- 1Soybean Research Institute, Shenyang Agricultural University, Shenyang, China
- 2Key Laboratory of Soybean Cultivation of Ministry of Agriculture, Soybean Research Institute, Heilongjiang Academy of Agricultural Sciences, Harbin, China
In maize-soybean intercropping system, soybean plants will be affected by the wide light-fluctuation, which resulted from the shading by maize plants, as the shading of maize the light is not enough for soybean in the early morning and late afternoon, but at noon, the light is strong as the maize shading disappeared. The objective of this study is to evaluate the photosynthetic response of soybean leaf to the wide light-fluctuation. The data of diurnal variation of photosynthetic characters showed that the photosynthetic rate of intercropped soybean was weaker than that of monocropped soybean. The chlorophyll content, ratio of chlorophyll a/b, and AQE (apparent quantum efficiency) were increased and Rd (dark respiration rate) was decreased for the more efficient interception and absorption of light and carbon gain in intercropping. δRo (The efficiency/probability with which an electron from the intersystem electron carriers was transferred to reduce end electron acceptors at the PSI acceptor side) and φRo (the quantum yield for the reduction of the end electron acceptors at the PSI acceptor side) in intercropped soybean leaf were lower compared to those in monocropped one, which showed that the acceptor side of PSI might be inhibited, and also it was the main reason that soybean plants showed a low photosynthetic capacity in intercropping. ψEo (the efficiency/probability with an electron moves further than QA-) in monocropping and intercropping decreased 5.8, and 35.7%, respectively, while φEo (quantum yield for electron transport) decreased 27.7 and 45.3% under the high radiation at noon, which suggested that the acceptor side of PSII was inhibited, while the NPQ became higher. These were beneficial to dissipate excess excitation energy in time, and protect the photosynthetic apparatus against photo-damage. The higher performance index on the absorption basis (PIABS) and lower δRo, φRo, ψEo, and φEo of intercropped soybeans compared to monocropping under high radiation indicated that the electron transfer of intercropped soybean was inhibited more seriously and intercropped soybean adjusted the electron transport between PSII to PSI to adapt the light-fluctuation. Higher NPQ capacity of intercropped soybeans played a key role in keeping the leaf with a better physiological flexibility under the high radiation.
Introduction
Light is one of the most important factors affecting plants growth and development (Li A. et al., 2016), with changes in irradiance having impacts on plant growth, morphology, physiology, etc. Maize-soybean intercropping is one of major planting patterns in China, and has contributed significantly to soybean production and to maintain the yield of maize (Yang et al., 2008; Yan et al., 2010; Li et al., 2014). In this intercropping, soybean grow in the rows between maize, and the light situation of soybean canopy is changed by maize (Awal et al., 2006; Yang et al., 2014). The light environment of soybean survived is very complicated. The soybean is shaded by maize at early morning and late afternoon, and exposed to high radiation that higher than light saturation point (LSP) at midday in intercropping (Gong et al., 2015).
The effect of shade on soybean was extensively investigated. In general, plant leaf grown in shade condition was thinner, had a lower net CO2 assimilation rate (An) (Tateno and Taneda, 2007), CO2 assimilation rate saturated at lower photosynthetic photon flux density (Zhang et al., 2004), and lower amounts of electron transfer carriers than those in unshade condition (Jiang et al., 2011). However, soybean plants grown in intercropping were not only affected by shade, but also affected by high radiation. In this study little was known about the effect of high radiation stress on soybean leaf in intercropping.
High radiation is one of the most frequently stresses that was encountered by plant during growth period. Under high radiation condition, the light energy absorbed by the plant leaf often exceeded the energy required to fix the CO2. If the excess excitation energy could not be dissipated in time, it resulted in energy overflow and excessive reactive oxygen species (Foyer and Noctor, 2005; Li et al., 2013; Ruban, 2012). This could be destructive to photosynthetic apparatus. Plants had several regulatory mechanisms to adjust a well-balanced performance of PSI and PSII, and protect photosynthetic apparatus against high radiation (Kono and Terashima, 2014; Kromdijk et al., 2016; Mishanin et al., 2016). Down-regulation of PSII performance is one of the most efficient mechanisms of photoprotection (Müller et al., 2001; Mishanin et al., 2016). This mechanism decreased in the quantum yield of PSII, the capacity of photosynthetic electron transport and photochemical quenching, while increased in NPQ, which provided enhanced dissipation of energy in the light-harvesting complex (Ruban et al., 2012; Niyogi and Truong, 2013; Mishanin et al., 2016). It is significant that plant dissipate excess solar radiation through NPQ to maintain optimal rates of photosynthesis and provide the plant against oxidative damage (Mishanin et al., 2016).
The leaf of intercropped soybean was exposed to high radiation for several hours at midday. However, little was known about the acclimation of soybean plants grown in intercropping to high radiation. And more effort should be done to study the mechanisms of photoprotection of PSI and PSII to strong fluctuations of environment light (Allakhverdiev and Murata, 2004; Allahverdiyeva et al., 2014). Chlorophyll a fluorescence is an important method for studying PSII function and reaction under different environmental conditions (Allakhverdiev and Murata, 2004; Strasser et al., 2004; Kalaji et al., 2017), and it can be used to analyze the changes of reaction center, the efficiency of electron transfer from PSII to the acceptor side of PSI in the intersystem chain under different growth conditions (Tóth et al., 2007; Tsimilli-Michael and Strasser, 2008; Strasser et al., 2010; Kalaji et al., 2017). Therefore, chlorophyll a fluorescence is used to study the effect of fluctuation light on plant.
In this study, the diurnal variation of photosynthesis characteristics, fast and slow chlorophyll fluorescence, morphological characteristic of soybean leaf grown in intercropping and monocropping were measured to understand light acclimation of soybean grown under different planting pattern. The objective of this study is to evaluate the photosynthetic response of soybean leaf to the wide light-fluctuation in intercropping. This study provides insights into the physiological flexibility of soybean adapt to light-fluctuation in intercropping.
Materials and Methods
Plant Material and Experimental Design
Field experiments was carried out from May 2015 to October 2015 at the experimental farm of Shenyang Agricultural University, Shenyang, Liaoning Province, China, The experiment was laid out a completely randomized block design with two cropping (maize-soybean intercropping and soybean monocropping). The row direction was north–south layout. Soybean and maize were sown on May 3rd, 2015. Soybean cultivar Liaodou32 was used, and maize cultivar used was Zhengdan958. The intercropping used wide-narrow row planting, and the ratio of maize to soybean rows in the intercropping was 2:2. The distance between the maize and soybean was 80 cm, and the distance between two rows of maize or two rows of soybean was 40 cm. The densities of sole cropping soybean, intercropped soybean and intercropped maize were 150000, 150000, and 60000 plants ha-1. The uppermost and fully expanded leaves were used for measurements at R2 stage (full flowering).
Determination of Light Conditions
The average PAR and maximum PAR of soybean canopy changes of different cropping were measured in a sunny day using a light meter (AccuPAR LP-80, United States) according to the method of Yang et al. (2014), and listed on Figure 1.
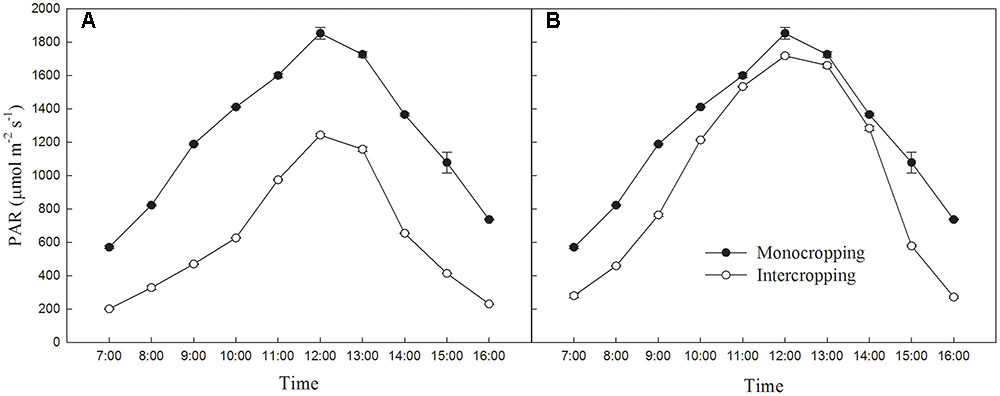
FIGURE 1. (A) Diurnal variation of average PAR on the soybean canopy during July 25th, 2015 in Shenyang. (B) Diurnal variation of maximum PAR on the soybean leaf during July 25th, 2015 in Shenyang.
Photosynthetic Parameters
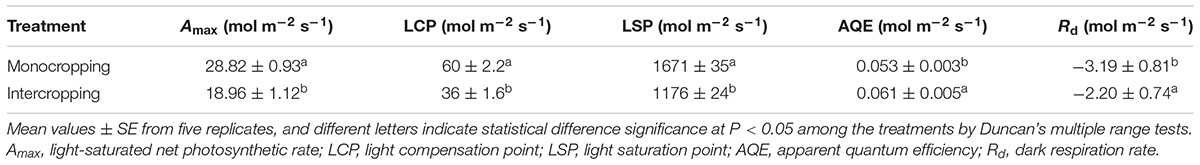
Light response curves of Photosynthesis were measured using a LI-6400XT (Li-Cor, United States). The parameters were measured on uppermost and fully expanded leaves from 09:00 to 11:30 am on a clear day. The temperature and CO2 concentration of leaf chamber were maintained at 25°C and 380 μmol mol-1, respectively. PAR was increased from 0 to 1500 μmol photons m-2 s-1 (0, 20, 50, 80, 100, 200, 400, 600, 800, 1000, 1200, 1500 μmol m-2 s-1, 36 min). And then, after linear fitting, light compensation point (LCP), LSP and light-saturated net photosynthetic rate (Amax), apparent quantum efficiency (AQE) and dark respiration rate (Rd) were estimated by the method of Ye (2007).
Diurnal variation of leaf gas exchange was measured on a clear sunny day. Photosynthesis was measured with a LI-6400XT (Li-Cor, United States) equipped with 2 cm × 3 cm clear chamber. Pn and Ci were recorded at intervals of 2 h from 08:30 am to 16:30 pm. The measured leaves were kept at their natural angle of posture exposing to direct irradiance outside leaf chamber. The temperature and CO2 concentration of leaf chamber were kept at natural environment.
Chlorophyll Fluorescence
Light response curves for fluorescence were monitored by PAM-2500 chlorophyll fluorometer (Heinz Walz GmbH, Germany), and according to the method of Chen et al. (2014). Rapid light curves were performed with gradually increasing irradiance in 11 steps with 180 s intervals. For each step, the irradiance is 0, 198, 363, 619, 785, 981, 1160, 1386, and 1663 μmol m-2 s-1, and the fluorescence signal was recorded, respectively. The data were recorded and read data from the PamWin V3.12g (system control and data acquisition system).
Diurnal variation of leaf chlorophyll fluorescence was measured on a clear sunny day by PAM-2500 chlorophyll fluorometer (Heinz Walz GmbH, Germany). The fluorescence signals were recorded at intervals of 2 h from 08:30 am to 16:30 pm. The measured leaves were kept at their natural angle of posture exposing to direct irradiance outside leaf chamber. Then, the Y(II) and other parameters were calculated as described by Baker (2008).
Chlorophyll a Fluorescence Transient
After a dark adaptation for 30 min, chlorophyll a fluorescence transient (OJIP) of soybean leaves were measured by the plant efficiency analyzer (Hansatech Instruments Ltd., Norfolk, United Kingdom) in a solar day at 10:00 am to 12:30 pm. The uppermost and fully expanded leaves were used for measurements. We obtained the parameters of chlorophyll a fluorescence which could reflect the PSII activity of soybean leaves. Then, the PSII parameters derived from the OJIP transient were analyzed based on the method of Strasser et al. (2004, 2010).
Leaf Chlorophyll Content, Morphological and Anatomical Features
After the measurements described above completed, the leaves were collected for determination of chlorophyll content (Chl a, Chl b, Chl a+b, Chl a/b). Chlorophyll pigments were extracted by grinding leaves in 80% acetone in the dark at room temperature and were expressed as mg/g FW from the equations of Porra (2002). The leaf area was measured by a portable leaf area meter (LI-3100C, LI- COR, United States).
The middle segments of the uppermost and fully expanded leaves were sampled and fixed in a formaldehyde solution (FAA). Leaf segments were dehydrated, cleared and embedded in paraffin. Then these samples were cut by RM2235 rotary microtome (Leica Microsystems Ltd., Germany) at thickness of 10 μm. Sections were stained with Safranin O and Fast green, then observed and captured by Axio Imager A2 microscope (Zeiss, Germany). Leaf thickness, palisade tissue thickness and spongy tissue thickness were quantified by using ZEN imaging software (Zeiss, Germany).
Determination of Malondialdehyde (MDA) Content and Activity of Antioxidant Enzymes
The middle segments of the uppermost and fully expanded leaves were collected at 12:30, and immediately stored in liquid nitrogen, and then kept at -80°C. Leaf sample was homogenized with 50 mM phosphate buffer (pH 7.8) containing 10 mM Polyvinylpyrrolidone (PVP) and 0.2 mM EDTA in an ice bath, and centrifuged at 12,000 × g and 4°C for 20 min. The supernatant was used for MDA and enzyme analysis. The MDA content was assayed by the thiobarbituric acid test (Hodges et al., 1999). Activity of antioxidant enzymes was measured according to Samantary (2002). The activity of superoxide dismutase (SOD) was assayed by measuring its ability to inhibit the photochemical reduction of NBT at 560 nm, and was expressed as units per g of fresh weight. The activity of catalase (CAT) was determined by measuring the decrease of oxidized phenols of H2O2 at 240 nm, and the activity of CAT was expressed as units per g of fresh weight during 1 min.
Data Analysis
The experiments were arranged in a completely randomized block design with three replications. One-way analysis of variance (ANOVA) and the Duncan’s multiple range tests were used to assess each of the parameters using SPSS statistics software (Version 20, SPSS, Chicago, IL, United States). The graphs were made using Sigmaplot (Version 12, Systat Software).
Results
Effect of Different Planting Pattern on PAR of Soybean Population
The light environment of different planting patterns was showed in Figure 1. The average PAR on the soybean canopy in intercropping was significantly lower than those in monocropping. The maximum PAR on the soybean leaf was significantly lower than those in monocropping in early morning and late afternoon, but was exposed to high radiation at noon.
Effect of Different Planting Pattern on Chlorophyll Content, Morphology of Soybean Leaf and Light Response Curve of Photosynthesis
Leaf in intercropping showed a significantly higher photosynthetic pigment concentration per fresh weight, and significantly lower chla/b than those under monocropping (Table 1). The leaf area per plant in intercropping was significantly lower than that in monocropping.
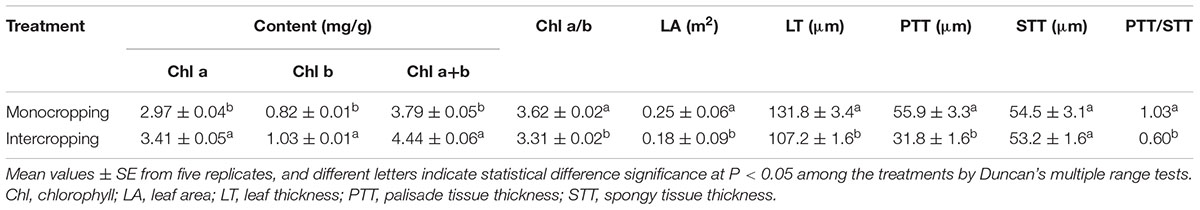
TABLE 1. The content of chlorophylls, leaf area and morphological characteristic of soybean leaves under monocropping and intercropping.
In contrast to soybean grown in monocropping, the leaf became thinner, and the thickness of both leaf and palisade tissue were significantly decreased, however, the spongy tissue thickness was little changed.
Pn increased rapidly as PAR increased to 600 μmol⋅m-2⋅s-1 and then increased slowly to saturation (Figure 2). Pn under intercropping was higher than that in monocropping at low PAR, while lower at high PAR. Amax (light-saturated net photosynthetic rate) of soybean leaf in intercropping was about 18.96 μmol⋅m-2⋅s-1, it was only about 65.79% of Amax in monocropping (28.82 μmol⋅m-2⋅s-1, Table 2). The LCP, LSP, and Rd (dark respiration rate) in intercropping were lower than those in monocropping, while AQE was higher than those in monocropping.
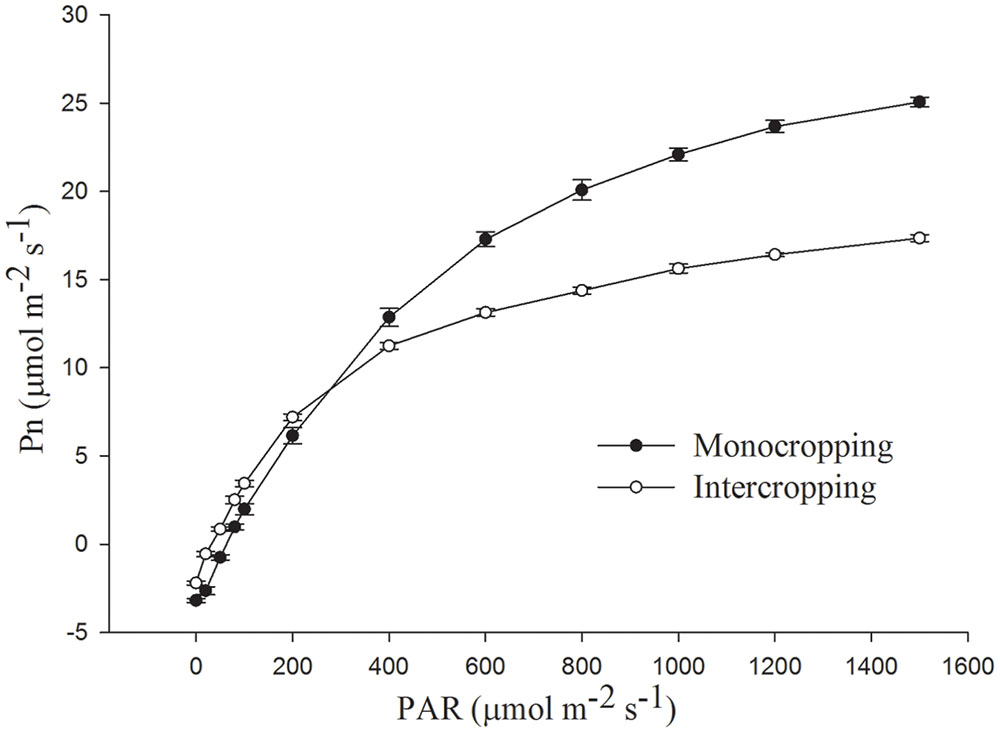
FIGURE 2. Net photosynthetic rate, measured as CO2 uptake in soybean leaf under monocropping and intercropping.
Effect of Different Planting Pattern on Rapid Light Response Curve of Soybean Leaf
Results obtained from rapid light curves showed that Y(II) (quantum yield of photochemical energy conversion in PS II), qP (coefficients estimating the fraction of open PS II reaction centers based on a puddle model), and qL (coefficients estimating the fraction of open PS II reaction centers based on a lake model) were decreased gradually with the increase of PAR (Figures 3A,C,E). And Y(II), qP and qL in intercropping were higher than those in monocropping. ETR (electron transport rate) increased significantly with the increase of PAR, and ETR in intercropping saturated at lower PAR than those in monocropping (Figure 3B). NPQ (non-photochemical quenching) and Y(NPQ), expressed the thermal dissipation of excitation energy, had a significant rise with the increase of PAR (Figures 3D,F).
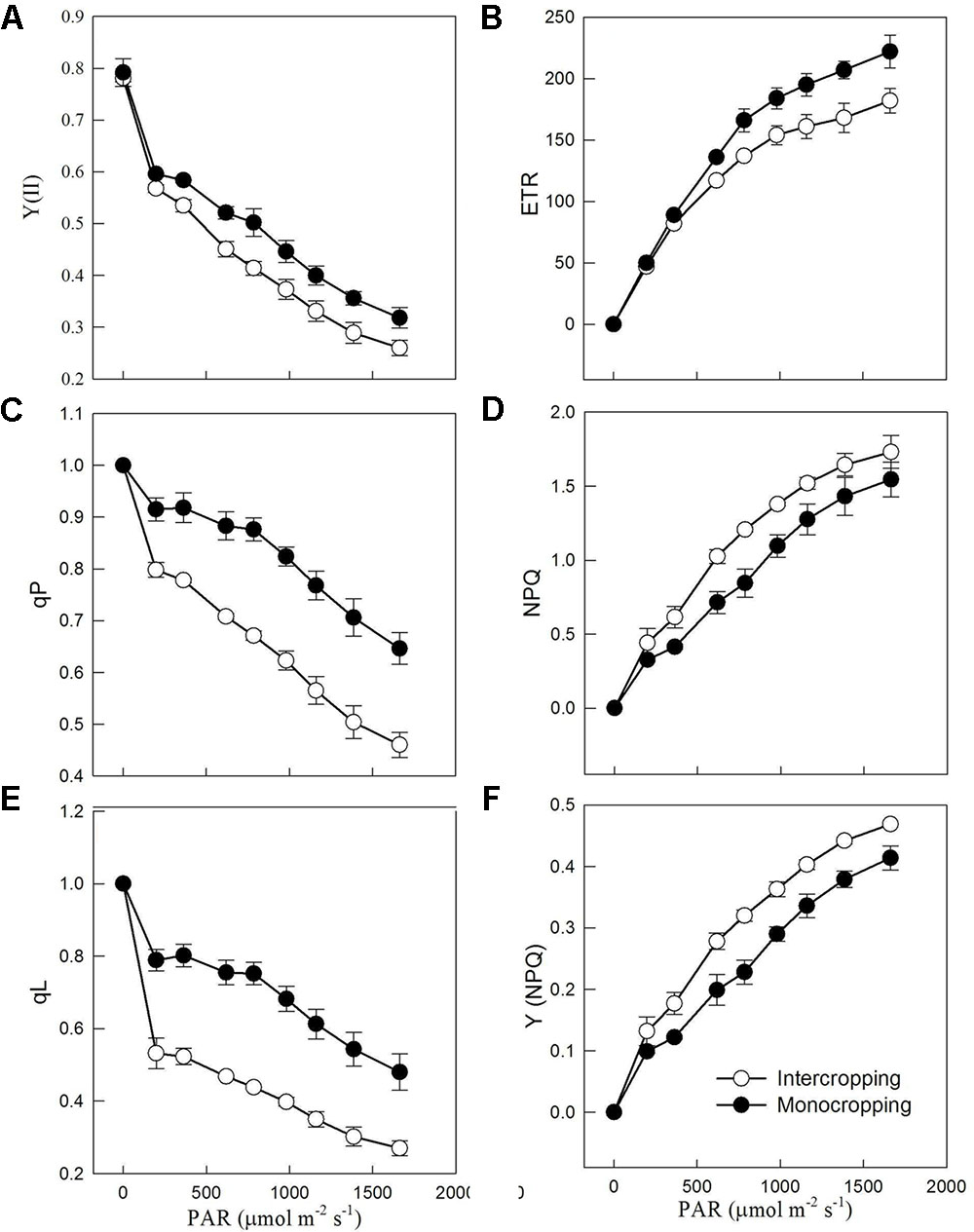
FIGURE 3. Chlorophyll a fluorescence parameters derived from the rapid light curves in monocropping and intercropping. (A) Y(II), the photochemical efficiency of PSII, (B) ETR, electron transport rate, (C) qP, coefficients estimating the fraction of open PS II reaction centers based on a puddle model, (D) NPQ, non-photochemical quenching, (E) qL, coefficients estimating the fraction of open PS II reaction centers based on a lake model, and (F) Y(NPQ), quantum yield of non-photochemical quenching.
Diurnal Variation of Leaf Gas Exchange and Chlorophyll a Fluorescence
Pn increased with the increase of light intensity, and reached maximum at 10:30, and then began to decrease. Pn in intercropping was significantly lower than that in monocropping (Figure 4A). Ci and Y(II) decreased with the increase of light intensity, and reached minimum at noon, and then began to recover. Ci in intercropping was significantly higher than that in monocropping. Y(II) in intercropping was significantly lower than that in monocropping at 10:30–14:30 (Figures 4B,C). NPQ increased with the increasing of light intensity, and reached maximum at noon, then began to decrease. And NPQ in intercropping was significantly higher than that in monocropping at 10:30–14:30 (Figure 4D).
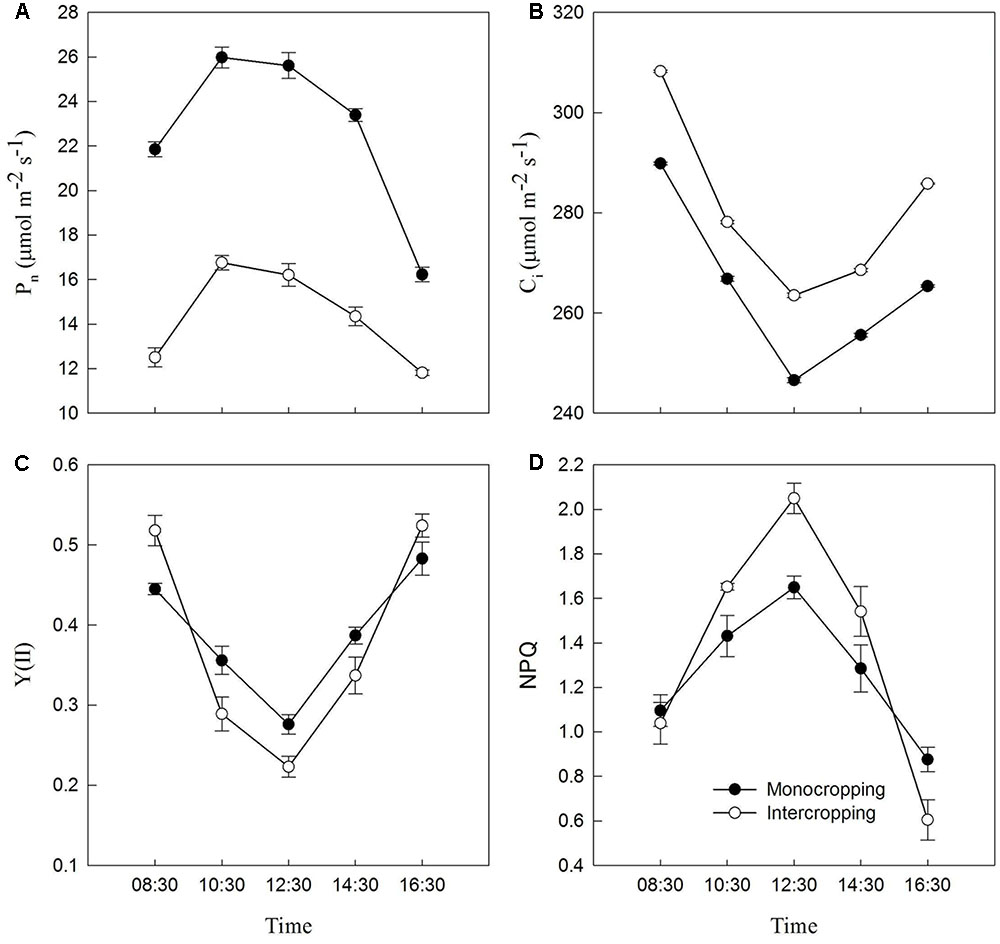
FIGURE 4. Diurnal variation of Pn, Ci, Y(II), and NPQ of soybean leaf in monocropping and intercropping. (A) Pn, net photosynthetic rate, (B) Ci, intercellular CO2 concentration, (C) Y(II), the photochemical efficiency of PSII, and (D) NPQ, non-photochemical quenching parameter.
Effect of High Radiation on Slow Kinetics of Chlorophyll a Fluorescence Induction at Noon
At noon, the NPQ (non-photochemical quenching) and qN (coefficients of non-photochemical quenching) in intercropping were significantly higher than those in monocropping, while qP (coefficients estimating the fraction of open PS II centers based on a puddle model) and qL (coefficients estimating the fraction of open PS II centers based on a lake model) in intercropping was significantly lower than those in monocropping (Table 3).
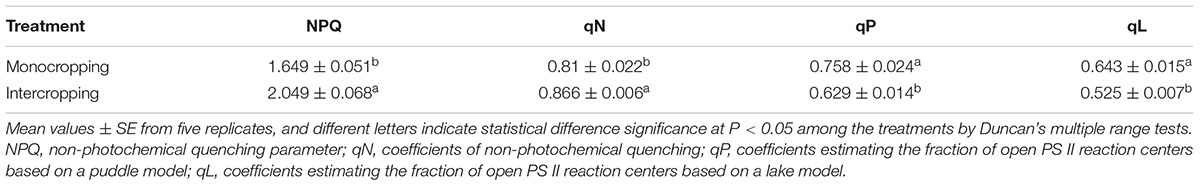
TABLE 3. Effect of high light on the mode of the yields for dissipative processes for the energy absorbed by PSII of soybean at midday (12:30 pm).
At noon, Y(II) in intercropping was significantly lower than those in monocropping, while Y(NPQ) in intercropping was significantly higher than those in monocropping. Y(NO) in intercropping was lower than those in monocropping, but there was no significant difference between them (Table 4).
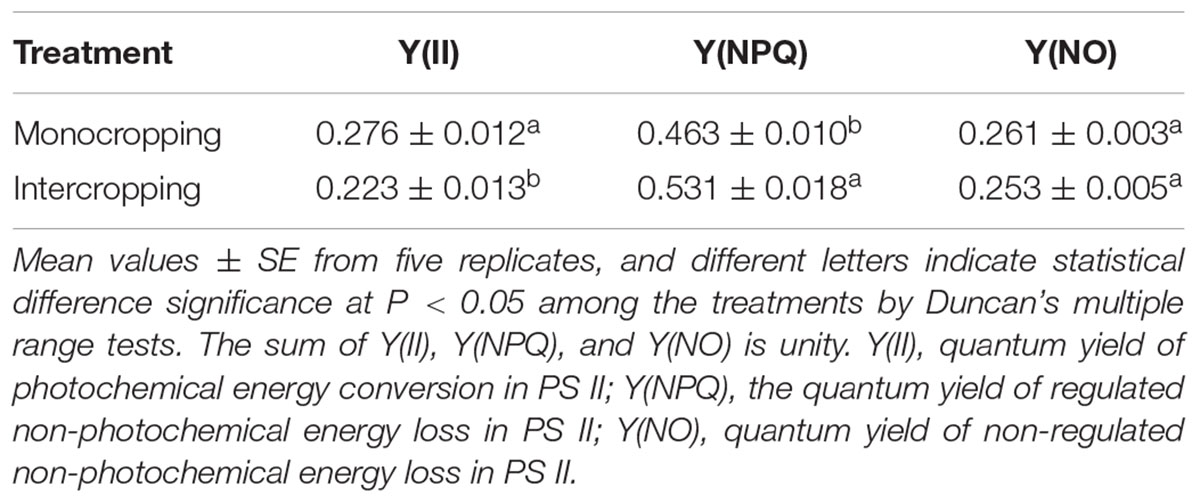
TABLE 4. Effect of high light on the mode of the yields for dissipative processes for the energy absorbed by PSII of soybean at midday (12:30 pm).
Effect of High Radiation on Fast Chlorophyll Fluorescence Kinetic in Monocropping and Intercropping
The fluorescence parameters derived from fast fluorescence kinetic are listed in Table 5. At 10:00 am, φPo (maximal quantum yield of primary photochemistry), ψEo (efficiency/probability that an electron moves further than QA-), φEo (quantum yield for electron transport), PIABS (performance index on the absorption basis) and Wk (the ratio of variable fluorescence at the K-step to the fluorescence difference Fj-Fo) in intercropping were significantly higher than those in monocropping, while δRo (efficiency/probability with which an electron from the intersystem electron carriers is transferred to reduce end electron acceptors at the PSI acceptor side) in intercropping was significantly lower than those in monocropping. At midday (12:30 pm), ψEo, φEo, δRo, and φRo in intercropping became lower than those in monocropping, while Wk in intercropping were higher than those in monocropping.
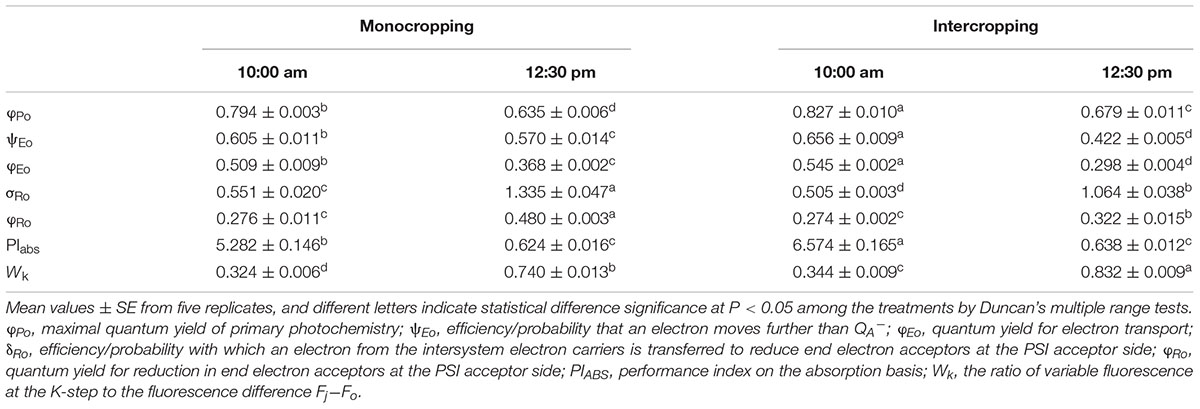
TABLE 5. Selected parameters derived from fast fluorescence kinetic measurements in soybean leaves at 10:00 am and 12:30 pm (the PAR of soybean leaf under intercropping and monocropping were 1213 and 1411 μmol m-2 s-1 at 10:00 am, while the PAR of soybean leaf under intercropping and monocropping were 1750 and 1860 μmol m-2 s-1 at 12:30 pm).
The Lipid Peroxidation and ROS Scavenging Metabolism
The MDA content and activity of antioxidant enzymes were showed in Table 6. The MDA content, activities of SOD and CAT in intercropping were significantly higher than those in monocropping at noon.
Discussion
The Change of Photosynthesis Capacity in Intercropping
In intercropping system, high crop significantly reduced the PAR for soybean, and soybean had to make some response to adapt the change of light environment. The decrease of LCP, LSP, and Amax (light-saturated net photosynthetic rate) in intercropping indicated that the photosynthetic capacity was limited. The increase of AQE indicated that the ability of light-intercepting gets promoted in light-limited environment conditions, and this was beneficial for higher light utilization efficiency in intercropping. The increase of Rd (dark respiration rate) indicated that soybean in intercropping dropped the energy expenditure. All these features contributed to the efficient interception and absorption of light and carbon gain in intercropping. And these were similar to that plant grew in shade condition (Zhang et al., 2004; Tateno and Taneda, 2007; Gong et al., 2014). Therefore, the shade of maize leaded to the decrease of photosynthetic capacity of soybean leaf in intercropping. And the shade-tolerant and high photosynthetic efficiency soybean cultivar could be choosed to improve the photosynthetic capacity and yield of soybean in intercropping (Liu et al., 2014; Cui et al., 2015).
The decrease of photosynthetic capacity was caused by stomatal or non-stomatal limitations (Gong et al., 2015). Previous study suggested that the decrease of photosynthetic capacity of spring barley in shade condition was not caused by stomatal effect (Zivcak et al., 2014). Our result showed that Pn was limited in intercropping, however, Ci inside the leaf in intercropping was higher than that in monocropping (Figure 4). This research showed the same result that the decrease of Pn in intercropping was not caused by stomatal effect.
Leaf photosynthetic rate is related to chlorophyll content (Shao et al., 2014). Chl a is essential for determining photosynthesis, and Chl b determine the wavelengths of light that can be absorbed by the organism (Field et al., 2013). Intercropped soybean leaf contained more chl a and chl b content per weight and had lower chl a/b than those in monocropping, which could broaden the wavelengths of light that could be absorbed, and effectively increase the ability of light capture (Gong et al., 2014). This is an important adaptation for plants growing in shaded environments.
The leaf and palisade tissue thickness of soybean leaf in intercropping became thinner, which resulted in the reduction of chloroplast, where carboxylation reactions of photosynthesis take place, mostly located in palisade tissue (Terashima et al., 2006; Gong et al., 2015). Therefore, thinner palisade tissue in intercropping decreased the photosynthetic capacity of soybean leaf.
The higher PIABS (performance index on the absorption basis) and φPo (maximal quantum yield of primary photochemistry) in intercropping indicated that the light-intercepting capacity and PSII activity was enhanced. But the δRo (the efficiency/probability with which an electron from the intersystem electron carrier s was transferred to reduce end electron acceptors at the PSI acceptor side) and φRo (the quantum yield for the reduction of the end electron acceptors at the PSI acceptor side) of the plants grown in intercropping were lower than those of the monocropped plants (Table 5). And this indicated that the quantum efficiency from PSII to PSI in intercropping were lower than that in monocropping, electron transport between QB and PSI and the acceptor side of PSI might be inhibited (Wang et al., 2006; Li et al., 2014; Li L. et al., 2016; Zivcak et al., 2014). Intercropped soybean plants increased the photochemical efficiency of PSII, but the electron transport was limited and the accepted capacity of PSI was low. This was one of the reasons that soybean growth was inhibited and showed a low photosynthetic capacity in intercropping. Therefore, the limitation of electron transport and the changing of morphology of soybean leaf in intercropping were the reason that the photosynthetic capacity of soybean cultivars decreased.
The Acclimation of Soybean Leaf on High Radiation at Noon
As shown in Figure 1B, intercropped soybean leaf were exposed to high radiation at noon, and had to take a series of reactions to adapt it. Leaf in intercropping exhibited higher Y(II) and lower NPQ than those in monocropping in the morning and afternoon, indicated the higher efficiency of light utilization at low radiation. However, leaf in intercropping showed lower Y(II) and higher NPQ than those in monocropping at noon (from 10:30 to 14:30), this indicated that the absorbed energy of PSII flux to photochemical processes reduced and this part of energy converted into the non-photochemical energy loss or non-photochemical quenching in high radiation. Higher NPQ indicates a higher transthylakoid proton gradient (ΔpH), which leads to more efficient downregulation of electron transport from PSII to PSI, hence, lower risk of hydroxyl radical production on PSI (Joliot and Johnson, 2011; Brestic et al., 2015). These all were beneficial for dissipating excess excitation energy in time and avoiding photo-damage. The lower qL in intercropping suggested that soybean plants grown in intercropping could close or inactivate more reaction centers to limit the energy input into PSII in high radiation (Table 3).
The fate of absorbed light energy was shown in Table 4. Y(NPQ) is an important indicator to reflect photoprotection. In high radiation, Y(II) in intercropping reduced, while Y(NPQ) increased significantly. The significant increase of Y(NPQ) suggested more absorbed energy flux from the photochemical energy conversion to the regulated non-photochemical energy loss in PSII in intercropping in order to adapt high radiation condition. Higher Y(NPQ) implied that there was still photochemical energy conversion or protective regulatory mechanisms to dissipate the light energy absorbed by soybean plants. Y(NO) is an important indicator of photo-damage. There is no significant difference in Y(NO) between intercropping and monocropping, which indicated that wide light-fluctuation in intercropping did not cause photo-damage. The high excitation pressure is considered to be directly related to the photo-damage (Kornyeyev et al., 2010; Zivcak et al., 2014), and is easy to happen at high light. Together with low LSP, Amax, and ETR in intercropping, we could expect severe photo-damage in intercropping. However, there were low differences in photo-damage. One possible explanation is that the photo-protection ability is increased to avoid photo-inhibition with the increasing of excitation pressure at high light (Niinemets and Kull, 2001).
The higher Wk in intercropping demonstrated that the donor side of PSII was seriously inhibited compared to monocropping in high radiation at noon (Chen et al., 2004; Li L. et al., 2016). The higher ψEo and φEo at 10:30 suggested that the quantum efficiencies in PSII electron transfer chain of soybean plants grown in intercropping were enhanced compared to the monocropped soybean. At 12:30, with the effect of high radiation, the ψEo and φEo in intercropping and monocropping decreased; the ψEo and φEo in intercropping were lower than those in monocropping. The higher decrease of parameters ψEo and φEo in intercropping reflects higher light susceptibility to high radiation. These indicated photo-inhibition of soybean leaf grown in intercropping caused a huge accumulation of QA- (Strasser et al., 2004). Excess electrons transported from PSII to the acceptor side of PSI may result in the occurring of photo-inhibition (Huang et al., 2015). Thus, we expected that soybean leaf grown in intercropping was more susceptible to photo-inhibition in high radiation. However, the lower PSII connectivity of shade leaves might keep the excitation pressure lower, physiologically more acceptable level and thus protected photosynthetic apparatus against high light (Zivcak et al., 2014).
MDA content is used as an indicator of lipid peroxidation (Sudhakar et al., 2001; Spicher et al., 2016). In our study, The MDA content of Intercropped soybean leaf was significantly higher than monocropped one. And this indicated that the higher accumulation of ROS led to much more membrane peroxidation within the thylakoid and chloroplasts in intercropping than this in monocropping. The higher excess excitation energy and the lower electron transportation activity between PSII and PSI in intercropping probably turns the photosynthetic apparatus into a stronger ROS source (Gill and Tuteja, 2010; Vanlerberghe et al., 2016). Antioxidative defense mechanisms can scavenge the ROS to protect the photosynthetic apparatus. In our study, intercropping increased the activities of SOD and CAT in soybean leaf to scavenge the higher production of ROS. And this was beneficial for the photosynthetic apparatus to against oxidative stress. Together with no significant difference in Y(NO) between intercropping and monocropping, these suggested that although there was a higher ROS in intercropping, the higher activity of antioxidant enzymes could scavenge the ROS in time to be not causing photo-damage.
Conclusion
Soybean leaf had a sufficient physiological flexibility to respond to change of light radiation. The photosynthetic capacity of soybean plants grown in intercropping was limited; and it was associated with the block of electron transport from PSII to PSI. In high radiation, the electron transport from PSII to PSI and NPQ were increased significantly, but acceptor side of PSII was inhibited, this was beneficial to keep the excitation pressure lower and protect the photosynthetic apparatus against photo-damage. Meanwhile, the activity of antioxidant enzymes were increased to against oxidative stress. Soybean leaf in intercropping showed a higher light susceptibility to high radiation and adapted the light-fluctuation by adjusting the electron transport between PSII to PSI.
Author Contributions
FX and XY conceived and designed research. XY performed the experiments, analyzed the data, wrote the manuscript. FX revised the manuscript. HoZ, and QZ helped in conducting the experiments and analyzing the data. CL, HuZ, and J-JW critically edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the project [2016YDF0300203-2] “Technological Innovation for High Yield and Efficiency of Grain Crops” of Ministry of Science and Technology, China and Natural Science Foundation of Liaoning Province [2016010657-301].
References
Allahverdiyeva, Y., Suorsa, M., Tikkanen, M., and Aro, E. M. (2014). Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 66, 2427–2436. doi: 10.1093/jxb/eru463
Allakhverdiev, S. I., and Murata, N. (2004). Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem ii in Synechocystis sp. pcc 6803. Biochim. Biophys. Acta 1657, 23–32.
Awal, M. A., Koshi, H., and Ikeda, T. (2006). Radiation interception and use by maize/peanut intercrop canopy. Agric. For. Meteorol. 139, 74–83. doi: 10.1016/j.agrformet.2006.06.001
Baker, N. R. (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113. doi: 10.1146/annurev.arplant.59.032607.092759
Brestic, M., Zivcak, M., Kunderlikova, K., Sytar, O., Shao, H., Kalaji, H. M., et al. (2015). Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 125, 151–166. doi: 10.1007/s11120-015-0093-1
Chen, H. X., Li, W. J., An, S. Z., and Gao, H. Y. (2004). Characterization of psii photochemistry and thermostability in salt-treated Rumex leaves. J. Plant Physiol. 161, 257–264. doi: 10.1078/0176-1617-01231
Chen, K., Sun, X., Amombo, E., Zhu, Q., Zhao, Z., Chen, L., et al. (2014). High correlation between thermotolerance and photosystem II activity in tall fescue. Photosynth. Res. 122, 305–314. doi: 10.1007/s11120-014-0035-3
Cui, L., Ben-Ying, S. U., Yang, F., and Yang, W. Y. (2015). Relationship between light interception and light utilization of soybean canopy in relay strip intercropping system. Sci. Agric. Sin. 48, 43–54.
Field, K. J., George, R., Fearn, B., Quick, W. P., and Davey, M. P. (2013). Best of both worlds: simultaneous high-light and shade-tolerance adaptations within individual leaves of the living stone Lithops aucampiae. PLOS ONE 8:e75671. doi: 10.1371/journal.pone.0075671
Foyer, C. H., and Noctor, G. (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gong, W., Qi, P., Du, J., Sun, X., Wu, X., Song, C., et al. (2014). Transcriptome analysis of shade-induced inhibition on leaf size in relay intercropped soybean. PLOS ONE 9:e98465. doi: 10.1371/journal.pone.0098465
Gong, W. Z., Jiang, C. D., Wu, Y. S., Chen, H. H., Liu, W. Y., and Yang, W. Y. (2015). Tolerance vs. avoidance: two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 53, 259–268. doi: 10.1007/s11099-015-0103-8
Hodges, D. M., Delong, J. M., Forney, C. F., and Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611.
Huang, W., Zhang, S. B., Zhang, J. L., and Hu, H. (2015). Photoinhibition of photosystem I under high light in the shade-established tropical tree species Psychotria rubra. Front. Plant Sci. 6:801. doi: 10.3389/fpls.2015.00801
Jiang, C. D., Wang, X., Gao, H. Y., Shi, L., and Chow, W. S. (2011). Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol. 155, 1416–1424. doi: 10.1104/pp.111.172213
Joliot, P., and Johnson, G. N. (2011). Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. U.S.A. 108, 13317–13322. doi: 10.1073/pnas.1110189108
Kalaji, H. M., Schansker, G., Brestic, M., Bussotti, F., Calatayud, A., Ferroni, L., et al. (2017). Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 132, 13–66. doi: 10.1007/s11120-016-0318-y
Kono, M., and Terashima, I. (2014). Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J. Photochem. Photobiol. B 137, 89–99. doi: 10.1016/j.jphotobiol.2014.02.016
Kornyeyev, D., Logan, B. A., and Holaday, A. S. (2010). Excitation pressure as a measure of the sensitivity of photosystem ii to photoinactivation. Funct. Plant Biol. 37, 943–951. doi: 10.1071/FP09276
Kromdijk, J., Głowacka, K., Leonelli, L., Gabilly, S. T., Iwai, M., Niyogi, K. K., et al. (2016). Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. doi: 10.1126/science.aai8878
Li, A., Li, S., Wu, X., Zhang, J., He, A., Zhao, G., et al. (2016). Effect of light intensity on leaf photosynthetic characteristics and accumulation of flavonoids in Lithocarpus litseifolius (Hance) Chun. (Fagaceae). Open J. For. 6, 445–459. doi: 10.4236/ojf.2016.65034
Li, L., Li, X. Y., Zeng, F. J., and Lin, L. S. (2016). Chlorophyll a, fluorescence of typical desert plant Alhagi sparsifolia, shap. at two light levels. Photosynthetica 54, 351–358. doi: 10.1007/s11099-016-0195-9
Li, L., Xiangyi, L., Xinwen, X., Lisha, L., Fanjiang, Z., and Fengli, C. (2014). Assimilative branches and leaves of the desert plant Alhagi sparsifolia shap. possesses a different adaptation mechanism to shade. Plant Physiol. Biochem. 74, 239–245. doi: 10.1016/j.plaphy.2013.11.009
Li, X. Q., Wang, J. G., Gen, C. X., and Jin, S. H. (2013). Gas exchange, chlorophyll fluorescence and antioxidant enzymes in leaves of centipede grass (Eremochloa ophiuroides) after barley stripe mosaic virus (bsmv) infection. J. Pure Appl. Microbiol. 7, 393–399.
Liu, W., Zou, J., Zhang, J., Yang, F., Wan, Y., and Yang, W. (2014). Evaluation of soybean (Glycine max) stem vining in maize-soybean relay strip intercropping system. Proc. Jpn. Acad. 91, 69–75.
Mishanin, V. I., Trubitsin, B. V., Benkov, M. A., Minin, A. A., and Tikhonov, A. N. (2016). Light acclimation of shade-tolerant and light-resistant Tradescantia, species: induction of chlorophyll a, fluorescence and P 700, photooxidation, expression of psbs and lhcb1 proteins. Photosynth. Res. 130, 275–291. doi: 10.1007/s11120-016-0252-z
Müller, P., Li, X. P., and Niyogi, K. K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566. doi: 10.1104/pp.125.4.1558
Niinemets, U., and Kull, O. (2001). Sensitivity of photosynthetic electron transport to photoinhibition in a temperate deciduous forest canopy: photosystem ii center openness, non-radiative energy dissipation and excess irradiance under field conditions. Tree Physiol. 21, 899–914. doi: 10.1093/treephys/21.12-13.899
Niyogi, K. K., and Truong, T. B. (2013). Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 16, 307–314. doi: 10.1016/j.pbi.2013.03.011
Porra, R. J. (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156. doi: 10.1023/A:1020470224740
Ruban, A. (2012). The Photosynthetic Membrane: Molecular Mechanisms and Biophysics of Light Harvesting. London: John Wiley. doi: 10.1002/9781118447628
Ruban, A. V., Johnson, M. P., and Duffy, C. D. (2012). The photoprotective molecular switch in the photosystem ii antenna. Biochim. Biophys. Acta 1817, 167–181. doi: 10.1016/j.bbabio.2011.04.007
Samantary, S. (2002). Biochemical responses of cr-tolerant and cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47, 1065–1072. doi: 10.1016/S0045-6535(02)00091-7
Shao, Q., Wang, H., Guo, H., Zhou, A., Huang, Y., Sun, Y., et al. (2014). Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLOS ONE 9:e85996. doi: 10.1371/journal.pone.0085996
Spicher, L., Glauser, G., and Kessler, F. (2016). Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front. Plant Sci. 7:167. doi: 10.3389/fpls.2016.00167
Strasser, R. J., Tsimilli-Michael, M., Qiang, S., and Goltsev, V. (2010). Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 1797, 1313–1326. doi: 10.1016/j.bbabio.2010.03.008
Strasser, R. J., Tsimilli-Michael, M., and Srivastava, A. (2004). “Analysis of the chlorophyll a fluorescence transient,” in Chlorophyll a Fluorescence, eds G. C. Papageorgiou and Govindjee (New York, NY: Springer), 321–362.
Sudhakar, C., Lakshmi, A., and Giridarakumar, S. (2001). Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under nacl salinity. Plant Sci. 161, 613–619. doi: 10.1016/S0168-9452(01)00450-2
Tateno, M., and Taneda, H. (2007). Photosynthetically versatile thin shade leaves: a paradox of irradiance-response curves. Photosynthetica 45, 299–302. doi: 10.1007/s11099-007-0049-6
Terashima, I., Hanba, Y. T., Tazoe, Y., Vyas, P., and Yano, S. (2006). Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57, 343–354. doi: 10.1093/jxb/erj014
Tóth, S. Z., Schansker, G., Garab, G., and Strasser, R. J. (2007). Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem ii. Biochim. Biophys. Acta 1767, 295–305. doi: 10.1016/j.bbabio.2007.02.019
Tsimilli-Michael, M., and Strasser, R. J. (2008). In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. Mycorrhiza 679–703. doi: 10.1007/978-3-540-78826-3_32
Vanlerberghe, G. C., Martyn, G. D., and Dahal, K. (2016). Alternative oxidase: a respiratory electron transport chain pathway essential for maintaining photosynthetic performance during drought stress. Physiol. Plant. 157, 322–337. doi: 10.1111/ppl.12451
Wang, G. G., Bauerle, W. L., and Mudder, B. T. (2006). Effects of light acclimation on the photosynthesis, growth, and biomass allocation in American chestnut ( Castanea dentata) seedlings. For. Ecol. Manag. 226, 173–180. doi: 10.1016/j.foreco.2005.12.063
Yan, Y., Gong, W., Yang, W., Wan, Y., Chen, X., Chen, Z., et al. (2010). Seed treatment with uniconazole powder improves soybean seedling growth under shading by corn in relay strip intercropping system. Plant Prod. Sci. 13, 367–374. doi: 10.1626/pps.13.367
Yang, F., Huang, S., Gao, R., Liu, W., Yong, T., Wang, X., et al. (2014). Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red: far-red ratio. Field Crops Res. 155, 245–253. doi: 10.1016/j.fcr.2013.08.011
Yang, W. Y., Yong, T. W., Ren, W. J., Fan, G. Q., and Lu, X. L. (2008). Develop relay-planting soybean, revitalize soybean industry. Soybean Sci. 27, 1–7.
Ye, Z. P. (2007). A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 45, 637–640. doi: 10.1007/s11099-007-0110-5
Zhang, J. Z., Shi, L., Shi, A. P., and Zhang, Q. X. (2004). Photosynthetic responses of four Hosta cultivars to shade treatments. Photosynthetica 42, 213–218. doi: 10.1023/B:PHOT.0000040592.10133.ee
Zivcak, M., Brestic, M., Kalaji, H. M., and Govindjee. (2014). Photosynthetic responses of sun- and shade-grown barley leaves to high light: is the lower psii connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 119, 339–354. doi: 10.1007/s11120-014-9969-8
Keywords: high radiation, stress responses, photosynthesis, PSII, photo-inhibition
Citation: Yao X, Zhou H, Zhu Q, Li C, Zhang H, Wu J-J and Xie F (2017) Photosynthetic Response of Soybean Leaf to Wide Light-Fluctuation in Maize-Soybean Intercropping System. Front. Plant Sci. 8:1695. doi: 10.3389/fpls.2017.01695
Received: 27 May 2017; Accepted: 14 September 2017;
Published: 28 September 2017.
Edited by:
Luis A. N. Aguirrezabal, National University of Mar del Plata, ArgentinaReviewed by:
Marek Zivcak, Slovak University of Agriculture, SlovakiaOscar Rodolfo Valentinuz, Instituto Nacional de Tecnología Agropecuaria (INTA), Argentina
Copyright © 2017 Yao, Zhou, Zhu, Li, Zhang, Wu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Futi Xie, c25zb3liZWFuQHNvaHUuY29t
 Xingdong Yao1
Xingdong Yao1 Futi Xie
Futi Xie