- Department of Biological Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI, USA
Natural floral organ degeneration or abortion results in unisexual or fully sterile flowers, while abiotic stresses lead to sterility after initiation of floral reproductive organs. Since normal flower development is essential for plant sexual reproduction and crop yield, it is imperative to have a better understanding of plant sterility under regular and stress conditions. Here, we review the functions of ABC genes together with their downstream genes in floral organ degeneration and the formation of unisexual flowers in Arabidopsis and several agriculturally significant cereal grains. We further explore the roles of hormones, including auxin, brassinosteroids, jasmonic acid, gibberellic acid, and ethylene, in floral organ formation and fertility. We show that alterations in genes affecting hormone biosynthesis, hormone transport and perception cause loss of stamens/carpels, abnormal floral organ development, poor pollen production, which consequently result in unisexual flowers and male/female sterility. Moreover, abiotic stresses, such as heat, cold, and drought, commonly affect floral organ development and fertility. Sterility is induced by abiotic stresses mostly in male floral organ development, particularly during meiosis, tapetum development, anthesis, dehiscence, and fertilization. A variety of genes including those involved in heat shock, hormone signaling, cold tolerance, metabolisms of starch and sucrose, meiosis, and tapetum development are essential for plants to maintain normal fertility under abiotic stress conditions. Further elucidation of cellular, biochemical, and molecular mechanisms about regulation of fertility will improve yield and quality for many agriculturally valuable crops.
Introduction
Flower development is a long and complex process, which is mainly classified into four stages: flowering transition, floral meristem identity, floral organ identity, and floral organ morphogenesis. Mainly using model species Arabidopsis thaliana and snapdragon (Antirrhinum majus), extensive molecular genetic studies have identified numerous genes required for flower development, particularly during early stages. Arabidopsis plants produce raceme-type indeterminate inflorescences where flowers are indefinitely generated. A typical Arabidopsis flower contains four protective sepals in the first whorl, four petals in the second whorl, six stamens (male reproductive organs) in the third whorl, and two fused carpels (female reproductive structure) that form the gynoecium in the fourth whorl (Figures 1A,B). Different from Arabidopsis, Poaceae plants, commonly known as grasses, produce determinate panicles where flowers (or florets) are organized into spikelets. In maize, these spikelets are grouped into separate male and female inflorescences (Figure 2A). The highly branched male inflorescence, the tassel, is composed of spikelet pairs, each of which comprises an upper and a lower floret surrounded by the leaf like structures known as glumes (Figure 2B). Similarly, spikelet pairs are formed in the female inflorescence, but the lower floret in each spikelet pair is aborted (Figure 2C) (Zhang and Yuan, 2014). Poaceae flowers have stamens and carpels similar to eudicot flowers, such as Arabidopsis. In maize, the male floret contains three stamens (Figures 1C,D), while the female floret produces three central carpels which are fused to form the pistil (Figures 1E,F) (Zhang and Yuan, 2014). Maize florets do not contain sepals and petals. Instead, the sepal-analogous organs lemma and palea are produced (Figures 1C–F) (Schmidt and Ambrose, 1998; Lombardo and Yoshida, 2015). Additionally, the petal analogous structures known as lodicules are essential for pollination via opening the bract organs (Yoshida, 2012).
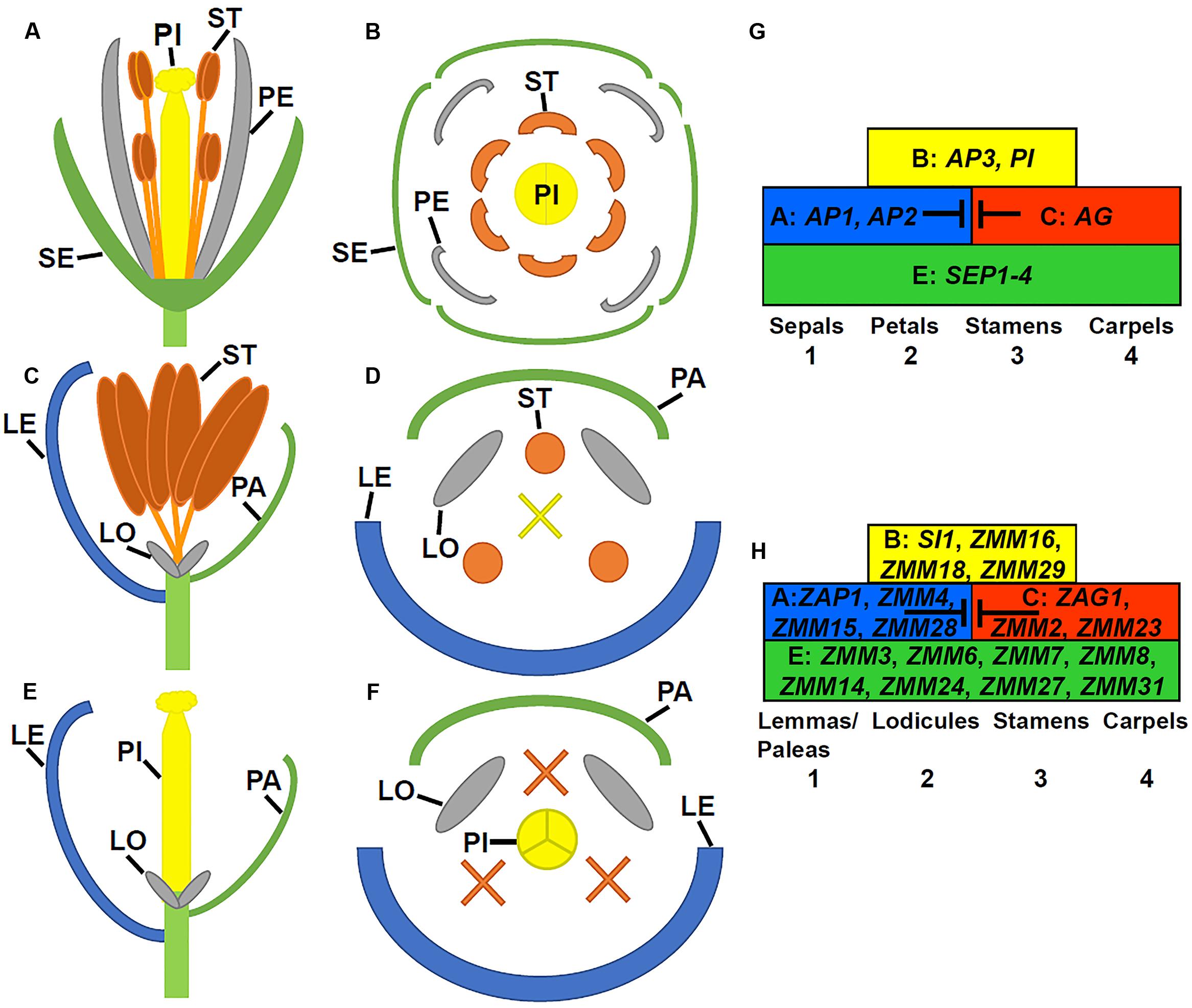
FIGURE 1. Flower structures in Arabidopsis and maize as well as ABCE model. (A) A longitudinal view through a mature Arabidopsis flower (only 2 of 4 long stamens shown). (B) A cross view through an Arabidopsis flower. (C) A longitudinal view through a mature male upper floret in maize. (D) A cross view through a male maize flower. (E) A longitudinal view through a mature female upper floret in maize. (F) A cross section view a female maize flower. LE, lemma; LO, lodicule; PA, palea; PE, petal; PI, pistil; SE, sepal; and ST, stamen. X indicates the aborted carpels. (G) The ABCE model in Arabidopsis. (H) The ABCE model in maize with most likely orthologous genes.
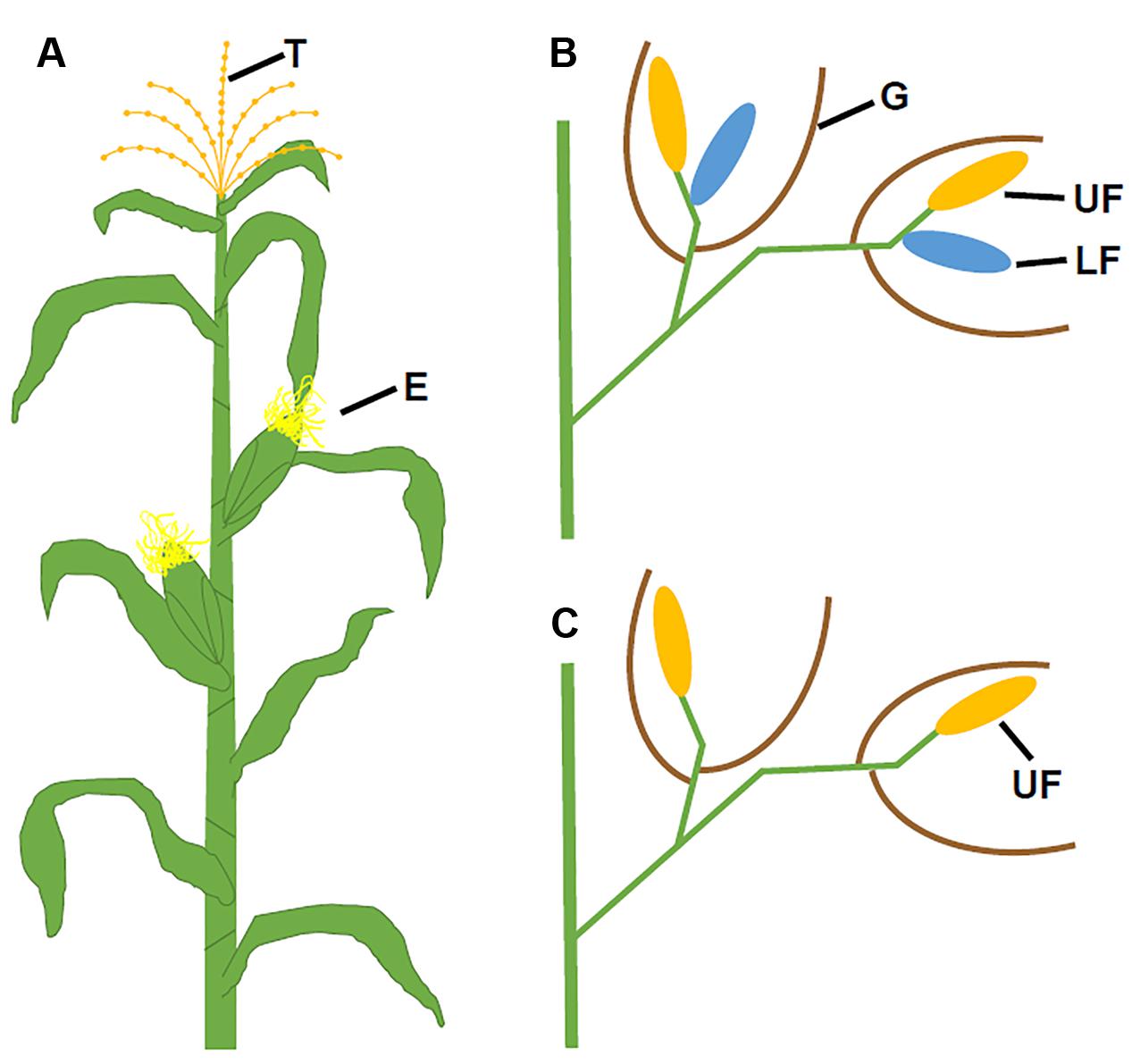
FIGURE 2. Positions of maize florets in ear and tassel. (A) A mature maize plant with positions of the tassel and ears. (B) Zoomed in view of male florets in the tassel showing the upper florets (yellow), lower florets (blue), and glumes (brown). (C) Enlarged view of female florets in the ear showing only the upper florets (yellow) after the degeneration of the lower florets. E, ears; G, glumes; LF, lower floret, T, tassel; and UF, upper floret. (B,C) Modified from Bortiri and Hake (2007).
Within cereal grains, floral organ degeneration is not unique to maize. During development in grain crops such as wheat (Triticum aestivum), rice (Oryza sativa), and sorghum (Sorghum bicolor), the arrest of stamen or carpel primordia, or both potentially results in reduced fertility or completely sterile flowers (Bommert et al., 2005; Yoshida and Nagato, 2011; Aryal and Ming, 2014). In the floret pair of sorghum, one floret is bisexually fertile, whereas the other one is bisexually sterile. Similarly, wild barley (Hordeum vulgare) produces a central fertile floret surrounded by a pair of sterile florets, and even oats (Avena sativa) are known to form sterile flowers at the apex of the rachilla (Schmidt and Ambrose, 1998). Additionally, abiotic stresses cause flower sterility, which consequently results in yield loss. In this review, we will focus on discussing molecular genetic and physiological mechanisms underlying sterility caused by floral organ degeneration and abiotic stresses mainly in Arabidopsis and key cereal grain plants.
Molecular Genetic Regulation of Floral Organ Degeneration
Degeneration or abortion of developing stamens and/or pistil is a main mechanism used by plants to produce unisexual flowers or sterile flowers. In Arabidopsis, there are four major classes of genes that specify floral organ identity. Class A genes [APETALA1 (AP1) and AP2], class B genes [AP3 and PISTILLATA (PI)], the class C gene [AGAMOUS (AG)], and the semi-redundant class E genes [SEPALATTA1-4 (SEP1-4)]. Class A and E genes are required for specifying sepals in the first whorl. Class A, B, and E genes in combination control petal identity in the second whorl. Class B, C, and E genes direct the stamen identity in the third whorl, and class C and E genes specify carpels in the fourth whorl (Figure 1G) (Bowman et al., 1991; Rounsley et al., 1995; Pelaz et al., 2000; Ditta et al., 2004). The ABCE model can also be applied to flower development in other plants including the Poaceae, although many variations exist (Figure 1H; Table 1). Altered expression patterns of B and C class genes can result in floral organ degeneration and sterility.
The extensive roles and interactions of ABC genes in flower development are summarized in Prunet and Jack (2014). What is less clear and receives less attention is that after the establishment of floral organ identity, how floral organ identity genes play a role in development of functional floral organs. AG is required throughout reproductive development for establishing fertility. Specifically, AG is expressed in stamen and carpel primordia initially, and later in specific cell types of stamens and carpels (Bowman et al., 1991). AG (along with PI and AP3) controls stamen development via directly activating the expression of SPOROCYTELESS/NOZZLE (SPL/NZZ), which in turn regulates microsporogenesis (Ito et al., 2004; Liu et al., 2009). AG also upregulates the expression of the DEFECTIVE IN ANTHER DEHISCENCE 1 (DAD1) gene that encodes a jasmonic acid (JA) biosynthesis enzyme (Ito et al., 2007). The dad1 mutant produces immature pollen, resulting in male sterility. If AG is not expressed in flowers prior to stage 7 in Arabidopsis, plants fail to undergo microsporogenesis, while increased duration of AG expression enhances normal stamen and pollen production (Ito et al., 2007). Similarly, in maize branched silkless1-2 (bd1-2) mutants, loss of expression of class C and D (ovule specification) genes like ZAG1 (Zea mays AG1), ZAG2, and ZMM2 (Zea mays MADS2) may cause female sterility (Colombo et al., 1998).
Flowers destined to be male or female often begin as hermaphroditic flowers, but later undergo a programmed degeneration of the gynoecium or androecium, respectively, in early reproductive development. This degeneration is often accompanied by down regulation of B and C class genes (Ainsworth et al., 2005). Unisexual flowers in plants like asparagus (Asparagus officinalis) undergo abortion late in development at the onset of meiosis, although remnants of male or female organs remain (Dellaporta and Calderon-Urrea, 1993; Aryal and Ming, 2014). In female asparagus flowers, the expression of B class gene AODEF (Asparagus officinalis DEFICIENS) is decreased in the stamen, which may cause stamen degeneration (Park et al., 2003). Loss of class B gene function also leads to stamen degeneration in the tulip (Tulipa gesneriana) mutant viridiflora (Kanno et al., 2007). In male sorrel (Rumex acetosa) flowers, both class B and C genes are present in early male flower formation. In later stages, the expression of a class C gene is not detectable in the region that would specify carpels in a female or hermaphroditic flower (Ainsworth et al., 2005). In white champion (Silene latifolia), the class C gene SLM1 (Silene latifolia MADS1) is expressed until meiosis in male and female floral organs. Later in female flower development, stamens undergo degeneration. The expression of SLM1 is not detected in aborted stamens, while its expression persists in the undeveloped gynoecium of male flowers (Hardenack et al., 1994). Moreover, in S. latifolia, Arabidopsis orthologs of SHOOT MERISTEMLESS (SLSTM1 and SLSTM2) and CUP SHAPED COTYLEDON (SLCUC1 and SLCUC2) likely control sex determination via regulating cellular proliferation in the third whorl (Zluvova et al., 2006).
Growing evidence supports that the early loss of class B and C genes leads to the arrest of development in reproductive organ primordia and ultimately the inability of these flowers to form functional carpels or stamens. It is clear that during development class B and C genes must be expressed in the correct location for a sufficient duration. Without the normal expression, flowers exhibit a wide array of phenotypes, ranging from floral organs present in incorrect whorls, to unisexual flower development, and even complete sterility.
Besides class B and C genes, many additional genes are essential for the establishment of the unisexual state in monoecious plants. Male and female flower development in plants like maize begins as identical, but degeneration of gynoecium primordia in the male flowers and degeneration of stamen primordia in the female flowers result in the production of two distinct flower types (Dellaporta and Calderon-Urrea, 1993; Irish, 1996). TASSEL SEED (TS) genes are responsible for normal pistil abortion in the tassel. In recessive ts1 and ts2 mutants, feminization of tassels occurs and pistillate flowers are formed. The ts1 mutant phenotype is attributable to a mutation in a lypoxygenase that produces JA (DeLong et al., 1993; Malcomber and Kellogg, 2006; Acosta et al., 2009). TS2 (a short-chain alcohol dehydrogenase) triggers the programmed cell death (PCD) of pistils (DeLong et al., 1993; Parkinson et al., 2007). In silkless1 (sk1) mutants, pistils are not developed in female florets, while male florets are unaffected (Malcomber and Kellogg, 2006). In the ear, SK1 protects pistils from undergoing PCD caused by TS2 (Calderon-Urrea and Dellaporta, 1999). Similarly, in the maize relative Tripsacum, the TS2 homolog GYNOMONOECIOUS SEX FORM1 is expressed in pistils prior to abortion (Li et al., 1997).
Moreover, in required to maintain repression6 (rmr6) mutants, pistils fail to abort, which causes the feminization in tassels (Parkinson et al., 2007). RMR6 (encoding the largest subunit of RNA polymerase IV, an orthologue of Arabidopsis NRPD1a) acts by limiting the activity of SK1 to the primary ear floret, resulting in PCD of the gynoecium in the tassel and the secondary ear floret (Parkinson et al., 2007; Erhard et al., 2009). In each of the dominant single mutants of Ts3, Ts5, and Ts6, as well as the recessive mutant ts4, a variety of phenotypes are observed in the tassel, such as reduced tassel size, bisexual flowers, and feminization of the tassel (Veit et al., 1993; Irish et al., 1994). Key genes involved in unisexual flower development in maize are summarized in Table 1.
In addition to the formation of unisexual flowers, completely sterile flowers are also commonly produced in grasses due to floral organ degeneration. In some cereal grains, a fertility conversion of sterile flowers is possible. In the sorghum multiseeded1 (msd1) mutant, the development of bisexually sterile flowers become normal, leading to the formation of all fertile flowers (Burow et al., 2014). In barley, the vrs1 (six-rowed spike1) mutation results in fully fertile barley known as six-rowed barley. In the wild-type barley, the VRS1 gene suppresses lateral spikelet development, causing a central fertile floret surrounded by two sterile florets (Komatsuda et al., 2007).
Roles of Hormones in Floral Organ Development
Hormones have strong influences on flower sexuality and fertility. Some hormones are essential for both male and female organ development, while others are male or female specific (Figure 3). Brassinosteroids (BRs) and JA promote male but suppress female organ development in both Arabidopsis and maize (Clouse et al., 1996; Szekeres et al., 1996; Clouse and Sasse, 1998; Stintzi and Browse, 2000; Zhao and Ma, 2000; Li et al., 2001; Nagpal et al., 2005; Mandaokar et al., 2006; Acosta et al., 2009; Yan et al., 2012). Ethylene has been shown to act as a feminizing agent in plants like cucumber, but its role in Arabidopsis and maize is less understood (Yin and Quinn, 1995; Duan et al., 2008; Wang et al., 2010). The function of gibberellic acid (GA) is conflicting, as it is critical for proper male organ development in Arabidopsis, but it antagonizes stamen development in the maize ear (Fujioka et al., 1988; Dellaporta and Calderon-Urrea, 1994; Bensen et al., 1995; Spray et al., 1996; Goto and Pharis, 1999; Michaels and Amasino, 1999; Cheng et al., 2004; Yu et al., 2004; Hu et al., 2008). Differently, auxin is necessary for both male and female floral organ development (Okada et al., 1991; Sessions et al., 1997; Nagpal et al., 2005; Cheng et al., 2006; Wu et al., 2006; Dong et al., 2013).
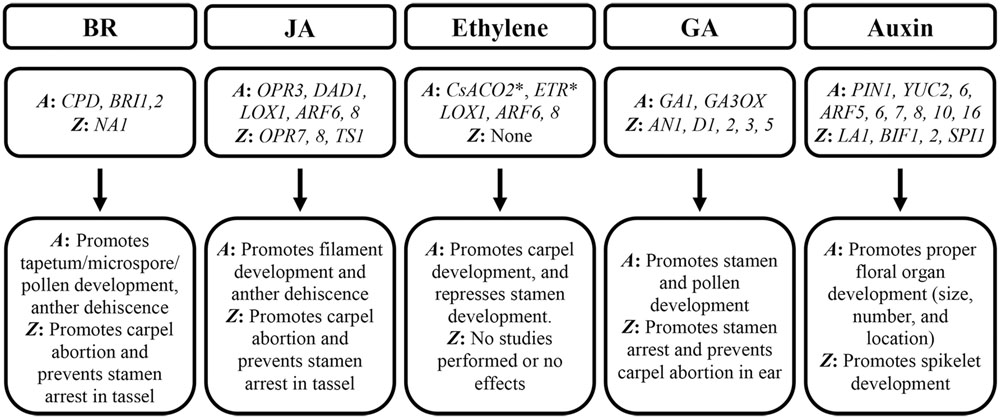
FIGURE 3. Effects of hormones on male and female flower development. Genes involved in hormone biosynthesis, transport, and perception are shown. A: Arabidopsis thaliana, Z: Zea mays, ∗ Indicates cucumber genes used in Arabidopsis studies.
Brassinosteroid
Brassinosteroids are widely involved in cell expansion, cell division, senescence, vascular differentiation, and stress responses. Overall, BRs promote the formation of stamens and pollen in both Arabidopsis and maize, and the abortion of pistils in staminate maize flowers. The constitutive photomorphogenesis and dwarfism (cpd) mutant which fails to form the ecdysone-like brassinosteroids, produces pollen defective in pollen tube elongation (Szekeres et al., 1996). Both cpd and brassinosteroid-insensitive1 (bri1) mutants make far fewer pollen grains per locule with limited viability. Similarly, the brassinosteroid-insensitive2 (bin2) mutant is male sterile (MS; Li et al., 2001). Further studies show that BRs control male fertility via regulating expression of genes critical for anther and pollen development, such as SPL/NZZ, DEFECTIVE IN TAPETAL DEVELOPMENT AND FUNCTION 1 (TDF1), ABORTED MICROSPORES (AMS), and MS1 and MS2 genes (Ye et al., 2010). Additionally, BRs are required for sex determination in maize. In the maize nana plant 1 (na1) mutant tassel, some carpels fail to abort, resulting in both staminate and pistillate flowers (Hartwig et al., 2011). The na1 mutation occurs in ZmDET2, a homolog of the Arabidopsis DE-ETIOLATED2 (DET2) which encodes the important BR biosynthesis enzyme 5α-steroid reductase, suggesting an important role of BRs in the formation of tassel flowers in maize.
Jasmonic Acid
In Arabidopsis and the maize tassel, JA is crucial for stamen and pollen maturation. In Arabidopsis, the 12-oxophydoienoic acid reductase 3 (opr3) mutant is deficient in JA synthesis at the conversion of linolenic acid to JA. The opr3 mutant produces stamens that are abnormal in filament elongation and dehiscence (Stintzi and Browse, 2000; Zhao and Ma, 2000; Mandaokar et al., 2006). Maize has a series of OPR genes, among which OPR7 and OPR8 represent the Arabidopsis OPR3 orthologs (Zhang et al., 2005; Yan et al., 2012). The opr7-5 opr8-2 double mutant plants form feminized tassels devoid of stamen formation and are capable of seed production if pollinated with wild-type pollen. This phenotype can be reversed by exogenous application of JA (Acosta et al., 2009; Yan et al., 2012). A similar phenotype is observed when the AG expression is lost, as AG promotes the DAD1 (JA biosynthesis enzyme) expression (Ito et al., 2007). AUXIN RESPONSE FACTOR (ARF) transcription factors ARF6 and ARF8 are required for JA production. Disruption of ARF6 and ARF8 genes results in delayed stamen development, and consequently the complete male sterility (Nagpal et al., 2005). As discussed above, feminization in the ts1 tassel is attributed to loss of JA synthesis (Acosta et al., 2009). In the tomato JAjas–insensitive1 (jai-1) mutant, the male fertility is also greatly affected with about 28% of pollen being viable and only 10% actually germinating (Li et al., 2004). However, it is believed that the additional female sterility may be caused by arrest in embryo/seed development.
The effects of JA on stamen development and male fertility in maize and Arabidopsis are consistent. In both organisms JA promotes male organ development, but suppresses female organ development. Unlike in Arabidopsis, JA is also important for female fertility in tomato, indicating potentially divergent and complex roles of JA in plant sexual reproduction that warrant further exploration. Of particular interest, AG is required for the DAD1 expression, suggesting interaction between the JA signaling and the class C gene AG.
Ethylene
Ethylene promotes the formation of female flowers in cucumber (Cucumis sativus). CsACO2 (OXIDASE GENE2) encodes an ACC OXIDASE which oxidizes ethylene intermediates to form ethylene. Transgenic Arabidopsis plants expressing CsACO2 under control of the AP3 promoter display repressed stamen development and male sterility (Yin and Quinn, 1995; Duan et al., 2008). Down-regulation of the ethylene receptor gene ETR1 results in the decrease of the ETR1-interacting kinase CTR1, which a repressor of the ethylene signaling. Loss of ETR1 fails the formation of ETR1-CTR1 complex. Consequently, de-suppression of the ethylene response pathway causes the production of female flowers in Arabidopsis (Wang et al., 2010). So far, little is known about the effects of ethylene on flower development in monocots, including maize.
Gibberellin
In Arabidopsis, gibberellin (GA) deficiencies greatly impact male fertility, resulting in partial or complete male sterility. Conversely, GA deficiencies promote stamen maturation in the maize ear, leading to the formation of perfectly bisexual flowers.
In Arabidopsis, the GA deficient mutant ga1-3, which fails to catalyze the first step in GA biosynthesis due to a deletion in ent-KAURENE SYNTHASE, exhibits abnormal microsporogenesis and retarded growth of all floral organs, e.g., stamens with greatly shortened filaments that cannot pollinate pistils (Michaels and Amasino, 1999; Cheng et al., 2004; Yu et al., 2004). Similarly, the ga1-1 mutant has severe defects in stamen and pollen maturation as well as petal and sepal growth (Goto and Pharis, 1999). DELLA proteins (transcriptional repressors), such as RGA, RGA-LIKE1 (RGL1), and RGL2, repress stamen development (Cheng et al., 2004). The DELLA degradation triggered by GA activates JA biosynthesis genes DAD1 and LIPOXYGENASE 1 (LOX1; Cheng et al., 2009).
In Arabidopsis, during GA biosynthesis, four GIBBERELLIN 3-OXIDASE (GA3OX) genes are responsible for the final GA activation. The ga3ox1 ga3ox3 double mutant shows high frequency of sterility on the lowest siliques with fertility restoration after the 20th to 25th silique, whereas triple mutants ga3ox1 ga3ox3 ga3ox4 and ga3ox1 ga3ox2 ga3ox3, on average, underwent a later conversion. This sterility is caused by abnormal anther dehiscence and shortened anther filaments, highlighting that GA is required for stamen development in Arabidopsis (Hu et al., 2008).
In the maize ear, GA promotes the arrest of stamens, but prevents carpel abortion. The maize ANTHER EAR1 (AN1) gene is necessary for the production of ent-kaurene during GA biosynthesis. Besides short stature and delayed maturity, the an1 mutant develops perfectly bisexual flowers in ears, indicating the inability of the an1 plant to successfully abort stamens in the ear (Bensen et al., 1995). In addition, maize dwarf mutants d1, d2, d3, and d5, which are deficient in GA production, also form stamens in flowers of the ear (Fujioka et al., 1988; Dellaporta and Calderon-Urrea, 1994; Spray et al., 1996).
Taken together, in both dicots and monocots the male organ development is sensitive to GA, however, its effects are opposite.
Auxin
In Arabidopsis and maize, auxin is required for the formation of all floral organs, as disruption of genes associated with auxin signaling, biosynthesis, and transport leads to flowers with various abnormalities (Okada et al., 1991; Nagpal et al., 2005; Cheng et al., 2006; Wu et al., 2006; Cecchetti et al., 2008). ARFs activate or repress expression of auxin response genes. In Arabidopsis, the arf6 arf8 double mutant and plants expressing miR167 resistant versions of ARF6 and ARF8 exhibit many flower defects, such as shortened petals, gynoecium, and stamen filaments, failure to release pollen, as well as abnormal ovules (Nagpal et al., 2005; Wu et al., 2006). In the arf3/ett (ettin) mutant, a decreased number of stamens are observed (Sessions et al., 1997). In the Arabidopsis floral organs in carpels (foc) mutant, increased expression of ARF10, 16, and 17 due to the lack of its negative regulator miR160 results in floral organ loss and abnormal female fertility (Liu et al., 2010). In rice, expressing the miR160 resistant version of OsARF18 causes the formation of abnormal flowers and reduced seed set (Huang et al., 2016a). Mutations in arf5/mp (monopteros) lead to either small or absent lateral flowers (Przemeck et al., 1996). YUCCA (YUC) genes in Arabidopsis encode auxin biosynthesis enzymes. Stamens in the yuc2yuc6 double mutant fail to elongate but produce pollen grains, while flowers in the yuc1yuc4 double mutant cannot form functional reproductive organs (Cheng et al., 2006). Ectopic expression of the small protein ligand TAPETUM DETERMINANT1 (TPD1) causes abnormal ovule and seed development via altering auxin signaling (Huang et al., 2016b,c). In maize, mutation in the SPARSE INFLORESCENCE1 (SPI1) gene, which functions as a YUC-like gene, results in tassels with small ears and few kernels (Gallavotti et al., 2008a).
Many genes in the auxin transport pathway play key roles in maintaining fertility and normal floral organ development. In Arabidopsis, PIN-FORMED (PIN) transporters function in polar auxin transport. In the pin1-1 mutant, various phenotypes, such as missing stamens, the formation of sterile pistil-like structures, and abnormal petal shape, are observed (Okada et al., 1991). In maize, BARREN INFLORESCENCE1 (BIF1) and BARREN INFLORESCENCE2 (BIF2) are involved in regulating polar auxin transport. BIF1 likely acts upstream of polar auxin transport via up-regulating the expression of ZmPIN1a (Gallavotti et al., 2008b). The tassels and ears in bif1 and bif2 mutants have reduced number of spikelets/florets and floral organs, and consequently fewer kernels (McSteen and Hake, 2001; Barazesh and McSteen, 2008; Skirpan et al., 2009). The rice gene LAZY1 (LA1), which encodes a novel grass specific protein, is a negative regulator of polar auxin transport (Li et al., 2007). In maize, the la1-ref mutant carries a mutation in the maize ortholog of LA1 (Dong et al., 2013). Spikelets in la1-ref either are not fully developed or undergo abortion especially in the tassel tip. Similarly, in the ear, silk production is decreased and spikelets are aborted in the ear tip (Dong et al., 2013).
The role of auxin in flower development is challenging to interpret. Due to the effects of auxin on the entirety of plant, it is possible that some phenotypes are the consequence of larger changes occurring in the plant. For example, as mentioned above, the LA1 gene regulates polar auxin transport in Arabidopsis. LA1 also affects plant architecture, since the tiller morphology is altered in mutants like la1-ZF802 in a pattern known as tiller-spreading (Li et al., 2007). These architectural changes may alter photosynthesis which consequently affects fertility and yield (Wu et al., 2013). Thus, more work should be done to look into specific roles of all involved hormones in flower development and fertility. Effects of hormones on female and male flower development are summarized in Figure 3.
Sterility and Abiotic Stresses
The loss of yield caused by abiotic stresses is partially attributed to defects in flower development. Even a mild or a short-term abiotic stress can cause a significant decrease in fertility. The majority of studies focus on the effects of abiotic stresses, including heat, cold, and drought, on fertility at the morphological level in Arabidopsis and cereal grains; however, the molecular mechanisms behind are not clear.
Heat Stress
Many stages of flower development, particularly the late stages of stamen development, are sensitive to heat stress. In Arabidopsis and cereal grains, sensitive stages include meiosis of pollen mother cells (PMC), tapetum development, anther dehiscence/pollen release, anthesis, and fertilization (Dupuis and Dumas, 1990; Kim et al., 2001; Abiko et al., 2005; Oshino et al., 2007; Thakur et al., 2010; Zinn et al., 2010; De Storme and Geelen, 2014). The overall effects of heat stress on male sterility depend on duration, timing, and temperature (Schoper et al., 1986, 1987a,b). The female organ is not as susceptible as the male organ to the heat stress.
The tapetum in the anther is particularly vulnerable to heat stress (Parish et al., 2012). In Arabidopsis, the tapetum consists of a monolayer of cells, which surrounds successive stages of microsporocytes, tetrads, microspores, and developing pollen as anther development progresses. Tapetal cells undergo three stages: differentiation, maturation, and PCD. First, the early differentiated tapetal cells secrete the callase enzyme that is required for the release of haploid microspores from meiotic tetrads. Second, mature tapetal cells produce a large amount of specialized non-photosynthetic plastids (elaioplasts) and tapetosomes, which provide lipids, proteins, and sporopollenin essential for pollen wall formation. Finally, tapetal cells are degenerated via PCD, and the remnants are important for the completion of pollen wall formation (McCormick, 1993; Wu and Cheung, 2000; Parish and Li, 2010).
The abnormal tapetum or altered timing of its degeneration causes pollen defects and consequently male sterility. Barley and wheat grown at elevated temperatures (barley: 30–35°C day/20–25°C night, wheat: 30°C for 1–3 days, or varied 30/20°C day/night at meiosis) display precocious tapetum degradation (Saini and Aspinall, 1982; Saini et al., 1984;Abiko et al., 2005; Oshino et al., 2007; Omidi et al., 2014). In rice, tapetal genes like YY1 and YY2 are down regulated following heat stress [39/30°C (day/night) for 5 days], affecting tapetum function and consequently pollen viability (Endo et al., 2009). Additionally in rice, male sterility in the thermos-sensitive genic male-sterile (TGMS) line 95850ms is caused by premature tapetum PCD and consequent pollen grain collapse (Ku et al., 2001, 2003). A recent study shows that the TGMS trait in the thermosensitive genic male sterile 5 (tms5) mutant is caused by the loss of function of RNase Zs1, which processes mRNAs of three ubiquitin fusion ribosomal protein genes (UbL40) (Zhou et al., 2014). At restrictive temperatures, high level of UbL40 results in abortive pollen and therefore male sterility. Arabidopsis plants under heat stress (31 and 33°C) show reduced expression of YUCCA genes especially in tapetum and PMC. Inactivation of YUC2 and YUC6 leads to decreased male fertility, while which can be reversed by exogenous application of auxin (Sakata et al., 2010, 2014). More work needs to be done to understand the genetic pathways leading to decreased fertility during heat stress, especially the role of auxin in male fertility and tapetum development.
Another sensitive stage is the PMC meiosis. Wheat and rice exposed to high or varied temperatures [wheat: high 30°C (1–3 days), varied 30°/20°C (day/night), rice: 39/30°C (day/night; 5 days)] at and prior to the onset of PMC meiosis exhibit greatly reduced grain set (Saini and Aspinall, 1982; Saini et al., 1984; Endo et al., 2009; Omidi et al., 2014). Impairments in rice PMC division occur even 5°C over the ambient temperature [28.3/21.3°C (day/night)], resulting in decreased pollen production especially in susceptible cultivars (Prasad et al., 2006).
Anther dehiscence, anthesis, and fertilization are sensitive to elevated temperatures too. Heat stress applied to wheat [two-day intervals of 36/31°C (day/night)] from floral emergence to 3 days post anthesis results in male sterility due to abnormal pollen grains (Tashiro and Wardlaw, 1990; Ferris et al., 1998). Similarly, rice that receives a short-term (33.7°C, 1 h) or a long-term heat stress (35°C, 38°C, and 41°C, 5 days) at anthesis display reduced fertility, but with a better fertility when stress was applied before or after anthesis (Satake and Yoshida, 1978; Jagadish et al., 2007). Heat stressed rice [35/25°C (day/night)] has decreased anther dehiscence and pollen count (Das et al., 2014). Pollen germination is also very vulnerable to high temperature stress. When maize tassels and rice spikelets are subjected to high heat stress [maize: 6 h of 40°C, rice: 35/25°C (day/night) or greater for 3 days], the ability of pollen to fertilize the ear is lost, which is attributed to the failure of pollen tube growth (Dupuis and Dumas, 1990; Das et al., 2014). In Arabidopsis, disruption of THERMOSENSITIVE MALE STERILE 1 (TMS1), which encodes the heat shock protein HSP40, causes pollen tubes to burst and decreased pollen tube length (Yang et al., 2009).
Although the cause of the heat-induced sterility is not clear, it might be related to heat shock proteins like HSP40 mentioned above (Yang et al., 2009). Mutations in the small heat shock protein gene BOBBER1 (BOB1) result in a range of phenotypes, such as irregular flowers and sterile siliques (Perez et al., 2009). In maize, pollen infertility may be due to the lack of production of major protective HSPs (Dupuis and Dumas, 1990; Hopf et al., 1992), supported by the fact that pollen grains do not express HSP RNAs at dehiscence (Dietrich et al., 1991; Young et al., 2001). In wheat, heat-stress induces many HSPs, including HSP17, HSP26, and HSP70, as well as microRNAs targeted HSP genes (Kumar et al., 2015).
Cold Stress
Extensive research has been done about the effects of below optimal temperature conditions on growth and development of Arabidopsis and cereal crops. In Arabidopsis, a large number of genes are identified with differential expression after chilling stress, and many of which play roles in pollen development (Lee et al., 2005; Zou and Yu, 2010). The COLD REGULATED (COR) genes are induced at low temperatures. The WRKY transcription factors repress COR expression via binding to their c-repeat binding factors (CBF; Zou et al., 2010). Plants harboring mutated WRKY genes show increased pollen viability under the chilling stress. In Arabidopsis, freezing treatment (0°C for 72 h) induces the acclimation of COR, lipid transfer proteins, and β-amylase in vegetative tissues, but not in pollen, which may explain the inability of pollen to withstand the chilling stress (Lee and Lee, 2003). In rice, DEHYDRATION RESPONSIVE ELEMENT BINDING PROTEIN1F (OsDREB1F) activates the expression of COR15a. Overexpression of OsDREB1F causes increased cold and drought tolerance, which aids spike development, further highlighting the role of COR genes in cold tolerance (Wang et al., 2008). For a review on freezing tolerance genes Thomashow (1999).
In cereal grains, the establishment of reproductive development, branching, and spikelet pair formation are sensitive to low temperature stress. Maize plants grown at cold conditions (10°C for 3 days or longer) during the reproductive transition produce less tassel branches and spikelet pairs (Bechoux et al., 2000). In the maize inbred line Dent11, chilling stress [17/6°C (day/night)] leads to the reduction of 43 and 29% of pollen when stress is applied at branch and spikelet initiation, respectively (Tranel et al., 2009).
In anthers, meiosis and tapetum development are particularly cold sensitive. Sorghum and rice display male sterility under cold conditions during meiosis and microspore development (Downes and Marshall, 1971; Mamun et al., 2006; Wood et al., 2006; Gothandam et al., 2007; Sakata et al., 2014). Abnormal tapetum development and degradation under chilling stress results in aberrant pollen (Sakata et al., 2014). Plants insensitive to GA or deficient in GA production exhibit more severe problems in tapetal cell hypertrophy and pollen production under chilling. In the tapetum, chilling stress represses both the cell wall bound acid invertase gene OSINV4 and the monosaccharide transporter gene OSMST8, which causes failed transport of sugar to the tapetum and developing pollen (Oliver et al., 2005; Mamun et al., 2006). ABA application also leads to abnormal pollen, possibly by repressing OSINV4 and OSMST8 (Oliver et al., 2005). More work needs to be done in the future to determine genes responsible for the abnormal development of tapetum under chilling conditions.
Later in development, anthesis and pollen germination are also cold sensitive. In wheat, chilling conditions [8/2°C (day/night)] applied to anthesis result in high levels of male sterility (Subedi et al., 1998). In Arabidopsis, freezing stress causes reduced pollen tube growth and decreased seed production. Similarly, mutations in G protein-coupled receptor-type G proteins (GTGs) lead to decreased pollen germination, abnormal pollen tube elongation, and consequent seed loss (Jaffé et al., 2012). In rice, the QTL COLD1, which encodes a regulator of G-protein signaling, acts to sense chilling (Ma et al., 2015). COLD1 is important for maintaining grain yield, further suggesting that G-protein signaling plays a key role in chilling tolerance during sexual reproduction. In young rice panicles, the Ctb1 QTL harbors an F-box protein gene that is responsible for chilling tolerance (Saito et al., 2010). Additionally, upregulation of the CORN CYSTATIN genes CC8 and CC9 is observed under cold stress (14 and 14/7°C). CC8 is found in kernel and the immature tassel, while CC9 is detected in immature and mature tassels, silk, and kernels (Massonneau et al., 2005). Future study looking into the roles of these cystatins in fertility could be valuable.
Collectively, similar to what is observed under heat stress, the stamen development is sensitive to cold stress, particularly during meiosis, tapetum development, pollen germination, and anthesis. The female organ development remains relatively unaffected to cold stress. However, not all studies agree with this finding. In maize, prolonged exposure to cold stress (10°C for 7 days) results in the abortion of the ear (Lejeune and Bernier, 1996). The effects of ear abortion may be prevented by applying benzyladenine (a synthetic cytokinin) exogenously (Lejeune et al., 1998). Genes like COR and those involved in GA and G-protein signaling may play important roles in chilling tolerance during sexual reproduction.
Drought Stress
Similar to heat, drought stress affects flower development and consequently impairs fertility. In stamen development, drought stress causes shortened anther filaments, delayed anther development and dehiscence, as well as reduced pollen viability (Su et al., 2013; Tunc-Ozdemir et al., 2013; Ma et al., 2014). Female fertility is less sensitive to drought stress (Su et al., 2013). Younger buds are sacrificed during early drought stress and water is likely allocated to older flowers (Su et al., 2013).
Under moderate and severe drought stresses, thousands of genes are differentially expressed (Ma et al., 2014). Genes like DREB1, ABA-RESPONSIVE ELEMENTS BINDING FACTORS (ABF), NAC DOMAIN CONTAINING PROTEIN019 (NAC019), RESPONSIVE TO DESSICATION20 (RED20), and RD29A were upregulated (Su et al., 2013). A great number of genes involved in ABA and JA signaling are also upregulated, which may affect stamen filament elongation as well as overall stamen and pistil development (Su et al., 2013). CYCLIC NUCLEOTIDE-GATED CHANNEL16 (CNGC16) is important for stress response, as disruption of CHGC16 leads to reduced pollen viability (Tunc-Ozdemir et al., 2013).
Some cereals like rice and wheat are sensitive to drought stress, whereas others such as sorghum are quite drought tolerant. In maize, female organ development, particularly prior to pollination, is sensitive to drought stress, which is often attributed to problems with carbohydrate transport and metabolism. When comparing well watered with drought treated plants, carbohydrate transport to ovary is decreased in drought conditions and expression of carbohydrate (e.g., starch and sucrose) metabolism genes is altered (Mäkelä et al., 2005; Kakumanu et al., 2012). Many genes in maize kernels show differential expression under drought stress, such as those important for carbohydrate metabolism (SU1P, ISA1, DULL1, FRK2, GLU1, and AAG1), stress response and regulation (ZmDJ1, SOD1, and STI1), and transcriptional regulation of drought inducible genes (EREBP1, MYB-IF35, MYB-IF25I, and RISBZ4; Marino et al., 2009). Under drought conditions, genes involved in cell cycle, cell division, and antioxidant formation are down regulated in ovaries, while genes essential for stress responses like ABA are expressed at higher levels. Increased ABA may lead to a reduction of invertase in ovaries, limiting sucrose use and subsequent ovary abortion (Zinselmeier et al., 1999; Andersen et al., 2002; Boyer and Westgate, 2004; McLaughlin and Boyer, 2004; Kakumanu et al., 2012).
During meiosis, drought stress results in differential expression of many genes, for example, genes encoding β-carotene hydroxylase and cytochrome P450 monooxygenase which might protect against oxidative damage. Altered expression was also observed in genes that encode histone H2A and dehydrin DHN1, suggesting the importance of chromatin stabilization and dehydration prevention under drought stress (Zhuang et al., 2008). After pollination, elevated expression of senescence genes may be the cause of embryo abortion (McLaughlin and Boyer, 2004). Interestingly, pollen development was relatively unaffected by drought stress in maize (Schoper et al., 1986; Westgate and Boyer, 1986).
Drought stress is detrimental to pollen production in wheat, resulting in a 40–50% of reduction in yield (Dorion et al., 1996). Drought-induced degeneration of tapetal cells may contribute to the failure of microspore and pollen development. The timing of tapetum degeneration is crucial, as its early degeneration results in loss of orientation, and late degeneration leads to microspores that do not receive essential nutrients (Saini et al., 1984; Lalonde et al., 1997; Ji et al., 2010). In addition, pollen developed under drought condition is devoid of starch, limiting fertilization, and pollen tube growth (Ji et al., 2010). In wheat, drought stress decreases the level of invertases in developing pollen and microspores, (Dorion et al., 1996; Lalonde et al., 1997; McLaughlin and Boyer, 2004; Koonjul et al., 2005). Drought tolerant lines have a normal invertase expression (Ji et al., 2010). In wheat, no effects are observed on female fertility under moderate drought stress (Saini and Aspinall, 1981; Ji et al., 2010).
In rice, male sterility is common under drought stress conditions. If drought conditions are applied during PMC meiosis, the pollen production is severely affected (Sheoran and Saini, 1996), which is potentially caused by tapetal cell vacuolization/degeneration and abnormal starch deposition (Nguyen and Sutton, 2009; Jin et al., 2013). Under drought conditions, the presence of reactive oxygen species (ROS) results in a depletion of ATP and therefore leads to PCD and pollen abortion in rice (Nguyen et al., 2009). Furthermore, expression of genes critical for tapetal cell PCD and pollen wall formation is altered along with increased ABA signaling and decreased GA signaling (Jin et al., 2013). Both invertase and starch synthase gene expression are reduced under drought stress (Sheoran and Saini, 1996; Nguyen et al., 2010). Conversely, genes involved in sugar transport are upregulated (Sheoran and Saini, 1996; Nguyen et al., 2010; Fu et al., 2011). The accumulation of sugar may help maintain water levels in the anther due to low water potential (Fu et al., 2011).
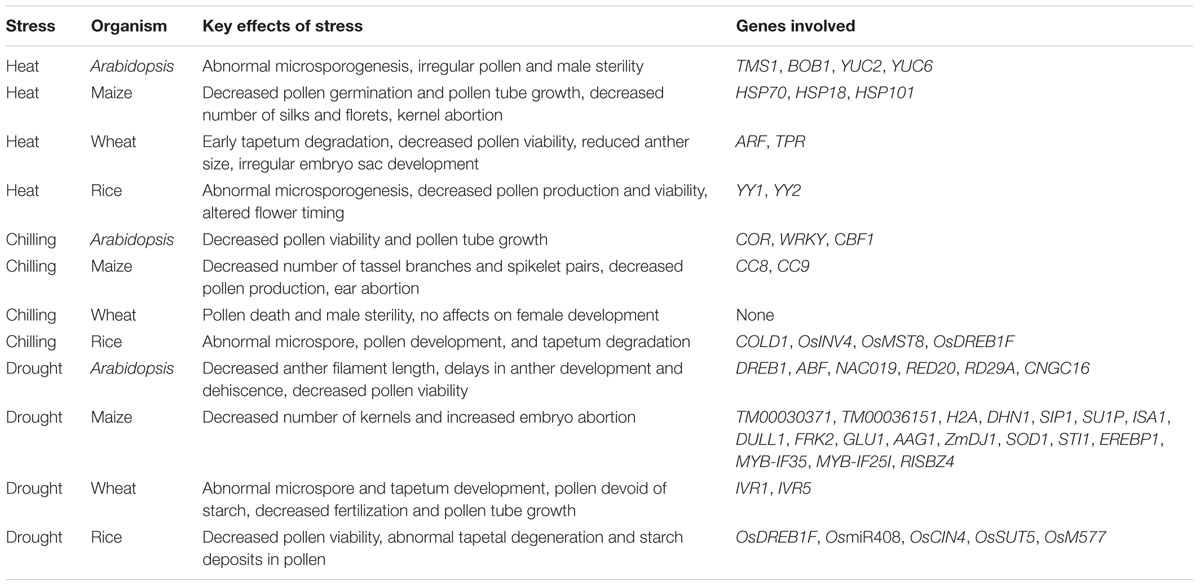
It is not clear why the stamen development and male fertility appear more susceptible to abiotic stresses in plants. It will be necessary to identify genes that first respond to abiotic stresses and genes that later build strength for plants to cope with long-term abiotic stresses. Hormones, such as ABA and auxin, are heavily involved in abiotic stresses. It is well known that cross-talks are important for plant hormonal signaling; however, little is known about cross-talks among hormones in response to different abiotic stresses. The effects on fertility and some potential genes involved under abiotic stresses are summarized in Table 2 and Figure 4.
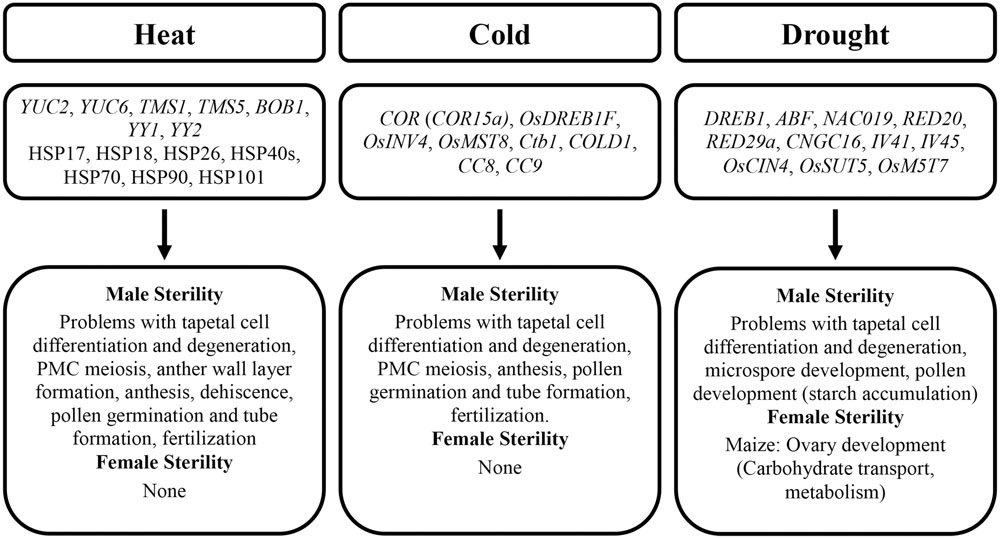
FIGURE 4. Effects of abiotic stresses on fertility in Arabidopsis and key cereals. Genes (italic) and proteins (non-italic) involved are shown.
Conclusion and Future Directions
Floral organ degeneration or abortion under the normal condition results in the formation of unisexual flowers, such as in maize; or completely sterile flowers, such as in sorghum. In addition to other genes, class B and C genes are involved in floral reproductive organ degeneration via losing their functions in floral organ identity or in regulating expression of downstream target genes. Moreover, hormones play important roles in establishing the male and female state. Genes underlying JA and BR signaling and their biosynthesis promote stamen development and carpel abortion, whereas genes involved in GA signaling and their biosynthesis induce carpel development and stamen abortion (with exception of the maize ear). Auxin is essential for the formation of all floral organs, including stamen and carpels. Interactions between flower and hormone regulation genes are essential for flower organ establishment and fertility.
It is evident that maintaining ideal temperature and soil moisture is crucial for fertility in Arabidopsis, maize, wheat, and rice. Abiotic stresses commonly lead to male sterility, while female viability is well maintained under most mild abiotic stresses. In nearly all plants under all observed abiotic stresses, the most sensitive stages causing sterility are during tapetum development, male meiosis, microsporogenesis, anthesis, and fertilization. Hormones play important roles in male and female organ development during abiotic stresses. Auxin application can reverse some effects of heat stress, whereas decreased GA in stressed plants worsens tapetum defects and consequently further reduces pollen production.
Overall, abiotic stress induced sterility causes the major loss of crop yield. By 2050 the global population is expected to reach 9.1 billion. Additionally, if the use of grains for biofuel production is intensified, the demand for crop products will be further increased. High-yield wheat ideotypes are currently being studied and improved based on long-term climate projections (Semenov and Stratonovitch, 2013). To develop high-yield crops that have ideal agronomic traits and can cope with anticipated environmental changes using traditional and molecular breeding approaches, it is necessary to decipher molecular genetic mechanisms that cause flower sterility under normal and stress conditions.
Author Contributions
AS and DZ conceived the idea and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our work was supported by the National Science Foundation (NSF IOS-0721192 and IOS-1322796), the Research Growth Initiative (RGI) at the University of Wisconsin-Milwaukee, and the UW-Madison/UW-Milwaukee Intercampus Research Incentive Grants Program. DZ also thankfully acknowledges supports of the Shaw Scientist Award from the Greater Milwaukee Foundation and the Bradley Catalyst Award from the UWM Research Foundation.
References
Abiko, M., Akibayashi, K., Sakata, T., Kimura, M., Kihara, M., Itoh, K., et al. (2005). High-temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition. Sex. Plant Reprod. 18, 91–100. doi: 10.1007/s00497-005-0004-2
Acosta, I. F., Laparra, H., Romero, S. P., Schmelz, E., Hamberg, M., Mottinger, J. P., et al. (2009). Tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323, 262–265. doi: 10.1126/science.1164645
Ainsworth, C., Rahman, A., Parker, J., and Edwards, G. (2005). Intersex inflorescences of rumex acetosa demonstrate that sex determination is unique to each flower. New Phytol. 165, 711–720. doi: 10.1111/j.1469-8137.2004.01281.x
Ambrose, B. A., Lerner, D. R., Ciceri, P., Padilla, C. M., Yanofsky, M. F., and Schmidt, R. J. (2000). Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 5, 569–579. doi: 10.1016/s1097-2765(00)80450-5
Andersen, M. N., Asch, F., Wu, Y., Jensen, C. R., Naested, H., Mogensen, V. O., et al. (2002). Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol. 130, 591–604. doi: 10.1104/pp.005637
Aryal, R., and Ming, R. (2014). Sex determination in flowering plants: papaya as a model system. Plant Sci. 217, 56–62. doi: 10.1016/j.plantsci.2013.10.018
Barazesh, S., and McSteen, P. (2008). Barren inflorescence1 functions in organogenesis during vegetative and inflorescence development in maize. Genetics 179, 389–401. doi: 10.1534/genetics.107.084079
Bechoux, N., Bernier, G., and Lejeune, P. (2000). Environmental effects on the early stages of tassel morphogenesis in maize (Zea mays L.). Plant Cell Environ. 23, 91–98. doi: 10.1046/j.1365-3040.2000.00515.x
Bensen, R. J., Johal, G. S., Crane, V. C., Tossberg, J. T., Schnable, P. S., Meeley, R. B., et al. (1995). Cloning and characterization of the maize An1 gene. Plant Cell 7, 75–84. doi: 10.1105/tpc.7.1.75
Bommert, P., Satoh-Nagasawa, N., Jackson, D., and Hirano, H. Y. (2005). Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol. 46, 69–78. doi: 10.1093/pcp/pci504
Bortiri, E., and Hake, S. (2007). Flowering and determinancy in maize. J. Exp. Bot. 58, 909–916. doi: 10.1093/jxb/erm015
Bowman, J. L., Drews, G. N., and Meyerowitz, E. M. (1991). Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower develompent. Plant Cell 3, 749–758. doi: 10.2307/3869269
Boyer, J. S., and Westgate, M. E. (2004). Grain yields with limited water. J. Exp. Bot. 55, 2385–2394. doi: 10.1093/jxb/erh219
Burow, G., Xin, Z., Hayes, C., and Burke, J. (2014). Characterization of a multiseeded (msd1) mutant of sorghum for increasing grain yield. Crop Sci. 54, 2030–2037. doi: 10.2135/cropsci2013.08.0566
Cacharrón, J., Saedler, H., and Theiβen, G. (1999). Expression of mads box genes ZMM8 and ZMM14 during inflorescence development of Zea mays discriminates between the upper and the lower floret of each spikelet. Dev. Genes Evol. 209, 411–420. doi: 10.1007/s004270050271
Calderon-Urrea, A., and Dellaporta, S. L. (1999). Cell death and cell protection genes determine the fate of pistils in maize. Development 126, 435–441.
Cecchetti, V., Altamura, M. M., Falasca, G., Costantino, P., and Cardarelli, M. (2008). Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20, 1760–1774. doi: 10.1105/tpc.107.057570
Cheng, H., Qin, L., Lee, S., Fu, X., Richards, D. E., Cao, D., et al. (2004). Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131, 1055–1064. doi: 10.1242/dev.00992
Cheng, H., Song, S., Xiao, L., Soo, H. M., Cheng, Z., Xie, D., et al. (2009). Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 5:e1000440. doi: 10.1371/journal.pgen.1000440
Cheng, Y., Dai, X., and Zhao, Y. (2006). Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20, 1790–1799. doi: 10.1101/gad.1415106
Chuck, G., Meeley, R., and Hake, S. (2008). Floral meristem initiation and meristem cell fate are regulated by the maize AP2 genes ids1 and sid1. Development 135, 3013–3019. doi: 10.1242/dev.024273
Chuck, G., Meeley, R. B., and Hake, S. (1998). The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 12, 1145–1154. doi: 10.1101/gad.12.8.1145
Clouse, S. D., Langford, M., and McMorris, T. C. (1996). A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 111, 671–678. doi: 10.1104/pp.111.3.671
Clouse, S. D., and Sasse, J. M. (1998). Brassinosteroids: essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 427–451. doi: 10.1146/annurev.arplant.49.1.427
Colombo, L., Marziani, G., Masiero, S., Wittich, P. E., Schmidt, R. J., Gorla, M. S., et al. (1998). BRANCHED SILKLESS mediates the transition from spikelet to floral meristem during Zea mays ear development. Plant J. 16, 355–363. doi: 10.1046/j.1365-313x.1998.00300.x
Das, S., Krishnan, P., Nayak, M., and Ramakrishnan, B. (2014). High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 101, 36–46. doi: 10.1016/j.envexpbot.2014.01.004
De Storme, N., and Geelen, D. (2014). The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ. 37, 1–18. doi: 10.1111/pce.12142
Dellaporta, S. L., and Calderon-Urrea, A. (1993). Sex determination in flowering plants. Plant Cell 5, 1241–1251. doi: 10.1105/tpc.5.10.1241
Dellaporta, S. L., and Calderon-Urrea, A. (1994). The sex determination process in maize. Science 266, 1501–1505. doi: 10.1126/science.7985019
DeLong, A., Calderon-Urrea, A., and Dellaporta, S. L. (1993). Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol-dehydrogenase required for stage-specific floral organ abortion. Cell 74, 757–768. doi: 10.1016/0092-8674(93)90522-r
Dietrich, P. S., Bouchard, R. A., Casey, E. S., and Sinibaldi, R. M. (1991). Isolation and characterization of a small heat-shock protein gene from maize. Plant Physiol. 96, 1268–1276. doi: 10.1104/pp.96.4.1268
Ditta, G., Pinyopich, A., Robles, P., Pelaz, S., and Yanofsky, M. F. (2004). The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 14, 1935–1940. doi: 10.1016/j.cub.2004.10.028
Dong, Z., Jiang, C., Chen, X., Zhang, T., Ding, L., Song, W., et al. (2013). Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol. 163, 1306–1322. doi: 10.1104/pp.113.227314
Dorion, S., Lalonde, S., and Saini, H. S. (1996). Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol. 111, 137–145.
Downes, R. W., and Marshall, D. R. (1971). Low temperature induced male sterility in Sorghum bicolor. Aus. J. Exp. Agric. Anim. Husb. 11, 352–356. doi: 10.1071/EA9710352
Duan, Q., Wang, D., Xu, Z., and Bai, S. (2008). Stamen development in Arabidopsis is arrested by organ-specific overexpression of a cucumber ethylene synthesis gene CsACO2. Planta 228, 537–543. doi: 10.1007/s00425-008-0756-7
Dupuis, I., and Dumas, C. (1990). Influence of temperature stress on invitro fertilization and heat-shock protein-synthesis in maize (Zea mays L.) reproductive tissues. Plant Physiol. 94, 665–670. doi: 10.1104/pp.94.2.665
Endo, M., Tsuchiya, T., Hamada, K., Kawamura, S., Yano, K., Ohshima, M., et al. (2009). High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 50, 1911–1922. doi: 10.1093/pcp/pcp135
Erhard, K. F. Jr., Stonaker, J. L., Parkinson, S. E., Lim, J. P., Hale, C. J., and Hollick, J. B. (2009). RNA polymerase IV functions in paramutationin Zea mays. Science 323, 1201–1205. doi: 10.1126/science.1164508
Ferris, R., Ellis, R. H., Wheeler, T. R., and Hadley, P. (1998). Effect of high temperature stress at anthesis on grain yield and biomass of field-grown crops of wheat. Ann. Bot. 82, 631–639. doi: 10.1006/anbo.1998.0740
Fischer, A., Baum, N., Saedler, H., and Theiβen, G. (1995). Chromosomal mapping of the MADS-box multigene family in Zea mays reveals dispersed distribution of allelic genes as well as transposed copies. Nucleic Acids Res. 23, 1901–1911. doi: 10.1093/nar/23.11.1901
Fu, G., Song, J., Xiong, J., Li, Y., Chen, H., Le, M., et al. (2011). Changes of oxidative stress and soluble sugar in anthers involve in rice pollen abortion under drought stress. Agric. Sci. China 10, 1016–1025. doi: 10.1016/s1671-2927(11)60089-8
Fujioka, S., Yamane, H., Spray, C. R., Gaskin, P., Macmillan, J., Phinney, B. O., et al. (1988). Qualitative and quantitative-analyses of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol. 88, 1367–1372. doi: 10.1104/pp.88.4.1367
Gallavotti, A., Barazesh, S., Malcomber, S., Hall, D., Jackson, D., Schmidt, R. J., et al. (2008a). sparse inflorescence1 encodes a monocot-specific YUCCA-like gene required for vegetative and reproductive development in maize. Proc. Natl. Acad. Sci. U.S.A. 105, 15196–15201. doi: 10.1073/pnas.0805596105
Gallavotti, A., Yang, Y., Schmidt, R. J., and Jackson, D. (2008b). The relationship between auxin transport and maize branching. Plant Physiol. 147, 1913–1923. doi: 10.1104/pp.108.121541
Gothandam, K. M., Kim, E. S., and Chung, Y. Y. (2007). Ultrastructural study of rice tapetum under low-temperature stress. J. Plant Biol. 50, 396–402. doi: 10.1007/BF03030674
Goto, N., and Pharis, R. P. (1999). Role of gibberellins in the development of floral organs of the gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can. J. Bot. 77, 944–954. doi: 10.1139/b99-090
Hardenack, S., Ye, D., Saedler, H., and Grant, S. (1994). Comparison of MADS box gene expression in developing male and female flowers of the dioecious plant white campion. Plant Cell 6, 1775–1787. doi: 10.1105/tpc.6.12.1775
Hartwig, T., Chuck, G. S., Fujioka, S., Klempien, A., Weizbauer, R., Potluri, D. P. V., et al. (2011). Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. U.S.A. 108, 19814–19819. doi: 10.1073/pnas.1108359108
Hopf, N., Plesofsky-Vig, N., and Brambl, R. (1992). The heat-shock response of pollen and other tissues of maize. Plant Mol. Biol. 19, 623–630. doi: 10.1007/bf00026788
Hu, J., Mitchum, M. G., Barnaby, N., Ayele, B. T., Ogawa, M., Nam, E., et al. (2008). Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20, 320–336. doi: 10.1105/tpc.107.057752
Huang, J., Li, Z., and Zhao, D. (2016a). Deregulation of the OsmiR160 target gene OsARF18 causes growth and developmental defects with an alteration of auxin signaling in rice. Sci. Rep. 6:29938. doi: 10.1038/srep29938
Huang, J., Wijeratne, A. J., Tang, C., Zhang, T., Fenelon, R. E., Owen, H. A., et al. (2016b). Ectopic expression of TAPETUM DETERMINANT 1 affects ovule development in Arabidopsis. J. Exp. Bot. 67, 1311–1326. doi: 10.1093/jxb/erv523
Huang, J., Zhang, T., Linstroth, L., Tillman, Z., Otegui, M., Owen, H. A., et al. (2016c). Control of anther cell differentiation by the TPD1 small protein ligand and its receptor EMS1 in Arabidopsis. PLoS Genet. 12:e1006147. doi: 10.1371/journal.pgen.1006147
Irish, E. E. (1996). Regulation of sex determination in maize. Bioessays 18, 363–369. doi: 10.1002/bies.950180506
Irish, E. E. (1997). Class II tassel seed mutations provide evidence for multiple types of inflorescence meristems in maize (Poaceae). Am. J. Bot. 84, 1502–1515. doi: 10.2307/2446611
Irish, E. E., Langdale, J. A., and Nelson, T. M. (1994). Interactions between tassel seed genes and other sex-determining genes in maize. Dev. Genet. 15, 155–171. doi: 10.1002/dvg.1020150206
Ito, T., Ng, K.-H., Lim, T.-S., Yu, H., and Meyerowitz, E. M. (2007). The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 19, 3516–3529. doi: 10.1105/tpc.107.055467
Ito, T., Wellmer, F., Yu, H., Das, P., Ito, N., Alves-Ferreira, M., et al. (2004). The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430, 356–360. doi: 10.1038/nature02733
Jaffé, F. W., Freschet, G. E., Valdes, B. M., Runions, J., Terry, M. J., and Williams, L. E. (2012). G protein-coupled receptor-type G proteins are required for light-dependent seedling growth and fertility in Arabidopsis. Plant Cell 24, 3649–3668. doi: 10.1105/tpc.112.098681
Jagadish, S. V. K., Craufurd, P. Q., and Wheeler, T. R. (2007). High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 58, 1627–1635. doi: 10.1093/jxb/erm003
Ji, X., Shiran, B., Wan, J., Lewis, D. C., Jenkins, C. L. D., Condon, A. G., et al. (2010). Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 33, 926–942. doi: 10.1111/j.1365-3040.2010.02130.x
Jin, Y., Yang, H., Wei, Z., Ma, H., and Ge, X. (2013). Rice male development under drought stress: phenotypic changes and stage-dependent transcriptomic reprogramming. Mol. Plant 6, 1630–1645. doi: 10.1093/mp/sst067
Kakumanu, A., Ambavaram, M. M., Klumas, C., Krishnan, A., Batlang, U., Myers, E., et al. (2012). Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-seq. Plant Physiol. 160, 846–867. doi: 10.1104/pp.112.200444
Kanno, A., Nakada, M., Akita, Y., and Hirai, M. (2007). Class B gene expression and the modified ABC model in nongrass monocots. Sci. World J. 7, 268–279. doi: 10.1100/tsw.2007.86
Kim, S. Y., Hong, C. B., and Lee, I. (2001). Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana. J. Plant Res. 114, 301–307. doi: 10.1007/pl00013991
Kobayashi, K., Maekawa, M., Miyao, A., Hirochika, H., and Kyozuka, J. (2010). PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Pysiol. 51, 47–57. doi: 10.1093/pcp/pcp166
Komatsuda, T., Pourkheirandish, M., He, C. F., Azhaguvel, P., Kanamori, H., Perovic, D., et al. (2007). Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc. Natl. Acad. Sci. U.S.A 104, 1424–1429. doi: 10.1073/pnas.0608580104
Koonjul, P. K., Minhas, J. S., Nunes, C., Sheoran, I. S., and Saini, H. S. (2005). Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat. J. Exp. Bot. 56, 179–190. doi: 10.1093/jxb/eri018
Ku, S., Cho, K. H., Choi, Y. J., Baek, W. K., Kim, S., Suh, H. S., et al. (2001). Cytological observation of two environmental genic male-sterile lines of rice. Mol. Cells 12, 403–406.
Ku, S. J., Yoon, H., Suh, H. S., and Chung, Y. Y. (2003). Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217, 559–565. doi: 10.1007/s00425-003-1030-7
Kumar, R. R., Pathak, H., Sharma, S. K., Kala, Y. K., Nirjal, M. K., Singh, G. P., et al. (2015). Novel and conserved heat-responsive microRNAs in wheat (Triticum aestivum L.). Funct. Integr. Genomics 15, 323–348. doi: 10.1007/s10142-014-0421-0
Lalonde, S., Beebe, D. U., and Saini, H. S. (1997). Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sex. Plant Reprod. 10, 40–48. doi: 10.1007/s004970050066
Lee, B. H., Henderson, D. A., and Zhu, J. (2005). The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17, 3155–3175. doi: 10.1105/tpc.105.035568
Lee, J. Y., and Lee, D. H. (2003). Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiol. 132, 517–529. doi: 10.1104/pp.103.020511
Lejeune, P., and Bernier, G. (1996). Effect of environment on the early steps of ear initiation in maize (Zea mays L.). Plant Cell Environ. 19, 217–224. doi: 10.1111/j.1365-3040.1996.tb00243.x
Lejeune, P., Prinsen, E., Van Onckelen, H., and Bernier, G. (1998). Hormonal control of ear abortion in a stress-sensitive maize (Zea mays) inbred. Aust. J. Plant Physiol. 25, 481–488. doi: 10.1071/PP97154
Li, D., Blakey, A., Dewaled, C., and Dellaporta, S. L. (1997). Evidence of a common sex determination mechanism for pistil abortion in maize and in its wild relative Tripsacum. Proc. Natl. Acad. Sci. U.S.A. 94, 4217–4222. doi: 10.1073/pnas.94.8.4217
Li, J., Nam, K. H., Vafeados, D., and Chory, J. (2001). BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 127, 14–22. doi: 10.1104/pp.127.1.14
Li, L., Zhao, Y., McCaig, B. C., Wingerd, B. A., Wang, J., Whalon, M. E., et al. (2004). The tomato homolog of CORONATINE-INSENSTIIVE1 is required for maternal control of seed maturation, jasmonate-signaled defense responses and glandular trichome develompent. Plant Cell 16, 126–143. doi: 10.1105/tpc.017954
Li, P., Wang, Y., Qian, Q., Fu, Z., Wang, M., Zeng, D., et al. (2007). LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 17, 402–410. doi: 10.1038/cr.2007.38
Liu, X., Huang, J., Parameswaran, S., Ito, T., Subert, B., Auer, M., et al. (2009). The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Phys. 151, 1401–1411. doi: 10.1104/pp.109.145896
Liu, X., Huang, J., Wang, Y., Khanna, K., Xie, Z., Owen, H., et al. (2010). The role of floral organs in carpels, an Arabidopsis loss-of-function mutation in MicroRNA160a, in organogenesis and the mechanism regulating its expression. Plant J. 62, 416–428. doi: 10.1111/j.1365-313X.2010.04164.x
Lombardo, F., and Yoshida, H. (2015). Interpreting lemma and palea homologies: a point of view from rice floral mutants. Front. Plant Sci. 6:61. doi: 10.3389/fpls.2015.00061
Ma, X., Sukiran, N. L., Ma, H., and Su, Z. (2014). Moderate drought causes dramatic floral transcriptomic reprogramming to ensure successful reproductive development in Arabidopsis. BMC Plant Biol. 14:164. doi: 10.1186/1471-2229-14-164
Ma, Y., Dai, X., Xu, Y., Luo, W., Zheng, X., Zeng, D., et al. (2015). COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221. doi: 10.1016/j.cell.2015.01.046
Mäkelä, P., McLaughlin, J. E., and Boyer, J. S. (2005). Imaging and quantifying carbohydrate transport to the developing ovaries of maize. Ann. Bot. 96, 939–949. doi: 10.1093/aob/mci246
Malcomber, S. T., and Kellogg, E. A. (2006). Evolution of unisexual flowers in grasses (Poaceae) and the putative sex-determination gene, TASSELSEED2 (TS2). New Phytol. 170, 885–899. doi: 10.1111/j.1469-8137.2006.01726.x
Mamun, E. A., Alfred, S., Cantrill, L. C., Overall, R. L., and Sutton, B. G. (2006). Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 30, 583–591. doi: 10.1016/j.cellbi.2006.03.004
Mandaokar, A., Thines, B., Shin, B., Lange, B. M., Choi, G., Koo, Y. J., et al. (2006). Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 46, 984–1008. doi: 10.1111/j.1365-313X.2006.02756.x
Marino, R., Ponnaiah, M., Krajewski, P., Frova, C., Gianfranceschi, L., Enrico Pé, M. E., et al. (2009). Addressing drought tolerance in maize by transcriptional profiling and mapping. Mol. Genet. Genomics 281, 163–179. doi: 10.1007/s00438-008-0401-y
Massonneau, A., Condamine, P., Wisniewski, J. P., Zivy, M., and Rogowsky, P. M. (2005). Maize cystatins respond to developmental cues, cold stress and drought. Biochim. Biophys. Acta 1729, 186–199. doi: 10.1016/j.bbaexp.2005.05.004
McCormick, S. (1993). Male gametophyte development. Plant Cell 5, 1265–1275. doi: 10.1105/tpc.5.10.1265
McLaughlin, J. E., and Boyer, J. S. (2004). Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann. Bot. 94, 675–689. doi: 10.1093/aob/mch193
McSteen, P., and Hake, S. (2001). barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 128, 2881–2891.
Mena, M., Mandel, M. A., Lerner, D. R., Yanofsky, M. F., and Schmidt, R. J. (1995). A characterization of the MADS-box gene family in maize. Plant J. 8, 845–854. doi: 10.1046/j.1365-313X.1995.8060845.x
Michaels, S. D., and Amasino, R. M. (1999). The gibberellic acid biosynthesis mutant ga1-3 of Arabidopsis thaliana is responsive to vernalization. Dev. Genet. 25, 194–198. doi: 10.1002/(SICI)1520-6408
Münster, T., Deleu, W., Wingen, L. U., Ouzunova, M., Cacharron, J., Faigl, W., et al. (2002). Maize MADS-box genes galore. Maydica 47, 287–301.
Münster, T., Wingen, L. U., Faigl, W., Werth, S., Saedler, H., and Theiβen, G. (2001). Characterization of three GLOBOSA-like MADS-box genes from maize: evidence for ancient paralogy in one class of floral homeotic B-function genes of grasses. Gene 262, 1–13. doi: 10.1016/s0378-1119(00)00556-4
Nagpal, P., Ellis, C. M., Weber, H., Ploense, S. E., Barkawi, L. S., Guilfoyle, T. J., et al. (2005). Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132, 4107–4118. doi: 10.1242/dev.01955
Nguyen, G. N., Hailstones, D. L., Wilkes, M., and Sutton, B. G. (2009). Drought-induced oxidative conditions in rice anthers leading to a programmed cell death and pollen abortion. J. Agron. Crop Sci. 195, 157–164. doi: 10.1111/j.1439-037X.2008.00357.x
Nguyen, G. N., Hailstones, D. L., Wilkes, M., and Sutton, B. G. (2010). Role of carbohydrate metabolism in drought-induced male sterility in rice anthers. J. Agron. Crop Sci. 196, 346–357. doi: 10.1111/j.1439-037X.2010.00423.x
Nguyen, G. N., and Sutton, B. G. (2009). Water deficit reduced fertility of young microspores resulting in a decline of viable mature pollen and grain set in rice. J. Agron. Crop Sci. 195, 11–18. doi: 10.1111/j.1439-037X.2008.00342.x
Okada, K., Ueda, J., Komaki, M. K., Bell, C. J., and Shimura, Y. (1991). Requirement of the auxin polar transport-system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. doi: 10.2307/3869249
Oliver, S. N., Van Dongen, J. T., Alfred, S. C., Mamun, E. A., Zhao, X., Saini, H. S., et al. (2005). Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 28, 1534–1551. doi: 10.1111/j.1365-3040.2005.01390.x
Omidi, M., Siahpoosh, M. R., Mamghani, R., and Modarresi, M. (2014). The influence of terminal heat stress on meiosis abnormalities in pollen mother cells of wheat. Cytologia 79, 49–58. doi: 10.1508/cytologia.79.49
Oshino, T., Abiko, M., Saito, R., Ichiishi, E., Endo, M., Kawagishi-Kobayashi, M., et al. (2007). Premature progression of anther early developmental programs accompanied by comprehensive alterations in transcription during high temperature injury in barley plants. Mol. Gen. 278, 31–42. doi: 10.1007/s00438-007-0229-x
Parish, R. W., and Li, S. F. (2010). Death of a tapetum: a programme of developmental altruism. Plant Sci. 178, 73–89. doi: 10.1016/j.plantsci.2009.11.001
Parish, R. W., Phan, H. A., Iacuone, S., and Li, S. F. (2012). Tapetal development and abiotic stress: a centre of vulnerability. Funct. Plant Biol. 39, 553–559. doi: 10.1071/fp12090
Park, J. H., Ishikawa, Y., Yoshida, R., Kanno, A., and Kameya, T. (2003). Expression of AODEF, a B-functional MADS-box gene, in stamens and inner tepals of the dioecoius species Asparagus officinalis L. Plant Mol. Biol. 51, 867–875. doi: 10.1023/A:1023097202885
Parkinson, S. E., Gross, S. M., and Hollick, J. B. (2007). Maize sex determination and abaxial leaf fates are canalized by a factor that maintains repressed epigenetic states. Dev. Biol. 308, 462–473. doi: 10.1016/j.ydbio.2007.06.004
Pelaz, S., Ditta, G. S., Baumann, E., Wisman, E., and Yanofsky, M. F. (2000). B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405, 200–203. doi: 10.1038/35012103
Perez, D. E., Hoyer, J. S., Johnson, A. I., Moody, Z. R., Lopez, J., and Kapinsky, N. J. (2009). BOBBER1 is a noncanonical Arabidopsis small heat shock protein required for both development and thermotolerance. Plant Phys. 151, 241–252. doi: 10.1104/pp.109.142125
Prasad, P. V. V., Boote, K. J., Allen, L. H. Jr., Sheehy, J. E., and Thomas, J. M. G. (2006). Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 95, 398–411. doi: 10.1016/j.fcr.2005.04.008
Prunet, N., and Jack, T. P. (2014). Flower development in Arabidopsis: there is more to it than learning your ABCs. Methods Mol. Biol. 1110, 3–33. doi: 10.1007/978-1-4614-9408-9_1
Przemeck, G. K. H., Mattsson, J., Hardtke, C. S., Sung, Z. R., and Berleth, T. (1996). Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200, 229–237. doi: 10.1093/emboj/17.5.1405
Rounsley, S. D., Ditta, G. S., and Yanofsky, M. F. (1995). Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7, 1259–1269. doi: 10.1105/tpc.7.8.1259
Saini, H. S., and Aspinall, D. (1981). Effect of water deficit on sporogenesis in wheat (Triticum aestivum L.). Ann. Bot. 48, 623–633.
Saini, H. S., and Aspinall, D. (1982). Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Ann. Bot. 49, 835–846.
Saini, H. S., Sedgley, M., and Aspinall, D. (1984). Development anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid. J. Plant Physiol. 11, 243–253.
Saito, K., Hayano-Saito, Y., Kuroki, M., and Sato, Y. (2010). Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci. 179, 97–102. doi: 10.1016/j.plantsci.2010.04.004
Sakata, T., Oda, S., Tsunaga, Y., Shomura, H., Kawagishi-Kobayashi, M., Aya, K., et al. (2014). Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol. 164, 2011–2019. doi: 10.1104/pp.113.234401
Sakata, T., Oshino, T., Miura, S., Tomabechi, M., Tsunaga, Y., Higashitani, N., et al. (2010). Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. U.S.A. 107, 8569–8574. doi: 10.1073/pnas.1000869107
Satake, T., and Yoshida, S. (1978). High temperature-induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 47, 6–17. doi: 10.1626/jcs.47.6
Schmidt, R. J., and Ambrose, B. A. (1998). The blooming of grass flower development. Curr. Opin. Plant Biol. 1, 60–67. doi: 10.1016/s1369-5266(98)80129-5
Schmidt, R. J., Veit, B., Mandel, M. A., Mena, M., Hake, S., and Yanofsky, M. F. (1993). Identification and molecular characterization of ZAG1, the maize homolog of the Arabidopsis floral homeotic gene AGAMOUS. Plant Cell 5, 729–737. doi: 10.1105/tpc.5.7.729
Schoper, J. B., Lambert, R. J., and Vasila, B. L. (1986). Maize pollen viability and ear receptivity under water and high temperature stress. Crop Sci. 26, 1029–1033. doi: 10.2135/cropsci1986.0011183X002600050038x
Schoper, J. B., Lambert, R. J., and Vasila, B. L. (1987a). Pollen viability, pollen shedding, and combining ability for tassel heat tolerance in maize. Crop Sci. 27, 27–31. doi: 10.2135/cropsci1987.0011183X002700010007x
Schoper, J. B., Lambert, R. J., Vasilas, B. L., and Westgate, M. E. (1987b). Plant factors controlling seed set in maize: the influence of silk, pollen, and ear-leaf water status and tassel heat-treatment at pollination. Plant Physiol. 83, 121–125. doi: 10.1104/pp.83.1.121
Semenov, M. A., and Stratonovitch, P. (2013). Designing high-yielding wheat ideotypes for a changing climate. Food Energy Sec. 2, 185–196. doi: 10.1002/fes3.34
Sessions, A., Nemhauser, J. L., McColl, A., Roe, J. L., Feldmann, K. A., and Zambryski, P. C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124, 4481–4491.
Sheoran, I. S., and Saini, H. S. (1996). Drought-induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sex. Plant Reprod. 9, 161–169. doi: 10.1007/bf02221396
Skirpan, A., Culler, A. H., Gallavotti, A., Jackson, D., Cohen, J. D., and McSteen, P. (2009). BARREN INFLORESCENCE2 interaction with ZmPIN1a suggests a role in auxin transport during maize inflorescence development. Plant Cell Physiol. 50, 652–657. doi: 10.1093/pcp/pcp006
Spray, C. R., Kobayashi, M., Suzuki, Y., Phinney, B. O., Gaskin, P., and MacMillan, J. (1996). The dwarf-1 (d1) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc. Natl. Acad. Sci. U.S.A. 93, 10515–10518. doi: 10.1073/pnas.93.19.10515
Stintzi, A., and Browse, J. (2000). The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. U.S.A. 97, 10625–10630. doi: 10.1073/pnas.190264497
Su, Z., Ma, X., Guo, H., Sukiran, N. L., Guo, B., Assmann, S. M., et al. (2013). Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25, 3785–3807. doi: 10.1105/tpc.113.115428
Subedi, K. D., Gregory, P. J., Summerfield, R. J., and Gooding, M. J. (1998). Cold temperatures and boron deficiency caused grain set failure in spring wheat (Triticum aestivum L.). Field Crops Res. 57, 277–288. doi: 10.1016/s0378-4290(97)00148-2
Szekeres, M., Németh, K., Koncz-Kálmán, Z., Mathur, J., Kauschmann, A., Altmann, T., et al. (1996). Brassinosteroids rescue the deficiency of cyp90, a cytochrome p450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85, 171–182. doi: 10.1016/s0092-8674(00)81094-6
Tashiro, T., and Wardlaw, I. F. (1990). The response to high-temperature shock and humidity changes prior to and during the early stages of grain development in wheat. Aust. J. Plant Physiol. 17, 551–561. doi: 10.1071/PP9900551
Thakur, P., Kumar, S., Malik, J. A., Berger, J. D., and Nayyar, H. (2010). Cold stress effects on reproductive development in grain crops: an overview. Environ. Exp. Bot. 67, 429–443. doi: 10.1016/j.envexpbot.2009.09.004
Theissen, G., Strater, T., Fischer, A., and Saedler, H. (1995). Structural characterization, chromosomal localization and phylogenetic evaluation of 2 pairs of AGAMOUS-like MADS-box genes from maize. Gene 156, 155–166. doi: 10.1016/0378-1119(95)00020-7
Thomashow, M. F. (1999). Plant acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. 50, 571–599. doi: 10.1146/annurev.arplant.50.1.571
Thompson, B. E., Bartling, L., Whipple, C., Hall, D. H., Sakai, H., Schmidt, R., et al. (2009). bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell 21, 2578–2590. doi: 10.1105/tpc.109.067751
Tranel, D., Knapp, A., and Perdomo, A. (2009). Chilling effects during maize tassel development and the lack of compensational plasticity. Crop Sci. 49, 1852–1858. doi: 10.2135/cropsci2008.10.0593
Tunc-Ozdemir, M., Tang, C., Ishka, M. R., Brown, E., Groves, N. R., Myers, C. T., et al. (2013). A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 161, 1010–1020. doi: 10.1104/pp.112.206888
Veit, B., Schmidt, R. J., Hake, S., and Yanofsky, M. F. (1993). Maize floral development: new genes and old mutants. Plant Cell 5, 1205–1215. doi: 10.1105/tpc.5.10.1205
Wang, D., Li, F., Duan, Q., Han, T., Xu, Z., and Bai, S. (2010). Ethylene perception is involved in female cucumber flower development. Plant J. 61, 862–872. doi: 10.1111/j.1365-313X.2009.04114.x
Wang, Q., Yucheng, G., Yaorong, W., Honglin, C., Chen, F., and Chu, C. (2008). Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 67, 589–602. doi: 10.1007/s11103-008-9340-6
Westgate, M. E., and Boyer, J. S. (1986). Reproduction at low and pollen water potentials in maize. Crop Sci. 26, 951–956. doi: 10.2135/cropsci1986.0011183X002600050023x
Whipple, C. J., Ciceri, P., Padilla, C. M., Ambrose, B. A., Bandong, S. L., and Schmidt, R. J. (2004). Conservation of B-class floral homeotic gene function between maize and Arabidopsis. Development 131, 6083–6091. doi: 10.1242/dev.01523
Wood, A. W., Tan, D. K. Y., Mamun, E. A., and Sutton, B. G. (2006). Sorghum can compensate for chilling-induced grain loss. J. Agron. Crop Sci. 192, 445–451. doi: 10.1111/j.1439-037X.2006.00233.x
Wu, H. M., and Cheung, A. Y. (2000). Programmed cell death in plant reproduction. Plant Mol. Biol. 44, 267–281. doi: 10.1023/a:1026536324081
Wu, M., Tian, Q., and Reed, J. W. (2006). Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133, 4211–4218. doi: 10.1242/dev.02602
Wu, X., Tang, D., Li, M., Wang, K., and Cheng, Z. (2013). Loose plant architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Phys. 161, 317–329. doi: 10.1104/pp.112.208496
Yan, Y., Christensen, S., Isakeit, T., Engelberth, J., Meeley, R., Hayward, A., et al. (2012). Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24, 1420–1436. doi: 10.1105/tpc.111.094151
Yang, K., Xia, C., Liu, X., Wang, W., Chen, L., Zhang, X., et al. (2009). A mutation in THERMOSENSITIVE MALE STERILE 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J. 57, 870–882. doi: 10.1111/j.1365-313X.2008.03732.x
Ye, Q., Zhu, W., Li, L., Zhang, S., Yin, Y., Ma, H., et al. (2010). Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. U.S.A. 107, 6100–6105. doi: 10.1073/pnas.0912333107
Yin, T., and Quinn, J. A. (1995). Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am. J. Bot. 82, 1537–1546. doi: 10.2307/2446182
Yoshida, H. (2012). Is the lodicule a petal: molecular evidence? Plant Sci. 184, 121–128. doi: 10.1016/j.plantsci.2011.12.016
Yoshida, H., and Nagato, Y. (2011). Flower development in rice. J. Exp. Bot. 62, 4719–4730. doi: 10.1093/jxb/err272
Young, T. E., Ling, J., Geisler-Lee, C. J., Tanguay, R. L., Caldwell, C., and Gallie, D. R. (2001). Developmental and thermal regulation of the maize heat shock protein, HSP101. Plant Phys. 127, 777–791. doi: 10.1104/pp.010160
Yu, H., Ito, T., Zhao, Y., Peng, J., Kumar, P., and Meyerowitz, E. M. (2004). Floral homeotic genes are targets of gibberellin signaling in flower development. Proc. Natl. Acad. Sci. U.S.A. 101, 7827–7832. doi: 10.1073/pnas.0402377101
Zhang, D., and Yuan, Z. (2014). Molecular control of grass inflorescence development. Annu. Rev. Plant Biol. 65, 553–578. doi: 10.1146/annurev-arplant-050213-040104
Zhang, J., Simmons, C., Yalpani, N., Crane, V., Wilkinson, H., and Kolomiets, M. (2005). Genomic analysis of the 12-oxo-phytodienoic acid reductase gene family of Zea mays. Plant Mol. Biol. 59, 323–343. doi: 10.1007/s11103-005-8883-z
Zhao, D., and Ma, H. (2000). Male fertility: a case of enzyme identity. Curr. biol. 10, R904–R907. doi: 10.1016/S0960-9822(00)00848-4
Zhou, H., Zhou, M., Yang, Y., Li, J., Zhu, L., Jiang, D., et al. (2014). RNase ZS1 processes UbL40 mRNAs and controls thermosensitive genic male sterility. Nat. Commin. 5:4884. doi: 10.1038/ncomms5884
Zhuang, Y., Ren, G., Zhu, Y., Hou, G., Qu, X., Li, Z., et al. (2008). Transcriptional profiles of immature ears and tassel in maize at early stage of water stress. Biol. Plant. 52, 754–758. doi: 10.1007/s10535-008-0146-9
Zinn, K. E., Tunc-Ozdemir, M., and Harper, J. F. (2010). Temperature stress and plant sexual reproduction: uncovering the weakest links. J. Exp. Bot. 61, 1959–1968. doi: 10.1093/jxb/erq053
Zinselmeier, C., Jeong, B. R., and Boyer, J. S. (1999). Starch and the control of kernel number in maize at low water potentials. Plant Physiol. 121, 25–36. doi: 10.1104/pp.121.1.25
Zluvova, J., Nicolas, M., Berger, A., Negrutiu, I., and Monéger, F. (2006). Premature arrest of the male flower meristem precedes sexual dimorphism in the dioecious plant Silene latifolia. Proc. Natl. Acad. Sci U.S.A. 103, 18854–18859. doi: 10.1073/pnas.0606622103
Zou, C., Jiang, W., and Yu, D. (2010). Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 61, 3901–3914. doi: 10.1093/jxb/erq204
Keywords: sterility, yield, floral organ degeneration/abortion, ABC genes, hormones, abiotic stresses, Arabidopsis, cereal crops
Citation: Smith AR and Zhao D (2016) Sterility Caused by Floral Organ Degeneration and Abiotic Stresses in Arabidopsis and Cereal Grains. Front. Plant Sci. 7:1503. doi: 10.3389/fpls.2016.01503
Received: 30 March 2016; Accepted: 21 September 2016;
Published: 14 October 2016.
Edited by:
Stefan De Folter, CINVESTAV, MexicoReviewed by:
Marcelo Carnier Dornelas, State University of Campinas, BrazilYuling Jiao, Institute of Genetics and Developmental Biology (CAS), China
Copyright © 2016 Smith and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dazhong Zhao, dzhao@uwm.edu
 Ashley R. Smith
Ashley R. Smith