- 1Department of Agronomy, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Bangladesh
- 2Department of Agricultural Botany, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Bangladesh
- 3Laboratory of Plant Stress Responses, Department of Applied Biological Science, Faculty of Agriculture, Kagawa University, Miki-cho, Japan
- 4Stress Physiology and Molecular Biology Laboratory, Centre for Biotechnology, Maharshi Dayanand University, Rohtak, India
- 5Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 6Department of Subtropical Agro-Environmental Sciences, Faculty of Agriculture, University of the Ryukyus, Nishihara, Japan
Cadmium (Cd) is considered as one of the most toxic metals for plant growth and development. In the present study, we investigated the role of externally applied hydrogen peroxide (H2O2) in regulating the antioxidant defense and glyoxalase systems in conferring Cd-induced oxidative stress tolerance in rapeseed (Brassica napus L.). Seedlings were pretreated with 50 μM H2O2 for 24 h. These pretreated seedlings as well as non-pretreated seedlings were grown for another 48 h at two concentrations of CdCl2 (0.5 and 1.0 mM). Both the levels of Cd increased MDA and H2O2 levels and lipoxygenase activity while ascorbate (AsA) declined significantly. However, reduced glutathione (GSH) content showed an increase at 0.5 mM CdCl2, but glutathione disulfide (GSSG) increased at any level of Cd with a decrease in GSH/GSSG ratio. The activities of ascorbate peroxidase (APX) and glutathione S-transferase (GST) upregulated due to Cd treatment in dose-dependent manners, while glutathione reductase (GR) and glutathione peroxidase (GPX) increased only at 0.5 mM CdCl2 and decreased at higher dose. The activity of monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), catalase (CAT), glyoxalase I (Gly I), and glyoxalase II (Gly II) decreased under Cd stress. On the other hand, H2O2 pretreated seedlings, when exposed to Cd, AsA and GSH contents and GSH/GSSG ratio increased noticeably. H2O2 pretreatment increased the activities of APX, MDHAR, DHAR, GR, GST, GPX, and CAT of Cd affected seedlings. Thus enhancement of both the non-enzymatic and enzymatic antioxidants helped to decrease the oxidative damage as indicated by decreased levels of H2O2 and MDA. The seedlings which were pretreated with H2O2 also showed enhanced glyoxalase system. The activities of Gly I, and Gly II and the content of GSH increased significantly due to H2O2 pretreatment in Cd affected seedlings, compared to the Cd-stressed plants without H2O2 pretreatment which were vital for methylglyoxal detoxification. So, the major roles of H2O2 were improvement of antioxidant defense system and glyoxalase system which protected plants from the damage effects of ROS and MG. The mechanism of H2O2 to induce antioxidant defense and glyoxalase system and improving physiology under stress condition is not known clearly which should be elucidated. The signaling roles of H2O2 and its interaction with other signaling molecules, phytohormones or other biomolecules and their roles in stress protection should be explored.
Introduction
Metal toxicity has been increasing considerably due to increase of toxic metal release as a result of urbanization and industrialization (Hasanuzzaman and Fujita, 2012). Cadmium (Cd) is considered as most toxic considering injurious effects on plant developmental processes and metabolism (Nouairi et al., 2009). Cd has nature to enter through roots readily and easily due to its high solubility in water. Cd content higher than 5–10 μg Cd g-1 leaf dry weight is considered toxic for plants, in general (White and Brown, 2010). Cd stress reduces growth and metabolism affecting plants’ basic physiological processes including water and nutrient translocation and assimilation, transpiration and photosynthesis (Hasanuzzaman and Fujita, 2012; Khan et al., 2015). At cellular level Cd provokes generation of ROS [may include superoxide anion (), hydroxyl radical (∙OH), alkoxyl (RO∙), peroxyl (ROO∙), hydrogen peroxide (H2O2), singlet oxygen (1O2), and so forth] which results in oxidative damages to lipids, proteins and fatty acid which disrupt biomembrane, ultrastructural cellular components, DNA, and causes programmed cell death (PCD; Gill and Tuteja, 2010; Nahar et al., 2015, 2016a). Plants’ antioxidant system scavenges ROS and keeps a state of balance under non-stress condition. Antioxidant machinery posses non-enzymatic antioxidants [AsA, GSH, flavonoids, phenolic compounds, carotenoids, alkaloids, Pro, non-protein amino acids, and α-tocopherols] and a bunch of antioxidant enzymes [CAT, APX, MDHAR, DHAR, GR, GPX, guaiacol peroxidase, and GST] which works in coordinated manner to scavenge ROS and to minimize oxidative stress (Apel and Hirt, 2004; Hasanuzzaman et al., 2012b). MG generation is an impulsive outcome of the glycolysis. Due to environmental stresses MG is overproduced many times higher than the normal growth condition to create toxic effects (Kaur et al., 2015). In glyoxalase system, utilizing GSH MG is transformed in to SLG by the activity of Gly I, while Gly II transforms SLG to D-lactic acid which is a MG detoxification process. At the end, GSH is regenerated. Tolerance against ROS and MG confers and improves abiotic stress adaptation and tolerance in different plants (Yadav et al., 2008; Hasanuzzaman et al., 2012a; Kaur et al., 2015).
Among the ROS, H2O2 has stability, being a versatile molecule shows signaling function (Quan et al., 2008; Saxena et al., 2016). It takes part of oxidative metabolism. It has been proved to involve in signaling cascades and metabolism which are vital for plants growth/developmental processes. Seed germination, initiation of root hair, strengthening of cell wall, cell wall loosening, xylem differentiation and stomatal movement were reported to link with H2O2 mediated signaling cascade (Dempsey and Klessig, 1995; Wojtyla et al., 2016). Interacting with other hormones and signaling molecules [abscisic acid (ABA) and ethylene], H2O2 regulates plant metabolism (Jubany-Mari et al., 2009; Chen et al., 2012). Recently it has been reported that nitric oxide (NO) and H2O2 regulate the salicylic acid (SA)- induced salvianolic acid B production (Guo et al., 2014). Thus, as a signaling molecule H2O2 regulates different metabolic pathways to develop stress tolerances (Mittler et al., 2004; Reczek and Chandel, 2015). H2O2-induced signal stimulates the expression and activation of stress tolerant genes (Prasad et al., 1994) which mediate stress acclimation and adaptation (Uchida et al., 2002). In different research findings, H2O2 mediated chilling (Prasad et al., 1994), salinity (Xu et al., 2008; Li et al., 2011), heat (Gao et al., 2010), osmotic stress (Liu et al., 2010), Cd (Hu et al., 2009), low light (Zhang et al., 2011), and multiple stress (Gong et al., 2001) tolerances were reported. Based on the results of previous studies we hypothesize that application of exogenous H2O2 might have a signaling function, influence antioxidant activities which can improve Cd stress tolerance. Very few research works demonstrated the beneficial roles of H2O2 on Cd or heavy metal stress (Chao et al., 2009; Hu et al., 2009). In the previous study, only few components of antioxidant defense system have been examined to show the effect of H2O2 under Cd stress (Chao et al., 2009; Hu et al., 2009). Moreover, effects of H2O2 on MG detoxification system under Cd stress were not reported. Many aspects of H2O2-induced Cd stress tolerance are yet to be elucidated. The present study provides a new insight into H2O2-induced coordinated effects on antioxidant defense and glyoxalase system to enhance the resistance to Cd toxicity in rapeseed seedlings. In this study, we will present several components of antioxidant defense and MG detoxification systems which were not mentioned in previous research findings.
Materials and Methods
Plant Material, Growth Condition, and Treatments
Healthy and uniform sized rapeseed (Brassica napus cv. BINA sharisha 3) seeds were dipped into 70% ethanol for 5 min, then washed with double distilled water (ddH2O). Seeds had been sown in Petri plates (9 cm) containing six layers of filter paper where filter papers were provided with 10 ml of ddH2O. The Petridishes containing seeds were kept in a dark germination chamber under controlled conditions, 72 h. Germinated seedlings were removed from the germinator and placed into growth chamber under control environment (providing with light 100 μmol photon m-2 s-1, temp 25 ± 2°C, RH 65–70%). Seedlings were supplied with 10,000-fold diluted Hyponex solution (Hyponex, Japan) as nutrient at regular interval. Eleven-day-old seedlings were pretreated with 50 μM H2O2 in their root for 24 h. Both H2O2-pretreated and non-pretreated seedlings were then exposed to Cd stress (0.5 and 1.0 mM CdCl2) for 48 h. Several trial experiments were conducted before selecting the present doses of treatments. Different doses of Cd were applied in combination with different doses of H2O2 and the present combination (0.5 and 1.0 mM CdCl2 with 50 μM H2O2; 48 h) showed the better result. We hypothesized that using two concentrations of Cd the trend how the H2O2 is affecting the Cd-stressed rapeseed seedlings could be understood better. The same experiment was repeated three times under the same treatment condition. There were 45 seedlings in each Petri dish. In total 6 × 3 = 18 dishes were used.
Measurement of Lipid Peroxidation
Lipid peroxidation had been determined by estimating MDA (a product of lipid peroxidation) using TBA (Heath and Packer, 1968; Hasanuzzaman et al., 2011).
Measurement of Hydrogen Peroxide Content
Hydrogen peroxide (H2O2) had been determined extracting leaves in potassium phosphate (K-P) buffer (pH 6.5; centrifuging at 11,500×g), then adding it to a mixture of TiCl4 in 20% H2SO4 (v/v). The supernatant was read spectrophotometrically at 410 nm (Yu et al., 2003).
Histochemical Detection of Hydrogen Peroxide and Superoxide
The H2O2 and were determined histochemically (Chen et al., 2010) in the leaves of rapeseed plants by staining leaves with 1% 3,3-diaminobenzidine (DAB; to get brown spots due to the reaction of DAB with H2O2) and 0.1% nitroblue tetrazolium chloride (NBT; to get deep blue spots appeared due to the reaction of NBT with ) solution, respectively. Then, leaves were blanched in boiling ethanol to visualize the spots.
Extraction and Measurement of Ascorbate and Glutathione
The leaves of rapeseed plant (0.5 g) had been homogenized in 5% meta-phosphoric acid containing 1 mM EDTA (centrifuged at 11,500 × g; 15 min at 4°C). Supernatant was collected for the assay of AsA and GSH pool. To determine total ascorbate, the oxidized fraction was reduced by adding 0.1 M dithiothreitol for 1 h at room temperature and then read at 265 nm using 1.0 unit AO. Oxidized ascorbate (DHA) content had been assayed by subtracting reduced AsA from total AsA (Hasanuzzaman et al., 2011; Nahar et al., 2016b). The glutathione pool had been determined according to previously described methods (Yu et al., 2003; Hasanuzzaman et al., 2011). Standard curves with known concentrations of GSH and GSSG had been used to calculate the unknown GSH and GSSG pool of plant sample. The content of reduced GSH had been calculated by subtracting GSSG from total GSH.
Protein Determination
Following the method of Bradford (1976) the protein content had been measured where we used BSA as a protein standard.
Enzyme Extraction and Assays
Leaves had been homogenized with 50 mM K-P buffer (pH 7.0) containing 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10% (w/v) glycerol in pre-chilled mortars. Homogenates were centrifuged at 11,500 × g. The supernatants were collected and used for the assay of enzyme activity.
Ascorbate peroxidase (EC: 1.11.1.11) activity: The reaction buffer solution contained 50 mM K-P buffer (pH 7.0), 0.5 mM AsA, 0.1 mM H2O2, 0.1 mM EDTA, and enzyme extract (final volume 700 μL). The reaction had been initiated adding H2O2. Absorbance had been monitored at 290 nm for 1 min and activity has been calculated using an extinction coefficient of 2.8 mM-1cm-1 (Nakano and Asada, 1981).
Monodehydroascorbate reductase (EC: 1.6.5.4) activity: The reaction mixture contained 50 mM Tris-HCl buffer (pH 7.5), 0.2 mM NADPH, 2.5 mM AsA, 0.5 unit of AO, and enzyme solution (final volume 700 μL). The reaction had been started by adding AO. Absorbance was taken at 340 nm; activity had been calculated from the change in absorbance for 1 min using an extinction coefficient of 6.2 mM-1cm-1 (Hossain et al., 1984).
Dehydroascorbate reductase (EC: 1.8.5.1) activity: The reaction buffer contained 50 mM K-P buffer (pH 7.0), 2.5 mM GSH, and 0.1 mM DHA. Activity had been calculated from the change in absorbance at 265 nm for 1 min using an extinction coefficient of 14 mM-1cm-1 (Nakano and Asada, 1981).
Glutathione reductase (EC: 1.6.4.2) activity: The reaction mixture contained 0.1 M K-P buffer (pH 7.0), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH, and enzyme solution (final volume 1 mL). The reaction had been started with GSSG; the decrease in absorbance at 340 nm was monitored for 1 min and activity had been calculated using an extinction coefficient of 6.2 mM-1cm-1 (Hasanuzzaman et al., 2011).
Glutathione S-transferase (EC: 2.5.1.18) activity: The reaction mixture had 100 mM Tris-HCl buffer (pH 6.5), 1.5 mM GSH, 1 mM CDNB, and enzyme solution (final volume 700 μL). The reaction had been started by CDNB; the raise of absorbance was monitored at 340 nm for 1 min. Activity had been calculated using an extinction coefficient of 9.6 mM-1cm-1 (Hossain et al., 2006).
Glutathione peroxidase (EC: 1.11.1.9) activity: The reaction mixture contained of 100 mM K-P buffer (pH 7.0), 1 mM EDTA, 1 mM NaN3, 0.12 mM NADPH, 2 mM GSH, 1 unit GR, 0.6 mM H2O2 (as a substrate), and 20 μL of sample solution. The oxidation of NADPH had been observed at 340 nm for 1 min and the activity was calculated using an extinction coefficient of 6.62 mM-1cm-1 (Hasanuzzaman et al., 2011).
Catalase (EC: 1.11.1.6) activity: Decrease of absorbance (by decomposition of H2O2) at 240 nm had been noticed for 1 min. The reaction had been started with enzyme extract; activity has been calculated using an extinction coefficient of 39.4 M-1cm-1 (Hasanuzzaman et al., 2011).
Glyoxalase I (EC: 4.4.1.5): The assay mixture contained 100 mM K-P buffer (pH 7.0), 15 mM magnesium sulfate, 1.7 mM GSH, and 3.5 mM MG (final volume 700 μL). Adding MG the reaction had been started; the increase in absorbance was recorded at 240 nm for 1 min. Activity had been calculated using an extinction coefficient of 3.37 mM-1cm-1 (Hasanuzzaman et al., 2011).
Glyoxalase II (EC: 3.1.2.6): Formation of GSH was monitored for 1 min at 412 nm. The reaction mixture contained 100 mM Tris-HCl buffer (pH 7.2), 0.2 mM DTNB, and 1 mM SLG (final volume of 1 mL). Reaction had been initiated by adding SLG; activity had been calculated using an extinction coefficient of 13.6 mM-1cm-1 (Principato et al., 1987).
Lipoxygenase (EC 1.13.11.12): LOX activity was estimated monitoring the increase of absorbance at 234 nm using linoleic acid as a substrate. Activity had been calculated using an extinction coefficient of 25 mM-1cm-1 and expressed as units (1 nmol of substrate oxidized per min) mg-1 protein (Doderer et al., 1992).
Statistical Analysis
All data were subjected to analysis of variance (ANOVA). Mean differences had been compared by Tukey’s HSD test using XLSTAT v. 2016.04.32525 software (Addinsoft, 2016). Differences at P ≤ 0.05 were considered significant.
Results
Production of ROS and Oxidative Stress
Cadmium stress imposition in the growing media caused oxidative damage in the seedlings. Membrane lipid peroxidation (increasing MDA levels) has been noticed in Cd-affected rapeseed seedlings (Figure 1A). Content of H2O2 increased by 37 and 60%, and activity of LOX increased by 62 and 145% under 0.5 and 1 mM CdCl2 stresses (Figures 1B,C), respectively, as compared with control plants. All these were responsible for peroxidation of membrane lipid. Exogenous H2O2 application reduced H2O2 content and LOX activity which are corroborating with the reduction of MDA contents by 23 and 25% in mild and severe Cd stresses when compared to stress treatments only (Figures 1A–C).
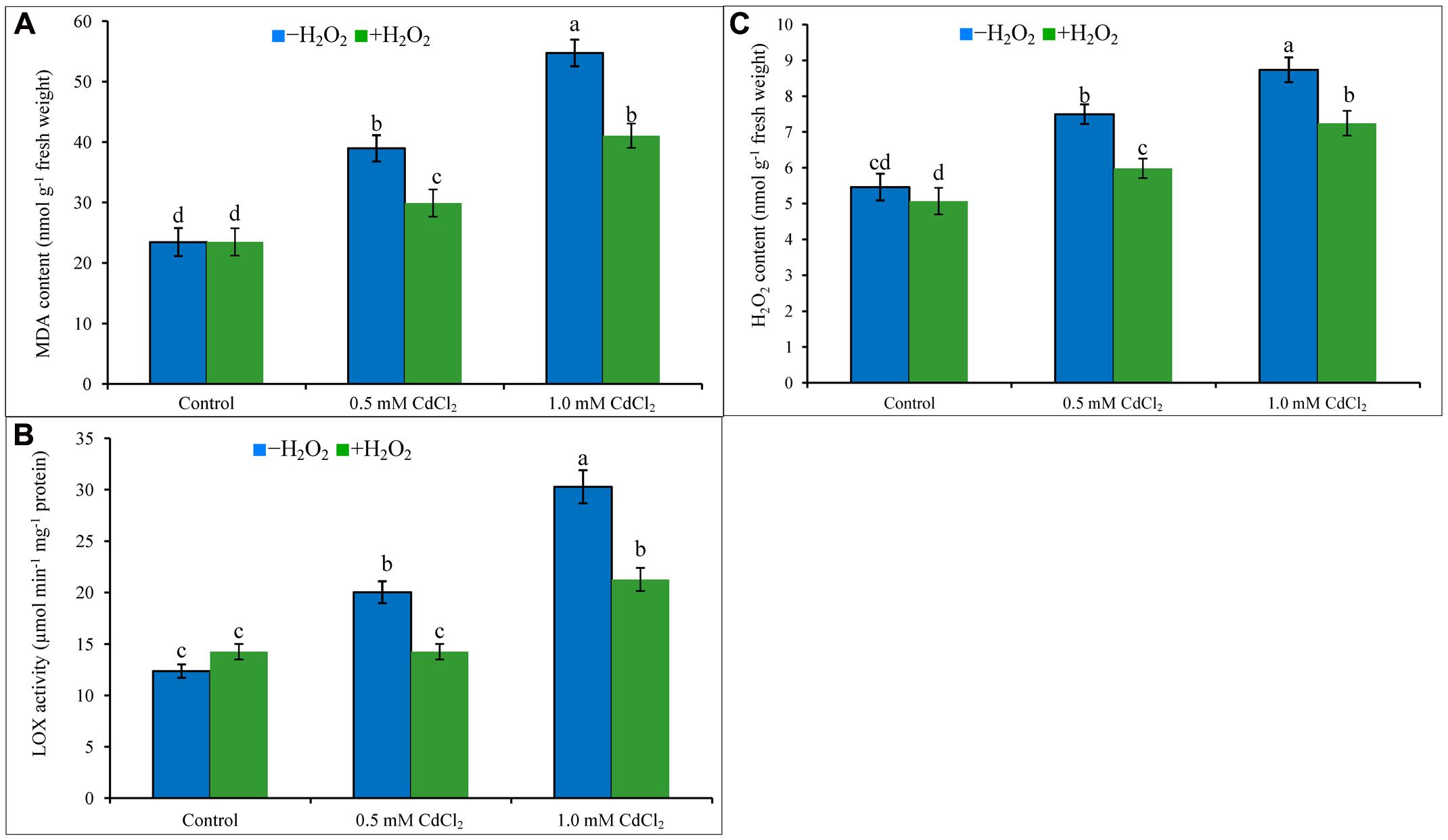
FIGURE 1. Malondialdehyde content (A), H2O2 content (B), and LOX activity (C) in rapeseed leaves induced by exogenous H2O2 under Cd stress. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 applying Tukey’s HSD test.
Histochemical Detection of ROS in Rapeseed Leaves
Leaves were dipped into DAB and NBT solution to visualize the generation and spots of H2O2 and , respectively. The leaves under Cd stress showed a high frequency of dark brown patches of H2O2 and deep blue spots of anions (Figures 2A,B). The spots were darker and larger in severe Cd stress, compared to the mild Cd stress. However, these spots of H2O2 and were somewhat reduced, compared to Cd stress alone when exogenous H2O2 was added with Cd stresses which are indicators for oxidative stress reduction (Figures 2A,B).
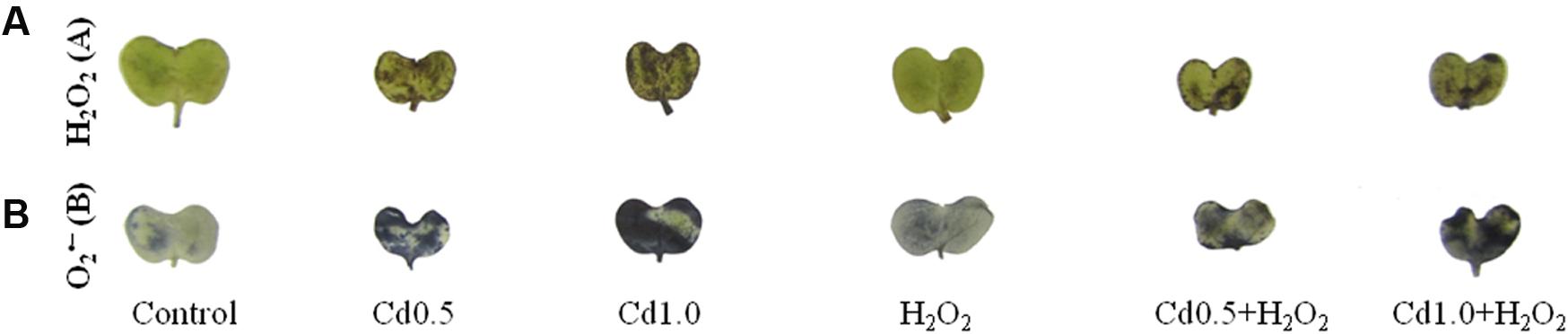
FIGURE 2. 3,3-Diaminobenzidine staining (A) of H2O2 and NBT staining (B) of superoxide in rapeseed leaves induced by exogenous H2O2 under Cd stress.
ASA-GSH Cycle
Ascorbate content decreased by 20 and 32%; in contrast, DHA content increased by 7 and 43% which resulted in 25 and 52% decrease of AsA/DHA ratio under mild and severe Cd stresses when compared to Cd untreated control. Increase of GSH pool and also with the high increase of GSSG resulted in decreased ratio of GSH/GSSG by 15 and 44%, respectively, under 0.5 and 1 mM CdCl2 stress, respectively, compared to control. Exogenous H2O2 addition inverted the AsA-GSH pool by increasing AsA content by 32 and 30% (Figure 3A), increasing GSH content by 38 and 25% (Figure 3D), decreasing DHA content by 12 and 21% (Figure 3B), and decreasing GSSG content by 17 and 8% (Figure 3E), under mild and severe Cd stresses, respectively. Alteration of AsA and GSH contents by H2O2 pretreatment were vital in improving AsA/DHA (Figure 3C) and GSH/GSSG (Figure 3F) ratios, compared to Cd stress alone.
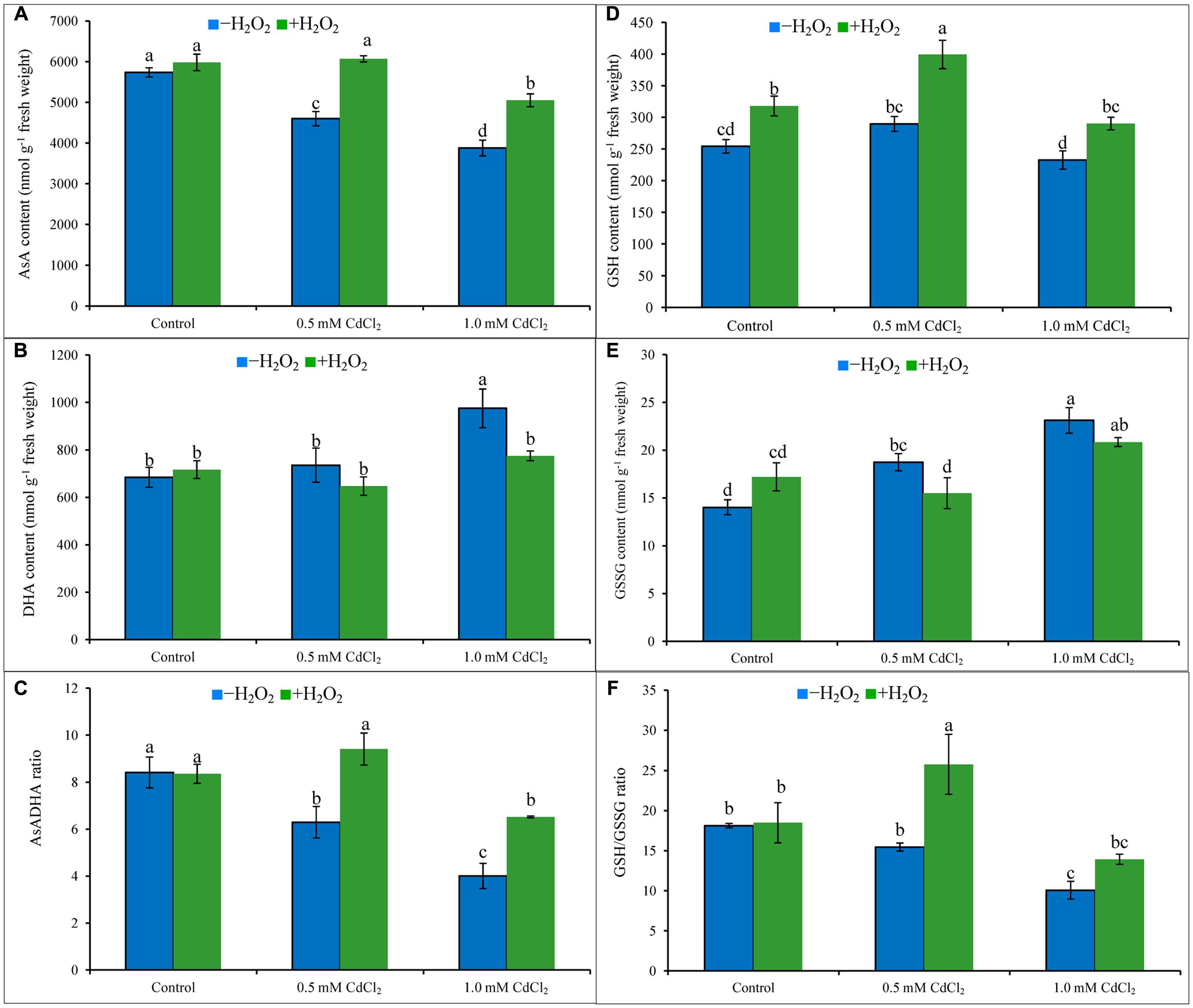
FIGURE 3. Ascorbate (AsA) content (A), DHA content (B), AsA/DHA ratio (C), GSH content (D), GSSG content (E), and GSH/GSSG ratio (F) in rapeseed leaves induced by exogenous H2O2 under Cd stress. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 applying Tukey’s HSD test.
The enzymes [APX, MDHAR, DHAR, and GR] (Figures 4A–D) of AsA-GSH cycle responded differentially in Cd-exposed seedlings. APX activity increased, MDHAR and DHAR activities reduced with the increase of Cd dose, compared to control, whereas, GR activity increased under mild Cd stress but reduced under severe Cd stress in comparison to their respective control (Figures 4A–D). External application of H2O2 under Cd stress increased activities of APX (40 and 39%), DHAR (77 and 67%), and GR (36 and 79%), respectively, under mild and severe Cd stresses, respectively, in contrast to Cd stress alone (Figures 4A–D).

FIGURE 4. Activities of AsA-GSH cycle enzymes, APX (A), MDHAR (B), DHAR (C), and GR (D) in rapeseed leaves induced by exogenous H2O2 under Cd stress. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 applying Tukey’s HSD test.
CAT, GPX, and GST Activities
Both levels of Cd stress-affected seedlings showed higher GST activity whereas GPX activity increased only under mild Cd stress level, but CAT activity decreased at both levels of Cd stresses when compared to control. Activity of GST upregulated by 115 and 145%, the activities of CAT reduced by 28 and 44% under mild and severe Cd stress, respectively; activity of GPX amplified by 23% under mild Cd stress but it decreased by 23% under severe Cd stress, compared to control (Figures 5A–C). Supplementation of H2O2 with Cd improved GST activities by 44 and 43%, and CAT activities by 79 and 47%, under mild and severe Cd stresses whereas augmented GPX activity by 40% under severe stress (Figures 5A–C), respectively, compared to Cd stress alone.
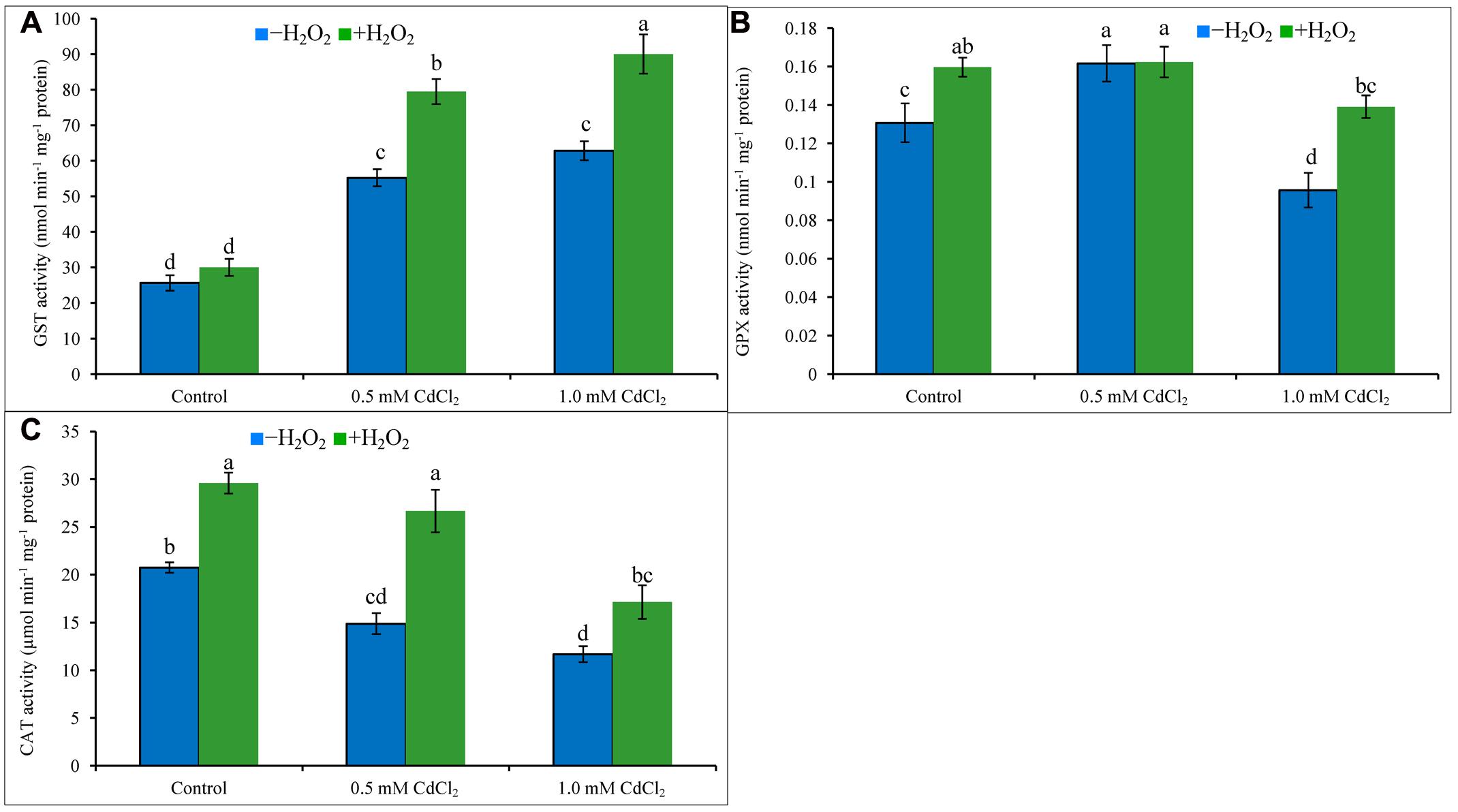
FIGURE 5. Activities of GPX (A), GST (B), and CAT (C) in rapeseed leaves induced by exogenous H2O2 under Cd stress. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 applying Tukey’s HSD test.
Glyoxalase System
Activities of Gly I and Gly II decreased due to exposure of Cd (Figures 6A,B). Their activities increased in both doses of Cd stress treatments supplemented with H2O2 except for Gly I activity at severe stress. The increase of Gly I activity under mild Cd stress was 35% after H2O2 supplementation, compared to Cd stress alone. Gly II activity increased by 47 and 55% in H2O2 added mild and severe Cd stresses, compared to Cd stress alone (Figures 6A,B).
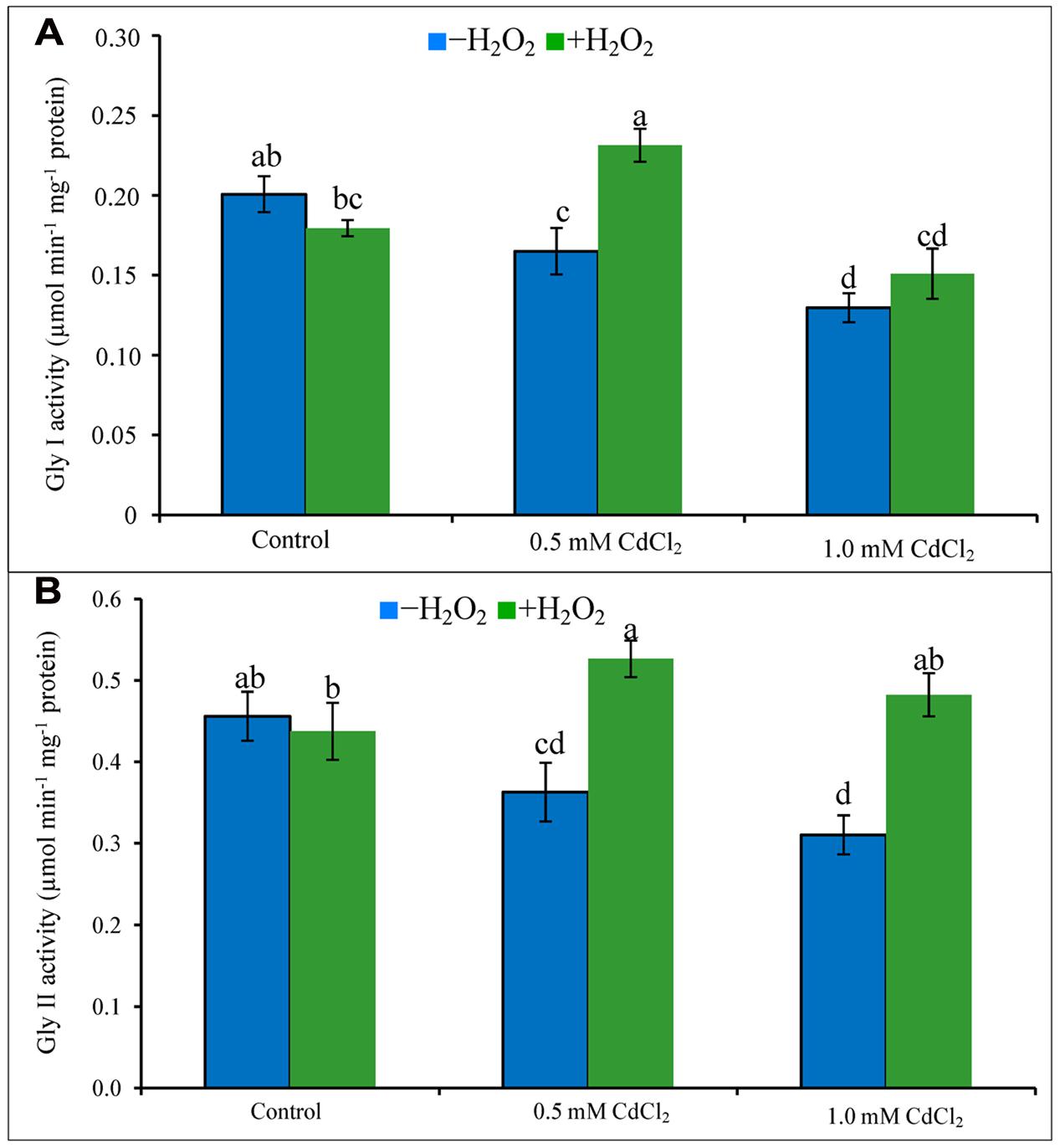
FIGURE 6. Activities of Gly I (A) and Gly II (B) in rapeseed leaves induced by exogenous H2O2 under Cd stress. Mean (±SD) was calculated from three replicates for each treatment. Bars with different letters are significantly different at P < 0.05 applying Tukey’s HSD test.
Discussion
Showing toxicity at higher concentration and acting as signaling molecule initiate, H2O2 plays a dual role and is considered as rival and comrade of stress tolerance development in plants. Due to dual roles of H2O2 and due to various unidentified roles of H2O2, recent research with H2O2 concentrate on diversified plausible mechanisms through which H2O2 is related to plant stress tolerance development. Present study has been executed to reveal the pivotal roles of H2O2 in relation to Cd stress tolerance in rapeseed.
The mechanism of Cd-induced oxidative stress is different from other stresses; Cd2+ cannot produce ROS directly as it through Fenton reaction or Haber Weiss reaction. Showing affinity to thiol Cd run downs GSH (Lopez et al., 2006). Cd enhances ROS production by weakening antioxidant defense mechanism (Srivastava et al., 2004; Gill and Tuteja, 2010), distressing photosystem II activity (Sigfridsson et al., 2004), disturbing functioning of vital enzymes (Dong et al., 2006). Cd displaces iron (Fe) from proteins and increases free Fe that is responsible for ROS generation. Cd also increases ROS production distorting mitochondrial function (Dorta et al., 2003). Spots of H2O2 and in leaves of rapeseed and the contents of H2O2 and MDA have been increased considerably in Cd affected rapeseed plants clearly indicating oxidative damage corroborating the results of previous studies (Dong et al., 2006; Hu et al., 2009; Hasanuzzaman et al., 2012a). H2O2 pretreatment reduced oxidative damage by decreasing the spots of and H2O2 and reducing the amount of H2O2 and MDA contents against Cd toxicity (Hu et al., 2009), reducing contents of MDA and in salinity affected wheat plants (Li et al., 2011), decreasing , H2O2 and MDA in chill affected cucumber seedlings (Zhang et al., 2011). The results of these previous reports indicate the decisive functions of H2O2 in reducing oxidative stress. At low concentration, H2O2 can as signaling molecule which modulates various genes related to stress defense mechanism. H2O2 implicated NO-mediated ABA-induced activation of mitogen-activated protein (MAP) kinase cascade which modulated antioxidant defense mechanism maize leaves. H2O2 can modulate NO and NO itself is an ROS scavenger (Zhang et al., 2007). In present study, the advantageous roles of H2O2 have been presented in later section where application of very low concentration exogenous H2O2 pretreatment induced and enhanced the antioxidant defense system components which in turn helped in decreasing the endogenous contents of ROS including H2O2 and and in decreasing the oxidative damage which is parallel with the results of the previous findings (Zhang et al., 2007, 2011). Both AsA and GSH presenting in chloroplast, cytoplasm, apoplast, mitochondria, peroxisome effectively scavenge H2O2. CAT, APX, GPX, and GST directly catalyze the reactions of H2O2 scavenging. Exogenous low dose of H2O2 in the present study enhanced the activities of these enzymes and increased the contents of AsA and GSH of Cd affected rapeseed seedlings which are directly related to H2O2 scavenging process and that is why H2O2 pretreatment decreased the endogenous H2O2 levels and subsequent oxidative damage of Cd affected seedlings (Mittler, 2002; Blokhina et al., 2003; Ashraf, 2009; Gill and Tuteja, 2010).
Ascorbate is water-soluble non-enzymatic antioxidant in cell decreasing oxidative stress scavenging and OH∙ (Gill and Tuteja, 2010). In rapeseed seedlings of present study, AsA level reduced and DHA level increased (because AsA is oxidized to DHA after scavenging ROS) due to reduced MDHAR and DHAR activities (which are AsA recycling enzymes) which decreased the AsA/DHA ratio and increased oxidative stress (Chao et al., 2010). APX activity upregulated due to Cd exposure, which is correlated to the reduced AsA content. H2O2 pretreatment followed by Cd exposure upregulated APX, MDHAR and DHAR activities in the seedlings which restored AsA and decreased oxidative stress which is supported by previous findings (Hu et al., 2009; Li et al., 2011; Zhang et al., 2011). In rapeseed seedlings, increased levels of GSH and GSSG but decreased GSH/GSSG ratio have been noticed in exposure to Cd which are supported by previous studies (Molina et al., 2008; Hu et al., 2009; Hasanuzzaman et al., 2012a). GSH is the thiol group of non-enzymatic antioxidant showing an imperative role in the stress signal, adaptation and defense mechanism of plants (Noctor et al., 2012). GSSG is recycled to GSH involving the GR activity (Gill and Tuteja, 2010). Increased GSH content after H2O2 application was found beneficial under Cd stress (Chao et al., 2009). The application of H2O2 upregulated AsA and GSH levels and improved CAT, POD, SOD, GPX, GR, MDHAR, and DHAR metabolism as reported in Al affected wheat seedlings (Xu et al., 2010). Seedlings pretreated with H2O2 increased GR activity (which recycles GSSG to GSH) which resulted in decreased GSSG level and increased GSH content which increased GSH/GSSG ratio in Cd affected rapeseed seedlings which is supported by previous findings (Hu et al., 2009; Li et al., 2011; Zhang et al., 2011).
The multifunctional isoenzymes GSTs are vital antioxidant enzymes, involved xenobiotic and toxic compound detoxification process (Polidoros and Scandalios, 1999). In the present investigation, GST activity and GSH amplified due to Cd exposure which were also observed in other studies (Hu et al., 2009; Hasanuzzaman et al., 2012a). Nonetheless, a further increase of GST activity and GSH content was noticed in H2O2 pretreated rapeseed seedlings under Cd stress which reduced adverse effects of Cd on physiology and growth which is similar to the findings of Hu et al. (2009) in rice. Increased Cd sequestration by H2O2 pretreatment in rice roots is an indication of crucial roles of H2O2 to further decline of Cd translocation to shoot (Hu et al., 2009). Cd stress reduced CAT and GPX activities in rapeseed seedlings that are correlated to a generation of high H2O2 which is comparable with previous findings (Hu et al., 2009; Hasanuzzaman et al., 2012a). The Cd has an affinity to proteins or –SH compounds and other side chains, Cd disturbs protein and enzymes synthesis which impair enzymatic activity (Sanitá di Toppi and Gabbrielli, 1999). CAT and GPX activities were restored and increased in H2O2 pretreated Cd-stressed seedlings which played vital roles in reducing the H2O2 level in Cd affected seedlings. Similar roles of H2O2 were observed in Cd affected rice seedlings (Hu et al., 2009), cucumber plants subjected to low light stress (Zhang et al., 2011) and in salt affected rice seedlings (Li et al., 2011).
Like other abiotic stresses, MG is overproduced within the plants under Cd stress (Hasanuzzaman et al., 2012a; Kaur et al., 2015; Nahar et al., 2015). Cd decreased both the activities of Gly I and Gly II that indicated the reduced MG detoxification via the glyoxalase system (Nahar et al., 2015). In this study, rapeseed seedlings exposed to Cd decreased Gly I and Gly II activities but pretreatment with H2O2 increased Gly I and Gly II activities and GSH content indicating the imperative roles of H2O2 in MG detoxification which are in the same line with the previous findings (Hasanuzzaman et al., 2012a; Nahar et al., 2016a,b).
Conclusion
In this study, we provided evidence for a specific pattern of ROS generation (H2O2 and ) and oxidative damage (MDA content) with the raise of Cd dose. Exogenous H2O2 treatment increased the amount of the most important ROS scavenging molecules AsA and GSH and increasing the antioxidant enzyme activities which enhanced ROS scavenging process. The Gly I and Gly II activities and content of GSH increased after H2O2 pretreatment indicating the roles of exogenous H2O2 in MG detoxification process. In contrast to the evidence of exogenous H2O2-induced ROS and MG detoxification in the present study, a number of unanswered questions still remain unclear. Why and how does H2O2 induce production of antioxidant molecules (AsA and GSH) and activities antioxidant enzymes? Previous reports support the notion that H2O2 induced signaling is involved with phytohormones and signaling molecules ABA, SA, JA (jasmonic acid), GA (gibberallic acid), ethylene, NO, Ca2+-mediated development of abiotic stress tolerances in plant (Mittler et al., 2004; Jubany-Mari et al., 2009; Chen et al., 2012; Guo et al., 2014; Reczek and Chandel, 2015; Saxena et al., 2016). In relation to the findings of present study, new questions arise: is there any signaling function of H2O2 in regulating the biosynthesis or degradation/metabolism of antioxidants components or, other hormones or signaling molecules affecting these processes? The possible mechanisms and signaling action of H2O2 in these aspects should be further elucidated.
Author Contributions
MH, MF, and KN conceived and designed the experiments; MH and KN performed the experiments; HA and BR analyzed the data; MF contributed reagents/materials/analysis tools; MH, KN, SG, HA, and BR wrote the manuscript. BR edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Dr. Anisur Rahman, Taufika Islam Anee, Mazhar Ul Alam and Farah Tasmin Bhuiyan of Laboratory of Plant Stress Responses, faculty of Agriculture, Kagawa University, Japan for the critical reading of the manuscript.
Abbreviations
AO, ascorbate oxidase; APX, ascorbate peroxidase; AsA, ascorbic acid (ascorbate); BSA, bovine serum albumin; CAT, catalase; CDNB, 1- chloro-2, 4-dinitrobenzene; Chl, chlorophyll; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; DTNB, 5,5′-dithio-bis (2-nitrobenzoic acid); EDTA, ethylenediaminetetraacetic acid; Gly I, glyoxalase I; Gly II, glyoxalase II; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; GST, glutathione S-transferase; HSD, honest significant difference; LOX, lipoxygenase; MDA, malondialdehyde; MDHA, monodehydroascorbate; MDHAR, MDHA reductase; MG, methylglyoxal; NADPH, nicotinamide adenine dinucleotide phosphate; NTB, 2-nitro-5-thiobenzoic acid; PEG, polyethylene glycol; Pro, proline; ROS, reactive oxygen species; RWC, relative water content; SLG, S-D-lactoylglutathione; TBA, thiobarbituric acid; TCA, trichloroacetic acid.
References
Addinsoft (2016). XLSTAT V. 2016.04.32525: Data Analysis and Statistics Software for Microsoft Excel. Paris: Addinsoft.
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Ashraf, M. (2009). Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 27, 84–93. doi: 10.1016/j.biotechadv.2008.09.003
Blokhina, O., Virolainen, E., and Fagerstedt, V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Chao, Y. Y., Hsu, Y. T., and Kao, C. H. (2009). Involvement of glutathione in heat shock– and hydrogen peroxide–induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 318, 37–45. doi: 10.1007/s11104-008-9815-x
Chao, Y. Y., Hong, C. Y., and Kao, C. H. (2010). The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol. Biochem. 48, 374–381. doi: 10.1016/j.plaphy.2010.01.009
Chen, F., Wang, F., Wu, F., Mao, W., Zhang, G., and Zhou, M. (2010). Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 48, 663–672. doi: 10.1016/j.plaphy.2010.05.001
Chen, H. J., Wu, S. D., Huang, G. J., Shen, C. Y., Afiyanti, M., Li, W. J., et al. (2012). Expression of a cloned sweet potato catalase SPCAT1 alleviates ethephon- mediated leaf senescence and H2O2 elevation. J. Plant Physiol. 169, 86–97. doi: 10.1016/j.jplph.2011.08.002
Dempsey, D. A., and Klessig, D. F. (1995). Signals in plant disease resistance. Bull. Inst. Pasteur 93, 167–186. doi: 10.1016/0020-2452(96)81488-6
Doderer, A., Kokkelink, I., van der Veen, S., Valk, B., Schram, A., and Douma, A. (1992). Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta 112, 97–104. doi: 10.1016/0167-4838(92)90429-H
Dong, J., Wu, F. B., and Zhang, G. P. (2006). Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64, 1659–1666. doi: 10.1016/j.chemosphere.2006.01.030
Dorta, D. J., Leite, S., DeMarco, K. C., Prado, I. M. R., Rodrigues, T., Mingatto, F. E., et al. (2003). A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem. 97, 251–257. doi: 10.1016/S0162-0134(03)00314-3
Gao, Y., Guo, Y. K., Lin, S. H., Fang, Y. Y., and Bai, J. G. (2010). Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Sci. Hortic. 126, 20–26. doi: 10.1016/j.scienta.2010.06.006
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gong, M., Chen, B., Li, Z. G., and Guo, L. H. (2001). Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J. Plant Physiol. 158, 1125–1130. doi: 10.1078/0176-1617-00327
Guo, H., Xiaolin, D., and Juane, D. (2014). Hydrogen peroxide and nitric oxide are involved ins alicylicacid-induced salvianolic acid B production in Salvia miltiorrhiza cell cultures. Molecules 19, 5913–5924. doi: 10.3390/molecules19055913
Hasanuzzaman, M., and Fujita, M. (2012). “Heavy metals in the environment: current status, toxic effects on plants and possible phytoremediation,” in Phytotechnologies: Remediation of Environmental Contaminants, eds N. A. Anjum, M. A. Pereira, I. Ahmad, A. C. Duarte, S. Umar, and N. A. Khan (Boca Raton: CRC Press), 7–73. doi: 10.1201/b12954-4
Hasanuzzaman, M., Hossain, M. A., and Fujita, M. (2011). Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 5, 353–365. doi: 10.1007/s11816-011-0189-9
Hasanuzzaman, M., Hossain, M. A., and Fujita, M. (2012a). Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Element Res. 149, 248–261. doi: 10.1007/s12011-012-9419-4
Hasanuzzaman, M., Hossain, M. A., Jaime, A., da Silva, T., and Fujita, M. (2012b). “Plant responses and tolerance to abiotic oxidative stress: antioxidant defense is a key factor,” in Crop Stress and its Management: Perspectives and Strategies, eds V. Bandi, A. K. Shanker, C. Shanker, and M. Mandapaka (Berlin: Springer), 261–316. doi: 10.1007/978-94-007-2220-0_8
Heath, R. L., and Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. doi: 10.1016/0003-9861(68)90654-1
Hossain, M. A., Nakano, Y., and Asada, K. (1984). Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 25, 385–395.
Hossain, M. Z., Hossain, M. D., and Fujita, M. (2006). Induction of pumpkin glutathione S-transferase by different stresses and its possible mechanisms. Biol. Plant. 50, 210–218. doi: 10.1007/s10535-006-0009-1
Hu, Y., Ge, Y., Zhang, C., Ju, T., and Cheng, W. (2009). Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul. 59, 51–61. doi: 10.1007/s10725-009-9387-7
Jubany-Mari, T., Munne-Bosch, S., Lopez-Carbonell, M., and Alegre, L. (2009). Hydrogen peroxide is involved in the acclimation of the mediterranean shrub, cistusalbidusl., to summer drought. J. Exp. Bot. 60, 107–120. doi: 10.1093/jxb/ern274
Kaur, C., Kushwaha, H. R., Mustafiz, A., Pareek, A., Sopory, S. K., and Singla-Pareek, S. L. (2015). Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front. Plant Sci. 6:682. doi: 10.3389/fpls.2015.00682
Khan, N. I. R., Nazir, F., Asgher, M., Per, T. S., and Khan, N. A. (2015). Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 173, 9–18. doi: 10.1016/j.jplph.2014.09.011
Li, J. T., Qiu, J. B., Zhang, X. W., and Wang, L. S. (2011). Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant. 33, 835–842. doi: 10.1007/s11738-010-0608-5
Liu, Z. J., Guo, Y. K., and Bai, J. G. (2010). Exogenous hydrogen peroxide changes antioxidant enzyme activities and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J. Plant Growth Regul. 29, 171–183. doi: 10.1007/s00344-009-9121-8
Lopez, E., Arce, C., Oset-Gasque, M. J., Cañadas, S., and González, M. P. (2006). Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 40, 940–951. doi: 10.1016/j.freeradbiomed.2005.10.062
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Mittler, R., Vanderauwera, S., Gollery, M., and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Molina, A. S., Nievas, C., Chaca, M. V. P., Garibotto, F., Gonza’lez, U., Marsa, S. M., et al. (2008). Cadmium induced oxidative damage and antioxidative defense mechanisms in Vigna mungo L. Plant Growth Regul. 56, 285–295. doi: 10.1007/s10725-008-9308-1
Nahar, K., Hasanuzzaman, M., Alam, M. M., Rahman, A., Suzuki, T., and Fujita, M. (2015). Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense, and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 126, 245–255. doi: 10.1016/j.ecoenv.2015.12.026
Nahar, K., Hasanuzzaman, M., Rahman, A., Alam, M. M., Mahmud, J.-A., Suzuki, T., et al. (2016b). Polyamines confer salt tolerance in mung bean by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense and methylgyoxal detoxification systems. Front. Plant Sci. 7:1104. doi: 10.3389/fpls.2016.01104
Nahar, K., Rohman, M. M., Hasanuzzaman, M., Alam, M. M., Rahman, A., Suzuki, T., et al. (2016a). Physiological and biochemical mechanism of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.). Environ. Sci. Pollut. Res. 23, 21206–21218. doi: 10.1007/s11356-016-7295-8
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880.
Noctor, G., Mhamdi, A., Chaouch, S., Han, Y., Neukermans, J., Marquez-Garcia, B., et al. (2012). Glutathione in plants: an integrated overview. Plant Cell Environ. 35, 454–484. doi: 10.1111/j.1365-3040.2011.02400.x
Nouairi, I., Ammar, W. B., Youssef, N. B., Miled, D. D. B., Ghorbal, M. H., and Zarrouk, M. (2009). Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant 31, 237–247. doi: 10.1007/s11738-008-0224-9
Polidoros, A. N., and Scandalios, J. G. (1999). Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.). Physiol. Plant. 106, 112–120. doi: 10.1034/j.1399-3054.1999.106116.x
Prasad, T. K., Anderson, M. D., Martin, B. A., and Stewart, C. R. (1994). Evidence for chilling induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6, 65–74. doi: 10.1105/tpc.6.1.65
Principato, G. B., Rosi, G., Talesa, V., Govannini, E., and Uolila, L. (1987). Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochem. Biophys. Acta 911, 349–355.
Quan, L. J., Zhang, B., Shi, W. W., and Li, H. Y. (2008). Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 50, 2–18. doi: 10.1111/j.1744-7909.2007.00599.x
Reczek, C. R., and Chandel, N. S. (2015). ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13. doi: 10.1016/j.ceb.2014.09.010
Sanitá di Toppi, L., and Gabbrielli, R. (1999). Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105–130. doi: 10.1016/S0098-8472(98)00058-6
Saxena, I., Srikanth, S., and Chen, Z. (2016). Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 7:570. doi: 10.3389/fpls.2016.00570
Sigfridsson, K. G. V., Bernát, G., Mamedov, F., and Styring, S. (2004). Molecular interference of Cd2+ with photosystem II. Biochim. Biophys. Acta 1659, 19–31. doi: 10.1016/j.bbabio.2004.07.003
Srivastava, S., Tripathi, R. D., and Dwivedi, U. N. (2004). Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa–an angiospermic parasite. J. Plant Physiol. 161, 665–674. doi: 10.1078/0176-1617-01274
Uchida, A., Jagendorf, A. T., Hibino, T., and Takabe, T. (2002). Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 163, 515–523. doi: 10.1016/S0168-9452(02)00159-0
White, P. J., and Brown, P. H. (2010). Plant nutrition for sustainable development and global health. Ann. Bot. 105, 1073–1080. doi: 10.1093/aob/mcq085
Wojtyla, L., Lechowska, K., Kubala, S., and Garnczarska, M. (2016). Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 7:731. doi: 10.3389/fpls.2016.00066
Xu, Q., Xu, X., Zhao, Y., Jiao, K., Herbert, S. J., and Hao, L. (2008). Salicylic acid, hydrogen peroxide and calcium-induced saline tolerance associated with endogenous hydrogen peroxide homeostasis innaked oat seedlings. Plant Growth Regul. 54, 249–259. doi: 10.1007/s10725-007-9247-2
Xu, F. J., Jin, C. W., Liu, W. J., Zhang, Y. S., and Lin, X. Y. (2010). Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J. Integr. Plant Biol. 54, 44–53. doi: 10.1111/j.1744-7909.2010.01008.x
Yadav, S. K., Singla-Pareek, S. L., and Sopory, S. K. (2008). An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metab. Drug Interact. 23, 51–68. doi: 10.1515/DMDI.2008.23.1-2.51
Yu, C. W., Murphy, T. M., and Lin, C. H. (2003). Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 30, 955–963. doi: 10.1071/FP03091
Zhang, X. L., Jia, X. F., Yu, B., Gao, Y., and Bai, J. G. (2011). Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci. Hortic. 129, 656–662. doi: 10.1016/j.scienta.2011.05.009
Zhang, A., Jiang, M., Zhang, J., Ding, H., Xu, S., Hu, X., et al. (2007). Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 175, 36–50. doi: 10.1111/j.1469-8137.2007.02071.x
Keywords: abiotic stress, antioxidant defense, cross tolerance, metal toxicity, methylglyoxal, oxidative stress, signaling molecule
Citation: Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN and Fujita M (2017) Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica napus L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems. Front. Plant Sci. 8:115. doi: 10.3389/fpls.2017.00115
Received: 09 September 2016; Accepted: 19 January 2017;
Published: 10 February 2017.
Edited by:
Vijay Pratap Singh, Government Ramanuj Pratap Singhdev Post Graduate College, IndiaReviewed by:
Weibiao Liao, Gansu Agricultural University, ChinaBarbara Hawrylak-Nowak, University of Life Sciences in Lublin, Poland
Copyright © 2017 Hasanuzzaman, Nahar, Gill, Alharby, Razafindrabe and Fujita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirza Hasanuzzaman, bWh6c2F1YWdAeWFob28uY29t
 Mirza Hasanuzzaman
Mirza Hasanuzzaman Kamrun Nahar
Kamrun Nahar Sarvajeet S. Gill
Sarvajeet S. Gill Hesham F. Alharby5
Hesham F. Alharby5 Masayuki Fujita
Masayuki Fujita