- 1Department of Vegetables and Field Crops, Institute of Plant Sciences, Agricultural Research Organization-Volcani Center, Rishon LeZion, Israel
- 2Department of Plant Pathology and Microbiology, The Robert H. Smith Faculty of Agriculture, Food and Environment, Hebrew University of Jerusalem, Rehovot, Israel
- 3The Robert H. Smith Faculty of Agriculture, Food and Environment, The Robert H. Smith Institute of Plant Sciences and Genetics in Agriculture, Hebrew University of Jerusalem, Rehovot, Israel
- 4Department of Evolutionary and Environmental Biology, Faculty of Natural Sciences, Institute of Evolution, University of Haifa, Haifa, Israel
The biotroph wheat powdery mildew, Blumeria graminis (DC.) E.O. Speer, f. sp. tritici Em. Marchal (Bgt), has undergone long and dynamic co-evolution with its hosts. In the last 10,000 years, processes involved in plant evolution under domestication, altered host-population structure. Recently both virulence and genomic profiling separated Bgt into two groups based on their origin from domestic host and from wild emmer wheat. While most studies focused on the Bgt pathogen, there is significant knowledge gaps in the role of wheat host diversity in this specification. This study aimed to fill this gap by exploring qualitatively and also quantitatively the disease response of diverse host panel to powdery mildew [105 domesticated wheat genotypes (Triticum turgidum ssp. dicoccum, T. turgidum ssp. durum, and T. aestivum) and 241 accessions of its direct progenitor, wild emmer wheat (T. turgidum ssp. dicoccoides)]. A set of eight Bgt isolates, originally collected from domesticated and wild wheat was used for screening this wheat collection. The isolates from domesticated wheat elicited susceptible to moderate plant responses on domesticated wheat lines and high resistance on wild genotypes (51.7% of the tested lines were resistant). Isolates from wild emmer elicited reciprocal disease responses: high resistance of domesticated germplasm and high susceptibility of the wild material (their original host). Analysis of variance of the quantitative phenotypic responses showed a significant Isolates × Host species interaction [P(F) < 0.0001] and further supported these findings. Furthermore, analysis of the range of disease severity values showed that when the group of host genotypes was inoculated with Bgt isolate from the reciprocal host, coefficient of variation was significantly higher than when inoculated with its own isolates. This trend was attributed to the role of major resistance genes in the latter scenario (high proportion of complete resistance). By testing the association between disease severity and geographical distance from the source of inoculum, we have found higher susceptibility in wild emmer close to the source. Both qualitative and quantitative assays showed a reciprocal resistance pattern in the wheat host and are well aligned with the recent findings of significant differentiation into wild-emmer and domesticated-wheat populations in the pathogen.
Introduction
Genetic patterns of host susceptibility and pathogen virulence variation are essential underlying factors influencing disease epidemiology. Moreover, the distribution of this variation in space and time may shed light on the co-evolutionary selection mode between pathogens and plants. Relatively little empirical data on how ecological and evolutionary processes interact to influence the generation and maintenance of spatial and temporal variation in natural host–pathogen interactions has been collected (Tack et al., 2012). Moreover, studying the dynamics of pathogen and host at the center of origin where both natural and domesticated habitats co-exist sympatricaly could be extremely valuable.
Wild emmer wheat [T. turgidum ssp. dicoccoides (Körn.) Thell.] is the allo-tetraploid (2n = 4x = 28, BBAA) progenitor of both the tetraploid durum wheat [T. turgidum ssp. durum (Desf.) MacKey] and the hexaploid (2n = 6x = 42; BBAADD) bread wheat (T. aestivum L.) (Figure S1; Feldman, 2001). Native stands of wild emmer are distributed throughout the Near-Eastern “Fertile Crescent,” in the transition zone between the Mediterranean and the steppe phytogeographic provinces (Harlan and Zohary, 1966). In Israel and surrounding regions, this species thrives across a wide ecological amplitude, in diverse primary and, to a limited extent, secondary habitats (Kimber and Feldman, 1987). In some geographical regions, natural habitats of wild emmer are distributed distributed sympatrically with agricultural wheat fields.
Powdery mildew caused by the parasitic fungus Blumeria graminis (DC.) E.O. Speer, f. sp. tritici Em. Marchal (designated Bgt below), is one of the most devastating diseases of wheat, causing yield losses of up to 34% (Johnson et al., 1979). The life cycle of powdery mildew includes sexual (between seasons) and asexual (within season) stages, a strategy that combines the benefits of both new allelic combinations and often-effective off-season survival mechanisms (through sexual reproduction) with the advantages of rapidly multiplying individual clonal lines (through asexual reproduction) that are particularly adapted to specific host habitats (McDonald and Linde, 2002). Since powdery mildew propagates very efficiently on wild plants it forms a huge reservoir of parasites deeply rooted in wild host populations—a reservoir that, occasionally or constantly, could serve as a source of inoculums to initiate epidemics in the fields (Dinoor, 1974). Bawden (1957) suggested that in natural habitats, where the hosts are genetically heterogeneous “each plant is a selective ecological niche favoring only a few parasites.” In the long term, the genetically diverse mixture of wild hosts, in contrast to the genetically uniform cultivated fields, enables recombination and development of differing pathotypes, and can influence the pathogenic profile of the inoculums (Dinoor, 1974).
An ex situ collection of Bgt isolates (the “Eshed–Dinoor mildew collection,” Ben-David et al., 2014), has been established and maintained for more than two decades. Availability of both host and pathogen ex situ genetic collections enables us to use phenotypic and genotypic assays to examine issues of host/parasite co-evolution. Recent investigation of this Bgt collection has shown that based on host, it is significantly differentiated into wild-emmer and domesticated-wheat populations (Ben-David et al., 2016; Menardo et al., 2016). However, the results did not support the existence of a separate B. graminis f. sp. dicocci (Ben-David et al., 2016). In the present study, we examined a diverse set of wild and domesticated wheat lines with respect to their responses to inoculation with Israeli Bgt isolates collected from both wild and domesticated hosts.
We hypothesize that disease responses of diverse host span will be in accordance to this Bgt differentiation at the center of origin and that quantitative characterization will improve our understanding of Wheat-Bgt co-evolution. A set of eight distinct Bgt isolates were selected for inoculation of diverse host panel which includes tetraploid and hexaploid domesticated wheat, as well as their wild emmer progenitor. The specific aims were to: (i) analyze the phenotypic reactions of domesticated and wild wheat germplasm to inoculation with Bgt isolates, using both qualitative and quantitative scales; (ii) characterize the virulence and aggressiveness of Bgt isolates originating from domesticated and wild hosts; and (iii) assess the association between disease severity and geographic distance between sources of pathogen isolates and wild host wheat genotypes. Our results highlight the evolutionary dynamics between wheat and its pathogen B. graminis at the center of origin, where wild and domesticated wheats grow in intimate mixtures. Thus, they shed light on the co-evolutionary process underlying the sympatric distribution of domesticated wheat and its wild progenitor across the Fertile Crescent.
Materials and Methods
Plant and Pathogen Material
A collection of 63 domesticated wheat genotypes, comprised of 16 bread wheats, 32 durum wheats, and 15 emmer wheats, together with 54 wild emmer accessions, was tested by means of detached-leaf assays at the seedling stage, for quantitative and qualitative responses to infection with powdery mildew (Table S1, Figure S2). These plant accessions comprise representative wheat collection of various habitats that encompass a wide eco-geographical range: 1–69.3°E; 8–52.5°N (Figure S3). The accessions originated from 18 different countries, the highest representations being from Israel (n = 25), Turkey (n = 14), Iran (n = 5), and Ethiopia (n = 5). The collection sites were not randomly distributed throughout the geographical area; the most numerous representations were comprised of wheat lines from the western arc of the Fertile Crescent (Figure S3). Hierarchical clustering (Ward, 1963) was used to identify discrete groups of sites, based on detailed eco-geographic profiles. Sites were selected to represent the six main clusters, which represent the maximum eco-geographic variance of the collection (Table S1). Hierarchical clustering was applied by using SPSS V21.0.0 (SPSS, 2004).
In addition, 42 wheat cultivars and 187 accessions of wild emmer were included in the qualitative phenotypic test. A set of differential wheat accessions carrying known Powdery mildew resistance genes (Pm) were obtained from the National Small Grain Collection (Aberdeen, Idaho, USA) and from Prof. F.J. Zeller (Technische Universität Munich, Institut für Pflanzenbau und Pflanzenzüchtung, Germany) (Table S2). This set of differential Pm wheat accessions is a common tool to characterize powdery mildew isolates based on virulence or avirulence against a specific Pm gene (e.g., Ben-David et al., 2016). All assays were performed on host plants at seedling stage. Pre-inoculation, plants were growing under controlled conditions, protected from pests and other pathogens.
Bgt isolates were collected from various wheat species (wild and domesticated) in various habitats across Israel (see Table 1 in Ben-David et al., 2010, Table S3). A single isolate—Bgt#GH—was collected from the cultivar Chinese Spring (designated CS below) in the greenhouse. Information regarding the origin of the isolates (excluding Bgt#GH) is presented in Table S2. In the present study, we have included six isolates for the quantitative assay: two isolates collected from durum wheat (Bgt#15 and Bgt#97), one isolate collected from bread wheat (Bgt#70) and three isolates collected from wild emmer (Bgt#58, Bgt#63, and Bgt#66). These six selected isolates represent both the virulence and genetic diversity of the Eshed—Dinoor mildew collection (Ben-David et al., 2016). In addition, two isolates collected from bread wheat (Bgt#101 and Bgt#GH) were included only in the qualitative phenotypic assay.
Qualitative Phenotypic Assay
Conditions of incubation, inoculation, and disease assessment were according to Hsam and Zeller (2002) and Ben-David et al. (2010), with some modifications: The tests for mildew resistance were conducted on 10- to 14-days-old primary leaf segments maintained on agar at 6 g/l supplemented with benzimidazole at 50 mg/l in polystyrene boxes. Each box of leaf segments included genotypes from the wheat collection and susceptible genotypes [e.g., bread wheat cv. CS (for Bgt#15, Bgt#70, Bgt#97, Bgt#101, and Bgt#GH), durum wheat cv. Inbar (for Bgt#58), and wild emmer accession Israel-A, (PI-481521, USDA-ARS Cereal Crops Research Unit, Fargo, ND, USA) (for Bgt#66 and Bgt#63)] as controls. Each wheat genotype was represented by three replicates (a column of three leaf segments). Spore and germ-tube densities and germination rates were calculated for each box (recorded 24 h post inoculation, six random measurements per box) and were related to the average disease index of the control lines that were present in each box, in order to exclude a possible correlation between initial inoculum condition and final infection rate. No correlation was found between the initial spore and germ-tube densities and disease severity, and no correlation between germination rate and levels of disease response was detected.
Results of the powdery mildew tests were scored about 12 days after inoculation (dpi) and once again 2–5 days later. The infection types (IT) of powdery mildew were recorded on the basis of symptoms on a scale of 0–4, with 0 representing no visible symptoms and 4 fully infected leaves (Mains and Dietz, 1930). The IT was divided into three main categories: 0–2, 2–3, and 3–4 for resistant (R), moderate (M), and susceptible (S) reactions, respectively. To examine the association between frequency data of the three categories and host genetic source (wild/domesticated) a χ2 test for discrete variables was applied.
Quantitative Phenotypic Assay
Conditions of incubation, inoculation and disease assessment were as described above. Quantitative assays were conducted on three separate dates, on each of which inoculation was performed with two different isolates: [Experiment I (Bgt#15 and Bgt#66); Experiment II (Bgt#70 and Bgt#58); and Experiment III (Bgt#97and Bgt#63)]. In each experiment, the leaf segments were scored quantitatively by counting the exact number of mildew pustules on each leaf segment 12 dpi. Area of leaf segments was measured by implementing the ImageJ software using the digital “Measure” function (http://rsb.info.nih.gov/ij/) and disease severity was calculated (No. of mildew pustules per Cm2).
Statistical Analyses
All statistical analyses were performed with the JMP statistical package (SAS Institute, Cary, NC, USA). ANOVA was applied to assess the effects of Bgt isolate and the source host, i.e., the wheat species from which mildew was sampled, on disease severity. An unbalanced nested factorial model was employed for all three experiments (see Supplementary File for model equation).
Results
Phenotypic Tests with Pm Differential Lines
The results of the phenotypic assays with seven Bgt isolates on a set of 18 differential wheat lines, that carry different Pm genes or gene combinations, are presented in Table S2. The Bgt isolates interacted differentially with the Pm lines, each eliciting a specific reaction profile representing the differing specificities of the seven tested Bgt isolates. Isolate Bgt#66, collected from wild emmer was avirulent on all 17 differential Pm lines. Bgt#58 (like Bgt#66 collected from the same wild population at Amiad site, Eastern Galilee) and Bgt#63 were more virulent, overcoming, respectively, eight and seven of the Pm resistance genes (Table S2). The four Bgt isolates which originated from domesticated hosts were highly virulent on the differential set of lines, overcoming most of the represented Pm resistance genes.
Qualitative Phenotypic Assay
Altogether, 1,692 interactions between wheat genotypes and Bgt isolates were qualitatively analyzed (Table 1, Figure 1, Figures S4, S5). When lines of specific wheat species were inoculated with Bgt isolates originating from the same species, low proportional rates of resistance to powdery mildew were detected, whereas higher resistance rates were evident when the pathogen isolates originated from different species. This trend was stronger when wild emmer wheat lines were exposed to isolates from domesticated hosts.
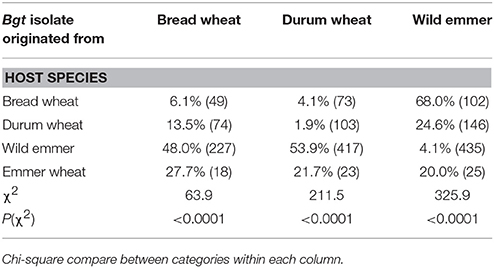
Table 1. The fraction of accessions (%), out of those tested (numbers), resistant to Bgt isolates collected from various wheat species.
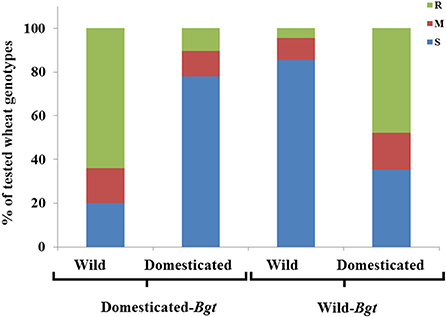
Figure 1. Distribution of qualitative disease resistance of wild and domesticated hosts. The distribution of phenotypic reactions of entries of wild and domesticated wheat lines [Resistant (R), Moderate (M), susceptible (s)], to Bgt isolates collected from domesticated wheat and wild emmer wheat.
Pooled data from all isolates and experiments can provide a general view of (wheat species × Bgt isolate) interactions (Table 2, Figure 1). A minute proportion (4.1%) of wild emmer accessions showed resistance when inoculated with Bgt isolates from wild emmer; the prominent χ2 value in this group [χ2 = 325.9; ≤ 0.0001] is due to the differing resistance proportions of the same lines when inoculated with Bgt isolates from bread wheat and durum wheat: 48 and 53.9%, respectively. Bread wheat genotypes were highly resistant (68%) to Bgt isolates from wild emmer, but very susceptible to Bgt isolates from cultivated wheats, with only 10.2% showing resistance [χ2 = 63.9; ≤ 0.0001]. Durum wheat varieties generally showed less resistance than bread wheats, but the general pattern was similar, with higher resistance against Bgt isolates from wild emmer. The frequency distribution of susceptibility among emmer wheat genotypes, the smallest group of wheat genotypes in the assay, does not show a differential pattern among the different origins of the Bgt isolates (Table 2).
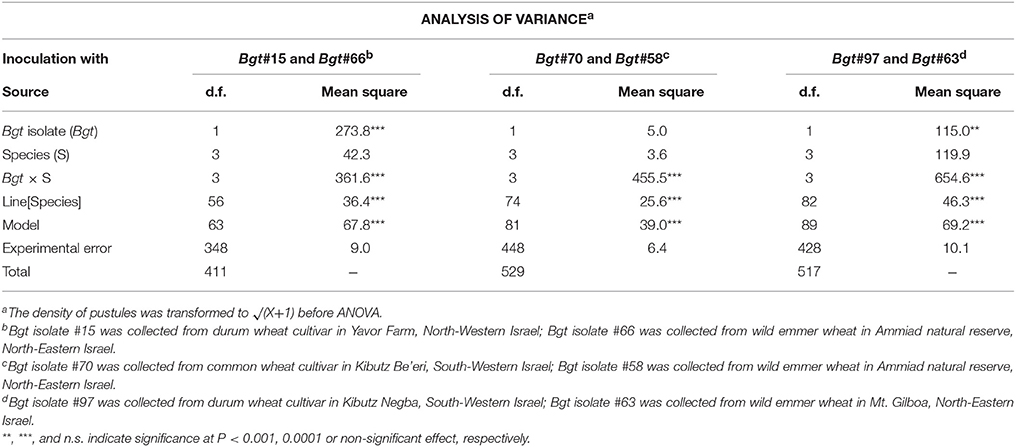
Table 2. Analysis of variance of the quantitative resistance of a collection of wheat lines representing four species (T. aestivum, T. durum, T. dicoccum, and or T. dicoccoides) inoculated with Bgt isolates #15 and #66, #70 and #58, #97 and #63.
Quantitative Phenotypic Assay
Analysis of variance (ANOVA) of the three experiments is summarized in Table 2. R-square values for the three independent experiments ranged from 0.52 to 0.58, which highlights the proportion of the variance of disease symptoms across the differing wheat lines that was accounted for by the tested ANOVA model. The (wheat species × Bgt isolate) interaction was significant in all three experiments (Table 2, Figure S6). Wild emmer genotypes showed significantly higher disease severity when inoculated with isolates originating from wild emmer than with isolates collected from domesticated wheat (Figure 2, Figure S6). For example, in wild emmer accessions the mean severity value of Bgt#63, the most aggressive isolate, was manifested in appearance of 123 pustules/cm2 in wild emmer leaves, whereas in bread wheat genotypes the mean value was only 9.86 pustules/cm2 (original values were extracted by inversing the transformed data set).
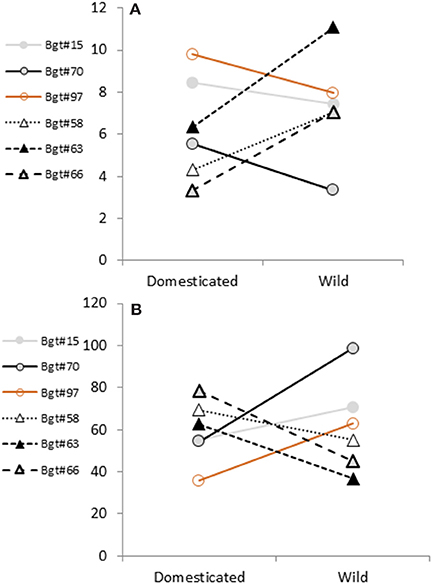
Figure 2. Mean comparison of quantitative disease responses of wild and domesticated hosts. Least square means (A) and CV values (B) showing the interaction between the pathogen (Bgt isolates) and host plants (domesticated/wild). Responses to Bgt from wild emmer host are shown as triangles and connected with dashed/dotted lines. Responses to Bgt from domesticated hosts bread wheat, durum wheat and wild emmer wheat are shown as open circles and connected with continuous lines.
A completely different pattern was evident when the domesticated lines were inoculated with Bgt originating from cultivated wheat fields: domesticated genotypes were heavily infected by inoculation with Bgt isolates collected from domesticated wheat species, as clearly exemplified by the high disease severity detected on bread wheat cultivars after inoculation with Bgt#70 isolate. In contrast, low levels of infection were evident in wild emmer wheat lines inoculated with powdery mildew isolates collected from domesticated wheat lines: disease severity values were 38.8, 10.1, and 39.1 pustules/cm2 for Bgt#15, Bgt#70, and Bgt#97, respectively (Figure S6).
In general, isolates sampled from modern wheat fields are showing a similar preference to domesticated host (Figure 2A). On the other hand, isolates sampled from wild wheat generated reciprocal reaction, showing reduced aggressiveness on domesticated germplasm and causing high disease severity in the wild material. To assess, variance estimates of disease severity CV values were compared (Figure 2B). Isolates from domesticated origin had a wider variance in pustules' density on wild emmer wheat. Their CV values where reduced when tested on domesticated hosts. All isolates originated from wild emmer wheat showed the exact reciprocal pattern.
The geographical distances of the hosts from the sources of inoculum ranged between 0–3,500 and 0–900 km for domesticated and wild wheat genotypes, respectively (Figure 3). No association was observed between disease severity in the domesticated wheat genotypes and the distance from the geographical origin of the inoculum; disease severity values varied randomly along the geographical distance axis (Figure 3). Bread wheat genotypes showed low to very low disease severity, i.e., high resistance, when inoculated with isolates originating from wild wheat (Figure 3, left-hand side). In general, the wild emmer collection is geographically subdivided into Israeli (0–300 km from inoculum origin site) and eastern Turkish (600–900 km from inoculum origin site) groups (Figure S2). Both groups were divided by the Hierarchical clustering analysis into two distinct eco-geographic clusters. When inoculated with Bgt isolates from domesticated wheat the Israeli wild emmer accessions showed higher proportions of complete resistance (disease severity = 0) than the Turkish accessions (Figure 3, right-hand side). Disease severity in wild emmer after inoculation with Bgt isolates originating from wild hosts showed little or no differences between the Israeli and the Turkish subgroups, e.g., Bgt#58 (Figure 3, right-hand side).
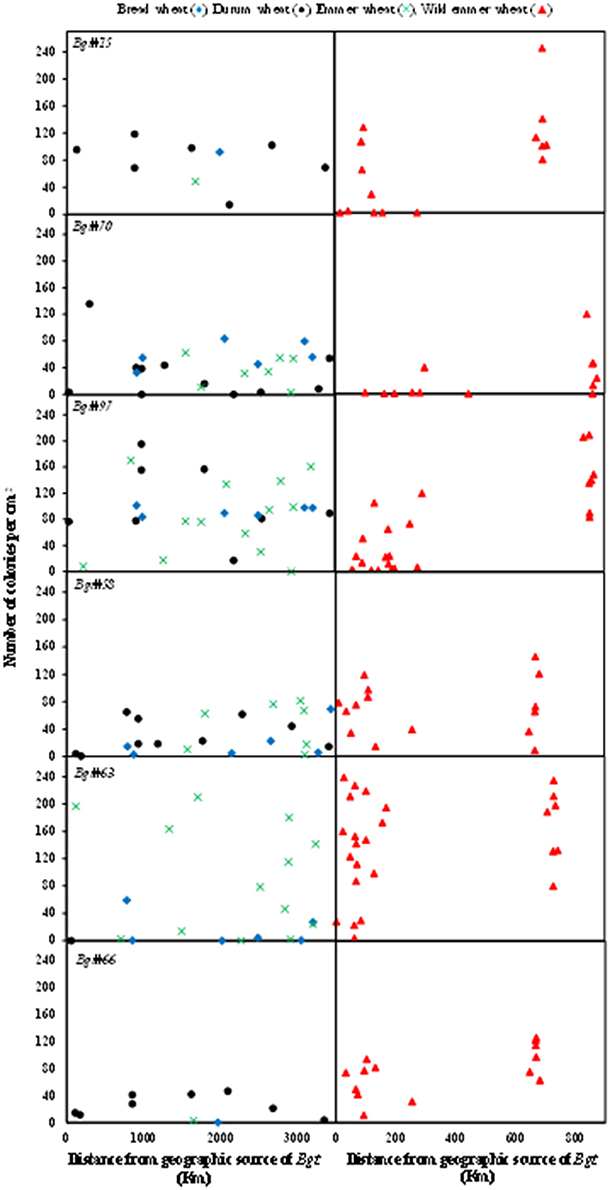
Figure 3. Association between geographic distance and disease response. Scatter chart showing the association between the geographic distances (km) of the host from the origin of the mildew isolate, and the number of mildew pustules/cm2 in the tests. Each row represents an individual mildew isolate that was used as inoculation source. The left column presents the disease severity of domesticated wheat lines. The right column represents disease reaction of wild emmer wheat accessions.
Discussion
The present study aims to characterize and investigate the wheat powdery mildew pathosystem and to test the hypothesis that disease responses of diverse host germplasm will correlate to the Bgt differentiation and adaptation to wild and domesticated host at the center of origin. Both qualitative and quantitative characterization have shown clear pattern of host differentiation in the response. The quantitative assay support biometrically the pattern of reciprocal hosts' responses to powdery mildew isolates originating from domesticated wheats.
Qualitative Phenotypic Assay
A selected set of eight Bgt isolates was used for screening a diverse wheat collection by means of qualitative and quantitative phenotypic scoring. The qualitative assay which summarizes over 1,600 different (wheat genotype × Bgt isolate) interactions has shown strong and significant interaction between the origins of the isolates and the genetic sources of the tested wheat lines (Table 1, Figure 1, Figures S2, S3).
In general, Bgt isolates collected from cultivated wheat fields were more successful in attacking domesticated wheat genotypes than those of wild wheats. Wild emmer, in contrast, was significantly more resistant to these isolates. The isolates collected in the wild elicited reciprocal reactions, showing reduced virulence on domesticated germplasm and causing higher disease severity in the wild material (Figures 1, 2A). This finding is in full accordance to what was found in previous virulence reports of Bgt from Israel (Ben-David et al., 2016).
Quantitative Phenotypic Assay
ANOVA of the quantitative test (Table 2) supported the finding of the qualitative assay by showing significant interaction between the pathogen (Bgt isolate) and the host (domesticated/wild wheat genotype). The symmetry of the reciprocal pattern was maintained despite the heterogeneity of the interaction slopes between isolates (Figure 2). These differences could be due to possible variance between the three independent experiments or to differing aggressiveness profiles of the different Bgt isolates. With regard to variance of disease severity a reciprocal trend was detected: all isolates originated from wild emmer showed wider variance in pustule density on domesticated than on wild hosts (in tests on wild emmer wheat the variance in pustule density was very small). On the other hand, the isolates originating from domesticated wheat showed an exactly reciprocal trend in CV values (Figure 2B). The factor responsible for high CV values is the high proportion of genotypes exhibiting complete resistance, i.e., IT = 0, which contributed to the overall within-group variance. Because this complete resistance is assumed to be conferred by major genes, it could serve as an additional evidence for host adaptation favoring accumulation of major resistance genes during the long host/pathogen co-evolution process. Together with the disease severity data, this finding strongly supports the differentiation between Bgt isolates from wild and domesticated origins, respectively, which was implied by Eshed et al. (1994). Likewise, Krupinsky (1997) showed that Stagonospora isolates from wild origins had lower aggressiveness than those from domesticated ones. The results obtained in the present study are also in full accord with the findings of Frenkel et al. (2008) in the Didymella rabiei/chickpea pathosystem. Didymella rabiei isolates from domesticated origin were significantly more aggressive on domesticated chickpea, than isolates from wild origin. In contrast but similarly to what we have found in our study on the wild host, C. judaicum, isolates from wild origin were generally more aggressive than isolates from domesticated origin. However, host specificity is not always detected. A recent investigation of a similar legume pathosystem of Peyronellaea pinodes of Pisum sp. suggested that Israel might be inhabited with a single metapopulational of the pathogen with no clear differentiation of wild and cultivated host (Golani et al., 2016).
The wild emmer Bgt isolates attacked the hexaploid wheat hardly at all, and attacked the domesticated tetraploid wheat only to an intermediate extent. The durum wheat Bgt attacked the full range of wheat species tested, but the wild tetraploid wheat to a somewhat lesser extent. The bread wheat Bgt was highly virulent on the hexaploid wheat, moderately virulent on domesticated tetraploid wheat, and was much less virulent on wild emmer. This quantitative reciprocal pattern provides solid support for the interpretations of Eshed and Wahl (1970) and Wyand and Brown (2003) regarding formae speciales in Bgt and in a more directed manner for the recently published evidence for bgt specialization (Ben-David et al., 2016; Menardo et al., 2016).
We have also examined the association between geographic distance and virulence. The Bgt capability to produce huge numbers of spores that are wind-borne from one susceptible host to another, is countered by the fact that long-distance dissemination of Bgt is limited to hundreds of kilometers on a multiseasonal time-scale and might involve a pronounced role of human-dependent dispersal of cleistothecia, (e.g., Bgt USA population; Brown and Hovmøller, 2002; Parks et al., 2009). The geographical distribution of the cultivated wheat varieties tested is much wider than that of the wild emmer samples tested. In addition, the wild emmer samples are derived from two distinct areas, which leads to a binomial distribution rather than a geographically continuous distribution of distances (Figure 3). Nevertheless, the results show that bread wheat cultivars exhibit high or complete resistance to Bgt derived from wild wheat populations. The higher portion of complete resistance of the Israeli wild emmer compared with the Turkish subgroup might imply that local adaptation of the host took place in the Israeli natural habitats. The high proportion of the wild accessions that exhibited complete resistance (disease severity = 0) when inoculated with Bgt from cultivated wheat might imply the involvement of R-genes in this local adaptation process. This trend was less evident when inoculation was done with Bgt from wild wheat populations which might imply wild host specialization (Figure 3). A recently developed demographic–genetic simulation model assumes co-existence of crop specialists and wild host specialists within a pathogen population. Based on the model this co-existence can occur under various scenarios of pathogen demography and pathogen dispersal (Papaïx et al., 2015). Our recent finding regarding Bgt differentiation based on host origin (see also Ben-David et al., 2016) may provide strong empirical support to such agro-ecological landscape models.
Conclusions
The reciprocal pattern, which was expressed throughout the interactions of wild compared with domesticated host species tested with eight Bgt isolates from various origins, probably results from long-term plant/pathogen co-evolution. Indeed, recent molecular analyses of widely diverse Bgt isolates obtained from sympatric wild and domesticated hosts have shed light upon ongoing differentiation (Ben-David et al., 2016; Menardo et al., 2016). Consistently with previous findings (Eshed et al., 1994) the present results indicate that in its center of origin, the wheat forma specialis of B. graminis might be in the process of diverging into two populations of mildew, one of emmer and one of domesticated wheat.
Author Contributions
RB-D, AD, ZP, and TF: Designed the experiments; RB-D, AD and ZP: Conducted the experiment; RB-D, AD, and ZP: Analyzed data and wrote the paper; All authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We dedicate this work to the late Dr. N. Eshed who contributed greatly to research on wheat powdery mildew in Israel. The authors thank A. Fahoum and Y. Gadri for skillful assistance in the experiments and to M. Gurevitch, A. Fadida-Myers, and K. Chandrasekhar for proof reading of the text. We thank the National Small Grains Collection (NSGC) of the USA, the Institute of Plant Genetics and Crop Plant Research (IPK) Genebank, Gatersleben, Germany, and Prof. F. Salamini, of the Max-Plank Institute, Köln, Germany, for providing some of the germplasm used in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00075/full#supplementary-material
Figure S1. Schematic chart illustrating the evolutionary relations between wheat species included in this study.
Figure S2. A graphical representation of the experimental plan.
Figure S3. Geographic distribution of wheat collection. Geographic distribution map of wheat lines collection sites across the Mediterranean and west Asia. Sites from which accessions originated are marked by black points. The pink circle represents Israel, the most intensively sampled region (Map generated by Map Send).
Figure S4. Distribution of qualitative disease reaction to Bgt from domesticated wheat. The distribution of phenotypic reactions of entries of wild and domesticated wheat lines [Resistant (R), Moderate (M), Susceptible (S)], to Bgt isolates collected from domesticated wheat. *, **, or *** indicate χ2 significance level P ≤ 0.05, 0.01, or 0.001, respectively.
Figure S5. Distribution of qualitative disease reaction to Bgt from wild wheat. The distribution of phenotypic reactions of entries of wild and domesticated wheat lines [Resistant (R), Moderate (M), Susceptible (S)] to Bgt isolates collected from wild wheat. *, **, and *** indicate χ2 significance level of P ≤ 0.05, 0.01, or 0.001, respectively.
Figure S6. Comparison of disease responses of wild and domesticated host's species. Comparison of means of disease severity (Transformed no. of mildew pustules/cm2) between wheat species inoculated with single Bgt isolates. For all isolates Tukey's LSD test was applied to the results. No result for Bgt#66 on bread wheat is presented because there were no symptoms of powdery mildew. (A) Inoculation with Bgt#15 and Bgt#66; (B) Inoculation with Bgt#70 and Bgt#58; (C) Inoculation with Bgt#97 and Bgt#63.
Table S1. Wheat accession name, Triticum species, country of origin and site of collection.
Table S2. The reactions of Pm differential wheat lines to a set of seven Bgt isolates originating from various wheat species.
Table S3. Phenotypic characterization of the two parental lines Langdon and G18-16 for powdery mildew resistance to 47 Bgt isolates.
Supplementary File. Statistical analyses.
References
Bawden, F. C. (1957). “The role of plant hosts in microbial ecology,” in Microbial Ecology, eds R. E. O. Williams and C. C. Spicer (London, UK: Cambridge University Press), 299–314.
Ben-David, R., Parks, R., Dinoor, A., Kosman, E., Wicker, T., Keller, B., et al. (2016). Differentiation among Blumeria graminis f. sp. tritici isolates originating from wild vs. domesticated Triticum species in Israel. Phytopathology 106, 861–870. doi: 10.1094/PHYTO-07-15-0177-R
Ben-David, R., Peleg, Z., Dinoor, A., Saranga, Y., Korol, A. B., and Fahima, T. (2014). Genetic dissection of quantitative powdery mildew resistance loci in tetraploid wheat. Mol. Breed. 34, 1647–1658. doi: 10.1007/s11032-014-0178-0
Ben-David, R., Xie, W., Peleg, Z., Saranga, Y., Dinoor, A., and Fahima, T. (2010). Identification and mapping of powdery mildew resistance gene PmG16, derived from wild emmer wheat, Triticum dicoccoides. Theor. Appl. Genet. 121, 499–510. doi: 10.1007/s00122-010-1326-5
Brown, J. K., and Hovmøller, M. S. (2002). Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297, 537–541. doi: 10.1126/science.1072678
Dinoor, A. (1974). Role of wild and cultivated plants in the epidemiology of plant diseases in Israel. Annu. Rev. Phytopathol. 12, 413–436. doi: 10.1146/annurev.py.12.090174.002213
Eshed, N., Dinoor, A., and Litwin, Y. (1994). The physiological specialization of wheat powdery mildew in Israel and the search for mildew resistance in wild wheat Triticum dicoccoides. Phytoparasitica 22:75.
Eshed, N., and Wahl, I. (1970). Host ranges and interrelations of Erysiphe graminis hordei, Erysiphe graminis tritici, and Erysiphe graminis avenae. Phytopathology 60, 628–634. doi: 10.1094/Phyto-60-628
Feldman, M. (2001). “The origin of cultivated wheat,” in The Wheat Book: A History of Wheat Breeding, eds A. P. Benjean and J. Angus (Paris: Lavoisier Publishing), 3–56.
Frenkel, O., Sherman, A., Abbo, S., and Shtienberg, D. (2008). Different ecological affinities and aggressiveness patterns among Didymella rabiei isolates from sympatric domesticated chickpea and wild Cicer judaicum. Phytopathology 98, 600–608. doi: 10.1094/PHYTO-98-5-0600
Golani, M., Abbo, S., Sherman, A., Frenkel, O., and Shtienberg, D. (2016). The temperature response and aggressiveness of Peyronellaea pinodes isolates originating from wild and domesticated Pisum sp. in Israel. Phytopathology 106, 824–832. doi: 10.1094/PHYTO-11-15-0306-R
Harlan, J. R., and Zohary, D. (1966). Distribution of wild wheat and barley. Science 153, 1074–1080. doi: 10.1126/science.153.3740.1074
Hsam, S. L. K., and Zeller, F. J. (2002). “Breeding for powdery mildew resistance in common wheat (Triticum aestivum L.),” in The Powdery Mildews, A Comprehensive Treatise, eds R. R. Belanger, W. R. Bushnell, A. J. Dik, and Carver (St. Paul, MN: APS PRESS), 219–238.
Johnson, J. W., Bäenziger, P. S., Yamazaki, W. T., and Smith, R. T. (1979). Effects of powdery mildew on yield and quality of isogenic lines of ‘Chancellor’ wheat. Crop Sci. 19, 349–352. doi: 10.2135/cropsci1979.0011183X001900030018x
Kimber, G., and Feldman, M. (1987). Wild Wheat: An Introduction. Special Report 353, College of Agriculture, University of Missouri, Columbia, MO.
Krupinsky, J. M. (1997). Aggressiveness of Stagonospora nodorum isolates obtained from wheat in the northern Great Plains. Plant Dis. 81, 1027–1031. doi: 10.1094/PDIS.1997.81.9.1027
Mains, E., and Dietz, S. (1930). Physiologic forms of barley mildew, Erysiphe graminis hordei Marchal. Phytopathology 20, 229–239.
McDonald, B. A., and Linde, C. (2002). Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 40, 349–379. doi: 10.1146/annurev.phyto.40.120501.101443
Menardo, F., Praz, C. R., Wyder, S., Ben-David, R., Bourras, S., Matsumae, H., et al. (2016). Hybridization of powdery mildew strains gives rise to pathogens on novel agricultural crop species. Nat. Genet. 48, 201–205. doi: 10.1038/ng.3485
Papaïx, J., Burdon, J. J., Zhan, J., and Thrall, P. H. (2015). Crop pathogen emergence and evolution in agro-ecological landscapes. Evol. Appl. 8, 385–402. doi: 10.1111/eva.12251
Parks, R., Carbone, I., Murphy, J. P., and Cowger, C. (2009). Population genetic analysis of an eastern U.S. wheat powdery mildew population reveals geographic subdivision and recent common ancestry with U.K. and Israeli populations. Phytopathology 99, 840–849. doi: 10.1094/PHYTO-99-7-0840
Tack, A. J., Thrall, P. H., Barrett, L. G., Burdon, J. J., and Laine, A. L. (2012). Variation in infectivity and aggressiveness in space and time in wild host–pathogen systems: causes and consequences. J. Evol. Biol. 25, 1918–1936. doi: 10.1111/j.1420-9101.2012.02588.x
Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244. doi: 10.1080/01621459.1963.10500845
Keywords: wheat domestication, Blumeria graminis tritici (Bgt), resistance, wild emmer wheat, powdery mildew
Citation: Ben-David R, Dinoor A, Peleg Z and Fahima T (2018) Reciprocal Hosts' Responses to Powdery Mildew Isolates Originating from Domesticated Wheats and Their Wild Progenitor. Front. Plant Sci. 9:75. doi: 10.3389/fpls.2018.00075
Received: 03 October 2017; Accepted: 15 January 2018;
Published: 23 February 2018.
Edited by:
Roberto Papa, Università Politecnica delle Marche, ItalyReviewed by:
Marco Maccaferri, University of Bologna, ItalyMariagiovanna Fragasso, Centro di Ricerca per la Cerealicoltura e le Colture Industriali, Consiglio per la Ricerca in Agricoltura e l'analisi dell'Economia Agraria (CREA-CI), Italy
Copyright © 2018 Ben-David, Dinoor, Peleg and Fahima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roi Ben-David, cm9pYkB2b2xjYW5pLmFncmkuZ292Lmls
 Roi Ben-David
Roi Ben-David Amos Dinoor
Amos Dinoor Zvi Peleg
Zvi Peleg Tzion Fahima
Tzion Fahima