- 1Chinese–German Joint Laboratory for Natural Product Research, Qinling-Bashan Mountains Bioresources Comprehensive Development C.I.C., College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, China
- 2Randall Division of Cell and Molecular Biophysics, King’s College London, London, United Kingdom
- 3Department of Integrative Plant Biology, Institute of Plant Genetics, Polish Academy of Sciences, Poznan, Poland
The particles within the size range of 1 and 100 nm are known as nanoparticles (NPs). NP-containing wastes released from household, industrial and medical products are emerging as a new threat to the environment. Plants, being fixed to the two major environmental sinks where NPs accumulate — namely water and soil, cannot escape the impact of nanopollution. Recent studies have shown that plant growth, development and physiology are significantly affected by NPs. But, the effect of NPs on plant secondary metabolism is still obscure. The induction of reactive oxygen species (ROS) following interactions with NPs has been observed consistently across plant species. Taking into account the existing link between ROS and secondary signaling messengers that lead to transcriptional regulation of secondary metabolism, in this perspective we put forward the argument that ROS induced in plants upon their interaction with NPs will likely interfere with plant secondary metabolism. As plant secondary metabolites play vital roles in plant performance, communication, and adaptation, a comprehensive understanding of plant secondary metabolism in response to NPs is an utmost priority.
Introduction
The National Science Foundation (NSF) projects that the global market for products incorporating nanotechnology could amount to three trillion USD by 2020 (Roco, 2011). Currently, more than 1000 commercial products containing nanoparticles (NPs) are available in the market (Vance et al., 2015). The NPs commonly found in household, industrial and healthcare products are Au (Gold), Ag (silver), ZnO (zinc oxide), CuO (copper oxide), TiO2 (titanium dioxide), Fe3O4/Fe2O3 (iron oxides), and CeO2 (cerium oxide). Similarly, incorporation of Ag, ZnO, TiO2, and SiO2 (silicon dioxide) NPs into agrochemicals (pesticides, fungicides, herbicides, fertilizers, etc.) is expected to have great potential in nanotechnology-driven smart agriculture (DeRosa et al., 2010; Khot et al., 2012; Parisi et al., 2015; Boxi et al., 2016; Fraceto et al., 2016). The expanding applications of nanotechnology in domestic, industrial and agricultural sectors are also increasing the possibilities of NPs reaching the environment as nanomaterial-containing wastes. As the consequences of NP pollutants reaching the environment in significant quantities are unknown, understanding the plant’s response to NPs is an intensive area of research.
Most studies with NPs indicated a certain degree of phytotoxicity, especially at high concentrations (Miralles et al., 2012). Depending on their size, NPs can enter plant cells from the apoplast, crossing the plasma membrane via endocytosis; subsequently they can be translocated from one part to another through symplastic flow (Rico et al., 2011). There is also evidence for the transport of NPs into subcellular organelles such as the nucleus, plastids, and vacuoles (Chichiriccó and Poma, 2015; Da Costa and Sharma, 2016).
Arabidopsis thaliana (L.) Heynh seedlings grown on soil treated with ZnONPs were observed to have reduced growth, chlorophyll content and rates of photosynthesis (Wang et al., 2016). These effects were concentration dependent with growth compromised 20 and 80%, respectively, with 200 and 300 mg/L treatments. At 300 mg/L, the chlorophyll content, net rate of photosynthesis, leaf stomatal conductance, intercellular CO2 concentration and transpiration rate were all reduced more than 50%. Similarly, an increasing concentration (0, 2.5, 10, 50, 100, and 1,000 mg/L) of CuONPs negatively affected Oryza sativa L. seedling growth in a hydroponic system (Da Costa and Sharma, 2016). Photosynthetic rate, transpiration rate, stomatal conductance, maximal quantum yield of PSII photochemistry, and photosynthetic pigment contents declined, with a complete loss of PSII photochemical quenching at 1,000 mg/L. ZnONPs inhibited the expression of genes involved in chlorophyll synthesis and photosystem structure (Wang et al., 2016). Accumulation of CuONPs in the chloroplasts was accompanied by a lower number of thylakoids per granum (Da Costa and Sharma, 2016). AgNPs inhibited Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity and the photo-protective capacity of PSII in the model aquatic higher plant Spirodela polyrhiza (L.) Schleid (Jiang et al., 2017).
In addition to reduced photosynthetic rates the growth inhibition caused by NPs has also been associated with increased oxidative stress (Da Costa and Sharma, 2016; Li et al., 2016; Jiang et al., 2017). However, whether the arrest of photosynthesis or the induction of oxidative stress is the dominant impact of NPs is a subject of debate, since both of them go hand in hand (Aarti et al., 2006). Although the accumulation of NPs in chloroplasts and damage to the photosynthetic apparatus (Da Costa and Sharma, 2016; Jiang et al., 2017) supports the former, the fact that to reach the chloroplast NPs must cross the plasma membrane, where they can induce reactive oxygen species (ROS) via NADPH oxidases (Sosan et al., 2016) argues the reverse. ROS production, damage to the membrane structure and function, and fluctuation in antioxidant enzymatic activities are documented across plant species as common responses to NPs (Thwala et al., 2013; Vannini et al., 2013; Fu et al., 2014; Mirzajani et al., 2014; Hossain et al., 2015; Xia et al., 2015; Jiang et al., 2017; Tripathi et al., 2017). A few studies have also demonstrated that treatment of plants and photosynthetic microorganisms with NPs resulted in increased production of phenolics (Comotto et al., 2014; Ghorbanpour and Hadian, 2015; Večeřová et al., 2016), which might act as antioxidants to scavenge the ROS (Dixon and Paiva, 1995; Franklin et al., 2009).
The possibility of NP-induced disturbance in ROS homeostasis and associated signaling pathways as a major factor underlying the changes in plant secondary metabolism is explored in this perspective.
“Oxidative Stress”- A Common Response of Plant to NPs Treatment
Oxidative burst has been consistently reported in plants exposed to toxic levels of NPs (Thwala et al., 2013; Hossain et al., 2015; Xia et al., 2015). Exposure to various NPs, for example Ag, ZnO, and Al2O3 (aluminum oxide), also induced reactive nitrogen species (∗NO, nitric oxide) and H2O2 in duckweed (Thwala et al., 2013), corn (Zhao et al., 2012) and tobacco bright yellow (BY2) cells (Poborilova et al., 2013). In tobacco BY2 cells, Al2O3NPs also induced the production of superoxide anion (), one of the highly reactive forms of ROS. Although it is debated whether ROS activation stems, actually, from intact particles or, rather, from ions released from NPs, recent studies supports the latter. In S. polyrhiza, internalized Ag, regardless of whether the exposure was Ag+ ions or AgNPs, had the same capacity to generate ROS supporting the hypothesis that intracellular AgNPs dissociate into highly toxic Ag+ ions (Jiang et al., 2017). Similarly, dissolution of ZnO, CuO, and CeO2 (cerium oxide) into their respective ions (Zn2+, Cu2+, or Ce4+) has been established in other studies (Ebbs et al., 2016; Bradfield et al., 2017).
The mechanisms through which NPs induce ROS production and trigger oxidative stress at the cellular level have also been investigated. AgNPs triggered Ca2+ and ROS signaling through the induction of Ca2+-permeable pores and direct oxidation of apoplastic L-ascorbic acid (Sosan et al., 2016). A. thaliana root hair defective 2 (rhd2) mutant lacking NADPH oxidase RBOHC showed a significantly lower level of ROS generation in response to AgNPs compared with wild type plants (Sosan et al., 2016), indicating that the accumulation of ROS in cells is mediated by plasma membrane-bound NADPH oxidases (RBOH) enzymes that produce ROS at the apoplast (Mittler, 2017). On the other hand, chloroplastic ROS generation was observed in S. polyrhiza, based on the ability of AgNPs to inhibit Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity and the photo-protective capacity of PSII (Jiang et al., 2017).
A common consequence of harmful levels of ROS is the damage to cellular macromolecules including membrane lipids that leads to cell death (Van Breusegem and Dat, 2006). Growth inhibition coupled with lipid peroxidation has been reported in O. sativa seedlings treated with 0.5, 1.0, and 1.5 mM CuONPs (Shaw and Hossain, 2013) and in 5 mg/L TiO2NPs treated Nitzschia closterium (Xia et al., 2015). NPs could also damage other macromolecules like DNA. AgNPs and AuNPs affected cell division in Allium cepa L. root tip cells (Kumari et al., 2009; Rajeshwari et al., 2016), the former causing chromatin bridge, chromosomal stickiness, disturbed metaphase, multiple chromosomal breaks, and cell disintegration (Kumari et al., 2009). DNA damage, mitochondrial dysfunction, and cell apoptosis were also observed in eggplant, as a consequence of oxidative stress induced by Co3O4 (Faisal et al., 2016).
In order to mitigate the effects of oxidative stress plants activate both enzymatic and non-enzymatic antioxidant defense machinery to scavenge excess ROS (Sewelam et al., 2016). Correspondingly, NP-mediated stress also activates plant’s antioxidant machinery/enzymes. Briefly, superoxide dismutase (SOD) that catalyzes detoxification of into either ordinary molecular oxygen (O2) or H2O2 and ascorbate peroxidase (APX), which detoxifies peroxides such as H2O2 using ascorbic acid (Asc) as a substrate, were up-regulated in plants upon treatment with NPs (Fu et al., 2014). Whereas, dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDAR) enzymes that regulate the cellular Asc redox state were downregulated (Fu et al., 2014). Proteomic analysis of AgNPs treated O. sativa roots revealed an increased abundance of SOD, APX, and glutathione-S-transferase (GST) (Mirzajani et al., 2014). These NPs also stimulated the activities of SOD and APX significantly, while inhibiting glutathione reductase (GR) and DHAR in Pisum sativum L. seedlings (Tripathi et al., 2017). Catalase (CAT), another enzyme that protects the cells from oxidative damage, was significantly elevated upon treatment of wheat roots with 500 mg/kg CuONPs (Dimkpa et al., 2012). Maize plants germinated and grown on soil amended with 0, 400, and 800 mg/kg CeO2NPs showed a concentration dependent increase in the accumulation of H2O2 when tested after 10 days, but on day 20 did not show any difference (Zhao et al., 2012). A similar pattern in the increase of CAT and APX activities protected CeO2NP treated maize seedlings from lipid peroxidation (Zhao et al., 2012).
As disruption of ROS homeostasis impairs plant growth and development, whereas maintenance of ROS levels within appropriate parameters promotes plant health (Mittler, 2017), it is emerging that the induction of antioxidant machinery by NPs might promote plant growth as reported in a few studies (Sharma et al., 2012; Burman et al., 2013; Kumar et al., 2013) as long as a harmful level of ROS is not reached in the cells, whereas, once breached, this may lead to impaired organelle function, membrane damage, and eventually phytotoxicity.
“NP-Induced ROS”- Can It Be An Inductive Signal for Plant Secondary Metabolism?
So far, a handful of studies have showed that NPs could affect microbial and plant secondary metabolism. For example, the concentration of phenolic compounds secreted to an extracellular medium was increased 127.5 and 22.1%, respectively, in Arthrospira platensis Gomont (cyanobacterium) and Haematococcus pluvialis Flotow (microalga) after treating with 100 mg/L TiO2NPs (Comotto et al., 2014). Artemisinin content was increased 3.9-fold in Artemisia annua L. hairy root cultures after 900 mg/L AgNPs treatment for 20 days (Zhang et al., 2013). This increase was associated with oxidative stress (H2O2 production), lipid peroxidation and CAT activity. A substantial increase in plant growth and diosgenin concentration was observed in fenugreek after 2 μg/kg AgNP treatment (Jasim et al., 2017). Ferulic acid and isovitexin were increased in barley plants exposed to CdO (cadmium oxide) NPs in air for 3 weeks at a concentration of 2.03 ± 0.45 × 105 particles cm-3 (Večeřová et al., 2016). In A. thaliana, anthocyanin and flavonoid biosynthetic genes were upregulated in response to AgNPs (Garcia-Sanchez et al., 2015).
Although all the studies discussed above provide evidence for NP-mediated modulation of plant secondary metabolism, the following studies provide an indirect link between ROS and secondary metabolism. Satureja khuzestanica Jamzad calli growth improved significantly with increasing concentrations of carbon nanotubes (CNTs) in culture medium up to 50 mg/L, and then began to decrease at 500 mg/L (Ghorbanpour and Hadian, 2015). At this toxic concentration (500 mg/L), the highest level of H2O2 was observed together with significantly higher polyphenol oxidase (PPO), peroxidase (POD), and secondary metabolic activities. Similarly, when A. thaliana was exposed to 250 and 1000 mg/L CeO2 and indium oxide (In2O3) NPs, in addition to excessive ROS production, the activities of phenylalanine ammonia lyase (PAL) and PPO were greatly induced (Ma et al., 2016) revealing a possible role of secondary metabolism in protection against oxidative stress. Furthermore, PAL is the first enzyme of the general phenylpropanoid pathway that catalyses the deamination of phenylalanine to cinnamic acid and play a key role in diverting aromatic amino acids from primary metabolism to phenylpropanoid pathway.
There are several lines of evidence available in the literature implicating ROS-mediated signaling events as inductive cues for plant secondary metabolism. ROS themselves are signaling molecules, capable of inducing plant secondary metabolism (Simon et al., 2010). This could be observed during the wound-induced activation of secondary metabolism where ROS plays a key role as signaling molecule (Jacobo-Velazquez et al., 2015). In addition, ROS can also serve as signals for other messengers like jasmonic acid (JA) (Wu and Ge, 2004), salicylic acid (SA) (Maruta et al., 2012; Noshi et al., 2012; Wrzaczek et al., 2013; Baxter et al., 2014), ethylene (ET) (Zhang et al., 2016a,b), NO (Wang et al., 2013; Lindermayr and Durner, 2015), brassinosteroids (BRs) (Xia et al., 2009), etc., which are capable of modulating secondary metabolisms directly or indirectly.
To support the notion that ROS induced by NPs acts as signals for secondary metabolism, many indirect lines of evidence are available. ZnONP treatment induced SA, whereas it suppressed JA in A. thaliana (Vankova et al., 2017). Moreover, SA-mediated systemic acquired resistance (SAR) against microbial pathogens was compromised in A. thaliana after treatment with Ag, TiO2NPs, and CNTs, resulting in an increased colonization by Pseudomonas syringae pv. tomato, Pst (Garcia-Sanchez et al., 2015). These authors further suggested that SA pathway repression is a common feature of NP exposure, as an inducible kinase in the pathway that activates basal immune response upon perception of bacterial flagellin namely FLG22-induced receptor-like kinase 1 (FRK1) was downregulated in response to NPs (Garcia-Sanchez et al., 2015). In addition to SA-mediated SAR, other signaling pathways such as ET, BRs, and NO were also affected by NPs. In A. thaliana plants treated with AgNPs expression of ET biosynthetic components 1-aminocyclopropane-1-carboxylate synthase ACC and ACC oxidase 2 was reduced (Syu et al., 2014), suggesting that these NPs could inhibit ET perception and affect its biosynthesis. ET is an important signaling molecule mediating sesquiterpenoid biosynthesis in the Atractylodes lancea (Thunb.) endophytic fungi Gilmaniella sp. AL12 interaction (Yuan et al., 2016). BRs, the steroidal phytohormones that play important role in plant growth, secondary metabolite accumulation, stress responses and adaptation (Çoban and Göktürk Baydar, 2016) could ameliorate ZnONP-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedlings (Li et al., 2016). NO, another universal signaling molecule that plays a central role in secondary metabolite production in plant cells (Zhang et al., 2012; Zeng et al., 2014), is also involved in plant–NP interactions. For instance, AgNP-induced phytotoxicity could be alleviated by NO in P. sativum seedlings (Tripathi et al., 2017). Correspondingly, O. sativa NO excess mutant (noe1) plants were tolerant to ZnONP treatment, whereas OsNOA1-silenced (noa1) plants were susceptible to ZnONP-induced phytotoxicity (Chen et al., 2015).
Possible Mechanisms of Modulation of Plant Secondary Metabolism By NPs
Although the aforementioned reports suggest that NPs are interfering with various signaling pathways and capable of modulating plant secondary metabolism, the exact mechanism through which this modulation could occur is not understood. We believe that the initial responses of plants to NPs might include elevated levels of ROS, cytoplasmic Ca2+ and upregulation of mitogen-activated protein kinase (MAPK) cascades similar to other abiotic stresses (Figure 1) because of the following reasons. Recognition of AgNPs by plasma membrane bound receptors triggered a Ca2+ burst and ROS induction in A. thaliana (Sosan et al., 2016). Ca2+ levels and associated signaling pathway proteins were found to be upregulated in the proteomic analysis of AgNP treated O. sativa roots (Mirzajani et al., 2014). These authors hypothesized that AgNPs, or ions released thereof, impede cell metabolism by binding to Ca2+ receptors, Ca2+ channels, and Ca2+/Na+ ATPases. As sensed by calcium binding proteins (CaBPs) or other NP-specific proteins, NPs either mimic Ca2+ or signaling molecules in the cytosol (Khan et al., 2017). MAPK phosphorylation, and activation of downstream transcription factors generally lead to the transcriptional reprogramming of secondary metabolism in plants (Vasconsuelo and Boland, 2007; Schluttenhofer and Yuan, 2015; Phukan et al., 2016). Although no direct evidence for the involvement of MAPK pathways in plant-NP interactions is available, animal and human cell line studies revealed that analogous pathways are involved in AgNP-induced signaling (Eom and Choi, 2010; Lim et al., 2012), and it has been postulated that plants may also utilize MAPK cascade upon exposure to Ag NPs (Kohan-Baghkheirati and Geisler-Lee, 2015).
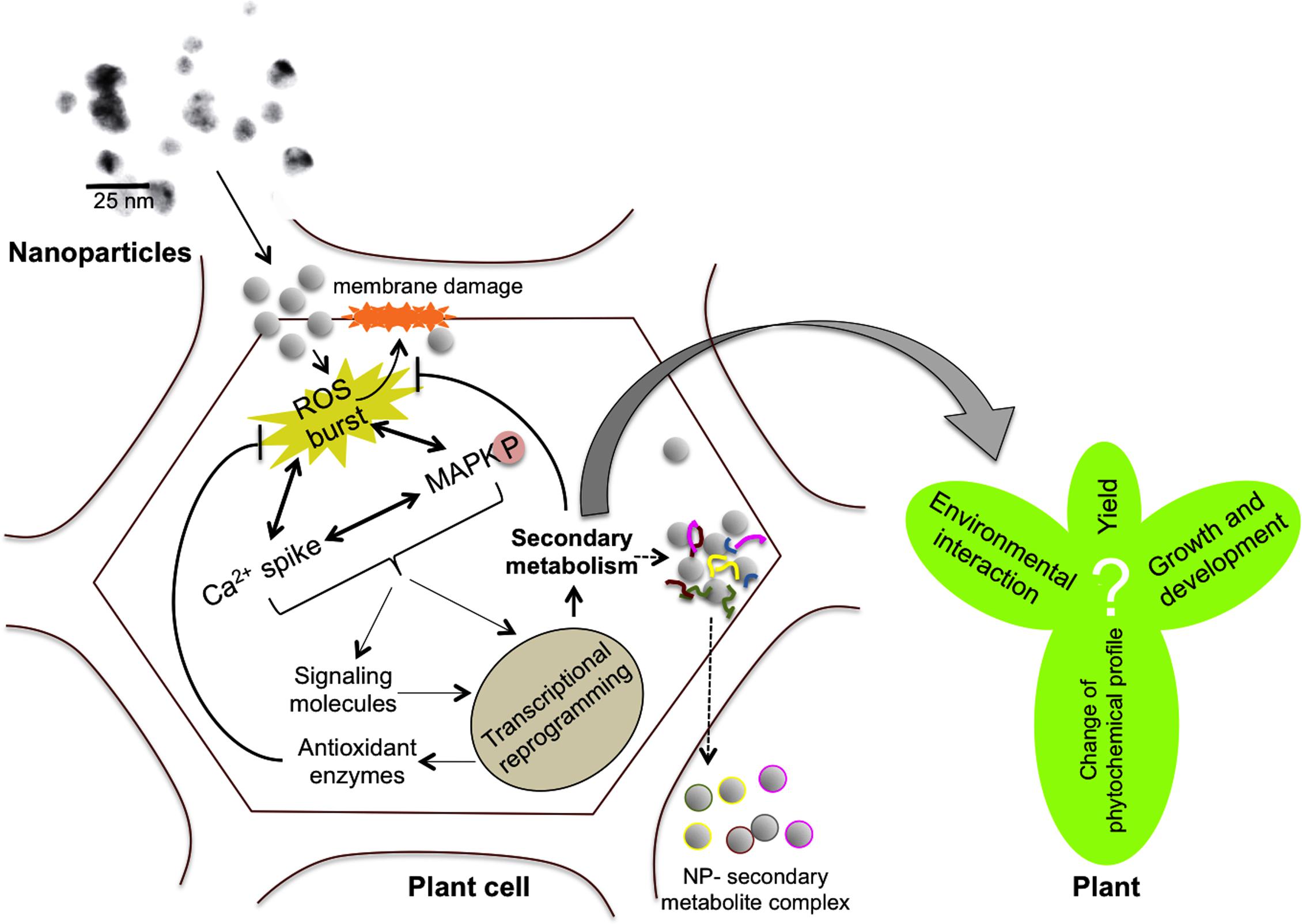
FIGURE 1. Scheme describing the possible mechanisms involved in nanoparticle (NP)-mediated modulation of plant secondary metabolism. NPs could enter plant cells through plasmodesmata causing physical damage to the plasma membrane. NPs can induce reactive oxygen species (ROS) production, calcium spikes, antioxidant machinery activation, mitogen-activated protein kinase (MAPK) cascades, etc., which could lead to transcriptional reprogramming of secondary metabolism. Activation of enzymatic antioxidant and non-enzymatic machineries including secondary metabolism might scavenge the ROS and protect the cells from oxidative damage. However, the exact consequences of changes in plant secondary metabolism on plant’s performance, environmental interaction, growth and yield are still unknown. On the other hand, the ability of NPs (e.g., anatase TiO2) to enter plant cells and exit as NP-secondary metabolite complexes could possibly be exploited for molecular pharming (dotted arrows).
Conclusion
As discussed in this article, exposure to NPs has the potential to alter plant secondary metabolism. Secondary metabolites can act as phytoalexins/phytoanticipins to protect plants from herbivores and pathogenic microbes, as signals for plant symbiotic interactions with beneficial microbes and as allelopathic agents to protect plants from rhizosphere competitors (Abdel-Lateif et al., 2012). In addition, they also serve as physical and chemical barriers to abiotic stressors and as antioxidants to scavenge ROS (Franklin et al., 2009; Ramakrishna and Ravishankar, 2011). Although NP-mediated changes in plant secondary metabolism would affect the optimal interaction of plants with their surrounding environment and possibly growth and productivity, substantial research is needed to understand the exact impact.
The presence of NPs in the environment might affect the pharmacological properties of medicinal plants, as many phytomedicines exert their beneficial effects through additive or synergistic actions of several compounds acting on single or multiple target sites associated with a physiological process (Briskin, 2000). While it is necessary to tackle these adverse effects, NP-mediated changes in secondary metabolism could also be beneficial if harnessed in such a way that NPs are used as elicitors in molecular pharming to enhance the production of desired secondary metabolites. For example, the content of important drugs like artemisinin (Zhang et al., 2013) and diosgenin (Jasim et al., 2017) were enhanced in plants treated with NPs. The ability of NPs to adsorb secondary metabolites (Kurepa et al., 2014) could be exploited for purification of precious compounds from plants via nanotrapping, if harnessed properly. Similarly, in vitro green synthesis of NPs using plant extracts can be further extended to develop high throughput tools to purify specific classes of compounds, as green synthesized NPs are often found as conjugates of secondary metabolites (Marslin et al., 2015).
Paucity of knowledge on the exact consequences of NP accumulation in the environment on plant metabolism is exacerbated by the fact that most of the studies have been conducted under controlled laboratory conditions and typically at much higher concentrations than what could be expected in the environment (Gottschalk et al., 2009; Baalousha et al., 2016). For instance, to induce statistically significant changes in the growth characteristics of A. thaliana plants, the minimum concentration of AgNPs was 300 mg/L under laboratory conditions (Sosan et al., 2016), a value much higher compared to the predicted environmental concentration of AgNPs in different environmental compartments: e.g., 1.3–4.4 mg/kg in sewage sludge (Gottschalk et al., 2009; Choi et al., 2017). Moreover, the ecologically relevant concentration of NPs largely depends on their environmental fate, plant species, characteristics of NPs, the medium through which it reaches the plant, etc. (Yin et al., 2012; Syu et al., 2014; Goswami et al., 2017), in addition to other, yet unknown, parameters. Although a recent study showed that ecologically relevant size and concentration of CdONPs could activate secondary metabolism in barley plants (Večeřová et al., 2016), it is difficult to generalize the impact of NPs on plant secondary metabolism in the environmental perspective. However, it is necessary to improve our understanding on the environmental fate of NPs and their hazards/risks, testing ecologically relevant conditions and concentrations in the context of plant secondary metabolism. Considering that plant secondary metabolism includes a vast array of compounds that are tightly controlled by signaling events and environmental cues, a case-by-case analysis might be necessary to have a deeper understanding.
Author Contributions
GM collected information on NPs and secondary metabolism. CS prepared the possible mechanisms of plant secondary metabolism induction by NPs. GF conceived the idea of this perspective and collected all other information on ROS, singling pathways, and phytotoxicity in response to NPs. All the authors participated in writing and approved the manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work has received funding from the National Science Center, Poland (2016/21/B/NZ9/01980). GM is supported by the Construction Project of Shaanxi Collaborative Innovation Center (Qinling-Bashan Mountains Bioresources Comprehensive Development and Collaborative Innovation) and university grant for the construction of Chinese–German Joint Laboratory for Natural Product Research. GF is supported by European Union’s 7th Framework Programme for research, technological development and demonstration under grant agreement n°621321 and co-financed by funds allocated for education through project no W26/7.PR/2015 [GA 3413/7.PR/2015/2] for the years 2015–2019. We thank Dr. William Truman for his critical reading of our manuscript.
References
Aarti, P., Tanaka, R., and Tanaka, A. (2006). Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 128, 186–197. doi: 10.1111/j.1399-3054.2006.00720.x
Abdel-Lateif, K., Bogusz, D., and Hocher, V. (2012). The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 7, 636–641. doi: 10.4161/psb.20039
Baalousha, M., Sikder, M., Prasad, A., Lead, J., Merrifield, R., and Chandler, G. T. (2016). EN15142 the concentration-dependent behaviour of nanoparticles. Environmental Chemistry 13. doi: 10.1071/EN15142
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
Boxi, S. S., Mukherjee, K., and Paria, S. (2016). Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 27:085103. doi: 10.1088/0957-4484/27/8/085103
Bradfield, S. J., Kumar, P., White, J. C., and Ebbs, S. D. (2017). Zinc, copper, or cerium accumulation from metal oxide nanoparticles or ions in sweet potato: yield effects and projected dietary intake from consumption. Plant Physiol. Biochem. 110, 128–137. doi: 10.1016/j.plaphy.2016.04.008
Briskin, D. P. (2000). Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 124, 507–514. doi: 10.1104/pp.124.2.507
Burman, U., Saini, M., and Kumar, P. (2013). Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 95, 605–612. doi: 10.1080/02772248.2013.803796
Chen, J., Liu, X., Wang, C., Yin, S.-S., Li, X.-L., Hu, W.-J., et al. (2015). Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J. Hazard. Mater. 297, 173–182. doi: 10.1016/j.jhazmat.2015.04.077
Chichiriccó, G., and Poma, A. (2015). Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 5, 851–873. doi: 10.3390/nano5020851
Choi, S., Johnston, M. V., Wang, G.-S., and Huang, C. P. (2017). Looking for engineered nanoparticles (ENPs) in wastewater treatment systems: qualification and quantification aspects. Sci. Total Environ. 59, 809–817. doi: 10.1016/j.scitotenv.2017.03.061
Çoban,Ö, and Göktürk Baydar, N. (2016). Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind. Crops Prod. 86, 251–258. doi: 10.1016/j.indcrop.2016.03.049
Comotto, M., Casazza, A. A., Aliakbarian, B., Caratto, V., Ferretti, M., and Perego, P. (2014). Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Sci. World J. 2014:9. doi: 10.1155/2014/961437
Da Costa, M. V. J., and Sharma, P. K. (2016). Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54, 110–119. doi: 10.1007/s11099-015-0167-5
DeRosa, M. C., Monreal, C., Schnitzer, M., Walsh, R., and Sultan, Y. (2010). Nanotechnology in fertilizers. Nat. Nanotechnol. 5:91. doi: 10.1038/nnano.2010.2
Dimkpa, C., Mclean, J., Latta, D., Manangón, E., Britt, D., Johnson, W., et al. (2012). CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 14, 1–15. doi: 10.1007/s11051-012-1125-9
Dixon, R. A., and Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 7, 1085–1097. doi: 10.1105/tpc.7.7.1085
Ebbs, S. D., Bradfield, S. J., Kumar, P., White, J. C., Musante, C., and Ma, X. (2016). Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ. Sci. Nano 3, 114–126. doi: 10.3389/fpls.2016.00188
Eom, H.-J., and Choi, J. (2010). p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ. Sci. Technol. 44, 8337–8342. doi: 10.1021/es1020668
Faisal, M., Saquib, Q., Alatar, A. A., Al-Khedhairy, A. A., Ahmed, M., Ansari, S. M., et al. (2016). Cobalt oxide nanoparticles aggravate DNA damage and cell death in eggplant via mitochondrial swelling and NO signaling pathway. Biol. Res. 49:20. doi: 10.1186/s40659-016-0080-9
Fraceto, L. F., Grillo, R., de Medeiros, G. A., Scognamiglio, V., Rea, G., and Bartolucci, C. (2016). Nanotechnology in agriculture: which innovation potential does it have? Front. Environ. Sci. 4:20. doi: 10.3389/fenvs.2016.00020
Franklin, G., Conceição, L. F. R., Kombrink, E., and Dias, A. C. P. (2009). Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 70, 60–68. doi: 10.1016/j.phytochem.2008.10.016
Fu, P. P., Xia, Q., Hwang, H. M., Ray, P. C., and Yu, H. (2014). Mechanisms of nanotoxicity: generation of reactive oxygen species. J. Food Drug Anal. 22, 64–75. doi: 10.1016/j.jfda.2014.01.005
Garcia-Sanchez, S., Bernales, I., and Cristobal, S. (2015). Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics 16:341. doi: 10.1186/s12864-015-1530-4
Ghorbanpour, M., and Hadian, J. (2015). Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 94, 749–759. doi: 10.1016/j.carbon.2015.07.056
Goswami, L., Kim, K.-H., Deep, A., Das, P., Bhattacharya, S. S., Kumar, S., et al. (2017). Engineered nano particles: nature, behavior, and effect on the environment. J. Environ. Manage. 196, 297–315. doi: 10.1016/j.jenvman.2017.01.011
Gottschalk, F., Sonderer, T., Scholz, R. W., and Nowack, B. (2009). Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 43, 9216–9222. doi: 10.1021/es9015553
Hossain, Z., Mustafa, G., and Komatsu, S. (2015). Plant responses to nanoparticle stress. Int. J. Mol. Sci. 16, 26644–26653. doi: 10.3390/ijms161125980
Jacobo-Velazquez, D. A., Gonzalez-Aguero, M., and Cisneros-Zevallos, L. (2015). Cross-talk between signaling pathways: the link between plant secondary metabolite production and wounding stress response. Sci. Rep. 5:8608. doi: 10.1038/srep08608
Jasim, B., Thomas, R., Mathew, J., and Radhakrishnan, E. K. (2017). Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 25, 443–447. doi: 10.1016/j.jsps.2016.09.012
Jiang, H. S., Yin, L. Y., Ren, N. N., Zhao, S. T., Li, Z., Zhi, Y., et al. (2017). Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 11, 157–167. doi: 10.1080/17435390.2017.1278802
Khan, M. N., Mobin, M., Abbas, Z. K., Almutairi, K. A., and Siddiqui, Z. H. (2017). Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 110, 194–209. doi: 10.1016/j.plaphy.2016.05.038
Khot, L. R., Sankaran, S., Maja, J. M., Ehsani, R., and Schuster, E. W. (2012). Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protect. 35, 64–70. doi: 10.1016/j.cropro.2012.01.007
Kohan-Baghkheirati, E., and Geisler-Lee, J. (2015). Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 5, 436–467. doi: 10.3390/nano5020436
Kumar, V., Guleria, P., Kumar, V., and Yadav, S. K. (2013). Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 46, 462–468. doi: 10.1016/j.scitotenv.2013.05.018
Kumari, M., Mukherjee, A., and Chandrasekaran, N. (2009). Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 407, 5243–5246. doi: 10.1016/j.scitotenv.2009.06.024
Kurepa, J., Nakabayashi, R., Paunesku, T., Suzuki, M., Saito, K., Woloschak, G. E., et al. (2014). Direct isolation of flavonoids from plants using ultra-small anatase TiO(2) nanoparticles. Plant J. 77, 443–453. doi: 10.1111/tpj.12361
Li, M., Ahammed, G. J., Li, C., Bao, X., Yu, J., Huang, C., et al. (2016). Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 7:615. doi: 10.3389/fpls.2016.00615
Lim, D., Roh, J. Y., Eom, H. J., Choi, J. Y., Hyun, J., and Choi, J. (2012). Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 31, 585–592. doi: 10.1002/etc.1706
Lindermayr, C., and Durner, J. (2015). Interplay of reactive oxygen species and nitric oxide: nitric oxide coordinates reactive oxygen species homeostasis. Plant Physiol. 167, 1209–1210. doi: 10.1104/pp.15.00293
Ma, C., Liu, H., Guo, H., Musante, C., Coskun, S. H., Nelson, B. C., et al. (2016). Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 3, 1369–1379. doi: 10.1039/C6EN00189K
Marslin, G., Selvakesavan, R. K., Franklin, G., Sarmento, B., and Dias, A. C. (2015). Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. Int. J. Nanomed. 10, 5955–5963. doi: 10.2147/IJN.S81271
Maruta, T., Noshi, M., Tanouchi, A., Tamoi, M., Yabuta, Y., Yoshimura, K., et al. (2012). H(2)O(2)-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 287, 11717–11729. doi: 10.1074/jbc.M111.292847
Miralles, P., Church, T. L., and Harris, A. T. (2012). Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 46, 9224–9239. doi: 10.1021/es202995d
Mirzajani, F., Askari, H., Hamzelou, S., Schober, Y., Rompp, A., Ghassempour, A., et al. (2014). Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol. Environ. Saf. 108, 335–339. doi: 10.1016/j.ecoenv.2014.07.013
Noshi, M., Maruta, T., and Shigeoka, S. (2012). Relationship between chloroplastic H(2)O(2) and the salicylic acid response. Plant Signal. Behav. 7, 944–946. doi: 10.4161/psb.20906
Parisi, C., Vigani, M., and Rodríguez-Cerezo, E. (2015). Agricultural nanotechnologies: what are the current possibilities? Nano Today 10, 124–127. doi: 10.1016/j.bios.2015.11.086
Phukan, U. J., Jeena, G. S., and Shukla, R. K. (2016). WRKY transcription factors: molecular regulation and stress responses in plants. Front. Plant Sci. 7:760. doi: 10.3389/fpls.2016.00760
Poborilova, Z., Opatrilova, R., and Babula, P. (2013). Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ. Exp. Bot. 91, 1–11. doi: 10.1016/j.envexpbot.2013.03.002
Rajeshwari, A., Suresh, S., Chandrasekaran, N., and Mukherjee, A. (2016). Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. RSC Adv. 6, 24000–24009. doi: 10.1039/C6RA04712B
Ramakrishna, A., and Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 6, 1720–1731. doi: 10.4161/psb.6.11.17613
Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2011). Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 59, 3485–3498. doi: 10.1021/jf104517j
Roco, M. C. (2011). The Long View of Nanotechnology Development: The National Nanotechnology Initiative at 10 years. Berlin: Springer.
Schluttenhofer, C., and Yuan, L. (2015). Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 167, 295–306. doi: 10.1104/pp.114.251769
Sewelam, N., Kazan, K., and Schenk, P. M. (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci. 7:187. doi: 10.3389/fpls.2016.00187
Sharma, P., Bhatt, D., Zaidi, M. G. H., Saradhi, P. P., Khanna, P. K., and Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167, 2225–2233. doi: 10.1007/s12010-012-9759-8
Shaw, A. K., and Hossain, Z. (2013). Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93, 906–915. doi: 10.1016/j.chemosphere.2013.05.044
Simon, C., Langlois-Meurinne, M., Bellvert, F., Garmier, M., Didierlaurent, L., Massoud, K., et al. (2010). The differential spatial distribution of secondary metabolites in Arabidopsis leaves reacting hypersensitively to Pseudomonas syringae pv. tomato is dependent on the oxidative burst. J. Exp. Bot. 61, 3355–3370. doi: 10.1093/jxb/erq157
Sosan, A., Svistunenko, D., Straltsova, D., Tsiurkina, K., Smolich, I., Lawson, T., et al. (2016). Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 85, 245–257. doi: 10.1111/tpj.13105
Syu, Y. Y., Hung, J. H., Chen, J. C., and Chuang, H. W. (2014). Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 83, 57–64. doi: 10.1016/j.plaphy.2014.07.010
Thwala, M., Musee, N., Sikhwivhilu, L., and Wepener, V. (2013). The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctata and the role of testing media parameters. Environ. Sci. Process. Impacts 15, 1830–1843. doi: 10.1039/c3em00235g
Tripathi, D. K., Singh, S., Singh, S., Srivastava, P. K., Singh, V. P., Singh, S., et al. (2017). Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 110, 167–177. doi: 10.1016/j.plaphy.2016.06.015
Van Breusegem, F., and Dat, J. F. (2006). Reactive oxygen species in plant cell death. Plant Physiol. 141, 384–390. doi: 10.1104/pp.106.078295
Vance, M. E., Kuiken, T., Vejerano, E. P., Mcginnis, S. P., Hochella, Jr., Rejeski, D., et al. (2015). Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 6, 1769–1780. doi: 10.3762/bjnano.6.181
Vankova, R., Landa, P., Podlipna, R., Dobrev, P. I., Prerostova, S., Langhansova, L., et al. (2017). ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 59, 535–542. doi: 10.1016/j.scitotenv.2017.03.160
Vannini, C., Domingo, G., Onelli, E., Prinsi, B., Marsoni, M., Espen, L., et al. (2013). Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 8:e68752. doi: 10.1371/journal.pone.0068752
Vasconsuelo, A., and Boland, R. (2007). Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 172, 861–875. doi: 10.1016/j.plantsci.2007.01.006
Večeřová, K., Večeřa, Z., Dočekal, B., Oravec, M., Pompeiano, A., Tříska, J., et al. (2016). Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 218, 207–218. doi: 10.1016/j.envpol.2016.05.013
Wang, X., Yang, X., Chen, S., Li, Q., Wang, W., Hou, C., et al. (2016). Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 6:1243. doi: 10.3389/fpls.2015.01243
Wang, Y., Loake, G. J., and Chu, C. (2013). Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front. Plant Sci. 4:314. doi: 10.3389/fpls.2013.00314
Wrzaczek, M., Brosche, M., and Kangasjarvi, J. (2013). ROS signaling loops - production, perception, regulation. Curr. Opin. Plant Biol. 16, 575–582. doi: 10.1016/j.pbi.2013.07.002
Wu, J., and Ge, X. (2004). Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol. Bioeng. 85, 714–721. doi: 10.1002/bit.10911
Xia, B., Chen, B., Sun, X., Qu, K., Ma, F., and Du, M. (2015). Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: growth inhibition, oxidative stress and internalization. Sci. Total Environ. 508, 525–533. doi: 10.1016/j.scitotenv.2014.11.066
Xia, X.-J., Wang, Y.-J., Zhou, Y.-H., Tao, Y., Mao, W.-H., Shi, K., et al. (2009). reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 150, 801–814. doi: 10.1104/pp.109.138230
Yin, L., Colman, B. P., Mcgill, B. M., Wright, J. P., and Bernhardt, E. S. (2012). Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 7:e47674. doi: 10.1371/journal.pone.0047674
Yuan, J., Sun, K., Deng-Wang, M. Y., and Dai, C. (2016). The mechanism of ethylene signaling induced by endophytic fungus Gilmaniella sp. AL12 mediating sesquiterpenoids biosynthesis in Atractylodes lancea. Front. Plant Sci. 7:361. doi: 10.3389/fpls.2016.00361
Zeng, F., Sun, F., Li, L., Liu, K., and Zhan, Y. (2014). Genome-scale transcriptome analysis in response to nitric oxide in birch cells: implications of the triterpene biosynthetic pathway. PLoS ONE 9:e116157. doi: 10.1371/journal.pone.0116157
Zhang, B., Zheng, L. P., and Wang, J. W. (2012). Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl. Microbiol. Biotechnol. 93, 455–466. doi: 10.1007/s00253-011-3658-8
Zhang, B., Zheng, L. P., Yi Li, W., and Wen Wang, J. (2013). Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 9, 363–370. doi: 10.2174/1573413711309030012
Zhang, H., Li, A., Zhang, Z., Huang, Z., Lu, P., Zhang, D., et al. (2016a). Ethylene response factor TERF1, regulated by ETHYLENE-INSENSITIVE3-like factors, functions in reactive oxygen species (ROS) scavenging in tobacco (Nicotiana tabacum L.). Sci. Rep. 6:29948. doi: 10.1038/srep29948
Zhang, M., Smith, J. A. C., Harberd, N. P., and Jiang, C. (2016b). The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 91, 651–659. doi: 10.1007/s11103-016-0488-1
Keywords: nanoparticles, nanopollution, reactive oxygen species, antioxidant enzymes, signaling pathways, plant secondary metabolism
Citation: Marslin G, Sheeba CJ and Franklin G (2017) Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 8:832. doi: 10.3389/fpls.2017.00832
Received: 29 January 2017; Accepted: 03 May 2017;
Published: 19 May 2017.
Edited by:
Raquel Esteban, University of the Basque Country, SpainReviewed by:
Oren Shelef, University of Nevada, Reno, United StatesBasil J. Nikolau, Iowa State University, United States
Copyright © 2017 Marslin, Sheeba and Franklin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory Franklin, ZmdyZUBpZ3IucG96bmFuLnBs
† These authors have contributed equally to this work.
 Gregory Marslin
Gregory Marslin Caroline J. Sheeba
Caroline J. Sheeba Gregory Franklin
Gregory Franklin