
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 21 March 2016
Sec. Plant Breeding
Volume 7 - 2016 | https://doi.org/10.3389/fpls.2016.00332
This article is part of the Research Topic Mechanisms of abiotic stress responses and tolerance in plants: physiological, biochemical and molecular interventions View all 121 articles
Tea plants (Camellia sinensis L.) possess high genetic diversity that is important for breeding. One cultivar, Baijiguan, exhibits a yellow leaf phenotype, reduced chlorophyll (Chl) content, and aberrant chloroplast structures under high light intensity. In contrast, under low light intensity, the flush shoot from Baijiguan becomes green, the Chl content increases significantly, and the chloroplasts exhibit normal structures. To understand the underlying molecular mechanisms for these observations, we performed de novo transcriptome sequencing and digital gene expression (DGE) profiling using Illumina sequencing technology. De novo transcriptome assembly identified 88,788 unigenes, including 1652 transcription factors from 25 families. In total, 1993 and 2576 differentially expressed genes (DEGs) were identified in Baijiguan plants treated with 3 and 6 days of shade, respectively. Gene Ontology (GO) and pathway enrichment analyses indicated that the DEGs are predominantly involved in the ROS scavenging system, chloroplast development, photosynthetic pigment synthesis, secondary metabolism, and circadian systems. The light-responsive gene POR (protochlorophyllide oxidoreductase) and transcription factor HY5 were identified. Quantitative real-time PCR (qRT-PCR) analysis of 20 selected DEGs confirmed the RNA-sequencing (RNA-Seq) results. Overall, these findings suggest that high light intensity inhibits the expression of photosystem II 10-kDa protein (PsbR) in Baijiguan, thus affecting PSII stability, chloroplast development and chlorophyll biosynthesis.
Tea plants (Camellia sinensis L.), whose leaves are used to make a traditional beverage consumed worldwide, possess great genetic diversity, with some cultivars exhibiting unique leaf colors. Leaf albinism, a common phenomenon in many ornamental and crop species, is caused by either genetic mutations or dramatic environmental factors, such as low temperature or high light intensity (Peng et al., 2012; Song et al., 2014). Several albino tea plant mutants have been identified in China; these mutants are classified into two categories: temperature-sensitive and light-sensitive mutants (Du et al., 2008). Anji Baicha is a temperature-sensitive tea cultivar; when the environmental temperature is below 20°C, the newly developed young shoots of this cultivar exhibits a white phenotype because of defective chloroplasts and reduced chlorophyll (Chl) content (Du et al., 2008; Ma et al., 2012). In contrast, Baijiguan is a light-sensitive tea cultivar; under high light intensity, the newly developed leaves of this cultivar exhibit a yellow phenotype, whereas if this cultivar is transferred to low intensity light, its yellow shoots turn green within several days. Temperature-sensitive albino tea plants have been extensively studied (Du et al., 2009; Li et al., 2011; Ma et al., 2012; Wei et al., 2012; Xiong et al., 2013; Feng et al., 2014); however, few studies regarding light-sensitive albino tea varieties have been conducted.
Previous studies have demonstrated that the albino or yellow phenotype can result from multiple factors, including Chl biosynthesis or degradation (Lin et al., 2014), tetrapyrrole synthesis for heme and phytochromobilin (Yaronskaya et al., 2003), carotenoid biosynthesis, or degradation (Qin et al., 2007; Wang et al., 2009), chloroplast development and biogenesis (Peng et al., 2012; Su et al., 2012), signal transduction during chloroplast development (Sheng et al., 2014), disease resistance (Nasir and Riazuddin, 2008), reduced light-harvesting Chl proteins (LHCPs) (Asakura et al., 2008), and photo-oxidative stress (Miura et al., 2010; Zhan et al., 2014). Digital gene expression (DGE) analysis of the tea cultivar Anji Baicha identified several genes involved in Chl biosynthesis or chloroplast development (Ma et al., 2012). Gene expression analysis identified that several photosynthesis-related genes were found to be suppressed during the albino stage, but increased dramatically when the shoots turned green. These differentially expressed genes (DEGs) include violaxanthin de-epoxidase (VDE), light harvesting Chl a/b-binding protein (Lhcb), Rubisco small subunit (RbcS), and Mg-chelatase subunit H (ChlH) (Ma et al., 2012). The albino phenotype of Anji Baicha has been reported to be caused by dysfunctional Chl biosynthesis (Ma et al., 2015). These studies have revealed some of the molecular mechanisms of leaf color formation in temperature-sensitive cultivars. In contrast, few transcriptome analyses of light-sensitive tea cultivars have been performed. The light-sensitive yellow leaf phenotype is a valuable trait that can be used to explore the molecular mechanisms underlying pigment metabolism, chloroplast development and leaf color formation. Therefore, we conducted RNA sequencing (RNA-Seq) and DGE analysis of a light-sensitive cultivar. The DEGs were assigned to diverse functional categories, and Gene Ontology (GO) annotation and pathway analyses revealed many light- and chloroplast-enriched categories. Twenty putative genes related to the light-induced yellow phenotype were selected for quantitative real-time PCR (qRT-PCR) analysis, the qRT-PCR results were in consistent with the DGE data. This study reveals the dynamic changes in gene expression in response to shade treatment and provides valuable insights into the genetic and genomic regulation of chloroplast development and chlorophyll biosynthesis in C. sinensis.
C. sinensis (L.) O. Kuntze cv. Baijiguan was planted in the Germplasm Tea Repository (Imperial Tea Garden) at the Tea Research Institute, Wuyi Mountain, China. Compared with other green tea cultivars, Baijiguan exhibits a yellow leaf phenotype and significantly reduced Chl content. Fully expanded second leaves of this cultivar were collected from the flush shoots at the one-bud and four-leaf stages. For the light-shading experiment, half of the second leaf was covered with aluminum foil for 3–6 days, from October 10-16, 2013. After the shade treatment, the two parts of the leaf were separately harvested. All samples were divided into three portions. One portion was immediately frozen in liquid nitrogen, and then stored at −80°C for RNA isolation. The second portion was cut into thin strips and immediately fixed in glutaraldehyde solution (2.5%) for ultra-structural characterization. The third portion was used for pigment and enzyme activity assays. Pooled leaf samples (three leaves per plant) were collected from three randomly selected tea plants.
For light and temperature treatments, 2-year-old tea plants were grown in potting soil in MGC-350HPY-2 climatic-controlled cabinets (Shanghai Yiheng Instruments Co., Ltd., Shanghai, China). The temperature was set to 25°C during the light period (12 h) and to 15°C during the dark period, with 75% humidity (Figures S1A–D). For the light treatment, the plants were grown under low-light conditions (240 μmol m−2 s−1) and medium-light conditions (600 μmol m−2 s−1); control plants were grown in a field (high-light conditions, 1400–1600 μmol m−2 s−1, Figures S1E,F). For the temperature experiments, tea plants were grown at 15, 20, and 25°C with 75% humidity. The second leaves were collected for imaging and pigment assays.
Photosynthetic pigment content was quantified as described previously (Arnon, 1949; Wei et al., 2012). The normal green tea cultivar C. sinensis (L.) O. Kuntze cv. Rougui was used as a control for pigment and ultra-structural analyses. For Chl analysis, we first performed a quick measurement of the relative amount of Chl content using a SPAD-502PLUS Chl meter (Spectrum Technologies, Konica Minolta, Japan) according to a previous report (Akita et al., 2013). Then, total Chl and carotenoids were extracted and quantified using a TU-1810PC spectrophotometer (Purkinje General Instrument Co. Ltd., Beijing, China). Lutein content was measured according to a previous report (Pan et al., 2007), and further confirmed by comparison with an authentic compound (Sigma-Aldrich, St. Louis, MO, USA) using an E2695 HPLC system (Waters, USA). The assays were conducted in triplicate.
Samples for ultra-structural observation were prepared as described previously (Du et al., 2008). Fresh tea leaves were cut into small pieces (1.0 × 2.0 mm) with a scalpel and fixed in glutaraldehyde solution (2.5%, v/v) overnight at 4°C. Ultrathin sections (70–90 nm) were prepared using a LKB Nova Ultra-microtome (LKB, Bromma, Sweden), examined and imaged using a JEM2100HC transmission electron microscope (JEOL, Tokyo, Japan).
Fresh leaves (0.5 g) were ground into a fine powder in liquid nitrogen, and then 4.5 mL phosphate-buffered saline (pH 7.4, 0.1 M) was then added to the leaves. The mixture was stirred on ice for 10 min and centrifuged at 5000 rpm for 10 min at 4°C. The supernatant was collected for enzyme activity assays. Superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) were assayed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and a TU-1810PC UV-visible spectrophotometer (Persee, Beijing, China) according to the manufacturers' instructions and a previous report (Li H. X. et al., 2013).
For Illumina HiSeq, total RNA was isolated from each sample using TRlzol reagent (Invitrogen™ Life Technologies, CA, USA) according to the manufacturer's recommendations and then purified using a Qubit® RNA Assay Kit with a Qubit® 2.0 Fluorometer (Life Technologies, CA, USA). RNA integrity was assessed using a 2100 Bio analyzer system (Agilent Technologies, CA, USA), with a minimum integrity value of 8.1. RNA degradation and contamination were monitored by 1% agarose gel electrophoresis. For RNA-Seq and DGE analyses, two biological replicates were used. Samples were harvested after the plants were shaded for 3 days (designated T3d_Z_1 and T3d_Z_2, T3d_W_1 and T3d_W_2) or 6 days (designated T6d_Z_1 and T6d_Z_2, T6d_W_1 and T6d_W_2). Total RNA (3 μg) per sample was pooled for 100-bp paired-end transcriptome sequencing. Four-microliter aliquots of each of the eight samples were used for expression profile analysis. cDNA library construction and Illumina sequencing were performed by Novogene Bioinformatics Technologies Co., Ltd. (Beijing, China).
Sequencing libraries were generated using a NEBNext Ultra RNA Library Prep Kit for Illumina® (NEB, USA), and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from 3 μg total RNA using poly-T oligo-attached magnetic beads. Fragmentation was performed using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). First-strand cDNA was synthesized using random hexamer primers, and second-strand cDNA was subsequently synthesized. Double-stranded cDNA was amplified by PCR with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. The PCR products were purified (AMPure XP system), and the library quality was assessed using an Agilent Bio analyzer 2100 system. The library preparations were sequenced from both the 5′ and 3′ ends using the Illumina HiSeq™ 2000 platform, and 100-bp paired-end reads were generated.
Raw sequencing reads were evaluated, and a unigene library was generated for C. sinensis. Raw data were preprocessed using in-house Perl scripts. Adaptor sequences, duplicated sequences, poly-N reads containing more than 10% “N,” and low-quality reads containing more than 50% bases with Q ≤ 5 were removed to obtain high-quality clean reads. After low-quality and ambiguous nucleotides were trimmed, de novo assemblies were created from the clean reads using Trinity (Grabherr et al., 2011). The data generated from the mixed samples were used to construct a whole transcriptome, which was used as a reference for further gene expression analysis.
For functional gene annotations, all non-redundant transcripts (≥ 200 bp) were used to search against public databases, including the National Center for Biotechnology Information (NCBI) non-redundant protein (Nr) and nucleotide (Nt) collections, Protein family (Pfam), Swiss-Prot, EuKaryotic Orthologous Groups (KOG), and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, with a significance threshold of E ≤ 10−5. For GO functional annotation analysis, GO terms were annotated according to molecular function, biological process and cellular component ontologies using Blast2GO software (Gotz et al., 2008).
For DGE analysis, the unigene dataset generated from the C. sinensis cv. Baijiguan transcriptome was used as a reference database. Clean reads were mapped to the reference sequences, and annotation information was obtained for each sample using RSEM software (Li and Dewey, 2011). To provide a relative assessment of transcript abundance, the fragments per kilobase of exon model per million mapped reads (FPKM) value was used as a measure of normalized gene expression (Mortazavi et al., 2008; Jakhesara et al., 2013). For samples with biological replicates, DEGs were identified with the DESeq R package using |log2(fold change)| ≥ 1 and a corrected P < 0.01 as the threshold for significant differential expression (Li C. et al., 2013; Sun et al., 2014). P-values were adjusted as described previously by Benjamini and Hochberg (1995).
GO enrichment analysis of all DEGs was implemented using the GOseq R package based on Wallenius non-central hyper-geometric distribution (Young et al., 2010). GO terms were assigned to the up- and down-regulated DEGs, with a corrected P = 0.01. For pathway enrichment analysis, all DEGs were mapped to pathways in the KEGG database using KOBAS software to identify significantly enriched KEGG pathways (Mao et al., 2005; Kanehisa et al., 2008). DEGs were considered significantly enriched in a metabolic pathway at a q ≤ 0.05 compared with the whole transcriptome background (Kanehisa et al., 2006).
To validate the results of RNA-Seq and DGE analyses, 20 DEGs associated with the light-harvesting complex, photosynthetic pigment, flavonoid biosynthesis and peroxisome pathways were selected for qRT-PCR. Specific primers were designed using Primer 5.0; the primer pair sequences are listed in Table S1. First-strand cDNAs (10-fold dilution) synthesized from RNA extracted from the second leaves were used as templates. qRT-PCR was performed using a SYBR Premix Ex Taq Kit (TaKaRa, Dalian, China) and an Eco™ Real-Time PCR System (Illumina, USA) according to the manufacturers' instructions. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was utilized as a loading control, and diethylpyrocarbonate (DEPC)-treated water in place of the template served as a negative control. PCR reaction efficiency was assayed for each primer set using a 10-fold dilution of cDNA. Each reaction was performed in triplicate along with an internal control reaction. Relative gene expression levels were calculated according to the 2−ΔΔCt comparative CT method (Livak and Schmittgen, 2001). The qRT-PCR and DGE analysis results are presented as fold changes in gene expression relative to the control samples. Therefore, the relative values of control samples were 1, and the relative values of the T3d_Z and T6d_Z samples were normalized to those of the control samples (T3d_W and T6d_W).
T-test was performed using the SPSS 17.0 program (SPSS Inc., Chicago, USA). The letters following the values listed in the tables indicate significant differences (capital letters represent P < 0.05, and lower case letters represent P < 0.01). Significant differences are indicated with either one asterisk (P < 0.05) or two asterisks (P < 0.01) in the figures. All data are presented as the means ± standard error (SE, n = 3).
Baijiguan plants showed an unusual leaf phenotype. In early spring, the first flush shoots were yellowish compared with a normal green tea cultivar (Figure 1A). In late spring, leaves of the Baijiguan plants retained a yellow phenotype at the one-bud and three-leaf stages, whereas leaves of most cultivars were dark green in color. On young shoots at the one-bud and four-leaf stages, the fourth leaf from the previous season gradually turned green, whereas the flush shoots remained yellow as the temperature and light intensity increased during the season. This phenomenon has also been reported for Huangjinya variety (Wang K. R. et al., 2013; Feng et al., 2014).
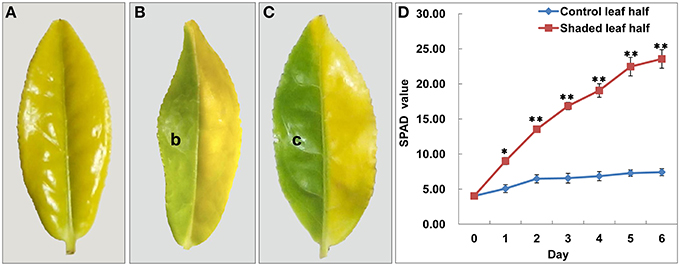
Figure 1. Shade treatment affected the leaf phenotype and Chl content in Baijiguan. (A) Phenotype of Baijiguan grown under field conditions. (B) Phenotype after 3 days of shading (b sector). The pale green leaf half was designated T3d_Z, and the control was designated T3d_W. (C) Phenotype after 6 days of shading (c sector). The green leaf half was designated T6d_Z, and the control was designated T6d_W. (D) Changes of relative chlorophyll content during the 6 days of shade treatment. All data points are presented as the means ± SE (n = 3).
To characterize the leaf color phenotype of Baijiguan, we compared its pigment content with that of Rougui, a normal green cultivar (Table S2). Under high light intensity conditions, the total Chl, Chl a, Chl b, and carotenoid contents of Baijiguan were significantly lower than those of Rougui; however, the ratios of Chl a/Chl b and carotenoid/total Chl in Baijiguan were higher than those in Rougui.
To test whether the yellow shoot phenotype of the Baijiguan variety is dependent on environmental factors, we grew the plants under different light and temperature conditions. Under low-light conditions, the leaf color of Baijiguan did not differ from that of the normal green cultivar. However, Baijiguan displayed green-yellow and yellow shoots under medium- and high- light intensity conditions, respectively (Figures S1A-F). The total Chl and carotenoid content also showed a decreasing trend as the light intensity increased (Figure S1G). In contrast, the yellow phenotype of Baijiguan was not affected when the plants were grown at different temperatures (15, 20, and 25°C).
To further confirm that the yellow leaf phenotype may result from exposure to high light intensity conditions, we conducted a light-shading experiment and observed that the sunlight-shielded portion of the leaf turned green, whereas the control portion remained yellow (Figures 1B,C). These observations demonstrated that Baijiguan is a light-hypersensitive variety.
Under shade conditions, the leaves of Baijiguan rapidly turned green, coinciding with the biosynthesis of Chl a, Chl b, and carotenoids (Table 1). The SPAD value, which reflects the visual perception of color differences, also increased by ~Six-fold in the shaded leaf half compared with the control half (Figure 1D). In contrast, the lutein content significantly decreased after 3 and 6 days of shade treatment (Table 1). These results indicated that the yellow phenotype in Baijiguan resulted from pigment composition changes.
To better understand the structural basis of the yellow phenotype, the leaf ultra-structures of C. sinensis cv. Baijiguan and Rougui were compared (Figure 2). Under high-intensity light, chloroplasts in Rougui developed a typical membrane system, with grana connected by stroma lamellae (Figures 2C,D). However, the grana stacks were less dense in Baijiguan compared with those in Rougui, and the thylakoid membrane system and spacing were disrupted (Figures 2A,B). Thus, the Baijiguan cultivar showed defects in chloroplast development.
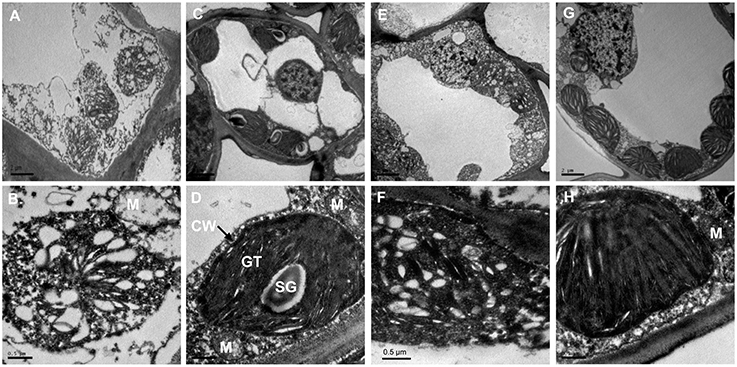
Figure 2. Chloroplast ultra-structural comparison between Baijiguan and Rougui under high-light and shade conditions. (A,B) Chloroplast ultra-structures in Baijiguan under high-light conditions. (C,D) Chloroplast ultra-structures in Rougui under high-light conditions. Chloroplasts in Rougui exhibit typical stacks and abundant starch granules compared with those in Baijiguan. (E,F) Chloroplast ultra-structures in the non-shaded part of Baijiguan at 6 days of shading. (G,H) Chloroplast ultra-structures in the shaded part of Baijiguan at 6 days of shading. Bar = 2 μm in (A), (C), (E), and (G); 0.5 μm in (B), (D), (F), and (H). SG: starch granule, GT: grana thylakoid, M: mitochondria, and CW: cell wall.
Under shade conditions, the chloroplast ultra-structure of the control leaf half showed disordered grana stacking, similar to that of the yellow leaf under high light conditions (Figures 2E,F). After 6 days of shading, the chloroplasts exhibited stacked thylakoids that were distributed around the cell wall similar to common green cultivars, except that fewer starch grains were found in Baijiguan (Figures 2G,H). These results indicated that the yellow phenotype in Baijiguan was induced by high light intensity and could be rescued by reducing the light intensity.
Antioxidant enzyme activities of SOD, CAT and POD at different leaf positions under high light conditions and in the second leaf under shade treatments were analyzed. Under high light conditions, SOD and CAT activities in the third and fourth leaves were higher than in the second leaf, whereas POD activity showed no significant changes. Overall, the antioxidant enzyme activities in the second leaf were the lowest compared with the other analyzed leaf positions (Figure 3A). Under shade treatment, SOD activity increased significantly, by ~10-fold compared to the control after 1 day of shading, and CAT activity was 2 times higher than the control value (Figures 3C,D). The activities of both SOD and CAT activities were decreased after 2 and 3 days of shading. Following 6 days of shading, slightly higher POD activity was observed (Figure 3B). These results indicated that the second leaf of Baijiguan might experience much higher oxidative stress under high light intensity conditions.
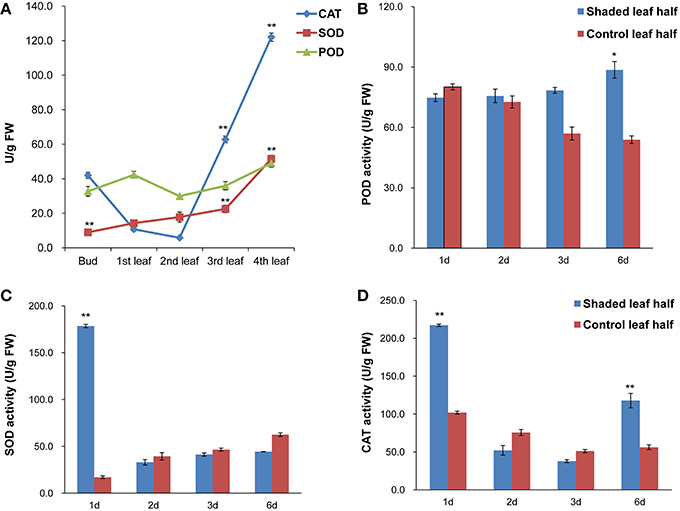
Figure 3. Antioxidant enzyme activities in C. sinensis cv. Baijiguan. (A) Antioxidant enzyme activities at different leaf positions under high-light conditions. The second leaf was utilized as the control. (B) Effects of shading on POD activity. (C) Effects of shading on SOD activity. (D) Effects of shading on CAT activity. * Represents a significant difference (P < 0.05) between the control and shade treatment. ** Represents a highly significant difference (P < 0.01) between the control and shade treatment. In Panel (A), other leaf position compared to the second leaf position, and in Panels (B–D), T3d_Z compared to T3d_W and T6d_Z compared to T6d_W.
To obtain an overview of transcriptome remodeling during shade treatments, a cDNA library was constructed using equal amounts of RNA from the second leaves of plants after 3 days (Figure 1B) and 6 days (Figure 1C) of shading. In total, we obtained 16.1 Gb of clean data (accounting for 97.34% of the raw data) from paired-end reads, with a single read length of ~100 bp and a Q20 percentage of over 97%. These clean reads were assembled into 88,788 unigenes representing 170,201 assembled unique transcripts, with an average length of 641 bp and an N50 length of 1021 bp (Table 2). Among these unigenes, 29,856 genes are longer than 500 bp and accounted for 33.63% of all unigenes. Additionally, 14,547 genes are longer than 1.0 kb and accounted for 16.38% of all unigenes. This dataset has been deposited at NCBI Short Read Archive (SRA) (accession number: SRX1078570).
To predict the functions of the assembled transcripts, the 88,788 unigenes were annotated by searching the Nr, Nt, Pfam, KOG, Swiss-Prot, GO, and KEGG public databases (Table 3). In total, transcriptome sequencing of all eight samples resulted in the annotation of 35,703 unigenes, accounting for 40.21% of all unigenes. Among these unigenes, 34.97% were annotated to the Nr databases. Of the Nr annotated unigenes, 46.67% had close homology to Vitis vinifera, followed by Populus trichocarpa and Ricinus communis (Figure S2).
GO analysis resulted in the classification of 24,846 unigenes into 47 sub-categories (Figure S3). Within the biological process category, “cellular process” (14,960 unigenes, 60.21%), and “metabolic process” (13,949 unigenes, 56.14%) were the top two GO terms. The most highly represented molecular function terms were “binding” (14,244 unigenes, 57.33%), “catalytic activity” (11,937 unigenes, 48.04%), and “transporter activity” (1773 unigenes, 7.14%). The top three GO terms in the cellular component category included “cell” (8975 unigenes, 36.12%), “cell part” (8946 unigenes, 13.00%), and “organelle” (6366 unigenes, 25.62%).
A total of 10,213 unigenes were subdivided into 26 groups according to the KOG database (Figure S4). Among these classifications, the “general functional prediction only” cluster (1984 unigenes, 19.43%) represented the largest group, followed by “post-translational modification, protein turnover, chaperone” (1345 unigenes, 13.17%), “signal transduction” (870 unigenes, 8.52%), and “translation” (705 unigenes, 6.90%). In addition, “lipid metabolism” and “cell wall/membrane/envelope biogenesis,” which are closely related to cell structure, were annotated.
KEGG provides a network diagram of cell metabolic pathways. A total of 8561 unigenes were assigned to specific pathways (Figure S5). The top enriched pathways were “carbohydrate metabolism” (792 unigenes), “translation” (772 unigenes), “folding, sorting and degradation” (640 unigenes), “signal transduction” (626 unigenes), and “energy metabolism” (621 unigenes).
TFs play significant roles in plant development and response to environmental stimuli. In this study, 1652 TFs were identified and categorized into 25 different common families (Figure 4). The MYB TF family was the most predominant (137 TFs, 8.29%), followed by the AP2 domain (126 TFs, 7.63%), homeobox (HB, 122 TFs, 7.38%), zinc finger (119 TFs, 7.20%), bZIP (112 TFs, 6.78%), and bHLH (107 TFs, 6.48%) families. The identification of these TFs provides opportunities for understanding their functions in light-regulated chloroplast development.
DGE analysis of T3d_Z, T3d_W, T6d_Z, and T6d_W was performed using an Illumina HiSeq™ 2000 sequencing platform with two biological replicates; the Pearson correlation coefficients between these two replicates ranged from 0.913 to 0.926 (Robles et al., 2012; Herzel and Neugebauer, 2015). A total of 1.59 Gb to 2.1 Gb of clean data was obtained, accounting for 99.7% of the raw data for each sample. The clean reads were mapped to reference sequences derived from the C. sinensis transcriptome data. The total proportion of mapped reads ranged from 91.05 to 92.42% (Table S3).
Two-group analysis was performed to identify C. sinensis genes differentially expressed after three and 6 days of shade treatment (Figure 5). The number of down-regulated genes exceeded the number of up-regulated genes following this treatment. As the shading time increased, the number of DEGs in C. sinensis gradually increased from 1993 to 2576. After 6 days of shading, a 1.29-fold increase in the number of induced DEGs was observed compared with that detected after 3 days of shading. Specifically, the up- and down-regulated genes increased in number by 4 and 579, respectively.
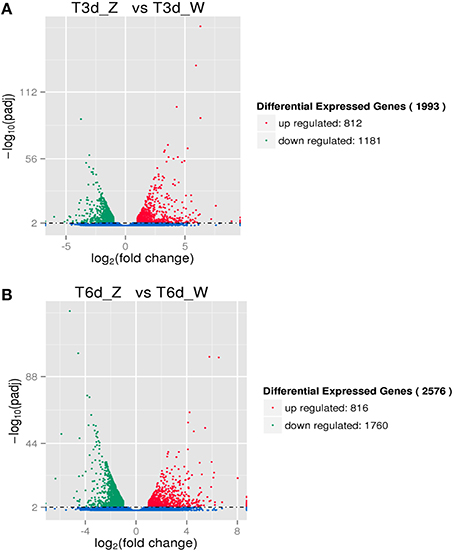
Figure 5. DEGs due to shade treatment. (A) Differentially expressed transcripts after 3 days of shading. (B) Differentially expressed transcripts after 6 days of shading.
The two shading treatment groups were subjected to multi-group differential expression analysis. The differentially co-expressed genes in the treatment and control groups are identified and illustrated in the Venn diagram shown in Figure 6. A total of 653 unique DEGs were identified in the T3d_Z vs. T3d_W group (346 up- and 307 down-regulated DEGs, Figure 6A), and 1236 unique DEGs were identified in the T6d_Z vs. T6d_W group (350 up- and 886 down-regulated). A comparison of the differentially co-expressed genes between the T6d_Z vs. T6d_W and T3d_Z vs. T3d_W groups resulted in the identification of 1340 DEGs (466 up- and 874 down-regulated DEGs, Table S4). Considering that the leaf color became greener with increased shading time (Figures 1B,C), these differentially co-expressed genes in the two shade-treated groups may play important roles in light-responsive regulation, leaf color formation and metabolic networks.
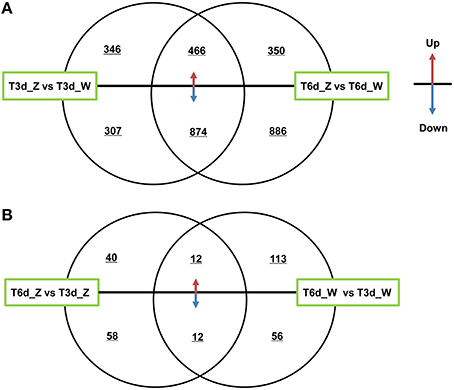
Figure 6. Differentially co-expressed genes at different time points after shading. (A) A comparison between the T3d_Z vs. T3d_W and T6d_Z vs. T6d_W groups. (B) A comparison between the T6d_Z vs. T3d_Z and T6d_W vs. T3d_W groups. The up or down arrows revealed that DEGs were up- or down-regulated.
Among the DEGs between the control and shade-treated groups, including both the T3d_Z vs. T3d_W and T6d_Z vs. T6d_W groups, 81 genes encoding putative TFs in C. sinensis were identified. Most of these TFs were classified into the bHLH (11), MYB (10), zinc finger (6), AP2 domain (5), bZIP (3), and homeobox (3) families (Table S5). MYB and bHLH TFs have crucial roles in secondary metabolism (particularly flavonoid biosynthesis) and abiotic stress (Roig-Villanova et al., 2007; Zhao et al., 2012; Wang et al., 2015).
The DEGs in the control and treatment groups were analyzed, and 98 unique DEGs were identified in the T6d_Z vs. T3d_Z group (40 up- and 58 down-regulated DEGs, Figure 6B). These data provide clear evidence that the genes identified as up-regulated are associated with oxidoreductase activity and that the down-regulated genes are associated with the light-harvesting complex and Chl A/B binding protein. These genes can be considered as candidate light-responsive genes in future studies (Table S6).
A comparison of the 3-day shaded samples with the control revealed 1993 DEGs, including 1181 down-regulated and 812 up-regulated genes, which were assigned to relevant functional terms. The top significantly enriched GO terms in the biological process category were “metabolic process,” “single-organism metabolic process,” and “biosynthetic process”; those in the cellular component category were “intracellular organelle” and “organelle”; and that in the molecular function category was “oxidoreductase activity” (Figure S6A). A comparison of the 6-day shaded samples with the control revealed 2576 DEGs, including 1760 down-regulated and 816 up-regulated genes; these DEGs were assigned to relevant functional terms. The results for the biological process and molecular function categories were similar to those of the above comparison, whereas “cytoplasm” and “cytoplasmic part” were significantly overrepresented in the cellular component category (Figure S6B). Overall, these data suggested that the terms “metabolic process,” “oxidoreductase activity,” “cytoplasm,” and “organelle” are strongly affected by shading. Other down-regulated GO terms, including “plastid,” “chloroplast,” and “thylakoid,” were highly enriched among the DEGs, supporting the efficiency of the light treatments and the reliability of the gene expression data.
All of the GO terms assigned to the group of up-regulated genes converged into two main categories (Figures S6C,D): “oxidation-reduction processes” in biological process and “oxidoreductase activity” in molecular function. Therefore, yellow leaves are highly susceptible to oxidative damage.
To determine whether light-responsive genes were enriched, the KEGG pathway database was searched using the DEGs to reveal the top 20 significantly enriched pathways (Figure 7 and Table S7). The following KEGG pathways were enriched after the 3-day shade treatment: 43 DEGs were enriched in “ribosome,” 29 DEGs were related to “carbon metabolism,” and 17 DEGs were related to “flavonoid biosynthesis” (Figure 7A), corresponding to ~12.8, 13.4, and 43.6% of the total DEGs, respectively. More than 20% of the DEGs were found to be associated with the “photosynthesis,” “porphyrin and Chl metabolism,” and “carotenoid biosynthesis” pathways, suggesting that low-light stress affects plant photosynthesis (Ho et al., 2009; Li C. et al., 2013). Similar results were observed for the 6-day shaded sample, in which more than 38.0% of the genes were enriched for “photosynthesis,” “porphyrin and Chl metabolism,” and “carotenoid biosynthesis” (Figure 7B). In addition, the “nitrogen metabolism,” “phenylpropanoid biosynthesis,” “glutathione metabolism,” “peroxisome,” and “circadian rhythm-plant” pathways were enriched in both groups, and these enrichments increased with time. Notably, 12 genes related to the light-harvesting complex were enriched in the “photosynthesis-antenna proteins” pathway in the 6-day shade group. Genes related to “plant hormone signal transduction,” “terpenoid backbone biosynthesis,” and “fatty acid degradation” were up-regulated, and these pathways were enriched in both the T3d_Z vs. T3d_W and T6d_Z vs. T6d_W groups (Figure S7), suggesting that they might be involved in the response to shading and in the formation of leaf color.
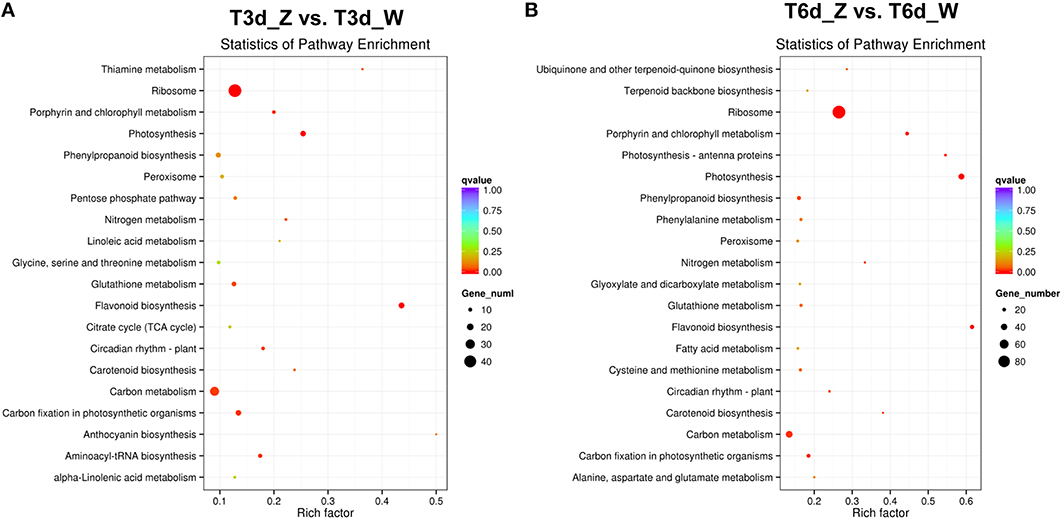
Figure 7. Top 20 enriched KEGG pathways among the annotated DEGs across two comparisons. (A) T3d_Z vs. control T3d_W; and (B) T6d_Z vs. control T6d_W. The Y-axis on the left represents KEGG pathways, and the X-axis indicates the enrichment factor. Low q-values are shown in red, and high q-values are depicted in blue.
Clustering analysis and heat map results for four representative pathways (photosynthetic pigment, flavonoid biosynthesis, glutathione metabolism and peroxisome) indicated clustering of the two treatment groups and of the two control groups (Figure 8). Overall, the shaded leaf portions appeared to lack gene products compared with the non-shaded portions. Notably, among those DEGs, POR (comp66695_c0, fold change = 3.23 in the 3-day shade group), encoding the Chl metabolism enzym protochlorophyllide oxidoreductase, and PsbR (comp38841_c0, fold change = 5.22 in the 3-day shade group, fold change = 4.03 in 6-day shade group), encoding the photosynthesis-related photosystem II 10-kDa protein, were significantly up-regulated; other DEGs were down-regulated (Table S4).
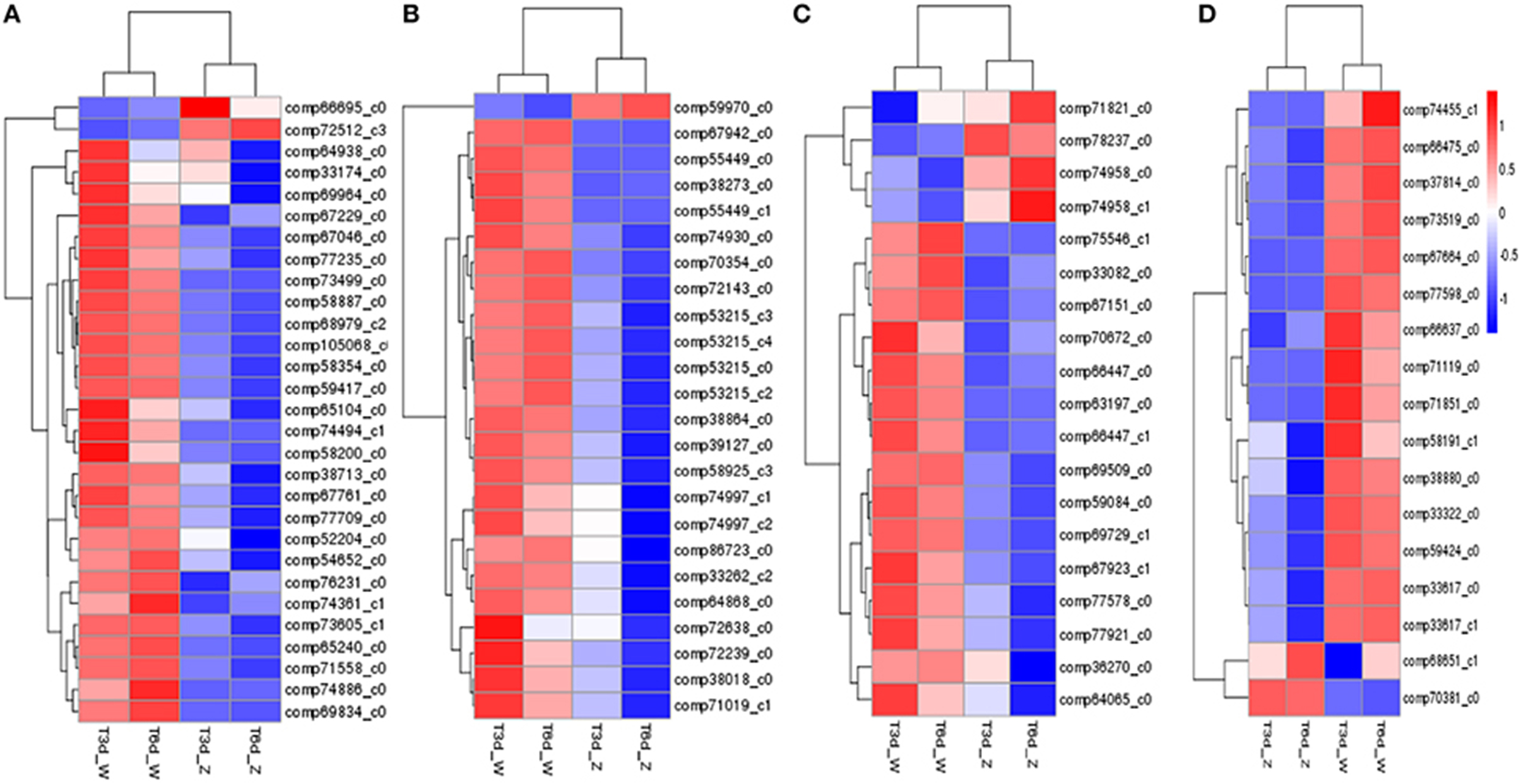
Figure 8. Expression profile clustering at different time points. (A) Photosynthetic pigment; (B) flavonoid biosynthesis; (C) glutathione metabolism; (D) peroxisome pathway. Expression ratios are expressed as log10 values, and each horizontal bar represents a single gene.
In summary, the transcriptome data were consistent with the Baijiguan phenotype. The abnormal leaf color may result from changes in the expression of genes involved in pigment biosynthesis and reactive oxygen species (ROS) scavenging.
Among all the identified DEGs, those genes involved in the light-harvesting complex, and those genes related to porphyrin and Chl metabolism, carotenoid biosynthesis, flavonoid biosynthesis and peroxisome are closely related to the observed changes in leaf color during shade treatment. Twenty of these genes were selected for qRT-PCR analysis. In the 3-day shade group, the expression patterns of 19 genes, which were detected by qRT-PCR, were similar to those observed in the DGE data, whereas the JAZ gene showed a different expression pattern (Figure 9A). In the 6-day shade group, three genes (COP1, ChlD, and ChlI) showed different expression patterns compared with the DGE data (Figure 9B). In general, the qRT-PCR data were consistent with the Illumina sequencing results, suggesting that the RNA-Seq data are reliable.
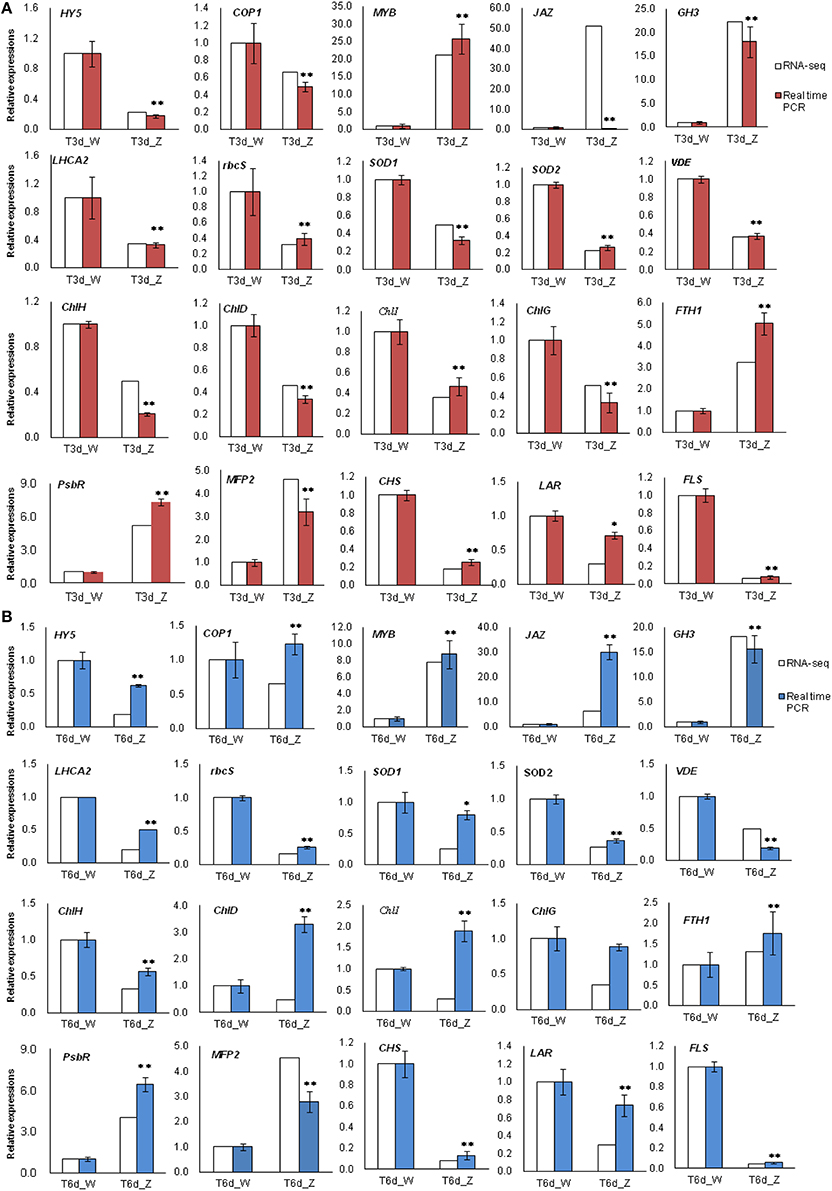
Figure 9. Real-time PCR results confirmed differentially expressed transcripts identified by RNA-Seq. Twenty genes involved in the light response were selected for validation. (A) T3d_Z compared to control T3d_W and (B) T6d_Z compared to control T6d_W. Data are normalized to GAPDH expression and are presented as the means ± SE (n = 3). * Represents a significant difference (P < 0.05) between the control and shade treatment. ** Represents a highly significant difference (P < 0.01) between the control and shade treatment.
The color variation of tea leaves has attracted much attention because the special chemical composition of these leaves affect tea quality. Light-shading experiments and ultra-structural observations have suggested that Baijiguan plants are hypersensitive to high-intensity light and have defects in Chl synthesis and chloroplast development. Therefore, this cultivar is an ideal system for studying the molecular mechanisms underlying leaf color formation.
The development of yellow leaves is related to pigment metabolism and chloroplast development. Leaf tissue has three major pigments: Chls, carotenoids and flavonoids. Chls are essential for photosynthesis, as these pigments are responsible for harvesting energy, charge separation and electron transport in antenna systems. Carotenoids, which are primarily synthesized in chloroplast membrane, form photosynthetic complexes and play critical roles in protecting Chl from destruction. Photosynthetic pigment measurements revealed that the yellow phenotype of Baijiguan positively correlated with the contents of Chls and carotenoids, particularly Chl a and Chl b (Table 1). Our results indicated that the yellow phenotype of Baijiguan might result from deficient Chl and carotenoid contents.
DEGs involved in carotenoid biosynthesis were identified based on the transcriptome and DGE data, and all 8 DEGs were down-regulated. One of these DEGs, VDE, which is involved in the xanthophyll cycle, which has a critical function in the photoprotection of photosystem II (Jahns and Holzwarth, 2012). Lutein is the most abundant xanthophyll and contributes to light harvesting by transferring excitation energy to Chl in higher plants. The lutein content was significantly decreased under shade conditions (Table 1). Moreover, the observed changes in the lutein content coincided with the VDE expression, thus suggesting that the xanthophyll cycle still functions normally under high-light intensity conditions and is not the cause for the yellow phenotype.
DEGs involved in Chl biosynthesis were also identified. Chl metabolism is a highly coordinated process that is catalyzed by numerous enzymes, particularly Mg-chelatase and protochlorophyllide reductase, via a series of cooperative reactions from glutamyl-tRNA to Chl a and Chl b (Zhang et al., 2006; Sakuraba et al., 2013). Mg-chelatase, which includes the subunits ChlD, ChlH, and ChlI, plays an important role in Chl production. Indeed, plants lacking Mg-chelatase show defective Chl and exhibit a yellow leaf phenotype (Jung et al., 2003). Thus, we examined the expression profiles of CsChlH, CsChlD, and CsChlI in the DGE data. Analysis of T3d_Z vs. T3d_W revealed that CsChlH (fold change = 0.49), CsChlI (fold change = 0.36), and CsChlD (fold change = 0.46) were down-regulated in Baijiguan. Analysis of T6d_Z vs. T6d_W showed that CsChlH (fold change = 0.33), CsChlI (fold change = 0.30), and CsChlD (fold change = 0.47) were also down-regulated. The expression levels of the three subunits of Mg-chelatase were not disrupted in Baijiguan; thus, they are not the causes of the yellow shoot phenotype. However, POR and ChlP, which encode protochlorophyllide oxidoreductase and a geranylgeranyl reductase, respectively, and which are essential for Chl synthesis under high light intensity conditions (Sakuraba et al., 2013; Zhou et al., 2013), were differentially expressed and identified in RNA-Seq data (comp66695_c0 and comp52204_c0). One study found that a light-induced yellow leaf mutant of rice is hypersensitive to high light intensity conditions and is defective in Chl synthesis. The light-induced leaf gene encoding geranylgeranyl reductase affects chlorophyll biosynthesis and light sensitivity (Zhou et al., 2013). However, we found that ChlP was down-regulated after shade treatment, suggesting that this gene may not be the cause for the yellow leaf development under high light intensity conditions. Compared with the non-shaded leaf, POR was significantly up-regulated after shading; Chl levels in an Arabidopsis PORC mutant have been shown to decrease dramatically under high light, and overexpression of AtPORC has been shown to increase the tolerance to photo-oxidative damage (Masuda et al., 2003; Pattanayak and Tripathy, 2011). These results suggest that POR was inhibited under high light intensity conditions, which might result in a reduced Chl content and the yellow-leaf phenotype.
Our light-shading experiments demonstrated that the Baijiguan cultivar displays increased sensitivity to high light intensity stress, which ROS and stimulates lipid peroxidation, resulting in the attacks on various cellular components (Li et al., 2015). In plants, ROS scavenging enzymes are induced in chloroplasts and mitochondria to detoxify ROS produced during abiotic stresses and to protect tissues from oxidative damage under stress conditions (Apel and Hirt, 2004; Cluis et al., 2004; Foyer and Noctor, 2005; Yoshimura et al., 2008). Genes encoding antioxidant enzymes, including superoxide dismutase (SOD, comp38880_c0, comp71851_c0), catalase (CAT, comp74455_c1), and glutathione peroxidase (GSH-PX, comp33082_c0, comp63197_c0), were identified among the DEGs (Figure 8D). Accordingly, we assayed the activities of SOD, CAT and POD. Among antioxidant enzymes, SOD plays a key role in cellular ROS detoxification. Our results showed that SOD activity increased markedly from 17.070 ± 1.42 to 178.52 ± 1.76 U/g FW after shade treatments (Figure 3). Therefore, this enzyme may be the first line of defense against the ROS that were generated in response to high light intensity stress, this enzyme clears harmful molecules such as O2− to reduce photo-oxidative stress.
Transmission electron microscopy analysis revealed that the ultra-structure of chloroplasts in Baijiguan was disrupted under high light intensity conditions, exhibiting poorly stacked grana (Figures 2A,B). However, the yellow leaves of Baijiguan changed to green when the light intensity was reduced; this color change coincided restoration of the typical chloroplast structure and an increase in the Chl content. Strong sunlight appears to suppress grana stacking and thylakoid development, resulting in underdeveloped chloroplasts. These results were consistent with the observed changes in transcriptional abundance. GO annotation revealed the enrichment of many light- and chloroplast-related categories, including “light-harvesting complex,” “plastid,” “chloroplast,” and “thylakoid.” Eight DEGs (comp38841_c0, comp66018_c0, comp28142_c0, comp76361_c1, comp61823_c0, comp37286_c0, comp67545_c0 and comp11002_c0) related to chloroplast (GO: 0009507) were significantly up-regulated in both the T3d_Z vs. T3d_W and T6d_Z vs. T6d_W groups, suggesting crucial roles for these DEGs in repairing chloroplast structure. KEGG pathway enrichment analysis revealed that “fatty acid degradation” was up-regulated. The modification of membrane fluidity is mediated by fatty acid desaturation (Upchurch, 2008; Chen and Thelen, 2013). One gene encoding a chloroplast ω-6 fatty acid desaturase has been identified in Arabidopsis thaliana, and mutation of this gene results in newly developed leaves becoming yellow at low temperature (Hugly and Somerville, 1992). Other genes (FAD3, FAD7 and FAD8) encoding ω-3 fatty-acid desaturases have been found to be differentially regulated in response to light (Collados et al., 2006). These results are consistent with the observations that non-shaded cells were devoid of thylakoid membranes and that light-shaded cells showed normal chloroplast structures (Figures 2E–H).
Pathway enrichment analysis revealed enrichment of both the “photosynthesis antenna proteins” and “photosynthesis” pathways. In the “photosynthesis antenna proteins” pathway, genes encoding light-harvesting complex I Chl a/b binding protein (LHCA) and light-harvesting complex II Chl a/b binding protein (LHCB) were down-regulated during shade treatment, suggesting that the yellow phenotype may not be related to the light-harvesting complex. However, PsbR, a 10-kDa photosystem II protein, was significantly up-regulated, whereas other genes involved in PSII stability were down-regulated after shade treatment (Figure 9 and Table S7). These results suggested that high light intensity inhibits PsbR expression in Baijiguan, thus affecting PSII stability and photosynthetic oxygen evolution (Suorsa et al., 2006; Liu et al., 2009). Up-regulation of the photosynthetic gene PsbR under shade conditions likely reduces photo-oxidative damage and restores chloroplast structure, ultimately turning the yellow leaves to a normal green color.
TFs play critical roles in coordinating development with environmental changes. In this study, 1652 TFs were identified. Many of these TFs belong to the MYB, AP2 domain, homeobox, zinc finger, bZIP, and bHLH families, whose expression levels are primarily affected by abiotic stress (Obertello et al., 2010; Wang X. C. et al., 2013).
A total of 11 genes encoding bHLH TFs were identified in both the T3d_Z vs. T3d_W and T6d_Z vs. T6d_W groups (8 up- and 3 down-regulated genes, Table S5). It is reported that bHLH135 is involved in light signaling in Arabidopsis (Castelain et al., 2012); in addition, two genes encoding bHLH factors that were rapidly up-regulated after shading; these genes played important roles in integrating shade and hormone transcriptional networks (Roig-Villanova et al., 2007). The MYB family has also been implicated in the light-mediated regulation of plant development, stress responses, and pigment biosynthesis (Yamagishi et al., 2012). In our study, 10 MYB family genes were identified in the above groups (6 up- and 4 down-regulated genes, Table S5). Expression of a light-regulated MYB gene has been shown to be induced following exposure of etiolated or dark-adapted Arabidopsis seedlings to light (Quaedvlieg et al., 1996). In Rehmannia glutinosa, seven MYB genes were found to be up-regulated in the leaf or tuberous root under shade conditions (Wang et al., 2015). In Litchi chinensis Sonn, nine and five genes were up- and down-regulated, respectively, in response to shading (Li C. et al., 2013). Thus, MYB and bHLH family TFs are positively induced by shading and may play crucial roles in transcriptome reprogramming and leaf color formation.
Photoreceptors (phytochromes and cryptochromes) mediate light perception and regulate the transcription or activity of TFs via post-translational modifications (Deng et al., 2014). PHYB (phytochrome B, comp53675_c0), CRY (cryptochrome, comp59933_c0), COP1 (constitutive photomorphogenic 1, comp74256_c0), HY5 (long hypocotyl 5, comp38740_c0), and LHY (late elongated hypocotyl transcription factor, comp71228_c0) were among the identified DEGs (see Table S7 and Figure 9). A previous in vitro analysis revealed that the HY5 binds directly to the promoter of several light-inducible genes (Hiltbrunner et al., 2006).
HY5 integrates the light and hormone signaling networks. A homolog gene of HY5, STF1, in Glycine max plays important roles in light and hormone signaling and in Chl accumulation (Song et al., 2014). HY5 has also been reported to be involved in the light and jasmonic acid signaling pathways (Prasad et al., 2012). In the present study, the expression level of HY5 was found to be down-regulated, whereas the expression levels of genes encoding auxin-responsive GH3 (comp72299_c0) and jasmonate ZIM domain-containing protein (JAZ, comp36449_c0) were significantly up-regulated under shade stress (Figure 9). Our data support the notion that HY5 promotes the expression of negative regulators of auxin signaling (Cluis et al., 2004), suggesting that the shade response of C. sinensis is also associated with jasmonic acid and auxin signaling networks.
Based on DEG analysis, some genes encoding key enzymes involved in secondary metabolism were found to be significantly affected by light shading (Kimura et al., 2003), with significant enrichment of genes associated with flavonoid biosynthesis. Catechin content decreases in tea plants under shade stress (Ku et al., 2010; Wang et al., 2012). Previous transcriptome analyses have revealed that genes involved in flavonoid biosynthesis are significantly down-regulated in Zhonghuang 2 compared with a green tea cultivar. In our study, the expression of genes involved in flavonoid biosynthesis was also repressed in Baijiguan following shade treatments (Figure 8B). This repression may be responsible for suppressing photosynthetic pathways, particularly “photosynthesis” and “carbon metabolism” pathways. Other secondary metabolic processes, such as phenylalanine metabolism, phenylpropanoid biosynthesis, and terpenoid backbone biosynthesis, were also significantly enriched (Figure 7). These data suggest that the levels of secondary metabolites in Baijiguan were altered; therefore, further studies of the metabolic profile of this cultivar are important.
Comparative transcriptome analysis of a yellow leaf tea plant variety was performed under shade conditions, and various genes and pathways potentially responsible for yellow leaf formation were identified. GO and pathway enrichment analyses revealed that these DEGs are involved in ROS scavenging system, chloroplast development and photosynthetic pigment synthesis pathways, and that gene expression alterations in these pathways might be responsible for the decreased Chl content and yellow phenotype of Baijiguan. Furthermore, the identification of a large number of DEGs offers a global view of light-induced albinism in tea plants, which will aid in the understanding of the molecular mechanisms of yellow leaf formation and will facilitate molecular breeding of tea plants.
Conceived and designed the experiments: WS, QW, and ZC. Performed the experiments: QW and ZC. Analyzed the data: QW, WS, MC, ZC, and TD. Contributed reagents/materials/analysis tools: QW, ZC, and TD. Contributed to the writing of the manuscript: QW, WS, and MC. Revised and approved the final version of the paper: QW, WS, ZC, MC, and TD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was partially supported by grants from the National High-Tech Research and Development Program (863 Program) (2013AA102600/2013AA102606/2013AA10260605), and Fujian University-Industry Cooperation Projects (2015N5008). We would like to thank Shan Li (Novogene Bioinformatics Technologies Co. Ltd., Beijing, China) for her help in RNA-Seq data analysis, Yang Wu (College of Horticulture, Fujian Agriculture and Forestry University, Fuzhou, China) for his help in sampling, and Chen Bingzhi (College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China) for his technical advice.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00332
Akita, S., Yano, A., Ishii, H., Satoh, C., Akai, N., and Nakata, M. (2013). Delayed fluorescence spectra of intact leaves photoexcited by sunlight measured with a multichannel Fourier-transform chemiluminescence spectrometer. Chem. Phys. Lett. 574, 120–123. doi: 10.1016/j.cplett.2013.04.062
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arnon, D. L. (1949). Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.1104/pp.24.1.1
Asakura, Y., Kikuchi, S., and Nakai, M. (2008). Non-identical contributions of two membrane-bound cpSRP components, cpFtsY and Alb3, to thylakoid biogenesis. Plant J. 56, 1007–1017. doi: 10.1111/j.1365-313X.2008.03659.x
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. 57, 289–300.
Castelain, M., Le Hir, R., and Bellini, C. (2012). The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiol. Plant. 145, 450–460. doi: 10.1111/j.1399-3054.2012.01600.x
Chen, M. J., and Thelen, J. J. (2013). ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 25, 1430–1444. doi: 10.1105/tpc.113.111179
Cluis, C. P., Mouchel, C. F., and Hardtke, C. S. (2004). The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 38, 332–347. doi: 10.1111/j.1365-313X.2004.02052.x
Collados, R., Andreu, V., Picorel, R., and Alfonso, M. (2006). A light-sensitive mechanism differently regulates transcription and transcript stability of omega3 fatty-acid desaturases (FAD3, FAD7 and FAD8) in soybean photosynthetic cell suspensions. FEBS Lett. 580, 4934–4940. doi: 10.1016/j.febslet.2006.07.087
Deng, Z. P., Oses-Prieto, J. A., Kutschera, U., Tseng, T. S., Hao, L. Z., Burlingame, A. L., et al. (2014). Blue light-induced proteomic changes in etiolated Arabidopsis seedlings. J. Proteome Res. 13, 2524–2533. doi: 10.1021/pr500010z
Du, Y. Y., Chen, H., Zhong, W. L., Wu, L. Y., Ye, J. H., Lin, C., et al. (2008). Effect of temperature on accumulation of chlorophylls and leaf ultrastructure of low temperature induced lbino tea plant. Afr. J. Biotechnol. 7, 1881–1885.
Du, Y. Y., Shin, S., Wang, K. R., Lu, J. L., and Liang, Y. R. (2009). Effect of temperature on the expression of genes related to the accumulation of chlorophylls and carotenoids in albino tea. J. Hortic. Sci. Biotechnol. 84, 365–369.
Feng, L., Gao, M. J., Hou, R. Y., Hu, X. Y., Zhang, L., Wan, X. C., et al. (2014). Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 155, 98–104. doi: 10.1016/j.foodchem.2014.01.044
Foyer, C. H., and Noctor, G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17, 1866–1875. doi: 10.1105/tpc.105.033589
Gotz, S., Garcia-Gomez, J. M., Terol, J., Williams, T. D., Nagaraj, S. H., Nueda, M. J., et al. (2008). High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36, 3420–3435. doi: 10.1093/nar/gkn176
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
Herzel, L., and Neugebauer, K. M. (2015). Quantification of co-transcriptional splicing from RNA-Seq data. Methods 85, 36–43. doi: 10.1016/j.ymeth.2015.04.024
Hiltbrunner, A., Tscheuschler, A., Viczian, A., Kunkel, T., Kircher, S., and Schafer, E. (2006). FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 47, 1023–1034. doi: 10.1093/pcp/pcj087
Ho, C. L., Teoh, S., Teo, S. S., Rahim, R. A., and Phang, S. M. (2009). Profiling the transcriptome of Gracilaria changii (Rhodophyta) in response to light deprivation. Mar. Biotechnol. 11, 513–519. doi: 10.1007/s10126-008-9166-x
Hugly, S., and Somerville, C. (1992). A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 99, 197–202. doi: 10.1104/pp.99.1.197
Jahns, P., and Holzwarth, A. R. (2012). The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 1817, 182–193. doi: 10.1016/j.bbabio.2011.04.012
Jakhesara, S. J., Koringa, P. G., Bhatt, V. D., Shah, T. M., Vangipuram, S., Shah, S., et al. (2013). RNA-Seq reveals differentially expressed isoforms and novel splice variants in buccal mucosal cancer. Gene 516, 24–32. doi: 10.1016/j.gene.2012.11.079
Jung, K. H., Hur, J., Ryu, C. H., Choi, Y., Chung, Y. Y., Miyao, A., et al. (2003). Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol. 44, 463–472. doi: 10.1093/pcp/pcg064
Kanehisa, M., Araki, M., Goto, S., Hattori, M., Hirakawa, M., Itoh, M., et al. (2008). KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484. doi: 10.1093/nar/gkm882
Kanehisa, M., Goto, S., Hattori, M., Aoki-Kinoshita, K. F., Itoh, M., Kawashima, S., et al. (2006). From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 34, D354–D357. doi: 10.1093/nar/gkj102
Kimura, M., Yamamoto, Y. Y., Seki, M., Sakurai, T., Sato, M., Abe, T., et al. (2003). Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem. Photobiol. 77, 226–233. doi: 10.1562/0031-8655(2003)0770226IOAGRB2.0.CO2
Ku, K. M., Choi, J. N., Kim, J., Kim, J. K., Yoo, L. G., Lee, S. J., et al. (2010). Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 58, 418–426. doi: 10.1021/jf902929h
Li, B., and Dewey, C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323
Li, C., Wang, Y., Huang, X., Li, J., Wang, H., and Li, J. (2013). De novo assembly and characterization of fruit transcriptome in Litchi chinensis Sonn and analysis of differentially regulated genes in fruit in response to shading. BMC Genomics 14:552. doi: 10.1186/1471-2164-14-552
Li, H. X., Xiao, Y., Cao, L. L., Yan, X., Li, C., Shi, H. Y., et al. (2013). Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE 8:e73380. doi: 10.1371/journal.pone.0073380
Li, H., Yao, W., Fu, Y., Li, S., and Guo, Q. (2015). De novo assembly and discovery of genes that are involved in drought tolerance in Tibetan Sophora moorcroftiana. PLoS ONE 10:e111054. doi: 10.1371/journal.pone.0111054
Li, Q., Huang, J. A., Liu, S. Q., Li, J., Yang, X. H., Liu, Y. S., et al. (2011). Proteomic analysis of young leaves at three developmental stages in an albino tea cultivar. Proteome Sci. 9:44. doi: 10.1186/1477-5956-9-44
Lin, Y. P., Lee, T. Y., Tanaka, A., and Charng, Y. Y. (2014). Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 80, 14–26. doi: 10.1111/tpj.12611
Liu, H., Frankel, L. K., and Bricker, T. M. (2009). Characterization and complementation of a psbR mutant in Arabidopsis thaliana. Arch. Biochem. Biophys. 489, 34–40. doi: 10.1016/j.abb.2009.07.014
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, C. L., Chen, L., Wang, X. C., Jin, J. Q., Ma, J. Q., Yao, M. Z., et al. (2012). Differential expression analysis of different albescent stages of 'Anji Baicha' (Camellia sinensis (L.) O. Kuntze) using cDNA microarray. Sci. Hortic. 148, 246–254. doi: 10.1016/j.scienta.2012.09.033
Ma, C.-L., Yao, M.-Z., Wang, X.-C., Jin, J.-Q., Ma, J.-Q., and Chen, L. (2015). Cloning and expression of three genes involved in chlorophyll biosynthesis at different albescent stages of tea plant variety “Baiye 1”. Acta Agron. Sin. 41:240. doi: 10.3724/SP.J.1006.2015.00240
Mao, X. Z., Cai, T., Olyarchuk, J. G., and Wei, L. P. (2005). Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21, 3787–3793. doi: 10.1093/bioinformatics/bti430
Masuda, T., Fusada, N., Oosawa, N., Takamatsu, K., Yamamoto, Y. Y., Ohto, M., et al. (2003). Functional analysis of isoforms of NADPH: protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol. 44, 963–974. doi: 10.1093/pcp/pcg128
Miura, E., Kato, Y., and Sakamoto, W. (2010). Comparative transcriptome analysis of green/white variegated sectors in Arabidopsis yellow variegated2: responses to oxidative and other stresses in white sectors. J. Exp. Bot. 61, 2433–2445. doi: 10.1093/jxb/erq075
Mortazavi, A., Williams, B. A., Mccue, K., Schaeffer, L., and Wold, B. (2008). Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628. doi: 10.1038/nmeth.1226
Nasir, I. A., and Riazuddin, S. (2008). In vitro selection for Fusarium wilt resistance in Gladiolus. J. Integr. Plant Biol. 50, 601–612. doi: 10.1111/j.1744-7909.2008.00656.x
Obertello, M., Krouk, G., Katari, M. S., Runko, S. J., and Coruzzi, G. M. (2010). Modeling the global effect of the basic-leucine zipper transcription factor 1 (bZIP1) on nitrogen and light regulation in Arabidopsis. BMC Syst. Biol. 4:111. doi: 10.1186/1752-0509-4-111
Pan, S. S., Lu, J. L., Yang, X. L., Zhang, X. Q., Du, Y. Y., Devijat, B., et al. (2007). Study on HPLC method for the analysis of trace pigments in ready-to-drink tea. J. Tea Sci. 27, 343–348. doi: 10.13305/j.cnki.jts.2007.04.014
Pattanayak, G. K., and Tripathy, B. C. (2011). Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE 6:e26532. doi: 10.1371/journal.pone.0026532
Peng, Y., Zhang, Y., Lv, J., Zhang, J., Li, P., Shi, X., et al. (2012). Characterization and fine mapping of a novel rice albino mutant low temperature albino 1. J. Genet. Genomics 39, 385–396. doi: 10.1016/j.jgg.2012.05.001
Prasad, B. R., Kumar, S. V., Nandi, A., and Chattopadhyay, S. (2012). Functional interconnections of HY1 with MYC2 and HY5 in Arabidopsis seedling development. BMC Plant Biol. 12:37. doi: 10.1186/1471-2229-12-37
Qin, G., Gu, H., Ma, L., Peng, Y., Deng, X. W., Chen, Z., et al. (2007). Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 17, 471–482. doi: 10.1038/cr.2007.40
Quaedvlieg, N., Dockx, J., Keultjes, G., Kock, P., Wilmering, J., Weisbeek, P., et al. (1996). Identification of a light-regulated MYB gene from an Arabidopsis transcription factor gene collection. Plant Mol. Biol. 32, 987–993. doi: 10.1007/BF00020495
Robles, J. A., Qureshi, S. E., Stephen, S. J., Wilson, S. R., Burden, C. J., and Taylor, J. M. (2012). Efficient experimental design and analysis strategies for the detection of differential expression using RNA-Sequencing. BMC Genomics 13:484. doi: 10.1186/1471-2164-13-484
Roig-Villanova, I., Bou-Torrent, J., Galstyan, A., Carretero-Paulet, L., Portoles, S., Rodriguez-Concepcion, M., et al. (2007). Interaction of shade avoidance and auxin responses: a role for two novel atypical bHLH proteins. EMBO J. 26, 4756–4767. doi: 10.1038/sj.emboj.7601890
Sakuraba, Y., Rahman, M. L., Cho, S. H., Kim, Y. S., Koh, H. J., Yoo, S. C., et al. (2013). The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J. 74, 122–133. doi: 10.1111/tpj.12110
Sheng, P., Tan, J., Jin, M., Wu, F., Zhou, K., Ma, W., et al. (2014). Albino midrib 1, encoding a putative potassium efflux antiporter, affects chloroplast development and drought tolerance in rice. Plant Cell Rep. 33, 1581–1594. doi: 10.1007/s00299-014-1639-y
Song, J., Wei, X. J., Shao, G. N., Sheng, Z. H., Chen, D. B., Liu, C. L., et al. (2014). The rice nuclear gene WLP1 encoding a chloroplast ribosome L13 protein is needed for chloroplast development in rice grown under low temperature conditions. Plant Mol. Biol. 84, 301–314. doi: 10.1007/s11103-013-0134-0
Su, N., Hu, M. L., Wu, D. X., Wu, F. Q., Fei, G. L., Lan, Y., et al. (2012). Disruption of a rice pentatricopeptide repeat protein causes a seedling-specific albino phenotype and its utilization to enhance seed purity in hybrid rice production. Plant Physiol. 159, 227–238. doi: 10.1104/pp.112.195081
Sun, S. M., Xuan, F. J., Ge, X. P., Fu, H. T., Zhu, J., and Zhang, S. Y. (2014). Identification of differentially expressed genes in hepatopancreas of oriental river prawn, Macrobrachium nipponense exposed to environmental hypoxia. Gene 534, 298–306. doi: 10.1016/j.gene.2013.10.036
Suorsa, M., Sirpio, S., Allahverdiyeva, Y., Paakkarinen, V., Mamedov, F., Styring, S., et al. (2006). PsbR, a missing link in the assembly of the oxygen-evolving complex of plant photosystem II. J. Biol. Chem. 281, 145–150. doi: 10.1074/jbc.M510600200
Upchurch, R. G. (2008). Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 30, 967–977. doi: 10.1007/s10529-008-9639-z
Wang, F., Suo, Y., Wei, H., Li, M., Xie, C., Wang, L., et al. (2015). Identification and characterization of 40 isolated Rehmannia glutinosa MYB family genes and their expression profiles in response to shading and continuous cropping. Int. J. Mol. Sci. 16, 15009–15030. doi: 10.3390/ijms160715009
Wang, K. R., Li, N. N., Du, Y. Y., and And Liang, Y. R. (2013). Effect of sunlight shielding on leaf structure and amino acids concentration of light sensitive albino tea plant. Afr. J. Biotechnol. 12, 5535–5539. doi: 10.5897/AJB12.857
Wang, M., Wang, G., Ji, J., and Wang, J. (2009). The effect of pds gene silencing on chloroplast pigment composition, thylakoid membrane structure and photosynthesis efficiency in tobacco plants. Plant Sci. 177, 222–226. doi: 10.1016/j.plantsci.2009.04.006
Wang, X. C., Zhao, Q. Y., Ma, C. L., Zhang, Z. H., Cao, H. L., Kong, Y. M., et al. (2013). Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genomics 14:415. doi: 10.1186/1471-2164-14-415
Wang, Y., Gao, L., Shan, Y., Liu, Y., Tian, Y., and Xia, T. (2012). Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 141, 7–16. doi: 10.1016/j.scienta.2012.04.013
Wei, K., Wang, L.-Y., Zhou, J., He, W., Zeng, J.-M., Jiang, Y.-W., et al. (2012). Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 130, 720–724. doi: 10.1016/j.foodchem.2011.07.092
Xiong, L., Li, J., Li, Y., Yuan, L., Liu, S., Huang, J., et al. (2013). Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 71, 132–143. doi: 10.1016/j.plaphy.2013.06.019
Yamagishi, M., Yoshida, Y., and Nakayama, M. (2012). The transcription factor LhMYB12 determines anthocyanin pigmentation in the tepals of Asiatic hybrid lilies (Lilium spp.) and regulates pigment quantity. Mol. Breed. 30, 913–925. doi: 10.1007/s11032-011-9675-6
Yaronskaya, E., Ziemann, V., Walter, G., Averina, N., Borner, T., and Grimm, B. (2003). Metabolic control of the tetrapyrrole biosynthetic pathway for porphyrin distribution in the barley mutant albostrians. Plant J. 35, 512–522. doi: 10.1046/j.1365-313X.2003.01825.x
Yoshimura, K., Masuda, A., Kuwano, M., Yokota, A., and Akashi, K. (2008). Programmed proteome response for drought avoidance/tolerance in the root of a C(3) xerophyte (wild watermelon) under water deficits. Plant Cell Physiol. 49, 226–241. doi: 10.1093/pcp/pcm180
Young, M. D., Wakefield, M. J., Smyth, G. K., and Oshlack, A. (2010). Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11:R14. doi: 10.1186/gb-2010-11-2-r14
Zhan, G. M., Li, R. J., Hu, Z. Y., Liu, J., Deng, L. B., Lu, S. Y., et al. (2014). Cosuppression of RBCS3B in Arabidopsis leads to severe photoinhibition caused by ROS accumulation. Plant Cell Rep. 33, 1091–1108. doi: 10.1007/s00299-014-1597-4
Zhang, H., Li, J., Yoo, J. H., Yoo, S. C., Cho, S. H., Koh, H. J., et al. (2006). Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol. Biol. 62, 325–337. doi: 10.1007/s11103-006-9024-z
Zhao, L., Gao, L., Wang, H., Chen, X., Wang, Y., Yang, H., et al. (2012). The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct. Integr. Genomics 13, 75–98. doi: 10.1007/s10142-012-0301-4
Keywords: light-shading, Camellia sinensis, differentially expressed genes, yellow phenotype, chloroplast development, chlorophyll synthesis, antioxidant enzymes
Citation: Wu Q, Chen Z, Sun W, Deng T and Chen M (2016) De novo Sequencing of the Leaf Transcriptome Reveals Complex Light-Responsive Regulatory Networks in Camellia sinensis cv. Baijiguan. Front. Plant Sci. 7:332. doi: 10.3389/fpls.2016.00332
Received: 11 July 2015; Accepted: 04 March 2016;
Published: 21 March 2016.
Edited by:
David John Burritt, University of Otago, New ZealandReviewed by:
Mohammad Shameem Al Mamun, Bangladesh Tea Research Institute, BangladeshCopyright © 2016 Wu, Chen, Sun, Deng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijiang Sun, c3dqODEwM0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.