- State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing, China
Although the increasing concentration of atmospheric carbon dioxide (CO2) accelerates the accumulation of carbohydrates and increases the biomass and yield of C3 crop plants, it also reduces their nitrogen concentration. The consequent changes in primary and secondary metabolites affect the palatability of host plants and the feeding of herbivorous insects. Aphids are phloem feeders and are considered the only feeding guild that positively responds to elevated CO2. In this review, we consider how elevated CO2 modifies host defenses, nutrients, and water-use efficiency by altering concentrations of the phytohormones jasmonic acid, salicylic acid, ethylene, and abscisic acid. We will describe how these elevated CO2-induced changes in defenses, nutrients, and water statusfacilitate specific stages of aphid feeding, including penetration, phloem-feeding, and xylem absorption. We conclude that a better understanding of the effects of elevated CO2 on aphids and on aphid damage to crop plants will require research on the molecular aspects of the interaction between plant and aphid but also research on aphid interactions with their intra- and inter-specific competitors and with their natural enemies.
Introduction
Since the industrial revolution, atmospheric CO2 concentrations have increased from 280 ppm to approximately 400 ppm due to anthropogenic effects, i.e., deforestation and fossil fuel combustion. These increases in atmospheric CO2 concentrations have serious implications for global warming and climate change (Stocker et al., 2013). Although changes in climate have been anticipated to greatly affect agricultural ecosystems (Fuhrer, 2003), increases in atmospheric CO2 concentration alone can also be very important because they can directly affect plant physiology and indirectly alter interactions between plants and herbivores and plant pathogens (Robinson et al., 2012). These altered interactions may then lead to more severe and frequent outbreaks of pest insects and plant diseases in agricultural ecosystems (Percy et al., 2002).
To understand how elevated concentrations of atmospheric CO2 could increase pest problems, we must first recognize that increases in CO2 tends to increase the growth of plants by enhancing their photosynthetic rate, resulting in higher yields for most C3 crops (Ainsworth and Rogers, 2007). Under elevated CO2, however, C3 crop plants exhibit decreases in nitrogen (N) and other trace elements, i.e., zinc and iron (Bloom et al., 2010). These decreases reduce the nutritional value for herbivorous insects and may therefore change their feeding behaviors (Myers et al., 2014). For those insects that chew leaves, a reduction in the N concentration in crop tissue and the resulting increase in the carbon/nitrogen ratio (C:N ratio) under elevated CO2 could cause these insect pests to consume more leaves to meet their N needs (Bezemer and Jones, 1998; Sun and Ge, 2011). In addition, leaves grown under elevated CO2 decrease their ability to produce jasmonic acid (JA), a hormone that contributes to plant defenses against chewing insects (Zavala et al., 2008).
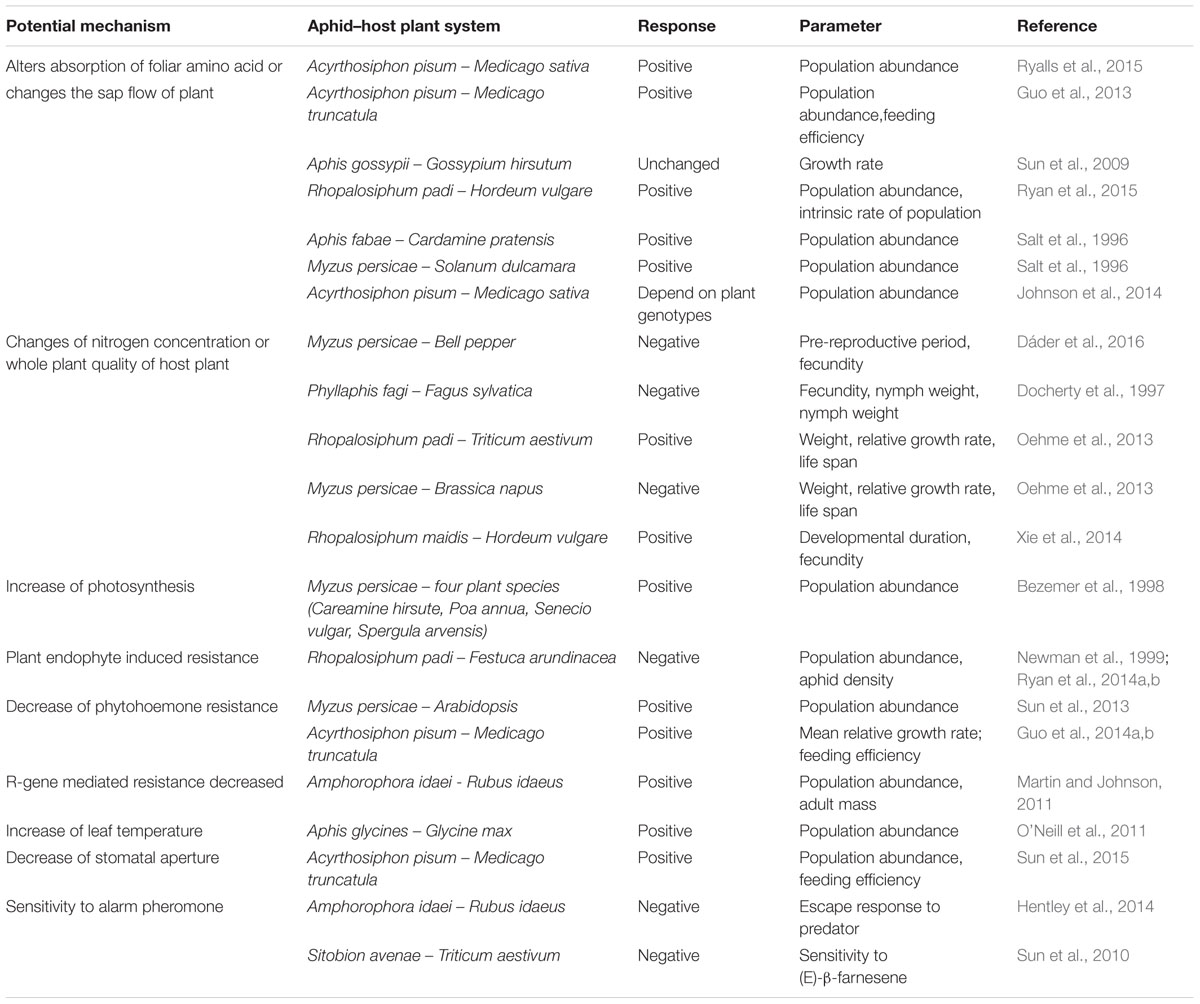
Elevated CO2 may also increase the damage to crops caused by phloem-sucking insects including aphids. Aphids feed exclusively on the phloem sap and are very sensitive to changes in plant quality caused by climate change (Pritchard et al., 2007). Recent meta-analysis result shows that aphids tend to perform better under elevated CO2 on average (Robinson et al., 2012). The conclusions from many statsitically signficant researches, however, exhibit idiosyncratic responses of aphids in terms of population abundance, fecundity as well as survival (summarized in Table 1). Although predictions are difficult, it is nevertheless useful to determine why some aphids are more fit while others are less fit under elevated CO2. A mechanistic understanding can help make sense of these contradictory results. Previous study demonstrates that the effect of elevated CO2 on plant, which includes C and N assimilation, secondary metabolism, plant stomatal conductance as well as leaf temperature, could in turn affect aphid population numbers and growth (Ainsworth et al., 2006; May et al., 2013). Futhermore, the feeding behavior of aphids and their interaction with host plant under elevated CO2 are largely ignored but should be crucial to the understanding of idiosyncratic responses. The aim of this review is to highlight overlooked processes and new discoveries that how elevated CO2 affects the components of plant leaves and how these effects alter the different feeding phases of aphids. We also suggest some possible molecular mechanisms underlying the interactions between aphids and their host plants under elevated CO2.
Aphid Feeding Behavior
Recent advances indicate that complex molecular interactions occur when aphids feed on plants. Unlike chewing insects that remove large pieces of plant tissues, aphids use their flexible and long stylets to obtain nutrients from the phloem sap and only inflict slight physical damage (Jaouannet et al., 2014). The specialized feeding behavior of aphids can be detected with electrical penetration graph (EPG) methods, i.e., EPG methods can be used to determine the locations and activities of aphid stylets, including pooled pathway phase activities, probing, salivation into sieve elements, passive uptake of the phloem sap, and xylem absorption (Tjallingii and Esch, 1993). Data on the initiation and duration of these feeding phases provide valuable cues regarding aphid activities and plant responses (Alvarez et al., 2006). Rather than simply withdrawing food from hosts, aphids can change their feeding location to avoid plant defenses or can secrete ‘effector’ proteins to suppress plant defenses (Hogenhout and Bos, 2011). To enhance their feeding, aphids can also alter host physiological traits, e.g., they can induce changes in host primary metabolism and in stomatal movement, and suppress the plant defenses (Giordanengo et al., 2010). Thus, a better understanding of aphid feeding behavior, its effects on hosts, and host responses is critical for understanding how elevated CO2 is likely to affect plant–aphid interactions.
Aphid Probing and Penetration Stage and its Relation to Plant Resistance
Influence of Plant Physical Barriers
Once they have arrived on a plant leaf, aphids must conquer host physical defenses including trichomes and waxes before they can insert their stylets into the host (Wang et al., 2004). Surface resistance is the first barrier of plant defense against aphid attack. The time that aphids spend between arriving on a leaf and making their first probe mainly reflects the physical barriers of the leaf surface including trichomes, repellent volatiles, and a thick or tough leaf surface (van Helden and Tjallingii, 1993). Plants can deter aphid attack by releasing secondary metabolites such as glucose esters and sesquiterpenes from glandular trichomes (Avé et al., 1987; Goffreda et al., 1989; Neal et al., 1990). Furthermore, a specifically expressed gene, NtLTP1, in the glandular trichomes of Nicotiana tabacum could enhance the plant’s defense against aphids (Choi et al., 2012). The changes in trichome density in response to CO2 are idiosyncratic. For example, trichome density increased in Brassica rape and Medicago truncatula (Karowe and Grubb, 2011; Guo et al., 2014a) but decreased in Arabidopsis and wheat under elevated CO2 (Masle, 2000; Bidart-Bouzat et al., 2005; Lake and Wade, 2009). In the legume M. truncatula under elevated CO2, the increased density of non-glandular and glandular trichomes caused aphids to spend more time before they made their first probe and to experience a prolonged pathway phase (Guo et al., 2014a). CO2 concentrations may affect trichome development by affecting the levels of gibberellic acid (GA), JA, and the microRNA molecule miR156. Elevated CO2 tends to increase plant GA content and decrease plant JA content (Teng et al., 2006; Zavala et al., 2008) and to decrease expression of miR156 (May et al., 2013). Additional research is needed, however, to clarify whether the effects of elevated CO2 on glandular trichome development and surface resistance to aphids is due to changes in GA, JA, and miR156.
Phytohormone-Mediated Defenses
When the aphid stylet penetrates the plant epidermis and mesophyll, it forms a channel that permits the delivery of saliva into the phloem (Jaouannet et al., 2014). On the one hand, elicitors in aphid saliva could trigger the formation of reactive oxygen species (ROS), which in turn could induce plant defenses (Giordanengo et al., 2010). On the other hand, “effectors” in aphid saliva could suppress plant resistance and manipulate host cell processes to favor aphid feeding and colonization (Bos et al., 2010a,b; McLellan et al., 2013; Gimenez-Ibanez et al., 2014; King et al., 2014). Parameters of aphid feeding behavior revealed by EPG could reflect the intensity of plant resistance; these parameters include the minimum duration of pathway phase activity, the number of test probes, and the total time before phloem ingestion begins (Alvarez et al., 2006). In the M. truncatula–pea aphid system, elevated CO2 increased the number of test probes but decreased the total time before phloem ingestion began (Guo et al., 2014a). The inconsistent effects of elevated CO2 on aphid feeding parameters may result from the contrasting effects of elevated CO2 on the defense signaling pathways involving the phytohormones JA, salicylic acid (SA), and ethylene (ET) (Guo et al., 2014a). Elevated CO2 tends to enhance SA-dependent defense but reduce JA- and ET-dependent defenses in plants (Zavala et al., 2009; Guo et al., 2012; Sun et al., 2013). Furthermore, the enhanced SA signaling pathway under elevated CO2 caused aphids to spend more time before the first probe and reduced aphid fitness (Casteel et al., 2012; Guo et al., 2014a). The suppression of the JA signaling pathway under elevated CO2, however, reduces the time required by aphids to reach the phloem. In addition, elevated CO2 down-regulates the expression of the ET signaling pathway genes ACC, SKL, and ERF in M. truncatula under attack by the pea aphid system; this downregulation, decreases the accumulation of H2O2 and the activities of key enzymes related to ROS (Guo et al., 2014b).
Moreover, elevated CO2 potentially disrupts the homeostatic cross-talk between SA and JA/ET pathways by directly activating the NPR1 (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1) gene (DeLucia et al., 2012; Zavala et al., 2013). NPR1-mediated suppression of JA signaling is regulated by glutathione biosynthesis (Spoel and Loake, 2011). Elevated CO2 changes the expression of genes that encode thioredoxins and glutathione S-transferase, which may activate the expression of NPR1 (DeLucia et al., 2012). However, Sun et al. (2013) found that when the NPR1 gene was knocked down, the JA-dependent defenses of Arabidopsis were not enhanced by elevated CO2, suggesting that the activation of NPR1 may not explain the response of SA, JA, and ET signaling pathways to elevated CO2. Clearly, the upstream network regulating plant immunity against aphids is complex. The elicitors secreted from aphid salivary glands were recognized by the host co-receptor BRI-ASSOCIATED RECEPTOR KINASE 1 (BAK1) which subsequently phosphorylates BOTRYTIS INDUCED KINASE1 (BIK1). The BAK1 and the BIK1 complexes could jointly modulate the downstream phytohormone-mediated defense signaling pathway (Chaudhary et al., 2014; Lei et al., 2014; Prince et al., 2014). In addition to BAK/BIK, other kinases such as mitogen protein kinases (MAPKs) are also important for regulating plant defense responses against insect herbivores (Hettenhausen et al., 2015). A number of studies reported that MAPKs could regulate the JA, SA, and ET signaling pathways by activating WRKY genes (Zavala et al., 2013). It is still unknown whether elevated CO2 affects the JA- and SA-dependent signaling pathways by regulating upstream BAK/BIK or MAPK signaling. Thus, additional research is needed to determine how elevated CO2 affects these regulatory molecules in phytohormone signaling networks.
Secondary Metabolite-Mediated Resistance
Many plant secondary metabolites may help plants resist aphid attack by negatively affecting the penetration pathway stage of aphid feeding. These secondary metabolites include alkaloids, steroids, foliar phenolic esters (rutin, cholorogenic acid, etc.), terpenoids, cyanogenic glycosides, glucosinolates, saponins, flavonoids, and pyrethrins (Sharma et al., 2000; Urbanska et al., 2002). For example, aphids that fed on high-saponin lines of alfafa required a prolonged time to penetrate the epidermis and mesophyll (pattern C wave) and showed a significant reduction in phloem sap ingestion (Goławska, 2007). Furthermore, different phenolic compounds seem to have different effects on the feeding parameters of aphids. Caffeic and gallic acids in cereals, for example, drastically shortened the probing phase of the grain aphid, whereas catechin prolonged the pathway phase and also decreased the number of probes by the grain aphid (Urbanska et al., 2002). On average, elevated CO2 increases the total phenolicsin plants by an average of 19%, condensed tannins by 22%, and flavonoids by 27% (Robinson et al., 2012). The excess of secondary metabolites in plants may help explain the increased epidermis and mesophyll resistance of plants during pathway and probing feeding stages of aphids under elevated CO2 (Guo et al., 2014a). Despite of increasing tannin content and phenolic compounds in whole host plant leaves, the bird cherry-oat aphid Rhopalosiphum padi performed better under elevated CO2 (Bezemer and Jones, 1998; Zhang et al., 2003). This result suggested that the tricky feeding strategy of the aphid allows it to avoid some potential defensive components. Thus, it is hard to predict the impact on aphid fitness only through surface or pathway effects.
Phenylalanine ammonialyase (PAL) and polyphenol oxidase (PPO) are two key enzymes involved in the synthesis of phenolic compounds that may be absorbed by the salivary sheath of the aphid stylet. The further polymerization of phenolic compounds causes browning of cells in contact with the saliva; such browning was associated with aphid probing activity during penetration of the epidermal and mesophyll tissues (Jiang and Miles, 1993; Urbanska et al., 1998; Han et al., 2009). PAL and PPO activities are changed by elevated CO2. For example, elevated CO2 tends to increase PAL activity but decrease PPO activity in M. truncatula. However, it is still unknown how changes in PPO and PAL activities under elevated CO2 affect the penetration phase of aphid feeding (Guo et al., 2014b).
Resistance Expressed in the Phloem
After overcoming defenses associated with the plant epidermis and mesophyll, the aphid stylet may finally reach the phloem, but plants have ways to prevent or reduce the ingestion of phloem sap. Phloem sap contains carbohydrates, proteins, and amino acids that are essential for plant development (Gündüz and Douglas, 2009). If the phloem is impaired, plants could suffer loss of nutrients, disturbance of translocation, and increased vulnerability to infection by microbial pathogens (Dinant et al., 2010). Therefore, plants have evolved a range of defenses to inhibit phloem feeding by aphids (Will et al., 2013). The most common defense involves the occlusion of sieve tubes by the plugging of sieve pores (Knoblauch and van Bel, 1998). Two groups of sieve-tube occlusion mechanisms can be found in plants: callose deposition and protein plugging (e.g., Will and van Bel, 2006; Furch et al., 2007). The Ca2+ signaling pathway in plants plays a key role in sieve-tube occlusion during aphid penetration. When the stylet penetrates the sieve membrane, the high concentration gradient of Ca2+ between the apoplast and the sieve element lumen leads to an influx of Ca2+into the sieve element lumen, which induces occlusion (Knoblauch and van Bel, 1998). When this occurs, aphids must secret watery saliva into the phloem; the saliva contains proteins that bind Ca2+ and counteract sieve element occlusion. Thus, the time spent during salivary secretion into sieve elements reflects the defenses located in the phloem (Will et al., 2013).
Phloem resistance against aphids may be affected by elevated CO2. The key gene involved in callose biosynthesis is up-regulated in Arabidopsis under elevated CO2 (Li et al., 2008). Furthermore, cytosolic free Ca2+ is increased by elevated CO2 in Commelina communis (Webb et al., 1996). The increased production of callose and free Ca2+ in cells may cause aphids to spend more time in overcoming phloem resistance. EPG data consistently showed that elevated CO2 increased the time of salivary secretion into sieve elements when pea aphids fed on M. truncatula (Guo et al., 2013). Still, there is no direct evidence confirming that elevated CO2 increases the phloem resistance against pea aphids because of increases in callose deposition and in the Ca2+ signaling pathway.
Aphid Phloem Ingestion and its Relation to Plant Nutrition
The efficiency with which aphids feed on phloem sap is determined by the nutritional composition of the sap (Douglas, 2003). Sucrose is the dominant organic compound in the phloem sap and is a crucial C source for aphids (Fisher and Cash-Clark, 2000; Douglas, 2003). Sucrose is the principal energy source for aphids and also provides the C skeleton for lipid, amino acid, and protein synthesis (Rhodes et al., 1996; Febvay et al., 1999). In potato, a mutation of the sucrose transporter StSUT1 gene reduced the phloem sucrose content and simultaneously reduced the performance of the potato aphid (Pescod et al., 2007). Nevertheless, high concentrations of soluble carbohydrates in plant tissues often reduce aphid performance because they dilute other nutrients such as amino acids and proteins; as a consequence, the aphids must increase their consumption of phloem sap and excrete the excess sucrose as honeydew (Wilkinson et al., 1997). In contrast to carbohydrate-based nutrients, N nutrition is a limiting factor for aphid growth. The phloem sap ingested by aphids has a protein/carbohydrate ratio (w/w) as low as 0.1 while the leaf tissue ingested by chewing insects has a protein/carbohydrate ratio ranging from 0.8 to 1.5 (Behmer, 2008).
Increases in atmospheric CO2 accelerate photosynthesis and synthesize and transport of sucrose into the phloem, which dilutes the N concentration and increases the C:N ratio in the phloem of non-legumes (Barbehenn et al., 2004). The decreased nitrogen concentration of plants could prolonged the pre-reproductive period and decrease the fecundity of some aphids under elevated CO2 (Dáder et al., 2016). However, Sun et al. (2009) found that although amino acid relative concentration in the phloem of cotton plants was lower under elevated CO2 than under ambient CO2, higher amounts of free amino acids were found in cotton aphids fed on cotton grown in elevated CO2. These results suggested that cotton aphids under elevated CO2 will ingest increased quantities of phloem sap to satisfy their nutritional requirements. Moreover, the relative concentrations of predominantly essential amino acids in the phloem of barley are increased under elevated CO2 (Ryan et al., 2015). The latter result is consistent with the large increases in the levels of minor amino acids (most of which are considered essential) in tobacco seedlings under elevated CO2 (Geiger et al., 1998). These results suggest that although the total N concentration of plants is decreased, amino acids biosynthesis and translation in some non-legumes may increase under elevated CO2. Moreover, the mathematic model constructed by Newman et al. (2003) predicted that aphid populations tend to be larger under elevated CO2 if host plants have higher N supplementation, that the nitrogen requirement of aphids is low and that the density-dependent response is weak. Thus, a general explanation for the species-specific responses of aphids to elevated CO2 remains to be elucidated.
In legumes, elevated CO2 leads to a 38% increase in the quantity of N fixed from the atmosphere, which can compensate for decreases in plant N under elevated CO2 and cause the legumes to maintain a C:N ratio similar to that under ambient CO2 (Lam et al., 2012). When M. truncatula was infested by pea aphids, elevated CO2 significantly increased the concentration of total amino acids in leaves and of most individual amino acids in the phloem by enhancing the enzyme activities of N transamination (Guo et al., 2013). The increased amino acids, however, are mostly nonessential, and require the aphid endosymbiont Buchnera to convert them into essential amino acids (Nikoh et al., 2010). When the N-fixation ability was reduced by artificially induced mutation, the individual amino acid relative concentration in the phloem of M. truncatula was decreased such that Buchnera could no longer convert the nonessential amino acids into essential amino acids (Guo et al., 2013). These results with legumes suggest that elevated CO2 may increase the phloem feeding time of the pea aphid by altering amino acid metabolism, and that this response depends on a functional N fixation system. Responses of different cultivars, varieties, or genotypes of the same species to elevated CO2, however, can also vary. For example, Johnson et al. (2014) found elevated CO2 increased 86% and 56% essential amino acid concentrations and pea aphid colonization success on the high resistant cultivar ‘Sequel’ of M. sativa. However, elevated CO2 decreased 53% and 33% essential amino acid concentrations and aphid colonization on the moderate resistant cultivar ‘Genesis’. This result suggested some cultivars may become more or less susceptible to aphid attack under climate change conditions, an important consideration for determining future outcomes (McKenzie et al., 2013).
The ability to fix N is regulated by several hormone signaling pathways including the ET signaling pathway (Ma et al., 2002; Penmetsa et al., 2008). When the key gene Mtskl in the ET-perception pathway was mutated in M. truncatula, the nitrogenase activity was increased about two times (Penmetsa and Cook, 1997). Previous study has shown that elevated CO2 decreases the ET signaling pathway in Arabidopsis (Sun et al., 2013). The suppression of the ET signaling pathway in M. truncatula increased nodulation and N fixation ability, which thereby satisfied the increased demand for N by plants growing under elevated CO2. The down-regulation of the ET signaling pathway, however, is accompanied by decreased ET-mediated host resistance against the pea aphid (Guo et al., 2014b). This result suggested that in the M. truncatula–pea aphid system under elevated CO2, both nutritional and resistance effects would increase the fitness of the pea aphid by suppressing the ET signaling pathway (Figure 1).
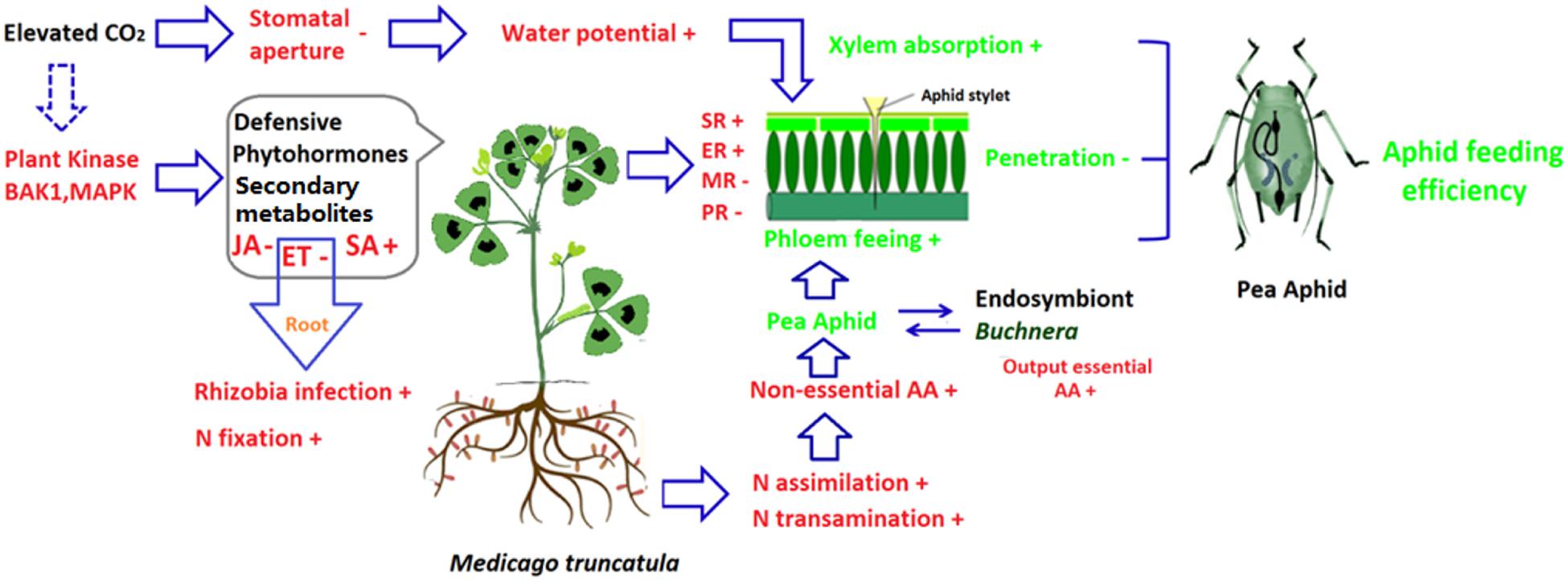
FIGURE 1. Potential effects of elevated CO2 on host plant, and the cascading effects on aphid feeding using Medicago truncatula-pea aphid as examples. Elevated CO2 affects aphid feeding efficiency in three ways. First, elevated CO2 modifies the phytohormone-dependent induced defenses and plant secondary metabolites derived defense. Enhancement of the salicylic acid-dependent defense pathway increased surface and epidermis resistance while the impairment of the jasmonic acid/ethylene-dependent defense pathway decreased mesophyll and phloem resistance. The changes of resistance facilitate the penetration feeding phase (the feeding phase that occurs before the stylet reaches the phloem). Second, the impairment of ethylene signaling pathway enhanced N fixation inroot, elevated CO2 tends to increase N assimilation and non-essential amino acid supple in the phloem. Furthermore, the aphid endosymbiont Buchnera could transform non-essential amino acid into essential amino acids, which increases the output of essential amino acid for aphids. Therefore, the increased amino acid supply benefits the phloem feeding of aphids. Third, elevated CO2 decreases stomatal conductance and transpiration, which increases the water potential in M. truncatula. As a result, aphid xylem feeding and osmotic pressure regulation are enhanced under elevated CO2. These three effects of elevated CO2 (alteration of host plant defenses, of amino acid supply in the phloem, and of host and aphid water status) greatly affect aphid feeding efficiency. AA, amino acid; BAK1, BRI-ASSOCIATED RECEPTOR KINASE 1; ER, epidermis resistance; ET, Ethylene; MAPK, mitogen protein kinases; JA, jasmonic acid; MR, mesophyll resistance; PR, phloem resistance; SA, Salicylic acid; SR, surface resistance; +, positively affected by elevated CO2; -, negatively affected by elevated CO2.
Aphid Xylem Absorption and its Relation to Plant Water Status
Aphids occasionally ingest xylem to increase their phloem feeding efficiency (Tjallingii and Esch, 1993; Douglas, 2006). Because the sugar-enriched phloem sap has an osmotic pressure that is as much as 4 to 5 times greater than that of the aphid’s haemolymph, continuously passive uptake of the phloem sap could result in aphid dehydration. To avoid self-dehydration and osmotic stress in the haemolymph during the phloem-feeding phase (i.e., to balance haemolymph osmolarity), aphids must consume a certain amount of xylem sap, which has a lower osmolarity than phloem sap (Pompon et al., 2011). This xylem-feeding behavior requires that the host plant has a relatively high plant water potential because the feeding is passive, i.e., fluid moves from plant to aphid because of a water potential gradient (Huberty and Denno, 2004; Daniels et al., 2009; Nalam et al., 2012). Aphids, like pathogens, can trigger stomatal closure, decrease leaf transpiration, and maintain the water content of the host plant by up-regulating the ABA signaling pathway. This manipulation of host stomata helps aphids absorb water from the xylem to neutralize phloem osmotic pressure (Sun et al., 2015).
Under elevated CO2, plants also exhibit reduced stomatal apertures and stomatal conductance. In FACE experiments, elevated CO2 has decreased stomatal conductance by an average of 22% for five functional plant groups that include 285 plant species (Ainsworth and Rogers, 2007). As noted, the decreased stomatal conductance reduces water loss from plants and increases plant water potential and water content (Wullschleger et al., 2002; Pritchard et al., 2007). Sun et al. (2015) found that the decreases in stomatal aperture and increases in plant water potential induced by elevated CO2 facilitated xylem feeding by aphids and thereby decreased aphid haemolymph osmolarity, which indicated a decreased cost of osmoregulation in aphids under elevated CO2.
Conclusion and Perspectives
Recent studies have provided evidence that elevated CO2 alters plant resistance, nutritional value, and water status and that these changes affect certain feeding stages of aphids (Figure 1). The evidence also indicates that such changes and effects could be mediated by the phytohormones JA, SA, ET, and ABA (Guo et al., 2013, 2014a,b; Sun et al., 2015). In these and related studies, elevated CO2 stimulated the SA signaling pathway and thereby increased the epidermis and mesophyll resistance of plants. However, elevated CO2 decreased JA and ET signaling pathways, which reduced the total time required by aphids to reach the phloem. The decreased ET signaling pathway also increased the N fixation ability of legumes and thereby increased their synthesis of amino acids, which in turn increased amino acid acquisition by aphids (Guo et al., 2013). Moreover, elevated CO2 decreased stomatal aperture and increased plant water potential, which thereby increased aphid xylem absorption and enhanced aphid osmoregulation (Sun et al., 2015). Nevertheless, transcriptomic evidence shows that elevated CO2 has a wide range of effects on plant metabolism (including C and N assimilation, secondary metabolism, and transportation), all of which may affect aphid performance (Ainsworth et al., 2006; May et al., 2013). Thus, the effects of elevated CO2 on the interaction between plants and aphids cannot be understood by simply relating one aspect of plant quality to a specific feeding phase of the aphid. Because the responses to elevated CO2 differ among plant species, it is currently difficult to generalize about how further increases in concentrations in atmospheric CO2 affects aphid feeding and damage. An increased understanding of the molecular mechanisms underlying the recognition and interactions between plants and aphids should increase our ability to predict aphid damage under elevated CO2.
In addition to changes in aphid feeding behavior, changes in aphid physiology must be considered to understand how aphid performance is affected by elevated CO2. Some studies have reported increases in aphid growth rate and fecundity under elevated CO2, which suggests that elevated CO2 increases aphid fitness and increases the probability of aphid outbreaks. At present, we have some understanding of what happens but we do not know how it happens. Like chewing insects, aphids could sense and respond to nutritional changes in host plants by regulating a complex regulatory network involving the insulin-related peptides, the target of rapamycin (TOR), ecdysteroids, and juvenile hormone (Badisco et al., 2013). For example, TOR acts as a central regulator of protein synthesis by sensing and integrating signals from amino acid nutrition, while the insulin signaling pathway is responsible for sensing carbohydrate-derived nutrients (Grewal, 2009). Thus, research is needed on how these two nutrient-sensing and regulatory pathways in aphids affect vitellogenins and juvenile hormone/ecdysone when aphids feed on plants with increased C:N ratios under elevated CO2.
Herbivorous insects can be affected by environmental change via changes in host physiology and chemical composition or via changes in competitors or natural enemies (Awmack and Leather, 2002). Elevated CO2 affects aphid performance from the level of individual physiology or even molecular function to the level of the ecosystem (Sun and Ge, 2011). The effects of elevated CO2 on individual plants and aphids may differ from the effects involving the entire ecosystem and multiple trophic levels because responses to elevated CO2 may differ among trophic levels. It is well known that elevated CO2 has bottom-up effects on the feeding behavior and population size of aphids, but the situation becomes more complicated when aphid–aphid interactions or top–down effects involving natural enemies are considered. For example, aphid species, or even different genotypes within the same species, differ in their responses to elevated CO2 (Mondor et al., 2005), and these differences might affect the outcome of intra- or inter-specific competition between aphid species or genotypes (Stacey and Fellowes, 2002; Sun et al., 2009). Furthermore, some reports indicate that parasitoids and predators are more abundant or effective under elevated CO2 (Percy et al., 2002; Chen et al., 2005) and that aphids are less sensitive to alarm pheromones under elevated CO2 (Awmack et al., 1997; Mondor et al., 2004). It seems that enhanced top-down effects on aphids under elevated CO2 may strongly alter the effects of aphids on host plants (Hentley et al., 2014).
The different feeding strategies evident in aphid responses to environmental changes are possibly driven by synchronous adaptation to host and environment. Because it directly affects herbivorous only weakly, elevated CO2 mainly influences herbivorous insect by altering the host plant (Yin et al., 2010). Thus, understanding plant–aphid interactions is likely to be central to understanding how aphids respond to elevated CO2. We suggest that molecular tools be used to better understand how the host plant ‘recognizes’ the aphid and vice versa; this research might focus on salivary secretions (the most obvious ‘signal’ available), which could trigger various molecular responses in the host that then affect the aphid in various ways. Although the knowledge from literatures shows that aphids may have species-specific molecular interaction with the hosts, it is believed that the genetics and physiology governing plant–aphid interactions have many commonalities rooted in their phylogenies so that understanding the complexity of interaction will provide meaningful insights into aphids acting on different kinds of plants and aid us in using them to our best advantage. Given increasing concentrations of atmospheric CO2 and climate change, new crop varieties will be needed that can produce sustainable yields in spite of the changing environment and the potential for increased pressure from aphids and other pests. The development of such crop varieties will be facilitated by a better understanding of the interactions between plants and aphids at molecular, community, and ecosystem levels.
Author Contributions
YS and HG wrote this article, FG revised it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB11050400) and the National Nature Science Fund of China (nos. 31500332 and 31221091).
References
Ainsworth, E. A., and Rogers, A. (2007). The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270. doi: 10.1111/j.1365-3040.2007.01641.x
Ainsworth, E. A., Rogers, A., Vodkin, L. O., Walter, A., and Schurr, U. (2006). The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 142, 135–147.
Alvarez, A. E., Tjallingii, W. F., Garzo, E., Vleeshouwers, V., Dicke, M., and Vosman, B. (2006). Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 121, 145–157. doi: 10.1111/j.1570-8703.2006.00464.x
Avé, D. A., Gregory, P., and Tingey, W. M. (1987). Aphid repellent sesquiterpenes in glandular trichomes of Solanum berthaultii and S. tuberosum. Entomol. Exp. Appl. 44, 131–138. doi: 10.1111/j.1570-7458.1987.tb01057.x
Awmack, C., Harrington, R., and Leather, S. (1997). Host plant effects on the performance of the aphid Aulacorthum solani (Kalt.) (Homoptera: Aphididae) at ambient and elevated CO2. Glob. Chang. Biol. 3, 545–549. doi: 10.1046/j.1365-2486.1997.t01-1-00087.x
Awmack, C. S., and Leather, S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844. doi: 10.1146/annurev.ento.47.091201.145300
Badisco, L., Van Wielendaele, P., and Broeck, J. V. (2013). Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 4:202. doi: 10.3389/fphys.2013.00202
Barbehenn, R. V., Chen, Z., Karowe, D. N., and Spickard, A. (2004). C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2. Glob. Chang. Biol. 10, 1565–1575. doi: 10.1111/j.1365-2486.2004.00833.x
Behmer, S. T. (2008). Insect herbivore nutrient regulation. Ann. Rev. Entomol. 54, 165–187. doi: 10.1146/annurev.ento.54.110807.090537
Bezemer, T. M., and Jones, T. H. (1998). Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82, 212–222. doi: 10.2307/3546961
Bezemer, T. M., Jones, T. H., and Knight, K. J. (1998). Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia 116, 128–135. doi: 10.1007/s004420050571
Bidart-Bouzat, M. G., Mithen, R., and Berenbaum, M. R. (2005). Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 145, 415–424. doi: 10.1007/s00442-005-0158-5
Bloom, A. J., Burger, M., Asensio, J. S. R., and Cousins, A. B. (2010). Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899–903. doi: 10.1126/science.1186440
Bos, J. I. B., Armstrong, M. R., Gilroy, E. M., Boevink, P. C., Hein, I., Taylor, R. M., et al. (2010a). Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc. Natl. Acad. Sci. U.S.A. 107, 9909–9914. doi: 10.1073/pnas.0914408107
Bos, J. I. B., Prince, D. C., Pitino, M., Maffei, M. E., Win, J., and Hogenhout, S. A. (2010b). A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (Green Peach Aphid). PLoS Genet. 6:e1001216. doi: 10.1371/journal.pgen.1001216
Casteel, C. L., Segal, L. M., Niziolek, O. K., Berenbaum, M. R., and DeLucia, E. H. (2012). Elevated carbon dioxide increases salicylic acid in Glycine max. Environ. Entomol. 41, 1435–1442. doi: 10.1603/EN12196
Chaudhary, R., Atamian, H. S., Shen, Z., Briggs, S. P., and Kaloshian, I. (2014). GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc. Natl. Acad. Sci. U.S.A. 111, 8919–8924. doi: 10.1073/pnas.1407687111
Chen, F. J., Ge, F., and Parajulee, M. N. (2005). Impact of elevated CO2 on tri-trophic interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environ. Entomol. 34, 37–46. doi: 10.1603/0046-225X-34.1.37
Choi, Y. E., Lim, S., Kim, H. J., Han, J. Y., Lee, M. H., Yang, Y., et al. (2012). Tobacco NtLTP1, a glandular-specific lipid transfer protein, is required for lipid secretion from glandular trichomes. Plant J. 70, 480–491. doi: 10.1111/j.1365-313X.2011.04886.x
Dáder, B., Fereres, A., Moreno, A., and Trębicki, P. (2016). Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6:19120. doi: 10.1038/srep19120
Daniels, M., Bale, J. S., Newbury, H. J., Lind, R. J., and Pritchard, J. (2009). A sublethal dose of thiamethoxam causes a reduction in xylem feeding by the bird cherry-oat aphid (Rhopalosiphum padi), which is associated with dehydration and reduced performance. J. Insect Physiol. 55, 758–765. doi: 10.1016/j.jinsphys.2009.03.002
DeLucia, E. H., Nabity, P. D., Zavala, J. A., and Berenbaum, M. R. (2012). Climate change: resetting plant-insect interactions. Plant Physiol. 160, 1677–1685. doi: 10.1104/pp.112.204750
Dinant, S., Bonnemain, J. L., Girousse, C., and Kehr, J. (2010). Phloem sap intricacy and interplay with aphid feeding. C. R. Biol. 333, 504–515. doi: 10.1016/j.crvi.2010.03.008
Docherty, M., Wade, F., Hurst, D., Whittaker, J., and Lea, P. (1997). Responses of tree sap-feeding herbivores to elevated CO2. Glob. Chang. Biol. 3, 51–59. doi: 10.1046/j.1365-2486.1997.00096.x
Douglas, A. E. (2003). The nutritional physiology of aphids. Adv. Insect Physiol. 31, 73–140. doi: 10.1016/S0065-2806(03)31002-1
Douglas, A. E. (2006). Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57, 747–754. doi: 10.1093/jxb/erj067
Febvay, G., Rahbé, Y., Rynkiewicz, M., Guillaud, J., and Bonnot, G. (1999). Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J. Exp. Biol. 202, 2639–2652.
Fisher, D. B., and Cash-Clark, C. E. (2000). Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 123, 125–138. doi: 10.1104/pp.123.1.125
Fuhrer, J. (2003). Agroecosystem responses to combinations of elevated CO2, ozone, and global climate change. Agric. Ecosyst. Environ. 97, 1–20. doi: 10.1016/S0167-8809(03)00125-7
Furch, A. C. U., Hafke, J. B., Schulz, A., and van Bel, A. J. E. (2007). Ca2+ mediated remote control of reversible sieve tube occlusion in Vicia faba. J. Exp. Bot. 58, 2827–2838. doi: 10.1093/jxb/erm143
Geiger, M., Walch-Liu, P., Engels, C., Harnecker, J., Schulze, E.-D., Ludewig, F., et al. (1998). Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ. 21, 253–268. doi: 10.1046/j.1365-3040.1998.00277.x
Gimenez-Ibanez, S., Boter, M., Fernández-Barbero, G., Chini, A., Rathjen, J. P., and Solano, R. (2014). The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 12:e1001792. doi: 10.1371/journal.pbio.1001792
Giordanengo, P., Brunissen, L., Rusterucci, C., Vincent, C., Van Bel, A., Dinant, S., et al. (2010). Compatible plant-aphid interactions: how aphids manipulate plant responses. C. R. Biol. 333, 516–523. doi: 10.1016/j.crvi.2010.03.007
Goffreda, J. C., Mutschler, M. A., Avé, D. A., Tingey, W. M., and Steffens, J. C. (1989). Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomato, Lycopersicon pennellii. J. Chem. Ecol. 15, 2135–2147. doi: 10.1007/BF01207444
Goławska, S. (2007). Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J. Chem. Ecol. 33, 1598–1606. doi: 10.1007/s10886-007-9333-y
Grewal, S. S. (2009). Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 41, 1006–1010. doi: 10.1016/j.biocel.2008.10.010
Gündüz, E. A., and Douglas, A. E. (2009). Symbiotic bacteria enable insect touse a nutritionally inadequate diet. Proc. R. Soc. Lond. B Biol. Sci. 276, 987–991. doi: 10.1098/rspb.2008.1476
Guo, H., Sun, Y., Li, Y., Liu, X., Wang, P., Zhu-Salzman, K., et al. (2014a). Elevated CO2 alters the feeding behaviour of the pea aphid by modifying the physical and chemical resistance of Medicago truncatula. Plant Cell Environ. 37, 2158–2168. doi: 10.1111/pce.12306
Guo, H., Sun, Y., Li, Y., Liu, X., Zhang, W., and Ge, F. (2014b). Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytol. 201, 279–291. doi: 10.1111/nph.12484
Guo, H., Sun, Y., Li, Y., Tong, B., Harris, M., Zhu-Salzman, K., et al. (2013). Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Glob. Chang. Biol. 19, 3210–3223. doi: 10.1111/gcb.12260
Guo, H., Sun, Y., Ren, Q., Zhu-Salzman, K., Kang, L., Wang, C., et al. (2012). Elevated CO2 reduces the resistance and tolerance of tomato plants to Helicoverpa armigera by suppressing the JA signaling pathway. PLoS ONE 7:e41426. doi: 10.1371/journal.pone.0041426
Han, Y., Wang, Y., Bi, J. L., Yang, X. Q., Huang, Y., Zhao, X., et al. (2009). Constitutive and induced activities of defense-related enzymes in aphid-resistant and aphid-susceptible cultivars of wheat. J. Chem. Ecol. 35, 176–182. doi: 10.1007/s10886-009-9589-5
Hentley, W. T., Vanbergen, A. J., Hails, R. S., Jones, T. H., and Johnson, S. N. (2014). Elevated atmospheric CO2 impairs aphid escape responses to predators and conspecific alarm signals. J. Chem. Ecol. 40, 1110–1114. doi: 10.1007/s10886-014-0506-1
Hettenhausen, C., Schuman, M. C., and Wu, J. (2015). MAPK signaling: a key element in plant defense response to insects. Insect Sci. 22, 157–164. doi: 10.1111/1744-7917.12128
Hogenhout, S. A., and Bos, J. I. (2011). Effector proteins that modulate plant–insect interactions. Cur. Opin. Plant Biol. 14, 422–428. doi: 10.1016/j.pbi.2011.05.003
Huberty, A. F., and Denno, R. F. (2004). Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85, 1383–1398. doi: 10.1890/03-0352
Jaouannet, M., Rodriguez, P. A., Thorpe, P., Lenoir, C. J., MacLeod, R., Escudero-Martinez, C., et al. (2014). Plant immunity in plant–aphid interactions. Front. Plant Sci. 5:663. doi: 10.3389/fpls.2014.00663
Jiang, Y., and Miles, P. W. (1993). Responses of a compatible lucerne variety to attack by spotted alfalfa aphid: changes in the redox balance in affected tissues. Entomol. Exp. Appl. 67, 263–274. doi: 10.1111/j.1570-7458.1993.tb01677.x
Johnson, S. N., Ryalls, J. M. W., and Karley, A. J. (2014). Global climate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 165, 62–72. doi: 10.1111/aab.12115
Karowe, D. N., and Grubb, C. (2011). Elevated CO2 increases constitutive phenolics and trichomes, but decreases inducibility of phenolics in Brassica rapa (Brassicaceae). J. Chem. Ecol. 37, 1332–1340. doi: 10.1007/s10886-011-0044-z
King, S. R. F., Mclellan, H., Boevink, P. C., Armstrong, M. R., Bukharova, T., Sukarta, O., et al. (2014). Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKKε to suppress plant immune signaling. Plant Cell 26, 1345–1359. doi: 10.1105/tpc.113.120055
Knoblauch, M., and van Bel, A. J. E. (1998). Sieve tubes in action. Plant Cell 10, 35–50. doi: 10.1105/tpc.10.1.35
Lake, J. A., and Wade, R. N. (2009). Plant–pathogen interactions and elevated CO2: morphological changes in favour of pathogens. J. Exp. Bot. 60, 3123–3131. doi: 10.1093/jxb/erp147
Lam, S. K., Chen, D., Norton, R., and Armstrong, R. (2012). Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop Pasture Sci. 63, 53–62. doi: 10.1071/CP11296
Lei, J., Finlayson, S. A., Salzman, R. A., Shan, L., and Zhu-Salzman, K. (2014). BOTRYTIS-INDUCED KINASE1 modulates Arabidopsis resistance to green peach aphids via PHYTOALEXIN DEFICIENT4. Plant Physiol. 165, 1657–1670. doi: 10.1104/pp.114.242206
Li, P., Ainsworth, E. A., Leakey, A. D., Ulanov, A., Lozovaya, V., Ort, D. R., et al. (2008). Arabidopsis transcript and metabolite profiles: ecotype-specific responses to open air elevated [CO2]. Plant Cell Environ. 31, 1673–1687. doi: 10.1111/j.1365-3040.2008.01874.x
Ma, W., Penrose, D. M., and Glick, B. R. (2002). Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 48, 947–954. doi: 10.1139/w02-100
Martin, P., and Johnson, S. N. (2011). Evidence that elevated CO2 reduces resistance to the European large raspberry aphid in some raspberry cultivars. J. Appl. Entomol. 135, 237–240. doi: 10.1111/j.1439-0418.2010.01544.x
Masle, J. (2000). The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiol. 122, 1399. doi: 10.1104/pp.122.4.1399
May, P., Liao, W., Wu, Y., Shuai, B., McCombie, W. R., Zhang, M. Q., et al. (2013). The effects of carbon dioxide and temperature on microRNA expression in Arabidopsis development. Nat. Commun. 4:2145. doi: 10.1038/ncomms3145
McKenzie, S. W., Hentley, W. T., Hails, R. S., Jones, T. H., Vanbergen, A. J., and Johnson, S. N. (2013). Global climate change and above-belowground insect herbivore interactions. Front. Plant Sci. 4:412. doi: 10.3389/fpls.2013.00412
McLellan, H., Boevink, P. C., Armstrong, M. R., Pritchard, L., Gomez, S., Morales, J., et al. (2013). An RxLR effector from Phytophthora infestans prevents localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 9:e1003670. doi: 10.1371/journal.ppat.1003670
Mondor, E. B., Tremblay, M., Awmack, C. S., and Lindroth, R. L. (2004). Divergent pheromone-mediated insect behaviour under global atmospheric change. Glob. Chang. Biol. 10, 1820–1824. doi: 10.1111/j.1365-2486.2004.00838.x
Mondor, E. B., Tremblay, M. N., Awmack, C. S., and Lindroth, R. L. (2005). Altered genotypic and phenotypic frequencies of aphid populations under enriched CO2 and O3 atmospheres. Glob. Chang. Biol. 11, 1990–1996.
Myers, S. S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, A. D. B., Bloom, A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510, 139–143. doi: 10.1038/nature13179
Nalam, V. J., Keeretaweep, J., Sarowar, S., and Shah, J. (2012). Root-derived oxylipins promote green peach aphid performance on Arabidopsis foliage. Plant Cell 24, 1643–1653. doi: 10.1105/tpc.111.094110
Neal, J. J., Tingey, W. M., and Steffens, J. C. (1990). Sucrose esters of carboxylic acids in glandular trichomes of Solanum berthaultii deter settling and probing by green peach aphid. J. Chem. Ecol. 16, 487–497. doi: 10.1007/BF01021780
Newman, J. A., Gibson, D. J., Hickam, E., Lorenz, M., Adams, E., Bybee, L., et al. (1999). Elevated carbon dioxide results in smaller populations of the bird cherry- oat aphid Rhopalosiphum padi. Ecol. Entomol. 24, 486–489. doi: 10.1046/j.1365-2311.1999.00210.x
Newman, J. A., Gibson, D. J., Parsons, A. J., and Thornley, J. H. M. (2003). How predictable are aphid population responses to elevated CO2. J. Anim. Ecol. 72, 556–566. doi: 10.1046/j.1365-2656.2003.00725.x
Nikoh, N., McCutcheon, J. P., Kudo, T., Miyagishima, S. Y., Moran, N. A., and Nakabachi, A. (2010). Bacterial genes in the aphid genome: absence of functional gene transfer from Buchnera to its host. PLoS Genet. 6:e1000827. doi: 10.1371/journal.pgen.1000827
Oehme, V., Högy, P., Zebitz, C. P., and Fangmeier, A. (2013). Effects of elevated atmospheric CO2 concentrations on phloem sap composition of spring crops and aphid performance. J. Plant Interact. 8, 74–84. doi: 10.1080/17429145.2012.736200
O’Neill, B. F., Zangerl, A. R., DeLucia, E. H., Casteel, C., Zavala, J. A., and Berenbaum, M. R. (2011). Leaf temperature of soybean grown under elevated CO2 increases Aphis glycines (Hemiptera: Aphididae) population growth. Insect Sci. 18, 419–425. doi: 10.1111/j.1744-7917.2011.01420.x
Penmetsa, R. V., and Cook, D. R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530. doi: 10.1126/science.275.5299.527
Penmetsa, R. V., Uribe, P., Anderson, J., Lichtenzveig, J., Gish, J. C., Nam, Y. W., et al. (2008). The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 55, 580–595. doi: 10.1111/j.1365-313X.2008.03531.x
Percy, K. E., Awmack, C. S., Lindroth, R. L., Kubiske, M. E., Kopper, B. J., Isebrands, J. G., et al. (2002). Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 420, 403–407. doi: 10.1038/nature01028
Pescod, K. V., Quick, W. P., and Douglas, A. E. (2007). Aphid responses to plants with genetically manipulated phloem nutrient levels. Physiol. Entomol. 32, 253–258. doi: 10.1111/j.1365-3032.2007.00577.x
Pompon, J., Quiring, D., Goyer, C., Giordanengo, P., and Pelletier, Y. (2011). A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 57, 1317–1322. doi: 10.1016/j.jinsphys.2011.06.007
Prince, D. C., Drurey, C., Zipfel, C., and Hogenhout, S. A. (2014). The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 164, 2207–2219. doi: 10.1104/pp.114.235598
Pritchard, J., Griffiths, B., and Hunt, E. J. (2007). Can the plant-mediated impacts on aphids of elevated CO2 and drought be predicted? Glob. Chang. Biol. 13, 1616–1629. doi: 10.1111/j.1365-2486.2007.01401.x
Rhodes, J., Croghan, P., and Dixon, A. (1996). Uptake, excretion and respiration of sucrose and amino acids in the pea aphid Acyrthosiphon pisum. J. Exp. Biol. 199, 1269–1276.
Robinson, E. A., Ryan, G. D., and Newman, J. A. (2012). A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 194, 321–336. doi: 10.1111/j.1469-8137.2012.04074.x
Ryalls, J. M., Moore, B. D., Riegler, M., Gherlenda, A. N., and Johnson, S. N. (2015). Amino acid-mediated impacts of elevated carbon dioxide and simulated root herbivory on aphids are neutralized by increased air temperatures. J. Exp. Bot. 66, 613–623. doi: 10.1093/jxb/eru439
Ryan, G. D., Emiljanowicz, L., Haerri, S. A., and Newman, J. A. (2014a). Aphid and host-plant genotype × genotype interactions under elevated CO2. Ecol. Entomol. 39, 309–315. doi: 10.1111/een.12101
Ryan, G. D., Shukla, K., Rasmussen, S., Shelp, B. J., and Newman, J. A. (2014b). Phloem phytochemistry and aphid responses to elevated CO2, nitrogen fertilization and endophyte infection. Agri. For. Entomol. 16, 273–283. doi: 10.1111/afe.12055
Ryan, G. D., Sylvester, E. V. A., Shelp, B. J., and Newman, J. A. (2015). Towards an understanding of how phloem amino acid composition shapes elevated CO2-induced changes in aphid population dynamics. Ecol. Entomol. 40, 247–257. doi: 10.1111/een.12181
Salt, D. T., Fenwick, P., and Whittaker, J. B. (1996). Interspecific herbivore interactions in a high CO2 environment: root and shoot aphids feeding on Cardamine. Oikos 77, 182–237. doi: 10.2307/3546072
Sharma, H. C., Sharma, K. K., Seetharama, N., and Ortiz, R. (2000). Prospects for using transgenic resistance to insects in crop improvement. Electron. J. Biotechnol. 3, 21–22. doi: 10.2225/vol3-issue2-fulltext-3
Spoel, S. H., and Loake, G. J. (2011). Redox-based protein modifications: the missing link in plant immune signalling. Cur. Opin. Plant Biol. 14, 358–364. doi: 10.1016/j.pbi.2011.03.007
Stacey, D. A., and Fellowes, M. E. (2002). Influence of elevated CO2 on interspecfic interactions at higher trophic levels. Glob. Chang. Biol. 8, 668–678. doi: 10.1046/j.1365-2486.2002.00506.x
Stocker, T. F., Qin, D., Plattner, G. K., Tignor, M., Allen, S. K., Boschung, J., et al. (2013). IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, MA: Cambridge University Press.
Sun, Y., and Ge, F. (2011). How do aphids respond to elevated CO2? J. Asia-Pac. Entomol. 14, 217–220. doi: 10.1016/j.aspen.2010.08.001
Sun, Y., Guo, H., Yuan, L., Wei, J., Zhang, W., and Ge, F. (2015). Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Chang. Biol. 21, 2739–2748. doi: 10.1111/gcb.12858
Sun, Y., Guo, H., Zhu-Salzman, K., and Ge, F. (2013). Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 210, 128–140. doi: 10.1016/j.plantsci.2013.05.014
Sun, Y., Su, J. W., and Ge, F. (2010). Elevated CO2 reduces the response of Sitobion avenae (Homoptera: Aphididae) to alarm pheromone. Agric. Ecosyst. Environ. 135, 140–147. doi: 10.1016/j.agee.2009.09.011
Sun, Y. C., Jing, B. B., and Ge, F. (2009). Response of amino acid changes in Aphis gossypii (Glover) to elevated CO2 levels. J. Appl. Entomol. 133, 189–197. doi: 10.1111/j.1439-0418.2008.01341.x
Teng, N., Wang, J., Chen, T., Wu, X., Wang, Y., and Lin, J. (2006). Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 172, 92–103. doi: 10.1111/j.1469-8137.2006.01818.x
Tjallingii, W. F., and Esch, T. H. (1993). Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 18, 317–328. doi: 10.1111/j.1365-3032.1993.tb00604.x
Urbanska, A., Leszczynski, B., Tjallingii, W. F., and Matok, H. (2002). Probing behaviour and enzymatic defence of the grain aphid against cereal phenolics. Electron. J. Polish Agric. Univ. Biol. 5,
Urbanska, A., Tjallingii, W. F., Dixon, A. F. G., and Leszczynski, B. (1998). Phenol oxidising enzymes in the grain aphid’s saliva. Entomol. Exp. Appl. 86, 197–203. doi: 10.1046/j.1570-7458.1998.00281.x
van Helden, M., and Tjallingii, W. F. (1993). Tissue localisation of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 68, 269–278. doi: 10.1111/j.1570-7458.1993.tb01713.x
Wang, E., Hall, J. T., and Wagner, G. J. (2004). Transgenic Nicotiana tabacum L. with enhanced trichome exudate cembratrieneols has reduced aphid infestation in the field. Mol. Breed. 13, 49–57. doi: 10.1023/B:MOLB.0000012328.04974.fb
Webb, A. A. R., McAinsh, M. R., Mansfield, T. A., and Hetherington, A. M. (1996). Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J. 9, 297–304. doi: 10.1046/j.1365-313X.1996.09030297.x
Wilkinson, T., Ashford, D., Pritchard, J., and Douglas, A. (1997). Honeydew sugars and osmoregulation in the pea aphid Acyrthosiphon pisum. J. Exp. Biol. 200, 2137–2143.
Will, T., Furch, A. C., and Zimmermann, M. R. (2013). How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 4:336. doi: 10.3389/fpls.2013.00336
Will, T., and van Bel, A. J. E. (2006). Physical and chemical interactions between aphids and plants. J. Exp. Bot. 57, 729–737. doi: 10.1093/jxb/erj089
Wullschleger, S. D., Tschaplinski, T. J., and Norby, R. J. (2002). Plant water relations at elevated CO2–implications for water-limited environments. Plant Cell Environ. 25, 319–331. doi: 10.1046/j.1365-3040.2002.00796.x
Xie, H., Zhao, L., Wang, W., Wang, Z., Ni, X., Cai, W., et al. (2014). Changes in life history parameters of Rhopalosiphum maidis (Homoptera: Aphididae) under four different elevated temperature and CO2 combinations. J. Econ. Entomol. 107, 1411–1418. doi: 10.1603/EC13302
Yin, J., Sun, Y., Wu, G., and Ge, F. (2010). Effects of elevated CO2 associated with maize on multiple generations of the cotton bollworm, Helicoverpa armigera. Entomol. Exp. Appl. 136, 12–20. doi: 10.1111/j.1570-7458.2010.00998.x
Zavala, J. A., Casteel, C. L., DeLucia, E. H., and Berenbaum, M. R. (2008). Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc. Natl. Acad. Sci. U.S.A. 105, 5129–5133. doi: 10.1073/pnas.0800568105
Zavala, J. A., Casteel, C. L., Nabity, P. D., Berenbaum, M. R., and DeLucia, E. H. (2009). Role of cysteine proteinase inhibitors in preference of Japanese beetles (Popillia japonica) for soybean (Glycine max) leaves of different ages and grown under elevated CO2. Oecologia 161, 35–41. doi: 10.1007/s00442-009-1360-7
Zavala, J. A., Nabity, P. D., and DeLucia, E. H. (2013). An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58, 79–97. doi: 10.1146/annurev-ento-120811-153544
Zhang, J., Xing, G. M., Liao, J. X., Hou, Z. D., Wang, G. X., and Wang, Y. F. (2003). Effects of different atmospheric CO2 concentrations and soil moistures on the populations of bird cherry-oat aphid (Rhopalosiphum padi) feeding on spring wheat. Eur. J. Entomol. 100, 521–530. doi: 10.14411/eje.2003.080
Keywords: elevated CO2, aphid, nitrogen metabolism, plant defenses, water potential, legumes
Citation: Sun Y, Guo H and Ge F (2016) Plant–Aphid Interactions Under Elevated CO2: Some Cues from Aphid Feeding Behavior. Front. Plant Sci. 7:502. doi: 10.3389/fpls.2016.00502
Received: 07 December 2015; Accepted: 29 March 2016;
Published: 13 April 2016.
Edited by:
Scott Nicholas Johnson, University of Western Sydney, AustraliaReviewed by:
James Michael William Ryalls, Hawkesbury Institute for the Environment, AustraliaJonathan A. Newman, University of Guelph, Canada
Copyright © 2016 Sun, Guo and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ge, Z2VmQGlvei5hYy5jbg==
†These authors have contributed equally to this work.
 Yucheng Sun
Yucheng Sun Huijuan Guo
Huijuan Guo Feng Ge*
Feng Ge*