- Department of Biological Sciences, Canadian Centre for World Hunger Research, University of Toronto, Toronto, ON, Canada
Although deemed a “non-essential” mineral nutrient, silicon (Si) is clearly beneficial to plant growth and development, particularly under stress conditions, including salinity and drought. Here, we review recent research on the physiological, biochemical, and molecular mechanisms underlying Si-induced alleviation of osmotic and ionic stresses associated with salinity and drought. We distinguish between changes observed in the apoplast (i.e., suberization, lignification, and silicification of the extracellular matrix; transpirational bypass flow of solutes and water), and those of the symplast (i.e., transmembrane transport of solutes and water; gene expression; oxidative stress; metabolism), and discuss these features in the context of Si biogeochemistry and bioavailability in agricultural soils, evaluating the prospect of using Si fertilization to increase crop yield and stress tolerance under salinity and drought conditions.
Introduction
Although appreciated by biologists for >150 years, the benefits of silicon (Si) to plants, particularly under stress, have been studied intensively only in recent decades. This is largely due to silicon’s “non-essential" designation by early plant nutritionists (e.g., Sachs, 1860; Arnon and Stout, 1939; see also Epstein, 1999; Liang et al., 2015, for historical overview). Indeed, Si is not considered “essential" for higher plants, as they can fulfil their life cycles without it (Epstein, 1999; Liang et al., 2015). Nevertheless, Si is considered to be “quasi-essential" (Epstein and Bloom, 2005), due to the far-reaching benefits it confers on plants, including enhanced growth, yield and crop quality, photosynthesis, N2 fixation, particularly in response to abiotic and biotic stresses such as infectious disease, herbivory, gravity, metal toxicity, high and low temperature, UV radiation, nutrient deficiency and excess, drought, and salinity (for review, see Epstein, 1994, 1999, 2009; Richmond and Sussman, 2003; Ma, 2004; Liang et al., 2007, 2015; Cooke and Leishman, 2011; Guntzer et al., 2012; Van Bockhaven et al., 2013).
However, despite much recent research, the mechanisms underlying these effects are not well understood, although important new insights into the membrane transport of Si (Ma and Yamaji, 2006, 2015; cf. Exley, 2015), and the alleviatory role of Si in biotic stress (Ma, 2004; Van Bockhaven et al., 2013; Liang et al., 2015) have been gained. Mechanistic understanding of the role of Si in abiotic stress resistance, however, is relatively limited (Liang et al., 2015), but important avenues of research in salinity and drought contexts are emerging (for review, see Ma, 2004; Liang et al., 2007; Zhu and Gong, 2014). Salinity stress affects over 800 million hectares globally – up to a third of all agricultural land and nearly half of all irrigated land, which produces roughly a third of the world’s food (Zhu, 2001; Rengasamy, 2010). Drought stress, which shares many features with salinity stress (Munns, 2002), is even more pervasive and damaging to agricultural production, particularly in arid and semi-arid regions, which account for approximately 30% of the world’s land area (Boyer, 1982; Eneji et al., 2008; Farooq et al., 2009). Both problems are predicted to be aggravated by anthropogenic climate change (Yeo, 1999; Schmidhuber and Tubiello, 2007).
In this mini-review, we focus on current understanding (and gaps therein) of the mechanisms underlying silicon-induced alleviation of salinity and drought stress in higher plants, and will also discuss the feasibility of Si amendments in arid or saline agricultural fields. Where possible, we distinguish apoplast effects (i.e., suberization, lignification, and silicification of the extracellular matrix; transpirational bypass flow of solutes and water), from those of the symplast (i.e., transmembrane transport of solutes and water; gene expression; oxidative stress; metabolism), and consider where mechanistic overlaps exist.
Si-Induced Changes to the Extracellular Matrix (Apoplast)
Silicon is well documented to strengthen cell walls and provide mechanical support for monocots and pteridophytes (much less is known about dicots), by enhancing suberization, lignification, and silicification (for a recent review, see Guerriero et al., 2016). Improved structural stability has been attributed to the binding of Si with cell-wall hemicellulose (He et al., 2013, 2015; Ma et al., 2015), which is clearly beneficial under water deficit. In addition, biosilicification in plants, involving the polymerization of silicic acid within the apoplast, leads to the formation of an amorphous silica barrier (Exley, 2015), which can help alleviate both biotic and abiotic stresses, hindering pathogen infection and the penetration of potential toxicants such as aluminum (Al), manganese (Mn), cadmium (Cd), zinc (Zn), and sodium (Na), into the symplast and/or transpiration stream (Rogalla and Römheld, 2002; Wang et al., 2004; Fauteux et al., 2005; Saqib et al., 2008; Ma et al., 2015; Guerriero et al., 2016). In roots of salt-sensitive and -tolerant wheat, for example, Si increased cell-wall binding of Na+ in the root while decreasing its transport to the shoot (Ahmad et al., 1992; Saqib et al., 2008; see also below); however, direct evidence of Na+ complexation by Si, which may underlie this potentially important salt-tolerance mechanism, is lacking.
Silicon has also been shown to promote Casparian band development in the root endodermis and exodermis (Fleck et al., 2011, 2015). For example, in rice, Si treatment resulted in enhanced suberization, and lignification of sclerenchyma, in these tissues (Fleck et al., 2011; cf. Suzuki et al., 2012), which coincided with reduced radial oxygen loss and oxidation power in the mature root (Fleck et al., 2011). Si also triggered the transcription of genes related to lignin and suberin synthesis (Fleck et al., 2011; see below). These components can form barriers to apoplastic Na+ transport in roots, correlating with higher salt tolerance in rice (Krishnamurthy et al., 2011). In particular, Si deposition in the endodermis is proposed to restrict Na+ transport along a “transpirational bypass” route from root to shoot in rice (Gong et al., 2006), as we shall now discuss.
Transpirational Bypass Flow
Limiting shoot Na+ and Cl- accumulation is critical to salt tolerance in many species, as it may prevent leaf metabolic disorders, ion imbalances, and the desiccation of leaf tissue via osmotic stress (Oertli, 1968; Flowers et al., 1991; Kronzucker et al., 2013). This is particularly important in rice, where, in addition to normal transpiration, involving xylem loading via the symplast, there is a pronounced transpirational bypass flow, i.e., a bypassing of the symplast in regions where endodermal barriers are underdeveloped or absent (root tips, or zones of lateral root emergence; Yeo et al., 1999; Gong et al., 2006; Shi et al., 2013; see also Speer and Kaiser, 1991; Flowers, 2004; Saqib et al., 2008; Coskun et al., 2013a; Shazad et al., 2013). Si provision has been shown to reduce root-to-shoot translocation of both Na+ and Cl- in salt-stressed rice, despite increasing transpiration and stomatal conductance, indicating that Si does not act to reduce sodium translocation by reducing transpiration per se, but rather by blocking bypass flow (Yeo et al., 1999; Gong et al., 2006; Figure 1, inset). The proposal that Si deposition in endodermal and exodermal Casparian bands forms physical barriers to Na+ and Cl- translocation is supported by X-ray localization patterns of Si deposition, and the greatly reduced translocation, with Si treatment, of the apoplastic dye trisodium-8-hydroxy-1,3,6-pyrenetrisulphonic acid (PTS) (Gong et al., 2006; Shi et al., 2013; see also Lux et al., 2003). Whether this mechanism is peculiar to rice, or is taxonomically widespread, awaits investigation in other species.
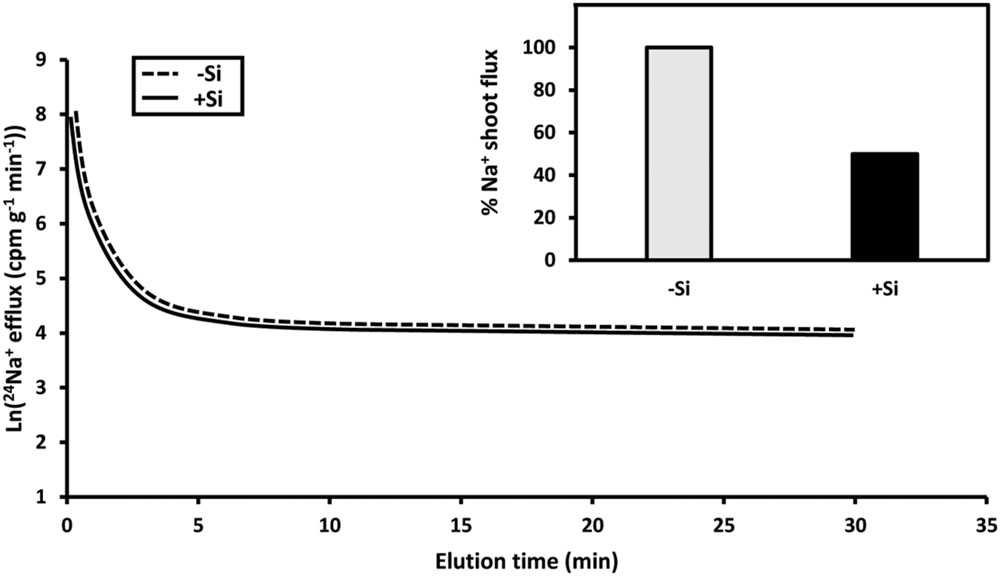
FIGURE 1. Contrasting responses of plant Na+ fluxes to Si. In pre-labeled roots of intact rice seedlings, 24Na+ efflux shows no difference in plants grown with or without Si in the presence of high salinity (main panel; redrawn from Malagoli et al., 2008). By contrast, Na+ fluxes from root to shoot are highly sensitive to Si supply (inset; redrawn from Gong et al., 2006).
Effects of Si on transpiration depend on species and environmental conditions. While Si increased transpiration in both drought and salt-stressed rice (Chen et al., 2011; see above), Si decreased transpiration in non-stressed rice (Ma and Takahashi, 1993; Agarie et al., 1998). Similar observations were found in drought-stressed wheat (Gong et al., 2005) and sorghum (Hattori et al., 2005; Ahmed et al., 2011), while, by contrast, Si reduced transpiration in drought-stressed maize (Gao et al., 2004, 2006), and had no effect in cucumber (Hattori et al., 2008). Such variability suggests divergent strategies among species, as they balance rates of water uptake and those of leaf-level water loss. The mechanisms underlying these strategies and responses to Si require much more discovery and analysis.
Water Transport and Plant Water Status
Salt- and drought-stressed plants have reduced water uptake and content, both of which can be alleviated by Si provision, which leads to improved water status and water-use efficiency in many species (Gao et al., 2004; Liu et al., 2014; Wang et al., 2015; Zhu et al., 2015; Shi et al., 2016). In sorghum, for example, Si increased root and whole-plant hydraulic conductance, transpiration, stomatal conductance, and leaf water content under osmotic stress (Liu et al., 2014, 2015). The increase in root hydraulic conductance coincided with a 2- to 4-fold increased expression of plasma-membrane intrinsic protein (PIP) aquaporins, and increased PIP-mediated water transport was suggested by inhibition of the water flux by mercury (Hg2+) (Liu et al., 2014, 2015; see also Zhu et al., 2015). The use of Hg2+ as an aquaporin inhibitor should be taken with caution, however, as it can also inhibit influx of K+ (Coskun et al., 2012), which can affect water transport due to the important osmotic role of K+ (Dolan and Davies, 2004). Regardless, the mechanisms by which Si nutrition affects aquaporin expression and activity have yet to be resolved.
It is interesting that Si transport is also mediated by aquaporins, specifically members of the Nod26-like major intrinsic protein (NIP) III subgroup (Ma and Yamaji, 2015). The expression pattern of Lsi1, a NIP homolog encoding a Si influx transporter, shows varying responses to Si nutrition in different species. For example, its expression in response to Si provision is downregulated in rice and soybean, unaffected in maize, barley, and wheat, and upregulated in cucumber (Ma and Yamaji, 2015; and references therein). Interestingly, under salinity stress, OsLsi1 expression was upregulated in roots of both a salt-sensitive and -tolerant variety of rice (1.82- and 2.12-fold, respectively; Senadheera et al., 2009). The authors proposed that this may result in greater Si uptake in the salt-tolerant variety compared to the salt-sensitive one, with enhanced Si deposition in the transpirational bypass route, and thus restricted shoot Na+ translocation. Given that rice OsLsi1 expression shows opposing responses to Si supply and salinity stress separately, it would be interesting to see how expression responds to co-application in this important, salt-sensitive, and highly water-demanding species.
Besides affecting hydraulic conductance and water transport by modulating aquaporin expression/activity, Si can affect water transport by adjusting the osmotic potential of cells through increased osmolyte accumulation (e.g., proline, soluble sugars, inorganic ions, etc.; Pei et al., 2010; Sonobe et al., 2010; Ming et al., 2012; Liu et al., 2014). Increased root hydraulic conductance may also be attributed to Si-induced reductions in oxidative stress and membrane damage (Shi et al., 2016; see below).
Ion Transport
The reduction of Na+ influx from the external solution into the cytosol, and the increase of Na+ efflux in the opposite direction (or from cytosol to vacuole) have been proposed to be major salt-tolerance mechanisms; both work toward lowering cytosolic Na+ pools (Munns and Tester, 2008). In addition, homeostatic maintenance of intracellular K+ pools under salt stress is critical to maintain proper cell function (Kronzucker et al., 2013). Si may alleviate salinity stress by influencing these aspects of Na+ and K+ transport and accumulation (for review, see Zhu and Gong, 2014; Rizwan et al., 2015). In salt-stressed barley, activities of root plasma membrane H+-ATPase, and tonoplast H+-ATPase and H+-PPase, are stimulated under Si supply (Liang, 1999; Liang et al., 2005, 2006). These changes in cellular H+ pumps have been proposed to enhance Na+ efflux via the Na+-H+ exchangers HvSOS1 and HvNHX1 (in the plasma membrane and tonoplast, respectively), and K+ influx via K+-H+ symporters such as HvHAK1, as they are secondarily active fluxes driven by electrochemical H+ gradients (Liang et al., 2005, 2006). However, proton-pump stimulation may be indirect, as H+-ATPase activity was unaffected by Si in plasma membrane vesicles from leaves of salt-stressed barley (Liang et al., 2006). Moreover, we have found no evidence for an effect of Si on putatively SOS1-mediated Na+ efflux in our own laboratory, in roots of intact rice seedlings under salinity stress (Figure 1, main panel; see also Malagoli et al., 2008). Nevertheless, this is an interesting potential mechanism of Si-mediated salt tolerance that requires further investigation by various means, including measurements of Na+ fluxes in root tips, where recent physiological evidence of Na+-H+ antiport activity has been demonstrated (Hamam et al., 2016), as well as in planta K+ fluxes (Coskun et al., 2014).
Silicon can stimulate synthesis and accumulation of polyamines (PA) such as putrescine, spermidine, and spermine, in salt-stressed plants, and this has also been proposed to help mediate salt tolerance (Gill and Tuteja, 2010a; Wang et al., 2015; Yin et al., 2016; see also below). PA production may function in this way via regulating K+ and Na+ transport, improving antioxidant ability, and modifying osmotic potential (Kusano et al., 2008; Alcazar et al., 2010). Patch-clamp analysis in root epidermal and cortical protoplasts from salt-stressed barley showed that PAs blocked inward and outward Na+ and K+ currents via non-selective cation channels (NSCCs; Zhao et al., 2007), suggesting that this may prevent toxic intracellular accumulation of Na+; however, it is important to note that in planta evidence of NSCC-mediated fluxes is currently lacking (Kronzucker and Britto, 2011; Coskun et al., 2013b), and such claims should be interpreted cautiously.
Oxidative Stress
Lipid peroxidation by reactive oxygen species (ROS) is another major mechanism of salt toxicity in higher plants (Hernandez et al., 1993; Fadzilla et al., 1997; Zhu, 2001; Gill and Tuteja, 2010b). Si has been shown to decrease the concentration of malondialdehyde (MDA), the end-product of lipid peroxidation, in salt-stressed barley (Liang et al., 2003), maize (Moussa, 2006), and grapevine rootstock (Soylemezoglu et al., 2009), and thus may help to maintain membrane integrity and decrease permeability (Liang et al., 2015). Si has also been shown to increase the activity of key antioxidant defense enzymes superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), as well as glutathione reductase (GR) activities and the glutathione (GSH) concentration in salt-stressed plants (Liang et al., 2003, 2006; Al-Aghabary et al., 2004; Zhu et al., 2004; Shi et al., 2016). Khoshgoftarmanesh et al. (2014) showed that MDA concentrations were positively correlated with Na+ uptake in salt-stressed cucumber but negatively correlated with Ca2+ and K+ uptake, and with Si supply. How Si mediates this response is unclear, but the explanation that, under Si supply, stabilized membranes lead to symplastic [Na+] reductions, and [K+] and [Ca2+] increases, is more parsimonious than one invoking altered ion transporters such as NSCCs (see above).
Si Fertilization and Agricultural Gains
Silicon is the second most abundant soil element after oxygen, comprising ∼29% of the Earth’s crust (Haynes, 2014). This is mostly as insoluble crystalline aluminosilicates, which are not plant-available. Soluble, bioavailable Si, by contrast (i.e., monosilicic/orthosilicic acid; H4SiO4), normally ranges between 0.1 and 0.6 mM in soils (Epstein, 1994). H4SiO4 is weakly acidic (pKa1 = 9.84, pKa2 = 13.2), and thus is largely undissociated in most soils. The traditional view, that bioavailable Si derives from solvation of primary and secondary minerals and buffered by the adsorption and desorption of silicate onto sesquioxides, has been supplanted by the idea that phytogenic cycling of Si (uptake by plants, silica formation mainly in leaves, and return to the soil as plant litter) is the main determinant of bioavailable soil Si in natural ecosystems (Haynes, 2014; see also Pii et al., 2015; Gattullo et al., 2016). Si pools in agricultural soils are often low due to the regular removal of Si-rich litter during harvest, a practice which may be altering terrestrial and global Si cycling (Savant et al., 1997; Struyf et al., 2010; Vandevenne et al., 2015).
Use of Si fertilizers began in the 1950s in Japan and is now widespread (Guntzer et al., 2012), the most common sources being industrial slags (Haynes, 2014), as well as plant straw, typically from rice (Hossain et al., 2001). These applications have been effective in enhancing the yield and quality of many agricultural crops, including both monocots such as rice, wheat, maize, barley, millet, sorghum, and sugarcane, and dicots such as cotton and soybean (Liang et al., 2015; and references therein).
It has been claimed that Si primarily benefits stressed plants, with minor effects on unstressed plants (Fauteux et al., 2006; Chain et al., 2009; Epstein, 2009; Van Bockhaven et al., 2013). For example, Si addition showed little alteration of the transcriptome of unstressed Arabidopsis (Fauteux et al., 2006), wheat (Chain et al., 2009), and rice (Watanabe et al., 2004; cf. Brunings et al., 2009; Van Bockhaven et al., 2013), and the proteome of rice (Nwugo and Huerta, 2011). However, such changes do not necessarily indicate the lack of a beneficial role. In long-term studies, Si was shown to increase crop yields, even in unstressed rice; this was attributed to lower transpiration by spikelets (Tamai and Ma, 2008; Detmann et al., 2012, 2013). Si was also shown to increase yields of rice growing under non-stressed conditions by altering source-sink relationships and increasing photosynthesis, mesophyll conductance, N-use efficiency, and mobilization of photoassimilates and amino acids from vegetative tissues to grains (Detmann et al., 2012, 2013). The authors suggested that Si may act as a signaling factor redirecting the primary metabolism of plants, although the mechanism by which this is achieved is not known; this is an exciting and promising new avenue of Si research.
Lastly, a new prospect of Si research involves “seed priming,” whereby exposing seeds to Si for only a few hours fortifies plants against future stress events. Currently, the priming literature is focused on biotic stress resistance (van Hulten et al., 2006; Conrath, 2011; Van Bockhaven et al., 2013), and relatively few studies have investigated effects on performance under abiotic stress; however, several interesting and promising findings have emerged. For example, wheat seeds treated with Si for 6–8 h showed significant increases in germination rate, vegetative growth, and crop yield under salinity and osmotic stresses, compared to non-primed seeds (Hameed et al., 2013; Azeem et al., 2015; Ahmed et al., 2016). Similarly, maize plants grown from seeds treated for 12 h in Si showed increased growth, leaf relative water content, and levels of photosynthetic pigments, soluble sugars, soluble proteins, total free amino acids, potassium, and activities of SOD, CAT, and POD enzymes, compared to plants that were not primed (Latef and Tran, 2016). Moreover, Si-primed seedlings showed decreased proline, MDA, and Na+ contents. Although the underlying mechanisms are unknown, and much more investigation is required, seed priming appears to be a promising and cost-effective procedure to confer resistance to major stresses such as drought and salinity.
Conclusion
Plant silicon research has made great strides since the designation of Si as “non-essential.” While this element is still generally excluded from most plant growth-media formulations, it is now widely accepted to benefit many plant species, including many agriculturally prominent crops. While these benefits may be particularly pronounced under stresses such as drought and salinity, growing evidence indicates that Si may also improve growth under relatively benign conditions. Today, the multiple threats faced by our species, including rapid human population growth, changing climate, and increasing salinity, add urgency to the investigation of crop improvement by Si.
Author Contributions
DC wrote the manuscript, with input and editing from DB, WH, and HK.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
Agarie, S. H. U., Agata, W., Kubota, F., and Kaufman, P. B. (1998). Effects of silicon on transpiration and leaf conductance in rice plants (Oryza sativa L.). Plant Prod. Sci. 1, 89–95. doi: 10.1626/pps.1.89
Ahmad, R., Zaheer, S. H., and Ismail, S. (1992). Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci. 85, 43–50. doi: 10.1016/0168-9452(92)90092-Z
Ahmed, M., Fayyaz Ul, H., Qadeer, U., and Aslam, M. A. (2011). Silicon application and drought tolerance mechanism of sorghum. Afr. J. Agr. Res. 6, 594–607.
Ahmed, M., Qadeer, U., Ahmed, Z. I., and Fayyaz-Ul, H. (2016). Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon. Arch. Acker Pflanzenbau Bodenkd. 62, 299–315.
Al-Aghabary, K., Zhu, Z., and Shi, Q. H. (2004). Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J. Plant Nutr. 27, 2101–2115. doi: 10.1081/PLN-200034641
Alcazar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0
Arnon, D. I., and Stout, P. R. (1939). The essentiality of certain elements in minute quantity for plants, with special reference to copper. Plant Physiol. 14, 371–375. doi: 10.1104/pp.14.2.371
Azeem, M., Iqbal, N., Kausar, S., Javed, M. T., Akram, M. S., and Sajid, M. A. (2015). Efficacy of silicon priming and fertigation to modulate seedling’s vigor and ion homeostasis in wheat (Triticum aestivum L.) under saline environment. Environ. Sci. Pollut. Res. Int. 22, 14367–14371. doi: 10.1007/s11356-015-4983-8
Boyer, J. S. (1982). Plant productivity and environment. Science 218, 443–448. doi: 10.1126/science.218.4571.443
Brunings, A. M., Datnoff, L. E., Ma, J. F., Mitani, N., Nagamura, Y., Rathinasabapathi, B., et al. (2009). Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann. Appl. Biol. 155, 161–170. doi: 10.1111/j.1744-7348.2009.00347.x
Chain, F., Côté-Beaulieu, C., Belzile, F., Menzies, J. G., and Bélanger, R. R. (2009). A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol. Plant Microbe Interact. 22, 1323–1330. doi: 10.1094/MPMI-22-11-1323
Chen, W., Yao, X., Cai, K., and Chen, J. (2011). Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 142, 67–76. doi: 10.1007/s12011-010-8742-x
Conrath, U. (2011). Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. doi: 10.1016/j.tplants.2011.06.004
Cooke, J., and Leishman, M. R. (2011). Is plant ecology more siliceous than we realise? Trends Plant Sci. 16, 61–68. doi: 10.1016/j.tplants.2010.10.003
Coskun, D., Britto, D. T., Hamam, A. M., and Kronzucker, H. J. (2014). Measuring fluxes of mineral nutrients and toxicants in plants with radioactive tracers. J. Vis. Exp. 90:e51877. doi: 10.3791/51877
Coskun, D., Britto, D. T., Jean, Y.-K., Kabir, I., Tolay, I., Torun, A. A., et al. (2013a). K+ efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes of rice (Oryza sativa L.). PLoS ONE 8:e57767. doi: 10.1371/journal.pone.0057767
Coskun, D., Britto, D. T., Jean, Y. K., Schulze, L. M., Becker, A., and Kronzucker, H. J. (2012). Silver ions disrupt K+ homeostasis and cellular integrity in intact barley (Hordeum vulgare L.) roots. J. Exp. Bot. 63, 151–162. doi: 10.1093/jxb/err267
Coskun, D., Britto, D. T., Li, M., Oh, S., and Kronzucker, H. J. (2013b). Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol. 162, 496–511. doi: 10.1104/pp.113.215913
Detmann, K., Araújo, W., Martins, S., Fernie, A. R., and DaMatta, F. (2013). Metabolic alterations triggered by silicon nutrition: is there a signaling role for silicon? Plant Signal. Behav. 8:e22523. doi: 10.4161/psb.22523
Detmann, K. C., Araújo, W. L., Martins, S. C., Sanglard, L. M., Reis, J. V., Detmann, E., et al. (2012). Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 196, 752–762. doi: 10.1111/j.1469-8137.2012.04299.x
Dolan, L., and Davies, J. (2004). Cell expansion in roots. Curr. Opin. Plant Biol. 7, 33–39. doi: 10.1016/j.pbi.2003.11.006
Eneji, A. E., Inanaga, S., Muranaka, S., Li, J., Hattori, T., An, P., et al. (2008). Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 31, 355–365. doi: 10.1080/01904160801894913
Epstein, E. (1994). The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. U.S.A. 91, 11–17. doi: 10.1073/pnas.91.1.11
Epstein, E. (1999). Silicon. Ann. Rev. Plant Physiol. Plant Mol. Biol. 50, 641–664. doi: 10.1146/annurev.arplant.50.1.641
Epstein, E. (2009). Silicon: its manifold roles in plants. Ann. Appl. Biol. 155, 155–160. doi: 10.1111/j.1744-7348.2009.00343.x
Epstein, E., and Bloom, A. J. (2005). Mineral Nutrition of Plants: Principles and Perspectives, 2nd Edn. Sunderland: Sinauer Associates Inc.
Exley, C. (2015). A possible mechanism of biological silicification in plants. Front. Plant Sci. 6:853. doi: 10.3389/fpls.2015.00853
Fadzilla, N. M., Finch, R. P., and Burdon, R. H. (1997). Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J. Exp. Bot. 48, 325–331. doi: 10.1093/jxb/48.2.325
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212. doi: 10.1051/agro:2008021
Fauteux, F., Chain, F., Belzile, F., Menzies, J. G., and Bélanger, R. R. (2006). The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc. Natl. Acad. Sci. U.S.A. 103, 17554–17559. doi: 10.1073/pnas.0606330103
Fauteux, F., Rémus-Borel, W., Menzies, J. G., and Bélanger, R. R. (2005). Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Letts. 249, 1–6. doi: 10.1016/j.femsle.2005.06.034
Fleck, A. T., Nye, T., Repenning, C., Stahl, F., Zahn, M., and Schenk, M. K. (2011). Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J. Exp. Bot. 62, 2001–2011. doi: 10.1093/jxb/erq392
Fleck, A. T., Schulze, S., Hinrichs, M., Specht, A., Wassmann, F., Schreiber, L., et al. (2015). Silicon promotes exodermal Casparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PLoS ONE 10:e0138555. doi: 10.1371/journal.pone.0138555
Flowers, T. J. (2004). Improving crop salt tolerance. J. Exp. Bot. 55, 307–319. doi: 10.1093/jxb/erh003
Flowers, T. J., Hajibagheri, M. A., and Yeo, A. R. (1991). Ion accumulation in the cell walls of rice plants growing under saline conditions – Evidence for the Oertli hypothesis. Plant Cell Environ. 14, 319–325. doi: 10.1111/j.1365-3040.1991.tb01507.x
Gao, X., Zou, C., Wang, L., and Zhang, F. (2006). Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 29, 1637–1647. doi: 10.1080/01904160600851494
Gao, X. P., Zou, C. Q., Wang, L. J., and Zhang, F. S. (2004). Silicon emproves water use efficiency in maize plants. J. Plant Nutr. 27, 1457–1470. doi: 10.1081/PLN-200025865
Gattullo, C. E., Allegretta, I., Medici, L., Fijan, R., Pii, Y., Cesco, S., et al. (2016). Silicon dynamics in the rhizosphere: connections with iron mobilization. J. Plant Nutr. Soil Sci. 179, 409–417. doi: 10.1002/jpln.201500535
Gill, S. S., and Tuteja, N. (2010a). Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 5, 26–33. doi: 10.4161/psb.5.1.10291
Gill, S. S., and Tuteja, N. (2010b). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gong, H. J., Randall, D. P., and Flowers, T. J. (2006). Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 29, 1970–1979. doi: 10.1111/j.1365-3040.2006.01572.x
Gong, H. J., Zhu, X. Y., Chen, K. M., Wang, S. M., and Zhang, C. L. (2005). Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 169, 313–321. doi: 10.1016/j.plantsci.2005.02.023
Guerriero, G., Hausman, J.-F., and Legay, S. (2016). Silicon and the plant extracellular matrix. Front. Plant Sci. 7:463. doi: 10.3389/fpls.2016.00463
Guntzer, F., Keller, C., and Meunier, J.-D. (2012). Benefits of plant silicon for crops: a review. Agron. Sustain. Dev. 32, 201–213. doi: 10.1007/s13593-011-0039-8
Hamam, A. M., Britto, D. T., Flam-Shepherd, R., and Kronzucker, H. J. (2016). Measurement of differential Na+ efflux from apical and bulk root zones of intact barley and Arabidopsis plants. Front. Plant Sci. 7:272. doi: 10.3389/fpls.2016.00272
Hameed, A., Sheikh, M. A., Jamil, A., and Basra, S. M. A. (2013). Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak. J. Life Soc. Sci. 11, 19–24.
Hattori, T., Inanaga, S., Araki, H., An, P., Morita, S., Luxova, M., et al. (2005). Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 123, 459–466. doi: 10.1111/j.1399-3054.2005.00481.x
Hattori, T., Sonobe, K., Araki, H., Inanaga, S., An, P., and Morita, S. (2008). Silicon application by sorghum through the alleviation of stress-induced increase in hydraulic resistance. J. Plant Nutr. 31, 1482–1495. doi: 10.1080/01904160802208477
Haynes, R. J. (2014). A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 177, 831–844. doi: 10.1002/jpln.201400202
He, C., Ma, J., and Wang, L. (2015). A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 206, 1051–1062. doi: 10.1111/nph.13282
He, C., Wang, L., Liu, J., Liu, X., Li, X., Ma, J., et al. (2013). Evidence for ’silicon’ within the cell walls of suspension-cultured rice cells. New Phytol. 200, 700–709. doi: 10.1111/nph.12401
Hernandez, J. A., Corpas, F. J., Gomez, M., Delrio, L. A., and Sevilla, F. (1993). Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant. 89, 103–110. doi: 10.1111/j.1399-3054.1993.tb01792.x
Hossain, K. A., Horiuchi, T., and Miyagawa, S. (2001). Effects of silicate materials on growth and grain yield of rice plants grown in clay loam and sandy loam soils. J. Plant Nutr. 24, 1–13. doi: 10.1081/PLN-100000308
Khoshgoftarmanesh, A. H., Khodarahmi, S., and Haghighi, M. (2014). Effect of silicon nutrition on lipid peroxidation and antioxidant response of cucumber plants exposed to salinity stress. Arch. Agron. Soil Sci. 60, 639–653. doi: 10.1080/03650340.2013.822487
Krishnamurthy, P., Ranathunge, K., Nayak, S., Schreiber, L., and Mathew, M. K. (2011). Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 62, 4215–4228. doi: 10.1093/jxb/err135
Kronzucker, H. J., and Britto, D. T. (2011). Sodium transport in plants: a critical review. New Phytol. 189, 54–81. doi: 10.1111/j.1469-8137.2010.03540.x
Kronzucker, H. J., Coskun, D., Schulze, L. M., Wong, J. R., and Britto, D. T. (2013). Sodium as nutrient and toxicant. Plant Soil 369, 1–23. doi: 10.1007/s11104-013-1801-2
Kusano, T., Berberich, T., Tateda, C., and Takahashi, Y. (2008). Polyamines: essential factors for growth and survival. Planta 228, 367–381. doi: 10.1007/s00425-008-0772-7
Latef, A. A. A., and Tran, L.-S. P. (2016). Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 7:243. doi: 10.3389/fpls.2016.00243
Liang, Y., Nikolic, M., Bélanger, R., Gong, H., and Song, A. (2015). Silicon in Agriculture. Dordrecht: Springer.
Liang, Y., Sun, W., Zhu, Y.-G., and Christie, P. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ. Pollut. 147, 422–428. doi: 10.1016/j.envpol.2006.06.008
Liang, Y., Zhang, W., Chen, Q., Liu, Y., and Ding, R. (2006). Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 57, 212–219. doi: 10.1016/j.envexpbot.2005.05.012
Liang, Y. C. (1999). Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209, 217–224. doi: 10.1023/A:1004526604913
Liang, Y. C., Chen, Q., Liu, Q., Zhang, W. H., and Ding, R. X. (2003). Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 160, 1157–1164. doi: 10.1078/0176-1617-01065
Liang, Y. C., Zhang, W. H., Chen, Q., and Ding, R. X. (2005). Effects of silicon on H+-ATPase and H+-PPase activity, fatty acid composition and fluidity of tonoplast vesicles from roots of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 53, 29–37. doi: 10.1016/j.envexpbot.2004.02.010
Liu, P., Yin, L., Deng, X., Wang, S., Tanaka, K., and Zhang, S. (2014). Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 65, 4747–4756. doi: 10.1093/jxb/eru220
Liu, P., Yin, L., Wang, S., Zhang, M., Deng, X., Zhang, S., et al. (2015). Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 111, 42–51. doi: 10.1093/jxb/eru220
Lux, A., Luxova, M., Abe, J., Tanimoto, E., Hattori, T., and Inanaga, S. (2003). The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 158, 437–441. doi: 10.1046/j.1469-8137.2003.00764.x
Ma, J., Cai, H., He, C., Zhang, W., and Wang, L. (2015). A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 206, 1063–1074. doi: 10.1111/nph.13276
Ma, J. F. (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50, 11–18. doi: 10.1080/00380768.2004.10408447
Ma, J. F., and Takahashi, E. (1993). Interaction between calcium and silicon in water-cultured rice plants. Plant Soil 148, 107–113. doi: 10.1007/BF02185390
Ma, J. F., and Yamaji, N. (2006). Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. doi: 10.1016/j.tplants.2006.06.007
Ma, J. F., and Yamaji, N. (2015). A cooperative system of silicon transport in plants. Trends Plant Sci. 20, 435–442. doi: 10.1016/j.tplants.2015.04.007
Malagoli, P., Britto, D. T., Schulze, L. M., and Kronzucker, H. J. (2008). Futile Na+ cyling at the root plasma membrane in rice (Oryza sativa L.) – kinetics, energetics, and relation to salinity tolerance. J. Exp. Bot. 59, 4109–4117. doi: 10.1093/jxb/ern249
Ming, D. F., Pei, Z. F., Naeem, M. S., Gong, H. J., and Zhou, W. J. (2012). Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop Sci. 198, 14–26. doi: 10.1111/j.1439-037X.2011.00486.x
Moussa, H. R. (2006). Influence of exogenous application of silicon on physiological response of salt-stressed maize (Zea mays L.). Int. J. Agric. Biol. 8, 293–297.
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nwugo, C. C., and Huerta, A. J. (2011). The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium stress. J. Prot. Res. 10, 518–528. doi: 10.1021/pr100716h
Oertli, J. J. (1968). Extracellular salt accumulation a possible mechanism of salt injury in plants. Agrochimica 12, 461–469.
Pei, Z. F., Ming, D. F., Liu, D., Wan, G. L., Geng, X. X., Gong, H. J., et al. (2010). Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 29, 106–115. doi: 10.1007/s00344-009-9120-9
Pii, Y., Cesco, S., and Mimmo, T. (2015). Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol. Biochem. 94, 48–56. doi: 10.1016/j.plaphy.2015.05.002
Rengasamy, P. (2010). Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37, 613–620. doi: 10.1071/FP09249
Richmond, K. E., and Sussman, M. (2003). Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 6, 268–272. doi: 10.1016/S1369-5266(03)00041-4
Rizwan, M., Ali, S., Ibrahim, M., Farid, M., Adrees, M., Bharwana, S. A., et al. (2015). Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ. Sci. Pollut. Res. 22, 15416–15431. doi: 10.1007/s11356-015-5305-x
Rogalla, H., and Römheld, V. (2002). Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 25, 549–555. doi: 10.1046/j.1365-3040.2002.00835.x
Sachs, J. V. (1860). Vegetationsversuche mit ausschluss des bodens über die nährstoffe und sonstigen ernährungsbedingungen von mais, bohnen, und anderen pflanzen. Landw. Versuchsst. 2, 219–268.
Saqib, M., Zoerb, C., and Schubert, S. (2008). Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Funct. Plant Biol. 35, 633–639. doi: 10.1071/FP08100
Savant, N. K., Snyder, G. H., and Datnoff, L. E. (1997). Silicon management and sustainable rice production. Adv. Agron. 58, 151–199. doi: 10.1016/S0065-2113(08)60255-2
Schmidhuber, J., and Tubiello, F. N. (2007). Global food security under climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19703–19708. doi: 10.1073/pnas.0701976104
Senadheera, P., Singh, R. K., and Maathuis, F. J. M. (2009). Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J. Exp. Bot. 60, 2553–2563. doi: 10.1093/jxb/erp099
Shazad, M., Zörb, C., Geilfus, C. M., and Mühling, K. H. (2013). Apoplastic Na+ in Vicia faba leaves rises after short-term salt stress and is remedied by silicon. J. Agron. Crop Sci. 199, 161–170. doi: 10.1111/jac.12003
Shi, Y., Wang, Y., Flowers, T. J., and Gong, H. (2013). Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 170, 847–853. doi: 10.1016/j.jplph.2013.01.018
Shi, Y., Zhang, Y., Han, W., Feng, R., Hu, Y., Guo, J., et al. (2016). Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 7:196. doi: 10.3389/fpls.2016.00196
Sonobe, K., Hattori, T., An, P., Tsuji, W., Eneji, A. E., Kobayashi, S., et al. (2010). Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr. 34, 71–82. doi: 10.1080/01904167.2011.531360
Soylemezoglu, G., Demir, K., Inal, A., and Gunes, A. (2009). Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci. Hortic. 123, 240–246. doi: 10.1016/j.scienta.2009.09.005
Speer, M., and Kaiser, W. M. (1991). Ion relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L. under salinity. Plant Physiol. 97, 990–997. doi: 10.1104/pp.97.3.990
Struyf, E., Smis, A., Van Damme, S., Garnier, J., Govers, G., Van Wesemael, B., et al. (2010). Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 1, 129. doi: 10.1038/ncomms1128
Suzuki, S., Ma, J. F., Yamamoto, N., Hattori, T., Sakamoto, M., and Umezawa, T. (2012). Silicon deficiency promotes lignin accumulation in rice. Plant Biotechnol. 29, 391–394. doi: 10.5511/plantbiotechnology.12.0416a
Tamai, K., and Ma, J. F. (2008). Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 307, 21–27. doi: 10.1007/s11104-008-9571-y
Van Bockhaven, J., De Vleesschauwer, D., and Hofte, M. (2013). Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J. Exp. Bot. 64, 1281–1293. doi: 10.1093/jxb/ers329
van Hulten, M., Pelser, M., Van Loon, L. C., Pieterse, C. M. J., and Ton, J. (2006). Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 5602–5607. doi: 10.1073/pnas.0510213103
Vandevenne, F. I., Barao, L., Ronchi, B., Govers, G., Meire, P., Kelly, E. F., et al. (2015). Silicon pools in human impacted soils of temperate zones. Glob. Biogeochem. Cycles 29, 1439–1450. doi: 10.1002/2014GB005049
Wang, S., Liu, P., Chen, D., Yin, L., Li, H., and Deng, X. (2015). Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front. Plant Sci. 6:759. doi: 10.3389/fpls.2015.00759
Wang, Y. X., Stass, A., and Horst, W. J. (2004). Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 136, 3762–3770. doi: 10.1104/pp.104.045005
Watanabe, S., Shimoi, E., Ohkama, N., Hayashi, H., Yoneyama, T., Yazaki, J., et al. (2004). Identification of several rice genes regulated by Si nutrition. Soil Sci. Plant Nutr. 50, 1273–1276. doi: 10.1080/00380768.2004.10408603
Yeo, A. (1999). Predicting the interaction between the effects of salinity and climate change on crop plants. Sci. Hortic. 78, 159–174. doi: 10.1016/S0304-4238(98)00193-9
Yeo, A. R., Flowers, S. A., Rao, G., Welfare, K., Senanayake, N., and Flowers, T. J. (1999). Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 22, 559–565. doi: 10.1046/j.1365-3040.1999.00418.x
Yin, L., Wang, S., Tanaka, K., Fujihara, S., Itai, A., Den, X., et al. (2016). Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 39, 245–258. doi: 10.1111/pce.12521
Zhao, F., Song, C.-P., He, J., and Zhu, H. (2007). Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 145, 1061–1072. doi: 10.1104/pp.107.105882
Zhu, J. K. (2001). Plant salt tolerance. Trends Plant Sci. 6, 66–71. doi: 10.1016/S1360-1385(00)01838-0
Zhu, Y., and Gong, H. (2014). Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 34, 455–472. doi: 10.1007/s13593-013-0194-1
Zhu, Y.-X., Xu, X.-B., Hu, Y.-H., Han, W.-H., Yin, J.-L., Li, H.-L., et al. (2015). Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 34, 1629–1646. doi: 10.1007/s00299-015-1814-9
Keywords: silicon, salinity stress, drought stress, sodium toxicity, osmotic stress, apoplast, water transport, ion transport
Citation: Coskun D, Britto DT, Huynh WQ and Kronzucker HJ (2016) The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 7:1072. doi: 10.3389/fpls.2016.01072
Received: 17 May 2016; Accepted: 07 July 2016;
Published: 18 July 2016.
Edited by:
Fernando Carlos Gómez-Merino, Colegio de Postgraduados, Montecillo, MexicoReviewed by:
Ryoung Shin, Riken Center for Sustainable Resource Science, JapanYoury Pii, Free University of Bozen-Bolzano, Italy
Copyright © 2016 Coskun, Britto, Huynh and Kronzucker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herbert J. Kronzucker, aGVyYmVydC5rcm9uenVja2VyQHV0b3JvbnRvLmNh
 Devrim Coskun
Devrim Coskun Dev T. Britto
Dev T. Britto Wayne Q. Huynh
Wayne Q. Huynh Herbert J. Kronzucker
Herbert J. Kronzucker