- 1Department of Chemistry, Life Sciences, and Environmental Sustainability, University of Parma, Parma, Italy
- 2Department of Biological, Geological and Environmental Sciences, University of Bologna, Bologna, Italy
Italy is recognized as one of the prominent hot spot areas for plant diversity at regional and global scale, hosting a rich range of ecosystems and habitat types. This is especially true considering aquatic habitats, which represent a major portion of the total water surfaces in the Mediterranean region. Nevertheless, only a scant attention was paid to clarify the species richness of aquatic plant and its contribution to the total diversity at the country scale, despite such plants are seriously threatened at multiple scales. This paper provided the first comprehensive inventory of aquatic plants at the whole country scale, collecting data on species’ distribution, trends, and explanatory determinants of species richness. We confirmed the key contribution of Italy to the regional and global aquatic plant diversity with a total of 279 species recorded since 2005, equal to the 88.5%, 55.9% and ∼10% of the richness estimated at European/Mediterranean, Palearctic and global scale, respectively. Ten species are considered extinct in the wild [among which Aldrovanda vesiculosa L., Caldesia parnassifolia (Bassi ex L.) Parl., Helosciadium repens (Jacq.) W.J.D. Koch, and Pilularia globulifera L.], four were doubt [among which Luronium natans (L.) Raf., Utricularia intermedia Hayne, and U. ochroleuca R.W. Hartman.], and eight were erroneously reported in the past, among which Isoëtes lacustris L., Myosotis rehsteineri Wartm., and Ranunculus aquatilis L. Only 18 species – mainly helophytes (14) – were present in all the 20 Italian regions, whereas hydrophytes showed most scanty regional frequencies. Temperature, latitude, area and water resources availability are the main drivers of aquatic plant spatial arrangement and diversity. Furthermore, the number of inhabitants per km2 well described the number of “lost species” since 2000. The findings of the present survey call for an urgent elaboration of large-scale strategies to ensure the survival of aquatic plants, stressing on multiple functions played by aquatic plants in supporting national economy and human well-being. In this context, Italy can play a fundamental role guaranteeing temporary refuge for projected or expected species migrations along latitude and longitude gradients. Besides, in hyper-exploited landscapes man-made water bodies can further enhance the achievement of minimum conservation targets.
Introduction
Italy is the focal axis of the Mediterranean basin region: a biodiversity hotspot that host a very rich range of ecosystems and habitats, and about the 10% of the world’s higher plants (Médail and Quézel, 1999; Thompson, 2005; Cuttelod et al., 2008). Within this region, the Italian peninsula is among major centers of species richness, with a high number of endemic species, especially in terms of vascular plants (Rossi et al., 2013; Peruzzi et al., 2014). This is due to the presence of multiple key alert areas for plant diversity (e.g., Maritime and Ligurian Alps, Tyrrhenian Islands), acting both as refuge and exchanging floral areas, supporting active plant speciation (Médail and Quézel, 1999). This is due not only through the preservation of genotypes during glacial periods, but also thanks to the intensity and accumulation of multiple processes in a patchy landscape based on a complex topographic matrix (Nieto Feliner, 2011).
Bilz et al. (2011) and Chappuis et al. (2012) have recently assessed the key contribution of Italy in maintaining a prevalent share of aquatic plants at the European and Mediterranean scale. In fact, Italy incorporates a large part of the total water bodies in the region, encompassing ∼80% of the deep lakes within the Mediterranean coastal areas and 42% of the area occupied by deep lakes. In addition, its Northern corner includes a large part of the Alpine chain and the Northern Apennine sector that feed a complex system of rivers and lakes, and groundwater aquifers (Azzella et al., 2014; Bocchiola, 2014).
Aquatic plants play complex interconnected functions, including – among others – C and nutrient cyclization, sediment and riparian sectors stabilization, and the provision of food and habitats for a variety of animal species (Chambers et al., 2008; O’Hare et al., 2017). They act as “engineering species” (Bouma et al., 2010; Bolpagni et al., 2015), and their disappearance causes drastic effects on trophic and functional status of the habitats within water bodies (Scheffer et al., 2003; Soana and Bartoli, 2014). Nevertheless, aquatic plants remain often unrecognized in broad-scale investigations, a condition that can lead to wrong or to conflicting evaluations in analyzing their current spatial patterns and rarity (Alahuhta et al., 2017 and references therein). In this context, Italy thanks to its great heterogeneity in hydro-ecoregions and habitats, covering a wide latitudinal and altitudinal range, is of focal importance for aquatic plant conservation at the continental and global scales. This imposes a number of challenges in supporting effective management plans, including the streamlining of the available data concerning the current distribution of aquatic plants, the consistency of their populations, and the impacts they suffer from human activities.
Hence, Italy is subjected to extremely high rates of human perturbations, with huge effects on plant diversity, especially in lowland areas where the collapse of the traditional agro-sylvo-pastoral system, the land use changes and the soil artificialization have led to a major loss of natural and semi-natural patches (Falcucci et al., 2007; Bolpagni and Piotti, 2015, 2016). Among others, in lowlands aquatic and riparian vegetation has shown a rapid and constant decline due to the impairment of river discharge regimes and land reclamation (Bolpagni et al., 2013). Therefore, species-poor, ruderal or simplified plant communities have replaced pristine complex aquatic vegetation (Bolpagni and Piotti, 2015, 2016). As a result, frequently, less-demanding aquatic primary producers, such as cyanobacteria, soft-bodied benthic algae dominate remnant aquatic ecosystems (Scheffer et al., 2003; Barrett et al., 2010).
In the recently updated Red List of Italian Flora (focused on the Policy Species and the extremely threatened plants), Rossi et al. (2013) have largely stressed the critical conservation status of plant species ecologically connected with inland water ecosystems. Currently, the only Italian policy plant species that are extinct in the wild are two lowland obligated aquatic plants [Aldrovanda vesiculosa L., and Caldesia parnassifolia (Bassi ex L.) Parl.], and – furthermore – the 50% of the critically endangered “probably extinct” species (=six species) are hydrophytes (Rossi et al., 2013; Ercole and Giacanelli, 2014).
Additionally, over the last years frequent significant anomalies both in terms of thermal and rainfall regimes were detected, especially in northern sectors, reinforcing the general awareness on the extreme vulnerability of Italian peninsula to climate change (Hoff, 2013; Marchina et al., 2017). Despite this, no systematic inventory was made to deepen the spatial patterns of aquatic species across Italy, and to acquire information on their trends and main drivers.
In this work, the hydro- and hygrophilous plant diversity was investigated at regional scale at the national scale. In agreement with Chappuis et al. (2012) and Alahuhta et al. (2017), a strong dependence of aquatic plant richness on environmental heterogeneity, mainly supported by climate-related restrictions (altitudinal-grown limitation) and water quality gradients (at low altitudes) was hypothesized. Furthermore, the amount of water bodies potentially available for colonization is expected to have a central role in driving aquatic plant geographical distribution. In this context, we aimed to add new insights on: (1) an up-to-date species richness assessment of aquatic plant flora and its regional distribution; (2) the incidence of hydrophytes and not-obligate aquatic plants (e.g., helophytes or amphibian plants with a predominant hydrophytic phase) in the aquatic plant flora; and (3) the main determinants of aquatic plant diversity, focusing on environmental drivers (including climate, habitat conditions and human impacts).
Materials and Methods
Study Area
All the 20 administrative regions that constitute the territory of Italy were investigated, considering a total area of 301,338 km2. Among Mediterranean countries, Italy is the richer in water resources, it counts 69 natural lakes equal to or larger than 0.5 km2, 183 artificial basins larger than 1 km2, and more than 230 rivers and streams of particular relevance: 58 exceeding 100 km in length, and 75 with average daily discharges greater than 10 m3 s-1. Nevertheless, since Roman age almost all of the national wetland complexes and the riverine landscapes have been progressively reclaimed, and transformed into productive lands, especially in lowlands (Barone et al., 1985; de Haas, 2015). Recent estimates indicate a total area occupied by wetlands (including ponds and small shallow lakes < 3 ha) and riverscapes of about 6,000 km2, just over the 2% of the national territory, compared to the pristine wetland area in Roman age estimated to 30,000 km2 (equal to the 10.0% of the area). At the same time, the quality status of surface waters is far from the objectives set by the Water Framework Directive (WFD), with more than half of the water bodies being in less than good ecological status or potential (Carré et al., 2017).
The climate remarkably varies along the wide latitudinal range encompassed in the national borders due to the structural and altitudinal complexity of its territory that includes two main mountain chains: Alps (in the North) and Apennines (in Central and South). Accordingly, climate covers a broad spectrum of types ranging from polar cold and glacial (Köppen climate classification ET and EF) to Mediterranean (Cs) (Peel et al., 2007). Precipitation is moderate in the range of 350–3,500 mm per year, typically with a peak in spring and autumn, and two relative minima in winter and in summer. Italy falls into the temperate region, with a mean long-term annual air temperature of 12.6°C, spanning from ∼0°C on the Alps to around 20°C in Sicily. Additionally, a distinct continental character typifies Italy, especially the northern sectors with differences between summer and winter more than 17°C (Costantini et al., 2013).
Aquatic Plant Data
Historical Italian floristic records (Pignatti, 1982) was compared with those reported by Conti et al. (2005, 2006), updated with the data by the “Notulae to the Italian native Vascular Flora” (NINVF) for the 2005–2015 period [from number 37(1) to number 47(1)], and with the data by the “Acta Plantarum notes” (APN) for the 2013–2015 period (from number 1 to 3). The NINVF were published by the Informatore Botanico Italiano, whereas the APN were published online by the “Acta Plantarum forum.”1
The historical (up to ∼2000) and the updated regional plant databases (for the period 2005–2015) ware explored in order to retrieve the number of aquatic species based on: (1) the biological form as reported by Pignatti (1982), focusing on “hydrophytes” sensu Raunkiær (1934); (2) the Ellenberg ecological indicator for “humidity” (U), as reported by Pignatti et al. (2005) and Guarino et al. (2012); and (3) the preferential colonized habitats, as indicated by Pignatti (1982). According to Baattrup-Pedersen et al. (2005) all species with an Ellenberg’s humidity value U ≥ 10 have been considered for the present analysis, being ecologically strictly related to “aquatic” habitats with permanently saturated substrates and therefore influenced by periodical submersion and/or by constant saturation of colonized sediments. Specifically, a U value of 10 refers to plant species adapted to transient submersion, 11 to aquatic plants rooted in waterlogged sediments but with emergent or floating organs, and 12 to submerged plants, constantly or at least for long periods (Pignatti et al., 2005). Additionally, few species with Ellenberg indicator values U = 9 were also considered. These species (e.g., Montia, Elatine, and Juncus genera and the Veronica anagallis-aquatica aggregate), although closely connected to aquatic ecosystems – have been classified in the past into non-aquatic life forms, or considered not obligatorily adapted to saturated sediments. Currently, new data on their ecology have permit to confirming their “aquatic life strategy.”
All the plant occurrence data were summarized into a matrix reporting the presence of each species/taxon within each region (Supplementary Table 1). Plant species were classified as follows: (1) species “no longer recorded at the local scale” since 2000 (lost; 0); (2) “dubious,” species whose confirmation requires further evaluation (?); (3) species “erroneously reported in the past,” based on exsiccata material re-examination and/or field surveys (-); and (4) species recently confirmed (+).
Environmental Drivers
To investigate the regional arrangement of aquatic plants, a series of environmental drivers were used as explanatory variables, focusing on climatic conditions (both rainfall and temperature) and the availability of preferential habitats (Table 1). These data were summarized at the regional scale, the same used for assembly the plant occurrence data. Climatic variables were derived from the temperature and precipitation data sets of the ISTAT and CREA (Agricultural Mechanic Experimental Institute) 2000–2010 monthly climate time-series. This interval was used as climate reference period because it is the only period for which we have methodologically comparable and accessible data at the regional scale. Furthermore, we believe that it well describes the climatic conditions in which the floristic data in analysis were collected (2005–2015), considering a minimum/reasonable delay between the records collection and their publication of about 3–5 years.
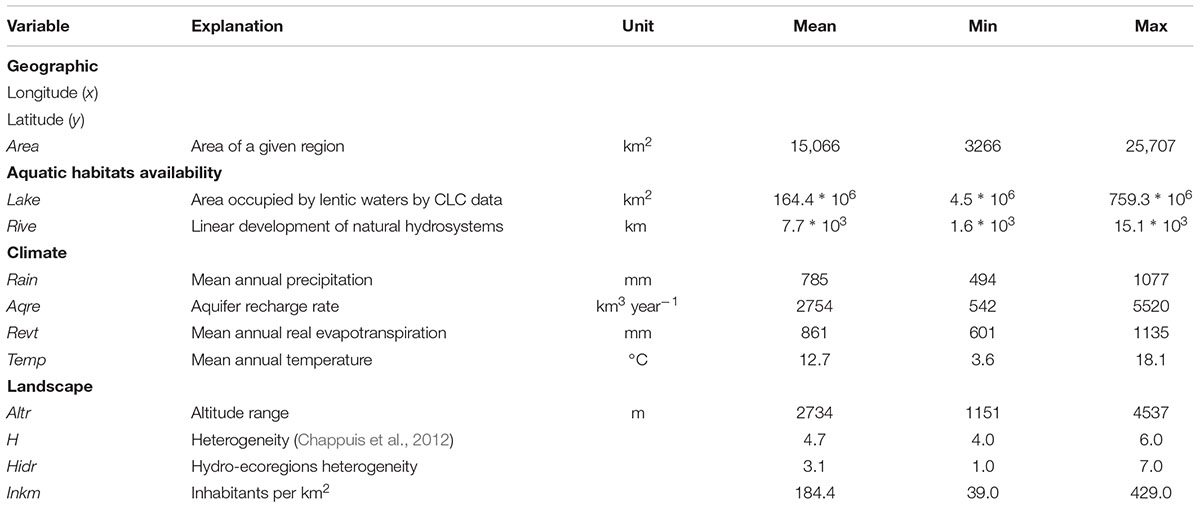
TABLE 1. Explanatory variables used to analyze the representativeness and spatial distribution of aquatic plants in the 20 Italian regions.
Actual evapotranspiration was indirectly calculated based on the monthly hydrological budget model proposed by Thornthwaite–Mather (Mather, 1979); whereas, the aquifer recharge rate was estimated by the basic outflow produced by waterways integrated with the direct supply of groundwater in agreement with Castany (1982). The abundance of available aquatic habitats for the establishment and growth of aquatic plants was estimated: (1) by the total surface occupied by lakes – based on the CORINE Land Cover data2 (Lake, km2) – and (2) by the total linear development of natural hydrosystems – based on GIS data provided by SINAnet3 (Rive, km).
In agreement with Chappuis et al. (2012), we considered the following explanatory variables: latitude (hereafter, y) and longitude (x) (y and x coordinates of the centroid of each region); area (Area, km2); Lake (km2); Rive (km); mean annual precipitation (Rain, mm); aquifer recharge rate (Aqre, km3 year-1); mean annual temperature (Temp, °C); mean annual real evapotranspiration (Revt, mm); altitude range (Altr, m). Additionally, we considered the heterogeneity index as proposed by Chappuis et al. (2012; H), and a hydro-ecoregion heterogeneity index (Hidr) calculated as the sum of hydro-ecoregions presented at regional scale in agreement with Wasson et al. (2002). Finally, as proxy of the potential exposure rate to human perturbations we considered the population of the region (Inkm, inhabitants per km2 for 2015; ISPRA data).
Statistical Analyses
For testing species richness patterns (updated for the period 2005–2015), we calculated three dependent variables per each region: species richness of all aquatic plants (SAP), species richness of hydrophytes (SHY), and species richness of not-obligate aquatic plants (SNO). Additionally, we also calculated the species richness of lost (SLO), dubious (SDU) and erroneously reported species (SER) to investigate their dependence on principal environmental drivers.
The co-variation among environmental drivers was tested by the spearman rank correlation prior to data analysis and those selected were used as covariates in a generalized linear modeling framework. The collinearity levels between our predictors were relatively high (Supplementary Table 2). X and y had a significant correlation with climatic variables, especially Revt (r = 0.63, and r = -0.95, respectively) and Temp (r = 0.61, and r = -0.93, respectively). Area was strictly correlated with water resources proxies (Lake and Rive; r = 0.66, and r = 0.89, respectively); whereas, Rain was strictly correlated with Temp (r = -0.82). Similarly, Revt and Altr had a significant correlation with Temp (r = 0.97, and r = -0.69, respectively), while, Aqre and Hidr were strictly correlated with InKm (r = 0.43, and r = 0.62, respectively). Based on these results, we excluded x, y, Rain, Aqre, Revt, Altr, and Hidr from variables included in the GLM analyses devoted to detect the better predictor of aquatic plant richness.
Non-metric multidimensional scaling (NMDS) was used to study the community structure of all aquatic plants, hydrophytes and not-obligate aquatic plants among the investigated regions, with Jaccard as the dissimilarity index and stress as the measure of goodness of fit, according to Oksanen et al. (2017). Vectors of environmental variables were fitted onto the ordination obtained by NMDS and the squared correlation coefficient (r2) calculated in order to assess the goodness of fit.
Since dependent variables were discrete, we used the Poisson family for the error distribution and logarithm as link function. In order to select the best model, a multi-model inference approach was used (Burnham and Anderson, 2002). The fit of all candidate models was thus compared using Bayesian Information Criterion (BIC) and the model with the lowest BIC was retained. Correlation analysis was also performed to verify specific relations between species richness variables and environmental drivers.
All analyses were performed with the R statistical software (R Core Team, 2017), and the packages bestglm (Oksanen et al., 2017) and vegan (McLeod and Xu, 2017).
Results
Aquatic Plant Diversity, Distribution, and Trends
A total of 279 aquatic plant species was recognized as present in Italy in the period 2005–2015. Ten species are to be considered extinct in the wild [among which Aldrovanda vesiculosa L., Caldesia parnassifolia (Bassi ex L.) Parl., Helosciadium repens (Jacq.) W.J.D. Koch, and Pilularia globulifera L.], four were doubt [among which Luronium natans (L.) Raf., Utricularia intermedia Hayne, and U. ochroleuca R.W. Hartman.], and eight were erroneously reported in the past, among which Isoëtes lacustris L., Myosotis rehsteineri Wartm., and Ranunculus aquatilis L. (Table 2). Focusing on the contribution of obligate aquatic plants, the 56.5% of the total diversity (158 species) is represented by hydrophytes, whereas 43.5% (121 species) were not-obligate aquatic species. These included: 65 geophytes [equal to the 53.7%; among which Berula erecta (Huds.) Coville, Cladium mariscus (L.) Pohl, Eleocharis palustris (L.) Roem. & Schult. subsp. palustris; Typha latifolia L.], 33 hemicryptophytes (27.3%; among which Nasturtium officinale R. Br. subsp. officinale; Veronica anagallis-aquatica L., V. beccabunga L.), 12 therophytes [9.9%; Montia fontana L. subsp. chondrosperma (Fenzel) Walters], ten helophytes (8.3%; Carex riparia Curtis), and one chamaephyte [0.8%; Potentilla palustris (L.) Scop.].
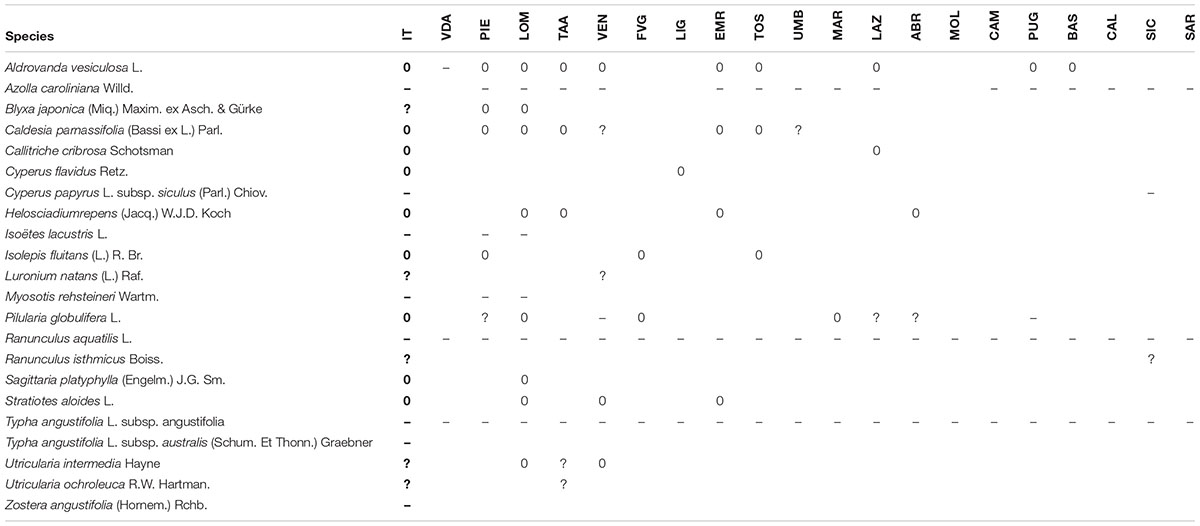
TABLE 2. List of the lost (0), dubious (?), and erroneously reported in the past (–) aquatic species since 2000 in Italy (IT, Italy, general evaluation), and their regional distribution (VDA, Valle d’Aosta; PIE, Piedmont; LOM, Lombardy; TAA, Trentino-Alto Adige/Südtirol; VEN, Veneto; FVG, Friuli Venezia Giulia; LIG, Liguria; EMR, Emilia-Romagna; TOS, Tuscany; UMB, Umbria; MAR, Marche; LAZ, Latium; ABR, Abruzzo; MOL, Molise; CAM, Campania; PUG, Apulia; BAS, Basilicata; CAL, Calabria; SIC, Sicily; SAR, Sardinia).
Regarding the distribution of species, 18 species were present in all the regions, but only four of them are hydrophytes (Glyceria notata Chevall., Lemna minor L., Potamogeon crispus L., and P. natans L.). 19 different species were recorded at least in the 90–95% of the investigated regions (18 and 19 regions over 20), of which seven hydrophytes: Alisma lanceolatum With., Callitriche stagnalis Scop., Lemna gibba L., Myriophyllum spicatum L., M. verticillatum L., Potamogeton pectinatus L. (=Stuckenia pectinata (L.) Börner), and Ranunculus trichophyllus Chaix subsp. trichophyllus.
Many species had a much more restricted distribution, with 32 species being limited to a only one single region (unique species), with 17 of them being hydrophytes (as Isoëtes sabatina Troia & Azzella, a recently described species from Lake Bracciano, Lazio region), and 15 being not-obligate aquatic species (as Eleocharis mamillata H.Lindb. subsp. mamillata, and Schoenoplectus carinatus (Sm.) Palla from Friuli Venezia Giulia and Veneto regions).
Species richness (SAP) per region ranged from a minimum of 68 (Valle d’Aosta) to a maximum of 197 (Lombardy) and averaged 117 plants. Species richness of hydrophytes (SHY) and not-obligate aquatic species (SNO) per region ranged from 27 and 35 species up to 109 and 88, respectively, with very similar richness patterns across the 20 regions (Figure 1). Dubious species (SDU) ranged from 0 (Tuscany) to 14 (Piedmont), whereas “species locally no longer recorded” (SLO) ranged from 0 (Molise and Umbria regions) to 26 (Campania) (Figure 2).
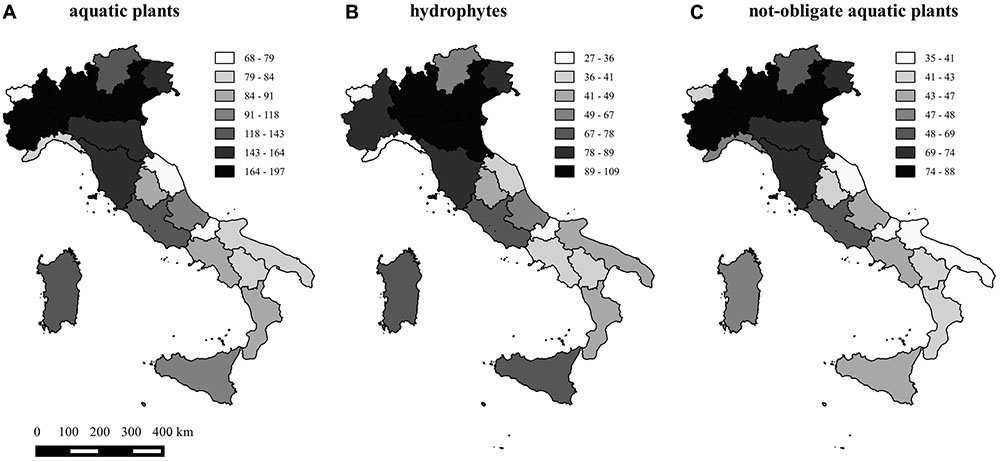
FIGURE 1. Map of the aquatic plant richness, considering the total number of species (A), the total number of hydrophytes (B), and the total number of not-obligate aquatic species (C) in the 20 regions of Italy.

FIGURE 2. Map of the number of dubious (A) and lost (B) aquatic species since 2005 in the 20 regions of Italy.
In terms of species composition, the scatterplots of aquatic plant and not-obligate aquatic species overlapped considerably in the NMDS ordination (Figures 3A,C). In contrast, hydrophyte plot exhibited a peculiar ordination with a lesser separation among regions (Figure 3B). In any case, it was not possible to identify a zonation between regions with clear differentiated clusters as an effect of rather similar aquatic plant assemblages.
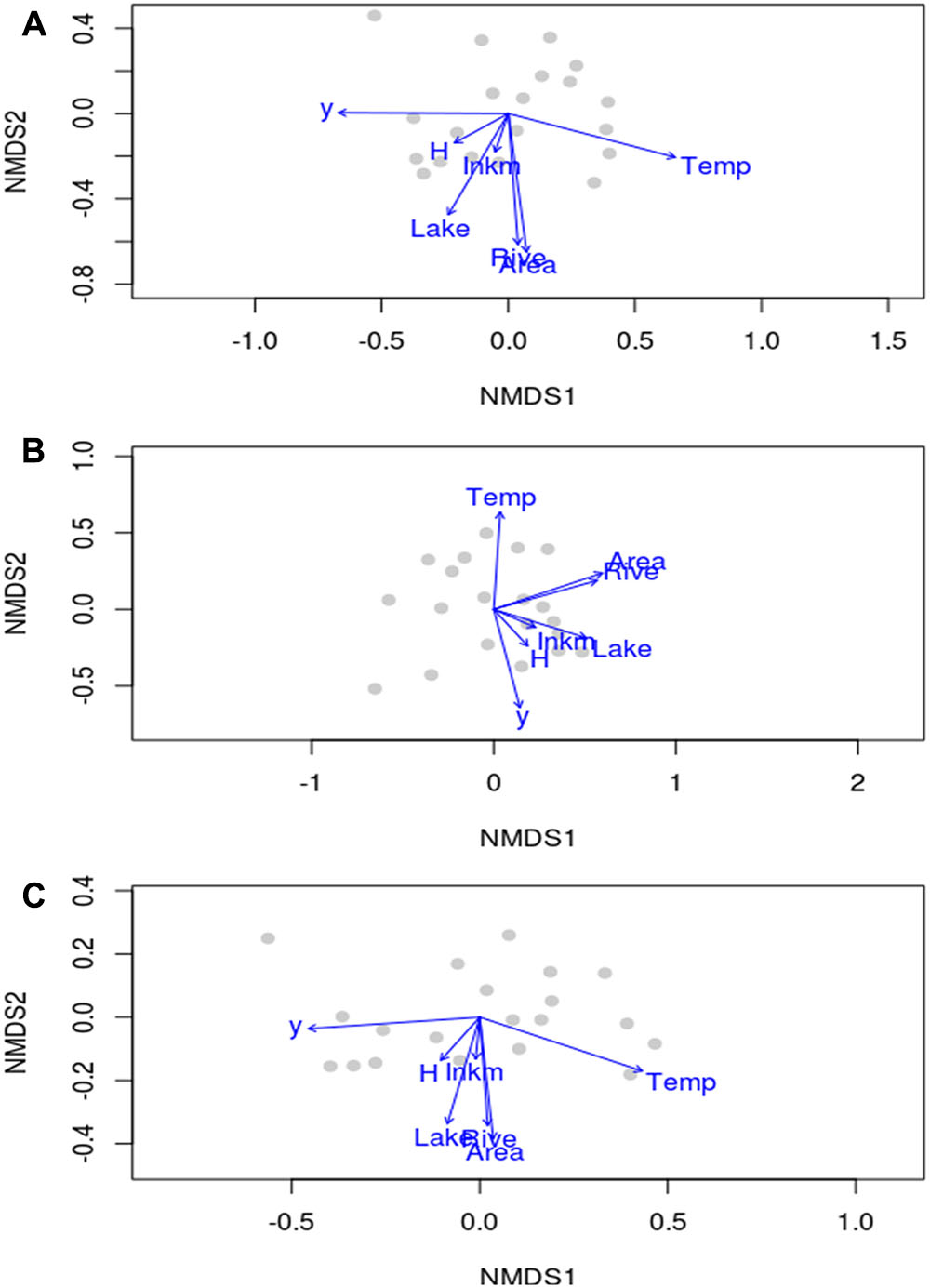
FIGURE 3. Non-metric multidimensional scaling plots of aquatic plant (A), hydrophyte (B) and not-obligate aquatic species (C) composition at the regional scale in Italy, reporting the location of regions, the direction and the magnitude of environmental drivers mapped onto the ordination space. y, latitude; Area, area of a given region; Lake, regional total surface occupied by lakes; Rive, the total regional linear development of natural hydrosystems; Temp, regional mean annual temperature; H, regional heterogeneity index by Chappuis et al. (2012); Inkm, regional inhabitants per km2.
Aquatic Plant Drivers
Temp (i.e., mean temperature values), latitude (y) and Area were the most important drivers of regional species compositional dissimilarity by NMDS, both for the total species richness of aquatic plants, and those of hydrophytes, and not-obligate aquatic species (Table 3). Similarly, Lake (i.e., available lentic habitats for colonization) and Rive (i.e., available lotic habitats for colonization) exhibited R2 values ≥ 0.49, suggesting a pivotal role in driving the spatial arrangement of aquatic plants across Italy (Table 3). On the contrary, H and Inkm did not statistically affect the regional distribution of the overall aquatic plants, and of hydrophytes and non-obligate aquatic species separately (Table 3). A significant dependence on the number of inhabitants per km2 as a proxy of local human perturbations was found exclusively for the “species locally no longer recorded” (r = 0.64, p = 0.002). Conversely, H seemed to have a very little influence on the total aquatic plant richness, but also considering the “species locally no longer recorded” (r = 0.26, p = 0.271), the dubious (r = 0.29, p = 0.208), and the erroneously reported species (r = 0.01, p = 0.967).
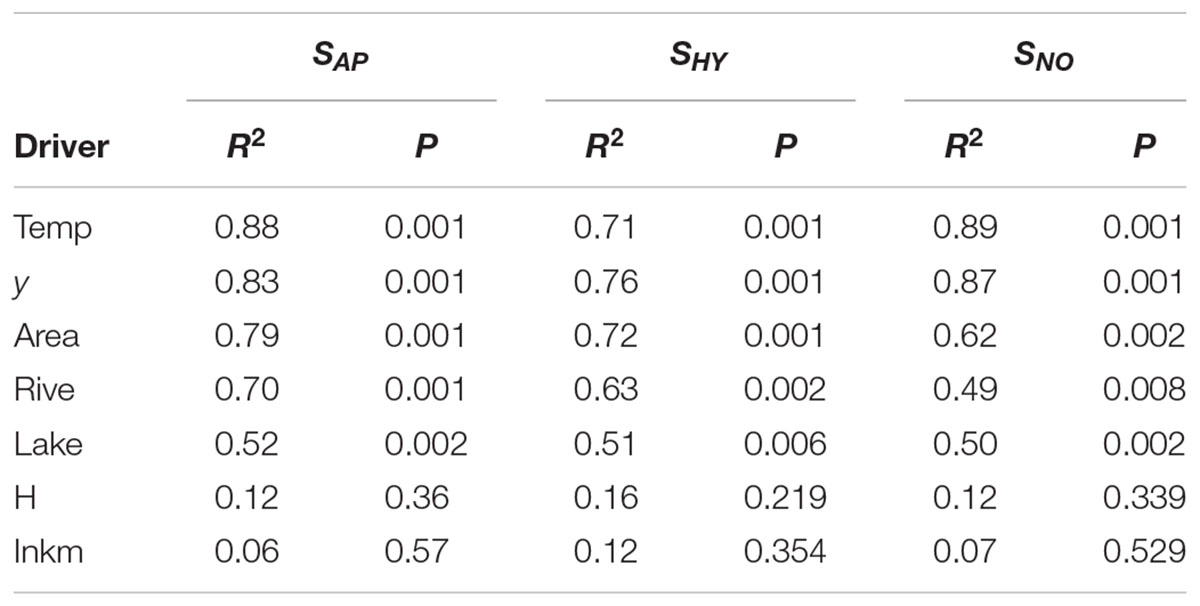
TABLE 3. Relationships between aquatic plant (SAP), hydrophyte (SHY), and not-obligate aquatic plant (SNO) regional composition (as represented in the two-dimensional NMDS space) and the following environmental drivers using ‘envfit’ in the R package ‘vegan: y (latitude), Area (area of a given regions), Lake (total surface occupied by lakes at the regional scale), Rive (the linear development of natural hydrosystems at the regional scale), Temp (regional mean annual temperature), H (regional heterogeneity index by Chappuis et al., 2012), Inkm (regional inhabitants per km2).
Based on the GLMs, we confirmed the results obtained by NMDS emphasizing the strong effect of Lake, Rive and Temp on aquatic plant diversity (Table 4). From the best models, the regional arrangement of overall aquatic plant, hydrophyte and not-obligate aquatic species diversity related positively with Lake and Rive. Additionally, Temp showed a significant positive relationship with overall aquatic plant and not-obligate aquatic diversity (Table 4).
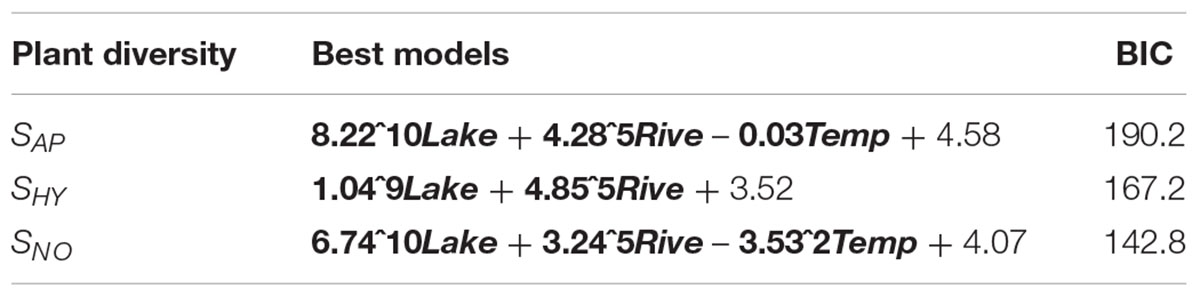
TABLE 4. GLM results between regional aquatic plant diversity – considering the total aquatic plant diversity (SAP), and the hydrophytic (SHY), and the not-obligate aquatic plants (SNO) diversity separately – and environmental drivers. BIC = Bayesian information criterion (means), Lake, regional total surface occupied by lakes; Rive, the total regional linear development of natural hydrosystems; and Temp, regional mean annual temperature. In bold the significant drivers.
Discussion
Aquatic Plant Diversity, Distribution, and Trends
The main findings of the present work confirm the key contribution of Italy to regional and global aquatic plant diversity, hosting a prevalent share (equal to the 88.5%) of the species richness present in European and Mediterranean areas, estimated at 314 different species (Chappuis et al., 2012). The species richness here check-listed also demonstrated that Italy hosts approximately 55.9% of the overall species richness of aquatic plants of the Palearctic region, and more than the 10% of the global diversity (Chambers et al., 2008). This calls for an urgent rational strategy (both at national and regional scale) to preserve aquatic plant diversity, shedding new light on the potentially dramatic consequences of local extinctions on higher spatial scales.
The high number of species recognized is due to the great variety of eco-regions and hydro-ecoregions sensu Wasson et al. (2002) present in Italy, able to guarantee optimal conditions for the establishment and growth of aquatic plants. Furthermore, Italy is also characterized by the presence of multiple climatic and edaphic conditions – from alpine to strictly Mediterranean – that exponentially increase the range of potential habitats for the life of plants. In addition, historical climate dynamics – coupled with habitat and landscape complexity – may be called into question to account for the observed aquatic plant richness rates (Nieto Feliner, 2011; Chappuis et al., 2012). Similarly, Alahuhta et al. (2013) had put emphasis on the potential “refugium” role-played by lakes occurred in supra-aquatic areas during the last Glaciation period in Finland in contributing to the current aquatic plant diversity. However, further evidences are needed to confirm the contribution of the historical aquatic plant distributions to the current ones considering their efficient dispersal mechanisms (Santamaria, 2002), as confirmed by their typical wide distributional ranges (Chambers et al., 2008).
Bilz et al. (2011) obtained very similar results to ours, indicating 270 aquatic species for Italy. However, these results are poorly comparable, considering the great methodological differences between these two studies. Hence, the key question – as stressed by Bilz et al. (2011) – is how to overcome the difficulties related to a proper selection of plants adapted to aquatic life. To do this, we decided to follow the ecological approach by Ellenberg, based on the plant “humidity” indicator values (Baattrup-Pedersen et al., 2005; Pignatti et al., 2005; Guarino et al., 2012). In this way, we have excluded several species that can be considered “tolerant” to submersion or to “saturated sediments” – as well as species of the genera Agrostis, Arundo, Lysimachia, and Lythrum, or Mentha pulegium L. – but which, in fact, do not have a predominant aquatic life form. It follows that the species number we obtained (279) can be considered far greater (in a certain sense, more reliable) than that reported by Bilz et al. (2011), even though this reasoning requires validation in future investigations, both at European and global scale.
The highest values of aquatic species richness have been recorded for the northern regions (Lombardy, 197; Veneto, 186; and Piedmont, 168), in line with the evidences found by Bolpagni et al. (2013) for the lowland wetlands of Lombardy. However, intermediate values of aquatic species richness were also recorded for the two major Italian islands, Sicily (116) and Sardinia (119), suggesting a non-negligible role played by local water resources availability in close relation to climatic variables (i.e., rather high mean annual temperatures). This is in agreement with several previous works that verified the contribution of both local (i.e., habitat heterogeneity), and regional (i.e., climate) drivers to macrophyte community composition in lakes (Alahuhta, 2015 and references therein).
We confirmed the high species richness of plants that can be considered “extinct in the wild” at the national scale, reinforcing the evidences of the critical status of conservation of Italian aquatic ecosystems, especially in lowlands (Bolpagni and Piotti, 2015, 2016). At regional scale, the number of lost species was well explained by the density of inhabitants, a good proxy for human disturbance. It is generally acknowledged that aquatic plants, despite represent a small fraction of the total vascular plant diversity (∼1%), are one of the most critical groups of threatened species worldwide (Saunders et al., 2002; Chambers et al., 2008). This is especially true for aquatic plants adapted e/o restricted to low altitudes, where human pressure on aquatic ecosystems is more intense (Alahuhta et al., 2013). Hence, the most impacted aquatic ecosystems are largely located at low altitudes, even in coastal sectors and along valley bottoms (below 500 m a.s.l), and show very poor water quality, as synthetized by Carré et al. (2017). They present turbid waters and an excess in nutrient availability, which favor the dominance of micro- and macroalgae, including cyanobacteria (Bolpagni et al., 2017b; Bresciani et al., 2017).
Several species were classified as “dubious” with huge variation at the regional scale (from 0 in Tuscany to 14 in Piedmont), suggesting that the present knowledge is still incomplete and there is need to fill it by future field surveys. A number of technical and practical limitations affect the effectiveness of survey campaigns in water ecosystems compared to terrestrial ones, justifying, in part, the current lack of updated information on these systems (Azzella et al., 2013). Despite this, at national level a remarkable revival of interest in aquatic flora was stimulated by the enactment of the WFD (Bolpagni et al., 2017a). It has resulted into a renewed attention for inland aquatic habitats in general, and it has favored the integration of the available aquatic plant knowledge (Testi et al., 2009; Ceschin et al., 2010; Azzella et al., 2013, 2014; Villa et al., 2015; Abati et al., 2016; Bolpagni et al., 2016). Nevertheless, much work has to be done in order to facilitate the comparison and sharing of information gathered by the various institutional actors involved in the monitoring programs.
A non-negligible number of species “erroneously reported in the past” was also recognized. In general, these species that are quite difficult to be properly identified, due to the extreme “lability” of the morphological characters used for classification, or because they could be considered cryptic species. This may explain why these entities – recognized in the past – have been not recently confirmed. Examples in this sense are given by M. rehsteineri, Ranunculus aquatilis L., and Isoetes lacustris L. M. rehsteineri is a very rare species that is very similar to the congeneric Myosotis scorpioides L., to which should be assigned the Italian records of M. rehsteineri, before 1980s. R. aquatilis was detailed investigated by Desfayes (2008) and, on the basis of comprehensive collections from North Italy and Sardinia, this author suggested that this species must be excluded from the Italian flora, and its historical records should largely be assigned to Ranunculus penicillatus (Dumort.) Bab. s.l. or R. peltatus Schrank s.l. (Desfayes, 2011). I. lacustris was erroneously indicated for specimens collected in the Lake Orta in the mid-19th century by De Notaris (1848), which actually are to be reported to Isoetes echinospora Durieu, as well as all the subsequent records of the species at the national scale (Troia and Greuter, 2015).
Ecological Drivers of Aquatic Plant Diversity
The relative availability of water resources emerged as a major driver in explaining the high level of species diversity of aquatic plants observed at regional scale. In fact, the regional surface occupied by lakes and the regional length of natural hydrosystems were found to be the predominant factors in the GLM models predicting aquatic species richness (Table 4). It may seem obvious, considering the strictly dependence of aquatic plants to water (see Chappuis et al., 2012). Even though, in none of the models rainfall and other climate variables – with the exception of mean annual temperature – were statistically significant. Chappuis et al. (2012) collected similar evidences, highlighting a strong interplay between temperature and water availability, in turn intimately interconnected with latitude. This is probably due to the peculiar geographic structure of the Italian peninsula, strictly oriented along the latitudinal North–South gradient, and characterized by the presence of significant mountain chains in almost all the Italian regions. The lowest altitude range recorded was equal to 1151 m, with a mean value of 2734 m (Table 1), suggesting the presence of a complex mosaic of habitats (i.e., high level of heterogeneity) within each considered region, that is a relatively narrow geographic context. Hence, the observations by Alahuhta et al. (2017) verified the pivotal contribution of environmental heterogeneity in driving the global pattern of macrophyte species richness among lakes, stressing on the role of climate-related restrictions (altitudinal-grown limitation) and water quality gradients (at low altitudes). Despite this, our results tended to minimize the role of heterogeneity on aquatic plant arrangement. This is probably due to the high rate of shared heterogeneity among regions, masking its contribution to the observed patterns of plant diversity.
Based on the present data, the regional patterns of aquatic plant in Italy seem, in the small, to mirror the general spatial models elaborated to explain aquatic plant distribution at larger scales. A quite clear geographical trend was observed: moving from the North to the South of the peninsula a progressive reduction in aquatic plant diversity was noted. This complements the findings by Chappuis et al. (2012), which verified a peak of aquatic plant diversity around 50° N. Additionally, we confirmed the tight overlap between the predictable latitudinal trend and the climate gradients (i.e., temperature and precipitation). Similarly, the water resources (Rive and Lake) were intimately linked to region’s area (Figure 3 and Table 3). Hence, as generally expected the aquatic plant richness was strictly positively related to the area investigated, and to the sampling effort carried out (Chappuis et al., 2012; Alahuhta et al., 2013).
Implications for Global Aquatic Plants Conservation in a Changing World: Suggestions from the Italian Case
The findings of this survey confirm the combined effects of climate change and the direct human impacts on aquatic ecosystems as the leading driver for the long-term conservation of aquatic plants both at regional and global scale (Li et al., 2006; Chambers et al., 2008). Based on historical and current evidences, Italy can play a non-negligible role in guarantee medium to high levels of species diversity for aquatic plans at multiple scales, acting as temporary refuge for projected or expected species migrations along the latitude and longitude gradients. Future scenarios for Italy suggest large changes in precipitation patterns and temperatures with huge effects on river discharges (Billi and Fazzini, 2017). This is expected, especially, for the Alpine and pre-Alpine sectors in the northern Italian regions (Coppola and Giorgi, 2010; Marchina et al., 2017), that host the prevalent share of the national aquatic plant diversity. Focusing on the Po plain, mid-term (average increases, with a Representative Concentration Pathway of 4.5, at 2050, mean forecast) predictive models suggest a clear increase in temperature descriptors. This is true, for example, in terms of the “highest temperature in the warmer month” that should range from 29.3 (current conditions) to 31.7–32.4°C range (depending on the forecast model chosen, CNRM-CM5 or MPI-ESM-LR, respectively; WorldClim 2 dataset; Fick and Hijmans, 2017).
In the short and mid-terms, these predictions imply worse conditions for aquatic plants, suggesting the need of urgent “conservation actions” to lower the current loss of aquatic plant diversity. In this context, all the aquatic ecosystems presented in a specific area, including the man-made water bodies, must be considered crucial for plants conservation overcoming classical paradigms in biodiversity conservation. In other words, the production system (mainly farming and agriculture, but also recreational areas in urban settlements) must play a central role in taking care the diversity of aquatic ecosystems considering its advantages in terms of products value, as well as functional services rendered to human. For example, the artificial network of ditches and channels in agricultural areas – especially if intensive – could act as a temporary refuge for rare and threatened plant species (Bolpagni et al., 2013; Bolpagni and Piotti, 2016). Similarly, artificial basins such as quarry lakes or irrigation reservoirs along river courses can mimic the alternating phases of formation and destruction of marginal aquatic environments counteracting the loss of fluvial dynamics. Accordingly, the use of more proper management practices for the secondary hydrographic network and artificial water bodies is a key strategy to both guarantee local plant diversity and improve more rapid and effective adaptive responses to future critical conditions. Focusing on lowlands, a strategic option is to valorise and support organic farming systems, which has largely proven to be winning in reducing energy consumption, local pollution (especially at ground- and superficial water level) and supporting diversity (Dalzochio et al., 2016; Martínez-Eixarch et al., 2017). In addition, it is also essential to elaborate lasting strategies able to counteract effectively the future critical conditions. To do this, a better integration among disciplines would be desirable, for example reinforcing the relations between legislative policies devoted to valorise natural resources. In Europe, a paradigmatic example of such a situation is the potential synergies between the WFD and the Habitats Directive in the management of water bodies, as well as aquatic habitats and biodiversity (Ecke et al., 2010; Bolpagni et al., 2017a).
A better comprehension of the contribution of aquatic plants to habitats functioning is equally fundamental, considering the key processes regulated by primary producers in water as sediment re-oxygenation, C and nutrient cyclization, sediment stabilization, algal bloom control, etc. (Chambers et al., 2008; Soana and Bartoli, 2014; O’Hare et al., 2017). This can support a new awareness on the importance of aquatic plants in high-stressed areas such as lowlands or wetland contexts where frequently aquatic ecosystems do not meet minimum quality standards. Hence, the maintenance of submerged plant meadows in drainage channels can improve the capability of semi-natural secondary hydrographic network to control N and P availability with enormous benefits for both natural and human uses (Castaldelli et al., 2015; Soana et al., 2017). Similarly, a plenty of studies have verified the pivotal role of hydrophytes in controlling the oxygen and C balances in stagnant waters (Bolpagni et al., 2007; Pierobon et al., 2010).
Based on these evidences, we are quite convinced that the ability to rise up the awareness by the stakeholders and the general public is essential to break down the slow but inexorable loss of aquatic plants. All that is strictly related to our ability to emphasize the crucial contribution of aquatic ecosystems (and hydrophytes) to local and global economy and human well-being.
Author Contributions
RB designed the research, contributed and coordinated the assembly of the Italian aquatic plant database. CS contributed to assembly the database, and AL processed and analyzed the data with help from RB. RB coordinated the writing of the manuscript, with contribution by all authors, especially by AC, who is the project coordinator of this work.
Funding
RB and AL were supported by fellowships co-funded by the University of Parma.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
A special thank goes to Ms. A. Tria for her valuable support in the implementation of the database concerning the Italian aquatic species, that was carried out during her Bachelor’s degree at the University of Bologna (2014–2015 academic year). We also wish to thank Dr. F. Tassara for his valuable information concerning the Utricularia’ species arrangement in Italy; Prof. S. Marchiori (University of Lecce) for the clarifications regarding the records of Pilularia globulifera for the Apulian region; Dr. G. Galasso (Milan Museum of Natural History) and Dr. N. M. G. Ardenghi (University of Pavia) for the clarifications regarding the records of Blyxa japonica for Lombardy.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00116/full#supplementary-material
Footnotes
- ^http://www.actaplantarum.org/flora/flora.php
- ^https://www.eea.europa.eu/data-and-maps/
- ^http://www.sinanet.isprambiente.it/it
References
Abati, S., Minciardi, M. R., Ciadamidaro, S., Fattorini, S., and Ceschin, S. (2016). Response of macrophyte communities to flow regulation in mountain streams. Environ. Monit. Assess. 188:414. doi: 10.1007/s10661-016-5420-2
Alahuhta, J. (2015). Geographic patterns of lake macrophyte communities and species richness at regional scale. J. Veg. Sci. 26, 564–575. doi: 10.1111/jvs.12261
Alahuhta, J., Kanninen, A., Hellsten, S., Vuori, K.-M., Kuoppala, M., and Hamalainen, H. (2013). Environmental and spatial correlates of community composition, richness and status of boreal lake macrophytes. Ecol. Indic. 32, 172–181. doi: 10.1016/j.ecolind.2013.03.031
Alahuhta, J., Kosten, S., Akasaka, M., Auderset, D., Azzella, M. M., Bolpagni, R., et al. (2017). Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J. Biogeogr. 44, 1758–1769. doi: 10.1111/jbi.12978
Azzella, M. M., Ricotta, C., and Blasi, C. (2013). Aquatic macrophyte diversity assessment: validation of a new sampling method for circular-shaped lakes. Limnologica 43, 492–499. doi: 10.1016/j.limno.2013.04.001
Azzella, M. M., Rosati, L., Iberite, M., Bolpagni, R., and Blasi, C. (2014). Changes in aquatic plants in the Italian volcanic-lake system detected using current data and historical records. Aquat. Bot. 112, 41–47. doi: 10.1016/j.aquabot.2013.07.005
Baattrup-Pedersen, A., Friberg, N., Larsen, S. E., and Riss, T. (2005). The influence of channelization on riparian plant assemblages. Freshw. Biol. 50, 1248–1261. doi: 10.1111/j.1365-2427.2005.01383.x
Barone, G., Gambi, L., and Rossi Doria, M. (1985). La storia delle bonifiche in Italia. Elementi per un dibattito. Stud. Stor. 26, 961–975.
Barrett, R., Nielsen, D. L., and Croome, R. (2010). Associations between the plant communities of floodplain wetlands, water regime and wetland type. River Res. Appl. 26, 866–876. doi: 10.1002/rra.1299
Billi, P., and Fazzini, M. (2017). Global change and river flow in Italy. Glob. Planet. Change 155, 234–246. doi: 10.1016/j.gloplacha.2017.07.008
Bilz, M., Kell, S. P., Maxted, N., and Lansdown, R. V. (2011). European Red List of Vascular Plants. Luxembourg: Publications Office of the European Union.
Bocchiola, D. (2014). Long term (1921–2011) hydrological regime of Alpine catchments in Northern Italy. Adv Water Resour. 70, 51–64. doi: 10.1016/j.advwatres.2014.04.017
Bolpagni, R., and Piotti, A. (2015). Hydro-hygrophilous vegetation diversity and distribution patterns in riverine wetlands in an agricultural landscape: a case study from the Oglio River (Po plain, Northern Italy). Phytocoenologia 45, 69–84. doi: 10.1127/0340-269X/2014/0044
Bolpagni, R., and Piotti, A. (2016). The importance of being natural in a human-altered riverscape: role of wetland type in supporting habitat heterogeneity and the functional diversity of vegetation. Aquat. Conserv. 26, 1168–1183. doi: 10.1002/aqc.2604
Bolpagni, R., Azzella, M. M., Agostinelli, C., Beghi, A., Bettoni, E., Brusa, G., et al. (2017a). Integrating the water framework directive into the habitats directive: analysis of distribution patterns of lacustrine EU habitats in lakes of Lombardy (northern Italy). J. Limnol. 76, 75–83. doi: 10.4081/jlimnol.2017.1627
Bolpagni, R., Bartoli, M., and Viaroli, P. (2013). Species and functional plant diversity in a heavily impacted riverscape: implications for threatened hydro-hygrophilous flora conservation. Limnologica 43, 230–238. doi: 10.1016/j.limno.2012.11.001
Bolpagni, R., Laini, A., and Azzella, M. M. (2016). Short-term dynamics of submerged aquatic vegetation diversity and abundance in deep lakes. Appl. Veg. Sci. 19, 711–723. doi: 10.1111/avsc.12245
Bolpagni, R., Laini, A., Soana, E., Tomaselli, M., and Nascimbene, J. (2015). Growth performance of Vallisneria spiralis under oligotrophic conditions supports its potential invasiveness in mid-elevation freshwaters. Weed Res. 55, 185–194. doi: 10.1111/wre.12128
Bolpagni, R., Pierobon, E., Longhi, D., Nizzoli, D., Bartoli, M., Tomaselli, M., et al. (2007). Diurnal exchanges of CO2 and CH4 across the water–atmosphere interface in a water chestnut meadow (Trapa natans L.). Aquat. Bot. 87, 43–48. doi: 10.1016/j.aquabot.2007.02.002
Bolpagni, R., Racchetti, E., and Laini, A. (2017b). Fragmentation and groundwater supply as major drivers of algal and plant diversity and relative cover dynamics along a highly modified lowland river. Stoten 568, 875–884. doi: 10.1016/j.scitotenv.2016.06.070
Bouma, T. J., De Vries, M. B., and Herman, P. M. J. (2010). Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. Ecology 91, 2696–2704. doi: 10.1890/09-0690.1
Bresciani, M., Giardino, C., Lauceri, R., Matta, E., Cazzaniga, I., Pinardi, M., et al. (2017). Earth observation for monitoring and mapping of cyanobacteria blooms. Case studies on five Italian lakes. J. Limnol. 76, 127–139. doi: 10.4081/jlimnol.2016.1565
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach. New York: Springer-Verlag.
Carré, C., Meybeck, M., and Esculier, F. (2017). The Water framework directive’s “percentage of surface water bodies at good status”: unveiling the hidden side of a “hyperindicator”. Ecol. Indic. 78, 371–380. doi: 10.1016/j.ecolind.2017.03.021
Castaldelli, G., Soana, E., Racchetti, E., Vincenzi, F., Fano, E. A., and Bartoli, M. (2015). Vegetated canals mitigate nitrogen surplus in agricultural watersheds. Agric. Ecosyst. Environ. 212, 253–262. doi: 10.1016/j.agee.2015.07.009
Ceschin, S., Zuccarello, V., and Caneva, G. (2010). Role of macrophyte communities as bioindicators of water quality: application on the Tiber river basin (Italy). Plant Biosyst. 144, 528–536. doi: 10.1080/11263500903429221
Chambers, P. A., Lacoul, P., Murphy, K. J., and Thomaz, S. M. (2008). Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595, 9–26. doi: 10.1111/gcb.13004
Chappuis, E., Ballesteros, E., and Gacia, E. (2012). Distribution and richness of aquatic plants across Europe and Mediterranean countries: patterns, enviornmental driving factors and comparison with total plant richness. J. Veg. Sci. 23, 985–997. doi: 10.1111/j.1654-1103.2012.01417.x
Conti, F., Abbate, G., Alessandrini, A., and Blasi, C. (2005). An Annotated Check-list of Italian Vascular Flora. Roma: Palombi Editore.
Conti, F., Alessandrini, A., Bacchetta, G., Banfi, E., Barberis, G., Bartolucci, F., et al. (2006). Updating of the checklist of the Italian vascular flora. Nat. Vicent 10, 5–74.
Coppola, E., and Giorgi, F. (2010). An assessment of temperature and precipitation change projections over Italy from recent global and regional climate model simulations. Int. J. Climatol. 30, 11–32.
Costantini, E. A. C., Fantappié, M., and L’Abate, G. (2013). “Climate and pedoclimate of Italy,” in The Soils of Italy, eds E. A. C. Costantini and C. Dazzi (Heidelberg: Springer), 19–37.
Cuttelod, A., García, N., Abdul Malak, D., Temple, H., and Katariya, V. (2008). “The Mediterranean: a biodiversity hotspot under threat,” in The 2008 Review of The IUCN Red List of Threatened Species, eds J.-C. Vié, C. Hilton-Taylor, and S. N. Stuart (Gland: IUCN), 89–102.
Dalzochio, M. S., Baldin, R., Stenert, C., and Maltchik, L. (2016). Can organic and conventional agricultural systems affect wetland macroinvertebrate taxa in rice fields? Basic Appl. Ecol. 17, 220–229. doi: 10.1016/j.baae.2015.10.009
de Haas, T. (2015). Managing the marshes: an integrated study of the centuriated landscape of the Pontine plain. J. Archaeol. Sci. Rep. 15, 470–481. doi: 10.1016/j.jasrep.2016.07.012
De Notaris, G. (1848). Index Seminum Quae Hortus Botanicus R. Archiginnasii Genuensis pro Commutatione Offert. Genova: Technical Institute.
Desfayes, M. (2008). Flore vasculaire herbacée des eaux douces et des milieus humides de la Sardaigne. Fl. Medit. 18, 247–331.
Desfayes, M. (2011). Notulae alla checklist della flora vascolare italiana 11: 1778. Inform. Bot. Ital. 43, 123–150.
Ecke, F., Hellsten, S., Mjelde, M., Kuoppala, M., and Schlacke, S. (2010). Potential conflicts between environmental legislation and conservation exemplified by aquatic macrophytes. Hydrobiologia 656, 107–115. doi: 10.1007/s10750-010-0424-3
Ercole, S., and Giacanelli, V. (2014). “Flora,” in Specie e Habitat di Interesse Comunitario in Italia: Distribuzione, Stato di Conservazione e Trend, eds P. Genovesi, P. Angelini, E. Bianchi, E. Dupré, S. Ercole, V. Giacanelli, et al. (Roma: ISPRA), 17–70.
Falcucci, A., Maiorano, L., and Boitani, L. (2007). Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landsc. Ecol. 22, 617–631. doi: 10.1007/s10980-006-9056-4
Fick, S. E., and Hijmans, R. J. (2017). Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. Inter. J. Climatol. 37, 4302–4315. doi: 10.1002/joc.5086
Guarino, R., Domina, G., and Pignatti, S. (2012). Ellenberg’s Indicator values for the Flora of Italy – first update: Pteridophyta, Gymnospermae and Monocotyledoneae. Fl. Medit. 22, 197–209. doi: 10.7320/FlMedit22.197
Hoff, H. (2013). “Vulnerability of ecosystem services in the Mediterranean region to climate change in combination with other pressures,” in Regional Assessment of Climate Change in the Mediterranean, Vol. 51, eds A. Navarra and L. Tubiana (Dordrecht: Springer), 9–20.
Li, Z., Yu, D., Xiong, W., Wang, D., and Tu, M. (2006). Aquatic plants diversity in arid zones of northwest China: patterns, threats and conservation. Biodivers. Conserv. 15, 3417–3444. doi: 10.1007/s10531-005-0769-5
Marchina, C., Natali, C., Fazzini, M., Fusetti, M., Tassinari, R., and Bianchini, G. (2017). Extremely dry and warm conditions in northern Italy during the year 2015: effects on the Po river water. Rend. Lincei 28, 281–290. doi: 10.1007/s12210-017-0596-0
Martínez-Eixarch, M., Curcó, A., and Ibáñez, C. (2017). Effects of agri-environmental and organic rice farming on yield and macrophyte community in Mediterranean paddy fields. Paddy Water Environ. 15, 457–468. doi: 10.1007/s10333-016-0563-x
Mather, J. R. (1979). “Use of the climatic water budget to estimate streamflow” in Use of the Climatic Water Budget in Selected Environmental Water Problems, Vol. 32, ed. J. R. Mather (Certerton, AR: C.W. Thornthwaite Associates Publications in Climatology), 1–52.
McLeod, A. I., and Xu, C. (2017). Bestglm: Best Subset GLM. R package version 0.36. Available at: https://CRAN.R-project.org/package=bestglm
Médail, F., and Quézel, P. (1999). Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conserv. Biol. 13, 1510–1513. doi: 10.1046/j.1523-1739.1999.98467.x
O’Hare, M. T., Aguiar, F. C., Asaeda, T., Bakker, E. S., Chambers, P. A., Clayton, J. S., et al. (2017). Plants in aquatic ecosystems: current trends and future directions. Hydrobiologia 153, 1–11 doi: 10.1007/s10750-017-3190-7
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2017). Vegan: Community Ecology Package. R package version 2.4-3. Available at: https://CRAN.R-project.org/package=vegan
Peel, M. C., Finlayson, B. L., and McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644. doi: 10.5194/hess-11-1633-2007
Peruzzi, L., Conti, F., and Bartolucci, F. (2014). An inventory of vascular plants endemic to Italy. Phytotaxa 168, 1–75. doi: 10.11646/phytotaxa.168.1.1
Pierobon, E., Bolpagni, R., Bartoli, M., and Viaroli, P. (2010). Net primary production and seasonal CO2 and CH4 fluxes in a Trapa natans L. meadow. J. Limnol. 69, 225–234. doi: 10.4081/jlimnol.2010.225
Pignatti, S., Menegoni, P., and Pietrosanti, S. (2005). Biondicazione attraverso le piante vascolari. Valori di indicazione secondo Ellenberg (Zeigerwerte) per le specie della Flora d’Italia. Braun Blanquetia 39, 1–97.
R Core Team. (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raunkiær, C. (1934). The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer. Oxford: Oxford University Press.
Rossi, G., Montagnani, C., Gargano, D., Peruzzi, L., Abeli, T., Ravera, S., et al. (2013). Lista Rossa della Flora Italiana. 1. Policy Species e altre Specie Minacciate. Roma: Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
Santamaria, L. (2002). Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol. 23, 137–154. doi: 10.1016/S1146-609X(02)01146-3
Saunders, D. L., Meeuwig, J. J., and Vincent, A. C. J. (2002). Freshwater protected areas: strategies for conservation. Biol. Conserv. 16, 30–41. doi: 10.1046/j.1523-1739.2002.99562.x
Scheffer, M., Szabó, S., van Nes, E. H., Rinaldi, S., Kautsky, N., Norberg, J., et al. (2003). Floating plant dominance as a stable state. Proc. Natl. Acad. Sci. U.S.A. 100, 4040–4045. doi: 10.1073/pnas.0737918100
Soana, E., Balestrini, R., Vincenzi, F., Bartoli, M., and Castaldelli, G. (2017). Mitigation of nitrogen pollution in vegetated ditches fed by nitrate-rich spring waters. Agric. Ecosyst. Environ. 243, 74–82. doi: 10.1016/j.agee.2017.04.004
Soana, E., and Bartoli, M. (2014). Seasonal regulation of nitrification in a rooted macrophyte (Vallisneria spiralis L.) meadow under eutrophic conditions. Aquat. Ecol. 48, 11–21. doi: 10.1007/s10452-013-9462-z
Testi, A., Bisceglie, S., Guidotti, S., and Fanelli, G. (2009). Detecting river environmental quality through plant and macroinvertebrate bioindicators in the Aniene River (Central Italy). Aquat. Ecol. 43, 477–486. doi: 10.1007/s10452-008-9205-8
Villa, P., Bresciani, M., Bolpagni, R., Pinardi, M., and Giardino, C. (2015). A rule-based approach for mapping macrophyte communities using multi-temporal aquatic vegetation indices. Remote Sens. Environ. 171, 218–233. doi: 10.1016/j.rse.2015.10.020
Keywords: macrophytes, vascular plants, spatial distribution, environmental drivers, freshwater ecosystems, human impacts
Citation: Bolpagni R, Laini A, Stanzani C and Chiarucci A (2018) Aquatic Plant Diversity in Italy: Distribution, Drivers and Strategic Conservation Actions. Front. Plant Sci. 9:116. doi: 10.3389/fpls.2018.00116
Received: 30 August 2017; Accepted: 22 January 2018;
Published: 13 February 2018.
Edited by:
Richard K. F. Unsworth, Swansea University, United KingdomReviewed by:
José Antonio Molina, Complutense University of Madrid, SpainAgnieszka Piernik, Nicolaus Copernicus University in Toruñ, Poland
Copyright © 2018 Bolpagni, Laini, Stanzani and Chiarucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossano Bolpagni, cm9zc2Fuby5ib2xwYWduaUB1bmlwci5pdA==
 Rossano Bolpagni
Rossano Bolpagni Alex Laini1
Alex Laini1