
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 25 March 2015
Sec. Plant Pathogen Interactions
Volume 6 - 2015 | https://doi.org/10.3389/fpls.2015.00170
This article is part of the Research Topic Salicylic acid signaling networks View all 20 articles
Transcriptional regulation is a central process in plant immunity. The induction or repression of defense genes is orchestrated by signaling networks that are directed by plant hormones of which salicylic acid (SA) and jasmonic acid (JA) are the major players. Extensive cross-communication between the hormone signaling pathways allows for fine tuning of transcriptional programs, determining resistance to invaders and trade-offs with plant development. Here, we give an overview of how SA can control transcriptional reprogramming of JA-induced genes in Arabidopsis thaliana. SA can influence activity and/or localization of transcriptional regulators by post-translational modifications of transcription factors and co-regulators. SA-induced redox changes, mediated by thioredoxins and glutaredoxins, modify transcriptional regulators that are involved in suppression of JA-dependent genes, such as NPR1 and TGA transcription factors, which affects their localization or DNA binding activity. Furthermore, SA can mediate sequestering of JA-responsive transcription factors away from their target genes by stalling them in the cytosol or in complexes with repressor proteins in the nucleus. SA also affects JA-induced transcription by inducing degradation of transcription factors with an activating role in JA signaling, as was shown for the ERF transcription factor ORA59. Additionally, SA can induce negative regulators, among which WRKY transcription factors, that can directly or indirectly inhibit JA-responsive gene expression. Finally, at the DNA level, modification of histones by SA-dependent factors can result in repression of JA-responsive genes. These diverse and complex regulatory mechanisms affect important signaling hubs in the integration of hormone signaling networks. Some pathogens have evolved effectors that highjack hormone crosstalk mechanisms for their own good, which are described in this review as well.
The activation of inducible immune responses in the plant is tightly regulated, ensuring an effective and cost-efficient response to pathogenic microbes and herbivorous insects (Vos et al., 2013a). Recognition of an attacker leads to accumulation of signaling molecules like the plant hormones salicylic acid (SA) and jasmonic acid (JA) and its derivatives, which play major roles in the activation of downstream defense responses (reviewed by Pieterse et al., 2012). Generally speaking, SA activates resistance against biotrophic pathogens, while JA is critical for activation of defense against herbivorous insects and necrotrophic pathogens. The SA- and JA-responsive signaling pathways are interdependent and act in complex networks. Other hormones participate in these defense signaling networks as well and can consequently modulate the outcome of the activated defense arsenal. Abscisic acid (ABA) and ethylene can act synergistically with distinct JA-regulated responses, while they generally antagonize SA responses. Auxin, gibberellins, and cytokinins can repress defense-related processes to prioritize growth of the plant, and vice versa their action can be suppressed by SA or JA leading to activation of defense at the expense of plant growth (Pieterse et al., 2012).
Most knowledge on hormone signaling pathways stems from work on the molecular genetic model plant Arabidopsis thaliana. Consequently, this review is based primarily on research with Arabidopsis, but we are aware that other plant species may regulate the interplay between hormone signaling pathways differently. We aim to focus on general mechanisms affecting transcriptional regulation that could also apply to other plant species. Hormone-modulated regulation of disease resistance is primarily achieved through effects on gene transcription. Activation or repression of target genes is accomplished by physical interaction between trans-acting proteins, such as transcription factors, and cis-acting DNA elements. Transcription factors and co-regulators can themselves be controlled at the transcriptional level, but they are also subject to post-translational modification through reduction or oxidation, sequestration, phosphorylation, degradation, or interaction with other transcription factors or co-factors (Moore et al., 2011). Moreover, transcriptional activation is determined by the accessibility of cis-acting elements, which can be influenced by remodeling of chromatin through modifications of histones (Liu et al., 2014).
Transcriptional and post-translational regulatory mechanisms are important in both SA- and JA-controlled signaling pathways. In the SA pathway, activity of NPR1, which was identified as a master transcriptional co-regulator of SA-dependent genes, is tightly regulated by several SA-dependent modifications (reviewed by Fu and Dong, 2013). SA induces a biphasic fluctuation in the cellular redox state that can be sensed by NPR1, which then switches from an oligomer to monomer form by reduction of intermolecular disulfide bonds. Thioredoxins TRX-h5 and TRX-h3 catalyze the formation of NPR1 monomers, which translocate to the nucleus (Figure 1A). Regulation of NPR1 monomer levels in the nucleus is also dependent on SA. NPR1 and NPR1-homologs NPR3 and NPR4 were described to be SA-receptors (Fu et al., 2012; Wu et al., 2012). NPR3 and NPR4 act as CUL3 ligase adapter proteins in proteasome-mediated degradation of NPR1. NPR3 and NPR4 differ in both their binding affinity for SA and binding capacity to NPR1, so that SA levels determine when NPR1 is targeted for degradation. When SA levels are low, NPR4 interacts with NPR1, leading to its degradation, and in this way untimely transcriptional activation in absence of SA is prevented. High SA levels facilitate binding between NPR1 and NPR3, again leading to removal of NPR1 (Fu et al., 2012). This degradation of NPR1 is thought to help activate programmed cell death, of which NPR1 is a negative regulator. When SA levels are intermediate, interaction between NPR1 and NPR3 is prevented, allowing NPR1 to accumulate and activate SA-dependent defenses. By interacting with transcription factors of the TGA family, NPR1 acts as a co-activator of SA-induced gene transcription, activating SA marker genes such as PR1, but also several WRKY transcription factor genes, which then fine-tune and amplify downstream transcriptional responses (Wang et al., 2006; Eulgem and Somssich, 2007).
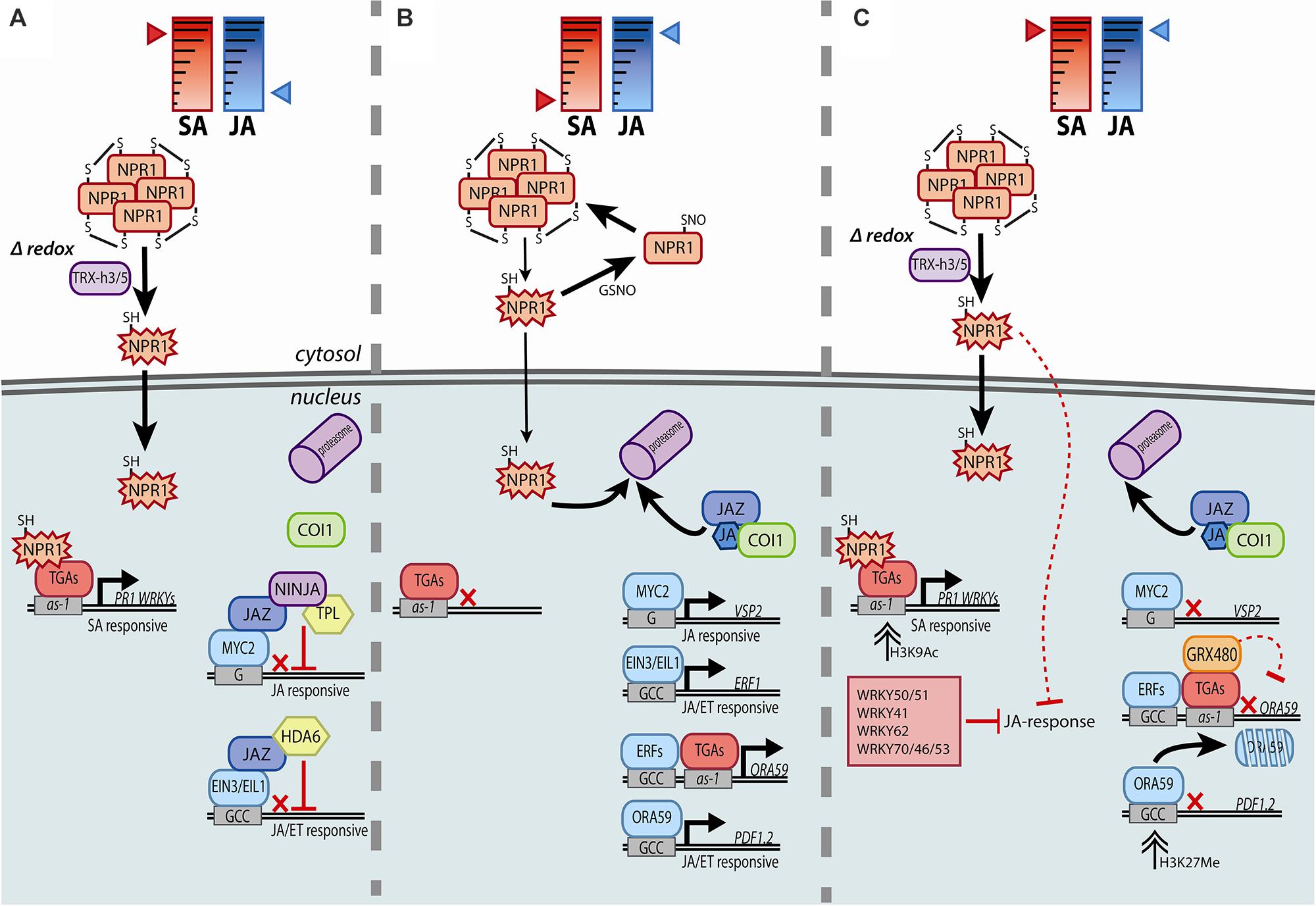
FIGURE 1. Simplified model of the molecular machinery involved in the transcriptional regulation of the SA signaling pathway (A), the JA signaling pathway (B), or the antagonism of SA on the JA signaling pathway (C). By inducing reduction and monomerization of NPR1, SA activates NPR1 (star-shaped), which then triggers gene expression in the nucleus. JA-responsive genes are kept in check by JAZ repressors in the absence of JA. In the presence of JA, MYC or ERF transcription factors activate JA-responsive genes, but only if SA is absent. Activation of both the SA and JA signaling pathways leads to antagonism of JA-responsive gene expression by SA. There are indications for roles in SA/JA crosstalk for cytosolic NPR1, and nuclear localized TGAs, GRX480, and WRKYs. See text for details on the molecular processes underlying the transcriptional control, like redox signaling, sequestration, degradation, phosphorylation, and chromatin modification. Solid lines indicate established (in)activities and dashed lines hypothesized (in)activities, where black arrows specify activation and red blocks suppression. Red crosses indicate that gene transcription is hampered.
Master regulators of the JA signaling pathway are the F-box protein COI1 and the JAZ repressor proteins. In the absence of JA, JAZ repressor proteins associate with the co-repressor TPL via the adapter protein NINJA, or with HDA6, thereby repressing various transcription factors, among which MYC2, EIN3, and EIL1 (Figure 1A; reviewed by Song et al., 2014). COI1 binds to JA-Ile, the bioactive form of JA, which leads to targeting of JAZ repressor proteins for degradation by the proteasome. The successive release of transcriptional activators then leads to activation of JA-responsive genes (Figure 1B). Two branches are distinguished in JA-dependent signaling: (i) MYC2 is the master regulator of the MYC branch, which is co-regulated by JA and ABA, activating downstream marker genes VSP2 and LOX2 (Lorenzo et al., 2004; Vos et al., 2013b), while (ii) EIN3, EIL1, and ERF transcription factors like ERF1 and ORA59 regulate the ERF branch, which is co-regulated by JA and ET, activating the downstream marker gene PDF1.2 (Zhu et al., 2011; Pieterse et al., 2012; Wasternack and Hause, 2013).
Recent work indicates that suppression of the JA-responsive pathway by SA (hereafter also referred to as SA/JA crosstalk) is predominantly regulated at the level of gene transcription (Van der Does et al., 2013). First, SA/JA crosstalk proved to be independent of downregulation of JA biosynthesis itself, as the SA-mediated suppression of MeJA-induced PDF1.2 was intact in the JA biosynthesis mutant aos/dde2 (Leon-Reyes et al., 2010b). Using the JA-receptor mutant coi1-1 ectopically expressing ERF1 to constitutively express downstream JA-responsive genes, Van der Does et al. (2013) further demonstrated that SA can suppress ERF1-activated PDF1.2 independently of COI1. Moreover, using GCC:GUS reporter lines, the GCC-box, which is a crucial cis-element in the regulation of PDF1.2 expression, was shown to be sufficient for SA/JA crosstalk. This indicates that SA antagonizes JA signaling downstream of COI1, possibly by interfering with JA-regulated transcription factors. The ERF transcription factor ORA59 was then demonstrated to be degraded by SA. At the SA signaling side, using mutant npr1-1, master regulator NPR1 was previously shown to be essential for suppression of JA-responsive gene expression (Spoel et al., 2003). Further, several WRKY and TGA transcription factors have been shown to be important for SA/JA crosstalk (Pieterse et al., 2012; Gimenez-Ibanez and Solano, 2013). However, the ways by which these transcriptional regulators down-regulate JA signaling in the presence of SA are largely unknown. In this review, we discuss the regulatory mechanisms that SA employs to repress JA-regulated transcriptional activity. Where relevant, examples of how other hormones interfere with hormone-dependent transcriptional regulation will be given.
The activation of the immune response in plants is associated with rapid production of reactive oxygen intermediates (ROI) and increased levels of nitric oxide (NO). Redox-sensing small-molecule couples, such as reduced and oxidized glutathione, can limit damage from these redox active molecules. Moreover, these redox sensors transduce changes in ROI and NO levels into posttranslational modifications by reduction or oxidation of cysteine residues of transcriptional regulators, causing changes in transcriptional activity (Frederickson and Loake, 2014). Redox signaling is important in SA signaling and moreover, SA-induced redox changes are associated with the suppression of JA responses as well.
In SA signaling, master regulator NPR1 is subject to several redox-dependent modifications. It sequesters in the cytoplasm as an oligomer, formed by intermolecular disulfide bonds, which are facilitated by S-nitrosylation of cysteine residues via NO donor S-nitrosoglutathione (GSNO; Figure 1B). SA triggers cycles of cellular reduction and oxidation, measurable for example by enhanced total glutathione levels and a higher ratio of reduced to oxidized glutathione after SA treatment (Spoel and Loake, 2011). In response to activation of the SA pathway, thioredoxins catalyze the reduction of intermolecular disulphide bonds, causing a conformational change of NPR1 to its monomeric form. As a monomer, NPR1 is able to translocate from the cytosol to the nucleus and activate downstream signaling (Figure 1A) (Mou et al., 2003; Koornneef et al., 2008a; Tada et al., 2008). Other transcriptional regulators functioning in the SA pathway are also redox controlled. Transcription factor TGA1 contains intramolecular disulfide bonds that prevent its interaction with NPR1. Only after reduction of these bonds under high SA conditions, TGA1 is able to interact with NPR1. Further S-nitrosylation and S-glutathionylation of the cysteine residues of TGA1 result in enhanced binding to DNA and activation of transcription (Figure 1A; Després et al., 2003; Lindermayr et al., 2010).
Redox-mediated reduction of transcriptional regulators is not only essential for SA signaling, but is also implicated in SA/JA crosstalk. The enhancement in glutathione levels after SA treatment was shown to coincide exactly with the window of opportunity in which SA could suppress JA-induced PDF1.2 expression, i.e., within 30 h after application of SA. In addition, treatment with glutathione synthesis inhibitor BSO blocked SA-mediated antagonism of PDF1.2 expression (Koornneef et al., 2008a). Interestingly, JA can also influence the redox state of cells, but, in contrast to SA, it decreases the total amount of glutathione, and shifts the ratio between reduced and oxidized glutathione toward the oxidized state (Spoel and Loake, 2011). When SA and JA were applied simultaneously, the pattern of glutathione increase was the same as after treatment with SA alone, suggesting a role for redox regulation in prioritization of the SA pathway over the JA pathway (Koornneef et al., 2008a). So far, it is unclear how the SA-induced cellular reduction can influence JA-inducible responses.
Master regulator NPR1 is essential for SA/JA crosstalk and, therefore, the importance of SA-induced redox changes in SA/JA crosstalk could be related to reduction and translocation of NPR1 to the nucleus. However, the nuclear localization of NPR1 that follows SA-induced monomerization is, although essential for SA-responsive gene expression, not needed for SA-mediated suppression of JA-dependent genes (Spoel et al., 2003; Leon-Reyes et al., 2009). This was shown with Arabidopsis plants that overexpress a fusion protein of NPR1 that was retained in the cytosol: stimulation of the SA pathway in these plants resulted in a wild-type level of suppression of JA-induced PDF1.2 (Spoel et al., 2003). The role of NPR1 in the cytoplasm for SA/JA crosstalk was confirmed in rice (Oryza sativa), where overexpression of OsNPR1 suppressed JA-responsive gene expression and defense against insects. However, when a mutated form of OsNPR1 was overexpressed that was constitutively present in the nucleus, herbivore resistance and expression of a JA-responsive gene were not affected (Yuan et al., 2007). Although NPR1 is exclusively needed in the cytosol for SA/JA crosstalk, it is still possible that redox-mediated modification of NPR1 is important in SA/JA crosstalk, for example if there is a role for the monomeric form of NPR1 in the cytosol to suppress JA signaling (Spoel et al., 2003; Beckers and Spoel, 2006). Alternatively, redox signaling may be important for post-translational modification of other factors with a role in SA/JA crosstalk, as described below.
The importance of redox regulation in SA/JA crosstalk is supported by the role of glutaredoxins (GRXs) in this phenomenon. GRXs are small ubiquitous redox enzymes that use glutathione to reduce their targets (Ndamukong et al., 2007; Ströher and Millar, 2012). SA is known to induce the expression of at least two GRXs, namely GRX480 and GRXS13, which are members of the group III class of GRXs in Arabidopsis. Overexpression of GRX480 blocks the induction of PDF1.2 by JA, and overexpression of GRXS13 makes plants more susceptible to the necrotrophic fungus Botrytis cinerea, suggesting a role for both GRXs in suppression of JA signaling (Ndamukong et al., 2007; Camera et al., 2011). In fact, 10 more group III GRXs, which are also called ROXYs, are able to suppress activation of the ORA59 promoter and are thus potentially involved in suppression of the JA pathway (Zander et al., 2012). Their antagonistic action on JA responses is likely downstream of NPR1, because expression of GRX480 is reduced in the npr1-1 mutant and overexpression of GRX480 in the npr1-1 background still results in suppression of PDF1.2 expression (Zander et al., 2012; Herrera-Vásquez et al., 2014). TGA transcription factors that are implicated in different hormonal signaling pathways and in SA/JA crosstalk (described more in-depth in SA-Inducible Expression of Transcription Factor Genes that Suppress JA Responses) are possible targets of group III GRXs, as they are shown to interact with each other (Figure 1C). Moreover, JA-induced PDF1.2 expression is not impaired when GRX480 is overexpressed in the triple mutant tga2/tga5/tga6 background, showing that the function of this GRX in suppression of JA-responses is dependent on these TGA transcription factors (Ndamukong et al., 2007; Zander et al., 2012).
Salicylic acid could antagonize JA signaling by preventing accessibility of JA-responsive transcriptional regulators to their target genes. This could be achieved by sequestering transcription factors in inactive complexes or by degradation of positive regulators.
By directing transcription factors to the cytosol, the possibility to activate transcription is obviously obstructed. In addition, transcription factors can be kept in check in the nuclear compartment as well, by inducing complex formation with other proteins that inhibit binding to the DNA, resulting in reduced transcription. There are no examples yet of SA-mediated sequestration of transcription factors leading to antagonism of JA signaling. However, some other plant hormone signaling interactions have been reported to be partly regulated via this mechanism, of which an example is the interaction between the SA and the ABA signaling pathways. The transcription factor WRKY40 is induced by SA and suppresses expression of the ABA-responsive genes ABI4 and ABI5. After ABA treatment, the ABA receptor ABAR interacts with WRKY40, which is then recruited to the cytosol. By this recruitment, binding of WRKY40 to ABA responsive promoters is inhibited and repression of ABA responsive genes is lifted (Shang et al., 2010; Liu et al., 2012).
In animal cells, cytosolic sequestration of a transcriptional regulator was shown to control the antagonistic interaction between SA and prostaglandin signaling, which shares several aspects with SA/JA crosstalk in plants. SA and aspirin block the formation of prostaglandins in animal cells, which are considered structural analogs of JA in plants. SA induces retention of transcription factor NF-κB in the cytoplasm by enforcing its interaction with IκB. In response to stress, IκB kinase is activated and degrades IκB, leading to nuclear localization of NF-κB, which then activates gene expression, necessary for the production of prostaglandins. In cells that are exposed to SA, degradation of IκB is inhibited, which prevents the nuclear translocation of NF-κB. Interestingly, IκB in animals has structural similarity with NPR1 (reviewed by Spoel and Dong, 2012). In plants, the cytosolic location of NPR1 is important for SA-mediated antagonism of JA-responsive gene expression (Spoel et al., 2003; Stein et al., 2008). One possible function for cytosolic NPR1 is that it may sequester JA-regulated transcriptional activators in the cytoplasm, thereby preventing them from moving to the nucleus and activating transcription. However, whether SA can interfere with translocation of JA-responsive transcription factors to the nucleus remains to be demonstrated.
In the nucleus, transcription factors can be prevented from binding DNA and thus activating gene expression by interacting with repressor proteins, which have been reported to function as important regulators in several hormone signaling pathways (Robert-Seilaniantz et al., 2011). JAZ proteins in the JA pathway are examples of such repressors. JA-induced ubiquitination of JAZ proteins mediates their degradation via the 26S proteasome, which releases their repressive effect on positive transcriptional regulators. By increasing the stability of repressor proteins, hormones can antagonize another hormone’s action. An example of this crosstalk mechanism is found in the SA-auxin interaction. Parallel to JAZ repressor proteins in the JA pathway, AUX-IAA proteins are the negative regulators that bind and inactivate activators of auxin signaling. Binding of auxin to F-box proteins TIR1 and TIR1-related proteins, which act as auxin receptors, leads to degradation of AUX-IAA repressors. SA was shown to inhibit the auxin signaling pathway through stabilization of AUX/IAA repressor proteins, probably indirectly through repression of TIR1. In this way, SA could lift the disease promoting effect of auxin in the infection of Arabidopsis by Pseudomonas syringae (Wang et al., 2007). Also crosstalk between JA and GA pathways is regulated through interaction with their key repressor proteins, JAZs and DELLAs, respectively. In the absence of GA, stabilized DELLA can interact with JAZ proteins, thus reducing the repressive effect of JAZ on JA-responsive gene expression. DELLAs are degraded when GA levels rise, leading to enhanced suppression of JA signaling by JAZs (Hou et al., 2010; Pieterse et al., 2014). On the other hand, JA delays GA-mediated degradation of DELLAs, which is associated with a reduction in growth, suggesting that the trade-off between JA-dependent defense and GA-dependent growth can be regulated by the DELLA-JAZ signaling module (Yang et al., 2012). There is no evidence, however, that SA interferes with the stability of JAZs to antagonize JA signaling. First, JAZ1 and JAZ9, two of the most important JAZ proteins, are still degraded in JA-treated Arabidopsis when plants are additionally treated with SA. Second, SA was shown to antagonize the JA signaling pathway downstream of COI1, the F-box protein that interacts with JAZ repressor proteins to target them for ubiquitination (Van der Does et al., 2013).
Salicylic acid-induced degradation of activating transcription factors of JA signaling could contribute to the repression of JA-responsive genes. Recently, SA was shown to lead to degradation of ORA59, a positive regulator in the ERF branch of the JA pathway. A whole-genome expression profiling analysis showed that the GCC-box was overrepresented in MeJA-induced genes that were antagonized by SA at 24 h after treatment with a combination of the hormones. The GCC-box was subsequently shown to be sufficient for suppression by SA (Van der Does et al., 2013). Similarly, the GCC-box was enriched in promoters of ethylene-induced genes that were suppressed by SA (Zander et al., 2014). The GCC-box is an essential promoter element for activation of PDF1.2 expression and ERF transcription factor ORA59 is an important regulator in this activation (Zarei et al., 2011). Van der Does et al. (2013) suggested that downregulation of transcription of ORA59 is not essential for SA/JA crosstalk, but showed that protein levels of ORA59 diminished after SA treatment, suggesting that SA could target positive regulators in the JA pathway for degradation. So far, degradation of other positive regulators of JA signaling has not been reported. The degradation rate of MYC2, master regulator of the MYC branch in the JA pathway, is likely not influenced by SA (Chico et al., 2014).
Perception of pathogenic microbes by the plant leads to activation of mitogen-activated protein kinases (MPKs) that can subsequently phosphorylate transcriptional regulators. Phosphorylation of transcription factors influences gene transcription by changing the binding strength to DNA, or affecting sequestration or stability (Tena et al., 2011). In particular MPK3, MPK4, and MPK6, which act at the last step of MAPK signaling cascades, are known to phosphorylate transcription factors and have been implicated in immune signaling (Meng and Zhang, 2013). For example, phosphorylation of WRKY33 by MPK3 and MPK6 is likely responsible for the WRKY33-mediated induction of the WRKY33 gene itself and of PAD3, which is a camalexin biosynthesis gene (Mao et al., 2011). It has also been suggested that WRKY33 is controlled by sequestration in a complex with MKS1 and MPK4. Upon bacterial pathogen attack the activated MAPK signaling cascade phosphorylates MKS1, which leads to disassociation from MPK4 so that WRKY33 is released from the complex and could bind to the promoter of PAD3 (Qiu et al., 2008).
There is not much known about the role of MAPK cascades in the interplay between different hormone pathways. MAPK cascades are important in the JA pathway, so inhibition of MAPK cascades by SA could be an effective way to antagonize JA signaling. For example, JA activates MPK6 and many AP2/ERFs transcription factors are phosphorylated and activated by MPK6, among which positive regulators ERF6 and ERF104 (Takahashi et al., 2007; Bethke et al., 2009; Popescu et al., 2009; Meng et al., 2013). It is not known if SA can prevent this phosphorylation to inhibit activation of the JA-regulated AP2/ERF transcription factors. MPK4 was thought to function as an integrator of SA and JA signaling as the mutant mpk4 constitutively expresses SA-inducible PR genes and fails to express PDF1.2, which correlates with enhanced resistance to biotrophic pathogens and increased susceptibility to necrotrophic pathogens (Petersen et al., 2000; Brodersen et al., 2006). However, recently it was suggested that MPK4 is guarded by the R protein SUMM2. Reduction of the kinase activity of MPK4 by the bacterial effector HopAI1 is monitored by SUMM2, and leads to activation of SA-dependent defense responses (Zhang et al., 2012b). The effects of MPK4 on SA signaling are thus indirect, and this makes a role for MPK4 as an integrator of SA and JA signaling unlikely. However, whether MPK4’s role in JA signaling is a direct or indirect one needs to be studied further.
Salicylic acid may also antagonize JA-inducible gene transcription by inducing the expression of genes encoding transcriptional regulators that interfere with JA signaling. These SA-induced regulators could inhibit a positive regulator of JA-inducible gene expression by interacting with it, as is described for the GRX480-TGA interaction in “Role of Reduction of Transcriptional Regulators in SA Signaling.” Alternatively, SA could induce transcription of suppressive transcription factors that directly bind to the promoter of JA responsive genes to repress their expression. Examples of TGA, ERF, WRKY, and bHLH transcription factors that are induced by SA and inhibit JA-dependent transcription are reviewed below.
TGA transcription factors have a role in various hormone-regulated transcriptional responses. They can generally activate SA-dependent gene expression, but are also known to have both positive and negative effects on JA/ethylene-dependent responses. TGA transcription factors are a class of bZIP transcription factors that bind to the as-1 element (TGACG) in promoters. In Arabidopsis, 10 TGAs exist of which several have been shown to interact with NPR1 (reviewed by Gatz, 2013). The PR1 promoter contains an as-1 element, and the triple mutant tga2/tga5/tga6 is, like npr1, compromised in SAR and does not express PR1 upon treatment with the SA-mimic INA (Zhang et al., 2003). In response to SA, a ternary complex of TGA, NPR1, and DNA is formed that can activate transcription of PR1 (Figure 1A). In non-induced conditions, suppression of PR1 by TGAs has also been reported (Rochon et al., 2006; Pape et al., 2010). TGAs are important for activation of JA/ethylene-dependent genes as well. Although mutant tga2/tga5/tga6 adult plants responded with PDF1.2 induction upon treatment with JA, they did not express PDF1.2 in response to ethylene or B. cinerea infection (Zander et al., 2010).
In addition, TGAs can be essential for suppression of JA responsive genes by SA, as JA-induced PDF1.2 is not suppressed after a combination treatment with SA in mutant tga2/tga3/tga5/tga6 (Leon-Reyes et al., 2010a). Microarray analysis comparing wild-type and tga2/tga5/tga6 mutant plants showed that after treatment with ethylene precursor ACC, 374 genes were induced in wild-type plants, of which 136 were dependent on TGA2/TGA5/TGA6. Half of these ACC-inducible TGA-dependent genes were, in wild-type plants, suppressed by SA after a combination treatment of ACC with SA. This suggests a role for TGAs in both activation of ethylene-responsive genes and SA-mediated repression of these genes (Zander et al., 2014). The PDF1.2 promoter contains an as-1 element, but this was shown not to be important for the antagonistic effect on JA-induced PDF1.2 expression by SA (Spoel et al., 2003). However, Zander et al. (2014) showed that the TGAs directly target the as-1 element in the promoter of ORA59 and could regulate both induction of ORA59 by ACC treatment and suppression of ORA59 by SA (Figure 1C). Transcriptional regulation of ORA59 by TGAs is in line with the observation that the GCC-box is enriched in the promoter elements of ACC-induced, SA-suppressed genes. How can TGA factors act as both activators and repressors in different hormone signaling pathways? Possibly, different co-factors can be recruited to TGA factors depending on both the promoter context and the hormonal context. In the case of activation of transcription by SA, TGAs have been shown to interact with transcriptional activators NPR1 and GRAS protein SLC14 (Rochon et al., 2006; Fode et al., 2008). Upon JA accumulation, TGAs may interact with so-far unknown JA signaling regulators to promote JA responsive gene expression. When SA/JA crosstalk is activated, SA induces GRXs, which could interact with TGAs on the ORA59 promoter leading to repression of JA-inducible genes (Figure 1C). GRXs were shown to down-regulate ORA59 expression in a TGA-dependent manner, as discussed in “Role of Reduction of Transcriptional Regulators in SA Signaling” (Zander et al., 2012).
Both Zander et al. (2014) and Van der Does et al. (2013) point to ORA59 as a major target of antagonism by SA. However, while the first show that SA targets expression of ORA59, the protein levels of ORA59 were shown to be influenced by SA by the latter. The apparent discrepancy between these two studies could partly be explained by the different combination of hormones that both groups studied, SA-ethylene or SA–JA, respectively. Support for differences in crosstalk mechanisms depending on hormonal context comes from the observation that in an ethylene-rich environment the SA-antagonized expression of JA-inducible PDF1.2 became independent of NPR1 (Leon-Reyes et al., 2009) or was even completely impaired when plant tissue was exposed to high levels of ethylene prior to treatment with SA (Leon-Reyes et al., 2010a). However, it is very well possible that ORA59 is regulated by SA at both the transcriptional and post-translational level, and that both mechanisms complement each other (Figure 1C).
Transcription factors of the ERF subfamily of AP2/ERF family of transcription factors can bind the GCC-box and can act as activators, such as ORA59, but also as repressors of transcription. Fourteen of the 122 ERFs in Arabidopsis contain an EAR domain, which is an active repressor domain that interacts with the general co-repressor TPL (Nakano et al., 2006). EAR-domain-containing ERF4 and ERF9 were shown to be able to suppress PDF1.2 expression (McGrath et al., 2005; Maruyama et al., 2009). Because of the importance of the GCC-box in SA/JA crosstalk, the suppression of JA-responsive genes may, besides through negative regulation of ORA59 by SA (covered in SA-Mediated Degradation of JA-Regulated Transcription Factors and TGA Transcription Factor Family), in part be regulated by suppressive SA-induced ERFs. This hypothesis has up to now not been tested.
WRKY transcription factors are foremost known for their inducibility by SA and pathogens, and their role in regulating SA-dependent gene expression. There are, however, also examples of WRKYs that positively regulate other hormone-regulated genes, including JA-responsive defense genes (Journot-Catalino et al., 2006; Xu et al., 2006). The W-box (C/TTGACC/T) is a DNA element that is bound by WRKY transcription factors (Eulgem and Somssich, 2007). Importantly, the W-box motif was reported to be enriched in JA-responsive genes that were antagonized by SA (Van der Does et al., 2013), suggesting the involvement of WRKYs in SA/JA crosstalk as well. Indeed, several WRKYs have been implicated in suppression of JA-induced PDF1.2 expression (Figure 1C). Overexpression of SA-induced WRKY70 suppressed MeJA-induced PDF1.2 expression (Li et al., 2004, 2006). However, in a wrky70 mutant, JA-dependent genes were induced by JA and suppressed by the combination treatment, indicating that WRKY70 is sufficient but not required for SA/JA crosstalk (Ren et al., 2008; Leon-Reyes et al., 2010a). Redundancy of different WRKYs could possibly explain the lack of a crosstalk phenotype of the single wrky70 mutant, as double and triple mutants of wrky70 with wrky46 and wrky53 did show enhanced PDF1.2 expression after MeJA treatment (Hu et al., 2012). Overexpression of the transcription factor MYB44 also led to suppression of the JA marker genes VSP1 and PDF1.2, which is likely established through activation of WRKY70. MYB44 is inducible by SA and binds to the WRKY70 promoter leading to its expression (Shim et al., 2013; Zou et al., 2013). Furthermore, WRKY62 was suggested to function in suppression of JA responses, because a wrky62 mutant displayed enhanced expression of JA responsive genes, while an overexpressor exhibited reduced expression. WRKY62 is induced by SA and was suggested to act downstream of cytosolic NPR1 (Mao et al., 2007). To end, WRKY41 has been implicated in suppression of JA responsiveness, since overexpression of WRKY41 led to increased PR5 and reduced PDF1.2 expression. However, in contrast to the aforementioned WRKY genes, WRKY41 is likely not a direct target of NPR1 and SA only slightly induces WRKY41 expression (Higashi et al., 2008).
Studies on the ssi2 mutant revealed two other WRKYs that are involved in SA/JA crosstalk. The ssi2 mutant was initially identified in a screen for npr1 suppressors and displays high SA responses while JA responses are repressed (Shah et al., 2001). The increased SA levels were not needed for the repression of JA responses, but instead lowered levels of 18:1 fatty acids appeared to regulate the repression of JA signaling (Kachroo et al., 2001, 2003; Nandi et al., 2005). In ssi2 mutants, 19 WRKYs were induced, of which five in a SA-independent manner. Double mutants of ssi2 with wrky50 or wrky51 restored the induction of PDF1.2 and resistance against B. cinerea without altering the 18:1 fatty acid levels. WRKY50 and WRKY51 thus negatively regulate JA responses under low 18:1 conditions. Single and double mutants of wrky50 and wrky51 also failed to suppress PDF1.2 and VSP2 after a combination treatment with SA and JA (Gao et al., 2011). Therefore, these two WRKYs seem to play important roles in the suppression of JA responses.
How can WRKY transcription factors repress JA responses? After their induction by SA, they could bind to W-boxes in JA-responsive genes to inhibit their expression directly or indirectly (Van der Does et al., 2013). There is no experimental proof of this repressive mechanism under the influence of SA yet, but recently WRKY51 has been reported to interact with JAV1, a VQ-motif containing protein that negatively regulates JA responses and acts in the nucleus (Hu et al., 2013).
Transcription factors of the bHLH family, including MYC2, play crucial roles in the JA signaling pathway. MYC2 is a master regulator of JA responses (reviewed by Kazan and Manners, 2013). The last 2 years have witnessed an boost in bHLHs that function as negative regulators in the JA signaling pathway (Nakata et al., 2013; Sasaki-Sekimoto et al., 2013; Song et al., 2013; Fonseca et al., 2014). Whether these repressive bHLHs are manipulated by SA to establish SA/JA crosstalk is currently unknown, but they are not obviously regulated at the transcription level by SA (BAR public database).
Salicylic acid can further control gene expression by remodeling of chromatin around target genes. Chromatin is the complex of DNA and histones and its condensed structure can reduce accessibility of DNA and thus inhibit transcription. Modifications of chromatin can result in local loosening of this structure, which creates access for transcriptional machinery and regulatory proteins to the DNA. Chromatin modifications include methylation, acetylation, phosphorylation, ubiquitination, or sumoylation of histones (Iwasaki and Paszkowski, 2014). Acetylation of histones is associated with activation of genes, while deacetylation of histones is correlated with gene repression. Enzymes called histone acyltransferases and histone deacetylases (HDA) can carry out these respective histone modifications (Liu et al., 2014). HDA6 and HDA19 were described to interfere with JA signaling. HDA6 interacts with JAZ1, JAZ3, and JAZ9 and is recruited to repress EIN3/EIL1-dependent transcription (Zhu et al., 2011). In contrast, HDA19 was reported to have a positive role in the ERF branch and in defense against Alternaria brassicicola (Zhou et al., 2005). HDA19 also targets SA signaling by binding to the PR1 and PR2 promoters leading to their repression (Choi et al., 2012), and by reducing transcriptional activity of WRKY38 and WRKY62 (Kim et al., 2008). Since chromatin remodeling plays an important role in SA and JA signaling, it could also well be manipulated by SA to antagonize JA signaling. However, Koornneef et al. (2008b) showed that at the PDF1.2 promoter there was no change in acetylation of histones after exogenous application of a combination of SA and MeJA.
Chromatin modifications are also described to be an important mechanism to prime plants for enhanced defense (Conrath, 2011). Interestingly, it was suggested that priming and SA/JA crosstalk could be carried over to offspring through acetylation and methylation of histones as well. Luna et al. (2012) showed that Arabidopsis plants that were inoculated with the bacterial pathogen P. syringae in the first generation, were more resistant to P. syringae and the oomycete pathogen Hyaloperonospora arabidopsidis in the next generation, and more susceptible to the necrotrophic pathogen A. brassicicola. This correlated with increased PR1 expression and reduced VSP2 and PDF1.2 expression in the second generation and was dependent on NPR1. Acetylation of histone H3 at Lys-9 (H3K9) at the PR1 promoter, which is associated with increased transcription, was enhanced in these plants. Conversely, tri-methylation of H3K27, which is associated with transcriptional silencing, was enriched at the PDF1.2 promoter (Figure 1C), suggesting that histone modifications were responsible for the observed increased or decreased transcription (Luna et al., 2012). It is not clear yet how these changes can be transmitted to offspring, since there is no evidence that histone modifications are inherited. DNA methylation, which is often associated with histone modifications, is a possible modification that could be passed on to next generations. DNA methylation was shown to have an effect on SA- and JA-regulated responses: epiRIL lines, which are identical at the DNA sequence level but highly variable at the level of DNA methylation, showed differences in responsiveness to both treatments (Latzel et al., 2012).
In the evolutionary arms race, pathogens have evolved effectors that are secreted into plant cells upon infection to reduce disease resistance or increase plant susceptibility (reviewed by Kazan and Lyons, 2014). Interestingly, several pathogen effectors can highjack a plant’s intricate hormonal crosstalk mechanism for their own good, resulting in lower induction of effective defenses. Some effectors are hormones themselves or are hormone-mimics that disturb the hormone balance in plants. The most famous example of such an effector is the JA-mimic coronatine, that is secreted by Pseudomonas pathogens and suppresses SA signaling (Zheng et al., 2012). More recently, effectors that interfere with signaling hubs in transcriptional regulation of JA signaling, such as JAZs, have been discovered. Effectors HopZ1a and HopX1 of two different Pseudomonas pathogen strains bind to and degrade JAZ repressor proteins, leading to activation of JA signaling and concomitant suppression of SA-regulated defense signaling (Jiang et al., 2013; Gimenez-Ibanez et al., 2014).
Other effectors can establish antagonism of SA signaling by manipulating the plant transcriptional machinery via interference with Mediator subunits. Mediator is a multi-protein transcriptional co-activator complex, which functions as a bridge between transcription factors and RNA polymerase II. Mediator recruits RNA polymerase II to promoters in response to different signals and controls the polymerase activity during transcription initiation and elongation (Conaway and Conaway, 2011). Several Mediator subunits have been implicated in SA- and/or JA-dependent gene expression. Mediator subunit MED16 was shown was shown to be important in defense against both biotrophic and necrotrophic pathogens by regulating SA- and JA/ethylene-responsive transcription and could therefore be viewed as a node of convergence between SA- and JA/ethylene-dependent pathways (Wathugala et al., 2012; Zhang et al., 2012a). Subunit MED25 was shown to be important for activation of JA-dependent genes, and likely acts through interaction with JA-responsive transcription factors, including ERF1, ORA59, and MYC2 (Çevik et al., 2012). The subunit MED19 positively regulates SA-dependent resistance that is effective against H. arabidopsidis. MED19 was shown to be targeted for degradation by the H. arabidopsidis effector HaRxL44. Expression of HaRxL44 in plants led to induction of JA-responsive genes, a response that is observed in med19 plants as well (Caillaud et al., 2013). These data suggest that HaRxL44 induces degradation of MED19 to rewire transcription from SA-responsive to JA-responsive, leading to enhanced infection by H. arabidopsidis. This example illustrates the highly sophisticated manner in which effectors manipulate the plant transcriptional machinery to influence hormonal signaling.
In the last years, knowledge on the interplay between different plant hormone signaling pathways has vastly increased. In this review we focused on the molecular mechanisms (potentially) underlying antagonistic effects of SA on JA-mediated transcriptional responses and highlighted several transcriptional regulators (like NPR1, TGA, WRKY, and ORA59) as signal integrators. However, there is still much unknown about hormonal crosstalk mechanisms. The use of whole-transcriptome sequencing techniques after combinatorial hormone treatment or pathogen infection will aid in the identification and characterization of additional transcriptional regulators that can act as nodes of convergence in multiple signaling pathways (Van Verk et al., 2013). Combining transcriptome data with ChIP-seq or DNase-seq studies, which can identify DNA sites occupied by transcription factors, can provide more detailed knowledge on the mechanisms by which these crosstalk transcriptional regulators rewire hormonal signaling. In addition, more intensive proteomic studies are necessary to get a full scale picture of the posttranslational modifications that influence the action of key transcriptional regulators. The knowledge gained from pharmacological experiments, in which combinations of hormones are applied exogenously, should be corroborated under biological conditions that trigger hormonal crosstalk, like (combinatorial) pathogen infection. Insights into the crosstalk signaling hubs that function in complex hormonal signaling networks will not only increase our fundamental knowledge on plant immune signaling but can also provide leads to develop crops with multi-attacker resistance and optimal growth.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors of this review are supported by VIDI grant no. 11281 of the Dutch Technology Foundation STW, which is part of the Netherlands Organization of Scientific Research, and ERC Advanced Investigator Grant no. 269072 of the European Research Council.
Beckers, G. J. M., and Spoel, S. H. (2006). Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol. 8, 1–10. doi: 10.1055/s-2005-872705
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bethke, G., Unthan, T., Uhrig, J. F., Poschl, Y., Gust, A. A., Scheel, D.,et al. (2009). Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 8067–8072. doi: 10.1073/pnas.0810 206106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brodersen, P., Petersen, M., Bjorn Nielsen, H., Zhu, S., Newman, M.-A., Shokat, K. M.,et al. (2006). Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 47, 532–546. doi: 10.1111/j.1365-313X.2006.02806.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Caillaud, M.-C., Asai, S., Rallapalli, G., Piquerez, S., Fabro, G., and Jones, J. D. G. (2013). A downy mildew effector attenuates salicylic acid-triggered immunity in Arabidopsis by interacting with the host mediator complex. PLoS Biol. 12:e1001919. doi: 10.1371/journal.pbio.1001732
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Camera, S. L., L’Haridon, F., Astier, J., Zander, M., Abou-Mansour, E., Page, G.,et al. (2011). The glutaredoxin ATGRXS13 is required to facilitate Botrytis cinerea infection of Arabidopsis thaliana plants. Plant J. 68, 507–519. doi: 10.1111/j.1365-313X.2011.04706.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Çevik, V., Kidd, B. N., Zhang, P., Hill, C., Kiddle, S., Denby, K. J.,et al. (2012). MEDIATOR25 acts as an integrative hub for the regulation of jasmonate-responsive gene expression in Arabidopsis. Plant Physiol. 160, 541–555. doi: 10.1104/pp.112.202697
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chico, J.-M., Fernández-Barbero, G., Chini, A., Fernández-Calvo, P., Díez-Díaz, M., and Solano, R. (2014). Repression of jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. Plant Cell 26, 1967–1980. doi: 10.1105/tpc.114.125047
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Choi, S.-M., Song, H.-R., Han, S.-K., Han, M., Kim, C.-Y., Park, J.,et al. (2012). HDA19 is required for the repression of salicylic acid biosynthesis and salicylic acid-mediated defense responses in Arabidopsis. Plant J. 71, 135–146. doi: 10.1111/j.1365-313X.2012.04977.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Conaway, R. C., and Conaway, J. W. (2011). Function and regulation of the Mediator complex. Curr. Opin. Genet. Dev. 21, 225–230. doi: 10.1016/j.gde.2011.01.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Conrath, U. (2011). Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. doi: 10.1016/j.tplants.2011.06.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Després, C., Chubak, C., Rochon, A., Clark, R., Bethune, T., Desveaux, D.,et al. (2003). The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15, 2181–2191. doi: 10.1105/tpc.012849
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eulgem, T., and Somssich, I. E. (2007). Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10, 366–371. doi: 10.1016/j.pbi.2007.04.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fode, B., Siemsen, T., Thurow, C., Weigel, R., and Gatz, C. (2008). The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 20, 3122–3135. doi: 10.1105/tpc.108.058974
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fonseca, S., Fernández-Calvo, P., Fernández, G. M., Díez-Díaz, M., Gimenez-Ibanez, S., López-Vidriero, I.,et al. (2014). bHLH003, bHLH013 and bHLH017 are new targets of JAZ repressors negatively regulating JA responses. PLoS ONE 9:e86182. doi: 10.1371/journal.pone.0086182
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Frederickson, D. E., and Loake, G. J. (2014). Redox regulation in plant immune function. Antioxid. Redox Signal. 21, 1373–1388. doi: 10.1089/ars.2013.5679
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fu, Z. Q., and Dong, X. (2013). Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863. doi: 10.1146/annurev-arplant-042811-105606
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fu, Z. Q., Yan, S., Saleh, A., Wang, W., Ruble, J., Oka, N.,et al. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232. doi: 10.1038/nature11162
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gao, Q.-M., Venugopal, S., Navarre, D., and Kachroo, A. (2011). Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 155, 464–476. doi: 10.1104/pp.110.166876
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gatz, C. (2013). From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant Microbe Interact. 26, 151–159. doi: 10.1094/MPMI-04-12-0078-IA
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gimenez-Ibanez, S., Boter, M., Fernandez-Barbero, G., Chini, A., Rathjen, J. P., and Solano, R. (2014). The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 12:e1001792. doi: 10.1371/journal.pbio.1001792
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gimenez-Ibanez, S., and Solano, R. (2013). Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Front. Plant Sci. 4:72. doi: 10.3389/fpls.2013.00072
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Herrera-Vásquez, A., Carvallo, L., Blanco, F., Tobar, M., Villarroel-Candia, E., Vicente-Carbajosa, J.,et al. (2014). Transcriptional control of glutaredoxin GRXC9 expression by a salicylic acid-dependent and NPR1-independent pathway in Arabidopsis. Plant Mol. Biol. Rep. doi: 10.1007/s11105-014-0782-5
Higashi, K., Ishiga, Y., Inagaki, Y., Toyoda, K., Shiraishi, T., and Ichinose, Y. (2008). Modulation of defense signal transduction by flagellin-induced WRKY41 transcription factor in Arabidopsis thaliana. Mol. Genet. Genomics 279, 303–312. doi: 10.1007/s00438-007-0315-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hou, X., Lee, L. Y. C., Xia, K., Yen, Y., and Yu, H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19, 884–894. doi: 10.1016/j.devcel.2010.10.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, P., Zhou, W., Cheng, Z., Fan, M., Wang, L., and Xie, D. (2013). JAV1 controls jasmonate-regulated plant defense. Mol. Cell 50, 504–515. doi: 10.1016/j.molcel.2013.04.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, Y., Dong, Q., and Yu, D. (2012). Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci. 185, 288–297. doi: 10.1016/j.plantsci.2011.12.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Iwasaki, M., and Paszkowski, J. (2014). Epigenetic memory in plants. EMBO J. 33, 1987–1998. doi: 10.15252/embj.201488883
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jiang, S., Yao, J., Ma, K.-W., Zhou, H., Song, J., He, S. Y.,et al. (2013). Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog. 9:e1003715. doi: 10.1371/journal.ppat.1003715
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Journot-Catalino, N., Somssich, I. E., Roby, D., and Kroj, T. (2006). The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18, 3289–3302. doi: 10.1105/tpc.106. 044149
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kachroo, P., Kachroo, A., Lapchyk, L., Hildebrand, D., and Klessig, D. F. (2003). Restoration of defective cross talk in ssi2 mutants: role of salicylic acid, jasmonic acid, and fatty acids in SSI2-mediated signaling. Mol. Plant Microbe Interact. 16, 1022–1029. doi: 10.1094/MPMI.2003.16.11.1022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kachroo, P., Shanklin, J., Shah, J., Whittle, E. J., and Klessig, D. F. (2001). A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. U.S.A. 98, 9448–9453. doi: 10.1073/pnas.151258398
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kazan, K., and Lyons, R. (2014). Intervention of phytohormone pathways by pathogen effectors. Plant Cell 26, 2285–2309. doi: 10.1105/tpc.114.125419
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kazan, K., and Manners, J. M. (2013). MYC2: the master in action. Mol. Plant 6, 686–703. doi: 10.1093/mp/sss128
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, K.-C., Lai, Z., Fan, B., and Chen, Z. (2008). Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 20, 2357–2371. doi: 10.1105/tpc.107.055566
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koornneef, A., Leon-Reyes, A., Ritsema, T., Verhage, A., Den Otter, F. C., Van Loon, L. C.,et al. (2008a). Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 147, 1358–1368. doi: 10.1104/pp.108.121392
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koornneef, A., Rindermann, K., Gatz, C., and Pieterse, C. M. J. (2008b). Histone modifications do not play a major role in salicylate-mediated suppression of jasmonate-induced PDF1.2 gene expression. Commun. Integr. Biol. 1, 143–145. doi: 10.4161/cib.1.2.6997
Latzel, V., Zhang, Y., Karlsson Moritz, K., Fischer, M., and Bossdorf, O. (2012). Epigenetic variation in plant responses to defence hormones. Ann. Bot. 110, 1423–1428. doi: 10.1093/aob/mcs088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leon-Reyes, A., Du, Y., Koornneef, A., Proietti, S., Körbes, A. P., Memelink, J.,et al. (2010a). Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol. Plant Microbe Interact. 23, 187–197. doi: 10.1094/MPMI-23-2-0187
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leon-Reyes, A., Van der Does, D., De Lange, E. S., Delker, C., Wasternack, C., Van Wees, S. C. M.,et al. (2010b). Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232, 1423–1432. doi: 10.1007/s00425-010-1265-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leon-Reyes, A., Spoel, S. H., De Lange, E. S., Abe, H., Kobayashi, M., Tsuda, S.,et al. (2009). Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 149, 1797–1809. doi: 10.1104/pp.108.133926
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, J., Brader, G., Kariola, T., and Palva, E. T. (2006). WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 46, 477–491. doi: 10.1111/j.1365-313X.2006.02712.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, J., Brader, G., and Palva, E. T. (2004). The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16, 319–331. doi: 10.1105/tpc.016980
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lindermayr, C., Sell, S., Müller, B., Leister, D., and Durner, J. (2010). Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22, 2894–2907. doi: 10.1105/tpc.109.066464
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, X., Yang, S., Zhao, M., Luo, M., Yu, C.-W., Chen, C.-Y.,et al. (2014). Transcriptional repression by histone deacetylases in plants. Mol. Plant 7, 764–772. doi: 10.1093/mp/ssu033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, Z.-Q., Yan, L., Wu, Z., Mei, C., Lu, K., Yu, Y.-T.,et al. (2012). Cooperation of three WRKY-domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA-responsive genes ABI4 and ABI5 in Arabidopsis. J. Exp. Bot. 63, 6371–6392. doi: 10.1093/jxb/ers293
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lorenzo, O., Chico, J. M., Sanchez-Serrano, J. J., and Solano, R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16, 1938–1950. doi: 10.1105/tpc.022319
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Luna, E., Bruce, T. J. A., Roberts, M. R., Flors, V., and Ton, J. (2012). Next-generation systemic acquired resistance. Plant Physiol. 158, 844–853. doi: 10.1104/pp.111.187468
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mao, G., Meng, X., Liu, Y., Zheng, Z., Chen, Z., and Zhang, S. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23, 1639–1653. doi: 10.1105/tpc.111.084996
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mao, P., Duan, M., Wei, C., and Li, Y. (2007). WRKY62 transcription factor acts downstream of cytosolic NPR1 and negatively regulates jasmonate-responsive gene expression. Plant Cell Physiol. 48, 833–842. doi: 10.1093/pcp/pcm058
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maruyama, Y., Yamoto, N., Suzuki, Y., Chiba, Y., Yamazaki, K.-I., Sato, T.,et al. (2009). The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci. 213, 79–87. doi: 10.1016/j.plantsci.2013.08.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McGrath, K. C., Dombrecht, B., Manners, J. M., Schenk, P. M., Edgar, C. I., Maclean, D. J.,et al. (2005). Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol. 139, 949–959. doi: 10.1104/pp.105.068544
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meng, X., Xu, J., He, Y., Yang, K.-Y., Mordorski, B., Liu, Y.,et al. (2013). Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25, 1126–1142. doi: 10.1105/tpc.112.109074
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meng, X., and Zhang, S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266. doi: 10.1146/annurev-phyto-082712-102314
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moore, J. W., Loake, G. J., and Spoel, S. H. (2011). Transcription dynamics in plant immunity. Plant Cell 23, 2809–2820. doi: 10.1105/tpc.111.087346
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mou, Z., Fan, W. H., and Dong, X. N. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944. doi: 10.1016/S0092-8674(03)00429-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakano, T., Suzuki, K., Fujimura, T., and Shinshi, H. (2006). Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 140, 411–432. doi: 10.1104/pp.105.073783
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakata, M., Mitsuda, N., Koo, M. H. A. J. K., Moreno, J. E., Suzuki, K., Howe, G. A.,et al. (2013). A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25, 1641–1656. doi: 10.1105/tpc.113.111112
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nandi, A., Moeder, W., Kachroo, P., Klessig, D. F., and Shah, J. (2005). Arabidopsis ssi2-conferred susceptibility to Botrytis cinerea is dependent on EDS5 and PAD4. Mol. Plant Microbe Interact. 18, 363–370. doi: 10.1094/MPMI-18-0363
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ndamukong, I., Abdallat, A. A., Thurow, C., Fode, B., Zander, M., Weigel, R.,et al. (2007). SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 50, 128–139. doi: 10.1111/j.1365-313X.2007.03039.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pape, S., Thurow, C., and Gatz, C. (2010). The Arabidopsis PR-1 promoter contains multiple integration sites for the coactivator NPR1 and the repressor SNI. Plant Physiol. 154, 1805–1818. doi: 10.1104/pp.110.165563
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Petersen, M., Brodersen, P., Naested, H., Andreasson, E., Lindhart, U., Johansen, B.,et al. (2000). Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103, 1111–1120. doi: 10.1016/S0092-8674(00)00213-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pieterse, C. M. J., Pierik, R., and Van Wees, S. C. M. (2014). Different shades of JAZ during plant growth and defense. New Phytol. 204, 261–264. doi: 10.1111/nph.13029
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pieterse, C. M. J., Van der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Popescu, S. C., Popescu, G. V., Bachan, S., Zhang, Z., Gerstein, M., Snyder, M.,et al. (2009). MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 23, 80–92. doi: 10.1101/gad.1740009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Qiu, J.-L., Fiil, B. K., Petersen, K., Nielsen, H. B., Botanga, C. J., Thorgrimsen, S.,et al. (2008). Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 27, 2214–2221. doi: 10.1038/emboj.2008.147
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ren, C.-M., Zhu, Q., Gao, B.-D., Ke, S.-Y., Yu, W.-C., Xie, D.-X.,et al. (2008). Transcription factor WRKY70 displays important but no indispensable roles in jasmonate and salicylic acid signaling. J. Integr. Plant Biol. 50, 630–637. doi: 10.1111/j.1744-7909.2008.00653.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Robert-Seilaniantz, A., Grant, M., and Jones, J. D. G. (2011). Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 49, 317–343. doi: 10.1146/annurev-phyto-073009-114447
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rochon, A., Boyle, P., Wignes, T., Fobert, P. R., and Després, C. (2006). The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell 18, 3670–3685. doi: 10.1105/tpc.106.046953
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sasaki-Sekimoto, Y., Jikumaru, Y., Obayashi, T., Saito, H., Masuda, S., Kamiya, Y.,et al. (2013). Basic Helix-Loop-Helix transcription factors JA-ASSOCIATED MYC2-LIKE 1 (JAM1), JAM2 and JAM3 are negative regulators of jasmonate responses in Arabidopsis. Plant Physiol. 163, 291–234. doi: 10.1104/pp.113.220129
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shah, J., Kachroo, P., Nandi, A., and Klessig, D. F. (2001). A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J. 25, 563–574. doi: 10.1046/j.1365-313x.2001.00992.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shang, Y., Lu, Y., Liu, Z.-Q., Cao, Z., Mei, C., Xin, Q.,et al. (2010). The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22, 1909–1935. doi: 10.1105/tpc.110.073874
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shim, J. S., Jung, C., Lee, S., Min, K., Lee, Y.-W., Choi, Y.,et al. (2013). AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 73, 483–495. doi: 10.1111/tpj.12051
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Song, S., Qi, T., Fan, M., Zhang, X., Gao, H., Huang, H.,et al. (2013). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9:e1003653. doi: 10.1371/journal.pgen.1003653
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Song, S., Qi, T., Wasternack, C., and Xie, D. (2014). Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 21, 112–119. doi: 10.1016/j.pbi.2014.07.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spoel, S. H., and Dong, X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. doi: 10.1038/nri3141
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spoel, S. H., Koornneef, A., Claessens, S. M. C., Korzelius, J. P., Van Pelt, J. A., Mueller, M. J.,et al. (2003). NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15, 760–770. doi: 10.1105/tpc.009159
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spoel, S. H., and Loake, G. J. (2011). Redox-based protein modifications: the missing link in plant immune signalling. Curr. Opin. Plant Biol. 14, 358–364. doi: 10.1016/j.pbi.2011.03.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stein, E., Molitor, A., Kogel, K. H., and Waller, F. (2008). Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol. 49, 1747–1751. doi: 10.1093/pcp/pcn147
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ströher, E., and Millar, A. H. (2012). The biological roles of glutaredoxins. Biochem. J. 446, 333–348. doi: 10.1042/BJ20112131
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tada, Y., Spoel, S. H., Pajerowska-Mukhtar, K., Mou, Z., Song, J., Wang, C.,et al. (2008). Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956. doi: 10.1126/science.1156970
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takahashi, F., Yoshida, R., Ichimura, K., Mizoguchi, T., Seoe, S., Yonezawac, M.,et al. (2007). The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19, 805–818. doi: 10.1105/tpc.106.046581
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tena, G., Boudsocq, M., and Sheen, J. (2011). Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14, 519–529. doi: 10.1016/j.pbi.2011.05.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van der Does, D., Leon-Reyes, A., Koornneef, A., Van Verk, M. C., Rodenburg, N., Pauwels, L.,et al. (2013). Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25, 744–761. doi: 10.1105/tpc.112. 108548
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Verk, M. C., Hickman, R., Pieterse, C. M. J., and Van Wees, S. C. M. (2013). RNA-Seq: revelation of the messengers. Trends Plant Sci. 18, 175–179. doi: 10.1016/j.tplants.2013.02.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vos, I. A., Pieterse, C. M. J., and Van Wees, S. C. M. (2013a). Costs and benefits of hormone-regulated plant defences. Plant Pathol. 62, 43–55. doi: 10.1111/ppa.12105
Vos, I. A., Verhage, A., Schuurink, R. C., Watt, L. G., Pieterse, C. M. J., and Van Wees, S. C. M. (2013b). Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant Sci. 4:539. doi: 10.3389/fpls.2013.00539
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, D., Amornsiripanitch, N., and Dong, X. (2006). A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2:e123. doi: 10.1371/journal.ppat.0020123
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, D., Pajerowska-Mukhtar, K., Hendrickson Culler, A., and Dong, X. (2007). Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 17, 1784–1790. doi: 10.1016/j.cub.2007.09.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wasternack, C., and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058. doi: 10.1093/aob/mct067
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wathugala, D. L., Hemsley, P. A., Moffat, C. S., Cremelie, P., Knight, M. R., and Knight, H. (2012). The Mediator subunit SFR6/MED16 controls defence gene expression mediated by salicylic acid and jasmonate responsive pathways. New Phytol. 195, 217–230. doi: 10.1111/j.1469-8137.2012. 04138.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wu, Y., Zhang, D., Chu, J. Y., Boyle, P., Wang, Y., Brindle, I. D.,et al. (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 1, 639–647. doi: 10.1016/j.celrep.2012.05.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, X., Chen, C., Fan, B., and Chen, Z. (2006). Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18, 1310–1326. doi: 10.1105/tpc.105.037523
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, D. L., Yao, J., Mei, C. S., Tong, X. H., Zeng, L. J., Li, Q.,et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. U.S.A. 109, E1192–E1200. doi: 10.1073/pnas.1201616109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yuan, Y., Zhong, S., Li, Q., Zhu, Z., Lou, Y., Wang, L.,et al. (2007). Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 5, 313–324. doi: 10.1111/j.1467-7652.2007.00243.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zander, M., Chen, S., Imkampe, J., Thurow, C., and Gatz, C. (2012). Repression of the Arabidopsis thaliana jasmonic acid/ethylene-induced defense pathway by TGA-interacting glutaredoxins depends on their C-terminal ALWL motif. Mol. Plant 5, 831–840. doi: 10.1093/mp/ssr113
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zander, M., La Camera, S., Lamotte, O., Métraux, J.-P., and Gatz, C. (2010). Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J. 61, 200–210. doi: 10.1111/j.1365-313X.2009.04044.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zander, M., Thurow, C., and Gatz, C. (2014). TGA transcription factors activate the salicylic acid-suppressible branch of the ethylene-induced defense program by regulating ORA59 expression. Plant Physiol. 165, 1671–1683. doi: 10.1104/pp.114.243360
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zarei, A., Korbes, A. P., Younessi, P., Montiel, G., Champion, A., and Memelink, J. (2011). Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 75, 321–331. doi: 10.1007/s11103-010-9728-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, X., Wang, C., Zhang, Y., Sun, Y., and Mou, Z. (2012a). The Arabidopsis mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell 24, 4294–4309. doi: 10.1105/tpc.112.103317
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Z., Wu, Y., Gao, M., Zhang, J., Kong, Q., Liu, Y.,et al. (2012b). Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11, 253–263. doi: 10.1016/j.chom.2012.01.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y. L., Tessaro, M. J., Lassner, M., and Li, X. (2003). Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15, 2647–2653. doi: 10.1105/tpc.014894
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zheng, X.-Y., Spivey, N. W., Zeng, W., Liu, P.-P., Fu, Z. Q., Klessig, D. F.,et al. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11, 587–596. doi: 10.1016/j.chom.2012.04.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, C., Zhang, L., Duan, J., Miki, B., and Wu, K. (2005). HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 17, 1196–1204. doi: 10.1105/tpc.104.028514
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhu, Z., An, F., Feng, Y., Li, P., Xue, L., Mu, A.,et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108, 12539–12544. doi: 10.1073/pnas.1103959108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: hormone crosstalk, transcription factors, regulation of gene expression, plant immunity, post-translational modifications
Citation: Caarls L, Pieterse CMJ and Van Wees SCM (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 6:170. doi: 10.3389/fpls.2015.00170
Received: 16 January 2015; Accepted: 03 March 2015;
Published online: 25 March 2015.
Edited by:
Jean Toby Greenberg, The University of Chicago, USAReviewed by:
Selena Gimenez-Ibanez, Centro Nacional de Biotecnologia, SpainCopyright © 2015 Caarls, Pieterse and Van Wees. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saskia C. M. Van Wees, Plant-Microbe Interactions, Department of Biology, Faculty of Science, Utrecht University, P.O. Box 800.56, 3508 TB Utrecht, Netherlandscy52YW53ZWVzQHV1Lm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.