
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 July 2017
Sec. Plant Genetics and Genomics
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.01062
This article is part of the Research Topic Integrating plant genetics and genomics for delineating climate resilience and health benefitting characteristics from millets View all 23 articles
The effect of photoperiod (day:night ratio) on flowering time was investigated in the wild species, Setaria viridis, and in a set of recombinant inbred lines (RILs) derived from a cross between foxtail millet (S. italica) and its wild ancestor green foxtail (S. viridis). Photoperiods totaled 24 h, with three trials of 8:16, 12:12 and 16:8 light:dark hour regimes for the RIL population, and these plus 10:14 and 14:10 for the experiments with S. viridis alone. The response of S. viridis to light intensity as well as photoperiod was assessed by duplicating photoperiods at two light intensities (300 and 600 μmol.m-2.s-1). In general, day lengths longer than 12 h delayed flowering time, although flowering time was also delayed in shorter day-lengths relative to the 12 h trial, even when daily flux in high intensity conditions exceeded that of the low intensity 12 h trial. Cluster analysis showed that the effect of photoperiod on flowering time differed between sets of RILs, with some being almost photoperiod insensitive and others being delayed with respect to the population as a whole in either short (8 or 12 h light) or long (16 h light) photoperiods. QTL results reveal a similar picture, with several major QTL colocalizing between the 8 and 12 h light trials, but with a partially different set of QTL identified in the 16 h trial. Major candidate genes for these QTL include several members of the PEBP protein family that includes Flowering Locus T (FT) homologs such as OsHd3a, OsRFT1, and ZCN8/12. Thus, Setaria is a short day plant (flowering quickest in short day conditions) whose flowering is delayed by long day lengths in a manner consistent with the responses of most other members of the grass family. However, the QTL results suggest that flowering time under long day conditions uses additional genetic pathways to those used under short day conditions.
Changes in photoperiod are potent signals for plant development, controlling patterns of germination and growth as well as time to flowering and other yield related traits. Photoperiodic response has been studied in many plants, and its manipulation has been of particular importance in the domestication and spread of crops, with a trend toward breeding lines whose flowering times are increasingly photoperiod insensitive (Vergara and Chang, 1985). Decreased photoperiod sensitivity allows crops to be grown in a wider range of environments and increases the utility of elite germplasm. Our knowledge of the effects of photoperiod on flowering time is best developed in Arabidopsis thaliana, but much is also known about photoperiodic effects on flowering time in rice and other grasses. We present here a quantitative genetic analysis of flowering time in multiple photoperiod regimes for the panicoid C4 model system Setaria, using a mapping population derived from a cross between domesticated foxtail millet (S. italica) and its wild progenitor green foxtail (S. viridis).
The effect of photoperiod on flowering time in the grasses has been best studied in rice, where increasing day-length leads to delays in flowering (Lee and An, 2015). There are two photoperiod pathways in rice, one mediated through the CONSTANS ortholog OsHD1, and the other through the grass-specific OsGhd7-OsEhd1 pathway (Nunez and Yamada, 2017). Both OsEhd1 and OsHd1 control homologs of Flowering Locus T (FT), the gene whose protein product is the signal transported from the leaf to the shoot apical meristem to initiate the floral transition. There are two FT homologs in rice, OsHd3a and OsRFT1, the product of a recent tandem duplication (Kojima et al., 2002; Ishikawa et al., 2005; Tamaki et al., 2007; Komiya et al., 2008, 2009). The OsHd1 pathway activates OsHd3a in short days and represses OsHD3a in long days. The OsEhd1 pathway activates OsHd3a in short days and OsRFT1 in long days. Thus, OsHd3a predominantly upregulates flowering in short days while OsRFT1 upregulates flowering in long days. Therefore, even though rice is generally considered a short day plant, it manages to flower in both short days and, eventually, in longer days by two partially distinct genetic pathways (Lee and An, 2015).
The effect of photoperiod on flowering time in other grasses is similar to that of rice, with a general delay of flowering time with longer day-lengths. The pooid grasses are the exception to the general short-day flowering pattern, as most also require vernalization to become competent to flower (Ream et al., 2014; Woods et al., 2016). Thus, the pooid grasses, including Brachypodium, barley and wheat, are long day flowering plants, that natively require winter exposure before becoming competent to flower, although mutations in the vernalization pathway have allowed the production of spring-flowering varieties that have expanded the range of pooid crops (Ream et al., 2014; Woods et al., 2016).
Breeding has in general reduced the photoperiod sensitivity of crops. Breeding for insensitivity was an early step in the improvement of maize, with selection first occurring in pre-historic times by early farmers as they took maize north and south from its site of domestication in central Mexico (Romero Navarro et al., 2017). Similar efforts were made by sorghum breeders in the last century as they moved African tropical germplasm to temperate latitudes in the United States, Australia and Europe (Stephens et al., 1967). Manipulating photoperiod sensitivity is of importance also in the development of new crops such as switchgrass and Miscanthus for biofuels production (Casler et al., 2004, 2007).
The genetic regulation of flowering in panicoid grasses is less well-known than that of rice. Most research has centered on maize and sorghum, which have both been selected for decreased photoperiod sensitivity. However, domestication and improvement history, coupled with the large size and experimentally difficult nature of most panicoid crops has made it difficult to effectively test the effect of different photoperiods in controlled environmental settings. A useful model to test the effects of photoperiod on flowering time in the panicoid grasses is the Setaria system, comprising two species (often considered con-specific), the domesticated cereal foxtail millet (S. italica) and its wild progenitor green foxtail (S. viridis) (Doust et al., 2009; Li and Brutnell, 2011). The C4 grass genus Setaria is a widespread genus in the subfamily Panicoideae, tribe Paniceae, closely related to switchgrass (Panicum virgatum) and pearl millet (Pennisetum glaucum) within the tribe Paniceae, and to maize (Zea mays) and sorghum (Sorghum bicolor), in the sister tribe, Andropogoneae (Kellogg et al., 2009; Layton and Kellogg, 2014). Species within the genus have been domesticated several times (Austin, 2006), but the only present-day domesticate is foxtail millet, an ancient grain domesticated in Northern China over 10,000 years ago. Its wild relative, green foxtail, is one of the world’s most widespread weeds (Dekker, 2003), and shows remarkable local adaptation to a wide variety of growing environments (Douglas et al., 1985). One of the more predictable factors that changes over the range of S. viridis is photoperiod, with day to night ratios varying between 16 h of daylight at high latitudes to 13 h or even less nearer the equator, making changes in photoperiod likely a primary signal for flowering. Indeed, Setaria has been shown to respond to both small and large changes in photoperiod, and genotypes with very different responses to photoperiod are grown at different latitudes in the various foxtail millet growing regions in China (Li and Yang, 2008; Jia et al., 2013; Diao and Jia, 2017). Accessions of S. viridis can be fast-cycling and small-statured, making the system an excellent model for studying photoperiod response in the panicoid grasses.
Flowering time in a Setaria recombinant inbred line (RIL) population has previously been assessed in field, greenhouse, and growth chamber trials (Mauro-Herrera et al., 2013; Doust, 2017), and preliminary studies of flowering time and other traits quantitative trait locus (QTL) analyses have identified several overlapping and several distinct QTL regions, suggesting that flowering time is positively correlated with both height and biomass (Doust, 2017). Here we examine whether flowering time is controlled by different pathways in long and short day regimes in Setaria, using both S. viridis and a mapping population between S. viridis and its domesticated relative, S. italica.
Two experiments were performed. In the first, plants of green foxtail (S. viridis accession A10.1) were grown in five growth chambers, each set at one of the following photoperiod ratios (light:dark): 8:16, 10:14, 12:12, 14:10 and 16:8. Day and night temperatures were 28°C and 22°C, respectively, and humidity was kept at approximately 30%. Each growth chamber had two shelves, and these were individually adjusted to give an illumination at the level of the soil surface of 600 and 300 μmol.m-2.s-1, respectively, to investigate the effect of different light fluxes on flowering time. For each combination of photoperiod and light level, there were twelve replicates of S. viridis grown in 10 cm × 10 cm × 10 cm square pots and spaced approximately 10 cm apart. Plants were irrigated as needed with an aqueous complete fertilizer mix (Jack’s mix: Nitrogen, Phosphorous and Potassium (20-20-20), JR Peters, Allentown, PA, United States.
In the second experiment, a total of 182 F7 RILs from an interspecific cross between S. italica accession B100 x S. viridis accession A10.1 (Bennetzen et al., 2012), together with their parents, were evaluated for flowering time in a walk-in growth chamber at Oklahoma State University (Stillwater, OK, United States). Three trials were performed, at photoperiod ratios (light:dark) of 8:16, 12:12, and 16:8. The chamber was kept at 30% humidity and day and night temperatures were 28 and 22°C, respectively. The different photoperiod ratios combined with the different day and night temperatures resulted in slightly different average temperatures per trial, with an average of 24°C in the first trial, 25°C in the second trial, and 26°C in the third trial. Illumination from full spectrum fluorescent tubes averaged 200 μmol.m-2.s-1 at the soil surface, but, owing to the short distance from the light source to the growing plants, actual illumination just before flowering was as much as 400 μmol.m-2.s-1. Three replicate plants of each RIL were grown in each experiment, with each pot having a single plant. Pots were randomized, and plants were spaced 8.5 cm apart. Pot volume was approximately 215 cm3, and pots were filled with Metro-Mix 366 (Sun Gro Horticulture Canada Ltd.). Plants were irrigated as needed with an aqueous complete fertilizer mix (Jack’s mix: Nitrogen, Phosphorous and Potassium (20-20-20), JR Peters, Allentown, PA, United States.
We used days to heading as the measurement of flowering, with plants recorded as flowering when the inflorescence on the main culm was first visible in the sheath of the flag leaf (Mauro-Herrera et al., 2013).
SPSS version 23 was used to assess the distribution of flowering times for normality. Trait differences between photoperiods were analyzed using analysis of variance (ANOVA). The model fitted for the ANOVA of photoperiod differences between S. viridis plants grown at five different photoperiods consisted of two factors, Photoperiod (fixed) and Light Intensity (fixed) and their interaction. The model fitted for the ANOVA of photoperiod differences between the three RIL trials consisted of two factors, Photoperiod (fixed) and RIL (random) and their interaction. η2 values were calculated to assess the size of the significant effects of each factor (η2 = SS factor/SS total, with η2 values summing to 1) (Levine and Hullett, 2002). Bivariate Pearson correlations between photoperiods in the RIL population were examined, and a cluster analysis of the rank order of flowering times of the RILs in the RIL population was conducted using the gplots library in R to detect if rank order remained constant or significantly changed between trials (R-Core-Team, 2016; Warnes et al., 2016).
For QTL analyses we used the previously published 684 marker genetic map (Mauro-Herrera et al., 2013). QTL Cartographer Unix version 1.16 (Basten et al., 1994, 2002) was used for QTL analyses with the composite interval mapping (CIM) method, a genome scan interval of 1 cM, a window size of 10, and the forward and backward regression method (Jansen and Stam, 1994; Zeng, 1994). QTL analyses were conducted for flowering time in each photoperiod trial as well as in a joint analysis. LOD threshold values were estimated via 1000 permutations (Churchill and Doerge, 1994; Doerge and Churchill, 1996).
Candidate genes within identified QTL regions were sought through literature searches, and their position relative to the QTL intervals assessed through comparison with the SNP markers in the map.
The distribution of flowering times in all trials was approximately normal, and therefore all analyses were carried out with the original data. The relationship of flowering time to photoperiod in the first experiment (S. viridis growth chamber experiment) was not linear, with plants in the 12 h trial flowering first, followed by 10 and 14 h trials, then 8 h and finally the 16 h trial (Figure 1A). In the ANOVA analysis both photoperiod and light level had significant effects on flowering time (Table 1), and post hoc Tukey HSD analyses on ANOVAs performed at each light level indicate that, for the lower light intensity, all flowering times were significantly different except for the 10 and 14 h light periods. At the higher light intensity, 8 vs. 10, 8 vs. 14, and 10 vs. 14 h light periods were not significantly different. However, at both light levels, the 12 and 16 h photoperiods were significantly different from all others (Figure 1). η2 values indicate that most of the effect on flowering time was due to differences in photoperiod (87%), and relatively little to differences in light intensity or the interaction between the two factors (Table 1).
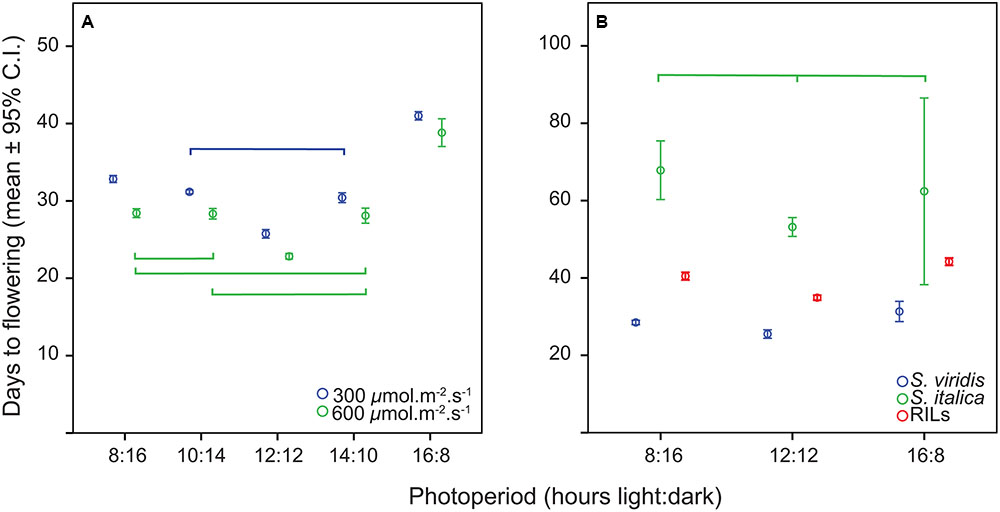
FIGURE 1. Means and 95% confidence intervals for flowering time in the two trials. (A) S. viridis photoperiod trial, (B) S. viridis, S. italica, and RILs in QTL trials. Connecting brackets between points indicate which treatments were not significantly different for flowering time, and color indicates to which group the brackets pertain.
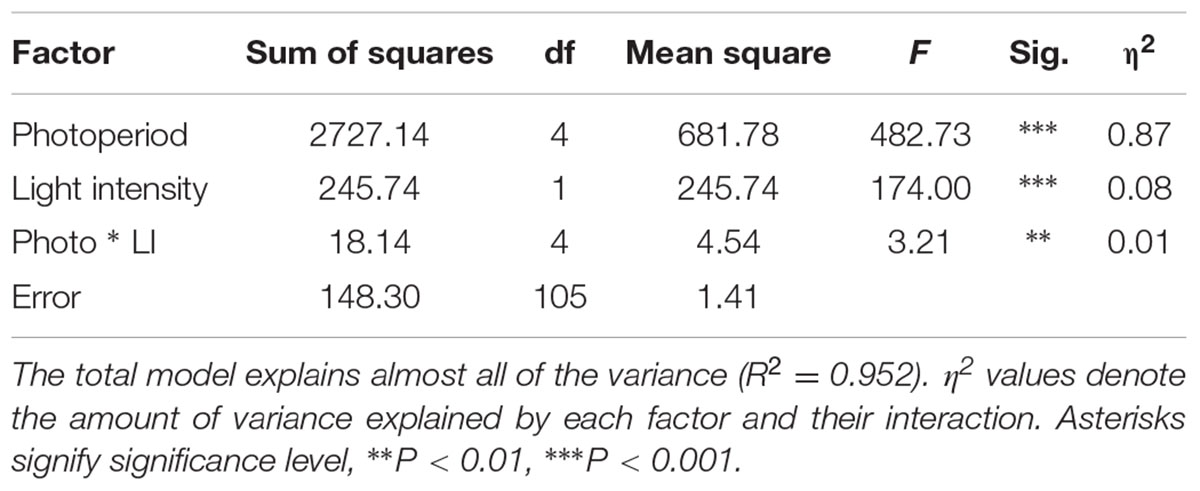
TABLE 1. Analysis of variance of Setaria viridis flowering time differences between the five photoperiod and two light intensities (see Materials and Methods), showing significant effects for both factors and their interaction.
The relationship of flowering time to photoperiod for S. viridis and S. italica in the RIL trial (where both parents were included for comparison) was also not linear, with the 12:12 h trial plants flowering first, followed by the 8:16 and 16:8 h trial plants (Figure 1B). This was also the case for the mean of the RIL population (Figure 1B). However, the “trough” at the 12:12 h photoperiod regime was much less evident than in the growth chamber trials for S. viridis, accounting for only a 11% dip between 8 and 12 h as opposed to a 19.5% dip between the same two photoperiod regimes in the growth chamber experiment.
Pearson bivariate correlations of flowering time between photoperiod trials for the RIL population were significant for each pair of trials tested, but explained much more of the variance in the 8 and 12 h trials (R2 = 0.85) than between the 8 and 16 h (R2 = 0.04) or the 12 and 16 h (R2 = 0.05). The cluster analysis of ranked flowering times in the three trials reveals a similar pattern, with different orders of early and late flowering RILs in the 16:8 h trial as compared to the 8:16 and 12:12 h trials (Figure 2). In addition, some RILs show the same relative ranking across all three trials (same color in each column of Figure 2) whereas others change in ranking across trials, indicating that differents sets of RILs are either photoperiod insensitive or sensitive, respectively.
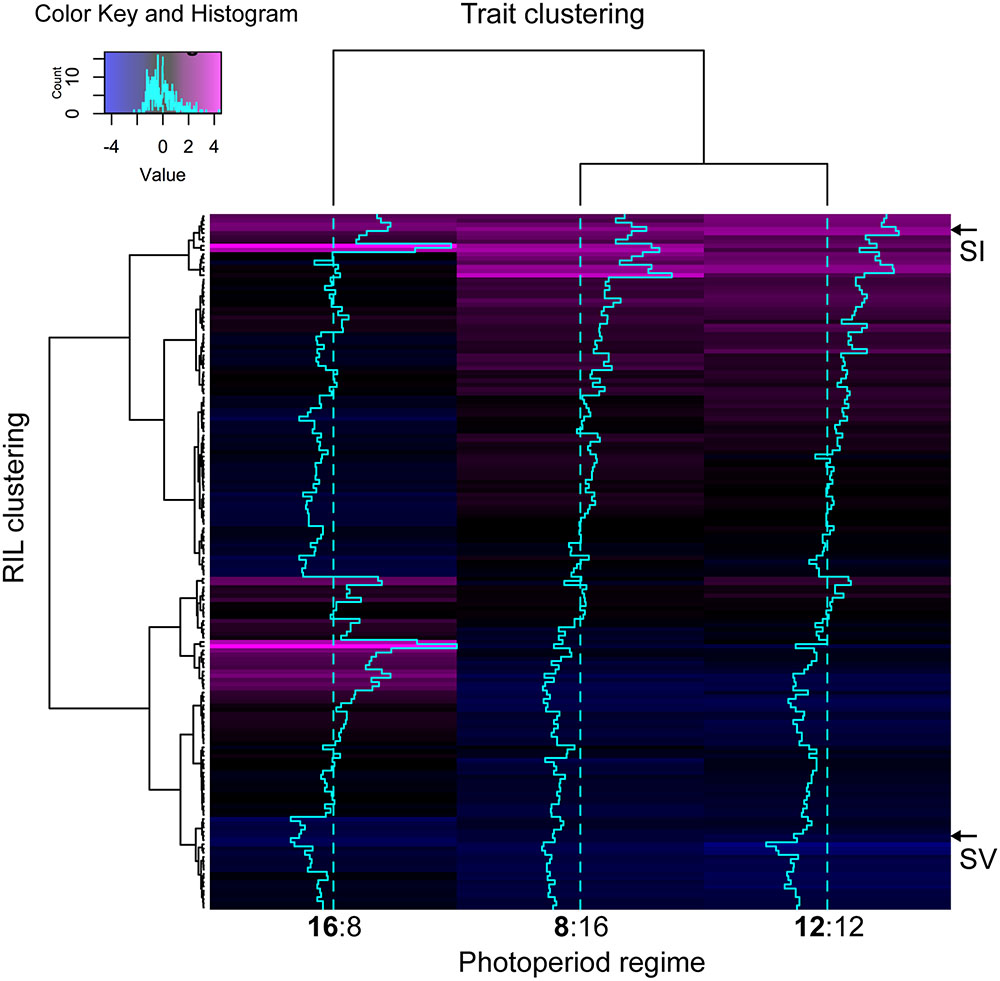
FIGURE 2. Heatmap visualizing the underlying structure of heading date phenotypes both between individual accessions (rows), and treatment groups (columns). Trace-lines down each treatment group (turquoise) show the degree to which each accession deviates from the mean (dashed line). The A10 S. viridis and B100 S. italica parents are indicated as ‘SV’ and ‘SI’ respectively. Black or blue coloration indicates that the RIL is early flowering with respect to the rest of the population in that trial, whereas magenta indicates relatively late flowering.
Analysis of variance of flowering time in the RIL population (with photoperiod as a fixed factor and RIL as a random factor) showed that flowering time differed significantly between photoperiods (Table 2). Post hoc tests found all flowering times to be significantly different from one another for both S. viridis and for the RIL population in all photoperiods. This was not so for S. italica, because of the wide spread of flowering times for S. italica (Figure 1B). The η2 values indicate that genotype (RIL) and genotype X photoperiod accounts for most of the variation (50 and 31%, respectively), with photoperiod accounting for only 11%.
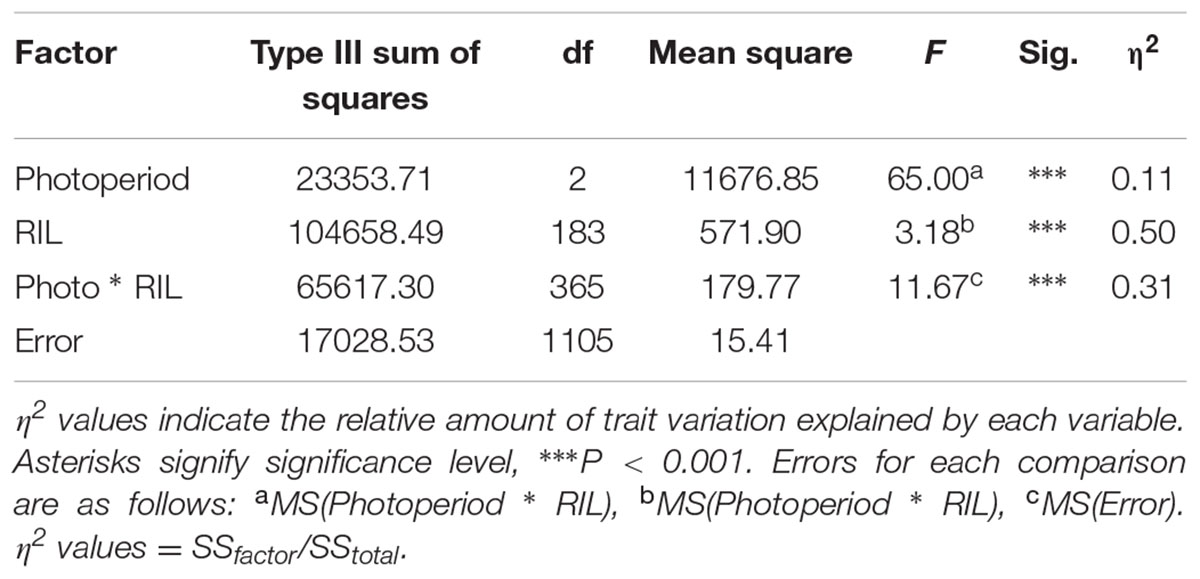
TABLE 2. Analysis of variance of the RIL population flowering time differences in the three photoperiod regimes, with Photoperiod (fixed) and RIL genotype (random).
The QTL analyses of flowering time in each photoperiod revealed multiple significant QTL that explained significant proportions of the phenotypic variance (PVE) (Figure 3 and Table 3). The 8 and 12 h trials share a large QTL peak on chromosome IV, whose interval overlaps with a QTL in the 16 h trial. However, the maximum LOD peak is slightly different in the 16 h trial, and the direction of the additive effect is opposite, strongly suggesting that this is not under the same genetic control. The 8 and 12 h trials also share a QTL region on chromosome V that is not found in the 16 h trial. All three trials share a QTL region on chromosome VII with similar additive effects, making this the most stable QTL occurring across trials. The 12 and 16 h trial share a QTL interval on chromosome VIII, and the 8 and 12 h trials share a QTL region on chromosome IX. Finally, the 16 h trial has unique QTL on chromosomes II and III. Almost all of the QTL regions have S. italica alleles that increase flowering time, with the only exception being that on chromosome IV in the 16:8 h trial. The joint QTL analysis identified main effect QTL on chromosomes IV, VII, and VIII, and genotype by environment QTL on chromosomes II and IV.
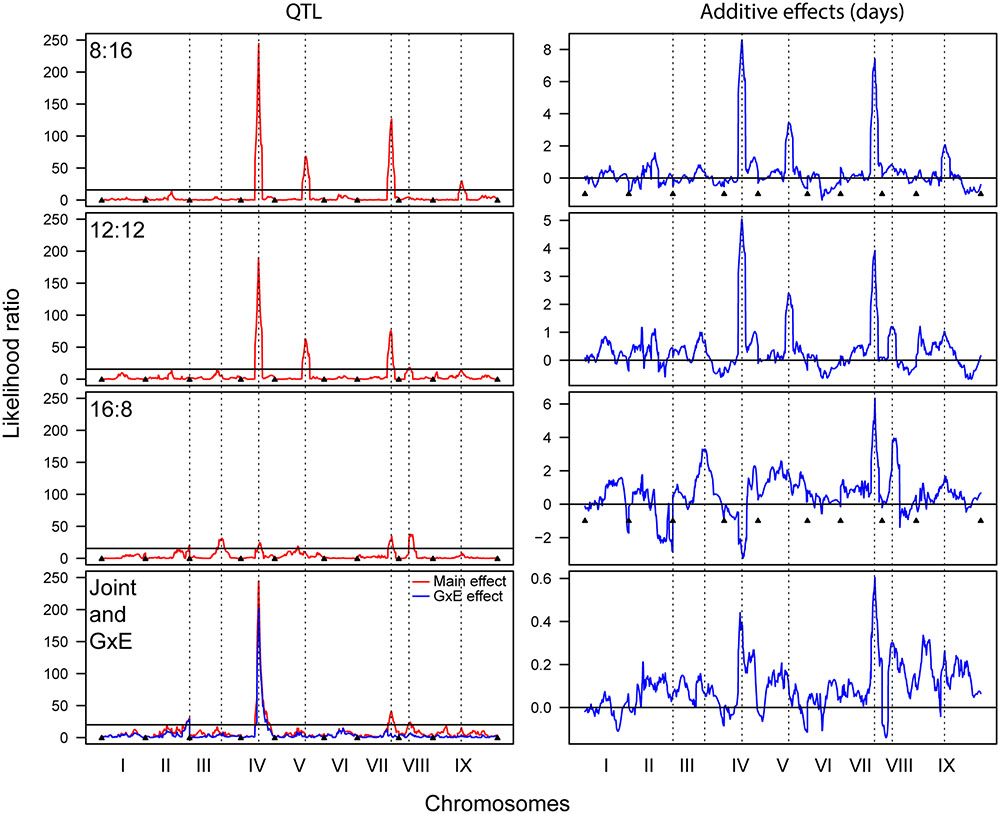
FIGURE 3. Quantitative trait locus analysis for 8:16, 12:12, and 16:8 photoperiod trials, as well as for a joint analysis showing both main and GxE QTL (Left column), and the corresponding additive effects (Right column). Dashed lines denote significant QTL and indicate whether their positions correspond between trials.
There are many genes associated with flowering time that map to these QTL intervals (Table 3). The large QTL interval on chromosome IV has previously been shown to contain homologs of a variety of flowering time genes, including FT-like and CONSTANS-like genes that are syntenic between maize, sorghum, and Setaria (Mauro-Herrera et al., 2013). The interval on Chromosome V in the 8 and 12 h trials contains the Setaria ortholog of ZCN12, an FT-like gene. Other members of the PEBP family to which FT belongs where found on chromosome VIII, and include an FT-like and a TFL-like gene.
This study examined the behavior of S. viridis in different photoperiods and light intensities as well as the behavior of S. viridis and S. italica and an F7 RIL population constructed from a cross between them. The means of all trials (parents and mean of the RIL population) showed a pattern of shortest flowering time at the 12:12 photoperiod, followed by longer flowering times at 8, 10, and 14 h light, and longest flowering times at 16 h of light. This data indicates that Setaria is typical for grasses in being a short day species that is also able to flower at longer daylengths, albeit with a delay in flowering time. However, the shortest flowering time is at 12 rather than 10 or 8 h light, in both the RIL experiment and at both light intensities for the growth chamber experiments with S. viridis. The simplest explanation is that the shorter daylengths provided insufficient photon flux for optimal development, delaying flowering as a result of a delay in carbon gain. This may be the case for the RIL trials, where average light levels were lower. However, in the S. viridis growth chamber experiments, both the 8 and 10 h daylength trials at high light intensity provided a higher flux than in the 12 h low light trial, and yet the 8 and 10 h trials flowered later. This may suggest that the flowering response is gated by a set of genes that have evolved for the naturally shortest light interval, and that shorter light intervals may hinder their interactions. The ANOVA results indicate that most of the variance in flowering time is explained by the difference in photoperiod and less by the difference in light intensity. However, the fact that light intensity can affect flowering time at all speaks to the necessity for a certain net carbon gain by the plant through photosynthesis before transition to an inflorescence meristem can occur. The interaction of light intensity and photoperiod can most clearly be seen between the high intensity 8 h trial and the low intensity 16 h trial, where both trials averaged almost identical light fluxes per day, and where the mean difference in flowering time of 12.6 days was highly significant.
The ANOVA results for the RIL population show that major portions of the variance for the traits are accounted for by the effect of genotype, as well as the interaction between genotype and photoperiod environment. The effect of genotype was expected, given the significant QTL identified for flowering time in a preliminary analysis of genetic map data associated with the RILs (Doust, 2017). The large amount of variance explained by the genotype by photoperiod interaction suggests that there are subsets of the RILs that react differently to the different photoperiod environments. This is in accord with the cluster analysis, which showed that the order of flowering time within the RILs was very different in the 16 h trial from that in the 8 and 12 h trials. Two main patterns are obvious in the cluster analysis, one of which is relative insensitivity to photoperiod, with accessions having a similar ranking in flowering time in all three trials. The other is photoperiodic sensitivity, where accessions flower early in 8 and 12 h trials and late in the 16 h trial or vice versa. The cluster analysis also identified the S. italica parent as being consistently among the RILs with the longest time to flowering in all three photoperiod regimes, suggesting that it is relatively photoperiod insensitive. These results accord with previous field and greenhouse studies of flowering time and vegetative architecture in this RIL population, which found significant genotype by environment interactions, including QTL specific for different photoperiod environments (Mauro-Herrera et al., 2013; Mauro-Herrera and Doust, 2016).
The QTL results support the results of the other statistical analyses but also provide a link between underlying genetic patterns and phenotypic effects. There is only one QTL position, that on chromosome VII, that is shared between all three photoperiods and which has an additive effect of the same sign. For this QTL the S. italica allele leads to a 4–6 day delay in flowering. In addition to the QTL on chromosome VII, the 8 and 12 h trials share QTL on chromosomes IV and V, with the S. italica allele delaying flowering by 4–8 days and 2–4 days, respectively. The 16 h trial has several unique QTL, including those on chromosomes III and V, where the S. italica allele delays flowering by 4 and 2 days, respectively. There is also a QTL on chromosome IV, where the interval overlaps with the large QTL in the 8 and 12 h light trial, but the effect is opposite, with the S. viridis allele delaying flowering by approximately 3 days. The QTL peaks for the 16 h trial are not as prominent as for the 8 and 12 h trials, but this is partially a result of the QTL graph being scaled to match the 8 and 12 h trials. In fact, each QTL in the 16 h trial explains between 6 and 17% of the variation in flowering time, with effect sizes of between 2 and 6 days. The joint analyses show both main and GxE effects for the QTL on chromosome IV and main effects for the QTL on chromosomes VII and VIII. There is a QTL with a GxE effect only on chromosome II. The QTL results in general mirror those found in field and greenhouse trials (Mauro-Herrera et al., 2013) but the effect sizes vary, suggesting that the size of the effects are greatly affected by genotype by photoperiod interactions.
The candidate genes identified in the various QTL regions include many known to be involved in flowering time control in other grass species. Of these, the most interesting are those that may control the different responses to short and long day photoperiods. The QTL on chromosome V in the 8 and 12 h trials contains the Setaria ortholog of ZCN12, an FT-like gene that is known to be functional in sorghum (Wolabu et al., 2016). The QTL on chromosome II in the joint analysis contains the Setaria ortholog of CDF1, a repressor of CONSTANS, known to be active in grasses grown under long day conditions (Higgins et al., 2010). However, other FT-like genes do not map to QTL in our mapping population, including an FT-like gene that has been reported to have a large effect on photoperiodic control of flowering in sorghum (Cuevas et al., 2016). The QTL region on chromosome VII, common to all trials contains a distant paralog of HAP5B, that may play a role in regulating CONSTANS expression (Ben-Naim et al., 2006; Wenkel et al., 2006). All of these candidates need functional characterization in Setaria, especially those in the 16:8 h trial, but the different QTL patterns indicate that photoperiodic control of flowering in Setaria, and possibly other panicoid grasses, may utilize additional genetic regulation pathways under long day conditions.
The weakly photoperiod sensitive nature of the domesticated parent and highly photoperiod sensitivity of the wild parent of the RIL population are likely representative of extremes that may be found in many accessions of both foxtail and other under-utilized millets. This is unlike their panicoid relatives, maize and sorghum, where the tropical germplasm is the most sensitive to photoperiod, and where there has been intense selection for photoperiod insensitivity to allow for plant growth and flowering at temperate latitudes. These trends are seen in other grasses, such as maize and wheat, where there appears to be a significant shift in newer varieties to more photoperiod insensitive genotypes, as breeders strive to produce varieties that maintain yield in multiple locations (Vergara and Chang, 1985; Bentley et al., 2011; Montesino-San Martin et al., 2014; Jones et al., 2017). Breeding for photoperiod insensitivity is also a goal for millet improvement, and a detailed understanding of the effects of photoperiod on flowering time and the underlying genetic pathways that control photoperiod effects could accelerate such efforts. Understanding the genetic regulation and phenotypic effects of photoperiod variation will also contribute to the production of less photoperiod sensitive elite varieties of switchgrass and other biofuels grasses. The value of the Setaria system, demonstrated in the analysis presented here, is that photoperiod, light flux, temperature, and other variables affecting growth and reproduction can be manipulated so that the effect of each and their interaction, can be assessed. The identification of sets of RILs within the RIL population that vary in their response to different photoperiods will be key to genetically dissecting these factors, along with further exploration of several of the high impact QTL that have been uncovered.
AD designed the study, interpreted data, wrote and revised manuscript. MM-H designed experiment, acquired and interpreted data, revised manuscript. JS acquired and interpreted data, revised manuscript. JH interpreted data, revised manuscript for intellectual content.
Funding to AD from NSF IOS 1339332 and OCAST PSB8-07 and PSB11-035.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Chris Wood for help in programming and maintaining the walk-in growth room and growth chambers, NSF-RET participants Morrison high school science teacher Tammy Will and education major Caitlin Snider for flowering time measurements in the S. viridis growth chamber trial, and members of the Doust lab for general help and insightful comments on this manuscript.
Ausin, I., Alonso-Blanco, C., Jarillo, J. A., Ruiz-Garcia, L., and Martinez-Zapater, J. M. (2004). Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 36, 162–166. doi: 10.1038/ng1295
Austin, D. F. (2006). Fox-tail millets (Setaria: Poaceae) - Abandoned food in two hemispheres. Econ. Bot. 60, 143–158. doi: 10.1663/0013-0001(2006)60[143:FMSPFI]2.0.CO;2
Baek, I. S., Park, H. Y., You, M. K., Lee, J. H., and Kim, J. K. (2008). Functional conservation and divergence of FVE genes that control flowering time and cold response in rice and Arabidopsis. Mol. Cells 26, 368–372.
Basten, C. J., Weir, B. S., and Zeng, Z. B. (1994). “Zmap-a QTL Cartographer,” in Proceedings of the 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software. Guelph, ON: Organizing Committee.
Basten, C. J., Weir, B. S., and Zeng, Z. B. (2002). QTL Cartographer. Raleigh, NC: North Carolina State University.
Ben-Naim, O., Eshed, R., Parnis, A., Teper-Bamnolker, P., Shalit, A., Coupland, G., et al. (2006). The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 46, 462–476. doi: 10.1111/j.1365-313X.2006.02706.x
Bennetzen, J. L., Schmutz, J., Wang, H., Percifield, R., Hawkins, J., Pontaroli, A. C., et al. (2012). Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30, 555–561. doi: 10.1038/nbt.2196
Bentley, A. R., Turner, A. S., Gosman, N., Leigh, F. J., Maccaferri, M., Dreisigacker, S., et al. (2011). Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breed. 130, 10–15. doi: 10.1007/s00122-008-0898-9
Casler, M. D., Vogel, K. P., Taliaferro, C. M., Ehlke, N. J., Berdahl, J. D., Brummer, E. C., et al. (2007). Latitudinal and longitudinal adaptation of switchgrass populations. Crop Sci. 47, 2249–2260. doi: 10.2135/cropsci2006.12.0780
Casler, M. D., Vogel, K. P., Taliaferro, C. M., and Wynia, R. L. (2004). Latitudinal adaptation of switchgrass populations. Crop Sci. 44, 293–303. doi: 10.2135/cropsci2004.2930
Churchill, G. A., and Doerge, R. W. (1994). Empirical threshold values for quantitative trait mapping. Genetics 138, 963–971.
Cuevas, H. E., Zhou, C., Tang, H., Khadke, P. P., Das, S., Lin, Y.-R., et al. (2016). The evolution of photoperiod-insensitive flowering in sorghum, a genomic model for panicoid grasses. Mol. Biol. Evol. 33, 2417–2428. doi: 10.1093/molbev/msw120
Danilevskaya, O. N., Meng, X., Hou, Z. L., Ananiev, E. V., and Simmons, C. R. (2008). A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol. 146, 250–264. doi: 10.1104/pp.107.109538
Dekker, J. (2003). The foxtail (Setaria) species-group. Weed Sci. 51, 641–656. doi: 10.1614/P2002-IR
Diao, X. M., and Jia, G. Q. (2017). “Foxtail Millet Breeding in China,” in The Genetics and Genomics of Setaria. Plant Genetics and Genomics: Crops and Models, eds A. N. Doust and X. M. Diao (New York: Springer-Verlag).
Doerge, R. W., and Churchill, G. A. (1996). Permutation tests for multiple loci affecting a quantitative character. Genetics 142, 285–294.
Douglas, B. J., Thomas, A. G., Morrison, I. N., and Maw, M. G. (1985). The biology of Canadian weeds. 70. Setaria viridis (L.) Beauv. Can. J. Plant Sci. 65, 669–690. doi: 10.4141/cjps85-089
Doust, A. N. (2017). “The effect of photoperiod on flowering time, plant architecture, and biomass in Setaria,” in The Genetics and Genomics of Setaria. Plant Genetics and Genomics: Crops and Models, eds A. N. Doust and X. M. Diao (New York, NY: Springer-Verlag), 19.
Doust, A. N., Kellogg, E. A., Devos, K. M., and Bennetzen, J. L. (2009). Foxtail millet: a sequence-driven grass model system. Plant Physiol. 149, 137–141. doi: 10.1104/pp.108.129627
Guo, H. W., Yang, W. Y., Mockler, T. C., and Lin, C. T. (1998). Regulations of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363. doi: 10.1126/science.279.5355.1360
Gustafsonbrown, C., Savidge, B., and Yanofsky, M. F. (1994). Regulation of the Arabidopsis floral homeotic gene apetala1. Cell 76, 131–143. doi: 10.1016/0092-8674(94)90178-3
Higgins, J. A., Bailey, P. C., and Laurie, D. A. (2010). Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS ONE 5:e10065. doi: 10.1371/journal.pone.0010065
Ishikawa, R., Tamaki, S., Yokoi, S., Inagaki, N., Shinomura, T., Takano, M., et al. (2005). Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17, 3326–3336. doi: 10.1105/tpc.105.037028
Jansen, R. C., and Stam, P. (1994). High-resolution of quantitative traits into multiple loci via interval mapping. Genetics 136, 1447–1455.
Jeon, J. S., Lee, S., Jung, K. H., Yang, W. S., Yi, G. H., Oh, B. G., et al. (2000). Production of transgenic rice plants showing reduced heading date and plant height by ectopic expression of rice MADS-box genes. Mol. Breed. 6, 581–592. doi: 10.1023/A:1011388620872
Jia, G. Q., Huang, X. H., Zhi, H., Zhao, Y., Zhao, Q., Li, W. J., et al. (2013). A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 45, 957–961. doi: 10.1038/ng.2673
Jones, H. E., Lukac, M., Brak, B., Martinez-Eixarch, M., Alhomedhi, A., Gooding, M. J., et al. (2017). Photoperiod sensitivity affects flowering duration in wheat. J. Agric. Sci. 155, 32–43. doi: 10.1017/S0021859616000125
Kang, H. G., and An, G. H. (1997). Isolation and characterization of a rice MADS box gene belonging to the AGL2 gene family. Mol. Cells 7, 45–51.
Kellogg, E. A., Aliscioni, S. S., Morrone, O., Pensiero, J., and Zuloaga, F. (2009). A phylogeny Of Setaria (Poaceae, Panicoideae, Paniceae) and related genera based on the chloroplast gene ndhF. Int. J. Plant Sci. 170, 117–131. doi: 10.1086/593043
Kojima, S., Takahashi, Y., Kobayashi, Y., Monna, L., Sasaki, T., Araki, T., et al. (2002). Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43, 1096–1105. doi: 10.1093/pcp/pcf156
Komiya, R., Ikegami, A., Tamaki, S., Yokoi, S., and Shimamoto, K. (2008). Hd3a and RFT1 are essential for flowering in rice. Development 135, 767–774. doi: 10.1242/dev.008631
Komiya, R., Yokoi, S., and Shimamoto, K. (2009). A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136, 3443–3450. doi: 10.1242/dev.040170
Laubinger, S., Marchal, V., Gentilhomme, J., Wenkel, S., Adrian, J., Jang, S., et al. (2006). Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133, 3213–3222. doi: 10.1242/dev.02481
Layton, D. J., and Kellogg, E. A. (2014). Morphological, phylogenetic, and ecological diversity of the new model species Setaria viridis (Poaceae: Paniceae) and its close relatives. Am. J. Bot. 101, 539–557. doi: 10.3732/ajb.1300428
Lee, J. H., Park, S. H., and Ahn, J. H. (2012). Functional conservation and diversification between rice OsMADS22/OsMADS55 and Arabidopsis SVP proteins. Plant Sci. 185, 97–104. doi: 10.1016/j.plantsci.2011.09.003
Lee, Y. S., and An, G. (2015). Regulation of flowering time in rice. J. Plant Biol. 58, 353–360. doi: 10.1007/s12374-015-0425-x
Levine, T. R., and Hullett, C. R. (2002). Eta squared, partial Eta squared and the Misreporting of effect size in communication research. Hum. Commun. Res. 28, 612–625. doi: 10.1111/j.1468-2958.2002.tb00828.x
Li, H.-Y., and Yang, Y.-F. (2008). Phenotypic plasticity of life history characteristics: quantitative analysis of delayed reproduction of green foxtail (Setaria viridis) in the Songnen Plain of China. J. Integrat. Plant Biol. 50, 641–647. doi: 10.1111/j.1744-7909.2008.00646.x
Li, P. H., and Brutnell, T. P. (2011). Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J. Exp. Bot. 62, 3031–3037. doi: 10.1093/jxb/err096
Liu, B. B., Yang, Z. H., Gomez, A., Liu, B., Lin, C. T., and Oka, Y. (2016). Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J. Plant Res. 129, 137–148. doi: 10.1007/s10265-015-0782-z
Luo, M., Platten, D., Chaudhury, A., Peacock, W. J., and Dennis, E. S. (2009). Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol. Plant 2, 711–723. doi: 10.1093/mp/ssp036
Matsubara, K., Kono, I., Hori, K., Nonoue, Y., Ono, N., Shomura, A., et al. (2008). Novel QTLs for photoperiodic flowering revealed by using reciprocal backcross inbred lines from crosses between japonica rice cultivars. Theor. Appl. Genet. 117, 935–945. doi: 10.1007/s00122-008-0833-0
Matsubara, K., Ogiso-Tanaka, E., Hori, K., Ebana, K., Ando, T., and Yano, M. (2012). Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol. 53, 709–716. doi: 10.1093/pcp/pcs028
Mauro-Herrera, M., and Doust, A. N. (2016). Development and genetic control of plant architecture and biomass in the Panicoid Grass, Setaria. PLoS ONE 11:e0151346. doi: 10.1371/journal.pone.0151346
Mauro-Herrera, M., Wang, X. W., Barbier, H., Brutnell, T. P., Devos, K. M., and Doust, A. N. (2013). genetic control and comparative genomic analysis of flowering time in Setaria (Poaceae). G3 (Bethesda) 3, 283–295. doi: 10.1534/g3.112.005207
Montesino-San Martin, M., Olesen, J. E., and Porter, J. R. (2014). A genotype, environment and management (GxExM) analysis of adaptation in winter wheat to climate change in Denmark. Agric. For. Meteorol. 187, 1–13. doi: 10.1016/j.agrformet.2013.11.009
Murakami, M., Ashikari, M., Miura, K., Yamashino, T., and Mizuno, T. (2003). The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol. 44, 1229–1236. doi: 10.1093/pcp/pcg135
Murakami, M., Matsushika, A., Ashikari, M., Yamashino, T., and Mizuno, T. (2005). Circadian-associated rice pseudo response regulators (OsPRRs): insight into the control of flowering time. Biosci. Biotechnol. Biochem. 69, 410–414. doi: 10.1271/bbb.69.410
Murphy, R. L., Klein, R. R., Morishige, D. T., Brady, J. A., Rooney, W. L., Miller, F. R., et al. (2011). Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc. Natl. Acad. Sci. U.S.A. 108, 16469–16474. doi: 10.1073/pnas.1106212108
Nakagawa, M., Shimamoto, K., and Kyozuka, J. (2002). Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J. 29, 743–750. doi: 10.1046/j.1365-313X.2002.01255.x
Noh, B., Lee, S.-H., Kim, H.-J., Yi, G., Shin, E.-A., Lee, M., et al. (2004). Divergent roles of a pair of homologous jumonji/zinc-finger–class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell 16, 2601–2613. doi: 10.1105/tpc.104.025353
Nunez, F. D. B., and Yamada, T. (2017). Molecular regulation of flowering time in grasses. Agronomy 7, 17. doi: 10.3390/agronomy7010017
Peng, L. T., Shi, Z. Y., Li, L., Shen, G. Z., and Zhang, J. L. (2007). Ectopic expression of OsLFL1 in rice represses Ehdl by binding on its promoter. Biochem. Biophys. Res. Commun. 360, 251–256. doi: 10.1016/j.bbrc.2007.06.041
Peng, L. T., Shi, Z. Y., Li, L., Shen, G. Z., and Zhang, J. L. (2008). Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J. Plant Physiol. 165, 876–885. doi: 10.1016/j.jplph.2007.07.010
R-Core-Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ream, T. S., Woods, D. P., Schwartz, C. J., Sanabria, C. P., Mahoy, J. A., Walters, E. M., et al. (2014). Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 164, 694–709. doi: 10.1104/pp.113.232678
Romero Navarro, J. A., Willcox, M., Burgueno, J., Romay, C., Swarts, K., Trachsel, S., et al. (2017). A study of allelic diversity underlying flowering-time adaptation in maize landraces. Nat. Genet. 49, 476–480. doi: 10.1038/ng.3784
Shannon, S., and Meekswagner, D. R. (1991). A mutation in the Arabidopsis Tfl1 gene affects inflorescence meristem development. Plant Cell 3, 877–892. doi: 10.1105/tpc.3.9.877
Stephens, J. C., Miller, F. R., and Rosenow, D. T. (1967). Conversion of alien sorghums to early combine genotypes. Crop Sci. 7, 396. doi: 10.2135/cropsci1967.0011183X000700040036x
Tamaki, S., Matsuo, S., Wong, H. L., Yokoi, S., and Shimamoto, K. (2007). Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036. doi: 10.1126/science.1141753
Vergara, B. S., and Chang, T. T. (1985). The Flowering Response of the Rice Plant to Photoperiod: a Review of the Literature. Los Baños: The International Rice Research Institute.
Warnes, G. R., Bolker, B., Bonebakker, L., Gentleman, R., Huber, W., Liaw, A., et al. (2016). gplots: Various R Programming Tools for Plotting Data. R Package Version 3.0.1. Available at: https://CRAN.R-project.org/package=gplots.
Wenkel, S., Turck, F., Singer, K., Gissot, L., Le Gourrierec, J., Samach, A., et al. (2006). CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18, 2971–2984. doi: 10.1105/tpc.106.043299
Wolabu, T. W., Zhang, F., Niu, L. F., Kalve, S., Bhatnagar-Mathur, P., Muszynski, M. G., et al. (2016). Three FLOWERING LOCUS T-like genes function as potential florigens and mediate photoperiod response in sorghum. New Phytol. 210, 946–959. doi: 10.1111/nph.13834
Woods, D. P., McKeown, M. A., Dong, Y. X., Preston, J. C., and Amasino, R. M. (2016). Evolution of VRN2/Ghd7-Like genes in vernalization-mediated repression of grass flowering. Plant Physiol. 170, 2124–2135. doi: 10.1104/pp.15.01279
Yang, S., Murphy, R. L., Morishige, D. T., Klein, P. E., Rooney, W. L., and Mullet, J. E. (2014). Sorghum Phytochrome B Inhibits flowering in long days by activating expression of SbPRR37 and SbGHD7, repressors of SbEHD1, SbCN8 and SbCN12. PLoS ONE 9:e105352. doi: 10.1371/journal.pone.0105352
Yano, M., Katayose, Y., Ashikari, M., Yamanouchi, U., Monna, L., Fuse, T., et al. (2000). Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12, 2473–2483. doi: 10.1105/tpc.12.12.2473
Keywords: Setaria, foxtail millet, green foxtail, photoperiod, short day, long day, flowering time, heading date
Citation: Doust AN, Mauro-Herrera M, Hodge JG and Stromski J (2017) The C4 Model Grass Setaria Is a Short Day Plant with Secondary Long Day Genetic Regulation. Front. Plant Sci. 8:1062. doi: 10.3389/fpls.2017.01062
Received: 08 November 2016; Accepted: 01 June 2017;
Published: 06 July 2017.
Edited by:
Marion S. Röder, Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), GermanyReviewed by:
Xiaoyu Weng, University of Texas Austin, United StatesCopyright © 2017 Doust, Mauro-Herrera, Hodge and Stromski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew N. Doust, YW5kcmV3LmRvdXN0QG9rc3RhdGUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.