- 1Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Institute of Botany (CAS), Guangzhou, China
- 2College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
- 3Guangdong Provincial Key Laboratory of Applied Botany, South China Institute of Botany (CAS), Guangzhou, China
- 4Department of Microbiology and Plant Biology, University of Oklahoma, Norman, OK, USA
- 5Guangxi Institute of Botany (CAS), Guilin, China
A clear understanding of hydraulic regulation in cultivated plants is crucial for addressing challenges to forest water cycling due to climate changes in low subtropical China. Experiments were conducted to determine the hydrologic balance of a Eucalyptus urophylla plantation in response to periodic drought. Trees displayed lower stomatal conductance (GS) and leaf water potentials (ΨL) during the dry periods. A decrease of 22.4% was found for the maximum reference GS (GS at D = 1 kPa; GSref-max). Accordingly, specific hydraulic conductivity (ks) decreased by 45.3 – 65.6% from the wet to the dry season, depending on the tree size. Fairly stable leaf stomatal conductance (gs) with decreasing ΨL (ΨL < -1.6 MPa) contributed to the high water-use efficiency (WUE) of this Eucalyptus species. Additionally, the lower stomatal sensitivity (-m = 0.53) in the dry season might also be responsible for the high WUE, since we found an anisohydric behavior that was associated with photosynthetically active radiation (Q0). Larger trees were found to use water more efficiently than small trees, due to the higher sensitivity of ks to decreasing ΨL. This was also verified by the decreasing leaf carbon isotope discrimination (Δ13C) with increasing tree diameter. However, further studies are needed to determine the universality of these results for other Eucalyptus species in this region.
Introduction
Due to anthropogenic climate changes, the survival of tropical and subtropical forest communities may be threatened in a variety of ways, including increased frequency of severe droughts caused by changes in precipitation pattern (Davidson et al., 2012), especially in the southern region of China (Zhai et al., 2005; Zhou et al., 2011). As previously reported, long term drought may lead to increased tree mortality, and decreased productivity and forest biomass carbon sinks for natural forests (Chaves et al., 2002; Zhou et al., 2013). Recently, planted forests have been suggested to be more vulnerable to severe environmental stress because of their weaker ecological resilience (Bleby et al., 2012).
Under light-saturating conditions and a high vapor pressure deficit (D), most plants reduce stomatal conductance (GS) to limit transpiration and to slow down the development of potentially damaging low leaf water potential (ΨL) (Meinzer, 1993), ultimately leading to a decrease in net primary productivity (NPP) (Ryan and Waring, 1992). However, some plants have been hypothesized to follow optimal trajectories to maximize their carbon gain (Hölttä et al., 2011; Rosen, 2013). For example, Eucalyptus species have been reported to have high photosynthetic capacity, WUE, and growth rate (Whitehead and Beadle, 2004), which may imply a high canopy GS. These characteristics may minimize and counteract the effects of extreme climate events, and reinforce community resilience (Lloret et al., 2012). However, how these species coordinate decreases in GS to avoid hydraulic failure with the demand to maximize carbon assimilation in dry conditions is poorly understood and less reported.
Generally, plants have been classified into two broad categories based on the ability of stomata to regulate ΨL: isohydric and anisohydric (Jones, 1998; Martínez-Vilalta et al., 2014). Isohydric species adjust their stomatal opening in such a way as to maintain midday ΨL relatively stable as environmental conditions change. In contrast, anisohydric species have less strict stomatal control, with no discernible threshold of minimum ΨL (Martínez-Vilalta et al., 2014). It has been found that the slope of the relationship between GS and ln (D) is closely related to the magnitude of GS at D = 1.0 kPa (GSref), and can be used as an empirical relationship to describe isohydric behavior (Oren et al., 1998). The stomatal sensitivity to D of isohydric plants is reported to be linearly proportional to GSref (i.e., -m = 0.6) (Pou et al., 2012), which provides insight into stomatal regulation. Some Eucalyptus (e.g., Eucalyptus gomphocephala) have been reported to allow a greater ΨL range than typical isohydric species and to occupy more drought-prone habitats, since they have xylem that is more resistant to negative water potentials (Franks et al., 2007; West et al., 2008). However, little is known about whether this behavior will facilitate the maximization of carbon assimilation for Eucalyptus.
Except for stomatal regulation, trees mainly respond to soil drought-induced water stress by changes to hydraulic properties, in order to adapt to the environment in the long term (Schäfer et al., 2000; Forrester, 2015). These changes are highly related to stomatal regulation. One of the most commonly observed strategies is that the hydraulic limitation of GS by increasing path length can be mitigated by structural compensation, particularly a reduction in AL/AS (Becker et al., 2000; McDowell et al., 2002; Delzon et al., 2004). Some other compensating strategies have been observed, such as larger trees possessing deeper roots and larger conduit diameters (Mokany et al., 2003; Anderegg et al., 2012). Köstner et al. (2002) claimed that the increased path length and gravitational head concomitant with height (H) growth must be offset either by a reduction in GS, the ratio of leaf area to sapwood area (AL/AS), ΨL, or by an increase in hydraulic conductivity (Ks), to maintain hydraulic balance for any given soil water potential (Ψs) and D. Eucalyptus species usually have large leaf area to support rapid stem growth (White et al., 1998), and thus have high transpiration demands at both the leaf and canopy levels during drought periods (Dawson, 1996). Therefore, we hypothesized that Eucalyptus are likely to maintain a constant Gs and support a higher AL as trees grow in order to meet the great growth demands, while other strategies are employed to compensate for the increased hydraulic limitation with tree height and transpiration demand. To shed light on this aspect of Eucalyptus, it is necessary to quantify the effect of tree size on tree growth, as soil water decreases (Feichtinger et al., 2014).
Eucalyptus urophylla is the most widely planted forest tree in southern China and its area is still rapidly expanding (Shi et al., 2012). Our study is focused on how fast growing Eucalyptus forests in low subtropical China balance hydraulic safety and carbon assimilation under periodical drought. Specifically we asked: (1) How does this species coordinate decreased Gs with changes in hydraulic conductivity to maximize carbon assimilation in dry conditions? (2) How do stomatal regulation and hydraulic conductivity converge with tree structural changes so as to maintain fast growth?
Materials and Methods
Study Site and Plant Material
This study was conducted at the Huangmian state forest farm (24°66′N, 109°87′E), which is located approximately 60 km southwest of Guilin city in South China. This farm is planted with E. urophylla for lumber and pulp production. All of the measurements took place in a 3–5 year old E. urophylla stand on a hill with an inclination of approximately 30° facing southwest. The forest density was 1375 trees ha-1. The soil of this forest is characterized as heavy loam. This area is characterized by a low subtropical monsoon climate with annual precipitation ranging from 1750 to 2000 mm and an average annual temperature of 19°C. Rainfall is unevenly distributed throughout the year, producing wet (March to September) and dry (October to February of the next year) seasons. Measurements were carried out from June 2012 to May 2013 on 15 trees. The mean tree height and diameter at breast height (DBH) of the sampled trees was 11.5 ± 2.9 m and 10.1 ± 2.2 cm, respectively. An instrument tower 23 m tall was set up within the plantation, providing access to the canopy of the forest stand.
Sap Flow and Environmental Factors
Self-made Granier-type sensors (20 mm in length; Granier et al., 1996) consisting of a heated (constant heat flow) probe and an unheated thermocouple probe were used to monitor the sap flow density (Fd, g m-2 s-1) of the sampled 15 trees. The probes were inserted into the xylem at breast height (1.3 m) on the north side of tree stems. The upper probe was supplied with a constant power of 120 mA. The temperature difference between both probes was measured and converted to Fd, according to Granier et al. (1996). More details of sensor installation have been described in Zhu et al. (2015) and Zhang et al. (2016). The Fd of E. urophylla is assumed to be isotropic in terms of evenly distributed leaf transpiration around the tree crown (Burgess and Dawson, 2008). Fd was used to estimate transpiration after it was converted into a spatially weighted mean flux based on the radial variation in sap flow density observed in another study of E. urophylla (n = 38, Zhou et al., 2004). They found that the variation in sapflow density from the outmost of the stem for 3–4 year old E. urophylla can be expressed as Fd = ax3+bx2+cx+d, where x is the ratio of the sensor depth to the radial sapwood thickness. We combined the results of the two plots in their study and obtained the equation Fd = 4.33x3-8.31x2+4.07x+0.52. Natural temperature gradients can lead to large potential errors of sap flow measurements (Do and Rocheteau, 2002), however, temperature gradients were found to be negligible in our study (Zhang et al., 2016).
A micro-meteorological station was built on the top of the tower. Photosynthetic photon flux density (Q0, μmol m-2 s-1), temperature (T, °C), relative humidity (RH, %), and wind speed (u, m s-1) were measured simultaneously with the sap flow measurements (Zhu et al., 2015; Zhang et al., 2016). Rainfall data (in Guilin) during the study period were obtained from the China Metrological Data Sharing Service System1. Soil water content (SWC, m3 m-3) was monitored with three soil water probes (SM300, UK) that were buried 30 cm under the ground surface.
Tree Morphological Features
For each sap flow tree, DBH was measured with a diameter tape, and tree height (h) was estimated with a tape dropped from the top of the tower. Leaf area (AL) was estimated using an allometric relationship between DBH and the AL that was constructed by harvesting seven trees outside (but near) the experimental plot. During the harvest, five small leaf sub-samples from each tree were scanned with a portable leaf area meter (LICOR-3000, USA) and weighed (fresh weight) to estimate specific leaf area (area/fresh mass ratio). Then all leaves of the harvested trees were collected, weighed and multiplied by the specific leaf area to obtain an estimate of whole tree AL. The DBH of the harvested trees ranged from 6.6 to 11.1 cm, while those for sap flow trees ranged from 8.5 to 16.1 cm (i.e., the range of DBH for harvest trees didn’t cover the full range of trees used for sap flow measurements). Thus, we combined our DBH and AL measurements with those from Zhu et al. (2009) (E. urophylla; n = 9) to derive an AL – DBH relationship [AL = 43.43∗ (1-exp (-0.15DBH))4.93, R2 = 0.93, n = 16]. Data was obtained from the table in their paper. Sapwood depth and bark thickness were determined from stem cores (5 mm in diameter) obtained with an increment borer from selected trees outside the sap flux measurement plots (5 m away, in the same stand). The sapwood depth was visually distinguished from heartwood based on color, and was used to calculate sapwood area (As), which can be expressed as AS = -0.008 + 0.0015DBH, R2 = 0.97, n = 27. Since sample trees were estimated based on the relationship between DBH and As, we used the fitted relationship between DBH and As and the AL from the harvested trees together to scale up whole tree transpiration (ET). Nocturnal sap flux (ET-NOC) was defined as ET that occurred when Q0 = 0. Since Eucalyptus are reported to have thick, tough and long lived leaves with weak seasonal dynamics (Reich et al., 1999), thus the variation in leaf area would not be accounted for these relationships.
Hydraulic Properties of Stem Xylem
The physical limitations on water transport in the xylem determine the stomatal behavior and transpiration in trees. This relationship is usually expressed as a combination of Darcy’s law with a simple expression for transpiration which is equated to liquid transport in wood (Whitehead and Jarvis, 1981):
Where ks is the effective hydraulic conductivity from soil to leaves (whole-plant conductance per unit sapwood), and ΔΨ is the water potential difference between root and leaf.
In order to determine the wet-dry seasonal variation in ΔΨ, the leaf water potentials at pre-dawn (Ψpre-dawn, 5:00), pre-night (Ψpre-night, 19:00) and midday (Ψmidday, 13:00) were measured with a portable pressure chamber (PMS 1000, Corvallis, OR, USA) on sunny days in the wet (5 days) and dry (4 days) seasons. Five trees were selected for measurement. ΨL were the mean of three replicate shoots with fully expanded leaves, sampled from the mid-crown of each tree. Since soil moisture variation within a single day is small, Ψpre-dawn was treated as a substitute for the water potential in the soil (Ψs) (Kim et al., 2008; Bleby et al., 2012). Thus, ΔΨ was estimated as the difference between Ψpre-dawn and Ψmidday.
Canopy Stomatal Conductance
If forest transpiration is well-coupled with atmospheric factors, the mean stomatal conductance can be estimated based on a simplified equation (Köstner et al., 1992), which is derived from Whitehead and Jarvis (1981). Due to low LAI, the canopy was found to meet the assumptions necessary to adopt this equation (Zhu et al., 2015). It is assumed that the Fd-scaled transpiration combined with As/AL is a proxy for transpiration rate per unit of leaf area (EL). Therefore, mean stomatal conductance (Gs) for each tree, can be expressed as:
where EL is whole-tree transpiration per unit leaf area (g m-2 s-1), GV is the universal gas constant adjusted for water vapor (0.462 m3kPa K-1 kg-1), Ta is the air temperature (K), ρ is the density of water (998 kg m-3), and D is in kPa. GSi is in units of mmol m-2 s-1 (Monteith and Unsworth, 2013).
The forest had an LAI of 1.68 ± 0.28 m2 m-2 and did not show significant seasonal changes (p = 0.78) (Zhu et al., 2015). Therefore, GSi calculation is not subject to errors caused by leaf area dynamics. GSi was estimated after (1) performing a cross-correlation analysis between D and Fd, and using the most appropriate time lag to implement a time-corrected Fd and (2) filtering out data where D < 0.6 kPa, in the hours of early morning and late afternoon (Oren et al., 1998).
Along with the ΨL measurements, we also measured leaf stomatal conductance (gs) at mid-day on the abaxial surface of sun-exposed leaves with a steady-state porometer (SC-1, DECAGON, USA). Three leaves of each tree were chosen randomly for these measurements.
Stomatal Sensitivity to Vapor Pressure Deficit
Stomatal sensitivity is proportionally related to the magnitude of Gs at low D (D = 1 kPa) when soil moisture is not limiting (Granier et al., 1996), and it can be derived as:
where GSref is the intercept (i.e., the value of Gs at D = 1 kPa in a log-linear relationship), and -m represents the slope of the regression fit representing stomatal sensitivity to D (i.e., dGs/dln D). By analyzing data from a variety of sources, including both porometric and sap flux derived Gs, -m was demonstrated to be approximately 0.6 (Oren et al., 1998). In our study, the -m and GSref for E. urophylla in the wet and dry seasons was calculated to characterize the response of Gs to drought.
A boundary line analysis of the relationship between D and Gs was performed for the dry and wet seasons. The datasets of Gs for each tree were binned by radiation (nine levels from 0 to 1600 μmol m-2 s-1). The data at night (Q0 = 0) were excluded because plant physiological response at night is much more complicated than that in the daytime (Oren et al., 2001). The relationship between the mean lnD and Gs of each subset was linearly fitted, and the intercept and slope corresponded to the GSref (Gs at D = 1 kPa) and sensitivity in response to D (dGs/dlnD, mmol m-2 s-1 kPa-1), respectively (Oren et al., 1998). Then, the relationship between GSref and -dlnd/dGs for the two seasons was fitted.
In order to determine the radiation regulation of stomatal conductance under different water conditions, the GSref under different light conditions was normalized by the value of the maximum Q0 of each tree in both seasons, and the relationship between the GSref and mean Q0 at that level for all of the trees was fitted with an exponential function expressed as:
Where a refers to the maximum dependent variable, i.e., the max GSref (GSref-max).
Leaf Stable Carbon Isotopes
After the experiments, we randomly collected leaves at the top of the crown for 11 trees with DBH ranging from 5.2 to 20.4 cm near the sap flow measurement trees. Leaves were dried at 65°C to constant mass, and leaf dry mass was determined to the nearest mg. The dry leaves were then ground to a fine, homogeneous powder (Cernusak and Hutley, 2011). The leaf carbon isotope ratio was determined using a stable isotope ratio mass spectrometer (Isoprime 100, Isoprime, UK) on a subsample of approximately 3 mg leaf material. These analyses were performed in the Public Laboratory of South China Botanical Garden, Guangzhou, China. Carbon isotope discrimination (∆13C) in dry leaf matter was calculated as ∆13C = (δ13Ca - δ13Cp)/(1 + δ13Cp), where δ13Cp is δ13C of dry leaf matter, and δ13Ca is that of atmospheric CO2. We assumed a value of -5.5‰ for δ13Ca, according to the previous measurements in low subtropical China (Zou et al., 2009).
Data Analysis
Boundary-line analysis was conducted in Excel (version 2010, Microsoft Office Excel) to set up the relationship between environmental conditions and maximum canopy stomatal conductance or Fd. The upper boundary line was derived by: (1) partitioning data of independent variables (VI) into specific intervals, (2) calculating the mean and standard deviation of dependent variables (Vd) in each interval, (3) removing outliers (P < 0.05; Dixon’s test), (4) selecting the data falling above the mean plus one standard deviation, and (5) averaging the selected data for each VI interval with n ≥ 5 remaining Vd values. The intervals with n < 5 was excluded to prevent VI intervals with too little information from affecting the relationship.
Statistical analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC, USA). A multiple regression analysis was conducted to determine the effect of hydraulic architecture on tree water use in the wet and dry seasons. A paired t-test was used to compare the differences in environmental and plant physiological responses between wet and dry seasons. Origin pro (version 8.6, Origin Lab, USA) was used to draw graphs.
Results
Water Conditions and Tree Water Use in Different Seasons
The precipitation (P) in the research site totaled 2167.6 mm from June 2012 to May 2013 (Figure 1). The water input was mainly contributed by precipitation in late spring and early summer (i.e., from April to June), which accounted for 55.4% of the annual total, while that from October to February (typical dry season in low subtropical China) was only 16%. The SWC in the dry season decreased 32.6% from the wet season, demonstrating a significant difference in the soil water conditions between the wet and dry seasons.
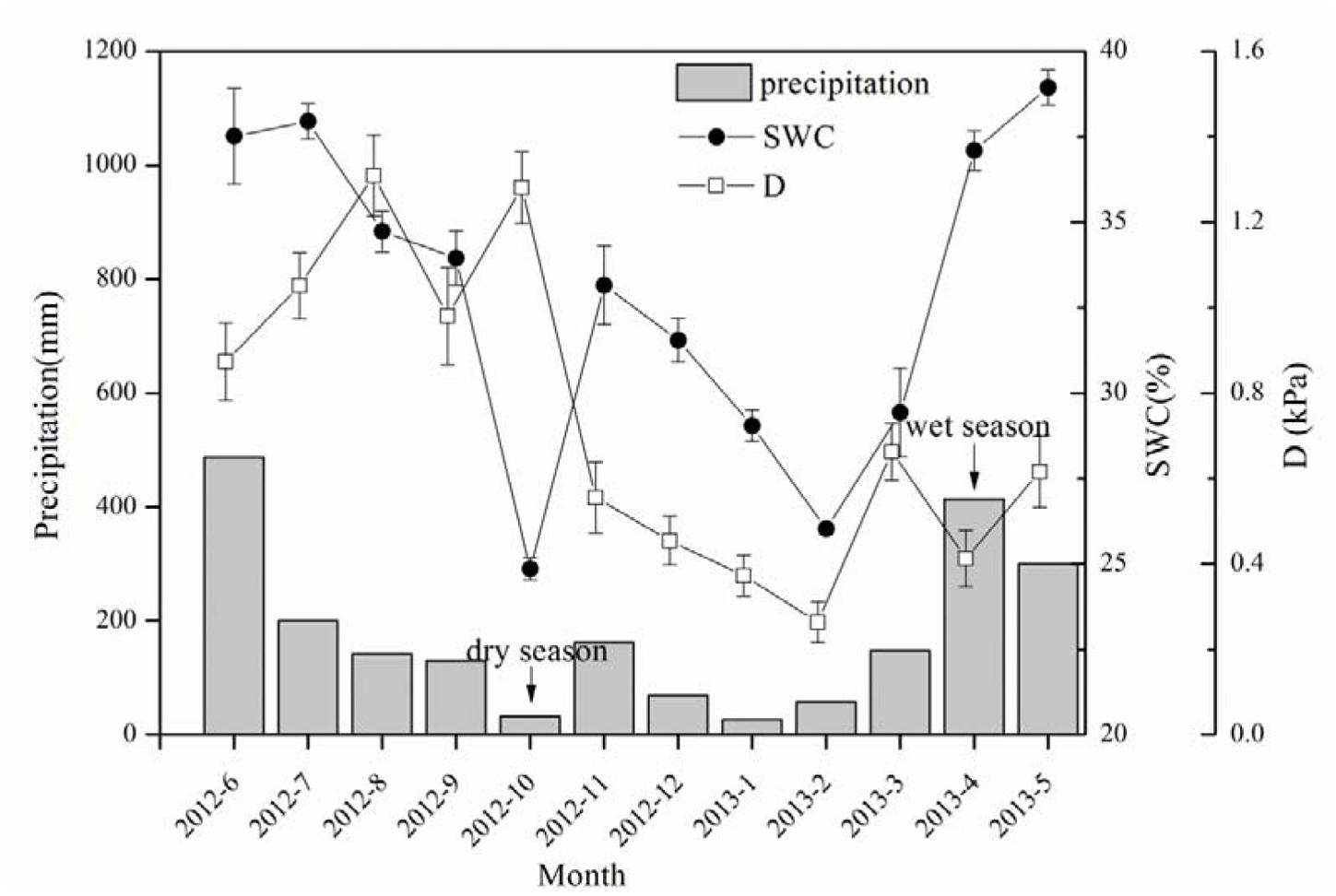
FIGURE 1. Precipitation (P), soil water content (SWC) and evaporative demand (D) during the period of sap flow measurement. SWC data shown in the figure are daily mean ± SE of that month, n = 28–31.
A boundary line analysis of the relationship between Fd and Q0 was conducted, and the maximum Fd was derived from the exponential relationship. The mean Fd of the 15 trees was 41.03 ± 7.97 and 38.82 ± 13.16 g m-2 s-1 in the dry and wet seasons, respectively, consistent with the pattern of D (Figure 1). Overall, Fd was not affected by tree size, although it was weakly related to DBH in the dry season (R2 = 0.19, p = 0.06). The wet/dry ratio of Fd varied from 0.4 to 0.8 and was not significantly related to the tree size (R2 = 0.03). Average ET in the dry season (5.7 ± 2.9 kg d-1) was 58.0% higher than that in the wet season (3.6 ± 2.3 kg d-1) (Figure 2A, p < 0.01), and linearly increased with tree size (p = 0.003). ET-NOC was 0.18 ± 0.021 kg d-1 in wet and 0.11 ± 0.01 kg d-1 in dry seasons (Figure 2B, p = 0.047), which contributed 1.82 ± 0.45% and 4.51 ± 1.34% to daily ET in dry and wet seasons, respectively. ET-NOC was also linearly related with tree size (p < 0.01).
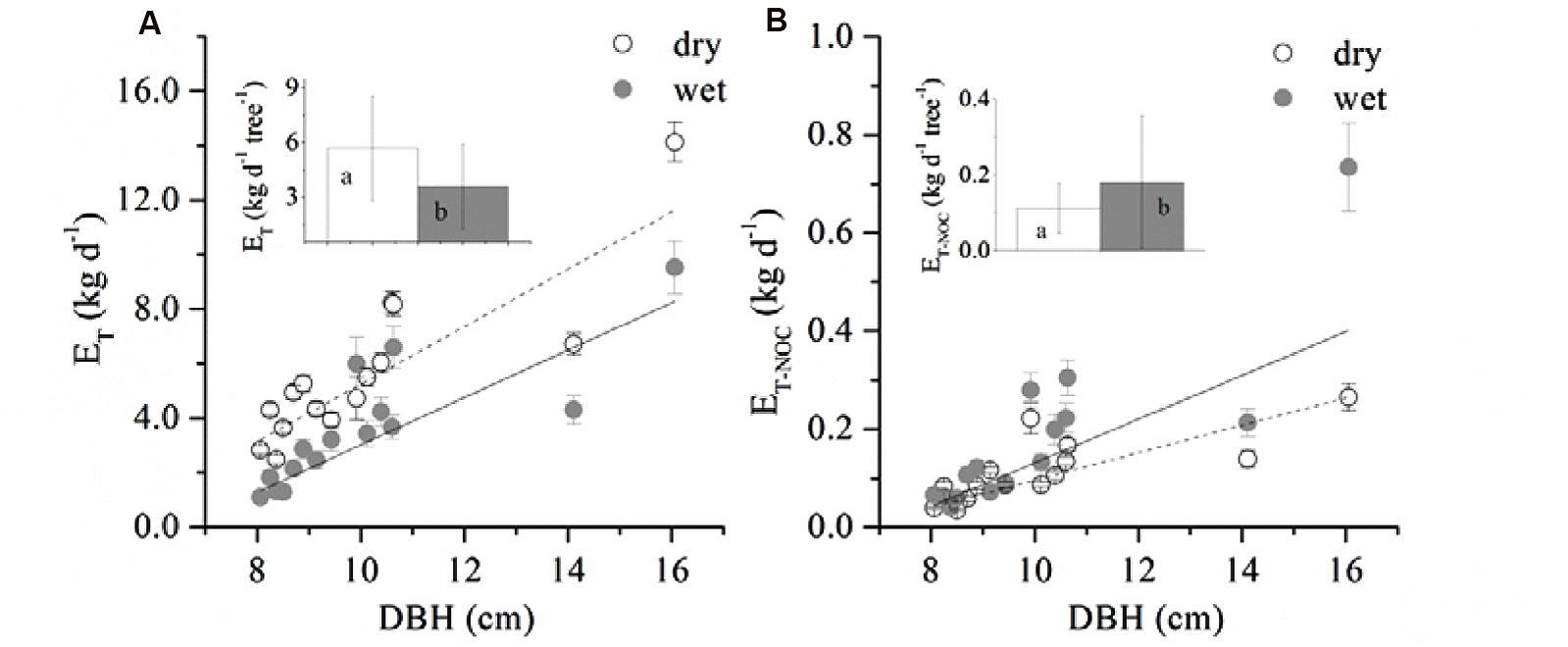
FIGURE 2. Relationship between DBH and (A) averaged daily transpiration (ET), (B) averaged total nocturnal sap flow (ET-NOC). Data are mean ± SE, and all linear fittings are significant at the p < 0.05 level. The insets in the figure represent the mean ET (A) and ET-NOC (B) of all 15 trees in dry and wet seasons respectively. Different letters indicate a significant difference between dry and wet seasons.
The average Ψpre-dawn and Ψpre-night was -0.24 ± 0.04 and -0.29 ± 0.02 MPa in the dry season and -0.21 ± 0.03 and -0.31 ± 0.05 MPa in the wet season. Seasonal differences for both were not significant (p > 0.05). The average Ψnoon was higher (-0.75 ± 0.23 MPa) in the wet season than that in the dry season (-1.46 ± 0.23 MPa) (p < 0.01). Ψpre-dawn and Ψnoon versus tree size in both seasons are shown in Figure 3. Tree size was not related to variations in either Ψpre-dawn and Ψnoon. Accordingly, the water potential difference at midday (ΔΨ) had a mean of 0.62 ± 0.66 (wet) and 1.22 ± 0.10 MPa (dry). ΔΨ was much higher in wet than in dry season (p < 0.01). No difference existed among the five trees for the parameters above (p > 0.05).
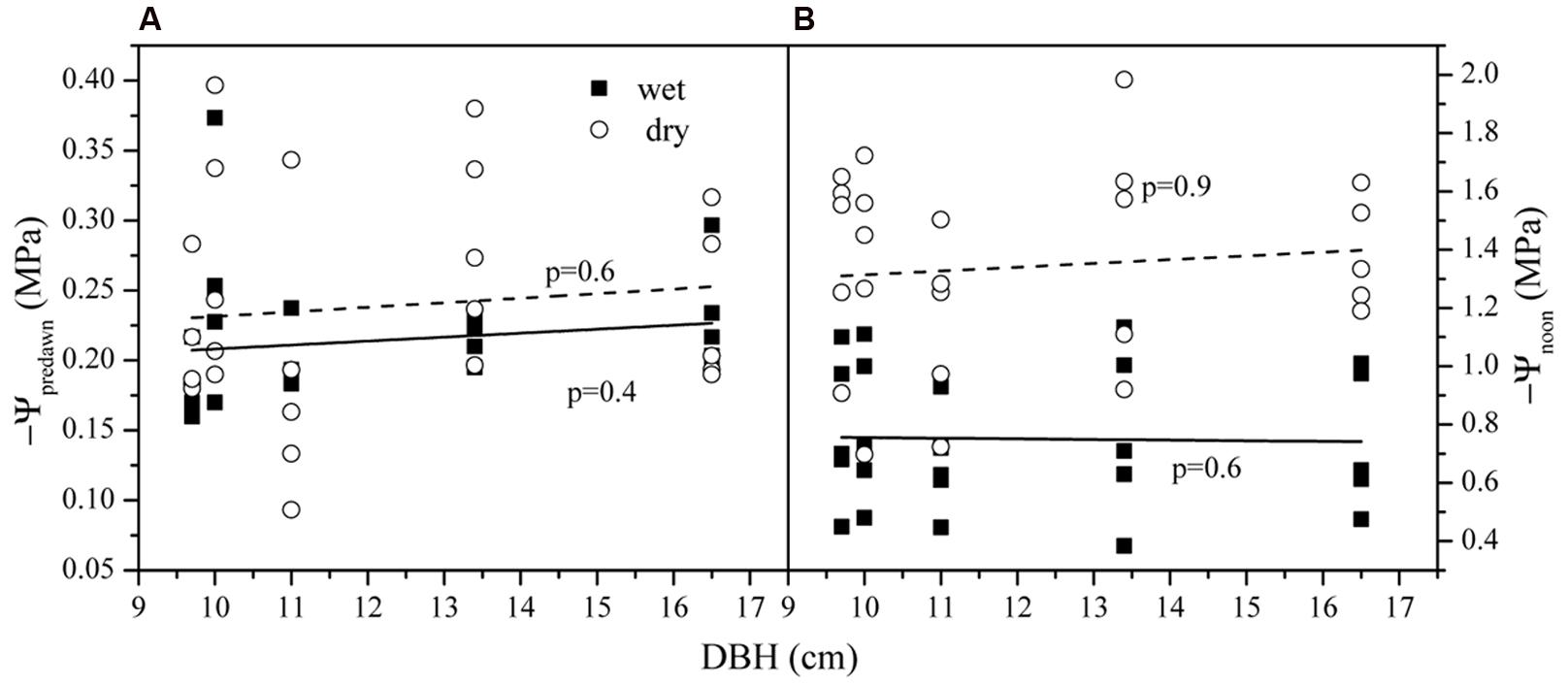
FIGURE 3. The leaf water potential at (A) pre-dawn (Ψpre-dawn) and (B) noon (Ψnoon) along the tree size gradient in dry and wet seasons.
VPD Regulated Gs under Different Light Conditions
The time lag between D and Gs was found to be 1.3 and 0.5 h in dry and wet seasons respectively. Thus, time-lagged Gs was used to calculate GSref. GSref had a linear relationship with -dGs/dLnD in both dry and wet seasons, but significant differences of the slopes were observed under different light levels (ANOVA, p < 0.01, Figure 4). Normalized GSref of all of the trees increased rapidly as Q0 rose until maximum (Figure 5A). GSref reached 90% of the maximum (GS90) when Q0 was 287.8 and 167.1 μmol m-2 s-1 in the dry and wet seasons, respectively. This revealed that GSref was more sensitive to light in wet season, leading to a lower saturation point than that in the dry season (p < 0.01). It was found that -m at different light levels had a weak relationship with tree size (not shown; p = 0.33). The effect of Q0 on –m was also quantified in both seasons (Figure 5B). –m gradually decreased with Q0 before a short increase under low light conditions (ANOVA, Duncan, p < 0.01), i.e., the sensitivity was not constant within a single day when the light intensity varied substantially. When the data under low light conditions (Q0< 200 μmol m-2 s-1) were removed, a linear decrease in -m ranging from 0.32 to 0.83 (dry season) and 0.22 to 1.10 (wet season) with radiation was observed for the 15 trees (Figure 5B). Mean -m was substantially higher in the wet season (0.58 ± 0.01) than in the dry season (0.53 ± 0.007) (p = 0.038).
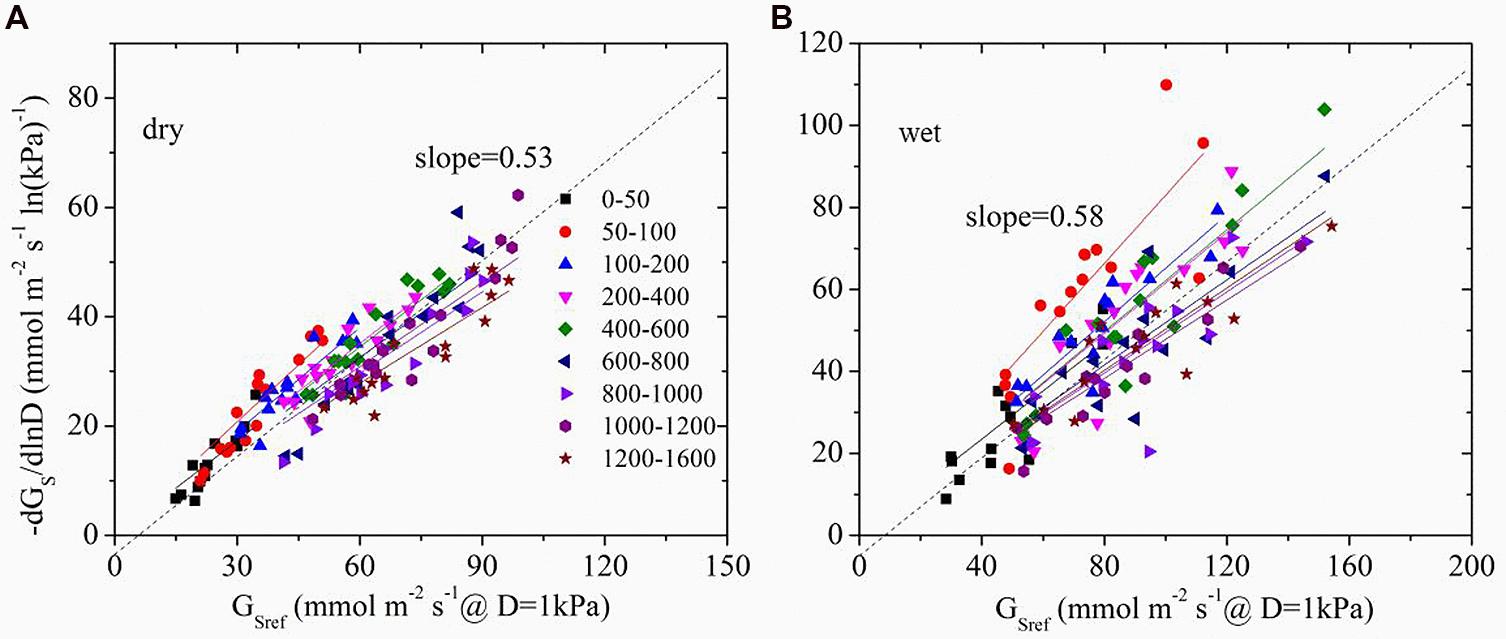
FIGURE 4. The sensitivity of average stomatal conductance of tree individuals at each light level in response to increasing vapor pressure deficit (-dGs/dlnD) as a function of the canopy stomatal conductance at D = 1 kPa (GSref) in dry (A, October) and wet season (B, April). Different symbols represent the different light levels.
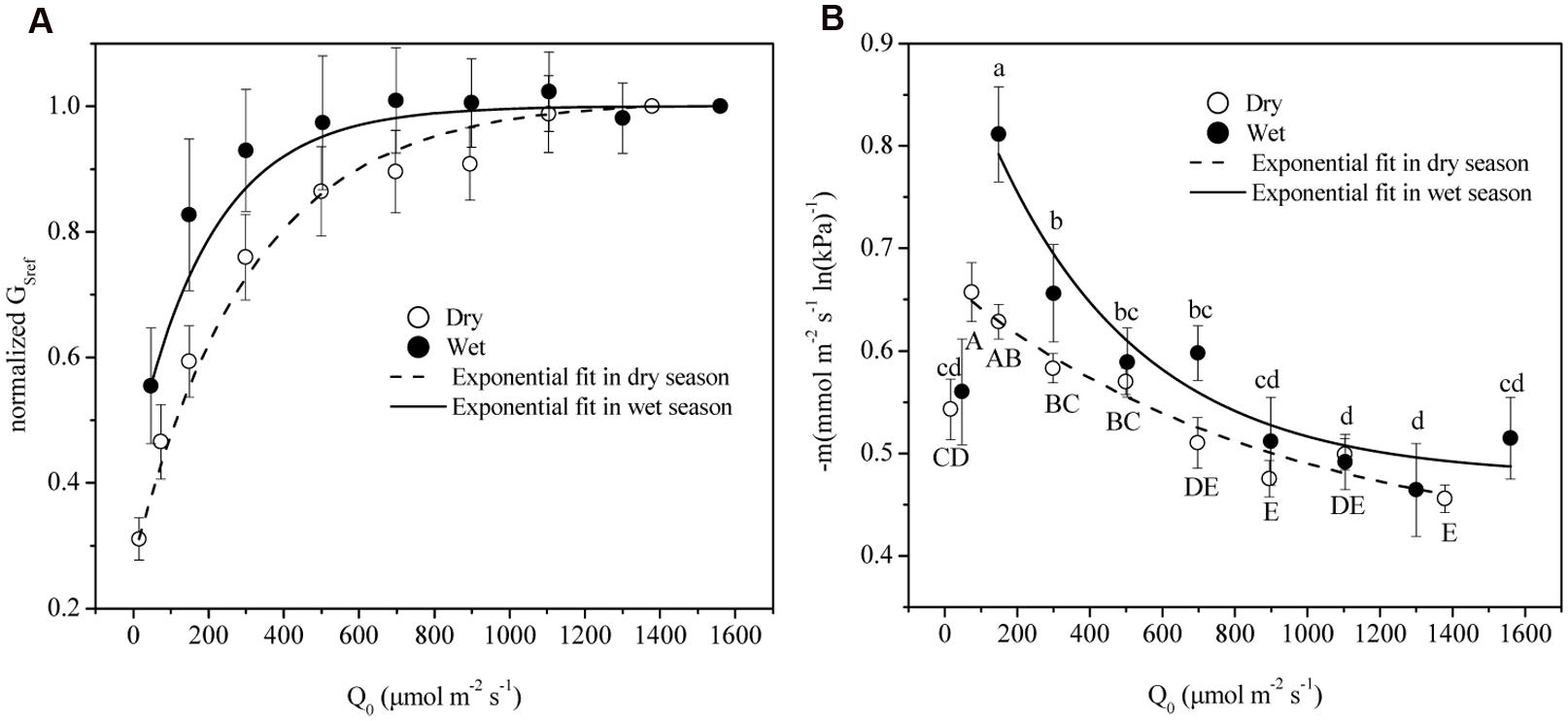
FIGURE 5. (A) GSref (Gs of each tree at D = 1 kPa) normalized based on the highest value in relation to mean photosynthetically active radiation (Q0). (B) The stomatal sensitivity (–m) of each tree in relation to Q0 in dry (open symbols) and wet seasons (solid symbols). Symbols are the mean ± SD of all 15 trees for each light group. Lines are least-square fit through the entire data. Symbols with same letters shown in (a) indicate non-significant differences among light groups, where capital/lower cases refer to dry/wet season.
Oren et al. (1998) reported that as long as stomata regulate the leaf potential near a constant value, a slope close to 0.6 is expected. The exact slope depends on the D range, boundary layer conductance (gbl), and changes in hydraulic conductance associated with D. To determine the effect of the D range, boundary analysis was conducted with the data shown in Figure 4 when D = 1 kPa – 2 kPa for all light levels. -m derived from this range was compared with that from all of the data, and it turned out to be insignificantly different from the full range in both dry and wet seasons (p = 0.43 and 0.14, respectively, ANOVA). In addition, since E. urophylla has narrow leaves, gbl in the stand during the wet and dry seasons was found to be 930.1 and 1149.8 mmol m-2s-1 (unpublished data), respectively, which led to a ratio of gbl/Gs higher than 2 in the dry season. It is claimed that -m is negatively related to gbl/Gs and equals 0.55 when the gbl/Gs is 10 (Oren et al., 1998). Our lower value will produce a higher -m value (>0.55) in the dry season for E. urophylla. Thus, it is the changes in hydraulic conductance that are responsible for the -m variation.
Stomatal Regulation in Relation to Tree Size and Leaf Water Potential
We estimated the max Gs (GSref-max) of each tree from the exponential function (equation 4, corresponding to a) before it was normalized and fitted to DBH (Figure 6). GSref-max was found to increase with DBH in the wet season before reaching the maximum when DBH > 9 cm. While no clear relationship in the dry season was observed, the mean GSref-max was higher in the wet season (88.6 mmol m-2 s-1) than in the dry season (68.8 mmol m-2 s-1)(p < 0.01). The ratio of GSref-max in the dry to wet season ranged from 0.58 to 1.26 (0.81 on average), decreasing rapidly when DBH < 9 cm and stabilizing when DBH > 9 cm.
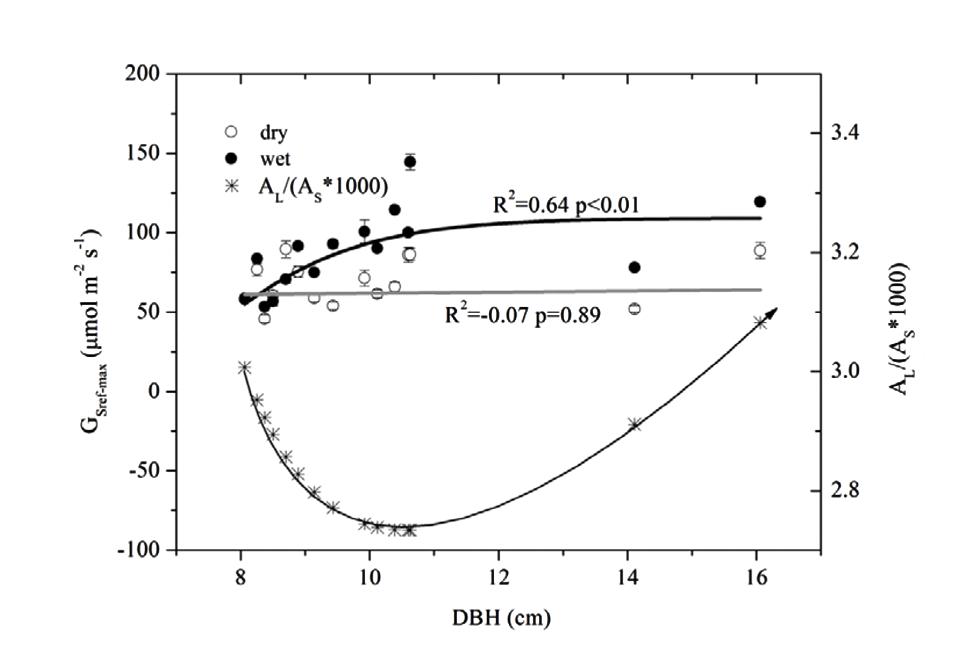
FIGURE 6. Relationship between DBH and maximum stomatal conductance at reference D (=1 kPa) (GSref-max) derived from Figure 4, and, the AL/(As∗1000) based on the predicted AL and As with DBH. Lines represent least square fits for dry (white circle) and wet (black circle) seasons respectively; data are mean ± SE.
We also related Ψnoon with the corresponding mid-day gs (Figure 7A) and found a positive relationship between gs and ΨL when light was low. Based on cell turgor theory, the change in ΨL is caused by the Gs-promoted water loss from the leaf (Dow and Bergmann, 2014). However, this value peaked and was maintained from -0.6 to -0.9 MPa before a gradual decrease.
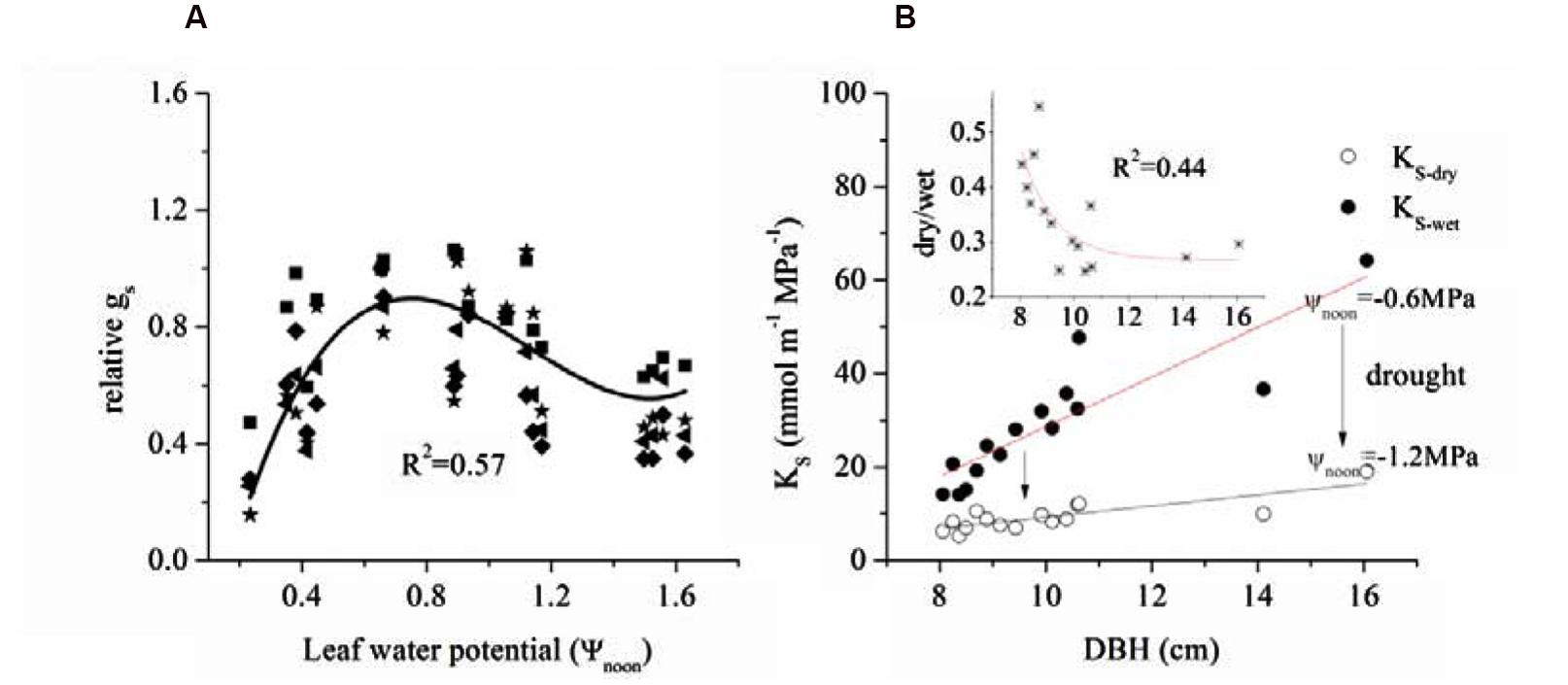
FIGURE 7. (A) Limitation of leaf water potential on relative gs during mid-day (at noon, 12:00-13:00) and (B) relationship between tree size and specific hydraulic conductivity ks of 15 sample trees in dry and wet seasons.
Hydraulic Conductivity
The combined effects of AL/As, H and ΨL on stomatal conductance of the 15 trees were described using Equation (1) to quantify the tree hydraulic aspects of the plant physiological response (Table 1). The AL/As gradually decreased up to approximately DBH = 10 cm, then started to increase when DBH > 11cm (Figure 6). We evaluated the seasonal change in ks with Equation (1) when D = 1 kPa (Figure 7B). It was observed that ks in the wet season was much higher than that in the dry season (29.1 ± 13.1 vs. 9.4 ± 3.2 mmol m-1 MPa-1). In addition, larger trees had a much higher seasonal variation in ks (p < 0.01). As shown in Figure 7B, the dry to wet season ratio of ks decreased rapidly with size growth, becoming constant for DBH > 10 cm.
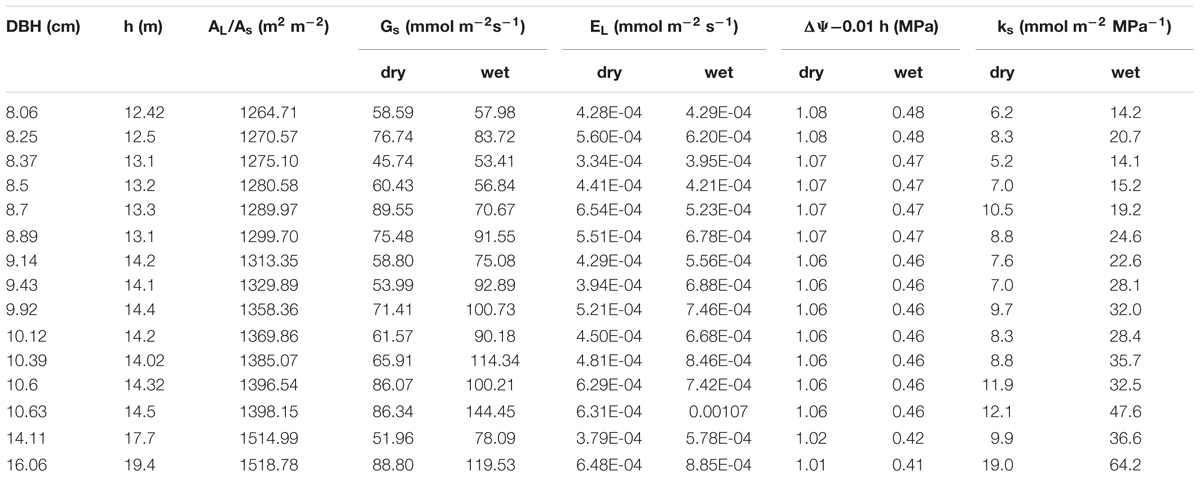
TABLE 1. Parameters that were employed to calculate ks based on Equation 1 for each tree during the dry and wet seasons.
Discussion
Stomatal Regulation in Response to Leaf Water Potential
The mechanism of stomatal closure is viewed as a direct response to the change in leaf water potential that is related closely to cell turgor (Martorell et al., 2014). In this study, it appears that Gs was not held constant in order to maximize carbon assimilation for E. urophylla in dry conditions. We found that Gs decreased by 22.4% following a two-fold decrease in ΔΨ in the dry season (Figure 6). According to equation (2), we assumed that if Gs does not change, a 234.4% enhancement of EL or ET would be expected with increased D, since no significant difference in Ta and AL was observed between the two seasons (p > 0.05). Thus, a 22.4% decrease in Gs eventually induced an increase in EL or ET of 159.5%.
That 50∼60% of maximum gs was maintained at -1.6 MPa implies that E. urophylla was capable of optimizing carbon assimilation under stressed leaf water conditions. gs peaked between -0.6 and -0.9 MPa after which it decreased gradually (Figure 6A), which is similar to the relationship between ΨL and gs across 70 tree species including Eucalyptus (Klein, 2014). Another example reported by Mielke et al. (2000) showed that Gs of Eucalyptus grandis maintained ∼40% of the maximum when ΨL < -2.45 MPa before it reached the minimum ΨL (-2.8 MPa). It was observed that the stomata maintained 50–60% of the maximum Gs when ΨL reached the minimum (1.6 MPa) (Figure 7A), which was consistent with the stabilized Gs (∼37.5% of the maximum) when predawn ΨL < -2.37 for three allopatric Eucalyptus species (White et al., 2000). It was shown that the ΨL has a range of -2.2 MPa to -1.0 MPa when Gs decreased to 50% of the maximum for most tree species (Klein, 2014). Thus, it was claimed that the Gs of Eucalyptus species tended to be less sensitive to the decrease of ΔΨ than most other woody species. This had already been argued for Eucalyptus gomphocephala, since ΨL is not fixed at or above any particular value (co-varies with monthly rainfall) in a manner that is consistent with typical anisohydric behavior (Franks et al., 2007). Nevertheless, whether this behavior (i.e., not complete stomatal closure under low leaf water potentials) is general among all Eucalpytus or not needs further studies (Martorell et al., 2014).
We further quantified this anisohydric behavior via the variation of -m (Figure 5A). When light is limited, the increase of Q0 will stimulate the opening of stomas as shown in Figure 4B, until the threshold is reached. Meanwhile, -m shared the same turning point of Q0 with GSref before gradual decrease. Despite the stabilized GSref, the stomas became less sensitive to increased D as Q0 increased, illustrated by the lower -m, i.e., anisohydric behavior. Thus, light plays a significant role in controlling the stomatal response to D and the carbon assimilation ability. Evidence has shown that taller trees with lower GSref sensitivity could maintain higher CO2 uptake rates over the wide diurnal range of D, which serves to support carbon exchange (Schäfer et al., 2000). Evidently, it is the different perception ability of abscisic acid (ABA) that contributes to the plants isohydric or anisohydric behavior (Tardieu and Simonneau, 1998; Schultz, 2003; Sade et al., 2012), and the release of ABA tends to be activated only when pH is low (such as high Ci) (Ackerson, 1982). For species with higher photosynthetic capacity, lower Ci will be predicted under high light conditions, thus the higher Gs and the lower sensitivity to D.
Hydraulic Conductivity of E. urophylla
Gsref-max showed a decline of 22.5% from ΨL = -0.6 MPa to -1.2 MPa, which is less than the 45.3–65.6% decrease of ks, implying a more important role of ks regulation in restricting excessive transpiration. As we know, Gs as well as ks coincide with decreased ΨL and start to decrease when xylem water refill cannot not balance canopy water loss (Franks et al., 2007). However, the extent of loss of hydraulic conductivity as ΨL decreases varies greatly across a variety of species, habitats, and climates (Hacke, 2014). Generally, species growing with high water supply tend to have larger vessels to promote a high hydraulic conductivity in the conducting tissue rather than to minimize the risk of drought-induced xylem embolism (Zach et al., 2010). In Eucalyptus grandis and the hybrid of Eucalyptus grandis × camaldulensis, vessel diameter and length increased from the dry to wet conditions as water uptake through transpiration increased (February et al., 1995). Because of high annual rainfall in our study site (2167.6 mm, Figure 1), growth of the xylem vessel of E. urophylla was found to have a mean diameter of 91.4 ± 10.1 μm (Zhao et al., 2014), which favors the significant decrease of ks (45.3–65.6%) in dry conditions.
ET was observed to increase significantly both in dry and wet season with tree size, which implied improved carbon assimilation (Kim et al., 2008). It was found that the changes of AL/As didn’t follow consistent pattern as other studies have reported (increase or decrease, Figure 6) (Buckley and Roberts, 2006). However, the GSref-max in the wet season was observed to increase with tree size (Figure 6), and the increased tree height will lead to stomatal regulation (decreased gs, Schäfer et al., 2000). Thus, the increased ks with DBH (Figure 7B) was thought to contribute to the gradually increased ET and Gs (Figure 2A and 6). In a tropical old-growth forest, for a variety of species, ks was found to significantly increase with tree height because of the increased mean vessel diameter both in trunk and twig xylem (Zach et al., 2010). Finally, our results also revealed that there was no significant difference of ΨL among tree sizes (Figure 3). Bleby et al. (2012) reported that the decreased ΨL of Eucalyptus marginata usually occurred under natural conditions where resources were limited. At the same time, AL/As was also found to decline in order to maintain hydraulic homeostasis. If the modification of Darcy’s Law for plant water translocation is correct, we can conclude that the increased burden on water transport due to increased Gs, AL/As and tree height were well compensated by the increased ks. However, increased ks will lead to more vulnerable xylem in the face of drought stress (Thomas et al., 2004; Ladjal et al., 2005). As observed in Figure 7B, ks decreased 45.3 and 65.6% respectively for the smallest and largest tree, which implies a lower resistance for suppressed trees with decreasing ΨL (Ambrose et al., 2009).
Enhanced WUE with Moderate Periodical Drought
The substantial increase of transpirational demand in the dry season led to the decrease of ks and Gs (Figure 7B). We found that Gs decreased by 22.4% from the wet to dry seasons, while ks decreased by 45.3–65.6% more than Gs. If leaves in both seasons have the same demand for CO2, the WUE should be higher in the dry season. Such a water-use strategy may contribute to high water-use efficiency for E. urophylla, especially under dry conditions because water flux in the xylem is reduced more than Gs in the leaves. This possibility had been observed in some other studies (Brienen et al., 2011; Maseyk et al., 2011; Liu et al., 2012). In addition, the ks ratio of dry/wet decreased from 0.55 to 0.30 with increased tree size (Figure 7B), implying a higher decrease in water loss for large trees. This meant that E. urophylla in our stands tended to improve their WUE in the dry period. To verify this hypothesis, we conducted leaf carbon isotope analysis along the tree size gradient. Consistently, Δ13C showed a clear decrease with DBH (Figure 8). A number of studies have argued that moderate drought favors high WUE of tree species from different ecosystems, including forest and desert (Maseyk et al., 2011; Liu et al., 2012). Otto et al. (2014) argued that dominant clonal Eucalyptus grandis × urophylla trees use water more efficiently compared with native species. However, the underlying mechanism responsible for higher WUE is unclear. Our results indicated possible mechanisms for high WUE in dominant trees, which might be verified by further studies on the annual net biomass yield/annual transpiration, since isotope discrimination only provides an estimate of intrinsic WUE rather than the ratio of uptaken CO2 to actual fluxes of water vapor.
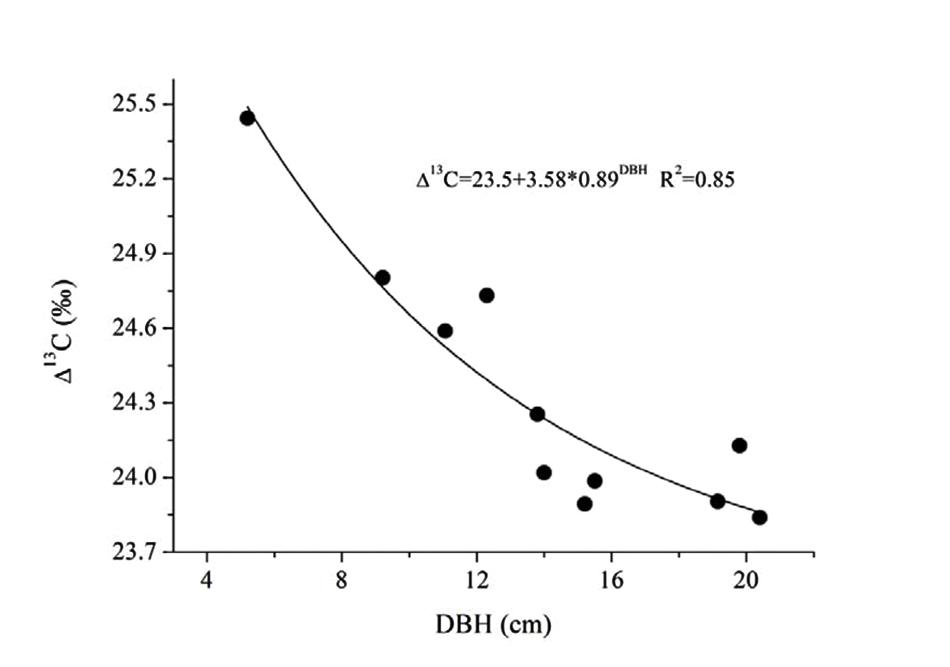
FIGURE 8. Relationship between DBH and the leaf carbon isotope discrimination (13C) of 11 trees for E. urophylla.
Conclusion
In a moist low subtropical area in South China with periodic drought, E. urophylla was observed to close stomata under lower water supply conditions with decreased ΨL, but showed anisohydric behavior with gradually stabilizing high Gs at low ΨL, especially under high light conditions (deceased -m). ks decreased 45.3–65.6% from wet to dry season, which is significantly higher than the proportional decline of Gs (22.4%) and may be responsible for the high WUE of Eucalyptus species. As tree size increased, greater sensitivity of ks to water loss was synchronous with improved WUE (decreased Δ13C), which contributed to the constant stomatal conductance in the dry season (GSref-max) and the persistent increase of AL/As with DBH and insignificant change in ΨL among trees for maintaining the hydraulic balance. We are uncertain of the generality of the above behavior for other Eucalyptus species, which deserves further studies.
Author’s Note
We declare that the previous version of this manuscript “Water use strategies of a young Eucalyptus urophylla forest in response to seasonal change of climatic factors in South China” submitted to Biogeosciences Discussion (doi: 10.5194/bgd-12-10469-2015) and appearing as a preprint service, was finally rejected.
Author Contributions
ZZ and PZ organized and supported the entire study. HM, LO, JN, LZ, GN, and YH performed the sap flow and physiological measurements. ZZ also wrote this manuscript, PZ and HM edited this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the National Nature Science Foundation of China (Grant No. 41630752, 41030638, 41275169), and the CAS/SAFEA International Partnership Program for Creative Research Teams, Guangxi Key Sci & Tech Foundation (GuiKeZhong 1347001). We thank Prof. Ram Oren for his substantial advice and comments on the writing of this manuscript. We are indebted to Dr. Syed Ashraful Alam, who worked both at South China Botanical Garden/CAS and Department of Physics of the University of Helsinki, for his comments and efforts to improve the language of the manuscript. Data used in this study were collected by the author and are available from the author upon request.
Footnotes
References
Ackerson, R. C. (1982). Synthesis and movement of abscisic acid in water-stressed cotton leaves. Plant Physiol. 69, 609–613. doi: 10.1104/pp.69.3.609
Ambrose, A. R., Sillett, S. C., and Dawson, T. E. (2009). Effects of tree height on branch hydraulics, leaf structure and gas exchange in California redwoods. Plant Cell Environ. 32, 743–757. doi: 10.1111/j.1365-3040.2009.01950.x
Anderegg, W. R. L., Berry, J. A., Smith, D. D., Sperry, J. S., Anderegg, L. D. L., and Field, C. B. (2012). The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. U.S.A. 109, 233–237. doi: 10.1073/pnas.1107891109
Becker, P., Meinzer, F. C., and Wullschleger, S. D. (2000). Hydraulic limitation of tree height: a critique. Funct. Ecol. 14, 4–11. doi: 10.1046/j.1365-2435.2000.00397.x
Bleby, T. M., Colquhoun, I. J., and Adams, M. A. (2012). Hydraulic traits and water use of Eucalyptus on restored versus natural sites in a seasonally dry forest in southwestern Australia. For. Ecol. Manage. 274, 58–66. doi: 10.1016/j.foreco.2012.02.029
Brienen, R. J. W., Wanek, W., and Hietz, P. (2011). Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species. Trees 25, 103–113. doi: 10.1007/s00468-010-0474-1
Buckley, T. N., and Roberts, D. W. (2006). How should leaf area, sapwood area and stomatal conductance vary with tree height to maximize growth? Tree Physiol. 26, 145–157. doi: 10.1093/treephys/26.2.145
Burgess, S. S. O., and Dawson, T. E. (2008). Using branch and basal trunk sap flow measurements to estimate whole-plant water capacitance: a caution. Plant Soil 305, 5–13. doi: 10.1007/s11104-007-9378-2
Cernusak, L. A., and Hutley, L. B. (2011). Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol. 155, 515–523. doi: 10.1104/pp.110.163337
Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P. P., Osorio, M. L., et al. (2002). How plants cope with water stress in the field. photosynthesis and growth. Ann. Bot. 89, 907–916. doi: 10.1093/aob/mcf105
Davidson, E. A., de Araújo, A. C., Artaxo, P., Balch, J. K., Foster Brown, I., Bustamante, M. M. C., et al. (2012). The amazon basin in transition. Nature 481, 321–328. doi: 10.1038/nature10717
Dawson, T. E. (1996). Determining water use by trees and forests from isotopic, energy balance and transpiration analyses: the roles of tree size and hydraulic lift. Tree Physiol. 16, 263–272. doi: 10.1093/treephys/16.1-2.263
Delzon, S., Sartore, M., Burlett, R., Dewar, R., and Loustau, D. (2004). Hydraulic responses to height growth in maritime pine trees. Plant Cell Environ. 27, 1077–1087. doi: 10.1111/gcb.13148
Do, F., and Rocheteau, A. (2002). Influence of natural temperature gradients on measurements of xylem sap flow with thermal dissipation probes, 1. Field observations and possible remedies. Tree Physiol. 22, 641–648. doi: 10.1093/treephys/22.9.641
Dow, G. J., and Bergmann, D. C. (2014). Patterning and processes: how stomatal development defines physiological potential. Curr. Opin. Plant Biol. 21, 67–74. doi: 10.1016/j.pbi.2014.06.007
February, E. C., Stock, W. D., Bond, W. J., and Leroux, D. J. (1995). Relationships between water availability and selected vessel characteristics in Eucalyptus grandis and two hybrids. Iawa J. 16, 269–276. doi: 10.1163/22941932-90001410
Feichtinger, L. M., Eilmann, B., Buchmann, N., and Rigling, A. (2014). Growth adjustments of conifers to drought and to century-long irrigation. For. Ecol. Manage. 334, 96–105. doi: 10.1016/j.foreco.2014.08.008
Forrester, D. I. (2015). Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiol. 35, 289–304. doi: 10.1093/treephys/tpv011
Franks, P. J., Drake, P. L., and Froend, R. H. (2007). Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ. 30, 19–30. doi: 10.1111/j.1365-3040.2006.01600.x
Granier, A., Biron, P., Kostner, B., Gay, L. W., and Najjar, G. (1996). Comparisons of xylem sap flow and water vapour flux at the stand level and derivation of canopy conductance for Scots pine. Theor. Appl. Climatol. 53, 115–122. doi: 10.1007/BF00866416
Hacke, U. G. (2014). Variable plant hydraulic conductance. Tree Physiol. 34, 105–108. doi: 10.1093/treephys/tpu007
Hölttä, T., Mencuccini, M., and Nikinmaa, E. (2011). A carbon cost-gain model explains the observed patterns of xylem safety and efficiency. Plant Cell Environ. 34, 1819–1834. doi: 10.1111/j.1365-3040.2011.02377.x
Jones, H. G. (1998). Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 49, 387–398. doi: 10.1093/jexbot/49.suppl_1.387
Kim, H.-S., Oren, R., and Hinckley, T. M. (2008). Actual and potential transpiration and carbon assimilation in an irrigated poplar plantation. Tree Physiol. 28, 559–577. doi: 10.1093/treephys/28.4.559
Klein, T. (2014). The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 28, 1313–1320. doi: 10.1111/1365-2435.12289
Köstner, B., Falge, E., and Tenhunen, J. D. (2002). Age-related effects on leaf area/sapwood area relationships, canopy transpiration and carbon gain of Norway spruce stands (Picea abies) in the Fichtelgebirge, Germany. Tree Physiol. 22, 567–574. doi: 10.1093/treephys/22.8.567
Köstner, B. M. M., Schulze, E. D., Kelliher, F. M., Hollinger, D. Y., Byers, J. N., Hunt, J. E., et al. (1992). Transpiration and canopy conductance in a pristine broad-leaved forest of Nothofagus: an analysis of xylem sap flow and eddy correlation measurements. Oecologia 91, 350–359. doi: 10.1007/BF00317623
Ladjal, M., Huc, R., and Ducrey, M. (2005). Drought effects on hydraulic conductivity and xylem vulnerability to embolism in diverse species and provenances of Mediterranean cedars. Tree Physiol. 25, 1109–1117. doi: 10.1093/treephys/25.9.1109
Liu, R., Pan, L.-P., Jenerette, G. D., Wang, Q.-X., Cieraad, E., and Li, Y. (2012). High efficiency in water use and carbon gain in a wet year for a desert halophyte community. Agric. For. Meteorol. 162, 127–135. doi: 10.1016/j.agrformet.2012.04.015
Lloret, F., Escudero, A., Maria Iriondo, J., Martinez-Vilalta, J., and Valladares, F. (2012). Extreme climatic events and vegetation: the role of stabilizing processes. Glob. Change Biol. 18, 797–805. doi: 10.1111/j.1365-2486.2011.02624.x
Martínez-Vilalta, J., Poyatos, R., Aguadé, D., Retana, J., and Mencuccini, M. (2014). A new look at water transport regulation in plants. New Phytol. 204, 105–115. doi: 10.1111/nph.12912
Martorell, S., Diaz-Espejo, A., Medrano, H., Ball, M. C., and Choat, B. (2014). Rapid hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant Cell Environ. 37, 617–626. doi: 10.1111/pce.12182
Maseyk, K., Hemming, D., Angert, A., Leavitt, S. W., and Yakir, D. (2011). Increase in water-use efficiency and underlying processes in pine forests across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia 167, 578–585. doi: 10.1007/s00442-011-2010-4
McDowell, N. G., Phillips, N., Lunch, C., Bond, B. J., and Ryan, M. G. (2002). An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol. 22, 763–774. doi: 10.1093/treephys/22.11.763
Meinzer, F. C. (1993). Stomatal control of transpiration. Trends Ecol. Evol. 8, 289–294. doi: 10.1016/0169-5347(93)90257-P
Mielke, M. S., Oliva, M. A., de Barros, N. F., Penchel, R. M., Martinez, C. A., da Fonseca, S., et al. (2000). Leaf gas exchange in a clonal eucalypt plantation as related to soil moisture, leaf water potential and microclimate variables. Trees Struct. Funct. 14, 263–270. doi: 10.1007/s004680050012
Mokany, K., McMurtrie, R. E., Atwell, B. J., and Keith, H. (2003). Interaction between sapwood and foliage area in alpine ash (Eucalyptus delegatensis) trees of different heights. Tree Physiol. 23, 949–957. doi: 10.1093/treephys/23.14.949
Monteith, J., and Unsworth, M. (2013). Principles of Environmental Physics: Plants, Animals, and the Atmosphere. New York: Academic Press.
Oren, R., Phillips, N., Katul, G., Ewers, B. E., and Pataki, D. E. (1998). Scaling xylem sap flux and soil water balance and calculating variance: a method for partitioning water flux in forests. Ann. For. Sci. 55, 191–216. doi: 10.1051/forest:19980112
Oren, R., Sperry, J. S., Ewers, B. E., Pataki, D. E., Phillips, N., and Megonigal, J. P. (2001). Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia 126, 21–29. doi: 10.1007/s004420000497
Otto, M. S. G., Hubbard, R. M., Binkley, D., and Stape, J. L. (2014). Dominant clonal Eucalyptus grandis× urophylla trees use water more efficiently. For. Ecol. Manage. 328, 117–121. doi: 10.1016/j.foreco.2014.05.032
Pou, A., Medrano, H., Tomàs, M., Martorell, S., Ribas-Carbó, M., and Flexas, J. (2012). Anisohydric behaviour in grapevines results in better performance under moderate water stress and recovery than isohydric behaviour. Plant Soil 359, 335–349. doi: 10.1007/s11104-012-1206-7
Reich, P. B., Ellsworth, D. S., Walters, M. B., Vose, J. M., Gresham, C., Volin, J. C., et al. (1999). Generality of leaf trait relationships: a test across six biomes. Ecology 80, 1955–1969. doi: 10.1890/0012-9658(1999)080[1955:GOLTRA]2.0.CO;2
Ryan, M. G., and Waring, R. H. (1992). Maintenance respiration and stand development in a subalpine lodgepole pine forest. Ecology 73, 2100–2108. doi: 10.2307/1941458
Sade, N., Gebremedhin, A., and Moshelion, M. (2012). Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 7, 767–770. doi: 10.4161/psb.20505
Schäfer, K. V. R., Oren, R., and Tenhunen, J. D. (2000). The effect of tree height on crown level stomatal conductance. Plant Cell Environ. 23, 365–375. doi: 10.1046/j.1365-3040.2000.00553.x
Schultz, H. R. (2003). Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 26, 1393–1405. doi: 10.1046/j.1365-3040.2003.01064.x
Shi, Z., Xu, D., Yang, X., Jia, Z., Guo, H., and Zhang, N. (2012). Ecohydrological impacts of eucalypt plantations: a review. J. Food Agric. Environ. 10, 1419–1426.
Tardieu, F., and Simonneau, T. (1998). Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J. Exp. Bot. 49, 419–432. doi: 10.1093/jxb/49.Special_Issue.419
Thomas, D. S., Montagu, K. D., and Conroy, J. P. (2004). Changes in wood density of Eucalyptus camaldulensis due to temperature - the physiological link between water viscosity and wood anatomy. For. Ecol. Manag. 193, 157–165. doi: 10.1016/j.foreco.2004.01.028
West, A. G., Hultine, K. R., Sperry, J. S., Bush, S. E., and Ehleringer, J. R. (2008). Transpiration and hydraulic strategies in a pinon-juniper woodland. Ecol. Appl. 18, 911–927. doi: 10.1890/06-2094.1
White, D., Beadle, C., Worledge, D., Honeysett, J., and Cherry, M. (1998). The influence of drought on the relationship between leaf and conducting sapwood area in Eucalyptus globulus and Eucalyptus nitens. Trees 12, 414–414. doi: 10.1007/s004680050167
White, D. A., Turner, N. C., and Galbraith, J. H. (2000). Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol. 20, 1157–1165. doi: 10.1093/treephys/20.17.1157
Whitehead, D., and Beadle, C. L. (2004). Physiological regulation of productivity and water use in Eucalyptus: a review. For. Ecol. Manag. 193, 113–140. doi: 10.1016/j.foreco.2004.01.026
Whitehead, D., and Jarvis, P. (1981). “Coniferous forests and plantations,” in ‘Water Deficits and Plant Growth’, ed. T. T. Kozlowski (New York: Academic Press), 49–152.
Zach, A., Schuldt, B., Brix, S., Horna, V., Culmsee, H., and Leuschner, C. (2010). Vessel diameter and xylem hydraulic conductivity increase with tree height in tropical rainforest trees in Sulawesi, Indonesia. Flora 205, 506–512. doi: 10.1093/treephys/tps122
Zhai, P. M., Zhang, X. B., Wan, H., and Pan, X. H. (2005). Trends in total precipitation and frequency of daily precipitation extremes over China. J. Clim. 18, 1096–1108. doi: 10.1175/JCLI-3318.1
Zhang, Z. Z., Zhao, P., McCarthy, H. R., Zhao, X. H., Niu, J. F., Zhu, L. W., et al. (2016). Influence of the decoupling degree on the estimation of canopy stomatal conductance for two broadleaf tree species. Agric. For. Meteorol. 221, 230–241. doi: 10.1016/j.agrformet.2016.02.018
Zhao, P. Q., Zhao, P., Niu, J. F., Zhu, L. W., Ni, G. Y., Gao, J. G., et al. (2014). Relationship between vessel characteristics and sap flow of eight subtropical tree species. J. Trop. Subtrop. Bot. 22, 537–548.
Zhou, G., Peng, C., Li, Y., Liu, S., Zhang, Q., Tang, X., et al. (2013). A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Change Biol. 19, 1197–1210. doi: 10.1111/gcb.12128
Zhou, G., Wei, X. H., Wu, Y. P., Liu, S. G., Huang, Y., Yan, J., et al. (2011). Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Change Biol. 17, 3736–3746. doi: 10.1111/j.1365-2486.2011.02499.x
Zhou, G. Y., Yin, G. C., Morris, J., Bai, J. Y., Chen, S. X., Chu, G. W., et al. (2004). Measured sap flow and estimated evapotranspiration of tropical Eucalyptus urophylla plantations in south China. Acta Bot. Sin. 46, 202–210.
Zhu, H. G., Yang, Y. Q., Wen, Y. G., Liang, H. W., and Yang, Y. Q. (2009). Estimating model for leaf area of standing tree and stand of E. grandis× E. urophylla in in Guangxi. J. Anhui Agric. Sci. 37, 15069–15070.
Zhu, L. W., Zhao, P., Wang, Q., Ni, G. Y., Niu, J. F., Zhao, X. H., et al. (2015). Stomatal and hydraulic conductance and water use in a eucalypt plantation in Guangxi, southern China. Agric. For. Meteorol. 202, 61–68. doi: 10.1016/j.agrformet.2014.12.003
Keywords: anisohydric, Eucalyptus urophylla, hydraulic balance, stomatal conductance, water use efficiency
Citation: Zhang Z, Zhao P, McCarthy HR, Ouyang L, Niu J, Zhu L, Ni G and Huang Y (2016) Hydraulic Balance of a Eucalyptus urophylla Plantation in Response to Periodic Drought in Low Subtropical China. Front. Plant Sci. 7:1346. doi: 10.3389/fpls.2016.01346
Received: 05 March 2016; Accepted: 22 August 2016;
Published: 26 September 2016.
Edited by:
Boris Rewald, University of Natural Resources and Life Sciences, Vienna, AustriaReviewed by:
Maurizio Mencuccini, University of Edinburgh, UKMarina Shinkai Gentil Otto, 4Tree Agroflorestal, Brazil
Copyright © 2016 Zhang, Zhao, McCarthy, Ouyang, Niu, Zhu, Ni and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zhao, emhhb3BpbmdAc2NpYi5hYy5jbg==
 Zhenzhen Zhang
Zhenzhen Zhang Ping Zhao
Ping Zhao Heather R. McCarthy
Heather R. McCarthy Lei Ouyang1
Lei Ouyang1