- 1School of Life Sciences, Yunnan Normal University, Kunming, China
- 2Engineering Research Center of Sustainable Development and Utilization of Biomass Energy, Ministry of Education, Kunming, China
- 3Key Laboratory of Biomass Energy and Environmental Biotechnology, Yunnan Normal University, Kunming, China
For a long time, hydrogen sulfide (H2S) has been considered as merely a toxic by product of cell metabolism, but nowadays is emerging as a novel gaseous signal molecule, which participates in seed germination, plant growth and development, as well as the acquisition of stress tolerance including cross-adaptation in plants. Cross-adaptation, widely existing in nature, is the phenomenon in which plants expose to a moderate stress can induce the resistance to other stresses. The mechanism of cross-adaptation is involved in a complex signal network consisting of many second messengers such as Ca2+, abscisic acid, hydrogen peroxide and nitric oxide, as well as their crosstalk. The cross-adaptation signaling is commonly triggered by moderate environmental stress or exogenous application of signal molecules or their donors, which in turn induces cross-adaptation by enhancing antioxidant system activity, accumulating osmolytes, synthesizing heat shock proteins, as well as maintaining ion and nutrient balance. In this review, based on the current knowledge on H2S and cross-adaptation in plant biology, H2S homeostasis in plant cells under normal growth conditions; H2S signaling triggered by abiotic stress; and H2S-induced cross-adaptation to heavy metal, salt, drought, cold, heat, and flooding stress were summarized, and concluded that H2S might be a candidate signal molecule in plant cross-adaptation. In addition, future research direction also has been proposed.
Introduction
Cross-adaptation, widely existing in nature, is the phenomenon in which plants expose to a moderate stress can induce the resistance to other stresses (Li and Gong, 2011; Foyer et al., 2016; Hossain et al., 2016). For example, cold pretreatment can improve the heat tolerance of winter rye, salt shock can rapidly induce the cold tolerance in spinach and potato, ultraviolet radiation (UV-B) can enhance the heat tolerance in cucumber and the cold tolerance in Rhododendron, and mechanical stimulation can improve the heat tolerance and the chilling tolerance in tobacco cells (Knight, 2000; Li and Gong, 2011, 2013). Interestingly, Foyer et al. (2016) found that cross-adaptation also can be induced between abiotic and biotic stresses. Infection by mycorrhizal fungi can improve the resistance of tomato, sunflower, pea, and rice to drought, chilling, salinity, metal toxicity, and high temperature stress (Grover et al., 2011), while drought stress can reduce aphid fecundity in Arabidopsis (Pineda et al., 2016). Our previous work also showed that heat shock could improve the resistance of maize seedlings to heat, chilling, salt, and drought stress (Gong et al., 2001). Numerous studies found that the acquisition of stress tolerance including cross-adaptation was involved in a complex signal network consisting of many second messengers such as Ca2+, abscisic acid (ABA), hydrogen peroxide (H2O2) and nitric oxide (NO), as well as their crosstalk (Knight, 2000; Pandey, 2015; Li and Gu, 2016; Li and Jin, 2016; Niu and Liao, 2016; Wang et al., 2016). In tobacco, mechanical stimulation can successively trigger H2O2 and NO signaling (Li and Gong, 2011, 2013), heat shock can induce Ca2+ and ABA signaling one after the other (Gong et al., 1998a,b), which in turn induce cross-adaptation to heat and chilling stress, similar results were reported by Gong et al. (2001) in maize seedlings. These results indicate that the acquisition of cross-adaptation is involved in signal crosstalk among Ca2+, H2O2, NO, and ABA in plants. Recently, hydrogen sulfide (H2S) was also found to be a member of this signal network in plants (Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014; Fotopoulos et al., 2015; Guo et al., 2016), indicating that H2S might be a signal molecule in plant cross-adaptation.
For a long time, H2S has been considered as merely a toxic intermediate of cell metabolism due to its strong affinity to Fe2+-containing proteins such as cytochrome oxidase, hemoglobin and myoglobin, which may have been primary cause of the mass extinction of species in the Permian (Li, 2013; Lisjak et al., 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014; Fotopoulos et al., 2015; Guo et al., 2016; Yamasaki and Cohen, 2016). H2S can inhibit oxygen release from young seedlings of six rice cultivars (Bluebelle, Dawn, Norin 22, Saturn, Yubae, and Zenith) and nutrient uptake such as phosphorus (Li, 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014). But nowadays, H2S is found to function as gaseous signal molecule at low concentration similar to carbon monoxide (CO) and NO in plants, and it has been shown that plants can actively synthesize endogenous H2S under normal, especially biotic or abiotic stress conditions (Li, 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014; Yamasaki and Cohen, 2016). The accumulation of endogenous H2S has become a common response of plants to environmental stress, including salt, heavy metal (HM), drought, heat and cold stress, as well as pathogen infection, which may be closely associated with the acquisition of stress tolerance in plants (Li, 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014). More interestingly, exogenously applied H2S, releasing from its donors such as NaHS and morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate (GYY4137), shows significant positive effects on seed germination (Li et al., 2012a; Li and He, 2015; Wojtyla et al., 2016), organogenesis and growth (Lin et al., 2012; Fang T. et al., 2014), the regulation of senescence (Zhang et al., 2011), as well as the acquisition of stress tolerance such as salt (Christou et al., 2013), HM (Chen et al., 2013), drought (Christou et al., 2013), heat (Li et al., 2013a,b; Li, 2015c) and cold tolerance (Fu et al., 2013). These results indicate that H2S may be a candidate signal molecule in plant cross-adaptation. In addition, NaHS and GYY4137 are commonly used as H2S donors because they can release H2S when dissolved in water, but NaHS giving a relatively short burst of H2S, while GYY4137 giving a longer more prolonged exposure to H2S (Wang, 2012; Lisjak et al., 2013). However, whether H2S concentration in plant cells or tissues is consistent with that of NaHS and GYY4137 applied as well as actual H2S concentration triggering cross-adaptation need to be further investigated. In addition, H2S usually exist in the forms of H2S (approximately 20%) and HS- (approximately 80%) in water solution, exact physiological concentration of H2S in plant cells or subcellular organelles is not clear.
Though, there are a lot of excellent reviews which expound potential physiological function of H2S in seed germination, plant growth and development, as well as the acquisition of stress tolerance (Li, 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014, 2016; Jin and Pei, 2015; Guo et al., 2016; Scuffi et al., 2016; Yamasaki and Cohen, 2016), the role of H2S as a candidate signal molecule in plant cross-adaptation was not summarized in depth. Therefore, in this review, H2S homeostasis in plant cells under normal growth conditions, H2S signaling triggered by adverse environment and H2S-induced cross-adaptation to various abiotic stresses are summarized, which further uncovers that H2S may be a candidate signal molecule in plant cross-adaptation.
H2S Homeostasis in Plant Cells
As mentioned above, due to the dual role of H2S, that is, as cytotoxin at high concentration and as cell signal molecule at low concentration, H2S homeostasis in plant cells is very important to exert its physiological functions including cross-adaptation induction. In plant cells, there are many metabolic pathways to regulate H2S homeostasis, similar to other signal molecules like H2O2, NO. H2S homeostasis is closely regulated by L-cysteine desulfhydrase (LCD, EC 4.4.1.1), D-cysteine desulfhydrase (DCD, EC 4.4.1.15), sulfite reductase (SiR, EC 1.8.7.1), cyanoalanine synthase (CAS, EC 4.4.1.9), and cysteine synthase (CS, EC 4.2.99.8; Li, 2013, 2015a; Figure 1). LCD/DCD catalyzes the degradation of L-/D-cysteine to produce H2S, amine and pyruvate; SiR reduces sulfite to H2S using ferredoxin as electron donor; H2S can be released from cysteine in the present of hydrogen cyanide by CAS; CS, namely O-acetyl-(thiol)-serinelyase (OAS-TL), can incorporate H2S into O-acetyl-L-serine to form cysteine, and its reverse reaction can release H2S (Li, 2013, 2015a; Figure 1). Generally, plants synthesize H2S via LCD or DCD, which respond to environment stress and induce the acquisition of stress tolerance. In addition, excess H2S can be released to air (Li, 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014).
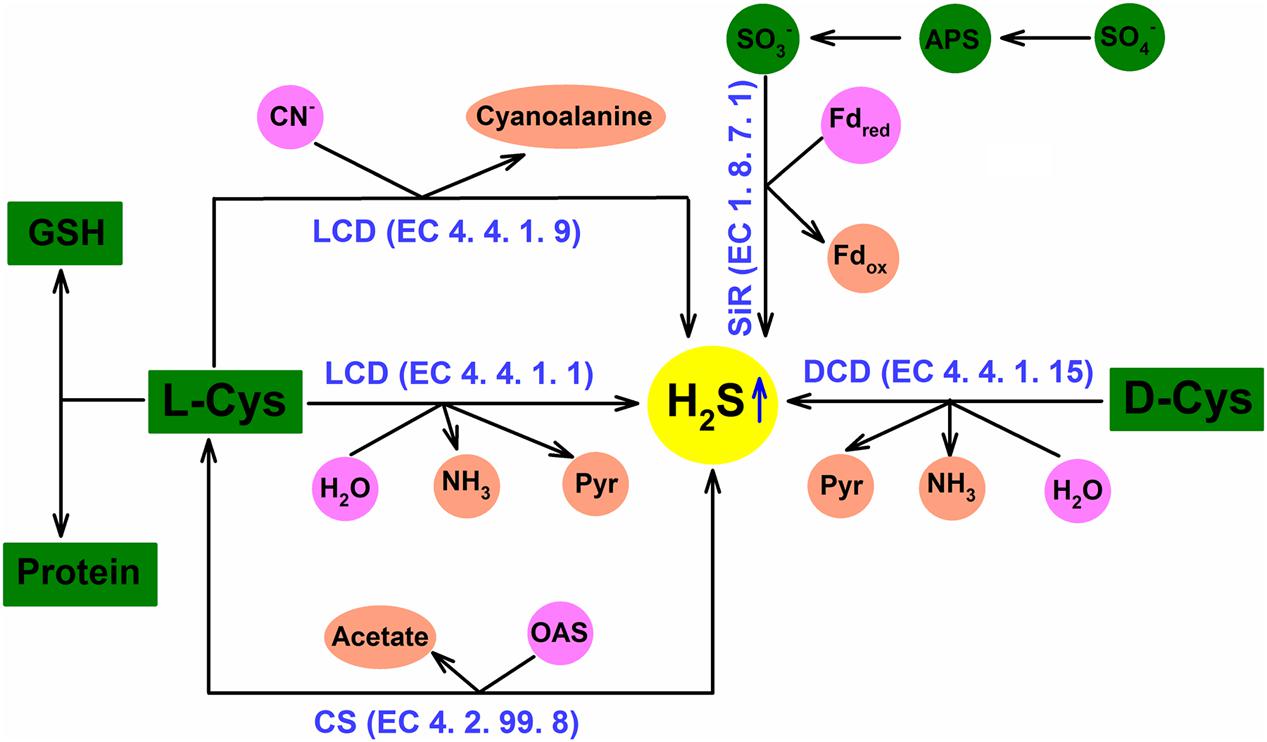
FIGURE 1. Hydrogen sulfide (H2S) homeostasis in plant cells. H2S homeostasis can be regulated by L-cysteine desulfhydrase (LCD), D-cysteine desulfhydrase (DCD), sulfite reductase (SiR), cyanoalanine synthase (CAS), and cysteine synthase (CS) pathways in plant cells (adapted from Li, 2015a).
H2S Signaling Triggered By Abiotic Stress
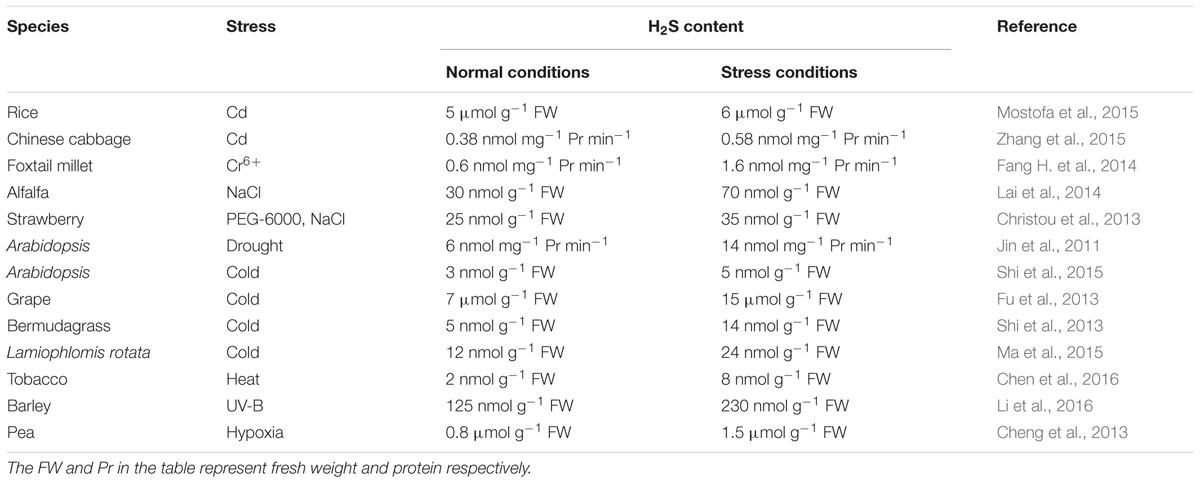
Similar to other second messengers such as Ca2+, H2O2, ABA and NO, the rapid production of endogenous H2S in many species of plant can be triggered by numerous stresses (Table 1; Figure 2), this is a common response of plants to various abiotic stresses, which is closely associated with the acquisition of cross-adaptation in plants.
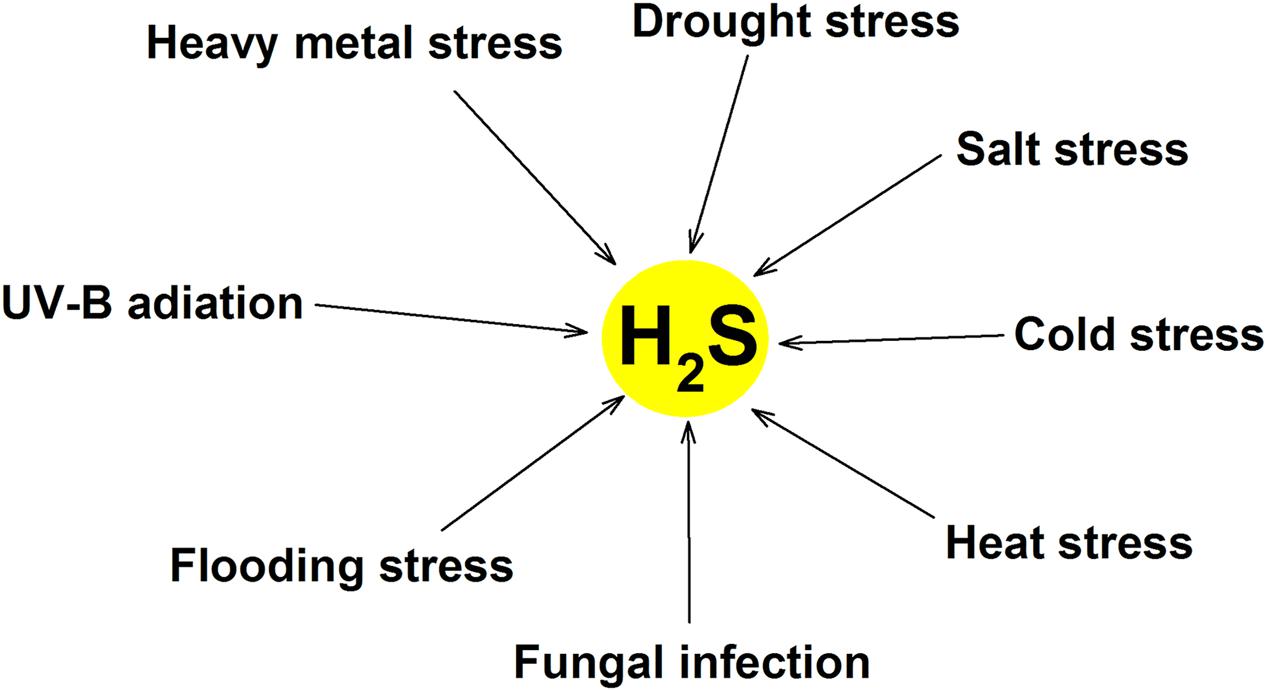
FIGURE 2. Mutiple environmental stress can induce endogenous H2S production in plants. Abiotic stress (heavy metal, drought, salt, cold, heat, flooding, and UV-B radiation) and biotic stress (fungal infection) induce the generation of endogenous H2S by mainly activating LCD.
H2S Signaling Triggered by Heavy Metal Stress
The rapid production of H2S has become a common response of plants to various HM stress, among HMs, Cd is the most severe stress due to its toxicity and stability (Ahmad, 2016). In rice seedlings, 0.5 mM Cd stress resulted in an increment of H2S content from approximately 5 μmol g-1 fresh weight (FW) to approximately 6 μmol g-1 FW. The addition of 0.1 mM NaHS caused an even further increase in the level of H2S (approximately 8 μmol g-1 FW) as compared with Cd treatment alone. Exposure to 0.2 mM hypotaurine (HT, H2S scavenger) with NaHS decreased H2S level compared with NaHS alone, indicating that this elevated level of H2S is correlated with the enhanced Cd tolerance (Mostofa et al., 2015). Zhang et al. (2015) found that the endogenous H2S emission was stimulated by Cd stress in Chinese cabbage. The relative expression of DCD1 and DES1 (cysteine desulfhydrase, OAS-TL homogenous family) genes (responsible for H2S synthesis) was up-regulated after treatment with Cd with a range of concentrations (0, 5, 10, and 20 mM) for 24 h. Expression of DES1 at 5 mM Cd already showed a significant increase, and at 20 mM Cd was 4.7 times of the control. Following a similar pattern, the endogenous H2S concentrations also significantly rose from 0.38 to 0.58 nmol mg-1 protein min-1 at 20 mM. Chromium (Cr), existing in the form of Cr3+ and Cr6+, is regarded as the second most common HM, both forms have become major environmental pollution sources. In foxtail millet seedlings, Fang H. et al. (2014) also reported that the expressions of H2S-emission related genes LCD, DCD2, and DES markedly increased during the first 12 h of Cr6+ exposure following decline at 24 h, while the expression of DCD1 was consistently increased from 0 to 24 h under 10 mM Cr6+ stress. Additionally, the H2S production rate is induced by Cr6+ stress in dose- and time-dependent manner, and this induction was the most significant with 24 h of 10 mM Cr6+ treatment (from 0.6 to 1.6 nmol mg-1 protein min-1). These results imply that endogenous H2S synthesis was activated by Cr6+ stress by activating its emission system in foxtail millet. Inconceivably, in compared with to other plant species, the both species Chinese cabbage and foxtail millet show a remarkable tolerance to HM (Cd and Cr6+). At 20 mM Cd for Chinese cabbage and 10 mM Cr6+ for foxtail millet, these treatment concentrations are far beyond the physiological level (generally micromolar concentrations) for many plant species, the precise physiological, biochemical, and molecular mechanisms are waiting for being uncovered.
H2S Signaling Triggered by Salt Stress
Salt stress commonly leads to an osmotic stress response, similar to drought stress, which triggers rapid generation of second messengers like H2S. In alfalfa seedlings, the increasing concentration of NaCl (from 50 to 300 mM) progressively caused the induction of total LCD activity and the increase of endogenous H2S production (from 30 to 70 nmol g-1 FW) (Lai et al., 2014). Exposure of strawberry seedlings to salinity (100 mM NaCl) and non-ionic osmotic stress (10% PEG-6000) greatly enhanced H2S concentration (48 and 50 nmol g-1 FW) in leaves, while 0.1 mM NaHS-pretreated plants subsequently exposed for 7 days to both stress factors were found to accumulate significantly higher amounts of H2S (55 nmol g-1 FW) in their leaves compared with NaCl-stressed plants (Christou et al., 2013).
H2S Signaling Triggered by Drought Stress
One of the most severe abiotic stresses being experienced world-wide is drought. In Arabidopsis seedlings, the results of Shen et al. (2013) showed that treating wild type with polyethylene glycol (PEG) 8000, to simulate drought stress, caused an increase in production rate of endogenous H2S (0.8 nmol mg-1 protein min-1). At early stage of osmotic exposure (PEG 6000 for 2 days), the endogenous H2S in wheat seeds rapidly increased from 1.5 to 3.5 μmol g-1 dry weight (DW) (Zhang et al., 2010a).
H2S Signaling Triggered by Low Temperature Stress
Low temperature is a major environmental stress factors that limit plant growth, development and distribution. In grape (Vitis vinifera L.) seedlings, chilling stress at 4°C induced the expression of L/DCD genes and increased the activities of L/DCD, which in turn enhanced endogenous H2S accumulation (from 7 to 15 μmol g-1 FW) (Fu et al., 2013). Similarly, Shi et al. (2013) also found that cold stress treatment at 4°C could induce the accumulation of endogenous H2S level (14 nmol g-1 FW) in bermudagrass [Cynodon dactylon (L). Pers.] seedlings. To uncover the adaptive strategies of alpine plants to the extremely cold conditions prevailing at high altitudes, Ma et al. (2015), using a comparative proteomics, investigated the dynamic patterns of protein expression in Lamiophlomis rotata plants grown at three different altitudes (4350, 4,800, and 5,200 m), and the results showed that the levels and enzyme activities of proteins (OAS-TL, CAS, L/DCD) involved in H2S biosynthesis markedly increased at higher altitudes (4,800 and 5,200 m), and that H2S accumulation increased to 12, 22, and 24 nmol g-1 FW, respectively, demonstrating that H2S plays a central role during the adaptation of L. rotata to environmental stress at higher altitudes.
H2S Signaling Triggered by High Temperature Stress
Similar to other stresses, high temperature also can induce endogenous H2S generation in many species of plant. In 3-week-old seedlings of tobacco, Chen et al. (2016) found that treatment with high temperature at 35°C increased the activity of LCD, which in turn induced the production of endogenous H2S (8 nmol g-1 FW) in tobacco seedlings, and that H2S production remained elevated level after 3 days of high temperature exposure. More interestingly, H2S production by high temperature can induce the accumulation of jasmonic acid, followed by promoting nicotine synthesis. These data suggest that H2S and nicotine biosynthesis is linked in tobacco plants subjected to high temperature stress. Additionally, heat stress caused a marked modulation in H2S content in strawberry seedlings, as indicated in a significant increase after 1, 4, and 8 h of exposure to 42°C compared with control plants. A significant increase in H2S content was also observed in 0.1 mM NaHS-pretreated plants after 1 h exposure to heat stress, gradually lowering to control levels thereafter (Christou et al., 2014).
H2S Signaling Triggered by UV-B Radiation
Recently, Li et al. (2016) found that UV-B radiation could induce H2S production in leaves of barley seedlings, reaching a peak of approximately 230 nmol g-1 FW after 12 h of exposure, which in turn promoted the accumulation of UV-absorbing compounds flavonoids and anthocyanins. H2S began to decline with time, but it is overall significantly higher than that of the control (approximately 125 nmol g-1 FW) at 48 h of exposure. A similar trend was observed for LCD activity, which was corroborated by the application of DL-propargylglycine (PAG, an inhibitor of LCD) that resulted in complete inhibition of the H2S production and the accumulation of UV-absorbing compounds induced by UV-B radiation (Li et al., 2016).
H2S Signaling Triggered by Hypoxia and Fungal Infection
Flooding often leads to hypoxia in plant roots, which significantly limits agriculture production. In pea (Pisum sativum L.) seedlings, Cheng et al. (2013) found that hypoxia could activate H2S biosynthesis system (LCD, DCD, OAS-TL, and CS), which in turn induced the accumulation of endogenous H2S from approximately 0.9 (control) to 5.1 μmol g-1 FW (hypoxia for 24 h), indicating that H2S might be a hypoxia signaling that triggers the tolerance of the pea seedlings to hypoxic stress, this hypothesis was further supported by exogenously applied NaHS.
Pathogen infection is a common biotic stress in plants. In oilseed rape (Brassica napus L.) seedlings, fungal infection with Sclerotinia sclerotiorum led to an even stronger increase in H2S, reaching a maximum of 3.25 nmol g-1 DW min-1 2 days after infection, suggesting that the release of H2S seems to be part of the response to fungal infection (Bloem et al., 2012).
H2S Signaling Triggered by Exogenously Applied NaHS or Up-regulating the Expression of L/DCD
In addition to above-described abiotic and biotic stressors, H2S signaling in plant cells also can be triggered by exogenously applying NaHS (H2S donor) or up-regulating the expression of genes involved in H2S biosynthesis like L/DCD under normal growth conditions. In strawberry seedlings, treatment of root with 0.1 mM NaHS resulted in significantly elevated H2S concentration (35 nmol g-1 FW) in leaves compared with control plants (25 nmol g-1 FW) (Christou et al., 2013). In wheat seeds, the endogenous H2S level [4.5 μmol g-1 dry weight (DW)] in NaHS-treated seed was slightly higher than that of control (1.7 mol g-1 DW) (Zhang et al., 2010a). These results indicated that H2S is easy to enter into plant cells and follow on being transported to other tissues or organs due to its highly lipophilic property, which in turn exert its physiological role in plants.
Additionally, Jin et al. (2011) found that the Arabidopsis seedlings expressing L/DCD showed higher endogenous H2S content under both normal (6 nmol mg-1 protein min-1) and drought stress conditions (14 nmol mg-1 protein min-1) compared with the control (3 nmol mg-1 protein min-1), and the expression pattern of L/DCD was similar to the drought associated genes dehydration-responsive element-binding proteins (DREB2A, DREB2B, CBF4, and RD29A) induced by dehydration, while exogenous application of H2S (80 μM) was also found to stimulate further the expression of drought associated genes. In addition, drought stress significantly induced endogenous H2S production in both transgenetic plant and wild type, a process that was reversed by re-watering (Jin et al., 2011). Interestingly, Arabidopsis seedlings overexpressing LCD or pre-treated with NaHS exhibited higher endogenous H2S level (from 2 to 10 nmol g-1 FW), followed by improving abiotic stress (drought, salt, and chilling) tolerance and biotic stress (bacteria) resistance, while LCD knockdown plants or HT (H2S scavenger) pre-treated plants displayed lower endogenous H2S level and decreased stress resistance (Shi et al., 2015).
In conclusion, above-mentioned researches in this section display that: (1) under normal growth conditions, the content of endogenous H2S or production rate in various plant species are different, ranging from 2 nmol g-1 FW to 7 μmol g-1 FW or 0.38 to 6 nmol mg-1 protein min-1. These differences may be relative to measurement methods, plant species and development stage, and experiment system. (2) Under abiotic stress conditions, the level of endogenous H2S in various plant species is averagely increased by 2∼2.5-fold, indicating that different environment stresses can trigger the H2S signaling, which may be a trigger that induces the acquisition of cross-adaptation in plants.
H2S-Induced Cross-Adaptation
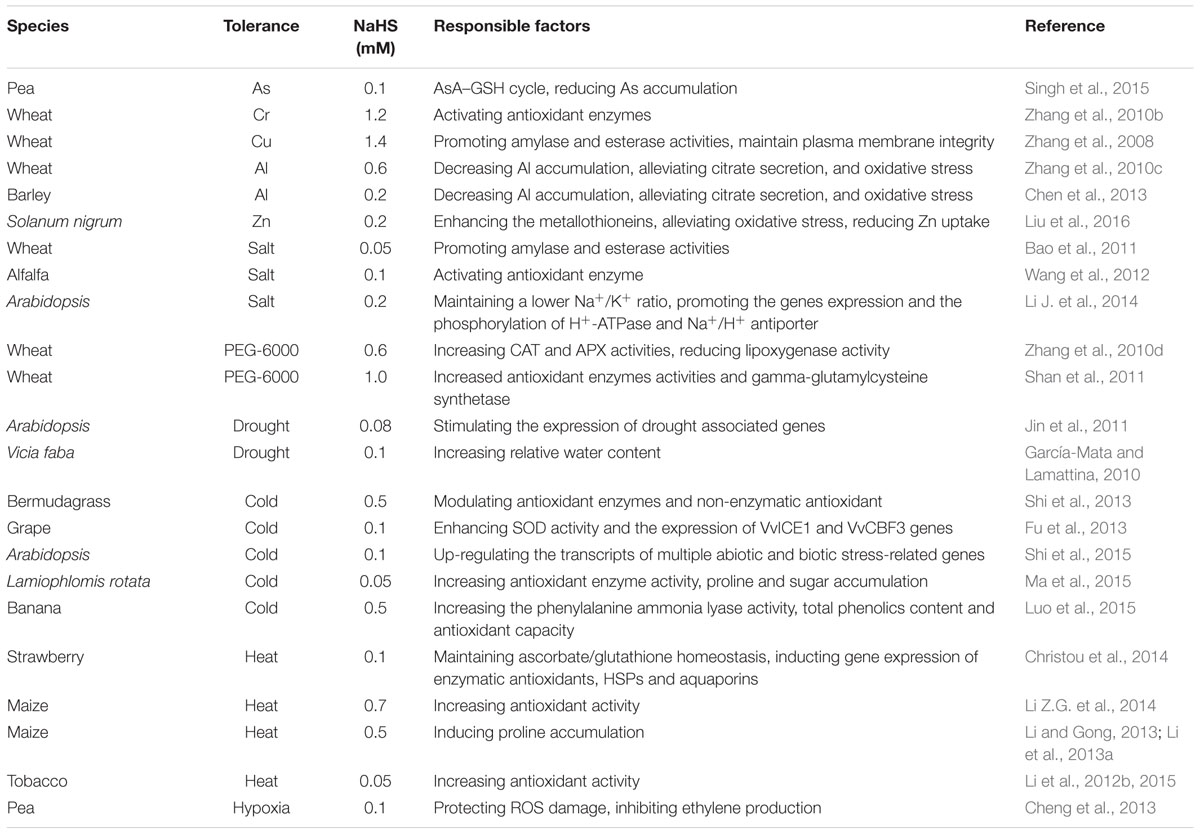
As described above, not only there are a broad range of environmental stressors can trigger H2S signaling in plants, but pretreating plants with exogenously applied H2S can provide additional resistance to subsequent stress exposure. The next section explores the role of H2S as an important signaling molecule for cross-adaptation to HM, salt, drought, cold, heat and flooding stress by enhancing antioxidant system activity, accumulating osmolyte, synthesizing heat shock proteins (HSPs), as well as maintaining ion and nutrient balance (Table 2; Figure 3), which may be common mechanism of cross-adaptation induced by H2S.
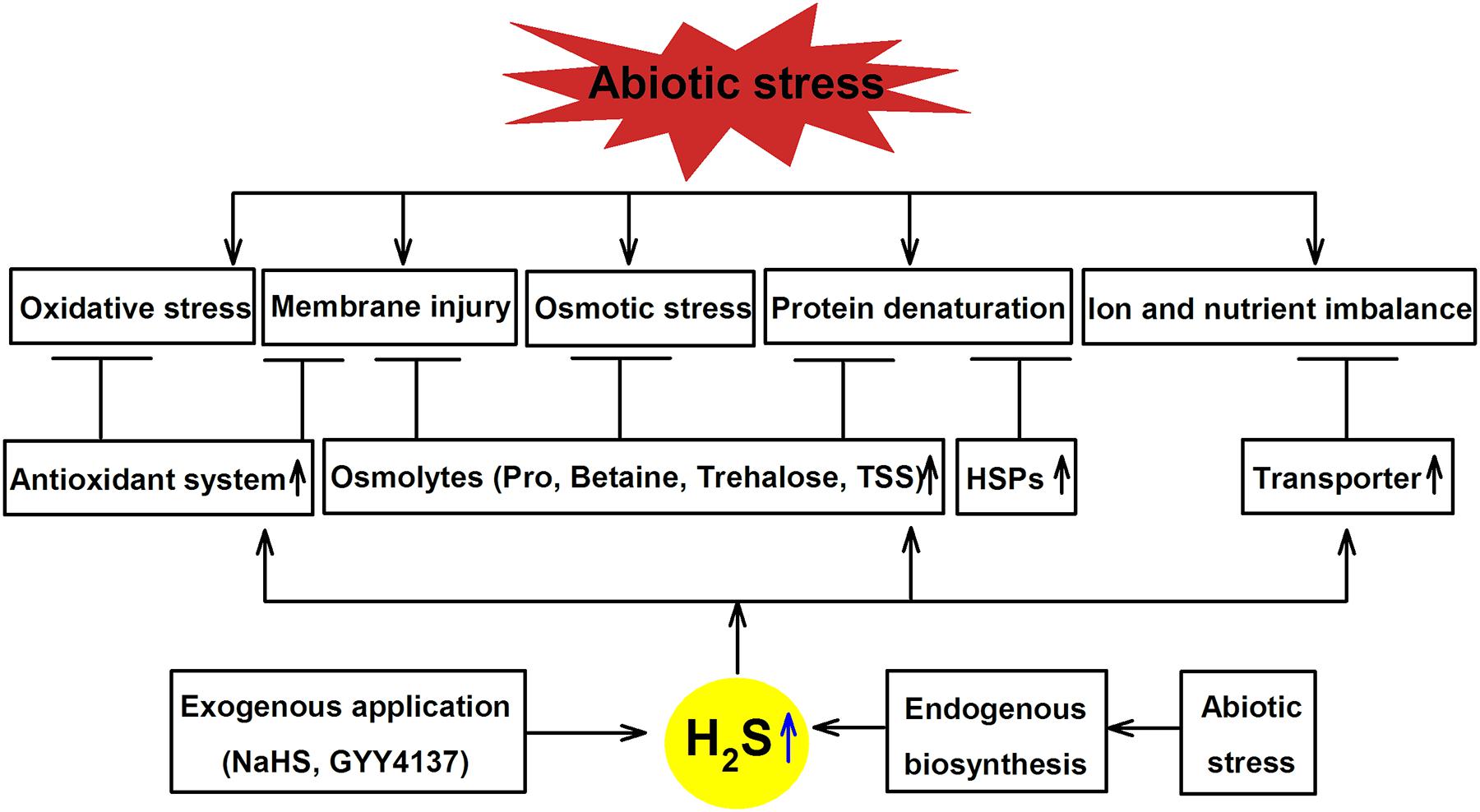
FIGURE 3. Mechanisms underlining H2S-induced abiotic tolerance in plants. Abiotic stress causes oxidative stress, membrane injury, osmotic stress, protein denaturation, as well as ion and nutrient imbalance, while exogenously applied or endogenously synthesized H2S can alleviate these damages by enhancing the activity of antioxidant system, synthesizing osmolytes and heat shock proteins (HSPs) and regulating ion and nutrient balance (adapted from Min et al., 2016).
H2S-Induced Metal and Metalloid Tolerance
Heavy metals refer to a group of metal elements with a density greater than 6 g/cm3, including Cr, Cu, Zn, and so forth (Gupta et al., 2013; Ahmad, 2016). Due to their toxicity and stablility, HM has become the major abiotic stress in plants, and even threatens human health by way of the food chain. HM stress commonly results in oxidative stress, that is, the excessive accumulation of ROS, which leads to lipid peroxidation, protein oxidation, enzyme inactivation, and DNA damage (Yadav, 2010; Gupta et al., 2013; Ahmad, 2016). However, higher plants have evolved a sophisticated antioxidant defense system to scavenge excessive ROS and maintain its homeostasis in plants (Foyer and Noctor, 2009, 2011).
Arsenic (As) is a highly toxic metalloid, it is major pollutant in the soil. In pea seedlings, As treatment increased the accumulation of ROS, which in turn damage to lipids, proteins and biomembranes. Meanwhile, higher cysteine level was observed in As-stress seedlings in comparison to all other treatments (As-free; NaHS; As + NaHS), while these effects were alleviated by the addition of NaHS (Singh et al., 2015). Further experiments showed that As treatment inhibited the activity of the enzymes involved in the ascorbic acid (AsA)–glutathione (GSH) cycle, whereas their activities were enhanced by application of NaHS (Singh et al., 2015). In addition, the redox status of AsA and GSH was disturbed, as indicated by a steep decline in their reduced/oxidized ratios. However, exogenously applied NaHS restored the redox status of the AsA and GSH pools under As stress (Singh et al., 2015). Furthermore, NaHS treatment ameliorated As toxicity, which was coincided with the increased accumulation of H2S. The results demonstrated that H2S might counterbalance ROS-mediated damage to macromolecules by reducing the accumulation of As and triggering up-regulation of the AsA–GSH cycle, further suggesting that H2S plays a crucial role in plant priming, and in particular for pea seedlings in mitigating As stress.
Under Cr stress, exogenous application of NaHS could improve the germination rate of wheat seeds in a dose-dependent manner and the activities of amylase, esterase as well as antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione peroxidase (GPX), whereas reduced the activity of lipoxygenase and over-production of malondialdehyde (MDA) as well as H2O2 induced by Cr, and sustained higher endogenous H2S level (Zhang et al., 2010b). Additionally, NaHS pretreatment increased the activities of SOD and CAT, but decreased that of lipoxygenase in wheat under Cu stress (Zhang et al., 2008), these results were consisted with the response of wheat to Cr stress (Zhang et al., 2010b).
Also, NaHS could alleviate the inhibitory effect of Cu stress in wheat in a dose-dependent manner, and H2S or HS- derived from NaHS rather than other sulfur-containing components (S2-, SO42-, SO32-, HSO4-, and HSO3-) attribute to the potential role in promoting seed germination under Cu stress (Zhang et al., 2008). Further experiments showed that NaHS could increase amylase and esterase activities, reduced the disturbance of plasma membrane integrity induced by Cu in the radicle tips, and sustain lower MDA and H2O2 levels in germinating seeds (Zhang et al., 2008), similar to the reports by (Zhang et al., 2010b).
Aluminium (Al), a non-essential element for plants, adversely affects plant growth, development and survival, especially in acid soil. In barley (Hordeum vulgare L.) seedlings, Al stress inhibited the elongation of roots, while pretreatment with NaHS partially rescued the inhibition of root elongation induced by Al, and this rescue was closely correlated with the decrease of Al accumulation in seedlings (Chen et al., 2013). Additionally, application of NaHS significantly alleviated citrate secretion and oxidative stress (as indicated in lipid peroxidation as well as ROS burst) induced by Al by activating the antioxidant system (Chen et al., 2013). Similar results were reported by Zhang et al. (2010c) in wheat (Triticum aestivum L.).
Though zinc (Zn) is an essential element for plants, its toxic effects can be observed when being excessive accumulation in plants. In Solanum nigrum L. seedlings, H2S ameliorated the inhibition of growth by excess Zn, especially in roots, and an increase in free cytosolic Zn2+ content in roots, which was correlated well with the down-regulation of Zn uptake and homeostasis related genes expression like zinc-regulated transporter (ZRT), iron-regulated transporter (IRT)-like protein (ZIP) and natural resistance associated macrophage protein (NRAMP) (Liu et al., 2016). In addition, H2S further enhanced the expression of the metallothioneins to chelate excessive Zn and alleviated Zn-oxidative stress by regulating the genes expression of antioxidant enzymes (Liu et al., 2016).
H2S-Induced Salt Tolerance
Salts stress is negative effects of excessive salt on seed germination, plant growth and development, and even survival, which is a major abiotic stress in agriculture production world-wide. Salt stress commonly leads to direct and indirect injury, namely ion toxicity, osmotic stress, nutrient imbalance, and oxidative stress (Ahmad et al., 2013a,b). To combat with salt injury, plants have evolved many protective strategies, including osmotic adjustment by synthesizing osmolytes such as proline (Pro), glycine betaine (GB), trehalose (Tre), and total soluble sugar (TSS); ion and nutrient balance by regulating transporter; and enhancement of antioxidant capacity by activating the activity of antioxidant enzymes SOD, CAT, APX, GPX and glutathione reductase (GR), as well as by synthesizing antioxidants like AsA and GSH (Ahmad et al., 2013a,b). In salt-sensitive wheat cultivar LM15, the results of Bao et al. (2011) showed that wheat seed priming with different concentrations of NaHS (0.01, 0.05, 0.09, 0.13 mM) for 12 h could significantly alleviate the inhibition of seed germination and seedling growth induced by 100 mM NaCl in a concentration-dependent manner, as indicated in germination rate, germination index, vigor index and growth of seedlings of wheat. In alfalfa (Medicago sativa), NaHS pretreatment differentially activate total and isoenzymatic activities as well as corresponding transcripts of antioxidant enzymes (SOD, CAT, POD, and APX) under 100 mM NaCl stress, thus resulting in the alleviation of oxidative damage induced by NaCl (Wang et al., 2012). In addition, NaCl stress inhibited seed germination and seedling growth, but pretreatment with NaHS could significantly attenuate this inhibitive effect and increase the ratio of potassium (K) to sodium (Na) in the root parts (Wang et al., 2012). Also, under 100 mM NaCl stress, Arabidopsis roots displayed a great increase in electrolyte leakage and Na+/K+ ratio, indicating that Arabidopsis was sensitive to salt stress, while treatment with NaHS enhanced the salt tolerance by maintaining a higher K+/Na+ ratio (Li J. et al., 2014). In addition, the level of gene expression and the phosphorylation of plasma membrane H+-ATPase and Na+/H+ antiporter protein was promoted by H2S, while the effect of H2S on the plasma membrane Na+/H+ antiporter system was removed by diphenylene iodonium (DPI, a PM NADPH oxidase inhibitor) or dimethylthiourea (DMTU, an ROS scavenger) (Li J. et al., 2014), suggesting that H2S can maintain ion homeostasis in salt-stress Arabidopsis root in the H2O2-dependent manner.
H2S-Induced Drought Tolerance
Similar to other stressors, drought stress, namely water deficiency, usually leads to osmotic stress and oxidative stress, which adversely affects plant growth, development and production (Iqbal et al., 2016). Plants can maintain water balance and ROS homeostasis by osmotic adjustment and antioxidant system (Foyer and Noctor, 2009, 2011; Iqbal et al., 2016). Zhang et al. (2010d) found that the germination rate reduced gradually with the increasing concentrations of PEG-6000, which mimicked osmotic stress, while NaHS treatment could promote wheat seed germination under osmotic stress in a dose-dependent manner, Na+ and other sulfur-containing components (S2-, SO42-, SO32-, HSO4-, and HSO3-) were not able to replace NaHS, confirming H2S or HS- derived from NaHS contribute to the protective roles (Zhang et al., 2010d). Further experiments showed that NaHS treatment significantly increased CAT and APX activities, reduced that of lipoxygenase as well as the accumulation of MDA and H2O2 in seeds (Zhang et al., 2010d). Additionally, exogenously applied NaHS increased the activities of APX, GR, dehydroascorbate reductase (DHAR) and gamma-glutamylcysteine synthetase in wheat seedlings, as well as the contents of AsA, GSH, total ascorbate and total glutathione under water stress compared to the control without NaHS treatment, which in turn decreased the MDA content and electrolyte leakage induced by water deficiency in wheat seedlings (Shan et al., 2011). In Arabidopsis seedlings, under drought stress, the expression pattern of L/DCD was similar to the drought associated genes, whose express was stimulated further by H2S (Jin et al., 2011). Also, seedlings treated with NaHS exhibited a higher survival rate and a significant reduction in the size of the stomatal aperture compared to the control (Jin et al., 2011). In addition to these, García-Mata and Lamattina (2010) also found that, in Vicia faba (L.) var. major and Impatiens walleriana Hook. f., H2S treatment could increase relative water content (RWC) and protect plants against drought stress.
H2S-Induced Cold Tolerance
Low temperature stress includes chilling stress (>0°C) and freezing stress (<0°C). Low temperature usually leads to osmotic stress and oxidative stress, plants can reduce the low temperature injury by osmotic adjustment and activating antioxidant system (Foyer and Noctor, 2009, 2011; Iqbal et al., 2016). Shi et al. (2013) found that exogenous application of NaHS conferred multiple stress tolerance including freezing tolerances in bermudagrass, in reflected in decreased electrolyte leakage and increased survival rate under freezing conditions. Additionally, NaHS treatment mitigated the ROS burst and cell damage induced by freezing stress via modulating the activities of antioxidant enzymes CAT, GPX and GR, as well as non-enzymatic GSH pool and redox state (Shi et al., 2013). In grape (Vitis vinifera L) seedlings, Fu et al. (2013) reported that treatment with NaHS showed the high activity of SOD and gene expression of VvICE1 and VvCBF3, lowed superoxide radical and MDA levels as well as cell membrane permeability under chilling stress at 4°C, while HT treatment displayed contrary effect under the chilling stress. Also, Arabidopsis seedlings overexpressing LCD or pretreating with NaHS exhibited higher endogenous H2S level and stronger chilling stress tolerance, while LCD knockdown or HT pre-treated plants displayed lower endogenous H2S level and weaker stress resistance. Moreover, H2S could up-regulate the expression of genes involved in multiple abiotic and biotic stress and inhibited ROS accumulation (Shi et al., 2015). Ma et al. (2015) found that the levels and enzyme activities of proteins involved in H2S biosynthesis (L/DCD, CAS, OAS-TL) markedly increased at higher altitudes at 4800 and 5200 m, which in turn maintained higher H2S level. Exogenous H2S application reduced ROS and RNS (reactive nitrogen species) damage by increasing antioxidant enzyme and GSNOR (S-nitrosoglutathione reductase) activities, activated the downstream defense response, resulting in protein degradation as well as Pro and SS accumulation. However, such defense responses could be reversed by HT and PAG, respectively. These results illustrated that H2S plays a central role in L. rotata uses multiple strategies to adapt to the alpine stress environment. Also, H2S fumigation maintained higher values of lightness and peel firmness of banana fruit and reduced the accumulation of MDA under chilling stress (Luo et al., 2015). In addition, H2S could increase the activities of GPX, SOD, CAT, APX, GR and the phenylalanine ammonia lyase and total phenolics content, which in turn improved antioxidant capacity of banana fruits, reducing H2O2 and superoxide anion accumulation (Luo et al., 2015). Further experiments also found that H2S fumigation elevated Pro content by activating P5CS activity and decreasing that of ProDH, which might be related to chilling injury tolerance improvement (Luo et al., 2015), similar to the report by Li and Gong (2013). These data indicate that H2S alleviated the chilling injury may be achieved through the enhancement of antioxidant system and Pro accumulation in banana fruit.
H2S-Induced Heat Tolerance
Along with global warming, high temperature has already become a noticeable abiotic stress worldwide, and the mechanisms of high temperature injury and heat tolerance have attracted much attention (Wahid et al., 2007; Asthir, 2015; Hemmati et al., 2015). Christou et al. (2011) found that pre-treatment of roots with NaHS effectively alleviated the decrease in leaf chlorophyll fluorescence, stomatal conductance and relative leaf water content in strawberry (Fragaria x ananassa cv. Camarosa) under heat stress at 42°C, as well as an increase in ion leakage and MDA accumulation in comparison with plants directly subjected to heat stress. In addition, NaHS pretreatment preserved AsA/GSH homeostasis, as evidenced by lower AsA and GSH pool redox disturbances and enhanced transcription of AsA and GSH biosynthetic enzymes, 8 h after heat stress exposure. Furthermore, NaHS root pretreatment increased the gene expression of antioxidant enzymes (cAPX, CAT, MnSOD, GR), heat shock proteins (HSP70, HSP80, HSP90), and aquaporins (PIP) (Christou et al., 2014). These results suggest that H2S root pretreatment activates a coordinated network of heat shock defense-related pathways at a transcriptional level and systemically protects strawberry plants from heat stress-induced damage. Our previous study also showed that 0.7 mM NaHS treatment increased the activities of CAT, GPX, SOD and GR, and the contents of GSH and AsA, as well as the ratio of reduced antioxidants to total antioxidants [AsA/(AsA+DHA) and GSH/(GSH +GSSG)] in maize seedlings under normal culture conditions compared with the control (Li Z.G. et al., 2014). Under heat stress, antioxidant enzymes activities, antioxidants contents and the ratio of the reduced antioxidants to total antioxidants in control and treated seedlings all decreased, but NaHS-treated seedlings maintained higher antioxidant enzymes activities and antioxidants levels as well as reduced antioxidants/total antioxidants ratio (Li Z.G. et al., 2014), similar results also were found in tobacco cells (Li et al., 2015). In addition, NaHS pretreatment significantly increased the survival percentage of tobacco cells under heat stress and regrowth ability after heat stress, alleviated a decrease in vitality of cells and an increase in electrolyte leakage and MDA accumulation (Li et al., 2012b). Meanwhile, the heat tolerance induced by NaHS was markedly enhanced by exogenous application of Ca2+ and its ionophore A23187, respectively, while was weakened by addition of Ca2+ chelator ethylene glycol-bis(b-aminoethylether)- N,N,N′,N′-tetraacetic acid, plasma membrane channel blocker La3+, as well as calmodulin antagonists chlorpromazine and trifluoperazine, respectively (Li et al., 2012b). Similarly, in maize, pretreatment with NaHS markedly improved the germination percentage of seeds and the survival percentage of seedlings under heat stress, alleviated an increase in electrolyte leakage of roots and a decrease in tissue vitality and accumulation of MDA in coleoptiles of maize seedlings (Li et al., 2013a). Furthermore, NaHS pretreatment could improve the activity of Δ1-pyrroline-5-carboxylate synthetase (P5CS) and lowered that of Pro dehydrogenase (ProDH), which in turn induced the accumulation of endogenous Pro in maize seedlings (Li et al., 2013a). Also, exogenously applied Pro could increase endogenous Pro content, followed by increase in the survival percentage of maize seedlings under heat stress (Li et al., 2013a). These results suggest that NaHS pretreatment can improve the heat tolerance in plants and the acquisition of heat tolerance induced by NaHS may require the synergistic effect of antioxidant system, calcium messenger system, HSPs and Pro.
H2S-Induced Flooding Tolerance and Pathogen Resistance
Flooding stress usually causes hypoxia, and even anoxia in plant roots, plants can improve hypoxia tolerance by reducing oxidative damage (van Dongen and Licausi, 2014). Cheng et al. (2013) found that hypoxia could induce root tip death of pea seedlings, while pretreatment with exogenous H2S dramatically alleviated cell death by protecting root tip cell membranes from ROS damage induced by hypoxia and by inhibiting ethylene production. Conversely, root tip death induced by hypoxia was strongly enhanced by inhibiting the key enzymes responsible for endogenous H2S biosynthesis (adding hydroxylamine to inhibit LCD activity). These results demonstrated that H2S can enhance the tolerance of the plant to hypoxic stress by alleviating hypoxia-induced root tip death in pea seedlings.
More interestingly, H2S also could transcriptionally regulate MIR393-mediate auxin signaling, including MIR393a/b and their target genes (TIR1, AFB1, AFB2, and AFB3), and this regulation was related with H2S-induced antibacterial resistance (Shi et al., 2015).
All of the above studies in this section show exogenous application of NaHS (a H2S donor) can induce cross-adaptation to HM, salt, osmosis, drought, cold, heat and hypoxia stresses in different plant species, and the optimal NaHS concentration range from 0.05 to 1.5 mM (Table 2), while higher NaHS concentration (>1.5 mM) exhibits negative effect on plant growth, development, survival, and even the acquisition of stress tolerance. Therefore, the optimal concentration of NaHS should be carefully selected according to plant species and experimental system.
Conclusion and Future Prospective
In general, after undergoing a moderate stress, plants not only can improve the resistance to this stress, but also can increase the tolerance to subsequent other stresses, which known as cross-adaptation. Many studies found that signaling triggered by a moderate stress, such as Ca2+, ABA, H2O2, and NO signaling, is a common response of plants to abiotic and biotic stress, which in turn induces the acquisition of cross-adaptation. In addition, exogenously applied these signal molecules also can trigger corresponding signaling, followed by improving stress tolerance of plants, thus Ca2+, ABA, H2O2, and NO are considered to be candidate signal molecules in cross-adaptation in plants (Knight, 2000; Gong et al., 2001; Li and Gong, 2011; Li et al., 2012b; Fang H. et al., 2014; Qiao et al., 2015; Chen et al., 2016). More recently, many research groups found that a number of abiotic stresses also can trigger H2S signaling, while exogenously applied H2S can induce cross-adaptation to multiple stresses, indicating that H2S represents a potential candidate signal molecule in cross-adaptation in plants (Li, 2013; Lisjak et al., 2013; Calderwood and Kopriva, 2014; Hancock and Whiteman, 2014; Fotopoulos et al., 2015; Guo et al., 2016). However, H2S acts as a signal molecule in plants cross-adaptation, the following questions need to be further answered: (1) Receptor or target of H2S. Due to H2S is easy to penetrate the cell membrane, maybe there is no H2S receptor in plant cells, but Li et al. (2011) and Aroca et al. (2015) found that H2S could modify the activity of some proteins with sulfhydryl (-SH) by sulfhydrylation (-SSH), whether these proteins are the receptors of H2S needs to be further research. (2) Physiological concentration of H2S. Many assay methods for H2S including colorimetric, fluorescence-based, gas chromatographic and electrochemical methods give highly contrasting results (Table 2; Peng et al., 2012; Li, 2015b), so accurate physiological concentration of H2S in plant cells or organelles is waiting for uncovering. It will be important to design the stress treatments closer to physiologically relevant stress intensities, thus low micromolar rather than millimolar HM concentration should be investigated in order to strengthen the conclusions. (3) Crosstalk between H2S and other signal molecules in cross-adaptation. The acquisition of abiotic tolerance is involved in a signal network consisting of many signal molecules including H2S, interaction among signal molecules needs to be updated and perfected. (4) Physiological, biochemical and molecular mechanisms of H2S-induced cross-adaptation. The study on H2S-induced abiotic tolerance including cross-adaptation has just started, many physiological, biochemical and molecular mechanisms require being expounded using transcriptome, proteome and metabolome approaches.
Author Contributions
Z-GL wrote and revised the paper, XM and Z-HZ provided the idea.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SM and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
This research is supported by National Natural Science Foundation of China (31360057) and Doctor Startup Foundation of Yunnan Normal University China (01200205020503099). We appreciate the reviewers and editors for their exceptionally helpful comments about the manuscript.
References
Ahmad, P., Azooz, M. M., and Prasad, M. N. V. (2013a). Ecophysiology and Responses of Plants under Salt Stress. New York, NY: Springer.
Ahmad, P., Azooz, M. M., and Prasad, M. N. V. (2013b). Salt Stress in Plants: Signalling, Omics and Adaptations. New York, NY: Springer.
Aroca, A., Serna, A., Gotor, C., and Romero, L. C. (2015). S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol. 168, 334–342. doi: 10.1104/pp.15.00009
Asthir, B. (2015). Mechanisms of heat tolerance in crop plants. Biol. Plant. 59, 620–628. doi: 10.1007/s10535-015-0539-5
Bao, J., Ding, T. L., Jia, W. J., Wang, L. Y., and Wang, B. S. (2011). Effect of exogenous hydrogen sulfiocde on wheat seed germination under salt stress. Modern Agric. Sci. Technol. 20, 40–42.
Bloem, E., Haneklaus, S., Kesselmeier, J., and Schnug, E. (2012). Sulfur fertilization and fungal infections affect the exchange of H2S and COS from agricultural crops. J. Agric. Food Chem. 60, 7588–7596. doi: 10.1021/jf301912h
Calderwood, A., and Kopriva, S. (2014). Hydrogen sulfide in plants: from dissipation of excess sulfur to signalling molecule. Nitric Oxide 41, 72–78. doi: 10.1016/j.niox.2014.02.005
Chen, J., Wang, W. H., Wu, F. H., You, C. Y., Liu, W. T., Dong, X. K., et al. (2013). Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362, 301–318. doi: 10.1007/s11104-012-1468-0
Chen, X., Chen, Q., Zhang, X., Li, R., Jia, Y., Ef, A., et al. (2016). Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol. Biochem. 104, 174–179. doi: 10.1016/j.plaphy.2016.02.033
Cheng, W., Zhang, L., Jiao, C. J., Su, M., Yang, T., Zhou, L. N., et al. (2013). Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol. Biochem. 70, 278–286. doi: 10.1016/j.plaphy.2013.05.042
Christou, A., Filippou, P., Manganaris, G. A., and Fotopoulos, V. (2014). Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 14:42. doi: 10.1186/1471-2229-14-42
Christou, A., Manganaris, G., Papadopoulos, I., and Fotopoulos, V. (2011). “The Importance of hydrogen sulfide as a systemic priming agent in strawberry plants grown under key abiotic stress factors,” in Proceedings of the 4th International Conference: Plant Abiotic Stress: From Systems Biology to Sustainable Agriculture, Limassol, 47.
Christou, A., Manganaris, G. A., Papadopoulos, I., and Fotopouls, V. (2013). Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 64, 1953–1966. doi: 10.1093/jxb/ert055
Fang, H., Jing, T., Liu, Z., Zhang, L., Jin, Z., and Pei, Y. (2014). Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 56, 472–481. doi: 10.1016/j.ceca.2014.10.004
Fang, T., Cao, Z. Y., Li, J. L., Shen, W. B., and Huang, L. Q. (2014). Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 76, 44–51. doi: 10.1016/j.plaphy.2013.12.024
Fotopoulos, V., Christou, A., Antoniou, C., and Manganaris, G. A. (2015). Hydrogen sulphide: a versatile tool for the regulation of growth and defence responses in horticultural crops. J. Hortic. Sci. Biotechnol. 90, 227–234.
Foyer, C. H., and Noctor, G. (2009). Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox. Signal. 11, 861–905. doi: 10.1089/ars.2008.2177
Foyer, C. H., and Noctor, G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155, 2–18. doi: 10.1104/pp.110.167569
Foyer, C. H., Rasool, B., Davey, J. W., and Hancock, R. D. (2016). Cross-tolerance to biotic and abiotic stresses in plants: a focus on resistance to aphid infestation. J. Exp. Bot. 67, 2025–2037. doi: 10.1093/jxb/erw079
Fu, P. N., Wang, W. J., Hou, L. X., and Liu, X. (2013). Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc. Bot. Pol. 82, 295–302. doi: 10.5586/asbp.2013.031
García-Mata, C., and Lamattina, L. (2010). Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 188, 977–984. doi: 10.1111/j.1469-8137.2010.03465.x
Gong, M., Chen, B., Li, Z. G., and Guo, L. H. (2001). Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J. Plant Physiol. 158, 1125–1130. doi: 10.1078/0176-1617-00327
Gong, M., Li, Y. J., and Chen, S. Z. (1998a). Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 153, 488–496. doi: 10.1016/S0176-1617(98)80179-X
Gong, M., van der Luit, A. H., Knight, M. R., and Trewavas, A. J. (1998b). Heat-shock-induced changes in intracellular Ca2+ Level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437. doi: 10.1104/pp.116.1.429
Grover, M., Ali, S. Z., Sandhya, V., Rasul, A., and Venkateswarlu, B. (2011). Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 27, 1231–1240. doi: 10.1007/s11274-010-0572-7
Guo, H., Xiao, T., Zhou, H., Xie, Y., and Shen, W. (2016). Hydrogen sulfide: a versatile regulator of environmental stress in plants. Acta Physiol. Plant. 38:16. doi: 10.1007/s11738-015-2038-x
Gupta, D. K., Corpas, F. J., and Palma, J. M. (2013). Heavy Metal Stress in Plants. Berlin: Springer.
Hancock, J. T., and Whiteman, M. (2014). Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol. Biochem. 78, 37–42. doi: 10.1016/j.plaphy.2014.02.012
Hancock, J. T., and Whiteman, M. (2016). Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 1365, 5–14. doi: 10.1111/nyas.12733
Hemmati, H., Gupta, D., and Basu, C. (2015). “Molecular physiology of heat stress responses in plants,” in Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Gerspectives, Vol. 2, ed. G. K. Pandey (New York, NY: Springer), 109–142.
Hossain, M. A., Burritt, D. J., and Fujita, M. (2016). “Cross-stress tolerance in plants: molecular mechanisms and possible involvement of reactive oxygen species and methylglyoxal detoxi?cation systems,” in Abiotic Stress Response in Plants, eds N. Tuteja and S. S. Gill (Chennai: Wiley), 323–375.
Iqbal, N., Nazar, R., and Khan, N. A. (2016). Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. London: Springer.
Jin, Z., and Pei, Y. (2015). Physiological implications of hydrogen sulfide in plants: pleasant exploration behind its unpleasant odour. Oxid. Med. Cell Longev. 2015, 1–6. doi: 10.1155/2015/758358
Jin, Z., Shen, J., Qiao, Z., Yang, G., Wang, R., and Pei, Y. (2011). Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem. Biophy. Res. Commun. 414, 481–486. doi: 10.1016/j.bbrc.2011.09.090
Knight, H. (2000). Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 195, 269–324. doi: 10.1016/S0074-7696(08)62707-2
Lai, D., Mao, Y., Zhou, H., Li, F., Wu, M., Zhang, J., et al. (2014). Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci. 225, 117–129. doi: 10.1016/j.plantsci.2014.06.006
Li, J., Jia, H., Wang, J., Cao, Q., and Wen, Z. (2014). Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251, 899–912. doi: 10.1007/s00709-013-0592-x
Li, L., Rose, P., and Moore, P. K. (2011). Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 51, 169–187. doi: 10.1146/annurev-pharmtox-010510-100505
Li, Q., Wang, Z., Zhao, Y., Zhang, X., Zhang, S., Bo, L., et al. (2016). Putrescine protects hulless barley from damage due to UV-B stress via H2S- and H2O2-mediated signaling pathways. Plant Cell Rep. 35, 1155–1168. doi: 10.1007/s00299-016-1952-8
Li, Z. G. (2013). Hydrogen sulfide: a multifunctional gaseous molecule in plants. Russ. J. Plant Physiol. 60, 733–740. doi: 10.1134/S1021443713060058
Li, Z. G. (2015a). Analysis of some enzymes activities of hydrogen sulfide metabolism in plants. Methods Enzymol. 555, 253–269. doi: 10.1016/bs.mie.2014.11.035
Li, Z. G. (2015b). Quantification of hydrogen sulfide concentration using methylene blue and 5,5′-dithiobis (2-nitrobenzoic acid) methods in plants. Methods Enzymol. 554, 101–110. doi: 10.1016/bs.mie.2014.11.031
Li, Z. G. (2015c). Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal. Behav. 10:e1051278. doi: 10.1080/15592324.2015.1051278
Li, Z. G., Ding, X. J., and Du, P. F. (2013a). Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J. Plant Physiol. 170, 741–747. doi: 10.1016/j.jplph.2012.12.018
Li, Z. G., and Gong, M. (2011). Mechanical stimulation-induced cross-adaptation in plants: an overview. J. Plant Biol. 54, 358–364. doi: 10.1007/s12374-011-9178-3
Li, Z. G., and Gong, M. (2013). Mechanical stimulation-induced chilling tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and its relation to proline. Russ. J. Plant Physiol. 60, 149–154. doi: 10.1134/S1021443712060118
Li, Z. G., Gong, M., and Liu, P. (2012a). Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha curcas. Acta Physiol. Plant. 34, 2207–2213. doi: 10.1007/s11738-012-1021-z
Li, Z. G., Gong, M., Xie, H., Yang, L., and Li, J. (2012b). Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 185–186, 185–189. doi: 10.1016/j.plantsci.2011.10.006
Li, Z. G., and Gu, S. P. (2016). Hydrogen sulfide as a signal molecule in hematin-induced heat tolerance of tobacco cell suspension. Biol. Plant. 60, 595–600. doi: 10.1007/s10535-016-0612-8
Li, Z. G., and He, Q. Q. (2015). Hydrogen peroxide might be a downstream signal molecule of hydrogen sulfide in seed germination of mung bean (Vigna radiata). Biologia 70, 753–759. doi: 10.1515/biolog-2015-0083
Li, Z. G., and Jin, J.-Z. (2016). Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tiss. Organ. Cult. 125, 207–214. doi: 10.1007/s11240-015-0939-4
Li, Z. G., Long, W. B., Yang, S. Z., Wang, Y. C., Tang, J. H., Wen, L., et al. (2015). Endogenous hydrogen sulfide regulated by calcium is involved in thermotolerance in tobacco Nicotiana tabacum L. suspension cell cultures. Acta Physiol. Plant. 37:219. doi: 10.1007/s11738-015-1971-z
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X., and Shen, Z. Z. (2013b). Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 36, 1564–1572. doi: 10.1111/pce.12092
Li, Z. G., Yi, X. Y., and Li, Y. T. (2014). Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 69, 1001–1009. doi: 10.2478/s11756-014-0396-2
Lin, Y. T., Li, M. Y., Cui, W. T., Lu, W., and Shen, W. B. (2012). Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 31, 519–528. doi: 10.1007/s00344-012-9262-z
Lisjak, M., Teklic, T., Wilson, I. D., Whiteman, M., and Hancock, J. T. (2013). Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ. 36, 1607–1616. doi: 10.1111/pce.12073
Liu, X., Chen, J., Wang, G. H., Wang, W. H., Shen, Z. J., Luo, M. R., et al. (2016). Hydrogen sulfide alleviates zinc toxicity by reducing zinc uptake and regulating genes expression of antioxidative enzymes and metallothioneins in roots of the cadmium/zinc hyperaccumulator L. Plant Soil 400, 177–192. doi: 10.1007/s11104-015-2719-7
Luo, Z., Li, D., Du, R., and Mou, W. (2015). Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 183, 144–151. doi: 10.1016/j.scienta.2014.12.021
Ma, L., Yang, L., Zhao, J., Wei, J., Kong, X., Wang, C., et al. (2015). Comparative proteomic analysis reveals the role of hydrogen sulfide in the adaptation of the alpine plant Lamiophlomis rotate to altitude gradient in the Northern Tibetan Plateau. Planta 241, 887–906. doi: 10.1007/s00425-014-2209-9
Min, X., Zhou, Z. H., and Li, Z. G. (2016). The metabolism of signal molecule hydrogen sulfide and its role in the acquisition of heat tolerance in plants. Plant Physiol. J. 52, 37–46.
Mostofa, M. G., Rahman, A., Ansary, M. M. U., Watanabe, A., Fujita, M., and Tran, L. P. (2015). Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 5:14078. doi: 10.1038/srep14078
Niu, L., and Liao, W. (2016). Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front. Plant Sci. 7:230. doi: 10.3389/fpls.2016.00230
Pandey, G. K. (2015). Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives. New York, NY: Springer.
Peng, H. J., Chen, W. X., and Wang, B. H. (2012). “Methods for the detection of gasotransmitters,” in Gasotransmitters: Physiology and Pathophysiology, eds A. Hermann, G. F. Sitdikova, and T. M. Weiger (Heidelberg: Springer), 99–137.
Pineda, A., Pangesti, N., Soler, R., van Dam, N. M., van Loon, J. J. A., and Dicke, M. (2016). Negative impact of drought stress on a generalist leaf chewer and a phloem feeder is associated with, but not explained by an increase in herbivore-induced glucosinolates. Environ. Exp. Bot. 123, 88–97. doi: 10.1016/j.envexpbot.2015.11.007
Qiao, Z., Jing, T., Liu, Z., Zhang, L., Jin, Z., Liu, D., et al. (2015). H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 393, 137–146. doi: 10.1007/s11104-015-2475-8
Scuffi, D., Lamattina, L., and García-Mata, C. (2016). Gasotransmitters and stomatal closure: is there redundancy, concerted action, or both? Front. Plant Sci. 7, 277. doi: 10.3389/fpls.2016.00277
Shan, C. J., Zhang, S. L., Li, D. F., Zhao, Y. Z., Tian, X. L., Zhao, X. L., et al. (2011). Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol. Plant. 33, 2533–2540. doi: 10.1007/s11738-011-0746-4
Shen, J., Xing, T., Yuan, H., Liu, Z., Jin, Z., Liu, Z., et al. (2013). Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS ONE 8:e77047. doi: 10.1371/journal.pone.0077047
Shi, H., Ye, T., and Chan, Z. (2013). Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 71, 226–234. doi: 10.1016/j.plaphy.2013.07.021
Shi, H., Ye, T., Han, N., Bian, H., Liu, X., and Chan, Z. (2015). Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Integr. Plant Biol. 57, 628–640. doi: 10.1111/jipb.12302
Singh, V. P., Singh, S., Kumar, J., and Prasad, S. M. (2015). Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: possible involvement of nitric oxide. J. Plant Physiol. 181, 20–29. doi: 10.1016/j.jplph.2015.03.015
van Dongen, J. T., and Licausi, F. (2014). Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia. Wien: Springer.
Wahid, A., Gelani, S., Ashraf, M., and Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wang, L., Wan, R., Shi, Y., and Xue, S. (2016). Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ modules. Mol. Plant 9, 489–491. doi: 10.1016/j.molp.2015.11.010
Wang, R. (2012). Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896. doi: 10.1152/physrev.00017.2011
Wang, Y. Q., Li, L., Cui, W. T., Xu, S., Shen, W. B., and Wang, R. (2012). Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351, 107–119. doi: 10.1007/s11104-011-0936-2
Wojtyla, L., Lechowska, K., Kubala, S., and Garnczarska, M. (2016). Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 7:66. doi: 10.3389/fpls.2016.00066
Yadav, S. K. (2010). Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 76, 167–179. doi: 10.1016/j.sajb.2009.10.007
Yamasaki, H., and Cohen, M. F. (2016). Biological consilience of hydrogen sulfide and nitric oxide in plants: gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 5, 91–100. doi: 10.1016/j.niox.2016.04.002
Zhang, H., Dou, W., Jiang, C. X., Wei, Z. J., Liu, J., and Jones, R. L. (2010a). Hydrogen sulide stimulates β-amylase activity during early stages of wheat grain germination. Plant Signal. Behav. 5, 1031–1033. doi: 10.4161/psb.5.8.12297
Zhang, H., Hu, L. Y., Li, P., Hu, K. D., Jiang, C. X., and Luo, J. P. (2010b). Hydrogen sulfide alleviated chromium toxicity in wheat. Biol. Plant. 54, 743–747. doi: 10.1007/s10535-010-0133-9
Zhang, H., Hu, L. Y., Hu, K. D., He, Y. D., Wang, S. H., and Luo, J. P. (2008). Hydrogen sulide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 50, 1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x
Zhang, H., Hu, S. L., Zhang, Z. J., Hu, L. Y., Jiang, C. X., Wei, Z. J., et al. (2011). Hydrogen sulfide acts as a regulator of ?ower senescence in plants. Postharv. Biol. Technol. 60, 251–257. doi: 10.1016/j.postharvbio.2011.01.006
Zhang, H., Tan, Z. Q., Hu, L. Y., Wang, S. H., Luo, J. P., and Jones, R. L. (2010c). Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J. Integr. Plant Biol. 52, 556–567. doi: 10.1111/j.1744-7909.2010.00946.x
Zhang, H., Wang, M. F., Hua, L. Y., Wang, S. H., Hua, K. D., Bao, L. J., et al. (2010d). Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ. J. Plant Physiol. 57, 532–539. doi: 10.1134/S1021443710040114
Keywords: cross-adaptation, hydrogen sulfide, signal crosstalk, stress tolerance
Citation: Li Z -G, Min X and Zhou Z -H (2016) Hydrogen Sulfide: A Signal Molecule in Plant Cross-Adaptation. Front. Plant Sci. 7:1621. doi: 10.3389/fpls.2016.01621
Received: 07 May 2016; Accepted: 13 October 2016;
Published: 26 October 2016.
Edited by:
Hanjo A. Hellmann, Washington State University, USAReviewed by:
Karl-Josef Dietz, Bielefeld University, GermanySutton Mooney, Washington State University, USA
Copyright © 2016 Li, Min and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-Guang Li, emhvbmdndWFuZ19saUAxNjMuY29t
 Zhong-Guang Li
Zhong-Guang Li Xiong Min
Xiong Min Zhi-Hao Zhou
Zhi-Hao Zhou