- 1Vegetable Research Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, Jeonju, South Korea
- 2Department of Vegetable Crops, Korea National College of Agriculture and Fisheries, Jeonju, South Korea
Silicon (Si), the quasi-essential element occurs as the second most abundant element in the earth's crust. Biological importance of Si in plant kingdom has become inevitable particularly under stressed environment. In general, plants are classified as high, medium, and low silicon accumulators based on the ability of roots to absorb Si. The uptake of Si directly influence the positive effects attributed to the plant but Si supplementation proves to mitigate stress and recover plant growth even in low accumulating plants like tomato. The application of Si in soil as well as soil-less cultivation systems have resulted in the enhancement of quantitative and qualitative traits of plants even under stressed environment. Silicon possesses several mechanisms to regulate the physiological, biochemical, and antioxidant metabolism in plants to combat abiotic and biotic stresses. Nevertheless, very few reports are available on the aspect of Si-mediated molecular regulation of genes with potential role in stress tolerance. The recent advancements in the era of genomics and transcriptomics have opened an avenue for the determination of molecular rationale associated with the Si amendment to the stress alleviation in plants. Therefore, the present endeavor has attempted to describe the recent discoveries related to the regulation of vital genes involved in photosynthesis, transcription regulation, defense, water transport, polyamine synthesis, and housekeeping genes during abiotic and biotic stress alleviation by Si. Furthermore, an overview of Si-mediated modulation of multiple genes involved in stress response pathways such as phenylpropanoid pathway, jasmonic acid pathway, ABA-dependent or independent regulatory pathway have been discussed in this review.
Introduction
The surface of earth is covered with 27.70% of silicon (Si) next to oxygen, but the existence of Si in its pure form is rare (Mitra, 2015). Silicon is deposited in the form of quartz (SiO2), sand, and sand stone in the earth crust (Rédei, 2008). In biological organisms, Si occurs in the form of amorphous silica (SiO2 nH2O) and soluble silicic acid (Si(OH)4) (Das and Chattopadhyay, 2000). Moreover in eukaryotes, Si is important for bones, cartilage, connective tissue formation, enzymatic activities, and lymphocyte proliferation (Carlisle, 1988, 1997). In plants, Si is absorbed as an uncharged monomeric silicic acid in the pH range below 9 (Knight and Kinrade, 2001; Ma and Yamaji, 2006). The level of Si accumulation by plants can be directly correlated with the beneficial effects attributed by Si. Among the plants, monocots like rice, sugarcane, maize, and cereals absorb Si in large quantities on comparison with dicots due to the presence of Si transporters (Ma et al., 2016). The absorption and transportation of Si in plants is a complex process which involves influx and efflux Si transporters belonging to aquaporin family with specific selectivity properties. For instance, the high Si accumulator like rice consists of low silicon rice 1 (Lsi1) transporter, a nodulin 26-like intrinsic protein (NIP) in roots.
Recently, several putative silicon transporters have been identified in monocot and dicot plants by Deshmukh et al. (2015). According to the report, uptake of Si is particularly confined to the plant species consisting of NIP type aquaporins with GSGR selectivity filter along with an exact distance of 108 amino acids between the asparagine-proline-alanine (NPA) domain (Deshmukh et al., 2015). The exogenous supplementation of Si proves to be beneficial for plants particularly under abiotic and biotic stress conditions (Supplementary Table 1). Silicon nutrition resulted in the improvement of growth and development (Eneji et al., 2008; Soundararajan et al., 2014; Zhang et al., 2015), increase in yield (Epstein, 1999), abiotic and biotic stress tolerance (Ma, 2004; Zhu et al., 2004; Liang et al., 2007; Muneer et al., 2014), management of macro and micro nutrients (Tripathi et al., 2014), resistance against pest and pathogens (Lanning, 1966; Cookson et al., 2007).
Apart from the abovementioned advantages, Si augmentation in soil-less cultivation of corn salad improved the edible yield, quality, and shelf life of baby leaf vegetable corn salad by the regulation of nutrient acquisition, uptake of nitrate/iron, phenoloxidase gene expression, and protection of chlorophyll degradation (Gottardi et al., 2012). Likewise, Si inclusion in tissue culture medium resulted in the enhancement of axillary shoot induction (Manivannan et al., 2017), alleviation of hyperhydricity (Soundararajan et al., 2017a), callus induction (Islam et al., 2005), and root morphogenesis (Asmar et al., 2013). Even though, the effect of Si in plants was studied for several years, the mechanisms behind the physiological responses or molecular regulation in plants upon Si nutrition under normal and stressed conditions is still under study.
Broadly, Si-mediated tolerance to stress can be interpreted either in the form of mechanical barrier through Si(OH)4 polymerization in cell walls to prevent the penetration of host tissue by pest or pathogen (Yoshida et al., 1962) or by triggering the chemical resistance mechanism (Fawe et al., 1998). According to Chérif et al. (1992), in cucumber the Si treatment increased the activities of chitinases, peroxidases, and polyphenoloxidases against Pythium ultimum. Similarly, Si nutrition enhanced the plant growth by the regulation of antioxidant and nutrient uptake in salt stressed in Salvia (Soundararajan et al., 2014). Moreover, Si retarded the Na+ and Cl− transportation due to silicon deposition to cope up the plants under salinity stress (Gong et al., 2006; Shi et al., 2013). Likewise, Si supplementation decreased metal toxicity such as toxicity of aluminum (Al) (Wang et al., 2004), boron (B) (Gunes et al., 2007), cadmium (Cd) (Liang et al., 2005), chromium (Cr) (Tripathi et al., 2012) copper (Cu) (Li et al., 2008), and zinc (Zn) (Neumann and Zur Nieden, 2001). Recently Debona et al. (2017), has elaborately reviewed the possible stress tolerance mechanisms attributed by Si upon abiotic and biotic stresses. According to the review, upon metal toxicity, silicon tends to modulate the pH range of soil, changes the metal speciation, compartmentalization and co-precipitation of metals, and sequestration strategies to combat the metal stress (Debona et al., 2017). In addition, the Si-fortified fertilizers are gaining interest in recent days due to its beneficial results particularly in the improvement of growth, photosynthesis, and maintenance of electrolyte leakage even under stressed conditions (Chen et al., 2011).
Overall, the inclusion of Si is important for plant growth and numerous reports and reviews illustrated the Si dependent modulations of antioxidant enzymes, nutrient contents, homeostasis in reactive oxygen species however, very few studies have dealt with the Si-mediated molecular regulation of genes in plants under abiotic and biotic stresses (Brunings et al., 2009; Song et al., 2014; Yin et al., 2016). The modern high-throughput approaches can aid in deciphering the important genes involved in the Si-mediated stress response in plants (Tables 1–3). The Si-dependent expression of genes was first investigated in rice using the microarray approach by Watanabe et al. (2004). According to the results, the addition of Si up-regulated the abundance of a zinc finger protein homolog and down-regulated the expressions of chlorophyll a/b binding protein, metallothione-like protein, Xa21 gene family member, and carbonic anhydrase homolog (Watanabe et al., 2004). In general, the zinc finger proteins act as the major transcription factors for stress responsible genes and the enhancement of its expression can increase the regulation of stress responsible genes which might increase the stress tolerance in Si treated plants (Watanabe et al., 2004). In the following sections, the Si-mediated regulations of genes involved in several physiological processes have been discussed.
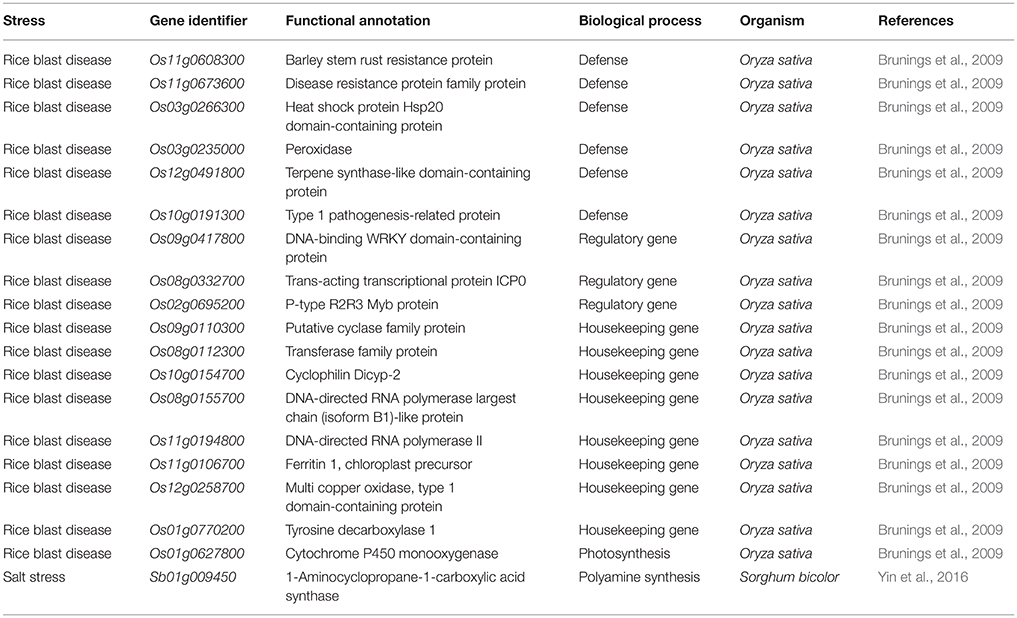
Table 3. List of genes down regulated upon the supplementation of Si under abiotic and biotic stresses.
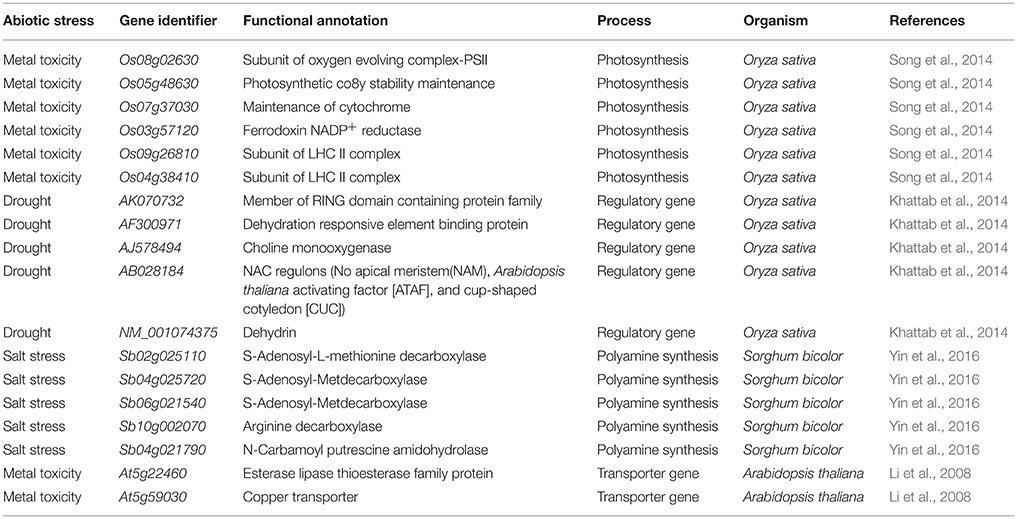
Silicon Regulated the Genes Involved in Photosynthesis upon Metal Toxicity
Among several mechanism of Si-mediated stress amelioration, the primary stress-combating strategies utilized by Si is the enhancement of photosynthesis process in the stressed plants. Broadly, the oxidative stress resulting from both abiotic and biotic stress target photosynthesis by affecting the major enzymes in calvin cycle and photosynthetic electron transport chain (Nwugo and Huerta, 2008; Gong and Chen, 2012; Muneer et al., 2014). Even though, various studies have evidenced the beneficial effects of Si on photosynthesis, only a few have examined the molecular rationale behind the gene expression upon Si addition, particularly in rice. The report by Song et al. (2014) illustrated the transcriptional regulation of photosynthesis related genes under Si amendment and zinc stress. Supplementation of Si increased the transcript levels of PsbY (Os08g02630), a vital polyprotein involved in photosystem II (PSII) whereas, the Zn in higher concentration retarded the PsbY expression. In detail, the PsbY is a subunit of oxygen-evolving complex of PSII with manganese-binding polypeptide consisting L-arginine metabolizing enzyme activity (Kawakami et al., 2007). Furthermore, the Si-mediated increase in the level of PsbY transcripts could activate the manganese-binding capacity, oxidation of water that might improve the efficiency of PS II and electron transfer rate (Song et al., 2014). Likewise, the application of Si has improved the abundance of PsaH which encodes the vital polypeptide subunits in the PSI dimer (Pfannschmidt and Yang, 2012). The PsaH knockout mutant damaged the LCH-II complex resulting in the energy transition delay between PS II and PS I (Lunde et al., 2000).
Similarly, the Zn toxicity resulted in the down regulation of PetC which has been recovered by Si supplementation. In general, PetC codes Rieske Fe-S center-binding polypeptide of cytochrome bf complex which is responsible for the proper functioning of cytochrome (Breyton et al., 1994). Hence, the Si mediated up-regulation of PetC could augment the structural integrity of the chloroplast (Song et al., 2014). Moreover, Si treatment increased the expression of PetH in similar manner with PetC. The product of PetH is ferredoxin NADP+ reductase, an important enzyme involved in the synthesis of NADPH via photosynthetic electron transport chain. Furthermore, reducible glutathione content in the cells is maintained by PetH (Song et al., 2014). In addition to the above listed genes, the supplementation of Si resulted in the up-regulation of genes (Os03g57120 and Os09g26810) involved in the light harvesting complex. Thus, the molecular insight into Si-dependent up-regulation of genes associated with PS I and PS II illustrate the positive effects rendered by Si on photosynthesis process. The physiological improvement of photosynthetic apparatus and reduction in the degradation of chlorophyll pigmentation reported by several researches can be correlated with the genic regulation of photosynthetic genes by Si at molecular level. A putative model representing the Si-mediated regulation of photosynthesis associated genes discussed above have been illustrated in Figure 1. Overall, the augmentation of Si instigated the expression levels of important genes in both photosystems to increase the efficiency of photosynthesis particularly under stressful environment.
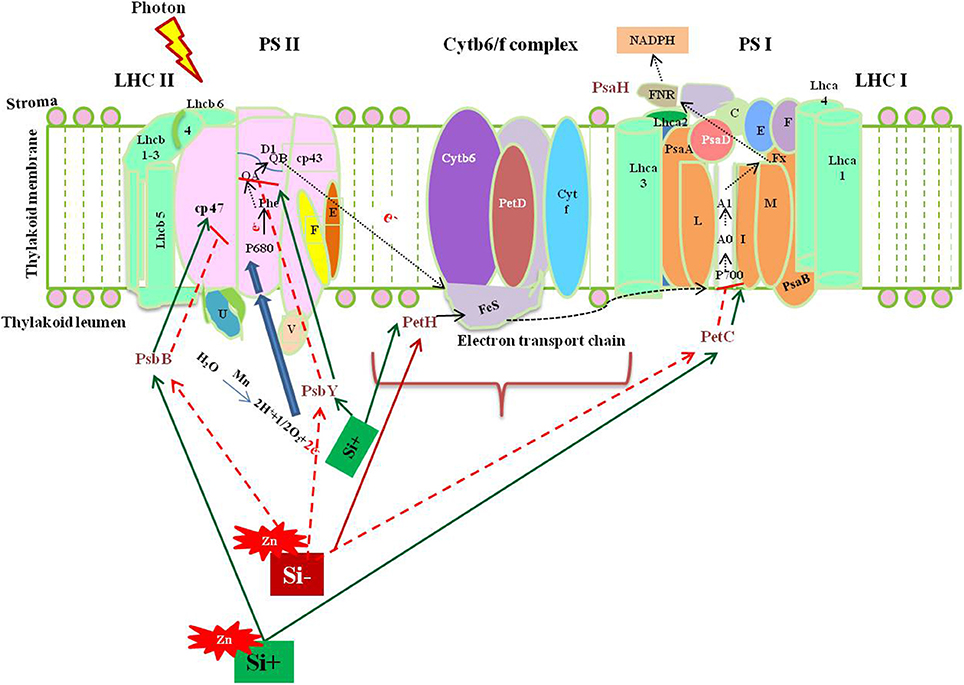
Figure 1. A putative model representing the regulation of photosynthesis related genes upon metal stress with or without Si supplementation. The green standard arrows represent the up-regulation of genes progressing with normal function in the presence of Si, blue standard arrows represent the entry of electrons into the PS II, flow of electron in the photosynthetic cascade has been represented by dotted black arrows, and red dotted arrows indicate the down-regulation of photosynthetic genes and corresponding gene functions upon metal stress in Si- plant. PSII, Photosystem II; Cytb6/f, cytochrome b6/f complex; PSI, photosystem 1; LHC I, light harvesting complex I. The diagram was conceived based on the interpretation from the following literatures (Lunde et al., 2000; Kawakami et al., 2007; Song et al., 2014).
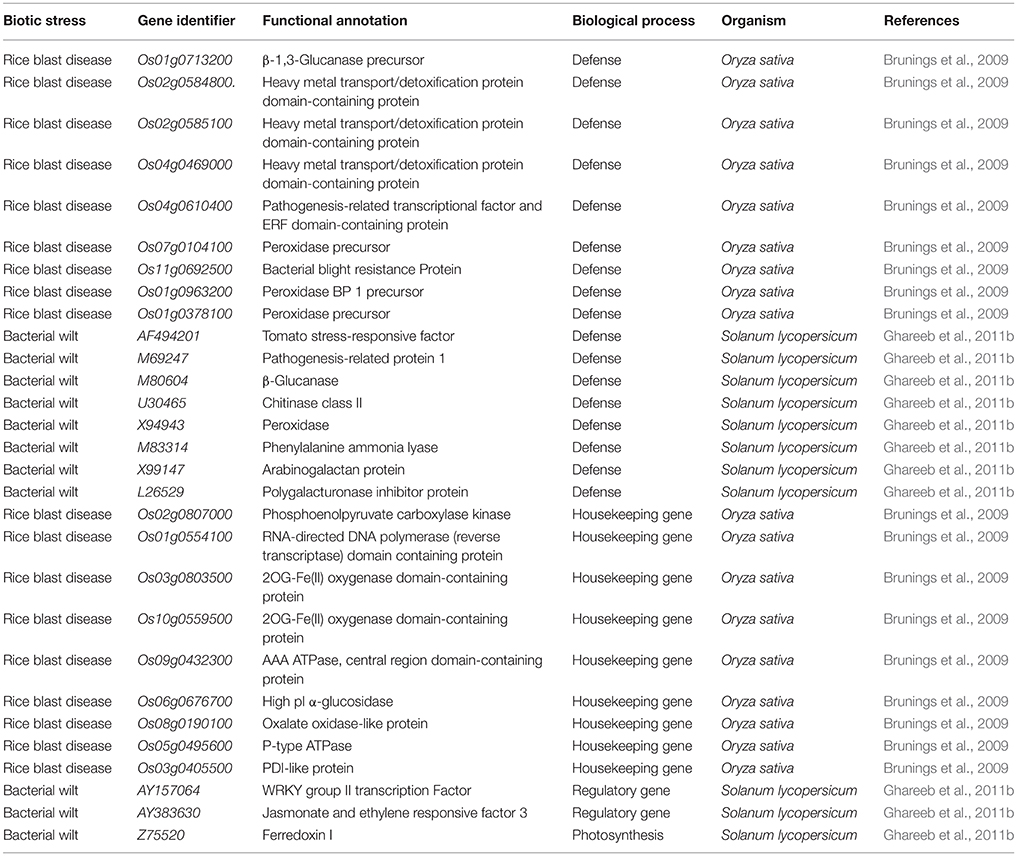
Silicon Modulated the Expression of Housekeeping Genes upon Pathogen Infection
In general, housekeeping genes are expressed constitutively in all cells regardless of its patho-physiological state and these genes are vital for the maintenance of proper functioning of cells. Although, the expression of housekeeping genes is constant, several studies illustrated their loss of stability under stressed conditions (Nicot et al., 2005; Jain et al., 2006). According to Brunings et al. (2009), the supplementation of Si down regulated the expression of important housekeeping genes in rice under normal condition however, upon pathogen infection Si up-regulated the housekeeping genes to maintain the cellular functions. Similarly, Ghareeb et al. (2011a) observed the Si-mediated up-regulation of housekeeping genes such as actin (ACT), alpha-tubulin (TUB), and phosphoglycerate kinase (PGK) in Ralstonia solanacearum infected tomato. According to Jarosch et al. (2005) actin cytoskeleton provided the basal resistance against the R. solanacearum. Therefore, the Si dependent up-regulation of actin in tomato plants induced the host resistance (Ghareeb et al., 2011a). Tomato is considered as the low-level silicon accumulator (~0.2% dry weight) because of the lack of high density Si transporter (Ma and Yamaji, 2006). Moreover, the impermeability of Si by nodulin 26-like intrinsic protein (NIP) in tomato (SINIP2-1) has been postulated due to the difference in the spacing between two NPA domains which forms the half helix inserts in SINIP2-1. However, the meager uptake of Si by low silicon accumulating plants is unclear but it might be possible that the lesser uptake of Si by tomato plants particularly under stressed environment might be due to the existence of a passive uptake mechanism. Moreover, the application of Si even in less biological concentration in the low accumulating species such as tomato (Romero-Aranda et al., 2006), capsicum (Jayawardana et al., 2015), and roses (Soundararajan et al., 2017b) has rendered abiotic and biotic stress tolerance. Despite the constant expression nature of housekeeping genes, variation in the expression levels upon Si amendment and pathogen infection could induce the basal defense mechanism in the host plant to protect from the pathogen. Taken together, the silicon amendment regulated the expression of vital housekeeping genes to alleviate the biotic stress.
Silicon Altered the Expression of Regulatory Elements Associated with Stress Response Genes
Stressful environment can induce the expression of myriads of genes involved in stress tolerance, metabolic processes, and signal transduction, etc. in plants (Shinozaki and Yamaguchi-Shinozaki, 2000; Xiong et al., 2002; Rabbani et al., 2003; Shinozaki et al., 2003). Amongst the stress induced genes, transcription factors (TF) are the primary regulators of the downstream genes important for plant tolerance against biotic and abiotic stresses (Gao et al., 2007; Lucas et al., 2011). In general, TFs are facilitated by particular cis-elements called regulons that are located in the promoter section of the target gene (Nakashima et al., 2009; Qin et al., 2011). Broadly, plants consists of a diverse number of regulons responding to stress, for example dehydration-responsive element binding protein (DREB2) are triggered by temperature and drought stress (Mizoi et al., 2012). Similarly, the NAC regulons [no apical meristem (NAM), Arabidopsis thaliana activating factor (ATAF), and cup-shaped cotyledon (CUC)] can be activated by osmotic stress in plants (Nakashima et al., 2009; Fujita et al., 2011). Moreover, the increase in the expression levels of TFs can stimulate a wide range of signal transduction pathways resulting in stress tolerance (Chaves and Oliveira, 2004; Umezawa et al., 2006). According to Khattab et al. (2014), in rice the addition of Si resulted in the up-regulation of TFs involved in the expression of DREB2A, NAC5, Oryza sativa RING domain containing protein (OsRDCP1), Oryza sativa choline monooxygenase (OsCMO), and dehydrin OsRAB16b (Figure 2). In rice, the OsDREB triggers the expression of stress-responsive genes that impart tolerance against osmotic stress in abscisic acid (ABA)—independent manner (Figure 2A) (Dubouzet et al., 2003; Hussain et al., 2011). In addition, the elevated levels of OsDREB2A provided drought resistance in rice (Chen et al., 2008; Wang et al., 2008). Similarly, NACs are TFs with various roles in development and stress response of plants (Tran et al., 2010). According to Fang et al., the rice genome consists of ~140 putative NAC or NAC-like genes among them 20 genes including OsNAC5 are classified as stress responsive genes involved detoxification, redox homeostasis, and macromolecule fortification (Hu et al., 2008). Hence, the Si-mediated enhancement of OsNAC5 transcripts led to prevention of lipid peroxidation and generation of excess hydrogen peroxide (H2O2). The abovementioned metabolic modulations shield the plants from dehydration and oxidative damages caused in stressed conditions (Takasaki et al., 2010; Song et al., 2011). Furthermore, in rice the up regulation of the OsNAC5 stimulated the stress tolerance by increasing the levels of stress inducible rice genes like LEA3 (Takasaki et al., 2010; Figure 2B). Moreover, OsRAB16b belongs to LEA genes that are expressed in response to abiotic stresses in both somatic and reproductive tissues (Tunnacliffe and Wise, 2007; Bies-Etheve et al., 2008). Broadly, LEA proteins encoded by the LEA genes render the property of acclimatization to the plants particularly under stressful conditions (Lenka et al., 2011).
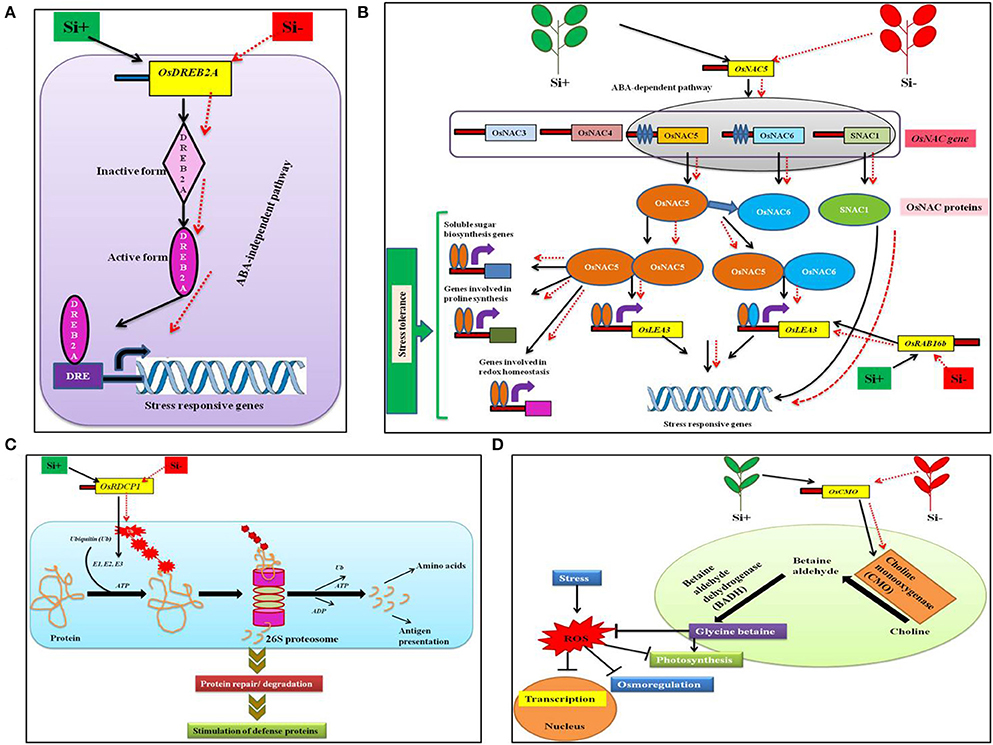
Figure 2. A schematic representation of the regulation of transcription factors under abiotic stress condition with or without Si supplementation. (A) Model displaying the OsDREB2A regulation in ABA-independent pathway to combat stress. (B) Regulation of OsNAC5 transcription factor in ABA-dependent pathway to trigger stress tolerance related genes. (C) The OsRDCP1 mediated stress tolerance response via the ubiquitin-proteosome degradation pathway. (D) Improvement of glycine betaine biosynthesis by OsCMO to combat ROS generation. The black standard arrows represent the up-regulation of gene in the presence of Si and red dotted arrows indicate the down-regulation of gene and corresponding functions upon stress in Si- plant. DRE, Dehydration responsive element; DREB2A; dehydration-responsive element binding protein 2A; NAC, no apical meristem (NAM), Arabidopsis thaliana activating factor (ATAF), and cup-shaped cotyledon (CUC) regulons; OsRDCP1, Oryza sativa RING domain containing protein; OsCMO, Oryza sativa choline monooxygenase; SNAC1, stress-responsive NAC protein, OsLEA3, Oryza sativa late embryogenesis abundant protein; E1, ubiquitin activating; E2, ubiquitin conjugating; E3,ubiquitin ligating enzymes, ATP, adenosine triphosphate; ADP, adenosine diphosphate. The diagram was conceived based on the interpretation from the following literatures (Mizoi et al., 2012; Nakashima et al., 2012; Khattab et al., 2014).
In eukaryotes, the protein turnover is maintained by the Ubiquitin (Ub)-26S proteasome pathway. During the process of ubiquitination, the target proteins are linked to multiple Ub chains by ubiquitin ligases such as E1, E2, and E3 (Kraft et al., 2005; Stone et al., 2005). According to previous reports, the RING E3 Ub ligases play a vital role particularly in response to drought stress in rice (Bae et al., 2011; Ning et al., 2011; Park et al., 2011). Till date, five homologs of OsRDCP were identified in rice which possesses a single RING motif in their N-terminal regions (Khattab et al., 2014). The OsRDCP1 is one among the five homologs which combated the dehydration stress in rice was up-regulated by the application of Si (Bae et al., 2011; Khattab et al., 2014; Figure 2C). Similarly, choline mono oxygenase the product of OsCMO is a primary enzyme involved in the biosynthesis of glycine betaine (Burnet et al., 1995). The glycine betaine is widely known for its osmolytic property that renders abiotic stress tolerance in several plants. Hence, the Si-mediated enhancement of OsCMO gene levels improved the stress tolerance in rice (Burnet et al., 1995; Figure 2D). The silicon-dependent up-regulation of transcription factors could interact with the cis-elements located in the promoter region of genes involved in stress resistance and trigger the stress tolerance against abiotic and biotic stresses. These regulatory genes might also induce the transcription of genes associated with the defense related or stress responsive pathways such as phenylpropanoid pathway, ABA-dependent or ABA-independent regulatory pathways to protect the plants from stress.
Modulation of Genes Involved in Water Uptake and Transportation upon Si Nutrition
Aquaporins are essential transmembrane proteins that maintain the uptake and movement of water molecules across cell membranes, particularly under abiotic stress condition (Boursiac et al., 2005). However, the function of aquaporin has been implicated by several factors such as abscisic acid, level of calcium ions, free radicals, and ethylene (Azad et al., 2004; Parent et al., 2009; Hu et al., 2012). According to Boursiac et al. (2008) the activity of aquaporin is susceptible to a mere change in the level of ROS, for instance the H2O2 stimulated by salt stress resulted in the prevention of aquaporin function by modulating the oxidant gating, phosphorylation condition, and re-localization of aquaporin. The amendment of Si enhanced the uptake of water particularly under salinity stress in several plants however the molecular mechanism behind the improvement of water status is unclear until recently. In Sorghum bicolor, the application of Si enhanced water uptake by increasing the activity of aquaporin by the up-regulation of SbPIP1;6, SbPIP2;2, and SbPIP2;6 encoding plasma membrane intrinsic protein (PIP), the copious aquaporin in root (Liu et al., 2015; Figure 3). In addition, the higher expression of genes related to aquaporin results in the rapid water uptake which also dilutes the excess concentration of Na+ ions lethal for the plants (Gao et al., 2010). In accordance with the existing reports on the uses of aquaporin up regulation, the findings of Sutka et al. (2011) illustrated that the abundance of aquaporin genes in the roots balance the water uptake by the plants even under water-deficit conditions. In both normal and stressful environment the regulation of aquaporins plays a vital role in maintaining the proper uptake and transportation of water and solutes in plants. The enhancement of aquaporin related genes by silicon might substantiate the improvement of water status in plants treated with Si in salinity and drought stressed plants. The improvement of water status and ion balance aid in the reclamation of plants from stress.
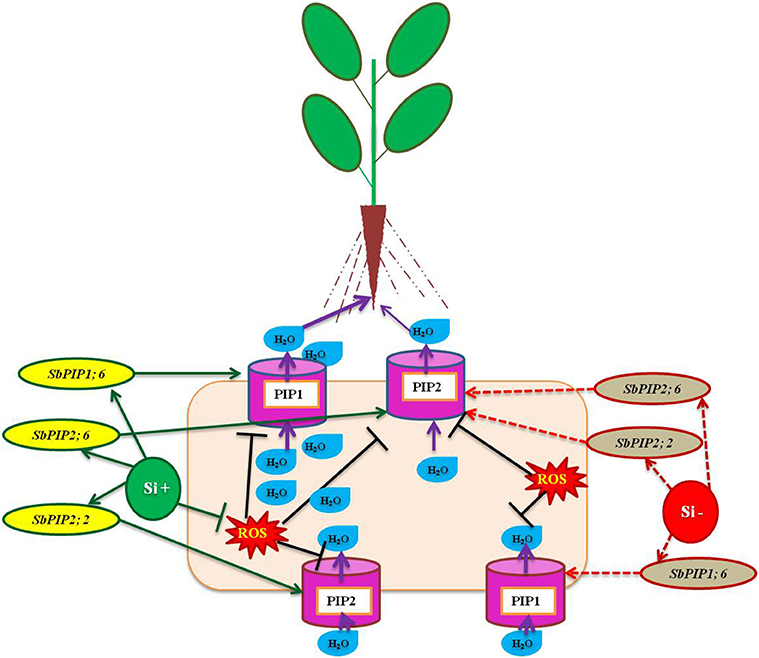
Figure 3. A model representation of aquaporin related genes regulation under osmotic stress condition with or without Si supplementation. The green standard arrows represent the up-regulation of genes in Si+ and red dotted arrows indicate the down-regulation of genes and corresponding functions upon stress in Si- conditions. The down regulation of PIP genes could result in the lesser activity of Aquaporin mediated transportation however upon Si augmentation the up-regulation of PIP genes improve the water status of the plants under stress. SbPIP1, Sorghum plasma membrane intrinsic protein, and PIP (plasma membrane intrinsic protein). The model was conceived based on the interpretation from the following literatures (Liu et al., 2015; Maurel et al., 2015).
Regulation of Polyamine Biosynthesis Genes by Si Supplementation
Plants with higher levels of polyamines like putrescine, spermidine, and spermine reported to possess more resistance against environmental onslaughts like salinity (Liu et al., 2006; Chai et al., 2010; Quinet et al., 2010; Pottosin and Shabala, 2014). Furthermore, the elevated levels of genes responsible for the synthesis of polyamines mitigates the negative effects of oxidative stress (Roy and Wu, 2001; Tang et al., 2007). Therefore, the role of polyamines in stress resistance is becoming inevitable and the molecular insight into the Si dependent modulation of polyamines has been reported in Sorghum bicolor (Yin et al., 2016). The augmentation of Si elevated the expression level of S-adenosyl-L-methionine decarboxylase (SAMDC) gene which encodes a vital enzyme involved in the biosynthesis of polyamines. In addition, the report also hypothesized that the Si-mediated salt tolerance in sorghum has been associated with the polyamines and ethylene synthesis. On the contrary to SAMDC, the Si application retarded the synthesis of ethylene in sorghum under salinity stress. Since the polyamines such as spermidine and spermine share a common precursor, S-adenosyl-L-methionine (SAM) with ethylene, it is considered as the presence of a competitive environment amongst the polyamines and ethylene (Pandey et al., 2000). Therefore, in order to reduce the competitive condition, Si could have reduced the ethylene level by the inhibition, 1-aminocyclopropane-1-1-carboxylic acid (ACC) an important ethylene precursor (Yin et al., 2016). The supplementation of Si balanced the metabolism of polyamines and ethylene to mitigate abiotic stress (Figure 4). Polyamines are involved in various vital processes such as replication, transcription and translation, stabilization of membranes, and modulation of enzyme activities in addition to stress tolerance. Hence, the regulation of polyamine biosynthesis genes by Si could not only helps in the stress alleviation but also improves the fundamental processes in cells upon stress and increase the growth and development of plants.
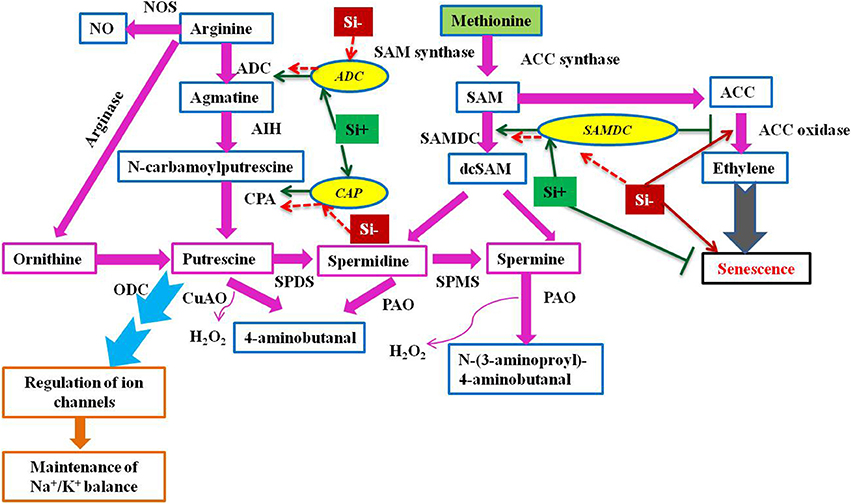
Figure 4. A schematic illustration of polyamine biosynthesis gene regulation under stress condition with or without Si supplementation. The green standard arrows represent the up-regulation of genes in Si+ and red dotted arrows indicate the down-regulation of genes and corresponding functions upon stress in Si- conditions. SAMDC, S-adenosyl-L-methionine decarboxylase; ADC, arginine decarboxylase; CAP, N-carbamoylputrescine amidohydrolase; ACC, 1-aminocyclopropane- 1-carboxylic acid; SAM, S-adenosyl-L-methionine; ODC, ornithine decarboxylase; SPDS, spermidine synthase; SPMS, spermine synthase; CuAO, copper amine oxidase; PAO, polyamine oxidase; AIH, agmatine iminohydrolase; PAO, polyamine oxidase; NOS, nitric oxide synthase. The model was conceived based on the interpretation from the following literatures (Mizoi et al., 2012; Khattab et al., 2014; Shi and Chan, 2014; Kurepin et al., 2015; and Yin et al., 2016).
Silicon-Mediated Expression of Defense Responsive Genes
The defensive role of Si against biotic and abiotic stresses has been evidenced by several plant biologists. Especially, the Si-mediated protection against potential plant diseases such as powdery mildew and rice blast disease has been studied widely (Figure 5). The extensive study by Rodrigues et al. (2004) elucidated the positive regulation of genes related to the defense mechanism such as chalcone synthase (CHS), phenylalanine-ammonia lyase (PAL), pathogenesis related protein (PR1), peroxidase (POX), chitinases, and β-1, 3-glucanases by Si upon Magnaporthe grisea infection. Among the listed genes, CHS is a rate limiting enzyme in the flavonoid biosynthesis pathway and PAL plays a vital role in the synthesis of secondary metabolites with potential chemical defense property via phenylpropanoid pathway (Rodrigues et al., 2004). Furthermore, the peroxidases enzymes are important for lignin biosynthesis which acts as the potential mechanical barrier against pathogens (Rhodes, 1994). Similarly, the pathogenesis related (PR-1) protein in combination with genes related to secondary metabolism acts as the primary outcome of the plant defense response (Zeier et al., 2004). Moreover, the supplementation of Si altered the expression pattern of defense genes in rice to render resistance against Magnaporthe oryzae (Brunings et al., 2009). In addition, Si application in rice also induced differential expression of heavy metal transport and detoxification related genes to mitigate the heavy metal toxicity (Brunings et al., 2009). Altogether, Si regulates the genes responsible for vital physiological functions in plants particularly under stressed environment. Among the several mechanism of stress tolerance reported to exhibit by silicon, instigation of defense mechanism is considered as the pivotal one. Particularly, Si-mediated induction of cascade of reactions via phenypropanoid pathway is responsible for the synthesis and accumulation of chemical defense molecules such as phenols, and flavanoids against pathogens. Apart from the phenypropanoid pathway, Si can also induce the systemic acquired resistance (SAR) by the regulation of genes involved in hypersensitivity response and jasmonic acid mediated defense pathway to protect against pathogen attack.
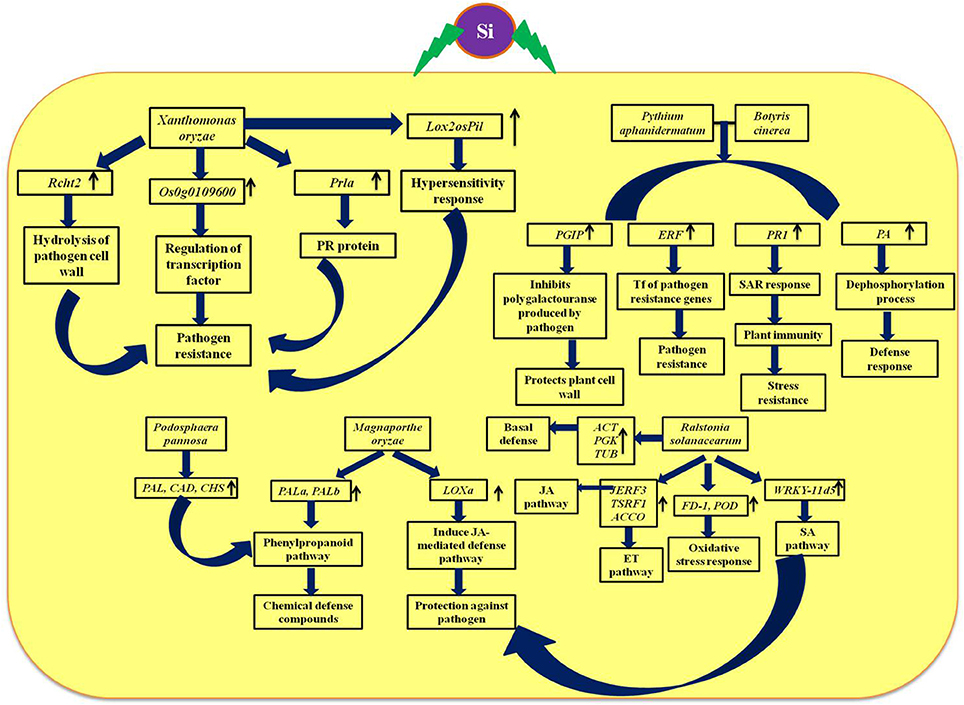
Figure 5. Schematic representation of Si-mediated regulation of vital genes associated with defense and phytohormones upon biotic stress. Rcht2, Chitinase; Prla, PR-1; Lox, Lipoxygenase; PAL, phenylalanine ammonia lyase; CAD, cinnamyl alcohol dehydrogenase; CHS, Chalcone synthase; PGIP, Polygalactouranase inhibitor protein; PA, phosphatase associated to defense; PR-1, pathogenesis-related protein; ERF, Ethylene response factor; JERF, Jasmonate and ethylene responsive factor 3; TSRF, Tomato stress-responsive factor; ACCO, 1-aminocyclopropane-1-carboxylate oxidase; FD-1, Ferredoxin-I; POD, Peroxidase; WRKY II, WRKY group II transcription factor; SA, Salicylic acid; JA, Jasmonic acid. The diagram was conceived based on the interpretation from the following literatures (Ghareeb et al., 2011b; Shetty et al., 2011; Rahman et al., 2015; El-Garhy et al., 2016; Song et al., 2016).
Conclusions
Silicon is the modest and a major element of soil with enormous benefits to plants especially in the mitigation of abiotic and biotic stress. Owing to its numerous advantages, the inclusion of Si in modern cultivation systems likes soil-less cultivation system has been blooming in several areas. In recent days, the modernization of technology allows us to investigate the molecular level regulation of compounds which has been extended to study the role of silicon in gene level by plant biologists under different stress conditions. Even though, the research on understanding of molecular rationale behind the Si-mediated stress tolerance is in rudimentary stage, upcoming outcomes from the recent studies have shed light into several possible ways of Si-dependent stress tolerance in plants. Based on the current reports it is evident that silicon possess multifaceted role in the regulation of genes involved in photosynthesis, secondary metabolism, polyamine biosynthesis, transcription, and water uptake. The molecular level modulations triggered by Si supplementation under stressed environment corresponded to the physiological improvement of plant growth and recovery from stress. In addition, several other novel molecular mechanisms behind the stress alleviation by Si have to be unraveled in the future.
Author Contributions
AM, collected the literatures and wrote the manuscript; YA proof-read, finalized, and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research work was supported by the National Agricultural Genome Program (NAGP) [Project No. PJ010449], Rural Development Administration, Republic of Korea.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01346/full#supplementary-material
References
Asmar, S. A., Castro, E. M., Pasqual, M., Pereira, F. J., and Soares, J. D. R. (2013). Changes in leaf anatomy and photosynthesis of micropropagated banana plantlets under different silicon sources. Sci. Hortic. 161, 328–332. doi: 10.1016/j.scienta.2013.07.021
Azad, A. K., Sawa, Y., Ishikawa, T., and Shibata, H. (2004). Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol. 45, 608–617. doi: 10.1093/pcp/pch069
Bae, H., Kim, S. K., Cho, S. K., Kang, B. G., and Kim, W. T. (2011). Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci. 180, 775–782. doi: 10.1016/j.plantsci.2011.02.008
Bies-Etheve, N., Gaubier-Comella, P., Debures, A., Lasserre, E., Jobet, E., Raynal, M., et al. (2008). Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol., 67, 107–124. doi: 10.1007/s11103-008-9304-x
Boursiac, Y., Chen, S., Luu, D. T., Sorieul, M., van den Dries, N., and Maurel, C. (2005). Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 139, 790–805. doi: 10.1104/pp.105.065029
Boursiac, Y., Prak, S., Boudet, J., Postaire, O., Luu, D.-T., Tournaire-Roux, C., et al. (2008). The response of Arabidopsis root water transport to a challenging environment implicates reactive oxygen species-and phosphorylation-dependent internalization of aquaporins. Plant Signal. Behav. 3, 1096–1098. doi: 10.4161/psb.3.12.7002
Breyton, C., de Vitry, C., and Popot, J. L. (1994). Membrane association of cytochrome b6f subunits. The Rieske iron-sulfur protein from Chlamydomonas reinhardtii is an extrinsic protein. J. Biol. Chem. 269, 7597–7602.
Brunings, A. M., Datnoff, L. E., Ma, J. F., Mitani, N., Nagamura, Y., Rathinasabapathi, B., et al. (2009). Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann. Appl. Biol. 155, 161–170. doi: 10.1111/j.1744-7348.2009.00347.x
Burnet, M., Lafontaine, P. J., and Hanson, A. D. (1995). Assay, purification, and partial characterization of choline monooxygenase from spinach. Plant Physiol. 108, 581–588. doi: 10.1104/pp.108.2.581
Carlisle, E. M. (1997). “Silicon,” in Handbook of Nutritionally Essential Mineral Elements, eds B. L. O'Dell and R. A. Sunde (New York, NY: Marcel Dekker Inc.), 603–608
Chai, Y. Y., Jiang, C. D., Shi, L., Shi, T. S., and Gu, W. B. (2010). Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol. Plant. 54, 145–148. doi: 10.1007/s10535-010-0023-1
Chaves, M. M., and Oliveira, M. M. (2004). Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J. Exp. Bot. 55, 2365–2384. doi: 10.1093/jxb/erh269
Chen, J. Q., Meng, X. P., Zhang, Y., Xia, M., and Wang, X. P. (2008). Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol. Lett. 30, 2191–2198. doi: 10.1007/s10529-008-9811-5
Chen, W., Yao, X., Cai, K., and Chen, J. (2011). Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 142, 67–76. doi: 10.1007/s12011-010-8742-x
Chérif, M., Benhamou, N., Menzies, J. G., and Bélanger, R. R. (1992). Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol. Mol. Plant Pathol. 41, 411–425. doi: 10.1016/0885-5765(92)90053-X
Cookson, L. J., Scown, D. K., McCarthy, K. J., and Chew, N. (2007). The effectiveness of silica treatments against wood-boring invertebrates. Holzforschung 61, 326–332. doi: 10.1515/HF.2007.045
Das, S., and Chattopadhyay, U. K. (2000). Role of silicon in modulating the internal morphology and growth of Mycobacterium tuberculosis. Ind. J. Tub 47, 87–91.
Debona, D., Rodrigues, F. A., and Datnoff, L. E. (2017). Silicon's role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 55, 4.1–4.23. doi: 10.1146/annurev-phyto-080516-035312
Deshmukh, R. K., Vivancos, J., Ramakrishnan, G., Guérin, V., Carpentier, G., Sonah, H., et al. (2015). A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 83, 489–500. doi: 10.1111/tpj.12904
Dubouzet, J. G., Sakuma, Y., Ito, Y., Kasuga, M., Dubouzet, E. G., Miura, S., et al. (2003). OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 33, 751–763. doi: 10.1046/j.1365-313X.2003.01661.x
El-Garhy, H. A. S., Rashid, I. A. S., Abou-Ali, R. M., and Moustafa, M. M. A. (2016). Field application of safe chemical elicitors induced the expression of some resistance genes against grey mold and cottony rot diseases during snap bean pods storage. Gene 576, 358–365. doi: 10.1016/j.gene.2015.10.048
Eneji, A. E., Inanaga, S., Muranaka, S., Li, J., Hattori, T., An, P., et al. (2008). Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 31, 355–365. doi: 10.1080/01904160801894913
Epstein, E. (1999). Silicon. Annu. Rev. Plant Biol. 50, 641–664. doi: 10.1146/annurev.arplant.50.1.641
Fawe, A., Abou-Zaid, M., Menzies, J. G., and Bélanger, R. R. (1998). Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 88, 396–401. doi: 10.1094/PHYTO.1998.88.5.396
Fujita, Y., Fujita, M., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2011). ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 124, 509–525. doi: 10.1007/s10265-011-0412-3
Gao, J. P., Chao, D. Y., and Lin, H. X. (2007). Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. J. Integr. Plant Biol. 49, 742–750. doi: 10.1111/j.1744-7909.2007.00495.x
Gao, Z., He, X., Zhao, B., Zhou, C., Liang, Y., Ge, R., et al. (2010). Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Physiol. 51, 767–775. doi: 10.1093/pcp/pcq036
Ghareeb, H., Bozsó, Z., Ott, P. G., Repenning, C., Stahl, F., and Wydra, K. (2011b). Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 75, 83–89. doi: 10.1016/j.pmpp.2011.02.003
Ghareeb, H., Bozsó, Z., Ott, P. G., and Wydra, K. (2011a). Silicon and Ralstonia solanacearum modulate expression stability of housekeeping genes in tomato. Physiol. Mol. Plant Pathol. 75, 176–179. doi: 10.1016/j.pmpp.2011.02.003
Gong, H., and Chen, K. (2012). The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 34, 1589–1594. doi: 10.1007/s11738-012-0954-6
Gong, H. J., Randall, D. P., and Flowers, T. J. (2006). Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 29, 1970–1979. doi: 10.1111/j.1365-3040.2006.01572.x
Gottardi, S., Iacuzzo, F., Tomasi, N., Cortella, G., Manzocco, L., Pinton, R., et al. (2012). Beneficial effects of silicon on hydroponically grown corn salad (Valerianella locusta (L.) Laterr) plants. Plant Physiol. Biochem. 56, 14–23. doi: 10.1016/j.plaphy.2012.04.002
Gunes, A., Inal, A., Bagci, E. G., Coban, S., and Sahin, O. (2007). Silicon increases boron tolerance and reduces oxidative damage of wheat grown in soil with excess boron. Biol. Plant. 51, 571–574. doi: 10.1007/s10535-007-0125-6
Hu, H., You, J., Fang, Y., Zhu, X., Qi, Z., and Xiong, L. (2008). Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 67, 169–181. doi: 10.1007/s11103-008-9309-5
Hu, W., Yuan, Q., Wang, Y., Cai, R., Deng, X., Wang, J., et al. (2012). Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 53, 2127–2141. doi: 10.1093/pcp/pcs154
Hussain, S. S., Kayani, M. A., and Amjad, M. (2011). Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol. Prog. 27, 297–306. doi: 10.1002/btpr.514
Islam, M. M., Ahmed, M., and Mahaldar, D. (2005). In vitro callus induction and plant regeneration in seed explants of rice (Oryza Sativa L.). Res. J. Agric. Biol. Sci. 1, 72–75.
Jain, M., Nijhawan, A., Tyagi, A. K., and Khurana, J. P. (2006). Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 345, 646–651. doi: 10.1016/j.bbrc.2006.04.140
Jarosch, B., Collins, N. C., Zellerhoff, N., and Schaffrath, U. (2005). RAR1, ROR1, and the actin cytoskeleton contribute to basal resistance to Magnaporthe grisea in barley. Mol. Plant-Microbe Interacti. 18, 397–404. doi: 10.1094/MPMI-18-0397
Jayawardana, H. A. R. K., Weerahewa, H. L. D., and Saparamadu, M. D. J. S. (2015). Enhanced resistance to anthracnose disease in chili pepper (Capsicum annuum L.) by amendment of the nutrient solution with silicon. J. Horticult. Sci. Biotechnol. 90, 557–562. doi: 10.1080/14620316.2015.11668714
Kawakami, K., Iwai, M., Ikeuchi, M., Kamiya, N., and Shen, J. R. (2007). Location of PsbY in oxygen-evolving photosystem II revealed by mutagenesis and X-ray crystallography. FEBS Lett. 581, 4983–4987. doi: 10.1016/j.febslet.2007.09.036
Khattab, H. I., Emam, M. A., Emam, M. M., Helal, N. M., and Mohamed, M. R. (2014). Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plant. 58, 265–273. doi: 10.1007/s10535-014-0391-z
Knight, C. T., and Kinrade, S. D. (2001). A primer on the aqueous chemistry of silicon. Studies Plant Sci. 8, 57–84. doi: 10.1016/S0928-3420(01)80008-2
Kraft, E., Stone, S. L., Ma, L., Su, N., Gao, Y., Lau, O. S., et al. (2005). Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 139, 1597–1611. doi: 10.1104/pp.105.067983
Kurepin, L. V., Ivanov, A. G., Zaman, M., Pharis, R. P., Allakhverdiev, S. I., Hurry, V., et al. (2015). Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosyn. Res. 126, 221–235. doi: 10.1007/s11120-015-0125-x
Lanning, F. C. (1966). Relation of silicon in barley to disease, cold, and pest resistance. J. Agric. Food Chem. 14, 636–638. doi: 10.1021/jf60148a026
Lenka, S. K., Katiyar, A., Chinnusamy, V., and Bansal, K. C. (2011). Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 9, 315–327. doi: 10.1111/j.1467-7652.2010.00560.x
Li, J., Leisner, S. M., and Frantz, J. (2008). Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J. Am. Soc. Horticult. Sci. 133, 670–677.
Liang, Y., Sun, W., Zhu, Y. G., and Christie, P. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ. Pollut. 147, 422–428. doi: 10.1016/j.envpol.2006.06.008
Liang, Y., Wong, J. W. C., and Wei, L. (2005). Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58, 475–483. doi: 10.1016/j.chemosphere.2004.09.034
Liu, J. H., Nada, K., Honda, C., Kitashiba, H., Wen, X. P., Pang, X. M., et al. (2006). Polyamine biosynthesis of apple callus under salt stress: importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 57, 2589–2599. doi: 10.1093/jxb/erl018
Liu, P., Yin, L., Wang, S., Zhang, M., Deng, X., Zhang, S., et al. (2015). Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 111, 42–51. doi: 10.1016/j.envexpbot.2014.10.006
Lucas, S., Durmaz, E., Akpınar, B. A., and Budak, H. (2011). The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol. Biochem. 49, 346–351. doi: 10.1016/j.plaphy.2011.01.016
Lunde, C., Jensen, P. E., Haldrup, A., Knoetzel, J., and Scheller, H. V. (2000). The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615. doi: 10.1038/35046121
Ma, D., Sun, D., Wang, C., Qin, H., Ding, H., Li, Y., et al. (2016). Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 35, 1–10. doi: 10.1007/s00344-015-9500-2
Ma, J. F. (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50, 11–18. doi: 10.1080/00380768.2004.10408447
Ma, J. F., and Yamaji, N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. doi: 10.1016/j.tplants.2006.06.007
Manivannan, A., Soundararajan, P., Cho, Y. S., Park, J. E., and Jeong, B. R. (2017). Sources of silicon influence photosystem and redox homeostasis-related proteins during the axillary shoot multiplication of Dianthus caryophyllus. Plant Biosyst. 1–7. doi: 10.1080/11263504.2017.1320312
Maurel, C., Boursiac, Y., Luu, D. T., Santoni, V., Shahzad, Z., and Verdoucq, L. (2015). Aquaporins in plants. Physiol. Rev. 95, 1321–1358. doi: 10.1152/physrev.00008.2015
Mizoi, J., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2012). AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819, 86–96. doi: 10.1016/j.bbagrm.2011.08.004
Muneer, S., Park, Y. G., Manivannan, A., Soundararajan, P., and Jeong, B. R. (2014). Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int. J. Mol. Sci. 15, 21803–21824. doi: 10.3390/ijms151221803
Nakashima, K., Ito, Y., and Yamaguchi-Shinozaki, K. (2009). Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 149, 88–95. doi: 10.1104/pp.108.129791
Nakashima, K., Takasaki, H., Mizoi, J., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2012). NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819, 97–103. doi: 10.1016/j.bbagrm.2011.10.005
Neumann, D., and Zur Nieden, U. (2001). Silicon and heavy metal tolerance of higher plants. Phytochemistry 56, 685–692. doi: 10.1016/S0031-9422(00)00472-6
Nicot, N., Hausman, J. F., Hoffmann, L., and Evers, D. (2005). Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 56, 2907–2914. doi: 10.1093/jxb/eri285
Ning, Y., Jantasuriyarat, C., Zhao, Q., Zhang, H., Chen, S., Liu, J., et al. (2011). The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 157, 242–255. doi: 10.1104/pp.111.180893
Nwugo, C. C., and Huerta, A. J. (2008). Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311, 73–86. doi: 10.1007/s11104-008-9659-4
Pandey, S., Ranade, S. A., Nagar, P. K., and Kumar, N. (2000). Role of polyamines and ethylene as modulators of plant senescence. J. Biosci. 25, 291–299. doi: 10.1007/BF02703938
Parent, B., Hachez, C., Redondo, E., Simonneau, T., Chaumont, F., and Tardieu, F. (2009). Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol. 149, 2000–2012. doi: 10.1104/pp.108.130682
Park, J. J., Yi, J., Yoon, J., Cho, L. H., Ping, J., Jeong, H. J., et al. (2011). OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 65, 194–205. doi: 10.1111/j.1365-313X.2010.04416.x
Pfannschmidt, T., and Yang, C. (2012). The hidden function of photosynthesis: a sensing system for environmental conditions that regulates plant acclimation responses. Protoplasma 249, 125–136. doi: 10.1007/s00709-012-0398-2
Pottosin, I., and Shabala, S. (2014). Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 5:154. doi: 10.3389/fpls.2014.00154
Qin, F., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2011). Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 52, 1569–1582. doi: 10.1093/pcp/pcr106
Quinet, M., Ndayiragije, A., Lefèvre, I., Lambillotte, B., Dupont-Gillain, C. C., and Lutts, S. (2010). Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J. Exp. Bot. 61, 2719–2733. doi: 10.1093/jxb/erq118
Rabbani, M. A., Maruyama, K., Abe, H., Khan, M. A., Katsura, K., Ito, Y., et al. (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 133, 1755–1767. doi: 10.1104/pp.103.025742
Rahman, A., Wallis, C. M., and Uddin, W. (2015). Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 105, 748–757. doi: 10.1094/PHYTO-12-14-0378-R
Rédei, G. P. (2008). “Silicon (Si),” in Encyclopedia of Genetics, Genomics, Proteomics, and Informatics (Springer), 1817.
Rhodes, M. J. C. (1994). Physiological roles for secondary metabolites in plants: some progress, many outstanding problems. Plant Mol. Biol. 24, 1–20. doi: 10.1007/BF00040570
Rodrigues, F. Á., McNally, D. J., Datnoff, L. E., Jones, J. B., Labbé, C., Benhamou, N., et al. (2004). Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology 94, 177–183. doi: 10.1094/PHYTO.2004.94.2.177
Romero-Aranda, M. R., Jurado, O., and Cuartero, J. (2006). Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 163, 847–855. doi: 10.1016/j.jplph.2005.05.010
Roy, M., and Wu, R. (2001). Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 160, 869–875. doi: 10.1016/S0168-9452(01)00337-5
Shetty, R., Fretté, X., Jensen, B., Shetty, N. P., Jensen, J. D., Jørgensen, H. J. L., et al. (2011). Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 157, 2194–2205. doi: 10.1104/pp.111.185215
Shi, H., and Chan, Z. (2014). Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 56, 114–121. doi: 10.1111/jipb.12128
Shi, Y., Wang, Y., Flowers, T. J., and Gong, H. (2013). Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 170, 847–853. doi: 10.1016/j.jplph.2013.01.018
Shinozaki, K., and Yamaguchi-Shinozaki, K. (2000). Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3, 217–223. doi: 10.1016/S1369-5266(00)00067-4
Shinozaki, K., Yamaguchi-Shinozaki, K., and Seki, M. (2003). Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410–417. doi: 10.1016/S1369-5266(03)00092-X
Song, A., Li, P., Fan, F., Li, Z., and Liang, Y. (2014). The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE 9:e113782. doi: 10.1371/journal.pone.0113782
Song, A., Xue, G., Cui, P., Fan, F., Liu, H., Yin, C., et al. (2016). The role of silicon in enhancing resistance to bacterial blight of hydroponic-and soil-cultured rice. Sci. Rep. 6:24640. doi: 10.1038/srep24640
Song, S. Y., Chen, Y., Chen, J., Dai, X. Y., and Zhang, W. H. (2011). Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234, 331–345. doi: 10.1007/s00425-011-1403-2
Soundararajan, P., Manivannan, A., Cho, Y. S., and Jeong, B. R. (2017a). Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Front. Plant Sci. 8:738. doi: 10.3389/fpls.2017.00738
Soundararajan, P., Manivannan, A., Ko, C. H., and Jeong, B. R. (2017b). Silicon enhanced redox homeostasis and protein expression to mitigate the salinity stress in rosa hybrida ‘Rock Fire’. J. Plant Growth Regul. 1–19. doi: 10.1016/j.pmpp.2011.02.003
Soundararajan, P., Sivanesan, I., Jana, S., and Jeong, B. R. (2014). Influence of silicon supplementation on the growth and tolerance to high temperature in Salvia splendens. Horticul. Environ. Biotechnol. 55, 271–279. doi: 10.1007/s13580-014-0023-8
Stone, S. L., Hauksdóttir, H., Troy, A., Herschleb, J., Kraft, E., and Callis, J. (2005). Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 137, 13–30. doi: 10.1104/pp.104.052423
Sutka, M., Li, G., Boudet, J., Boursiac, Y., Doumas, P., and Maurel, C. (2011). Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 155, 1264–1276. doi: 10.1104/pp.110.163113
Takasaki, H., Maruyama, K., Kidokoro, S., Ito, Y., Fujita, Y., Shinozaki, K., et al. (2010). The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genomics 284, 173–183. doi: 10.1007/s00438-010-0557-0
Tang, W., Newton, R. J., Li, C., and Charles, T. M. (2007). Enhanced stress tolerance in transgenic pine expressing the pepper CaPF1 gene is associated with the polyamine biosynthesis. Plant Cell Rep. 26, 115–124. doi: 10.1007/s00299-006-0228-0
Tran, L. S. P., Nishiyama, R., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2010). Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach. GM Crops 1, 32–39. doi: 10.4161/gmcr.1.1.10569
Tripathi, D. K., Singh, V. P., Gangwar, S., Prasad, S. M., Maurya, J. N., and Chauhan, D. K. (2014). “Role of silicon in enrichment of plant nutrients and protection from biotic and abiotic stresses,” in Improvement of Crops in the Era of Climatic Changes, eds P. Ahmad, M. R. Wani, M. M. Azooz and L. S. P. Tran (New York, NY: Springer), 39–56.
Tripathi, D. K., Singh, V. P., Kumar, D., and Chauhan, D. K. (2012). Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol. Plant. 34, 279–289. doi: 10.1007/s11738-011-0826-5
Tunnacliffe, A., and Wise, M. J. (2007). The continuing conundrum of the LEA proteins. Naturwissenschaften 94, 791–812. doi: 10.1007/s00114-007-0254-y
Umezawa, T., Fujita, M., Fujita, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2006). Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 17, 113–122. doi: 10.1016/j.copbio.2006.02.002
Wang, Q., Guan, Y., Wu, Y., Chen, H., Chen, F., and Chu, C. (2008). Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 67, 589–602. doi: 10.1007/s11103-008-9340-6
Wang, Y., Stass, A., and Horst, W. J. (2004). Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 136, 3762–3770. doi: 10.1104/pp.104.045005
Watanabe, S., Shimoi, E., Ohkama, N., Hayashi, H., Yoneyama, T., Yazaki, J., et al. (2004). Identification of several rice genes regulated by Si nutrition. Soil Sci. Plant Nutr. 50, 1273–1276. doi: 10.1080/00380768.2004.10408603
Xiong, L., Schumaker, K. S., and Zhu, J. K. (2002). Cell signaling during cold, drought, and salt stress. Plant Cell 14, S165–S183.
Yin, L., Wang, S., Tanaka, K., Fujihara, S., Itai, A., Den, X., et al. (2016). Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 39, 245–258. doi: 10.1111/pce.12521
Yoshida, S., Ohnishi, Y., and Kitagishi, K. (1962). Histochemistry of silicon in rice plant: III. The presence of cuticle-silica double layer in the epidermal tissue. Soil Sci. Plant Nutr. 8, 1–5. doi: 10.1080/00380768.1962.10430982
Zeier, J., Delledonne, M., Mishina, T., Severi, E., Sonoda, M., and Lamb, C. (2004). Genetic elucidation of nitric oxide signaling in incompatible plant-pathogen interactions. Plant Physiol. 136, 2875–2886. doi: 10.1104/pp.104.042499
Zhang, Q., Liu, J., Lu, H., Zhao, S., Wang, W., Du, J., et al. (2015). Effects of silicon on growth, root anatomy, radial oxygen loss (ROL) and Fe/Mn plaque of Aegiceras corniculatum (L.) Blanco seedlings exposed to cadmium. Environ. Nanotechnol. Monitor. Manage. 4, 6–11. doi: 10.1016/j.enmm.2015.04.001
Keywords: defense response, gene regulation, photosynthesis, polyamine biosynthesis, regulatory elements
Citation: Manivannan A and Ahn Y-K (2017) Silicon Regulates Potential Genes Involved in Major Physiological Processes in Plants to Combat Stress. Front. Plant Sci. 8:1346. doi: 10.3389/fpls.2017.01346
Received: 24 April 2017; Accepted: 19 July 2017;
Published: 03 August 2017.
Edited by:
Rupesh Kailasrao Deshmukh, Laval University, CanadaReviewed by:
Stefano Cesco, Free University of Bozen-Bolzano, ItalyAmit A. Deokar, University of Saskatchewan, Canada
Copyright © 2017 Manivannan and Ahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yul-Kuyn Ahn, YXlreXVuQGtvcmVhLmty
 Abinaya Manivannan
Abinaya Manivannan Yul-Kuyn Ahn1,2*
Yul-Kuyn Ahn1,2*