou province, China, 2014), and Wenjiang (WJ, N30°97, E103°81′, Chengdu, Sichuan province, China, 2014). The 257 inbred lines were sown in a randomized complete block design in two replications. Each plot consisted of a single row (14 plants) that was 3 m in length and 0.75 m from the next row, and the plant density was approximately 62,000 individuals per hectare. Each line was grown in a single-row.
At the flowering stage, 10 plants from each line from each replication were randomly selected for phenotyping and their mean values were computed for the three traits: SD, SBS, and RPR, as detailed in Wang L. M. et al. (2012). Briefly, a Vernier caliper was used to measure the SD (mm) of the 15-cm region above ground. A plant stalk strength appliance SY-S03 with a measuring range from 5 to 500 N and a resolution ratio of 0.1 N (Shijiazhuang Shiya Technology Co., Ltd) was used to measure RPR and SBS, and the units of RPR and SBS are N/mm2 and N, respectively.
Statistical Analysis of the Phenotype
SPSS version 21.0 (IBM, Armonk, NY, 2012) was used to analyze the phenotypic data, including descriptive statistics (mean, range, standard deviation, skewness, kurtosis) and the correlation analysis. To obtain the best linear unbiased prediction (BLUP) of the three lodging-related traits, the R package lme4 (version 3.4.2, https://www.r-project.org/) was fitted to each genotype: Phenotype ~ (1|Genotype) + (1|Repeat%in%Environment) + (1|Genotype&Environment). Broad-sense heritability (h2) for each trait was estimated as described by Knapp (Knapp et al., 1985) as: h2 = σg2/(σg2+σgy2/r+σe2/yr), where σg2, σgy2, and σe2 are genetic, genotype-by-environment interaction and residual error variances, respectively, r is the number of replications, and y is the number of environments. All the variances were calculated using a general linear model in SPSS.
Genotyping and ML-GWAS
Using publicly available genotypic data from previous studies, all the 257 lines of the association panel were genotyped using the Maize SNP50 BeadChip (Illumine, San Diego, CA), which contains 56,110 SNP loci (Ganal et al., 2011; Yang et al., 2011; Li et al., 2012). A total of 48,193 high-quality SNPs with a minor allele frequency (MAF) ≥0.05 were used in this study (http://www.maizego.org/Resources.html). A total of 500 SNPs for each chromosome were randomly selected to calculate population structure, as described by (Pritchard et al., 2009). Briefly, five independent simulations with 500,000 Markov Chain Monte Carlo (MCMC) replications and 5,000 SNPs were performed with the number of subpopulations (k) ranging from 1 to 12. The results calculated by STRUCTURE software were submitted to the website http://taylor0.biology.ucla.edu/structureHarvester/, and the optimal k was inferred. The relative kinship (K matrix) between the lines was calculated as previously described in Wang et al. (2016) and Zhang J. et al. (2017). Four multi-locus GWAS methods including mrMLM, FASTmrEMMA, pLARmEB, and ISIS EM-BLASSO were used in this study. All parameters were set at default values (Wang et al., 2016; Tamba et al., 2017; Wen et al., 2017; Zhang J. et al., 2017).
Annotation of Candidate Genes and Pathway Enrichment Analysis
Those genes with common SNPs in the GWAS result were selected as candidate genes. The maize inbred line B73 assembly v2 that was used as the reference genome for the candidate gene analyses was publicly available on the MaizeGDB genome browser (Andorf et al., 2010). The methods of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for these candidate genes were annotated as described by Zhang Y. et al. (2017).
Results
Diversity and Heritability of the Three Lodging-Related Traits
The phenotypic characteristics for SD, SBS, and RPR across the three environments are shown in Table 1 and Figure 1. As shown in Table 1, the skewness and kurtosis were less than 1 for SD and RPR, indicating that SD and RPR followed a normal distribution. SBS was slightly skewed to the left (Figures 1A,C,E). For the above three traits, the means of phenotypic values decreased from XSBN, WJ, to GZ; the coefficients of variation ranged from 11.87~14.2, 36.42~49.29, and 19.43~23.45 (%), respectively (Table 1, Figures 1B,D,F).
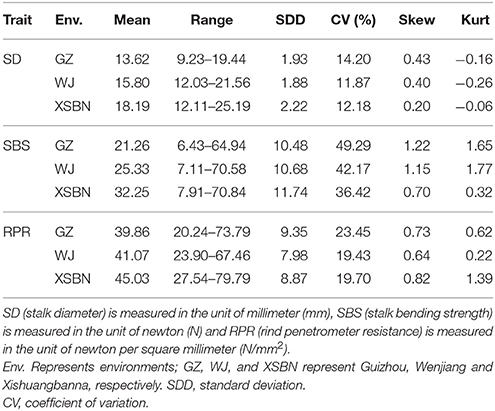
Table 1. Phenotypic performance of the three lodging resistance-related traits in 257 inbred lines under three environments.
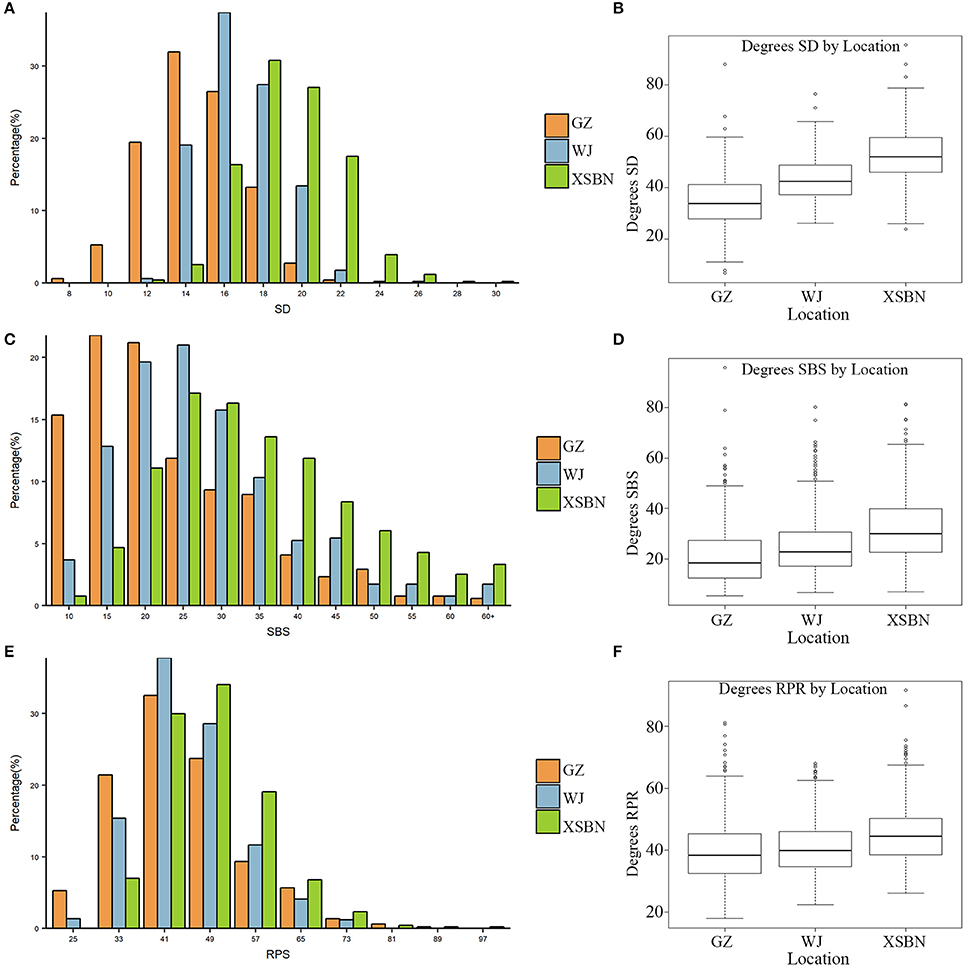
Figure 1. Frequency distributions of SD (A), SBS (C), RPR (E) in 257 maize inbred lines and the boxplots for SD (B), SBS (D), RPR (F) in the three environments.
The results of correlation analysis were showed in Table 2. Significant correlations between the traits across three environments were observed. For example, the correlation coefficients between SD and SBS in GZ, WJ, and XSBN were 0.762, 0.615 and 0.668 (P-values <0.01), respectively; the correlations between SD and RPR across three environments were relatively smaller (0.219 < r < 0.308, P < 0.01) than those between SBS and RPR (0.507 < r < 0.652, P < 0.01). In addition, a significant correlation between different environments was observed for each of the three traits (Table 2).
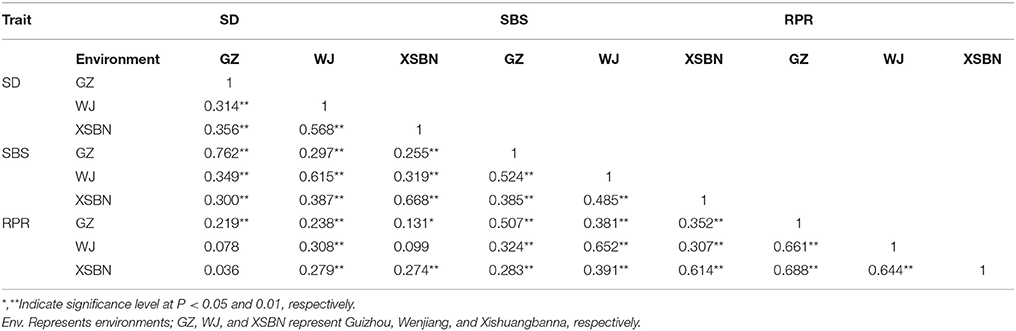
Table 2. Phenotypic correlation coefficients between lodging resistance-related traits across three environments.
In the analysis of variance for the three traits, highly significant variations for genotypes (G) and environments (E) and significant variation for genotype-by-environment interaction were found (Table 3). This indicates the important roles of both environment and G × E interaction. The broad-sense heritabilities (h2) for SD, SBS, and RPR across the three environments in the 257 inbred lines ranged from 0.679 (SD) to 0.854 (RPR), indicating the predominant role of genetic factors for these traits (Table 3).
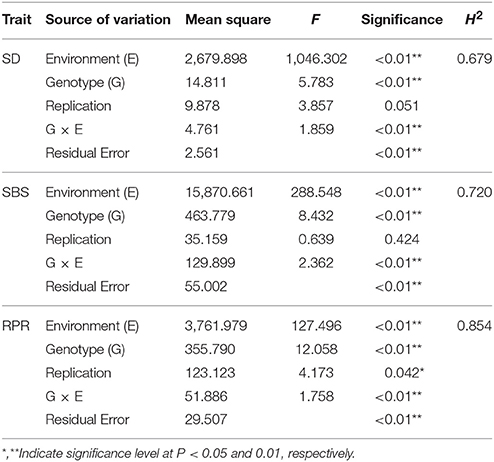
Table 3. Analysis of variance (ANOVA) for lodging resistance-related traits of 257 lines in three environments.
QTNs Identified by ML-GWAS
The ΔK calculation of STRUCTURE indicated a peak (K = 2) in the broken line graph reflecting the number of subpopulations (K) (Figures S1A,B), indicating that the 257 maize inbred lines could be divided into two subpopulations. Owning to significant variations for each of the three lodging-related traits in 257 maize inbred lines across the three locations, BLUP values across the three locations were also used for the GWAS. In total, 423 significant QTNs were identified at the critical logarithm of odds (LOD) score (≥3) for these traits in the three environments using mrMLM, FASTmrEMMA, PLARmEB and ISIS EM-BLASSO (Table S2, Figure S2).
A total of 126 significant QTNs, mainly distributed on chromosomes 1, 2, 3, 5, 6, 8, and 9, were detected to be associated with SD (Table S2, Figure S2A). Among them, 29 QTNs were common across the methods or the locations. The LOD of these 32 QTNs identified by mrMLM ranged from 3.03 to 6.25, and the percentage of phenotypic variation explained by each QTN (PVE) in GZ, WJ, XSBN, and BLUP was 30.96, 40.90, 44.21, and 54.38 (%), respectively. The LOD scores of the significant 21 QTNs identified by FASTmrEMMA ranged from 3.08 to 6.21, and the PVE in GZ, WJ, XSBN, and BLUP for SD was 19.51, 20.51, 22.31, and 21.25 (%), respectively. For PLARmEB, the LOD scores of the 43 QTNs ranged from 3.01 to 7.83 in GZ, WJ, XSBN, and BLUP, and PVE was 13.84, 35.20, 31.17, and 36.84 (%), respectively. The LOD scores of the 66 QTNs detected by ISIS EM-BLASSO ranged from 3.00 to 14.08, and the PVE in GZ, WJ, XSBN and BLUP was 51.15, 41.37, 48.99, and 44.37 (%), respectively.
In total, 148 significant QTNs were correlated with SBS, and were evenly distributed on 10 chromosomes under the environments and BLUP model. Among them, 35 QTNs were common across the methods or the locations. The LOD values of the 148 QTNs identified by mrMLM, FASTmrEMMA, PLARmEB, and ISIS EM-BLASSO were in the range of 3.01~8.78, 3.32~10.75, 3.09~8.69, and 3.05~12.18, respectively (Table S2, Figure S2B). Among these QTNs, 43 identified by mrMLM explained 58.31, 53.47, 52.02, and 57.69 (%) of the phenotypic variation in GZ, WJ, XSBN, and BLUP for SBS, respectively. Conversely, 26.98, 43.87, 17.02, and 26.10 (%) of the phenotypic variation was separately explained by 28 QTNs using FASTmrEMMA. Using PLARmEB, the PVE was 28.77, 30.40, 17.31, and 49.24 (%) in the different environments, respectively. The PVE in GZ, WJ, XSBN, and BLUP for SBS was 53.88, 64.19, 56.12, and 49.64 (%), respectively, for the 73 QTNs by ISIS EM-BLASSO.
We detected a total of 149 RPR-associated QTNs with LODs ranging from 3.01 to 14.39 in the three environments and BLUP model, and were mainly located on chromosomes 1, 2, 4, 5, 7, 8, and 9 (Table S2, Figure S2C). And 47 QTNs were common across the methods or the locations. Among these, four QTNs were also detected in SBS traits. Of the 149 RPR-associated QTNs, 54, 31, 57, and 74 QTNs were separately identified by mrMLM, FASTmrEMMA, PLARmEB, and ISIS EM-BLASSO, which explained 60.91~67.76, 23.53~35.38, 30.90~56.86, and 45.28~63.77 (%) of the phenotypic variation, respectively.
Verification of the Common QTNs by Multi-Methods or Across Environments
A total of 107 QTNs were co-identified by at least two of the methods or across different environments, among which 29, 34, and 48 were associated with SD, SBS, and RPR, respectively (Table S3 and Figure 2). To verify the significance of each common QTN, we divided the population into two groups according to their allele types and compared the mean phenotypic values between the two groups. For SD, the average of the group containing the superior alleles was significantly greater than the group containing inferior alleles, with the exception of the SNPs SYN35339, SYN6428, PZE-102085765, and PZE-101121408 (Table S4). As for SBS and RPR, the group with the superior alleles showed a significantly larger mean than the group with inferior alleles for every common SNP (Table S4). These results verified the reliability and significance of the common QTNs identified by these ML-GWAS methods.
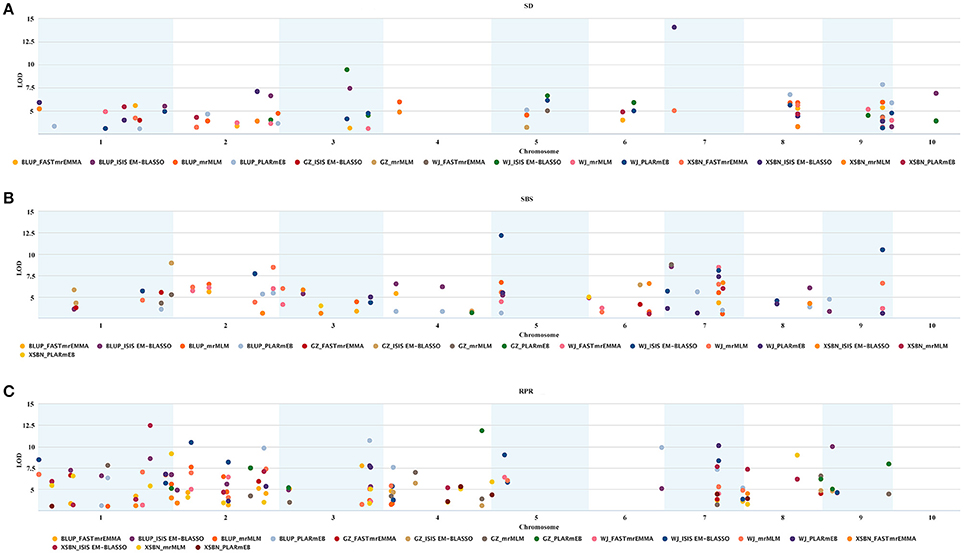
Figure 2. Repeatability and significance of the SNPs associated with the three lodging resistance-related traits in the three environments and BLUP. The significance threshold is LOD = 3.0. (A–C) Represent SD, SBS, and RPR, respectively.
Utilization of Superior Alleles in Elite Maize Lines
Thirty elite inbred lines from China and America that have excellent agronomic traits and serve as the parents of commercialized hybrid varieties were included in the maize population, which enabled us to evaluate the utilization of the superior alleles for maize breeding. The results indicated that the percentage of SD superior alleles in the elite lines ranged from 27.59 to 55.17% (Table S5). The lines with >15 superior alleles indicated a significantly higher SD phenotypic value, with an average of 14.50 in GZ, 16.75 in WJ, and 20.72 in XSBN, whereas the lines with 0~10 superior alleles had average SD values of 12.78, 14.13, and 16.61 in GZ, WJ, and XSBN, respectively (Table S5, Figure 3A). The utilization of the SBS superior alleles in the elite lines ranged from 25.71 to 65.71% (Table S5). The phenotypic averages of the lines with >20 superior alleles were 30.56, 53.68, and 44.83 in GZ, WJ, and XSBN, respectively, whereas those with 15~20 superior alleles had a lower average of 15.14, 15.87, and 25.85 in GZ, WJ, and XSBN, respectively (Table S5, Figure 3B). As for RPR, the elite lines contained 29.17~66.67% of the superior alleles (Table S5). The average RPR in the lines with >30 superior alleles were 45.06, 50.69, and 49.01 in GZ, WJ, and XSBN, respectively; however, those lines with <20 superior alleles had average RPR values of 29.05, 32.43, and 35.97 in GZ, WJ, and XSBN, respectively (Table S5, Figure 3C). The results suggested that these superior alleles had an additive effect on the lodging resistance-related traits. Further analysis indicated that only eight of the 30 elite inbred lines had more than 50% utilization of all the superior alleles (Table S5, Figure 4), implying that the superior alleles were not efficiently selected during maize breeding. In future work, integrated utilization of the superior alleles would be an efficient approach for lodging-resistance breeding in maize by marker-assisted selection (MAS).
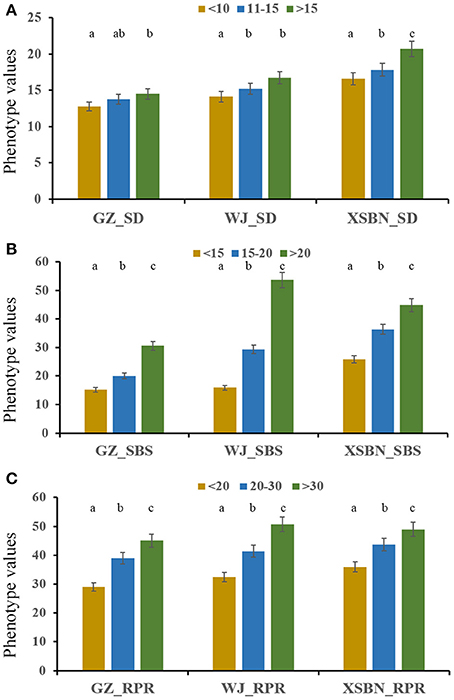
Figure 3. The phenotypic values in the maize elite inbred lines with different numbers of superior alleles for SD (A), SBS (B), and RPR (C).
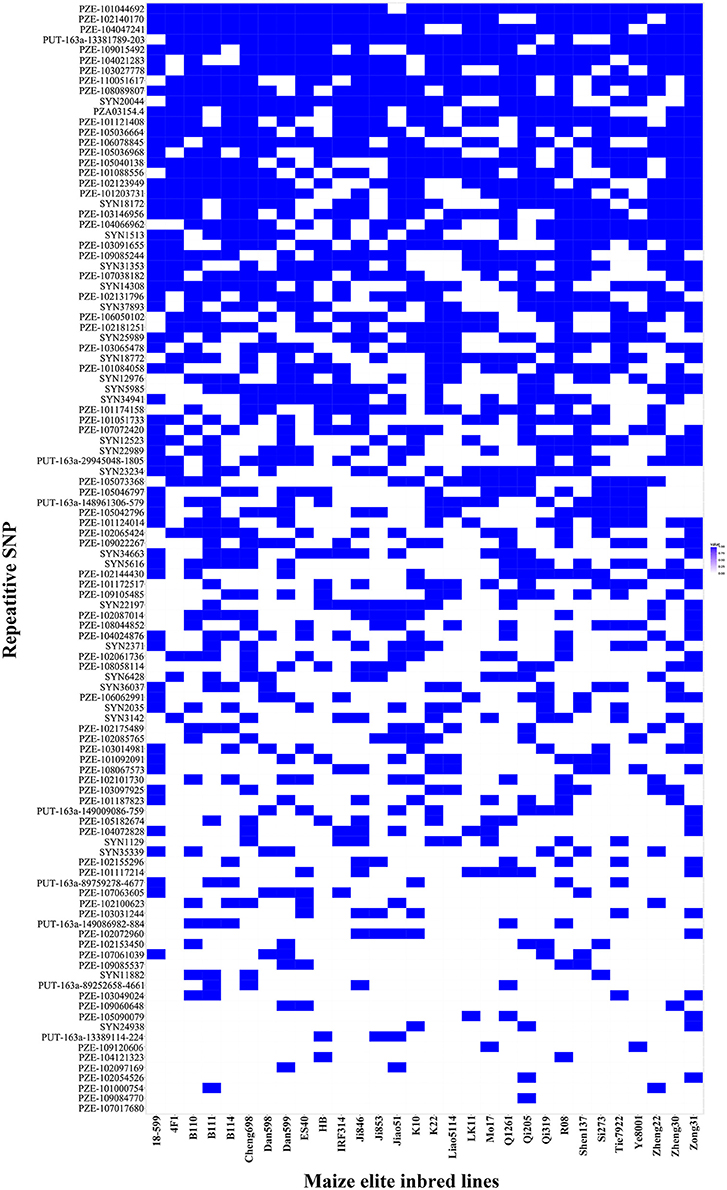
Figure 4. The superior allele SNP distributions in the 30 maize elite inbred lines. Blue and white colors represent superior and inferior alleles, respectively.
Candidate Genes Associated With Common QTNs
To further understand the molecular basis of lodging-related traits, we focused on the candidate genes that were directly associated with the common QTNs. As a result, 19, 17, and 30 candidate genes around their common QTNs were found to be associated with SD, SBS, and RPR, respectively. The annotations for the candidate genes are displayed in Table S3, with seven transcription factors, eight kinase-related proteins, and four transport proteins involved. These genes mainly participate in metabolic pathway, genetic information processing, environmental information processing, cellular processes, and organismal systems (Table 4).
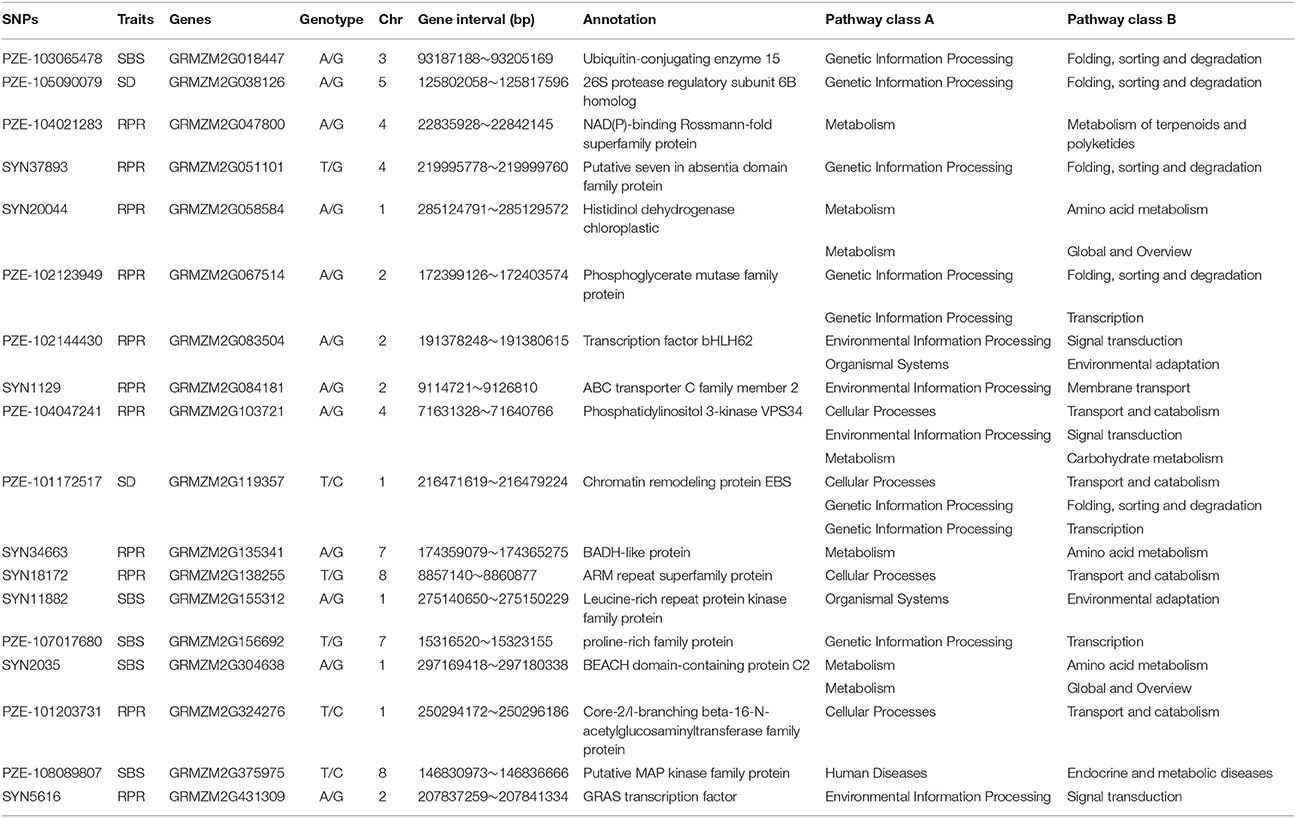
Table 4. SNPs, chromosomal position and the pathway of candidate genes significantly associated with three lodging resistance-related traits identified by multi GWAS methods across all environments.
Discussion
According to previous studies, the strength of the maize stalk depends on the tissue and morphology, and the morphology of the stalk is largely determined by the mechanical stresses in maize (Von et al., 2015). SD, SBS, and RPR were demonstrated to show potential as selective breeding indexes for improving lodging resistance (Liu et al., 2011; Xiang et al., 2016). The heritability and genetic models vary among different studies since the calculations depend on the experimental populations, design, and conditions (Lynch and Walsh, 1998). The genetic architecture of lodging resistance-related traits has been illustrated in diverse maize populations by linkage mapping. (Kashiwagi et al., 2008; Hu et al., 2012, 2013). However, the genetic basis and the molecular pathways underlying lodging resistance-related traits, as well as the major genes associated with the traits, remain largely unknown. In this study, we interpreted the natural variation and revealed the genetic architecture of three lodging resistance-related traits based on 257 maize inbred lines by ML-GWAS analysis. And identified the candidate genes and their possible pathways for stalk lodging resistance.
Genetic Basis of Lodging-Related Traits
In this study, the three lodging-related traits exhibited wide phenotypic variation and were normally distributed. ANOVA showed that the genetic effects and interactive effects between the genetics and environment were both significant for these traits, and the heritability (h2) was very high for SD, SBS, and RPR. Previous studies on SD in different crops mainly focused on the phenotypic correlations with stalk mechanical strength and the identification of QTLs for SD, whereas the heritability of SD has not been investigated (Lin et al., 2005; Kashiwagi et al., 2008). In our study, h2 was 0.679 across the three environments for SD. The Fmax, Mmax, and σmax can be used as tools to determine SBS accreditation (Timoshenko and Gere, 1972). An h2 of 0.84 for Fmax, was reported in rice, and in maize the h2 for Fmax, Mmax, and σmax were 0.81, 0.79, and 0.75, respectively (Sun, 1987; Hu et al., 2013). Both these estimates are in close agreement with the estimates of SBS (h2 = 0.720) in our study (Table 3). The h2 estimates were previously found to range from 0.81 to 0.92 for RPR in different maize populations (Flintgarcia et al., 2003; Hu et al., 2012), which corroborates our value of 0.854 across the three environments. In combination with previous results, our findings suggest that all the measured lodging-related traits showed high precision and that the three lodging resistance-related traits generally exhibited high heritability.
Phenotypic correlations were observed among the three lodging-related traits. For instance, the correlation coefficient between SBS and RPR was 0.507 in GZ, 0.652 in WJ, and 0.614 in XSBN, respectively (Table S2). Meanwhile, we identified four QTNs (PZE-101187823, SYN31353, PZE-105036664, and PZE-107063605), all of which were associated with both SBS and RPR (Table S3). The above results suggested that some genetic factors were shared among these lodging resistance-related traits.
Common Candidate Genes Reveal the Possible Molecular Basis of Lodging Resistance
No previous studies have reported on GWAS for SD, SBS, and RPR in maize. However, some studies have evaluated the QTLs. Hu et al. (2013) detected two, three, and two QTLs for Fmax, Mmax, and σmax, respectively, using 216 recombinant inbred lines and 129 SSR markers. Among them, a QTL of σmax, an important parameter for characterizing SBS, was located in markers umc1993 and bulg1450. In the present research, a QTN on Chr10 (position: 137282081 bp) for SBS locates exactly in the interval of the σmax QTL reported by Hu et al. (2013) (Table S2). The remaining QTNs in the present study are the first to be reported as associated with lodging resistance-related traits in maize.
Furthermore, we identified 63 common candidate genes in total that were around common QTNs for lodging-related traits. Notably, GRMZM5G856734 encodes Membrane steroid-binding protein 1 (MSBP1) (Table S3), whose homologous gene MSBP1 in Arabidopsis thaliana was proven to be involved in the inhibition of cell elongation (Yang et al., 2005). Interestingly, the candidate gene GRMZM2G116885 that encodes cyclin-dependent kinase inhibitor 1 was associated with both SBS and RPR. The homologous gene of GRMZM2G116885 in Arabidopsis was reported to be involved in coordinated cell growth or cell division (Bemis and Torii, 2007). It is generally known that cell elongation and cell wall thickening regulate plant lodging resistance (Fan et al., 2017). According to RNA-Seq data from the previous study, the candidate genes GRMZM5G856734 and GRMZM2G116885 had high expression levels in maize stems, with the FPKM are 115.5 and 58.0, respectively (Sekhon et al., 2012). In addition, more than 90% of the candidate genes found in our study were expressed in maize stems, especially the expression levels of GRMZM2G038126, GRMZM2G073934, GRMZM2G058584, and GRMZM2G084181 were extremely high (Table S3). The functional validation of these genes should be addressed in future work.
Additionally, seven candidate genes were classified into transcription factors based on their functional annotation, including ethylene-responsive transcription factor 12, bHLH-transcription factor 105, bHLH-transcription factor 65, GRAS transcription factor, transcription factor VOZ1, and MYB 9 transcription factor (Table S3). Transcription factors are a group of proteins that regulate targeted gene expression in particular cells at a certain time, and are vital for cell division, growth, and death (Latchman, 1997; Riechmann and Meyerowitz, 1998; Guilfoyle and Hagen, 2007).
The Superiority of the New ML-GWAS
Previous studies demonstrated that the single-locus GWAS was useful to dissect complex agronomic trait by using general linear models (GLMs) and mixed linear models (MLMs) (Zhang et al., 2010; Wang M. et al., 2012). High false positive rates are an obvious shortcoming for GLMs, because there is no kinship among materials as covariate (Pace et al., 2015). The screening criteria of significance for single-locus GWAS is P = 0.05/m (m is the number of markers) (Perneger, 1998). Owning to large number of SNPs (Gordon et al., 2016), some important loci might be excluded under the stringent criteria in MLM. To remedy the shortcomings of the methods mentioned above, ML-GWAS methods have recently been explored, including mrMLM (Wang et al., 2016), pLARmEB (Zhang J. et al., 2017), ISIS EM-BLASSO (Tamba et al., 2017), and FASTmrEMMA (Wen et al., 2017). Several studies have individually analyzed published data using the multi-locus methods, and have indicated that these methods constituted effective approaches with high detection power and less stringent criteria (Wang et al., 2016; Tamba et al., 2017; Wen et al., 2017; Zhang J. et al., 2017). In our study, a total of 126, 77, 176, and 230 significant QTNs were detected for three lodging-related traits using mrMLM, FASTmrEMMA, pLARmEB, and ISIS EM-BLASSO, respectively (Figure S2, Table S2). A comparison of the four methods showed that ISIS EM-BLASSO was more powerful than the other three multi-locus methods in the identification of QTNs for lodging resistance-related traits (Table S2, Figure S2). Furthermore, some stably expressed QTNs were detected in the multi-environment and BLUP model using multi-methods (Tables s2, s3). Notably, the candidate genes GRMZM5G856734 and GRMZM2G116885, were proven to inhibit cell elongation and division, which regulates lodging resistance. However, only 4, 4, and 7 SNPs were detected for SD, SBS, and RPR, respectively, from FarmCPU (R packages FarmCPU, K and PCA calculated by SPAGeDi software and GAPIT package, respectively. The threshold is P-value = 0.05/48193) (Table S6). In addition, six of these SNPs were also be detected by ML-GWAS methods. Using GAPIT (R packages GAPIT) method, only one SD-associated SNP was found in XSBN, which was also detected in the ML-GWAS methods (Table S6). Our study demonstrated that improved efficiency and accuracy could be achieved by a combination of the new multi-locus methods for identification of lodging resistance-related QTNs in maize.
Conclusions
SD, SBS, and RPR were used in this study to dissect the genetic basis of stalk lodging resistance in maize using ML-GWAS methods. Among all the significantly associated QTNs for the three traits, 107 were commonly identified across multiple methods or environments. Around these common QTNs, sixty-three candidate genes were responsive for maize lodging resistance. These QTNs provide the important information for the marker-assisted selection, and these candidate genes should improve our understanding of the molecular basis of maize lodging resistance.
Author Contributions
YS and PL designed the experiments. YLZ, XZ, QZ, MC, FG, ZL, WS, and YZ performed the analysis. YLZ, YS, ZG, TL, YZ, XT, CZ, HP, and GP drafted the manuscript. All the authors critically revised and approval the final version of this manuscript.
Funding
This work was supported by the National Basic Research Program of China (2014CB138200) and the Young Scientists Fund of Sichuan Province (2016JQ0008).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jianbing Yan (Huazhong Agricultural University) for providing the maize population.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00611/full#supplementary-material
Figure S1 Population structure of the 257 maize inbred lines based on 48,193 SNP markers. (A) Plot of delta.K against putative K ranging from 1 to 12. (B) Stacked bar plot of ancestry relationship of the natural population.
Figure S2 Manhattan plots showing all the significant SNPs associated with lodging resistance-related traits using four ML-GWAS methods across three environments and BLUP. (A–C) represent SD, SBS, and RPR, respectively. Points of different colors represent different methods and environments.
Table S1 Pedigree information of the maize accessions used in this study.
Table S2 ML-GWAS detected significant signals associated with SD, SBS, and RPR across the three environments and BLUP.
Table S3 Repetitive SNPs and their information by ML-GWAS consistently identified in multiple methods or environments.
Table S4 Distribution of the important SNPs superior alleles in the 257 inbred lines.
Table S5 Distribution and utilization percentage of the important SNPs superior alleles in the 30 maize elite inbred lines.
Table S6 Significant signals associated with SD, SBS, and RPR, detected by FarumCPU and GAPIT.
References
Adelana, B. O. (1980). Relationship between lodging, morphological characters and yield of tomato cultivars. Sci. Hortic. 13, 143–148. doi: 10.1016/0304-4238(80)90078-3
Andorf, C. M., Lawrence, C. J., Harper, L. C., Schaeffer, M. L., Campbell, D. A., and Sen, T. Z. (2010). The Locus Lookup tool at MaizeGDB: identification of genomic regions in maize by integrating sequence information with physical and genetic maps. Bioinformatics 26, 434. doi: 10.1093/bioinformatics/btp556
Bemis, S. M., and Torii, K. U. (2007). Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev. Biol. 304, 367. doi: 10.1016/j.ydbio.2006.12.049
Cook, J. P., McMullen, M. D., Holland, J. B., Tian, F., Bradbury, P., Ross-Ibarra, J., et al. (2012). Genetic architecture of maize kernel composition in the nested association mapping and inbred association panels. Plant Physiol. 158, 824. doi: 10.1104/pp.111.185033
Fan, C., Li, Y., Hu, Z., Hu, H., Wang, G., Li, A., et al. (2017). Ectopic expression of a novel OsExtensin-like gene consistently enhances plant lodging resistance by regulating cell elongation and cell wall thickening in rice. Plant Biotechnol. J. 16, 254–263. doi: 10.1111/pbi.12766
Flintgarcia, S. A., Jampatong, C., Darrah, L. L., and Mcmullen, M. D. (2003). Quantitative Trait Locus Analysis of Stalk Strength in Four Maize Populations. Crop Sci. 43, 13–22. doi: 10.2135/cropsci2003.0013
Ganal, M. W., Durstewitz, G., Polley, A., Bérard, A., Buckler, E. S., Charcosset, A., et al. (2011). A large maize (Zea mays L.) SNP genotyping array: development and germplasm genotyping, and genetic mapping to compare with the B73 reference genome. PLoS ONE 6:e28334. doi: 10.1371/journal.pone.0028334
Gordon, D., Huddleston, J., Chaisson, M. J., Hill, C. M., Kronenberg, Z. N., Munson, K. M., et al. (2016). Long-read sequence assembly of the gorilla genome. Science 352:aae0344. doi: 10.1126/science.aae0344
Guilfoyle, T. J., and Hagen, G. (2007). Auxin response factors. J. Plant Growth Regul. 10:453. doi: 10.1016/j.pbi.2007.08.014
Hu, H., Liu, W., Fu, Z., Homann, L., Technow, F., Wang, H., et al. (2013). QTL mapping of stalk bending strength in a recombinant inbred line maize population. Theor. Appl. Genet. 126, 2257–2266. doi: 10.1007/s00122-013-2132-7
Hu, H., Meng, Y., Wang, H., Hai, L., and Chen, S. (2012). Identifying quantitative trait loci and determining closely related stalk traits for rind penetrometer resistance in a high-oil maize population. Theor. Appl. Genet. 124, 1439–1447. doi: 10.1007/s00122-012-1799-5
Kashiwagi, T., Togawa, E., Hirotsu, N., and Ishimaru, K. (2008). Improvement of lodging resistance with QTLs for stem diameter in rice (Oryza sativa L.). Theor. Appl. Genet. 117, 749–757. doi: 10.1007/s00122-008-0816-1
Knapp, S. J., Stroup, W. W., and Ross, W. M. (1985). Exact Confidence Intervals for Heritability on a Progeny Mean Basis. Crop Sci. 25, 192–194. doi: 10.2135/cropsci1985.0011183X002500010046x
Korte, A., and Farlow, A. (2013). The advantages and limitations of trait analysis with GWAS: a review. Plant Methods 9:29. doi: 10.1186/1746-4811-9-29
Latchman, D. S. (1997). Transcription factors: an overview. Int. J. Exp. Pathol. 29:1305. doi: 10.1016/S1357-2725(97)00085-X
Li, H., Peng, Z., Yang, X., Wang, W., Fu, J., Wang, J., et al. (2013). Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 45, 43–50. doi: 10.1038/ng.2484
Li, Q., Yang, X., Xu, S., Cai, Y., Zhang, D., Han, Y., et al. (2012). Genome-wide association studies identified three independent polymorphisms associated with α-tocopherol content in maize kernels. PLoS ONE 7:e36807. doi: 10.1371/journal.pone.0036807
Lin, H., Guo, H., Xiao, S., Jiang, G., Zhang, X., Yan, C., et al. (2005). Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of wheat (Triticum aestivum L.). Euphytica 141, 1–9. doi: 10.1007/s10681-005-4713-2
Ling, G. (2008). Bending mechanical properties of stalk and lodging-resistance of maize (Zea mays L.): bending mechanical properties of stalk and lodging-resistance of Maize (Zea mays L.). Acta Agron. Sin. 34, 653–661. doi: 10.3724/SP.J.1006.2008.00653
Liu, W. W., Zhao, H. J., Hong-Qi, L. I., Zhao, X. J., Yuan, L. G., and Wei-Wei, H. U. (2011). Effects of planting densities and modes on stem lodging resistance of summer maize. J. Henan Agric. Sci. 40, 75–78. doi: 10.15933/j.cnki.1004-3268
Liu, X., Huang, M., Fan, B., Buckler, E. S., and Zhang, Z. (2016). Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet. 12:e1005767. doi: 10.1371/journal.pgen.1005767
Lynch, M., and Walsh, B. (1998). Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer Associates.
Misra, G., Badoni, S., Anacleto, R., Graner, A., Alexandrov, N., and Sreenivasulu, N. (2017). Whole genome sequencing-based association study to unravel genetic architecture of cooked grain width and length traits in rice. Sci. Rep. 7:12478. doi: 10.1038/s41598-017-12778-6
Ookawa, T., Hobo, T., Yano, M., Murata, K., Ando, T., Miura, H., et al. (2010). New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat. Commun. 1:132. doi: 10.1038/ncomms1132
Pace, J., Yu, X., and Lübberstedt, T. (2015). Genomic prediction of seedling root length in maize (Zea mays L.). Plant J. Cell Mol. Biol. 83:903. doi: 10.1111/tpj.12937
Perneger, T. V. (1998). What's wrong with Bonferroni adjustments. Br. Med. J. 316, 1236–1238. doi: 10.1136/bmj.316.7139.1236
Pritchard, J. K., Wen, X., and Falush, D. (2009). Documentation for STRUCTURE software: version 2.3. The University of Chicago Press.
Remison, U., and Dele Akinleye, S. (1978). Relationship between lodging, morphological characters and yield of varieties of maize (Zea mays L.). J. Agric. Sci. 91, 633–638. doi: 10.1017/S0021859600060019
Riechmann, J. L., and Meyerowitz, E. M. (1998). The AP2/EREBP family of plant transcription factors. Biol. Chem. 379:633.
Sekhon, R. S., Childs, K. L., Santoro, N., Foster, C. E., Buell, C. R., Leon, N. D., et al. (2012). Transcriptional and metabolic analysis of senescence induced by preventing pollination in maize. Plant Physiol. 159, 1730–1744. doi: 10.1104/pp.112.199224
Sibale, E. M., Darrah, L. L., and Zuber, M. S. (1992). Comparison of two rind penetrometers for measurement of stalk strength in maize. Maydica 37, 111–114.
Sun, X. (1987). Studies on the resistance of the culm of rice to lodging. Sci. Agric. Sin. 20, 32–37.
Tamba, C. L., Ni, Y. L., and Zhang, Y. M. (2017). Iterative sure independence screening EM-Bayesian LASSO algorithm for multi-locus genome-wide association studies. PLoS Comput. Biol. 13:e1005357. doi: 10.1371/journal.pcbi.1005357
Tesso, T., and Ejeta, G. (2011). Stalk strength and reaction to infection by Macrophomina phaseolina of brown midrib maize (Zea mays) and sorghum (Sorghum bicolor). Fuel Ener. Abstr. 120, 271–275. doi: 10.1016/j.fcr.2010.10.015
Tian, F., Bradbury, P. J., Brown, P. J., Hung, H., Sun, Q., Flint-Garcia, S., et al. (2011). Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat. Genet. 43, 159–162. doi: 10.1038/ng.746
Timoshenko, S. P., and Gere, J. M. (1972). Mechanics of Materials. Daejeon-si: Van Nostrand Reinhold Company.
Von, F. G., Robertson, D., Lee, S. Y., and Cook, D. D. (2015). Preventing lodging in bioenergy crops: a biomechanical analysis of maize stalks suggests a new approach. J. Exp. Bot. 66:4367. doi: 10.1093/jxb/erv108
Wang, L. M., Jian-Sheng, L. I., and Yao, G. Q. (2012). Characterizations of resistance to stalk and root lodging in maize. J. Maize Sci. 20, 69–74,81. doi: 10.13597/j.cnki.maize.science.2012.02.015
Wang, M., Yan, J., Zhao, J., Song, W., Zhang, X., Xiao, Y., et al. (2012). Genome-wide association study (GWAS) of resistance to head smut in maize. Plant Sci. Int. J. Exper. Plant Biol. 196:125. doi: 10.1016/j.plantsci.2012.08.004
Wang, S. B., Feng, J. Y., Ren, W. L., Huang, B., Zhou, L., Wen, Y. J., et al. (2016). Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci. Rep. 6:19444. doi: 10.1038/srep19444
Wen, Y. J., Zhang, H., Ni, Y. L., Huang, B., Zhang, J., Feng, J. Y., et al. (2017). Methodological implementation of mixed linear models in multi-locus genome-wide association studies. Brief. Bioinform. doi: 10.1093/bib/bbw145
Weng, J., Xie, C., Hao, Z., Wang, J., Liu, C., Li, M., et al. (2011). Genome-wide association study identifies candidate genes that affect plant height in Chinese elite maize (Zea mays L.) inbred lines. PLoS ONE 6:e29229. doi: 10.1371/journal.pone.0029229
Wu, X., Li, Y., Shi, Y., Song, Y., Zhang, D., Li, C., et al. (2016). Joint-linkage mapping and GWAS reveal extensive genetic loci that regulate male inflorescence size in maize. Plant Biotechnol. J. 14, 1551–1562. doi: 10.1111/pbi.12519
Xiang, D. B., Zhao, G., Wan, Y., Tan, M. L., Song, C., and Song, Y. (2016). Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 19, 1–10. doi: 10.1080/1343943X.2016.1188320
Xu, C., Gao, Y., Tian, B., Ren, J., Meng, Q., and Wang, P. (2017). Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 200, 71–79. doi: 10.1016/j.fcr.2016.10.011
Yang, X., Gao, S., Xu, S., Zhang, Z., Prasanna, B. M., Li, L., et al. (2011). Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 28, 511–526. doi: 10.1007/s11032-010-9500-7
Yang, X. H., Xu, Z. H., and Xue, H. W. (2005). Arabidopsis membrane steroid binding protein 1 is involved in inhibition of cell elongation. Plant Cell 17:116. doi: 10.1105/tpc.104.028381
Zhang, J., Feng, J., Ni, Y., Wen, Y., Niu, Y., Tamba, C. L., et al. (2017). pLARmEB: integration of least angle regression with empirical Bayes for multilocus genome-wide association studies. Heredity (Edinb). 118, 517–524. doi: 10.1038/hdy.2017.8
Zhang, Y., Ge, F., Hou, F., Sun, W., Zheng, Q., Zhang, X., et al. (2017). Transcription factors responding to Pb stress in maize. Genes 8:231. doi: 10.3390/genes8090231
Keywords: maize, stalk lodging resistance, multi-locus GWAS, QTNs, candidate gene
Citation: Zhang Y, Liu P, Zhang X, Zheng Q, Chen M, Ge F, Li Z, Sun W, Guan Z, Liang T, Zheng Y, Tan X, Zou C, Peng H, Pan G and Shen Y (2018) Multi-Locus Genome-Wide Association Study Reveals the Genetic Architecture of Stalk Lodging Resistance-Related Traits in Maize. Front. Plant Sci. 9:611. doi: 10.3389/fpls.2018.00611
Received: 31 January 2018; Accepted: 18 April 2018;
Published: 07 May 2018.
Edited by:
Yuan-Ming Zhang, Huazhong Agricultural University, ChinaReviewed by:
Xiaohong Yang, China Agricultural University, ChinaYang Xu, Yangzhou University, China
Copyright © 2018 Zhang, Liu, Zhang, Zheng, Chen, Ge, Li, Sun, Guan, Liang, Zheng, Tan, Zou, Peng, Pan and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaou Shen, c2hlbnlhb3VAc2ljYXUuZWR1LmNu
 Yanling Zhang1
Yanling Zhang1 Peng Liu
Peng Liu Yaou Shen
Yaou Shen