
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 07 November 2016
Sec. Plant Nutrition
Volume 7 - 2016 | https://doi.org/10.3389/fpls.2016.01664
 Yanliang Wang1,2
Yanliang Wang1,2 Tore Krogstad2*
Tore Krogstad2* Jihong L. Clarke1
Jihong L. Clarke1 Moritz Hallama3
Moritz Hallama3 Anne F. Øgaard4
Anne F. Øgaard4 Susanne Eich-Greatorex2
Susanne Eich-Greatorex2 Ellen Kandeler3
Ellen Kandeler3 Nicholas Clarke4*
Nicholas Clarke4*Many arable lands have accumulated large reserves of residual phosphorus (P) and a relatively large proportion of soil P is less available for uptake by plants. Root released organic anions are widely documented as a key physiological strategy to enhance P availability, while limited information has been generated on the contribution of rhizosphere organic anions to P utilization by crops grown in agricultural soils that are low in available P and high in extractable Ca, Al, and Fe. We studied the role of rhizosphere organic anions in P uptake from residual P in four common crops Triticum aestivum, Avena sativa, Solanum tuberosum, and Brassica napus in low- and high-P availability agricultural soils from long-term fertilization field trials in a mini-rhizotron experiment with four replications. Malate was generally the dominant organic anion. More rhizosphere citrate was detected in low P soils than in high P soil. B. napus showed 74–103% increase of malate in low P loam, compared with clay loam. A. sativa had the greatest rhizosphere citrate concentration in all soils (5.3–15.2 μmol g−1 root DW). A. sativa also showed the highest level of root colonization by arbuscular mycorrhizal fungi (AMF; 36 and 40%), the greatest root mass ratio (0.51 and 0.66) in the low-P clay loam and loam respectively, and the greatest total P uptake (5.92 mg P/mini-rhizotron) in the low-P loam. B. napus had 15–44% more rhizosphere acid phosphatase (APase) activity, ~0.1–0.4 units lower rhizosphere pH than other species, the greatest increase in rhizosphere water-soluble P in the low-P soils, and the greatest total P uptake in the low-P clay loam. Shoot P content was mainly explained by rhizosphere APase activity, water-soluble P and pH within low P soils across species. Within species, P uptake was mainly linked to rhizosphere water soluble P, APase, and pH in low P soils. The effects of rhizosphere organic anions varied among species and they appeared to play minor roles in improving P availability and uptake.
In order to produce enough food to feed the increasing global population, large amounts of mineral phosphorus (P) fertilizers have been used. Soluble P applied to agricultural soils as P fertilizers is readily sorbed to aluminum (Al) and iron (Fe) (hydr)oxides (in acid soils) or calcium (Ca) (in calcareous soils) exposed at the surfaces of soil constituents, leading to up to 80% of soil P being unavailable for uptake by most plants (Raghothama, 1999; Vance et al., 2003). For example, in Norwegian agricultural soils, although the average P surplus has been reduced by nearly 50% since 1985, the surplus is still about 15 kg P ha−1 year−1 (Eurostat, http://ec.europa.eu/eurostat/web/agri-environmental-indicators/farm-management-practices). Therefore, many arable lands have accumulated large reserves of residual P: In Norway, a relatively large proportion of cultivated soils is classified as high to very high in extractable P (Singh and Subramaniam, 1996). Only 3–25% of total P was extractable in ammonium lactate (PAL), which is an estimate of the plant-available P, whereas 34–68% of P was sorbed to Al, Fe, and Ca. In addition, organic P accounted for 17–51% of total P in different Norwegian soils (Singh et al., 2005). Improving the utilization of residual P accumulated in agricultural soils would help to reduce P fertilizer application and environmental stress. Hence, studies on how to mobilize the less-available residual P and improve P-acquisition efficiency are needed.
Higher plants have evolved root morphological and physiological strategies to improve P availability and enhance P uptake (Lambers et al., 2006; Faucon et al., 2015). Root morphological strategies include altered root mass distribution, root length, root surface area, and number and length of root hairs and lateral roots (Lambers et al., 2006, 2008; Lynch, 2011; Pedas et al., 2011). Root physiological traits that play active roles in enhancing P availability and uptake include the release of protons to change rhizosphere pH, which in turn changes soil P adsorption and desorption; the exudation of organic anions such as citrate and malate, which can mobilize both inorganic and organic P (Lambers et al., 2015); and the release of phosphatase enzymes, which hydrolyse soil organic P to release inorganic P (Tarafdar et al., 2001; George et al., 2004). Moreover, P uptake can also be improved by root-colonizing arbuscular mycorrhizal fungi (AMF), which increase the volume of the soil that can be explored by plant roots (Smith and Read, 2010). For some plant and AMF species, P uptake by mycorrhizal hyphae has been shown to be the dominant pathway for P acquisition when plant roots are colonized by AMF (Smith et al., 2003; Watts-Williams et al., 2015). Root released organic anions may play a key role in mobilizing less-available P (Ryan et al., 2001; Lambers et al., 2006), as shown by root released organic anions increasing under P deficiency (Hoffland et al., 1989; Gahoonia et al., 2000) and over-expression of organic anion synthase genes and organic anion transporters in several plants leading to enhanced P uptake ability (Lü et al., 2012; Wang et al., 2013). On the other hand, higher root organic anion exudation does not necessarily result in higher grain yield (Pandey et al., 2014). Moreover, root exudates do not relate consistently to P uptake from less available P sources (Pearse et al., 2007). Field experiments using near isogenic lines of wheat that differed in citrate efflux indicated that citrate efflux provided no consistent advantage for biomass production or yield (Ryan et al., 2014). The above reports suggest that further study is necessary to elucidate the role of root exudates in mobilizing plant less-available P.
In our previous study, we conducted a hydroponic experiment and found contrasting responses of root morphology and root-exuded organic acids to low P availability in three important food crops, Brassica napus, Hordeum vulgare, and Solanum tuberosum, which have divergent root traits (Wang et al., 2015). In the current study, we carried out an experiment using a mini-rhizotron culture system as described by James et al. (1985) and selected two dicot and two monocot widely cultivated crops (Triticum aestivum, Avena sativa, Solanum tuberosum and Brassica napus) to investigate the contribution of root-exuded organic anions to improving P uptake in agricultural soils low in P availability.
For these four crops grown in three soils obtained from long-term fertilization field plots in Norway, we addressed three hypotheses: (1) Low P availability will stimulate plant roots to release more organic anions and APase to rhizosphere soil; (2) the amounts of rhizosphere organic anions and APase will have positive correlations with rhizosphere plant-available P fractions and P uptake by plants in low P soils; (3) different crops will show differences in root released organic anions and APase in terms of using residual P from agricultural soils. The ultimate goal of this study is to increase understanding of the contribution of root-released organic anions and APases to P uptake in low P availability agricultural soils in common crops.
The soils for the rhizotron experiment were collected from the plow layer (0–20 cm) of a clay loam and a loam of two long-term fertilization trials in southeastern Norway (Kristoffersen and Riley, 2005). The clay loam (26% clay, 38% silt, 36% sand) was collected from field plots at Ås, Akershus county (59°39′ N, 10°45′ E), which had received either 48 (P high, soil AHP) or 0 (P low, soil ALP) kg P ha−1 year−1 as single superphosphate since 1966. Both P treatments received 100 kg N ha−1 year−1 as calcium nitrate and 100 kg K ha−1 year−1 as potassium chloride. The loam (soil B:14% clay, 34% silt, 52% sand) was collected from a field in Møystad, Hedmark county (60°47′ N, 11°10′ E), which had only received nitrogen (N) and potassium (K) fertilizers since 1922 (100 kg N ha−1 year−1 as calcium nitrate and 120 kg K ha−1 year−1 as potassium chloride).
The soils were air-dried at 30°C, passed through a 3.15 mm sieve, homogenized, and subsequently analyzed in order to make an initial characterization of the soils (Table 1). The pH was measured in water extract, solid: solution ratio of 1:2.5 (v/v), plant-available P (ammonium lactate extractable P, i.e., PAL) was determined according to Egnér et al. (1960) and easily releasable P was extracted in 0.0025 M CaCl2, solid: solution ratio of 1:20 (w/v), hereafter called water-soluble P, WSP). According to the guidelines used in Norway (Krogstad et al., 2008), PAL-values below 30 mg kg−1 are considered low, whilst those above 140 mg kg−1 are considered very high. This standard was used in the present study. The pH of soils AHP and ALP was adjusted from 5.4 and 5.0, respectively to ~6.5 by adding moderate amounts of CaCO3before use. No P fertilizers were applied to the soils but all other basic nutrients were provided as follows, in mg kg−1 soil: K, 70.2; N, 70; Mg, 9.6; S, 29.9; Zn, 0.65; Mo, 0.48; Cu, 0.32; Fe, 5.6; Mn, 1.1; B, 0.11; Na, 0.12. For N, K, Mg, and S, weighed K2SO4, NH4NO3, and MgCl2 powders were mixed thoroughly with the soils assuring a homogeneous distribution of nutrients in the soils. For micronutrients, chemicals were dissolved in water, and then applied to each pot to ensure that plant growth was not limited by these micronutrients.
Canola (B. napus cv. MARIE), wheat (T. aestivum cv. AINO), oat (A. sativa cv. BELINDA), and micropropagated seedlings of potato (S. tuberosum cv. PIMPERNEL) were grown in 20 cm × 20 cm × 1 cm mini-rhizotrons consisting of Plexiglas plates (James et al., 1985). The experiment was conducted in a greenhouse with 18°C/15°C day/night temperature with a 16 h photoperiod at a light intensity of 200 ± 20 μmol m−2 s−1 and 50–75% relative humidity. After filling each mini-rhizotron with ~0.5 kg fertilized homogenized soils, water was added to achieve a soil moisture level of 25% (w/w). Mini-rhizotrons were wrapped in black plastic bags to avoid light exposure. Five surface-sterilized seeds were sown in each mini-rhizotron and three uniform seedlings were kept in each mini-rhizotron after germination. For potato, three tissue culture-derived seedlings of around 10 cm height were used. There were four replicates and one mini-rhizotron without plant for each soil was set as control-unplanted bulk soil. Deionized water (15–40 mL) was given daily to keep the soil surface moist. Through the whole experimental period, the plants were irrigated according to weight loss, and the position of the mini-rhizotrons was changed randomly every week.
Three plants in each mini-rhizotron were harvested 5 weeks after germination and no potato root tuber was produced during the 5-week experiment. At harvest, the intact plants were carefully removed from the mini-rhizotrons and divided into shoots and roots. The roots were first shaken slightly to remove excess soil. The soil remaining attached to the roots was defined as rhizosphere soil (e.g., Veneklaas et al., 2003). For each mini-rhizotron, about 30 g rhizosphere fresh soil was carefully sampled using tweezers and spoons. The rhizosphere soil was divided into two groups, one air-dried for analysis of rhizosphere soil pH, PAL and water-soluble P, and another stored at −20°C for microbial P immobilization and soil enzyme activity measurements.
After subsamples of rhizosphere soil were obtained, the entire root system with the remaining rhizosphere soil was transferred into a container with a known volume of 0.2 mM CaCl2 solution to ensure cell integrity. Roots were then gently and carefully dunked for 60–90 s to get rhizosphere extract (Pearse et al., 2007; Pang et al., 2015). A subsample of the rhizosphere extract was taken and filtered through a Phenex regenerated cellulose syringe filter (pore size 0.45 μm, filter diameter 15 mm) (Phenomenex, Torrance, CA, USA). Micropur (0.01 g L−1, Katadyn Products, Kemptthal, Switzerland) was then added to the solution to inhibit the activity of microorganisms (Cheng et al., 2014). The collected rhizosphere extracts were immediately frozen and stored at −20°C until analysis with liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS, Waters, Milford, MA, USA and Micromass, Manchester, UK) was carried out. The root systems were then washed thoroughly to remove remaining soil and sub-sampled for AMF detection. The remaining extract containing the rhizosphere soil in the container was centrifuged at 4000 rpm and the supernatant transferred to a new container. The container with soil was then placed in a 65°C oven for 2 weeks. The rhizosphere soil inside the container was then weighed.
Before analysis with LC-MS/MS, 910 μL extract was taken out of each sample to a separate vial, 50 μL deuterium-labeled succinic acid (0.2 μg) were added to be used as an internal standard (IS), and each vial was acidified with 40 μL concentrated formic acid. The LC-MS/MS analysis was performed as described previously (Wang et al., 2015). The concentrations were determined by comparison with their standard concentration measurements and further calculated based on the root dry weight or rhizosphere soil dry weight.
After sampling of rhizosphere extract, fresh root subsamples were randomly taken and examined for AMF and other root inhabiting fungi (non-AMF). Roots were maintained in 10% (w/v) KOH for 3 days at ~25°C, and then stained in a 0.1% aniline blue solution for 1 h and distained/stored in lactoglycerol (Vierheilig et al., 1998). The line intersect method was used to assess the percentage of root length colonized by AMF and non-AMF (Giovanetti and Mosse, 1980). For each root sample, ten 1-cm pieces were randomly selected and five fields of vision were examined in each 1-cm root section at 100x microscopy; thus, 50 fields of vision were examined for each sample. The colonization percentage was calculated as the ratio of the colonized sections to the total sections examined. Identification of AMF was based on observations of arbuscules, and roots where only intraradical or extraradical hyphae or vesicles were observed were defined as non-AMF.
Shoots and roots were dried at 65°C for 48 h, and dry weight (DW) was measured. Root mass ratio was calculated as the ratio of root DW to the total plant DW. Shoot and root P concentrations were determined by inductively coupled plasma atomic emission spectroscopy (AtomComp 1100, Thermo Jarrell-Ash, MA, USA) according to Ogner et al. (1999) after digestion in a mixture of 65% (v/v) HNO3/72% (v/v) HClO4 (5: 1, v/v) at 220°C in a microwave oven. Shoot and root P contents were calculated by P concentrations × shoot or root DW.
Acid phosphomonoesterase (EC 3.1.3.2), β-glucosidase (EC 3.2.1.21), β-xylosidase (EC 3.2.1.37), and N-acetyl-β-glucosaminidase (EC 3.2.1.52) activities were measured by multi-substrate microplate-scale fluorometric assays based on the use of 4-methylumbelliferone (MUF) as described by Giacometti et al. (2014). A subsample of 1 g moist soil from the rhizosphere was placed into a 100 mL beaker with 50 mL de-ionized water and the soil was then dispersed with an ultrasonic bar for 2 min. Fifty microliter of soil suspension, 50 μL MES buffer, and 100 μL substrate (4-methylumbelliferyl-phosphate, 4-methylumbelliferyl-β-D-glucoside, 4-methylumbelliferyl-β-D-xylopyranoside, and 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide, all 1 mM) were pipetted into one well of a 96-well black polystyrene microplate. The microplates were covered and incubated in the dark at 30°C. Fluorescence intensity was measured using a microplate reader (Flx® 200, Biotek Instruments, Winooski, USA) with 360 nm excitation and 460 nm emission filters. Measurements were made immediately after the plate was set up and then every 30 min over a 3 h incubation period. Each sample had three analytical replicates and the concentrations were determined by comparison with their standard concentration measurements. Rates of fluorescence increase were converted to enzyme activity (nmol MUF gDM−1 h−1) according to German et al. (2011).
Concentration of P held in the microbial biomass (Pmic) was determined by the anion exchange membrane-based fumigation extraction method as described by Kouno et al. (1995) with slight modifications. Moist soil (equivalent to 2 g dry matter) samples were shaken (160 rpm) with two 1 cm × 2 cm resin membrane strips in (1) 30 mL distilled water, (2) 30 mL distilled water + 1 mL hexanol, and (3) 30 mL distilled water + 1 mL P solution with a known P concentration as a spike to correct for soil sorption of P released during fumigation-extraction. After shaking for 17 h at room temperature, P adsorbed on resin strips was extracted by transferring them into a clean tube, followed by adding 20 mL 0.5 M HCl and shaking for 1 h under the same conditions. Finally, P concentration in HCl solution was determined photometrically (Murphy and Riley, 1962).
R software (version 3.2.3) was used for data analyses. Two-way ANOVAs were used to study main effects of soil, plant species, and their interaction on all parameters involved in this study, followed by pairwise Tukey's honest significant difference tests for multiple comparisons, along with the minimum significant difference (MSD) at p < 0.05 (presented in figure captions). Simple linear regressions were used to estimate the relationships among response variables. Within each soil type, linear regressions were made across species. Within species, linear regressions were made across either similar soil properties (AHP and ALP) or similar P availability (ALP and B), or for all three soils.
No P was applied in our system. In order to confirm that P was the limiting factor for plant growth in low P soils, we calculated the N:P ratio in aboveground plant tissues (Table 2 and Figure 1). The N:P ratio varied from 6.9 to 10.5, 15.3 to 25.8, and 13.5 to 21.6 when soils AHP, ALP, and B were used as growth medium, respectively. According to van Duivenbooden et al. (1995), the N:P ratio of agricultural crops is in general between 6 and 8, and plants can be diagnosed as P limited when the N:P ratio is above 14. Hence, our soils ALP and B were P deficient and soil AHP was P sufficient.
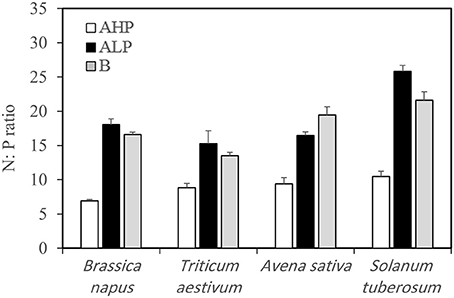
Figure 1. N:P ratios of the aboveground tissues of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for N:P ratio (p < 0.001, MSD 0.05 = 1.80).
Shoot and total dry biomass varied significantly among soils and species (Table 2 and Figures 2A,B). All crops showed significant decrease of both shoot and total biomass in soil ALP, compared with soil AHP; however, crops grown in soil B showed almost the same total biomass as those grown in soil AHP, except T. aestivum (Figure 2). Dicots had similar shoot biomass in soil AHP and soil B while monocots had lower shoot biomass in soil B, compared with soil AHP. B. napus accumulated the most shoot biomass in all soil types (average 2.81 g, 1.79 g, and 2.74 g in soils AHP, ALP, and B, respectively) while S. tuberosum accumulated the least shoot biomass (average 1.27 g, 0.71 g, and 1.31 g in soils AHP, ALP, and B, respectively); T. aestivum and A. sativa had similar amounts of shoot biomass in all soils.
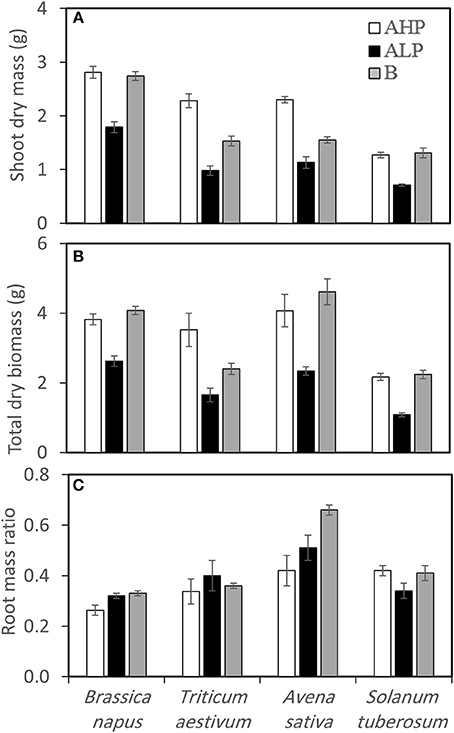
Figure 2. (A) Shoot dry weight, (B) total dry weight, and (C) root mass ratio (dry matter basis) of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for shoot dry weight (p < 0.001, MSD 0.05 = 0.17 g), total dry weight (p < 0.01, MSD 0.05 = 0.48 g) and root mass ratio (p < 0.01, MSD 0.05 = 0.07).
Different root mass ratio (root DW: total DW) patterns were observed in the four crops (Figure 2C). Compared with soil AHP, B. napus showed a significant increase of root mass ratio in soil ALP and soil B, by 23 and 27%, respectively while A. sativa showed 21 and 57% increase, respectively. T. aestivum did not show any significant differences in root mass ratios between soils and S. tuberosum showed a decrease of root mass ratio by 19% when plants were grown in soil ALP compared with AHP, but showed about the same value when plants were grown in soil B and soil AHP.
Shoot and root P concentrations varied significantly, and were unsurprisingly highest when plants were grown in soil AHP for all crops (Table 2 and Figures 3A,B). For all three soils, S. tuberosum had the highest shoot and root P concentrations, while B. napus had the lowest shoot and root P concentrations in soils ALP and B. Generally, the shoot P concentrations in soil B were equal (S. tuberosum and T. aestivum) or a little bit lower (B. napus and A. sativa) than those in soil ALP, whereas the root P concentrations in soil B tended to be higher than in soil ALP with the exception of S. tuberosum.
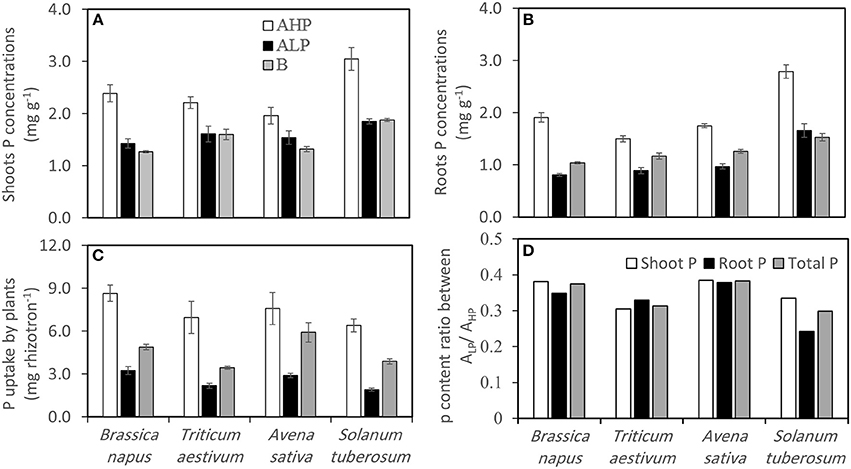
Figure 3. (A) Shoot P concentrations, (B) root P concentrations, (C) total P uptake of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars) and B (gray bars), and (D) P content ratio between soils ALP/AHP for shoot P (white bars), root P (black bars), and total P (gray bars). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for shoot P concentrations (p < 0.01, MSD 0.05 = 0.23 mg g−1), and root P concentrations (p < 0.001, MSD 0.05 = 0.14 mg g−1).
Shoot and root P content followed the order soil AHP > B > ALP for all species, except for root P content in A. sativa (Figure S1). The total P acquired by plants also varied significantly among soils and species (Table 2 and Figure 3C). Unsurprisingly, the greatest amount of P was removed from soil AHP, and the least was removed from soil ALP. For soil ALP, B. napus acquired the most P (3.24 mg/mini-rhizotron) while S. tuberosum obtained least P (1.91 mg/mini-rhizotron), whilst for soil B, A. sativa obtained the greatest P (5.92 mg/mini-rhizotron) and T. aestivum obtained the least (3.44 mg/mini-rhizotron). The P uptake ratio between ALP/AHP indicated that B. napus and A. sativa performed better in acquiring P under low P conditions than the two other species (Figure 3D).
Organic anions accumulated in the rhizosphere varied significantly among species (Table 2 and Figures 4A–C). Malate, citrate, succinate, and tartrate were detected and malate was the dominant organic anion in the present study. A. sativa had the greatest rhizosphere citrate concentration in all soils (Figure 4A), as well as malate (Figure 4B), and total organic anions (Figure 4C) in soil AHP, compared with the other three species. In soil B, B. napus had the greatest rhizosphere malate and total organic anion concentrations of all the studied species. Generally, more rhizosphere citrate was detected in low P soils than in high P soil while a contrasting pattern was found for malate except for B. napus, which showed an increase of malate in soil B, compared with soils AHP and ALP (by 74 and 103%, respectively). Similar patterns were found if the concentrations were calculated based on rhizosphere soil weight, except for S. tuberosum (Figure S2).
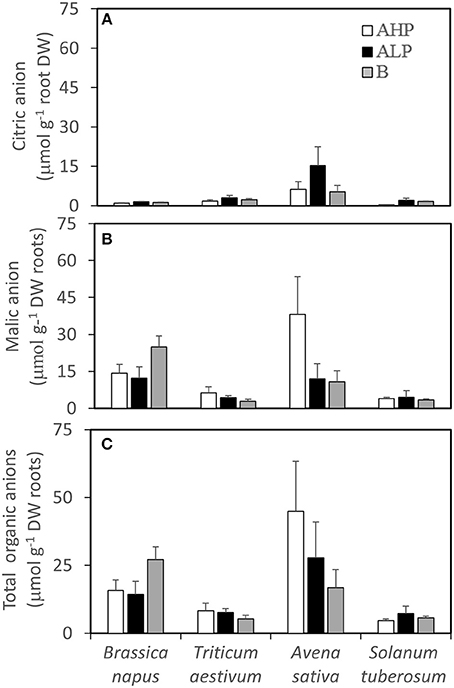
Figure 4. (A) Rhizosphere citrate concentrations, (B) rhizosphere malate concentrations, and (C) rhizosphere total organic anion concentrations of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars) based on root dry weight. Error bars indicate SE (n = 4). There was a significant interaction between species and soils for rhizosphere malate concentrations (p < 0.05, MSD 0.05 = 11.47 μmol g−1 DW roots).
In soils AHP and ALP (with the same soil texture but different P availability), a significant negative linear correlation was found between the amount of citrate in the rhizosphere and the shoot P concentration for B. napus (r = −0.82, p < 0.01) and S. tuberosum (r = −0.71, p < 0.05). However, a positive correlation was found between the amount of rhizosphere malate and shoot P concentration for S. tuberosum (r = 0.83, P < 0.01, n = 8).
The pH of the rhizosphere varied significantly among species and soils (Table 2 and Figure 5). All species generally had a rhizosphere water extract pH between 5.5 and 6.3. In soil AHP, T. aestivum had the highest rhizosphere pH (6.1) while S. tuberosum had the lowest pH (5.9). In soil ALP and soil B, A. sativa, followed by T. aestivum, had the highest rhizosphere pH and B. napus had the lowest pH.
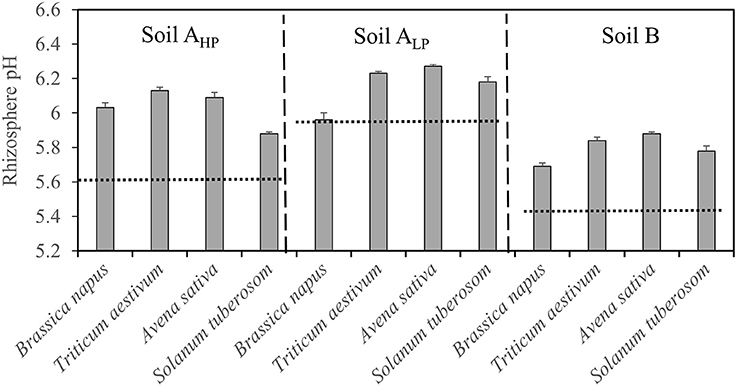
Figure 5. Rhizosphere soil pH of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP, ALP, and B. Error bars indicate SE (n = 4). There was a significant interaction between species and soils for rhizosphere pH (p < 0.001, MSD 0.05 = 0.1). Dashed lines indicate the soil pH values in unplanted mini-rhizotrons.
The plant-available P (determined as PAL) in the rhizosphere increased slightly in soil B but decreased in soil ALP in all species, compared with bulk soils (Figure 6A). The PAL of T. aestivum rhizosphere increased slightly in soil AHP, while decreasing slightly for B. napus.
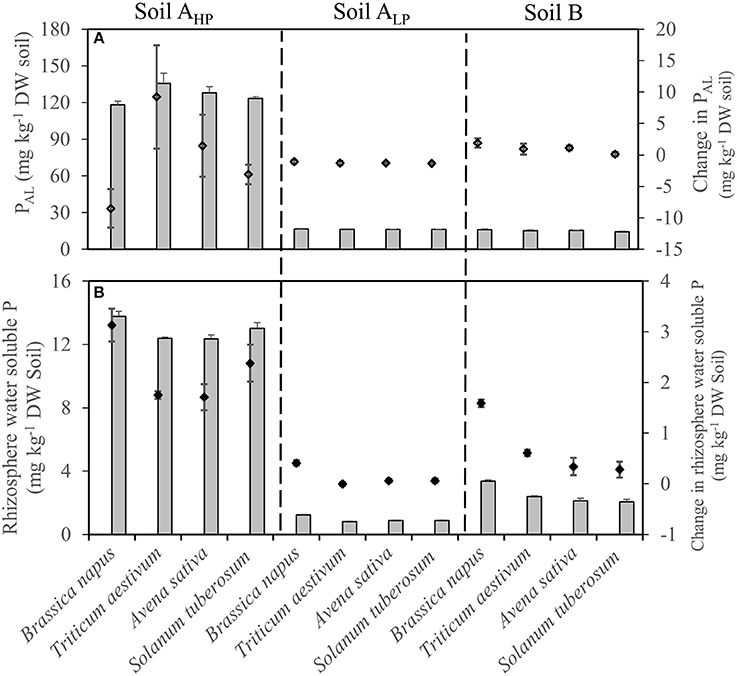
Figure 6. (A) Rhizosphere AL-extractable P and (B) rhizosphere water soluble P of Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP, ALP, and B (gray bars), as well as changes compared to unplanted bulk soils (black diamonds). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for change in PAL (p < 0.05, MSD 0.05 = 5.63 mg kg−1), and change in rhizosphere water soluble P (p < 0.01, MSD 0.05 = 0.34 mg kg−1).
Water-soluble P (WSP) is the most easily available P for plants. In the present study, almost all the rhizosphere WSP concentrations increased, compared with unplanted bulk soils. However, rhizosphere WSP varied significantly among soils and species (Table 2 and Figure 6B). For the bulk soils, soil AHP had the highest WSP concentrations (10.64 mg kg−1 DW soil), and the lowest WSP concentrations were observed in soil ALP (0.81 mg kg−1 DW soil). In all the three soils, B. napus showed the highest rhizosphere WSP concentrations compared with the other three species. Compared with the unplanted bulk soils, it increased by 3.13 mg kg−1 DW soil (29.4%), 0.41 mg kg−1 DW soil (50.6%), and 1.59 mg kg−1 DW soil (89.3%) in soils AHP, ALP, and B, respectively (Figure 6B).
Rhizosphere acid phosphatase activity (APase) was not affected by plant species when grown in the soil AHP, while in the low P soils, ALP and B, B. napus rhizosphere soil had the highest APase activity (Table 2 and Figure 7). The other three enzymes measured in the present study (β-glucosidase, β-xylosidase, and N-acetyl-β-glucosaminidase) were mainly influenced by soil type (Figure S3). Moreover, B. napus rhizosphere soil had greater ratios of APase to the other three enzymes in soil ALP than other soils and species (Figure S4).
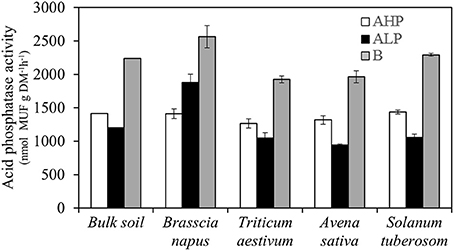
Figure 7. Acid phosphatase activities of bulk soil, and rhizosphere soil for Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for acid phosphatase activities (p < 0.001, MSD 0.05 = 153 nmol MUF g−1 DM−1h−1).
The soil microbial biomass P content (Pmic) was mainly affected by soil type (Table 2 and Figure 8) but showed an interaction with plant species. For bulk soils, Pmic declined in the order soil B > AHP > ALP. In the plant rhizosphere, soil ALP showed always the lowest and B the highest Pmic (except for A. sativa). The dicots (S. tuberosum and B. napus) supported a similar low amount of microbial P when growing in soils ALP and AHP, whereas under cereals, soil AHP supported a higher Pmic than ALP. In soils AHP and B, A. sativa had the greatest rhizosphere Pmic. No significant difference was found for plants grown in soil ALP. In addition, B. napus rhizosphere soil had greater ratios of APase to Pmic in soil ALP than other soils and species (Figure S4).
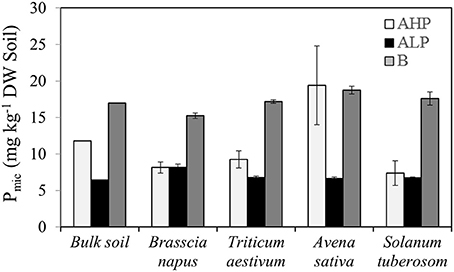
Figure 8. Phosphorus content immobilized by microbial biomass (Pmic) of bulk soil, and rhizosphere soil for Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4). There was a significant interaction between species and soils for Pmic (p < 0.01, MSD 0.05 = 3.28 mg kg−1 DW soil).
For mycorrhizal plant species, non-AMF were more abundant than AMF in roots (Table 3). Neither non-AMF nor AMF were influenced by soil P availability for any of the three species. The percentage of root length colonized differed among the plant species in all soils. A. sativa had the highest colonization of both AMF (32–40%) and non-AMF (58–75%) and colonization was lowest in S. tuberosum (22–36% of non-AMF and no AMF were detected).
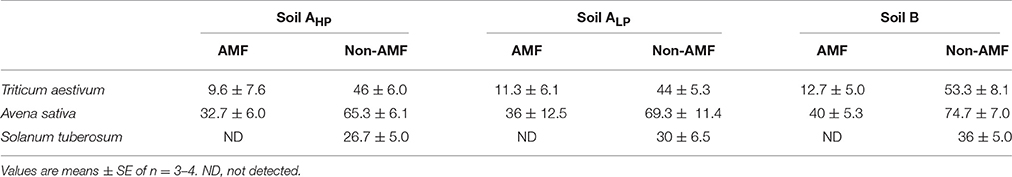
Table 3. The percentage of root length colonized by arbuscular mycorrhizal fungi (AMF) and non-arbuscular mycorrhizal fungi (non-AMF) in mycorrhizal species.
In within-soil analyses, significant correlations were found in low P availability soils ALP and B (Table 4). Plant shoot P content showed strong correlations with rhizosphere water-soluble P, rhizosphere pH, and APase. Total P uptake had weak positive correlations with rhizosphere APase and water-soluble P in soil ALP. Moreover, shoot P content and total P content showed strong positive correlations with root mass ratio in soil B. Positive correlations between rhizosphere citrate concentration and root P content were found in soil ALP and soil B and rhizosphere malate correlated weakly with root P content in soil ALP. In addition, rhizosphere water-soluble P showed strong correlations with rhizosphere APase and pH. A weak correlation between rhizosphere malate concentration and water-soluble P was also found in soil B. Rhizosphere APase correlated negatively with rhizosphere pH.
As shown in Table 5, within each species across all three soils, plant P uptake had a strong positive correlation with rhizosphere WSP for all species. Rhizosphere citrate, APase, and root mass ratio explained the WSP and P uptake in B. napus. In A. sativa, P uptake was linked to rhizosphere malate, APase and pH while rhizosphere APase was linked to root mass ratio. For S. tuberosum, P mobilization and uptake was mainly explained by rhizosphere citrate and pH. Across low P soils ALP and B, plant P uptake was mostly explained by rhizosphere APase, pH, and rhizosphere WSP (data not shown) for all species. Rhizosphere pH also correlated significantly with rhizosphere APase and WSP for all species. In addition, rhizosphere APase showed significant correlation with root mass ratio in A. sativa and S. tuberosum. The only significant correlation between total P uptake and root mass ratio was found in A. sativa.
The results of this experiment suggested that rhizosphere organic anions made a minor contribution to P mobilization and uptake for the studied crops in the studied low P clay loam and loam soils. Different plant species may have different growth potential in a mini-rhizotron and different needs for P to support their development. In addition to plant species, rhizosphere WSP, APase, and pH were likely to affect P availability and uptake. Unsurprisingly, rhizosphere WSP appears to have contributed greatly to P uptake in both high and low P availability soils. B. napus took up P efficiently, possibly due to greater rhizosphere APase activities and lower rhizosphere pH. A. sativa was another crop that could use P efficiently in our study, possibly due to its greater root mass ratio and higher percentage of root colonizing AMF. A. sativa had a larger proportion of root length colonized by AMF than T. aestivum and S. tuberosum, which might benefit its P acquisition. The implications of these findings and other points of interest are discussed below.
When studying rhizosphere organic anions, the great challenge is how to extract them effectively from soil samples due to their ability to interact with soil particles (Valentinuzzi et al., 2015). Low concentrations of Ca are often added to the extractant to ensure cell integrity, limit osmotic stress, and possible leakage/diffusion. Although Micropur can inhibit organic anion degradation by microorganisms, it also influences the exudation and degradation processes and hence affects the results (Valentinuzzi et al., 2015). Moreover, root exudates have complicated interactions with soil microbial communities (Badri and Vivanco, 2009). Therefore, it is very hard to extract all the rhizosphere organic anions and to perform accurate analytical determination. In addition, not all the rhizosphere soil in this study was taken to extract organic anions due to other soil measurements. Taking all the above factors into account, our data probably underestimated organic anion concentrations but may still reflect the relative differences between the plant species.
Generally, more citrate was detected in ALP than in AHP while the opposite was the case for malate for all species, and more total organic anions were detected in ALP than AHP only for S. tuberosum. Hence, our hypothesis (1) that low P availability stimulates plant roots to release more organic anions to the soil was not fully supported in our system. Inconsistent with our earlier results using hydroponic culture (Wang et al., 2015), we detected citrate in S. tuberosum rhizosphere, which may be due to longer-term accumulation in the soil experiment while we collected root exudates for only 2 h in the hydroponic system; alternatively, it may have been produced by soil microbes. We found that B. napus had higher rhizosphere total organic anion concentrations than T. aestivum and S. tuberosum but lower than those of A. sativa. Considering that A. sativa also showed highest AMF colonization and rhizosphere Pmic, these soil microbes might explain large amounts of the rhizosphere organic anions found. AMF-released carbon can trigger phosphate-solubilizing bacterium growth and activity (Zhang et al., 2016) and these bacteria can release organic anions to the soil (Jones, 1998). In addition, in soils AHP and ALP (soils with the same soil texture but different P availabilities), shoot P concentrations of B. napus and S. tuberosum had negative correlations with rhizosphere citrate concentration. Therefore, our hypothesis (3) was supported.
Root-released organic anions have been widely documented as a key physiological strategy to mobilize P from plant less-available P sources (e.g., Raghothama, 1999; Lambers et al., 2006, 2015). In our study, no simple linear relationships were found between rhizosphere organic anions and WSP, except that in soil B, malate concentration had weak correlation with rhizosphere WSP. Within species, B. napus and S. tuberosum showed weak negative correlations between total P content, root P content and rhizosphere WSP with citrate across the three soils but not in low P soils ALP and B. These correlations might therefore be due to the influence of data from high P availability soil AHP. We found that the rhizosphere citrate and malate concentrations had weak positive correlations with root P content in low P soils ALP and B, but no correlations with shoot and total P content. This was not consistent with some previous reports which reported strong positive correlations between total P content and rhizosphere organic anions in legumes and 12 Kennedia species (Ryan et al., 2012; Pang et al., 2015). These two studies were conducted in river sand to minimize interference of soil with P availability and varyingly soluble P was applied. However, our results compared well with the findings of Nazeri et al. (2014), who used agricultural soil. These findings suggested that rhizosphere organic anions may play a minor role in improving soil P availability and plant P uptake. Although organic acids have often been referred to as a possible source of rhizosphere acidification (Hinsinger, 2001), the effects of organic anion exudation on pH are complex (Roelofs et al., 2001). No correlation between rhizosphere organic anions and rhizosphere pH compared well with previous reports (Pang et al., 2010, 2015) and suggested that other factors were more important in influencing soil pH. A similar result was reported in B. napus; P deficiency induced decrease of rhizosphere pH was not associated with the increase of extractable rhizosphere organic anions but mainly from root released H+ (Hedley et al., 1982a). Overall, our results suggesting that rhizosphere organic anions make a minor contribution to P uptake agree with other reports (Pearse et al., 2007; Pandey et al., 2014; Ryan et al., 2014). In many cases, a single trait like larger amounts of rhizosphere organic anions did not result in improved P uptake and other factors such as root morphology (which we did not investigate) and pH may play key roles. Hence, hypothesis (2) that rhizosphere organic anions correlate with P availability and uptake was not supported, while hypothesis (3) was partly supported.
Compared with AHP, rhizosphere APase activity decreased in ALP for all species except B. napus, hence, our hypothesis (1) that low P availability will induce plant roots to release more APase to the soil was rejected. APase increased greatly in soil B compared with AHP and ALP indicating that other soil properties (possibly for example texture) have strong influence on plant rhizosphere APase. Across different crops, plant shoot P uptake, and rhizosphere water-soluble P had a strong positive correlation with rhizosphere APase in low P soils. In addition, total P uptake had a significant correlation with rhizosphere APase across crops in low P soil ALP and within all species across low P soils. Acid phosphatase, which originates from both plants and soil microbes (Lambers et al., 2006), could hydrolyze organic P compounds in soil and increase P availability, thereby enhancing plant P uptake (Tarafdar et al., 2001; George et al., 2004; Richardson et al., 2009). Tarafdar et al. (2001) suggested that microbes are responsible for the dominant contribution to soil phosphatases. This seems to be the case in our study as the bulk soil analysis also showed high APase concentrations. However, some reports have indicated that plant roots have a major impact on the composition and function of the rhizosphere microbial community, and phosphatase in the rhizosphere is mainly secreted by plant roots rather than by microbial activity in response to P limitation (Kandeler et al., 2002; Wasaki et al., 2008). For B. napus exposed to P deficiency, rhizosphere APase activity increased 10 times compared with bulk soil and appeared to be a response to increasing root density and no evidence was found for significant hydrolysis of (Hedley et al., 1982b, 1983). We also found that APase had significant negative correlation with rhizosphere WSP and positive correlation with root mass ratio in B. napus across all soils. Therefore, compared with the bulk soils, the increase in APase activities in rhizosphere soils of B. napus in low P soils might be from the roots. In addition, rhizosphere APase had a strong negative correlation with rhizosphere pH in low P availability soils, which was consistent with previous reports (Mobley et al., 1984; Šarapatka et al., 2004). Taking all of the above together, our hypothesis (2) that APase correlates with P availability and uptake and hypothesis (3) were supported. Further study is needed to prove whether APase can hydrolyze soil organic P.
In the present study, both mycorrhizal and non-mycorrhizal species were selected. Significant differences were found for AMF colonization among the three mycorrhizal plant species but this was not affected by soil P availability. Results reported in the literature vary. According to a previous review, high P application can decrease both root and soil AMF biomass per plant (Smith et al., 2011). In a semi-arid grassland experiment, AMF colonization was reduced significantly when soil P was high in 1 year, but not in the following year, probably due to the effects of other environmental factors (Klabi et al., 2015). Moreover, a 15 mg kg−1 P pulse treatment did not affect the percentage of root length colonized in five legumes grown in sandy soil with moderate P content of 11 mg kg−1 Olsen P (Nazeri et al., 2014). Our results that soil P did not affect AMF colonization are thus in accordance with the second year's results of the semi-arid grassland experiment and also consistent with the report by Nazeri et al. (2014). Nazeri et al. (2014) also reported that inoculation with AMF in five legumes decreased the amount of rhizosphere carboxylates by 52%, raised the rhizosphere pH by 0.2–0.7 pH units and was associated with higher rhizosphere Colwell P (bicarbonate-extractable P). Although our results show that A. sativa, which had a higher coverage percent of root colonizing AMF, had higher rhizosphere pH and lower APase in low P soils than the non-mycorrhizal plant B. napus, we could not reach any conclusions due to lack of non-mycorrhizal control treatments and enough non-mycorrhizal species. It would be necessary to carry out new experiments that include proper non-mycorrhizal control to reveal the mechanisms involved.
In our study, WSP correlated highly with P uptake. Hence, WSP can be used to evaluate a soil's P availability, as well as PAL, Olsen P, and Colwell P. We observed an increase in rhizosphere WSP, which is different from other reports which showed decreases in rhizosphere Olsen P or Colwell P during plant growth (Nazeri et al., 2014; Li et al., 2016). Therefore, WSP can be used as a parameter to study P mobilization. Root morphology is also a key factor that affects P uptake (Lambers et al., 2006, 2008; Lynch, 2011; Pedas et al., 2011). We measured root morphology in our previous experiment (Wang et al., 2015) but not in this study so it will not be discussed here. Rhizosphere pH is another important factor involved in this study, lower rhizosphere pH may result in higher APase activity (Mobley et al., 1984; Šarapatka et al., 2004) and altered P sorption/desorption (Singh et al., 2005), thereby affecting P uptake through changing plant available P. Indeed, the rhizosphere pH decreased almost 2.4 units after 2 weeks and was raised slightly after 5 weeks when B. napus was grown in thin layers of a P-deficient soil, and the pH decrease was associated with an increase in rhizosphere available P (Grinsted et al., 1982; Hedley et al., 1982b). We found significant correlations of pH with WSP and APase within or across species in low P soils.
Moreover, it has been reported that phosphorus adsorption by soil was enhanced with an increase in clay content in suspension (Syers et al., 1973; Ullah et al., 1983), since with increasing clay content there is often increased content of Fe- and Al-(hydro)oxides, which are important constituents for P sorption. A lower content of clay in soil B suggests that this soil might adsorb less P (lower P sorption capacity) and organic anions than soil ALP and that plants may therefore grow better in soil B than in soil ALP. Another explanation is that more sand in soil B makes it more porous, which probably gives better aeration of the soil and thereby more oxygen to the roots. Further studies in long-term field experiments under varied agricultural soil textures might help to clarify this.
Through this experiment using four common crops and three agricultural soils, we found that plant P uptake may be linked to rhizosphere WSP, APase activity, and pH. Rhizosphere organic anions appear to play a minor role in improving P uptake. We conclude that our hypothesis (1) that low P availability soils will stimulate plant roots to release more organic anions and phosphatase enzymes to rhizosphere soil was not supported. Our hypothesis (2) that the amounts of rhizosphere APase will have positive correlations with rhizosphere plant-available P fractions and P uptake by plants in low P soils was supported, but this was not the case for rhizosphere organic anions. WSP can be used to study P mobilization and assess soil P availability. Hypothesis (3) that different crops will show differing root released organic anions and APase in terms of using residual P from agricultural soils was supported. We found that both B. napus and A. sativa are good candidates to study P utilization. The results and information generated in this study are valuable for understanding P mobilization and P uptake in low P agricultural soils, and for future effective utilization of P and improving the productivity of the studied crops.
NC, TK, JC, AØ, SE, and YW made contributions to the design of the study. YW conducted the experiment, collected and analyzed the samples, and drafted the manuscript. MH and EK made a contribution to analysis of soil enzyme activities and Pmic. All authors participated in preparing the manuscript.
This study was supported by the strategic institute program on “Opportunities for sustainable use of phosphorus in food production” at the Norwegian Institute of Bioeconomy Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer IJ and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Many thanks to Hans Lambers (University of Western Australia) for valuable suggestions concerning the study. We thank Sissel Haugslien, Jan Erik Jacobsen, Monica Fongen, Marit Almvik, Toril Drabløs Eldhuset, Erik Joner, Belachew Asalf Tadesse, and Torfinn Torp (NIBIO) for their valuable help with seed collection, phosphorus determinations, LC-MS/MS analysis, a preliminary rhizobox experiment, AMF observation and statistical analysis. Thanks also should go to Paula Gruner and Pascal Nassal (University of Hohenheim) for help with Pmic/MUF analysis.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01664/full#supplementary-material
Figure S1. (A) Shoot P content and (B) root P content of Brassica napus, Triticum aestivum, Avena sativa and Solanum tuberosum grown in soils AHP (stippled bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4).
Figure S2. (A) Rhizosphere citrate concentrations, (B) rhizosphere malate concentrations, and (C) rhizosphere total organic anion concentrations for Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars) based on rhizosphere soil dry weight. Error bars indicate SE (n = 4).
Figure S3. (A) β-glucosidase activity, (B) β-xylosidase activity, and (C) N-acetyl-β-glucosaminidase activity of bulk soil for Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars) and B (gray bars). Error bars indicate SE (n = 4).
Figure S4. (A) Ratio of acid phosphatase activity to Pmic, (B) ratio of acid phosphatase activity to β-glucosidase activity, (C) ratio of acid phosphatase activity to N-acetyl-β-glucosaminidase activity, and (D) ratio of acid phosphatase activity to β-xylosidase activity of bulk soil, for Brassica napus, Triticum aestivum, Avena sativa, and Solanum tuberosum grown in soils AHP (white bars), ALP (black bars), and B (gray bars). Error bars indicate SE (n = 4).
Badri, D. V., and Vivanco, J. M. (2009). Regulation and function of root exudates. Plant Cell Environ. 32, 666–681. doi: 10.1111/j.1365-3040.2009.01926.x
Cheng, L., Tang, X., Vance, C. P., White, P. J., Zhang, F., and Shen, J. (2014). Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). J. Exp. Bot. 65, 2995–3003. doi: 10.1093/jxb/eru135
Egnér, H., Riehm, H., and Domingo, W. (1960). Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 26, 199–215.
Faucon, M.-P., Houben, D., Reynoird, J.-P., Mercadal-Dulaurent, A.-M., Armand, R., and Lambers, H. (2015). “Advances and perspectives to improve the phosphorus availability in cropping systems for agroecological phosphorus management.” Adv. Agron. 134, 51–79. doi: 10.1016/bs.agron.2015.06.003
Gahoonia, T. S., Asmar, F., Giese, H., Gissel-Nielsen, G., and Nielsen, N. E. (2000). Root-released organic acids and phosphorus uptake of two barley cultivars in laboratory and field experiments. Eur. J. Agron. 12, 281–289. doi: 10.1016/S1161-0301(00)00052-6
George, T., Richardson, A., Hadobas, P., and Simpson, R. (2004). Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: growth and P nutrition in laboratory media and soil. Plant Cell Environ. 27, 1351–1361. doi: 10.1111/j.1365-3040.2004.01225.x
German, D. P., Weintraub, M. N., Grandy, A. S., Lauber, C. L., Rinkes, Z. L., and Allison, S. D. (2011). Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43, 1387–1397. doi: 10.1016/j.soilbio.2011.03.017
Giacometti, C., Cavani, L., Baldoni, G., Ciavatta, C., Marzadori, C., and Kandeler, E. (2014). Microplate-scale fluorometric soil enzyme assays as tools to assess soil quality in a long-term agricultural field experiment. Appl. Soil Ecol. 75, 80–85. doi: 10.1016/j.apsoil.2013.10.009
Giovanetti, M., and Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x
Grinsted, M., Hedley, M., White, R., and Nye, P. (1982). Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings. I. pH change and the increase in P concentration in the soil solution. New Phytol. 91, 19–29. doi: 10.1111/j.1469-8137.1982.tb03289.x
Hedley, M. J., Nye, P. H., and White, R. E. (1982a). Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings. II. Origin of the pH change. New Phytol. 91, 31–44.
Hedley, M. J., White, R. E., and Nye, P. H. (1982b). Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings. III. Changes in L value, soil phosphate fractions and phosphatase activity. New Phytol. 91, 45–56.
Hedley, M. J., White, R. E., and Nye, P. H. (1983). Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings. IV. The effect of rhizosphere phosphorus status on the pH, phosphatase activity and depletion of soil phosphorus fractions in the rhizosphere and on the cation-anion balance in the plants. New Phytol. 95, 69–82.
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi: 10.1023/A:1013351617532
Hoffland, E., Findenegg, G. R., and Nelemans, J. A. (1989). Solubilization of rock phosphate by rape. Plant Soil 113, 155–160. doi: 10.1007/BF02280175
James, B. R., Bartlett, R. J., and Amadon, J. F. (1985). A root observation and sampling chamber for pot studies. Plant Soil 85, 291–293. doi: 10.1007/BF02139633
Jones, D. L. (1998). Organic acids in the rhizosphere–a critical review. Plant Soil 205, 25–44. doi: 10.1023/A:1004356007312
Kandeler, E., Marschner, P., Tscherko, D., Gahoonia, T., and Nielsen, N. (2002). Structural and functional diversity of soil microbial community in the rhizosphere of maize. Plant Soil 238, 301–312. doi: 10.1023/A:1014479220689
Klabi, R., Bell, T. H., Hamel, C., Iwaasa, A., Schellenberg, M., Raies, A., et al. (2015). Plant assemblage composition and soil P concentration differentially affect communities of AM and total fungi in a semi-arid grassland. FEMS Microbiol. Ecol. 91, 1–13. doi: 10.1093/femsec/fiu015
Kouno, K., Tuchiya, Y., and Ando, T. (1995). Measurement of soil microbial biomass phosphorus by an anion exchange membrane method. Soil Biol. Biochem. 27, 1353–1357. doi: 10.1016/0038-0717(95)00057-L
Kristoffersen, A. Ø., and Riley, H. (2005). Effects of soil compaction and moisture regime on the root and shoot growth and phosphorus uptake of barley plants growing on soils with varying phosphorus status. Nutr. Cycl. Agroecosyst. 72, 135–146. doi: 10.1007/s10705-005-0240-8
Krogstad, T., Øgaard, A. F., and Kristoffersen, A. Ø. (2008). New PRecommendations for Grass and Cereals in Norwegian Agriculture. NJF Rep. 4, 42–46.
Lambers, H., Hayes, P. E., Laliberté, E., Oliveira, R. S., and Turner, B. L. (2015). Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 20, 83–90. doi: 10.1016/j.tplants.2014.10.007
Lambers, H., Raven, J. A., Shaver, G. R., and Smith, S. E. (2008). Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 23, 95–103. doi: 10.1016/j.tree.2007.10.008
Lambers, H., Shane, M. W., Cramer, M. D., Pearse, S. J., and Veneklaas, E. J. (2006). Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot. 98, 693–713. doi: 10.1093/aob/mcl114
Li, C., Dong, Y., Li, H., Shen, J., and Zhang, F. (2016). Shift from complementarity to facilitation on P uptake by intercropped wheat neighboring with faba bean when available soil P is depleted. Sci. Rep. 6:18663. doi: 10.1038/srep18663
Lü, J., Gao, X., Dong, Z., Yi, J., and An, L. (2012). Improved phosphorus acquisition by tobacco through transgenic expression of mitochondrial malate dehydrogenase from Penicillium oxalicum. Plant Cell Rep. 31, 49–56. doi: 10.1007/s00299-011-1138-3
Lynch, J. P. (2011). Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 156, 1041–1049. doi: 10.1104/pp.111.175414
Maguire, R., Foy, R., Bailey, J., and Sims, J. (2001). Estimation of the phosphorus sorption capacity of acidic soils in Ireland. Eur. J. Soil Sci. 52, 479–487. doi: 10.1046/j.1365-2389.2001.00394.x
Mobley, D. M., Chengappa, M. M., Kadel, W. L., and Stuart, J. G. (1984). Effect of pH, temperature and media on acid and alkaline phosphatase activity in “clinical” and “nonclinical” isolates of Bordetella bronchiseptica. Can. J. Comp. Med. 48, 175–178.
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Nazeri, N. K., Lambers, H., Tibbett, M., and Ryan, M. H. (2014). Moderating mycorrhizas: arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant Cell Environ. 37, 911–921. doi: 10.1111/pce.12207
Ogner, G., Wickstrøm, T., Remedios, G., Gjelsvik, S., Hensel, G. R., Jacobsen, J. E., et al. (1999). The Chemical Analysis Program of the Norwegian Forest Research Institute 2000. Ås: Norwegian Forest Research Institute.
Pandey, R., Meena, S. K., Krishnapriya, V., Ahmad, A., and Kishora, N. (2014). Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 33, 919–928. doi: 10.1007/s00299-014-1570-2
Pang, J., Ryan, M. H., Tibbett, M., Cawthray, G. R., Siddique, K. H., Bolland, M. D., et al. (2010). Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 331, 241–255. doi: 10.1007/s11104-009-0249-x
Pang, J., Yang, J., Lambers, H., Tibbett, M., Siddique, K. H., and Ryan, M. H. (2015). Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol. Plant. 154, 511–525. doi: 10.1111/ppl.12297
Pearse, S. J., Veneklaas, E. J., Cawthray, G., Bolland, M. D., and Lambers, H. (2007). Carboxylate composition of root exudates does not relate consistently to a crop species' ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol. 173, 181–190. doi: 10.1111/j.1469-8137.2006.01897.x
Pedas, P., Husted, S., Skytte, K., and Schjoerring, J. K. (2011). Elevated phosphorus impedes manganese acquisition by barley plants. Front. Plant Sci. 2:37. doi: 10.3389/fpls.2011.00037
Raghothama, K. (1999). Phosphate acquisition. Ann. Rev. Plant Biol. 50, 665–693. doi: 10.1146/annurev.arplant.50.1.665
Richardson, A. E., Hocking, P. J., Simpson, R. J., and George, T. S. (2009). Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 60, 124–143. doi: 10.1071/CP07125
Roelofs, R., Rengel, Z., Cawthray, G., Dixon, K., and Lambers, H. (2001). Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant Cell Environ. 24, 891–904. doi: 10.1046/j.1365-3040.2001.00741.x
Ryan, M. H., Tibbett, M., Edmonds-Tibbett, T., Suriyagoda, L. D., Lambers, H., Cawthray, G. R., et al. (2012). Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 35, 2170–2180. doi: 10.1111/j.1365-3040.2012.02547.x
Ryan, P., Delhaize, E., and Jones, D. (2001). Function and mechanism of organic anion exudation from plant roots. Ann. Rev. Plant Biol. 52, 527–560. doi: 10.1146/annurev.arplant.52.1.527
Ryan, P. R., James, R. A., Weligama, C., Delhaize, E., Rattey, A., Lewis, D. C., et al. (2014). Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol. Plant. 151, 230–242. doi: 10.1111/ppl.12150
Šarapatka, B., Dudová, L., and Kršková, M. (2004). Effect of pH and phosphate supply on acid phosphatase activity in cereal roots. Biol. Bratislava 59, 127–131.
Singh, B. R., and Subramaniam, V. (1996). Phosphorus supplying capacity of heavily fertilized soils II. Dry matter yield of successive crops and phosphorus uptake at different temperatures. Nutr. Cycl. Agroecosyst. 47, 123–134. doi: 10.1007/BF01991544
Singh, B. R., Krogstad, T., Shivay, Y. S., Shivakumar, B. G., and Bakkegard, M. (2005). Phosphorus fractionation and sorption in P-enriched soils of Norway. Nutr. Cycl. Agroecosyst. 73, 245–256. doi: 10.1007/s10705-005-2650-z
Smith, S. E., Jakobsen, I., Grønlund, M., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156, 1050–1057. doi: 10.1104/pp.111.174581
Smith, S. E., Smith, F. A., and Jakobsen, I. (2003). Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133, 16–20. doi: 10.1104/pp.103.024380
Syers, J., Browman, M., Smillie, G., and Corey, R. (1973). Phosphate sorption by soils evaluated by the Langmuir adsorption equation. Soil Sci. Soc. Am. J. 37, 358–363. doi: 10.2136/sssaj1973.03615995003700030015x
Tarafdar, J. C., Yadav, R. S., and Meena, S. C. (2001). Comparative efficiency of acid phosphatase originated from plant and fungal sources. J. Plant Nutr. Soil Sci. 164, 279–282. doi: 10.1002/1522-2624(200106)164:3<279::AID-JPLN279>3.0.CO;2-L
Ullah, M., Jabbar, A., and Khan, M. (1983). The influence of soil pH and texture on the adsorption of phosphorus by soils. Pakistan J. Agr. Res. 4, 41–46.
Valentinuzzi, F., Cesco, S., Tomasi, N., and Mimmo, T. (2015). Influence of different trap solutions on the determination of root exudates in Lupinus albus L. Biol. Fertil. Soils 51, 757–765. doi: 10.1007/s00374-015-1015-2
Vance, C. P., Uhde-Stone, C., and Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
van Duivenbooden, N., de Wit, C. T., and van Keulen, H. (1995). Nitrogen, phosphorus and potassium relations in five major cereals reviewed in respect to fertilizer recommendations using simulation modelling. Fert. Res. 44, 37–49. doi: 10.1007/BF00750691
Veneklaas, E. J., Stevens, J., Cawthray, G. R., Turner, S., Grigg, A. M., and Lambers, H. (2003). Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248, 187–197. doi: 10.1023/A:1022367312851
Vierheilig, H., Coughlan, A. P., Wyss, U., and Piché, Y. (1998). Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 64, 5004–5007.
Wang, Y.-L., Almvik, M., Clarke, N., Eich-Greatorex, S., Øgaard, A. F., Krogstad, T., et al. (2015). Contrasting responses of root morphology and root-exuded organic acids to low phosphorus availability in three important food crops with divergent root traits. AoB Plants 7:plv097. doi: 10.1093/aobpla/plv097
Wang, Y., Xu, H., Kou, J., Shi, L., Zhang, C., and Xu, F. (2013). Dual effects of transgenic Brassica napus overexpressing CS gene on tolerances to aluminum toxicity and phosphorus deficiency. Plant Soil 362, 231–246. doi: 10.1007/s11104-012-1289-1
Wasaki, J., Kojima, S., Maruyama, H., Haase, S., Osaki, M., and Kandeler, E. (2008). Localization of acid phosphatase activities in the roots of white lupin plants grown under phosphorus-deficient conditions. Soil Sci. Plant Nutr. 54, 95–102. doi: 10.1111/j.1747-0765.2007.00207.x
Watts-Williams, S. J., Smith, F. A., McLaughlin, M. J., Patti, A. F., and Cavagnaro, T. R. (2015). How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 390, 157–166. doi: 10.1007/s11104-014-2374-4
Keywords: phosphorus, rhizosphere organic anions, rhizosphere APase, rhizosphere pH, rhizosphere water-soluble P
Citation: Wang Y, Krogstad T, Clarke JL, Hallama M, Øgaard AF, Eich-Greatorex S, Kandeler E and Clarke N (2016) Rhizosphere Organic Anions Play a Minor Role in Improving Crop Species' Ability to Take Up Residual Phosphorus (P) in Agricultural Soils Low in P Availability. Front. Plant Sci. 7:1664. doi: 10.3389/fpls.2016.01664
Received: 29 May 2016; Accepted: 21 October 2016;
Published: 07 November 2016.
Edited by:
Jan Kofod Schjoerring, University of Copenhagen, DenmarkReviewed by:
Iver Jakobsen, University of Copenhagen, DenmarkCopyright © 2016 Wang, Krogstad, Clarke, Hallama, Øgaard, Eich-Greatorex, Kandeler and Clarke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tore Krogstad, dG9yZS5rcm9nc3RhZEBubWJ1Lm5v
Nicholas Clarke, bmljaG9sYXMuY2xhcmtlQG5pYmlvLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.