- 1Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Hyderabad, India
- 2Department of Applied Biological Science, Tokyo University of Science, Chiba, Japan
Plants use stomatal closure mediated by elicitors as the first step of the innate immune response to restrict the microbial entry. We present a comprehensive study of the effect of cryptogein and harpin, two elicitors from microbial pathogens of tobacco, on stomatal closure and guard cell signaling components in Arabidopsis thaliana, a model plant. Cryptogein as well as harpin induced stomatal closure, while elevating the levels of reactive oxygen species (ROS) and nitric oxide (NO) in the guard cells of A. thaliana. Kinetic studies with fluorescent dyes revealed that the rise in ROS levels preceded that of NO in guard cells, when treated with these two elicitors. The restriction of NO levels in guard cells, even by ROS modulators indicates the essentiality of ROS for NO production during elicitor-triggered stomatal closure. The signaling events during elicitor-induced stomatal closure appear to converge at NADPH oxidase and ROS production. Our results provide the first report on stomatal closure associated with rise in ROS/NO of guard cells by cryptogein and harpin in A. thaliana. Our results establish that A. thaliana can be used to study stomatal responses to the typical elicitors from microbial pathogens of other plants. The suitability of Arabidopsis opens up an excellent scope for further studies on signaling events leading to stomatal closure by microbial elicitors.
Introduction
Stomata, the microscopic pores on the epidermis are the gateways for not only CO2 and H2O (Hetherington and Woodward, 2003) but also for microbes (Agurla et al., 2014). Stomatal closure therefore is essential to prevent pathogen entry into leaves and forms a part of their innate immune response (Zeng et al., 2010; Sawinski et al., 2013; Melotto et al., 2014). Production of elicitors has been considered as one of the mechanisms to induce stomatal closure in response to microbial pathogens. The elicitors (derived from either pathogens or from plants) induce several defense responses, besides stomatal closure, including ROS production, HR, cell death and production of antimicrobial secondary metabolites (Boller and Felix, 2009; Cui et al., 2009; Tsuda and Katagiri, 2010; Schwessinger and Ronald, 2012; Spoel and Dong, 2012; Newman et al., 2013). Compared to the extensive literature on elicitor effects on plant tissues, particularly cell cultures, the studies on stomatal closure by elicitors are limited.
The stomatal aperture is determined by the turgor status of guard cells and is mediated by the modulation of ion fluxes (Kim et al., 2010). The mechanism and signaling components of stomatal closure by ABA have been extensively studied in plants, such as Arabidopsis thaliana, Pisum sativum, and Vicia faba. ABA induced stomatal closure is invariably associated with marked increase in ROS, NO, and pH of guard cells (Gayatri et al., 2013; Agurla and Raghavendra, 2016). Further, several signaling components, are also involved in ABA-induced stomatal closure such as PYR/RCAR-PP2C-SnRK2 complexes, G-proteins, phospholipids, OST1, free Ca2+ and finally cation/anion channels (Hubbard et al., 2010; Raghavendra et al., 2010; Lee and Luan, 2012; Zhu et al., 2012; Murata et al., 2015). The transduction of both biotic and abiotic signals appears to share some common signaling components in guard cells, such as ROS and NO (Melotto et al., 2006; Srivastava et al., 2009; Khokon et al., 2010a,b; Zeng et al., 2010; Zhang et al., 2012; Agurla et al., 2014).
In view of the importance of ROS and NO as key signaling components during stomatal closure, extensive studies have been made on their sources. In guard cells ROS production can be mediated by enzymes such as NADPH oxidase, cell wall peroxidases, amine oxidases, and other flavin containing enzymes (Song et al., 2014). Most of these observations were made during stomatal closure by ABA, MeJA, methylglyoxal, and bicarbonate (Allan and Fluhr, 1997; Hoque et al., 2012). Very few studies were made on the RBOH dependency in elicitor mediated stomatal closure. Stomatal closure by flg22 and harpin were prevented in tobacco deficient in NADPH oxidase (Zhang et al., 2009; Mersmann et al., 2010). In contrast, stomatal closure and ROS production by YEL in Arabidopsis were dependent on SHAM sensitive peroxidases but not NADPH oxidase (Khokon et al., 2010a,b).
Two enzymes, NR and nitric oxides synthase like enzyme (NOA) act as NO sources in guard cells (Gayatri et al., 2013). While the role of NR in contribution to NO-generation is widely accepted, the role of NOA is debated (Neill et al., 2008; Gupta et al., 2011; Agurla et al., 2014). Most of the reports on stomatal closure by elicitors focused on the role of H2O2 and very few studies were made on the role of NO and its interactions with other signaling components (Melotto et al., 2006; Srivastava et al., 2009; Hao et al., 2010).
There has been great interest to understand the elicitor-induced stomatal closure and the signaling components involved in the process. Most of the work on microbial elicitor-induced stomatal closure was carried out with epidermis of Nicotiana (Ye and Murata, 2016). Stomatal closure by harpin, INF1, boehmerin, and Nep1 was reported in N. benthamiana (Zhang et al., 2009, 2010, 2012). It would be useful to study the effects of different microbial elicitors on stomatal closure in the same plant, so to assess the common components of signaling pathway. Oligogalacturonic acid and chitosan induced the stomatal closure in tomato, Commelina communis, pea, and Brassica napus (Lee et al., 1999; Li et al., 2009; Srivastava et al., 2009). Cryptogein and harpin, elicitors from microbial pathogens of tobacco, were shown to induce HR responses and stomatal closure in tobacco (Kadota et al., 2004; Higaki et al., 2007; Zhang et al., 2009, 2012; Kurusu et al., 2013).
Arabidopsis thaliana would be an ideal plant for such studies on elicitor triggered stomatal closure. There have been no reports on stomatal closure by cryptogein in A. thaliana. Most of the studies with other elicitors were limited to selected signaling components in guard cells of A. thaliana, e.g., either ROS or NO, but not with both (Table 1). We attempted a comprehensive study on the effects of cryptogein and harpin on stomatal closure as well as key signaling components of guard cells in epidermis of A. thaliana, a model plant. We have also examined the kinetics of changes in levels of ROS and NO in guard cells in response to cryptogein and harpin. We extended our studies to evaluate the responses of stomatal guard cells in mutants of A. thaliana deficient in signaling components involved in ROS/NO production.
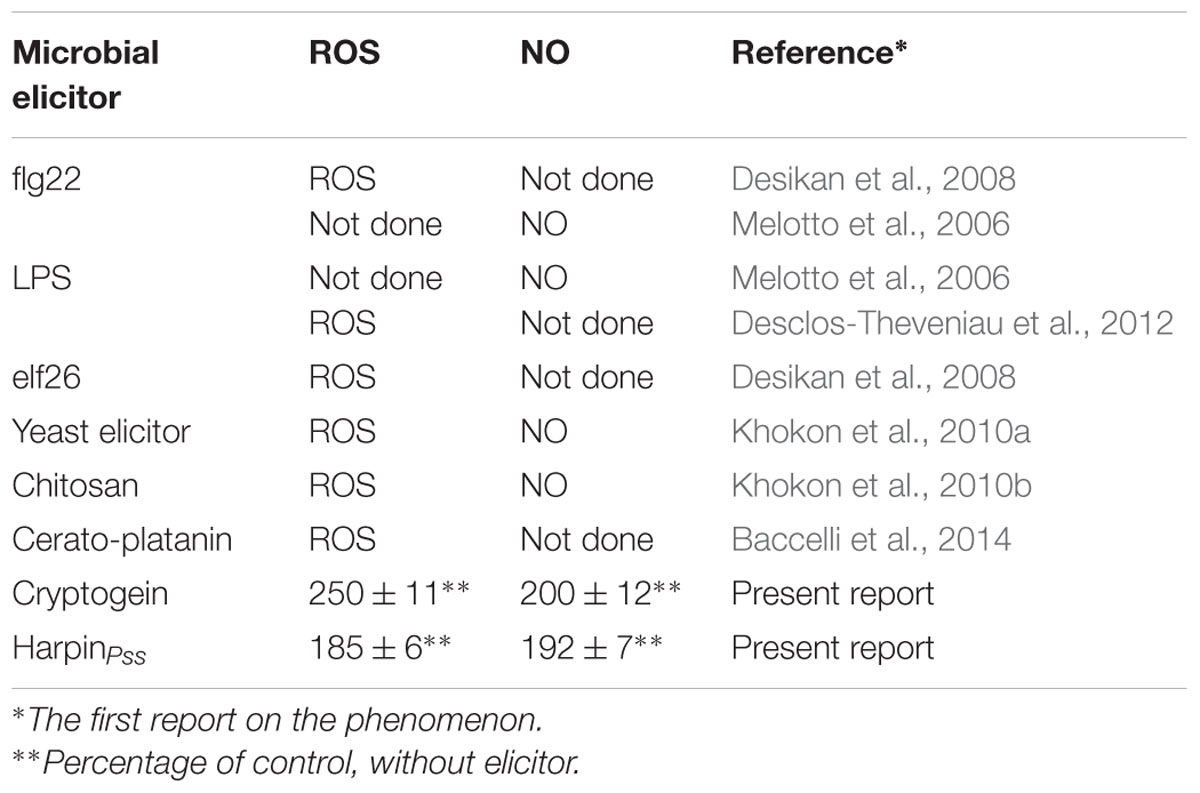
TABLE 1. Reports on ROS or NO production in guard cells during stomatal closure by microbial elicitors in the epidermis of Arabidopsis thaliana.
Materials and Methods
Plant Materials and Growth Conditions
Seeds of Arabidopsis thaliana were sown in a 1:1:1 mixture of vermiculite, perlite and soilrite in plastic disposable containers and kept at 4°C in dark for 3 days and then were transferred to 22–23°C to allow germination. The seedlings were grown in controlled environment growth rooms, under an 8 h light (125–150 mmol m-2 s-1) and 16 h dark photoperiod, with an average temperature of 22–23°C. The plants were supplied with a nutrient solution (Somerville and Ogren, 1982) or full strength Murashige and Skoog medium (salt mixture obtained from HIMEDIA), on alternate days twice a week. The plants were watered on other days.
Chemicals
Among the two microbial elicitors, cryptogein was prepared from Phytophthora cryptogea (Kadota et al., 2004). Harpin was prepared from Pseudomonas syringae pv. syringae and was the same as the full length harpinPss used by Anil and Podile (2012). Both the microbial elicitors were dissolved in milli-Q water, and stock solutions were stored at -20°C. The fluorescent probes of CM-H2DCFDA and DAF-FM DA from Invitrogen-Molecular Probes were dissolved in DMSO. cPTIO, L-NAME, and DPI from Calbiochem were dissolved in milli-Q water.
Bioassay of Stomatal Closure
Leaves from 5- to 6-week-old A. thaliana plants were detached and incubated in opening medium (10 mM MES-KOH, pH 6.15 and 50 mM KCl) for 3 h under light. A light intensity of 200 μmol m-2 s-1 was maintained with the help of a bank of tungsten lamps, and light filtered through a water jacket. The photon flux was measured with a Li-Cor quantum sensor (Li-Cor Instruments Ltd, Lincoln, NE, United States). The temperature was maintained at 25 ± 1°C. After 3 h of light incubation, the leaves were incubated in the medium containing effectors and/or modulators. After treatment for 2 h with the effectors under light, the abaxial epidermis of the leaf was stuck to the cover slip with the help of medical adhesive Telesis V (Premiere Products Inc., Pacoima, CA, United States). The remaining leaf tissue was removed and the stuck epidermis was washed immediately with water. Stomatal apertures were measured with the help of a pre-calibrated research microscope (Olympus CX21) by using NIH image for windows. Approximately 30 stomatal apertures were measured for each experiment and for each treatment.
Monitoring ROS or NO
The levels of ROS and NO were monitored by using CM-H2DCFDA and DAF-FM DA (Excitation 488 nm, Emission 510–550 nm), respectively. The leaves of A. thaliana were incubated in an opening medium (10 mM MES-KOH, pH 6.15 and 50 mM KCl) under light for 3 h to allow the opening of the stomata. The abaxial epidermis from leaves were mounted on the cover slips with the help of silicone adhesive and were loaded separately with the 20 μM CM-H2DCFDA or 30 μM DAF-FM DA fluorescent probes for 30 min in dark. After incubation, the epidermis of leaves was washed with the buffer to remove the excess dye and was treated with the effectors or modulators.
The fluorescence in the treated guard cells was observed by using confocal laser scanning microscope (Leica, TCS-SP-2, AOBS 4 channel UV and visible, Heidelberg, Germany) for every 5 min up to 30 min, and the images of the guard cells were captured. Average fluorescence of 30 stomata was quantified from the captured images by using NIH Image for Windows (Gonugunta et al., 2008). Averages from three different experiments on different days were presented. The fluorescence of the guard cells without the effectors was taken as control (100%) and the relative fluorescence of the guard cells for different treatment at different time points were calculated and plotted.
Replication
All the experiments were repeated at least on three different days. The presented data are averages with standard errors.
Results
Stomatal Closure by Cryptogein and Harpin, Associated with Rise in ROS or NO of Guard Cells
Cryptogein and harpin caused marked stomatal closure in epidermis of A. thaliana. Maximum closure already occurred at 5 μM of cryptogein, while harpin induced maximum stomatal closure at 0.5 μM (Figure 1).
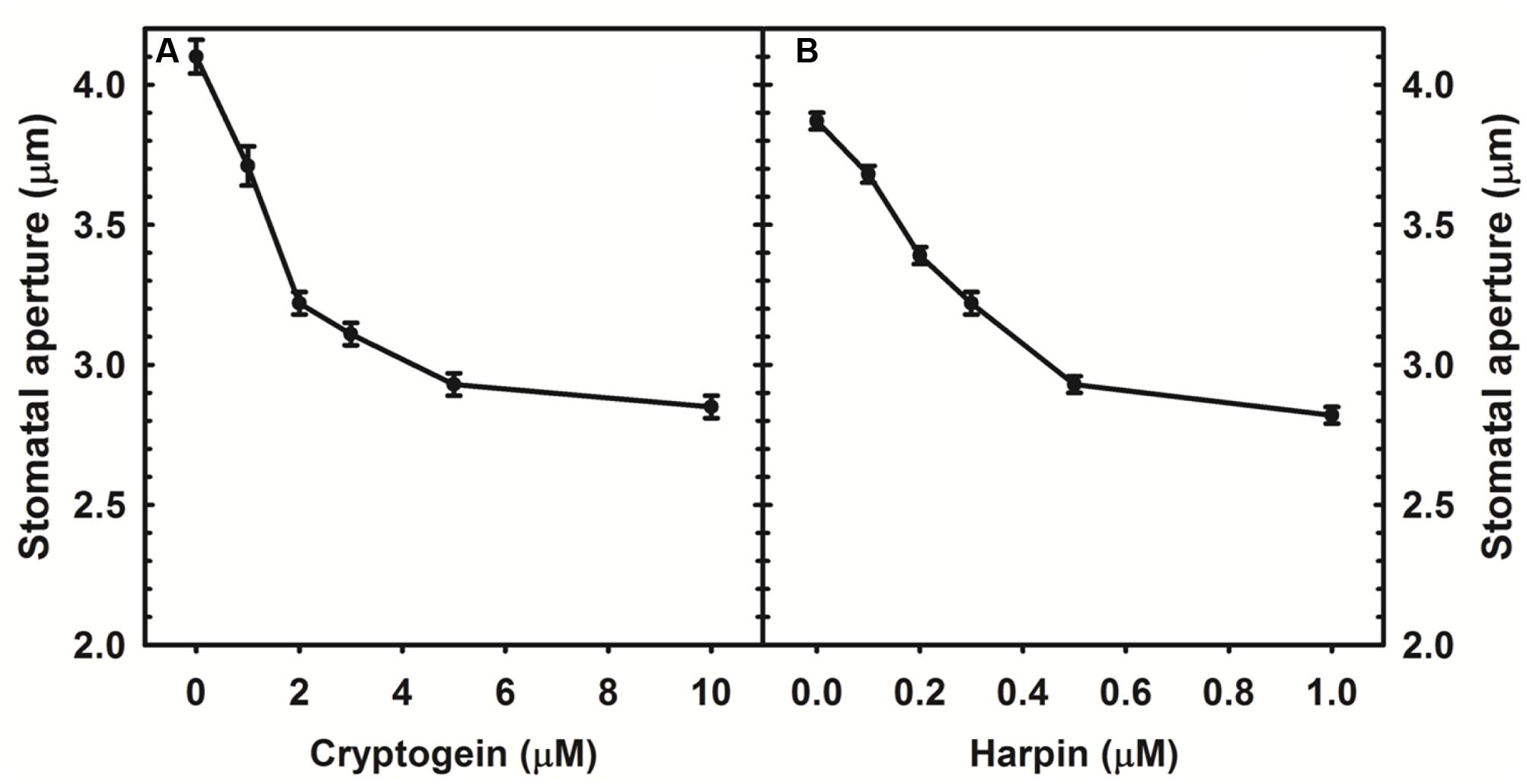
FIGURE 1. Stomatal closure in response to varying concentrations of microbial elicitors, cryptogein and harpin in Arabidopsis thaliana. The two elicitors induced the stomatal closure in a concentration dependant manner and maximum closure was observed at 5 μM of cryptogein (A), 500 nM of harpin (B). Averages of three different experiments from three different days with SE are plotted.
The levels of ROS or NO in guard cells were monitored by using fluorescent probes of CM-H2DCFDA and DAF-FM DA, respectively. When treated with the cryptogein, the levels of both ROS and NO increased remarkably, very similar to the effects of harpin (Figure 2). Real time monitoring of ROS or NO revealed that maximum accumulation of ROS occurred at 15 min after exposure to cryptogein and harpin. Similarly, levels of NO in the guard cells increased at 20 min after exposure to these two elicitors (Figure 3).
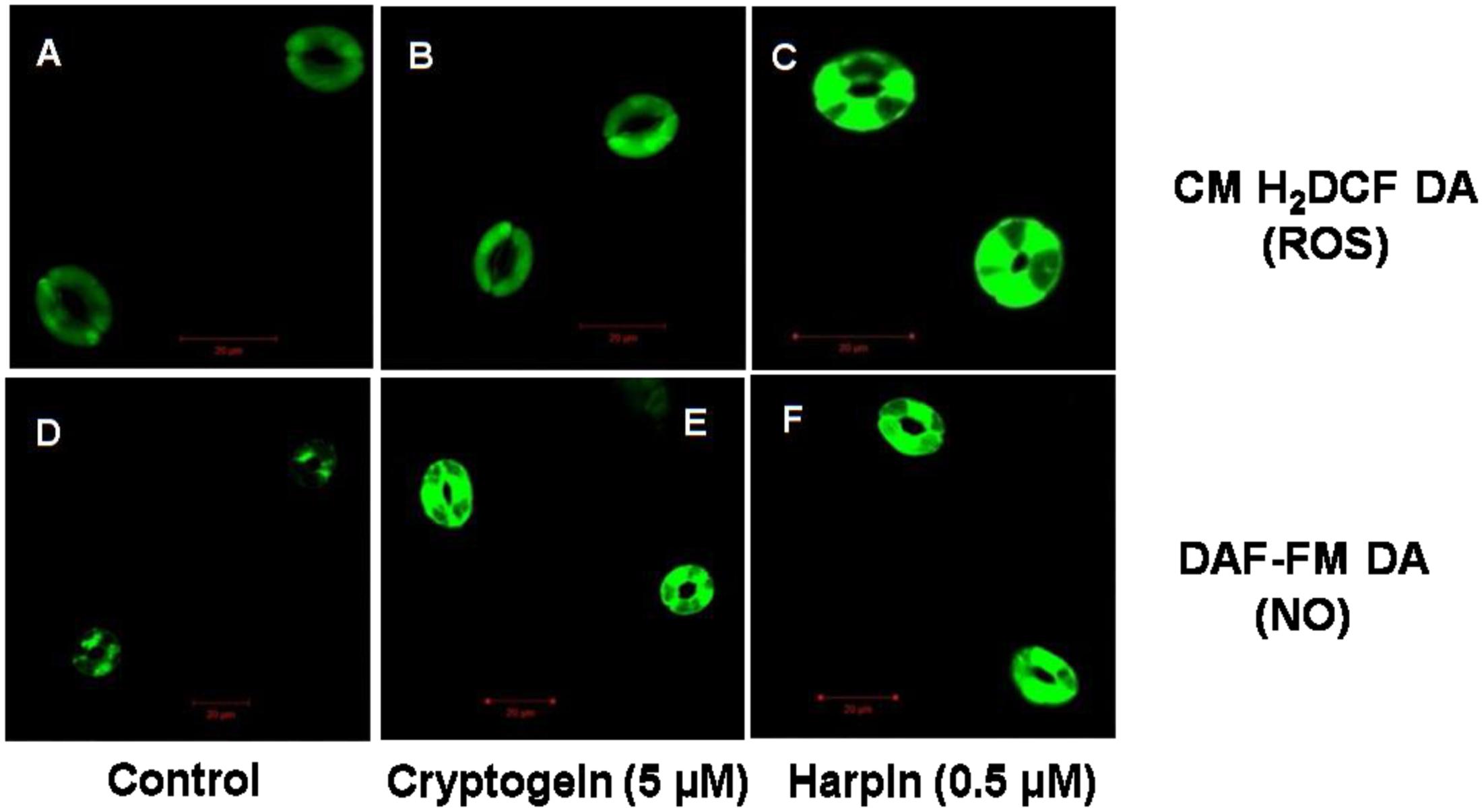
FIGURE 2. Confocal images of ROS or NO levels in guard cells of A. thaliana, treated with cryptogein and harpin. The upper panel (A–C) represents the representative confocal images of the guard cells showing ROS levels indicated by CM-H2DCFDA fluorescent probe, in response to the microbial elicitors, cryptogein and harpin. The lower panel (D–F) represents the confocal images of the NO expressing guard cells by molecular probe DAF-FM DA, in response to the cryptogein and harpin. The levels of ROS or NO were more in the guard cells treated with the two microbial elicitors, were compared to guard cells which were untreated, i.e., control (A,D).
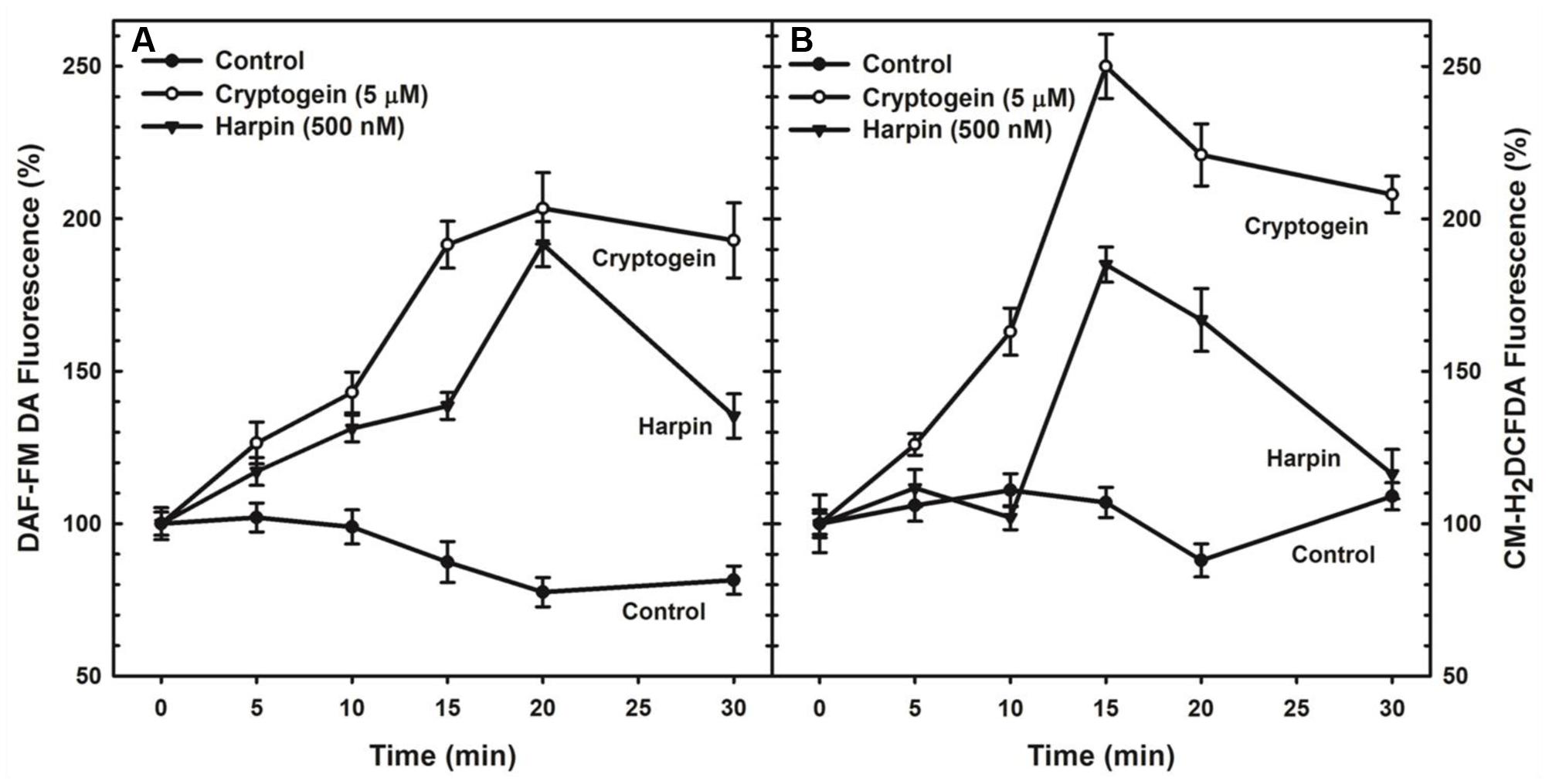
FIGURE 3. Kinetic studies of NO (A) or ROS (B) production in guard cells in response to cryptogein and harpin. The levels of NO or ROS raised from 0 to 30 min in the guard cells in response to two microbial elicitors compared with that of untreated guard cells. The fluorescence which is emitted by fluorescent probes, DAF-FM DA or CM-H2DCFDA in response to cryptogein and harpin was quantified for every 5 min and plotted in the graph. The data plotted in the graph are the averages of three different experiments on three different days. Further details are in section “Materials and Methods.”
Effect of ROS or NO Modulators on Stomatal Closure and ROS or NO in Guard Cells
The role of ROS or NO during stomatal closure by microbial elicitors, was assessed by using modulators of either ROS or NO. Cryptogein and harpin induced stomatal closure was partially restricted in the presence of ROS modulators, catalase (ROS scavenger), and DPI (NADPH oxidase inhibitor) (Figure 4A). Among the modulators of NO, cPTIO (NO scavenger), could reverse the stomatal closure by cryptogein or harpin. In contrast, L-NAME (inhibitor of NOS) and sodium tungstate (NR inhibitor) could restrict the stomatal closure by cryptogein or harpin only to a partial extent (Figure 4B).
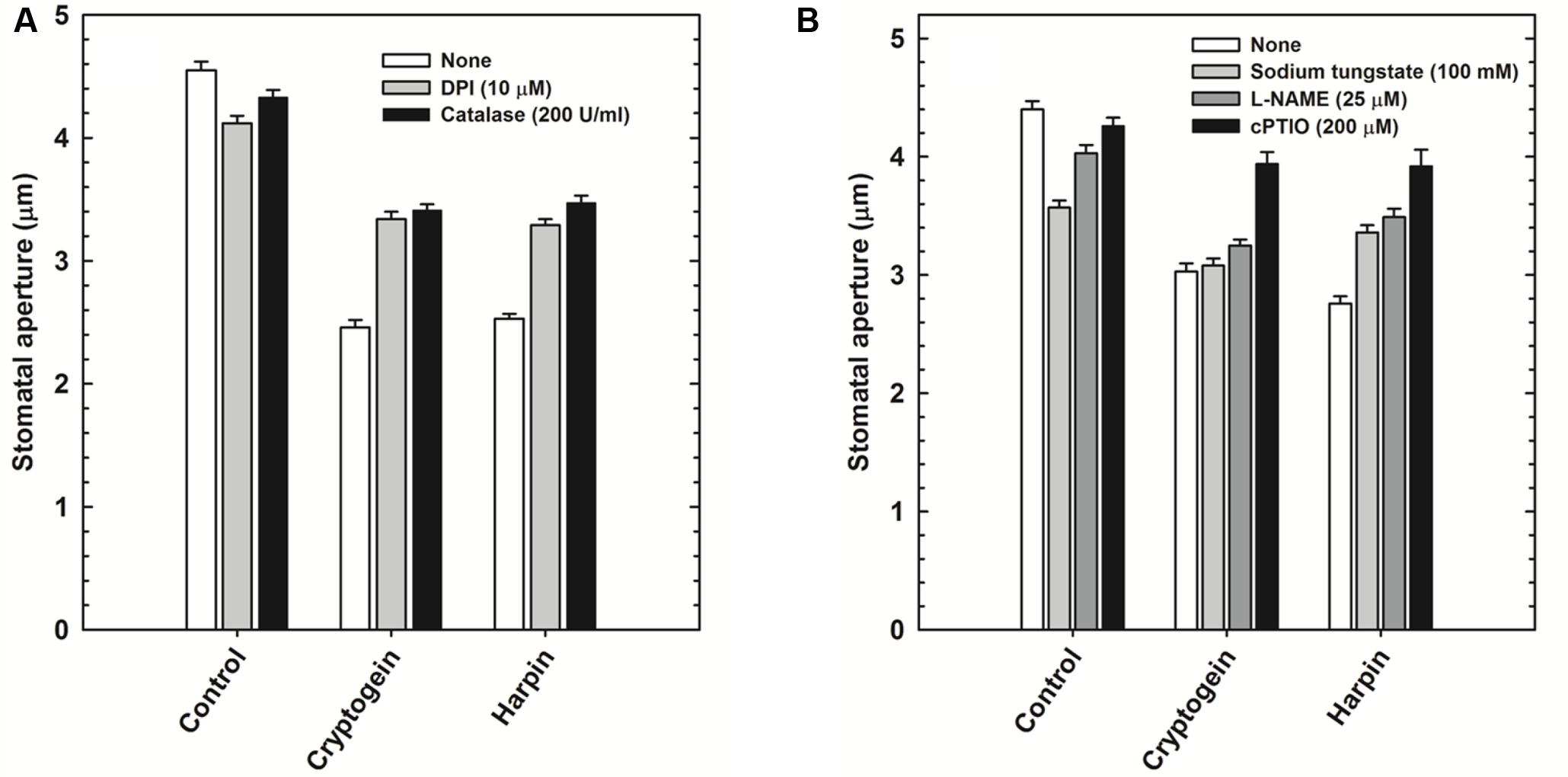
FIGURE 4. Effect of ROS or NO modulators on stomatal closure by cryptogein and harpin. ROS modulators 10 μM DPI (NADPH oxidase inhibitor) and 200 U/ml catalase (ROS scavenger) relieved the effect of two microbial elicitors, partially (A). Cryptogein and harpin showed the effect on stomatal closure, in the absence of NO modulators, 100 μM sodium tungstate (NR inhibitor), 25 μM L-NAME (NOS inhibitor) and 200 μM cPTIO (NO scavenger). All the NO modulators partially relieved the effect of two microbial elicitors on stomatal closure (B). Averages of three different experiments from three different days with SE are plotted.
The modulators of ROS (catalase and DPI) restricted the elevation of not only ROS (Figure 5A) but also NO (Figure 6A) in the guard cells, pretreated with elicitors cryptogein and harpin. In contrast, the modulators of NO (cPTIO and L-NAME) prevented the production of NO (Figure 6B), but did not affect the rise in levels of ROS, in the guard cells (Figure 5B).
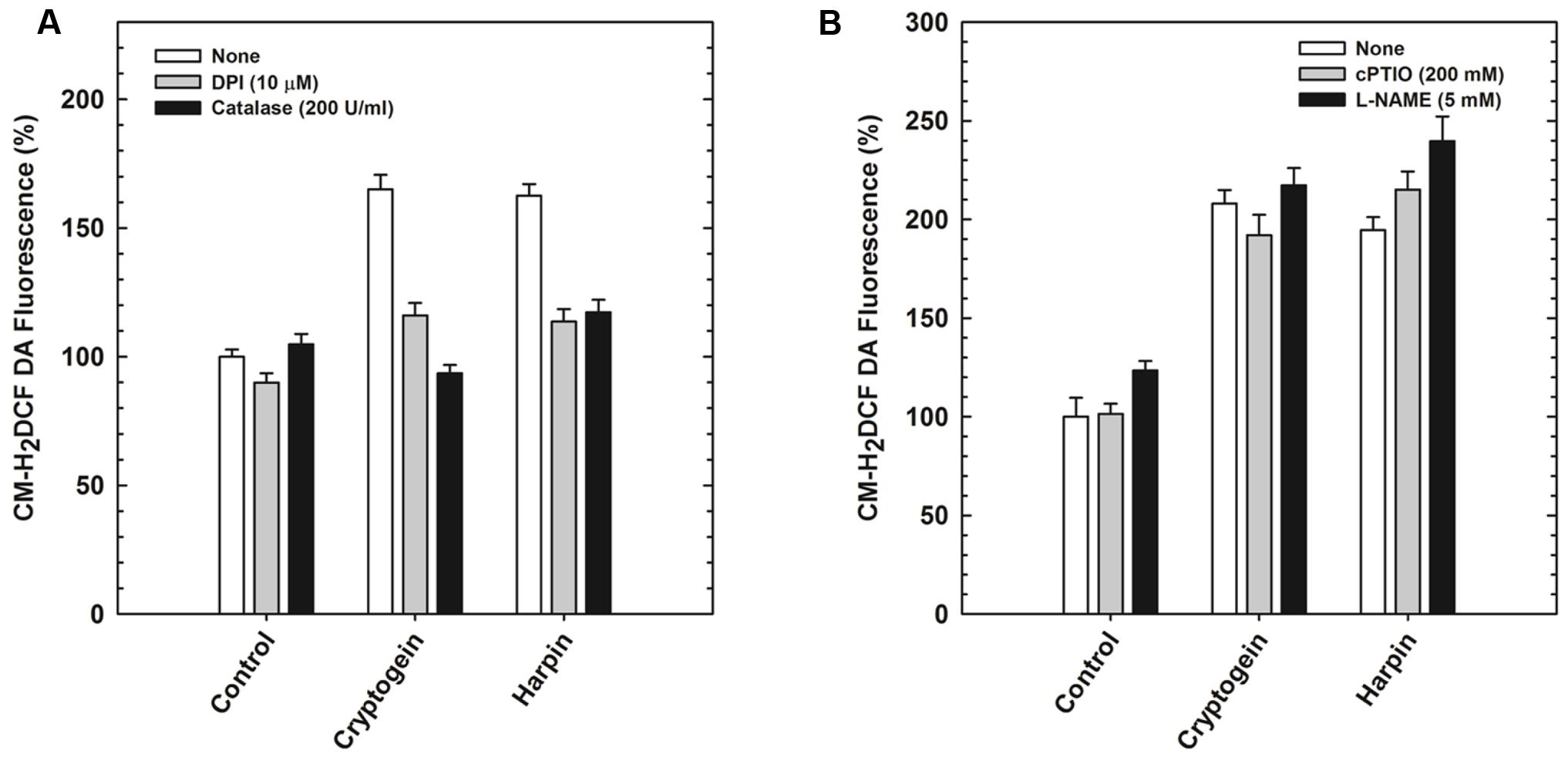
FIGURE 5. The elevation of ROS levels, (indicated by CM-H2DCFDA) by cryptogein or harpin, in the absence or presence of ROS/NO modulators. The levels of ROS in guard cells were more, when treated with cryptogein and harpin, than their respective controls. The extent of increase in ROS by cryptogein or harpin was reduced by ROS modulators (catalase, ROS scavenger and DPI, NADPH oxidase inhibitor) (A). In contrast, elicitor-induced increase was unaffected by NO modulators (cPTIO, NO scavenger and L-NAME, NOS inhibitor) (B). Further details are as in Figure 3 and “Materials and Methods.”
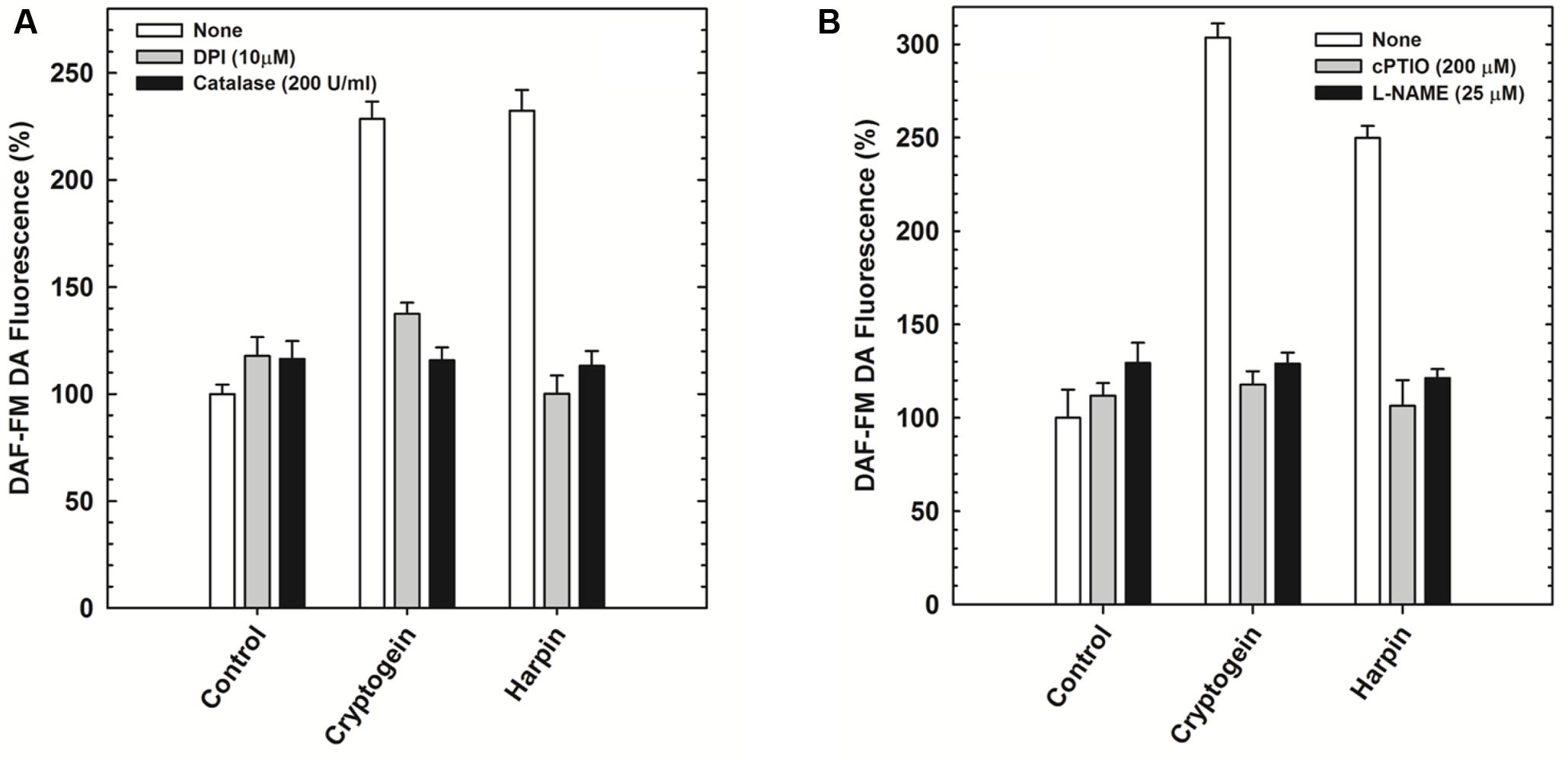
FIGURE 6. The effect of ROS/NO modulators on the NO production in the guard cells in response to cryptogein or harpin. The levels of NO were indicated by the fluorescence of DAF-FM DA. The ROS modulators, catalase, and DPI restricted the rise of NO in the guard cells when treated with the two microbial elicitors (A). NO modulators, cPTIO and L-NAME relieved the effect of elictors on NO production, where as the rise in NO were observed in the guard cells treated with cryptogein and harpin alone (B). Further details are as in Figure 3.
Elicitor-Induced Stomatal Closure in Arabidopsis Mutants
The effect of cryptogein and harpin on stomatal closure was studied in Arabidopsis mutants, deficient in NADPH oxidase (atrbohD/F) or NR (nia1 and nia2) or protein phosphatase ABI (abi1 and abi2). The results were compared with wild type, Col-0 or Ler. The stomatal closure by cryptogein and harpin was partially impaired in atrbohD/F mutant plants, compared to wild type (Figure 7A). Stomatal closure by cryptogein and harpin was partially relieved in the nia1 and nia2 mutants compared with that of their respective wild type Ler and Col-0 plants (Figure 7C). The stomatal closure by cryptogein or harpin was completely reversed in abi1 mutants (Figure 7B), compared to partial impairment in abi2 mutants.
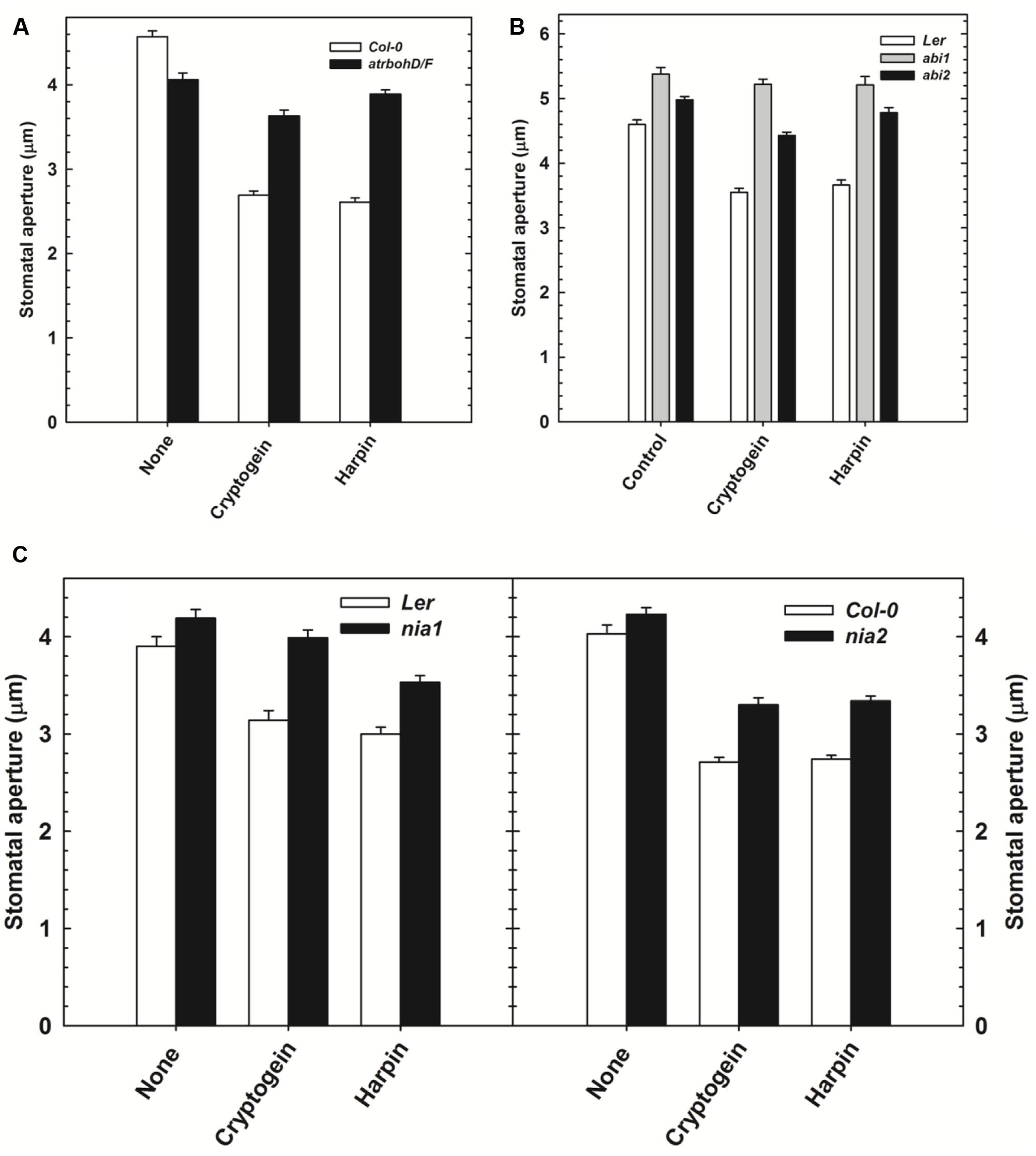
FIGURE 7. The effect of microbial elicitors cryptogein and harpin on stomatal closure in Arabidopsis mutants. The stomatal closure by elicitors was partial in atrbohD/F (NADPH oxidase deficient mutant) plants compared with Col-0 (wild type, WT) (A). In contrast, the stomatal closure by elicitors was impaired in the abi1 (PP2C deficient) and partially impaired in abi2 (B). The effects of microbial elicitors, cryptogein and harpin were partially relieved in the NR deficient mutants, nia1 and nia2 (C). The data plotted in the graph are the averages of three different experiments on three different days. Further details are as in Figure 1.
Discussion
Arabidopsis thaliana as a Model Plant to Study Stomatal Responses to Microbial Elicitors
The stomatal responses to various biotic and abiotic stress factors have been studied in different plant species including tobacco, Vicia, Pisum, Brassica napus, tomato, and Commelina. Nevertheless, A. thaliana offers as an excellent model compared to the other plant species due to the availability of a large collection of mutants, deficient in the different signaling components. Our article emphasizes the marked stomatal closure by cryptogein and harpin in A. thaliana (Figure 1). Cryptogein and harpin are well known as elicitors of microbial pathogens of tobacco. Our studies provide for the first time a detailed examination of cryptogein and harpin induced stomatal closure in epidermis of A. thaliana. Further, the kinetics and modulation of ROS/NO in guard cells by elicitors are all being reported for the first time in Arabidopsis (Figures 3, 5, 6). We suggest that A. thaliana offers a good model for comprehensive studies on stomatal signaling events, including the responses to microbial elicitors. Since several mutants of Arabidopsis are available, our article can trigger further interest to use Arabidopsis for elicitor-induced stomatal closure.
Rise in ROS or NO in Guard Cells by Cryptogein and Harpin
Reactive oxygen species and NO are the important signaling components during stomatal closure by a variety of signals: ABA, MeJA, elicitors like chitosan or even bicarbonate (Kolla and Raghavendra, 2007; Kolla et al., 2007; Gonugunta et al., 2009; Srivastava et al., 2009). The increase in the levels of ROS and NO in the guard cells treated with the cryptogein and harpin (Figure 2), confirms their signaling role during closure. There have been reports on increase in ROS as well as NO by cryptogein in epidermal peels of tobacco (Allan and Fluhr, 1997; Foissner et al., 2000) and tobacco BY-2 cells (Kadota et al., 2004; Leborgne-Castel et al., 2008; Stanislas et al., 2009) while inducing cell death and plant defense responses. But the increase in ROS or NO levels in guard cells by cryptogein has so far not been reported.
Harpins from different sources are known to induce production of ROS and NO in Arabidopsis suspension cells (Desikan et al., 1996; Krause and Durner, 2004) and in guard cells of tobacco (Zhang et al., 2009, 2012). Again our article is the first attempt of a comprehensive study on the production of both ROS and NO in guard cells during stomatal closure by harpin in Arabidopsis (Figure 2).
Rise in ROS Occurs before NO Production during Stomatal Closure by Elicitors
Real-time monitoring of fluorescence in guard cells indicated that on exposure to cryptogein and harpin, the levels of ROS rise and reach a peak before that of NO (Figure 3). Although the levels of ROS or NO in the guard cells were observed earlier in response to elicitors, such as chitosan, flg22, harpin, boehmerin (Lee et al., 1999; Melotto et al., 2006; Zhang et al., 2009, 2012), studies on kinetics of ROS/NO production are very few. While endorsing the suggestion that ROS production is essential for NO rise in the guard cells during closure by ABA or elicitors (Bright et al., 2006; Srivastava et al., 2009), we conclude that the ROS acts upstream of NO during cryptogein and harpin triggered stomatal closure.
Interactions of NO and ROS
In guard cells ROS, NO, cytosolic pH, and intracellular calcium are the major points of action, leading to the loss of ions/turgor and subsequent stomatal closure (Agurla and Raghavendra, 2016). Besides their direct effects, there seems to be a strong network of interactions among them. Studies with mutants suggested the upstream action of the ROS to MAP kinases, ABI2 and intracellular Ca2+ (Murata et al., 2001; Jammes et al., 2009; Wang et al., 2013). Among the components downstream of ROS are ABI2, NO production, K+in/SLAC channels (Murata et al., 2001; Zhang et al., 2001; Vahisalu et al., 2008; Srivastava et al., 2009). A rise in NO is considered as the early signaling event in guard cell signaling toward ABA, MJ, bicarbonate (Gayatri et al., 2013). The marked interactions of NO with ROS, phospholipase D and G-protein in guard cells during stomatal closure are known (Gonugunta et al., 2008; Li et al., 2009; Distéfano et al., 2012; Zhang et al., 2012). Further studies on these interactions using elicitors as signals would be extremely interesting.
Another point of interest is the role of mitochondrial AOX. The levels of ROS and NO in mitochondria are minimized by an active AOX (Cvetkovska and Vanlerberghe, 2012; Vanlerberghe, 2013). Unlike cytochrome c oxidase (CytOX), NO resistant AOX plays a major role in preventing excessive NO generation in guard cells, thus regulating the stomatal closure (Cvetkovska et al., 2014; Gupta et al., 2014). Further experiments are warranted to understand the role of AOX in relation to other sources of NO production during stomatal closure by elicitors.
Cryptogein and Harpins as tools to Induce Stomatal Closure
Cryptogein, a microbial elicitor triggered stomatal closure, and elevated ROS/NO levels in guard cells of A. thaliana (Figures 1, 2). Besides guard cells, cryptogein has been known to be a potent microbial elicitor in inducing several defense responses, but mostly in tobacco (Montillet et al., 2005; Sawai et al., 2010; Kurusu et al., 2013; Rosnoblet et al., 2017). Similarly, harpin has also been extensively documented for its defense responses again mostly in tobacco (Chen et al., 2008; Boureau et al., 2011; Chang et al., 2016). The marked stomatal closure associated with the rise in ROS/NO of guard cells along with the suitability of A. thaliana for these studies opens up an excellent scope for further use of cryptogein and harpin as tools to study stomatal function. The ability of cryptogein and harpin, two elicitors from microbial pathogens of tobacco, to close stomata markedly in Arabidopsis reaffirms the role of stomatal closure as a typical component of innate immunity responses of plants.
Conclusion
It is quite interesting to note the efficacy of cryptogein and harpin, studied extensively with tobacco, on stomatal closure even in Arabidopsis, a model plant. Our work emphasizes also the ability of these microbial elicitors to induce a marked stomatal closure at very low concentrations, while increasing the levels of ROS and NO in guard cells, A. thaliana. We are sure that our work would trigger further use of Arabidopsis to examine the guard cell signal transduction mechanisms during stomatal closure.
Author Contributions
AR and KK conceptualized the topic and designed the experiments. GG performed most of the experiments. SA and KA conducted some experiments. AR, KK, and AP evaluated the data and drafted the skeleton of manuscript. GG, SA, KK, KA, AP, and AR revised and finalized the manuscript. All the authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RD and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The work is supported by a J C Bose National Fellowship (No. SR/S2/JCB-06/2006) from the Department of Science and Technology, New Delhi, CSIR project [No. 38 (1195)/08/EMR-II], DBT project (BT/PR/11674/PBD/16/838/2008) to AR. This work is a part of collaboration between AR and KK supported by a DST-JSPS project No. DST/INT/JSPS/P-121/2011. GG and SA were holders of University Grants Commission-Senior Research Fellowship. We also thank DBT-CREBB, DST-FIST, and UGC-SAP-CAS, for support of infrastructure in Department/School.
Abbreviations
ABA, abscisic acid; abi1, abscisic acid insensitive1; abi2, abscisic acid insensitive2; AOX, alternative oxidase; atrboh, Arabidopsis thaliana respiratory burst oxidase homolog; BY-2, bright yellow-2; CM-H2DCF DA, 5-(and-6)-chloro methyl-2′,7′-dichloro dihydro fluorescein diacetate; Col, Columbia; cPTIO, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl3-oxide; DAF-FM DA, 4-amino-5-methyl amino-2′,7′-difluorofluorescein diacetate; DMSO, dimethyl sulfoxide; DPI, diphenylene iodonium chloride; flg22, flagellin 22; HR, hypersensitive response; INF1, Phytophthora infestans elicitin, infestin 1; Ler, Landsberg erecta; L-NAME, N-nitro-L-arginine methyl ester; LPS, lipopolysaccharide; MAP kinase, mitogen-activated protein kinase; MeJA, methyl jasmonate; Nbrboh, Nicotiana benthamiana respiratory burst oxidase homolog; Nep1, necrosis- and ethylene-inducing peptide 1; nia1, nitrate reductase1; nia2, nitrate reductase2; NO, nitric oxide; NOA, nitric oxide associated; NOS, nitric oxide synthase; NR, nitrate reductase; OST1, open stomata1; PYR/PYL/RCAR, pyrabactin resistance1/PYR1-like/regulatory component of ABA receptor; RBOH, respiratory burst oxidase homolog; ROS, reactive oxygen species; SHAM, salicylhydroxamic acid; SLAC, slow anion channel; YEL, yeast elicitor.
References
Agurla, S., Gayatri, G., and Raghavendra, A. S. (2014). Nitric oxide as a secondary messenger during stomatal closure as a part of plant immunity response against pathogens. Nitric Oxide 43, 89–96. doi: 10.1016/j.niox.2014.07.004
Agurla, S., and Raghavendra, A. S. (2016). Convergence and divergence of signaling events in guard cells during stomatal closure by plant hormones or microbial elicitors. Front. Plant Sci. 7:1332. doi: 10.3389/fpls.2016.01332
Allan, A. C., and Fluhr, R. (1997). Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–1572. doi: 10.1105/tpc.9.9.1559
Anil, K., and Podile, A. R. (2012). HarpinPss-mediated enhancement in growth and biological control of late leaf spot in groundnut by a chlorothalonil-tolerant Bacillus thuringiensis SFC24. Microbiol. Res. 167, 194–198. doi: 10.1016/j.micres.2011.07.002
Baccelli, I., Lombardi, L., Luti, S., Bernardi, R., Picciarelli, P., Scala, A., et al. (2014). Cerato-platanin induces resistance in Arabidopsis leaves through stomatal perception, over expression of salicylic acid-and ethylene-signalling genes and camalexin biosynthesis. PLoS ONE 9:e100959. doi: 10.1371/journal.pone.0100959
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Boureau, T., Siamer, S., Perino, C., Gaubert, S., Patrit, O., Degrave, A., et al. (2011). The HrpN effector of Erwinia amylovora, which is involved in type III translocation, contributes directly or indirectly to callose elicitation on apple leaves. Mol. Plant Microbe Interact. 24, 577–584. doi: 10.1094/MPMI-09-10-0212
Bright, J., Desikan, R., Hancock, J. T., Weir, I. S., and Neill, S. J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45, 113–122. doi: 10.1111/j.1365-313X.2005.02615.x
Chang, X., Seo, M., Takebayashi, Y., Kamiya, Y., Riemann, M., and Nick, P. (2016). Jasmonates are induced by the PAMP flg22 but not the cell death-inducing elicitor harpin in Vitis rupestris. Protoplasma 254, 271–283. doi: 10.1007/s00709-016-0941-7
Chen, L., Qian, J., Qu, S., Long, J., Yin, Q., Zhang, C., et al. (2008). Identification of specific fragments of HpaGXooc, a harpin from Xanthomonas oryzae pv. oryzicola, that induce disease resistance and enhance growth in plants. Phytopathology 98, 781–791. doi: 10.1094/PHYTO-98-7-0781
Cui, H., Xiang, T., and Zhou, J.-M. (2009). Plant immunity: a lesson from pathogenic bacterial effector proteins. Cell. Microbiol. 11, 1453–1461. doi: 10.1111/j.1462-5822.2009.01359.x
Cvetkovska, M., Dahal, K., Alber, N. A., Jin, C., Cheung, M., and Vanlerberghe, G. C. (2014). Knockdown of mitochondrial alternative oxidase induces the ‘stress state’of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytol. 203, 449–461. doi: 10.1111/nph.12773
Cvetkovska, M., and Vanlerberghe, G. C. (2012). Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol. 195, 32–39. doi: 10.1111/j.1469-8137.2012.04166.x
Desclos-Theveniau, M., Arnaud, D., Huang, T.-Y., Lin, G. J.-C., Chen, W.-Y., Lin, Y.-C., et al. (2012). The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 8:e1002513. doi: 10.1371/journal.ppat.1002513
Desikan, R., Hancock, J. T., Coffey, M. J., and Neill, S. J. (1996). Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 382, 213–217. doi: 10.1016/0014-5793(96)00177-9
Desikan, R., Horák, J., Chaban, C., Mira-Rodado, V., Witthöft, J., Elgass, K., et al. (2008). The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS ONE 3:e2491. doi: 10.1371/journal.pone.0002491
Distéfano, A. M., Scuffi, D., García-Mata, C., Lamattina, L., and Laxalt, A. M. (2012). Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta 236, 1899–1907. doi: 10.1007/s00425-012-1745-4
Foissner, I., Wendehenne, D., Langebartels, C., and Durner, J. (2000). In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 23, 817–824. doi: 10.1046/j.1365-313X.2000.00835.x
Gayatri, G., Agurla, S., and Raghavendra, A. S. (2013). Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 4:425. doi: 10.3389/fpls.2013.00425
Gonugunta, V. K., Srivastava, N., Puli, M. R., and Raghavendra, A. S. (2008). Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant Cell Environ. 31, 1717–1724. doi: 10.1111/j.1365-3040.2008.01872.x
Gonugunta, V. K., Srivastava, N., and Raghavendra, A. S. (2009). Cytosolic alkalinization is a common and early messenger preceding the production of ROS and NO during stomatal closure by variable signals, including abscisic acid, methyl jasmonate and chitosan. Plant Signal. Behav. 4, 561–564. doi: 10.4161/psb.4.6.8847
Gupta, K. J., Fernie, A. R., Kaiser, W. M., and van Dongen, J. T. (2011). On the origins of nitric oxide. Trends Plant Sci. 16, 160–168. doi: 10.1016/j.tplants.2010.11.007
Gupta, K. J., Mur, L. A., and Ratcliffe, R. G. (2014). Guarding the guard cells? New Phytol. 203, 349–351. doi: 10.1111/nph.12882
Hao, F., Zhao, S., Dong, H., Zhang, H., Sun, L., and Miao, C. (2010). Nia1 and Nia2 are involved in exogenous salicylic acid induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 52, 298–307. doi: 10.1111/j.1744-7909.2010.00920.x
Hetherington, A. M., and Woodward, F. I. (2003). The role of stomata in sensing and driving environmental change. Nature 424, 901–908. doi: 10.1038/nature01843
Higaki, T., Goh, T., Hayashi, T., Kutsuna, N., Kadota, Y., Hasezawa, S., et al. (2007). Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol. 48, 1414–1425. doi: 10.1093/pcp/pcm109
Hoque, T. S., Uraji, M., Ye, W., Hossain, M. A., Nakamura, Y., and Murata, Y. (2012). Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J. Plant Physiol. 169, 979–986. doi: 10.1016/j.jplph.2012.02.007
Hubbard, K. E., Nishimura, N., Hitomi, K., Getzoff, E. D., and Schroeder, J. I. (2010). Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 24, 1695–1708. doi: 10.1101/gad.1953910
Jammes, F., Song, C., Shin, D., Munemasa, S., Takeda, K., Gu, D., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 20520–20525. doi: 10.1073/pnas.0907205106
Kadota, Y., Goh, T., Tomatsu, H., Tamauchi, R., Higashi, K., Muto, S., et al. (2004). Cryptogein-induced initial events in tobacco BY-2 cells: pharmacological characterization of molecular relationship among cytosolic Ca2+ transients, anion efflux and production of reactive oxygen species. Plant Cell Physiol. 45, 160–170. doi: 10.1093/pcp/pch020
Khokon, M. A. R., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., Mori, I. C., et al. (2010a). Yeast elicitor induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol. 51, 1915–1921. doi: 10.1093/pcp/pcq145
Khokon, M. A. R., Uraji, M., Munemasa, S., Okuma, E., Nakamura, Y., Mori, I. C., et al. (2010b). Chitosan induced stomatal closure accompanied by peroxidase mediated reactive oxygen species production in Arabidopsis. Biosci. Biotechnol. Biochem. 74, 2313–2315.
Kim, T.-H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Kolla, V. A., and Raghavendra, A. S. (2007). Nitric oxide is a signalling intermediate during bicarbonate induced stomatal closure in Pisum sativum. Physiol. Plant. 130, 91–98. doi: 10.1111/j.1399-3054.2007.00887.x
Kolla, V. A., Vavasseur, A., and Raghavendra, A. S. (2007). Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 225, 1421–1429. doi: 10.1007/s00425-006-0450-6
Krause, M., and Durner, J. (2004). Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol. Plant Microbe Interact. 17, 131–139. doi: 10.1094/MPMI.2004.17.2.131
Kurusu, T., Saito, K., Horikoshi, S., Hanamata, S., Negi, J., Yagi, C., et al. (2013). An S-type anion channel SLAC1 is involved in cryptogein-induced ion fluxes and modulates hypersensitive responses in tobacco BY-2 cells. PLoS ONE 8:e70623. doi: 10.1371/journal.pone.0070623
Leborgne-Castel, N., Lherminier, J., Der, C., Fromentin, J., Houot, V., and Simon-Plas, F. (2008). The plant defense elicitor cryptogein stimulates clathrin-mediated endocytosis correlated with reactive oxygen species production in bright yellow-2 tobacco cells. Plant Physiol. 146, 1255–1266. doi: 10.1104/pp.107.111716
Lee, S., Choi, H., Suh, S., Doo, I.-S., Oh, K.-Y., Choi, E. J., et al. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121, 147–152. doi: 10.1104/pp.121.1.147
Lee, S. C., and Luan, S. (2012). ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. doi: 10.1111/j.1365-3040.2011.02426.x
Li, Y., Yin, H., Wang, Q., Zhao, X., Du, Y., and Li, F. (2009). Oligochitosan induced Brassica napus L. production of NO and H2O2 and their physiological function. Carbohydr. Polym. 75, 612–617. doi: 10.1016/j.carbpol.2008.09.005
Melotto, M., Panchal, S., and Roy, D. (2014). Plant innate immunity against human bacterial pathogens. Front. Microbiol. 5:411. doi: 10.3389/fmicb.2014.00411
Melotto, M., Underwood, W., Koczan, J., Nomura, K., and He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. doi: 10.1016/j.cell.2006.06.054
Mersmann, S., Bourdais, G., Rietz, S., and Robatzek, S. (2010). Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154, 391–400. doi: 10.1104/pp.110.154567
Montillet, J. L., Chamnongpol, S., Rustérucci, C., Dat, J., Van De Cotte, B., Agnel, J. P., et al. (2005). Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138, 1516–1526. doi: 10.1104/pp.105.059907
Murata, Y., Mori, I. C., and Munemasa, S. (2015). Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66, 369–392. doi: 10.1146/annurev-arplant-043014-114707
Murata, Y., Pei, Z. M., Mori, I. C., and Schroeder, J. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523. doi: 10.1105/tpc.13.11.2513
Neill, S., Barros, R., Bright, J., Desikan, R., Hancock, J., Harrison, J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59, 165–176. doi: 10.1093/jxb/erm293
Newman, M. A., Sundelin, T., Nielsen, J. T., and Erbs, G. (2013). MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 4:139. doi: 10.3389/fpls.2013.00139
Raghavendra, A. S., Gonugunta, V. K., Christmann, A., and Grill, E. (2010). ABA perception and signaling. Trends Plant Sci. 15, 395–401. doi: 10.1016/j.tplants.2010.04.006
Rosnoblet, C., Bègue, H., Blanchard, C., Pichereaux, C., Besson-Bard, A., Aimé, S., et al. (2017). Functional characterization of the chaperon-like protein Cdc48 in cryptogein-induced immune response in tobacco. Plant Cell Environ. 40, 491–508. doi: 10.1111/pce.12686
Sawai, Y., Tamotsu, S., Kuchitsu, K., and Sakai, A. (2010). Effects of growth phase and cell density on cryptogein-induced programmed cell death in suspension-cultured tobacco BY-2 cells: development of a model system for 100% efficient hypersensitive cell death. Cytologia 75, 389–396. doi: 10.1508/cytologia.75.389
Sawinski, K., Mersmann, S., Robatzek, S., and Böhmer, M. (2013). Guarding the green: pathways to stomatal immunity. Mol. Plant Microbe Interact. 26, 626–632. doi: 10.1094/MPMI-12-12-0288-CR
Schwessinger, B., and Ronald, P. C. (2012). Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 63, 451–482. doi: 10.1146/annurev-arplant-042811-105518
Somerville, C. R., and Ogren, W. L. (1982). “Isolation of photorespiration mutants in Arabidopsis thaliana,” in Methods in Chloroplast Biology, eds M. Edelman, R. B. Hallick, and N. H. Chua (Amsterdam: Elsevier), 129–138.
Song, Y., Miao, Y., and Song, C. P. (2014). Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol. 201, 1121–1140. doi: 10.1111/nph.12565
Spoel, S. H., and Dong, X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. doi: 10.1038/nri3141
Srivastava, N., Gonugunta, V. K., Puli, M. R., and Raghavendra, A. S. (2009). Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229, 757–765. doi: 10.1007/s00425-008-0855-5
Stanislas, T., Bouyssie, D., Rossignol, M., Vesa, S., Fromentin, J., Morel, J., et al. (2009). Quantitative proteomics reveals a dynamic association of proteins to detergent-resistant membranes upon elicitor signaling in tobacco. Mol. Cell. Proteomics 8, 2186–2198. doi: 10.1074/mcp.M900090-MCP200
Tsuda, K., and Katagiri, F. (2010). Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 13, 459–465. doi: 10.1016/j.pbi.2010.04.006
Vahisalu, T., Kollist, H., Wang, Y. F., Nishimura, N., Chan, W. Y., Valerio, G., et al. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452, 487–491. doi: 10.1038/nature06608
Vanlerberghe, G. C. (2013). Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 14, 6805–6847. doi: 10.3390/ijms14046805
Wang, Y., Chen, Z. H., Zhang, B., Hills, A., and Blatt, M. R. (2013). PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl- channels through reactive oxygen species-mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells. Plant Physiol. 163, 566–577. doi: 10.1104/pp.113.219758
Ye, W., and Murata, Y. (2016). Microbe associated molecular pattern signaling in guard cells. Front. Plant Sci. 7:583. doi: 10.3389/fpls.2016.00583
Zeng, W., Melotto, M., and He, S. Y. (2010). Plant stomata: a check point of host immunity and pathogen virulence. Curr. Opin. Plant Biol. 21, 599–603. doi: 10.1016/j.copbio.2010.05.006
Zhang, H., Dong, S., Wang, M., Wang, W., Song, W., Dou, X., et al. (2010). The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor-triggered hypersensitive response and stomatal closure. J. Exp. Bot. 61, 3799–3812. doi: 10.1093/jxb/erq189
Zhang, H., Fang, Q., Zhang, Z., Wang, Y., and Zheng, X. (2009). The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hyper-sensitive response in Nicotiana benthamiana. J. Exp. Bot. 60, 3109–3122. doi: 10.1093/jxb/erp146
Zhang, H., Wang, M., Wang, W., Li, D., Huang, Q., Wang, Y., et al. (2012). Silencing of G proteins uncovers diversified plant responses when challenged by three elicitors in Nicotiana benthamiana. Plant Cell Environ. 35, 72–85. doi: 10.1111/j.1365-3040.2011.02417.x
Zhang, X., Miao, Y. C., An, G. Y., Yun, Z. H. O. U., Shangguan, Z. P., Gao, J. F., et al. (2001). K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res. 11, 195–202. doi: 10.1038/sj.cr.7290086
Keywords: Arabidopsis, guard cells, innate immunity, microbial elicitors, nitric oxide, reactive oxygen species, signal transduction, stomatal closure
Citation: Gayatri G, Agurla S, Kuchitsu K, Anil K, Podile AR and Raghavendra AS (2017) Stomatal Closure and Rise in ROS/NO of Arabidopsis Guard Cells by Tobacco Microbial Elicitors: Cryptogein and Harpin. Front. Plant Sci. 8:1096. doi: 10.3389/fpls.2017.01096
Received: 12 April 2017; Accepted: 06 June 2017;
Published: 21 June 2017.
Edited by:
Girdhar Kumar Pandey, University of Delhi, IndiaReviewed by:
Renu Deswal, University of Delhi, IndiaJagadis Gupta Kapuganti, National Institute of Plant Genome Research, India
Copyright © 2017 Gayatri, Agurla, Kuchitsu, Anil, Podile and Raghavendra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agepati S. Raghavendra, YXNfcmFnaGF2ZW5kcmFAeWFob28uY29t; YXNyc2xAdW9oeWQuZXJuZXQuaW4=
 Gunja Gayatri
Gunja Gayatri Srinivas Agurla
Srinivas Agurla Kazuyuki Kuchitsu
Kazuyuki Kuchitsu Kondreddy Anil
Kondreddy Anil Appa R. Podile
Appa R. Podile Agepati S. Raghavendra
Agepati S. Raghavendra