
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 24 May 2017
Sec. Plant Abiotic Stress
Volume 8 - 2017 | https://doi.org/10.3389/fpls.2017.00871
 Ghazala Nawaz
Ghazala Nawaz Hunseung Kang*
Hunseung Kang*The yields and productivity of crops are greatly diminished by various abiotic stresses, including drought, cold, heat, and high salinity. Chloroplasts and mitochondria are cellular organelles that can sense diverse environmental stimuli and alter gene expression to cope with adverse environmental stresses. Organellar gene expression is mainly regulated at posttranscriptional levels, including RNA processing, intron splicing, RNA editing, RNA turnover, and translational control, during which a variety of nucleus-encoded RNA-binding proteins (RBPs) are targeted to chloroplasts or mitochondria where they play essential roles in organellar RNA metabolism. DEAD-box RNA helicases (RHs) are enzymes that can alter RNA structures and affect RNA metabolism in all living organisms. Although a number of DEAD-box RHs have been found to play important roles in RNA metabolism in the nucleus and cytoplasm, our understanding on the roles of DEAD-box RHs in the regulation of RNA metabolism in chloroplasts and mitochondria is only at the beginning. Considering that organellar RNA metabolism and gene expression are tightly regulated by anterograde signaling from the nucleus, it is imperative to determine the functions of nucleus-encoded organellar RBPs. In this review, we summarize the emerging roles of nucleus-encoded chloroplast- or mitochondria-targeted DEAD-box RHs in organellar RNA metabolism and plant response to diverse abiotic stresses.
The biggest threat to the increasing population worldwide is the scarcity of food due to reducing crop yields caused by various abiotic and biotic stresses such as drought, cold, heat, high salinity, UV, bacteria, fungi, and viruses (Zsigmond et al., 2008; Nouri et al., 2015). As sessile organisms, plants have evolved various strategies to withstand these adverse environmental conditions (Komatsu and Hossain, 2013; Ranty et al., 2016). The survival of plants against these environmental stresses depends on their ability to recognize stress stimuli and adapt to such stresses by regulating the expression of stress-responsive genes in cellular organelles, nucleus, and cytoplasm. Photosynthesis in chloroplasts (Biswal et al., 2011; Ashraf and Harris, 2013; Komatsu and Hossain, 2013) and energy metabolism in mitochondria (Zsigmond et al., 2008) are particularly important cellular processes necessary for plant growth and survival under stressful and normal growth conditions. Therefore, gene expression affecting photosynthesis and energy metabolism in chloroplasts and mitochondria should be tightly regulated for plant growth and survival under stress conditions.
Expression of genes in chloroplasts and mitochondria is regulated mainly at posttranscriptional levels, including RNA processing, intron splicing, RNA editing, RNA turnover, and translational control (del Campo, 2009; Hammani and Giegé, 2014; Sun and Guo, 2016). Although the genomes of chloroplast and mitochondrion harbor less than 150 genes, more than 3,000 and 2,000 nucleus-encoded proteins are transported to the chloroplast and mitochondrion, respectively, and play essential roles in posttranscriptional RNA metabolism in cellular organelles (Millar et al., 2006; Nott et al., 2006; Pesaresi et al., 2007; del Campo, 2009). Therefore, fine-tuning communications between chloroplasts or mitochondria and the nucleus via anterograde signaling and retrograde signaling is essential for organellar gene expression, biogenesis, and function (Zsigmond et al., 2008; del Campo, 2009; Sun and Guo, 2016). Regulation of RNA metabolism in chloroplasts and mitochondria mediated by nucleus-encoded proteins is important for plants to adapt to deleterious biotic and abiotic stresses (Simpson and Filipowicz, 1996; Meierhoff et al., 2003; Williams and Barkan, 2003; Rocak and Linder, 2004; Floris et al., 2009). Many recent studies have demonstrated that diverse RNA-binding proteins (RBPs) play central roles in plant growth, development, and stress responses (Lorković, 2009; Cook et al., 2011; Ambrosone et al., 2012; Kang et al., 2013; Lee and Kang, 2016). Considering that organellar RNA metabolism and gene expression largely depend on nucleus-encoded RBPs, it is imperative to understand the functions of nucleus-encoded organellar RBPs to provide us deeper insights into how plants respond to diverse environmental stresses. Among RBPs are DEAD-box RNA helicases (RHs) that can assist the formation of functional mature RNAs in chloroplasts and mitochondria (Cordin et al., 2006; Kohler et al., 2010). In this review, we will focus on the emerging roles of nucleus-encoded chloroplast- or mitochondria-targeted DEAD-box RHs in organellar RNA metabolism and plant responses to diverse abiotic stresses.
RHs are enzymes implicated in a number of cellular processes involving alteration of RNA structures. Based on their shared sequence motifs, RHs are classified into six super families (SF1–SF6). Superfamily II (SF2), the largest helicase family, is mainly composed of DEAD-box RHs (Gorbalenya and Koonin, 1993; Fairman-Williams et al., 2010). Unwinding of double-stranded nucleic acids (DNAs and RNAs) requires energy. Hence, all DEAD-box RHs contain a nucleoside triphosphate (NTP) binding motif. Besides this NTP motif, DEAD-box RHs harbor eight motifs called Q, I, Ia, Ib, II, III, IV, V, and VI (Caruthers and McKay, 2002; Silverman et al., 2003; Tuteja and Tuteja, 2004; Kwong et al., 2005; Singleton et al., 2007) falling under two helicase domains: domain I and domain II. They span over a conserved core region of 400–700 amino acids (de la Cruz et al., 1999; Rocak and Linder, 2004; Figure 1). Each motif has specific function for helicase activity. The Q-motif is the most recently discovered motif. It has highly conserved glutamine residue and regulates ATP binding and hydrolysis (Tanner, 2003; Tanner et al., 2003). Motif I (AxxGxGKT) forms a loop structure (P loop) that accommodates ATP and facilitates interactions with Mg2+ ions (Tanner and Linder, 2001). Motif Ia forms the groove for facilitating single-stranded DNA/RNA binding (Velankar et al., 1999). Motif II contains a so-called “DEAD” (Asp-Glu-Ala-Asp) box. Its highly conserved first Asp (D) residue interacts with Mg2+ ion which is required for NTP binding (Gorbalenya and Koonin, 1989). On the basis of sequence alteration in motif II, SF2 members are classified into three sub-groups: Ski2, DEAD-box, and DEAH-box. Both DEAD-box and DEAH-box RHs have close sequence similarities except alterations in conserved amino acid sequences at motif II (Tanner and Linder, 2001). Motif III (SAT) is required for NTPase and helicase activities and performs the unwinding of RNA (Pause and Sonenberg, 1992). Motif VI (HxxGRxxR) is the third most conserved segment (Gorbalenya and Koonin, 1989). It is part of the ATP-binding cleft and also involved in coupling between helicase and NTPase activities of the protein (Pause and Sonenberg, 1992). The structures of RHs indicate that the remaining motifs (Ib, IV, and V) are probably involved in RNA binding (Rocak and Linder, 2004), although biochemical data are still lacking.
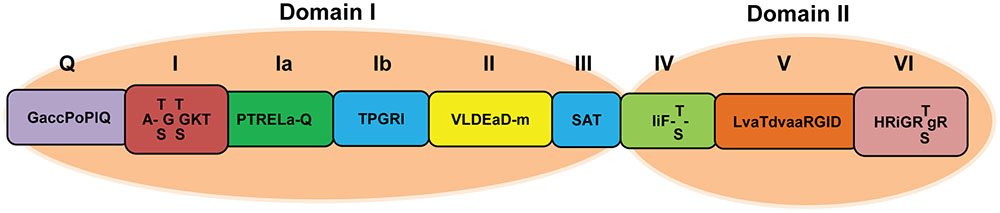
FIGURE 1. Schematic representation of the domain structure of SF2 family DEAD-box RH. The Q, I, Ia, Ib, II, and III motifs are included in domain I, and motifs IV, V, and VI are in domain II. Capital letters represent highly conserved amino acid sequences with >80% homology while small letters indicate less conserved amino acid sequences with 50–80% homology.
Although numerous reports have demonstrated that the plant genome encodes a large number of RHs which play significant roles in the regulation of various cellular metabolic pathways, their occurrence and functions in chloroplasts and mitochondria have not been well-documented yet. The genomes of Caenorhabditis elegans and Drosophila melanogaster encode 30 and 34 DEAD-box RHs, respectively, which are only half of the number of DEAD-box RHs found in plants (Boudet et al., 2001). In Arabidopsis thaliana, at least 120 RH members have been predicted using TAIR database1 (Mingam et al., 2004; Umate et al., 2010), and 58 DEAD-box RHs have been identified so far (Aubourg et al., 1999; Boudet et al., 2001; Zybailov et al., 2008). Among the identified 58 DEAD-box RHs, 10 DEAD-box RHs target chloroplasts and eight DEAD-box RHs are localized in the mitochondria (Table 1). The genome of rice (Oryza sativa) harbors more than 60 genes encoding DEAD-box RHs (Umate et al., 2010). Among the identified rice DEAD-box RHs, one RH, OsABP, has been confirmed to be localized in chloroplasts (Macovei et al., 2012), and one RH, OsSUV3, has been confirmed to be localized in mitochondria (Tuteja et al., 2013). To date, functions of only one DEAD-box RH have been determined in the chloroplasts of tobacco (Nicotiana tabacum), barely (Hordeum vulgare), and maize (Zea mays) (Table 1).
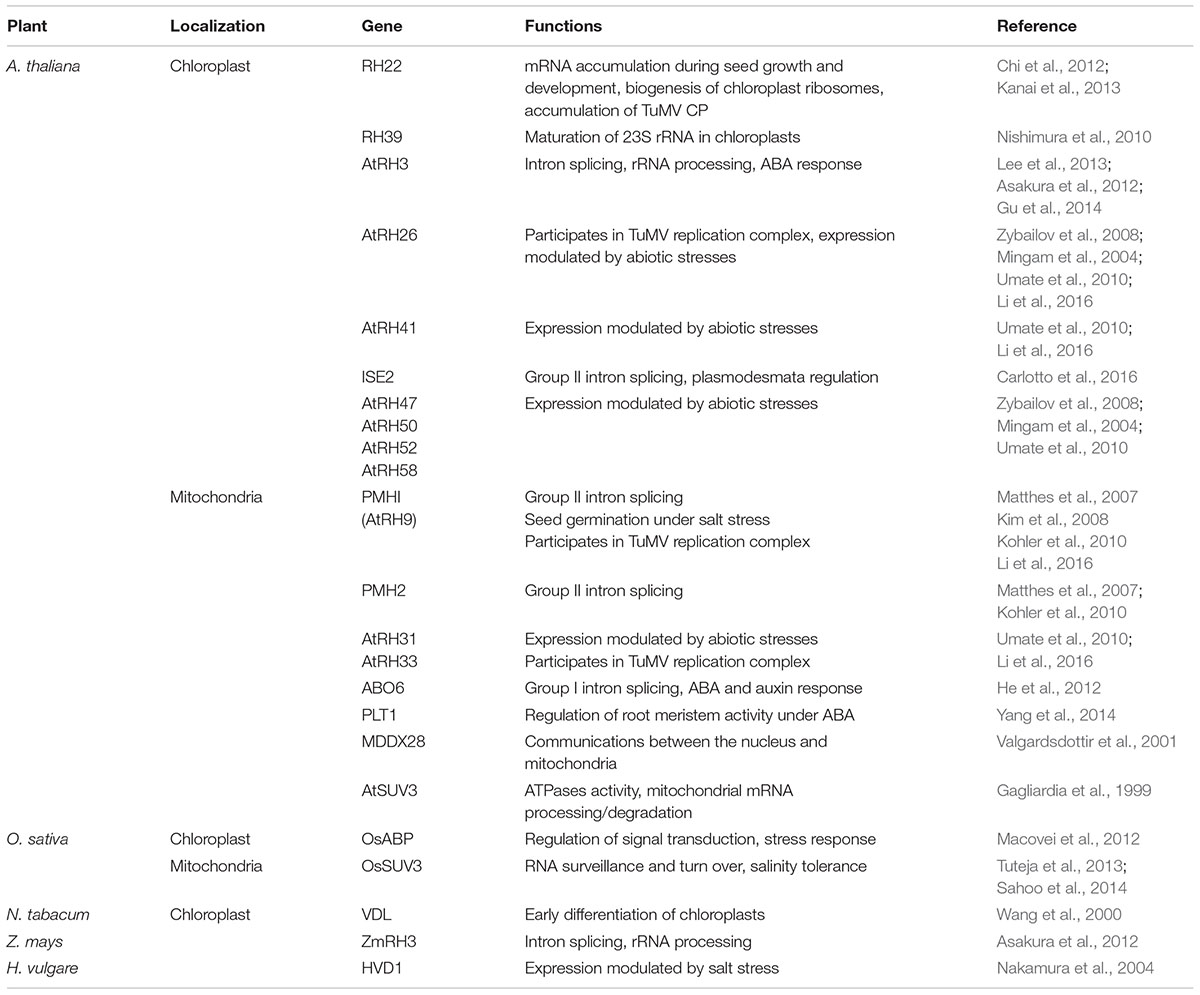
TABLE 1. List of DEAD-box RHs associated with RNA metabolism in chloroplasts or mitochondria and stress responses in various plants.
In addition to these previously identified DEAD-box RHs, our search for DEAD-box RHs harboring potential target sequences for chloroplasts or mitochondria localization revealed more potential chloroplast- or mitochondria-targeted DEAD-box RHs. Using web-based software and database2,3 ,4 ,5 ,6, we identified 12 DEAD-box RHs with putative chloroplast transit peptide (cTP) sequence and four DEAD-box RHs with putative mitochondrial targeting sequences in rice. We also identified seven DEAD-box RHs containing cTP sequences and five DEAD-box RHs harboring mitochondrial targeting sequences in maize. In addition, we found eight DEAD-box RHs with potential cTP sequences and seven DEAD-box RHs having mitochondrial targeting sequences in wheat (Triticum aestivum; Table 2). These findings suggest that approximately 7–12 DEAD-box RHs and 4–7 DEAD-box RHs are targeted to the chloroplast and mitochondria, respectively, which are much larger numbers than previously reported, indicating that DEAD-box RHs might play significant roles in gene expression regulation and functions in chloroplasts and mitochondria.
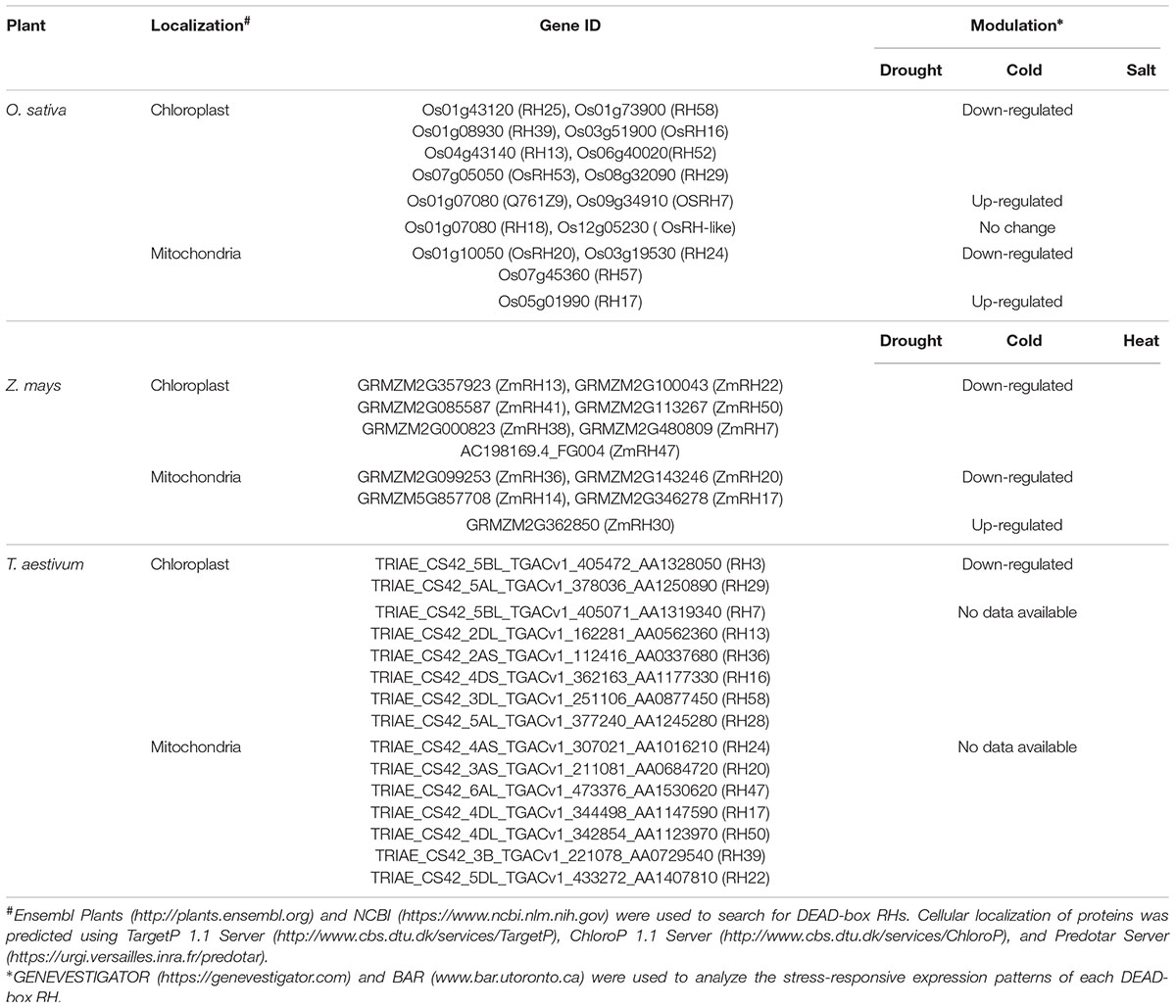
TABLE 2. List of predicted chloroplast- or mitochondria-targeted DEAD-box RHs and stress-responsive expression patterns.
Increasing numbers of recent reports have pointed to the importance of DEAD-box RHs in the regulation of RNA metabolism in chloroplasts and mitochondria (Figure 2). Arabidopsis RH3, RH22, and ISE2 are involved in the splicing of group I and II introns and the processing of 23S and 16S rRNAs in chloroplasts to help assemble the 50S ribosomal subunit (Chi et al., 2012; Kanai et al., 2013; Gu et al., 2014; Carlotto et al., 2016). In particular, Arabidopsis RH39 has been found to introduce a hidden break in chloroplast 23S rRNA, which is an essential process for the maturation of 23S rRNA (Nishimura et al., 2010). A recent report has demonstrated that chloroplast-targeted ZmRH3 is involved in rRNA biogenesis and the splicing of chloroplast introns in maize (Asakura et al., 2012). Several mitochondria-localized DEAD-box RHs such as ABO6, PHM1, and PHM2 can regulate the splicing of intron-containing genes in Arabidopsis (Matthes et al., 2007; Kohler et al., 2010; He et al., 2012). Mitochondria-localized AtSUV3 and OsSUV3 are involved in mRNA processing and RNA degradation to regulate gene expression in Arabidopsis and rice, respectively (Gagliardia et al., 1999; Tuteja et al., 2013). A mitochondrial MDDX28 is involved in mRNA transport, which is important for communications between the nucleus and mitochondria (Valgardsdottir et al., 2001). Recently, several chloroplast-localized DEAD-box RHs (such as RH58, RH47, RH26, RH50, RH41, and RH52) and mitochondria-targeted DEAD-box RHs (such as RH33 and RH31) have been shown to regulate transcription in response to multiple abiotic stresses (Umate et al., 2010). Moreover, chloroplast-targeted AtRH26 and AtRH41 and mitochondria-localized DEAD-box RHs, AtRH9, AtRH33, and AtRH31 have been found to interact with proteins involved in the formation of replication complex in turnip mosaic virus (Li et al., 2016). All these reports emphasize that DEAD-box RHs are essential for RNA metabolism in chloroplasts and mitochondria.
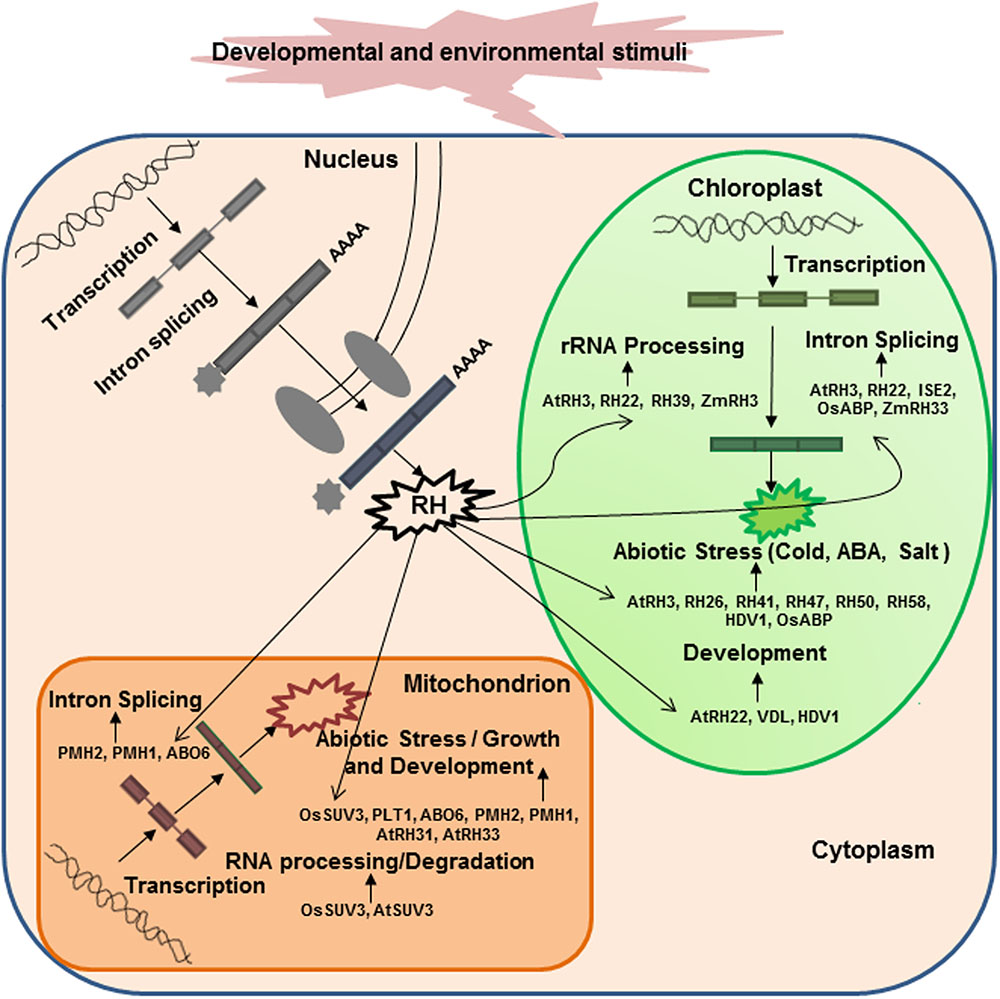
FIGURE 2. Cellular functions of diverse DEAD-box RHs involved in RNA metabolism in chloroplasts or mitochondria. A variety of nucleus-encoded RHs are targeted to chloroplasts or mitochondria and play essential roles in RNA processing, intron splicing, and translation, crucial for organellar biogenesis, function, and stress responses. Examples of the experimentally verified DEAD-box RHs that play unique roles in chloroplasts and mitochondria are shown in parenthesis. The functions and cellular roles of each RH are described in Table 1.
Although the functions of many DEAD-box RHs in chloroplasts or mitochondria await further experimental verification, their possible cellular roles can be inferred from the studies on nuclear RHs in plants. Two rice DEAD-box RHs, OsRH2 and OsRH34, are essential components of exon junction complex (Huang et al., 2016), and rice TOGR1 (for thermo-tolerant growth required 1) is involved in pre-rRNA homeostasis (Wang et al., 2016). Arabidopsis DEAD-box RHs (AtRH7, AtRH36, and AtRH57) and maize ZmDRH1 play essential roles in rRNA processing (Huang et al., 2010; Hsu et al., 2014; Liu et al., 2016). Notably, the CARPEL FACTORY, an Arabidopsis DEAD-box RH, is involved in the biogenesis of microRNAs (miRNAs; Park et al., 2002) and nuclear DEAD-box RHs play a crucial role in the metabolism of aberrant and silencing RNAs (reviewed in Linder and Owttrim, 2009). Considering that chloroplasts and mitochondria also contain small non-coding RNAs (ncRNAs) and miRNAs (Lung et al., 2006; reviewed in Hotto et al., 2012), it is of keen interest to determine whether DEAD-box RHs are involved in the processing and biogenesis of ncRNAs or miRNAs in chloroplasts and mitochondria.
Several recent studies have demonstrated that chloroplast- or mitochondria-targeted DEAD-box RHs play essential roles in plant growth and development under normal conditions (Figure 2). A chloroplast-targeted RH, INCREASED SIZE EXCLUSION LIMIT 2, is required for group II intron splicing and is involved in chloroplast pigmentation and plasmodesmata regulation during embryogenesis of Arabidopsis (Carlotto et al., 2016). A tobacco DEAD-box RH, VDL (for variegated and distorted leaf), is involved in early chloroplast maturation and regulates flower and root growth (Wang et al., 2000). Several mitochondria-localized RHs, PMHI (AtRH9), PMHII, and ABO6, regulate seed germination in Arabidopsis (Kim et al., 2008; Kohler et al., 2010; He et al., 2012). These results clearly show that chloroplast- or mitochondria-targeted DEAD-box RHs play important roles in plant growth and development. As nuclear DEAD-box RHs are involved in regulating programmed cell death in rice (Li et al., 2011) and two DEAD-box RHs in rice, OsRH2 and OsRH34, are necessary for pollen and seed development (Huang et al., 2016), further determination of the functions of chloroplast or mitochondrial DEAD-box RHs in plant development is necessary.
Although the functions of chloroplast- or mitochondria-targeted DEAD-box RHs in plant growth and development are known for only a few cases, potential roles of DEAD-box RHs in abiotic stresses are increasingly being discovered (Table 1 and Figure 2). Chloroplast-localized RH3 (Gu et al., 2014) and mitochondria-targeted PMHI (AtRH9; Matthes et al., 2007; Kim et al., 2008; Kohler et al., 2010) have been found to confer freezing tolerance in Arabidopsis. A chloroplast-localized HVD1 has been found to be up-regulated by cold and salt stress and affected photosynthetic activity under stress in barley (Nakamura et al., 2004). A chloroplast-localized OsABP is involved in the response of rice to diverse abiotic stresses (Macovei et al., 2012). Several studies have demonstrated that mitochondria-localized rice OsSUV3 and Arabidopsis AtOsSUV3 can regulate the expression of various stress-induced genes and confer plants tolerance to salt stress (Gagliardia et al., 1999; Tuteja et al., 2013; Sahoo et al., 2014). Recently, mitochondrial PLT1 (PLETHORA1) and ABO6 (ABA overly sensitive) have been found to play important roles in the regulation of primary root growth and root meristem activity by modulating ABA and auxin signaling (He et al., 2012; Yang et al., 2014). A chloroplast-targeted RH3 is reported to be essential for carbon fixation and the maintenance of ABA level in Arabidopsis under environmental stresses (Lee et al., 2013).
In addition to these already-verified chloroplast- or mitochondria-targeted DEAD-box RHs, our analysis using GENEVESTIGATOR7 and Bio-Analytic Resource for Plant Biology8 servers showed that the expression of potential chloroplast- or mitochondria-targeted DEAD-box RHs found in rice, maize, and wheat was high modulated by various abiotic stresses (Table 2), implying that more DEAD-box RHs in chloroplasts or mitochondria might be involved in abiotic stress responses. Interestingly, the expression of majority of chloroplast- or mitochondria-targeted DEAD box-RHs in rice, maize, and wheat is down-regulated under diverse abiotic stresses (Table 2). Although the physiological significance of such stress-responsive expression patterns of DEAD-box RHs remains unclear, these findings suggest that a large number of nucleus-encoded chloroplast- or mitochondria-targeted DEAD-box RHs might be involved in plant responses to diverse abiotic stresses. Considering that photosynthesis in chloroplasts functions as a global sensor of abiotic stresses (Biswal et al., 2011) and that expression of genes in chloroplasts is mainly regulated at posttranscriptional RNA metabolism (del Campo, 2009; Stern et al., 2010), it is interesting to determine how DEAD-box RHs affect the processing, splicing, and decay of chloroplast transcripts involved in photosynthesis, which will provide further insights into the importance of DEAD-box RHs in plant growth and survival under stress conditions.
Although recent progress on the analysis of plant genomes and proteomes has revealed the presence of a large number of DEAD-box RHs targeted to chloroplasts or mitochondria, cellular roles of DEAD-box RHs in organellar RNA metabolism and function remain unclear. It is evident that DEAD-box RHs targeted to either chloroplasts or mitochondria is crucial for the regulation of gene expression and RNA metabolism in these cellular organelles. However, more analyses are needed to determine the functions of each DEAD-box RH family member. Considering that communications between the nucleus and chloroplasts or mitochondria via anterograde and retrograde signaling are essential for organellar gene expression, biogenesis, and function (Zsigmond et al., 2008; del Campo, 2009; Sun and Guo, 2016), it would be of great interest to determine whether chloroplast- or mitochondria-targeted DEAD-box RHs can affect the expression of nuclear genes under stress conditions. In addition, it is necessary to determine the mechanistic roles of DEAD-box RHs in organellar RNA metabolism. Given that several RHs function as RNA chaperones to aid RNA folding via structural rearrangement of substrate RNAs (Kang et al., 2013; Lee and Kang, 2016), it is likely that these chloroplast- or mitochondria-targeted DEAD-box RHs might also function as RNA chaperones in organellar RNA metabolism. Major future tasks should be focused on identifying RNA targets and understanding how DEAD-box RHs recognize substrate RNAs to regulate posttranscriptional RNA metabolism in cellular organelles. Such studies will provide further insights into the importance of DEAD-box RHs targeted to chloroplasts and mitochondria in plant growth and survival.
GN and HK contributed equally writing the review.
This work was supported by a grant from the Next-Generation BioGreen21 Program (PJ01105401), Rural Development Administration, Republic of Korea, and by a grant from the Mid-career Researcher Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1A2B4009172), Republic of Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ambrosone, A., Costa, A., Leone, A., and Grillo, S. (2012). Beyond transcription: RNA-binding proteins as emerging regulators of plant response to environmental constraints. Plant Sci. 182, 12–18. doi: 10.1016/j.plantsci.2011.02.004
Asakura, Y., Galarneau, E., Watkins, K. P., Barkan, A., and van Wijk, K. J. (2012). Chloroplast RH3 DEAD-box RNA helicases in Zea mays and Arabidopsis thaliana function in splicing of specific group II introns and affect chloroplast ribosome. Plant Physiol. 159, 961–974. doi: 10.1104/pp.112.197525
Ashraf, M., and Harris, P. J. C. (2013). Photosynthesis under stressful environments: an overview. Photosynthetica 51, 163–190. doi: 10.1007/s11099-013-0021-6
Aubourg, S., Kreis, M., and Lecharny, A. (1999). The DEAD box RNA helicase family in Arabidopsis thaliana. Nucleic Acids Res. 27, 628–636. doi: 10.1093/nar/27.2.628
Biswal, B., Joshi, P. N., Raval, M. K., and Biswal, U. C. (2011). Photosynthesis, a global sensor of environmental stress in green plants: stress signaling and adaptation. Curr. Sci. 101, 47–56. doi: 10.1371/journal.pone.0032124
Boudet, N., Aubourg, S., Toffano-Nioche, C., Kreis, M., and Lecharny, A. (2001). Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 11, 2101–2114. doi: 10.1101/gr.200801
Carlotto, N., Wirth, S., Furman, N., Ferreyra-Solari, N., Ariel, F., Crespi, M., et al. (2016). The chloroplastic DEVH-box RNA helicase INCREASED SIZE EXCLUSION LIMIT 2 involved in plasmodesmata regulation is required for group II intron splicing. Plant Cell Environ. 39, 165–173. doi: 10.1111/pce.12603
Caruthers, J. M., and McKay, D. B. (2002). Helicase structure and mechanism. Curr. Opin. Struct. Biol. 12, 123–133. doi: 10.1016/s0959-440x(02)00298-1
Chi, W., He, B., Mao, J., Li, Q., Ma, J., Ji, D., et al. (2012). The function of RH22, a DEAD RNA helicase, in the biogenesis of the 50S ribosomal subunits of Arabidopsis chloroplasts. Plant Physiol. 158, 693–707. doi: 10.1104/pp.111.186775
Cook, K. B., Kazan, H., Zuberi, K., Morris, Q., and Hughes, T. R. (2011). RBPDB: a database of RNA-binding specificities. Nucleic Acids Res. 39, D301–D308. doi: 10.1093/nar/gkq1069
Cordin, O., Banroques, J., Tanner, N. K., and Linder, P. (2006). The DEAD-box protein family of RNA helicases. Gene 367, 17–37. doi: 10.1016/j.gene.2005.10.019
de la Cruz, J., Kressler, D., and Linder, P. (1999). Unwinding RNA in Saccharomyces cerevisiae: DEAD box proteins and related families. Trends Biochem. Sci. 24, 192–198. doi: 10.1016/s0968-0004(99)01376-6
del Campo, E. M. (2009). Post-transcriptional control of chloroplast gene expression. Gene Regul. Syst. Biol. 3, 31–47.
Fairman-Williams, M. E., Guenther, U. P., and Jankowsky, E. (2010). SF1 and SF2 helicases: family matters. Curr. Opin. Struct. Biol. 20, 313–324. doi: 10.1016/j.sbi.2010.03.011
Floris, M., Mahgoub, H., Lanet, E., Robaglia, C., and Menand, B. (2009). Posttranscriptional regulation of gene expression in plants during abiotic stress. Int. J. Mol. Sci. 10, 3168–3185. doi: 10.1101/gad.11.14.1864
Gagliardia, D., Kuhn, J., Spadinger, U., Brennicke, A., Leaver, C. J., and Binder, S. (1999). An RNA helicase (AtSUV3) is present in Arabidopsis thaliana mitochondria. FEBS Lett. 458, 337–342. doi: 10.1016/s0014-5793(99)01168-0
Gorbalenya, A. E., and Koonin, E. V. (1989). Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 17, 8413–8440. doi: 10.1093/nar/gki578
Gorbalenya, A. E., and Koonin, E. V. (1993). Helicases: amino acids sequence comparisons and structure–function relationship. Curr. Opin. Struct. Biol. 3, 419–429. doi: 10.1016/s0959-440x(05)80116-2
Gu, L., Xu, T., Lee, K., Lee, K. H., and Kang, H. (2014). A chloroplast-localized DEAD-box RNA helicase AtRH3 is essential for intron splicing and plays an important role in the growth and stress response in Arabidopsis thaliana. Plant Physiol. Biochem. 82, 309–318. doi: 10.1016/j.plaphy.2014.07.006
Hammani, K., and Giegé, P. (2014). RNA metabolism in plant mitochondria. Trends Plant Sci. 19, 380–389. doi: 10.1016/j.tplants.2013.12.008
He, J., Duan, Y., Hua, D., Fan, G., Wang, L., and Liu, Y. (2012). DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24, 1815–1833. doi: 10.1105/tpc.112.098707
Hotto, A. M., Germain, A., and Stern, D. B. (2012). Plastid non-coding RNAs: emerging candidates for gene regulation. Trends Plant Sci. 17, 737–744. doi: 10.1016/j.tplants.2012.08.002
Hsu, Y. F., Chen, Y. C., Hsiao, Y. C., Wang, B. J., Lin, S. Y., Cheng, W. H., et al. (2014). AtRH57, a DEAD-box RNA helicase, is involved in feedback inhibition of glucose mediated abscisic acid accumulation during seedling development and additively affects pre-ribosomal RNA processing with high glucose. Plant J. 77, 119–135. doi: 10.1111/tpj.12371
Huang, C. K., Huang, L. F., Huang, J. J., Wu, S. J., Yeh, C. H., and Lu, C. A. (2010). A DEAD- box protein, AtRH36, is essential for female gametophyte development and is involved in rRNA biogenesis in Arabidopsis. Plant Cell Physiol. 51, 694–706. doi: 10.1093/pcp/pcq045
Huang, C. K., Si, Y. S., Chen, Y. F., Huang, T. S., and Lu, C. A. (2016). Two highly similar DEAD-box proteins, OsRH2 and OsRH34, homologous to eukaryotic initiation factor 4AIII, play roles of the exon junction complex in regulating growth and development in rice. BMC Plant Biol. 16:84. doi: 10.1186/s12870-016-0769-5
Kanai, M., Hayashi, M., Kondo, M., and Nishimura, M. (2013). The plastidic DEAD-box RNA helicase 22, HS3, is essential for plastid functions both in seed development and in seedling growth. Plant Cell Physiol. 54, 1431–1440. doi: 10.1093/pcp/pct091
Kang, H., Park, S. J., and Kwak, K. J. (2013). Plant RNA chaperones in stress response. Trends Plant Sci. 18, 100–106. doi: 10.1016/j.tplants.2012.08.004
Kim, J. S., Kim, K. A., Oh, T. R., Park, C. M., and Kang, H. (2008). Functional characterization of DEAD-box RNA helicases in Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol. 49, 1563–1571. doi: 10.1093/pcp/pcn125
Kohler, D., Schmidt-Gattung, S., and Binder, S. (2010). The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol. Biol. 72, 459–467. doi: 10.1007/s11103-009-9584-9
Komatsu, S., and Hossain, Z. (2013). Organ-specific proteome analysis for identification of abiotic stress response mechanism in crop. Front. Plant Sci. 4:71. doi: 10.3389/fpls.2013.00071
Kwong, A. D., Rao, B. G., and Jeang, K. T. (2005). Viral and cellular RNA helicases as antiviral targets. Nat. Rev. Drug Discov. 4, 845–853. doi: 10.1038/nrd1853
Lee, K., and Kang, H. (2016). Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol. Cells 39, 179–185. doi: 10.14348/molcells.2016.2359
Lee, K. H., Park, J., Williams, D. S., Xiong, Y., Hwang, I., and Kang, B. H. (2013). Defective chloroplast development inhibits maintenance of normal levels of abscisic acid in a mutant of the Arabidopsis RH3 DEAD-box protein during early post-germination growth. Plant J. 73, 720–732. doi: 10.1111/tpj.12055
Li, X., Gao, X., Wei, Y., Deng, L., Ouyang, Y., Chen, G., et al. (2011). Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-Box adenosine 5’-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell 23, 1416–1434. doi: 10.1105/tpc.110.082636
Li, Y., Xiong, R., Bernards, M., and Wang, A. (2016). Recruitment of Arabidopsis RNA helicase AtRH9 to the viral replication complex by viral replicase to promote turnip mosaic virus replication. Sci. Rep. 6:30297. doi: 10.1038/srep30297
Linder, P., and Owttrim, G. W. (2009). Plant RNA helicases: linking aberrant and silencing RNA. Trends Plant Sci. 14, 344–352. doi: 10.1016/j.tplants.2009.03.007
Liu, Y., Tabata, D., and Ima, R. (2016). A cold-inducible DEAD-box RNA helicase from Arabidopsis thaliana regulates plant growth and development under low temperature. PLoS ONE 11:e0154040. doi: 10.1371/journal.pone.0154040
Lorković, Z. J. (2009). Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 14, 229–236. doi: 10.1016/j.tplants.2009.01.007
Lung, B., Zemann, A., Madej, M. J., Schuelke, M., Techritz, S., Ruf, S., et al. (2006). Identification of small non-coding RNAs from mitochondria and chloroplasts. Nucleic Acids Res. 34, 3842–3852. doi: 10.1093/nar/gkl448
Macovei, A., Vaid, N., Tula, S., and Tuteja, N. (2012). A new DEAD-box helicase ATP-binding protein (OsABP) from rice is responsive to abiotic stress. Plant Signal. Behav. 7, 1138–1143. doi: 10.4161/psb.21343
Matthes, A., Schmidt-Gattung, S., Kohler, D., Forner, J., Wildum, S., Raabe, M., et al. (2007). Two DEAD-box proteins may be part of RNA-dependent high-molecular-mass protein complexes in Arabidopsis mitochondria. Plant Physiol. 145, 1637–1646. doi: 10.1104/pp.107.108076
Meierhoff, K., Felder, S., Nakamura, T., Bechtold, N., and Schuster, G. (2003). HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 15, 1480–1495. doi: 10.1105/tpc.010397
Millar, A. H., Whelan, J., and Small, I. (2006). Recent surprises in protein targeting to mitochondria and plastids. Curr. Opin. Plant Biol. 9, 610–615. doi: 10.1016/j.pbi.2006.09.002
Mingam, A., Toffano, N. C., Brunaud, V., Boudet, N., Kreis, M., and Lecharny, A. (2004). DEAD-box RNA helicases in Arabidopsis thaliana, establishing a link between quantitative expression, gene structure and evolution of a family of genes. Plant Biotechnol. J. 2, 401–415. doi: 10.1111/j.1467-7652.2004.00084.x
Nakamura, T., Muramoto, Y., Yokota, S., Ueda, A., and Takabe, T. (2004). Structural and transcriptional characterization of a salt-responsive gene encoding putative ATP-dependent helicase in barley. Plant Sci. 167, 63–70. doi: 10.1016/j.plantsci.2004.03.001
Nishimura, K., Ashida, H., Ogawa, T., and Yokota, A. (2010). A DEAD box protein is required for formation of a hidden break in Arabidopsis chloroplast 23S rRNA. Plant J. 63, 766–777. doi: 10.1111/j.1365-313x.2010.04276.x
Nott, A., Jung, H. S., Koussevitzky, S., and Chory, J. (2006). Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 57, 739–759. doi: 10.1146/annurev.arplant.57.032905.105310
Nouri, M. Z., Ali, M., and Komatsu, S. (2015). Abiotic stresses: insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 16, 20392–20416. doi: 10.3390/ijms160920392
Park, W., Li, J., Song, R., Messing, J., and Chen, X. (2002). CARPEL FACTORY, a dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495. doi: 10.1016/S0960-9822(02)01017-5
Pause, A., and Sonenberg, N. (1992). Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 11, 2643–2654.
Pesaresi, P., Schneider, A., Kleine, T., and Leister, D. (2007). Interorganellar communication. Curr. Opin. Plant Biol. 10, 600–606. doi: 10.1016/j.pbi.2007.07.007
Ranty, B., Aldon, D., Cotelle, V., Galaud, J. P., Thuleauand, P., and Mazars, C. (2016). Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 7:327. doi: 10.3389/fpls.2016.00327
Rocak, S., and Linder, P. (2004). DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 5, 232–241. doi: 10.1038/nrm1335
Sahoo, R. K., Ansari, M. W., Tuteja, R., and Tuteja, N. (2014). OsSUV3 transgenic rice maintains higher endogenous levels of plant hormones that mitigates adverse effects of salinity and sustains crop productivity. Rice 7, 17. doi: 10.1186/s12284-014-0017-2
Silverman, E., Edwalds-Gilbert, G., and Lin, R. J. (2003). DExD/H-box proteins and their partners: helping RNA helicases unwind. Gene 312, 1–16. doi: 10.1016/s0378-1119(03)00626-7
Simpson, G. G., and Filipowicz, W. (1996). Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol. Biol. 32, 1–41. doi: 10.1007/BF00039375
Singleton, M. R., Dillingham, M. S., and Wigley, D. B. (2007). Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 76, 23–50. doi: 10.1146/annurev.biochem.76.052305.115300
Stern, D. B., Goldschmidt-Clermont, M., and Hanson, M. R. (2010). Chloroplast RNA metabolism. Annu. Rev. Plant Biol. 61, 125–155. doi: 10.1146/annurev-arplant-042809-112242
Sun, A. Z., and Guo, F. Q. (2016). Chloroplast retrograde regulation of heat stress responses in plants. Front. Plant Sci. 7:398. doi: 10.3389/fpls.2016.00398
Tanner, N. K. (2003). The newly identified Q motif of DEAD box helicases is involved in adenine recognition. Cell Cycle 2, 18–19. doi: 10.4161/cc.2.1.296
Tanner, N. K., Cordin, O., Banroques, J., Doère, M., and Linder, P. (2003). The Q motif. A newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol. Cells 11, 127–138. doi: 10.1016/s1097-2765(03)00006-6
Tanner, N. K., and Linder, P. (2001). DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol. Cell 8, 251–262. doi: 10.1016/s1097-2765(01)00329-x
Tuteja, N., Sahoo, R. K., Garg, B., and Tuteja, R. (2013). OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J. 76, 115–127. doi: 10.1111/tpj.12277
Tuteja, N., and Tuteja, R. (2004). Unraveling DNA helicases: motif, structure, mechanism and function. Eur. J. Biochem. 271, 1849–1863. doi: 10.1111/j.1432-1033.2004.04094.x
Umate, P., Tuteja, R., and Tuteja, N. (2010). Genome-wide analysis of helicase gene family from rice and Arabidopsis: a comparison with yeast and human. Plant Mol. Biol. 73, 449–465. doi: 10.1007/s11103-010-9632-5
Valgardsdottir, R., Brede, G., Eide, L. G., Frengen, E., and Prydz, H. (2001). Cloning and characterization of MDDX28, a putative DEAD-box helicase with mitochondrial and nuclear localization. J. Biol. Chem. 276, 32056–32063. doi: 10.1074/jbc.M011629200
Velankar, S. S., Soultanas, P., Dillingham, M. S., Subramanya, H. S., and Wigley, D. B. (1999). Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell 97, 75–84. doi: 10.1016/s0092-8674(00)80716-3
Wang, D., Qin, B., Li, X., Tang, D., Zhang, Y., Cheng, Z., et al. (2016). Nucleolar DEAD-box RNA helicase TOGR1 regulates thermos-tolerant growth as a pre-rRNA chaperone in rice. PLoS Genet. 12:e1005844. doi: 10.1371/journal.pgen.1005844
Wang, Y., Duby, G., Purnelle, B., and Boutry, M. (2000). Tobacco VDL gene encodes a plastid DEAD box RNA helicase and is involved in chloroplast differentiation and plant morphogenesis. Plant Cell 12, 2129–2142. doi: 10.1105/tpc.12.11.2129
Williams, P. M., and Barkan, A. (2003). A chloroplast-localized PPR protein required for plastid ribosome accumulation. Plant J. 3, 675–686. doi: 10.1046/j.1365-313x.2003.01915.x
Yang, L., Zhang, J., He, J., Qin, Y., Hua, D., Duan, Y., et al. (2014). ABA-Mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 10:e1004791. doi: 10.1371/journal.pgen.1004791
Zsigmond, L., Rigó, G., Szarka, A., Szekely, G., Otvos, K., Darula, Z., et al. (2008). Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 146, 1721–1737. doi: 10.1104/pp.107.111260
Keywords: chloroplast, DEAD-box RNA helicase, mitochondria, RNA metabolism, stress response
Citation: Nawaz G and Kang H (2017) Chloroplast- or Mitochondria-Targeted DEAD-Box RNA Helicases Play Essential Roles in Organellar RNA Metabolism and Abiotic Stress Responses. Front. Plant Sci. 8:871. doi: 10.3389/fpls.2017.00871
Received: 09 February 2017; Accepted: 10 May 2017;
Published: 24 May 2017.
Edited by:
Andy Pereira, University of Arkansas, United StatesReviewed by:
Li-Fen Huang, Yuan Ze University, TaiwanCopyright © 2017 Nawaz and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hunseung Kang, aHNrYW5nQGpudS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.