- International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India
High temperature is one of the biggest abiotic stress challenges for agriculture. While, Nitric oxide (NO) is gaining increasing attention from plant science community due to its involvement in resistance to various plant stress conditions, its implications on heat stress tolerance is still unclear. Several lines of evidence indicate NO as a key signaling molecule in mediating various plant responses such as photosynthesis, oxidative defense, osmolyte accumulation, gene expression, and protein modifications under heat stress. Furthermore, the interactions of NO with other signaling molecules and phytohormones to attain heat tolerance have also been building up in recent years. Nevertheless, deep insights into the functional intermediaries or signal transduction components associated with NO-mediated heat stress signaling are imperative to uncover their involvement in plant hormone induced feed-back regulations, ROS/NO balance, and stress induced gene transcription. Although, progress is underway, much work remains to define the functional relevance of this molecule in plant heat tolerance. This review provides an overview on current status and discuss knowledge gaps in exploiting NO, thereby enhancing our understanding of the role of NO in plant heat tolerance.
Introduction
An increase in temperature above the plant's optimum growth temperature that causes an irreversible damage to the growth is defined as heat stress (Wahid et al., 2007). Plants often encounter high temperature stress which disrupts plant metabolism and cellular homeostasis. More so in the coming decades, since ensuing steady increase in global temperature is predicted to have greater impact on biological processes (IPCC Climate Change, 2007; Johkan et al., 2011). Several reports indicate adverse effects of high and low temperatures on the molecular, biochemical, and physiological characteristics of plants (Suzuki and Mittler, 2006; Wahid, 2007; Mathur et al., 2011; Khan et al., 2013a; Chinthapalli et al., 2014; Brestic et al., 2016). Heat stress can cause oxidative burst, peroxidation of membrane lipids, pigment bleaching, protein degradation followed by enzyme inactivation and macromolecule damage in plants (Pospíšil et al., 2003; Suzuki and Mittler, 2006; Awasthi et al., 2016; Prasad et al., 2016; Santisree et al., 2017b). Heat stress has also been shown to disturb coordination of plant organelles, damage to the cytoskeleton, thereby altering cell differentiation and elongation (Smertenko et al., 1997). At times, high temperature lead to activation of silenced gene clusters nearer to the centromeric regions by inducing temporary loss of epigenetic gene silencing as shown in case of Arabidopsis thaliana (Lang-Mladek et al., 2010).
Plant responses to heat stress depend on plant developmental stage and strength of the stress condition (Brestic et al., 2012; Yamori et al., 2014). For example, in Cicer arietinum, exposure to high temperatures during the reproductive stage is more detrimental to yield when compared to the vegetative stage. Higher temperatures during anthesis lead to greater number of aborted and malformed buds, while at vegetative stage heat stress can negatively impact the development, morphology and nutrient uptake of the plant (Devasirvatham et al., 2012). Nevertheless, the optimum temperature varies among different compartments within the cell and species with in the genus (Pospíšil and Tyystjarvi, 1999; Hasanuzzaman et al., 2013; Chinthapalli et al., 2014). At times heat stress might lead to replacement of sensitive species by more heat tolerant species (Devasirvatham et al., 2012). A more recent research on 12 cultivars of Oryza sativa revealed a strong negative and differential impact of high night temperature on plant metabolism (Sharma et al., 2017). Hence, exploring the molecules that have the potential to protect plants from harmful effects of climate adversaries is gaining increasing interest among plant researchers (Hossain and Fujita, 2013; Mostofa and Fujita, 2013; Nahar et al., 2014; Wang et al., 2014; Savvides et al., 2016). Exogenous application of osmoprotectants, phytohormones, signaling molecules, trace elements have shown beneficial effects on plants under high temperatures, mostly due to their growth promoting and antioxidant capabilities (Uchida et al., 2002; Song et al., 2006; Khan et al., 2013b; Sagor et al., 2013; Mostofa et al., 2014; Nahar et al., 2014; Chan and Shi, 2015).
Nitric oxide (NO), a free radical gaseous molecule has been shown to be involved in diverse biological functions in plants (Domingos et al., 2015). Being a small diatomic molecule with short half-life, and absence of charge qualifies NO as an ideal diffusible molecular messenger in plant signaling (Yu et al., 2014). Although, initial discoveries in plants recognized NO as an atmospheric toxic pollutant for plant foliage (Wellburn, 1990), it was eventually considered as a modulator of plant defense during pathogen attacks (Durner et al., 1998). In due course, accumulated evidence suggested its key role in diverse plant physiological processes including seed germination (Bethke et al., 2007; Popova and Tuan, 2010), plant maturation and senescence (Mishina et al., 2007), multiple abiotic (Gould et al., 2003; Neill et al., 2008; Molassiotis et al., 2010; Tossi et al., 2012; Sehrawat et al., 2013; Ziogas et al., 2013), and biotic stress responses in plants (Durner et al., 1998; Schlicht and Kombrink, 2013). During the past decade number of studies focused on describing the crucial role of NO in moderating various plant hormone-mediated development and stress responses (Pagnussat et al., 2002; Gould et al., 2003; Freschi, 2013; Asgher et al., 2016). Further, accumulation of NO has been shown to induce gene expression of defense proteins during stress episodes and recovery (Romero-Puertas et al., 2013; Fancy et al., 2017). Mounting evidence suggests the role of NO in maintaining cellular homeostasis by acting as an antioxidant and negating the intensity of oxidative damage caused by various stress treatments (Sang et al., 2008; Karpets et al., 2011; Hasanuzzaman et al., 2012; Groß et al., 2013). Since the functional roles of NO in plants are being explored in parallel under various environmental challenges, it is imperative to recapitulate the current status of research with respect to individual stress conditions to have more focused future investigations addressing gaps in NO-mediated stress signaling in plants. During the past decade, the implication of NO in the mechanism of heat tolerance has been reported in many plant species (Figure 1; Uchida et al., 2002; Song et al., 2006). Despite the growing knowledge about NO-mediated plant heat responses such as decreasing ROS levels by the stimulation of antioxidants, protecting membranes from damage, osmolyte accumulation, and regulation of various hormone–mediated signaling events, its functional status has been far from clarity. Therefore, this review will focus on the current state-of-the-art regarding the emerging functions of NO during plant acclimation to heat stress.
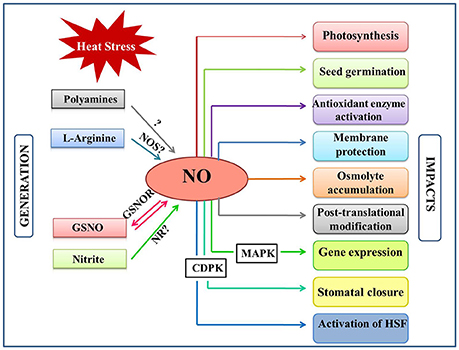
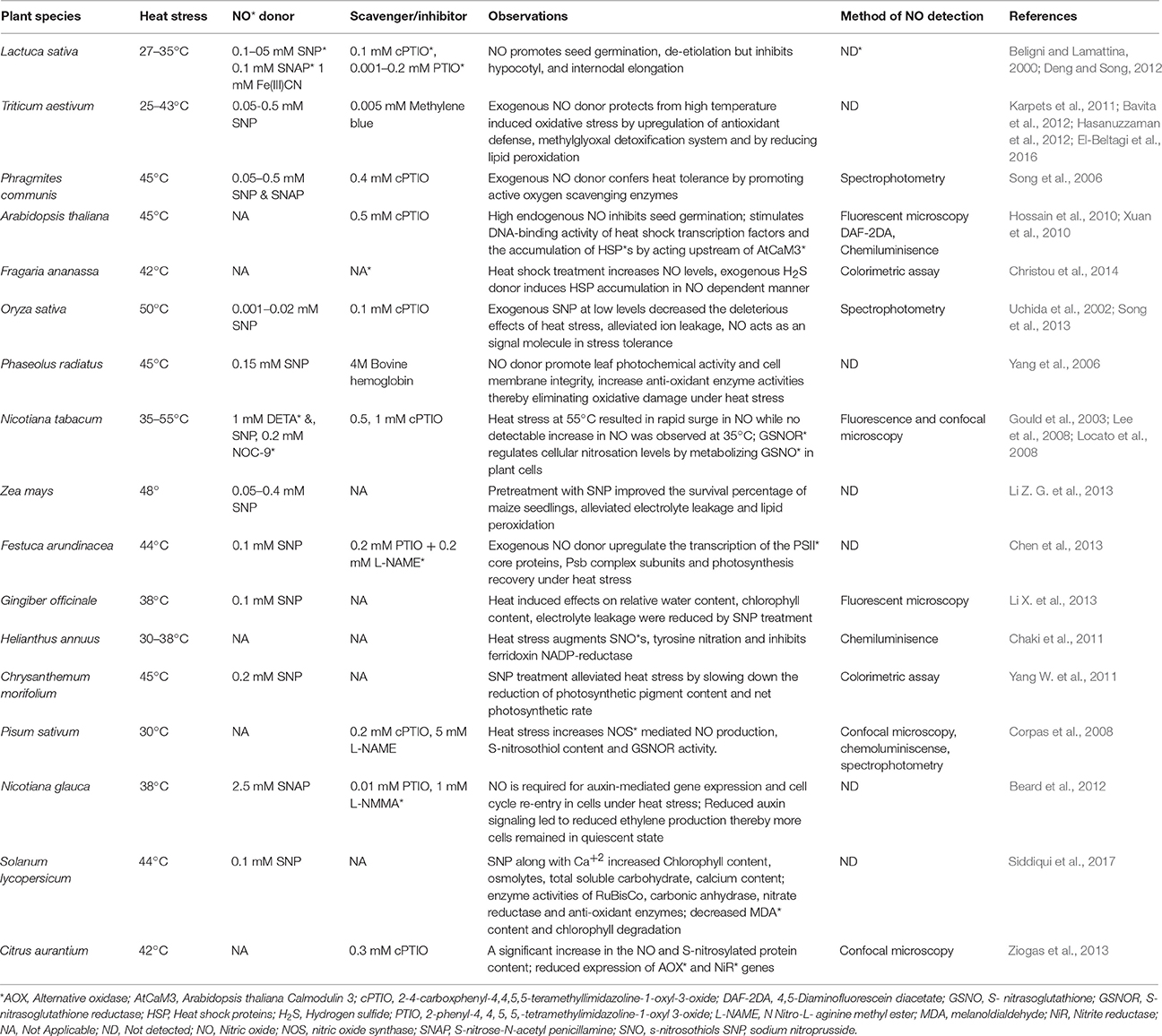
Figure 1. Overview of the NO generation and impacts in plants under heat stress. Heat stress induce NO accumulation majorly by a non-identified NOS-pathway, nitrite-dependent pathway. NO can also be generated by polyamines and by reversible regulation of S-nitrosoglutathione (GSNO) by the action of GSNO reductase (GSNOR). Besides, NO levels are also influenced by various plant hormones under heat stress. This figure also indicates the major plant functions that are mediated by NO-generation/signaling when exposed to heat stress. CDPK, Calcium dependent protein kinase; GSNO, s-ntrosoglutathione; GSNOR, GSNO reductase; HSFs, Heat shock factors; MAPK, mitogen activated protein kinase; NO, nitric oxide; NOS, Nitric oxide synthase; NR, nitratereductase.
NO in Connection with Heat Stress
The literature describes an increase in NO accumulation in various plant species in response to high temperature treatments (Leshem, 2001; Yu et al., 2014). Although, these studies underline the emission of heat induced NO as a vital response for increasing plant adaptation to stress (Bouchard and Yamasaki, 2008; Yu et al., 2014), NO burst depends on the severity and duration of heat treatment. For instance, Nicotiana tabacum suspension cells exhibited less NO burst upon heat shock at 35°C compared to cells at 55°C (Locato et al., 2008). Moreover, even a short–term heat treatment at 45°C increased NO levels in leaves of Medicago sativa and N. tabacum leaf peels (Leshem et al., 1998; Leshem, 2001; Gould et al., 2003). Meanwhile, the possibility of NO release as a generalized stress response has been ruled out and its functional specificity has been corroborated by scavenging endogenous NO levels by 2-4-carboxphenyl-4,4,5,5-teramethyllimidazoline-1-oxyl-3-oxide (cPTIO) that eliminated its beneficial effects under heat stress (Zhao et al., 2004; Song et al., 2006). Exogenous application of NO donors has also been able to reduce heat-induced cellular damage further underlining the involvement of NO in plant heat response (Song et al., 2006; Hasanuzzaman et al., 2013). Diverse cellular sites of NO synthesis have been documented previously under heat stress, mostly based on the fluorescent dyes such as 5,6- diaminofluorescein diacetate (Beard et al., 2012). For instance, heat stress induced NO-fluorescence was observed in diverse cell types in N. tabacum, including palisade mesophyll cells, all epidermal cell types, such as guard cells, subsidiary cells, long and short trichomes. However, the first appearance of heat induced NO fluorescence occurred in the plastids, followed by nucleus and subsequently spread across the cytosol (Gould et al., 2003). Other than 5,6- diaminofluorescein, electron paramagnetic resonance and chloroflurosence measurements have also been deployed to determine NO levels in thylakoid membrane complexes of Pisum sativum (Corpas et al., 2008; Wodala et al., 2008). Nonetheless, few reports contravene this heat induced NO accumulation in plants. Although, in M. sativa, heat treatment at 37°C for 2 h resulted in great NO emission, a reduced NO content was observed in P. sativam leaves and Chrysanthemum morifolium seedlings exposed at 38°C for 4 h (Corpas et al., 2008; Chaki et al., 2011). Furthermore, the reduced NO levels in C. morifolium seedlings was followed by a sequence of events such as reduced S- nitrasoglutathione reductase (GSNOR) activity, accumulation of SNOs, an increase in peroxynitrites and consequently intensified protein tyrosine nitration (Chaki et al., 2011). Similarly, heat stress at 38°C for 16 h reduced NO accumulation in the guard cell protoplasts of tree Nicotiana glauca (Beard et al., 2012). Abolishing internal NO levels by the treatment with a well- known NO synthase (NOS) inhibitor, L-N(G)-nitroarginine methyl ester, also resulted in similar effects of heat stress in these cells. A comprehensive list of heat stress studies related to NO are provided in Table 1. Although, it is clear that the modulation of intracellular NO levels are crucial under heat stress, the contradictory effects might depend on the type of plant tissues or species and severity of heat treatment.
NO Synthesis and Signaling in Plant Heat Stress
Despite evidence on functional existence of NO in plants, its metabolic origin and mode of its involvement in signaling pathways is yet unresolved (Gupta et al., 2011). Some of the pathways in NO production include NOS pathway (Delledonne, 2005; Negi et al., 2010), nitrate reductase (NR) pathway (Morot-Gaudry-Talarmain et al., 2002), other enzymatic and non-enzymatic pathways (Gupta et al., 2011). Although, the source of heat stress-induced NO accumulation is not clearly understood, few studies have reported an L-arginine-dependent production of NO through NOS-like activity (Corpas et al., 2008; Wodala et al., 2008) and NR-mediated production (Siddiqui et al., 2017). However, other studies demonstrated heat-induced accumulation of NO rather independent of enzymes (Hancock, 2012). NOS-dependent increase in the NO production was observed in Zooxanthellae (Symbiodinum microadriaticum), a marine microalgae, in a temperature-dependent manner (Bouchard and Yamasaki, 2008). Similarly, heat stress-induced increase in endogenous NO production in mycelial cells of edible fungi (Pleurotus eryngii) was found sensitive to the NOS inhibitor nitro-L- aginine methyl ester (L-NAME), while the NR suppressor tungstate had no effect, suggesting a NOS-dependent response to heat stress (Kong et al., 2012). High temperature induced NOS and GSNOR activities followed by the accumulation of NO and S-nitrosothiols without much change in the concentration of nitrites and nitrates in P. sativum leaves (Wodala et al., 2008). While this suggests that NOS activity is responsible for the NO produced in P. sativum leaves, the possibility of NO production from sources other than NOS activity cannot be ruled out.
While the source of NO under high temperature is yet to be defined in plants, the endogenous levels of NO in plant cells is regulated by many other factors such as S- nitrasoglutathione (GSNO) (Leterrier et al., 2012). This GSNO is a natural reservoir of NO, being transported over long distances through the phloem. The turnover of GSNO has been known to be controlled under stress conditions by GSNOR1, impacting the NO hemostasis in plant cells (Liu et al., 2001; Cheng et al., 2015). The A. thaliana loss-of function mutations in sensitive to hot temperatures 5 HOT5 GSNOR1 locus resulted in high heat sensitivity and increased s-nitrosothiols (SNO) and nitrate concentrations (Larkindale et al., 2005; Crawford et al., 2006; Lee et al., 2008). Moreover, heat sensitivity in these mutants was rescued using a NO scavenger (Lee et al., 2008). While the mutation in NOA1 locus encoding a GTPase, CUE1 gene encoding a chloroplast phosphoenolpyruvate/phosphate translocator, ARGAH1 or ARGAH2 encoding arginine amidohydrolase-1 and -2, respectively, PHB3 gene encoding prohibition are known to regulate NO levels in A. thaliana, their heat stress responses need further exploration (Flores et al., 2008; Lee et al., 2008; Moreau et al., 2008; Wang et al., 2010; Fröhlich and Durner, 2011). Besides, generation of peroxynitrite (ONOO−) from the reaction between NO· and and NO dioxygenase activity that produces from NO and also contribute for the elimination of NO from plant cells (Sanz-Luque et al., 2015). Good progress has been made in the recent years toward understanding the post-translational modifications (PTMs) mediated by NO in plants such as protein S-nitrosylation and tyrosine nitration, that have immense influence in the activity of various enzymes that confer stress tolerance (Chaki et al., 2011; Romero-Puertas et al., 2013; Fancy et al., 2017).
Heat Amelioration by Exogenous NO Donors
Exogenous NO donors have been often deployed successfully as priming agents to ward off abiotic stress induced losses in plants (Uchida et al., 2002; Zhao et al., 2007; Sang et al., 2008; Hasanuzzaman et al., 2012; Santisree et al., 2015; Savvides et al., 2016). Different donors/inhibitors of NO have been deployed in plant heat studies using various application methods (Tables 1, 2). In Triticum aestivum seedlings, pretreatment with 0.25 mM sodium nitroprusside (SNP) enhanced ascorbate and glutathione contents and activities of monodehydroascorbate reductase, dehydroascorbate reductase, and glyoxalase I and II under heat stress at 38°C given for 48 h (Hasanuzzaman et al., 2012). Likewise, Zea mays seedlings pretreated with 0.15 mM SNP enhanced the heat stress survival percentage at 48°C for 18 h as a result of reduced electrolyte leakage and malondialdehyde (MDA) content (Li Z. G. et al., 2013). The extent of its impact depends on factors like timing of application, developmental stage of the plant, combination of stress strength and donor concentration, tissue analyzed, and plant species (Chaki et al., 2011). Usually, exogenous application of selected dose of NO donors is a cost-effective approach in protecting plants from heat stress. However, it is very essential to establish the suitable NO donor, dosage, toxicity, complete NO releasing mechanism, byproducts and their bioactivities before large scale agricultural applications (Santisree et al., 2015). While, the use of SNOs, such as GSNO, the natural reservoir of NO in plants, could be encouraged under heat stress conditions, the release of NO from donors such as SNP often relies on the environmental factors like light, temperature, pH of the cells etc. and released toxic byproducts need to be carefully considered. Although, it is difficult to apply and measure the real cellular concentrations of NO donors/inhibitors, this pharmacological approach seems inevitable until the complete elucidation of NO generation in plants. In spite of technical difficulties, exploration of specific targets of these donors (Santisree et al., 2017a), cautious interpretation of results and combination of genetic and pharmacological approach seems more promising to give insights into NO-mediated heat stress regulation in future.
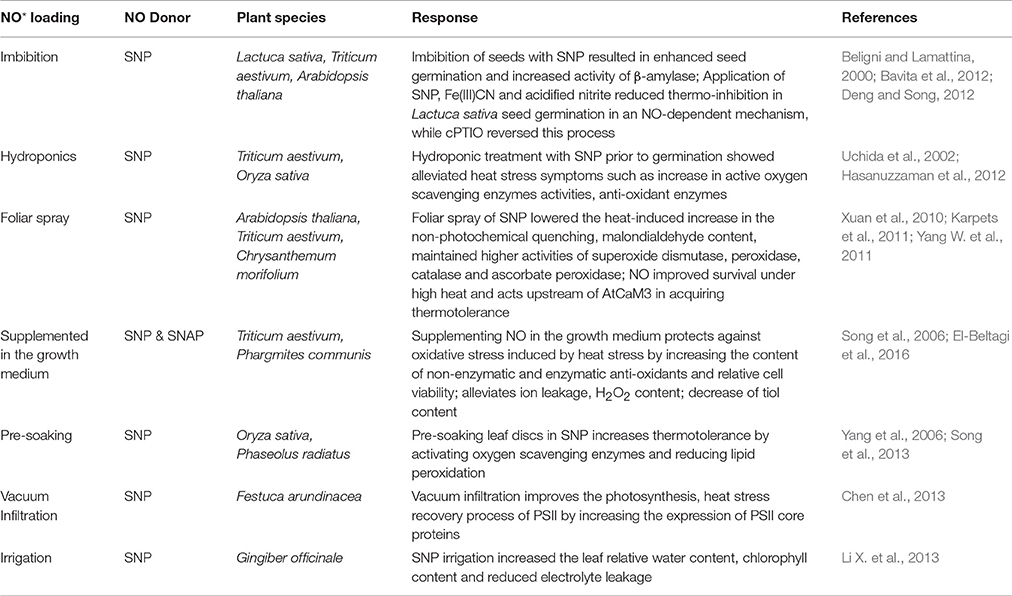
Table 2. Various methods deployed for exogenous nitric oxide treatment in plant heat stress studies.
NO as Antioxidant
Heat stress induces an increased generation of reactive oxygen species (ROS) such as the superoxide anion radicle () and hydrogen peroxide (H2O2) resulting in oxidative stress that causes a disturbance in the redox balance of cells (Khan et al., 2013a). These free radicals damage membranes and macromolecules that subsequently have detrimental effects on plant metabolism and yield (Santisree et al., 2015; Awasthi et al., 2016). Thus, the faster removal of ROS by antioxidant enzymes or molecules is often the deciding factor for plant survival under heat stress condition. The antioxidant function of NO has been well-documented under a wide range of stress responses including heat stress (Beligni and Lamattina, 1999; Uchida et al., 2002; Tian et al., 2007; Song et al., 2008; Palmieri et al., 2010; Santisree et al., 2015). Two significant roles of NO has been established in mitigating heat induced oxidative stress response, one is the maintenance of cellular redox homoeostasis due to its ability to neutralize the harmful ROS, and another is NO-induced protection against oxidative stress through moderating the carotenoids under heat stress. Carotenoids are known as protecting agents against photo-oxidative damage due to their ability to neutralize free radicals. Hence, enhanced levels of carotenoids offer better protection under heat stress. Foliar application of SNP enhanced the carotenoid content in heat-stressed C. morifolium which can be attributed to enhanced heat tolerance (Yang W. et al., 2011).
The most demonstrated ability of NO in reducing ROS levels is by enhancing antioxidant enzyme activities under unfavorable temperatures (Neill et al., 2002; Karpets et al., 2011). For instance, stimulation of ascorbate peroxidase and glutathione reductase enzyme activities by heat stress-induced NO signal in T. aestivum plays an important role in the metabolism of H2O2 (El-Beltagi et al., 2016). Likewise, 0.5 mM SNP-activated antioxidant enzymes superoxide dismutase, catalase and soluble peroxidase, were positively correlated with elevated tolerance in T. aestivum coleoptiles heat shocked at 43°C for 10 min (Karpets et al., 2011). Exogenous application of NO donor also conferred heat tolerance to the seedlings of Phaseolus radiatus and Phragmites communis by activating scavenging enzymes (Yang et al., 2006; Song et al., 2008). However, pretreatment of young O. sativa plants with 1 μM SNP alleviated salt or heat stress while high concentrations of SNP (>100 μM) decreased growth rate (Uchida et al., 2002). Promotion of antioxidant activity during heat stress period is the common NO-mediated protective response observed in most species, nonetheless there are some cases where NO sustained the antioxidant defense even after the stress episode. For instance, treatment with SNP not just enhanced heat-induced antioxidant enzymes activities, but also maintained their level during later stages as well in heat-stressed C. morifolium (Yang W. et al., 2011). Treatment of T. aestivum coleoptiles with scavenger of superoxide reduced generation and reversed heat resistance induced by SNP (Karpets et al., 2011). This suggests the importance of superoxide generation in SNP-induced heat resistance. However, prolonged and much sever heat stress episodes lead to the nitrosative stress due to substantially increased SNO content in P. sativum (Corpas et al., 2008). Hence, maintaining cellular NO status is essential to acquire thermotolerance in plants. GSNOR regulates endogenous NO levels by metabolizing GSNO, which is a well-known cytoplasmic NO reservoir in both plants and animals (Liu et al., 2001). The absence of GSNOR undeniably affects NO/nitroso levels and subsequently affects heat sensitivity. Hence, it was demonstrated that GSNOR function is required for acclimation to high temperature and for normal plant growth and fertility (Lee et al., 2008; Xu et al., 2013). The importance of GSNOR has been highlighted by a null mutation (atgsnor1-3/hot5-2) or RNAi line that reduced GSNOR expression and lead to over-accumulation of NO that subsequently correlated with the heat-sensitive phenotype in A. thaliana. Moreover, these thermotolerance defects has been rescued with NO scavengers further supporting the link between excess NO-related nitrosation and plant heat sensitivity in plants (Lee et al., 2008). Apart from this, NO overproducing nox1 mutant seedlings were defective in heat tolerance while, noa1 and nia1/nia, mutants producing less endogenous NO (Crawford et al., 2006) were similar to wild-type seedlings in their heat tolerance. Although, the available information made it clear that maintaining cellular levels of NO is an important component of plant heat stress response, the fine regulation of ROS/NO using exogenous NO donors under heat stress needs further investigations.
NO and Membrane Integrity
One of the important components of heat tolerance is the maintenance of membrane integrity. Heat stress induced oxidative burst significantly increase the membrane lipid peroxidation consequently reduce the membrane thermostability and increase electrolyte leakage in plants (Hasanuzzaman et al., 2013; Khan et al., 2013a). By virtue of its free radical nature, NO may interact with lipid hydroperoxyl or superoxide radicals that promote lipid peroxidation and acts as a chain breaker to ensure the protection of membranes (O'Donnell et al., 1997). It was found that application of 0.2 mM SNP and S-nitrose-N-acetyl penicillamine dramatically reduced heat induced ion leakage, reduction in growth, and loss of cell viability in callus of P. communis treated with 45°C for 2 h (Song et al., 2006). High temperature causes enhanced accumulation of thiobarbituric acid reactive substances by reducing membrane protein thiol level (Bhattacharjee, 2009). While the thiobarbituric acid reactive substances content was substantially reduced in 0.15 mM SNP-treated P. radiatus leaf discs, that was partially reversed by adding equal concentration of NO scavenger, thereby suggesting NO-induced protection from lipid peroxidation (Yang et al., 2006). 0.2 mM SNP also lessened lipid peroxidation in the leaves of C. morifolium plants subjected to 45°C for 24 h (Yang W. et al., 2011). Reduced lipid peroxidation by SNP treatment was also evident by the reduction in membrane leakage by up to 48% in comparison with the non-treated controls in P. radiatus leaf discs (Yang et al., 2006). Similarly, alleviation of heat-induced lipid peroxidation and electrolyte leakage by pretreatment with SNP was also demonstrated in T. aestivum seedlings (Hasanuzzaman et al., 2012). Heat stress-induced MDA content in hydroponically grown T. aestivum seedlings was brought down substantially by SNP treatment (Hasanuzzaman et al., 2012). Moreover, enhanced membrane thermostability and reduced lipid peroxidation by NO were more pronounced in the shoots over roots of heat tolerant T. aestivum that could suggest site-specific regulation of NO effects under heat stress (Bavita et al., 2012). Similarly in another study, exogenous application of SNP recovered relative water content, chlorophyll(chl) concentration, electrolyte leakage in heat stressed Gingiber officinale leaves consequently normalized chl fluorescence parameters (Li X. et al., 2013). Taken together, these studies provided good evidence that NO donor treatment can enhance membrane stability and thus reduce electrolyte leakage under heat stress.
NO and Photosynthesis Under Heat Stress
Photosynthesis, a vital plant process that forms sole basis for all assimilates is highly vulnerable to heat stress (Allakhverdiev et al., 2008; Khan et al., 2013b; Mathur et al., 2014). High temperature affects the physicochemical properties and functional organization of thylakoid membrane thus irreversibly damaging the chloroplast protein complexes including photosystem II (PSII) (Brestic et al., 2012). Heat stress also induce some reversible effects such as increase in photorespiration, decreased activities of Calvin–Benson cycle enzymes including RuBisCo, RuBisCO activase. Both reversible and irreversible effects of heat often lead to yield penalty as well as longer impacts on plant metabolism (Brestic et al., 2014, 2016; Mathur et al., 2014). Hence, increasing photosynthetic efficiency by enhancing heat acclimation of photosynthetic apparatus is one of the most desirable future target (Yamori et al., 2014). To achieve this goal, it is vital to understand the processes that limit the photosynthetic productivity under heat stress and the role of NO in ameliorating these processes.
Plants show reduced chl biosynthesis and damage of photosynthetic apparatus such as swelling of grana stacks followed by ion leakage form leaf cells when exposed to high temperatures (Allakhverdiev et al., 2008). Several studies suggest the role of NO in heat acclimation of photosynthesis at various levels. In several species such as C. morifolium, Quercus pubescens, O. sativa, Vicia faba, and Solanum lycopersicon, heat stress induced decrease in photosynthesis rate (Haldimann and Feller, 2004) and chl bleaching (Misra, 1980, 1981; Misra et al., 1997; Brestic et al., 2012) got partially alleviated by SNP treatment by delaying the reduction of photosynthetic pigment content and net photosynthetic rate (Yang Q. et al., 2011). Literature suggests the inactivation of both electron acceptor and donor sides of PSII by high temperature (Pospíšil and Tyystjarvi, 1999; Mathur et al., 2011, 2014; Brestic et al., 2012). NO has been shown to prevent this heat induced chl loss and maintain the activity of PSII, thereby mitigating the reduction in photosynthesis (Misra, 1981). SNP treatment also helped to retain the level of Fv/Fm and inhibited the rise in Fo (Yang Q. et al., 2011). As an evidence, pre-treatment with 100 μM SNP resulted in enhanced photosynthetic electron transport in Festuca arundinacea at 44°C (Chen et al., 2013). Similarly, increased survival rate of T. aestivum and Z. mays seedlings was also observed under extreme temperatures due to SNP treatment. The involvement of NO in heat acclimation of photosynthesis was further confirmed by the temperature-dependent synthesis of chl as well as RuBisCo by overexpressing NOA1 in O. sativa plants (Yang Q. et al., 2011). In addition to its role in enhancing chl biosynthesis, NO is also shown to reduce chl degradation (Kong et al., 2014). The reduced chl loss in NO treated plants can also attribute to its close relation to iron metabolism which has a direct correlation with chl biosynthesis and plant productivity. It has been observed earlier that exogenous application of SNP promoted uptake, translocation and internal availability of iron in plants (Graziano et al., 2002; Zhang et al., 2012; Kong et al., 2014). In addition, exogenous NO donor was also known to revert the chlorotic phenotype of Z. mays mutants yellow stripe1 and yellow stripe3 defective in iron uptake mechanism providing genetic evidence for its role in NO uptake(Graziano et al., 2002). Furthermore, heat-induced structural and functional changes in the thylakoid membrane often results in ROS formation (Pospíšil et al., 2007; Pospíšil and Prasad, 2014; Pospíšil, 2016). Heat stress induced oxidative stress slowdown carbon assimilation by inhibiting the activities of ferredoxin–NADP oxidoreductase and carbonic anhydrase by 31 and 43%, respectively. Indeed, the antioxidant property of NO has been demonstrated by many studies where NO acts as a signal to induce the antioxidative enzyme activity, consequently protect plants from heat-induced oxidative damage (Song et al., 2008). Besides, NO has been shown to influence reversible inhibition of key enzymes of photosynthesis by tyrosine nitration (Chaki et al., 2011, 2013). For instance, the combined application of SNP and calcium resulted in higher levels of Chl a and b due to reduced Chl degradation, and also enhanced activities of RuBisCo, carbonic anhydrase and NR under heat stress in Solanum lycopersicum (Siddiqui et al., 2017). Non-photochemical quenching is a mechanism of protection to the photosynthetic apparatus by dissipating excess energy, while also reducing the excitation energy necessary for PSII execution (Gilmore, 1997). Treatment with 5 mM SNP reduced the rate of Non-photochemical quenching in T. aestivum leaf discs heat shocked for 2 h at 35°C and thus diverted more energy to PSII (Hossain et al., 2011). Similarly, a T-DNA insertional mutant of Arabidopsis thaliana glb3 that has knocked-out for class 3 hemoglobin gene responsible to scavenge excess NO, exhibited severe heat induced reduction in non-photochemical quenching due to over accumulation of NO (Hossain et al., 2011). While this was completely prevented by cPTIO, exogenous NO donors photocopied the observed response. These results clearly demonstrate the role of NO in heat induced decline of non-photochemical quenching (Hossain et al., 2011).
Despite of its protective effects on photosynthesis uncontrolled burst of NO due to high temperatures results in damage to the photosynthetic machinery (Zhang et al., 2009). Excess NO inhibits electron transport by reversibly binding to thylakoid membrane complexes of P. sativum (Wodala et al., 2008). Similarly, the levels of NO, S-nitrosothiols (SNO), and were also enhanced leading to nitrosative stress under high heat in Citrus aurantium plants (Ziogas et al., 2013). This increment is associated with reduced chl content and increased electrolytic leakage. In Symbiodinium microadriaticum, heat-induced NO production caused a decline in the photosynthetic efficiency of PSII (Fx/Fm), which correlated with the coral bleaching phenomenon (Bouchard and Yamasaki, 2008). However, unlike other free radicals, the generated NO can diffuse rapidly. In a situation like heat stress where photosynthetic electron transport is restricted, the cytosolic nitrite is converted to NO by NR in presence of NAD(P)H and diffuses out easily into the atmosphere (Yamasaki, 2000). Hence, the emission of NO has been speculated as an effective way to dissipate excess free radicals under heat stress.
Seed Germination and Osmolyte Accumulation
Although, slightly higher than optimum growth temperature is needed for seed germination, heat stress exerts negative impact on seed germination (Essemine et al., 2007; Essamine et al., 2010; Brunel-Muguet et al., 2015). High temperature affects the rate of germination, percentage of germination, seedling emergence in many plant species. At times heat stress completely prohibited seed germination due to embryo damage and cell death (Johkan et al., 2011). Data have shown the promotion of seed germination by exogenous application of NO-releasing compounds in various plants (Bethke et al., 2006; Arc et al., 2013). NO has been implicated in promotion of seed germination in many species such as A. thaliana, Hordeium vulgare, Panicum virgatum, Lactuca sativa, Stellaria media, Malus domestica either by reducing seed dormancy, or by reducing the effects of adverse environmental conditions (Giba et al., 1992; Beligni and Lamattina, 2000; Zhang et al., 2005; Bethke et al., 2007; Krasuska et al., 2017). One mechanism of reduced seed germination by heat stress is by decreasing the mobilization and utilization efficiency of seed reserves (Blum and Sinmena, 1994). SNP has been found to promote seed germination by inducing the activity of β-amylase during early stages of seed germination in many plant species such as A. thaliana, T. aestivum, M. sativa (Zhang et al., 2005; Duan et al., 2007; Maurice et al., 2016). NO has been implicated in loss of dormancy by decreasing seed sensitivity to abscisic acid (ABA), while acting upstream of gibberellic acid during vacuolation of protein storage vacuoles (Bethke et al., 2006, 2007). However, according to a recent study excessive NO production under heat stress is speculated to be involved in thermo-inhibition of seed germination in A. thaliana (Hossain et al., 2010). Further, the seeds of A. thaliana T-DNA insertion mutant glb3 compromised in NO scavenging activity, fail to remove heat-induced excessive NO and hence, show high temperature sensitivity during germination at 32°C, while NO scavenger cPTIO partially restored the germination up to 40% in comparison with 100% in the wild type (Hossain et al., 2010). Similarly, application of SNP, Fe(III)CN, and acidified nitrite reduced thermo-inhibition in L. sativa seed germination in an NO-dependent mechanism, while cPTIO reversed the stimulation of seed germination by these compounds (Deng and Song, 2012). Although, the optimum temperatures for L. sativa seed germination were 11–19°C in light, SNP promoted germination up to 25°C in light in a dose-dependent manner (Deng and Song, 2012). Not only that, seed germination of L. sativa seeds at 25°C was independent of light in NO donor imbibed seeds (Beligni and Lamattina, 2000). Based on these results the relevance of NO emission within the deeper soil layers has been speculated to stimulate light responses such as germination where photoperception is low being even more potent than gibberellin. This notion was supported by the involvement of NO in the phytochrome controlled seed germination in Paulownia tomentosa (Giba et al., 1998). Seed dormancy due to soil temperature has been recently linked to the regulation of ABA metabolism and signaling genes (Arc et al., 2013). Indeed, A. thaliana copper amineoxidase, cuo1 mutant seedlings defective in ABA-induced NO synthesis, exhibit a reduced sensitivity to exogenous ABA during germination (Wimalasekera et al., 2011).
Several lines of evidence have reinforced the importance of accumulation of osmolytes such as proline, glycine-betaine, and soluble sugars to maintain osmotic balance in heat stressed plants (Hasanuzzaman et al., 2013). SNP pre-treatment up-regulated the P5CS gene in O. sativa seedlings that helped in survival after exposition to heat stress (Uchida et al., 2002). Similarly, transcriptional induction of P5CR by heat and salt stress was observed in A. thaliana (Hua et al., 2001). Indeed, a 92 bp fragment of the P5CR 5′UTR was found as a regulator to control its mRNA stability under heat stress (Hua et al., 2001). The combined application of SNP along with calcium enhanced proline as well as glycine-betaine, osmolyte content observed in heat-stressed S. lycopersicum seedlings (Siddiqui et al., 2017). From previous studies, it is known that while exogenous application of polyamines to A. thaliana seedlings induced NO production, exogenous application of NO donor also promoted polyamine metabolism in plants. For example, SNP treatment reduced putricine/PAs ratio in G. officinale leaves under heat stress (Li X. et al., 2013). Global metabolite profile of NO-treated A. thaliana seedlings also supported the accumulation of polyamines by NO as a possible strategy to attenuate stress-induced cell death (León et al., 2016). Although, direct link between polyamines and NO is yet to be revealed under heat stress, a general concentration-dependent proline and polyamine metabolite accumulation was reported by SNP treatment in Medicago truncatula leaves (Filippou et al., 2013). In fact, increased polyamine biosynthesis by any means can also result in increased proline content (Minocha et al., 2014). Heat stress often decreases soluble sugars and proteins that are also implicated in the loss of fertility due to failed pollen germination in crops such as C. arietinum (Kaushal et al., 2013). While exogenous NO enhanced soluble sugars and proteins in various stress responses such as salinity, drought etc., increased soluble sugar content under heat stress is yet to be studied (Ahmad et al., 2016).
NO Regulation at Gene and Protein Level
NO trigger plant responses at multiple levels, from gene to the metabolites to achieve heat stress tolerance (Palmieri et al., 2010; León et al., 2016). Few studies using the mutational and transgenic approaches have provided molecular insights into the role of NO in thermotolerance in plants (Table 3). In mutants gsnor1/hot5 and nox1/cue1, unusual thermotolerance was observed due to the accumulation of NO (Fancy et al., 2017). As discussed earlier, A. thaliana seeds of glb3 mutant lacking the hemoglobin gene also accumulated high cellular NO exhibit high temperature sensitivity akin to NO donor treated wild type plants. Although these mutants increased the momentum of NO exploration in heat stress studies, complete characterization of such responses is imperative to elucidate NO role in heat stress. Similarly, transgenic efforts using nNOS lines of N. tabacum, antisense suppression of GSNOR or overexpression of non-symbiotic hemoglobins in A. thaliana also confirmed the role of NO in heat stress tolerance (Table 3; Lee et al., 2008; Hossain et al., 2010; Shi et al., 2014).
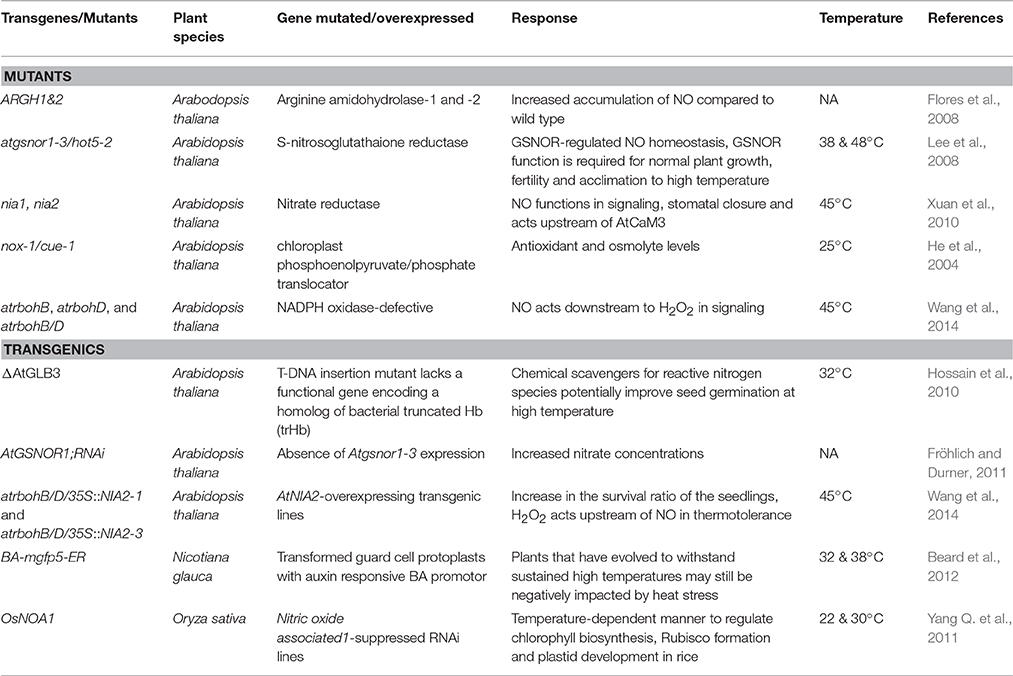
Table 3. List of few mutants and transgenic lines used in elucidation of nitric oxide (NO) role in plant heat tolerance.
During the last decade, few studies demonstrated implications of NO on modulation of gene transcription and post-translational modifications of proteins during stress conditions in plants by using exogenous donors (Ahlfors et al., 2009; Besson-Bard et al., 2009; Palmieri et al., 2010). NO induced transcription of many stress-inducible genes improved the plant survival and yield under heat stress (Begara-Morales et al., 2014). Similarly, SNP pretreatment improved heat stress tolerance in O. sativa seedlings by inducing gene transcription of sucrose-phosphate synthase, small heat shock protein (HSP) 26 and d-pyrroline-5- carboxylate synthase (Uchida et al., 2002). Similarly, the expression of alternative oxidase and nitrite reductase were observed to be reduced in the heat-treated C. aurantium plants (Ziogas et al., 2013). While, transcriptomic analysis of GSNO-responsive genes in A. thaliana roots showed up-regulation of various heat stress transcription factors and HSPs (Begara-Morales et al., 2014), the heat induced accumulation of Hsp70 corresponded to the endogenous NO level in S. lycopersicum leaf discs (Piterková et al., 2013) pointing toward an important role of HSPs in protecting cellular membranes and protein folding and aggregation during heat stress. Another study indicated the NO induced stimulation of A. thaliana calmodulin 3(AtCaM3), which in turn activates its heat shock factor-DNA binding activity and HSP gene expression imparting heat tolerance (Xuan et al., 2010). There have been pointers on NO in a signaling cascade together with other signaling molecules such as ABA, H2O2, Ca2+, and calmodulin, stimulate DNA-binding activity of heat shock factors and abundance of HSPs, leading to heat stress resistance in plants (Khan M. N. et al., 2015).
NO directly alters a wide variety of key proteins through PTMs resulting in remarkable change in their activity, ionic state, aggregation, and cellular location establishing the functional relevance of NO signaling in plants (Martinez-Ruiz et al., 2011). Recent studies demonstrated these PTMs as the most effective means through which NO exerts its global impact on most of the proteins and enzymes during various stress conditions (Romero-Puertas et al., 2013). NO-mediated post-translational modifications such as S-nitrosylation and tyrosine nitration has been involved in heat stress responses in plants (Fancy et al., 2017). Protein tyrosine nitration was intensified by heat stress in P. sativum leaves indicating the nitrosative stress caused by high temperatures (Corpas et al., 2008). Similarly, the heat induced nitrosative stress lead to enhanced protein nitration in C. morifolium seedlings. Detailed analysis of the nitroproteome revealed the induction of 13 tyrosine-nitrated proteins by heat stress including enzymes like ferredoxin–NADP oxidoreductase and carbonic anhydrase (Chaki et al., 2011). Further, it was found by in silico analysis that Tyr205 is the most likely potential target for nitration in P. sativum CA sequence (Chaki et al., 2013). Similarly there have been reports on NO-induced post-translational modifications in antioxidant enzymes such as catalse, superoxide dismutase, peroxiredoxins, as well as enzymes of the ascorbate-glutathione cycle during stress conditions (Santisree et al., 2015). S-nitrosylation of antioxidant enzymes not just stimulates their activities, but also protects Cys residues from ROS-mediated irreversible oxidations. Similarly, inhibition of ascorbate peroxidase activity by S-nitrosylation also acts as signaling PTM due to subsequent degradation by ubiquitination followed by programmed cell death in N. tabacum BY2 cells under heat stress (de Pinto et al., 2013). Several other proteins such as GSNOR and NADPH are also prone to S-nitrosylation, with S-nitrosylation of GSH forming GSNO, that in turn mediates transnitrosylation reactions under stress (Lee et al., 2008; Xu et al., 2013). A detailed global molecular profiling by omics approaches could potentially increase this knowledge on the effect of NO and NO-mediated PTMs at subcellular level in order to mitigate nitrosative stress impacts induced by high heat.
NO and Other Signaling Molecules Under Heat Stress
NO signaling under heat stress involves a cross talk with other signaling molecules such as cyclic adenosine diphosphate ribose, mitogen activated protein kinases, Ca2+, and phytohormones (Figure 2; Mioto et al., 2014; Khan M. N. et al., 2015; Asgher et al., 2016). Although, only few reports explored the link between them under heat stress, action of NO as a downstream component in hormone-mediated pathways appears to be the common factor in thermotolerance (Mioto et al., 2014; Asgher et al., 2016). A possible role for NO as a downstream component in the auxin signaling pathway has been proposed in a series of plant growth and stress responses (Pagnussat et al., 2002; Hu et al., 2005; Chen et al., 2010; Kolbert et al., 2012; Freschi, 2013; Asgher et al., 2016). In addition to the impact of auxin on NO production, NO has been shown to influence auxin metabolism, transport and signaling (Fernández-Marcos et al., 2011; Terrile et al., 2012). Although, literature supports an intricate interconnection between these signaling pathways in various plant processes, their link has not been established under heat stress. However, in heat-stressed N. tabacum guard cell protoplasts, reduced NO levels lead to the suppression of auxin signaling suggesting NO as the central determinant of auxin-mediated cell fate (Beard et al., 2012). Further treatment with NOS-inhibitor L-N(G)-nitroarginine, photocopied the effects of heat stress in these protoplasts and seedlings by limiting cell division, expansion and dedifferentiation, and repressing the auxin-responsive promoter eventually resulting in reduced lateral root elongation, petiole length, and leaf expansion (Beard et al., 2012). This heat induced reduction of NO levels lead to the reduction of auxin signaling, that subsequently reduced ethylene production making most of cells quiescent. Thus far, a general antagonism has been observed between NO and ethylene for most of the physiological processes (Manjunatha et al., 2012; Melo et al., 2016) besides the indication of synergistic effects (Gniazdowska et al., 2007; Arc et al., 2013). In agreement with the reported antagonism of ethylene and NO, it is speculated that loss of root hair formation in N. glauca under heat treatment might be due to the scavenging effect of heat induced ethylene on NO (Beard et al., 2012). Numerous studies have implicated the role of ethylene in thermo-inhibition of seed germination (Arc et al., 2013). Induction of thermo-dormancy is correlated with reduced ethylene levels and ethylene biosynthetic gene expression, while exogenous ethylene could overcome the thermo-dormancy in C. arietinum and A. thaliana (Arc et al., 2013). One can attribute the observed inhibition of seed germination due to the increased NO levels as an indirect effect of the reduced ethylene levels under heat stress, a notion supported by increased seed germination upon NO scavenging. NO can modulate ethylene biosynthesis by reversibly inhibition of methionine adenosyltransferase through S-nitrosylation, leading to the reduction of the S-Adenosyl methionine pool or by stimulation of 1-aminocyclopropane 1-carboxylic acid-malonyltransferase activity thus diverting S-Adenosyl methionine pool toward the polyamine pathway (Lindermayr et al., 2006; Arc et al., 2013). Polyamines also have a significant role in heat tolerance in plants as evident by the altered heat sensitivity of transgenic plants overexpressing spermine synthase and a spermine deficient mutant plant of A. thaliana (Sagor et al., 2013). These results revealed the positive correlation between higher spermine content and thermotolerance. The reported functional overlap between polyamines and NO under other stress conditions and their co-occurrence in heat stress responses represents an rich scope for further interaction studies under heat stress (Tun et al., 2006; Fan et al., 2013; Nahar et al., 2016).
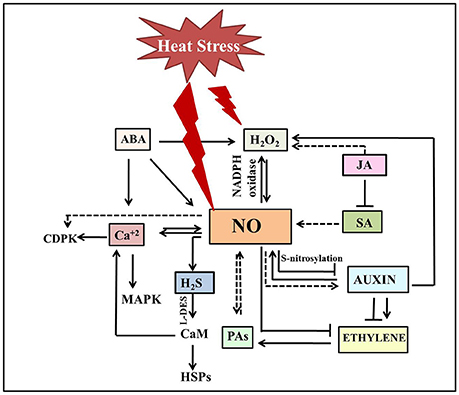
Figure 2. Schematic illustration depicting cross talk of NO with other signaling molecules under heat stress. Heat stress triggers NO accumulation which in turn either stimulates (normal end arrow) or inhibits (blunt end arrow) hormone-mediated heat stress signaling. NO mostly acts downstream to phytohormones under heat stress. Plant heat tolerance not just include crosstalk between H2O2, NO, and H2S, but also involve activation of Ca2+ channels, and Ca2+-CaM-dependent protein phosphatase along with other factors to induce DNA-binding activity of the heat shock factors and subsequent accumulation of HSPs. The dotted line denotes the pathways not studied clearly. The double-sided arrow indicates the mutual regulation of molecules. ABA, Abscisic acid; Ca2+, calcium ion; CaM, calmodulin; CDPK, calcium dependent protein kinase; H2O2, hydrogen peroxide; HSPs, heat shock proteins; H2S, Hydrogen sufide; JA, jasmonic acid; MAPK, mitogen activated protein kinase; NO, nitric oxide; SA, salicylic acid; PAs, polyamines.
In addition, hydrogen sulfide (H2S)donor was also shown to improve heat tolerance in N. tabacum suspension culture cells (Li et al., 2015; Li and Jin, 2016) by enhancing calcium and proline levels NO has been suggested to act upstream to H2S in the acquisition of heat tolerance in Z. mays seedlings (Li Z. G. et al., 2013). SNP promoted the accumulation of H2S by stimulating the activity of L-cysteine disulfhydrase, that has resulted in enhanced heat survival percentage of Z. mays seedlings. While SNP-induced heat tolerance was augmented by the application of H2S donors such as sodiumhydrosulphideor GYY4137, its inhibitors/scavengers like DL-propargylglycine, aminooxyacetic acid, potassium pyruvate, hydroxylamine, and hypotaurine diminish heat tolerance (Li Z. G. et al., 2013). Sodiumhydrosulphide-pretreatment in Fragaria ananassa plants preserved photosynthesis by reducing MDA, H2O2 content and increasing the gene expression of antioxidants without any difference in NO levels under heat stress (Christou et al., 2014), thereby suggesting the operation of downstream to NO.
In another study, pretreatment with salicylic acid inhibited ethylene synthesis and increased nitrate reductase and γ-glutamyl kinase in T. aestivum leaves under heat stress. Although, not supported yet by experimental evidence, the observed reduction in ethylene levels and increased NR activity drives us to suggest the probable involvement of NO in this signaling cascade (Khan et al., 2013a,b; Khan M. I. R. et al., 2015). Stomatal closure is the other response where a possible downstream operation of NO and H2O2 has been suggested (Khokon et al., 2011; Zandalinas et al., 2016). Notably, the details of the actual mechanisms of NO and salicylic acid interaction in heat stress mitigation is not fully known. Heat induced stomatal closure in the leaves of A. thaliana has been observed due to enhanced NO levels triggered by the heat-induced H2O2, jasmonic acid, and repression of salicylic acid. Interestingly, NO-induced stomatal closure was also observed in ABA signaling mutant (abi1-1) suggesting an ABA-independent signaling triggered by NO (Zandalinas et al., 2016). Interestingly, in heat-stressed P. communis calli, inhibition of ABA synthesis by fluridone while showed no influence on the protective effect of exogenous NO, inhibition of NO accumulation by cPTIO and nito-L-arginine blocked the protective effect of exogenous ABA. Exogenous ABA further increased the heat induced NOS activity and NO release (Song et al., 2008) suggesting the role of NO as a key downstream molecule in ABA-dependent and -independent heat stress mitigation.
A broad spectrum of environmental stresses including heat stress trigger the accumulation of endogenous H2O2 (Lopez-Delgado et al., 1998). Exogenous H2O2 also enhanced thermotolerance in Agrostis stolonifera and S. lycopersicum microplants (Lopez-Delgado et al., 1998; Larkindale and Huang, 2005). Both NO and H2O2 operate in synergistic as well as antagonistic manner to serve as stress signals in plants (Neill et al., 2002) where their synthesis is synchronous during stress responses. Crosstalk between NO, H2S, and H2O2 has been reported in acquisition of heat tolerance in Z. mays seedlings (Li Z. G. et al., 2013). Nevertheless, feedback inhibition of H2O2 through the stimulation of antioxidant enzymes has conferred thermotolerance in plants (Wu et al., 2015). For example, SNP presoaking of P. radiatus leaf discs reduced subsequent production of H2O2 (Yang et al., 2006; Hasanuzzaman et al., 2013). While, increasing endogenous NO levels improved the heat tolerance in H2O2-deficient NADPH-oxidase mutants of A. thaliana, atrbohB, atrbohD, and atrbohB/D, enhanced endogenous H2O2 levels did not affect the NO-deficient noa1 mutant, designating NO as a downstream factor in H2O2 signaling. Further, overexpression of AtNIA2 or AtNOA1 also restored stress acquisition in atrbohB/D mutant further confirming the relationship between NO and H2O2 in heat stress tolerance (Wang et al., 2014). In another study, pretreatment of Z. mays seedlings with H2O2 rapidly induced endogenous H2O2, NO, and H2S accumulation under heat stress, and this accumulation was reversed by H2O2 scavenger dimethylthiourea and NO scavenger cPTIO, indicating that H2O2 induced heat stress tolerance was involved in the crosstalk between downstream components NO and H2S (Li Z. G. et al., 2013).
Plant heat tolerance not just include crosstalk between H2O2, NO, and H2S, but also involve activation of Ca2+ channels, and Ca2+-CaM-dependent protein phosphatase along with other factors to induce DNA-binding activity of the heat shock factors and subsequent accumulation of HSPs (Wang et al., 2014). NO acted upstream of AtCaM3 in A. thaliana, inducing the DNA-binding activity of heat stress transcription factors and the abundance of HSP18.2 (Xuan et al., 2010) suggesting that NO acts in a systematic signaling pathway recruiting other signaling molecules timely at the right location in order to achieve heat stress tolerance. So far, while the crosstalk between these signaling molecules and events has been well studied in drought stress, not much information is available under heat stress. Hence, further investigations in this direction are imperative to develop a complete network of signal transduction in response to heat stress.
Hypothetical Mechanism of NO Induced Heat Tolerance in Plants
Plants adapt to high temperatures by a series of events beginning with the stress perception and end at the expression of target genes. Plants perceive heat stress by perturbation to cellular homeostasis that triggers stress signaling, probably by transducers such as NO, H2O2, Ca2+, and other stress hormones like ABA. These signaling molecules in turn induce the synthesis of selected protein kinases that stimulate the downstream gene expression. The altered gene expression often leads to cascade of events including changes in plant metabolism, synthesis of antioxidants, accumulation of osmoprotectants, synthesis of HSP, and enhanced survival under heat stress (Farooq et al., 2011).
Previous works have provided evidence for the involvement of NO at various levels of thermo-tolerance (Table 1). Various signaling components/molecules, transcription regulation elements, candidate genes, and proteins are involved in NO-mediated stress response, including the stress induced morphological, physiological, biochemical, and molecular changes. Reports suggest that NO acts downstream of H2O2 in the heat stress signaling in A. thaliana seedlings (Wang et al., 2014). However, as mentioned earlier, a feedback inhibition of H2O2 levels by the action of antioxidant enzymes by NO is known to inhibit heat shock factor activity and HSP production. Network of H2O2, NO, Ca2+ channels, and Ca2+-CaM dependent protein phosphatase was also reported to mediate heat shock factor activity and HSP accumulation in heat stress tolerance (Wang et al., 2014). Exogenous NO donors such as SNP might activate NADPH oxidases, subsequently enhancing the production of Ca2+ and ROS. H2S-induced expression of NiR and mitochondrial NAD(P)H dehydrogenases has been reported in Citrus aurantinum roots. However, the NO production by this pathway still needs to be proven under heat stress (Gupta et al., 2011). A recent study in S. lycopersicum seedlings subjected to chilling stress indicated that an exogenous treatment with NO enhanced putrescine and spermidine levels and application of spermine and spermidine stimulated NO production in a H2O2-dependent manner through both NR and NOS-like pathways. Although, NO has short half-life, its functionality can be extended in the form of SNOs which can release NO in a controlled manner. Heat stress induced a significant increase in the total SNOs content in C. morifolium while decreasing the NO and GSNOR levels (Chaki et al., 2011). Moreover, the functional existence of NO-mediated PTMs add more complexity and interaction nodes in heat stress signaling. The experimental proofs so far obtained under heat stress, and the predicted possible interactions with other signaling molecules strongly support the central role played by the NO in plant heat stress signaling. However, an intensive future research is required to have complete picture that mechanistically explain “how and where” NO fits in the heat stress signaling.
Future Perspectives
Elevated temperatures have greater and negative impact on plants. Despite of the methodological difficulties, recent literature clearly indicates the various roles played by NO in mediating heat stress responses in plants (Figure 2). Owing to its concentration dependent cytoprotective and toxic role, it is essential to have controlled release of NO in plant cells especially under stress conditions. Some recent reviews suggest encapsulation of NO donors in nanomaterials for an improved efficiency and controlled release over direct exogenous applications (Seabra et al., 2014; Savvides et al., 2016). This kind of target site application of nanoparticles also offer high potential due to reduced cost of application, precise dosing, decreased decomposition due to environmental factors, reduced runoff of unutilized excess chemicals into environment. Further exploration of smart technological approaches and deployment of these nanoparticles can allow effective release of NO minimizing side effects. While pretreatment with NO donors has shown promise in increasing heat tolerance in plants, it is critical to understand the methods of stress treatment and NO loading, and the tissue type used to explain the contradictions in reported data. Since the sensitivity to heat varies from species to species, mere extrapolation to other species is not practical to achieve heat stress tolerance at field level.
Several studies have used NO at different plant growth and developmental stages to understand its role in plant heat stress tolerance (Table 4). Although, these efforts do add to our understanding, we advocate the importance of developmental stage-specific studies of NO-mediated thermotolerance, especially in crop plants because many legumes and cereals show high heat sensitivity during reproductive development due to restricted water and nutrient transport under stress (Young et al., 2004; Devasirvatham et al., 2012). Hence, it is essential to put more efforts in NO-mediated stress tolerance research in crop plants to validate its implications at the field level by moving from lab to field experiments. This would allow stress ameliorating effects of NO to be translated to yield benefit in the context of anticipated climate change. Indeed, the most attributed NO function in heat tolerance is the stimulation of antioxidants (Bavita et al., 2012; Hasanuzzaman et al., 2012), however, merely increasing survival without enhanced biomass and yield again might not be practical for crop improvement.
Table 4. Different types of plant tissues used for nitric oxide treatment in plant heat stress studies.
A major knowledge gap in NO-heat stress studies is the lack of in depth molecular understanding. So far, most of the reports on the exploration of beneficial potential of NO under heat stress have been based on the physiological and biochemical studies. Hence, it is crucial to develop high-throughput genomic, proteomic, and metabolomics data and its integration at the systems level to understand the impact of heat induced accumulation of NO in plant tissues. Not only it is worth for establishing correlations between NO content and the gene/protein expression, with special emphasis on its subcellular location; but also the role of NO-mediated PTMs in heat stress mitigation. It is also essential to address the change in scenario of heat stress signaling mechanism and the NO-interacting partners when plants simultaneously challenged by other stress conditions in the field. Moreover, it is important to develop metabolite profiles of various durations might potentially provide deep insights into the functional intermediary or signal transduction components involved in exogenous NO-mediated response of plants to heat stress and various donors (León et al., 2016). Futuristic tools based on the emerging genome editing technologies also have tremendous potential in uncovering the NO signaling components and identification of the key genes involved in the NO-mediated thermotolerance. Since the accumulating data is establishing NO as a key role player in heat acclimation, clear insights into NO signaling networks underpinning the plant heat stress responses using newer tools and technologies might offer novel opportunities for rational crop design to ameliorate heat stress impacts.
Author Contributions
SP conceived the idea and wrote the first draft. SP, SA, PB and KS contributed to the writing and refining of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a financial grant to PS through the INSPIRE Faculty Award (IFA12-LSPA-08) from the Department of Science and Technology, Government of India, and partial funding from the CGIAR Research Program on Grain Legumes.
References
Ahlfors, R., Brosché, M., Kollist, H., and Kangasjärvi, J. (2009). Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. Plant J. 58, 1–12. doi: 10.1111/j.1365-313X.2008.03756.x
Ahmad, P., Abdel-Latef, A. A., Hashem, A., Abd-Allah, E. F., Gucel, S., and Tran, L. S. P. (2016). Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 7:367. doi: 10.3389/fpls.2016.00347
Allakhverdiev, I., Kreslavski, V. D., Klimov, V. V., Los, D. C., and Mohanty, R. P. (2008). Heat stress: an overview of molecular responses in photosynthesis. Photosynth. Res. 98, 541–550. doi: 10.1007/s11120-008-9331-0
Arc, E., Sechet, J., Corbineau, F., Rajjou, L., and Marion-Poll, A. (2013). ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 4:63. doi: 10.3389/fpls.2013.00063
Asgher, M., Per, T. S., Masood, A., Fatma, M., Freschi, L., and Corpas, F. J. (2016). Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 24, 2273–2285. doi: 10.1007/s11356-016-7947-8
Awasthi, R., Bhandari, K., and Nayyar, H. (2016). Temperature stress and redox homeostasis in agricultural crops. Redox homeostasis managers in plants under environmental stresses. Front. Environ. Sci. 3:11 doi: 10.3389/fenvs.2015.00011
Bavita, A., Shashi, B., and Navtej, S. B. (2012). Nitric oxide alleviates oxidative damage induced by high temperature stress in wheat. Indian J. Exp. Biol. 50, 372–378.
Beard, R. A., Anderson, D. J., Bufford, J. L., and Tallman, G. (2012). Heat reduces nitric oxide production required for auxin-mediated gene expression and fate determination in tree tobacco guard cell protoplasts. Plant Physiol. 159, 1608–1623. doi: 10.1104/pp.112.200089
Begara-Morales, J. C., Sánchez-Calvo, B., Luque, F., Leyva-Pérez, M. O., Leterrier, M., Corpas, F. J., et al. (2014). Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol. 55, 1080–1095. doi: 10.1093/pcp/pcu044
Beligni, M. V., and Lamattina, L. (1999). Is nitric oxide toxic or protective? Trends Plant Sci. 4, 299–300. doi: 10.1016/S1360-1385(99)01451-X
Beligni, M. V., and Lamattina, L. (2000). Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210, 215–221. doi: 10.1007/PL00008128
Besson-Bard, A., Astier, J., Rasul, S., Wawer, I., Dubreuil-Maurizi, C., Jeandroz, S., et al. (2009). Current view of nitric oxide-responsive genes in plants. Plant Sci. 177, 302–309. doi: 10.1016/j.plantsci.2009.06.006
Bethke, P. C., Libourel, I. G., Aoyama, N., Chung, Y. Y., Still, D. W., and Jones, R. L. (2007). The Arabidopsis aleurone layer responds to nitric oxide, gibberellins and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 143, 1173–1188. doi: 10.1104/pp.106.093435
Bethke, P. C., Libourel, I. G. L., Reinohl, V., and Jones, R. L. (2006). Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223, 805–812. doi: 10.1007/s00425-005-0116-9
Bhattacharjee, S. (2009). Involvement of calcium and calmodulin in oxidative and temperature stress of Amaranthus lividus L. during early germination. J. Environ. Biol. 30, 557–562.
Blum, A., and Sinmena, B. (1994). Wheat seed endosperm utilization under heat stress and its relation to thermotolerance in the autotrophic plant. Field Crops Res. 37, 185–191. doi: 10.1016/0378-4290(94)90097-3
Bouchard, J. N., and Yamasaki, H. (2008). Heat stress stimulates nitric oxide production in Symbiodinium microadriaticum: a possible linkage between nitric oxide and the coral bleaching phenomenon. Plant Cell Physiol. 49, 641–652. doi: 10.1093/pcp/pcn037
Brestic, M., Zivcak, M., Kalaji, H. M., Carpentier, R., and Allakhverdiev, S. I. (2012). Photosystem II thermostability in situ: environmentally induced acclimation and genotype specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 57, 93–105. doi: 10.1016/j.plaphy.2012.05.012
Brestic, M., Zivcak, M., Kunderlikova, K., and Allakhverdiev, S. I. (2016). High temperature specifically affects the photoprotective responses of chlorophyll b deficient wheat mutant lines. Photosynth. Res. 130, 251–266. doi: 10.1007/s11120-016-0249-7
Brestic, M., Zivca, M., Olsovaka, K., Kalaji, H. M., Shao, H., and Hakeem, K. R. (2014). “Heat signaling and stress responses in photosynthesis,” in Plant Signalling: Understanding the Molecular Crosstalk, eds K. Hakeem, R. Rehman, and I. Tahir (New Delhi: Elsevier), 241–256. doi: 10.1007/978-81-322-1542-4_12
Brunel-Muguet, S., D'Hooghe, P., Bataillé, M. P., Larré, C., Kim, T. H., and Trouverie, J. (2015). Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 6:213. doi: 10.3389/fpls.2015.00213
Chaki, M., Carreras, A., López-Jaramillo, J., Begara-Morales, J. C., Sánchez-Calvo, B., Valderrama, R., et al. (2013). Tyrosine nitration provokes inhibition of sunflower carbonic anhydrase (β-CA) activity under high temperature stress. Nitric Oxide 29, 30–33. doi: 10.1016/j.niox.2012.12.003
Chaki, M., Valderrama, R., Fernández-Ocaña, A. M., Carreras, A., Gómez-Rodríguez, M. V., López-Jaramillo, J., et al. (2011). High temperature triggers the metabolism of S-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin–NADP reductase by tyrosine nitration. Plant Cell Environ. 34, 1803–1818. doi: 10.1111/j.1365-3040.2011.02376.x
Chan, Z., and Shi, H. (2015). Improved abiotic stress tolerance of bermudagrass by exogenous small molecules. Plant. Signal. Behav. 10:e991577. doi: 10.4161/15592324.2014.991577
Chen, K., Chen, L., Fan, J., and Fu, J. (2013). Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 116, 21–31. doi: 10.1007/s11120-013-9883-5
Chen, W. W., Yang, J. L., Qin, C., Jin, C. W., Mo, J. H., Ye, T., et al. (2010). Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 154, 810–819. doi: 10.1104/pp.110.161109
Cheng, T. L., Chen, J. H., Abd Allah, E. F., Wang, P., Wang, G. P., Hu, X. Y., et al. (2015). Quantitative proteomics analysis reveals that S-nitrosoglutathione reductase (GSNOR) and nitric oxide signaling enhance poplar defense against chilling stress. Planta 242, 1361–1390. doi: 10.1007/s00425-015-2374-5
Chinthapalli, B., Chitra, D. S. V., and Radhavendra, A. S. (2014). Temperature modulation of the activity and malate inhibition of the phosphoenolpyruvate carboxylase from leaves of Alternanthera pengens, compared to that of Lycoperisican esculentum. Am. J. Biosci. 2, 238–243. doi: 10.11648/j.ajbio.20140206.18
Christou, A., Filippou, P., Manganaris, G. A., and Fotopoulos, V. (2014). Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 14:42. doi: 10.1186/1471-2229-14-42
Corpas, F. J., Chaki, M., Fernández-Ocaña, A., Valderrama, R., Palma, J. M., Carreras, A., et al. (2008). Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 49, 1711–1722. doi: 10.1093/pcp/pcn144
Crawford, N. M., Gally, M., Tischner, R., Heimer, Y. M., Okamoto, M., and Mack, A. (2006). Plant nitric oxide synthase: back to square one. Trends Plant Sci. 11, 526–527. doi: 10.1016/j.tplants.2006.09.007
de Pinto, M. C., Locato, V., Sgobba, A., del Carmen Romero-Puertas, M., Gadaleta, C., Delledonne, M., et al. (2013). S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol. 163, 1766–1775. doi: 10.1104/pp.113.222703
Delledonne, M. (2005). NO news is good news for plants. Curr. Opin. Plant Biol, 8, 390–396. doi: 10.1016/j.pbi.2005.05.002
Deng, Z., and Song, S. (2012). Sodium nitroprusside, ferricyanide, nitrite and nitrate decrease the thermo-dormancy of lettuce seed germination in a nitric oxide-dependent manner in light. S. Afr. J. Bot. 78, 139–146. doi: 10.1016/j.sajb.2011.06.009
Devasirvatham, V., Gaur, P. M., Mallikarjuna, N., Tokachichu, R. N., Trethowan, R. M., and Tan, D. K. Y. (2012). Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 39, 1009–1018. doi: 10.1071/FP12033
Domingos, P., Prado, A. M., Wong, A., Gehring, C., and Feijo, J. A. (2015). Nitric oxide: a multitasked signaling gas in plants. Mol. Plant. 8, 506–520. doi: 10.1016/j.molp.2014.12.010
Duan, P., Ding, F., Wang, F., and Wang, B. S. (2007). Priming of seeds with nitric oxide donor sodium nitroprusside (SNP) alleviates the inhibition on wheat seed germination by salt stress. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 33, 244–250.
Durner, J., Wendehenne, D., and Klessig, D. F. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. U.S.A. 95, 10328–10333. doi: 10.1073/pnas.95.17.10328
El-Beltagi, H. S., Ahmed, O. K., and Hegazy, A. E. (2016). Protective effect of nitric oxide on high temperature induced oxidative stress in wheat. Not. Sci. Biol. 8, 192. doi: 10.15835/nsb.8.2.9807
Essamine, J., Ammar, S., and Bouzid, S. (2010). Impact of heat stress on germination and growth in higher plants: physiological, biochemical and molecular repercussion and mechanisms of defense. J. Biol. Sci. 10, 565–572. doi: 10.3923/jbs.2010.565.572
Essemine, J., Ammar, S., Jbir, N., and Bouzid, S. (2007). Sensitivity of two wheat species's seeds (Triticum durum, Variety Karim and Triticum aestivum, Variety Salambo) to heat constraint during germination. Pak. J. Biol. Sci. 10, 3762–3768. doi: 10.3923/pjbs.2007.3762.3768
Fan, H. F., Du, C. X., and Guo, S. R. (2013). Nitric oxide enhances salt tolerance in cucumber seedlings by regulating free polyamine content. Environ. Exp. Bot. 86, 52–59. doi: 10.1016/j.envexpbot.2010.09.007
Fancy, N. N., Bahlmann, A. K., and Loake, G. J. (2017). Nitric oxide function in plant abiotic stress. Plant Cell Environ. 4, 462–472. doi: 10.1111/pce.12707
Farooq, M., Bramley, H., Palta, J. A., and Siddique, K. H. (2011). Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant. Sci. 30, 491–507. doi: 10.1080/07352689.2011.615687
Fernández-Marcos, M., Sanz, L., Lewis, D. R., Muday, G. K., and Lorenzo, O. (2011). Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc. Natl. Acad. Sci. U.S.A. 108, 18506–18511. doi: 10.1073/pnas.1108644108
Filippou, P., Antoniou, C., and Fotopoulos, V. (2013). The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 56, 172–183. doi: 10.1016/j.freeradbiomed.2012.09.037
Flores, T., Todd, C. D., Tovar-Mendez, A., Dhanoa, P. K., Correa-Aragunde, N., Hoyos, M. E., et al. (2008). Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 147, 1936–1946. doi: 10.1104/pp.108.121459
Freschi, L. (2013). Nitric oxide and phytohormone interactions: current status and perspectives. Front. Plant Sci. 4:398. doi: 10.3389/fpls.2013.00398
Fröhlich, A., and Durner, J. (2011). The hunt for plant nitric oxide synthase (NOS): is one really needed? Plant Sci. 181, 401–404. doi: 10.1016/j.plantsci.2011.07.014
Giba, Z., Grubisic, D., and Konjevic, R. (1992). Sodium nitroprusside-stimulated germination of common chick weed (Stellaria media L.) seeds. Arch. Biol. Sci. 44, 17–18.
Giba, Z., Grubisic, D., Todorovic, S., Sajc, L., Stoja-kovic, D., and Konjevic, T. (1998). Effect of nitric oxide-re-leasing compounds on phytochrome-controlled germination of Empress tree seeds. Plant Growth Regul. 26, 175–181. doi: 10.1023/A:1006131215297
Gilmore, A. M. (1997). Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol. Plant. 99, 197–209. doi: 10.1111/j.1399-3054.1997.tb03449.x
Gniazdowska, A., Dobrzynska, U., Babanczyk, T., and Bogatek, R. (2007). Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 225, 1051–1057. doi: 10.1007/s00425-006-0384-z
Gould, K. S., Lamotte, O., Klinguer, A., Pugin, A., and Wendehenne, D. (2003). Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ. 26, 1851–1862. doi: 10.1046/j.1365-3040.2003.01101.x
Graziano, M., Beligni, M. V., and Lamattina, L. (2002). Nitric oxide improves internal iron availability in plants. Plant Physiol. 130, 1852–1859. doi: 10.1104/pp.009076
Groß, F., Durner, J., and Gaupels, F. (2013). Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 4:419. doi: 10.3389/fpls.2013.00419
Gupta, K. J., Fernie, A. R., Kaiser, W. M., and van Dongen, J. T. (2011). On the origins of nitric oxide. Trends Plant Sci. 16, 160–168. doi: 10.1016/j.tplants.2010.11.007
Haldimann, P., and Feller, U. (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 27, 1169–1183. doi: 10.1111/j.1365-3040.2004.01222.x
Hasanuzzaman, M., Nahar, K., Alam, M. M., and Fujita, M. (2012). Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Agric. Res. 6, 1314–1323.
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R., and Fujita, M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. doi: 10.3390/ijms14059643
He, Y., Tang, R.-H., Hao, Y., Stevens, R. D., Cook, C. W., Ahn, S. M., et al. (2004). Nitric oxide represses the Arabidopsis floral transition. Science 305, 1968–1971. doi: 10.1126/science.1098837
Hossain, K. K., Itoh, R. D., Yoshimura, G., Tokuda, G., Oku, H., Cohen, M. F., et al. (2010). Effects of nitric oxide scavengers on thermoinhibition of seed germination in Arabidopsis thaliana. Russ. J. Plant Physiol. 57, 222–232. doi: 10.1134/S1021443710020093
Hossain, K. K., Nakamura, T., and Yamasaki, H. (2011). Effect of nitric oxide on leaf non-photochemical quenching of fluorescence under heat-stress conditions. Russ. J. Plant Physol. 58:629. doi: 10.1134/S1021443711030046
Hossain, M. A., and Fujita, M. (2013). Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.). Plant Gene Trait 4, 109–123. doi: 10.5376/pgt.2013.04.0020
Hu, X. Y., Neill, S. J., Tang, Z. C., and Cai, W. M. (2005). Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 137, 663–670. doi: 10.1104/pp.104.054494
Hua, X. J., Van De cotte, B., Van Montagnu, M., and Verbruggen, N. (2001). The 5 untranslated region of the At-P5Rgene is involved in both transcriptional and post-transcriptional regulation. Plant J. 26, 157–169. doi: 10.1046/j.1365-313x.2001.01020.x
IPCC Climate Change (2007). The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
Johkan, M., Oda, M., Maruo, T., and Shinohara, Y. (2011). “Crop production and global warming,” in Global Warming Impacts-Case Studies on the Economy, Human Health, and on Urban and Natural Environments, ed C. Stefano (Rijeka: InTech), 139–152.
Karpets, Y. V., Kolupaev, Y. E., and Yastreb, T. O. (2011). Effect of sodium nitroprusside on heat resistance of wheat coleoptiles: dependence on the formation and scavenging of reactive oxygen species. Russ. J. Plant Physiol. 58:1027. doi: 10.1134/S1021443711060094
Kaushal, N., Awasthi, R., Gupta, K., Gaur, P. M., Siddique, K. H. M., and Nayyar, H. (2013). Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 40, 1334–1349. doi: 10.1071/FP13082
Khan, M. I. R., Asgher, M., and Khan, N. A. (2013a). Rising temperature in the changing environment: a serious threat to plants. Climate Change Environ. Sustain. 1, 25–36. doi: 10.5958/j.2320-6411.1.1.004
Khan, M. I. R., Fatma, M., Per, T. S., Anjum, N. A., and Khan, N. A. (2015). Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6:462. doi: 10.3389/fpls.2015.00462
Khan, M. I. R., Iqbal, N., Masood, A., Per, T. S., and Khan, N. A. (2013b). Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 8:e26374. doi: 10.4161/psb.26374
Khan, M. N., Mobin, M., and Abbas, K. Z. (2015). “Nitric oxide and High temperature stress: a physiological perspective,” in Nitric Oxide Action in Abiotic Stress Responses in Plants, eds M. Khan, M. Mobin, F. Mohammad, and F. Corpas (Cham: Springer), 77–93. doi: 10.1007/978-3-319-17804-2_5
Khokon, M. D., Okuma, E. I. J. I., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., et al. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34, 434–443. doi: 10.1111/j.1365-3040.2010.02253.x
Kolbert, Z., Petö, A., Lehotai, N., Feigl, G., and Erdei, L. (2012). Long-term copper (Cu2+) exposure impacts on auxin, nitric oxide (NO) metabolism and morphology of Arabidopsis thaliana L. Plant Growth Regul. 68, 151–159. doi: 10.1007/s10725-012-9701-7
Kong, J., Dong, Y., Xu, L., Liu, S., and Bai, X. (2014). Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot. Stud. 55:9. doi: 10.1186/1999-3110-55-9
Kong, W., Huang, C., Chen, Q., Zou, Y., and Zhang, J. (2012). Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 49, 15–20. doi: 10.1016/j.fgb.2011.12.003
Krasuska, U., Ciacka, K., and Gniazdowska, A. (2017). Nitric oxide-polyamines cross-talk during dormancy release and germination of apple embryos. Nitric Oxide 68, 38–50. doi: 10.1016/j.niox.2016.11.003
Lang-Mladek, C., Popova, O., Kiok, K., Berlinger, M., Rakic, B., Aufsatz, W., et al. (2010). transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol. Plant. 3, 594–602. doi: 10.1093/mp/ssq014
Larkindale, J., Hall, J. D., Knight, M. R., and Vierling, E. (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138, 882–897. doi: 10.1104/pp.105.062257
Larkindale, J., and Huang, B. (2005). Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul. 47:17. doi: 10.1007/s10725-005-1536-z
Lee, U., Wie, C., Fernandez, B. O., Feelisch, M., and Vierling, E. (2008). Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 20, 786–802. doi: 10.1105/tpc.107.052647
León, J., Costa, Á., and Castillo, M. C. (2016). Nitric oxide triggers a transient metabolic reprogramming in Arabidopsis. Sci. Rep. 6:37945. doi: 10.1038/srep37945
Leshem, Y. Y. (2001). Nitric Oxide in Plants. Occurance, Function and Use. Boston, MA: Kulwer Academic Publishers.
Leshem, Y. Y., Wilisr, B. H., and Ku, V. V. (1998). Evidence for the function of the free radical gas nitric oxide as an endogenous matu-ration and senescence regulating factor in higher plant. Plant Physiol. Biochem. 36, 825–833. doi: 10.1016/S0981-9428(99)80020-5
Leterrier, M., Airaki, M., Palma, J. M., Chaki, M., Barroso, J. B., and Corpas, F. J. (2012). Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 166, 136–143. doi: 10.1016/j.envpol.2012.03.012
Li, X., Baio, G., and Yun, W. (2013). Heat stress mitigation by exogenous nitric oxide application involves polyamine metabolism and psII physiological strategies in ginger leaves. Agric. Sci. China 47, 1171–1179. doi: 10.3864/j.issn.0578-1752.2014.06.013
Li, Z. G., and Jin, J. Z. (2016). Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tissue Organ Cult. 125, 207–214. doi: 10.1007/s11240-015-0939-4
Li, Z. G., Luo, L. J., and Sun, Y. F. (2015). Signal crosstalk between nitric oxide and hydrogen sulfide may be involved in hydrogen peroxide –induced thermotolerance in maize seedlings. Russ. J. Plant Physiol. 62, 507–514. doi: 10.1134/S1021443715030127
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X., and Shen, Z. Z. (2013). Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 36, 1564–1572.
Lindermayr, C., Saalbach, G., Bahnweg, G., and Durner, J. (2006). Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J. Biol. Chem. 281, 4285–4291. doi: 10.1074/jbc.M511635200
Liu, L., Hausladen, A., Zeng, M., Que, L., Heitman, J., and Stamler, J. S. (2001). A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410, 490–494. doi: 10.1038/35068596
Locato, V., Gadaleta, C., De Gara, L., and de Pinto, M. C. (2008). Production of reactive species modulation of antioxidant network in response to heat shock: a critical balance for cell fate. Plant Cell Environ. 31, 1606–1619. doi: 10.1111/j.1365-3040.2008.01867.x
Lopez-Delgado, H., Dat, J. F., Foyer, C. H., and Scott, I. M. (1998). Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J. Exp. Bot. 49, 713–720. doi: 10.1093/jxb/49.321.713
Manjunatha, G., Gupta, K. J., Lokesh, V., Mur, L. A., and Neelwarne, B. (2012). Nitric oxide counters ethylene effects on ripening fruits. Plant Signal. Behav. 7, 476–483. doi: 10.4161/psb.19523
Martinez-Ruiz, A., Cadenas, S., and Lamas, S. (2011). Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 51, 17–29. doi: 10.1016/j.freeradbiomed.2011.04.010
Mathur, S., Agrawal, D., and Jajoo, A. (2014). Photosynthesis: response to high temperature stress. J. Photochem. Photobiol. B. 137, 116–126. doi: 10.1016/j.jphotobiol.2014.01.010
Mathur, S., Allakhverdiev, S. I., and Jajoo, A. (2011). Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). Biochim. Biophys. Acta 1807, 22–29. doi: 10.1016/j.bbabio.2010.09.001
Maurice, N., Ping, C. Y., Miaomiao, Q., Constantine, U., Bo, Y., and Qi, K. Y. (2016). Effects of exogenous nitric oxide on germination and carbohydrates mobilization in alfalfa seedlings under cadmium stress. Int. J. Environ. Sci. Technol. 4, 2337–2350.
Melo, N. K., Bianchetti, R. E., Lira, B. S., Oliveira, P. M., Zuccarelli, R., Dias, D. L., et al. (2016). Nitric oxide, ethylene and auxin crosstalk mediates greening and plastid development in deetiolating tomato seedlings. Plant Physiol. 170, 2278–2294. doi: 10.1104/pp.16.00023
Minocha, R., Majumdar, R., and Minocha, S. C. (2014). Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 5:175. doi: 10.3389/fpls.2014.00175
Mioto, P. T., Freschi, L., and Mercier, H. (2014). “Phytohormones and nitric oxide interactions during abiotic stress responses,” in Nitric Oxide in Plants: Metabolism and Role in Stress Physiology, eds M. Khan, F. Mohammad, and F. Corpas (Cham: Springer), 211–224. doi: 10.1007/978-3-319-06710-0_13
Mishina, T. E., Lamb, C., and Zeier, J. (2007). Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ. 30, 39–52 doi: 10.1111/j.1365-3040.2006.01604.x
Misra, A. N. (1980). Dark and thermal stress induced changes in the level of chlorophyll during aging of attached and detached leaves, and of isolated chloroplasts. J. Sci. Res. 2, 48–49.
Misra, A. N. (1981). Effect of temperature on chlorophyll degradation of senescing rice leaves. J. Sci. Res. 3, 9–10.
Misra, A. N., Ramaswamy, N. K., and Desai, T. S. (1997). Thermoluminescence studies on photoinhibition of potato leaf discs at chilling, room and high temperature. J. Photochem. Photobiol. B. 38, 164–168. doi: 10.1016/S1011-1344(96)07439-8
Molassiotis, A., Tanou, G., and Diamantidis, G. (2010). NO says more than ‘YES’ to salt tolerance: salt priming and systemic nitric oxide signaling in plants. Plant Signal. Behav. 5, 209–212. doi: 10.4161/psb.5.3.10738
Moreau, M., Lee, G. I., Wang, Y., Crane, B. R., and Klessig, D. F. (2008). AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 283, 32957–32967. doi: 10.1074/jbc.M804838200
Morot-Gaudry-Talarmain, Y., Rockel, P., Moureaux, T., Quillere, I., Leydecker, M., Kaiser, W., et al. (2002). Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta 215, 708–715. doi: 10.1007/s00425-002-0816-3
Mostofa, M., Yoshida, N., and Fujita, M. (2014). Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 73, 31–44. doi: 10.1007/s10725-013-9865-9
Mostofa, M. G., and Fujita, M. (2013). Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22, 959–973. doi: 10.1007/s10646-013-1073-x
Nahar, K., Hasanuzzaman, M., Alam, M. M., and Fujita, M. (2014). Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 112, 44–54. doi: 10.1016/j.envexpbot.2014.12.001
Nahar, K., Hasanuzzaman, M., Alam, M. M., Rahman, A., Suzuki, T., and Fujita, M. (2016). Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 126, 245–255. doi: 10.1016/j.ecoenv.2015.12.026
Negi, S., Santisree, P., Kharshiing, E. V., and Sharma, R. (2010). Inhibition of the ubiquitin—proteasome pathway alters cellular levels of nitric oxide in tomato seedlings. Mol. Plant. 3, 854–869. doi: 10.1093/mp/ssq033
Neill, S., Barros, R., Bright, J., Desikan, R., Hancock, J., Harrison, J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59, 165–176. doi: 10.1093/jxb/erm293
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D., and Hancock, J. T. (2002). Hydrogen peroxide and nitric oxide as signaling molecules in plants. J. Exp. Bot. 53, 1237–1247. doi: 10.1093/jexbot/53.372.1237
O'Donnell, V. B., Chumley, P. H., Hogg, N., Bloodsworth, A., Darley-Usmar, V. M., and Freeman, B. A. (1997). Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with α-tocopherol. Biochem. J. 36, 15216–15223. doi: 10.1021/bi971891z
Pagnussat, G. C., Simontacchi, M., Puntarulo, S., and Lamattina, L. (2002). Nitric oxide is required for root organogenesis. Plant Physiol. 129, 954–956. doi: 10.1104/pp.004036
Palmieri, M. C., Lindermayr, C., Bauwe, H., Steinhauser, C., and Durner, J. (2010). Regulation of plant glycine decarboxylase by S-nitrosylation and glutathionylation. Plant Physiol. 152, 1514–1528. doi: 10.1104/pp.109.152579
Piterková, J., Luhová, L., Mieslerová, B., Lebeda, A., and Petřivalský, M. (2013). Nitric oxide and reactive oxygen species regulate the accumulation of heat shock proteins in tomato leaves in response to heat shock and pathogen infection. Plant Sci. 207, 57–65. doi: 10.1016/j.plantsci.2013.02.010
Popova, L., and Tuan, T. (2010). Nitric oxide in plants: properties, biosynthesis and physiological functions. Iran J. Sci. Technol. 34, 173–183.
Pospíšil, P. (2016). Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 7:1950. doi: 10.3389/fpls.2016.01950
Pospíšil, P., and Prasad, A. (2014). Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 137, 39–48. doi: 10.1016/j.jphotobiol.2014.04.025
Pospíšil, P., and Tyystjarvi, E. (1999). Molecular mechanism of high-temperature-induced inhibition of acceptor side of Photosystem II. Photosynth. Res. 62, 55–66. doi: 10.1023/A:1006369009170
Pospíšil, P., Haumann, M., Dittmer, J., Sole, V. A., and Dau, H. (2003). Stepwise transition of the tetra-manganese complex of photosystem II to a binuclear Mn-2(mu-O)(2) complex in response to a temperature jump: a time-resolved structural investigation employing X-ray absorption spectroscopy. Biophys. J. 84, 1370–1386. doi: 10.1016/S0006-3495(03)74952-2
Pospíšil, P., Šnyrychová, I., and Nauš, J. (2007). Dark production of reactive oxygen species in photosystem II membrane particles at elevated temperature: EPR spin-trapping study. Biochim. Biophys. Acta 1767, 854–859. doi: 10.1016/j.bbabio.2007.02.011
Prasad, A., Ferretti, U., Sedlářová, M., and Pospíšil, P. (2016). Singlet oxygen production in Chlamydomonas reinhardtii under heat stress. Sci. Rep. 6:20094. doi: 10.1038/srep20094
Romero-Puertas, M. C., Rodríguez-Serrano, M., and Sandalio, L. M. (2013). Protein S-nitrosylation in plants under abiotic stress: an overview. Front. Plant Sci. 4:373. doi: 10.3389/fpls.2013.00373
Sagor, G. H. M., Berberich, T., Takahashi, Y., Niitsu, M., and Kusano, T. (2013). The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res. 22, 595–605. doi: 10.1007/s11248-012-9666-3
Sang, J., Jiang, M., Lin, F., Xu, S., Zhang, A., and Tan, M. (2008). Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J. Integr. Plant Biol. 50 231–243. doi: 10.1111/j.1744-7909.2007.00594.x
Santisree, P., Bhatnagar-Mathur, P., and Sharma, K. K. (2015). NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci. 239, 44–55. doi: 10.1016/j.plantsci.2015.07.012
Santisree, P., Bhatnagar-Mathur, P., and Sharma, K. K. (2017a). Molecular insights into the functional role of nitric oxide (NO) as a signal for plant responses in chickpea. Funct. Plant Biol. doi: 10.1071/FP16324. [Epub ahead of print].
Santisree, P., Bhatnagar-Mathur, P., and Sharma, K. K. (2017b). Heat responsive proteome changes reveal molecular mechanisms underlying heat tolerance in chickpea. Environ. Exp. Bot. 141, 132–144. doi: 10.1016/j.envexpbot.2017.07.007
Sanz-Luque, E., Ocaña-Calahorro, F., Montaigu, A., Chamizo-Ampudia, A., Llamas, Á., Galván, A., et al. (2015). THB1, a truncated hemoglobin, modulates nitric oxide levels and nitrate reductase activity. Plant J. 81, 467–479. doi: 10.1111/tpj.12744
Savvides, A., Ali, S., Tester, M., and Fotopoulos, V. (2016). Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 21, 329–340. doi: 10.1016/j.tplants.2015.11.003
Schlicht, M., and Kombrink, E. (2013). The role of nitric oxide in the interaction of Arabidopsis thaliana with the biotrophic fungi, Golovinomyces orontii and Erysiphe pisi. Front. Plant Sci. 4:351. doi: 10.3389/fpls.2013.00351
Seabra, A. B., Rai, M., and Durán, N. (2014). Nano carriers for nitric oxide delivery and its potential applications in plant physiological process: a mini review. J. Plant Biochem. Biotechnol. 23, 1–10. doi: 10.1007/s13562-013-0204-z
Sehrawat, A., Gupta, R., and Deswal, R. (2013). Nitric oxide-cold stress signaling cross-talk, evolution of a novel regulatory mechanism. Proteomics 13, 1816–1835. doi: 10.1002/pmic.201200445
Sharma, N., Yadav, A., Khetarpal, S., Anand, A., Sathee, L., Kumar, R. R., et al. (2017). High day–night transition temperature alters nocturnal starch metabolism in rice (Oryza sativa L.). Acta Physiol Plant. 39, 74. doi: 10.1007/s11738-017-2370-4
Shi, H., Ye, T., Zhu, J. K., and Chan, Z. (2014). Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 65, 4119–4131. doi: 10.1093/jxb/eru184
Siddiqui, M., Alamri, S. A., Mutahhar, Y. Y., Al-Khaishany, M. A., Al-Qutami, H. M., and Nasir Khan, M. A. (2017). Nitric Oxide and calcium induced physiobiochemical changes in tomato (Solanum Lycopersicum) plant under heat stress. Fresen. Environ. Bull. 26, 1663–1672.
Smertenko, A., Dráber, P., Viklický, V., and Opatrný, Z. (1997). Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell Environ. 20, 1534–1542. doi: 10.1046/j.1365-3040.1997.d01-44.x
Song, L., Ding, W., Shen, J., Zhang, Z., Bi, Y., and Zhang, L. (2008). Nitric oxide mediates abscisic acid induced thermotolerance in the calluses from two ecotypes of reed under heat stress. Plant Sci. 175, 826–832. doi: 10.1016/j.plantsci.2008.08.005
Song, L., Ding, W., Zhao, M., Sun, B., and Zhang, L. (2006). Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci. 171, 449–458. doi: 10.1016/j.plantsci.2006.05.002
Song, L., Zhao, H., and Hou, M. (2013). Involvement of nitric oxide in acquired thermotolerance of rice seedlings. Russ. J. Plant Physiol. 60, 785–790. doi: 10.1134/S1021443713060149
Suzuki, N., and Mittler, R. (2006). Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol. Planta 126, 45–51. doi: 10.1111/j.0031-9317.2005.00582.x
Terrile, M. C., París, R., Calderón-Villalobos, L. I., Iglesias, M. J., Lamattina, L., Estelle, M., et al. (2012). Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 70, 492–500. doi: 10.1111/j.1365-313X.2011.04885.x
Tian, Q. Y., Sun, D. H., Zhao, M. G., and Zhang, W. H. (2007). Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol. 174, 322–331. doi: 10.1111/j.1469-8137.2007.02005.x
Tossi, V., Lombardo, C., Cassia, R., and Lamattina, L. (2012). Nitric oxide and flavonoids are systemically induced by UV-B in maize leaves. Plant Sci. 193, 103–109. doi: 10.1016/j.plantsci.2012.05.012
Tun, N. N., Santa-Catarina, C., Begum, T., Silveira, V., Handro, W., Floh, E. I. S., et al. (2006). Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47, 346–354. doi: 10.1093/pcp/pci252
Uchida, A., Jagendorf, A., Hibino, T., and Takabe, T. (2002). Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 163, 515–523. doi: 10.1016/S0168-9452(02)00159-0
Wahid, A. (2007). Physiological implications of metabolites biosynthesis in net assimilation and heat stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 120, 219–228. doi: 10.1007/s10265-006-0040-5
Wahid, A., Gelani, S., Ashraf, M., and Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wang, L., Guo, Y., Jia, L., Chu, H., Zhou, S., Chen, K., et al. (2014). Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiol. 164, 2184–2196. doi: 10.1104/pp.113.229369
Wang, Y., Ries, A., Wu, K., Yang, A., and Crawford, N. M. (2010). The Arabidopsis prohibitin gene PHB3 functions in nitric oxide–mediated responses and in hydrogen peroxide–induced nitric oxide accumulation. Plant Cell 22, 249–259. doi: 10.1105/tpc.109.072066
Wellburn, A. R. (1990). Tansley Review No. 24 Why are atmospheric oxides of nitrogen usually phytotoxic and not alternative fertilizers? New Phytol. 115, 395–429.
Wimalasekera, R., Villar, C., Begum, T., and Scherer, G. F. (2011). Copper amine oxidase1 (CuAO1) of Arabidopsis thaliana contributes to abscisic acid- and polyamine-induced nitric oxide biosynthesis and abscisic acid signal transduction. Mol. Plant. 4, 663–678. doi: 10.1093/mp/ssr023
Wodala, B., Deák, Z., Vass, I., Erdei, L., Altorjay, I., and Horváth, F. (2008). In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol. 146, 1920–1927. doi: 10.1104/pp.107.110205
Wu, D., Chu, H. Y., Jia, L. X., Chen, K. M., and Zhao, L. Q. (2015). A feedback inhibition between nitric oxide and hydrogen peroxide in the heat shock pathway in Arabidopsis seedlings. Plant Growth Regul. 75, 503–509. doi: 10.1007/s10725-014-0014-x
Xu, S., Guerra, D., Lee, U., and Vierling, E. (2013). S-nitrosoglutathione reductases are low-copy number, cysteine-rich proteins in plants that control multiple developmental and defense responses in Arabidopsis. Front. Plant Sci. 4:430. doi: 10.3389/fpls.2013.00430
Xuan, Y., Zhou, S., Wang, L., Cheng, Y., and Zhao, L. (2010). Nitric oxide functions as a signal and acts upstream of atcam3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 153, 1895–1906. doi: 10.1104/pp.110.160424
Yamasaki, H. (2000). Nitrite–dependent nitric oxide production pathway: implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1477–1488. doi: 10.1098/rstb.2000.0708
Yamori, W., Hikosaka, K., and Way, D. A. (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res. 119, 101–117. doi: 10.1007/s11120-013-9874-6
Yang, J. D., Yun, J. Y., Zhang, T. H., and Zhao, H. L. (2006). Presoaking with nitric oxide donor SNP alleviates heat shock damages in mung bean leaf discs. Bot. Stud. 47, 129–136.
Yang, Q., He, H., Li, H., Tian, H., Zhang, J., Zhai, L., et al. (2011). NOA1 functions in a temperature-dependent manner to regulate chlorophyll biosynthesis and RuBisCo formation in rice. PLoS ONE 6:e20015. doi: 10.1371/journal.pone.0020015
Yang, W., Sun, Y., Chen, S., Jiang, J., Chen, F., Fang, W., et al. (2011). The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed Chrysanthemum. Biol. Plant. 55, 737–740. doi: 10.1007/s10535-011-0178-4
Young, L. W., Wilen, R. W., and Bonham-Smith, P. C. (2004). High temperature stress of Brassica napus during flowering reduces micro and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 55, 485–495. doi: 10.1093/jxb/erh038
Yu, M., Lamattina, L., Spoel, S. H., and Loake, G. J. (2014). Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 202, 1142–1156. doi: 10.1111/nph.12739
Zandalinas, S. I., Balfagón, D., Arbona, V., Gómez-Cadenas, A., Inupakutika, M. A., and Mittler, R. (2016). ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 18, 5381–5390. doi: 10.1093/jxb/erw299
Zhang, H., Shen, W. B., Zhang, W., and Xu, L. L. (2005). A rapid response of β-amylase to nitric oxide but not gibberellin in wheat seeds during the early stage of germination. Planta 220, 708–716. doi: 10.1007/s00425-004-1390-7
Zhang, W., Zhou, R. G., Gao, Y. J., Zheng, S. Z., Xu, P., Zhang, S. Q., et al. (2009). Molecular and genetic evidence for the key role of atcam3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 149, 1773–1784. doi: 10.1104/pp.108.133744
Zhang, X. W., Dong, Y. J., Qiu, X. K., Hu, G. Q., Wang, Y. H., and Wang, Q. H. (2012). Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on calcareous soil. Plant Soil Environ. 58, 111–120.
Zhao, L., Zhang, F., Guo, J., Yang, Y., Li, B., and Zhang, L. (2004). Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 134, 849–857. doi: 10.1104/pp.103.030023
Zhao, M. G., Tian, Q. Y., and Zhang, W. H. (2007). Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 144, 206–217. doi: 10.1104/pp.107.096842
Keywords: nitric oxide, heat stress, sodium nitroprussside, photosynthesis, antioxidants, lipid peroxidation, climate change
Citation: Parankusam S, Adimulam SS, Bhatnagar-Mathur P and Sharma KK (2017) Nitric Oxide (NO) in Plant Heat Stress Tolerance: Current Knowledge and Perspectives. Front. Plant Sci. 8:1582. doi: 10.3389/fpls.2017.01582
Received: 06 June 2017; Accepted: 29 August 2017;
Published: 13 September 2017.
Edited by:
Avinash Mishra, Central Salt & Marine Chemicals Research Institute (CSIR), IndiaReviewed by:
Ankush Prasad, Palacký University, Olomouc, CzechiaMarian Brestic, Slovak University of Agriculture, Slovakia
Nafees A. Khan, Aligarh Muslim University, India
Copyright © 2017 Parankusam, Adimulam, Bhatnagar-Mathur and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Santisree Parankusam, cy5wYXJhbmt1c2FtQGNnaWFyLm9yZw==; c2FudGhpa2lubnVAZ21haWwuY29t
 Santisree Parankusam
Santisree Parankusam Srivani S. Adimulam
Srivani S. Adimulam Pooja Bhatnagar-Mathur
Pooja Bhatnagar-Mathur Kiran K. Sharma
Kiran K. Sharma