
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 11 February 2015
Sec. Plant Genetics and Genomics
Volume 6 - 2015 | https://doi.org/10.3389/fpls.2015.00056
This article is part of the Research Topic Natural diversity in the new millennium View all 12 articles
 Alfredo S. Negri1
Alfredo S. Negri1 Domenico Allegra2
Domenico Allegra2 Laura Simoni2
Laura Simoni2 Fabio Rusconi2
Fabio Rusconi2 Chiara Tonelli3
Chiara Tonelli3 Luca Espen1*
Luca Espen1* Massimo Galbiati2,3*
Massimo Galbiati2,3*Strawberry is one of the most valued fruit worldwide. Modern cultivated varieties (Fragaria × ananassa) exhibit large fruits, with intense color and prolonged shell life. Yet, these valuable traits were attained at the cost of the intensity and the variety of the aroma of the berry, two characteristics highly appreciated by consumers. Wild species display smaller fruits and reduced yield compared with cultivated varieties but they accumulate broader and augmented blends of volatile compounds. Because of the large diversity and strength of aromas occurring in natural and domesticated populations, plant breeders regard wild strawberries as important donors of novel scented molecules. Here we report a comprehensive metabolic map of the aroma of the wild strawberry Profumata di Tortona (PdT), an ancient clone of F. moschata, considered as one of the most fragrant strawberry types of all. Comparison with the more renowned woodland strawberry Regina delle Valli (RdV), an aromatic cultivar of F. vesca, revealed a significant enrichment in the total level of esters, alcohols and furanones and a reduction in the content of ketones in in the aroma of PdT berries. Among esters, particularly relevant was the enhanced accumulation of methyl anthranilate, responsible for the intensive sweetish impression of wild strawberries. Interestingly, increased ester accumulation in PdT fruits correlated with enhanced expression of the Strawberry Alcohol Acyltransferase (SAAT) gene, a key regulator of flavor biogenesis in ripening berries. We also detected a remarkable 900-fold increase in the level of mesifurane, the furanone conferring the typical caramel notes to most wild species.
Garden strawberries (Fragaria × ananassa) are among the most appreciated fruits and represent a valuable economic crop with a global annual production that exceeds 4.5 Mt (FAOSTAT, 2014). Berries of high yield modern varieties are characterized by large size, attractive color and prolonged shell life (Hancock, 1999). Yet, the sensory quality of traded strawberries is often criticized, as they lack flavor and fragrance. Sensory perceptions originate from the combination of sweetness, texture and aroma (Christensen, 1983). Among these features, aroma remains the most valued quality indicator for consumers worldwide (Azodanlou et al., 2003; Colquhon et al., 2012). Just as in other fruits, strawberry's aroma is a complex blend of volatile organic compounds (VOCs). These compounds only represent 0.001 to 0.01% of the berry fresh weight but have a major effect on its flavor and fragrance (Buttery, 1983). As many as 360 volatiles have been identified in ripe strawberries; these include esters, aldehydes, ketones, alcohols, terpenes and furanones (Menager et al., 2004; Jetti et al., 2007). Individual compounds, although often present in minute quantities, may have a significant impact on the aroma. The reduced fragrance of most garden strawberries derives from the relatively limited accumulation of esters molecules, frequently combined with an excess of lactones, which usually cause a disproportionate peach note.
As opposite to garden varieties, wild strawberries are renowned for their intense flavor and fragrance. Most spontaneous species bear small fruits which accumulate higher levels and wider assortments of volatile molecules, compared with cultivated varieties (Honkanen and Hirvi, 1990). The ample natural variation occurring among the wild ancestors of garden strawberries provides a valuable source of novel volatile compounds for breeding new commercial strawberries with improved aroma properties (Ulrich and Hoberg, 2000a). Over 20 wild species are found within the Fragaria genus, of which the diploid woodland strawberry (F. vesca) is the most common (Rousseau-Gueutin et al., 2009). Among other species, musk strawberries (F. moschata) are recognized for their distinguished and extraordinary strong aroma. Native to highland areas from France to Siberia, musk strawberries were widely cultivated in Europe to the mid-1900, when they were replaced by firmer, higher yielding and more remunerative F. × ananassa cultivars (Darrow, 1966).
Today, only few musk strawberries survive in farm plantings, although on a very small scale. Noteworthy is the Italian clone Profumata di Tortona (PdT), regarded as one of the most fragrant strawberry types of all (Urruty et al., 2002). PdT is a dioecious strawberry having the male and female reproductive organs in separate flowers on separate plants. Berries, distinguishable for the intense red color of the peel and the whitish flesh, posses a delightful sour–sweet, slightly astringent flavor, with green, caramel and clove-like notes (Pet'ka et al., 2012). Hallmark of PdT is its peculiar floral, spicy aroma, with hints of honey, musk and wine. The fragrance of this strawberry is so intense that a few ripe berries can perfume an entire room with a penetrating mango-like, tropical scent. Differently from most cultivated strawberries, the harvesting season for PdT is extremely limited. Berries are only available for a period of 10–15 days, coinciding with the second half of June. Currently, the commercial cultivation of PdT is restricted to the municipality of Tortona, in the Pedimont region in Northern Italy. Remarkably, the first historical evidence of musk strawberries in this area dates back to year 1411 (Bergaglio, 2007). Cultivation lingered into the early 1960s, when strawberry fields succumbed to urban development. Most recently, there has been a renewed interest for the Profumata di Tortona, considered a delicacy both for fresh consumption and gourmet preparations.
Diversity of volatile patterns in woodland strawberries in comparison to cultivated garden varieties has been extensively investigated (Drawert et al., 1973; Ulrich et al., 1997). Recent surveys of aroma profiles across 16 F. vesca accessions and five F. × ananassa cultivars, identified significant differences in the accumulation of individual esters, ketones and terpinoids between the two strawberries (Ulrich and Olbricht, 2013, 2014). In particular, small esters, including ethyl hexanoate, methyl butanoate and methyl hexanoate, were found in higher amounts in garden strawberries compared with woodland accessions. Conversely, the key ester methyl antranilate (MA) was more abundant in F. vesca. Similarly, ketones (e.g., 2-pentanone, 2-heptanone, and 2-nonanone) and terpinoids (e.g., myrtenal, myrtenil acetate, α-terpineol) occurred at higher levels in wild berries, with the exception of the monoterpene linalool, which was more abundant in garden strawberries (Ulrich and Olbricht, 2013, 2014).
Detailed profiling of the aroma composition in musk strawberries has only been reported for few spontaneous populations (Ulrich et al., 2007; Pet'ka et al., 2012). Urruty and colleagues performed a first partial assessment of the volatile compounds produced by ripe PdT berries (Urruty et al., 2002). These authors determined the abundance of 23 preselected VOCs in the aroma of two F. moschata clones (Capron Royal and PdT) compared with 15 garden varieties. Selected compounds, representing major constituents of the strawberry aroma, included esters (e.g. methyl hexanoate, MA), monoterpenes (e.g., linalool, nerolidol), ketones (e.g., 2-pentanone, 2-heptanone), aldehydes (e.g., 2-hexenal), lactones and furanones (e.g., γ-decalactone, mesifurane). Among the volatiles analyzed, Capron Royal and PdT displayed lower levels of small esters as methyl hexanoate, compared with cultivated strawberries. In contrast, both F. moschata clones revealed exceptionally high levels of MA, which was barely detectable in most garden varieties (Urruty et al., 2002). Despite the relevance of these findings, a more comprehensive analysis of the aroma profile of PdT is required to fully uncover the volatile composition of ripe PdT berries and gain more insights into its extraordinary aromatic properties.
Here we report a re-assessment of the VOCs composition of PdT berries, based on a non-targeted Solid-Phase Micro-Extraction/Gas chromatography-Mass Spectrophotometry (SPME/GC-MS) approach (Ulrich and Hoberg, 2000b). We compared the aroma of PdT with that of the woodland strawberry Regina delle Valli (RdV). The latter represents a widely cultivated cultivar of F. vesca, renowned for its intense and pleasant aroma. Most importantly, the aroma composition of this strawberry has not been investigated in previous studies. In total, we identified 131 VOCs in the headspace of the two strawberries, which provide a comprehensive picture of the aroma patterns of PdT and RdV berries. As a whole, our results contribute to shed new light on the natural variation occurring in the aroma of wild strawberry species.
Plants of Profumata di Tortona were provided by “Consorzio per la valorizzazione e la tutela della Fragola Profumata di Tortona,” Tortona, Italy. Regina delle Valli plants were purchased from Azienda Agricola Ortomio, Forlì, Italy (http://www.ortomio.it/). Both strawberries were grown in the production area of Tortona (Italy) under commercial conditions, accordingly to the standards adopted by the “Consorzio per la valorizzazione e la tutela della Fragola Profumata di Tortona.” Fruits for the analysis were harvested with the assistance of local producers, to ensure selection of uniform, healthy and fully ripe berries. Five randomized samples, composed of 15 individual ripe fruits each, were collected the early morning in a single harvest. The extremely reduced harvesting season of PdT did not justify the adoption of multiple harvests. Berries employed in our analysis represent a faithful sample of the commercial fruits that are normally available to consumers.
Harvested fruits samples were immediately frozen at −20°C and stored at −80°C. Prior to the analysis fruits were powdered in liquid nitrogen and 1 g of fresh weight for each sample was incubated at 30°C for 5 min. Following addition of 300 μ L of a NaCl saturated solution, 900 μ L of the homogenized mixture were transferred to a10 mL screw cap headspace vial. Three technical replicas were performed for each sample.
Volatiles were sampled by SPME with a 2 cm × 50/30-micron DVB/Carboxen/PDMS Stable Flex fiber (Sigma, Milano, Italy). Extraction and desorption of the volatiles were performed automatically by a CombiPAL autosampler (CTC Analytics, Zwingen, Switzerland) as described (Zorrilla-Fontanesi et al., 2012). Chromatography was performed on a DB-5 ms (30 m × 0.25 mm × 1 mm) column (Sigma, Milano, Italy) with Helium at a constant flow of 1.2 mL/min, accordingly to Zorrilla-Fontanesi et al. (2012). Mass spectra were recorded in scan mode in the 35 to 220 mass-to-charge ratio range by a 5975B mass spectrometer (Agilent Technologies, Cernusco sul Naviglio, Italy) (ionization energy 70 eV; scanning speed 7 scans/s). The Enhanced ChemStation software (Agilent Technologies, Cernusco sul Naviglio, Italy) was used for recording and processing of chromatograms and spectra. Three technical replicas were conducted for each sample.
Compound abundance was determined using the software MET-IDEA that directly extracts ion intensities exploiting a list of ions coupled with their relative retention time values (Broeckling et al., 2006). The ion list was built as following. For each biological replicate, a randomly chosen chromatogram was analyzed by Automated Mass Spectral Deconvolution and Identification System (AMDIS; http://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:amdis) comparing the deconvoluted spectra with entries at the National Institute of Standards and Technology MS library (NIST08) (Type of Analysis, Simple; Resolution, Medium; Sensitivity, Medium; Shape Requirement, Low; Component Width, 12; Minimum Match Factor, 70). The 113 entries with a match score larger than 800 on the NIST Search software were extracted and added to a.msl file containing the 1537 plant derived metabolites of the VOC BinBase library (Skogerson et al., 2011) to assemble the library used for the analysis of the 10 randomly-chosen chromatograms. During this second cycle of AMDIS analysis, the Minimum match Factor was set to 90 and the resulting.fin files were joined in a single ion list, manually eliminating overlapping entries. The resulting ion list was employed for Met-IDEA analysis over all the 30 chromatograms. Peak areas of selected specific ions were integrated for each compound. The relative content (R.C.) of each tentatively identified metabolite (expressed as percentage) was calculated as the ratio between each peak area and the sum of all the peak areas present in the chromatogram, multiplied by 100 [R.C. = (Areapeak/ΣAreaspeak) × 100]. CAS numbers and flavoring descriptors were retrieved from the web-based Chemical Search Engine (http://www.chemindustry.com/apps/chemicals) and from the online edition of the “Specifications for Flavorings” database (http://www.fao.org/ag/agn/jecfa-flav/), respectively.
Differences in volatiles accumulation between the two cultivars were investigated through the Soft Independent Modeling of Class Analogies (SIMCA) provided by the software Unscrambler (Camo Process AS, Oslo, Norway), trough the construction of a Principal Component Analysis (PCA) model for each cultivar. Samples were projected on the orthogonal system constituted by the two models to assess their object-to-model distance and to judge their membership to one of the two classes. The capability of variable k in discriminating between model PdT and RdV (fitting samples from model PdT onto model RdV) was described by the Discrimination Power (DiscrPower), computed as:
The significance of the differences in volatile levels in the two strawberries was tested through a t-Student test considering as significant variations with p < 0.01.
RNA was isolated from Small Green, Turning and Red fruits according to Schultz et al. (1994). Reverse transcription, and qPCR analysis were performed as previously described (Galbiati et al., 2011). SAAT expression was analyzed using primers SAAT-F1 (5′-TTGGATGGGGGAGGACATCAT-3′) and SAAT -R1 (5′-CACCCACGCTTCAATTCCAGTA-3′). Gene expression was normalized using the ACTIN gene (GenBank: JN616288.1), amplified with primers ACT-F1 (5′-ATGTTGCCCTTGACTACGAACAA-3′) and ACT-R1 (5′- TGGCCGTCGGGAAGCTCATA-3′). Primers efficiency was first assessed on both genomic DNA and cDNAs derived from PdT and RdV fruits, to avoid differences in amplification efficiency in the two genotypes. Changes in SAAT gene expression were calculated relative to ACTIN using the ΔΔCt method (Livak and Schmittgen, 2001).
Fully ripe berries of F. moschata, clone Profumata di Tortona (PdT) and F. vesca, cv Regina delle Valli (RdV) were analyzed by SPME/GC-MS. In total, through the construction of a non-redundant ion list collecting information from the AMDIS analysis of 10 different chromatograms, 131 VOCs were tentatively identified in the headspace of the two strawberries. GC-MS data obtained from individual biological and technical replicas were analyzed using the Soft Independent Modeling of Class Analogies (SIMCA) based on the models relative to the two genotypes built with the Principal Component Analysis (PCA) (Svante and Michael, 1977). The SIMCA Cooman's plot showed full differentiation of PdT and RdV, based on their aroma components. All the samples grouped in the relative membership class, as determined by a significance level of 5% (Figure 1). The model assigned the highest discriminating power to the ketones heptan-2-one and nonan-2-one (Table 1). A third ketone molecule, undecan-2-one, also scored among the most significant volatiles for the discrimination between the two strawberries. Additional molecules with high discriminating power included several esters, such as hexyl butanoate, methyl benzoate, ethyl hexanoate, pinocarvyl acetate, the furanone γ-hexalactone, and the terpenes pinocarveol and myrtenyl acetate (Table 1).
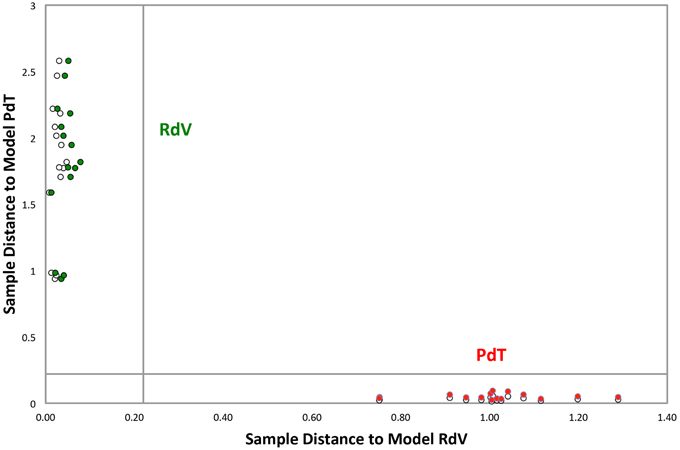
Figure 1. Cooman's plot of the classification analysis between PdT and RdV. Green and red circles represent calibration samples belonging to RdV and PdT, respectively. White circles represent test set samples of the two classes. Graph inner lines represent the significance level of 5%. The analysis employed 131 peak signals. Among these, 95 peaks were tentatively identified by MS library search.
Among the 131 volatiles identified in the aroma of the two strawberries, 47 were classified as esters, which are long known to represent the most abundant VOCs found in ripe strawberries (Latrasse, 1991) (Table 2). Terpenoids included 20 mono- and 4 sesqui-terpenes (Table 3). Amongst the remaining aroma constituents we identified 5 alcohols, 9 aldehydes (Table 4), 6 ketones, and 4 lactones (Table 5). Additional compounds comprised a single fatty acid (hexanoic acid), a single alkane hydrocarbon (tetradecane), and 36 volatiles of unknown chemical identity.
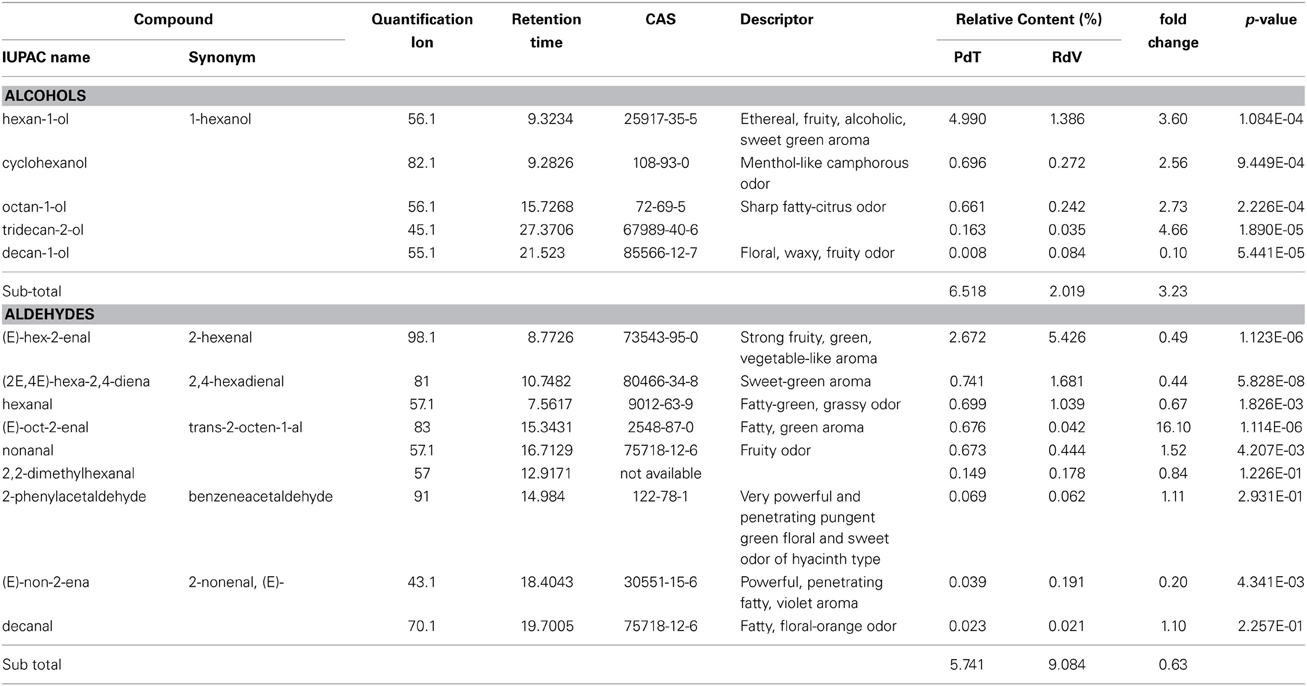
Table 4. Alcohol and aldehyde molecules identified in the headspace of PdT and RdV berries by GC-MS.
The relative abundance of individual chemical classes significantly differed between the two strawberries (Supplementary Figure 1). Quantitatively, esters were the predominant volatiles in berries of PdT, accounting for nearly 50% of the aroma. In comparison, their relative amount was drastically reduced in the aroma of RdV, only representing 25% of the total volatiles. Terpenes were the second most abundant class of compounds found in PdT (24%). A comparable level of total terpenes was identified in RdV (21%). Conversely, the two strawberries disclosed a marked difference in the relative level of ketones, which were severely reduced in PdT (4%), while highly abundant in RdV (27%). We also observed a disparity in the relative abundance of alcohols, which were far more copious in PdT compared with RdV, representing 7and 2% of the total volatile molecules, respectively. No substantial differences were detected in the relative amount of aldehydes and lactones. Aldehydes accounted for 6 and 9% of total volatiles in PdT and RdV, respectively, whilst lactones were the least represented molecules, only covering 1.4 and 0.4% of the aroma of PdT and RdV, respectively. Finally, compounds of uncertain identity were slightly more abundant in RdV (14%) compared with PdT (8%) (Supplementary Figure 1).
Analysis of individual constituents of the aroma identified the monoterpene myrtenyl acetate as the most abundant volatile in both PdT and RdV (Table 3). This finding is in line with a previous work, which demonstrated that this molecule dominates the terpenoid profile of wild strawberries (Aharoni et al., 2004). Interestingly, the level of myrtenyl acetate was significantly augmented in PdT (20.1%) compared with RdV (15.4%). The relative quantities of two esters molecules were also preeminent in the aroma of PdT, namely octyl acetate (12.7%) and 4-acetyloxybutyl acetate (11.6%) (Table 2). Together with myrtenyl acetate these two compounds constituted 45% of the total volatiles found in this strawberry. Both esters were also detected in the aroma of RdV, even tough their relative abundance was drastically reduced compared with PdT (4.6 and 3.6%, respectively). Two ketones, 2-heptanone, and 2-nonanone, were the most abundant molecules, after myrtenyl acetate, in the aroma of RdV, accounting for 12.7 and 11.9% of the total volatiles, respectively (Table 5). Conversely, the amount of these two molecules was significantly reduced in PdT (0.7 and 0.2%, respectively).
Additional abundant components of the aroma of PdT included, 1-hexanol (5%) (Table 4) and several other esters, as hexyl formate (4.5%), methyl anthranilate (MA) (3.4%) and hexyl acetate (2.7%) (Table 2). On average, the content of these volatiles was significantly higher in the aroma of PdT compared with RdV. The only aldehyde found in relatively high amounts in both strawberries was 2-hexenal (Table 4). Its content was significantly greater in RdV (5.4%) compared with PdT (2.7%). The most abundant ketone in the aroma of PdT was 2-tridecanone, reaching 2.3% of the total volatiles (Table 5). As opposite to the other ketones, the level of this molecule was significantly reduced in RdV (0.6%) compared with PdT.
Among less abundant molecules (<1% of total volatiles), major differences between the two strawberries were observed within terpenes, esters, and furanones. In particular, the levels of α-pinene, a monterpene specifically identified in the aroma of F. vesca (Aharoni et al., 2004), linalool, known to dominate the terpenoid profile of cultivated strawberry (Aharoni et al., 2004) and α-citronellol, were significantly reduced in the aroma of PdT in comparison with RdV (Table 3). Contrariwise, the level of megastigma-3,7(E),9-triene, the major terpene found in the essential oil of some Eucalyptus species (El-Mageed et al., 2011), was 18 times higher in PdT compared with RdV. We also observed a 74- and 108-fold increase in the accumulation of the terpenes 3-cyclohexen-1-ol,5-methylene-6-(1-methylethyl)-(9CI) and 1H-Cyclopropa[a]naphthalene, in the aroma of RdV compared with PdT (Table 3). Among minor esters, the most striking differences were detected for 2-methylbutanoic acid and methyl 2-methylbutanoate, whose content was 120- and 150-times more abundant in berries from PdT relatively to RdV, respectively (Table 2). Additional esters over-represented in the aroma of RdV included [(E)-3-phenylprop-2-enyl] acetate (24-fold), octyl 3-methylbutanoate (16-fold), tridecan-2-yl acetate (14-fold) and methyl tiglate (13-fold) (Table 2).
Even if present in lower amount compared to other volatiles, lactones and furanones, were more copious in the aroma of PdT compared to RdV. Remarkably, mesifurane, the typical furanone conferring sweet caramel notes to wild strawberries, was nearly 900 times more abundant in PdT compared with RdV (Table 5).
In strawberries only very few genes have been directly associated with aroma biogenesis in ripening fruits. Among them, the Strawberry Alcohol Acyltransferase gene (SAAT), controlling a key step in esters biosynthesis (Aharoni et al., 2000), is of particular interest. The enzyme AAT catalyzes the transfer of an acyl moiety from acyl-CoA onto specific alcohols, resulting in the production of ester molecules (Harada et al., 1985). Intriguingly, octyl-acetate, the most abundant ester in both PdT and RdV aroma, has been demonstrated to be a genuine AAT product (Aharoni et al., 2000). The activation of SAAT expression during berry development has been positively correlated with the on-set of esters accumulation. In F. × ananassa SAAT expression is induced at early stage during fruit ripening, it peaks in correspondence of the turning stage, and it is rapidly down-regulated in red fruits (Aharoni et al., 2000). We compared the expression profile of the SAAT gene in developing berries from PdT and RdV (Supplementary Figure 2) to unravel potential differences in the level of gene expression and/or in the kinetic of SAAT activation between the two strawberries.
As shown in Figure 2, both RdV and PdT accumulated comparable low levels of SAAT transcripts in small green fruits. At the turning stage, both strawberries displayed very strong activation of SAAT expression. Interestingly, at this stage, the degree of gene activation was significantly enhanced in PdT compared with RdV (t-test, p < 0.01). This finding is conceivable with the increased accumulation of esters observed in PdT relatively to RdV fruits. Finally, in red fruits SAAT expression was down-regulated to the same extent in both berries (Figure 2).
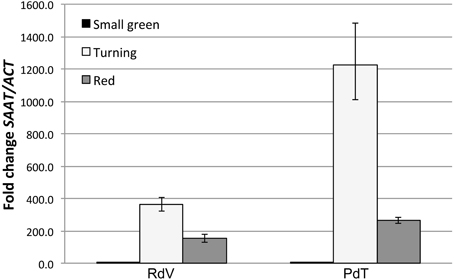
Figure 2. Comparative analysis of SAAT gene expression in developing berries. qPCR analysis of SAAT expression in small green, turning and red fruits of RdV and PdT. Relative SAAT transcript levels were determined using SAAT-specific primers and normalized to the expression of a strawberry ACTIN gene (GenBank: JN616288.1).
The vast array of wild species found in the genus Fragaria provide an exceptionally large and conveniently located germplasm, which can serve to breeding novel quality traits into garden strawberries (Hancock and Luby, 1993). Key to the successful exploitation of the natural variation occurring in the Fragaria genus is the detailed characterization of the aroma profile of individual species, ecotypes and clones. This study unraveled the volatile composition of two domesticated wild strawberries: F. moschata clone Profumata di Tortona and F. vesca cv. Regina delle Valli. Both strawberries are regarded as highly aromatic, although the scent of PdT is far more intense and persistent (Urruty et al., 2002).
Sampling procedures are key to the analysis of aroma composition in strawberries, as significant changes in VOCs profiles can occur among harvest dates within a single season (Schwieterman et al., 2014). Different strategies employing multiple harvests or the laborious harvest of all the available fruits throughout the season have been proposed to overcome this obstacle (Ulrich and Olbricht, 2013; Schwieterman et al., 2014). Our results rely on the analysis of biological replicas from a single harvest. In contrast to perpetual flowering accession characterized by prolonged harvesting seasons, the seasonal flowering PdT clone, only bears fruit for less than 2 weeks. Environmental changes and differences in plant physiology, known to affect fruit quality over months (Schwieterman et al., 2014), are unlike to influence the aroma composition of PdT berries within a period of 15 days.
We identified 131 volatiles in ripe berries of PdT and RdV, a number exceeding the aroma compounds usually found in commercial strawberries. A recent survey of the chemical diversity of the aromas of 35 different garden varieties recognized no more than 80 VOCs even in the most fragrant commercial genotypes (Schwieterman et al., 2014). Comparative analysis of aroma patterns revealed the significant enrichment in esters and alcohols along with the severe reduction in ketones in berries from PdT compared with RdV. Conversely, the two strawberries disclosed comparable levels of terpenes, aldehydes and lactones (Supplementary Figure 1). Over 130 different ester molecules have been identified in strawberries (Latrasse, 1991). In F. × ananassa, the chemical composition of ester volatiles is usually dominated by methyl and ethyl esters even though the abundance of each form varies with cultivar (Forney et al., 2000). Methyl esters were the prevalent form in the aroma of both PdT and RdV (22 molecules), whereas ethyl esters were poorly represented (3 molecules) (Table 2).
Even if it is generally difficult to establish a direct correlation between individual aroma constituents and specific sensory impressions, the fragrance of wild strawberries largely depend upon the relatively high amounts of methyl anthranilate (MA) (Ulrich et al., 1997). The intensive sweetish-flowery impression of this ester is at the basis of the definition of strawberry aroma chemo-types, which are essentially subdivided into MA-containing and MA-free types (Ulrich et al., 1997). Our analysis uncovered a nine-fold increase in the level of MA in berries from PdT compared with RdV, thus emphasizing the role of this key ester in determining the unique fragrance of musk strawberry. This conclusion is corroborated by previous studies, which reported an exceedingly higher concentration of MA in F. moschata relatively to F. vesca (Urruty et al., 2002). It is also interesting to note that, MA and γ-decalactone can directly inhibit the in vitro growth of relevant strawberry pathogens, thus implying that these volatiles may contribute to the healthiness of the berry at harvest (Chambers et al., 2013).
We also detected significant differences in the accumulation of low abundant esters between the two strawberries, as for instance methyl 2-methylbutanoate, which was 150 times more abundant in PdT compared with RdV (Table 2). Along with other esters of butanoic acid this molecule, conferring a sweet and fruity impact to the aroma, is found in higher amounts in garden strawberries compared with woodland accessions (Ulrich and Olbricht, 2013). We also detected a 16-fold increase in the relative level of octyl 3-methylbutanoate, in the headspace of PdT compared with RdV. This molecule, conferring an apple-pineapple odor, is present in the most flavored commercial varieties but undetected in the least flavorful. Evidence indicates that, octyl 3-methylbutanoate is an important component of strawberry aroma, potentially enhancing the perceived sweetness intensity, independently of individual sugars (Schwieterman et al., 2014).
Ester accumulation in ripening strawberries is directly associated with the expression of the SAAT gene, encoding a fruit-specific ALCOHOL ACYLTRANSFERASE (AAT) (Aharoni et al., 2000). It is intriguingly to speculate that the enhanced accumulation of ester molecules in the aroma of PdT, results from the hyper activation of SAAT expression observed in turning fruits from PdT, as compared with RdV (Figure 2). Further support to this hypothesis comes from the observation that genuine products of the SAAT enzyme, including octyl acetate, [(Z)-hex-3-enyl] acetate, 2-phenylethyl acetate and nonyl acetate (Aharoni et al., 2000), were found at higher levels in the headspace of PdT compared with RdV (Table 2).
The implication for the increased alcohol accumulation in PdT, as compared with RdV, on the quality of the berry is questionable, as these molecules have normally little impact on the aroma (Larsen and Watkins, 1995). Nevertheless, Schwieterman and colleagues recently reported a direct effect of the level of 1-hexanol on sweetness and flavor intensity in different garden cultivars (Schwieterman et al., 2014). Notably, we found a significant 4-fold increase in the relative amount of 1-hexanol in PdT compared with RdV, possibly suggesting a positive role for this molecule in determining the unique flavor of musk strawberries.
PdT and RdV displayed a similar terpene profile, largely dominated by myrtenyl acetate, by far the most abundant molecule found in the aroma of both strawberries. Higher concentrations of myrtenyl acetate are normally found in woodland strawberries compared with garden varieties (Ulrich and Olbricht, 2013). We observed a moderate, although significant, increased level of myrtenyl acetate in PdT compared with RdV (Table 3). This is in accord with previous comparative analysis of other F. moschata clones with F. vesca accessions (Ulrich et al., 1997). Major differences in the accumulation of low abundant terpenes involved linalool and 1H-Cyclopropa[a]naphthalene. The former, conferring pleasant flowery, citrus-like notes, represents the predominant monoterpene found in cultivated strawberries (Aharoni et al., 2004; Ulrich and Olbricht, 2014). Its relative concentration was 19 fold higher in RdV compared with PdT (Table 3). Conversely, 1H-Cyclopropa[a]naphthalene was 108 times more abundant in PdT in comparison with RdV. Interestingly, this molecule is among the major constituent of some agarwood oils, highly appreciated for their unique and intense fragrance and for their therapeutic properties (Takemoto et al., 2008). To our knowledge, this volatile compound has never been reported in previous analyses of strawberry aromas and could represent a novel target to enhancing the fragrance of traded strawberries.
Even if present in relative low amounts, furanones are considered as dominating components of strawberry aroma. In particular furaneol (DHF) and its methyl ether mesifurane (DMF), contribute to the typical caramel-like, sweet, floral and fruity aroma of the berry (Jetti et al., 2007). Interestingly, PdT strawberries revealed an enhanced accumulation of total furanones compared with RdT. In particular, we detected a nearly 900-fold increase in the relative amount of DMF compared with RdV (Table 5). Augmented levels of DMF in F. moschata accessions relatively to F. vesca have been previously reported, yet not to this very large extent (Ulrich et al., 1997). The identification of the genetic bases for such an increased mesifurane production is beyond the scope of this work. Yet it is important to note that, PdT may represent a valuable breeding material to enhance the DMF content of garden varieties. Our analysis did not reveal detectable amount of DHF neither in PdT nor in RdV berries. This is in contrast with a preceding work, which identified this furanone in different F. vesca and F. moschata genotypes (Urruty et al., 2002). One possible explanation for such a discrepancy could reside in differences in the analytic methods employed in previous studies and ours. It is interesting to note, that DHF accumulation has been negatively correlated to the quality of the berry, as DHF-type strawberries are generally characterized by medium to poor flavor (Ulrich et al., 1997). The lack of DHF in PdT and RdV berries could alternatively be correlated to the organoleptic excellence of their fruits.
As a whole, our data provide a comprehensive metabolic map of PdT, the most fragrant strawberry of all. Despite the fact that F. moschata is not a direct ancestor of commercial garden strawberries, the aroma profile of PdT could assist the exploitation of this ancient clone as breeding material to enhance fruit quality in commercial strawberries. The successful introgression of wild-derived traits into cultivated garden varieties largely depends upon the possibility to generate viable and fertile interspecific hybrids. Evidently, crosses between octoploid F. × ananassa and species at lower ploidy level, including hexaploid PdT, are rather difficult. Yet, breeders have successfully performed interploid crosses between cultivated strawberries with F. vesca and F. moschata, which produced viable hybrids with partial seed set (Luby et al., 1991). Synthetic octoploids containing varying levels of F. moschata have also been generated using colchicine-induced artificial doubling of chromosome number (Evans, 1982a,b). Finally, advancements in genetic engineering of cultivated strawberries have opened unprecedented possibilities for the breeding of new varieties with desirable traits. Genetic transformation has been successfully employed to enhance strawberry resistance to pests, herbicides, diseases, environmental stresses as well as fruit quality (reviewed in Qin et al., 2008). Further developments are expected in which metabolomic data, as those provided in this study, combined with genome-wide transcriptomic analysis and next generation genome-sequencing strategies will allow the identification of suitable molecular targets for engineering of volatile biosynthesis in strawberries. In this perspective, the genome of Profumata di Tortona will prove an invaluable source of genetic material.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the “Consorzio per la valorizzazione e la tutela della Fragola Profumata di Tortona” (Tortona, Italy) and “Progetto Derthona” (Tortona, Italy) for providing the plant material along with useful technical and historical information on the clone “Profumata di Tortona”; and “Agrodinamica s.r.l.” (Tortona, Italy) for technical support with field activities.
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fpls.2015.00056/abstract
Aharoni, A., Giri, A. P., Verstappen, F. W., Bertea, C. M., Sevenier, R., Sun, Z., et al. (2004). Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16, 3110–3131. doi: 10.1105/tpc.104.023895
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aharoni, A., Keizer, L. C., Bouwmeester, H. J., Sun, Z., Alvarez-Huerta, M., Verhoeven, H. A., et al. (2000). Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12, 647–662. doi: 10.1105/tpc.12.5.647
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Azodanlou, R., Darbellay, C., Luisier, J. L., Villettaz, J. C., and Amado, R. (2003). Quality assessment of strawberries (Fragaria species). J. Agric. Food Chem. 51, 715–721. doi: 10.1021/jf0200467
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Broeckling, C. D., Reddy, I. R., Duran, A. L., Zhao, X., and Sumner, L. W. (2006). MET-IDEA: data extraction tool for mass spectrometry-based metabolomics. Anal. Chem. 78, 4334–4341. doi: 10.1021/ac0521596
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Buttery, R. (1983). “Vegetable and fruit flavors,” in Flavor Reserach: Recent Advances, eds R. Teranishi, R. Flath, and H. Sugusawa (New York, NY: Marcel Dekker).
Chambers, A. H., Evans, S. A., and Folta, K. M. (2013). Methyl anthranilate and gamma-decalactone inhibit strawberry pathogen growth and achene germination. J. Agric. Food Chem. 61, 12625–12633. doi: 10.1021/jf404255a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Christensen, C. (1983). Effect of color on aroma, flavor and texture judgment of food. J. Food Sci. 48, 787–790. doi: 10.1111/j.1365-2621
Colquhon, T., Levin, L., Mosckowitz, H., Whiteker, V., Clark, D., and Folta, K. (2012). Framing the perfect strawberry: an excercise in consumer-assisted selection of fruit crops. J. Berry Res. 2, 45–61. doi: 10.3233/JBR-2011-027
Darrow, G. (1966). The Strawberry: History, Breeding and Physiology. New York, NY: Holt, Rinehart and Winston.
Drawert, F., Tressl, R., Staudt, G., and Koppler, H. (1973). Gaschromatographical-masspectrometrical differentiation of aroma subtances from strawberries. J. Biosci. C 28, 488–493.
El-Mageed, A. A., Osman, A. K., Tawfik, A. Q., and Mohammed, H. A. (2011). Chemical composition of the essential oils of four Eucalyptus species (Myrtaceae) from Egypt. Res. J. Phytochem. 5, 115–122. doi: 10.3923/rjphyto.2011.115.122
FAOSTAT. (2014). Available online at: Available: http://faostat3.fao.org/faostat-gateway/go/to/browse/Q/QC/E (Accessed September 15, 2014).
Forney, C., Kalt, W., and Jordan, M. (2000). The composition of strawberry aroma is influenced by cultivar, maturity and storage. Hort Sci. 35, 1022–1026.
Galbiati, M., Matus, J. T., Francia, P., Rusconi, F., Canon, P., Medina, C., et al. (2011). The grapevine guard cell-related VvMYB60 transcription factor is involved in the regulation of stomatal activity and is differentially expressed in response to ABA and osmotic stress. BMC Plant Biol. 11:142. doi: 10.1186/1471-2229-11-142
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hancock, J., and Luby, J. (1993). Genetic resources at our doorstep: the wild strawberries. Bioscience 43, 141–147. doi: 10.2307/1312017
Harada, M., Ueda, Y., and Iwata, T. (1985). Purification and some properties of alcohol acetyltransferase from banana fruit. Plant Cell Physiol. 26, 1067–1074.
Honkanen, E., and Hirvi, Y. (1990). “The flavour of berries,” in Food Flavours, eds I. Morton and A. Macleod (Amsterdam: Elsevier Scientific Publications), 125–193.
Jetti, R. R., Yang, E., Kurnianta, A., Finn, C., and Qian, M. C. (2007). Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 72, S487–S496. doi: 10.1111/j.1750-3841.2007.00445.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Larsen, M., and Watkins, C. B. (1995). Firmness and concentrations of acetalde- hyde, ethyl acetate and ethanol in strawberries stored in controlled and modified atmospheres. Postharvest Biol. Technol. 5, 39–50. doi: 10.1016/0925-5214(94)00012
Latrasse, A. (1991). “Fruits III,” in Volatile Compounds in Food and Beverages, ed H. Maarse (New York; Basel, Hong Kong: Marcel Dekker Inc.), 334–340.
Livak, K., and Schmittgen, T. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luby, J., Hancock, J. F., and Cameron, J. C. (1991). “Expansion of the strawberry germoplasm base in North America,” in The Strawberry into the 21st Century, eds A. Dale and J. Luby (Portland; Oregon: Timber Press), 65–75.
Menager, I., Jost, M., and Aubert, C. (2004). Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 52, 1248–1254. doi: 10.1021/jf0350919
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pet'ka, J., Leitner, E., and Parameswaran, B. (2012). Musk strawberies: the flavour of a formerly famous fruit reassessed. Flavour Fragr. J. 27, 273–279. doi: 10.1002/ffj.3095
Qin, Y., Silva, J. T. D., Zhang, L., and Zhang, S. (2008). Transgenic strawberry: state of the art for improved traits. Biotech. Adv. 26, 219–232. doi: 10.1016/j.biotechadv.2007.12.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rousseau-Gueutin, M., Gaston, A., Ainouche, A., Ainouche, M., Olbricht, K., Staudt, G., et al. (2009). Tracking the evolutionary history of polyploid strawberries (Fragaria L.): new insights from phylogenetic analyses of low-copy nuclear genes. Mol. Phylog. Evol. 51, 515–530. doi: 10.1016/j.ympev.2008.12.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schultz, D., Craig, R., Cox-Foster, D., Mumma, R., and Medford, J. (1994). RNA isolation from recalcitrant plant tissues. Plant Mol. Biol. Rep. 12, 310–316. doi: 10.1007/BF02669273
Schwieterman, M. L., Colquhoun, T. A., Jaworski, E. A., Bartoshuk, L. M., Gilbert, J. L., Tieman, D. M., et al. (2014). Strawberry flavor: diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 9:e88446. doi: 10.1371/journal.pone.0088446
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Skogerson, K., Wohlgemuth, G., Barupal, D. K., and Fiehn, O. (2011). The volatile compound BinBase mass spectral database. BMC Bioinform. 12:321. doi: 10.1186/1471-2105-12-321
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Svante, W., and Michael, S. (1977). SIMCA: a method for analyzing chemical data in terms of similarity and analogy. ACS Symp. Ser. 52, 243–282. doi: 10.1021/bk-1977-0052.ch012
Takemoto, H., Ito, M., Shiraki, T., Yagura, T., and Honda, G. (2008). Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 62, 41–46. doi: 10.1007/s11418-007-0177-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ulrich, D., and Hoberg, E. (2000a). “Flavour analysis in plant breeding research on strawberries,” in Frontiers of Flavour Sciences, eds P. Schieberle and K.-H. Engel. (Garching: Deutsche Forschungsanstalt Lebensmittelchemie), 161–163.
Ulrich, D., and Hoberg, E. (2000b). Rapid methods in plant aroma analysis-mass spectrometric sensor measurments on strawberries. Acta Hortic. 538, 443–446. Available online at: http://www.actahort.org/books/538/538_77.htm
Ulrich, D., Hoberg, E., Rapp, A., and Kecke, S. (1997). Analysis of strawberry flavour discrimination of aroma types by quantification of volatile compounds. Z.- Lebensm.-Unters. Forsch. A 205, 218–223. doi: 10.1007/s002170050154
Ulrich, D., Komes, D., Olbricht, K., and Horberg, E. (2007). Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet. Resour. Crop Evol. 54, 1185–1196. doi: 10.1007/s10722-006-9009-4
Ulrich, D., and Olbricht, K. (2013). Diversity of volatile patterns in sixteen Fragaria vesca L. accessions in comparison to cultivars of Fragaria x ananassa. J. App. Bot. Food Quality 86, 37–46. doi: 10.5073/JABFQ.2013.086.006
Ulrich, D., and Olbricht, K. (2014). Diversity of metabolite patterns and sensory characters in wild and cultivated strawberries. J. Berry Res. 4, 11–17. doi: 10.3233/JBR-140067
Urruty, L., Giraudel, J. L., Lek, S., Roudeillac, P., and Montury, M. (2002). Assessment of strawberry aroma through SPME/GC and ANN methods. Classification and discrimination of varieties. J. Agric. Food Chem. 50, 3129–3136. doi: 10.1021/jf0116799
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zorrilla-Fontanesi, Y., Rambla, J. L., Cabeza, A., Medina, J. J., Sanchez-Sevilla, J. F., Valpuesta, V., et al. (2012). Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 159, 851–870. doi: 10.1104/pp.111.188318
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: strawberry, Fragaria moschata, Fragaria vesca, gas chromatography-mass spectrometry, aroma, volatiles
Citation: Negri AS, Allegra D, Simoni L, Rusconi F, Tonelli C, Espen L and Galbiati M (2015) Comparative analysis of fruit aroma patterns in the domesticated wild strawberries “Profumata di Tortona” (F. moschata) and “Regina delle Valli” (F. vesca). Front. Plant Sci. 6:56. doi: 10.3389/fpls.2015.00056
Received: 27 October 2014; Accepted: 22 January 2015;
Published online: 11 February 2015.
Edited by:
Aleksandra Skirycz, Instituto Tecnológico Vale Desenvolvimento Sustentável/Vale Institute of Technology Sustainable Development, BrazilReviewed by:
Camila Caldana, Brazilian Bioethanol Science and Technology Laboratory (CTBE) - Centro Nacional de Pesquisa em Energia e Materiais/Associação Brasileira de Tecnologia de Luz Síncrotron, BrazilCopyright © 2015 Negri, Allegra, Simoni, Rusconi, Tonelli, Espen and Galbiati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Espen, Dipartimento di Scienze Agrarie e Ambientali - Produzione, Territorio, Agroenergia, Università degli Studi di Milano, Via Celoria 2, Milano 20133, Italy e-mail:bHVjYS5lc3BlbkB1bmltaS5pdA==;
Massimo Galbiati, Department of Life Sciences, Università degli Studi di Milano, Via Celoria 26, Milano 20133, Italy e-mail:bWFzc2ltby5nYWxiaWF0aUB1bmltaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.