- 1Department of Plant and Environmental Sciences, University of Copenhagen, Frederiksberg, Denmark
- 2GlycoSpot IVS, Frederiksberg, Denmark
Plant cell walls are highly complex structures composed of diverse classes of polysaccharides, proteoglycans, and polyphenolics, which have numerous roles throughout the life of a plant. Significant research efforts aim to understand the biology of this cellular organelle and to facilitate cell-wall-based industrial applications. To accomplish this, researchers need to be provided with a variety of sensitive and specific detection methods for separate cell wall components, and their various molecular characteristics in vitro as well as in situ. Cell wall component-directed molecular detection probes (in short: cell wall probes, CWPs) are an essential asset to the plant glycobiology toolbox. To date, a relatively large set of CWPs has been produced—mainly consisting of monoclonal antibodies, carbohydrate-binding modules, synthetic antibodies produced by phage display, and small molecular probes. In this review, we summarize the state-of-the-art knowledge about these CWPs; their classification and their advantages and disadvantages in different applications. In particular, we elaborate on the recent advances in non-conventional approaches to the generation of novel CWPs, and identify the remaining gaps in terms of target recognition. This report also highlights the addition of new “compartments” to the probing toolbox, which is filled with novel chemical biology tools, such as metabolic labeling reagents and oligosaccharide conjugates. In the end, we also forecast future developments in this dynamic field.
Introduction to Tools for Probing Cell Walls
Plant cell walls play an important role in numerous cellular functions and developmental processes including the mediation of cell-to-cell adhesion, provision of mechanical support, and interaction with pathogens and the environment (Albersheim et al., 2010; Wolf et al., 2012; Malinovsky et al., 2014; Chebli and Geitmann, 2017; Höfte and Voxeur, 2017). Cell walls can contribute up to 90% of the dry matter of a plant and are a crucial component of nature-derived industrial feedstock. This is becoming increasingly important for sustainable energy and novel biomaterials (Marriott et al., 2016). Due to the requirement for their multi-functionality, cell walls are complex and heterogeneous structures with a high level of spatiotemporal dynamics (Drakakaki, 2015; Barnes and Anderson, 2017; Höfte and Voxeur, 2017). In addition, cell wall composition and architecture varies between different cell types, developmental stages (Wilson et al., 2015; Monniaux and Hay, 2016; Mravec et al., 2017a), and also between species (Sørensen et al., 2011; Fangel et al., 2012; Popper et al., 2014; Leroux et al., 2015; Harholt et al., 2016). The cell wall is, for the most part, built from polysaccharides (cellulose, hemicelluloses, and pectins), proteoglycans (extensins and arabinogalactan proteins), and, in some cells, polyphenolics (lignin) and polyesters (cutin and suberin). Each of these components has its own particular function(s) within the intricate cell wall structure (Albersheim et al., 2010; Burton et al., 2010). However, scientists still face many open-ended questions regarding the biology of plant cell walls, particularly with respect to the compartmentalized synthesis of cell wall components, in muro dynamic co-ordination of the cell wall architecture, the relationship between different cell wall components, and the activity of regulatory loops coupled to intrinsic cellular, developmental and pathogenesis-related pathways.
The diverse techniques used to study cell walls relies heavily on detection probes that are specific for cell wall components (Cell Wall Probes-CWPs). In this review, a CWP will be defined as any molecule with the ability to specifically bind (or be incorporated into) a cell wall component, therefore allowing subsequent selective detection, quantification or visualization. Here it is important to note that “chemical probe,” in the field of chemical biology, is a wider term that can also be used to describe molecules that are able to influence the function of the target, e.g., by altering its activity via specific binding (Garbaccio and Parmee, 2016).
Although many biochemical and biophysical techniques, such as mass spectroscopy (MS) or nuclear magnetic resonance (NMR), can accurately decipher the detailed structure of cell wall components, they are not high-throughput (HTP) and usually require homogenization of samples, harsh pre-treatments or extractions, and a high-level of expertise from the operating personnel. By contrast, CWPs can be used to detect various cell wall components (specifically, their relative abundance and structural alterations) in a HTP manner, whilst simultaneously providing insights into their molecular structure and interactions with other glycans. In addition, this understanding of the cell wall material can often be acquired by detecting polymers in their native context without having to deconstruct the cell wall. As such, CWPs are vital for the study of different aspects of cell wall microstructure in muro (Knox, 2008; Lee et al., 2011), and can be used to follow the dynamics of the cell wall, and its components, in planta (Wallace and Anderson, 2012; Voiniciuc et al., 2018). The current set of available CWPs (Figure 1) consists of anti-glycan monoclonal antibodies (mAbs), phage display-based probes, carbohydrate-binding modules (CBMs), and small molecular probes such as fluorophores, oligosaccharide conjugates, and building blocks for metabolic labeling. In the following sections we will review the developments in this field, and discuss technical aspects and the important features of various classes of CWPs.
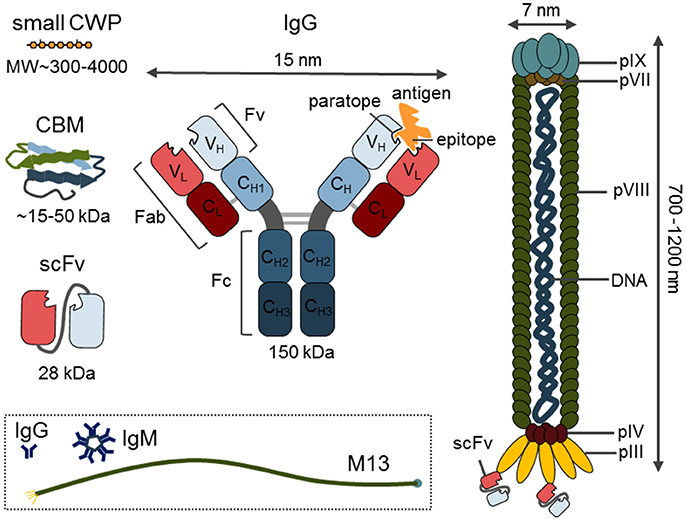
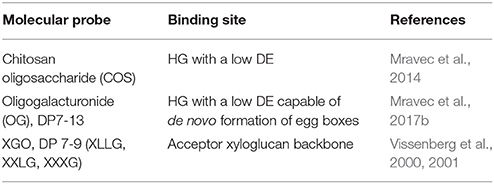
Figure 1. Overview of the different classes of probes with a size comparison. Depicted are small cell wall probe (CWP), carbohydrate-binding module (CBM), single chain variable fragment (scFV) and immunoglobulin G (IgG) structure with indicated regions and size in kDa. The structure of M13 phage with indicated capsid structural proteins (pIII, pIV, pVII, pVIII, pIX). The content of the dashed square indicates the real-size relation of immunoglobulins IgG and IgM to the M13 phage.
Proteinaceous Probes—Combining Diversity With Versatility
Antibodies—The Largest Compartment of the CWP Tool Box
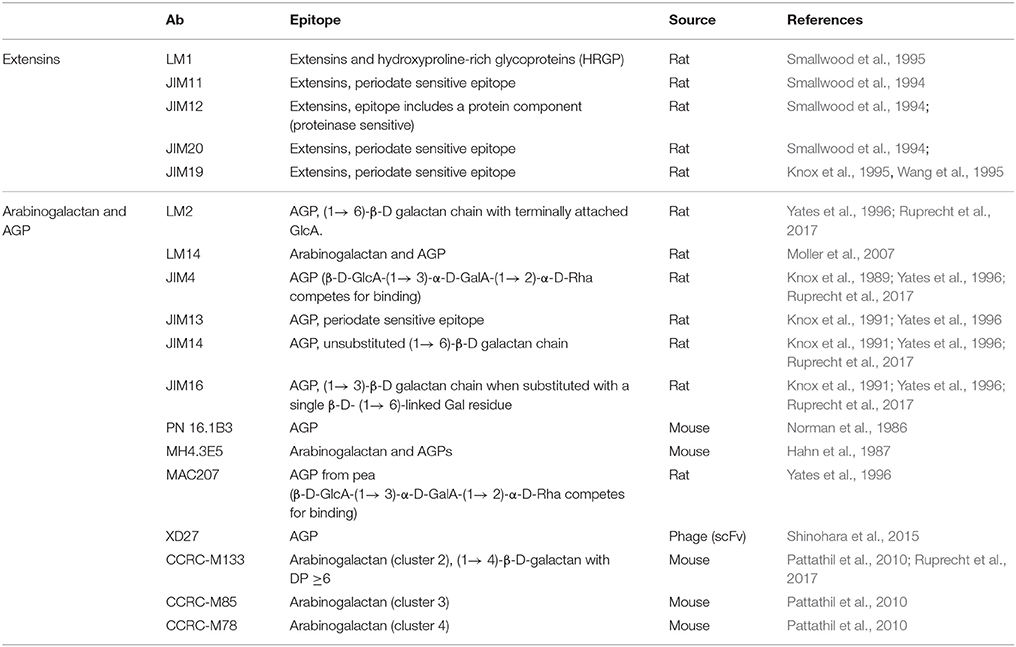
Antibodies are the largest, and possibly the most important, component of the CWP tool box. They are highly specific, versatile, and can be applied to a diverse range of techniques (Cummings et al., 2017; Figure 2). One of the biggest advantages of antibodies is that they can be developed against virtually any antigen, as long as there is a significant immune response in the host. The production of antibodies against plant carbohydrates was initiated in the 1980's and, to date, hundreds of plant cell wall-related antibodies have been reported, covering all classes of the major polymeric components and some of their molecular characteristics (Pattathil et al., 2012). Tables 1–5 provide an overview of the most notable antibodies related to pectins (Table 1), hemicelluloses (Table 2), proteoglycans (Table 3), cell wall phenolics (Table 4), and algal polysaccharides (Table 5). The website1 curated by the CCRC, at the University of Georgia, is another great resource for mining information regarding cell wall related antibodies, including their specificities.
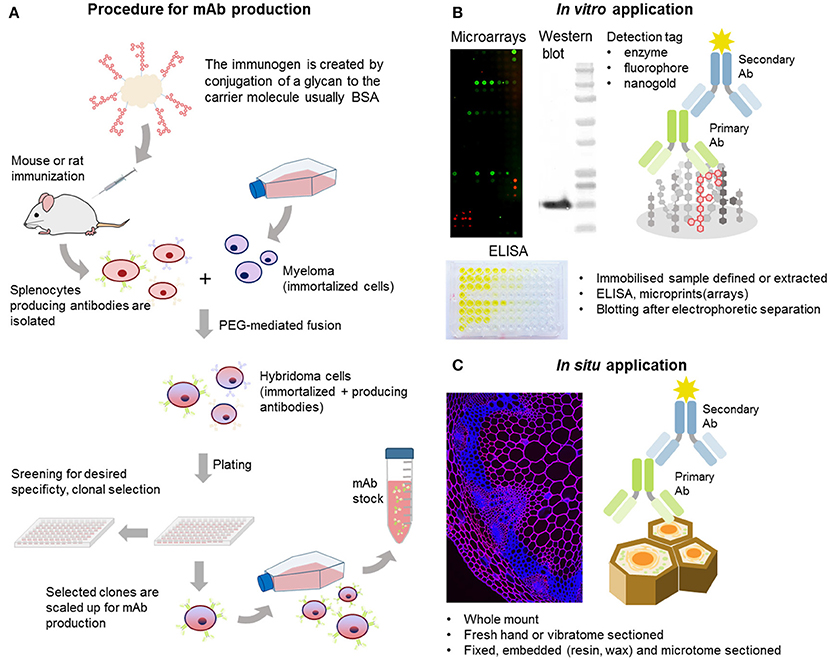
Figure 2. Generation and examples of mAb applications. (A) Simplified scheme of the standard procedure for anti-glycan mAb production. Unlike whole proteins, glycans are conjugated to a carrier molecule (typically BSA or KLH) before immunization. (B) Generated mAbs can be used in vitro to profile cell wall extracts in microarray applications or in ELISA-based methods like epitope detection chromatography providing also more structural information. (C) MAbs can be also used in situ for localization of the target molecules in situ on various types of plant material whole mount or sectioned by various methods.
The majority of these are mAbs produced from rat or mouse hybridoma cells, resulting from cell-based isolation procedures (Köhler and Milstein, 1975; Knox, 2008). However, a handful have also been generated as polyclonal Abs (pAbs). The standard procedure for the generation of an anti-glycan mAb is depicted in Figure 2A, where the immunogen is generated through conjugation of the target molecule to a carrier, which is usually bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH). The protocols used are not significantly different from those for the generation of anti-protein or anti-peptide mAbs. Hybridoma, as immortalized cells, can in theory be cultivated indefinitely, thus supplying high affinity mAbs in relatively large quantities. However, the loss of production capability or specificity in some hybridoma lines over time is a serious problem. To overcome these problems, switching to recombinant mAb production is recommended. In addition, new quality standards for the characterization, propagation, and tracking of cell lines are being implemented in the immuno-biotechnology field (Weller, 2016).
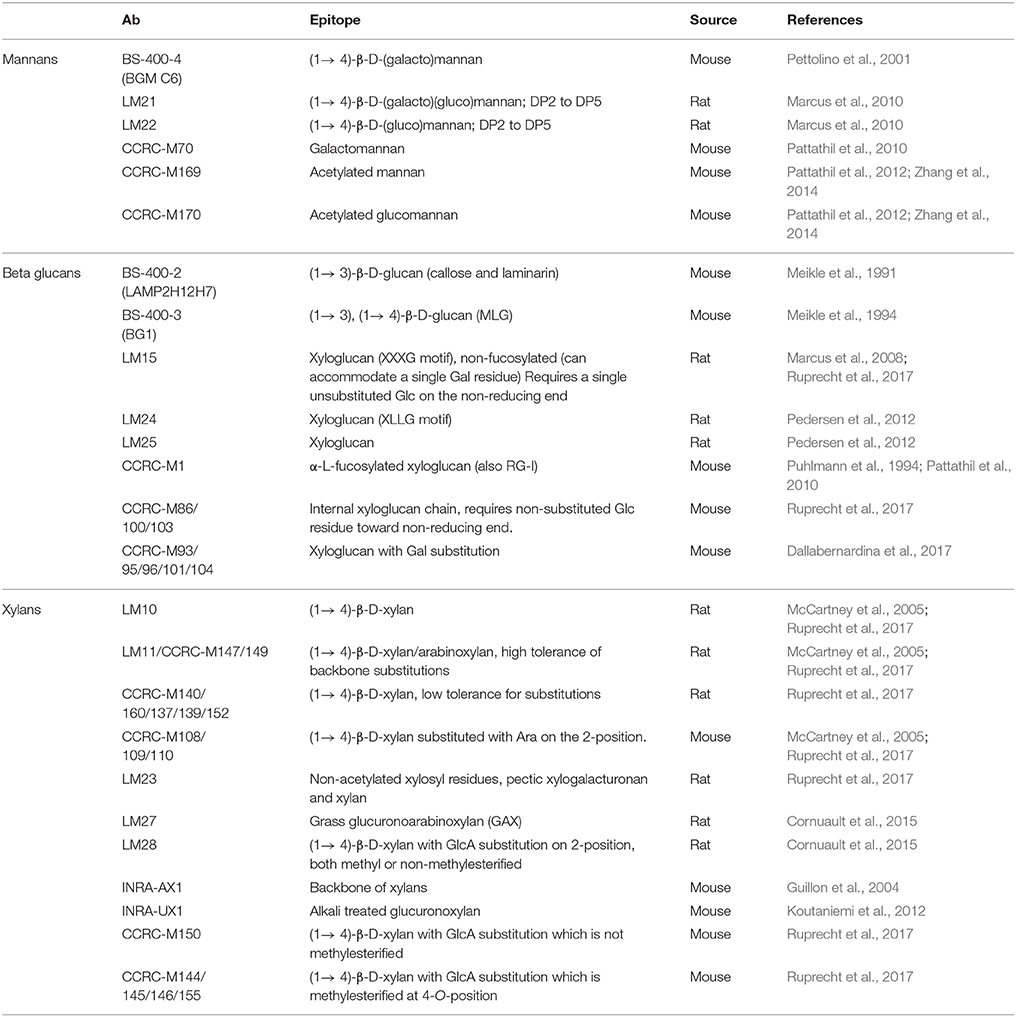
Unlike polyclonal sera, mAbs only bind one epitope (a recognized motif on the antigen molecule), a feature that is especially important in plant glycobiology. Many polysaccharides are not homogeneous polymers and instead exhibit various molecular alterations to their backbone. A classic example of this is homogalacturonan (HG), which can have different levels and patterns of methyl-esterification (Willats et al., 2001). The current set of anti-HG antibodies available can distinguish between these patterns (Verhertbruggen et al., 2009a) and some can even recognize HG in its supramolecular conformation (Table 1). For example, mAb 2F4 recognizes HG with a low DE but when it is in a complex with a divalent calcium (e.g., egg boxes, pectin gel) (Liners et al., 1989). The epitope of a carbohydrate-recognizing antibody varies in size depending on the mAb, from a single monosaccharide moiety up to a complex glycan or a long stretch of more than 10 monomeric units. Most mAbs used in the cell wall field recognize molecular structures consisting of 2–6 monosaccharide units, which is enough for them to distinguish between different types of polysaccharides. For mAbs that recognize cell wall proteoglycans, the epitope may contain a protein, a glycan part or a combination of both. See Figure 3A for an example of epitopes that can appear on one type of polysaccharide, in this case rhamnogalacturonan I (RG-I). It is important to note that some epitopes can be present on different classes of cell wall components, for example arabinogalactan chains, which are typical for both RG-I and arabinogalactan proteins (AGPs) (Pattathil et al., 2010).
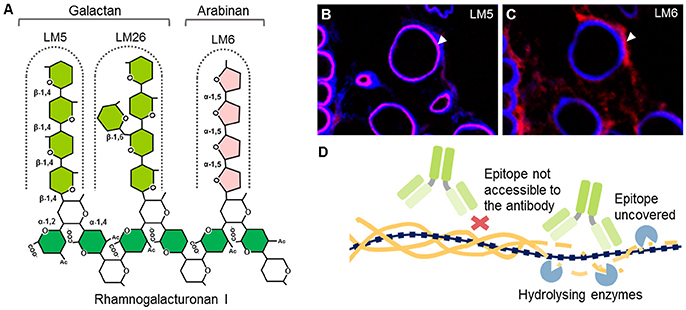
Figure 3. Example of mAb-recognized epitopes, cell wall heterogeneity, and masking. (A) Example of different epitopes recognized by mAbs on RG-I side chains. Both LM5 and LM26 mAbs bind to (1 → 4)-β-D galactan epitopes with a difference in requirement of a Gal substitution via α-D-(1 → 6) bond in the case of LM26. LM6 requires a linear chain of four α-(1 → 5) linked L-arabinose units. This epitope can be found also on AGPs. (A,B) LM5 and LM6 mAbs can have affinity toward different cell wall microdomains. An example of an in situ labeling of sections of resin embedded pea border cells with LM5 and LM6. Calcofluor White (blue channel) and signal from the secondary anti-rat antibody conjugated to Alexa Fluor 555 (red). (B) LM5 labels cell walls of released border cells, whereas (C) LM6 labels the shed cell wall material released to the environment (arrowheads). For the original experiments see Mravec et al. (2017a). (D) Phenomenon of masking of mAbs epitopes. Cell wall carbohydrates are arranged in tight arrays and this could prevent binding of mAbs. Pre-digestion with specific enzymes can reveal these “hidden” epitopes.
mAbs are relatively easy to manipulate or modulate, and can be used in various forms. For example, expressed and purified variable small fragments of native antibodies can be utilized as a version of mAbs with a significantly reduced size (~15–55 kDa as opposed to a 150 kDa full protein) (Figure 1). Depending on the end-application, the use of a secondary antibody, or protein A or G allows for different indirect detection methods. Microarray and ELISA-based applications usually allow detection through the use of a chromogenic enzymatic reaction via horse radish peroxidase (HRP) or alkaline phosphatase (AP) conjugated to the secondary antibody (Pattathil et al., 2015a,b; Kracun et al., 2017; Figure 2B). By contrast, for in situ immunohistochemistry, fluorescent conjugates of secondary antibodies are usually the first choice while for transmission electron microscopy (TEM), nanogold conjugates of secondary antibodies or protein A/G are required (Wilson and Bacic, 2012) (Figure 2C). A curious fact is that the names of most mAbs are based on the names of the institutes where they have been generated: JIM, John Innes Centre Monoclonal; LM, University of Leeds Monoclonal; INRA, Institut National de la Recherche Agronomique; CCRC, Complex Carbohydrate Research Center; BS, Biosupplies; KM- Kyoto University and, finally, INCh, a collaboration between INRA and the University of Copenhagen.
Recent Additions to the Repertoire of mAbs
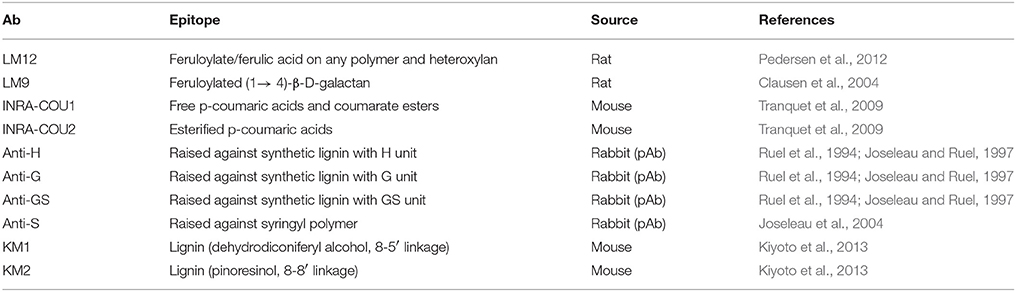
Several new additions to the repertoire of mAbs that recognize land plant polysaccharide structures have been reported over the last 5 years, such as the INRA-AGI-1 mAb, which recognizes the RGI-related arabinogalactan linear chain (Buffetto et al., 2015), and the new xylan-related mAbs LM27 and LM28 (Cornuault et al., 2015). Lignin is a highly heterogeneous phenolic polymer, which is of great scientific interest as it has a strong influence on the effectiveness of bioconversion of plant cell walls to biofuels (Li et al., 2016). However, the number of lignin immunological probes with well-defined specificities has been limited (Table 4). In 2013, it was reported that the KM1 and KM2 antibodies could recognize two distinct phenolic linkages present in lignin (Kiyoto et al., 2013), therefore representing an important, but hitherto relatively overlooked, addition to CWPs related to cell wall components of land plants.
At present, there is an appreciable shift of interest into research on algal cell walls. Increasing our knowledge about them is essential with respect to our basic understanding of the evolution of cell walls (Popper et al., 2014; Harholt et al., 2016), and the biology of algae as a whole. Algal cell wall polysaccharides are also becoming increasingly important as pharmaceuticals, nutraceuticals and food additives (Wells et al., 2016). Recently, two sets of mAb designated as Brown Alga Monoclonal (BAM) antibodies have been developed; one against fucoidans (Torode et al., 2015) and one set against alginates (Torode et al., 2016). The latest addition to the algal probe repertoire is a mAb against the green algae polysaccharide ulvan (Rydahl et al., 2017; Table 5). However, algal polysaccharides are highly diverse molecules and there are still many potential targets. For instance, no antibodies have been reported against red algae polysaccharides agarose and its derivative porphyran. The current set of mAbs should still be extended with those having well-defined specificities against special structural conformations of algal polysaccharides, substitutions on the backbone and other occurring variable molecular features. Recent advances in HTP screening platforms reviewed in the following paragraph could be highly beneficial in generating such CWPs.
Detailed Characterization of Recognized Epitopes by Chemical Synthesis and HTP Screening
One of the most challenging aspects of mAb development is the precise determination of the recognized epitopes. Large series of mAbs have been generated in the past but the descriptions of their binding patterns were often vague. This led to the development of HTP screening platforms of mAbs. Over the last 10 years, they have been already used for extensive hierarchical clustering analysis of antibody specificities (Moller et al., 2008; Pattathil et al., 2010), and in combination with chemical synthesis of pure oligosaccharides, have enabled a more detailed characterization of epitope structures (Moller et al., 2008; Pedersen et al., 2012; Andersen et al., 2016; Dallabernardina et al., 2017; Ruprecht et al., 2017; Torode et al., 2017). Notably, a large set of CCRC antibodies have recently been characterized in detail using 88 artificial oligosaccharides related to xylan, xyloglucan, RG-I, and arabinogalactan chains, and some patterns were resolved in high resolution (Ruprecht et al., 2017). Another recent study more deeply deciphered the epitopes of two galactan-specific mAbs: LM5 and LM26. This study showed that LM5 requires at least three galactose units at the non-reducing end of the galactan chain (Andersen et al., 2016) while LM26 requires a single substitution with a Gal residue via a β-(1 → 6) bond and at least three units of the backbone (Torode et al., 2017; Figure 3A). Both antibodies also exhibit interesting complementary labeling patterns in phloem (Torode et al., 2017) that relates to the mechanical properties of the cells in this tissue. Similarly, a group of xyloglucan-specific mAbs requiring Gal substitutions were characterized by Dallabernardina et al. (2017). We expect that these valuable efforts, which show the great potential of HTP platform in characterizing mAbs, will continue in providing a refined specificity data set for most mAbs.
Remaining Gaps in mAb Availability
Despite the existence of a large panel of mAbs, there are still significant gaps with respect to target recognition. These gaps limit research efforts to understanding the biological role of some polysaccharides but also their endogenous chemical modifications. This is especially true for O-acetylation, a common modification that has a significant negative effect on biomass bioconversion. Although acetyl esters appear on many polymers (Gille and Pauly, 2012; Nafisi et al., 2015), so far only mAbs against acetylated mannan have been reported (CCRCM169 and CCRC-M170) (Pattathil et al., 2012; Zhang et al., 2014).
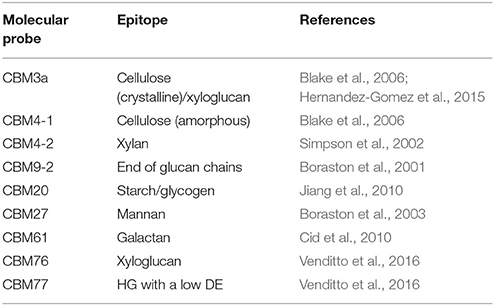
Interestingly, no antibody has yet been generated toward the most abundant polymer in nature—cellulose. CBM3a is currently used but lacks a high-degree of specificity toward cellulose (Blake et al., 2006; Hernandez-Gomez et al., 2015). The development of highly specific mAbs as tools truly capable of discriminating between amorphous and crystalline cellulose would be a major breakthrough, especially for the analysis of secondary cell wall formation, or for monitoring of biomass deconstruction and the saccharification process in its entirety. Hopefully, the recent success in the generation of anti-starch mAbs (Rydahl et al., 2017) might invoke a necessary momentum and optimism for the initiation of such efforts.
Polyesters, like cutin or suberin, are also deposited in the extracellular space of some specialized tissues where they play an essential role in water management and the formation of a semipermeable barrier between the plant and environment (Andersen et al., 2015; Fich et al., 2016). These compounds are constructed from various monomeric units and present in highly localized cell wall microdomains, such as the Casparian strip (Andersen et al., 2015). Currently, their visualization in situ relies solely on cytological staining. If developed in the future, CWPs for these special cell wall components and their numerous structural variants, could be used in conjunction with immunolocalization of relevant biosynthetic enzymes. This can help to unravel how these compounds are synthesized and spatially deposited in a very site-restrictive manner.
The lack of specific detection tools is not only limited to above mentioned cell wall components but also to their fine in muro arrangement. Cell walls are constructed as an intricate array where different polysaccharides are mutually interlinked to form desired spatial 3D configurations. These linkages can form through covalent bonding (Tan et al., 2013; Cornuault et al., 2015) or non-covalent association (e.g., hydrogen bonding) between various classes of polymers (Cosgrove, 2016). Visualization and quantification of these intermolecular linkages with probes would dramatically advance the study of cell walls and allow a deeper understanding their dynamics during development. This is especially true for the cell wall remodeling during cell elongation that involves rapid dissociation of xyloglucan and cellulose microfibrils after apoplastic acidification (Cosgrove, 2016; Höfte and Voxeur, 2017). To visualize this elusive biological phenomenon in real-time using CWPs, would require highly unorthodox approaches, some of which are discussed later in this review.
Non-conventional Approaches in the Generation of Novel mAbs
Antibody development is often limited because some molecules do not induce an adaptive immune response in the immune system of the host animal. The level of immunogenicity of plant polysaccharides is known to be much lower than for proteins, partially due to the fact that their 3D molecular structure is not as complex as those of proteins. In addition, different pathways are induced in response to carbohydrate and protein structures (Cunto-Amesty et al., 2001). The lack of a sufficient immune response to certain carbohydrate moieties can, in some cases, be explained by the fact that some of these molecules are a part of an animal's daily feed. Low immunogenicity is a well-known, although relatively surprising, problem in the case of rhamnogalacturonan II (RG-II) despite its highly complex molecular structure (Albersheim et al., 2010; Pabst et al., 2013). Although, polyclonal antibodies specific to RG-II have been produced (Matoh et al., 1998), no reliable anti-RG-II mAbs have been reported thus far. It has been speculated that this is due to the high-degree of variation in its structure and the level of methylation in different subdomains (Pabst et al., 2013).
One way to circumvent these obstacles could be to develop antibodies in a more randomized manner, where instead of using a single well-defined antigen, a complex mixture of antigens (e.g., whole cell wall extracts) is used in a form of “shotgun” immunization. An antigen overload, or presentation of the antigen in the context of the whole cell wall glycome, might hijack the host's immune response (Rydahl et al., 2017). This has recently been proven to be a successful way to overcome the barrier of the limited immunogenicity of starch (Rydahl et al., 2017). Such an approach could also be a way to remove the existing bias toward well-known carbohydrates, which are found in the primary and secondary cell walls of higher plants. Immunization using crude non-separated samples from e.g., algae, living fossils, less studied or rare species, followed by simultaneous characterization of the mAbs generated and the antigens by HTP analytical techniques may lead to the identification of completely new polysaccharides, or other cell wall components, by having probes for them already in hand.
In Vitro Applications of mAbs
One of the most powerful applications of anti-glycan mAbs is in HTP glycan profiling, either in nitrocellulose-based microarrays (also called comprehensive microarray polymer profiling, CoMPP) or ELISA-based methods (Moller et al., 2007; Pattathil et al., 2015a; Kracun et al., 2017). Since its introduction, HTP cell wall profiling has proved to be extremely beneficial in the characterization of the cell walls of different species (Sørensen et al., 2011; Fangel et al., 2012; Hervé et al., 2016), elucidation of the properties and composition of biomass (Pattathil et al., 2015b; Djajadi et al., 2017), analysis of the tissue specific distribution of given epitopes (Leroux et al., 2015; Wilson et al., 2015), and for studying enzymatic characteristics (Vidal-Melgosa et al., 2015; Walker et al., 2017). During CoMPP, the extracted material is printed in the form of a microarray using a nitrocellulose matrix. In the case of ELISA-based assays, the extractions are immobilized on an immunosorbent plate and probed. Glycan profiling can be done in tandem with other biochemical methods thus expanding the data obtained such as for example, more structural information. This includes, for instance, epitope detection chromatography (EDC), which couples size-exclusion or anion-exchange chromatography and immunodetection (Cornuault et al., 2014), or HTP analysis of enzymes with cell wall degrading activities using microarray printing and profiling of the reaction products (Vidal-Melgosa et al., 2015).
One of the major limitations of these applications is the semi-quantitative nature of mAb probing. The data produced are usually presented as normalized relative values but they do little to account for the different avidity and affinity of the various mAbs. This can lead to the risk of misinterpretation of results. Affinity is the strength of the interaction between an epitope and its corresponding paratope, while avidity is the total strength of the interaction between the antigen and the entire immunoglobulin and is dependent on its valence. Two antibodies can have different affinities and avidities even when recognizing the same epitope. In other words, value signals obtained for two different antibodies do not necessarily reflect the actual difference in epitope abundance. Moreover, the quantification of an antigen's absolute concentration by comparison to known standards is hindered by the non-linear nature of color product development in e.g., HRP or AP-based secondary probing. Taken together, caution should be exercised when comparing different antibodies, as well as when different detection methods are used for the same antibody.
In Situ Applications of mAbs
mAbs are also usually a researcher's first choice for use in in situ analysis (Hervé et al., 2011; Avci et al., 2012; Verhertbruggen et al., 2017). mAbs can be used either with whole mounts, for instance, for surface labeling of Arabidopsis roots (Larson et al., 2014) or pollen tubes (Chebli et al., 2012) on fresh hand- or vibratome-sectioned material, or on embedded and (ultra-, cryo-) microtome sectioned material. Figures 3B,C shows resin-embedded and sectioned pea root apices that have been probed with two mAbs (LM5 and LM6) as an example of in situ analysis of the heterogeneity of cell walls. There are considerable methodological differences between the various methods for sectioning and their applications to address particular scientific questions. These have been extensively compared by Verhertbruggen et al. (2017). The large size of mAbs is sometimes an obstacle for accurate analysis, even in very thinly sectioned material. A phenomenon described as “masking” can affect the results of in situ localization studies (Marcus et al., 2008). Cell wall polysaccharides are arranged in a very compact way in the cell wall, interacting though weak interactions (hydrogen bonds, van der Waals interactions), which limits the accessibility of the antibodies to the epitopes (Figure 3D). However, pre-treatment of samples with various hydrolytic enzymes can reveal, or “unmask,” the hidden epitopes (Marcus et al., 2008, 2010; Xue et al., 2013; Kozlova et al., 2014; Buffetto et al., 2015). Although masking is often considered to be an experimental hurdle, a detailed elucidation of this phenomenon may provide important, and otherwise elusive, information about the nature of tight arrays of polysaccharides and their mutual interaction in muro. mAbs are also less efficient than other smaller probes (discussed below) for in planta studies, not only because of their size. For instance, mAbs are produced at mammalian pH, which is much higher than the normal acidic pH of the apoplast (Cosgrove, 2005). This could limit the binding of some antibodies when used directly in growth media or generate artifacts. Furthermore, preservatives like sodium azide—present in commercial antibody preparations—may have a toxic effect on the living sample.
Phage Display—Generation of Cell Wall Binders Without Immunization
In the late 1980's, the expansion of molecular biology methods and DNA cloning also allowed the development of innovative ways to produce synthetic antibodies. Phage display technology is a recombinant technique for the screening and selection of recombinant paratopes against a desired compound (McCafferty et al., 1990). Unlike traditional antibody production, which uses the host system for antibody selection, this approach requires potential binders to be expressed and presented on the surface of a phage (bacterial virus; most commonly the M13 phage). A library of DNA sequences of vFc fragments are cloned into a phagemid vector and the fragment is then expressed in fusion with one of the phage capsid proteins (Figure 1, Nissim et al., 1994). Several rounds of panning steps with an immobilized antigen and E. coli host reinfection can allow selection of the strongest and most specific binders. In terms of advantages, this method of production can be targeted against any molecule. It is a valuable second choice when classical antibody production is unsuccessful due to a low immunological response in the host. Finally, the use of phage display has a strong bioethical advantage over classical antibody production as it does not use laboratory animals.
However, the current selection of reported phage display-based probes is not yet extensive. The CCRC-R1 phage was the first attempt to overcome the low immunogenicity of RG-II (Williams et al., 1996), and was followed by the better known and more widely used PAM1, which recognizes long stretches of HG lacking ester groups (more than 30) (Willats et al., 1999, 2000). Other phage display-generated probes include B3 (anti-carrageenan) (Liners et al., 2005), XD3 [anti-(1 → 4)-β-D-galactan], and XD27 (anti-AGP) (Shinohara et al., 2015). One of the main disadvantages of the phage display approach is that the panning selection requires the binding of whole phage particles (see Figure 1 dashed square for size comparison to mAbs) and, therefore, strongly relies on the strength of the paratope-epitope interaction. Experience shows that charged molecules (e.g., sulfated polysaccharides or HG with low DE) that are able to form strong polar interactions are usually the best target for phage display. Another limitation is that only small variable fragments, like scFv or random peptide libraries, are suitable for bacterial expression in phage display. Due to the size limitations of the phagemid vector, whole antibodies cannot be presented on phages (Steinwand et al., 2014).
CBMs—Proteinaceous Probes With a Moderate Size and High Manipulability
Some proteins, or parts of proteins, exhibit an affinity for carbohydrates as an essential feature of their activity. As such, they can be also used as probes (Figure 4; Table 6). For instance, carbohydrate-active enzymes (CAZYmes)2 are frequently appended with non-catalytic carbohydrate-binding modules (CBMs), typically 5–20 kDa in size (Gunnarsson et al., 2004), through a highly flexible linker (Gilbert et al., 2013; Figure 4A). CBMs are, by the CAZy database2 (The CAZypedia Consortium, 2018) definition, a contiguous amino acid sequence within a carbohydrate-active enzyme with a discrete fold having carbohydrate-binding activity. In some cases, large multi-enzyme complexes known as cellulosomes, with several CBMs appended, have also been described (>3 MDa) (Fontes and Gilbert, 2010; Smith et al., 2017). Currently, known CBMs are grouped into 83 families based on their amino acid sequence3 These can further be sub-grouped into CBM A-, B-, or C-type depending on their binding properties to polysaccharides (Figure 4B). Type A, B, and C CBMs are defined as binding crystalline surfaces, glycan chains, and short oligosaccharide sequences respectively (Boraston et al., 2004). Through substrate targeting, CBMs have the ability to enhance the activity of appended enzymes (Hervé et al., 2010). Although the convergent binding specificity may assign a CBM to a certain family, the function of the specific structure might be divergent (Montanier et al., 2009).
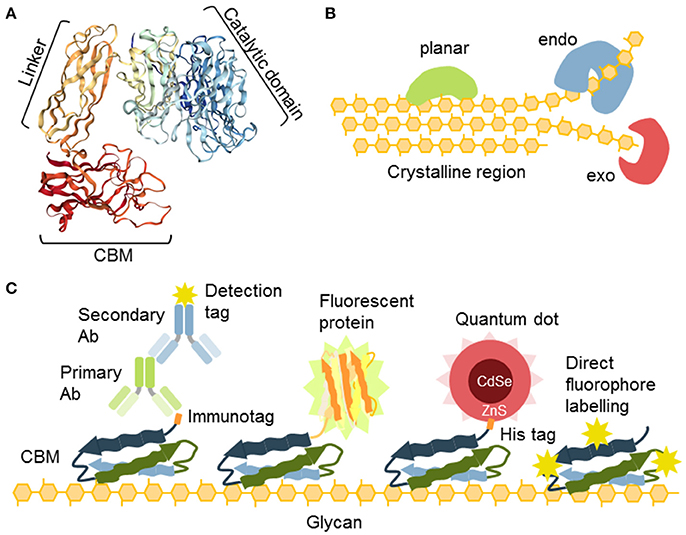
Figure 4. The nature and versatility of CBMs as probes. (A) CBMs are often a part of glycosyl hydrolases connected to the catalytic unit with a flexible linker. (B) Different types of cellulose-specific CBMs. Some binding is planar, such as to the crystalline part of cellulose. Some require amorphous parts and their binding can be characterized as “endo” and some bind ends of chains and can be characterized as “exo” binders. (C) CBMs offer a variety of secondary detection methods: (i) detection via immunotags (His, GST), (ii) as fusion constructs with fluorescent proteins, (iii) through coordination binding of the His tag with ZnS shell of quantum dots, (iv) CBMs can also be directly conjugated to fluorophores.
Expressed separately, these modules can be utilized in the context of the CWP as recombinant proteins (Figure 4C). Their detection is accomplished via an immunological tag (His, myc, GST, or other), or they can be used directly as fusion proteins with fluorescent proteins (Porter et al., 2007; Kawakubo et al., 2010; Badruna et al., 2017) or as conjugates to fluorophores (Dagel et al., 2011; Ding et al., 2012; Khatri et al., 2016). Interestingly, His-tagged CBMs can also be detected using quantum dots (brightly fluorescent semiconductor particles) with an outer shell that contains ZnS, which allows direct coordination by binding directly to a His-tag (Ding et al., 2006; Figure 4C).
The possibility of the mutational manipulation of the protein sequences of CBMs generates a momentum for engineering new specificities, which has already been explored to some extent leading to the creation of xylan and xyloglucan-specific CBMs (Gunnarsson et al., 2006), amongst others. This was accomplished by generating a combinatorial library of CBMs using limited substitution of specific amino acids in the binding cleft of the module (Gunnarsson et al., 2004, 2006). Consequently, the potential for diversification of CBMs as molecular probes has been shown. A major challenge in the post-genomic era is the analysis of ligand specificity for predicted CBMs, derived from genomic sequencing. While there is a growing list of over 80.000 modules that are assigned as CBMs in the CAZy database, only a minute fraction of these have been studied in detail and empirically tested (Gilbert et al., 2013; Venditto et al., 2016), and only a handful are regularly used as probes.
Labeled Cell Wall Degrading Enzymes as Probes
Cell wall component-degrading enzymes usually exhibit high levels of substrate specificity (Obeng et al., 2017) and the 3D molecular structure of many enzymes, and their different functional domains, have been described. Site-directed mutation can inactivate catalytic sites, leaving the binding site intact and functional, creating a possibility for novel proteinaceous probes. This has, for example, been explored by Dornez et al. (2011), who created an arabinoxylan-specific probe using an inactivated and fluorophore-labeled xylanase from Bacillus subtilis. It has also been shown that labeled active enzymes, like cellulases, can be used, particularly to study different aspects of the spatiotemporal dynamics of cellulose degradation (Ding et al., 2012; Wang et al., 2012; Luterbacher et al., 2013). Although these proteinaceous probes have been shown to provide high levels of resolution and specificity (Ding et al., 2012; Wang et al., 2012), they are still waiting for a more widespread usage in fields outside of biomass biotechnology.
Small Molecular CWPs—a New Set of Fine Tools
Many of the proteinaceous CWPs mentioned above are suitable for many applications but have serious limitations with respect to the tracking of polysaccharides in living systems, and in the real-time study of the dynamics of cell wall architecture. Small direct molecular probes can negate some of the shortcomings of typically much larger proteinaceous CWPs. Developments in this field have been very successful over the last decade with some innovative solutions.
Fluoro(Chromo)Phores—The Smallest Specific Binders Available
One of the most classical cytological methods to detect cell wall components is the use of dyes and stains. Although these stains are useful for morphological studies (especially Toluidine Blue which is dichromatic), they usually do not provide sufficiently high-resolution images and their specificity is often questioned. Despite this caveat, some are strongly favored and are not yet replaceable. Such an example of this would be the Yariv reagent for diagnostic detection and purification of arabinogalactan proteins (AGPs), or phloroglucinol (Wiesner stain), an easy and inexpensive reagent to detect lignin. For an overview of cell wall cytological staining methods see Krishnamurthy (1999).
Unlike classical stains for light microscopy, fluorophores, also called fluorochromes, or simply fluorescent dyes, can provide higher resolution images (Paës, 2014). Moreover, due to their low toxicity, they are sometimes suitable for in vivo imaging, even when using spinning disc or super-resolution confocal microscopy (Anderson et al., 2010; Liesche et al., 2013). They are relatively small but, although this is advantageous for in vivo studies, this feature can also significantly limit their specificity. The most commonly used fluorophores are listed in Table 7 and their structures and examples of their uses in situ are shown in Figure 5. Calcofluor White has been traditionally used as a general cell wall counterstain in immunohistological analyses. However, it is important to point out that it is not a cellulose dye as sometimes claimed—it binds all sorts of plant cell wall, bacterial, and fungal β-glucans including chitin. The advantages of Calcofluor White is its ease of visibility, rapid labeling, inexpensiveness, and its spectral properties that make it suitable for multichannel confocal imaging (Harrington and Hageage, 2003). Unlike Calcofluor White, some fluorochromes exhibit relatively high levels of specificity. Pontamine Scarlet 4B, for example, is more target-specific and its binding to cellulose can be successfully used for high-resolution in vivo studies (Anderson et al., 2010; Liesche et al., 2013), such as studying the deposition and orientation of cellulose microfibrils during cellular elongation (Anderson et al., 2010). Sirofluor is an essential fluorophore for in situ visualization of callose deposition (Evans and Hoyne, 1982; Stone et al., 1982). Propidium iodide (PI) has been used for a long time as a cell wall-illuminating counterstain for in vivo imaging of genetic markers with fluorescent proteins. One report more closely elaborated on PI binding and reported that PI competes with calcium in association with negatively charged pectins (Rounds et al., 2011). However, plant cell walls also contain other types of charged molecules and PI specificity has not been determined by more elaborate in vitro methods. Here it is important to note that PI staining of cell walls works only during in vivo imaging but not on fixed and sectioned material. PI exhibits a strong affinity for nucleic acids (this property of PI is used in other models), and prefers them over any cell wall material in fixed samples (Figure 5).
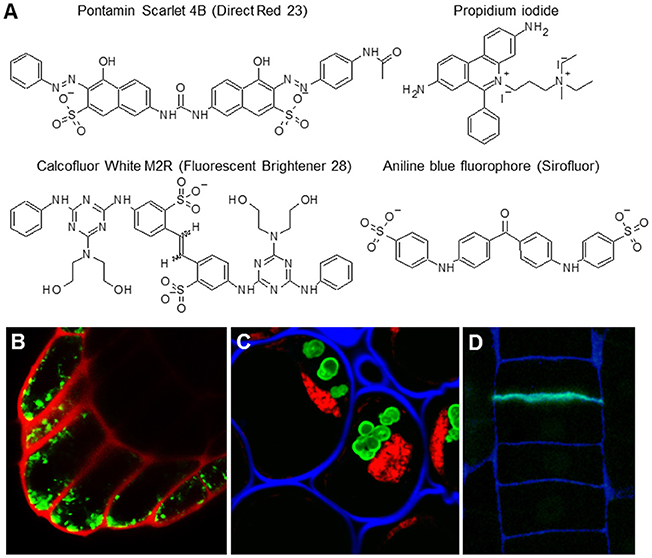
Figure 5. The most common cell wall-related fluorophores. (A) Molecular structures of four fluorophores. (B) Propidium iodide (PI) is traditionally used as cell wall counterstain for in vivo imaging of expression and localization of fluorescent proteins. Scan of the Arabidopsis columella root cap expressing YFP marker (green) counterstained with PI (red). (C,D) The fluorophores can be used in tandem and are compatible with other detection methods like immunolocalization. (C) Staining of the section of resin embedded pea root cap with INCh1 (anti-starch antibody, starch granules), Calcofluor White (blue; cell walls), and PI (red, staining nuclei). For the original experiments see Rydahl et al. (2017). (D) Sirofluor is specific for callose (Stone et al., 1982) which is present also in newly made cell walls. Example of a resin section of a root epidermis stained with Sirofluor (green) and Calcofluor White (blue). Note the septum-specific staining of Sirofluor.
Oligosaccharide Conjugates—Emerging Probes
Interestingly, certain oligosaccharides can also be used as CWPs (Table 8; Figure 6). For example, it has been known for a long time that defined fragments of xyloglucan (XGO) are substrates for “cut and paste” enzymes – xyoglucan endotransglycosylases (XETs) (Franková and Fry, 2013). When tagged XGOs are applied to plants their incorporation localizes the XET activity in vivo but also demonstrates the presence of the acceptor—the xyloglucan backbone (Vissenberg et al., 2000, 2001; Figure 6B). Particular physicochemical properties of oligosaccharides can be exploited as well. Recently, we introduced the chitosan oligosaccharide (COS) and oligogalacturonide (OG)-based probes (Mravec et al., 2014, 2017b; Figure 6A). Chitosan is a de-acetylated form of chitin and it bears positive charges in a spatial configuration that can interact with carboxyl groups on HG lacking methyl esters. Through a strong ionic interaction, the long OGs have been shown to be able to generate complexes with endogenous homogalacturonan in the presence of divalent cations, and can therefore mark sites with the ability for de novo egg box-formation (Mravec et al., 2017b; Figure 6A). This approach has demonstrated the dynamics of pectin complexation in tip growing structures: pollen tubes and root hairs. It is important to note that both probes can also be elicitors of cellular responses; chitin is a pathogen associated molecular pattern (PAMP) while OGs are damage-associated molecular patterns (DAMPs) (Malinovsky et al., 2014). Despite this disadvantage, they provide versatility in terms of different tags due to the possibility of conjugation to various florescent tags and even nanogold particles (Mravec et al., 2014). They are smaller than antibodies and exhibit a better penetration capacity than mAbs (Mravec et al., 2014). Unfortunately, these types of probes are currently limited to certain polysaccharides with special physicochemical properties. However, the association of two oligo(poly)saccharides can actually be governed by other molecular interactions, including hydrogen bonding or van der Waals forces. This means that structural modeling in combination with chemical synthesis of diverse artificial oligosaccharides might in theory expand this section of the toolbox in the future.
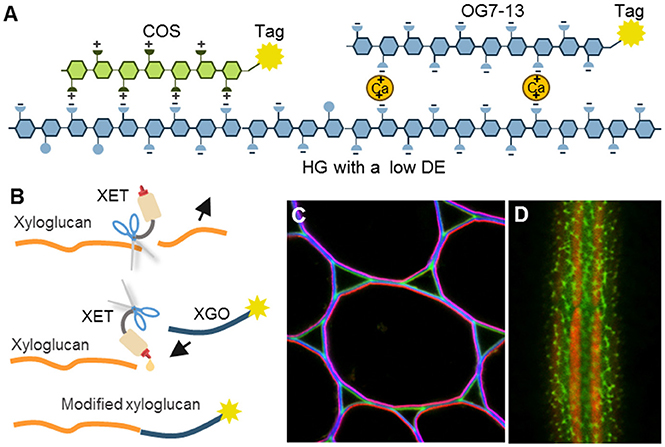
Figure 6. The mechanisms of oligosaccharide probes and examples of their usage. (A) Homogalacturonan (HG) with a low degree of esterification (DE) can be detected by positively charged chitosan oligosaccharides (COS) or by calcium-mediated complexation to long oligogalacturonides (OG7-13) as a form of artificial egg box formation (Mravec et al., 2014, 2017b). (B) Incorporation of tagged xyloglucan (XGO) oligosaccharides to xyloglucan backbone by an activity of xyloglucan endotransglycosylases (XETs). This can be used to visualize the presence of both, a xyloglucan backbone and XET activity. (C,D) Two examples of in situ usage of COS oligosaccharide probes. (C) Triple labeling of stem parenchyma with COS488 (green), Calcofluor White (blue) and JIM7 antibody (red). Note the specific labeling of the middle lamella and triangular junctions with COS488. (D) Labeling of intricate cell wall structures in single cell green alga Penium margaritaceum with COS488 (green). The red signal is due to chlorophyll autofluorescence. For the original experiments see Mravec et al. (2014).
New Toolbox Compartments Filled With the Metabolic Labeling-Ready Reagents
Tagging of cell wall components by so-called metabolic labeling involves the incorporation of the fluorescent analog/conjugate of a tagged building block into a native molecule in planta and subsequent tracking, a technique which is becoming increasingly important (Figure 7). The introduction of click chemistry technology to cell biology has been hugely effective, and facilitated the tracking of molecules in living models. Click chemistry is a term that was coined by Prof. Sharpless in the early 90's (Kolb et al., 2001). By this definition, the reaction can be called “click” when it is highly specific and efficient, and can be performed under mild conditions. For in situ probing, the most commonly used reaction is the Huisgen bipolar cycloaddition—a reaction between alkyne and azide in the presence of monovalent copper as a catalyst (Rostovtsev et al., 2002; Figure 7B). The ingenious idea behind the method is that one of these biorthogonal (biologically inert) groups can be part of a building block or a molecule of interest, and the other one can be the detection tag, usually a fluorophore. It is important to bear in mind that the copper catalyst is toxic for plants. However, this can be overcome by using a copper-free system, such as strained alkynes (Baskin et al., 2007), which can be used for tracking in live cells.
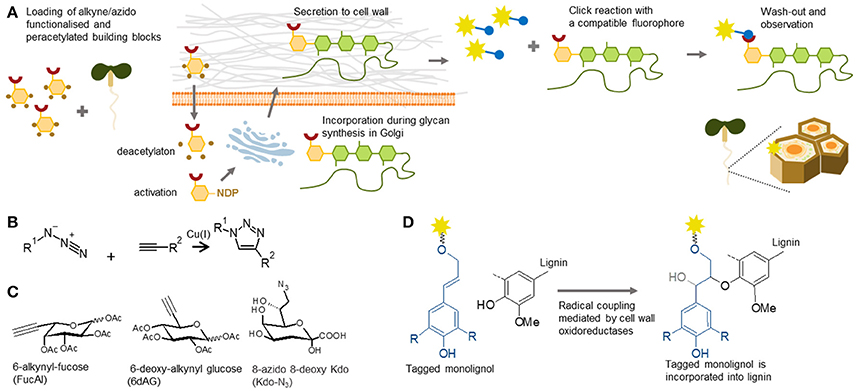
Figure 7. Metabolic labeling. (A) Principle of metabolic labeling or click chemistry-based tracking of polysaccharides in plants. Functionalized monosaccharides are loaded into plants. After entering the cell, they are incorporated by biosynthetic pathways pertinent to the respective glycan. The tracking analysis is enabled by a click reaction to a fluorophore containing a compatible reactive group. (B) Reaction scheme of Huisgen cycloaddition used in click chemistry. The reaction is catalyzed by Cu(I) and requires alkyne and azide groups, one present on the target molecule and one on the detection tag. (C) Three examples of click-chemistry-ready sugar analogs used in the cell wall field: 6-alkynyl-fucose (Anderson et al., 2012), 6-deoxy-alkynyl glucose (McClosky et al., 2016) and 8-azido 8-deoxy Kdo (Dumont et al., 2016). (D) Proposed mechanism of incorporation of functionalizes, in this case fluorophore-tagged monolignols, into lignin according Tobimatsu et al. (2013).
Metabolic Reagents for Polysaccharides and Proteoglycans
Click chemistry has numerous applications in glycobiology studies of animal and yeast systems (Yoon et al., 2017). In 2012, this method was also introduced into the field of plant cell wall biology (Anderson et al., 2012). An alkyne-containing derivative of fucose (FucAl), previously used in animal glycosylation, has been shown to be incorporated into pectin chains (as part of RG-I), which has enabled visualization of the arrangement and sites of pectin delivery at the plasma membrane-cell wall interface (Anderson et al., 2012). A dramatic increase in the panel of available click chemistry-enabled monomers has been reported in the past 2 years and now encompasses almost the whole spectrum of monosaccharides. For example, it now includes azido-KDO, which is incorporated into RG-II chains (Dumont et al., 2016), and alkyne-glucose (McClosky et al., 2016), which is incorporated into a non-specified component of the root hair cell walls and arrests their growth (Figure 7C). These constructs were recently followed by many more, including those that can be used to study O- and N-glycosylation of cell wall proteoglycans (Hoogenboom et al., 2016; Zhu and Chen, 2017). Although these monosaccharide analogs have been evaluated in situ, and some clearly generate cell wall staining, the precise targets have not yet been elucidated by other genetic, physiological, or analytical methods. We consider this a major challenge for the future work within the field of plant polysaccharide and proteoglycan metabolic labeling.
Metabolic Labeling Reagents for Lignin
The expansion of metabolic labeling tools can be also seen in the area of lignin biology. The first such reagents reported, were direct conjugates of fluorophores to monolignols (three types of hydroxycinnamyl alcohols). In glycan structures a large fluorophore molecule on the monomer would likely hinder the activity of the necessary glycosyltransferase as it would not be recognized as an appropriate natural donor. However, lignin polymerization is based on radical coupling and fluorescently labeled monolignols have been shown to be efficiently incorporated into lignin in stem cells undergoing formation of secondary cell walls (Tobimatsu et al., 2011, 2013; Figure 7D). Moreover, these reagents even enabled visualization of local deposition of polyphenolics within the Casparian strip of root endodermis (Tobimatsu et al., 2013). In addition to these fluorescent monolignols, several different click chemistry-ready monolignols, have been synthesized recently (Bukowski et al., 2014; Tobimatsu et al., 2014; Pandey et al., 2015). The latest publication (Lion et al., 2017) provided the most striking demonstration, how a variety of click chemistry reagents can be used for in situ studies of tissue specific lignification and formation of distinct types of lignin. The utilization of two types of clickable monolignols, H unit tagged with an azide group and G unit tagged with an alkyne group in tandem, enabled researchers to follow the dynamics of lignification in flax stems. We believe that by a similar approach, many of the less understood phenomena, such as turnover and salvage pathways of cell wall components (Barnes and Anderson, 2017), can be deciphered using either glycan or polyphenolic metabolic labeling.
Conclusions and Future Directions
Recent progress in plant glycobiology, chemical biology, and HTP analytical methods has expanded the probing tool box plant researchers can use in their studies. However, the development of new CWPs is still progressing, and will undoubtedly mimic the great boost in biomedical probing technologies that we have seen in the past 20 years. We expect that the antibody field will benefit from the recently explored approach of a more biology- than researcher-driven selection process, shifting the current paradigm of anti-glycan antibody production requiring predefined targets. The more widespread use of synthetic antibodies, using recombinant methods and expression, might pave the way for complex targets to be addressed, such as those for which the deconstruction of their native 3D context is an issue in conventional procedures. This is especially relevant for non-covalent interlinks within cell walls. The large collection of mAbs available, as well as CBMs, offer a hitherto unexplored resource for generating genetic probes. In this approach, the DNA coding sequences of the known proteinaceous binders are expressed in planta via stable transgenic constructs as fusion proteins with a small immunotag or fluorescent protein. Although this approach might be problematic for visualization of epitopes in the context of the whole organism, or tissues, due to the constant and ubiquitous expression, it might be useful for studying the dynamics of cell wall microdomains. It is well-known that the binding of an antibody can influence the antigen's expected biological activity so the genetic approach can also provide entirely new tools for functional analyses of cell wall components. Proteinaceous probes can be further engineered by random or via site-directed mutagenesis to widen the diversity of specificities or to modulate their affinity.
Other tools that have already found important uses in biomedical research, like aptamer and affimer technologies, can also be applied to cell wall components, thus opening new frontiers for replenishing the shortage of probes against supramolecular conformations, intermolecular linkages, and fine 3D arrays of polysaccharides in muro. Aptamers are DNA/RNA oligonucleotides (Zhang et al., 2016) while affimers are small proteins (Tiede et al., 2017) exhibiting levels of affinity and specificity similar to mAbs. Due to their small-size, they can be beneficial in applications where accessibility of the epitope is an issue. Furthermore, since they can be developed against a target in its native state, aptamers also offer the possibility for quantification using standard molecular biology methods like qPCR. Interestingly, several anti-glycan DNA aptamers have already been generated, such as those against cellulose (Yang et al., 1998). However, they have not yet been fully investigated as CWPs. The future work will certainly also involve further development of the HTP methods to analyze the specificity and sensitivity of the generated CWPs. HTP profiling platforms, especially those involving synthesized defined oligosaccharides, are a relatively recent advance and their potential has not been fully explored. The combination of HTP screening with large chemical libraries and combinatorial synthesis of fluorophores or artificial oligosaccharides can be another valuable source of exciting new tools for the cell wall probing toolbox.
Author Contributions
MR: drafted the part dealing with mAbs and CBMs; AH: drafted the part about phage display; SK and JM: drafted the parts dealing with small molecular probes while JM assembled the figures, and performed the compilation and editing.
Funding
The work in our laboratory is kindly supported by the Villum Foundation projects PLANET (00009283) and Instant architect (17489), and The Innovation Fund Denmark project BioValue SPIR (0603-00522B).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Claire Holland for her help in editing the manuscript.
Abbreviations
AGP, arabinogalactan protein; AP, alkaline phosphatase; CBM, carbohydrate-binding module; CDTA, cyclohexanediaminetetraacetic acid; CoMPP, comprehensive microarray polymer profiling; COS, chitosan oligosaccharide; DE, degree of esterification; DP, degree of polymerisation; HG, homogalacturonan; HRP, horse radish peroxidase; HTP, high throughput; KLH, keyhole limpet hemocyanin; KDO, 3-Deoxy-D-manno-oct-2-ulosonic acid; NDP, nucleotide diphosphate; OG, oligogalacturonide; PI, propidium iodide; PS4B, Pontamine Scarlet 4B; RG-I/-II, rhamnogalacturonan I/II; XET, xyloglucan endotransglycosylases; XGO, xyloglucan oligosaccharide.
Footnotes
References
Albersheim, P., Darvill, A., Roberts, K., Sederoff, R., and Staehelin, A. (2010). Plant Cell Walls. New York, NY: Garland Science.
Andersen, M. C., Boos, I., Marcus, S. E., Kračun, S. K., Rydahl, M. G., Willats, W. G., et al. (2016). Characterization of the LM5 pectic galactan epitope with synthetic analogues of β-1,4-d-galactotetraose. Carbohydr. Res. 436, 36–40. doi: 10.1016/j.carres.2016.10.012
Andersen, T. G., Barberon, M., and Geldner, N. (2015). Suberization - the second life of an endodermal cell. Curr. Opin. Plant Biol. 28, 9–15. doi: 10.1016/j.pbi.2015.08.004
Anderson, C. T., Carroll, A., Akhmetova, L., and Somerville, C. (2010). Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 152, 787–796. doi: 10.1104/pp.109.150128
Anderson, C. T., Wallace, I. S., and Somerville, C. R. (2012). Metabolic click-labeling with a fucose analog reveals pectin delivery, architecture, and dynamics in Arabidopsis cell walls. Proc. Natl. Acad. Sci. U.S.A. 109, 1329–1334. doi: 10.1073/pnas.1120429109
Avci, U., Pattathil, S., and Hahn, M. G. (2012). Immunological approaches to plant cell wall and biomass characterization: immunolocalization of glycan epitopes. Methods Mol. Biol. 908, 73–82. doi: 10.1007/978-1-61779-956-3_7
Badruna, L., Burlat, V., and Montanier, C. Y. (2017). CBMs as probes to explore plant cell wall heterogeneity using immunocytochemistry. Methods Mol. Biol. 1588, 181–197. doi: 10.1007/978-1-4939-6899-2_14
Barnes, W. J., and Anderson, C. T. (2017). Release, recycle, rebuild: cell wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant. 11, 31–46. doi: 10.1016/j.molp.2017.08.011
Baskin, J. M., Prescher, J. A., Laughlin, S. T., Agard, N. J., Chang, P. V., Miller, I. A., et al. (2007). Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U.S.A. 104, 16793–16797. doi: 10.1073/pnas.0707090104
Blake, A. W., McCartney, L., Flint, J. E., Bolam, D. N., Boraston, A. B., Gilbert, H. J., et al. (2006). Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J. Biol. Chem. 281, 29321–29329. doi: 10.1074/jbc.M605903200
Boraston, A. B., Bolam, D. N., Gilbert, H. J., and Davies, G. J. (2004). Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem. J. 382, 769–781. doi: 10.1042/BJ20040892
Boraston, A. B., Creagh, A. L., Alam, M. M., Kormos, J. M., Tomme, P., Haynes, C. A., et al. (2001). Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry 40, 6240–6247. doi: 10.1021/bi0101695
Boraston, A. B., Revett, T. J., Boraston, C. M., Nurizzo, D., and Davies, G. J. (2003). Structural and thermodynamic dissection of specific mannan recognition by a carbohydrate binding module, TmCBM27. Structure 1, 665–675. doi: 10.1016/S0969-2126(03)00100-X
Buffetto, F., Cornuault, V., Rydahl, M. G., Ropartz, D., Alvarado, C., Echasserieau, V., et al. (2015). The deconstruction of pectic rhamnogalacturonan I unmasks the occurrence of a novel Arabinogalactan Oligosaccharide Epitope. Plant Cell Physiol. 56, 2181–2196. doi: 10.1093/pcp/pcv128
Bukowski, N., Pandey, J. L., Doyle, L., Richard, T. L., Anderson, C. T., and Zhu, Y. (2014). Development of a clickable designer monolignol for interrogation of lignification in plant cell walls. Bioconjug. Chem. 25, 2189–2196. doi: 10.1021/bc500411u
Burton, R. A., Gidley, M. J., and Fincher, G. B. (2010). Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 6, 724–732. doi: 10.1038/nchembio.439
Chebli, Y., and Geitmann, A. (2017). Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell Biol. 44, 28–35. doi: 10.1016/j.ceb.2017.01.002
Chebli, Y., Kaneda, M., Zerzour, R., and Geitmann, A. (2012). The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 160, 1940–1955. doi: 10.1104/pp.112.199729
Cid, M., Pedersen, H. L., Kaneko, S., Coutinho, P. M., Henrissat, B., et al. (2010). Recognition of the helical structure of beta-1,4-galactan by a new family of carbohydrate-binding modules. J. Biol. Chem. 285, 35999–36009. doi: 10.1074/jbc.M110.166330
Clausen, M. H., Ralet, M.-C., Willats, W. G., McCartney, L., Marcus, S. E., Thibault, J.-F., et al. (2004). A monoclonal antibody to feruloylated-(1-4)-beta-D-galactan. Planta 219, 1036–1041. doi: 10.1007/s00425-004-1309-3
Clausen, M. H., Willats, W. G., and Knox, J. P. (2003). Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338, 1797–1800. doi: 10.1016/S0008-6215(03)00272-6
Cornuault, V., Buffetto, F., Rydahl, M. G., Marcus, S. E., Torode, T. A., Xue, J., et al. (2015). Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 242, 1321–1334. doi: 10.1007/s00425-015-2375-4
Cornuault, V., Manfield, I. W., Ralet, M.-C., and Knox, J. P. (2014). Epitope detection chromatography: a method to dissect the structural heterogeneity and inter-connections of plant cell-wall matrix glycans. Plant J. 78, 715–722. doi: 10.1111/tpj.12504
Cosgrove, D. J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861. doi: 10.1038/nrm1746
Cosgrove, D. J. (2016). Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67, 463–476. doi: 10.1093/jxb/erv511
Cummings, R. D., Darvill, A. G., Etzler, M. E., and Hahn, M. G. (2017). “Glycan-recognizing probes as tools,” in Essentials of Glycobiology, Chapter 48, 3rd Edn, eds A. Varki, R. D. Cummings, J. D. Esko, P. Stanley, G. W. Hart, M. Aebi, A. G. Darvill, T. Kinoshita, N. H. Packer, J. H. Prestegard, R. L. Schnaar, and P. H. Seeberger (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), 611–621.
Cunto-Amesty, G., Dam, T. K., Luo, P., Monzavi-Karbassi, B., Brewer, C. F., Van Cott, T. C., et al. (2001). Directing the immune response to carbohydrate antigens. J. Biol. Chem. 276, 30490–30498. doi: 10.1074/jbc.M103257200
Dagel, D. J., Liu, Y. S., Zhong, L. L., Luo, Y. H., Himmel, M. E.;, Xu, Q., et al. (2011). In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J. Phys. Chem. B 115, 635–641. doi: 10.1021/jp109798p
Dallabernardina, P., Ruprecht, C., Smith, P. J., Hahn, M. G., Urbanowicz, B. R., and Pfrengle, F. (2017). Automated glycan assembly of galactosylated xyloglucan oligosaccharides and their recognition by plant cell wall glycan-directed antibodies. Org. Biomol. Chem. 15, 9996–10000. doi: 10.1039/C7OB02605F
Ding, S. Y., Liu, Y. S., Zeng, Y., Himmel, M. E., Baker, J. O., and Bayer, E. A. (2012). How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338, 1055–1060. doi: 10.1126/science.1227491
Ding, S. Y., Xu, Q., Ali, M. K., Baker, J. O., Bayer, E. A., and Barak, Y. (2006). Versatile derivatives of carbohydrate-binding modules for imaging of complex carbohydrates approaching the molecular level of resolution. BioTechniques 41, 435–442. doi: 10.2144/000112244
Djajadi, D. T., Hansen, A. R., Jensen, A., Thygesen, L. G., Pinelo, M., Meyer, A. S., et al. (2017). Surface properties correlate to the digestibility of hydrothermally pretreated lignocellulosic Poaceae biomass feedstocks. Biotechnol. Biofuels 10:49. doi: 10.1186/s13068-017-0730-3
Dornez, E., Cuyvers, S., Holopainen, U., Nordlund, E., Poutanen, K., Delcour, J. A., et al. (2011). Inactive fluorescently labeled xylanase as a novel probe for microscopic analysis of arabinoxylan containing cereal cell walls. J. Agric. Food Chem. 59, 6369–6375. doi: 10.1021/jf200746g
Drakakaki, G. (2015). Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci. 236, 177–184. doi: 10.1016/j.plantsci.2015.03.018
Dumont, M., Lehner, A., Vauzeilles, B., Malassis, J., Marchant, A., Smyth, K., et al. (2016). Plant cell wall imaging by metabolic click-mediated labelling of rhamnogalacturonan II using azido 3-deoxy-D-manno-oct-2-ulosonic acid. Plant J. 85, 437–447. doi: 10.1111/tpj.13104
Evans, N. A., and Hoyne, P. A. (1982). A fluorochrome from aniline blue: structure, synthesis, and fluorescence properties. Aust. J. Chem. 35, 2571–2575. doi: 10.1071/CH9822571
Fangel, J. U., Ulvskov, P., Knox, J. P., Mikkelsen, M. D., Harholt, J., Popper, Z. A., et al. (2012). Cell wall evolution and diversity. Front. Plant Sci. 3:152. doi: 10.3389/fpls.2012.00152
Fich, E. A., Segerson, N. A., and Rose, J. K. (2016).The plant polyester cutin: biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 67, 207–233. doi: 10.1146/annurev-arplant-043015-111929
Fontes, C. M., and Gilbert, H. J. (2010). Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79, 655–681. doi: 10.1146/annurev-biochem-091208-085603
Franková, L., and Fry, S. C. (2013). Darwin review: biochemistry and physiological roles of enzymes that ‘cut and paste' plant cell-wall polysaccharides. J. Exp. Bot. 64, 3519–3550. doi: 10.1093/jxb/ert201
Garbaccio, R. M., and Parmee, E. R. (2016). The impact of chemical probes in drug discovery: a pharmaceutical industry perspective. Cell Chem. Biol. 23, 10–17. doi: 10.1016/j.chembiol.2015.11.011
Gilbert, H. J., Knox, J. P., and Boraston, A. B. (2013). Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struct. Biol. 23, 1–9. doi: 10.1016/j.sbi.2013.05.005
Gille, S., and Pauly, M. (2012). O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 3:12. doi: 10.3389/fpls.2012.00012
Guillon, F., Tranquet, O., Quillien, L., Utille, J. P., Ortiz, J. J. O., and Saulnier, L. (2004). Generation of polyclonal and monoclonal antibodies against arabinoxylans and their use for immunocytochemical location of arabinoxylans in cell walls of endosperm of wheat. J. Cereal Sci. 40, 167–182. doi: 10.1016/j.jcs.2004.06.004
Gunnarsson, L. C., Karlsson, E. N., Albrekt, A. S., Andersson, M., Holst, O., and Ohlin, M. (2004). A carbohydrate binding module as a diversity-carrying scaffold. Protein Eng. Des. Sel. 17, 213–221. doi: 10.1093/protein/gzh026
Gunnarsson, L. C., Zhou, Q., Montanier, C., Karlsson, E. N., Brumer, H., and Ohlin, M. (2006). Engineered xyloglucan specificity in a carbohydrate-binding module. Glycobiology 16, 1171–1180. doi: 10.1093/glycob/cwl038
Hahn, M. G., Lerner, D. R., Fitter, M. S., Norman, P. M., and Lamb, C. J. (1987). Characterization of monoclonal antibodies to protoplast membranes of Nicotiana tabacum identified by an enzyme-linked immunosorbent assay. Planta 171, 453–465. doi: 10.1007/BF00392292
Harholt, J., Moestrup, Ø., and Ulvskov, P. (2016). Why plants were terrestrial from the beginning. Trends Plant Sci. 21, 96–101. doi: 10.1016/j.tplants.2015.11.010
Harrington, B. J., and Hageage, G. J. (2003). Calcofluor white: a review of its uses and applications in clinical mycology and parasitology. Laboratory Medicine 34, 361–367. doi: 10.1309/EPH2TDT8335GH0R3
Hernandez-Gomez, M. C., Rydahl, M. G., Rogowski, A., Morland, C., Cartmell, A., Crouch, L., et al. (2015). Recognition of xyloglucan by the crystalline cellulose-binding site of a family 3a carbohydrate-binding module. FEBS Lett. 589, 2297–2303. doi: 10.1016/j.febslet.2015.07.009
Hervé, C., Marcus, S. E., and Knox, J. P. (2011). Monoclonal antibodies, carbohydrate-binding modules, and the detection of polysaccharides in plant cell walls. Methods Mol. Biol. 715, 103–113. doi: 10.1007/978-1-61779-008-9_7
Hervé, C., Rogowski, A., Blake, A. W., Marcus, S. E., Gilbert, H. J., and Knox, J. P. (2010). Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc. Natl. Acad. Sci. U.S.A. 107, 15293–15298. doi: 10.1073/pnas.1005732107
Hervé, C., Siméon, A., Jam, M., Cassin, A., Johnson, K. L., Salmeán, A. A., et al. (2016). Arabinogalactan proteins have deep roots in eukaryotes: identification of genes and epitopes in brown algae and their role in Fucus serratus embryo development. New Phytol. 209, 1428–1441. doi: 10.1111/nph.13786
Höfte, H., and Voxeur, A. (2017). Plant cell walls. Curr. Biol. 27, R865–R870. doi: 10.1016/j.cub.2017.05.025
Hoogenboom, J., Berghuis, N., Cramer, D., Geurts, R., Zuilhof, H., and Wennekes, T. (2016). Direct imaging of glycans in Arabidopsis roots via click labeling of metabolically incorporated azido-monosaccharides. BMC Plant Biol. 16:220. doi: 10.1186/s12870-016-0907-0
Jiang, S., Heller, B., Tagliabracci, V. S., Zhai, L., Irimia, J. M., and DePaoli-Roach, A. A. (2010). Starch binding domain-containing protein 1/genethonin 1 is a novel participant in glycogen metabolism. J. Biol. Chem. 285, 34960–34971. doi: 10.1074/jbc.M110.150839
Jones, L., Seymour, G. B., and Knox, J. P. (1997). Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1[->]4)-[beta]-D-galactan. Plant Physiol. 113, 1405–1412.
Joseleau, J. P., Faix, O., Kuroda, K., and Ruel, K. (2004). A polyclonal antibody directed against syringylpropane epitopes of native lignins. C. R. Biol. 327, 809–815. doi: 10.1016/j.crvi.2004.06.003
Joseleau, J. P., and Ruel, K. (1997). Study of lignification by noninvasive techniques in growing maize internodes. An investigation by Fourier transform infrared cross-polarization-magic angle spinning 13C-nuclear magnetic resonance spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiol. 114, 1123–1133. doi: 10.1104/pp.114.3.1123
Kawakubo, T., Karita, S., Araki, Y., Watanabe, S., Oyadomari, M., and Takada, R. (2010). Analysis of exposed cellulose surfaces in pretreated wood biomass using carbohydrate-binding module (CBM)-cyan fluorescent protein (CFP). Biotechnol. Bioeng. 105, 499–508. doi: 10.1002/bit.22550
Khatri, V., Hébert-Ouellet, Y., Meddeb-Mouelhi, F., and Beauregard, M. (2016). Specific tracking of xylan using fluorescent-tagged carbohydrate-binding module 15 as molecular probe. Biotechnol. Biofuels. 9:74. doi: 10.1186/s13068-016-0486-1
Kiyoto, S., Yoshinaga, A., and Takabe, K. (2013). Immunolocalization of 8-5′ and 8-8′ linked structures of lignin in cell walls of Chamaecyparis using monoclonal antibodies. Planta 237, 705–715. doi: 10.1007/s00425-012-1784-x
Knox, J. P. (2008). Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 11, 308–313. doi: 10.1016/j.pbi.2008.03.001
Knox, J. P., Day, S., and Roberts, K. (1989). A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 106, 47–56.
Knox, J. P., Linstead, P. J., King, J., Cooper, J. P. C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. doi: 10.1007/BF00193004
Knox, J. P., Linstead, P. J., Peart, J., Cooper, C., and Roberts, K. (1991). Developmentally-regulated epitopes of cell surface arabinogalactan-proteins and their relation to root tissue pattern formation. Plant J. 1, 317–326. doi: 10.1046/j.1365-313X.1991.t01-9-00999.x
Knox, J. P., Peart, J., and Neill, S. J. (1995). Identification of novel cell surface epitopes using a leaf epidermal-strip assay system. Planta 196, 266–270.
Köhler, G., and Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497. doi: 10.1038/256495a0
Kolb, H. C., Finn, M. G., and Sharpless, K. B. (2001). Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Koutaniemi, S., Guillon, F., Tranquet, O., Bouchet, B., Tuomainen, P., Virkki, L., et al. (2012). Substituent-specific antibody against glucuronoxylan reveals close association of glucuronic acid and acetyl substituents and distinct labeling patterns in tree species. Planta 236, 739–751. doi: 10.1007/s00425-012-1653-7
Kozlova, L. V., Ageeva, M. V., Ibragimova, N. N., and Gorshkova, T. A. (2014). Arrangement of mixed-linkage glucan and glucuronoarabinoxylan in the cell walls of growing maize roots. Ann. Bot. 114, 1135–1145. doi: 10.1093/aob/mcu125
Kracun, S. K., Fangel, J. U., Rydahl, M. G., Pedersen, H. L., Vidal-Melgosa, S., and Willats, W. G. (2017). Carbohydrate microarray technology applied to high-throughput mapping of plant cell wall glycans using comprehensive microarray polymer profiling (CoMPP). Methods Mol. Biol. 1503, 147–165. doi: 10.1007/978-1-4939-6493-2_12
Larson, E. R., Tierney, M. L., Tinaz, B., and Domozych, D. S. (2014). Using monoclonal antibodies to label living root hairs: a novel tool for studying cell wall microarchitecture and dynamics in Arabidopsis. Plant Methods 10:30. doi: 10.1186/1746-4811-10-30
Lee, K. J., Marcus, S. E., and Knox, J. P. (2011). Cell wall biology: perspectives from cell wall imaging. Mol. Plant. 4, 212–219. doi: 10.1093/mp/ssq075
Leroux, O., Sørensen, I., Marcus, S. E., Viane, R. L., Willats, W. G., and Knox, J. P. (2015). Antibody-based screening of cell wall matrix glycans in ferns reveals taxon, tissue and cell-type specific distribution patterns. BMC Plant Biol. 15:56. doi: 10.1186/s12870-014-0362-8
Li, M., Pu, Y., and Ragauskas, A. J. (2016). Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 4:45. doi: 10.3389/fchem.2016.00045
Liesche, J., Ziomkiewicz, I., and Schulz, A. (2013). Super-resolution imaging with Pontamine Fast Scarlet 4BS enables direct visualization of cellulose orientation and cell connection architecture in onion epidermis cells. BMC Plant Biol. 13:226. doi: 10.1186/1471-2229-13-226
Liners, F., Helbert, W., and Van Cutsem, P. (2005). Production and characterization of a phage-display recombinant antibody against carrageenans: evidence for the recognition of a secondary structure of carrageenan chains present in red algae tissues. Glycobiology 15, 849–860. doi: 10.1093/glycob/cwi072
Liners, F., Letesson, J. J., Didembourg, C., and Van Cutsem, P. (1989). Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiol. 91, 1419–1424. doi: 10.1104/pp.91.4.1419
Lion, C., Simon, C., Huss, B., Blervacq, A. S., Tirot, L., Toybou, D., et al. (2017). BLISS: a bioorthogonal dual-labeling strategy to unravel lignification dynamics in plants. Cell Chem. Biol. 24, 326–338. doi: 10.1016/j.chembiol.2017.02.009
Luterbacher, J. S., Walker, L. P., and Moran-Mirabal, J. M. (2013). Observing and modeling BMCC degradation by commercial inearize cocktails with fluorescently labeled Trichoderma reseii Cel7A through confocal microscopy. Biotechnol. Bioeng. 110, 108–117. doi: 10.1002/bit.24597
Malinovsky, F. G., Fangel, J. U., and Willats, W. G. (2014). The role of the cell wall in plant immunity. Front. Plant Sci. 5:178. doi: 10.3389/fpls.2014.00178
Manfield, I. W., Bernal, A. J., Moller, I., McCartney, I., Riess, N. P., Knox, J. P., et al. (2005). Re-engineering of the PAM1 phage display monoclonal antibody to produce a soluble, versatile anti-homogalacturonan scFv. Plant Sci. 169, 1090–1095. doi: 10.1016/j.plantsci.2005.07.008
Marcus, S. E., Blake, A. W., Benians, T. A., Lee, K. J., Poyser, C., Donaldson, L., et al. (2010). Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 64, 191–203. doi: 10.1111/j.1365-313X.2010.04319.x
Marcus, S. E., Verhertbruggen, Y., Hervé, C., Ordaz-Ortiz, J. J., Farkas, V., Pedersen, H. L., et al. (2008). Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 8:60. doi: 10.1186/1471-2229-8-60
Marriott, P. E., Gómez, L. D., and McQueen-Mason, S. J. (2016). Unlocking the potential of lignocellulosic biomass through plant science. New Phytol. 209, 1366–1381. doi: 10.1111/nph.13684
Matoh, T., Takasaki, M., Takabe, K., and Kobayashi, M. (1998). Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol. 39, 483–491.
McCafferty, J., Griffiths, A. D., Winter, G., and Chiswell, D. J. (1990). Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554.
McCartney, L., Marcus, S. E., and Knox, J. P. (2005). Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 53, 543–546. doi: 10.1369/jhc.4B6578.2005
McClosky, D. D., Wang, B., Chen, G., and Anderson, C. T. (2016). The click-compatible sugar 6-deoxy-alkynyl glucose metabolically incorporates into Arabidopsis root hair tips and arrests their growth. Phytochemistry 123, 16–24. doi: 10.1016/j.phytochem.2016.01.007
Meikle, P. J., Bonig, I., Hoogenraad, N. J., Clarke, A. E., and Stone, B. A. (1991). The location of (1–>3)-beta-glucans in the walls of pollen tubes of Nicotiana alata using a (1–>3)-beta-glucan-specific monoclonal antibody. Planta 185, 1–8.
Meikle, P. J., Hoogenraad, N. J., Bonig, I., Clarke, A. E., and Stone, B. A. (1994). A (1–>3,1–>4)-beta-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1–>3,1–>4)-beta-glucans. Plant J. 5, 1–9.
Moller, I., Marcus, S. E., Haeger, A., Verhertbruggen, Y., Verhoef, R., Schols, H., et al. (2008). High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj. J. 25, 37–48. doi: 10.1007/s10719-007-9059-7
Moller, I., Sørensen, I., Bernal, A. J., Blaukopf, C., Lee, K., Øbro, J., et al. (2007). High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 50, 1118–1128. doi: 10.1111/j.1365-313X.2007.03114.x
Monniaux, M., and Hay, A. (2016). Cells, walls, and endless forms. Curr. Opin. Plant Biol. 34, 114–121. doi: 10.1016/j.pbi.2016.10.010
Montanier, C., van Bueren, A. L., Dumon, C., Flint, J. E., Correia, M. A., Prates, J. A., et al. (2009). Evidence that family 35 carbohydrate binding modules display conserved specificity but divergent function. Proc. Natl. Acad. Sci. U.S.A. 106, 3065–3070. doi: 10.1073/pnas.0808972106
Mravec, J., Guo, X., Hansen, A. R., Schückel, J., Kračun, S. K., Mikkelsen, M. D., et al. (2017a). Pea border cell maturation and release involve complex cell wall structural dynamics. Plant Physiol. 174, 1051–1066. doi: 10.1104/pp.16.00097
Mravec, J., Kracun, S. K., Rydahl, M. G., Westereng, B., Miart, F., Clausen, M. H., et al. (2014). Tracking developmentally regulated post-synthetic processing of homogalacturonan and chitin using reciprocal oligosaccharide probes. Development 141, 4841–4850. doi: 10.1242/dev.113365
Mravec, J., Kracun, S. K., Rydahl, M. G., Westereng, B., Pontiggia, D., De Lorenzo, G., et al. (2017b). An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant J. 91, 534–546. doi: 10.1111/tpj.13574
Nafisi, M., Stranne, M., Fimognari, L., Atwell, S., Martens, H. J., and Pedas, P. R. (2015). Acetylation of cell wall is required for structural integrity of the leaf surface and exerts a global impact on plant stress responses. Front. Plant Sci. 6:550. doi: 10.3389/fpls.2015.00550
Nissim, A., Hoogenboom, H. R., Tomlinson, I. M., Flynn, G., Midgley, C., Lane, D., et al. (1994). Antibody fragments from a ‘single pot' phage display library as immunochemical reagents. EMBO J. 13, 692–698.
Norman, P. M., Wingate, V. P., Fitter, M. S., and Lamb, C. J. (1986). Monoclonal antibodies to plant plasma-membrane antigens. Planta. 167, 452–459.
Obeng, E. M., Adam, S. N. N., Budiman, C., Ongkudon, C. M., Maas, R., and Jose, J. (2017). Lignocellulases: a review of emerging and developing enzymes, systems, and practices. Bioresour. Bioprocess. 4:16. doi: 10.1186/s40643-017-0146-8
Pabst, M., Fischl, R. M., Brecker, L., Morelle, W., Fauland, A., Köfeler, H., et al. (2013). Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. Plant J. 76, 61–72. doi: 10.1111/tpj.12271
Paës, G. (2014). Fluorescent probes for exploring plant cell wall deconstruction: a review. Molecules 19, 9380–9402. doi: 10.3390/molecules19079380
Pandey, J. L., Wang, B., Diehl, B. G., Richard, T. L., Chen, G., and Anderson, C. T. (2015). A versatile click-compatible monolignol probe to study lignin deposition in plant cell walls. PLoS ONE 10:e0121334. doi: 10.1371/journal.pone.0121334
Pattathil, S., Avci, U., Baldwin, D., Swennes, A. G., McGill, J. A., Popper, Z., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153, 514–525. doi: 10.1104/pp.109.151985
Pattathil, S., Avci, U., Miller, J. S., and Hahn, M. G. (2012). “Immunological approaches to plant cell wall and biomass characterization: glycome profiling,” in Biomass Conversion: Methods and Protocols, ed M. Himmel (New York, NY: Springer Science + Business Media, LLC), 61–72.
Pattathil, S., Avci, U., Zhang, T., Cardenas, C. L., and Hahn, M. G. (2015a). Immunological approaches to biomass characterization and utilization. Front. Bioeng. Biotechnol. 3:173. doi: 10.3389/fbioe.2015.00173
Pattathil, S., Hahn, M. G., Dale, B. E., and Chundawat, S. P. (2015b). Insights into plant cell wall structure, architecture, and integrity using glycome profiling of native and AFEXTM-pre-treated biomass. J. Exp. Bot. 66, 4279–4294. doi: 10.1093/jxb/erv107
Pedersen, H. L., Fangel, J. U., McCleary, B., Ruzanski, C., Rydahl, M. G., Ralet, M. C., et al. (2012). Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 287, 39429–39438. doi: 10.1074/jbc.M112.396598
Pettolino, F. A., Hoogenraad, N. J., Ferguson, C., Bacic, A., Johnson, E., and Stone, B. A. (2001). A (1–>4)-beta-mannan-specific monoclonal antibody and its use in the immunocytochemical location of galactomannans. Planta 214, 235–242. doi: 10.1007/s004250100606
Popper, Z. A., Ralet, M. C., and Domozych, D. S. (2014). Plant and algal cell walls: diversity and functionality. Ann. Bot. 114, 1043–1048. doi: 10.1093/aob/mcu214
Porter, S. E., Donohoe, B. S., Beery, K. E., Xu, Q., Ding, S. Y., Vinzant, T. B., et al. (2007). Microscopic analysis of corn fiber using starch- and cellulose-specific molecular probes. Biotechnol. Bioeng. 98, 123–131. doi: 10.1002/bit.21409
Puhlmann, J., Bucheli, E., Swain, M. J., Dunning, N., Albersheim, P., Darvill, A. G., et al. (1994). Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1,2)-linked fucosyl-containing epitope. Plant Physiol. 104, 699–710.
Ralet, M.-C. C., Tranquet, O., Poulain, D., Moise, A., Guillon, F., Moïse, A., et al. (2010). Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 231, 1373–1383. doi: 10.1007/s00425-010-1116-y
Rostovtsev, V. V., Green, L. G., Fokin, V. V., and Sharpless, K. B. (2002). A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599. doi: 10.1002/1521-3773(20020715)41:14 < 2596::AID-ANIE2596>3.0.CO;2-4
Rounds, C. M., Lubeck, E., Hepler, P. K., and Winship, L. J. (2011). Propidium iodide competes with Ca(2+) to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol. 157, 175–187. doi: 10.1104/pp.111.182196
Ruel, K., Faix, O., and Joseleau, J. P. (1994). New immunogold probes for studying the distribution of the different lignin types during plant cell wall biogenesis. J. Trace Microprobe Tech. 12, 267–276.
Ruprecht, C., Bartetzko, M. P., Senf, D., Dallabernadina, P., Boos, I., Andersen, M. C., et al. (2017). A synthetic glycan microarray enables epitope mapping of plant cell wall glycan-directed antibodies. Plant Physiol. 175, 1094–1104. doi: 10.1104/pp.17.00737
Rydahl, M. G., Kracun, S. K., Fangel, J. U., Michel, G., Guillouzo, A., Génicot, S., et al. (2017). Development of novel monoclonal antibodies against starch and ulvan — implications for antibody production against polysaccharides with limited immunogenicity. Sci. Rep. 7:9326. doi: 10.1038/s41598-017-04307-2
Shinohara, N., Kakegawa, K., and Fukuda, H. (2015). Monoclonal antibody-based analysis of cell wall remodeling during xylogenesis. J. Plant Res. 128, 975–986. doi: 10.1007/s10265-015-0758-z
Simpson, P. J., Jamieson, S. J., Abou-Hachem, M., Karlsson, E. N., Gilbert, H. J., Holst, O., et al. (2002). The solution structure of the CBM4-2 carbohydrate binding module from a thermostable Rhodothermus marinus xylanase. Biochemistry 41, 5712–5719. doi: 10.1021/bi012093i
Smallwood, M., Beven, A., Donovan, N., Neill, S. J., Peart, J., Roberts, K., et al. (1994). Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 5, 237–246. doi: 10.1046/j.1365-313X.1994.05020237.x
Smallwood, M., Martin, H., and Knox, J. P. (1995). An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 196, 510–522. doi: 10.1007/BF00203651
Smith, S. P., Bayer, E. A., and Czjzek, M. (2017). Continually emerging mechanistic complexity of the multi-enzyme cellulosome complex. Curr. Opin. Struct. Biol. 44, 151–160. doi: 10.1016/j.sbi.2017.03.009
Sørensen, I., Pettolino, F. A., Bacic, A., Ralph, J., Lu, F., O'Neill, M. A., et al. (2011). The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 68, 201–211. doi: 10.1111/j.1365-313X.2011.04686.x
Steinwand, M., Droste, P., Frenzel, A., Hust, M., Dübel, S., and Schirrmann, T. (2014). The influence of antibody fragment format on phage display based affinity maturation of IgG. MAbs. 6, 204–218. doi: 10.4161/mabs.27227
Stone, B. A., Evans, N. A., Bonig, I., and Clarke, A. E. (1982). The application of Sirofluor, a chemically defined fluorochrome from aniline blue for the histochemical detection of callose. Protoplasma 122, 191–195. doi: 10.1007/BF01281696
Tan, L., Eberhard, S., Pattathil, S., Warder, C., Glushka, J., Yuan, C., Hao, Z., et al. (2013). An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 25, 270–287. doi: 10.1105/tpc.112.107334
The CAZypedia Consortium (2018). Ten years of CAZypedia: a living encyclopedia of carbohydrate-active enzymes. Glycobiology 28, 3–8. doi: 10.1093/glycob/cwx089
Tiede, C., Bedford, R., Heseltine, S. J., Smith, G., Wijetunga, I., Ross, R., AlQallaf, D., et al. (2017). Affimer proteins are versatile and renewable affinity reagents. Elife 6:e24903. doi: 10.7554/eLife.24903
Tobimatsu, Y., Davidson, C. L., Grabber, J. H., and Ralph, J. (2011). Fluorescence-tagged monolignols: synthesis, and application to studying in vitro lignification. Biomacromolecules 12, 1752–1761. doi: 10.1021/bm200136x
Tobimatsu, Y., Van de Wouwer, D., Allen, E., Kumpf, R., Vanholme, B., Boerjan, W., et al. (2014). A click chemistry strategy for visualization of plant cell wall lignification. Chem. Commun. 50, 12262–12265. doi: 10.1039/C4CC04692G
Tobimatsu, Y., Wagner, A., Donaldson, L., Mitra, P., Niculaes, C., Dima, O., et al. (2013). Visualization of plant cell wall lignification using fluorescence-tagged monolignols. Plant J. 76, 357–366. doi: 10.1111/tpj.12299
Torode, T. A., Marcus, S. E., Jam, M., Tonon, T., Blackburn, R. S., Hervé, C., et al. (2015). Monoclonal antibodies directed to fucoidan preparations from brown algae. PLoS ONE 10:e0118366. doi: 10.1371/journal.pone.0118366
Torode, T. A., O'Neill, R. E., Marcus, S. E., Cornuault, V., Pose-Albacete, S., Lauder, R. P., et al. (2017). Branched pectic galactan in phloem-sieve-element cell walls: implications for cell mechanics. Plant Physiol. 176, 1547–1558. doi: 10.1104/pp.17.01568
Torode, T. A., Siméon, A., Marcus, S. E., Jam, M., Le Moigne, M. A., Duffieux, D., et al. (2016). Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. J. Exp. Bot. 67, 6089–6100. doi: 10.1093/jxb/erw369
Tranquet, O., Saulnier, L., Utille, J.-P., Ralph, J., and Guillon, F. (2009). Monoclonal antibodies to p-coumarate. Phytochemistry 70, 1366–1373. doi: 10.1016/j.phytochem.2009.06.019
Venditto, I., Luis, A. S., Rydahl, M., Schückel, J., Fernandes, V. O., Vidal-Melgosa, S., et al. (2016). Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc. Natl. Acad. Sci. U.S.A. 113, 7136–7141. doi: 10.1073/pnas.1601558113
Verhertbruggen, Y., Marcus, S. E., Haeger, A., Ordaz-Ortiz, J. J., and Knox, J. P. (2009a). An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 344, 1858–1862. doi: 10.1016/j.carres.2008.11.010
Verhertbruggen, Y., Marcus, S. E., Haeger, A., Verhoef, R., Schols, H. A., McCleary, B. V., et al. (2009b). Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 59, 413–425. doi: 10.1111/j.1365-313X.2009.03876.x
Verhertbruggen, Y., Walker, J. L., Guillon, F., and Scheller, H. V. (2017). A comparative study of sample preparation for staining and immunodetection of plant cell walls by light microscopy. Front. Plant Sci. 8:1505. doi: 10.3389/fpls.2017.01505
Vidal-Melgosa, S., Pedersen, H. L., Schückel, J., Arnal, G., Dumon, C., Amby, D. B., et al. (2015). A new versatile microarray-based method for high throughput screening of carbohydrate-active enzymes. J. Biol. Chem. 290, 9020–9036. doi: 10.1074/jbc.M114.630673
Vissenberg, K., Fry, S. C., and Verbelen, J. P. (2001). Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycosylase action in Arabidopsis roots. Plant Physiol. 127, 1125–1135. doi: 10.1104/pp.010295
Vissenberg, K., Martinez-Vilchez, I. M., Verbelen, J.-P., Miller, J. G., and Fry, S. C. (2000). In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell 12, 1229–1237. doi: 10.1105/tpc.12.7.1229
Voiniciuc, C., Pauly, M., and Usadel, B. (2018). Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 176, 2590–2600. doi: 10.1104/pp.17.01776
Walker, J. A., Pattathil, S., Bergeman, L. F., Beebe, E. T., Deng, K., Mirzai, M., et al. (2017). Determination of glycoside hydrolase specificities during hydrolysis of plant cell walls using glycome profiling. Biotechnol. Biofuels 10:31. doi: 10.1186/s13068-017-0703-6
Wallace, I. S., and Anderson, C. T. (2012). Small molecule probes for plant cell wall polysaccharide imaging. Front. Plant Sci. 3:89. doi: 10.3389/fpls.2012.00089
Wang, L., Wang, Y., and Ragauskas, A. J. (2012). Determination of colocalization on cellulose fiber with quantitative FRET measured by acceptor photobleaching and spectrally unmixing fluorescence microscopy. Analyst 137, 1319–1324. doi: 10.1039/c2an15938d
Wang, M., Heimovaara-Dijkstra, S., van der Meulen, R. M., Knox, J. P., and Neill, S. J. (1995). The monoclonal antibody JIM19 modulates abscisic acid action in barley aleurone protoplasts. Planta 196, 271–276. doi: 10.1007/BF00201384
Weller, M. G. (2016). Quality issues of research antibodies. Anal. Chem. Insights 11, 21–27. doi: 10.4137/ACI.S31614
Wells, M. L., Potin, P., Craigie, J. S., Raven, J. A., Merchant, S. S., Helliwell, K. E., et al. (2016). Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29, 949–982. doi: 10.1007/s10811-016-0974-5
Willats, W. G., Limberg, G., Buchholt, H. C., van Alebeek, G. J., Benen, J., Christensen, T. M., et al. (2000). Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr. Res. 327, 309–320. doi: 10.1016/S0008-6215(00)00039-2
Willats, W. G., Marcus, S. E., and Knox, J. P. (1998). Generation of monoclonal antibody specific to (1 → 5)-alpha-L-arabinan. Carbohydr. Res. 308, 149–152. doi: 10.1016/S0008-6215(98)00070-6
Willats, W. G., McCartney, L., Steele-King, C. G., Marcus, S. E., Mort, A., Huisman, M., et al. (2004). A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 218, 673–681. doi: 10.1007/s00425-003-1147-8
Willats, W. G., Orfila, C., Limberg, G., Buchholt, H. C., van Alebeek, G. J., Voragen, A. G., et al. (2001). Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 276, 19404–19413. doi: 10.1074/jbc.M011242200
Willats, W. G., Gilmartin, P. M., Mikkelsen, J. D., and Knox, J. P. (1999). Cell wall antibodies without immunization: generation and use of de-esterified homogalacturonan block-specific antibodies from a naive phage display library. Plant J. 18, 57–65. doi: 10.1046/j.1365-313X.1999.00427.x
Williams, M. N., Freshour, G., Darvill, A. G., Albersheim, P., and Hahn, M. G. (1996). An antibody Fab selected from a recombinant phage display library detects deesterified pectic polysaccharide rhamnogalacturonan II in plant cells. Plant Cell 8, 673–685. doi: 10.1105/tpc.8.4.673
Wilson, M. H., Holman, T. J., Sørensen, I., Cancho-Sanchez, E., Wells, D. M., Swarup, R., et al. (2015). Multi-omics analysis identifies genes mediating the extension of cell walls in the Arabidopsis thaliana root elongation zone. Front. Cell Dev. Biol. 3:10. doi: 10.3389/fcell.2015.00010
Wilson, S. M., and Bacic, A. (2012). Preparation of plant cells for transmission electron microscopy to optimize immunogold labeling of carbohydrate and protein epitopes. Nat. Protoc. 7, 1716–1727. doi: 10.1038/nprot.2012.096
Wolf, S., Hématy, K., and Höfte, H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63, 381–407. doi: 10.1146/annurev-arplant-042811-105449
Xue, J., Bosch, M., and Knox, J. P. (2013). Heterogeneity and glycan masking of cell wall microstructures in the stems of Miscanthus x giganteus, and its parents M. sinensis and M. sacchariflorus. PLoS ONE 8:e82114. doi: 10.1371/journal.pone.0082114
Yang, Q., Goldstein, I., Mei, H. Y., and Engelke, D. R. (1998). DNA ligands that bind tightly and selectively to cellobiose. Proc. Natl. Acad. Sci. U.S.A. 95, 5462–5467. doi: 10.1073/pnas.95.10.5462
Yates, E. A., Valdor, J. F., Haslam, S. M., Morris, H. R., Dell, A., Mackie, W., et al. (1996). Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6, 131–139. doi: 10.1093/glycob/6.2.131
Yoon, H. Y., Koo, H., Kim, K., and Kwon, I. C. (2017). Molecular imaging based on metabolic glycoengineering and click chemistry. Biomaterials 132, 28–36. doi: 10.1016/j.biomaterials.2017.04.003
Zhang, H., Zhou, L., Zhu, Z., and Yang, C. (2016). Recent progress in aptamer-based functional probes for bioanalysis and biomedicine. Chemistry 22, 9886–98900. doi: 10.1002/chem.201503543
Zhang, X., Rogowski, A., Zhao, L., Hahn, M. G., Avci, U., Knox, J. P., et al. (2014). Understanding how the complex molecular architecture of mannan degrading hydrolyses contributes to plant cell wall degradation. J. Biol. Chem. 289, 2002–2012. doi: 10.1074/jbc.M113.527770
Keywords: cell wall, polysaccharide, molecular probe, monoclonal antibody, carbohydrate-binding module, metabolic labeling, glycan microarray, imaging
Citation: Rydahl MG, Hansen AR, Kračun SK and Mravec J (2018) Report on the Current Inventory of the Toolbox for Plant Cell Wall Analysis: Proteinaceous and Small Molecular Probes. Front. Plant Sci. 9:581. doi: 10.3389/fpls.2018.00581
Received: 11 January 2018; Accepted: 13 April 2018;
Published: 03 May 2018.
Edited by:
Maurice Bosch, Aberystwyth University, United KingdomReviewed by:
Paul Knox, University of Leeds, United KingdomCharles T. Anderson, Pennsylvania State University, United States
Copyright © 2018 Rydahl, Hansen, Kračun and Mravec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jozef Mravec, bXJhdmVjQHBsZW4ua3UuZGs=
 Maja G. Rydahl
Maja G. Rydahl Aleksander R. Hansen
Aleksander R. Hansen Stjepan K. Kračun
Stjepan K. Kračun Jozef Mravec
Jozef Mravec