
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Plant Sci., 02 July 2015
Sec. Plant Physiology
Volume 6 - 2015 | https://doi.org/10.3389/fpls.2015.00497
This article is part of the Research TopicPlant cell wall integrity in plant developmentView all 12 articles
Gas exchange is essential for multicellular organisms. In contrast to the circulatory systems of animals, land plants have tissues with intercellular spaces (ICSs), called aerenchyma, that are critical for efficient gas exchange. Plants form ICSs by two different mechanisms: schizogeny, where localized cell separation creates spaces; and lysogeny, where cells die to create ICSs. In schizogenous ICS formation, specific molecular mechanisms regulate the sites of cell separation and coordinate extensive reorganization of cell walls. Emerging evidence suggests the involvement of extracellular signaling, mediated by peptide ligands and leucine-rich repeat receptor-like kinases, in the regulation of cell wall remodeling during cell separation. Recent work on the liverwort Marchantia polymorpha has demonstrated a critical role for a plasma membrane-associated plant U-box E3 ubiquitin ligase in ICS formation. In this review, I discuss the mechanism of schizogenous ICS formation, focusing on the potential role of extracellular signaling in the regulation of cell separation.
Gas exchange is vital for all living organisms. Single-celled or small multicellular organisms carry out gas exchange via diffusion directly between cells and the environment or through the gastrovascular cavity. Complex multicellular organisms achieve gas exchange through specialized systems for gas uptake and circulation. For example, vertebrates have a closed circulatory system in which blood is pumped by a heart, and gas exchange occurs through lungs or gills. Although plants lack a circulatory system, many develop intercellular spaces (ICSs), which are connected directly to the external environment and provide efficient gas exchange via stomata or air pores (Raven, 1996; Jackson and Armstrong, 1999; Evans, 2004). Development of ICSs for gas exchange is critical for photosynthesis and transpiration in plants because the diffusion coefficients of carbon dioxide and oxygen are 10,000 times lower in water than in air (Voesenek et al., 2006). In the leaves of vascular plants, spongy mesophyll is a typical example of tissue containing ICSs for efficient gas exchange. As a result of ICS formation in mesophyll, the internal surface area of a leaf may be between 7 and 32 times larger than its external surface area, depending on the species and on environmental factors such as light intensity or quality (Dale, 1988).
Intercellular spaces are common and well developed in the roots and stems of wetland plants that grow in hypoxic soils (Seago et al., 2005; Joshi and Kumar, 2012). ICSs are also observed in bryophytes, such as the air chambers in the gametophyte of some liverworts, and sporophyte substomatal cavities of mosses and hornworts (Renzaglia et al., 2000). ICSs and stomata are recorded in fossils of early land plants found in the Rhynie chert, which is approximately 400–412 million years old, indicating that ICSs are among the most conservative characters in the evolution of land plants (Edwards et al., 1998). In addition to the evolution of stomata, the cuticle, lignified cell walls, and the water conduction system, internalization of the gas exchange surface by ICSs is likely one of the most critical land plant adaptations to terrestrial environments (Raven, 2002).
Intercellular spaces in plants are formed either via lysogeny or schizogeny. A lysigenous ICS is caused by spatially specified cell death to leave a space. Lysigenous ICS formation occurs in the roots of many crop species, including rice (Justin and Armstrong, 1991), maize (He et al., 1996; Gunawardena et al., 2001), and wheat (Trought and Drew, 1980). In contrast, schizogenous ICSs are formed by cell separation, instead of cell death. Schizogenous ICS formation is common in the roots and stems of wetland plants, such as species of Rumex and Sagittaria. In most vascular plants, ICSs in the leaf mesophyll are schizogenous, formed by the partial separation of cells following the breakdown of cell wall components (Sachs et al., 1882; Dale, 1988). Schizogenous ICSs may be formed by differential growth, resulting in separation of adjacent cells from one another at the middle lamella of the cell walls. This process involves the differentiation of specialized cells that undergo cell wall breakdown, then subsequently divide and enlarge differentially, to generate ICSs by cell separation. Although the process of schizogenous ICS formation has been described at the histological level in some species, its developmental regulation and molecular mechanisms remain largely unknown (Jackson and Armstrong, 1999; Evans, 2004).
Cell separation is a critical aspect of various developmental processes, including the emergence of lateral roots, the shedding of damaged or senescent organs, the release of pollen from anthers and seeds from pods, the softening of fruits, and the formation of ICSs in leaf mesophyll or in the aerenchyma of waterlogged roots. The process of cell separation involves the breakdown of cell walls in a defined area of tissue (Patterson, 2001). ICS formation appears to require specialized modifications of the cell wall in most reported cases (Knox, 1992). In pea root tissues, the initial stage of ICS formation is preceded by the formation of a defined cell separation layer to open the gap (Roland, 1978). In bean leaves, ICS formation occurs schizogenously at newly formed cell junctions and involves highly localized breakdown of the cell wall in the parent cells, which continues in the region of the middle lamella of the parent and daughter cells (Jeffree et al., 1986). In pea cotyledons, the extent of separation at cell junctions is associated with localized wall thickenings and electron-dense structures (Kollöffel and Linssen, 1984). The localized lysis of cell wall components initiates the development of extensive ICSs in maize leaf mesophyll. Subsequently, these gaps are extended by the mechanical stresses exerted by distinctive cell shapes. This process is accompanied by localized wall thickening (Apostolakos et al., 1991).
The process of organ abscission has been studied in diverse angiosperm species, including rice, tomato, and Arabidopsis thaliana. It provides a model for well-organized cell separation, which involves breakdown of cell wall material between adjacent cells (Patterson, 2001). In organ abscission, cell separation is restricted to the narrow band of cells that compose the abscission layer (Aalen et al., 2013). In the first stage of organ abscission, cells in the abscission zone (AZ) differentiate to form small, isodiametric, and cytoplasmically dense cells. The differentiation of AZ cells occurs simultaneously with the development of lateral organs from the apical meristem (Sexton and Roberts, 1982; Roberts et al., 2002). The second stage is activation of abscission, in which the AZ cells become capable of responding to abscission signals, and the organs prepare to separate from the plant. Plant hormones are the major endogenous regulators of this process. In general, ethylene and jasmonate act as signals to accelerate abscission, whereas auxin, gibberellins, and brassinosteroids inhibit the process (Estornell et al., 2013). After the activation of abscission, pectic polysaccharides are broken down in the AZ cell walls, followed by cell expansion in the AZ layer. Various enzymes act on structural polysaccharides, leading to hydrolysis of the middle lamella and cell walls of the AZ cells. These enzymes include expansins, xyloglucan endotransglucosylase/hydrolases, β-1,4-glucanases (cellulases), and polygalacturonases (PGs; Estornell et al., 2013). Activation of the molecular machinery of abscission involves regulating the expression of numerous gene families that encode cell-wall-remodeling enzymes (Cai and Lashbrook, 2008; Ogawa et al., 2009; Estornell et al., 2013). Mutational analyses indicate the significance of PGs in cell separation events, including abscission (Ogawa et al., 2009). Following the actual separation, lignification of the abscission wound occurs to protect the plant from pathogen attack.
The key genetic components that regulate cell separation have been studied using floral organ abscission mutants in A. thaliana. In wild-type flowers, the petals, sepals, and filaments abscise shortly after pollination or anthesis. The gene INFLORESCENCE DEFICIENT IN ABSCISSION (IDA), which encodes a short protein with an N-terminal signal peptide, has been identified as the causal gene of a mutant phenotype that causes flowers to be retained on a plant indefinitely (Butenko et al., 2003). The loss of function in genes encoding the leucine-rich repeat receptor-like kinase (RLK) HAESA (HAE) and its closely related, redundant partner HAESA-Like2 (HSL2) is sufficient to block organ abscission (Jinn et al., 2000; Cho et al., 2008). Morphological studies of floral organs show that specification of AZ cells is not affected in either the ida or hae hsl2 double mutant. This finding suggests that both IDA and HAE/HSL2 are expressed after the specification of AZ cells, and that they promote the activation of coordinated cell-wall-remodeling enzymes in AZ cells, which leads to cell separation. Considerable genetic and biochemical evidence supports a ligand–receptor relationship between IDA and HAE/HSL2 (Stenvik et al., 2006; Cho et al., 2008; Butenko et al., 2014). Several members of a mitogen-activated kinase cascade, namely, MITOGEN-ACTIVATED PROTEIN KINASE KINASE4 (MKK4), MKK5, MITOGEN-ACTIVATED PROTEIN KINASE3 (MAPK3) and MAPK6, act downstream of IDA and HAE/HSL2 signaling (Cho et al., 2008). The MADS-domain transcription factor AGAMOUS-like 15 (AGL15), which regulates expression of HAE through direct binding to the HAE promoter, is a direct target of MAPK phosphorylation. Phosphorylation by MAPK relieves AGL15 repression of HAE expression, leading to an increase in HAE receptor-mediated signaling, and thus completes a positive feedback loop controlling floral organ abscission (Patharkar and Walker, 2015). Overexpression of IDA confers early abscission, and production of a white substance in the floral AZs. The main components of the white substance are the cell wall monosaccharides arabinose and galactose, suggesting that IDA acts as a positive regulator of cell separation by promoting cell wall breakdown in the AZ (Stenvik et al., 2006; Cho et al., 2008). These effects of IDA overexpression are reversed when HAE and HSL2 activity is compromised (Stenvik et al., 2006; Cho et al., 2008). Microarray data suggest that the IDAHAE/HSL2 signaling module is involved in the regulation of cell-wall-remodeling genes (Cai and Lashbrook, 2008). Reduced transcription of cell-wall-modifying enzymes is observed in AZs of the hae hsl2 mutant (Cho et al., 2008). Additionally, the IDA-HAE/HSL2 signaling module regulates expression of cell-wall-remodeling genes, which promotes degradation of pectic polysaccharides during the cell separation process of lateral root emergence (Kumpf et al., 2013). These findings indicate that the IDA-HAE/HSL2 signaling module has been adapted to function in different root and shoot cell-separation processes.
The gametophyte thallus of the liverwort Marchantia polymorpha contains a multilayered tissue with air chambers on the dorsal surface (Smith, 1955). The air chambers have an ICS that contains chloroplast-rich filaments developed from the subepidermis, and the ICS is connected directly to the external atmosphere through air pores in the epidermis of the chamber (Barnes and Land, 1907; Apostolakos et al., 1982). The development of the air pores and air chambers of Marchantia starts with the formation of schizogenous ICSs, termed “initial apertures,” between the anticlinal walls of protodermal cells in the apical region of the thallus (Apostolakos et al., 1982). ICS expansion from the initial apertures, which results from cell division and protodermal and subprotodermal cell growth around the initial aperture, leads to the formation of an air chamber (Figure 1A; Apostolakos et al., 1982).
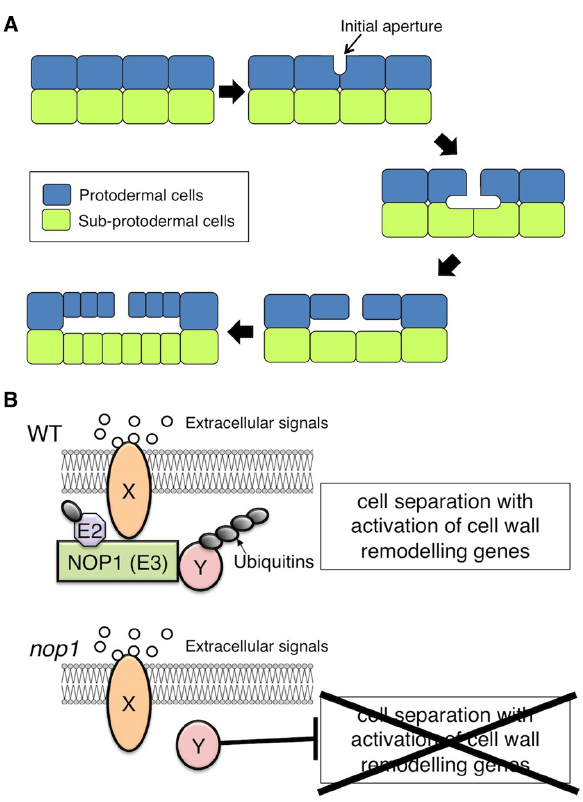
Figure 1. Schizogenous intercellular space (ICS) formation in Marchantia polymorpha. (A) Schematic representation of ICS formation during air chamber development. After a periclinal cell division to generate the protodermal and sub-protodermal cell layers, ICSs first appear as an initial aperture, between the anticlinal walls of protodermal cells. The base of the initial aperture broadens. The primary air chamber is formed via anticlinal cell divisions and protodermal and subprotodermal cell growth surrounding the ICS. (B) Hypothetical model of NOP1-mediated signaling involved in schizogenous ICS formation. Extracellular signals such as peptide ligands that are perceived by a receptor, Y, promote ICS formation via modulation of regulatory factor X that otherwise suppresses activation of cell-wall-remodeling enzymes. In the modulation of regulatory factor X, receptor Y signaling activates the E3 ligase NOP1 via phosphorylation of the ARM-repeat domain. NOP1 catalyzes the ubiquitination and subsequent degradation of regulatory factor X, which promotes cell separation through transcriptional activation of cell-wall-remodeling genes. In nop1, cell separation is constitutively suppressed by the action of regulatory factor X.
For more than a century, M. polymorpha has been the subject of developmental and physiological studies. It is regarded as an emerging model organism because of its ease of growth, basal evolutionary position among land plants, simple genetic architecture and genetic transformability (Ishizaki et al., 2008; Kubota et al., 2013), and the ease of gene-targeting (Ishizaki et al., 2013a; Sugano et al., 2014). Using an efficient Agrobacterium-mediated transformation protocol (Ishizaki et al., 2008), a mutant impaired in air chamber formation, nopperabo1 (nop1), was isolated from 10,000 T-DNA-tagged lines (Ishizaki et al., 2013b). In nop1, cell wall separation during initial aperture formation is impaired. The causal gene of nop1 was identified as NOPPERABO1 (NOP1), which encodes a Plant U-box (PUB) protein that carries tandem ARMADILLO (ARM) repeats in the C-terminus and localizes on the plasma membrane (Ishizaki et al., 2013b).
The U-box domain is similar in structure to the RING finger motif. U-box proteins function as E3 ubiquitin ligases and catalyze ubiquitin transfer from the ubiquitin-conjugating enzyme (E2) to the target for ubiquitination (Aravind and Koonin, 2000; Ohi et al., 2003). PUB proteins contain a variety of domain organizations and, in many cases, carry additional predicted domains, such as serine/threonine kinase, tetratricopeptide repeat, WD40 repeat, and ARM repeat domains. The ARM repeat domain is a highly conserved right-handed superhelix of α-helices involved in protein–protein interactions (Samuel et al., 2006; Tewari et al., 2010). Thus, the location of NOP1 in plasma membrane may depend on its C-terminus ARM repeats, via their interaction with regulatory proteins located on or near the plasma membrane.
Initiation of ICS in Marchantia occurs at the corners of three or more protodermal cells located only in the apical notch region (Apostolakos et al., 1982). To specify the position of cell wall remodeling for ICS formation, intercellular communication may participate in the mechanism to determine the relative position of the protodermal cells within the apical notch region.
Recent evidences point to connections between PUB-ARM proteins and RLK, and suggests a role for PUB-ARM proteins as potential signaling proteins for RLKs (Gu et al., 1998; Kim et al., 2003; Samuel et al., 2006, 2008; Mbengue et al., 2010; Lu et al., 2011). For example, in A. thaliana, the PUB-ARM proteins PUB12 and PUB13 diminish the activity of leucine-rich repeat RLK FLAGELLIN-SENSING 2 by ubiquitination and subsequent degradation (Lu et al., 2011). In Brassica and Arabidopsis, S-receptor kinase phosphorylates the ARM-repeat domain of the PUB-ARM protein ARK1, which acts as a positive regulator of the self-incompatibility response (Gu et al., 1998; Samuel et al., 2008).
NOP1 may be involved in a plasma membrane-localized RLK signaling pathway that regulates ICS formation in air chambers (Figure 1B). Because loss-of-function mutation of NOP1 confers impaired cell wall separation, NOP1 could catalyze ubiquitination and subsequent degradation of the regulatory factor(s) that suppress cell wall separation (Figure 1B). Identification of NOP1-associated proteins will be crucial to understanding the molecular mechanisms of ICS formation.
As discussed above, the IDA-HAE/HSL2 signaling module regulates cell separation through transcriptional activation of cell-wall-remodeling genes, such as expansins, xyloglucan endotransglucosylase/hydrolases, cellulases, and PGs in A. thaliana (Cai and Lashbrook, 2008; Cho et al., 2008; Kumpf et al., 2013). In the initial process of air chamber formation of M. polymorpha, a local thickening and subsequent detachment of the cell wall occurs to form an initial aperture at the junction of three to five protodermal cells (Apostolakos and Galatis, 1985). The PUB-ARM E3-ligase NOP1 is involved in this process (Ishizaki et al., 2013b). Bryophytes show some distinct features in terms of cell wall composition compared with vascular plants, i.e., the lack of a lignified secondary cell wall and a decreased amount of borate cross-linked rhamnogalacturonan II in the primary cell wall. However, the primary cell wall of bryophytes shares some common components with vascular plants, such as cellulose microfibrils, mannose-containing hemicelluloses, and xyloglucans (Matsunaga et al., 2004; Sarkar et al., 2009). Homologs of cell-wall-remodeling genes for expansins, xyloglucan endotransglucosylase/hydrolases, cellulases, and PGs have been identified in the moss Physcomitrella patens genome (Davison and Blaxter, 2005; Carey and Cosgrove, 2007; Eklöf and Brumer, 2010; McCarthy et al., 2014). To achieve well-organized ICS formation, NOP1 might be involved in RLK signaling in a similar manner to IDA-HAE/HSL2, which regulates the transcriptional activity of cell-wall-remodeling genes. Further comparative genomic studies are required to shed light on the genetic and biochemical processes of cell wall remodeling in ICS formation of M. polymorpha, and contribute to our understanding of the fundamental mechanism of the cell-wall-remodeling machinery and its evolution in land plants.
The development of ICSs is considered a significant event in the evolution of land plants, but little is known about the molecular mechanisms involved. The process of schizogenous ICS formation involves well-organized cell wall remodeling and may share a common regulatory mechanism with the process of organ abscission. In recent years, significant progress has been made in understanding many regulatory aspects of organ abscission, using the model plant A. thaliana. The extracellular signaling module that is mediated by peptide ligands and RLK molecules activates transcription of genes that encode cell-wall-remodeling enzymes, thus promoting organ abscission and the emergence of lateral roots. Knowledge of the mechanisms of organ abscission may be useful to design genetic and molecular strategies for understanding schizogenous ICS formation. Because M. polymorpha has a simple genome architecture, and is amenable both to transformation (Ishizaki et al., 2008; Kubota et al., 2013) and targeted genome modification (Ishizaki et al., 2013a; Sugano et al., 2014), it should be an effective model organism for studying ICS formation at the molecular level by examining air chamber development. Further investigations of NOP1-mediated air chamber development in M. polymorpha will contribute to our understanding of the fundamental regulatory mechanism and evolution of cell separation in land plants.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
I thank Miya Mizutani, Ryuichi Nishihama, and Takayuki Kohchi for helpful discussions. This work was supported by MEXT KAKENHI Grants-in-Aid for Scientific Research on Innovative Area (25114510 to KI).
Aalen, R. B., Wildhagen, M., Sto, I. M., and Butenko, M. A. (2013). IDA: a peptide ligand regulating cell separation processes in Arabidopsis. J. Exp. Bot. 64, 5253–5261. doi: 10.1093/jxb/ert338
Apostolakos, P., and Galatis, B. (1985). Studies on the development of the air pores and air chambers of Marchantia paleacea II. Ultrastructure of the initial aperture formation with particular reference to cortical microtubule organizing centres. Can. J. Bot. 63, 744–756.
Apostolakos, P., Galatis, B., and Mitrakos, K. (1982). Studies on the development of the air pores and air chambers of Marchantia paleacea I. light microscopy. Ann. Bot. 49, 377–396. doi: 10.1007/BF01276334
Apostolakos, P., Galatis, B., and Panteris, E. (1991). Microtubules in cell morphogenesis and intercellular space formation in Zea mays leaf mesophyll and Pilea cadierei epithem. J. Plant Physiol. 137, 591–601. doi: 10.1016/S0176-1617(11)80705-4
Aravind, L., and Koonin, E. V. (2000). The U box is a modified RING finger—a common domain in ubiquitination. Curr. Biol. 10, R132–R134. doi: 10.1016/S0960-9822(00)00398-5
Barnes, C. R., and Land, W. J. G. (1907). Bryological papers. I. the origin of air chambers. Bot. Gaz. 44, 197–213. doi: 10.1086/329317
Butenko, M. A., Patterson, S. E., Grini, P. E., Stenvik, G. E., Amundsen, S. S., Mandal, A., et al. (2003). Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 15, 2296–2307. doi: 10.1105/tpc.014365
Butenko, M. A., Wildhagen, M., Albert, M., Jehle, A., Kalbacher, H., Aalen, R. B., et al. (2014). Tools and strategies to match peptide-ligand receptor pairs. Plant Cell 26, 1838–1847. doi: 10.1105/tpc.113.120071
Cai, S., and Lashbrook, C. C. (2008). Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol. 146, 1305–1321. doi: 10.1104/pp.107.110908
Carey, R. E., and Cosgrove, D. J. (2007). Portrait of the expansin superfamily in Physcomitrella patens: comparisons with angiosperm expansins. Ann. Bot. 99, 1131–1141. doi: 10.1093/aob/mcm044
Cho, S. K., Larue, C. T., Chevalier, D., Wang, H., Jinn, T. L., Zhang, S., et al. (2008). Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 105, 15629–15634. doi: 10.1073/pnas.0805539105
Dale, J. E. (1988). The control of leaf expansion. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 267–295. doi: 10.1146/annurev.pp.39.060188.001411
Davison, A., and Blaxter, M. (2005). Ancient origin of glycosyl hydrolase family 9 cellulase genes. Mol. Biol. Evol. 22, 1273–1284. doi: 10.1093/molbev/msi107
Edwards, D., Kerp, H., and Hass, H. (1998). Stomata in early land plants: an anatomical and ecophysiological approach. J. Exp. Bot. 49, 255–278. doi: 10.1093/jxb/49.Special_Issue.255
Eklöf, J. M., and Brumer, H. (2010). The XTH gene family: an update on enzyme structure, function, and phylogeny in xyloglucan remodeling. Plant Physiol. 153, 456–466. doi: 10.1104/pp.110.156844
Estornell, L. H., Agustí, J., Merelo, P., Talón, M., and Tadeo, F. R. (2013). Elucidating mechanisms underlying organ abscission. Plant Sci. 199–200, 48–60. doi: 10.1016/j.plantsci.2012.10.008
Evans, D. E. (2004). Aerenchyma formation. New Phytol. 161, 35–49. doi: 10.1046/j.1469-8137.2003.00907.x
Gu, T., Mazzurco, M., Sulaman, W., Matias, D. D., and Goring, D. R. (1998). Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc. Natl. Acad. Sci. U.S.A. 95, 382–387. doi: 10.1073/pnas.95.1.382
Gunawardena, A. H., Pearce, D. M., Jackson, M. B., Hawes, C. R., and Evans, D. E. (2001). Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212, 205–214. doi: 10.1007/s004250000381
He, C., Finlayson, S. A., Drew, M. C., Jordan, W. R., and Morgan, P. W. (1996). Ethylene biosynthesis during aerenchyma formation in roots of Maize subjected to mechanical impedance and hypoxia. Plant Physiol. 112, 1679–1685.
Ishizaki, K., Chiyoda, S., Yamato, K. T., and Kohchi, T. (2008). Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 49, 1084–1091. doi: 10.1093/pcp/pcn085
Ishizaki, K., Johzuka-Hisatomi, Y., Ishida, S., Iida, S., and Kohchi, T. (2013a). Homologous recombination-mediated gene targeting in the liverwort Marchantia polymorpha L. Sci. Rep. 3, 1532. doi: 10.1038/srep01532
Ishizaki, K., Mizutani, M., Shimamura, M., Masuda, A., Nishihama, R., and Kohchi, T. (2013b). Essential role of the E3 ubiquitin ligase NOPPERABO1 in schizogenous intercellular space formation in the liverwort Marchantia polymorpha. Plant Cell 25, 4075–4084. doi: 10.1105/tpc.113.117051
Jackson, M. B., and Armstrong, W. (1999). Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1, 274–287. doi: 10.1111/j.1438-8677.1999.tb00253.x
Jeffree, C. E., Dale, J. E., and Fry, S. C. (1986). The genesis of intercellular spaces in developing leaves of Phaseolus vulgaris L. Protoplasma 132, 90–98. doi: 10.1007/BF01275795
Jinn, T. L., Stone, J. M., and Walker, J. C. (2000). HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 14, 108–117.
Joshi, R., and Kumar, P. (2012). Lysigenous aerenchyma formation involves non-apoptotic programmed cell death in rice (Oryza sativa L.) roots. Physiol. Mol. Biol. Plant 18, 1–9. doi: 10.1007/s12298-011-0093-3
Justin, S. H. F. W., and Armstrong, W. (1991). Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol. 118, 49–62. doi: 10.1111/j.1469-8137.1991.tb00564.x
Kim, M., Cho, H. S., Kim, D. M., Lee, J. H., and Pai, H. S. (2003). CHRK1, a chitinase-related receptor-like kinase, interacts with NtPUB4, an armadillo repeat protein, in tobacco. Biochim. Biophys. Acta 1651, 50–59. doi: 10.1016/S1570-9639(03)00234-6
Knox, J. P. (1992). Cell adhesion, cell separation and plant morphogenesis. Plant J. 2, 137–141. doi: 10.1111/j.1365-313X.1992.00137.x
Kollöffel, C., and Linssen, P. W. T. (1984). The formation of intercellular spaces in the cotyledons of developing and germinating pea seeds. Protoplasma 120, 12–19. doi: 10.1007/BF01287613
Kubota, A., Ishizaki, K., Hosaka, M., and Kohchi, T. (2013). Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci. Biotechnol. Biochem. 77, 167–172. doi: 10.1271/bbb.120700
Kumpf, R. P., Shi, C. L., Larrieu, A., Sto, I. M., Butenko, M. A., Peret, B., et al. (2013). Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proc. Natl. Acad. Sci. U.S.A. 110, 5235–5240. doi: 10.1073/pnas.1210835110
Lu, D., Lin, W., Gao, X., Wu, S., Cheng, C., Avila, J., et al. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442. doi: 10.1126/science.1204903
Matsunaga, T., Ishii, T., Matsumoto, S., Higuchi, M., Darvill, A., Albersheim, P., et al. (2004). Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 134, 339–351. doi: 10.1104/pp.103.030072
Mbengue, M., Camut, S., De Carvalho-Niebel, F., Deslandes, L., Froidure, S., Klaus-Heisen, D., et al. (2010). The Medicago truncatula E3 ubiquitin ligase PUB1 interacts with the LYK3 symbiotic receptor and negatively regulates infection and nodulation. Plant Cell 22, 3474–3488. doi: 10.1105/tpc.110.075861
McCarthy, T. W., Der, J. P., Honaas, L. A., dePamphills, C. W., and Anderson, C. T. (2014). Phylogenetic analysis of pectin-related gene families in Physcomitrella patens and nine other plant species yields evolutionary insights into cell walls. BMC Plant Biol. 14:79. doi: 10.1186/1471-2229-14-79
Ogawa, M., Kay, P., Wilson, S., and Swain, S. M. (2009). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21, 216–233. doi: 10.1105/tpc.108.063768
Ohi, M. D., Vander Kooi, C. W., Rosenberg, J. A., Chazin, W. J., and Gould, K. L. (2003). Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 10, 250–255. doi: 10.1038/nsb906
Patharkar, O. R., and Walker, J. C. (2015). Floral organ abscission is regulated by a positive feedback loop. Proc. Natl. Acad. Sci. U.S.A. 112, 2906–2911. doi: 10.1073/pnas.1423595112
Patterson, S. E. (2001). Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 126, 494–500. doi: 10.1104/pp.126.2.494
Raven, J. A. (1996). Into the voids: the distribution, function, development and maintenance of gas spaces in plants. Ann. Bot. 78, 137–142. doi: 10.1006/anbo.1996.0105
Raven, J. A. (2002). Selection pressures on stomatal evolution. New Phytol. 153, 371–386. doi: 10.1046/j.0028-646X.2001.00334.x
Renzaglia, K. S., Duff, R. J. T., Nickrent, D. L., and Garbary, D. J. (2000). Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 769–793. doi: 10.1098/rstb.2000.0615
Roberts, J. A., Elliott, K. A., and Gonzalez-Carranza, Z. H. (2002). Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 53, 131–158. doi: 10.1146/annurev.arplant.53.092701.180236
Roland, J. C. (1978). Cell wall differentiation and stages involved with intercellular gas space opening. J. Cell Sci. 32, 325–336.
Sachs, J., Bennett, A. W., Vines, S. H., and Thiselton-Dyer, W. T. (1882). Text-Book of Botany: Morphological and Physiological. Oxford: Clarendon Press.
Samuel, M. A., Mudgil, Y., Salt, J. N., Delmas, F., Ramachandran, S., Chilelli, A., et al. (2008). Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 147, 2084–2095. doi: 10.1104/pp.108.123380
Samuel, M. A., Salt, J. N., Shiu, S. H., and Goring, D. R. (2006). Multifunctional arm repeat domains in plants. Int. Rev. Cytol. 253, 1–26. doi: 10.1016/S0074-7696(06)53001-3
Sarkar, P., Bosneaga, E., and Auer, M. (2009). Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J. Exp. Bot. 60, 3615–3635. doi: 10.1093/jxb/erp245
Seago, J. L. Jr., Marsh, L. C., Stevens, K. J., Soukup, A., Votrubova, O., et al. (2005). A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann. Bot. 96, 565–579. doi: 10.1093/aob/mci211
Sexton, R., and Roberts, J. A. (1982). Cell biology of abscission. Annu. Rev. Plant Physiol. 33, 133–162. doi: 10.1146/annurev.pp.33.060182.001025
Smith, G. M. (1955). Cryptogamic Botany, Vol. 2, Bryophytes and Pteridophytes. London: McGraw-Hill Book Company, Inc.
Stenvik, G. E., Butenko, M. A., Urbanowicz, B. R., Rose, J. K., and Aalen, R. B. (2006). Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 18, 1467–1476. doi: 10.1105/tpc.106.042036
Sugano, S. S., Shirakawa, M., Takagi, J., Matsuda, Y., Shimada, T., Hara-Nishimura, I., et al. (2014). CRISPR/Cas9-mediated targeted mutagenesis in the liverwort Marchantia polymorpha L. Plant Cell Physiol. 55, 475–481. doi: 10.1093/pcp/pcu014
Tewari, R., Bailes, E., Bunting, K. A., and Coates, J. C. (2010). Armadillo-repeat protein functions: questions for little creatures. Trend. Cell Biol. 20, 470–481. doi: 10.1016/j.tcb.2010.05.003
Trought, M. C. T., and Drew, M. C. (1980). The development of waterlogging damage in young wheat plants in anaerobic solution cultures. J. Exp. Bot. 31, 1573–1585. doi: 10.1093/jxb/31.6.1573
Keywords: aerenchyma, cell separation, cell wall remodeling, extracellular signaling, intercellular space formation, Marchantia polymorpha
Citation: Ishizaki K (2015) Development of schizogenous intercellular spaces in plants. Front. Plant Sci. 6:497. doi: 10.3389/fpls.2015.00497
Received: 28 March 2015; Accepted: 22 June 2015;
Published: 02 July 2015.
Edited by:
Takumi Higaki, The University of Tokyo, JapanCopyright © 2015 Ishizaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kimitsune Ishizaki, Graduate School of Science, Kobe University, 1-1 Rokkodai, Nada, Kobe 657-8501, Japan,a2ltaUBlbWVyYWxkLmtvYmUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.