
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 18 July 2017
Sec. Plant Breeding
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.01209
 Chuanzhi Zhao1
Chuanzhi Zhao1 Jingjing Qiu1,2
Jingjing Qiu1,2 Gaurav Agarwal3,4
Gaurav Agarwal3,4 Jiangshan Wang1
Jiangshan Wang1 Xuezhen Ren1
Xuezhen Ren1 Han Xia1
Han Xia1 Baozhu Guo3
Baozhu Guo3 Changle Ma2
Changle Ma2 Shubo Wan1
Shubo Wan1 David J. Bertioli5
David J. Bertioli5 Rajeev K. Varshney4
Rajeev K. Varshney4 Manish K. Pandey4*
Manish K. Pandey4* Xingjun Wang1,2*
Xingjun Wang1,2*Despite several efforts in the last decade toward development of simple sequence repeat (SSR) markers in peanut, there is still a need for more markers for conducting different genetic and breeding studies. With the effort of the International Peanut Genome Initiative, the availability of reference genome for both the diploid progenitors of cultivated peanut allowed us to identify 135,529 and 199,957 SSRs from the A (Arachis duranensis) and B genomes (Arachis ipaensis), respectively. Genome sequence analysis showed uneven distribution of the SSR motifs across genomes with variation in parameters such as SSR type, repeat number, and SSR length. Using the flanking sequences of identified SSRs, primers were designed for 51,354 and 60,893 SSRs with densities of 49 and 45 SSRs per Mb in A. duranensis and A. ipaensis, respectively. In silico PCR analysis of these SSR markers showed high transferability between wild and cultivated Arachis species. Two physical maps were developed for the A genome and the B genome using these SSR markers, and two reported disease resistance quantitative trait loci (QTLs), qF2TSWV5 for tomato spotted wilt virus (TSWV) and qF2LS6 for leaf spot (LS), were mapped in the 8.135 Mb region of chromosome A04 of A. duranensis. From this genomic region, 719 novel SSR markers were developed, which provide the possibility for fine mapping of these QTLs. In addition, this region also harbors 652 genes and 49 of these are defense related genes, including two NB-ARC genes, three LRR receptor-like genes and three WRKY transcription factors. These disease resistance related genes could contribute to resistance to viral (such as TSWV) and fungal (such as LS) diseases in peanut. In summary, this study not only provides a large number of molecular markers for potential use in peanut genetic map development and QTL mapping but also for map-based gene cloning and molecular breeding.
Cultivated peanut or groundnut (Arachis hypogaea L.), is a source of high quality edible oil and protein, and is one of the most important oil crops worldwide. Peanut is widely planted in China, India and the United States. The world’s total consumption of peanut is about 29 million metric tons per year1, contributing to an estimated production value of about $35 billion (Guo et al., 2011). However, peanut is often grown on marginal soils with lesser inputs and usually intercropped with cereals in many countries. Peanut production and productivity is often constrained by several biotic and abiotic factors, such as drought, salinity, bacterial wilt disease, and leaf spot disease (Krishna et al., 2015). Cultivated peanut is an allotetraploid (AABB, 2n = 4 × = 40) derived from hybridization between A. duranensis (AA, 2n = 2 × = 20) and A. ipaensis (BB, 2n = 2 × = 20) (Lavia et al., 2011). During the past decade, significant progress has been made in developing genomic resources which facilitated several successful efforts of trait mapping and molecular breeding in peanut (Pandey et al., 2012, 2016; Varshney et al., 2013). These studies used low density genetic maps and hence could not provide the optimum level of resolution for trait dissection and discovery of candidate genes. Nevertheless, some of these studies produced very good results despite deploying the minimal genomic and genetic resources including SSRs available at that time (Varshney et al., 2013).
Microsatellites, or simple sequence repeats (SSRs), are DNA fragments consisting of tandemly repeated short units (1–6 bp) that are present in both protein coding and non-coding regions of the genome (Gupta et al., 1996; Squirrell et al., 2003; Haq et al., 2014). SSRs have become a common tool and were broadly used in plant genetics analysis and breeding programs, owing to their characteristics of simplicity, abundance, ubiquity, variation, co-dominance, and multi-allelism among genomes (Powell et al., 1996). The polymorphism of SSRs generated from the number of repeat units can easily be detected by PCR method using primers designed according to the flanking sequences. Although the development of SSR markers was previously expensive, labor intensive and time consuming (Varshney et al., 2002), the availability of low-cost sequencing data using next-generation sequencing (NGS) made this task faster and less expensive in identifying genome-wide structural variations including SSRs as potential genetic markers in many crops (Pandey et al., 2016).
Simple sequence repeats derived from expressed sequence tags, transcriptome libraries, and genomic libraries are referred to as EST-SSRs, transcriptome-SSRs and g-SSRs, respectively. EST-SSRs and transcriptome-SSRs are derived from the CDS region of genes with high selection pressure, leading to low polymorphism in contrast to SSRs from non-CDS regions of the genome (Cho et al., 2000; Shore et al., 2010). In bread wheat, it was shown that the polymorphism rate in EST-SSRs is lower than that in g-SSRs, suggesting that g-SSRs can serve as valuable complements to EST-SSRs and transcriptome-SSRs (Balfourier et al., 2007; Han et al., 2015). A number of studies reported development of SSR markers in peanut, for example, EST-SSRs derived from cDNA libraries (Liang et al., 2009; Song et al., 2010), SSRs from bacterial artificial chromosome (BAC)-end sequences (Wang et al., 2012), and transcriptome-SSRs from a transcriptome library of developing seeds (Zhang et al., 2012; Huang et al., 2016; Zhong et al., 2016; Zhou et al., 2016). Such studies were very limited and hence the available SSRs are insufficient for conducting moderate to high resolution genetic studies in peanut.
The peanut genomics research witnessed significant milestone in 2016 by completing genome sequencing of the diploid progenitors of cultivated peanut, i.e., A. duranensis and A. ipaensis (Bertioli et al., 2016; Chen et al., 2016). Although one of these sequencing efforts developed genome-wide SSRs for A. duranensis (Chen et al., 2016), the application of these SSRs is limited due to lack of pseudomolecule-level information. The high quality genome assemblies developed by the International Peanut Genome Initiative (IPGI) and the Peanut Genome Consortium (PGC) provided opportunity for developing genetic markers from genome-wide structural variations in peanut (Bertioli et al., 2016). As members of IPGI and the joint effort to accelerate marker-assisted selection (MAS) in peanut, we identified and developed g-SSRs from A. duranensis and A. ipaensis. The frequency and distribution of motif length, type, and repeat number of SSRs in the genomes of these two Arachis species were also compared. Using these SSRs, we constructed a high-density SSR physical map of wild peanuts. The positions of these SSRs were compared with the previously developed Arachis SSR markers. To evaluate the application value of these SSRs, we studied their polymorphism in different species. This study found these newly developed g-SSR markers very useful and could facilitate the advancement of many basic and applied genomic studies in peanut, including fine mapping of quantitative trait loci (QTLs) for important agronomic traits, positional-based gene cloning, molecular breeding, and diversifying the cultivated genepool using wild relatives of peanut.
Two cultivated peanut genotypes namely Tifrunner and Fenghua 1, two diploid ancestors namely A. duranensis and A. ipaensis, a synthetic amphidiploid namely IpaDur (A. ipaensis × A. duranensis), and a (Fenghua1 × IpaDur) F1 individual were used in this study for SSR identification and validation. Genomic DNA of A. duranensis, A. ipaensis, Tifrunner and (Fenghua1 × IpaDur) F1 was isolated from seeds using Plant Genomic DNA Extraction Kit (TIANGEN, Beijing, China) according to the instructions of the manufacturer2.
Polymerase chain reactions (PCRs) were performed in a total of volume of 20 μl that contained 0.2 mM dNTP Mix, 0.5 μM of each primers, 0.5 U of rTaq DNA polymerase (Takara, Dalian, China), 2.0 mM MgCl2, 1x PCR buffer and 70 ng of template DNA. The PCR program was as following: 94°C for 4 min, 35 cycles of 30 s at 94°C, 30 s at annealing temperatures (refer to Supplementary Tables S1, S2) and 30 s at 72°C, 7 min at 72°C. PCR products were separated via 6.5% denaturing polyacrylamide gels. Gels were fast silver stained and photographed.
The whole genome sequences of A. duranensis and A. ipaensis were downloaded from PeanutBase3. The genome sequences were used to identify SSR loci using Perl scripts software MISA4 with the default parameters. The identification criteria were: mono-nucleotide repeats motif with at least 12 repeats, di-nucleotide with six, tri- and quad-nucleotide with five, penta and hexa-nucleotide with four repeats. Compound microsatellites were defined as those with the interval between two repeats motifs shorter than 100 nt as previous reports (Wang et al., 2015; Deng et al., 2016; Liu et al., 2016).
Primers were designed from flanking sequences of the identified SSRs using Primer 3 software5 with the following parameters: 18–27 bp primer length, 57–63°C melting temperature, 30–70% GC content and 100–300 bp product size. In order to run Primer 3.0, another two Perl scripts, p3_in.pl and p3_out.pl were used. The p3_in.pl and p3_out.pl were used to create a primer3 input file and to calculate and merge all information.
The amplification efficiency of newly developed SSRs was evaluated using in silico PCR method. The genome sequences of A. duranensis, A. ipaensis and the scaffold sequences of Tifrunner (unpublished data) were used as templates. The software e-PCR (Version: 2.3.126) was used for in silico PCR with the default parameters: 2 bp mismatch, 1 bp gap, 50 bp margin, and 50–1000 bp product size (Shi et al., 2014; Deng et al., 2016). The polymorphism of SSR primers were tested by comparing the repeat numbers of the particular SSR in different Arachis species.
The newly developed SSRs were physically mapped on the 10 pseudomolecules each of A. duranensis and A. ipaensis according to their genomic location (Bertioli et al., 2016). For the available published SSR markers of Arachis genetic linkage maps (Shirasawa et al., 2013), the original sequences of ESTs, GSS, BAC and transcriptome sequences were downloaded from GenBank7 for sequence alignment. To integrate the new SSRs with other publicly available SSR maps (Shirasawa et al., 2013), the original sequences of public SSRs were mapped against Arachis genome sequences using BLAST software8.
The available genome sequences of A. duranensis and A. ipaensis were searched for microsatellites with different types of desirable repeat motifs from mono- to hexa-nucleotide. A total of 135,529 and 199,957 SSRs were identified from the 1,084.3 and 1,353.8 Mb genomic sequences of A. duranensis and A. ipaensis, respectively. The overall frequency occurrence of SSRs was 125.0 and 147.7 SSRs per Mb, or one SSR every 8.0 and 6.7 Kb in A. duranensis and A. ipaensis, respectively (Table 1). The length, type and repeat numbers of SSRs had high correlations between A. duranensis and A. ipaensis genomes.
A total of 378 and 392 types of SSR motifs were detected in these two Arachis species, respectively. Among them, there were 2, 4, 10, 26, 89, 247 types, and 2, 4, 10, 25, 93, 258 types of mono- to hexanucleotide repeats in A. duranensis and A. ipaensis, respectively. The type and number of mono-, di-, and tri- SSR motifs were similar in frequency in these two progenitor species. A total of 25 types of tetra- SSR motifs were found in both A. duranensis and A. ipaensis genomes except AGCC/CGGT, which could be detected only in A. duranensis. In addition, 10 types of penta- SSR motifs (three in A. duranensis, seven in A. ipaensis) and 89 types of hexa- SSR motifs (39 in A. duranensis, 50 in A. ipaensis) were found in only one of these two progenitor species.
Among the 135,529 SSRs obtained from A. duranensis, the dinucleotide repeats were most abundant (47,805) with a proportion of 35.3%, followed by tri- (42,529, 31.4%), mono- (29689, 21.9%), penta- (7,658, 5.7%), tetra- (4,988, 3.7%), and hexa- (2,860, 2.1%) nucleotide SSRs. For A. ipaensis, dinucleotide (75,334, 37.7%) also represented the most abundant SSR motifs, followed by mono- (57589, 28.8%), tri- (45,717, 22.5%), penta- (11,092, 5.5%), tetra- (6,736, 3.4%), and hexa- (3,489, 1.7%) nucleotide SSRs (Figure 1A). These results indicated that the distributions of the motif length in the assembled genomic sequences of A. duranensis and A. ipaensis were almost identical, i.e., mono-, di-, tri- repeats represented the most abundant motifs and accounted for similar proportions, whereas tetra-, penta-, and hexanucleotide repeats were relatively uncommon.
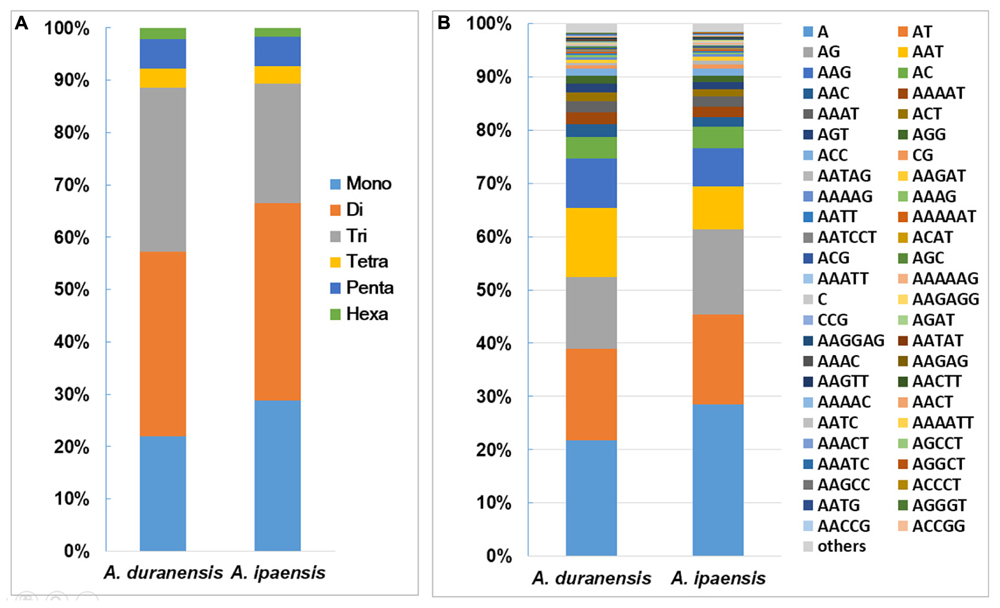
FIGURE 1. Distribution of SSR motifs in Arachis species. (A) Distribution of SSR motif length from mono- to hexanucleotide repeats in A. duranensis and A. ipaensis. Flexible relaxed criteria were used to identify SSR with minimum repeats number of 12, 6, 5, 5, 4, and 4 for mono- to hexanucleotide repeat motifs. (B) The vertical axis indicates the abundance (%) of SSRs with different type of motifs.
The distributions of the top 50 SSR types in the assembled genomic sequences of A. duranensis and A. ipaensis are presented in Figure 1B. Among them, A/T had the highest occurrence (21.8% in A. duranensis, 28.4% in A. ipaensis), followed by AT/AG (17.3% in A. duranensis, 16.9% in A. ipaensis), AG/CT (13.4% in A. duranensis, 16.0% in A. ipaensis) and AAT/ATT (9.3% in A. duranensis, 7.3% in A. ipaensis) (Figure 1B). Specifically, the major motifs of mono- to hexanucleotide repeats were A, AT, AAT, AAAT, AAAAT and AAAAAT, respectively, in which AT motifs were rich. SSRs with CG-rich repeats were rare in both Arachis species. In addition, we observed that the abundances of SSRs decreased with the motif repeat number increasing, and the rate of this change was the fastest for hexa- and pentanucleotide repeats, followed by tetra-, tri-, di, and monoucleotide repeats (Figure 2).
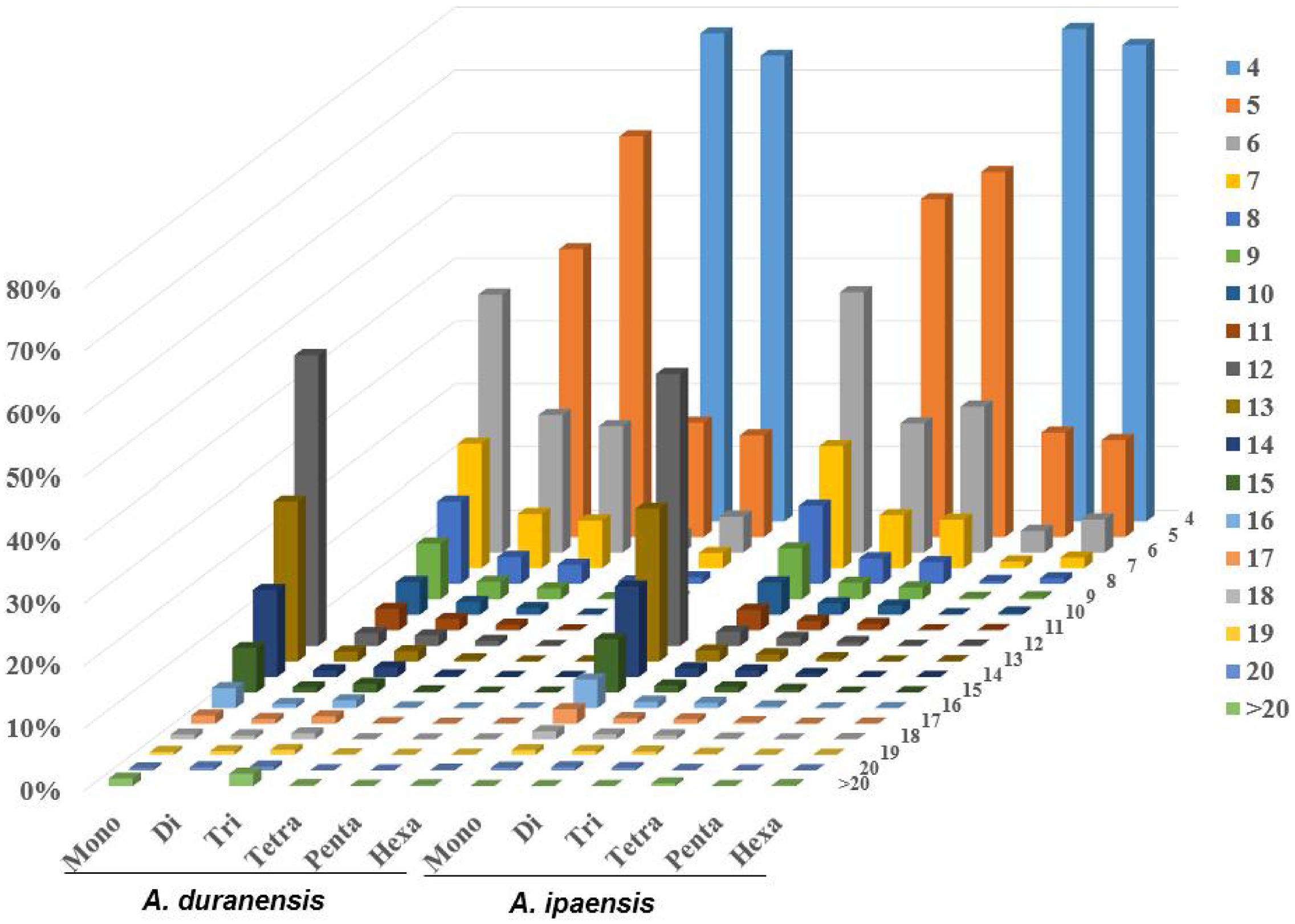
FIGURE 2. Distribution of SSR motif repeat number from mono- to hexanucleotide. The vertical axis shows the abundance of SSRs that have different motif repeat number (from 4 to >20), which are discriminated by legends of different colors.
We analyzed the distribution of SSRs on each chromosome of A. duranensis (A genome) and A. ipaensis (B genome). Among the 10 chromosomes of A. duranensis, chromosome A03 (16,546) had the largest number of SSRs, followed by chromosomes A05, A04, A09, A06, A01, A10, A02, A07, and A08. The top five largest chromosomes of A. duranensis are A03, A04, A09, A06 and A05, therefore, chromosome length was not associated with the number of SSRs per chromosome. The differences in densities of SSRs on different chromosomes were significant, ranging from 111.63 SSRs/Mb to 189.58 SSRs/Mb with average of 124.69 SSRs/Mb. The density on chromosome A08 was the highest (189.58 SSRs/Mb), while that on chromosome A10 was the lowest (111.63 SSRs/Mb). For A. ipaensis, chromosome B03 (21,680) had the largest number of SSRs, followed by chromosomes B05, B09, B06, B10, B01, B04, B08, B07, and B02. Similarly, the numbers of SSRs were not in accordance with the length of each chromosome. Compared to A. duranensis, the differences in densities of SSRs on different chromosomes were not significant in A. ipaensis, ranging from 159.28 SSRs/Mb to 136.49 SSRs/Mb with average 142.43 SSRs/Mb. As mentioned above, the average density of SSRs on the entire genome of A. ipaensis was higher than that of A. duranensis. We noted that the number and densities of SSRs in each chromosome of A. ipaensis was higher than that in the counterpart chromosome of A. duranensis, except for A08 and B08 (Figure 3).
According to the flanking sequences of the identified SSRs from A. duranensis and A. ipaensis, amplification primers for 51,354 and 60,893 SSRs were successfully designed, accounting for 39.40 and 31.58% of 130,346 and 192,821 SSR loci, respectively (Table 2). We failed to generate specific amplification primers for the remaining SSRs mostly due to the limited length of flanking sequences from each side of the SSRs, especially for mononucleotide type SSRs. The densities of the SSR markers that could be amplified were 49.13 per Mb (or one every 20.36 kb) and 44.98 per Mb (or one every 22.23 kb) in the A. duranensis and A. ipaensis genomes, respectively (Table 3). Among these SSR markers, dinucleotide repeats SSRs were most abundant (23595, 30304) with proportions of 45.95% and 49.60% in A. duranensis and A. ipaensis, respectively. Trinucleotide repeats were the second most popular SSRs in both diploid progenitor species, accounting for more than one-third of total SSRs, followed by hexa-, penta-, and tetranucleotide SSRs.
The motif types and the distributions of developed SSR markers were consistent with that of SSR loci in A. duranensis and A. ipaensis genomes, in which AT-rich repeat motifs were abundant, while the CG-rich repeat patterns were rare (Supplementary Tables S1, S2). For example, AT/TA (11,829, 16,227) repeats were the most abundant SSRs, contributing to 50.13% and 53.55% of total dinucleotide SSR markers in A. duranensis and A. ipaensis, respectively. Moreover, we analyzed the distribution of SSR markers on different chromosomes of A. duranensis and A. ipaensis (Table 3). For A. duranensis, chromosome A03 had the largest number (6,734) of SSR markers, followed by chromosomes A05 and A01, containing 5,548 and 5,305 SSR markers, respectively. In A. ipaensis, chromosome B03 (7,343) had more SSR markers than other chromosomes (Table 3). Chromosome A07 (4,056) and B02 (5,548) had the fewest SSR markers in A. duranensis and A. ipaensis, respectively (Table 3). Chromosome A08 had a density of 82.67 SSR markers per Mb, which was considerably higher than that of other chromosomes. The densities of SSRs on other chromosomes were not different and ranged from 41.82 (B01) to 53.59 per Mb (B03) (Table 3).
To analyze the amplification efficiency and specificity of the developed SSR markers, all SSR markers were subjected to in silico PCR analysis based on the genome sequences of A. duranensis, A. ipaensis and the scaffold sequences of Tifrunner (unpublished data), a tetraploid A. hypogaea variety which was selected for whole genome sequencing. The numbers of in silico PCR product(s) were recorded and summarized (Table 4). The in silico PCR results demonstrated that more than three-quarters of the SSR markers (39,922 out of 51,354) from AA genome only generated one specific PCR product in AA genome. And 4,617 (8.99%), 1,562 (3.04%), and 5,253 (10.23%) SSR markers from AA genome generated 2, 3, and >3 PCR products, respectively, in A. duranensis genome sequences. A total of 15,734 (30.64%), 2,187 (4.26%), 969 (1.89%), and 4,091 (7.97%) SSR markers from A. duranensis generated 1, 2, 3, and >3 PCR products, respectively, in A. ipaensis genome. More than half of the developed SSR markers from the AA genome could not generate PCR product(s) in the BB genome (Table 4).
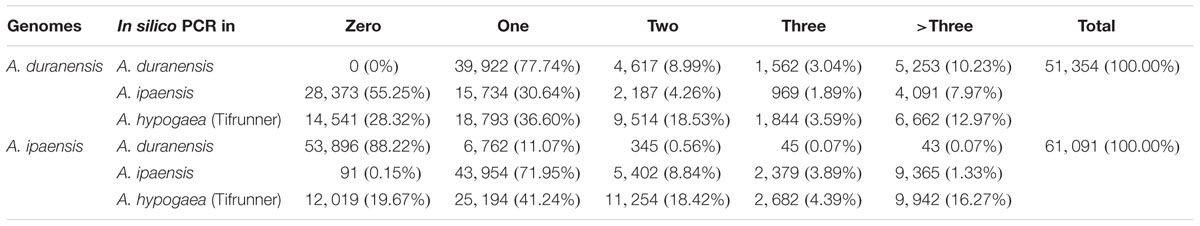
TABLE 4. Generated number of in silico PCR products by genome-wide SSR markers in A. duranensis, A. ipaensis, and A. hypogaea (Tifrunner) genomes.
For SSR markers from A. ipaensis, 91 (0.15%), 43,954 (71.95%), 5,402 (8.84%), 2,379 (3.89%), and 9,365 (1.33%) of them generated 0, 1, 2, 3, and >3 PCR products, respectively, in A. ipaensis genome. A total number of 53,896 (88.22%), 6,762 (11.07%), 345 (0.56%), 45 (0.07%), and 43 (0.07%) of them generated 0, 1, 2, 3, and >3 PCR products, respectively, from A. duranensis genome (Table 4).
Although all of these SSR markers were derived from the wild diploid progenitor species, we found that a large portion of them could be amplified in cultivated peanut Tifrunner. A total of 36,813 (71.68%) SSR markers from A. duranensis and 49,072 (80.33%) from A. ipaensis could generate at least one PCR product. A total of 18,793 (36.60%) and 25,194 (41.24%) SSR markers derived from A. duranensis and A. ipaensis, respectively, generated only one specific PCR product in Tifrunner (Table 4). These results suggested that SSR markers from wild Arachis species had high transferability between wild and cultivated Arachis species, implying that these SSR markers have high application value in peanut molecular breeding.
In total, 58,549, 84,081 and 85,885 SSR markers could generate at least one PCR product in diploid progenitors (A. duranensis, A. ipaensis), and cultivated tetraploid (A. hypogaea), respectively (Figure 4). Among them, 27,161 markers could be used in A. duranensis, A. ipaensis and Tifrunner.
To confirm amplification of the SSR markers, 113 of them were randomly selected for PCR amplification in A. duranensis, A. ipaensis and Tifrunner. Genomic DNA was used as the template. Our results showed that 111 (98.23%) of the 113 SSRs could produce clear amplification products. The results demonstrated that most of the SSRs displayed polymorphism in A. duranensis and A. ipaensis. Interestingly, many of these polymorphism sites could be observed in the tetraploid cultivated peanut (Figure 5A). These polymorphic markers can be used for distinguishing the alleles from the AA or BB ancestor species.
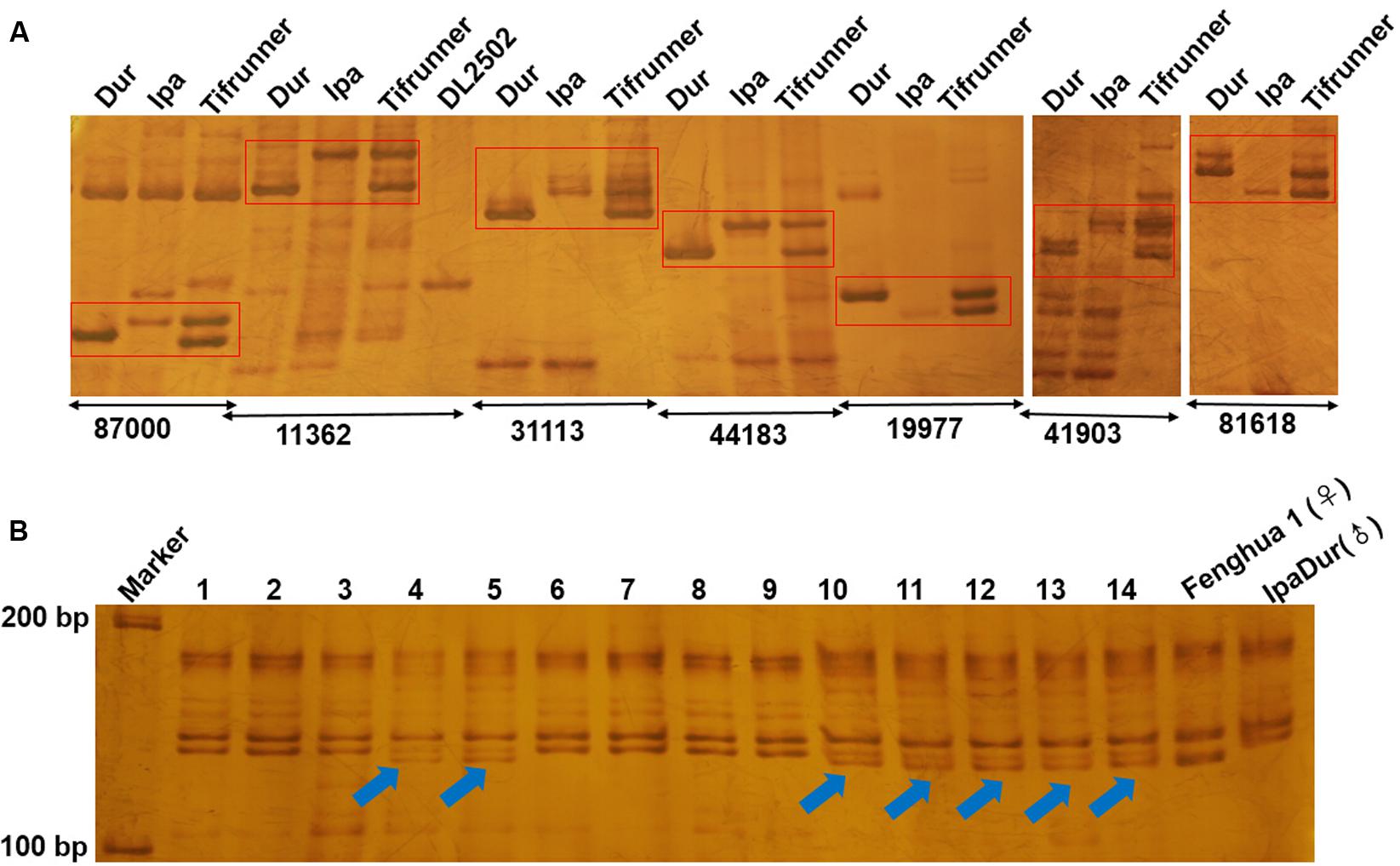
FIGURE 5. Electrophoresis analysis of genomic SSR in peanut. (A) Partial polymorphic SSR markers in A. duranensis, A. ipaensis and Tifrunner. (B) SSR detection using SSR marker Araip.B07_130994 in hybrid F1 of Fenghua1 x IpaDur. 1–14 represent harvest F1 offsprings, the arrows indicate the true hybrids.
All the developed SSR markers were anchored to the draft genome sequences of A. duranensis and A. ipaensis. As a result, 50,774 of 51,354 (98.9%) markers from A. duranensis and 60,970 of 61,091 (99.8%) markers from A. ipaensis were mapped to the 20 Arachis chromosomes, respectively (Supplementary Tables S1, S2). For each marker, the SSR repeat motifs, position in chromosomes, primer sequences, annealing temperature (Tm), and the length of PCR production, etc. listed in Supplementary Tables S1, S2. These maps provided a platform for marker assisted breeding, gene and QTL mapping in peanut. Recently, a reference integrated map was constructed using a comparative genomic method with 3,693 marker loci anchored to 20 consensus LGs of the peanut A and B sub-genomes (Shirasawa et al., 2013). The SSR-based physical maps were compared with the genetic linkage maps reported previously (Shirasawa et al., 2013). There were 84 marker loci in A01 linkage group, and most of them were SSRs developed from ESTs. A large portion (34 out of 84, 40.5%) of the EST-SSRs matched with the SSR markers from this study. The alignment of the physical map with the known linkage groups of peanut provided enough markers for increasing the density of genetic map for further fine mapping (Figure 6).
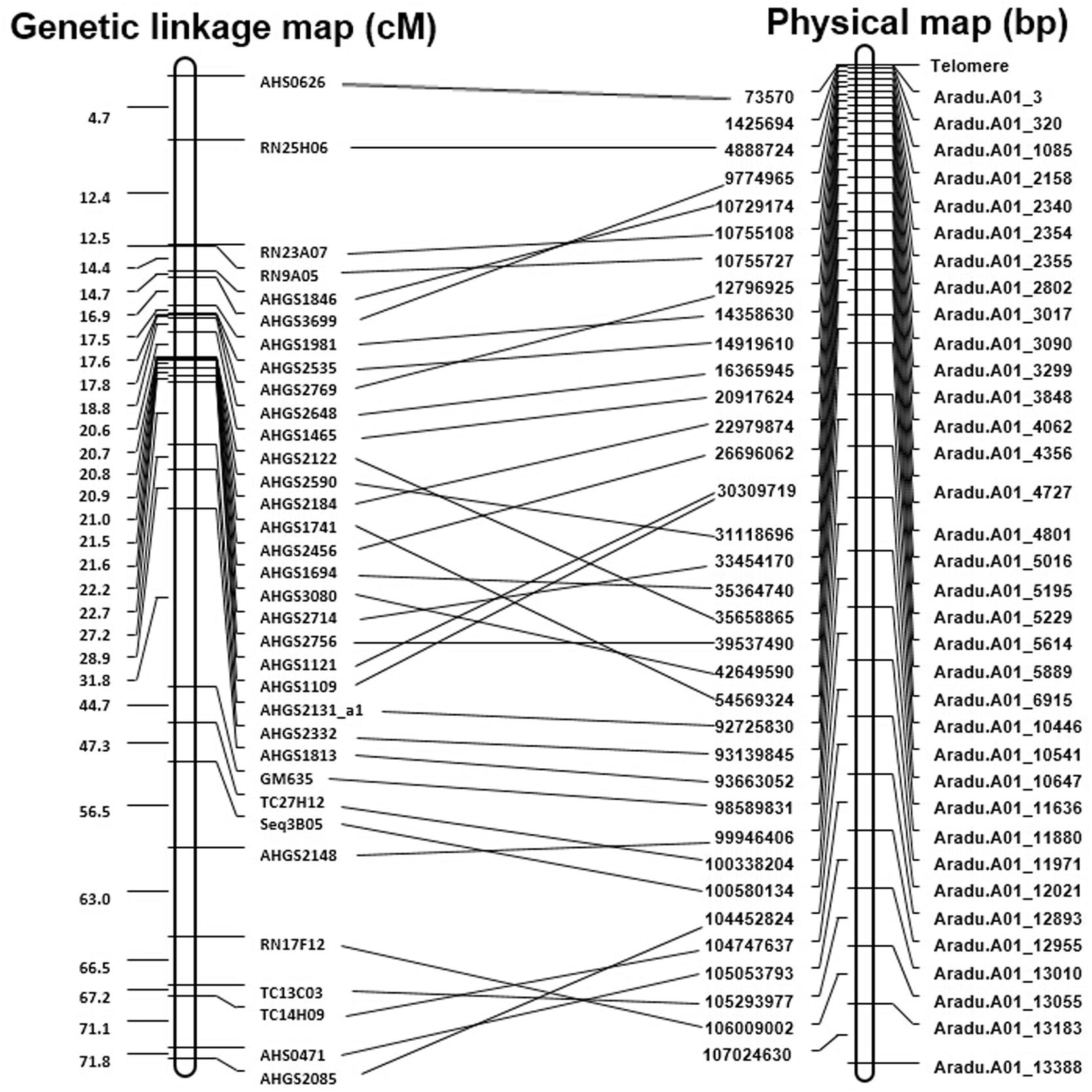
FIGURE 6. Comparison of the genetic linkage maps with physical map of A01 chromosome. This figure shows comparison on the genetic linkage map of Shirasawa et al. (2013) with the physical map.
SSRs markers have been very useful for genetic analysis, mapping and breeding in several crop species. Although the current trend is more toward using single nucleotide polymorphism (SNP) markers in advanced research organizations in peanut (Pandey et al., 2017), the majority of research organizations with low to moderate genomics facilities still use SSRs in their research. In this context, a large number of genome-wide SSRs were previously only available for A. duranensis (Chen et al., 2016) and their limitation for tracking to a physical location in a specific genomic region restricts their use in genetic studies. Further, there was no effort to make available a large number of SSRs from A. ipaensis. In this context, we identified large scale genome-wide SSRs from both diploid progenitor species, i.e., 135,529 SSRs from the A genome (A. duranensis) and 199,957 SSRs from the B genome (A. ipaensis). In order to deploy these important structural variations in multiple genetic and breeding studies, we also developed primers for 112,247 genome-wide SSRs (51,354 SSRs for A genome and 60,893 SSRs for B genome). These SSRs will be a great resource for conducting genetic and genomic studies such as genetic diversity, genetic mapping, marker-trait association, and molecular breeding.
In the last decade, several efforts were employed for identification of QTLs linked to multiple phenotypic traits of peanut, which provides the basis for MAS. However, due to the lack of molecular markers, most of the peanut QTLs were far away from the target traits in genetic distance. Here, the physical map of A. duranensis and A. ipaensis allowed identification of molecular markers closer to the target traits. For example, previous studies reported a genomic region (TC5A07-TC7G10) in peanut linkage group TA04, which harbored a single QTL for tomato spotted wilt virus (TSWV)and named as qF2TSWV5 (Wang et al., 2013). The QTL of qF2TSWV5 account for 23.02% of TWSV phenotypic variance (PV) (Wang et al., 2013). In this region, four QTLs for leaf spot (LS) were also identified and named as qF2LS6, accounting for 10.08%–24.19% of phenotype variation (Wang et al., 2013). Markers TC5A07 and TC7G10 were derived from EST clones DQ099196 and DQ099144, respectively. According to the sequences of DQ099196 and DQ099144, TC5A07 and TC7G10 were successfully located in region of 8.135 Mb of chromosome 4 in A. duranensis (A04). Upon comparing this region in the SSR-based physical map of A. duranensis, we found a sequence of 8.135 Mb between the Aradu.A04_43370 and Aradu.A04_45137 genes (Figure 7). This region contained 719 newly developed genomic-SSR markers (Figure 7 and Supplementary Table S3). These large number of SSRs provide the possibility for further fine mapping or even cloning of the QTLs or genes that confer resistance to TSWV and LS.
Genome sequence analysis of A. duranensis indicated presence of 652 genes in the above described region. Functional annotation of these genes demonstrated that 49 genes were defense related (Supplementary Table S4). Among them, there were two NB-ARC protein genes, three LRR receptor-like genes, three cytochrome P450 genes, four disease resistance-responsive (dirigent-like protein) genes, three WRKY family transcription factors, 15 pentatricopeptide (PPR) repeat protein genes, eight peroxidase genes, and 11 serine/threonine-protein phosphatase genes. The NBS-LRR family genes are known as the largest class of disease resistance (R) genes in plants (Meyers et al., 2003). The majority of R genes cloned by a map-based approach were NBS-LRR genes, for example, the rice blast disease resistance gene Pi9, Pi36, and Pi5 (Qu et al., 2006; Liu et al., 2007; Lee et al., 2009), wheat leaf rust disease resistance gene Lr10 and wheat powdery mildew resistance gene Pm3 (Feuillet et al., 2003; Yahiaoui et al., 2004). WRKY transcription factors act as key regulators of many processes in plants. Previous studies showed that WRKY transcription factors also play important roles in pathogen defense (Rushton et al., 2010). Our earlier study reported the identification of WRKY genes in A. duranensis and A. ipaensis, and found the expression of some WRKY genes respond to SA and JA treatment. This result suggested that these WRKY transcription factors might play important roles in peanut defense reaction (Song et al., 2016). Serine/threonine kinases are also important factors in plant defense system. In tomato, Pti1 encodes a serine/threonine kinase which was involved in the hypersensitive response (Zhou et al., 1995). In wheat, a serine/threonine kinase Stpk-V encoded by a powdery mildew resistance gene Pm21, conferred powdery mildew resistance in wheat (Cao et al., 2011). These results suggest that the NBS-LRR, WRKY and serine/threonine kinase genes in the genomic region could be the major genes for TWSV or LS resistance. To test this hypothesis, we need further fine mapping of these QTLs. The nearest markers for the defense related genes are listed in Supplementary Table S4.
Many of the wild relatives of peanut exhibit high resistance to diseases, that could be used to improve cultivars. For example, many wild Arachis species including A. duranensis (PI 219823) and their interspecific derivatives were resistant or immune to rust under both field and laboratory conditions (Wynne et al., 1991). A. duranensis was also highly resistant to tikka leaf spot disease caused by Cercospora arachidicola (Seetharam et al., 1973; Pande and Rao, 2001). In order to use the resistance genes of wild peanuts, several approaches were developed, such as distant hybridization and development of synthetic amphidiploids. Recent studies showed that the development of synthetic amphidiploids could help to overcome the crossing barrier caused by ploidy difference between tetraploid cultivated peanut and diploid wild relatives. Several synthetic amphidiploids were developed, including the artificial tetraploid IpaDur (A. ipaensis × A. duranensis V14167) and ISATGR278-18 (A. duranensis × A. batizocoi) (Mallikarjuna et al., 2004, 2011; Kumari et al., 2014; Bertioli et al., 2016). After hybridization between cultivars and synthetic amphidiploids, molecular markers are needed for detection and tracking the DNA fragments from the wild type peanuts. The amphidiploid IpaDur was crossed with Fenghua 1, a high-yielding variety in China. Using one of the SSR markers (Araip.B07_130994) developed in this study, true F1 hybrids were easily identified (Figure 5B). In the future, we will create A. hypogaea–A. duranensis near-isogenic lines (NILs), which will be important materials for both peanut breeding and genetic studies. Using these SSR markers, the introgression of resistant genes and large DNA fragments from A. duranensis could be examined in the NILs.
In summary, we developed a large number of genomic SSR markers from diploid progenitors of cultivated peanut for deployment in an array of genetic and breeding applications. This study also demonstrated high transferability for these SSRs in different Arachis species, indicating high potential for their useful applications in improving cultivated peanut.
XW, BG, MKP, SW, and RKV conceived and designed the experiments. CZ, MKP, JQ, GA, JW, DB, and XR performed the experiments. CZ, MKP, HX, and CM analyzed data. CZ, XW, MKP, and BG wrote and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by National High Tech Project (2013AA102602), Shandong Province Agricultural Crop Breeding Project (2016LZGC025), Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2016B02) and Young Talents Training Program of Shandong Academy of Agricultural Sciences. We would also like to thank the members of the International Peanut Genome Initiative (IPGI) and the Peanut Genome Consortium (PGC) for the support and work for the IPGI, and for the financial support for IPGI. The authors are also thankful to Bill & Melinda Gates Foundation (Tropical Legumes III), MARS Chocolate Inc., and The Peanut Foundation for financial assistance.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01209/full#supplementary-material
Balfourier, F., Roussel, V., Strelchenko, P., Exbrayat-Vinson, F., Sourdille, P., Boutet, G., et al. (2007). A worldwide bread wheat core collection arrayed in a 384-well plate. Theor. Appl. Genet. 114, 1265–1275.
Bertioli, D. J., Cannon, S. B., Froenicke, L., Huang, G., Farmer, A. D., Cannon, E. K., et al. (2016). The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48, 438–446. doi: 10.1038/ng.3517
Cao, A., Xing, L., Wang, X., Yang, X., Wang, W., Sun, Y., et al. (2011). Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. U.S.A. 108, 7727–7732. doi: 10.1073/pnas.1016981108
Chen, X., Li, H., Pandey, M. K., Yang, Q., Wang, X., Garg, V., et al. (2016). Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proc. Natl. Acad. Sci. U.S.A. 113, 6785–6790. doi: 10.1073/pnas.1600899113
Cho, Y. G., Ishii, T., Temnykh, S., Chen, X., Lipovich, L., McCouch, S. R., et al. (2000). Diversity of microsatellites derived from genomic libraries and GenBank sequences in rice (Oryza sativa L.). Theor. Appl. Genet. 100, 713–722.
Deng, P., Wang, M., Feng, K., Cui, L., Tong, W., Song, W., et al. (2016). Genome-wide characterization of microsatellites in Triticeae species: abundance, distribution and evolution. Sci. Rep. 6:32224. doi: 10.1038/srep32224
Feuillet, C., Travella, S., Stein, N., Albar, L., Nublat, A., and Keller, B. (2003). Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc. Natl. Acad. Sci. U.S.A. 100, 15253–15258.
Guo, B., Fedorova, N. D., Chen, X., Wan, C. H., Wang, W., Nierman, W. C., et al. (2011). Gene expression profiling and identification of resistance genes to Aspergillus flavus infection in peanut through EST and microarray strategies. Toxins 3, 737–753. doi: 10.3390/toxins3070737
Gupta, P. K., Balyan, H. S., Sharma, P. C., and Ramesh, B. (1996). Microsatellites in plants: a new class of molecular markers. Curr. Sci. 70, 45–54.
Han, B., Wang, C., Tang, Z., Ren, Y., Li, Y., Zhang, D., et al. (2015). Genome-wide analysis of microsatellite markers based on sequenced database in Chinese spring wheat (Triticum aestivum L.). PLoS ONE 10:e0141540. doi: 10.1371/journal.pone.0141540
Haq, S. U., Jain, R., Sharma, M., Kachhwaha, S., and Kothari, S. L. (2014). Identification and characterization of microsatellites in expressed sequence tags and their cross transferability in different plants. Int. J. Genomics 2014:863948. doi: 10.1155/2014/863948
Huang, L., Wu, B., Zhao, J., Li, H., Chen, W., Zheng, Y., et al. (2016). Characterization and transferable utility of microsatellite markers in the wild and cultivated Arachis species. PLoS ONE 11:e0156633. doi: 10.1371/journal.pone.0156633
Krishna, G., Singh, B. K., Kim, E. K., Morya, V. K., and Ramteke, P. W. (2015). Progress in genetic engineering of peanut (Arachis hypogaea L.)—A review. Plant Biotechnol. J. 13, 147–162. doi: 10.1111/pbi.12339
Kumari, V., Gowda, M. V. C., Tasiwal, V., Pandey, M. K., Bhat, R. S., Mallikarjuna, N., et al. (2014). Diversification of primary gene pool through introgression of resistance to foliar diseases from synthetic amphidiploids to cultivated groundnut (Arachis hypogaea L.). Crop J. 2, 110–119.
Lavia, G. I., Ortiz, A. M., Robledo, G., Fernández, A., and Seijo, G. (2011). Origin of triploid Arachis pintoi (Leguminosae) by autopolyploidy evidenced by FISH and meiotic behaviour. Ann. Bot. 108, 103–111. doi: 10.1093/aob/mcr108
Lee, S. K., Song, M. Y., Seo, Y. S., Kim, H. K., Ko, S., Cao, P. J., et al. (2009). Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil–nucleotide-binding–leucine-rich repeat genes. Genetics 181, 1627–1638. doi: 10.1534/genetics.108.099226
Liang, X., Chen, X., Hong, Y., Liu, H., Zhou, G., Li, S., et al. (2009). Utility of EST-derived SSR in cultivated peanut (Arachis hypogaea L.) and Arachis wild species. BMC Plant Biol. 9:35. doi: 10.1186/1471-2229-9-35
Liu, F., Hu, Z., Liu, W., Li, J., Wang, W., Liang, Z., et al. (2016). Distribution, function and evolution characterization of microsatellite in Sargassum thunbergii (Fucales, Phaeophyta) transcriptome and their application in marker development. Sci. Rep. 6:18947. doi: 10.1038/srep18947
Liu, X., Lin, F., Wang, L., and Pan, Q. (2007). The in silico map-based cloning of Pi36, a rice coiled-coil–nucleotide-binding site–leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 176, 2541–2549.
Mallikarjuna, N., Pande, S., Jadhav, D. R., Sastri, D. C., and Rao, J. N. (2004). Introgression of disease resistance genes from Arachis kempff-mercadoi into cultivated groundnut. Plant Breed. 123, 573–576.
Mallikarjuna, N., Senthilvel, S., and Hoisington, D. (2011). Development of new sources of tetraploid Arachis to broaden the genetic base of cultivated groundnut (Arachis hypogaea L.). Genet. Resour. Crop Evol. 58, 889–907.
Meyers, B. C., Kozik, A., Griego, A., Kuang, H., and Michelmore, R. W. (2003). Genome-wide analysis of NBS-LRR–encoding genes in Arabidopsis. Plant Cell 15, 809–834.
Pande, S., and Rao, J. N. (2001). Resistance of wild Arachis species to late leaf spot and rust in greenhouse trials. Plant Dis. 85, 851–855.
Pandey, M. K., Agarwal, G., Kale, S. M., Clevenger, J., Nayak, S. N., Sriswathi, M., et al. (2017). Development and evaluation of a high density genotyping ‘Axiom_Arachis’ array with 58K SNPs for accelerating genetics and breeding in groundnut. Sci. Rep. 7:40577. doi: 10.1038/srep40577
Pandey, M. K., Monyo, E., Ozias-Akins, P., Liang, X., Guimarães, P., Nigam, S. N., et al. (2012). Advances in Arachis genomics for peanut improvement. Biotechnol. Adv. 30, 639–651. doi: 10.1016/j.biotechadv.2011.11.001
Pandey, M. K., Roorkiwal, M., Singh, V. K., Ramalingam, A., Kudapa, H., Thudi, M., et al. (2016). Emerging genomic tools for legume breeding: current status and future prospects. Front. Plant Sci. 7:455. doi: 10.3389/fpls.2016.00455
Powell, W., Machray, G. C., and Provan, J. (1996). Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1, 215–222.
Qu, S., Liu, G., Zhou, B., Bellizzi, M., Zeng, L., Dai, L., et al. (2006). The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site–leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172, 1901–1914.
Rushton, P. J., Somssich, I. E., Ringler, P., and Shen, Q. J. (2010). WRKY transcription factors. Trends Plant Sci. 15, 247–258. doi: 10.1016/j.tplants.2010.02.006
Seetharam, A., Nayar, K. M. D., Sreekantaradhya, R., and Achar, D. K. T. (1973). Cytological studies on the interspecific hybrid of Arachis hypogaea × Arachis duranensis. Cytologia 38, 277–280.
Shi, J., Huang, S., Zhan, J., Yu, J., Wang, X., Hua, W., et al. (2014). Genome-wide microsatellite characterization and marker development in the sequenced Brassica crop species. DNA Res. 21, 53–68. doi: 10.1093/dnares/dst040
Shirasawa, K., Bertioli, D. J., Varshney, R. K., Moretzsohn, M. C., Leal-Bertioli, S. C., Thudi, M., et al. (2013). Integrated consensus map of cultivated peanut and wild relatives reveals structures of the A and B genomes of Arachis and divergence of the legume genomes. DNA Res. 20, 173–184. doi: 10.1093/dnares/dss042
Shore, J., Parida, S. K., Yadava, D. K., and Mohapatra, T. (2010). Microsatellites in Brassica unigenes: relative abundance, marker design, and use in comparative physical mapping and genome analysis. Genome 53, 55–67. doi: 10.1139/g09-084
Song, G. Q., Li, M. J., Xiao, H., Wang, X. J., Tang, R. H., Xia, H., et al. (2010). EST sequencing and SSR marker development from cultivated peanut (Arachis hypogaea L.). Electr. J. Biotechnol. 13, 7–8.
Song, H., Wang, P., Lin, J. Y., Zhao, C., Bi, Y., and Wang, X. (2016). Genome-wide identification and characterization of WRKY gene family in peanut. Front. Plant Sci. 7:534. doi: 10.3389/fpls.2016.00534
Squirrell, J., Hollingsworth, P. M., Woodhead, M., Russell, J., Lowe, A. J., Gibby, M., et al. (2003). How much effort is required to isolate nuclear microsatellites from plants? Mol. Ecol. 12, 1339–1348.
Varshney, R. K., Mohan, S. M., Gaur, P. M., Gangarao, N. V. P. R., Pandey, M. K., Bohra, A., et al. (2013). Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 31, 1120–1134. doi: 10.1016/j.biotechadv.2013.01.001
Varshney, R. K., Thiel, T., Stein, N., Langridge, P., and Graner, A. (2002). In silico analysis on frequency and distribution of microsatellites in ESTs of some cereal species. Cell. Mol. Biol. Lett. 7, 537–546.
Wang, H., Pandey, M. K., Qiao, L., Qin, H., Culbreath, A. K., He, G., et al. (2013). Genetic mapping and quantitative trait loci analysis for disease resistance using F2 and F5 generation-based genetic maps derived from ‘Tifrunner’ × ‘GT-C20’in peanut. Plant Genome 6, 1–10.
Wang, H., Penmetsa, R. V., Yuan, M., Gong, L., Zhao, Y., Guo, B., et al. (2012). Development and characterization of BAC-end sequence derived SSRs, and their incorporation into a new higher density genetic map for cultivated peanut (Arachis hypogaea L.). BMC Plant Biol. 12:10. doi: 10.1186/1471-2229-12-10
Wang, Q., Fang, L., Chen, J., Hu, Y., Si, Z., Wang, S., et al. (2015). Genome-wide mining, characterization, and development of microsatellite markers in Gossypium species. Sci. Rep. 5:10638. doi: 10.1038/srep10638
Wynne, J. C., Beute, M. K., and Nigam, S. N. (1991). Breeding for disease resistance in peanut (Arachis hypogaea L.). Annu. Rev. Phytopathol. 29, 279–303.
Yahiaoui, N., Srichumpa, P., Dudler, R., and Keller, B. (2004). Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 37, 528–538.
Zhang, J., Liang, S., Duan, J., Wang, J., Chen, S., Cheng, Z., et al. (2012). De novo assembly and characterization of the transcriptome during seed development, and generation of genic-SSR markers in Peanut (Arachis hypogaea L.). BMC Genomics 13:90. doi: 10.1186/1471-2164-13-90
Zhong, R., Zhou, M., Zhao, C., Hou, L., Li, C., Wang, X., et al. (2016). SSR marker development from peanut gynophore transcriptome sequencing. Plant Breed. 135, 111–117.
Zhou, J., Loh, Y. T., Bressan, R. A., and Martin, G. B. (1995). The tomato gene pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell 83, 925–935.
Keywords: wild peanut, genome sequence, microsatellites, molecular markers, quantitative trait locus (QTL)
Citation: Zhao C, Qiu J, Agarwal G, Wang J, Ren X, Xia H, Guo B, Ma C, Wan S, Bertioli DJ, Varshney RK, Pandey MK and Wang X (2017) Genome-Wide Discovery of Microsatellite Markers from Diploid Progenitor Species, Arachis duranensis and A. ipaensis, and Their Application in Cultivated Peanut (A. hypogaea). Front. Plant Sci. 8:1209. doi: 10.3389/fpls.2017.01209
Received: 02 May 2017; Accepted: 26 June 2017;
Published: 18 July 2017.
Edited by:
Nicolas Rispail, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Anne Frary, İzmir Institute of Technology, TurkeyCopyright © 2017 Zhao, Qiu, Agarwal, Wang, Ren, Xia, Guo, Ma, Wan, Bertioli, Varshney, Pandey and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingjun Wang, eGluZ2p1bndAaG90bWFpbC5jb20= Manish K. Pandey, bS5wYW5kZXlAY2dpYXIub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.