
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 15 January 2018
Sec. Plant Physiology
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.02237
 Hongwei Xu1,2
Hongwei Xu1,2 Tanya Y. Curtis2
Tanya Y. Curtis2 Stephen J. Powers3
Stephen J. Powers3 Sarah Raffan2
Sarah Raffan2 Runhong Gao1,2
Runhong Gao1,2 Jianhua Huang1*
Jianhua Huang1* Monika Heiner4
Monika Heiner4 David R. Gilbert5
David R. Gilbert5 Nigel G. Halford2*
Nigel G. Halford2*Asparagine synthetase activity in cereals has become an important issue with the discovery that free asparagine concentration determines the potential for formation of acrylamide, a probably carcinogenic processing contaminant, in baked cereal products. Asparagine synthetase catalyses the ATP-dependent transfer of the amino group of glutamine to a molecule of aspartate to generate glutamate and asparagine. Here, asparagine synthetase-encoding polymerase chain reaction (PCR) products were amplified from wheat (Triticum aestivum) cv. Spark cDNA. The encoded proteins were assigned the names TaASN1, TaASN2, and TaASN3 on the basis of comparisons with other wheat and cereal asparagine synthetases. Although very similar to each other they differed slightly in size, with molecular masses of 65.49, 65.06, and 66.24 kDa, respectively. Chromosomal positions and scaffold references were established for TaASN1, TaASN2, and TaASN3, and a fourth, more recently identified gene, TaASN4. TaASN1, TaASN2, and TaASN4 were all found to be single copy genes, located on chromosomes 5, 3, and 4, respectively, of each genome (A, B, and D), although variety Chinese Spring lacked a TaASN2 gene in the B genome. Two copies of TaASN3 were found on chromosome 1 of each genome, and these were given the names TaASN3.1 and TaASN3.2. The TaASN1, TaASN2, and TaASN3 PCR products were heterologously expressed in Escherichia coli (TaASN4 was not investigated in this part of the study). Western blot analysis identified two monoclonal antibodies that recognized the three proteins, but did not distinguish between them, despite being raised to epitopes SKKPRMIEVAAP and GGSNKPGVMNTV in the variable C-terminal regions of the proteins. The heterologously expressed TaASN1 and TaASN2 proteins were found to be active asparagine synthetases, producing asparagine and glutamate from glutamine and aspartate. The asparagine synthetase reaction was modeled using SNOOPY® software and information from the BRENDA database to generate differential equations to describe the reaction stages, based on mass action kinetics. Experimental data from the reactions catalyzed by TaASN1 and TaASN2 were entered into the model using Copasi, enabling values to be determined for kinetic parameters. Both the reaction data and the modeling showed that the enzymes continued to produce glutamate even when the synthesis of asparagine had ceased due to a lack of aspartate.
Asparagine is an important nitrogen storage and transport molecule in many plant species due to its relatively high nitrogen to carbon ratio (2:4, compared with 2:5 for glutamine, 1:5 for glutamic acid and 1:4 for aspartic acid, for example) and its relative chemical inertia (Lea et al., 2007). It accumulates in its free (non-protein) form in response to a range of abiotic and biotic stresses, as well as during normal physiological processes such as seed germination (Lea et al., 2007). In wheat grain it accumulates to very high levels in response to sulfur deficiency (Muttucumaru et al., 2006; Granvogl et al., 2007; Curtis et al., 2009, 2018) and poor disease control (Curtis et al., 2016). There are also large differences in the free asparagine concentration of grain from different wheat varieties (Curtis et al., 2018). Understanding the mechanisms that control free asparagine accumulation is important for improving crop yield and stress resistance. However, more pressingly, it also has implications for food safety because free asparagine is a precursor for acrylamide formation (reviewed by Halford et al., 2012; Curtis et al., 2014).
Acrylamide is a processing contaminant that forms during high-temperature cooking and processing, particularly as a result of frying, roasting, and baking. It is classed as a probable (Group 2a) carcinogen by the International Agency for Research on Cancer (1994) and has reproductive and neurotoxicological effects at high doses (Friedman, 2003). The European Food Safety Authority (EFSA) Expert Panel on Contaminants in the Food Chain (CONTAM) stated in its 2015 report that the margins of exposure for acrylamide indicate a concern for neoplastic effects (EFSA Panel on Contaminants in the Food Chain [Contam], 2015). The European Commission issued “Indicative Values” for the presence of acrylamide in food in 2011, based on results reported to EFSA (European Food Safety Authority, 2011), and reduced them for many product types in 2013 (European Commission, 2013). If a product is found to exceed the Indicative Value, the relevant food safety authority should take action to ensure that the manufacturer addresses the problem. Furthermore, the European Commission has just approved strengthened risk management measures including compulsory Codes of Practice and the renaming of Indicative Values as Benchmark Levels, with reduced Benchmark Levels for many products (European Commission, 2017). The proposals also include a specific reference to the setting of mandatory Maximum Levels for acrylamide in certain foods, stating that this should be considered following the adoption of the new regulations. The proposals will come before the European Parliament and European Council in 2017 and could be in force by early 2018.
The predominant route for the formation of acrylamide is via a Strecker-type degradation of free asparagine by highly reactive carbonyl compounds produced within the Maillard reaction (Mottram et al., 2002; Stadler et al., 2002; Zyzak et al., 2003), and free asparagine concentration is the main determinant of acrylamide-forming potential in cereal grains (Muttucumaru et al., 2006; Granvogl et al., 2007; Curtis et al., 2009, 2010; Postles et al., 2013). This has re-invigorated interest in the enzymes involved in asparagine synthesis and breakdown, and other metabolic pathways that could impact on free asparagine concentrations.
Asparagine synthesis is catalyzed by the enzyme asparagine synthetase, and occurs by the ATP-dependent transfer of the amino group of glutamine to a molecule of aspartate to generate glutamate and asparagine. Two asparagine synthetase genes were cloned from wheat (Triticum aestivum) by Wang et al. (2005) and called TaASN1 and TaASN2. TaASN1 expression in seedlings was shown to be up-regulated by treatment with abscisic acid, and by salt and osmotic stress (Wang et al., 2005). Subsequently, its expression in leaves was shown to be induced by sulfur deficiency, but to be greatly reduced when a general control non-derepressible-2-type protein kinase, TaGCN2, was over-expressed (Byrne et al., 2012). In 2016, two additional genes, TaASN3 and TaASN4, were identified (Gao et al., 2016), although TaASN4 was only discovered from wheat genome data and has not yet been cloned or characterized. The expression of TaASN1–3 was studied in different tissues and in response to nutrition (Gao et al., 2016). Notably, the expression of TaASN2 in the embryo and endosperm during mid to late grain development was shown to be the highest of any of the genes in any tissue, although TaASN1 was most responsive to sulfur supply.
Maize (Zea mays) and barley (Hordeum vulgare) also have four differentially-expressed asparagine synthetase genes (Todd et al., 2008; Avila-Ospina et al., 2015), suggesting that this is typical of the cereals. However, a full picture of the role of the different asparagine synthetases will only emerge when the kinetic parameters of the enzymes have been measured. This is problematic because asparagine synthetase activity in plant tissues is difficult to purify and measure (Joy et al., 1983; Snapp and Vance, 1986; Kudiyarova et al., 2013), probably because of the presence of asparaginase activities and natural inhibitors (Rognes, 1980). There has also been a scarcity of antibodies for immunological analysis of purified or expressed proteins. However, the enzymes encoded by three of the maize genes have been analyzed after heterologous expression in Escherichia coli and been shown to have significant differences in kinetic properties (Duff et al., 2011). The aim of this study was to characterize the wheat asparagine synthetase gene family and to compare the enzymes encoded by TaASN1 and TaASN2, the two genes that are most highly expressed in the grain.
Wheat (T. aestivum) cv. Spark seeds were surface-sterilized as described by Gao et al. (2016), and germinated in a growth room in small containers. After 7 days, seedlings were harvested, flash frozen in liquid nitrogen, and then stored at -80°C ready for use.
RNA was extracted using the hot phenol method (Verwoerd et al., 1989), with some modification, as described previously (Postles et al., 2016). It was used as a template for first-strand cDNA synthesis using SuperScript III® first-strand synthesis supermix (Invitrogen, supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). The full-length coding sequences of TaASN1, TaASN2, and TaASN3 were then amplified by polymerase chain reaction (PCR). “Forward” and “reverse” primers for TaASN1 were 5′-ccggaattcATGTGCGGCATACTGGC and 5′-ccgctcgagAACTCTCAATTGCGACACCAG (lower case letters denote additional nucleotides that were added to incorporate EcoRI and XhoI restriction sites at either end of the PCR product). “Forward” and “reverse” primers for TaASN2 were 5′-ccggaattcATGTGCGGCATACTAGCGGTG and 5′-ccgctcgagAAGTCTCAATGGCAAC, while for TaASN3 they were 5′-ccggaattcATGTGCGGCATCCTCGC and 5′-ataagaatgcggccgcAAACAGCAGCTGCTGGAACA. The additional nucleotides on the “reverse” primer for TaASN3 incorporated a NotI restriction site.
All products were amplified using Phusion® High-Fidelity DNA polymerase (New England Biolabs, Hitchin, United Kingdom). The cycling conditions were: 30 s denaturation at 98°C, followed by 35 cycles of 10 s at 98°C, 30 s at 63°C, and 30 s at 72°C, with a final extension period at 72°C for 10 min. The resulting PCR products were purified using the Wizard PCR Clean-up system (Promega, Southampton, United Kingdom) and ligated into the pGEM-T Easy Vector (Promega, Southampton, United Kingdom) using the restriction sites incorporated during the PCR. Nucleotide sequence analysis was performed by MWG Biotech (Wolverhampton, United Kingdom) and contigs were assembled using ContigExpress or Geneious Version 81 (Kearse et al., 2012). Amino acid sequence alignments were also performed using Geneious Version 8.
DeCypher Tera-BLASTN Search Nucleic Query vs. Nucleic Database was used to assess the wheat asparagine synthetase gene sequences. The NR_Gene_v0.4 scaffold wheat genome was used as the database of choice (PLANT_T.aestivum_NRgene_v0.4_scaf), and the cDNA nucleotide sequences for TaASN1 (GenBank BT009245), TaASN2 (GenBank BT009049), and TaASN3 (GenBank AK333183) were used as the query sequences.
The returned scaffolds were downloaded and aligned to the cDNAs using the Geneious Version 8 software package (pairwise alignment was run using the Geneious Alignment algorithm on its default settings; multiple alignments were run using the Consensus Align algorithm, again on its default settings). The aligned consensus sequences were then used to search the T. aestivum TGACv1 (Genomic sequence) database2 to assess chromosomal positioning. The returned genes from the TGAC database were then re-aligned to the original cDNA sequences to confirm gene identity.
TaASN4 was identified through its divergence from the other wheat asparagine synthetase sequences. The TGAC sequence was confirmed through re-alignments to both the TGAC and NR-Gene databases. BLAST searches using the PLANT_T.aestivum_nt_w7984 database were used to further confirm gene identity.
The PCR products were excised from the PGEM®-T vector and ligated into the specialist expression vector, pET-30a (Novagen, United Kingdom) to produce plasmids pET-30a–TaASN1, pET-30a–TaASN2, and pET-30a–TaASN3. These were maintained in E. coli NovaBlue cells (Novagen, United Kingdom), which carry recA and endA mutations, and transferred to RosettaBlueTM cells (Novagen, United Kingdom) for high levels of expression of the ASN1–3 proteins. Single colonies of the cells carrying the plasmids were inoculated into medium containing 15 μg/mL kanamycin and 34 μg/mL chloramphenicol. The bacteria were grown at 37°C with shaking until they had reached mid-log phase (OD 600 between 0.6 and 1.0). The culture was then split between two flasks, and isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to one of the flasks to a final concentration of 1 mM in order to induce expression of the asparagine synthetase gene carried by the plasmid. The other flask acted as an “un-induced” control. The bacteria were incubated with shaking at 27°C for a further 3 h, then harvested by centrifugation and stored at -80°C until further use.
The use of the pET30a plasmid meant that the asparagine synthetase proteins were synthesized with a six-residue histidine N-terminal tag, and could therefore be extracted and purified using the nickel-nitrilotriacetic acid (Ni-NTA) purification system (Invitrogen, supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). Bacterial cells were pelleted and lysed. Proteins in inclusion bodies were solubilized using NuPAGE® LDS-sample buffer and NuPAGE® Sample Reducing Agent and the proteins were separated on 4–12% Bis-Tris gels (Invitrogen, supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). Protein concentration was assayed using a Bicinchoninic Acid Kit (Sigma–Aldrich, Gillingham, United Kingdom).
Monoclonal antibodies were produced by Abmart (Shanghai, China). Peptides were synthesized corresponding to probable epitopes in the C-terminal region of the TaASN1, TaASN2, and TaASN3 proteins where the amino acid sequences show less similarity with each other. Antibodies were raised to four different peptides for each of the three proteins. For western analysis, soluble proteins were separated by electrophoresis on NuPAGE® Novex® 4–12% Bis-Tris gels and transferred to polyvinylidene fluoride membranes (13 cm × 8 cm) using the iBlot® Gel Transfer Device (Invitrogen, supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). Immunodetection was performed with the antibody in a 1:1000 dilution for 2 h at room temperature, after which the membrane was incubated for 1 h at room temperature with 1:15,000 horseradish peroxidase-conjugated goat anti-mouse IgG (Invitrogen, supplied by Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). Bands representing proteins that had reacted with the anti-asparagine synthetase antibody were visualized with ECLTM Western Blotting Detection Reagents (GE Healthcare, Amersham, United Kingdom), and signals were quantified by scanning densitometry using Quantity One software (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom).
Purified asparagine synthetase proteins, TaASN1 and TaASN2, were added to an assay buffer of 100 mM HEPES (pH 7.6), 1.6 mM aspartate, 10 mM glutamine, 10 mM ATP, 10 mM MgCl2, and 1 mM DTT and incubated at 30°C. Aliquots (100 μL) were removed after 1.5, 2.5, 3.5, 5, 15, 25, and 35 min, placed in a 96-well filter plate and mixed with 100 μL of 10% trichloroacetic acid to stop the reaction (Todd et al., 2008; Duff et al., 2011; Kudiyarova et al., 2013). Two replicate assays were done.
The free asparagine and glutamate produced in the reaction were detected after derivatization with o-phthalaldehyde reagent (Sigma–Aldrich, Gillingham, United Kingdom). The fluorescent derivative was measured by high performance liquid chromatography (HPLC) using a Waters Alliance 2795 HPLC system fitted with a Waters 474 Scanning Fluorescence Detector (Waters, Elstree, United Kingdom). A Symmetry C18, 4.6 mm × 150 mm column (for particle size 3.5–5 μm) (Waters, Elstree, United Kingdom) was used, and the fluorescence detector was set with an excitation wavelength of 340 nm and emission wavelength of 455 nm. For calibration, standards were used to provide areas under the HPLC peaks corresponding to asparagine and glutamate concentrations of 0, 5, 10, 15, and 20 nmol. The areas were modeled on the concentrations using linear regression, so that, by inverting the resulting linear equation, estimated concentrations of the amino acids and the asparagine synthetase enzyme could be made given HPLC areas for the sample aliquots taken at the seven sampling time points. Standards were run separately for each experiment.
A model for the reactions catalyzed by asparagine synthetases TaASN1 and TaASN2 was constructed using the SNOOPY® tool3 (Heiner et al., 2012) for designing, animating, and simulating Petri Nets. The model was then exported to Copasi 4.16 (Build 104; Hoops et al., 2006). Data for the reaction parameters were taken from the Brenda enzyme database4.
Gao et al. (2016) identified three distinct asparagine synthetase gene nucleotide sequences in the GenBank database: TaASN1 (Wang et al., 2005; GenBank AY621539; BT009245), TaASN2 (GenBank BT009049), and TaASN3 (GenBank AK333183). TaASN1 and TaASN2 were already annotated as asparagine synthetases, but the TaASN3 entry had not been up to that point. Gao et al. (2016) also identified a fourth gene, TaASN4, from a BLAST search of wheat genome data5 (Wilkinson et al., 2012). TaASN4 is present in cultivated and wild rice (Oryza sativa and Oryza brachyantha), Brachypodium distachyon, Aegilops tauschii, foxtail millet (Setaria italica), and maize (Z. mays), as well as wheat (Gao et al., 2016), but to date has not been cloned from or characterized in wheat.
Gao et al. (2016) described the differential expression of TaASN1, TaASN2, and TaASN3 in different wheat tissues and in response to nitrogen and sulfur feeding, with expression of TaASN2 in the embryo and to a lesser extent the endosperm of the grain during mid-development being far higher than the expression of any of the genes in any other tissue, although TaASN1 showed most response to nutrition. This suggests that TaASN2 expression in the grain is the primary determinant of asparagine levels, either for protein synthesis or accumulation in the free form, rather than import of free asparagine from other tissues, at least under normal (nutrient-sufficient) conditions, making TaASN2 a potential target for genetic interventions to reduce free asparagine accumulation in wheat grain. However, modeling of the processes controlling the accumulation of free asparagine in order to confirm this requires information on the kinetic parameters of asparagine synthetase enzymes to add to the data on gene expression, and this was the aim of the current study.
To that end, TaASN1, TaASN2, and TaASN3 PCR products were amplified from wheat cv. Spark using primers designed from published sequences (see section “Materials and Methods”). TaASN1 from this variety was found to encode a protein of 585 amino acid residues with a molecular weight of 65.49 kDa, while TaASN2 encoded a slightly smaller protein of 581 residues, molecular weight 65.06 kDa, and TaASN3 a slightly larger protein of 591 residues, molecular weight 66.24 kDa. The nucleotide sequences have been deposited in the GenBank database and been given accession numbers KY937995, KY937996, and KY937997. Comparisons of the derived amino acid sequences of the proteins with those encoded by the nucleotide sequences already in the database confirmed that the three PCR products were derived from TaASN1, TaASN2, and TaASN3 (Table 1).
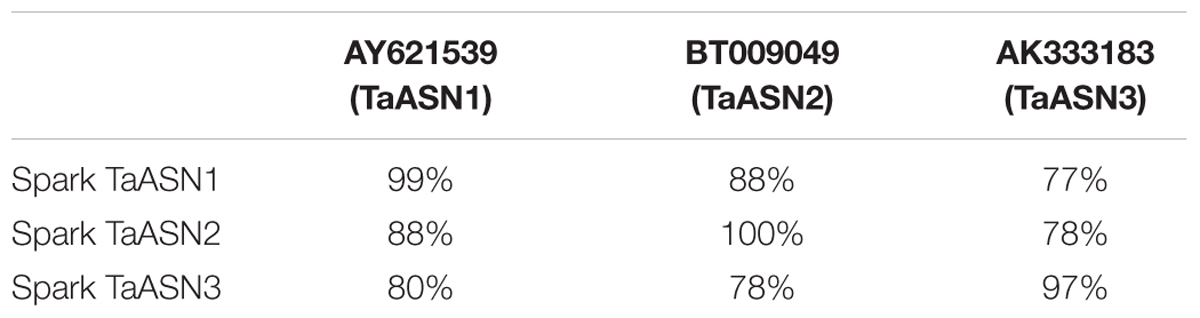
TABLE 1. Amino acid sequence identity between the asparagine synthetases encoded by the PCR products amplified from wheat cv. Spark and wheat asparagine synthetases from the GenBank database.
The amino acid sequences of the three proteins are aligned in Figure 1. All three share conserved amino acid residues typical of asparagine synthetases (highlighted in red in Figure 1), including the essential residues of a purF-type glutamine-binding site, Cys2, Asp34, and His104 (Mei and Zalkin, 1989), and other residues important for glutamine binding and positioning that have been identified from the E. coli AsnB enzyme (Arg50, Leu51, Ile53, Asn75, Gly76, Glu77, and Asp98; Larsen et al., 1999). Residues Thr316, Thr317, Arg319, and Cys523 are involved in the binding of aspartate and ATP (Boehlein et al., 1994a,b, 1997a,b), while Leu231, Val267, Ser341, and Gly342 have been recognized as the anchoring points for the AMP moiety (Larsen et al., 1999). Interestingly, Val267 is replaced with a different hydrophobic residue, Ile, in TaASN3. Lastly, the Ser, Gly, Gly, Leu, Asp, Ser motif beginning at position 233 is conserved in all of the asparagine synthetases characterized to date and may be involved in pyrophosphate binding (Richards and Schuster, 1998).
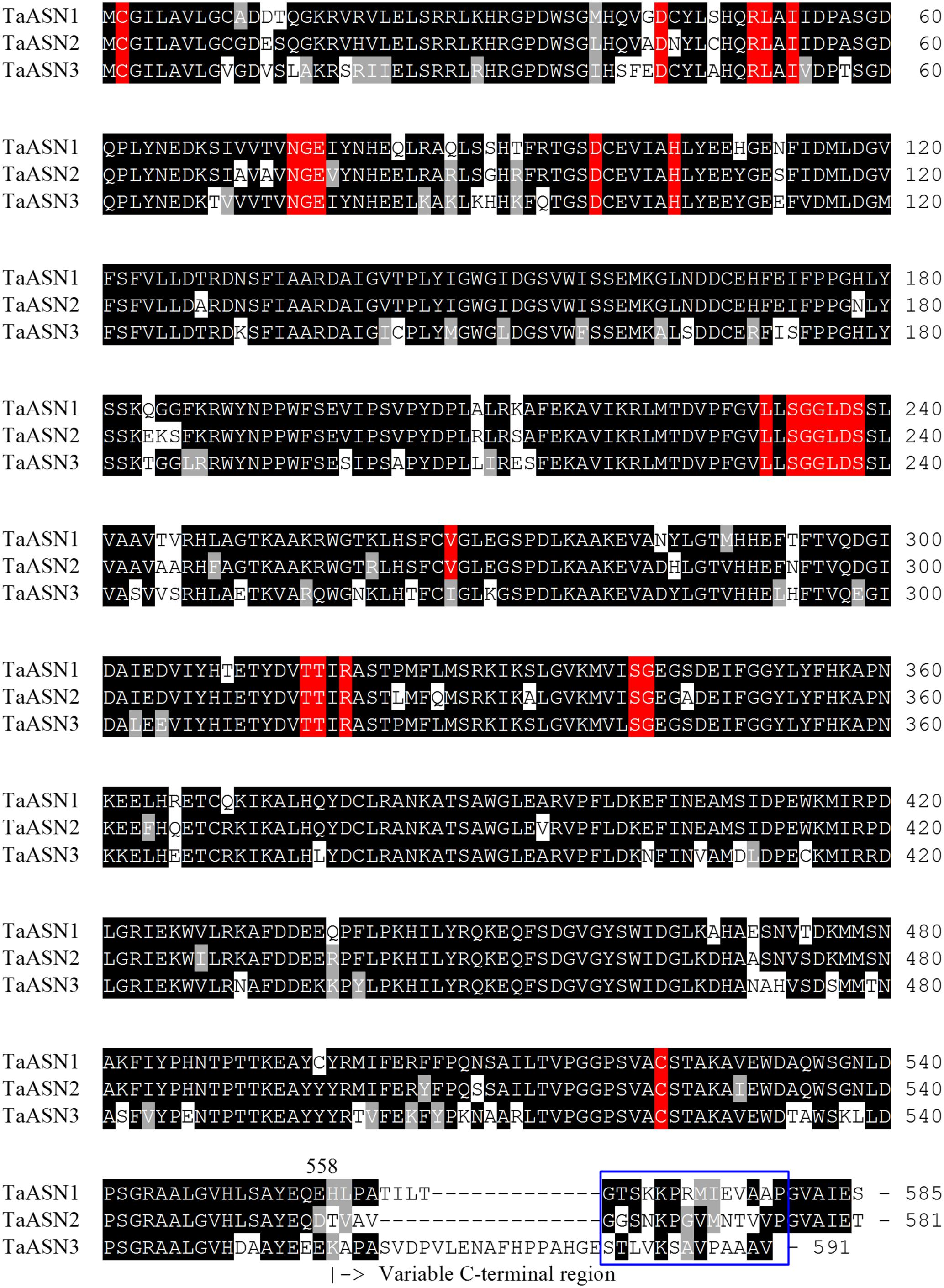
FIGURE 1. Amino acid sequence alignment of TaASN1, TaASN2, and TaASN3 proteins from wheat (Triticum aestivum) cv. Spark. Identical residues at the same position are highlighted in black, except for residues known to be critical for the function of the enzyme (see text), which are highlighted in red. Similar residues at the same position (conservative substitutions) are highlighted in gray. The region corresponding to peptides used to raise the two monoclonal antibodies that showed highest specificity for the asparagine synthetase proteins is indicated with a blue box.
BLAST searches were performed of the wheat (T. aestivum) scaffold genome in the non-redundant genome database (NR_Gene_v0.4) using the cDNA sequences for TaASN1, TaASN2, and TaASN3 (Gao et al., 2016). This search also identified scaffolds for TaASN4, which to date has not been cloned from bread wheat (Gao et al., 2016). The returned scaffolds were aligned to the cDNAs to identify exons and introns, and the results confirmed by searches of the T. aestivum TGACv1 genomic sequence. The consensus sequences derived from these alignments were then used to search the Ensembl wheat database to determine the chromosomal positioning of the genes. Note that both the NR and TGAC genome data are from variety Chinese Spring.
The chromosomal positions and scaffold references for the genes are given in Table 2. TaASN1, TaASN2, and TaASN4 were all found to be single copy genes, located on chromosomes 5, 3, and 4, respectively, of each genome (A, B, and D), except that TaASN2 was not present in the B genome. Analysis of unpublished wheat genome data (A. Huttly, Rothamsted Research, personal communication) suggests that not all wheat varieties lack a TaASN2 gene on chromosome 3B, but the relative prevalence of the presence or absence of a B genome TaASN2 gene cannot yet be assessed. In the case of TaASN3, there were two copies on chromosome 1 of each genome, and these were given the names TaASN3.1 and TaASN3.2.
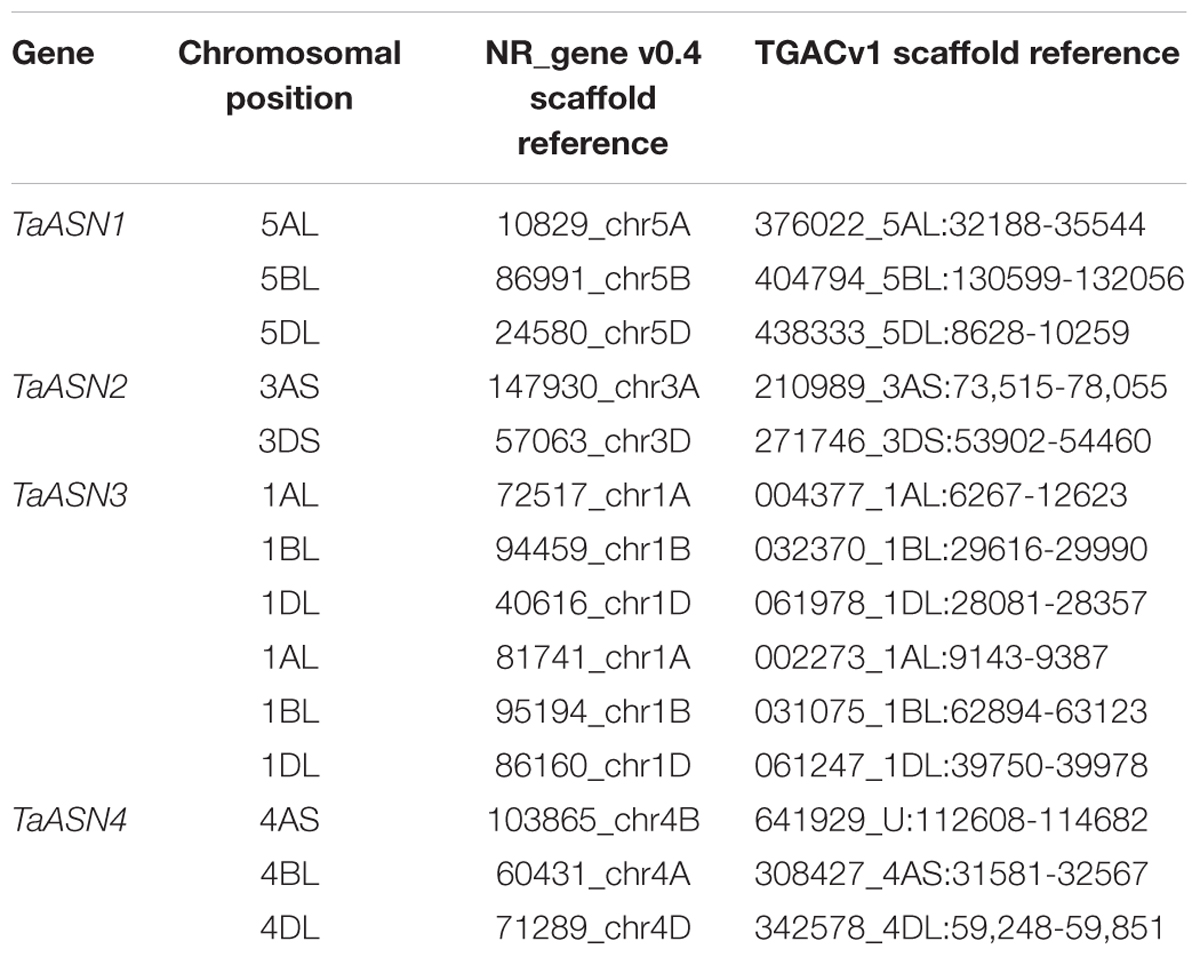
TABLE 2. Chromosomal position and scaffold references for wheat (Triticum aestivum) cv. Chinese Spring asparagine synthetase genes (TaASN1–4) (PLANT_T. aestivum_NRgene_v0.4_scaf).
The structures of the genes are shown in Figure 2, illustrating the considerable divergence of intron/exon patterns between the different genes, but conservation of structure within each group of homeologs. The three TaASN1 homeologs, on chromosomes 5A, B, and D, are the shortest at approximately 3 kb from the ATG translation start codon to the translation stop codon, including 12 exons. The two TaASN2 homeologs are approximately 4 kb in length, with 11 exons, and the three TaASN3.1 and TaASN3.2 homeologs just over and just under 6 kb, respectively, making them the longest group. The two TaASN3 genes share a similar intron/exon pattern, with 15 exons. The three TaASN4 homeologs are just under 4 kb in length, with 12 exons, except that the TaASN4 gene on chromosome 4B lacks exon 8. Clearly, this deletion may affect the activity of the enzyme encoded by the gene.
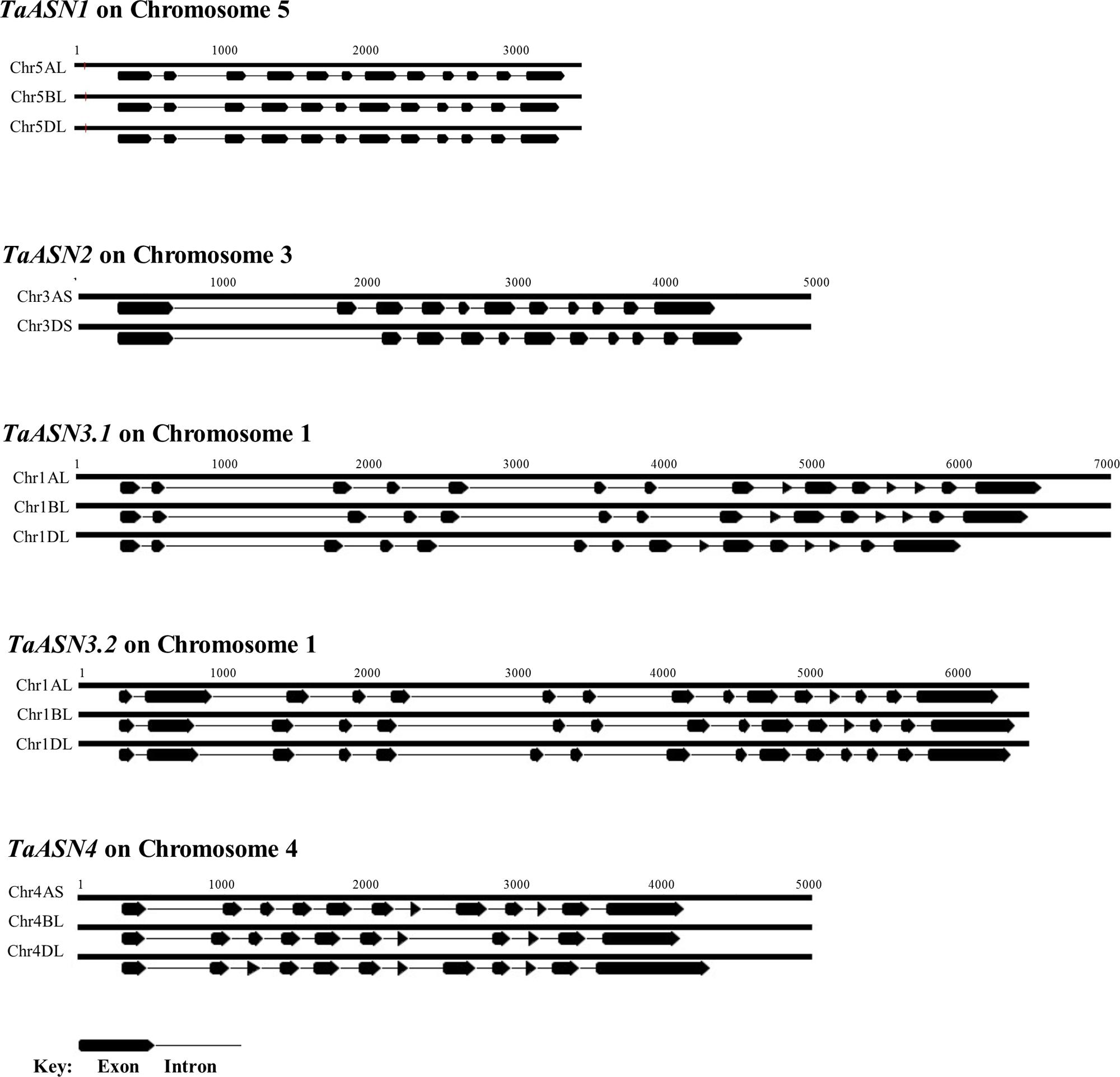
FIGURE 2. Diagrammatic representation of the gene structures of TaASN1, TaASN2, TaASN3.1, TaASN3.2, and TaASN4.
The TaASN1, TaASN2, and TaASN3 PCR products were sub-cloned into vector pET-30a and the resulting plasmids transformed into E. coli Rosetta DE3 cells to enable expression of the asparagine synthetase proteins. Use of this system meant that the proteins were synthesized with a six-residue histidine “tag,” enabling them to be purified on a Ni-NTA agarose column. Expression of the proteins was induced by addition of IPTG to the cell culture medium.
The result of SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of the expressed TaASN1, TaASN2, and TaASN3 proteins in both crude E. coli lysates and after purification on the column is shown in Figure 3. The proteins were solubilized by addition of NuPAGE® LDS-sample buffer, which contains lithium dodecyl sulfate at a pH of 8.4, and NuPAGE® Sample Reducing Agent, which contains dithiothreitol. An un-induced control is also shown (Figure 3) for each protein. The result showed the TaASN proteins to be highly expressed and readily purified.
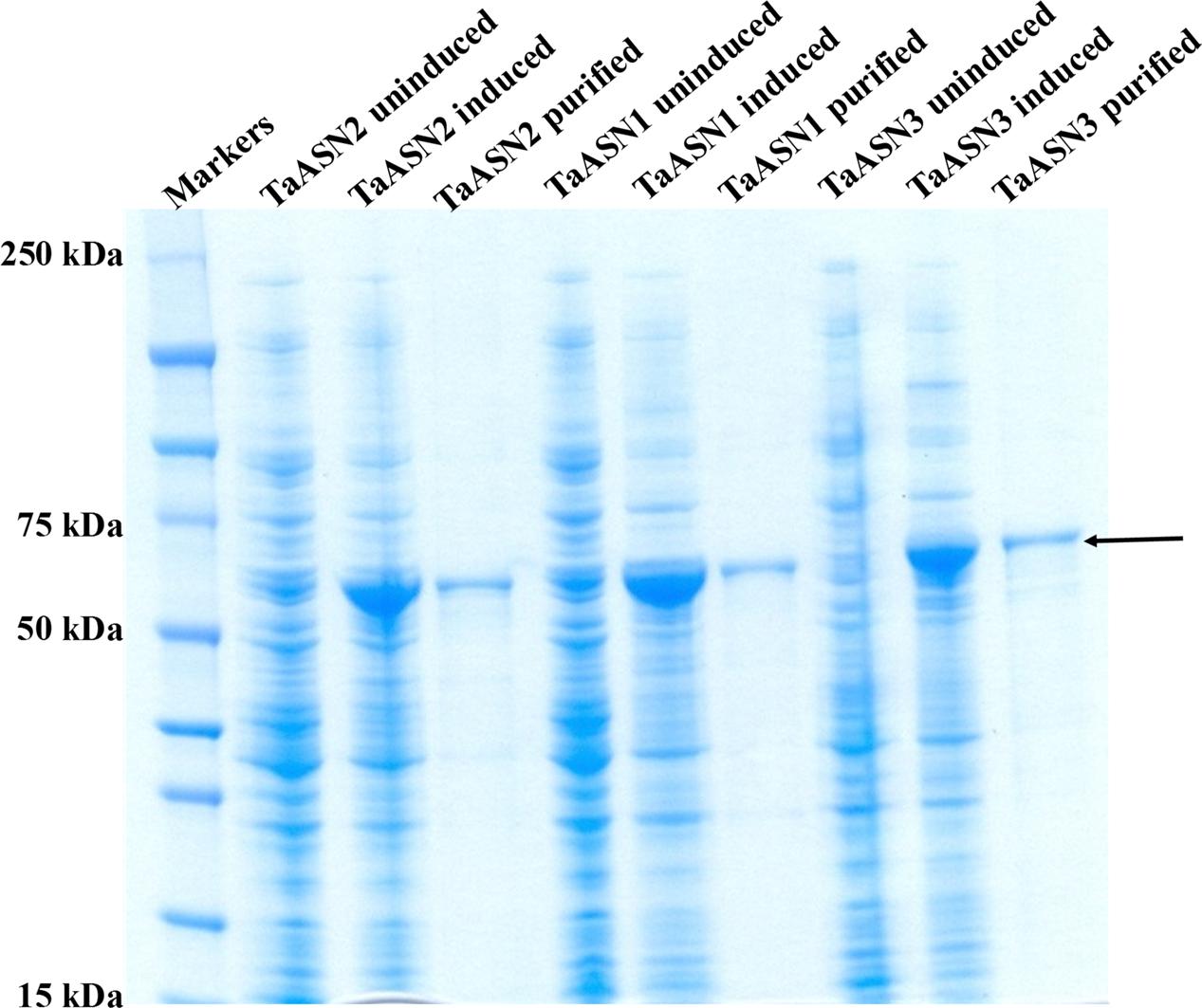
FIGURE 3. SDS-polyacrylamide gel electrophoresis of extracts of E. coli cells expressing wheat asparagine synthetases: TaASN1, TaASN2, and TaASN3. Expression of the proteins was induced by addition of IPTG to the bacterial cell cultures. An uninduced control is included for each protein, and each protein is also shown after purification on a nickel-nitrilotriacetic acid (Ni-NTA) agarose column. The arrow indicates the position of the expressed proteins.
A panel of monoclonal antibodies was raised to peptides (Table 3) corresponding to epitopes within the variable C-terminal regions of the proteins (Figure 1) with the aim of identifying antibodies that showed high specificity for the TaASN proteins and could distinguish between them. A western blot of the heterologously expressed TaASN1 proteins reacted with antibodies raised to two of the peptides, SKKPRMIEVAAP and GGSNKPGVMNTV, is shown in Figure 4. These two antibodies showed the highest specificity for the TaASN proteins, with the least non-specific binding, although all of the antibodies reacted with the TaASN proteins (not shown). However, none of the antibodies distinguished between the three asparagine synthetases. This was surprising because of the divergence of the amino acid sequences in this region (Figure 1). The SKKPRMIEVAAP epitope is present in TaASN1, while the GGSNKPGVMNTV epitope is at almost the same position in TaASN2 (indicated with a blue box in Figure 1). In each case there are only four identical residues and two conservative substitutions between the two proteins, and the similarity with TaASN3 is even lower. Nevertheless, TaASN1, TaASN2, and TaASN3 could be distinguished on the basis of size, with TaASN2 (65.06 kDa) migrating the furthest in the SDS-PAGE, followed by TaASN1 (65.49 kDa) and TaASN3 (66.24 kDa) (Figure 4).
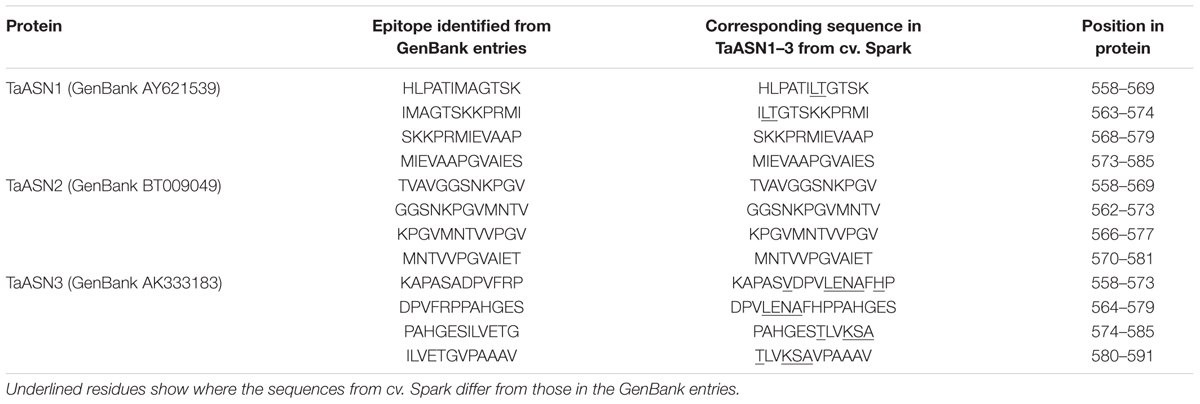
TABLE 3. Epitopes used for the production of monoclonal antibodies for asparagine synthetases TaASN1, TaASN2, and TaASN3 from wheat.
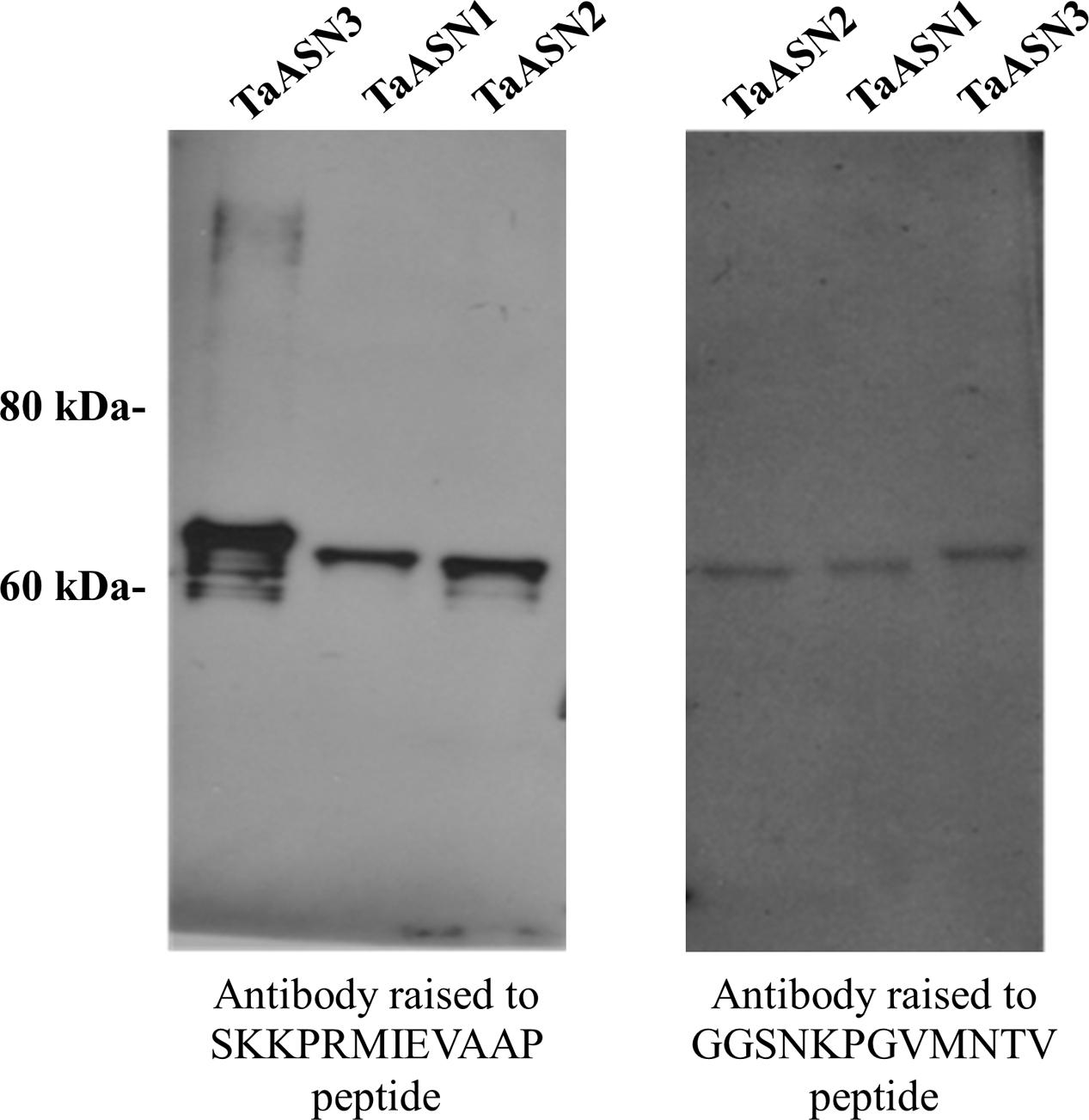
FIGURE 4. Western blot of heterologously expressed TaASN1, TaASN2, and TaASN3 proteins reacted with monoclonal antibodies raised to peptides SKKPRMIEVAAP and GGSNKPGVMNTV, as indicated.
The production of asparagine and glutamate from aspartate and glutamine by TaASN1 and TaASN2 was measured in standard assays adapted from those described by Todd et al. (2008), Duff et al. (2011), and Kudiyarova et al. (2013). The reactions were sampled at 0, 1.5, 2.5, 3.5, 5, 15, 25, and 35 min and the asparagine and glutamate produced in the reaction were detected after conversion to a fluorescent derivative using o-phthalaldehyde reagent, separation by HPLC and detection of the fluorescent derivative with a scanning fluorescence detector. Both enzymes produced asparagine and glutamate, confirming that both were asparagine synthetases. The concentrations measured for the reactions are given in Supplementary File S1 for the two replicate assays done. Data from the calibration of the HPLC areas using standard concentrations of asparagine and glutamate are also given. The results up to the 15 min time point, by which time the concentrations of both asparagine and glutamate had plateaued, are shown graphically in Figure 5.
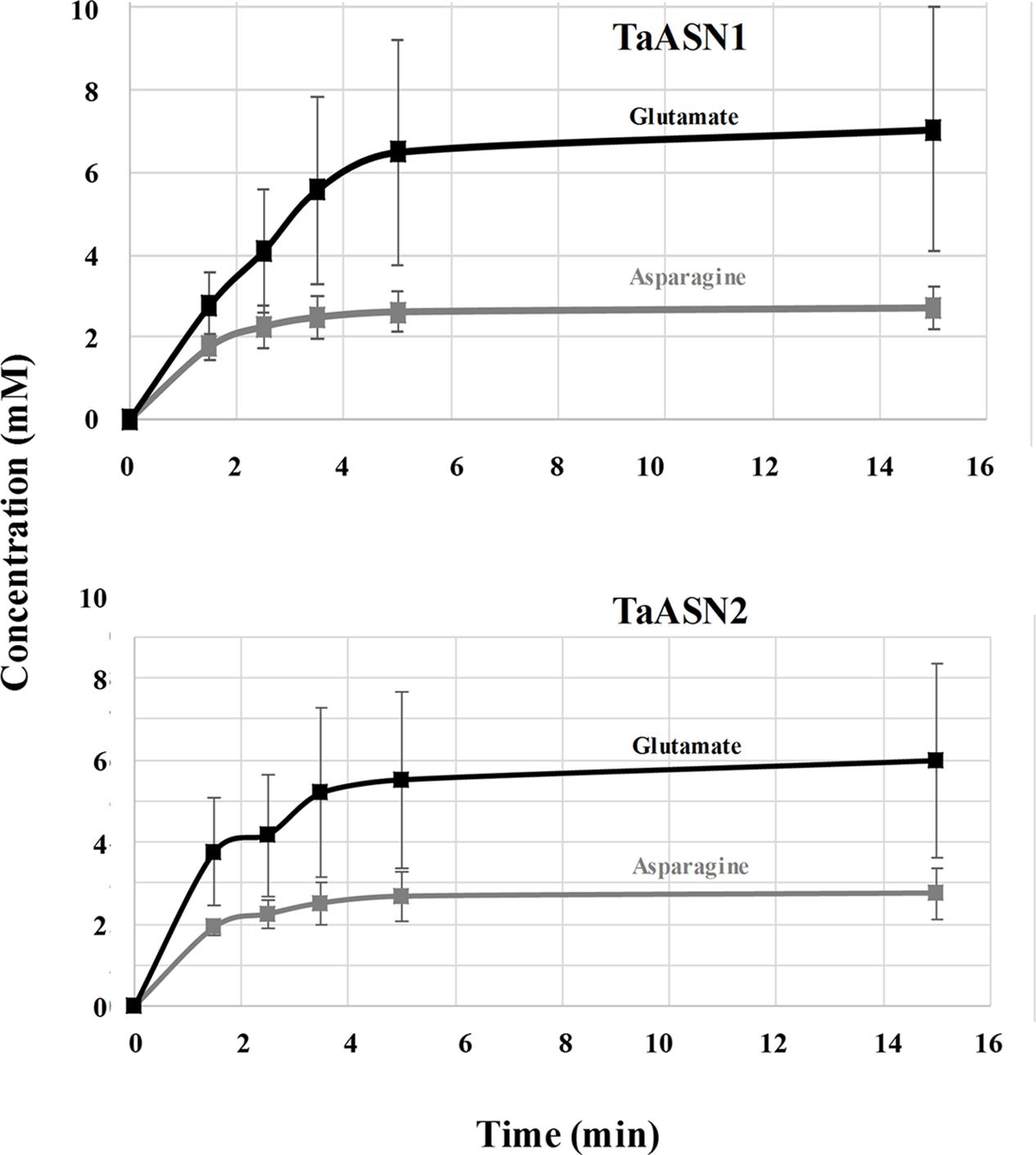
FIGURE 5. Plots (means with standard errors from two replicates) showing the synthesis of asparagine and glutamate, in reactions catalyzed by TaASN1 (top) and TaASN2 (bottom).
It was clear that the reactions catalyzed by both asparagine synthetases proceeded much more rapidly than had been reported for heterologously expressed maize or soybean enzymes (Todd et al., 2008; Duff et al., 2011). The reaction buffer contained 1.6 mM aspartate and 10 mM glutamine, meaning that glutamine was present in relative excess compared with aspartate. In both cases, the concentration of glutamate increased at a faster rate than the concentration of asparagine (Figure 5). By the 5-min time point in both reactions, the concentrations of both products were at or close to their maximum, indicating that the concentration of the reactants had become depleted. Notably, at this point, the concentration of glutamate was more than double that of asparagine, and the measured concentration of asparagine was actually slightly higher than would be expected given the starting concentration of aspartate.
The SNOOPY® tool was used to construct a continuous Petri Net model which incorporates underlying ordinary differential equations (ODEs), representing the reaction catalyzed by asparagine synthetases, TaASN1 and TaASN2. The model was based on the reaction stages proposed by Gaufichon et al. (2010), with some modifications, and the experimental data, assuming mass action kinetics. A schematic diagram of the model is given in Figure 6. It comprises metabolites (“places” in Petri Net terminology) indicated by circles, and reactions (“transitions”) indicated by squares, connected by arrows (“edges”). The concentration of metabolites is represented abstractly by numbers on places. The model comprises one compartment (cell) with eleven molecules (species): adenosine monophosphate (AMP), asparagine (Asn), asparagine synthetase enzyme (for the purpose of the modeling annotated as ASNe), asparagine synthetase enzyme complexed with glutamine (ASNe-Gln), asparagine synthetase enzyme complexed with ammonia (ASNe–NH3), aspartate (Asp), adenosine triphosphate (ATP), β-aspartyl-complex (βAsp–AMP–ASNe–NH3), glutamine (Gln), glutamate (Glu) and magnesium ions (Mg2+). The four elementary biochemical reactions involved in the formation of asparagine are represented by the following equations:
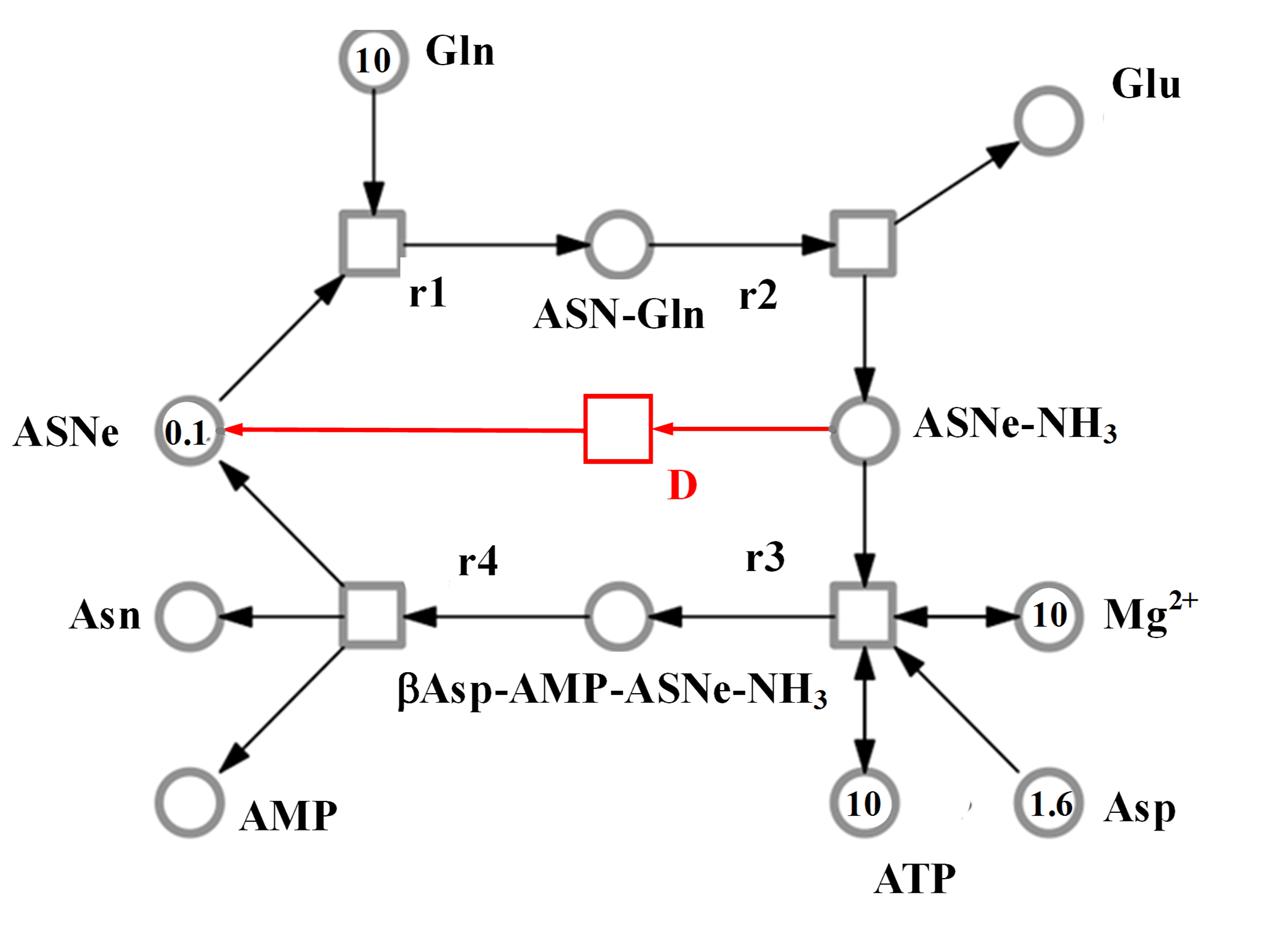
FIGURE 6. Model representing the reaction catalyzed by asparagine synthetase, comprising metabolites (circles), and reactions (squares). The concentration of metabolites is indicated abstractly by numbers in the circles. The model features 11 molecules: AMP, ATP, asparagine (Asn), glutamine (Gln), glutamate (Glu), aspartate (Asp), asparagine synthetase enzyme (ASNe), ASNe complexed with glutamine (ASNe–Gln), ASNe complexed with ammonia (ASNe–NH3), β-aspartyl-complex (βAsp–AMP–ASNe–NH3), and magnesium ions. Note that for clarity, water and pyrophosphate are not included. The model was generated assuming that the reactions follow mass action kinetics. The dissociation step for the ASNe–NH3 complex is highlighted in red.
• Reaction r1: ASNe + Gln → ASNe–Gln
• Reaction r2: ASNe–Gln → Glu + ASNe–NH3
• Reaction r3: ASNe–NH3 + Asp + ATP + Mg2+ (+ H2O) → βAsp–AMP–ASNe–NH3 + Mg2+ (+ PPi)
• Reaction r4: βAsp–AMP–ASNe–NH3 → Asn + ASNe + AMP
Note that H2O and PPi (pyrophosphate, P2O74-) are in parentheses because they are ubiquitous and therefore were not included in the model. The behavior of each reaction is dependent on the corresponding parameter values and the initial concentrations of the metabolites. The model additionally includes a dissociation step for the ASNe–NH3 complex (reaction “D” highlighted in red in Figure 6), because this better fits the observed experimental data: Reaction D: ASNe–NH3 → ASNe
The following ODEs were generated by the SNOOPY® Petri Net software (up to some naming adaptations to comply with the software requirements) to describe the mass action reactions determining the behavior of the 11 metabolites in the model:
The model structure and corresponding induced behavior indicates that: (a) asparagine synthesis is dependent on the aspartic acid (aspartate), glutamine, and ATP concentration; (b) when aspartic acid is depleted but glutamine is still available, asparagine synthetase will continue to hydrolyze glutamine to glutamic acid, which is consistent with the observed experimental data (Figure 5); (c) if all substrates are available except for ATP (i.e., ATP would only be an input to the system, unlike in the current model), the limiting factor becomes ATP.
The parameters (Table 4) were determined using the parameter estimation function of Copasi (version 4.16; Hoops et al., 2006) based on the Hooke and Jeeves (1961) method. The values for the TaASN1 enzyme were defined using the following concentrations (mg/mL): ASNe between 1e - 06 and 1e + 06 with start value = 0.1; Glu between 1e - 06 and 1e + 06 with start value = 0.0; Asn between 1e - 06 and 1e + 06 with start value = 0.0, based on the data for Glu, Asn and ASNe provided in Supplementary File S1. The values for TaASN2 were the same, except for the start value of ASNe which was set at 0.09. The parameter estimation tasks were run for both enzymes for 2100 s with 2000 steps, size 1.05, with resulting rate values in units of mg/mL/s. The corresponding plots showing the time-series simulation results from the parameter fitting against the experimental data are given in Figure 7 for TaASN1 and Figure 8 for TaASN2, using initial concentrations of TaASN1 = 2.03 nmol/mL and TaASN2 = 2.10 nmol/mL.
Wheat is now known to contain four classes of asparagine synthetase genes, TaASN1–4 (Gao et al., 2016). Our study established that there are single copies of TaASN1, TaASN2, and TaASN4, and two of TaASN3, and identified their chromosomal locations. The relatively simple structure of the gene family means that genetic interventions to reduce free asparagine accumulation and thereby acrylamide-forming potential in wheat grain are more likely to be successful. The antibodies raised in the study would be useful tools in the analysis of plants in which asparagine synthetase gene expression had been modified. The antibodies did not distinguish between TaASN1, TaASN2, and TaASN3, but it was possible to separate the enzymes on SDS-PAGE due to their slightly different sizes.
The study also showed that wheat asparagine synthetase enzymes, TaASN1 and TaASN2, can be expressed in E. coli and analyzed biochemically. Wheat asparagine synthetase activity has been measured before (Kudiyarova et al., 2013) but this was in plant extracts, so the results were not directly comparable to those obtained here. However, Todd et al. (2008) and Duff et al. (2011) analyzed heterologously expressed enzymes, the former from maize and the latter from maize and soybean. The reactions were not modeled, because their overall activity was very low, Todd et al. (2008) reporting a specific activity for asparagine production of 1–2 nmol/min/mg of protein. The reaction buffer used by Todd et al. (2008) contained 1.6 mM aspartate and 1 mM glutamine, while Duff et al. (2011) used a buffer containing 1.6 mM aspartate and 2 mM glutamine, and the reactions were sampled over a period of 60–90 min. In this study, a buffer was used containing 1.6 mM aspartate and 10 mM glutamine, as well as 10 mM ATP and 10 mM MgCl2, meaning that glutamine was present in relative excess compared with aspartate. The reactions catalyzed by the wheat asparagine synthetases proceeded much more rapidly than had been reported for the maize or soybean enzymes. By the 5-min time point, the concentrations of glutamate and asparagine were at or close to their maximum, indicating that the concentration of the reactants had become depleted.
A continuous Petri Net model based on mass-action kinetics was constructed using SNOOPY® software to describe the reaction catalyzed by asparagine synthetase, and a set of differential equations was generated to describe each part of the reaction. It was notable from the experimental data that the concentration of glutamate increased at a faster rate than the concentration of asparagine. Indeed, the product concentrations for both enzymes plateaued with the concentration of glutamate more than double that of asparagine, although the ratio of glutamate to asparagine was higher for TaASN1 than TaASN2. This indicates that the early stages of the reaction (r1 and r2 in the model) can proceed faster than and independently of the later stages (r3 and r4), consistent with the hypothesis proposed by Gaufichon et al. (2010) that steps r1 to r4 occur sequentially rather than simultaneously. So, despite the overall equation of the reaction being Glutamine + Aspartate + ATP → Glutamate + Asparagine + AMP + PPi, glutamate synthesis can proceed independently of asparagine synthesis when aspartate is not available.
Modeling of the reactions catalyzed by TaASN1 and TaASN2 showed the two enzymes to be biochemically very similar except for the rate parameter (kD) for the dissociation step (Table 4). The careful fitting resulted in parameter values k1–k4 which were within expected biochemical ranges (Meister, 1974). The dissociation reaction, D, which we postulate in order to be able to fit the overall model to the data, is currently not described in the literature. However, the higher kD value for TaASN1 could explain the higher ratio of glutamate to asparagine produced in the TaASN1 reaction compared with the TaASN2 reaction.
Gene expression analyses have shown TaASN1 and TaASN2 to be the most highly expressed asparagine synthetase genes in wheat grain, with TaASN2 expression rising to 10 times that of TaASN1 by mid-development (Gao et al., 2016). Given this and the similarity in the biochemical data obtained for the two asparagine synthetases in the present study, bearing in mind that this was a single experiment with heterologously expressed enzymes, we conclude that TaASN2 is the major enzyme synthesizing asparagine in wheat grain, and therefore an appropriate target for genetic interventions to reduce free asparagine accumulation.
HX performed molecular cloning, expression, and biochemical analysis of wheat asparagine synthetases. TC performed modeling. SP performed statistical analyses. SR performed genomic analyses. RG performed nucleotide sequence analysis of wheat asparagine synthetases. JH is a Joint project leader. MH and DG performed modeling. NH is a Joint project leader and lead author.
The project was supported by a consortium of companies and organizations from the wheat supply chain, comprising PepsiCo, Nestlé, Cereal Partners UK, Weetabix, Kelloggs, United Biscuits, Con Agra Foods, Lantmännen, Saaten Union, the Agriculture and Horticulture Development Board, CEEREAL, the Association of Cereal Food Manufacturers and SNACMA. This consortium did not participate in the experimental design or execution, or in the presentation or interpretation of the results in this report.
HX and RG were supported as visiting workers at Rothamsted Research by Shanghai Agriculture Applied Technology Development Program, China (Grant No. Z20160101) and overseas visiting grants from Shanghai Academy of Agricultural Sciences, Shanghai, China. TC was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom and a consortium of companies and organizations from the wheat supply chain through stand-alone LINK project BB/I020918/1 “Genetic improvement of wheat to reduce the potential for acrylamide formation during processing.” NH was supported at Rothamsted Research by the BBSRC via the 20:20 Wheat® and Designing Future Wheat Programmes.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.02237/full#supplementary-material
Avila-Ospina, L., Marmagne, A., Talbotec, J., and Krupinska, K. (2015). The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. J. Exp. Bot. 66, 2013–2026. doi: 10.1093/jxb/erv003
Boehlein, S. K., Richards, N. G., and Schuster, S. M. (1994a). Glutamine-dependent nitrogen transfer in Escherichia coli asparagine synthetase B. Searching for the catalytic triad. J. Biol. Chem. 269, 7450–7457.
Boehlein, S. K., Richards, N. G., Walworth, E. S., and Schuster, S. M. (1994b). Arginine 30 and asparagine 74 have functional roles in the glutamine dependent activities of Escherichia coli asparagine synthetase B. J. Biol. Chem. 269, 26789–26795.
Boehlein, S. K., Walworth, E. S., Richards, N. G., and Schuster, S. M. (1997a). Mutagenesis and chemical rescue indicate residues involved in beta-aspartyl-AMP formation by Escherichia coli asparagine synthetase B. J. Biol. Chem. 272, 12384–12392.
Boehlein, S. K., Walworth, E. S., and Schuster, S. M. (1997b). Identification of cysteine-523 in the aspartate binding site of Escherichia coli asparagine synthetase B. Biochemisty 36, 10168–10177.
Byrne, E. H., Prosser, I., Muttucumaru, N., Curtis, T. Y., Wingler, A., Powers, S., et al. (2012). Over-expression of GCN2-type protein kinase in wheat has profound effects on free amino acid concentration and gene expression. Plant Biotech. J. 10, 328–340. doi: 10.1111/j.1467-7652.2011.00665.x
Curtis, T. Y., Muttucumaru, N., Shewry, P. R., Parry, M. A., Powers, S. J., Elmore, J. S., et al. (2009). Effects of genotype and environment on free amino acid levels in wheat grain: implications for acrylamide formation during processing. J. Agric. Food Chem. 57, 1013–1021. doi: 10.1021/jf8031292
Curtis, T. Y., Postles, J., and Halford, N. G. (2014). Reducing the potential for processing contaminant formation in cereal products. J. Cer. Sci. 59, 382–392. doi: 10.1016/j.jcs.2013.11.002
Curtis, T. Y., Powers, S. J., Balagiannis, D., Elmore, J. S., Mottram, D. S., Parry, M. A. J., et al. (2010). Free amino acids and sugars in rye grain: implications for acrylamide formation. J. Agric. Food Chem. 58, 1959–1969. doi: 10.1021/jf903577b
Curtis, T. Y., Powers, S. J., and Halford, N. G. (2016). Effects of fungicide treatment on free amino acid concentration and acrylamide-forming potential in wheat. J. Agric. Food Chem. 64, 9689–9696. doi: 10.1021/acs.jafc.6b04520
Curtis, T. Y., Powers, S. J., Wang, R., and Halford, N. G. (2018). Effects of variety, year of cultivation and sulphur supply on the accumulation of free asparagine in the grain of commercial wheat varieties. Food Chem. 239, 304–313. doi: 10.1016/j.foodchem.2017.06.113
Duff, S. M. G., Qi, Q., Reich, T., Wu, X., Brown, T., Crowley, J. H., et al. (2011). A kinetic comparison of asparagine synthetase isozymes from higher plants. Plant Physiol. Biochem. 49, 251–256. doi: 10.1016/j.plaphy.2010.12.006
EFSA Panel on Contaminants in the Food Chain [Contam] (2015). Scientific opinion on acrylamide in food. EFSA J. 13:4104.
European Commission (2013). Commission Recommendation of 8 November 2013 on Investigations into the Levels of Acrylamide in Food. Brussels: European Commission.
European Commission (2017). Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Brussels: European Commission.
European Food Safety Authority (2011). Results on acrylamide levels in food from monitoring years 2007–2009 and exposure assessment. EFSA J. 9:2133. doi: 10.2903/j.efsa.2011.2133
Friedman, M. (2003). Chemistry, Biochem. and safety of acrylamide. A review. J. Agric. Food Chem. 51, 4504–4526. doi: 10.1021/jf030204
Gao, R., Curtis, T. Y., Powers, S. J., Xu, H., Huang, J., and Halford, N. G. (2016). Food safety: structure and expression of the asparagine synthetase gene family of wheat. J. Cer. Sci. 68, 122–131. doi: 10.1016/j.jcs.2016.01.010
Gaufichon, L., Reisdorf-Cren, M., Rothstein, S. J., Chardon, F., and Suzuki, A. (2010). Biological functions of asparagine synthetase in plants. Plant Sci. 179, 141–153. doi: 10.1016/j.plantsci.2010.04.010
Granvogl, M., Wieser, H., Koehler, P., Von Tucher, S., and Schieberle, P. (2007). Influence of sulfur fertilization on the amounts of free amino acids in wheat. Correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 55, 4271–4277. doi: 10.1021/jf070262l
Halford, N. G., Curtis, T. Y., Muttucumaru, N., Postles, J., Elmore, J. S., and Mottram, D. S. (2012). The acrylamide problem: a plant and agronomic science issue. J. Exp. Bot. 63, 2841–2851. doi: 10.1093/jxb/ers011
Heiner, M., Herajy, M., Liu, F., Rohr, C., and Schwarick, M. (2012). “Snoopy – a unifying Petri Net tool,” in Lecture Notes in Computer Science, (Hamburg: Springer), 398–407.
Hooke, R., and Jeeves, T. A. (1961). Direct search solution of numerical and statistical problems. J. ACM 8, 212–229. doi: 10.1145/321062.321069
Hoops, S., Sahle, S., Gauges, R., Lee, C., Pahle, J., Simus, N., et al. (2006). COPASI: a complex pathway simulator. Bioinformatics 22, 3067–3074. doi: 10.1093/bioinformatics/btl485
International Agency for Research on Cancer (1994). Some Industrial Chemicals; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 60. Lyon: International Agency for Research on Cancer (IARC).
Joy, K. W., Ireland, R. J., and Lea, P. J. (1983). Asparagine synthesis in pea leaves, and the occurrence of an asparagine synthetase inhibitor. Plant Physiol. 73, 165–168. doi: 10.1104/pp.73.1.165
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kudiyarova, Z. S., Muttucumaru, N., West, J. S., and Halford, N. G. (2013). Activity of enzymes associated with asparagine synthesis and breakdown in wheat under biotic and abiotic stress conditions. Aspects Appl. Biol. 116, 119–124.
Larsen, T. M., Boehlein, S. K., Schuster, S. M., Richards, N. G., Thoden, J. B., and Holden, H. M. (1999). Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product. Biochemisty 38, 16146–16157. doi: 10.1021/bi9915768
Lea, P. J., Sodek, L., Parry, M. A., Shewry, P. R., and Halford, N. G. (2007). Asparagine in plants. Ann. Appl. Biol. 150, 1–26. doi: 10.1111/j.1744-7348.2006.00104.x
Mei, B., and Zalkin, H. (1989). A cysteine-histidine-aspartate catalytic triad is involved in glutamine amide transferase function in purf-type glutamine amidotransferases. J. Biol. Chem. 264, 16613–16619.
Meister, L. (1974). “Asparagine synthesis,” in The Enzymes, 3rd Edn, Vol. 10, ed. P. D. Boyer (New York, NY: Academic Press), 561–580.
Mottram, D. S., Wedzicha, B. L., and Dodson, A. T. (2002). Acrylamide is formed in the Maillard reaction. Nature 419, 448–449. doi: 10.1038/419448a
Muttucumaru, N., Halford, N. G., Elmore, J. S., Dodson, A. T., Parry, M., Shewry, P. R., et al. (2006). Formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 54, 8951–8955. doi: 10.1021/jf0623081
Postles, J., Curtis, T. Y., Powers, S. J., Elmore, J. S., Mottram, D. S., and Halford, N. G. (2016). Changes in free amino acid concentration in rye grain in response to nitrogen and sulfur availability, and expression analysis of genes involved in asparagine metabolism. Front. Plant Sci. 7:917. doi: 10.3389/fpls.2016.00917
Postles, J., Powers, S. J., Elmore, J. S., Mottram, D. S., and Halford, N. G. (2013). Effects of variety and nutrient availability on the acrylamide forming potential of rye grain. J. Cer. Sci. 57, 463–470. doi: 10.1016/j.jcs.2013.02.001
Richards, N. G. J., and Schuster, S. M. (1998). Mechanistic issues in asparagine synthetase catalysis. Adv. Enzymol. Relat. Areas Molec. Biol. 72, 145–198.
Rognes, S. E. (1980). Anion regulation of lupine asparagine synthetase: chloride activation of the glutamine-utilizing reactions. Phytochemistry 19, 2287–2293. doi: 10.1016/S0031-9422(00)91013-6
Snapp, S. S., and Vance, C. P. (1986). Asparagine biosynthesis in alfalfa (Medicago sativa L.) root nodules. Plant Physiol. 82, 390–395. doi: 10.1104/pp.82.2.390
Stadler, R. H., Blank, I., Varga, N., Robert, F., Hau, J., Guy, P. A., et al. (2002). Acrylamide from Maillard reaction products. Nature 419, 449–450. doi: 10.1038/419449a
Todd, J., Screen, S., Crowley, J., Penga, J., Andersen, A., Brown, T., et al. (2008). Identification and characterization of four distinct asparagine synthetase (AsnS) genes in maize (Zea mays L.). Plant Sci. 175, 799–808. doi: 10.1016/j.plantsci.2008.08.004
Verwoerd, T. C., Dekker, B. M. M., and Hoekema, A. (1989). A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17:2362. doi: 10.1093/nar/17.6.2362
Wang, H., Liu, D., Sun, J., and Zhang, A. (2005). Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J. Plant Physiol. 162, 81–89. doi: 10.1016/j.jplph.2004.07.006
Wilkinson, P. A., Winfield, M. O., Barker, G. L. A., Allen, A. M., Burridge, A., Coghill, J. A., et al. (2012). CerealsDB 2.0: an integrated resource for plant breeders and scientists. BMC Bioinformatics 13:219.
Keywords: wheat, asparagine synthetase, acrylamide, food safety, enzyme activity, mathematical modeling
Citation: Xu H, Curtis TY, Powers SJ, Raffan S, Gao R, Huang J, Heiner M, Gilbert DR and Halford NG (2018) Genomic, Biochemical, and Modeling Analyses of Asparagine Synthetases from Wheat. Front. Plant Sci. 8:2237. doi: 10.3389/fpls.2017.02237
Received: 04 September 2017; Accepted: 20 December 2017;
Published: 15 January 2018.
Edited by:
Stanislav Kopriva, University of Cologne, GermanyReviewed by:
Andrea Mozzarelli, Università degli Studi di Parma, ItalyCopyright © 2018 Xu, Curtis, Powers, Raffan, Gao, Huang, Heiner, Gilbert and Halford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nigel G. Halford, bmlnZWwuaGFsZm9yZEByb3RoYW1zdGVkLmFjLnVr Jianhua Huang, c3cxQHNhYXMuc2guY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.