- 1Department of Life Sciences, Shaanxi Xueqian Normal University, Xi’an, China
- 2School of Life Sciences, Sun Yat-sen University, Guangzhou, China
- 3Research Institute of Sun Yat-sen University, Shenzhen, China
- 4College of Life Science, South China Agricultural University, Guangzhou, China
The plastid accD gene encodes a subunit of the acetyl-CoA carboxylase (ACCase) enzyme. The length of accD gene has been supposed to expand in Cryptomeria japonica, Taiwania cryptomerioides, Cephalotaxus, Taxus chinensis, and Podocarpus lambertii, and the main reason for this phenomenon was the existence of tandemly repeated sequences. However, it is still unknown whether the accD gene length in other cupressophytes has expanded. Here, in order to investigate how widespread this phenomenon was, 18 accD sequences and its surrounding regions of cupressophyte were sequenced and analyzed. Together with 39 GenBank sequence data, our taxon sampling covered all the extant gymnosperm orders. The repetitive elements and substitution rates of accD among 57 gymnosperm species were analyzed, the results show: (1) Reading frame length of accD gene in 18 cupressophytes species has also expanded. (2) Many repetitive elements were identified in accD gene of cupressophyte lineages. (3) The synonymous and non-synonymous substitution rates of accD were accelerated in cupressophytes. (4) accD was located in rearrangement endpoints. These results suggested that repetitive elements may mediate the chloroplast genome rearrangement and accelerated the substitution rates.
Introduction
Cupressophytes, also called non-Pinaceae conifers, comprise about 380 species in 58 genera of five families: Araucariaceae, Podocarpaceae, Sciadopityaceae, Taxaceae (including Cephalotaxaceae), and Cupressaceae (including Taxodiaceae) (Christenhusz et al., 2011). Most species of Araucariaceae and Podocarpaceae are usually distributed in Southern Hemisphere, while other three families are located in the Northern Hemisphere. Some of the cupressophytes species are of economic and ecological value to humans. For instance, most species of Cupressaceae are valued for the production of timbers or ornamentals. The secondary metabolite paclitaxel (taxol) extracted from the bark of Taxus is a chemotherapy drug to treat ovarian and breast cancer.
Dispersed repetitive DNA sequences are scattered throughout the chloroplast genome. Most of the studies concentrate on detecting repeat sequences on a chloroplast genome-wide degree (Saski et al., 2005; Guo et al., 2007; Haberle et al., 2008; Tangphatsornruang et al., 2011); while there are only very few reports about the presence and structure of the repetitive DNA of a specific gene among many lineages (Hipkins et al., 1995; Erixon and Oxelman, 2008). Some repeats exist in the coding regions of chloroplast gene. For instance, ycf1 and ycf2 in Panax ginseng (Zhao et al., 2014), Taxus chinensis (Zhang et al., 2014), Podocarpus lambertii (Vieira et al., 2014), Cephalotaxus oliveri (Yi et al., 2013), and Globe artichoke (Curci et al., 2015); as well as accD in T. chinensis (Zhang et al., 2014), P. lambertii (Vieira et al., 2014), Medicago truncatula (Gurdon and Maliga, 2014), C. oliveri (Yi et al., 2013), Capsicum annuum (Jo et al., 2011), Pisum sativum and Lathyrus sativus (Magee et al., 2010) all have repeat sequences. Many studies have suggested that highly rearranged chloroplast genome generally possess a great many repetitive sequences which are associated with rearrangement endpoints, and this phenomenon has been observed in some land plants: Douglas-fir (Pseudotsuga menziesii) (Tsai and Strauss, 1989), Pelargonium (Chumley et al., 2006), Trifolium (Cai et al., 2008), Trachelium (Haberle et al., 2008), Oleaceae (Lee et al., 2007), and Asteraceae (Kim et al., 2005; Timme et al., 2007). The size and number of repeats also correlate to the extent of genome rearrangement (Guisinger et al., 2011). The genome containing the most frequency of long repeats is usually the most reorganized.
Acetyl-CoA carboxylase (ACCase) facilitate the acetyl-CoA to form malonyl-CoA and is supposed to regulate de novo fatty acid biosynthesis (Konishi and Sasaki, 1994; Sasaki and Nagano, 2004). Most higher plants, except for Gramineae, have two forms of ACCase: a prokaryotic type made up of several subunits in the stroma of plastids and a eukaryotic form composed of an only multifunctional polypeptide located in the cytosol (Konishi et al., 1996). The prokaryotic ACCase form is organized by the α-carboxyl transferase, the biotin carboxyl carrier, the biotin carboxylase, and the β-carboxyl transferase subunit (Gornicki et al., 1997). Except for β-carboxyl transferase was encoded by the plastid accD gene, other three subunit are all nucleus encoded. The plastid-localized accD gene is essential for leaf growth and to maintain plastid compartment in tobacco (Kode et al., 2005). Elevation of accD expression successfully raised the entire ACCase amount in plastids, and significantly raised the fatty acid content in tobacco leaves (Madoka et al., 2002). Furthermore, expression of accD was considered to be essential at the stage of embryo development in Arabidopsis (Bryant et al., 2011).
AccD is widely distributed in plants, including the reduced chloroplast genome of parasitic and non-photosynthetic plants (Wolfe et al., 1992; de Koning and Keeling, 2006). However, accD has been lost several times from the chloroplast genomes of some angiosperm lineage: Acoraceae (Goremykin et al., 2005), Poaceae (Konishi and Sasaki, 1994; Harris et al., 2012), Campanulaceae (Haberle et al., 2008), Geraniaceae (Guisinger et al., 2008), and Fabaceae (Magee et al., 2010). In Poaceae, the plastid-located prokaryotic form ACCase is functionally replaced by the nuclear-encoded eukaryotic type (Konishi et al., 1996; Gornicki et al., 1997). The loss of accD gene from the chloroplast genomes of Campanulaceae and Fabaceae was also consistent with an additional ACCase counterpart in the nucleus (Magee et al., 2010; Rousseau et al., 2013). In Trifolium repens of Fabaceae, through scanning high-throughput EST sequence data, accD was found to fuse with a nuclear gene for plastid lipoamide dehydrogenase (LPD2) (Magee et al., 2010); in Trachelium caeruleum of Campanulaceae, a transit peptide is combined with an abridged accD gene, which includes only 331 amino acids (Rousseau et al., 2013). In contrast to the loss of this gene among the above species, the length of accD gene in cupressophyte species including Cryptomeria japonica, Taiwania cryptomerioides, Cephalotaxus wilsoniana, C. oliveri, T. chinensis, and P. lambertii have diversified in an increasing direction (Hirao et al., 2008; Wu et al., 2011; Yi et al., 2013; Vieira et al., 2014; Zhang et al., 2014). The extension of the accD gene length is mainly caused by the insertion of large number of tandem repeated sequences in this area. But the repetitive elements of the gene are different among Cephalotaxus, T. cryptomerioides, T. chinensis, and P. lambertii (Yi et al., 2013; Vieira et al., 2014; Zhang et al., 2014). Therefore, evolutionary mechanisms underlying the occurrence of repetitive elements in cupressophyte of accD gene remain poorly studied. Sequence data from a wider phylogenetic breadth of cupressophytes are needed to clarify the evolutionary history of accD gene.
In the study of four mammalian and a bird genome, it is suggested that regions surrounding tandem repeats evolve faster than other non-repeat-containing regions (Simon and Hancock, 2009). One explanation is that regions nearby repeat sequences have evolved under weaker negative selection than the remaining region they embedded in (Djian et al., 1996; Faux et al., 2007). Another explanation is that the repeat sequences give rise to more substitutions near the flanking sequences (Huntley and Clark, 2007). Recent evidence also suggests that the insertion of repeat sequence elevated substitution rate of the entire sequence (Huntley and Clark, 2007). It is also assured that repeat sequence themselves evolves faster than their flanking sequence (Huntley and Golding, 2000). With many repeat elements in accD, whether the substitution rates for the repeat sequences or their flanking sequences have accelerated is unknown. To elucidate the overall evolutionary history or patterns of the repeat sequences in chloroplast genome, substitution rate pattern of accD gene were identified in this study.
In order to have a better insight into the evolutionary trace of accD in cupressophytes, in this study, we have sequenced accD genes from 18 cupressophytes species. The aim of this study focuses on: (1) investigating whether accD gene length in cupressophytes tends to increase; (2) exploring if accD gene in other cupressophytes species have specific repetitive elements like Cephalotaxus, T. cryptomerioides, T. chinensis, and P. lambertii; (3) determining the substitution rates pattern of accD in cupressophytes; (4) identifying gene order states around accD gene and verifying the association of repetitive elements, substitution rates and genome rearrangement.
Materials and Methods
Plant Sampling
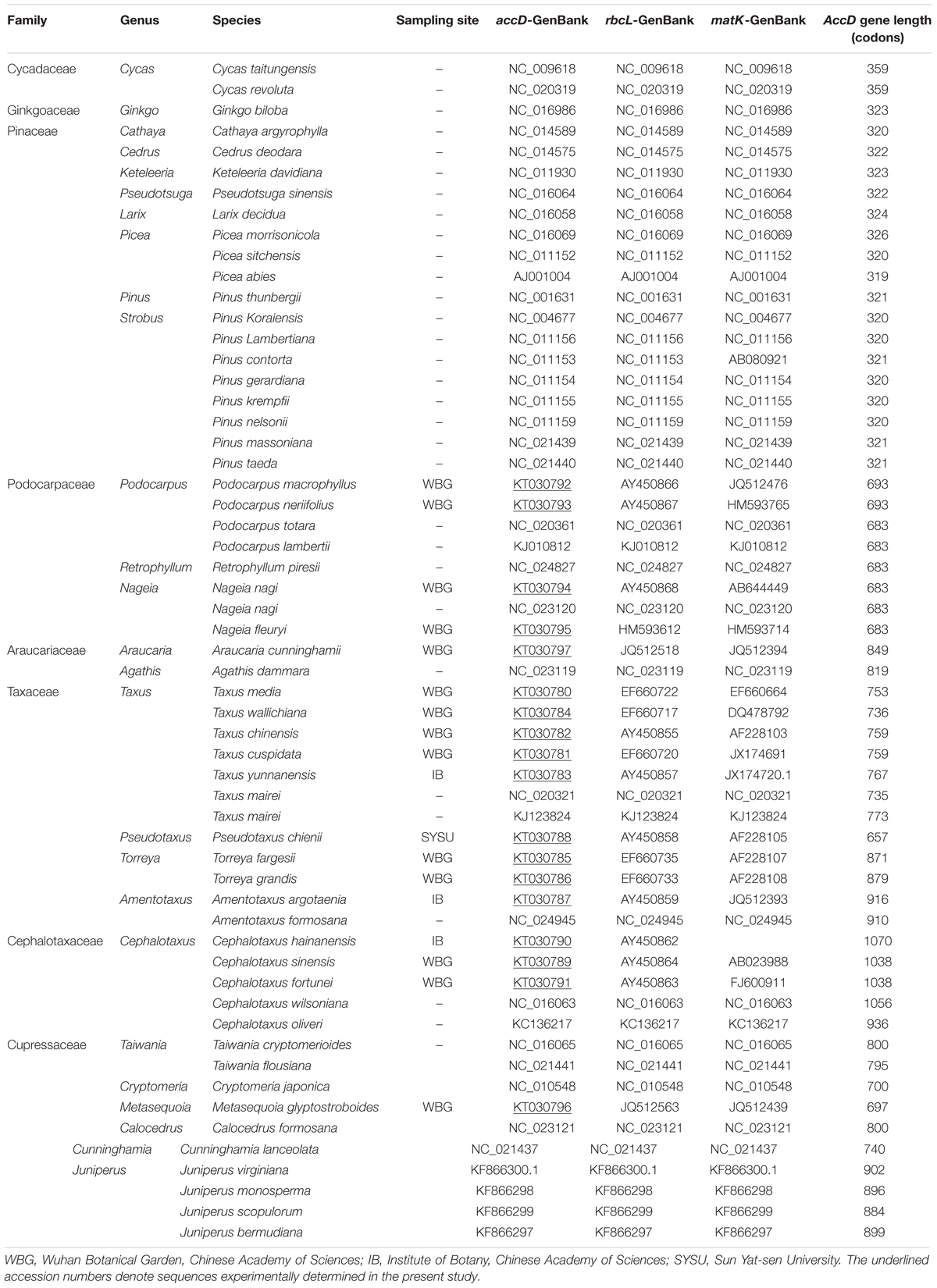
Fresh leaves of 18 conifer species were sampled from Wuhan Botanical Garden, Chinese Academy of Sciences (CAS), Institute of Botany, CAS, and Sun Yat-sen University, respectively (Table 1). The materials used for DNA extraction were saved in silica gel.
DNA Extraction and Sequencing
Total genomic DNA was isolated from the leaves of samples using the CTAB method (Gawel and Jarret, 1991). The quality of the genomic DNA was determined by 1% agarose gel electrophoresis. The accD gene investigated in this study was acquired using polymerase chain reaction (PCR). PCR primers (Supplementary Table 1) were designed from conserved region sequences in four gymnosperms (C. japonica, NC_010548; T. cryptomerioides, NC_016065; C. wilsoniana, NC_016063; C. oliveri, KC136217). The PCR system was as described in former study (Li et al., 2016). Then the PCR products were cloned into PCR 2.1 plasmid vector (Invitrogen, Carlsbad, CA, United States), and transformed to E. coli DH5α. At least three random positive clones were sequenced using ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, United States).
Sequence Assembly and Annotation
The sequences generated from different primers were assembled as a single sequence by BioEdit (Hall, 1999) with an overlapping of 150–300 bp. Contigs were initially annotated by DOGMA (Dual Organellar GenoMe Annotator). Genes that not be confirmed by DOGMA were recognized using Blastx1 and ORF Finder2. The tRNA genes were annotated by tRNAscan-SE v1.21 (Lowe and Eddy, 1997).
Repeat Sequence Analyses
The sequences were initially scanned by REPuter at a repeat length ≥20 bp with a similarity of above 90% (Kurtz et al., 2001). Sequences were further processed by the Tandem Repeats Finder software (Benson, 1999).
The Estimation of Substitution Rate
For the analysis in Figure 2, we first constructed a maximum likelihood (ML) tree using rbcL sequences. The analysis was performed in RaxML v8.1.x software with the GTR+I+G model. In addition, according to the strongly supported relationship published elsewhere (Lu et al., 2014), the Podocarpaceae and Araucariaceae were adjusted as a sister group for the rbcL ML tree. At last, this tree was used for the following substitution rate calculation. In order to compare the substitution rate of accD gene to two other widely used chloroplast gene marker rbcL and matK, we also downloaded these two gene sequences from GenBank. The branch lengths of non-synonymous (dN) and synonymous (dS) nucleotide substitutions for accD, matK, and rbcL trees were calculated using the free-ratio model implemented in PAML Codeml program.
Results
The General Features of accD Gene in Cupressophytes
The sequences acquired in this study were deposited in the GenBank with the accession number of KT30780-KT30797. A comparison of 57 gymnosperm accD sequences showed that the approximate 200 amino-acids at the end of this gene were highly conserved (Supplementary Figures 1-9, 1-10, the position of 1200–1400 in the alignment). This C-terminal region is functional importance for ACCD protein (Zhang et al., 2003). However, we found that the residues at the N-terminal and the middle region showed low similarities (Supplementary Figures 1-1 to 1-8). The major difference between 57 gymnosperm accD sequences is apparent as a large insertion sequences in the N-terminal and the middle region of cupressophyte accD sequence (Supplementary Figures 1-1 to 1-8). Furthermore, the open reading frame has not been destroyed by these insertion sequences.
The accD gene length in cupressophyte experienced an extraordinary expansion. The accD gene in Podocarpaceae lineage expands above 600 codons (Table 1). The Cephalotaxus hainanensis analyzed in this study shows the largest accD gene size, reaching 1070 codons (Table 1), which is approximately three times of the other Pinaceae species. The accD gene length also varies significantly within family. The accD gene in Taxus has experienced dramatic expansion, reaching as long as 735, 736, 753, 759, and 767 codons in T. mairei, T. wallichiiana, T. media, T. chinensis, and T. yunnanensis, respectively (Table 1); P. chienii has only 657 codons; T. fargesii and T. grandis have 871 codons and 879 codons, respectively; the accD gene length of A. argotaenia in Taxaceae is the longest, possessing 916 codons (Table 1). In general, our results support Hirao et al’s hypothesis that the accD gene length in cupressophytes has been expanded (Hirao et al., 2008).
Repetitive Amino Acid Elements in accD
To initiate our investigation into the mechanisms underlying accD gene length-associated mutation, REPuter and Tandem Repeat Finder were used to search repetitive sequences. As expected, accD gene length variation is explained by the insertions consisting of tandem repeated sequences. The repetitive sequences in accD gene are represented by a total of 31 categories present in 2–13 nearly identical copies, all of which are in the same (i.e., direct) orientation relative to each other (Supplementary Table 2 and Figure 1). Cycadaceae, Ginkgoaceae and the Pinaceae species with a relatively small gene size (Table 1) do not have repetitive elements. In comparison, the accD in cupressophytes investigated in this study possess a great many repetitive sequences. Ten repetitive elements were identified in the accD gene from the Cephalotaxaceae (Supplementary Table 2 and Figure 1A). Some repetitive elements, represented by R5, R9, and R8, were exclusively found either in C. wilsoniana or C. hainanensis, whereas the other repetitive elements such as R1, R2, R3, R4, R10 were found in all Cephalotaxus species. R1, R2, and R10 repetitive elements were all duplicated two times in the five Cephalotaxus species. The copy number of R3, R4, R6, and R7 varies in different species. For instance, the C. hainanensis has 13 repetitive elements of R3; while C. sinensis, C. Wilsoniana, and C. fortune have 12 copies of this repetitive element and C. oliveri has only six copies of R3 repetitive elements.
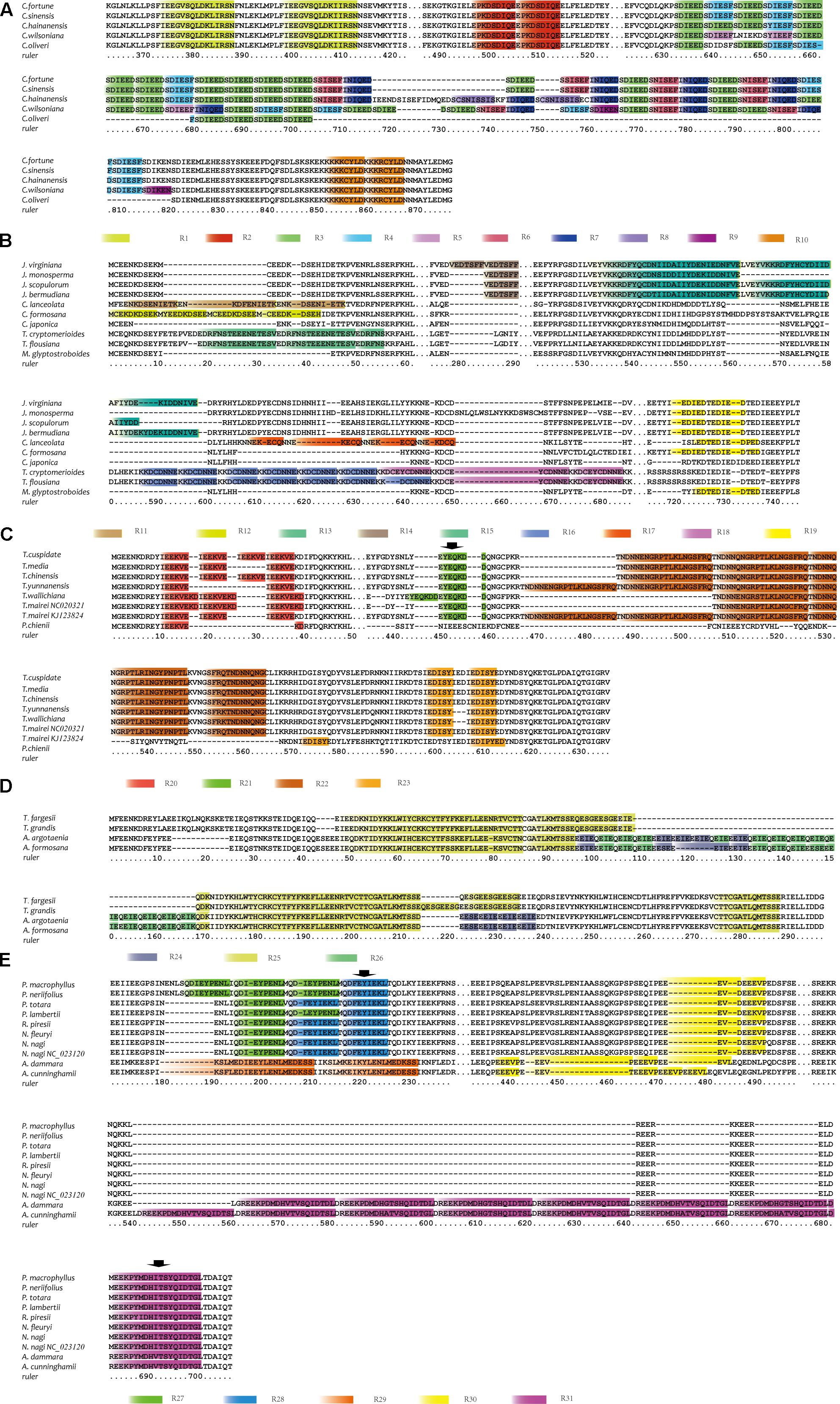
FIGURE 1. The repetitive elements of accD gene in five groups of species. Sequence alignments were performed individually in each group using MEGA. (A) The alignment of amino acid sequences of ACCD protein from five Cephalotaxaceae species. The figure only shows the regions from 364 to 422 and 488 to 876. (B) The alignment of amino acid sequences of ACCD protein from ten Cupressaceae species. The figure only shows the regions from 1 to 61, 276 to 290, and 522 to 744. (C) The alignment of amino acid sequences of ACCD protein from seven Taxus species and P. chienii. The figure only shows the regions from 1 to 50 and 436 to 636. (D) The alignment of amino acid sequences of ACCD protein from two Torreya and two Amentotaxus species. The figure only shows the regions from 1 to 297. (E) The alignment of amino acid sequences of ACCD protein from eight Podocarpaceae and two Araucariaceae species. The figure only shows the regions from 171 to 237, 434 to 497 and 530 to 707. Different repetitive elements were marked with different colored boxes. The arrows indicate the repetitive elements which has only one copy. The spacer between two fragments was divided by three dots.
Three repetitive elements of R11 and four of R12 were found in Cunninghamia lanceolata and Calocedrus formosana, respectively (Supplementary Table 2 and Figure 1B). Juniperus virginiana has two copies of R14 while the other Juniperus species have only one copy of this repetitive element (Supplementary Table 2 and Figure 1B). The main difference in repetitive elements between two Taiwania species was the copy number variations of R16 and R18. T. cryptomerioides has six copies of R16 and three copies of R18, while T. flousiana has seven copies of R16 and two copies of R18 (Supplementary Table 2 and Figure 1B). R19 repetitive element commonly exists in Cupressaceae species except for C. lanceolata and Taiwania (Supplementary Table 2 and Figure 1B).
In Taxus and Pseudotaxus, the accD gene contained four kinds of repetitive elements: R20, R21, R22 and R23 (Supplementary Table 2 and Figure 1C). Two copies of R21 were found in T. wallichiana while other Taxus species have only one copy of R21. T. chinensis and T. cuspidate both have four copies of R20 and T. yunnanensis has only two, while the remaining four Taxus species each have three copies of this repetitive elements. P. chienii also has R20 element but only one copy. The copy number of R22 in Taxus is also different, ranging from two in T. wallichiana to four in T. yunnanensis.
The largest tandem repetitive elements, spanning 59 amino acids, named as R25, exist in Torreya and Amentotaxus (Supplementary Table 2 and Figure 1D). Two copies of R25 were identified in Torreya and Amentotaxus. Amentotaxus has two genus-specific repetitive elements, R24 and R26, whose copy number are also different between Amentotaxus argotaenia and Amentotaxus formosana (Supplementary Table 2 and Figure 1D). Podocarpaceae has only a few repetitive elements (Supplementary Table 2 and Figure 1E). P. macrophyllus and P. neriifolius each contain three copies of R27, while P. lambertii contains two. Other Podocarpaceae species contain two copies of R28. A. cunninghamii and A. dammara each have eight and seven copies of R31, which is also lineage specific (Supplementary Table 2 and Figure 1E). The consensus sequences of R31 were also found in Podocarpaceae but all existing as single copy (not repeated), suggesting that R31 repetitive element was only duplicated in Araucariaceae. Furthermore, no pairs of direct repetitive sequences were identified in two sides of the inserted repetitive elements of cupressophytes.
Rapid Evolution of accD in Cupressophytes
The value of dN and dS for accD, rbcL, and matK gene were represented as branch lengths in Figure 2. In the dN tree, rbcL and matK gene has a relatively low substitute rate through the entire tree. The branch leading to the ancestry clade of Cupressaceae, Taxaceae, and Cephalotaxaceae in the accD dN tree is longer than other branches, suggesting that accD evolves faster in this clade. In addition, the branch leading to Podocarpaceae and Araucariaceae in the accD dN tree is longer than other gymnosperms (Figure 2). Interestingly, the accD gene length also starts to expand at the lineage of Podocarpaceae and Araucariaceae. For the matK and rbcL dS tree, most gymnosperm species evolve slowly and consistently except for the branch leading to Podocarpaceae. However, the dS value of accD gene for cupressophyte evolves much faster than many of the Pinaceae species. Compared with rbcL and matK, accD gene shows a high level of divergence among cupressophyte species. In general, accD has experienced substitution rates acceleration and this acceleration is locus and lineage specific.
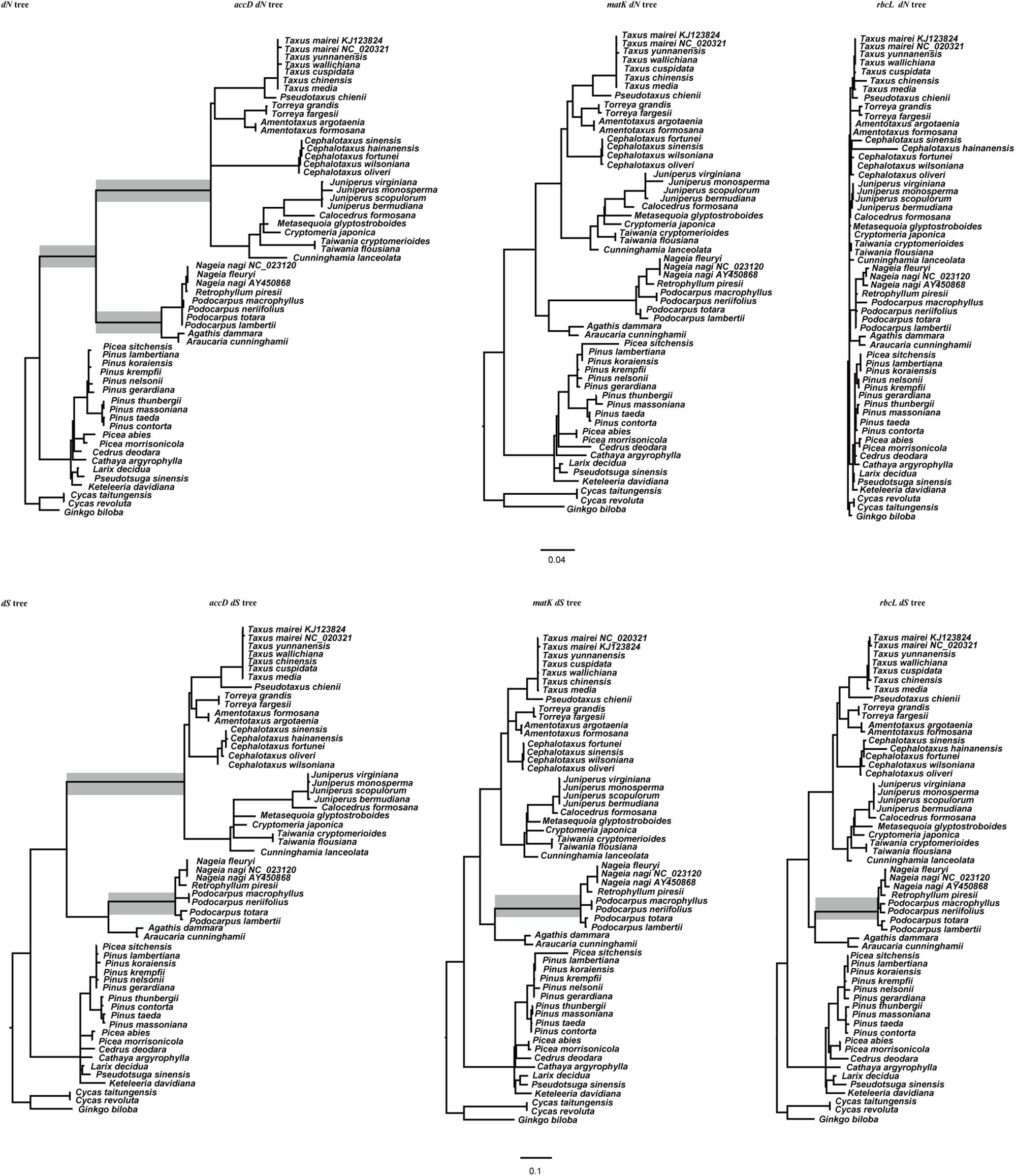
FIGURE 2. dN and dS trees for accD, matK, and rbcL gene. Branch lengths are in terms of dN and dS as estimated by PAML under a constrained topology. The topology of the accD dN tree, accD dS tree, rbcL dN tree, rbcL dS tree were identical to each other. The matK dN tree and dS tree were similar with rbcL and accD trees after removing C. hainanensis. The gray boxes denote the branch whose dN or dS has been accelerated.
Gene Order Around accD in Gymnosperms
The gene order around accD could be classified into six types (Figure 3). At high taxonomic levels, the gene order tends to be conserved across Cycadaceae, Ginkgoaceae and Pinaceae with a type of: rbcL-trnR-accD-psaI. Gene order in Araucariaceae and Podocarpaceae excluding Podocarpus totara is nearly identical to that of Cycadaceae, Ginkgoaceae and Pinaceae except that an extra trnD gene was found between rbcL and trnR. In P. totara, the gene order is: psbM-trnD-accD-psaI, which is different from that of the other three Podocarpus species, despite being members of the same genus. In Taxaceae, C. japonica, Taiwania, M. glyptostroboides and C. lanceolata, rbcL and clpP is near accD. The gene order of Cephalotaxus differs from that of Taxaceae by the inversion of clpP and translocation of rps16. Comparing with Taxaceae, the rpl23 takes the place of clpP making the gene order to be: rbcL-accD-rpl23 in Juniperus and C. formosana. It is amazing that gymnosperm chloroplast genomes have so much difference in gene organization surrounding accD, so we speculate that the accD gene must be involved in some rearrangement events of gymnosperm chloroplast genome.
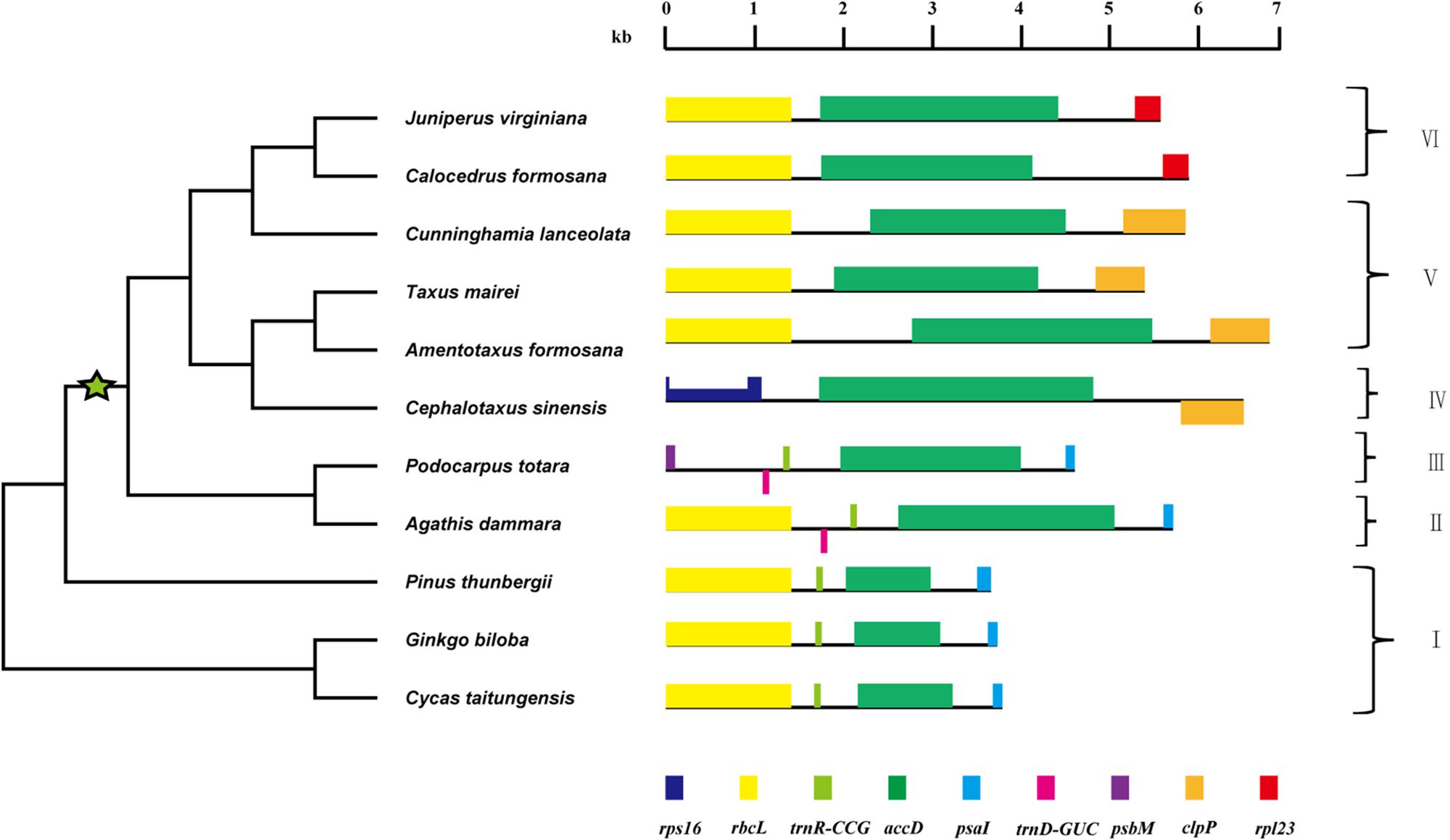
FIGURE 3. Gene organization around the accD locus in gymnosperms. Green star indicates the lineage where accD has expanded. Genes shown above line are transcribed from left to right, while those located below line are transcribed opposite direction. The half-height region in rps16 represents an intron. The topology (not drawn to scale) in the left side was the same as accD dN tree in Figure 2. The roman numbers I–VI denotes six types of gene organization around the accD.
Discussion
The accD Gene Length and Repetitive Elements
In gymnosperms, the reading frame lengths of accD vary considerably. At present, six complete chloroplast (cp) genomes of Gnetales have been published. However, accD could not be found in these cp genomes, suggesting that accD was lost from the cp genomes of Gnetales (Wu et al., 2009). The accD gene length of Cycas (359 codons) and Ginkgo (323 codons) is relatively short. In Pinaceae, the accD gene length range from 319 (Picea abies) to 326 (Picea morrisonicola) codons. However, we identified that the accD gene length in cupressophyte experienced an extraordinary expansion. From the alignment of 57 gymnosperm accD gene sequences, we can speculate that the enlarged accD gene size in cupressophytes is mainly caused by numerous amounts of insertion repetitive sequences in the middle region. Meanwhile, many different repetitive elements were identified in the inserted sequence. The repetitive elements have a relatively low similarity among different genus (Supplementary Table 2 and Figure 1), suggesting these repetitive elements likely do not have a common origin, and have formed independently.
The Function of Repetitive Elements in accD
In addition to cupressophyte, the repeat sequences in accD were also reported in two legume species (P. sativum and L. sativus) (Magee et al., 2010), pepper (C. annuum) (Jo et al., 2011) and M. truncatula (Gurdon and Maliga, 2014). This verifies the idea that some proteins are more easily generating repeats during evolution (Mularoni et al., 2010). The accD gene in both P. sativum and L. sativus contains many repeat sequences in their middle region, but the repetitive elements from these two species show low similarity. The repetitive elements in P. sativum and L. sativus were also different with those in cupressophyte, suggesting that repetitive elements were species-specific. In pepper, seven repeats of an 18 bp-long element sequences were observed. And interestingly, one pair of short direct repeat sequences was located nearby the inserted repeat sequences. But no such sequences were found near the inserted repeat sequences of accD gene in cupressophytes and legume, suggesting that these direct repeat sequences were not necessary for the formation of repeat sequences. The transcription of accD gene in pepper was confirmed by reverse transcriptase PCR, so the expanded accD gene in pepper is supposed to be functional. Furthermore, a large number of complex repeats were found in the different ecotype of M. truncatula. It is suggested that the function of these inserted repeat sequences is not very important for ACCase (Gurdon and Maliga, 2014). However, on the other hand, the reading frame in this gene was not destroyed; so we speculate that the repetitive elements in these species may play a role of regulation to protein function. All of these results suggest that accD is a specific gene that tends to be easily form independently repeat sequence. And these repeat sequences are species-specific, which were only detected in some species.
The accD gene encodes the carboxyltransferase β subunit of ACCase. It is essential for leaf development in tobacco, as knocking out accD gene may be lethal (Kode et al., 2005). Three points strongly indicate that the function of this gene has not been destroyed in cupressophytes. Firstly, despite containing repeats, the original reading frame of accD gene is maintained, revealing that the genes in cupressophytes work well. Secondly, three sites were considered to be important for accD gene in potato: an acetyl-CoA bonding site, a CoA-carboxylation catalytic site and a carboxy-biotin binding site. All these three sites were located at the C-terminal region of the protein in all gymnosperm species. Thirdly, only the lineages of cupressophytes contain a large number of complex repeats. The Cycas (359 amino acids), Ginkgo (323 amino acids) (Table 1) and most angiosperm accD genes have not been expanded and did not contain repeat sequences.
Yi et al. (2013) have confirmed that accD in C. oliveri still have function after expansion. From the alignment of accD gene in 57 gymnosperms, we could see that the accD gene is considerably conserved in the 3′ end. In comparison, the nucleus copies of accD in Trachelium and Trifolium each encodes only the 3′ end region of the chloroplastic gene (Rousseau et al., 2013). The 3′ end region of the accD gene encode the carboxyl transferase, which is the most important functional region discovered in this gene (Zhang et al., 2003). So it is reasonable to see a higher conservable 3′ end of the accD relative to the much variable 5′ end as their function restriction.
The Acceleration of Substitution Rate
Gene-specific rate acceleration is considered to be a common character of chloroplast genome evolution (Jansen et al., 2007; Park et al., 2017). Among three genes (rbcL, matK, accD) analyzed in this study, only accD gene shows an obviously acceleration in both dN and dS in cupressophyte (Figure 2), which was also the lineage full of abundant repeat sequences (Supplementary Table 2 and Figure 1). Meanwhile, the repetitive elements were also identified for rbcL and matK using Tandem Repeat Finder, but no repetitive elements were found. It seems that there is a positive correlation between repetitive elements and substitution rate acceleration. Many studies support that species-specific rate acceleration have relevant with genomic rearrangement (Jansen et al., 2007; Guisinger et al., 2008, 2010). However, the relationship between repeat sequence and rate acceleration has been little documented. Park et al. (2017) attribute the acceleration substitutate rate of accD gene in Geraniaceae to the insertion sequence. However, we found that repetitive elements also exist in these insertion sequences. Maybe the insertion of repetitive elements promoted the sequence to be more variable, thus leading to the acceleration of substitution rate.
Genome Rearrangement Happened Near accD
Dispersed repeat elements were supposed to locate in rearrangement endpoints. In this study, we also sequenced the gene nearby accD, and found that there are six kinds of gene order type near accD (Figure 3). This indicates that accD gene is located in inversion endpoints. In addition to the relocation or complete loss of trnD or trnR, rbcL is generally located on one side of accD. For Cephalotaxus, a large inversion has happened relative to other gymnosperms which relocated accD gene near rps16 rather than rbcL (Wu et al., 2011). We speculated that repetitive elements may induce the rearrangement near accD. An explanation for this correlation is that recombination between repeat sequence can lead to rearrangements of genome (Rogalski et al., 2006; Gray et al., 2009). In addition to accD, other rearrangement endpoints causing by inversions also exist. For instance, two inversions were identified between Agathis dammara and Nageia nagi chloroplast genome (Wu and Chaw, 2014), making the intergenic region of ycf1 and clpP, rpl23, rpl20, and petG as rearrangement endpoints. However, repeat elements were only detected in the intergenic region of ycf1 and clpP. Rpl23, rpl20, and petG did not have repeat sequence. These suggest that not all the rearrangement endpoints have repeat elements.
Conclusion
The accD gene in cupressophyte has undergone an extraordinary length expansion, which was mainly caused by abundant independent repetitive elements. Accompanied with repetitive elements, the dN and dS of accD are also accelerated. In addition, accD has been involved in many rearrangement events. All these results suggest that the repetitive elements may promote the acceleration of substitution rate and mediate the genome rearrangement. Our study provides a typical case for the research of relationship between repetitive elements, genome rearrangement and substitution rate.
Author Contributions
JL carried out the experiments, conducted data analysis, and wrote the manuscript. TW wrote the manuscript. YS designed the study and wrote the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31370364, 31570652, 31670200, and 31770587), the Natural Science Foundation of Guangdong Province, China (2016A030313320 and 2017A030313122), the Science and Technology Planning Project of Guangdong Province, China (2017A030303007), Project of Department of Science and Technology of Shenzhen City, Guangdong, China (JCYJ20160425165447211 and JCYJ20170413155402977), Science and Technology Planning Project of Guangzhou City, China (201804010389), the Doctoral Scientific Research Foundation of Shaanxi Xueqian Normal University (2016DS04), and Provincial Experimental Teaching Demonstration Center of Biology (2016).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with one of the authors YS.
Acknowledgments
We thank Shou-Jun Zhang in Wuhan Botanical Garden, Chinese Academy of Sciences, for sampling materials, and Lei Gao and Xuan Yi for the experimental assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00533/full#supplementary-material
Footnotes
References
Benson, G. (1999). Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580. doi: 10.1093/nar/27.2.573
Bryant, N., Lloyd, J., Sweeney, C., Myouga, F., and Meinke, D. (2011). Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis. Plant Physiol. 155, 1678–1689. doi: 10.1104/pp.110.168120
Cai, Z., Guisinger, M., Kim, H. G., Ruck, E., Blazier, J. C., McMurtry, V., et al. (2008). Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. J. Mol. Evol. 67, 696–704. doi: 10.1007/s00239-008-9180-7
Christenhusz, M. J. M., Reveal, J. L., Farjon, A., Gardner, M. F., Mill, R. R., and Chase, M. W. (2011). A new classification and linear sequence of extant gymnosperms. Phytotaxa 19, 55–70. doi: 10.11646/phytotaxa.19.1.3
Chumley, T. W., Palmer, J. D., Mower, J. P., Fourcade, H. M., Calie, P. J., Boore, J. L., et al. (2006). The complete chloroplast genome sequence of Pelargonium x hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 23, 2175–2190. doi: 10.1093/molbev/msl089
Curci, P. L., De Paola, D., Danzi, D., Vendramin, G. G., and Sonnante, G. (2015). Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS One 10:e0120589. doi: 10.1371/journal.pone.0120589
de Koning, A. P., and Keeling, P. J. (2006). The complete plastid genome sequence of the parasitic green alga Helicosporidium sp. is highly reduced and structured. BMC Biol. 4:12. doi: 10.1186/1741-7007-4-12
Djian, P., Hancock, J. M., and Chana, H. S. (1996). Codon repeats in genes associated with human diseases: fewer repeats in the genes of nonhuman primates and nucleotide substitutions concentrated at the sites of reiteration. Proc. Natl. Acad. Sci. U.S.A. 93, 417–421. doi: 10.1073/pnas.93.1.417
Erixon, P., and Oxelman, B. (2008). Whole-gene positive selection, elevated synonymous substitution rates, duplication, and indel evolution of the chloroplast clpP1 gene. PLoS One 3:e1386. doi: 10.1371/journal.pone.0001386
Faux, N. G., Huttley, G. A., Mahmood, K., Webb, G. I., de la Banda, M. G., and Whisstock, J. C. (2007). RCPdb: an evolutionary classification and codon usage database for repeat-containing proteins. Genome. Res. 17, 1118–1127. doi: 10.1101/gr.6255407
Gawel, N., and Jarret, R. (1991). A modified CTAB DNA extraction procedure for musa and ipomoea. Plant Mol. Biol. Rep. 9, 262–266. doi: 10.1007/bf02668371
Goremykin, V. V., Holland, B., Hirsch-Ernst, K. I., and Hellwig, F. H. (2005). Analysis of Acorus calamus chloroplast genome and its phylogenetic implications. Mol. Biol. Evol. 22, 1813–1822. doi: 10.1093/molbev/msi173
Gornicki, P., Faris, J., King, I., Podkowwinski, J., Gill, B., and Haselkorn, R. (1997). Plastid-localized acetyl-CoA carboxylase of bread wheat is encoded by a single gene on each of the three ancestral chromosome sets. Proc. Natl. Acad. Sci. U.S.A. 94, 14179–14184. doi: 10.1073/pnas.94.25.14179
Gray, B. N., Ahner, B. A., and Hanson, M. R. (2009). Extensive homologous recombination between introduced and native regulatory plastid DNA elements in transplastomic plants. Transgenic Res. 18, 559–572. doi: 10.1007/s11248-009-9246-3
Guisinger, M. M., Chumley, T. W., Kuehl, J. V., Boore, J. L., and Jansen, R. K. (2010). Implications of the plastid genome sequence of Typha (Typhaceae, Poales) for understanding genome evolution in Poaceae. J. Mol. Evol. 70, 149–166. doi: 10.1007/s00239-009-9317-3
Guisinger, M. M., Kuehl, J. V., Boore, J. L., and Jansen, R. K. (2008). Genome-wide analyses of Geraniaceae plastid DNA reveal unprecedented patterns of increased nucleotide substitutions. Proc. Natl. Acad. Sci. U.S.A. 105, 18424–18429. doi: 10.1073/pnas.0806759105
Guisinger, M. M., Kuehl, J. V., Boore, J. L., and Jansen, R. K. (2011). Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol. Biol. Evol. 28, 583–600. doi: 10.1093/molbev/msq229
Guo, X., Castillo-Ramirez, S., Gonzalez, V., Bustos, P., Fernandez-Vazquez, J. L., Santamaria, R. I., et al. (2007). Rapid evolutionary change of common bean (Phaseolus vulgaris L.) plastome, and the genomic diversification of legume chloroplasts. BMC Genomics 8:228. doi: 10.1186/1471-2164-8-228
Gurdon, C., and Maliga, P. (2014). Two distinct plastid genome configurations and unprecedented intraspecies length variation in the accD coding region in Medicago truncatula. DNA Res. 21, 417–427. doi: 10.1093/dnares/dsu007
Haberle, R. C., Fourcade, H. M., Boore, J. L., and Jansen, R. K. (2008). Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J. Mol. Evol. 66, 350–361. doi: 10.1007/s00239-008-9086-4
Hall, T. A. (1999). BioEdit:a user friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Harris, M. E., Meyer, G., Vandergon, T., and Vandergon, V. O. (2012). Loss of the acetyl-CoA carboxylase (accD) gene in Poales. Plant Mol. Biol. Rep. 31, 21–31. doi: 10.1007/s11105-012-0461-3
Hipkins, V. D., Marshall, K. A., Neale, D. B., Rottmann, W. H., and Strauss, S. H. (1995). A mutation hotspot in the chloroplast genome of a conifer (Douglas-fir: Pseudotsuga) is caused by variability in the number of direct repeats derived from a partially duplicated tRNA gene. Curr. Genet. 27, 572–579. doi: 10.1007/bf00314450
Hirao, T., Watanabe, A., Kurita, M., Kondo, T., and Takata, K. (2008). Complete nucleotide sequence of the Cryptomeria japonica D. Don. chloroplast genome and comparative chloroplast genomics: diversified genomic structure of coniferous species. BMC Plant Biol. 8:70. doi: 10.1186/1471-2229-8-70
Huntley, M., and Golding, G. B. (2000). Evolution of simple sequence in proteins. J. Mol. Evol. 51, 131–140. doi: 10.1007/s002390010073
Huntley, M. A., and Clark, A. G. (2007). Evolutionary analysis of amino acid repeats across the genomes of 12 Drosophila species. Mol. Biol. Evol. 24, 2598–2609. doi: 10.1093/molbev/msm129
Jansen, R. K., Cai, Z., Raubeson, L. A., Daniell, H., Depamphilis, C. W., Leebens-Mack, J., et al. (2007). Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. U.S.A. 104, 19369–19374. doi: 10.1073/pnas.0709121104
Jo, Y. D., Park, J., Kim, J., Song, W., Hur, C. G., Lee, Y. H., et al. (2011). Complete sequencing and comparative analyses of the pepper (Capsicum annuum L.) plastome revealed high frequency of tandem repeats and large insertion/deletions on pepper plastome. Plant Cell Rep. 30, 217–229. doi: 10.1007/s00299-010-0929-2
Kim, K. J., Choi, K. S., and Jansen, R. K. (2005). Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae). Mol. Biol. Evol. 22, 1783–1792. doi: 10.1093/molbev/msi174
Kode, V., Mudd, E. A., Iamtham, S., and Day, A. (2005). The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 44, 237–244. doi: 10.1111/j.1365-313x.2005.02533.x
Konishi, T., and Sasaki, Y. (1994). Compartmentalization of two forms of acetyl-CoA carboxylase in plants and the origin of their tolerance toward herbicides. Proc. Natl. Acad. Sci. U.S.A. 91, 3598–3601. doi: 10.1007/978-94-015-8394-7_15
Konishi, T., Shinohara, K., Yamada, K., and Sasaki, Y. (1996). Acetyl-CoA carboxylase in higher plants: most plants other than Gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol. 37:117–122. doi: 10.1093/oxfordjournals.pcp.a028920
Kurtz, S., Choudhuri, J., Ohlebusch, E., Schleiermacher, C., Stoye, J., and Robert, G. (2001). REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29, 4633–4642. doi: 10.1093/nar/29.22.4633
Lee, H. L., Jansen, R. K., Chumley, T. W., and Kim, K. J. (2007). Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol. Biol. Evol. 24, 1161–1180. doi: 10.1093/molbev/msm036
Li, J., Gao, L., Chen, S. S., Tao, K., Su, Y. J., and Wang, T. (2016). Evolution of short inverted repeat in cupressophytes, transfer of accD to nucleus in Sciadopitys verticillata and phylogenetic position of Sciadopityaceae. Sci. Rep. 6:20934. doi: 10.1038/srep20934
Lowe, T. M., and Eddy, S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964. doi: 10.1093/nar/25.5.955
Lu, Y., Ran, J. H., Guo, D. M., Yang, Z. Y., and Wang, X. Q. (2014). Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS One 9:e107679. doi: 10.1371/journal.pone.0107679
Madoka, Y., Tomizawa, K. I., Mizoi, J., Nishida, I., Nagano, Y., and Sasaki, Y. (2002). Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol. 43, 1518–1525. doi: 10.1093/pcp/pcf172
Magee, A. M., Aspinall, S., Rice, D. W., Cusack, B. P., Semon, M., Perry, A. S., et al. (2010). Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res. 20, 1700–1710. doi: 10.1101/gr.111955.110
Mularoni, L., Ledda, A., Toll-Riera, M., and Alba, M. M. (2010). Natural selection drives the accumulation of amino acid tandem repeats in human proteins. Genome Res. 20, 745–754. doi: 10.1101/gr.101261.109
Park, S., Ruhlman, T. A., Weng, M. L., Hajrah, N. H., Sabir, J. S. M., and Jansen, R. K. (2017). Contrasting patterns of nucleotide substitution rates provide insight into dynamic evolution of plastid and mitochondrial genomes of Geranium. Genome Biol. Evol. 9, 1766–1780. doi: 10.1093/gbe/evx124
Rogalski, M., Ruf, S., and Bock, R. (2006). Tobacco plastid ribosomal protein S18 is essential for cell survival. Nucleic Acids Res. 34, 4537–4545. doi: 10.1093/nar/gkl634
Rousseau, G. M., Huang, X., Higginson, E., Ayliffe, M., Day, A., and Timmis, J. N. (2013). Potential functional replacement of the plastidic acetyl-CoA carboxylase subunit (accD) gene by recent transfers to the nucleus in some angiosperm lineages. Plant Physiol. 161, 1918–1929. doi: 10.1104/pp.113.214528
Saski, C., Lee, S. B., Daniell, H., Wood, T. C., Tomkins, J., Kim, H. G., et al. (2005). Complete chloroplast genome sequence of Glycine max and comparative analyses with other legume genomes. Plant Mol. Biol. 59, 309–322. doi: 10.1007/s11103-005-8882-0
Sasaki, Y., and Nagano, Y. (2004). Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci. Biotechnol. Biochem. 68, 1175–1184. doi: 10.1271/bbb.68.1175
Simon, M., and Hancock, J. M. (2009). Tandem and cryptic amino acid repeats accumulate in disordered regions of proteins. Genome Biol. 10:R59. doi: 10.1186/gb-2009-10-6-r59
Tangphatsornruang, S., Uthaipaisanwong, P., Sangsrakru, D., Chanprasert, J., Yoocha, T., Jomchai, N., et al. (2011). Characterization of the complete chloroplast genome of Hevea brasiliensis reveals genome rearrangement, RNA editing sites and phylogenetic relationships. Gene 475, 104–112. doi: 10.1016/j.gene.2011.01.002
Timme, R. E., Kuehl, J. V., Boore, J. L., and Jansen, R. K. (2007). A comparative analysis of the Lactuca and Helianthus (Asteraceae) plastid genomes: identifcation of divergent regions and categorazation of shared repeats. Am. J. Bot. 94, 302–312. doi: 10.3732/ajb.94.3.302
Tsai, C. H., and Strauss, S. H. (1989). Dispersed repetitive sequences in the chloroplast genome of Douglas-fir. Curr. Genet. 16, 211–218. doi: 10.1007/bf00391479
Vieira, L. N., Faoro, H., Rogalski, M., Fraga, H. P., Cardoso, R. L., et al. (2014). The complete chloroplast genome sequence of Podocarpus lambertii: genome structure, evolutionary aspects, gene content and SSR detection. PLoS One 9:e90618. doi: 10.1371/journal.pone.0090618
Wolfe, K. H., Mordent, C. W., and Palmers, J. D. (1992). Function and evolution of a minimal plastid genome from a nonphotosynthetic parasitic plant. Proc. Natl. Acad. Sci. U.S.A. 89, 10648–10652. doi: 10.1073/pnas.89.22.10648
Wu, C. S., and Chaw, S. M. (2014). Highly rearranged and size-variable chloroplast genomes in conifers II clade (cupressophytes): evolution towards shorter intergenic spacers. Plant Biotechnol. J. 12, 344–353. doi: 10.1111/pbi.12141
Wu, C. S., Lai, Y. T., Lin, C. P., Wang, Y. N., and Chaw, S. M. (2009). Evolution of reduced and compact chloroplast genomes (cpDNAs) in gnetophytes: selection toward a lower-cost strategy. Mol. Phylogenet Evol. 52, 115–124. doi: 10.1016/j.ympev.2008.12.026
Wu, C. S., Wang, Y. N., Hsu, C. Y., Lin, C. P., and Chaw, S. M. (2011). Loss of different inverted repeat copies from the chloroplast genomes of Pinaceae and cupressophytes and influence of heterotachy on the evaluation of gymnosperm phylogeny. Genome Biol. Evol. 3, 1284–1295. doi: 10.1093/gbe/evr095
Yi, X., Gao, L., Wang, B., Su, Y. J., and Wang, T. (2013). The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): evolutionary comparison of Cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol. Evol. 5, 688–698. doi: 10.1093/gbe/evt042
Zhang, H., Yang, Z., Shen, Y., and Tong, L. (2003). Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase. Science 299, 2064–2067. doi: 10.2210/pdb3k8x/pdb
Zhang, Y., Ma, J., Yang, B., Li, R., Zhu, W., Sun, L., et al. (2014). The complete chloroplast genome sequence of Taxus chinensis var. mairei (Taxaceae): loss of an inverted repeat region and comparative analysis with related species. Gene 540, 201–209. doi: 10.1016/j.gene.2014.02.037
Keywords: accD, repeat sequences, substitution rates, rearrangement, cupressophytes
Citation: Li J, Su Y and Wang T (2018) The Repeat Sequences and Elevated Substitution Rates of the Chloroplast accD Gene in Cupressophytes. Front. Plant Sci. 9:533. doi: 10.3389/fpls.2018.00533
Received: 06 February 2018; Accepted: 05 April 2018;
Published: 20 April 2018.
Edited by:
Renchao Zhou, Sun Yat-sen University, ChinaReviewed by:
Xiaofang Jin, Nanchang Institute of Technology, ChinaPeng-Cheng Fu, Luoyang Normal University, China
Copyright © 2018 Li, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjuan Su, c3V5akBtYWlsLnN5c3UuZWR1LmNu Ting Wang, dGluZ3dhbmdAc2NhdS5lZHUuY24=
 Jia Li
Jia Li Yingjuan Su
Yingjuan Su Ting Wang
Ting Wang