- 1Academy of Scientific and Innovative Research, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, India
- 2Plant Omics Division, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, India
- 3Process Design and Engineering Division, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, India
Aim: Many countries import potassic fertilizers due to dearth of K-mineral deposits. Therefore processes to obtain K-nutrient sources from sea bittern were developed by our Institute. The present investigation evaluated the fertilizer potential of three different sea bittern-derived (SBD) potassium forms developed viz., potassium schoenite, potassium nitrate and potassium ammonium sulfate on maize productivity in two cropping seasons.
Methods: The pot and field experiments consisted of four treatments, wherein the three K forms were applied at the recommended rate of 40 kg K2O ha−1 and were compared with commercially used sulfate of potash. The effect of these fertilizers on different parameters of plant and soil were evaluated.
Results: The application of SBD-potassic fertilizers led to enhancement in growth, productivity and quality of maize which related well with higher photosynthesis, nutrient uptake and soil quality parameters. On an average all the three forms of sea bittern-derived potash enhanced yield of maize over control by 22.3 and 23.8%, respectively, in pot and field trials. The best performance was under SBD-KNO3, which also recorded the highest benefit: cost ratio of 1.76.
Conclusion: The K-fertilizers derived from sea-bittern—a waste product of salt industry—can thus be economically used to improve crop production sustainably.
Introduction
Potassium plays an important role in plant growth, development, defense, immunity, signaling, and transport processes (Beringer and Troldenier, 1980) and is therefore crucial for obtaining good yield and quality of crop plants (Zörb et al., 2014). It also helps plants to adapt to various environmental stresses such as drought and salinity (Anschütz et al., 2014). Despite being one of the most abundant elements in the earth's crust (2.3%), potassium is not easily available to the plants at once from the soil as most of it (90–98%) is present in chemically bound forms which is either unavailable or slowly available (Römheld and Kirkby, 2010). Thus soil reserve has to be supplemented with potassic fertilizers for crop production. Since 1980, about 25% increase has been found in the use of potassic fertilizers (Zörb et al., 2014). Cereals have the maximum share (FAO, 2011) for potassium use. Predominantly, potassium nutrient is applied to the crops through muriate of potash (KCl), which accounts for 96% of the world's potash capacity (Kinekar, 2011). However, other fertilizer forms such as sulfate of potash (SOP), potassium ammonium sulfate (PAS), potassium nitrate (KNO3) are also used depending upon the availability and cost. However, sufficient potassic mineral deposits are not present in many countries for producing potassic fertilizers; for example, India imports 100% of its potassic requirement from Canada, Belarus, CIS, Jordan, Israel, UK and Germany (Kinekar, 2011) which for 2013–14 was 1.926 million tonnes. China has to import about 55% of its potash requirement. Other Asian countries (except Middle East, India and China) also are fully dependent on import to fulfill their potassic demand. Similarly, the bulk of Brazilian potash is also shipped from countries like Belarus, Russia, Canada, Israel etc. Even though North American domestic production is high, it still has to rely on imports to meet some of its requirement (TFI World Fertilizer Conference, 2015). Thus it is pertinent to devise technologies to produce potassic fertilizers from other potash rich sources. In the pursuit of manufacturing potassic fertilizers through other feed stocks, we developed few processes of making potassium containing compounds using potash rich sea bittern which may have fertilizer potential (Ghosh et al., 2011, WO 2010109492 A8; Vohra et al., 2004, US 6776972 B2). Bittern is the mother liquor left after solar salt production from brines and/or seawater which is rich in potassium. The bittern also contains other elements like magnesium, chlorides, sulfates, bromides, iodides. For every 1 ton of common salt produced, at least 1.0 m3 of bittern is left behind (personal communication with Dr. Arvind Kumar). Given the fact that about a quarter billion tons of salt is produced in the world, of which about 40% is produced by solar evaporation technique (Sedivy, 2012), the amount of bittern generated offers tremendous scope to produce potassic fertilizers. The present investigation sought to evaluate the fertilizer potential of three different forms of sea bittern-derived (SBD) potassium viz., K schoenite (K2SO4.MgSO4), KNO3 and PAS for crop production. Detailed study was done to assess the efficacy of these K-compounds on plant and soil in a pot study followed by field trial using maize as a test crop so as to establish their fertilizer potential in crop production.
Materials and Methods
Production of Potassic Compounds
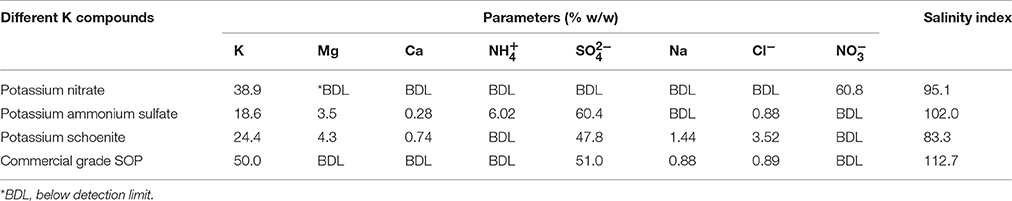
SBD-KNO3 was produced by selective precipitation method using tartaric acid as precipitant from sea bittern (Ghara et al., 2014; Maiti et al., 2014) Tartaric acid was treated with bittern upon which selectively, potassium was precipitated as potassium bitartrate. Potassium bitartrate was decomposed by Mg(OH)2 and HNO3 to yield magnesium tartrate that precipitated as a solid and KNO3 as liquor. Solid SBD-KNO3 was obtained through cooling crystallization of KNO3 liquor. SBD-Potassium ammonium sulfate was also produced by tartaric acid selective precipitation method from bittern. When potassium bitartrate was treated with MgSO4 and NH4OH, magnesium tartrate that precipitated as a solid and SBD-PAS in solution were produced. The SBD-PAS salt was then crystallized from solution through forced evaporation. SBD-K schoenite was produced through the decomposition of kainite mixed salt (KCl·MgSO4·3H2O) which was obtained from sea bittern (Ghosh et al., 2005; Dave and Ghosh, 2006; US 20050220698 A1). Elemental composition and salinity index (SI) of these compounds are presented in Table 1. SI was determined according to Jackson (1958) by following equation:
Study Area and Experimental Design
The experiment was carried out during two cropping seasons at varied locations during 2014–15. Both the trial locations are located 35 km apart. The region receives about 555 mm of rainfall annually and has a mean maximum and minimum temperature of 35 and 19°C, respectively.
Pot Experiment
The pot experiment using maize (sweet corn, variety Sugar 75, Syngenta) as test crop was conducted at the net house facility (21°44′57.6″N latitude, 72°08′39.3″E longitude) of CSMCRI, Bhavnagar district, Gujarat, India during Kharif season (July to October 2014). The treatments comprised of three different potassic compounds produced from sea bittern, viz., SBD-K schoenite (T2), SBD-KNO3 (T3) and SBD-PAS (T4) which were compared to commercial grade sulfate of potash (SOP) currently available in the market (control; T1). Each treatment was replicated five times and laid out in completely randomized design (CRD). All the pots were filled with 32 kg of soil to which chemical fertilizers at the recommended rate of 120:60 kg ha−1 of N: P2O5 were applied uniformly to all the treatments through urea and single super phosphate (SSP), respectively. Potassium as K2O was applied at 40 kg ha−1 to all the treatments through different fertilizers depending upon their potassium content. In the treatments receiving potassium nitrate and potassium ammonium sulfate, the balance amount of nitrogen was supplied through urea. Two liters of water was applied to each pot every third day. The soil of pot experiment was sandy loam in texture, having initial pH 7.88 with 0.22 dS m−1 electrical conductivity. Available N, P, and K were 49, 6.70, and 74 mg kg−1, respectively. Organic carbon was 1.71%. Four seeds were sown in each pot, which after successful germination was thinned to single plant per pot.
Field Experiment
A trial comprising the same treatments was repeated in field in bigger sized plots at Neswad farm (Altitude-314 ft, N 21°30′494″ E 072°02′185″) of Bhavnagar district, Gujarat, India during Rabi season (November 2014 to February 2015). The soil was Entisol, classified according to the USDA soil taxonomy as isohyperthermic, mixed-loamy kaolinitic, lithic ustorthents. It was also sandy loam in texture, having initial pH 6.97 and 0.14 dS m−1 electrical conductivity. Available N, P and K were 74, 1.39 and 117 mg kg−1, respectively. Ca and Mg were 3.62 and 2.22 meq 100g−1, while S was 99 mg kg−1. Organic carbon and organic matter were 0.41 and 0.71%, respectively. Here each treatment was replicated thrice and was laid out in randomized block design (RBD) with gross plot size of 4 × 3 m and the measurements were taken from net plot size of 3 × 2 m. Chemical fertilizers at the recommended rate of 120:60:40 kg ha−1 of N: P2O5: K2O were applied uniformly to all the treatments. Nitrogen was applied in three doses during the entire life cycle of maize. One fourth of nitrogen was applied as basal application before sowing while remaining quantities of nitrogen was divided into two equal doses and applied as top dressing at the knee-high stage and tasselling stage, respectively. The exact amount of applied fertilizers in g (gross plot)−1 were as follows: (1) 96 g Commercial-SOP, (2) 205.6 g SBD-K schoenite, (3) 102.4 g SBD-KNO3, (4) 156.3 g SBD-PAS, (5) 450 g SSP, (6) 313.0 g urea (in T1 and T2), (7) 294.5 g urea (in T3), and (8) 294.4 g urea (in T4). The spacing between two rows was maintained 60 cm and plant-to-plant distance was 20 cm, with a plant population of 83,333 ha−1. Data on agronomic parameters was collected from plants within the net plot area from each plot for further analysis. Five furrow irrigations at the rate of 50 mm were applied during the whole period of the experiment. Meteorological data for the field experimental is presented in the Supplementary Table.
Growth, Yield and Photosynthetic Parameters
The growth, yield and other yield attributes were recorded at harvest. Plant height was measured as the distance from soil to the last leaf collar, while stem diameter was measured at the base 3 cm above the soil surface. Leaf area was measured with leaf area meter (CI-202, CID Inc., USA). Fresh, sundried as well as oven dried weight of the plant parts (grain, leaf, stem and root) were recorded after harvest. Roots were carefully removed from soil and shaken by hand, sieved to remove the sand particles. Root fibers were then cleaned by moist paper towel until complete removal of the soil particles from root tissue. Fresh root weight was recorded at this point of time and after which root volume was measured by water displacement method. To measure root volume, samples were submerged into 1 liter graduated cylinder filled with 500 ml water. Water volumes in the graduated cylinder were recorded before as well as after submerging and root volume was calculated as follows: Root volume = volume of the water after submerging the roots into the cylinder—volume of the water before submerging the roots (Pang et al., 2011). Measurements on cob were done just after harvest of fresh cobs. The grains were separated from the cobs after sun-drying and were expressed as per plant and hectare basis in pot and field trial, respectively. In the field trial, yield data were collected from the net plot area of 3 × 2 m by manually harvesting the cobs. Further five plants were selected randomly from each plot and were used for recording data of plant height, fresh cob attributes (weight, length as well as for other measurements), root weight and other yield parameters like grain fill length. Photosynthetic rate (PR), transpiration rate (TR) and other gas exchange parameters were measured from the flag leaf of each replicates using infrared gas analyzer system (IRGA; Model Li-6400XT, LI-COR, USA) at the photosynthetic photon flux density (PPFD) of 1,000 μmol m−2 s−1. CO2 in the leaf chamber was maintained at a fixed concentration by allowing air to continuously pass through leaf chamber from an open end, it being an open photosynthesis gas exchange system. Water use efficiency (WUE) defined as the net productivity per-unit water transpired, was calculated as the ratio of photosynthesis and transpiration (PR/TR). Initial values of the minimum (Fo) and maximum (Fm) fluorescence yield were recorded in the dark state of leafs (before dawn). Remaining chlorophyll fluorescence parameters. viz., Fv/Fm, SC (stomatal conductance), qP (photochemical quenching), ΦPSII (quantum yield of PS II electron transport), NPQ (non-photochemical fluorescence quenching), ETR (photosynthetic electron transport rate), ΦCO2 (apparent quantum yield of CO2 assimilation), Ci (intercellular CO2 concentration), Ca (ambient CO2 concentration) and Ci/Ca ratio were calculated as reported by Maxwell and Johnson (2000). Chlorophyll index (CI) was measured using Chlorophyll content meter (Model CCM-200, Opti-Sciences Inc., USA). Three leaves per plant and total five plants in each treatment were used to measure chlorophyll content. The three leaves were flag leaf, one above and one below the flag leaf. Water content of the plant parts was estimated by gravimetric method after oven drying the material at 80°C till constant weight and expressed as ml per plant part.
Plant and Soil Analysis
All leaves, stem, root and grains of individual plants were collected, homogeneously crushed and powdered for nutrient content analysis. N, P and K were determined by digesting the plant samples with sulfuric acid-selenium-salicylic acid mixture as described by Novozamsky et al. (1983) followed by estimation using continuous flow analyzer (San++ SKALAR) where N was estimated by colorimetric Berthelot reaction (Krom, 1980; Searle, 1984), P was estimated by formation of a blue-colored phosphomolybdenum complex by reduction with ascorbic acid and K was estimated by flame photometric method described in Plant Analysis Procedures (Ed. Temminghoff and Houba, 2004). Ca and Mg were determined by digesting the powdered plant samples in di-acid mixture (nitric acid and perchloric acid) as described by Miller (1998) followed by estimation through inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 2000, PerkinElmer).
For soil analysis, immediately after harvest, core samples 5 cm diameter from 0 to 20 cm depth were drawn randomly from 3 regions, pooled to get a composite mixture per replicate. Samples were further sieved (< 2 mm) and stored at 4°C prior to biochemical analysis. All the enzymatic and microbial parameters were determined in pot experiment and analysis was carried out within 2 weeks of sample collection, while the other physico-chemical analyses were completed within 8 weeks. pH and electric conductivity (EC) were determined in 1:2.5 slurry of soil: water. Water holding capacity and moisture content were determined by gravimetric method (drying the soil at 105°C until the constant weight was achieved). Organic carbon, total-, available- nitrogen and available P were determined by Walkley and Black procedure as described in Nelson and Sommers (1982), modified Kjeldahl method using salicylic acid by Wilke (2005) and Olsen's method (Olsen and Sommers, 1982), respectively. Na and K were extracted by neutral normal ammonium acetate (Hanway and Heidel, 1952) and determined by using flame photometer. Ca and Mg were determined by EDTA titrimetric method as described by Estefan et al. (2013). S was extracted with 0.15% CaCl2 (Williams and Steinbergs, 1959) and estimated by turbidimetric method as described by Estefan et al. (2013). Organic matter was calculated from organic carbon using multiplication factor of 1.724 (Howard, 1965). Aryl sulphatase, acid and alkaline phosphomonoesterase and glucosidase activities in the field moist soil was assayed according to Tabatabai (1982) using 4-nitrophenol as standard. FDA activity was measured according to Schnürer and Rosswall (1982).
Statistical Analysis
Statistical analyses were carried out using MSTAT C software (Michigan State University, East Lansing, MI) suitably employing completely randomized design (CRD) and randomized block design (RBD) procedures for pot and field experimental data, respectively. Post hoc comparison of means was carried out using Tukey's HSD at a significance level of p < 0.05.
Economic Analysis
The general cost of cultivation comprised of all the costs incurred in maize cultivation sans potassic fertilizer and nitrogenous fertilizers. Notably, N through urea was applied to the SBD-KNO3 and SBD-PAS treatments after discounting the N amounts that gets inadvertently applied through these fertilizer forms. Thus, the incremental costs included the varying cost of N and K fertilizers for the purpose of economic calculation and together along with general cost was taken as the total cost of cultivation. The cost of commercially available NPK fertilizers, viz., urea, single superphosphate and sulfate of potash used in the study were taken as 6.27, 6.5, and 40 INR kg−1, while the cost of indigenous sea bittern derived K-fertilizers, viz., K-schoenite, KNO3 and PAS was deduced to be 10, 40, and 16 INR Kg−1 on the basis of process cost data generated at pilot plant in the Institute. The total gross returns included the cumulative price realized on account of maize grains, stone and stalks based on the prevailing market rates which were 13,650, 1,000, 1,000 INR Kg−1, respectively. The net return represented the difference between the total gross returns and the total cost incurred per hectare. The benefit: cost ratio (B : C ratio) was calculated as the ratio of total gross return and total cost of cultivation (Gahoonia et al., 2005).
Results
The effects of different bittern-derived potassic nutrient treatments on maize crop were examined in terms of crop growth attributes, photosynthetic efficiency, yield attributes, yield, nutrient uptake, soil physico-chemical and biochemical parameters and through economic analysis (Tables 2–6).
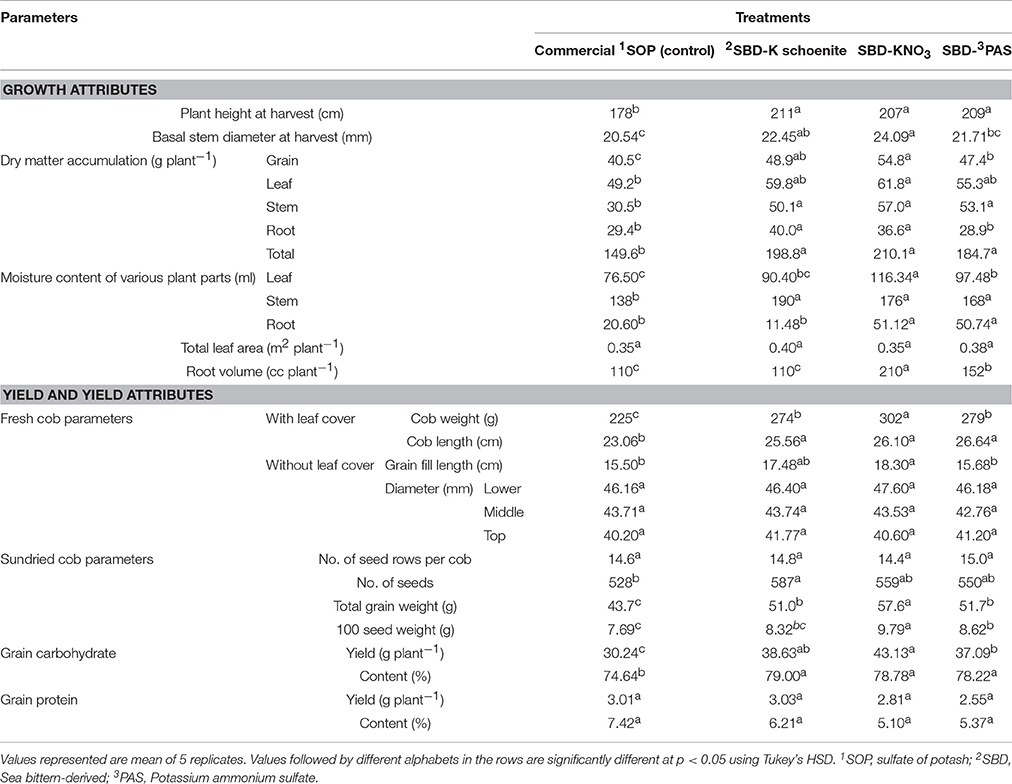
Table 2. Growth, yield and yield attributes of maize as affected by different sea bittern-derived potassic nutrient treatments in pot experiment.
Growth Attributes
Growth attributes of maize plant were altered significantly due to different K fertilizers (Tables 2, 3). In pot experiment, compared to control (commercial sulfate of potash) plant height and diameter at harvest were significantly increased by all the three bittern-derived potassic nutrient treatments, viz., SBD-K schoenite, SBD-KNO3 and SBD-PAS. Maximum plant height was observed in SBD-K schoenite treated plants which was at par to the other two bittern-derived potash treatments. Maximum basal stem diameter was recorded in SBD-KNO3 treated plants which was closely followed by SBD-K schoenite and both these treatments were superior to SBD-PAS and control. Dry matter accumulation in different plant parts (grain, leaf, stem and root) has also been presented in Table 2. The highest total plant biomass was found in SBD-KNO3, which was however at par with all other bittern-derived potassic nutrients, all of which were superior to control, connoting improved stover production. Other than in leaf, dry matter accumulation in other plant parts was lower in control as compared to that in SBD-K schoenite and SBD-KNO3 treated plants. SBD-PAS and control treatments were however at par to each other with respect to dry matter accumulation in roots and leaves. SBD-KNO3 treated plants had the highest degree of hydration in its vegetative biomass and contained about 45% higher water content (dry basis, w/w) compared to control. This treatment recorded the highest root volume compared to all other treatments and also recorded the highest water content in leaves. All the three bittern-derived K treatments were at par with each other with respect to water content in stem but were superior to control. There was no change in leaf area per plant due to any of the bittern-derived nutrient treatment (Table 2). Results of growth yield and yield attributes of field trial has also been shown in Table 3. In the field trial, the highest plant height was found in SBD-KNO3 treated plants which was at par with SBD-PAS and SBD-K schoenite applied treatments. However, SBD-K schoenite was found at par with control that recorded the lowest value for this parameter.
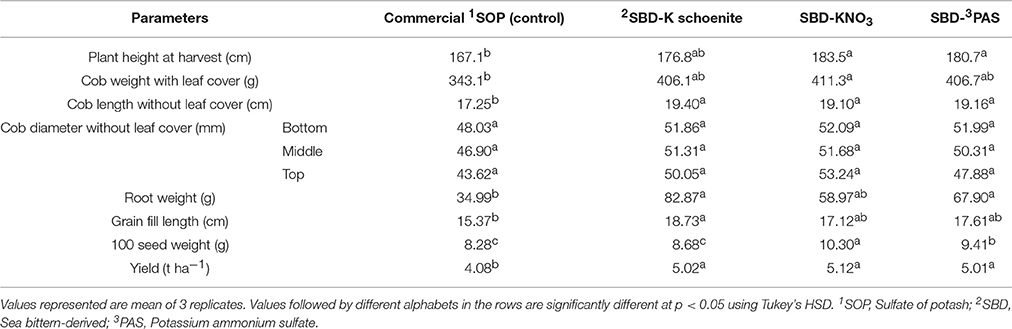
Table 3. Growth, yield and yield attributes of maize as affected by different sea bittern-derived potassic nutrient treatments in field trial.
Yield Attributes
The different bittern-derived nutrient treatments also significantly altered the yield parameters of maize plants in both the pot and field experiments (Tables 2, 3). In the pot experiment (Table 2), the green cob length and weight (inclusive of cob green leaf cover) were significantly higher in all the bittern-derived K treatments compared to that in control. The highest green cob weight was obtained under potassium nitrate treatment and this treatment was superior to all others, while in case of green cob length, the SBD-PAS treatment recorded maximum length, but was at par with all other bittern-derived K treatments. The grain fill length on the cob differed due to the different treatments and was the maximum under SBD-KNO3 treatment, which was at par with SBD-K schoenite. There was no change in cob diameter (without cob green leaf cover) and number of seed rows per cob due to the treatments. SBD-K schoenite was the only treatment that differed significantly in the number of grains formed per cob, compared to control. 100 seed weight was maximum in SBD-KNO3 treated plants, which was 27% higher over control. Test weight in SBD-PAS was significantly higher by 12% over control, while SBD-schoenite was at par for this parameter. Total grain weight was significantly higher in all the treatments compare to control. SBD-KNO3 gave the maximum grain weight (57.6g), which was 32% higher than commercial grade SOP. SBD-K schoenite and -PAS were at par to each other with respect to grain yield but were significantly higher than control (17 and 18%, respectively). Significantly the highest carbohydrate content, compared to control, was found in grains of SBD-K schoenite treated plants which was, however, at par with SBD-KNO3 and -PAS, while maximum carbohydrate yield was found in SBD-KNO3 treated plants, which was at par with SBD-K schoenite but significantly higher than SBD-PAS followed by control, which recorded the lowest. No change in protein content as well as protein yield was apparent due to any of the bittern-derive K treatments.
Similarly in field trial (Table 3), significantly highest green cob weight was also observed in SBD-KNO3 treated plants, while all others were at par to each other. Notably, length of cob (without its cob leaf cover) was significantly higher in all the bittern-derived potassic treatments compared to that in control. Similar to the results obtained in pot experiment, no change was found in cob diameter. Root weight was found higher in all bittern-derived potassic treatments compared to that in control. Cob fill length was found maximum in SBD-K schoenite treated plants compared to all others which were, however, at par among themselves. 100 seed weight was significantly highest in SBD-KNO3 treated plants, followed by SBD-PAS and SBD-K schoenite. SBD-K schoenite was found at par with control with respect to 100 seed weight. This was more or less similar to that in pot experiment. In comparison to control, yield was found significantly highest in SBD-KNO3 treatment, which was however, at par to other two bittern-derived potash treatments.
Gas Exchange Parameters
Photosynthetic rate and other gas exchange parameters were also significantly affected by different forms of bittern-derived potash (Table 4). Photosynthesis rate was found significantly highest in SBD-PAS treated plants, followed by SBD-K schoenite and SBD-KNO3, both of which were at par to each other. When compared to control, chlorophyll index (CI) was significantly higher in all bittern-derived potassic treatments, while they all were at par to each other. The quantum yield of PS II electron transport (ϕPS2), Fv/Fm ratio, apparent quantum yield of CO2 assimilation (ϕCO2), electron transport rate (ETR) and water use efficiency (WUE) were significantly enhanced in all the treatments when compared to control. Transpiration rate was the highest in control, being comparable to that in SBD-KNO3 and SBD-PAS, while it was significantly lowest in SBD-K schoenite treated plants. Intracellular CO2 concentration (Ci) and Ci/Ca ratio was also found significantly higher in different K fertilizers compared to that in control.
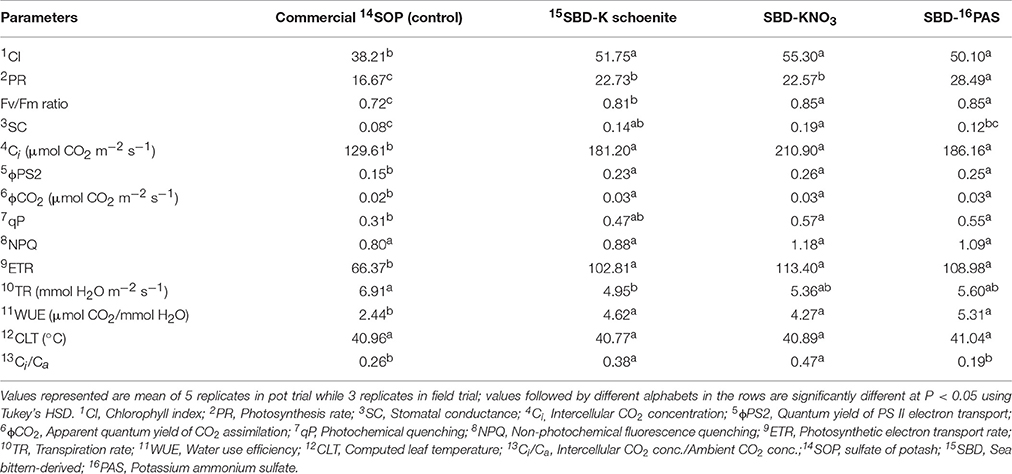
Table 4. Chlorophyll Index and gas exchange parameters of maize as affected by different sea bittern-derived potassic nutrient treatments in pot experiment.
Nutrient Content and Uptake
Nutrient content in the plant parts as well as their uptake in their respective plant parts were affected due to the different bittern-derived K treatments (Figures 1, 2). No specific pattern of changes were found in common for all these nutrients studied and it was apparent that different treatments brought out different levels of changes in different plant parts with respect to content and uptake. Total potassium uptake by plants was the highest in SBD-K schoenite treatment which was at par to other bittern-derived K compounds, all of which except SBD-PAS were statistically superior to control. Compared to control, K content in grains was found to be significantly higher in SBD-PAS treated plants, closely followed by SBD-K schoenite, -KNO3 and commercial SOP; however, these three treatments were at par to each other. Similar trend was also observed in the uptake of K in grains. The K content in leaf was found to be more or less similar in SBD-KNO3, -K schoenite and control, while SBD-PAS exhibited significantly lower leaf K content. Leaf K uptake however did not vary due to any of the treatments. Stem K content was at par in all the treatments, while the uptake was significantly higher in all the three bittern-derived potash treatments compared to that in control. Root K content was significantly higher in SBD-K schoenite treatment, followed by -KNO3, -PAS and control, the latter being at par to control. Similar trend was observed for the K uptake by roots. Among the different plant parts, N content varied only in roots, however, the differences were not significant enough to influence total N uptake by the plants due to various treatments. There was no change in P content and uptake in leaves and grains due to the various treatments. Significant changes were observed in content and uptake of P in stem and root. The significant difference in total P uptake in all the bittern-derived K treatments when compared to control was mainly due to differences in P uptake by stem. The total uptake of Ca and Mg was the highest in SBD-KNO3 treatments and the difference was significant compared to that in control. This treatment was however at par with SBD-K schoenite. The SOP and SBD-PAS were found statistically similar for total Ca and Mg uptake and were inferior to the other two treatments. Noticeably, the Ca and Mg content and uptake in grains were significantly higher in SBD-KNO3 treatment when compared to all other remaining treatments.
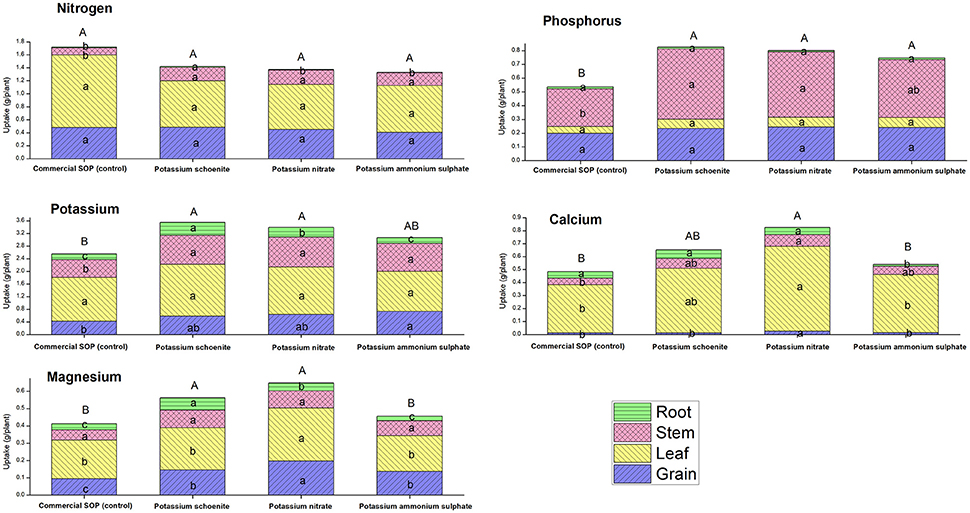
Figure 1. Nutrient uptake (g plant−1) of specific plant part viz. grain, leaf, stem and root of maize pot experiment as affected by different sea bittern-derived potassic nutrient treatments. Values are mean of 5 replicates. Bars followed by different alphabets within the treatment are significantly different at P < 0.05 using Tukey's HSD. Capital alphabets represents overall uptake of the particular nutrient in a treatment and small alphabets represents uptake of particular nutrient by the specific plant part viz. grain, leaf, stem and root.
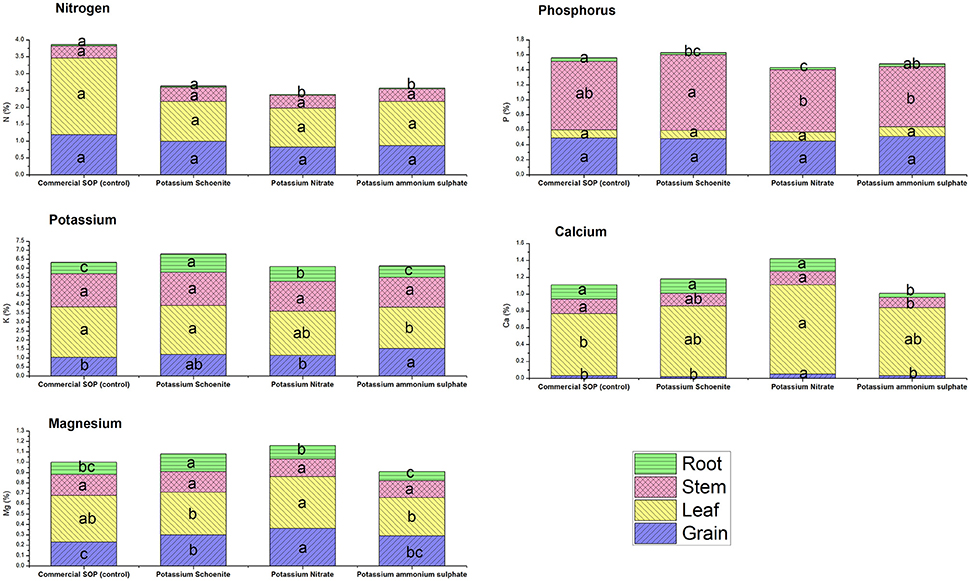
Figure 2. Nutrient content (%) of specific plant part viz. grain, leaf, stem and root of maize pot experiment as affected by different sea bittern-derived potassic nutrient treatments. Values are mean of 5 replicates. Bars followed by different alphabets within the treatment are significantly different at P < 0.05 using Tukey's HSD.
Soil Parameters
Effect of different potassic sources on soil properties has been shown in Table 5. Soil pH, moisture content (MC) and water holding capacity (WHC) were not affected by different treatments. Electrical conductivity was also not affected except in one treatment SBD-PAS, in which it decreased slightly as compared to commercial SOP. One of the explanations for the similar EC values in different treatments with differing quantities of applied fertilizers may be on account of soil buffering effect. Moreover, addition of K salts to the soil would not only result in a simple increase in the concentration of K, but might also modulate the concentrations of various other cations such as Ca, Mg, Na, Al, and H in the soil solution making it a complex phenomenon. The lowered EC in SBD- PAS may be as per the prevailing view, that if cationic nutrients as K and NH4 are added to soils, the bulk of them if not all, can be held as an exchangeable form by the negative charge of soils and hence would not be in the soil solution phase thus leading to lower EC values as opined by Yamasaki and Kishita (1972). Available K content of the soil measured after the harvest decreased in all the bittern-derived K treatments, which was significant in case of SBD-K Schoenite and SBD-PAS compared to control, however all the SBD-potassic forms were at par to each other. This might be due to higher uptake by the plants. Available nitrogen was significantly decreased in SBD-KNO3 and SBD-PAS sulfate treated soils, while available phosphorus was at par in all treatments. Soil organic carbon was also found at par among all the treatments. Soil enzymatic activity was also found to be significantly altered by different forms of bittern-derived potash treatments (Table 5). Activity of aryl sulphatase was significantly increased by all bittern-derived K treatments compared to SOP commercial, and all three of them were at par to each other. Similar trend was also recorded in acid phosphatase and FDA hydrolysis activity. The highest activity of alkaline phosphatase was recorded in SBD-KNO3 treated soil, followed by SBD-K schoenite, SBD-PAS and commercial SOP. Maximum glucosidase enzyme activity was found in SBD-KNO3 treatment, which was at par with SBD-K schoenite and significantly higher than SBD-PAS and control.
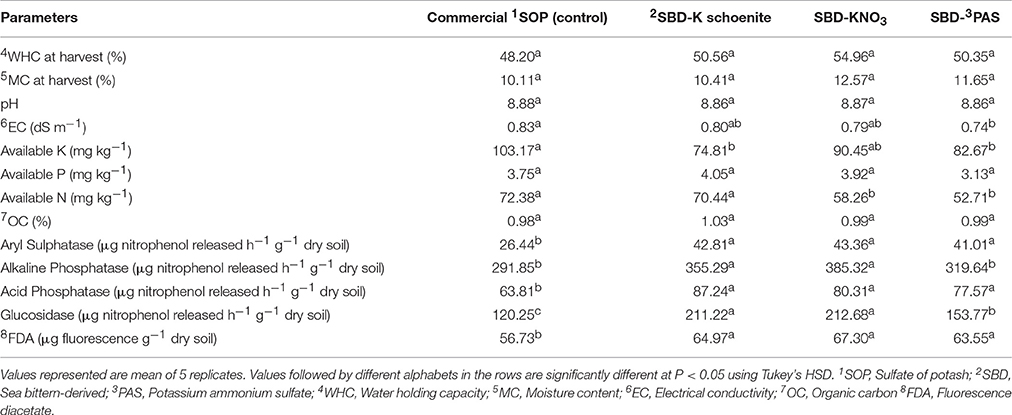
Table 5. Soil physico-chemical properties of maize as affected by different sea bittern-derived potassic nutrient treatments of pot experiment.
Economic Analysis
The highest net return per hectare was obtained in the SBD-KNO3 treatment (INR 33694), followed by SBD-K schoenite and SBD-PAS, with the benefit: cost ratio also following the same order. The commercial SOP had the lowest values for both these parameters. The incremental return realized over the control treatment per unit of incremental investment on account of K and the inadvertently adjusted N fertilizers was found the highest (3.8) in the schoenite treatment in spite of the lower enhancement in grain yield over commercial SOP, while SBD-KNO3 closely followed it recording a ratio of 3.7 (Table 6).
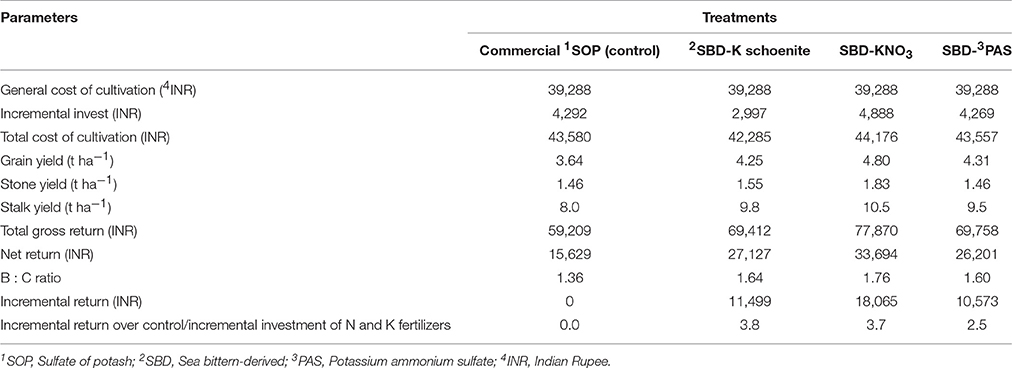
Table 6. Economic analysis of maize as affected by different sea bittern-derived potassic nutrient treatments in pot experiment.
Discussion
The experimental results revealed that the sea-bittern derived potassic nutrient sources were superior to the conventionally used sulfate of potash in terms of growth, yield, gas exchange parameters and nutrient uptake. Further, the application of these K sources also improved the soil enzymatic activity. We hypothesize that the three SBD-fertilizers used might have improved growth and yield by different mechanisms. The SBD-PAS and SBD-schoenite was apparently found to be more balanced among the three SBD-fertilizers as it had the presence of all the three secondary nutrients, viz., Ca, Mg, and S in it in contrast to control and KNO3.This might have enhanced various physiological parameters like photosynthetic rate, quantum yield of PS II electron transport, chlorophyll index (Table 4) which might have contributed to the growth and yield. On the other hand, the improvement in productivity brought out by the use of SBD-KNO3 might have been due to improved plant water status as evident from the measurements of water content in the plant parts and increase in root volume. This might be explained due to enhanced nitrate concentration around roots which induces alteration in root hydraulic properties which persists as long as nitrate levels are maintained (Gorska et al., 2008b). Further, they showed that the increased flux was linked to nitrate addition, but not to the addition of sulfate and phosphate anions or other forms of nitrogen which is commensurate with that observed in control treatment (Gorska et al., 2008a). It was confirmed that nitrate concentration within cells, rather than the products of nitrate assimilation, triggered the hydraulic response. There are evidences showing that nitrate can regulate transcript levels of some water channel (aquaporin) genes (Wang et al., 2001; Guo et al., 2007). In addition, higher root volume in SBD-KNO3 treated plants might have resulted in enhanced nutrient absorption area eventually leading to higher nutrient uptake. The enhanced hydration level together with the observed better stomatal conductance and consequent higher intercellular CO2 concentration led to least stressful leaf conditioning as evident by the highest Fv/Fm ratio. These might have led to better formation and assimilation of photosynthates that ultimately manifested into high dry matter accumulation by application of SBD-KNO3. Although leaf thickness was not carried out, we hypothesize that the leaf thickness might be higher for all the treatments compared to control. This emanates from the fact that although there was significant difference in dry leaf biomass plant−1 between control and other three treatments, there was no statistical difference in leaf area plant−1 among the treatments. This is also evident from specific leaf weight (data not shown) which was higher for all the treatments than control, wherein this value for SBD-KNO3 was 22.7% higher than that for control. This also probably manifested in having higher pigment concentration which was significantly higher in the three sea bittern-derived K treatments than control (Table 4). Although there was no statistical difference among all the treatments for cob diameter and number of seed rows per cob, the higher grain and carbohydrate yield obtained in SBD-KNO3 treated plants could be due to longer fill distance of seeds on the cob and bolder seeds as evidenced by the highest 100-seed weight compared to all other treatments. Heavier fresh cob with bigger seed size is important factor for getting premier price in market. Similarly, relatively higher yield obtained over control by application of SBD-PAS could be attributed to higher test weight, while higher yields by application of SBD-K schoenite was due to the highest number of seeds formed per cob and a modestly higher test weight. Similar results were obtained under field conditions as well, wherein all the bittern-derived potassic sources of nutrients were found significantly superior to traditional sulfate of potash. Slightly better response of SBD-K schoenite and SBD-PAS under field condition than under pot condition might be explained on account of higher pH in pot soils which might have made the ammonical or the urea form of N prone to greater volatilization losses.
The data on nutrient analysis revealed no significant change in nitrogen uptake among the various treatments while uptake of P, K and Mg was significantly higher over control in plants that received SBD-K schoenite and SBD-potassium nitrate. Interestingly, Ca and Mg uptake was significantly lower, though at par with control, under SBD-PAS treatments. This might have been due to the known negative impact of ammonium ions on the base cation uptake (Gloser and Gloser, 2000). This may be important in case the soil contains less than critical levels of these nutrients which would diminish the Mg uptake very adversely and thus application of SBD-PAS might not be suitable proposition for Ca and Mg deficient soils. This condition is especially possible in saline and sodic soils. Notably, NaCl content and thus Cl− concentration in SBD- K schoenite was on a higher side, i.e., 3.66 and 3.52%, respectively, and therefore its continuous use over the long run might cause damage to plant growth, especially to the chloride sensitive crops. It may be noted that the Na and Cl− content in SBD- K schoenite is dependent on the NaCl content in the feed composition of the kainite from which it is made.
The control treatment received N through urea which needs to be converted into ammonical form and then into nitrate form in which it is absorbed by maize plants. It is well known that this transformation is more prone to different kinds of volatilization and leaching losses resulting in lower fertilizer use efficiency. Compared to nitrate releaser SBD-KNO3, ammonium releaser SBD-PAS resulted in smaller root volume and lesser degree of hydration in plant. However, this disadvantage was partially offset by photon energy saving brought about by significantly higher net photosynthetic rate along with better water use efficiency also resulted in relatively higher grain yield compared to control. Similar results were also reported by Guo et al. (2007). Fertilizers effecting high water use efficiency yet giving modest yield, higher than usual, would certainly be instrumental for the crops grown in drought prone areas. Notably, K is also known to influence disease resistance and the enhanced availability and uptake of K by SBD K fertilizer treated plants might be other aspect that may be investigated in future.
Notably, there were no significant changes in most of the soil physico-chemical properties due to the application of bittern-derived potassic source of nutrients. There was slight decrease in electrical conductivity of the soil under SBD-PAS treatment. The highest available potash at crop harvest was found in the treatment receiving control which might be on the account of low potassium uptake from soil in this treatment. The lower available N in soil in SBD-KNO3 and SBD-PAS treated plants may be explained on account of being in more soluble forms resulting in greater exhaustion until harvest.
Activities of all the soil enzymes studied viz., aryl sulphatase, alkaline phosphatase, acid phosphatase, glucosidase and FDA which are involved in nutrient transformation cycle were significantly higher than that under control. Enzymes being the direct mediators for biological catabolism of soil organic and mineral fractions, allow a meaningful assessment of reaction rates for important soil processes and are closely related to microbial activity. Although it would require repeated application to assess the long term effect of these fertilizers on soil, nevertheless, soil enzymatic activity can reflect upon changes much sooner than other measurable parameters and therefore can give early indications of changes in soil health (Das and Varma, 2010). The favorable results of soil enzymatic activities obtained upon application of sea-bittern derived potassic nutrient sources unequivocally establishes the potential to use these compounds as fertilizer material to increase the productivity of field crops without harming soil health.
Conclusion
It can be concluded that there was no deleterious effect on plant and soil by application of these bittern-derived potassic nutrient sources and that they can be unequivocally used as a fertilizer for raising crop productivity in a sustainable and economic manner. The sustainability is also on account of huge saving in terms of global warming potential for transporting potassic fertilizer through oceanic transport in countries that do not have potassic mineral reserves (Singh et al., 2015). These fertilizers are superior to the traditionally used sulfate of potash on being manufactured from non-traditional feedstock—sea bittern—which otherwise is a waste material. Also, they either contain nitrogen in more available forms (SBD-KNO3 and SBD-PAS) or contain additional nutrients like sulfur, magnesium and calcium (SBD-K schoenite and SBD-PAS) which gets inadvertently added to the soil when added as fertilizer, thereby eliciting better crop response due to being more balanced in its composition.
Author Contributions
KT performed experimental work, analyzed and interpreted data and wrote the manuscript. DK and KA helped in soil analysis. KA helped in data interpretation and critically reviewed the manuscript. RK and HT helped in the photosynthesis data and plant analysis. KG and PM prepared characterized and provided the fertilizers. AG designed the experiment, coordinated the work, interpreted data and wrote the manuscript.
Funding
This work was supported by the Council of Scientific and Industrial Research, Delhi, India under the project “Potassic fertilizers toward empowering the nation (K-TEN)” (CSC0105).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge Council of Scientific and Industrial Research for funding the project CSC0105. Mr. M. R. Gandhi is acknowledged for providing schoenite prepared based on the CSIR-CSMCRI's developed process. Dr. Pradeep K. Agarwal is gratefully acknowledged for his support in the division. C. M. Jambucha, Bhavesh V. Baraiya and Harpal U. Chauhan are acknowledged for their assistance while conducting the field and pot experiments. We would also like to thank the reviewers for their valuable comments which helped in refining the manuscript. This manuscript bears CSIR-CSMCRI PRIS number 070/2016.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01541/full#supplementary-material
Abbreviations
PAS, Potassium ammonium sulfate; SOP, Sulfate of potash; KNO3, Potassium nitrate; SSP, Single Super Phosphate; SBD: Sea bittern derived; SI, Salinity Index; FDA, Fluorescence diacetate; INR, Indian Rupee; CC, Cubic centimeters.
References
Anschütz, U., Becker, D., and Shabala, S. (2014). Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 171, 670–687. doi: 10.1016/j.jplph.2014.01.009
Beringer, H., and Troldenier, G. (1980). “The influence of K nutrition on the response of plants to environmental stress. Potassium research-review and trends,” in Proceedings of 11th Congress of the International Potash Institute (Bern), 189–222.
Das, S. K., and Varma, A. (2010). “Role of enzymes in maintaining soil health,” in Soil Enzymology, Vol. 22, eds G. Shukla and A. Varma (Berlin; Heidelberg: Springer), 25–42.
Dave, R. H., and Ghosh, P. K. (2006). Efficient recovery of potassium chloride from liquid effluent generated during preparation of schoenite from kainite mixed salt and its reuse in production of potassium sulfate. Ind. Eng. Chem. Res. 45, 1551–1556. doi: 10.1021/ie050433g
Estefan, G., Sommer, R., and Ryan, J. (2013). Methods of Soil, Plant, and Water Analysis: A manual for the West Asia and North Africa Region. Beirut: ICARDA.
FAO (2011). Current World Fertilizer Trends and Outlook to 2015. Rome: Food and Agriculture Organization of the United Nations.
Gahoonia, T. S., Ali, O., Sarker, A., and Rahman, M. M. (2005). Root traits, nutrient uptake, multi-location grain yield and benefit–cost ratio of two lentil (Lens culinaris, Medikus.) varieties. Plant Soil 272, 153–161. doi: 10.1007/s11104-004-4573-x
Ghara, K. K., Korat, N., Bhalodia, D., Solanki, J., Maiti, P., and Ghosh, P. K. (2014). Production of pure potassium salts directly from sea bittern employing tartaric acid as a benign and recyclable K+ precipitant. RSC. Adv. 4, 34706–34711. doi: 10.1039/C4RA04360J
Ghosh, P., Langalia, K., Gandhi, M., Dave, R., Joshi, H., Vohra, R., et al. (2005). Process for Recovery of Sulphate of Potash. Available online at: http://www.google.com/patents/US20050220698 2005/10/06/
Ghosh, P. K., Mody, H. M., Chunawala, J., Gandhi, M. R., Bajaj, H. C., Maiti, P., et al. (2011). Process for Simultaneous Production of Potassium Sulphate, Ammonium Sulfate, Magnesium Hydroxide and/Or Magnesium Oxide from Kainite Mixed Salt and Ammonia. Available online at: https://www.google.co.in/patents/WO2010109492A8?cl=en 2011/03/24/
Gloser, V., and Gloser, J. (2000). Nitrogen and base cation uptake in seedlings of Acer pseudoplatanus and Calamagrostis villosa exposed to an acidified environment. Plant Soil 226, 71–77. doi: 10.1023/A:1026545415275
Gorska, A., Ye, Q., Holbrook, N. M., and Zwieniecki, M. A. (2008b). Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiol. 148, 1159–116710. doi: 10.1104/pp.108.122499
Gorska, A., Zwieniecka, A., Holbrook, N. M., and Zwieniecki, M. A. (2008a). Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta 228, 989–998. doi: 10.1007/s00425-008-0798-x
Guo, S., Zhou, Y., Shen, Q., and Zhang, F. (2007). Effect of ammonium and nitrate nutrition on some physiological processes in higher plants – growth, photosynthesis, photorespiration, and water relations. Plant Biol. 9, 21–29. doi: 10.1055/s-2006-924541
Hanway, J. J., and Heidel, H. (1952). Soil analysis method as used in Iowa state college soil testing Laboratory. Iowa Agric. 57, 1–31.
Howard, P. J. A. (1965). The carbon-organic matter factor in various soil types. Oikos 15, 229–236. doi: 10.2307/3565121
Jackson, M. L. (ed.). (1958). “Soluble salt analysis for soils and waters,” in Soil Chemical Analysis (Englewood Cliffs, NJ: Prentice-Hall, Inc.), 245.
Kinekar, B. K. (2011). Potassium fertilizer situation in India: current use and perspectives. Karnataka J. Agric. Sci. 24, 1–6.
Krom, M. (1980). Spectrophotometric determination of ammonia: a study of a modified berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105, 305–316. doi: 10.1039/an9800500305
Maiti, P., Ghosh, P. K., Ghara, K. K., Solanki, J., Brahmbhatt, H. R., Chunawala, J. R., et al. (2014). Selective Extraction of Potassium Chloride from Schoenite end Liquor Employing Tartaric Acid as Safe, Benign and Recyclable Extractant. Available online at: http://www.google.com/patents/EP2751028A1?cl=tr 2014/07/09/
Maxwell, K., and Johnson, G. N. (2000). Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668.
Miller, R. O. (1998). “Soil and plant analysis council,” in Handbook of Methods for Plant Analysis, ed Y. P. Kalra (Boca Raton, FL; Boston, MA; London; New York, NY; Washington, DC: CRC Press), 57–61.
Nelson, D. W., and Sommers, L. E. (1982). “Total carbon, organic carbon and organic matter,” in Methods of Soil Analysis, Part 2: Chemical and Microbial Properties, Agronomy, ed A. L. Page (Madison, WI: Soil Science Society of America), 570–571.
Novozamsky, I., Houba, V. J. G., van Eck, R., and van Vark, W. (1983). A novel digestion technique for multi-element plant analysis. Commun. Soil Sci. Plant Anal. 14, 239–248. doi: 10.1080/00103628309367359
Olsen, S. R., and Sommers, L. E. (1982). “Phosphorus,” in Methods of Soil Analysis, Part 2: Chemical and Microbial Properties e Agronomy, ed A. L. Page (Madison, WI: Soil Science Society of America), 421–422.
Pang, W., Crow, W. T., Luc, J. E., McSorley, R., Giblin-Davis, R. M., Kenworthy, K. E., et al. (2011). Comparison of water displacement and WinRHIZO software for plant root parameter assessment. Plant Dis. 95, 1308–1310. doi: 10.1094/PDIS-01-11-0026
Römheld, V., and Kirkby, E. A. (2010). Research on potassium in agriculture: needs and prospects. Plant Soil 335, 155–180. doi: 10.1007/s11104-010-0520-1
Schnürer, J., and Rosswall, T. (1982). Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 43, 1256–1261.
Searle, P. L. (1984). The berthelot or lndophenol reaction and its use in the analytical chemistry of nitrogen. Analyst 109, 549–568. doi: 10.1039/an9840900549
Sedivy (2012). Demand for Solar Salt in Recent Years Has Resulted in Tighter Supplies and Higher Prices. Zürich: Industrial Minerals.
Singh, S., Singh, M. K., Pal, S. K., Trivedi, K., Yesuraj, D., Singh, C. S., et al. (2015). Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J. Appl. Phycol. 28, 2099–2112. doi: 10.1007/s10811-015-0680-8
Tabatabai, M. A. (1982). “Soil enzymes,” in Methods of Soil Analysis. Part 2: Chemical and Microbial Properties e Agronomy, ed A. L. Page (Madison, WI: American Society of Agronomy, Soil Science Society of America), 903–947.
Temminghoff, E. J. M., and Houba, V. J. G. (2004). Plant Analysis Procedures, 2nd Edn. London: Kluwer Academic Publishers.
Vohra, R. N., Ghosh, P. K., Mohandas, V. P., Joshi, H. L., Deraiya, H. H., Dave, R. H., et al. (2004). Recovery of Common Salt and Marine Chemicals from Brine. Available online at: http://www.google.co.in/patents/US6776972 2004/08/17/
Wang, Y. H., Garvin, D. F., and Kochian, L. V. (2001). Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant. Physiol. 127, 345–359. doi: 10.1104/pp.127.1.345
Wilke, B. M. (2005). “Determination of physical and chemical properties,” in Manual for Soil Analysis, Soil Biology, vol. 5, eds R. Margesin and F. Schinner (Berlin; Heidelberg: Springer-Verlag), 79–82.
Williams, C. H., and Steinbergs, A. (1959). Soil sulphur fractions as chemical indices of available sulphur in some Australian soils. Aust. J. Agric. Res. 10, 340–352. doi: 10.1071/AR9590340
Yamasaki, S. I., and Kishita, A. (1972). Studies on soil solution with reference to nutrient availability: I. Effect of various potassium fertilizer on its behavior in the soil solution. Soil Sci. Plant Nutr. 18, 1–6. doi: 10.1080/00380768.1972.10433268
Keywords: sea bittern, schoenite, maize, potash, fertilizer, soil quality
Citation: Trivedi K, Kubavat D, Ghara KK, Kumar R, Trivedi H, Anand KGV, Maiti P and Ghosh A (2017) Evaluation of Fertilizer Potential of Different K Compounds Prepared Utilizing Sea Bittern as Feed Stock. Front. Plant Sci. 8:1541. doi: 10.3389/fpls.2017.01541
Received: 10 April 2017; Accepted: 23 August 2017;
Published: 07 September 2017.
Edited by:
David Bryla, U.S. Department of Agriculture, Agricultural Research Service, United StatesReviewed by:
Pangirayi Tongoona, University of KwaZulu-Natal, South AfricaRui Manuel Almeida Machado, University of Évora, Portugal
Copyright © 2017 Trivedi, Kubavat, Ghara, Kumar, Trivedi, Anand, Maiti and Ghosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arup Ghosh, arupghosh@csmcri.res.in
 Khanjan Trivedi
Khanjan Trivedi Denish Kubavat
Denish Kubavat Krishna K. Ghara
Krishna K. Ghara Ranjeet Kumar
Ranjeet Kumar Hardik Trivedi
Hardik Trivedi K. G. Vijay Anand
K. G. Vijay Anand Pratyush Maiti
Pratyush Maiti Arup Ghosh
Arup Ghosh