- 1College of Horticulture, Sichuan Agricultural University, Chengdu, China
- 2Institute of Pomology and Olericulture, Sichuan Agricultural University, Chengdu, China
Melatonin, a multiple signal molecule, plays important roles in delaying senescence during the development of plants. Because few species have been studied for the effect of exogenous melatonin on anti-aging, the plausible mechanism of melatonin of anti-aging effects on other plant species has remained largely unknown. In the present study, the effects of exogenous melatonin on leaf senescence in kiwifruit were examined during natural aging after melatonin (200 μM) or water (Control) pretreatment. The decreased membrane damage and lower hydrogen peroxide (H2O2) content due to the enhanced scavenging activity of antioxidant enzymes peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) demonstrated that melatonin effectively delayed the aging of kiwifruit leaves. Likewise, owing to up-regulated expression of chlorophyll a/b-binding protein (CAB) gene in the sampled leaves pretreated with melatonin, chlorophyll degradation decreased. Therefore, osmoregulatory substances in sampled leaves accumulated (e.g., soluble sugar and soluble protein) and seedling cell environment stability was maintained. Simultaneously, melatonin decreased H2O2 concentration owing to increased glutathione (GSH) and ascorbate (AsA) content, and the expression levels of glutathione reductase (GR), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR), dehydroascorbate reductase (DHAR) were up-regulated by melatonin application, indicating that the increase of GSH and AsA was attributed to the expression of these genes. In addition, a large amount of flavonoids accumulated in seedlings pretreated with melatonin, and transcript levels of eight genes involved in flavonoid synthesis, including phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxymate (C4H), chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), flavonol synthase (FNS), leucoanthocyanin reductase (LAR), anthocyanin reductase (ANR), flavonoid 3-O-glucosyltransferase (UFGT) were enhanced in response to melatonin application. These results indicated that melatonin delayed aging of kiwifruit leaves by activating the antioxidant capacity and enhancing flavonoid biosynthesis. All of these results can provide clear proof that melatonin plays a key roles in delaying leaf senescence.
Introduction
Senescence, the final stage in the leaf development, is programmed and complex process, which is regulated by developmental and environmental factors. Cells in those tissues experience several drastic changes in metabolism (Quirino et al., 2000; Lim and Nam, 2007; Kim et al., 2017). These changes include degradation of chlorophyll and active macromolecules, recycling of nutrients, transcriptional level of senescence-associated genes, and accumulation of excess of harmful free radicals (Inada et al., 1998; Thompson et al., 1998). Although a crucial and evolutionarily physiological process for plant fitness, senescence of plant leaves affect plant biomass accumulation and flower bud differentiation, and yield in future, resulting in economic losses. Therefore, an improved understanding of leaf senescence must be explored, and novel strategies for preventing or delaying this process in certain environment must be developed to enhance ecological and safety of plant.
Melatonin (N-acetyl-5-methoxytryptamine), an indole molecule, was extracted in 1958 from the bovine pineal gland (Lerner et al., 1958, 1959). Since the discovery of melatonin, much progress has been made in unraveling its role in plants. Previous studies speculated that mitochondria and chloroplasts are the original synthesis sites of melatonin according to melatonin’s primary function and evolution in eukaryotes. Mitochondria and chloroplasts are major producer of free radical. High levels of melatonin in mitochondria and chloroplasts are used to protect these important cellular organelles against oxidative stress and preserve their physiological functions. (Tan et al., 2013; Manchester et al., 2015; Reiter et al., 2017).
A primary function of melatonin in plants is to act as an antioxidant. Melatonin works in plant by decreasing ROS, reducing menbrane lipid peroxidation and up-regulating of antioxidant enzymes activity (glutathione peroxidase, superoxide dismutases, and catalase et at.) (Tan et al., 2002; Rodriguez et al., 2004; Reiter et al., 2014). It can act as a growth regulator in a similar way as the auxin, indole-3-acetic acid (IAA) does, governing the growth of roots, shoots, and explants (Murch et al., 2001; Hernandez-Ruiz et al., 2004, 2005; Arnao and Hernández-Ruiz, 2014). Chen et al. (2009) detected 0.1 μM melatonin stimulated the roots growth of young wild leaf mustard seedlings (Brassica juncea), while 100 μM inhibited. Furthermore, exogenous supplement of 0.1 μM melatonin improved the endogenous levels of free IAA in roots of young seedlings. In addition, melatonin can delay senescence as a biological stimulant, which was demonstrated by previous studies (Tal et al., 2011; Zhang et al., 2013; Wang et al., 2014). Moreover, melatonin protects plants against a variety of environmental stresses, such as cold (Ding et al., 2017), drought (Cui et al., 2017), salinity (Arnao and Hernandez-Ruiz, 2009; Wang et al., 2016), and heavy metal toxicity (Gu et al., 2017; Nawaz et al., 2018). A study utilized mRNA-seq technology to analyze the effect of melatonin on genome-wide gene expression, and monitored a large number of differentially expressed genes: (i) genes involved in plant stress defense: many stress receptors, kinases, and stress-associated calcium signals were up-regulated, chlorophyllase and PaO, involved in chlorophyll degradation, were both down-regulated; in addition, cell death associated genes were mostly down-regulated, (ii) genes involved in hormone signaling: most identified genes in ABA, ethylene, salicylic acid, and jasmonic acid pathways were up-regulated, while genes associated to auxin responses, homeostasis, signaling, peroxidases, and those associated with cell wall synthesis and modifications were mostly down-regulated (Weeda et al., 2014). The interesting results may indicate the reason why melatonin possesses antioxidant ability and the role of melatonin in stress defense and delaying senescence.
Excess and uncontrolled accumulation of reactive oxygen species (ROS) is responsible for the onset of senescence. ROS levels and their destructive effects are known as the common action the plant response to senescence (Apel and Hirt, 2004; Khanna-Chopra, 2012). Many researchers have demonstrated that melatonin can protect organisms against ROS (Tan et al., 2007; Li et al., 2012; Wang P. et al., 2012; Shi et al., 2015; Zhang et al., 2016; Gong et al., 2017). Its antioxidant activity appears to function via the following pathways: (i) scavenging free radicals directly, (ii) stimulating antioxidant enzymes, such as peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT), (iii) increasing the activities of other antioxidants, e.g., ascorbic acid (AsA), soluble sugars, and flavonoids, (iv) protecting antioxidant enzymes from oxidative damage, and (v) enhancing the efficiency of the mitochondrial electron transport chain, thereby easing electron leakage and, to reduce the content of free radicals (Li et al., 2012; Wang P. et al., 2012; Shi et al., 2015; Zhang et al., 2016; Zhou et al., 2016; Gong et al., 2017). However, regulation mechanisms involved in the senescence of leaves by melatonin is rarely reported in plants, particularly fruit trees.
The objective of the present study was to investigate the effects of melatonin in delaying senescence and to analyze the mechanisms induced by melatonin. Here, we were able to detect the changes in physiology and molecules, included chlorophyll concentrations, membrane lipid peroxidation degree, H2O2 content, et al. Specifically, we analyzed the content of GSH, AsA, and phenolic compounds, and studied the gene expression of related enzyme in the AsA-GSH cycle and the flavonoid biosynthetic pathway to highlight the exact mechanism of melatonin-induced antioxidant properties.
Materials and Methods
Sample Collection, Plant Material Preparation, and Melatonin Application
In the present study, kiwifruit seedlings were used as study materials. The plant seeds were collected in September 2016. We soaked the seeds in 800 mg⋅L-1 gibberellin solution for a day and stratified them at 4°C for 2 months. For inducing germination, the seeds were placed in incubator at 25°C for 8 h and at 4°C for 16 h for 2 weeks. Thereafter, the seeds were incubated at 25°C until sprouting occurred. The germinated seeds were planted in a seedling tray and covered with 2 mm nutrient soil layer. They were cultured in an artificial climate room (light/dark cycle: 12 h/12 h, temperature: 25°C/20°C). When the seedlings grew to have two to three true leaves, the seedlings with similar growth were transferred into plastic pots (20 cm in diameter) containing perlite and placed in a greenhouse before treatment. All the lateral branches were carefully removed.
After 4 months of growth in 2017, healthy and uniform plants were assigned to two conditions for pretreatment: (i) standard water supply (control) or (ii) solution of 200 μmol⋅L-1 melatonin in water (treatment). The pretreatment was conducted from September 14 to October 11 in an open experimental field. During this period, the seedlings were treated with melatonin or water every 7 days by root irrigation (20 mL per pot). After the fifth irrigation, the plants were sampled at day 0 (October 12), day 15 (October 5), day 30 (November 11), and day 45 (November 26), between 10:00 and 11:00 h, by removing the fifth to ninth leaves upward along the stem from five trees per treatment. Each treatment contained 45 pots (1 seedling per pot). The samples were quickly frozen after collection and stored in a cryogenic refrigerator at -80°C for subsequent index determination. All reactions were performed by using the leaf mixture of five kiwifruit seedlings with three technical and three biological replicates.
Measurement of Chlorophyll, Malondialdehyde (MDA), and Hydrogen Peroxide (H2O2) Concentration
The chlorophyll concentration was measured as described by Arnon (1949); chlorophyll was extracted with 80% acetone, and the concentrations of chlorophyll a and b were determined with a UV-1800 system (Shimadzu, Kyoto, Japan). MDA content was measured according to the thiobarbituric acid method (Hodges et al., 1999). Briefly, 0.3 g leaves was ground as homogenate using 5 ml cold 5% (w/v) trichloroacetic acid solution (TCA) and centrifuged at 10000 g for 10 min at 4°C. Then, 2 ml supernatant and 0.67% (w/v) thiobarbituric acid (TBA) were mixed, sealed and heated for 10 min in boiling water bath. Following boiling water bath, the absorbance of supernatant was measured at 450, 532, 600 nm after cooling. Determination of H2O2 concentration was based on the method of Lin et al. (1988). In brief, 0.3 g leaves was ground as homogenate with 5 ml cold acetone and centrifuged at 10000 g for 10 min for 4°C. Then, 1 ml supernatant was added to 0.1 ml 5% (w/v) titanium sulfate and 0.2 mL concentrated ammonia and the mixture was mixed. The mixture was centrifuged at 4000 g for 10 min for 4°C. After centrifuging, the precipitation was saved and washed for 3–5 times with acetone until the plant pigment was removed. Finally, the precipitation was dissolved with 5 mL 2 M concentrated sulfuric acid and fix the volume of distilled water to 10 ml. The absorbance was determined at 415 nm. The content of H2O2 was based on a standard curve generated with known H2O2 concentrations.
Determination of Osmotic Substance Content and Activity of Antioxidant Enzymes
Content of soluble protein and soluble sugar, as well as the activities of POD, SOD, CAT were determined using the method of Wang and Huang (2015) The content of soluble sugar was determined using anthrone colorimetry method. 0.2 g leaves and deionized water were extracted in boiling water bath for 30 min for twice. The extract was added to ethyl acetate containing anthrone and concentrated sulfuric acid and the mixture was mixed. Then the mixture was induced in boiling water bath about 1 min and the absorbance was determined at 630 nm. Soluble protein content was measured by Coomassie brilliant blue G-250 method. In brief, 0.3 g of sample leaves was ground as homogenate with 50 mM cold potassium phosphate buffer (PBS) (pH 7.8). The homogenate was centrifuged at 10000 g for 10 min at 4°C and the supernatant was saved for further analysis. The supernatant was added to coomassie brilliant blue G250 solution (dissolved in 90% ethanol and 85% (w/v) phosphoric acid) and the mixture was mixed. The absorbance was determined at 595 nm.
For the extraction of crude enzyme solution, 0.3g leaves was ground as homogenate in 8 mL cold 50 mM PBS (pH 7.8) containing 1% (w/v) polyvinyl pyrrolidone (PVP), 2 mM dithiothreitol (DTT) and 0.1 mM ethylenediaminetetraacetic acid (EDTA). Homogenates were then centrifuged at 10000 g at 4°C for 10 min and the supernatant was saved for further enzyme activity measurement. The guaiacol colorimetry was used for the measurement of POD activity. POD activity was measured in a reaction mixture containing 50 mM PBS (pH 5.5), guaiacol, 30% (v/v) H2O2, and the enzyme extract. The absorbance change in 470 nm was monitored and the result was expressed as U.g-1 (absorbance decrease of 0.01 per minute is 1 U at 470 nm). SOD activity was determined based on photochemical reduction of nitro blue tetrazolium (NBT) and assayed by monitoring the absorbance at 560 nm. The result was expressed as U.g-1 (reaction mixture absorbance of 1 g kiwifruit leaf at 470 nm decrease of 0.01 per minute is 1 U). The activity of CAT was determined by monitoring the decline in 240 nm and the result was expressed as U.g-1 (reaction mixture absorbance of 1 g kiwifruit leaf at 240 nm decrease of 0.1 per minute is 1 U). The reaction mixture containing 200 mM PBS (pH 7.8), 100 mM H2O2, and the enzyme extract.
Determination of AsA and GSH Content
AsA content was determined by the Fe3+ reduction method (Kampfenkel et al., 1995). In brief, 0.5 g of sample leaves was ground as homogenate using 5 mL cold 5% (w/v) TCA. The homogenate was centrifuged at 10000 g for 10 min at 4°C and the supernatant was saved for further analysis. For the measurement of total ascorbate (T-AsA), 0.2 mL supernatant was incubated in 200 mM PBS (pH 7.4) and 6 mM DTT mixture at 42°C for 20 min in a water bath. After incubation, 0.2 ml 0.4% (w/v) N-ethylmaleimide (NEM) was added to remove excess DTT. Then 1 mL 10% (w/v) TCA, 0.8 mL 42% (w/v) o-phosphoric acid, 0.8 mL 2% (w/v) 2, 2′-dipyridyl in 70% ethanol, and 0.4 mL 3% (w/v) FeCl3 were added to the reaction mixture. The reaction was incubated at 42°C for 50 min in a water bath. AsA content was measured by using the similar method described above except DTT and NEM were substituted with 0.4 mL deionized water. The absorbance of AsA and T-AsA reaction mixture were determined at 525 nm by an ultraviolet spectrophotometer. T-AsA and AsA contents were determined based on a standard curve generated with known AsA concentrations. Dehydroascorbate (DHA) was defined as the difference between T-AsA and AsA.
The measurement of GSH content was based on the method of Griffith (1980); Briefly, 0.5 g of leaf tissue was ground as homogenate using 5 mL cold 7% (w/v) sulfosalicylic acid. The homogenate was centrifuged at 10000 g for 10 min at 4°C and the supernatant was saved for further analysis. For total glutathione (T-GSH) content analysis, 0.1 mL supernatant was mixed with 2.0 mL 200 mM PBS (pH 7.0), 0.3 mL 3 mM dithiobis-2-nitrobenzoicacid (DTNB), 0.3 mL 0.5 mM NADPH (include 7 mM EDTA). The reaction was initiated by adding five units of GR and incubated at 27°C for 30 min in a water bath. Glutathione disulfide (GSSG) content was analyzed in a same method as above, except for that the volume of PBS was substituted with 1.7 ml and the 0.1 mL supernatant was first incubated with 0.3 mL 2-vinylpyridine (2-VP) at 27°C for 1 h to derivatize GSH. The absorbance was measured at 412 nm for the reaction mixture of T-GSH and GSSG. T-GSH and GSSG contents were determined based on a standard curve generated with known GSH concentrations. GSH content was the difference between T-GSH and GSSS.
Determination of the Content of Phenolic Compounds and Antioxidant Capacity
The methods of determining total phenolics (TPC), flavonoids (TFC), flavanols (TFAC), and anthocyanins (TMAC) were described by Wang (2015). In brief, 0.2 g of leaf tissue was ground as homogenate with cold 70% (v/v) methanol containing 2% (v/v) formic acid and 28% (v/v) ethanol. The homogenate was ultrasonically extracted for 30 min and shake at 250 rpm for 2 h at 30°C. Then the homogenate was centrifuged at 10000 g for 10 min at 4°C and the supernatant was filtrated by 0.45 μm filter membrane for further analysis. TPC was determined by folin-ciocaleu method, and the absorbance was measured using an ultraviolet spectrophotometer at 765 nm by using gallic acid as standard; the result was represented by gallic acid equivalency. The absorption of TFC was measured at 510 nm, and expressed as rutin equivalents. TFAC was determined by p-DMACA method; absorbance was determined at 640 nm, and the result was expressed as catechin equivalents. The pH differential was used to measure TMAC. The fruit extract was diluted to pH 1.0 and 4.5 using a buffer solution and the absorbance value at 510 and 700 nm was measured at each pH. The total anthocyanin content was calculated as the difference between them. The methods of Du (2009) were applied to measure free radical scavenging ability, including DPPH, ABTS, and FRAP methods. The results of these three methods were expressed as trolox equal antioxidant capacity.
Quantitative Polymerase Chain Reaction (PCR) Analysis
Quantitative PCR (qPCR) was used to analyze the transcript levels of genes involved in the synthesis of flavonoids and AsA-GSH cycle in naturally senescencing seedlings. The Primer3 INPUT1 was used to design primers (Table 1). OmniPlant RNA Kit (DNase I) (CoWin, China) was used to extract total RNA, according to the manufacturer’s instructions. One microgram total RNA was used to synthesize the first strand cDNA using PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Japan). The synthesis was performed according to the instructions of the manufacturer. A qPCR was performed with a SYBR Premix Ex TaqTM II Kit (TaKaRa, Japan) on Real-Time System (CFX96, Bio-Rad, ıHercules, CA, United States). The reaction mixture (20 μL) contained 1.5 μL cDNA (100 times dilution), 0.8 μL each primer (10 μmol L-1), 10 μL 2× SYBR Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa, Japan), and 6.9 μL ddH2O. The reaction conditions were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 10 s, and 58°C for 30 s. As an internal control, Actin (Atkinson et al., 2009) (Table 1) was used to normalize the relative expression levels of the genes studied. Three PCR replicates were conducted per sample and the 2-ΔΔCT method was applied to calculate the relative expression levels. Total RNA were extracted in the sample leaves with three biological replicates, three technical replicates were conducted when qPCR was performed.
Statistical Analysis
Excel 2010 was used for data processing. The data were plotted using SigmaPlot 12.5 (Systat, Santa Clara, CA, United States). Analysis of variance was performed by the statistical program SPSS 22.0 (SPSS, Inc., Chicago, IL, United States). Significant differences were detected by Duncan’s multiple range tests at the 0.05 level.
Result
Effect of Melatonin Application on the Physiological State of the Leaves of Kiwifruit During Senescence
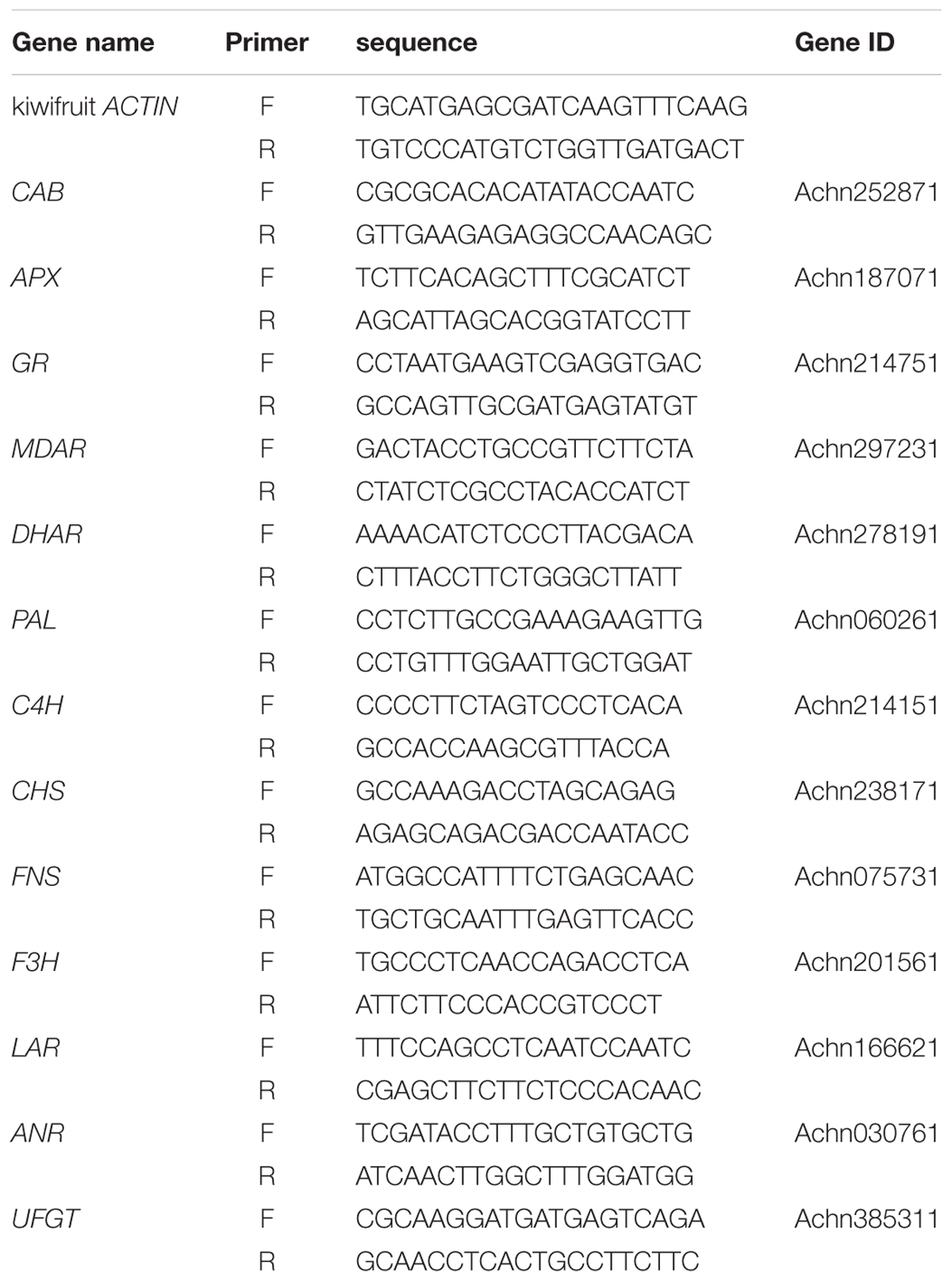
To determine whether supplemental melatonin can delay the senescence, we detected the chlorophyll content, soluble sugar, and soluble protein of kiwifruit leaves in different treatments. We found that melatonin pretreatment decreased chlorophyll degradation (Figure 1). During natural senescence, the concentrations both chlorophyll a and b of the melatonin-treated group were higher than that of control group after day 0; particularly on day 15, total chlorophyll concentration of the melatonin-treated group was 1.9 times of that of the control. However, in later senescence processes, the efficacy of melatonin application reduced gradually, indicated by the lack of any significant difference between the two groups. CAB was responsible for encoding chlorophyll a/b-binding protein. The expression of CAB was down-regulated during senescence, but the CAB expression in samples pretreated with melatonin was slightly higher than that in the control group. However, there was no significant difference between day 15 and 45 in the control group, and the result appeared consistent with the change in chlorophyll content.
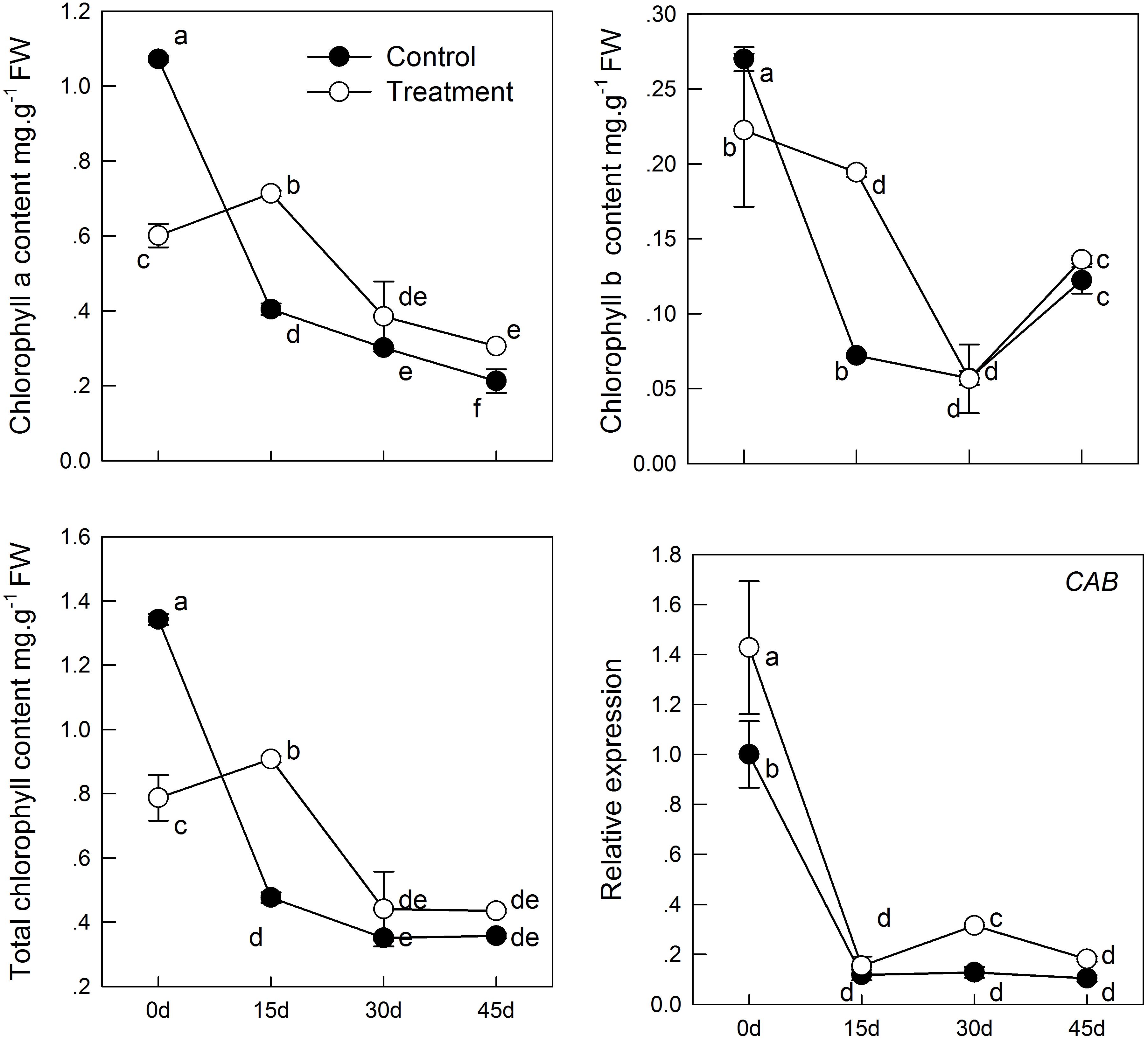
FIGURE 1. The effect of melatonin on leaves chlorophyll content and CAB gene analyzed by qPCR during kiwifruit senescence. Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
With aging, the content of soluble sugar in the leaf samples decreased continuously (Figure 2), which may induced by the decrease in total chlorophyll concentration, thereby causing reduction of carbohydrates synthesis by photosynthesis. However, melatonin treatment delayed the decrease in soluble sugar content. The soluble sugar content in the sampled leaves treated with melatonin was significantly higher in the treatment than in the group before 30 days. Furthermore, melatonin treatment promoted the content of soluble protein (Figure 2), which was 1.3 times as high in the melatonin-treated group as that in the control group on day 15. However, there was no significant difference between the treatment and control groups after 15 days.
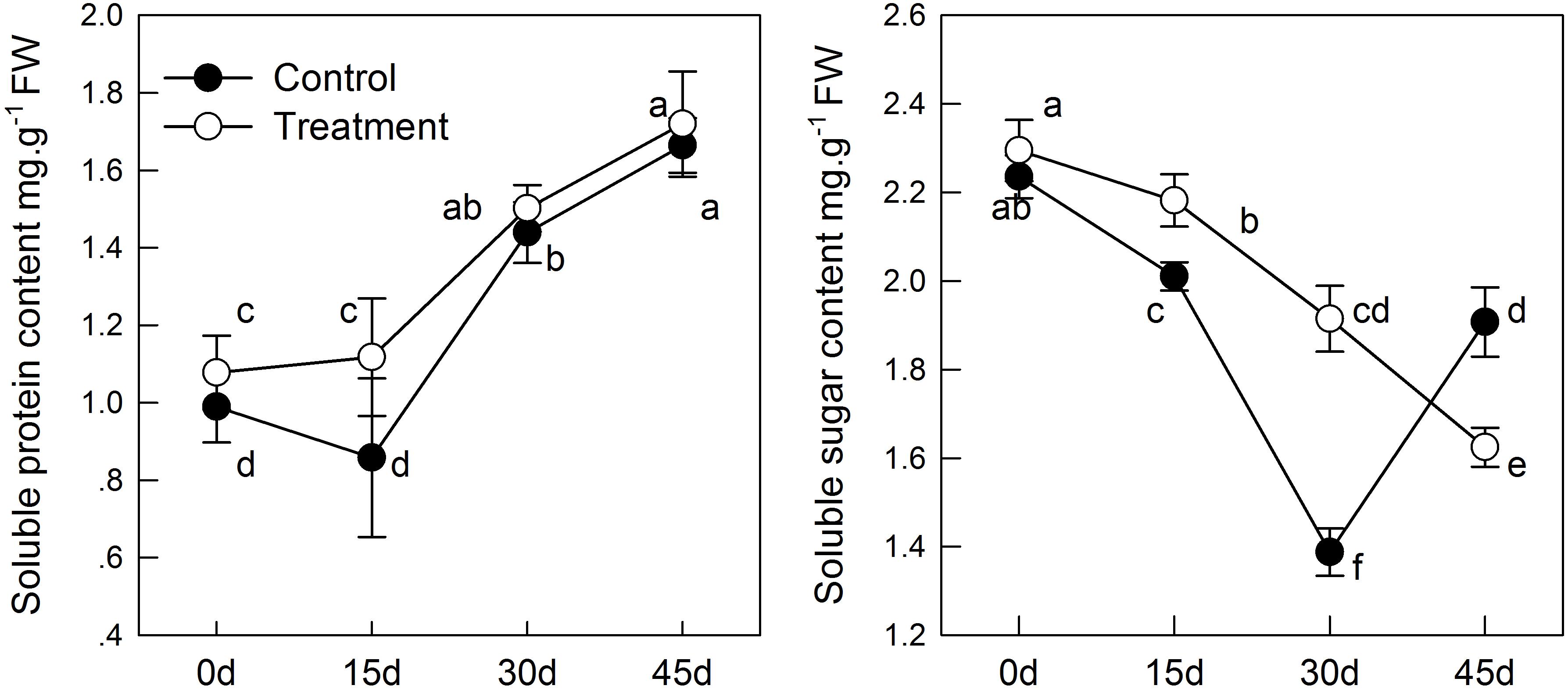
FIGURE 2. The effect of melatonin on leaves physiological state of kiwifruit during senescence. Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
Effects of Exogenous Melatonin Application on Antioxidant System of Kiwifruit Leaves During Natural Senescence
The MDA content indicates lipid peroxidation in plant cell membranes. In the senescence process, melatonin treatment can alleviate the membrane peroxidation process induced by aging; but the difference between the treatment and control groups was not significant before 30 days (Figure 3). Similar results as those mentioned above were obtained when monitoring H2O2 levels. H2O2 concentration increased with aging, whereas H2O2 was reduced in tissues sampled from pretreated seedlings. On day 45, the content of H2O2 in the melatonin-treated group was significantly lower than that in the control group (Figure 3). These results indicated that melatonin might partly control the ROS concentration in leaves and alleviate membrane lipid oxidation.
FIGURE 3. The effects of melatonin on antioxidant system of kiwifruit during senescence; Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
In the process of natural senescence, POD activity in the melatonin-treated group was significantly higher than that in the control group (Figure 3). When the melatonin- pretreated seedlings were at day 45, the activity of POD was higher than that in the control seedlings. CAT activity in pretreated seedlings was slightly higher than that in the control group, but the difference was not significant. Likewise, melatonin strongly activated the activity of SOD of treatment group; SOD had maximum activity on day 30. The results were consistent with the changes of H2O2 content, which indicated that melatonin could promote the activity of antioxidant enzymes and enhance the antioxidant ability of kiwifruit.
Effect of Melatonin Application on the AsA-GSH Cycle in Leaves of Kiwifruit During Senescence
The intracellular redox state conversion of ascorbic acid and glutathione plays an important role in resistance of plants to stress and senescence. In the process of senescence, T-GSH and GSSG content decreased constantly; simultaneously, melatonin pretreatment increased GSH content and decreased GSSG content (Figure 4). However, the T-GSH content in control was significantly higher than in the melatonin-treated group on day 30, which was the result of GSSG accumulation. Similarly, the content of both T-AsA and AsA were promoted in tissues sampled from pretreated seedlings. The resultant DHA produced in the melatonin-treated group was higher than that in the control on day 15 and 30 owing to continuous oxidation of AsA. Meanwhile, a higher rate of AsA/(AsA + DHA) and GSH/(GSH + GSSG) was found in the melatonin-treated samples with aging than in the untreated samples.
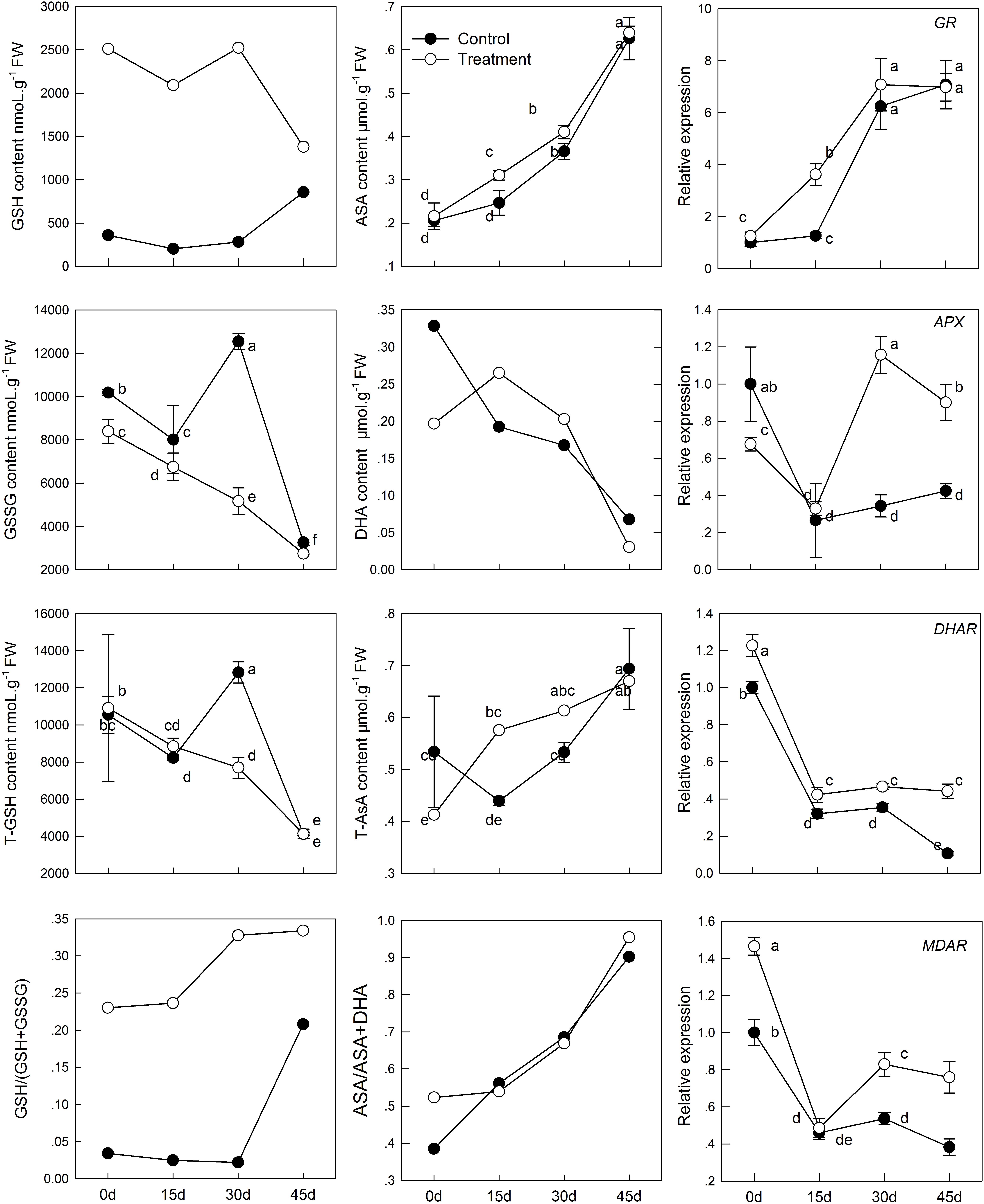
FIGURE 4. Effects of exogenous melatonin on AsA-GSH cycle and genes that analyzed by qPCR involved in AsA-GSH cycle during kiwifruit senescence; Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
The transcript levels of four genes related to AsA-GSH cycle (GR, APX, DHAR, and MDAR) in leaves of seedlings were found to be significantly activated by pretreatment with melatonin (Figure 4). The GR expression level of melatonin-treated group was slightly higher than that of the control group, but exhibited no significant difference, except at day 15. APX expression was lower in the melatonin-treated group than in the control group in the earlier period, but it was up-regulated by melatonin after day 15. The maximum expression was 3.37 times as that of the control group on day 30. The expression of DHAR and MDAR was down-regulated. However, it was markedly increased by melatonin treatment. On day 45, DHAR and MDAR of the melatonin-treated group was 4.18 and 1.98 fold higher than that of the control group, respectively.
Effect of Melatonin Application on Flavonoid Biosynthesis in Leaves of Kiwifruit During Senescence
Phenolic compounds are one of foremost and widely distributed secondary metabolites in plants, which can enhance plant stress resistance and natural antioxidant ability. We found that phenolic compound content in the course of natural senescence was significantly improved by melatonin application, as compared with that in the control group (Figure 5). In pretreated seedlings, The TPC reached the maximum on day 15 (increased by 21%); thereafter TPC began to decrease gradually, and at day 45, there was no significant difference from day 0. However, TFC constantly increased to levels that were 1.3 times higher in the melatonin-treated group that in the control group at day 45. Moreover, TFAC began to stabilize after reaching a maximum value on day 15. In accordance with TFC, TMAC in the melatonin-treated group was markedly higher than that in the control group. It increased 50.7% from day 0 to day 45. The anti-oxidation ability in sample leaves was also promoted by melatonin pretreatment. Both the DPPH, FRAP, ABTS, and antioxidant capacity of the melatonin-treated group was significantly higher than that in the control group, which demonstrated that melatonin can directly or indirectly regulate the antioxidant ability of plants as an antioxidant.
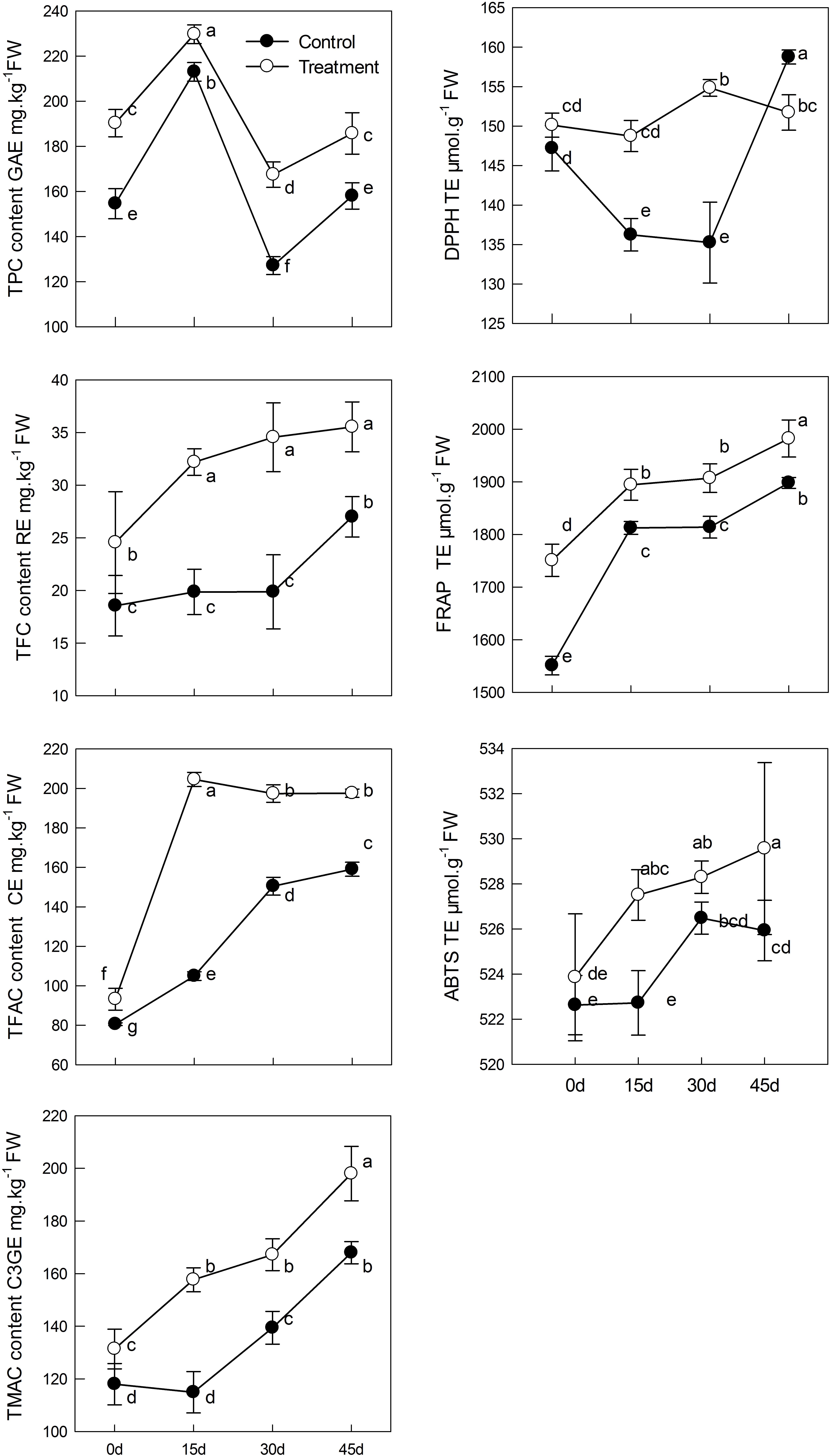
FIGURE 5. The effects of melatonin on phenolic compounds and antioxidant capacity determined by DPPH, ABTS, and FRAP assays of kiwifruit during senescence; Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
For further dissecting melatonin modulation of controlling flavonoids metabolism, we measured the mRNA levels of PAL, C4H, CHS, FNS, F3H, LAR, ANR, and UFGT by real-time quantitative PCR, both are the crucial genes related flavonoids metabolism (Figure 6). PAL and C4H are all rate-limiting enzymes in the first two steps of the flavonoid synthesis pathway; we observed that transcript levels of two genes induced by melatonin were significantly higher in the melatonin-treated group than in the control group. Similarly, the expression levels of TMAC biosynthesis structural genes (CHS, FNS, F3H, and UFGT) were markedly higher in melatonin pretreatment than in the control, but the expression level of CHS and F3H was down-regulated. There was no significant difference in CHS and F3H expression between the melatonin-treated group and the control group after day 30. Moreover, in the melatonin-treated group, FNS expression reached the maximum (approximately 10.87-fold of that of the control group) at 30 days. Melatonin also increased the transcript level of the proanthocyanidin biosynthesis genes, LAR and ANR, but it was significant only before day 15; thereafter, the effect of melatonin gradually decreased with the process of senescence. There was roughly no difference among the control and melatonin-treated groups. Various expression patterns were observed among the flavonoid biosynthesis pathway genes, but in all, melatonin played a catalytic role in the expression. This result also coincided with the significant increase of TPC, TFC, TFAC, and TMAC (Figure 5).
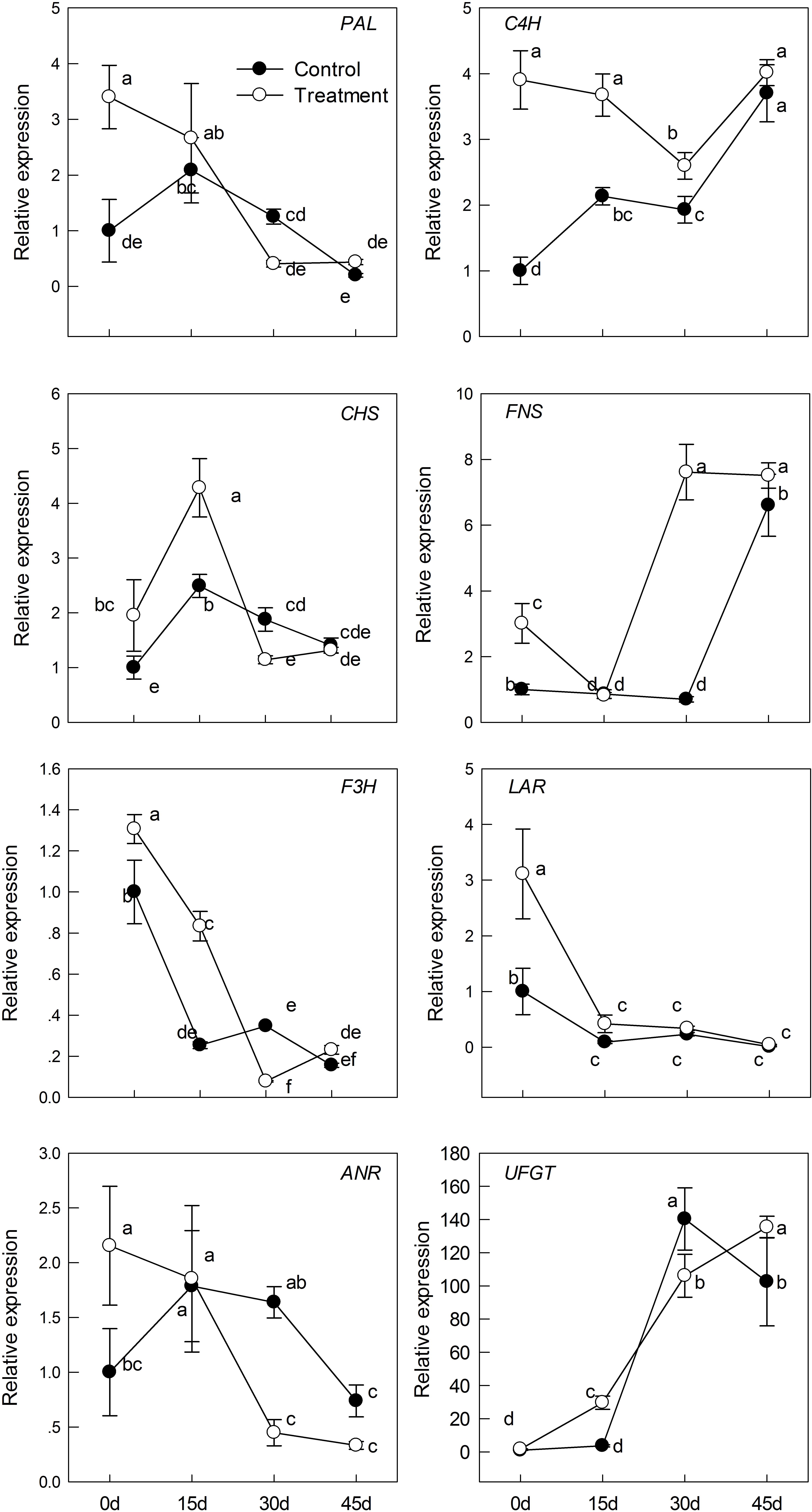
FIGURE 6. The effects of melatonin on flavonoids biosynthesis genes that analyzed by qPCR during kiwifruit senescence; Data are show as means ± SE (n = 9), different letters indicate significant differences at p < 0.05 level.
Discussion
The most obvious indicators of plant senescence are the change in leaf color, from green to yellow, and leaves falling off, while chloroplast disintegration and chlorophyll degradation appear at the cellular level. Variation in leaf chlorophyll concentration is an important indicator of leaf physiological activity, making it a valuable, intuitive method to determine the effect of an exogenous substance on leaf senescence. Present results showed that melatonin clearly has a role in delaying leaf senescence, as observed by the maintenance of chlorophyll content (Figure 1) in the treatment group. One key gene involved in chlorophyll biosynthesis, CAB, which encodes the chlorophyll a/b-binding protein, is down-regulated in all groups; however, supplement of melatonin promoted transcript level of CAB compared to control group. In previous study, short-term and long-term application of exogenous melatonin to apple trees resulted in more chlorophyll content and delayed leaf senescence (Wang et al., 2013b,c, 2016). Besides, the protective effect of melatonin on chlorophyll was also verified in barley (Arnao and Hernandez-Ruiz, 2009) and photosynthetic green alga (Ulva sp.) (Tal et al., 2011). All of these findings demonstrated that melatonin is a positive regulator of plant aging, and that exogenous applications delay senescence.
Leaf senescence is accompanied by many physiological processes, such as the accumulation of H2O2, increased membrane permeability, and release of protein and other contents. Our findings indicated that exogenous melatonin delayed senescence: seedlings pretreated with melatonin had lower H2O2 and MDA concentration. H2O2 is an important ROS in plants and is accumulated under stress. Accumulation of ROS can accelerate membrane liposylation of cells and produce the toxic substance, MDA, thus increasing membrane permeability of plants and damaging the integrity of the membrane structure. In the experiment, H2O2 and MDA content continuously increased with the aging process, but the content in pretreated seedlings was lower than that in CK. This result can be partly attributed to the fact that melatonin is a free radical scavenger and broad-spectrum antioxidant, and can remove H2O2 involved in the aging process directly and in time, which is helpful to maintain homeostasis in cells (Tan et al., 2007); this was similarly seen in apples (Wang P. et al., 2012), cucumber (Marta et al., 2016), and peach (Gao et al., 2016). SOD, POD, and CAT are three important protective enzymes in plant growth and defense. O2- in a plant can be reduced to H2O2 by SOD, and then H2O2 will be completely decomposed as H2O and O2 by POD and CAT; this will alleviate damage to the membrane system (Tao et al., 2008). We found that, during aging, the difference in SOD and POD activity was extremely significant between the melatonin-pretreated group and the control group. CAT activity was continuously decreased with the aging process, and no difference between control and treatment, but also higher than control slightly. This has been demonstrated in experiments with the Malus plant where melatonin directly scavenges H2O2 and enhances the activities of the antioxidant enzyme to detoxify H2O2 in plants under salt (Jiang et al., 2016; Li et al., 2017a), drought (Wang et al., 2013c), and alkaline (Gong et al., 2017) stress. In watermelon, local application of melatonin activates these enzymes to remove excess ROS and make plants tolerant to cold stress (Li et al., 2017b). These finding suggested that melatonin has a significant effect on delaying kiwifruit senescence.
The soluble sugar content decreased with age, but the soluble protein content showed the opposite trend. The soluble sugar and soluble protein content were higher in pretreated seedlings than in control seedlings (Figure 2), which is consistent the results of the study by Wang et al. who suggested that soluble sugar and soluble protein content in apples under salt stress changed in response to stress (Wang et al., 2013a). Likewise, melatonin treatment increased the content of soluble sugar and slowed its degradation during senescence, suggesting the potential function of melatonin in increasing crop yield. Overall, chlorophyll content in the melatonin-treated group was higher than in the control group. Chlorophyll converts energy (photons) into its own carbohydrates through photosynthesis. The up-regulation expression of CAB and higher chlorophyll content were beneficial for the accumulation of soluble sugars and soluble proteins. Both of which can balance the cell metabolism during leaf senescence, thus maintaining cellular homeostasis.
The AsA-GSH cycle is an important way for eliminating free radicals in plants. It has been reported that the production of GSH and AsA is induced by melatonin under drought stress and is associated with low H2O2 content in tomato (Liu et al., 2015). Under stress, AsA and GSH in the cells were oxidized, and there was a similar transformation during leaf senescence (Borraccino et al., 1994). Wang et al. also discovered that delayed senescence of apple leaves by exogenous melatonin treatment regulates the AsA-GSH cycle (Wang P. et al., 2012). In the present study, we observed T-AsA and AsA content in the melatonin-pretreated leaves were significantly higher than those in the untreated controls. Meanwhile, down-regulation of MDAR and DHAR expression were found, but melatonin markedly improved the transcript level of MDAR and DHAR. It indicated that higher expression of MDAR and DHAR guaranteed higher T-AsA and AsA content. In this experiment, melatonin also maintained higher GSH content and total GSH content in senescent leaves. The proportion of GSH in T-GSH was also higher in the melatonin-treated group than that in control. Up-regulation of GR gene was observed in melatonin-treated seedlings in this study, which corresponded with the decreased GSSG content. APX can utilize AsA to reduce H2O2 to H2O and produce DHA and MDA, the expression of which was promoted by melatonin as well. The decreased H2O2 content owing to treatment with melatonin may be related to the high expression of APX and the higher AsA and GSH content. Our findings suggest that melatonin plays a vital role in the biosynthesis of GSH and AsA and highlights the role of AsA-GSH and melatonin in ROS balance.
Phenolic compounds are the secondary metabolites with antioxidant activity in plants, and they may act as the second line of defense to participate in the scavenging process of ROS (Fini et al., 2012; Wang, 2015). In tomato, the application of exogenous melatonin increased the production of eight proteins involved in anthocyanin accumulation during fruit ripening; therefore, the total anthocyanin content was increased after melatonin treatment.(Sun et al., 2016). In the present study, our treatment evidently improved the accumulation of phenolic compounds. Moreover, we measured the mRNA levels of PAL, C4H, CHS, FNS, F3H, LAR, ANR, and UFGT by qPCR and found that melatonin was also involved in the regulation of those genes. PAL and C4H are rate-limiting enzymes in the first two steps of the flavonoid synthesis pathway. CHS, FNS, F3H, and UFGT are the structural genes of anthocyanin biosynthesis, and LAR and ANR are related to proanthocyanidins biosynthesis. We observed the genes PAL, C4H, CHS, F3H, LAR, ANR, and UFGT exhibited higher expression level in treatment group than control before 15 days (Figure 6). Moreover, the treatment content of TPC, TFC, TFAC, and TMAC reached the maximum or stable value and also significantly higher than control group on 15 days (Figure 5). Consequently, the accumulation of phenolic compounds attributed to the higher expression level of those genes before 15 days. While the gene FNS presented higher transcript level after 15 days, the result indicated the reason why TPC content constantly increased. Additionally, the expression level of genes PAL, CHS, F3H, and UFGT in treatment group presented no difference or lower than control after 15 days, the TPC and TFAC content decreased accordingly. The TMAC content reached the maximum on 45 days, which phenomenon may explicate by the interaction of gene LAR and ANR. Although these genes showed different expression patterns with aging, melatonin treatment improved in the melatonin-treated group than in the control group. Overall, our results indicated that the presence of direct or indirect cross-talk between melatonin and flavonoids for playing important roles in delaying leaf senescence. DPPH, ABTS, and FRAP are three commonly used methods for the determination of antioxidant activity, which can comprehensively evaluate the antioxidant capacity of plants (Wang X.Y. et al., 2012). We observed that antioxidant activity was enhanced in melatonin-pretreated leaves, which was in accordance with the trends of increased content of phenolic compounds.
In summary, 200 μM melatonin treatment significantly delayed natural-senescence of kiwifruit leaf. The exogenous application of melatonin effectively promoted the transcription of CAB, slowed chlorophyll degradation, resulted more soluble protein and soluble sugar accumulation and maintained the balance of cell metabolism. Supplementary of melatonin also promoted the activity of SOD, POD, and CAT. Melatonin may influence both antioxidant enzyme activity as an antioxidant and cellular mRNA levels for these enzymes (Rodriguez et al., 2004). In addition, the expression of genes involved in AsA-GSH cycle significantly promoted by melatonin-pretreatment, as a result the content of antioxidant substances (AsA, GSH) strongly increased. Moreover, a large amount of flavonoids accumulated in seedlings pretreated with melatonin, and transcript levels of eight genes involved in flavonoid synthesis were increased in response to melatonin application. Owing to the higher antioxidant enzymes activity and more antioxidant substances in leaves melatonin-pretreated, the treatment group had higher antioxidant ability to scavenging ROS, increase the stability of cell membrane as manifested by lower MDA content compared to control. Our results demonstrated that the mechanisms of melatonin delayed leaf natural-senescence through regulating antioxidant metabolism and biosynthesis of flavonoids, as depicted in Figure 7. The finding can provide clear proof that melatonin plays a key roles in delaying leaf senescence.
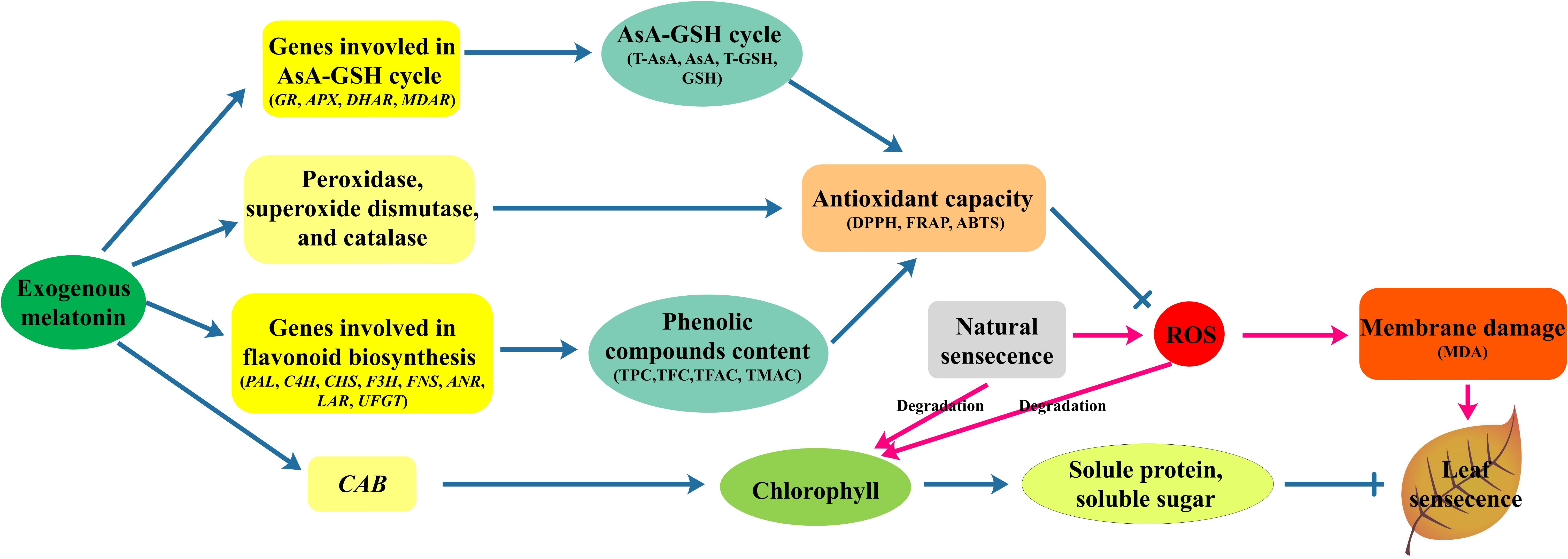
FIGURE 7. The mechanisms of melatonin regulation leaf natural-senescence derived from results involving antioxidant metabolism and biosynthesis of flavonoids.
Author Contributions
YS carried out the experiments with the help of NX, QW, and ZL. HX and DL provided all critical intellectual inputs into the study design. YS, DL, and ZN collected the experimental data and drafted the manuscript. QD, LL, XL, HX, and JW provided suggestions for the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by the Sichuan Science and Technology Project (2016NZ0105 and 2017JY0054).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Editage (www.editage.cn) for their help in writing.
References
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arnao, M. B., and Hernandez-Ruiz, J. (2009). Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 46, 295–299. doi: 10.1111/j.1600-079X.2008.00660.x
Arnao, M. B., and Hernández-Ruiz, J. (2014). Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19, 789–797. doi: 10.1016/j.tplants.2014.07.006
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.1104/pp.24.1.1
Atkinson, R. G., Johnston, S. L., Yauk, Y.-K., Sharma, N. N., and Schröder, R. (2009). Analysis of xyloglucan endotransglucosylase/hydrolase (XTH) gene families in kiwifruit and apple. Postharvest Biol. Technol. 51, 149–157. doi: 10.1016/j.postharvbio.2008.06.014
Borraccino, G., Mastropasqua, L., De Leonardis, S., and Dipierro, S. (1994). The role of the ascorbic acid system in delaying the senescence of oat (Avena sativa L.) leaf segments. J. Plant Physiol. 144, 161–166. doi: 10.1016/S0176-1617(11)80538-9
Chen, Q., Qi, W.-B., Reiter, R. J., Wei, W., and Wang, B.-M. (2009). Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 166, 324–328. doi: 10.1016/j.jplph.2008.06.002
Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C., and Xi, Y. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 118, 138–149. doi: 10.1016/j.plaphy.2017.06.014
Ding, F., Liu, B., and Zhang, S. (2017). Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 219, 264–271. doi: 10.1016/j.scienta.2017.03.029
Du, G. R. (2009). Study on the Total Antioxidant Capacity and Bioactive Compounds of Kiwi(Actinidia), Persimmon(Diospyros kaki L.) and Apple(Malus Domestica Borkh.) Fruits. Doctoral dissertation, Xianyang, Northwest A&F University.
Fini, A., Guidi, L., Ferrini, F., Brunetti, C., Di Ferdinando, M., Biricolti, S., et al. (2012). Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: an excess light stress affair? J. Plant Physiol. 169, 929–939. doi: 10.1016/j.jplph.2012.02.014
Gao, H., Zhang, Z. K., Chai, H. K., Cheng, N., Yang, Y., Wang, D. N., et al. (2016). Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 118, 103–110. doi: 10.1016/j.postharvbio.2016.03.006
Gong, X. Q., Shi, S. T., Dou, F. F., Song, Y., and Ma, F. W. (2017). Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by regulating the biosynthesis of polyamines. Molecules 22:e1542. doi: 10.3390/molecules22091542
Griffith, O. W. (1980). Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 106, 207–212. doi: 10.1016/0003-2697(80)90139-6
Gu, Q., Chen, Z., Yu, X., Cui, W., Pan, J., Zhao, G., et al. (2017). Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 261, 28–37. doi: 10.1016/j.plantsci.2017.05.001
Hernández-Ruiz, J., Cano, A., and Arnao, M. B. (2005). Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 39, 137–142. doi: 10.1111/j.1600-079X.2005.00226.x
Hernandez-Ruiz, J., Cano, A., and Arnao, M. B. (2004). Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220, 140–144. doi: 10.1007/s00425-004-1317-3
Hodges, D. M., DeLong, J. M., Forney, C. F., and Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. doi: 10.1007/s004250050524
Inada, N., Sakai, A., Kuroiwa, H., and Kuroiwa, T. (1998). Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Investigations of tissues and cells by fluorescence microscopy. Planta 205, 153–164. doi: 10.1007/s004250050307
Jiang, C., Cui, Q., Feng, K., Xu, D., Li, C., and Zheng, Q. (2016). Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 38:82. doi: 10.1007/s11738-016-2101-2
Kampfenkel, K., Vanmontagu, M., and Inze, D. (1995). Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 225, 165–167. doi: 10.1006/abio.1995.1127
Khanna-Chopra, R. (2012). Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 249, 469–481. doi: 10.1007/s00709-011-0308-z
Kim, J., Kim, J. H., Lyu, J. I., Woo, H. R., and Lim, P. O. (2017). New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 69, 787–799. doi: 10.1093/jxb/erx287
Lerner, A. B., Case, J. D., and Heinzelman, R. V. (1959). Structure of melatonin. J. Am. Chem. Soc. 81, 6084–6085. doi: 10.1021/ja01531a060
Lerner, A. B., Case, J. D., Takahashi, Y., Lee, T. H., and Mori, W. (1958). Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 80, 2587–2592. doi: 10.1021/ja01543a060
Li, C., Wang, P., Wei, Z. W., Liang, D., Liu, C. H., Yin, L. H., et al. (2012). The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 53, 298–306. doi: 10.1111/j.1600-079X.2012.00999.x
Li, H., Chang, J., Chen, H., Wang, Z., Gu, X., Wei, C., et al. (2017a). Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 8:295. doi: 10.3389/fpls.2017.00295
Li, H., Chang, J., Zheng, J., Dong, Y., Liu, Q., Yang, X., et al. (2017b). Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci. Rep. 7:40858. doi: 10.1038/srep40858
Lim, P. O., and Nam, H. G. (2007). Aging and senescence of the leaf organ. J. Plant Biol. 50, 291–300. doi: 10.1007/bf03030657
Lin, Z. F., Li, S. S., Lin, G. Z., and Guo, J. Y. (1988). Relationship between H2O2 accumulation and membrane lipid peroxidation in senescent leaves and chloroplasts. J. Plant Physiol. Mol. Biol. 14, 16–22.
Liu, J. L., Wang, W. X., Wang, L. Y., and Sun, Y. (2015). Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 77, 317–326. doi: 10.1007/s10725-015-0066-6
Manchester, L. C., Coto-Montes, A., Boga, J. A., Andersen, L. P. H., Zhou, Z., Galano, A., et al. (2015). Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59, 403–419. doi: 10.1111/jpi.12267
Marta, B., Szafrańska, K., and Posmyk, M. M. (2016). Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 7:575. doi: 10.3389/fpls.2016.00575
Murch, S. J., Campbell, S. S. B., and Saxena, P. K. (2001). The role of serotonin and melatonin in plant morphogenesis: regulation of auxin-induced root organogenesis in in vitro-cultured explants of st. John’s Wort (Hypericum perforatum L.). In Vitro Cell. Dev. Biol. Plant 37, 786–793. doi: 10.1007/s11627-001-0130-y
Nawaz, M. A., Jiao, Y., Chen, C., Shireen, F., Zheng, Z., Imtiaz, M., et al. (2018). Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 220, 115–127. doi: 10.1016/j.jplph.2017.11.003
Quirino, B. F., Noh, Y.-S., Himelblau, E., and Amasino, R. M. (2000). Molecular aspects of leaf senescence. Trends Plant Sci. 5, 278–282. doi: 10.1016/S1360-1385(00)01655-1
Reiter, R. J., Rosales-Corral, S., Tan, D. X., Jou, M. J., Galano, A., and Xu, B. (2017). Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74, 3863–3881. doi: 10.1007/s00018-017-2609-7
Reiter, R. J., Tan, D.-X., and Galano, A. (2014). Melatonin reduces lipid peroxidation and membrane viscosity. Front. Physiol. 5:377. doi: 10.3389/fphys.2014.00377
Rodriguez, C., Mayo, J. C., Sainz, R. M., Antolín, I., Herrera, F., Martín, V., et al. (2004). Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36, 1–9. doi: 10.1046/j.1600-079X.2003.00092.x
Shi, H., Jiang, C., Ye, T., Tan, D.-X., Reiter, R. J., Zhang, H., et al. (2015). Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694. doi: 10.1093/jxb/eru373
Sun, Q., Zhang, N., Wang, J., Cao, Y., Li, X., Zhang, H., et al. (2016). A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal Res. 61, 138–153. doi: 10.1111/jpi.12315
Tal, O., Haim, A., Harel, O., and Gerchman, Y. (2011). Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 62, 1903–1910. doi: 10.1093/jxb/erq378
Tan, D.-X., Manchester, L. C., Helton, P., and Reiter, R. J. (2007). Phytoremediative capacity of plants enriched with melatonin. Plant Signal. Behav. 2, 514–516. doi: 10.4161/psb.2.6.4639
Tan, D.-X., Manchester, L. C., Liu, X., Rosales-Corral, S. A., Acuna-Castroviejo, D., and Reiter, R. J. (2013). Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54, 127–138. doi: 10.1111/jpi.12026
Tan, D. X., Reiter, R. J., Manchester, L. C., Yan, M. T., Elsawi, M., Sainz, R. M., et al. (2002). Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2, 181–197. doi: 10.2174/1568026023394443
Tao, J., Yu, J., Yu, L. Q., Su, J. L., and Zhang, C. H. (2008). Effects of short-term high temperature stress on antioxidant system in poinsettia. Acta Hortic. Sin. 35, 1681–1684.
Thompson, J. E., Froese, C. D., Madey, E., Smith, M. D., and Hong, Y. (1998). Lipid metabolism during plant senescence. Prog. Lipid Res. 37, 119–141. doi: 10.1016/S0163-7827(98)00006-X
Wang, K., Zhang, L. X., Gao, M., Zhao, Y., Lu, L., Zhang, L., et al. (2013a). Effects of salinity stress on growth and organic osmolytes accumulation of callus and tissue culture seedlings of two Malus. Acta Agric. Boreali Occidentalis Sin. 22, 112–118.
Wang, L. Y., Liu, J. L., Wang, W. X., and Sun, Y. (2016). Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54, 19–27. doi: 10.1007/s11099-015-0140-3
Wang, P., Sun, X., Chang, C., Feng, F., Liang, D., Cheng, L., et al. (2013b). Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 55, 424–434. doi: 10.1111/jpi.12091
Wang, P., Sun, X., Li, C., Wei, Z., Liang, D., and Ma, F. (2013c). Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 54, 292–302. doi: 10.1111/jpi.12017
Wang, P., Sun, X., Xie, Y. P., Li, M. J., Chen, W., Zhang, S., et al. (2014). Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 57, 291–307. doi: 10.1111/jpi.12169
Wang, P., Yin, L. H., Liang, D., Li, C., Ma, F. W., and Yue, Z. Y. (2012). Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 53, 11–20. doi: 10.1111/j.1600-079X.2011.00966.x
Wang, X. K., and Huang, J. L. (2015). Principles and Techniques of Plant Physiology and Biochemistry Experiments. Beijing: Higher Education Press.
Wang, X. Q. (2015). Phenolic Metabolism of Red-Fleshed Apples and Its Response to Stress. Doctoral dissertation, Xianyang, Northwest A&F University.
Wang, X. Y., Du, G. R., and Li, H. (2012). Progress of analytical method for antioxidant capacity in vitro. J. Food Sci. Biotechnol. 31, 247–252.
Weeda, S., Zhang, N., Zhao, X., Ndip, G., Guo, Y., Buck, G. A., et al. (2014). Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS One 9:e93462. doi: 10.1371/journal.pone.0093462
Zhang, J., Li, H., Xu, B., Li, J., and Huang, B. (2016). Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 7:1500. doi: 10.3389/fpls.2016.01500
Zhang, N., Zhao, B., Zhang, H. J., Weeda, S., Yang, C., Yang, Z. C., et al. (2013). Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54, 15–23. doi: 10.1111/j.1600-079X.2012.01015.x
Keywords: melatonin, kiwifruit, natural senescence, flavonoid biosynthesis, antioxidant capacity
Citation: Liang D, Shen Y, Ni Z, Wang Q, Lei Z, Xu N, Deng Q, Lin L, Wang J, Lv X and Xia H (2018) Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 9:426. doi: 10.3389/fpls.2018.00426
Received: 05 February 2018; Accepted: 20 March 2018;
Published: 05 April 2018.
Edited by:
Vasileios Fotopoulos, Cyprus University of Technology, CyprusReviewed by:
Ana Margarida Fortes, Universidade de Lisboa, PortugalFrantisek Baluska, Universität Bonn, Germany
Copyright © 2018 Liang, Shen, Ni, Wang, Lei, Xu, Deng, Lin, Wang, Lv and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiulan Lv, NzU2MTQxOTU5QHFxLmNvbQ== Hui Xia, c3VzYW54aWFfMjAwMUAxNjMuY29t
†These authors have contributed equally to this work.
 Dong Liang
Dong Liang Yanqiu Shen1†
Yanqiu Shen1†