
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 23 June 2014
Sec. Plant Physiology
Volume 5 - 2014 | https://doi.org/10.3389/fpls.2014.00288
This article is part of the Research Topic Nitrogen biotransformation: an adventure through isotopic fractionation mechanisms View all 4 articles
Nitrogen isotopic studies have the potential to shed light on the structure of ancient ecosystems, agropastoral regimes, and human-environment interactions. Until relatively recently, however, little attention was paid to the complexities of nitrogen transformations in ancient plant-soil systems and their potential impact on plant and animal tissue nitrogen isotopic compositions. This paper discusses the importance of understanding nitrogen dynamics in ancient contexts, and highlights several key areas of archaeology where a more detailed understanding of these processes may enable us to answer some fundamental questions. This paper explores two larger themes that are prominent in archaeological studies using stable nitrogen isotope analysis: (1) agricultural practices (use of animal fertilizers, burning of vegetation or shifting cultivation, and tillage) and (2) animal domestication and husbandry (grazing intensity/stocking rate and the foddering of domestic animals with cultigens). The paucity of plant material in ancient deposits necessitates that these issues are addressed primarily through the isotopic analysis of skeletal material rather than the plants themselves, but the interpretation of these data hinges on a thorough understanding of the underlying biogeochemical processes in plant-soil systems. Building on studies conducted in modern ecosystems and under controlled conditions, these processes are reviewed, and their relevance discussed for ancient contexts.
This paper addresses the complexities of nitrogen (N) isotopic fractionations in plant-soil systems, drawing from modern field and experimental studies to address several key areas of archaeological investigation related to prehistoric agriculture and animal husbandry. While N isotopic compositions vary in a relatively predictable and consistent manner between animal species according to trophic level (Caut et al., 2009; Szpak et al., 2012c), a diverse array of biogeochemical processes exist that influence the natural abundance of 15N in plant-soil systems. These processes are instrumental in structuring isotopic variation in animal tissues at various spatial and temporal scales, and it is thus imperative that isotopic studies of ancient human and animal tissues adequately consider these aspects of N isotopic biogeochemistry.
Since the early 1980s, stable isotope analysis has become an extremely effective and prevalent tool for the reconstruction of the diet and ecology of human and animal species in archaeological and paleontological contexts. Stable isotope analysis is now widely used in areas of study such as foraging ecology of extinct species, large-scale shifts in ecosystems due to natural and anthropogenic processes, issues surrounding animal domestication and management, weaning behavior in past populations, agricultural practices, and the diet of prehistoric human populations in the most general sense (Schwarcz et al., 2010; Clementz, 2012). The number of archaeological studies that have used stable isotope analysis has been steadily increasing (Figure 1), but all too often the interpretations of these data rely only on the fundamental isotopic relationships established early on such as the differences between C3 and C4 plants or marine and terrestrial foods (DeNiro and Epstein, 1978, 1981; Schoeninger and DeNiro, 1984).
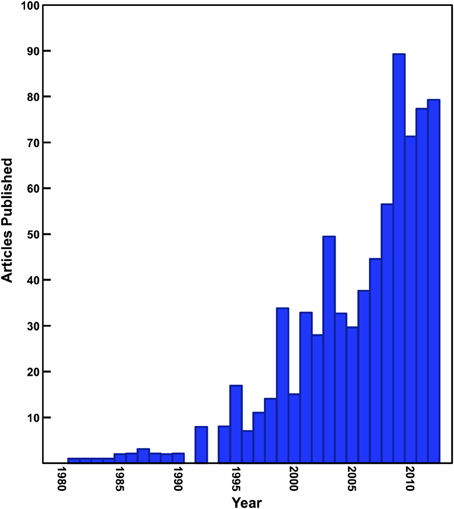
Figure 1. Number of papers published in archaeology and anthropology journals utilizing isotope ratio mass spectrometry. Data presented in this figure were generated using a simple keyword search of archaeology and anthropology journals indexed by Scopus.
Although the isotopic data that have been and continue to be generated are overwhelmingly derived from animal tissues, the basis for the interpretation of these data are the biogeochemical processes that influence isotopic fractionations at the base of the food web. Considerable progress has been made in assessing the complexities of isotopic variation in plant-soil systems, but these processes are still only beginning to be understood in a more comprehensive manner through extensive field and laboratory studies. While several studies have been initiated by archaeologists or anthropologists (Commisso and Nelson, 2006; Bogaard et al., 2007; Fraser et al., 2011; Szpak et al., 2012a,b, 2014), most have been conducted within the context of ecology, agricultural science, food chemistry, and geochemistry. This paper synthesizes this literature, highlighting several areas of research that have direct relevance to the study of prehistoric human subsistence economies as they are assessed via stable isotope analysis. The larger goal of this work is to underscore the need for archaeologists to consider N isotopic variation in a more comprehensive manner, paying particular attention to natural and anthropogenic processes that may impact plant and soil δ15N values. In a more general sense, I hope that the discussion of potentially productive areas of future research that focus on better understanding N isotope dynamics can serve as a call for archaeologists and anthropologists to prioritize such work in their own research programs.
The purpose of this paper is not to provide a detailed overview of the various processes influencing the N isotopic composition of plants and soils; for these purposes the reader is referred to many of the comprehensive reviews and syntheses that have already been published on this topic (Nadelhoffer and Fry, 1994; Handley and Scrimgeour, 1997; Högberg, 1997; Hobbie and Ouimette, 2009; Hobbie and Högberg, 2012). Nevertheless, a very brief synopsis of the major biogeochemical processes that drive variation in plant N isotopic compositions is provided below, with more detailed discussion of these processes throughout the paper where relevant.
Many factors influence plant N isotopic compositions at multiple scales. At the level of the plant, the N isotopic composition will be determined by: the type of N obtained (e.g., NO−3, NH+4, N2), the manner in which this N was obtained (i.e., through direct uptake of soil N or through uptake mediated by symbiotic microbes), where the N is assimilated (in the root or in the shoot), and the plant part (e.g., leaves, stem, fruit) to which the N is allocated. Beyond the level of the individual plant, foliar δ15N values vary across a number of spatial scales. The most significant patterns that have emerged are relationships between foliar δ15N and (1) plant functional type or mycorrhizal associations (Craine et al., 2009b; Hobbie and Högberg, 2012), (2) climate (Austin and Vitousek, 1998; Handley et al., 1999; Amundson et al., 2003; Murphy and Bowman, 2006), and (3) nutrient status (Stock et al., 1995; Fogel et al., 2008)—note that some of these may be strongly correlated with one another (e.g., nutrient status and mycorrhizal associations). Many studies have observed negative relationships between mean annual precipitation and foliar δ15N, with plants growing at arid sites being characterized by higher δ15N values than those growing at wetter sites (Austin and Vitousek, 1998; Handley et al., 1999; Amundson et al., 2003). Additionally, foliar δ15N values have been positively correlated with local temperature, such that warmer ecosystems are characterized by higher δ15N values than colder ecosystems (Martinelli et al., 1999; Amundson et al., 2003; Pardo et al., 2006), although this relationship deteriorates at the lowest temperatures (mean annual temperature ≤0.5°C; Craine et al., 2009b). The cause for this relationship is believed to be that hot and arid ecosystems tend to be more prone to N loss whereas colder and wetter ecosystems tend to conserve and recycle N (Handley et al., 1999). Because biogeochemical processes associated with N loss (e.g., NH3 volatilization and denitrification) are associated with large fractionations, enriching the remaining soil N in 15N, these processes drive the overall ecosystem δ15N values upwards. Foliar δ15N values have thus been used as a means of generalizing various aspects of N cycling at regional, continental, or even global scales (Amundson et al., 2003; McLauchlan et al., 2007).
When plants acquire N through symbiotic relationships with mycorrhiza, this tends to decrease plant δ15N values due to the retention of isotopically heavy N by the fungi (Figure 2), although these effects vary according to the mycorrhizal type as well as local environmental conditions. Mycorrhizae are mutualistic fungi that partner with plant roots, providing plants with N (as well as other nutrients, most notably P) in exchange for photosynthates. Mycorrhizal associations are extremely important for plant communities throughout the world and most plants are dependent on mycorrhizal fungi for some portion of their N (Brundrett, 2009). The three types of mycorrhizae that are significant for this discussion are outlined below (summarized from Read, 1991; Finlay, 2008; Craine et al., 2009b; Hobbie and Agerer, 2010; Hobbie and Högberg, 2012):
(1) Arbuscular mycorrhizae (AM) are the most common type and form relationships with the widest range of plant species. They are most common in areas with high rates of N cycling and lack the ability to decompose organic matter. AM plants tend to have the highest δ15N values of the three types, but usually have comparable or lower δ15N values relative to non-mycorrhizal plants.
(2) Ectomycorrhizae (ECM) are common partners of long-lived perennials or trees in boreal, temperate, and to a lesser extent tropical environments. There is substantial variation in the ability of ECM to decompose organic matter. ECM with morphologies that differ according to nutrient acquisition strategy (exploration type) tend to have distinct N isotopic compositions, but overall, ECM plants have intermediate N isotopic compositions of the three mycorrhizal types.
(3) Ericoid mycorrhizae (ERM) are not widely distributed, being largely limited to systems with low availability of mineralized N, low rates of N cycling, and high amounts of organic N in the soil. ERM have strong proteolytic capabilities and ERM plants tend to have the lowest δ15N values of the three mycorrhizal plant types.
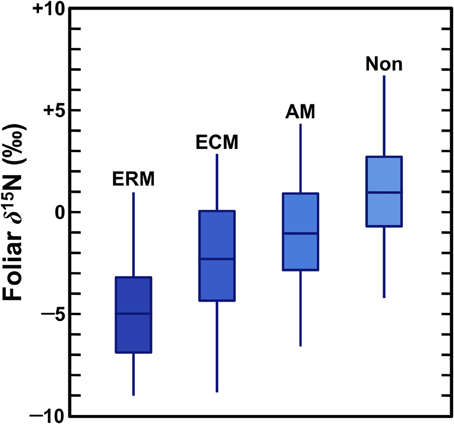
Figure 2. Foliar N isotopic compositions of plants according to mycorrhizal associations (Craine et al., 2009b). Boxes represent interquantile (25–75%) ranges, bars dividing boxes represent means, vertical lines represent 95% of the data. As described in Craine et al. (2009b) data were normalized to a common temperature, precipitation, and foliar [N]. ERM, ericoid; ECM, ectomycorrhizal; AM, arbuscular; Non, non-mycorrhizal.
A growing body of evidence has demonstrated that mycorrhizal fungi are enriched in 15N relative to their plant partners because of the transfer of 15N-depleted compounds to the plants (Hobbie and Högberg, 2012). A recent global survey by Craine et al. (2009b) found mycorrhizal associations significantly influence foliar N isotopic compositions, with mycorrhizal plant δ15N being lower than non-mycorrhizal plant δ15N by 2‰ for AM, 3.2‰ for ECM, and 5.9‰ for ERM. Thus, the relative importance of mycorrhizal associations plays a strong role in structuring spatial and temporal variation in plant δ15N values.
Nitrogen cycle openness and mycorrhizal associations appear to be the dominant factors controlling N isotopic variation in plants at various spatial scales (Craine et al., 2009b; Hobbie and Högberg, 2012). A generalized model of N cycling in plant-soil systems is presented in Figure 3. Three categories of processes are outlined: inputs, outputs, and transformations, all of which have the potential to influence soil and plant N isotopic compositions.
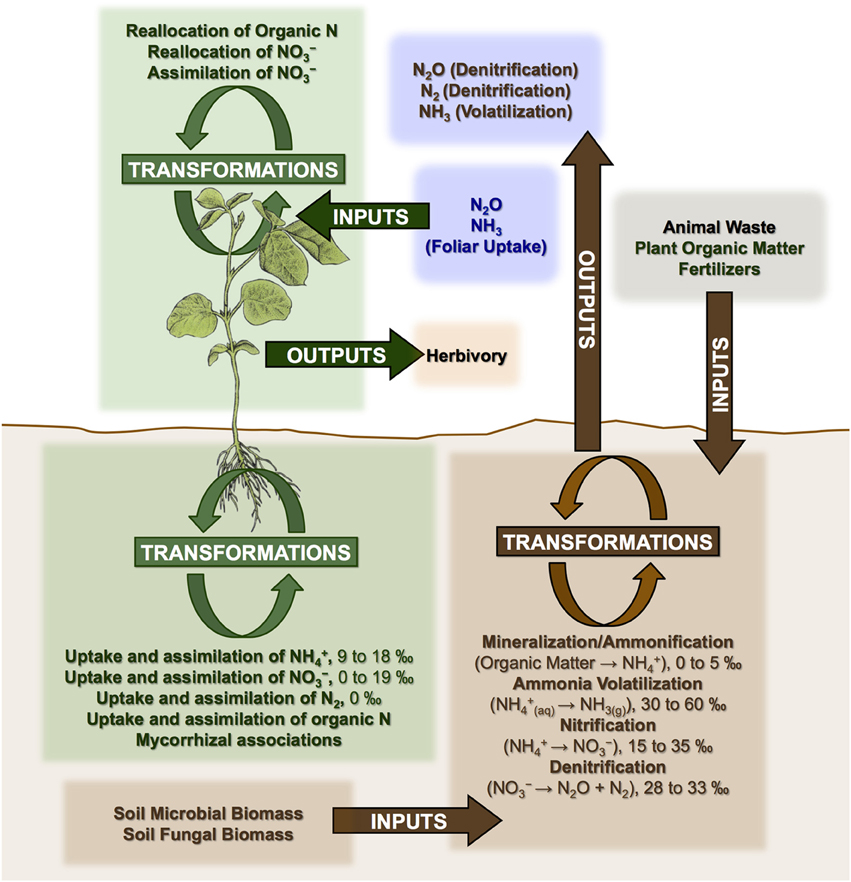
Figure 3. Generalized model of N cycling in plant-soil systems showing processes that may affect plant N isotopic compositions. Mineralization refers to the conversion of organic N into ammonium (NH+4). Nitrification refers to the conversion of ammonium into nitrate (NO−3). Denitrification is the reduction of nitrate into atmospheric nitrogen (N2). Fractionations (Δ15N) are shown for some processes (Robinson, 2001), while others with more complex and variable effects are discussed throughout the text (e.g., mycorrhizal associations, addition of animal fertilizers).
The origins and development of agriculture have been and continue to be a topic of major theoretical and practical interest in archaeology. Because the transition to agriculture is typically coincident with a change in diet, stable isotope analysis has been utilized extensively to address the development or adoption of agriculture in various parts of the world. In those regions where the transition to agriculture involved the cultivation of a C4 plant (such as maize in the Americas or millet in northern China) in a predominantly C3 plant-dominated area, carbon isotope compositions of human or animal tissues have been of primary importance. In other areas where the suite of agricultural products were C3 plants (Europe and the Near East) more importance has been place on N isotopic compositions of human remains with a reduction in the importance of some food sources with relatively high δ15N values (e.g., marine foods, freshwater fish, animal protein in general) with the adoption of agriculture (Bocherens et al., 2007; Borić and Price, 2013). Implicit in these interpretations is an assumption that the N isotopic composition of plants, and cultivated plants in particular, is relatively static and should be approximately 3–4‰ lower than contemporaneous herbivorous animals considering typical trophic level enrichments of 15N (Caut et al., 2009; Szpak et al., 2012c). Recent experimental work conducted with animal fertilizers has demonstrated that this assumption is an oversimplification and somewhat problematic in agricultural societies. This section will review three processes that were likely important in prehistoric agriculture (use of animal fertilizers, burning of vegetation or shifting cultivation, and tillage) and that have the potential to alter plant and soil N isotopic compositions.
The maintenance of soil fertility has been, and continues to be, a matter of utmost importance to agricultural societies. Fertilizers derived from animal excreta have long been significant sources of nutrients (including N) for cultivated plants at a global scale, likely for millennia (Jones, 2012). In prehistoric contexts, the detection of the use of animal-derived fertilizers is not straightforward and is often ambiguous, although several lines of evidence have been utilized to detect the presence of animal dung in archaeological deposits (see chapters in Jones, 2012). Nitrogen is the most important nutrient added to the soil via animal manure, and the N isotopic composition of animal manure is often higher than that of endogenous soil N. There is, therefore, potential for plant N isotopic compositions to be significantly altered by fertilization. This has important implications for the interpretation of isotopic data derived from human and animal remains, as well as for the potential detection of fertilization practices in prehistoric contexts.
Numerous studies in the fields of soil and plant sciences (Choi et al., 2002, 2003, 2006; Watzka et al., 2006; Yun et al., 2006; Lim et al., 2007; Yun and Ro, 2009; Kriszan et al., 2014), agricultural and food chemistry (Bateman et al., 2005; Nakano and Uehara, 2007; Del Amor et al., 2008; Rapisarda et al., 2010; Yun et al., 2011; Yuan et al., 2012; Zhou et al., 2013), and archaeology (Bogaard et al., 2007; Fraser et al., 2011; Szpak et al., 2012b) have demonstrated higher δ15N values for plants fertilized with animal manures relative to unfertilized plants, or plants treated with nitrogenous chemical fertilizers (Figure 4). The extent to which animal fertilizer application affects plant δ15N values is highly variable and depends on the type of fertilizer applied, the amount applied, and the duration of application. For the animal fertilizers that have been studied, a pattern of 15N enrichment in plants can be summarized as follows: poultry ≤ cattle < pig < seabird (Figure 5). In general, as the δ15N value of the manure increases, so too does the δ15N value of the fertilized plant. It is important to keep in mind, however, that the input of a new N source that occurs with fertilization is not the only factor that affects the δ15N values of soils and plants—the variation observed in plant δ15N for any particular fertilizer δ15N clearly demonstrates the importance of other factors (Figure 5). The extent to which a particular fertilizer will be expected to influence the N isotopic composition of a plant will also be strongly dependent on how the presence of that fertilizer impacts transformations and losses (outputs) of N, since these processes discriminate against 15N, in some cases very strongly (Figure 3). For instance, while the δ15N values of pig manure is typically higher than the δ15N values of cattle manure (Bateman and Kelly, 2007), the availability of mineralized N from pig manure is also much higher than for cattle manure—20 to 40% N available from cattle manure after 1 year compared to 75–90% for pig manure (Eghball et al., 2002). While the mineralization of organic N is not associated with any appreciable fractionation (Robinson, 2001), the availability of the fertilizer-derived N will be strongly affected by mineralization rates.
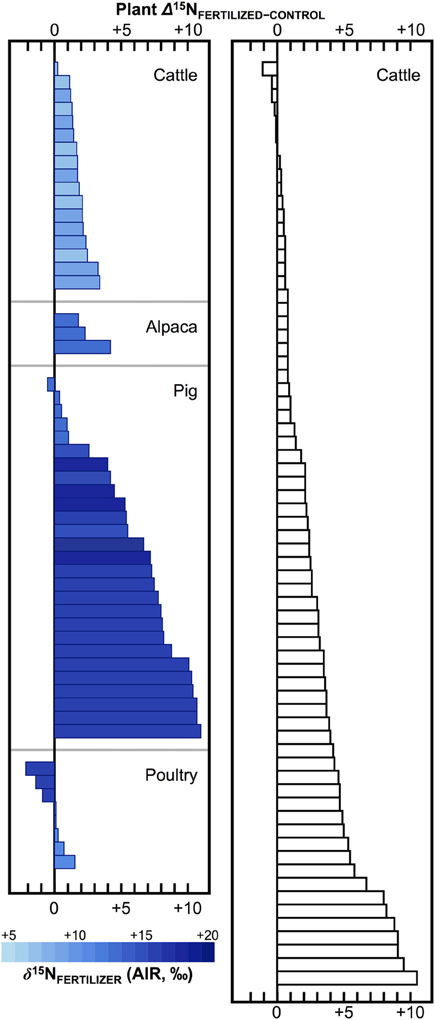
Figure 4. Effects of animal fertilizers on plant δ15N values. Bars represent differences between control plants (no fertilizer applied) and treatments receiving animal fertilizers. Bars are shaded according to δ15N of the fertilizer applied, with darker colors representing higher δ15N values; open bars indicate fertilizer δ15N value was not determined (right panel). Data are compiled from published literature (Choi et al., 2002; Watzka et al., 2006; Yun et al., 2006; Lim et al., 2007; Yun and Ro, 2009; Fraser et al., 2011; Szpak et al., 2012b; Yuan et al., 2012; Zhou et al., 2013). Studies contrasting chemically and organically fertilized plants without unfertilized controls were not included. For readability, results from plants fertilized with seabird guano (differences between fertilized and control plant δ15N +20 to +40‰) were excluded (Szpak et al., 2012a,b, 2014).
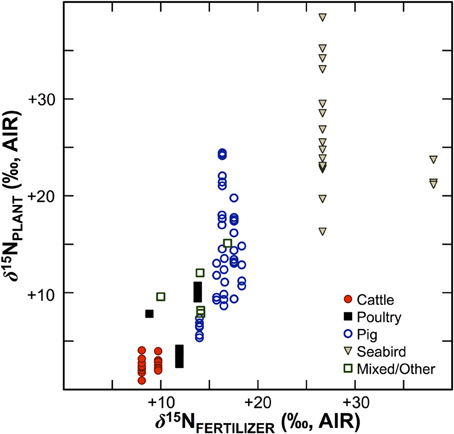
Figure 5. Bivariate plot of fertilizer δ15N vs. plant δ15N. Generally, plant δ15N increases with fertilizer δ15N, although there is considerable variation, which can be ascribed to the relative availability of N compounds in the fertilizer, the rate and duration of application, and interspecific differences in plant N acquisition strategies. Data are compiled from experimental studies (Choi et al., 2002, 2003; Watzka et al., 2006; Yun et al., 2006; Lim et al., 2007; Nakano and Uehara, 2007; Yun and Ro, 2009; Rapisarda et al., 2010; Szpak et al., 2012a,b, 2014; Yuan et al., 2012; Zhou et al., 2013).
The bulk N isotopic composition of mammalian urine tends to be depleted in 15N relative to the diet by about −2.5‰, while feces is typically enriched in 15N relative to the diet by +2.0‰ (Table 1). The δ15N values of animal manures typically are not consistent with the diet-feces or diet-urine 15N fractionations that have been observed for many species, and instead manures tend to be characterized by much higher δ15N values than would be expected. This is because of several important chemical processes that act on various N species in these manures at various stages between animal excretion, collection, composting (if applicable), storage, and decomposition after application. These processes include: ammonification (mineralization), immobilization, nitrification, dentrification, NH3 volatilization, and leaching (Petersen et al., 1998). Nitrogen losses from NH3 volatilization and denitrification tend to be particularly large during manure storage, with losses of 10–40% N being common (Kirchmann, 1985). Accordingly, there tends to be a considerable increase in manure δ15N values during composting and storage (Choi et al., 2007; Kim et al., 2008).
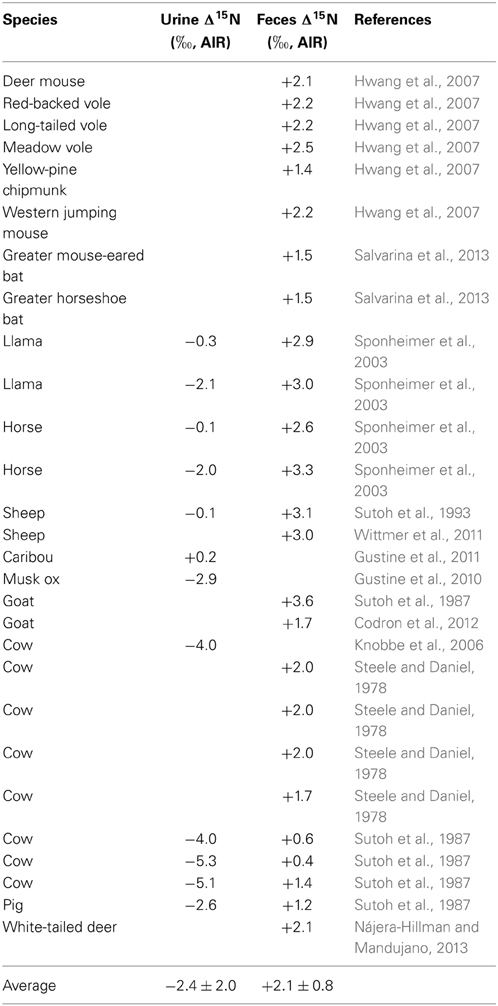
Table 1. Summary of studies presenting data for the differences in N isotopic compositions between the diet and urine and/or diet and feces (Δ15N) of different mammalian species.
While the potential effects of animal fertilizers on δ15N values in plant-soil systems have long been known (Riga et al., 1971; Kreitler and Jones, 1975), it has only been since the recent work of Bogaard et al. (2007) on the N isotopic compositions of plants from long-term agricultural stations that archaeologists have begun to more seriously consider the potential impact of fertilizers on plant, and in turn, human tissue δ15N values. Two key issues in archaeology have emerged out of this recent reconsideration of the effects of animal fertilizers on plant δ15N values, one a complication and one an opportunity. The complication comes with the interpretation of human δ15N values from archaeological contexts. Essentially, because of the reasonably consistent trophic level enrichment in 15N that occurs between herbivores and plants (Caut et al., 2009), all things being equal, herbivorous animal tissues should have δ15N values 3–4‰ higher than coeval plants. Working backwards from this general relationship, direct measurements of contemporaneous herbivore bone collagen δ15N values are used as a reference point for crop δ15N values. Considering a scenario where animal manure is used to fertilize plants and the δ15N values of those plants are 2–6‰ higher than would be expected without the addition of the manure there is a convergence of plant and animal δ15N values (provided the animals are not consuming significant quantities of fertilized crops) such that the relative contributions of plant and animal protein to the diet become less clear. Bulk collagen δ15N values of human bone collagen are thus insufficient in and of themselves to differentiate between terrestrial herbivore and fertilized plant consumption. Accordingly, additional isotopic markers are required. The isotopic analysis of individual amino acids isolated from bone collagen is particularly promising in this regard as there is evidence that the consumption of plant vs. animal protein can be resolved via the carbon isotopic composition of individual amino acids (Petzke et al., 2005), which should not be influenced by fertilization (Szpak et al., 2012a).
When considering N isotopic data derived from human tissues such as bulk bone collagen, direct evidence for the manuring of crops will be ambiguous. There is opportunity to more directly approach questions of crop management and manuring practices through the analysis of plant material preserved at archaeological sites. DeNiro and Hastorf (1985) demonstrated the potential of analyzing the N isotopic composition of charred plant remains for paleodietary baselines. More recently, a number of studies have utilized similar methods to investigate the manuring of ancient cereals, as well as various aspects of crop management that may affect plant δ15N values, at European and Near Eastern sites (Lightfoot and Stevens, 2012; Bogaard et al., 2013; Vaiglova et al., 2014; Kanstrup et al., in press). Interpreted within the context of data from long-term experimental agricultural stations, these data have indicated variable levels of manuring over thousands of years, although it is not possible to draw a direct causal link between the application of animal manure and elevated archaeobotanical δ15N values given the highly variable nature of plant N isotopic compositions, even at relatively small spatial scales. Experimental charring has demonstrated that this process does not substantially alter grain δ15N values (Kanstrup et al., 2012; Fraser et al., 2013; Styring et al., 2013), but it is less clear to what extent post-depositional processes may alter these values, if at all. Comparatively, a range of techniques and measures are available for assessing the “intactness” of collagen extracted from bone (C:N ratio, minimum %C and %N, collagen yield), which can be used to discard data unlikely to be representative of endogenous isotopic compositions. No such measures currently exist for charred plant remains and this no doubt has to do with the non-specific nature of bulk plant samples (relative to purified protein isolated from bone). Recent work by Styring et al. (2013) is an important first step in this direction, but additional studies are required to develop a more comprehensive and reliable set of quality indicators.
One of the most frequently discussed forms of land management by prehistoric human populations is swiddening (alternatively slash-and-burn or shifting cultivation). Vegetation is cleared by burning and crops are grown on the land until soil fertility sufficiently declines and this land is left fallow for a number of years and allowed to regenerate; this pattern is shifted to another location and repeated. It has been suggested that swidden agriculture was important in nearly every region of the globe where plants were cultivated prehistorically, and although its significance has been questioned in some parts of the world (Sherratt, 1980; Bogaard, 2002), swidden agriculture remains a prominent aspect of cultivation in the humid tropics today and was likely of some importance prehistorically even if not at a global scale. In addition to farmers, clearing vegetation through burning is also a behavior recorded for numerous groups of foragers to encourage the presence of particular species (Rowley-Conwy and Layton, 2011). Thus, within the context of human dietary studies, this issue requires consideration. Numerous studies have examined the potential consequences of vegetation burning on soil and plant N isotopic compositions (Herman and Rundel, 1989; Mordelet et al., 1996; Grogan et al., 2000; Cook, 2001; Saito et al., 2007; Schafer and Mack, 2010; Beghin et al., 2011; Johnson et al., 2011; Huber et al., 2013; Leduc et al., 2013), but to the best of my knowledge these effects have not been investigated directly as they are associated with swidden agriculture and are instead mostly limited to studies of forest soils that are not cultivated after burning. Nevertheless, some generalizations about N cycling in burned soils can be made.
In the process of clearing land by burning, there are alterations to the distribution and cycling of nutrients. Ash that is incorporated into the soil is typically rich in P and mineral nutrients (e.g., K, Ca, Mg), with C and N being largely lost due to volatilization (Juo and Manu, 1996). Aside from direct nutrient additions to the soil, the addition of charred organic material has been demonstrated to facilitate the retention of soil nutrients (such as those added by organic fertilizers) in both temperate (Laird et al., 2010) and tropical (Lehmann et al., 2003) environments. Although burning results in large losses of N, soil mineralized N pools (NH+4 especially) have been observed to increase significantly after burning (Grogan et al., 2000; Schafer and Mack, 2010; Huber et al., 2013). There are several possible reasons for this increase: (1) N is added directly through the contribution of burned vegetation, although this is not supported in most cases because the N content of the ash itself tends to be extremely low, (2) increased temperature in the soil enhances mineralization of organic N in the soil (Klopatek et al., 1990), and (3) the increase in soil pH caused by the addition of ash may enhance microbial activity (Grogan et al., 2000). The mechanism(s) by which additional mineralized N enters the burned area is significant because they will have variable consequences on the N isotopic composition of the soil N pools and in turn on foliar δ15N values. With respect to the organic material derived from the burned vegetation, the duration and intensity of the fire may have important consequences for the N isotopic composition of the resultant ash or char. For very high temperature fires, it has been suggested that there is little opportunity for fractionation because nearly all of the N is lost in gaseous form (Saito et al., 2007). Conversely, for low temperature fires resulting in charred organic material rather than ash, there is greater opportunity for fractionation and the charred material may be enriched in 15N relative to unburned vegetation (Saito et al., 2007; Huber et al., 2013). Therefore, where vegetation is relatively N rich and fire intensity is relatively low, the mineralized N derived from this organic matter may be enriched in 15N relative to the overall soil N pool.
Numerous studies have examined the consequences of vegetation burning on foliar and soil δ15N values with inconsistent results. Most studies have recorded either significantly higher foliar δ15N values in post-fire vegetation (Grogan et al., 2000; Saito et al., 2007; Beghin et al., 2011; Johnson et al., 2011; Huber et al., 2013; Leduc et al., 2013) or inconsistent changes between pre-and post-fire vegetation (Mordelet et al., 1996; Schafer and Mack, 2010; Beghin et al., 2011); comparatively few studies have found significantly lower foliar δ15N values following fire (Herman and Rundel, 1989; Cook, 2001). That there is not a consistent pattern in all cases is not surprising and likely reflects the diversity of processes responsible for the N available, which will vary on a case-by-case basis. Nonetheless, higher δ15N values in post fire vegetation initially, followed by a return to pre-fire δ15N values is the most common pattern recorded.
Two hypotheses have been put forth to explain the elevated foliar δ15N values following fire, which are not mutually exclusive. First, as discussed above, the burning of organic material may cause discrimination against 15N, leaving the residual organic matter relatively 15N enriched (Saito et al., 2007). This organic matter is mineralized in the soil to NH+4 and taken up by plants. As nitrification, which discriminates against 15N, proceeds, the NH+4 pool becomes more 15N-enriched and creates a situation where plants that use predominantly NH+4 are characterized by significantly higher δ15N values (Huber et al., 2013). Additionally, although both NH+4 and NO−3 are highly soluble, NH+4 has a greater capacity to adsorb to organics and minerals, and is thus less likely to be lost due to leaching (Johnson et al., 2011), which may further enriched the post-fire N pool in 15N. The second hypothesis was outlined by Högberg (1997) and focuses on the differential access of organic matter pools with variable depth in burned and unburned soils. In short, fire consumes the surface organic matter layer, which tends to be depleted of 15N relative to deeper horizons, especially in forest soils (Nadelhoffer et al., 1996; Hobbie and Ouimette, 2009). Following a fire, plants rely on mineralized N from deeper horizons, which is enriched in 15N relative to the litter, causing their tissues to also be enriched in 15N (Högberg, 1997). After vegetation reestablishes in the burned zone, foliar δ15N values gradually return to pre-fire levels (Leduc et al., 2013).
The magnitude of the difference observed in δ15N between pre- and post-fire vegetation, or between vegetation on control plots and plots subjected to fire can be quite large (Figure 6). For example, in California pine forest stands, Grogan et al. (2000) recorded differences in foliar δ15N between burned and mature forests of +8.6‰ (herb), +4.7‰ (N-fixer), +5.8‰ (re-sprouter), and +6.0‰ (pine). Approximately 1 year after wildfires in Australia, Huber et al. (2013) found increases in foliar δ15N relative to pre-fire values of: +4.1‰ (grassland), +4.8‰ (heathland), +5.5‰ (woodland). These differences in foliar δ15N are comparable to, or in some cases greater than, those reported between unfertilized plants and those fertilized with cattle manure. Accordingly, the potential impact of burning on crop δ15N values within the context of shifting cultivation requires additional investigation. Although the effects of burning on foliar δ15N values lessen after a period of several years or decades (Leduc et al., 2013), the relatively short period during which burned plots would be utilized in shifting cultivation (typically 1–5 years) (Bogaard, 2002) creates a strong possibility that plant δ15N values could be significantly altered in a systematic manner.
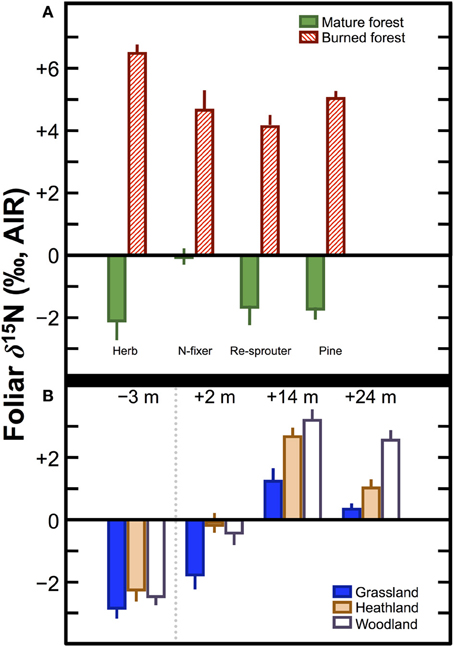
Figure 6. Effects of fire on plant δ15N values. (A) Comparisons between plants grown on mature and recently burned California pine forests (Grogan et al., 2000). (B) Change over time in three different Australian ecosystems following substantial wildfires. Values above indicate number of months prior to or after the wildfires. The broken vertical line separates the pre- and post-fire samples (Huber et al., 2013). In both (A,B) bars represent means ± one standard deviation.
The mixing of soil using plow technology was an important part of agriculture in the Old World from at least the fourth millennium B.C. (Sherratt, 1980). Even in the absence of draft animals, mechanical agitation of the soil through human tillage has likely been an important part of cultivation globally. The mechanical agitation of soil that occurs with tillage serves to disturb aggregated soil particles, increases aeration, and exposes previously buried soil surfaces (Erickson, 1982). Thus, in addition to bringing mineralized nutrients to the surface directly, tillage promotes mineralization of organic matter (Silgram and Shepherd, 1999). These practices may have significant implications for the N isotopic compositions of soils and plants because there is often strong variation in δ15N according to depth within a soil profile (Hobbie and Högberg, 2012). An increase in soil δ15N with depth has been observed in forests (Gebauer et al., 1994; Högberg et al., 1996; Koopmans et al., 1997; Emmett et al., 1998), grasslands (Steele et al., 1981; Mordelet et al., 1996; Frank and Evans, 1997), tundra (Nadelhoffer et al., 1996), and pastures (Steele et al., 1981; Ledgard et al., 1984; Piccolo et al., 1996). The magnitude of these changes appears to be consistently the greatest in forests relative to other environments (Hobbie and Ouimette, 2009). In some cases, although deeper layers are generally characterized by higher δ15N values than more shallow layers, a maximum δ15N value is reach at an intermediate depth in the soil profile (e.g., 20 cm) (Hobbie and Ouimette, 2009). Because of this depth-related variation in soil δ15N, it has been suggested that differences in rooting depth between plant species or functional types are responsible for some variation in wild plant δ15N values (Schulze et al., 1994), although these effects have not been investigated in agricultural contexts. Several mechanisms have been suggested to be responsible for the increase in soil δ15N values with depth:
(1) Plant tissues are depleted in 15N relative to surface soils and the return of 15N-depleted plant matter to the soil surface via litterfall creates a 15N-depleted surface layer (Högberg, 1997).
(2) Soil δ15N increases with the age of organic matter and the extent of decomposition. As organic matter undergoes mineralization, the ammonium that is produced is relatively depleted in 15N and the residual organic matter becomes increasingly 15N-enriched. Organic matter decomposes, reduces in size, and travels down the soil profile, creating the characteristic increase in δ15N with depth (Tiessen et al., 1984; Nadelhoffer and Fry, 1994).
(3) Through mycorrhizal transfer, plants acquire N that is relatively depleted in 15N and once incorporated into the litter layer, this decomposing plant material creates a 15N-depleted surface. Nitrogen derived from the tissues of the mycorrhizal fungi, which are enriched in 15N relative to their plant partners, accumulates at greater depths, leading to 15N-enriched soil N relative to the surface layers (Högberg et al., 1996; Hobbie et al., 1999; Hobbie and Ouimette, 2009; Hobbie and Högberg, 2012).
The redistribution of N throughout the soil profile associated with tillage has the potential to disturb the depth-related variation in soil δ15N. Specifically, the exposure of deeper layers that are likely enriched in 15N to the surface might cause plants growing in tilled fields to be characterized by higher δ15N values than those growing in no-till fields (see Emmett et al., 1998), although there are several issues with this notion. For the first of the three hypotheses discussed above (15N-depleted litterfall creates a 15N-depleted soil surface horizon), the incorporation of plant matter into a discrete layer of surface litter occurs in only limited circumstances as much of this above-ground organic matter is removed during harvest, or is redistributed through the soil profile during tilling. Thus, the abrupt shift in δ15N in the uppermost portions of the soil profile associated with forests (Figure 7A) is unlikely to occur in most cultivated fields (Figure 7B), and this is generally consistent with field measurements (Shearer et al., 1978; Karamanos et al., 1981; Selles et al., 1984).
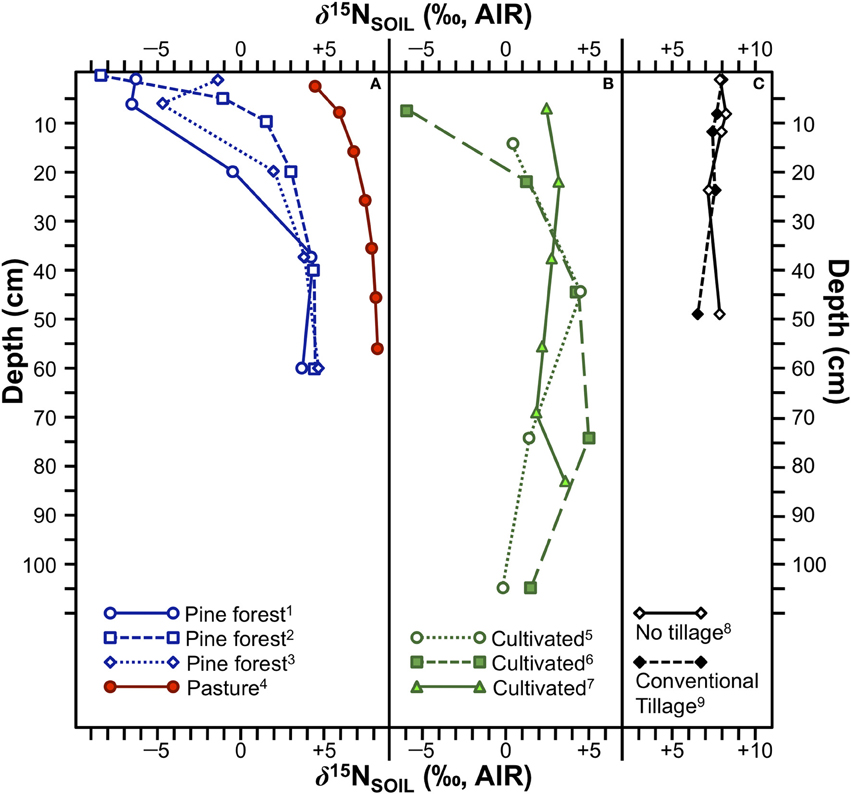
Figure 7. δ15N variation with depth in soil profiles under various conditions. (A) Soil profiles for three pine forests and one pasture. The much larger and abrupt shift in δ15N with depth is typical of N-limited forests. (1) pine forest, Netherlands (data from Koopmans et al., 1997), (2) pine forest, USA (redrawn from Hobbie and Ouimette, 2009), (3) pine forest, Netherlands (data from Koopmans et al., 1997), (4) native pasture, southeastern Australia (redrawn from Ledgard et al., 1984). (B) Soil profiles for three cultivated California soils. (5) Hanford sandy loam, (6) Yolo fine sandy loam, (7) Vernalis silt loam (data from Broadbent et al., 1980). (C) Comparison of soil profiles from experimental fields in Saskatchewan with no tillage (8) and conventional tillage (9) showing no significant differences in δ15N between the two fields (data from Selles et al., 1984).
Mycorrhizal associations (specifically arbuscular) are critical components of sustainable and organic farming systems (Gosling et al., 2006). The majority of agricultural plants with the exceptions of the Brassicaeceae (e.g., broccoli, cabbage, radish) and Chenopodiaceae (e.g., spinach, quinoa) form mycorrhizal associations (Newman and Reddell, 1987). That agricultural fields tend to be overwhelmingly characterized by arbuscular mycorrhizal communities is significant. The majority of temperate forests from which significant changes in soil δ15N with depth have been recorded are dominated by ectomycorrhizae, which tend to be more 15N enriched (and hence symbiotic plants are more 15N depleted) relative to AM (Craine et al., 2009b; Hobbie and Högberg, 2012). Communities dominated by ectomycorrhizae tend to be characterized by larger changes in soil δ15N with depth relative to arbuscular-dominated communities (Hobbie and Ouimette, 2009). Additionally, the increased N or P input through fertilization that characterizes many agricultural fields is likely to inhibit mycorrhizal colonization (Jensen and Jakobsen, 1980; Kahiluoto et al., 2001), although this may not apply equally well to the relatively low-input farming of the past. Further, the act of tilling the soil can decrease the prevalence of mycorrhizal root colonization and the contribution of mycorrhizae to overall fungal biomass in agricultural fields (Kabir et al., 1997; Mozafar et al., 2000; Van Groenigen et al., 2010). Thus, from a mycorrhizal perspective the variation in soil δ15N with depth should not be as pronounced in agricultural fields relative to forests, both for tilled and no-till soils.
In agricultural fields the increase in soil δ15N with depth is unlikely to be as strong as in forests and systematic depth-related variation in δ15N may be completely absent, reducing the likelihood that tillage would significantly increase plant δ15N values by bringing 15N-enriched compounds to the surface. Selles et al. (1984) tested this premise directly and found no significant change in soil δ15N at any depth due to tillage (Figure 7C). It is therefore unlikely that soil tillage is a significant contributor to variation in ancient plant N isotopic compositions.
A critical area of investigation within the context of human paleodietary studies involves understanding the range and variation of foods that may have been consumed. Most studies that have attempted to differentiate organically and chemically fertilized crops have found significantly lower δ15N values in the plants treated with chemical fertilizers (Bateman et al., 2005; Choi et al., 2006). Given the importance of chemical fertilizers in modern agriculture (Matson et al., 1997), this must be taken into account when data derived from modern surveys of cultivated plants are used as dietary baselines (e.g., Keegan and DeNiro, 1988; Szpak et al., 2013; Warinner et al., 2013). Many of the N isotopic compositions derived from modern plants may be of little or no value as paleodietary baselines if the fertilization method used is unknown or relies on chemical N fertilizers. Interpretations utilizing currently published data must therefore interpret these δ15N values cautiously.
Additional studies in controlled and field settings are essential to better understand the complexities of N cycling in ancient agricultural systems and their consequences on plant and soil N isotopic compositions. To do so, archaeologists must initiate controlled and field studies that have the greatest potential to yield results with direct relevance to archaeological contexts. In this respect, there is considerable potential for biogeochemically-oriented archaeologists to conduct collaborative research with ethnobiologists studying traditional land management systems. Moreover, the growing interest in organic and sustainable farming systems creates an additional layer of relevance to the investigation of nutrient cycling in ancient agricultural contexts. Importantly, we must not maintain a myopic focus on animal fertilization, and should instead look to a more diverse array of land management strategies and their effects on soil and plant N isotopic compositions.
To understand the nature of agricultural practices in the past, the greatest potential exists with the analysis of archaeobotanical materials as they provide a more direct (relative to animal tissues) view of N cycling. To effectively make sense of these data, however, additional work is required to resolve a wider range of factors that may influence cultigen N isotopic compositions. Additionally, where the interpretation of a particular agricultural practice (e.g., manuring) rests on the difference of a few ‰ in δ15N, we must be able to assess with a high degree of confidence whether the N isotopic compositions derived from archaeobotanical materials are in fact endogenous and not influenced by post-depositional alteration.
The nature of human-animal relationships has been an area of intensive study, both in anthropology and archaeology. The manner in which these relationships develop and change over time in domestic animals is particularly significant, and isotopic analysis has figured prominently in this context in recent years. Isotopic data have been used to examine specific types of plants consumed by animals, the scale of animal herding, demography, and the trade in animal products. The majority of domestic animals from archaeological contexts that have been subjected to isotopic analysis are herbivores (e.g., cattle, sheep, goats, camelids). Therefore, unlike omnivorous species (e.g., dogs, pigs), the N isotopic compositions of herbivore tissues will not be affected by trophic level, but principally by biogeochemical processes in plant-soil systems. It is therefore important to consider how these systems may be different for animals kept under different conditions (e.g., stabling vs. free-ranging) or fed particular diets (e.g., agricultural byproducts). This section focuses on the isotopic consequences of grazing intensity/stocking rate and the foddering of domestic animals with cultigens.
The presence of grazing animals has the capacity to alter nutrient cycling in plant-soil systems through several mechanisms: additions of nutrients derived from urine and feces, trampling and physical disturbances, and changes in floral community composition (Bardgett and Wardle, 2003; Singer and Schoenecker, 2003). Within the context of N isotope studies, the effects of herbivore waste on plant-soil systems have been studied in a number of natural and controlled settings. While this section concentrates on grazing intensity in domestic herbivore species, it is also applicable to wild species and has implications for paleoecological contexts.
Where animals deposit waste, the concentrated addition of mineral nutrients and organic matter to the soil has the potential to alter the N isotopic compositions of soils and plants. This is somewhat analogous to the effects of animal fertilizers on plant δ15N values in agricultural fields although the redistribution of N through herbivore activity is qualitatively and quantitatively different from the direct application of animal manure. Several archaeological studies have discussed the possibility that stocking rate or grazing intensity in animal populations may influence animal tissue δ15N values (Britton et al., 2008; Oelze et al., 2011; Makarewicz, 2014; Müldner et al., 2014). The effects of grazing on N cycling in plant communities is complex and a closer examination of the literature reveals that unlike the very consistent increase in plant δ15N values caused by manuring, there is not a simple relationship between grazing intensity or stocking rate and plant δ15N.
Studies presenting N isotopic compositions for plants and soils under different levels of grazing pressure or stocking rates are summarized in Table 2. Most studies analyzed above ground plant tissues and soil δ15N, and although results vary considerably across studies the general pattern observed is that more intensively grazed areas tend to have higher plant and soil δ15N values. This pattern of general 15N enrichment in more intensively grazed zones may be the product of increased N cycle openness (Ruess and McNaughton, 1987). Aside from the direct addition of mineralized or highly labile forms of N, more intensively grazed areas tend to be characterized by one or more of the following: higher rates of ammonification, leaching of NO−3, NH3 volatilization, and denitrification (McNaughton et al., 1988; Ruess and McNaughton, 1988; Hobbs, 1996; Frank and Zhang, 1997; Frank et al., 2004). All of these processes, with the exception of ammonification, are associated with 15N enrichment in the residual substrates (Robinson, 2001), and therefore by increasing both N inputs and outputs (Singer and Schoenecker, 2003), herbivores tend to cause higher δ15N values in soils and plants. These patterns fit with the general observation that ecosystem δ15N tends to be higher where inputs, cycling, and outputs are higher (Högberg and Johannisson, 1993; Högberg et al., 2011, 2014).
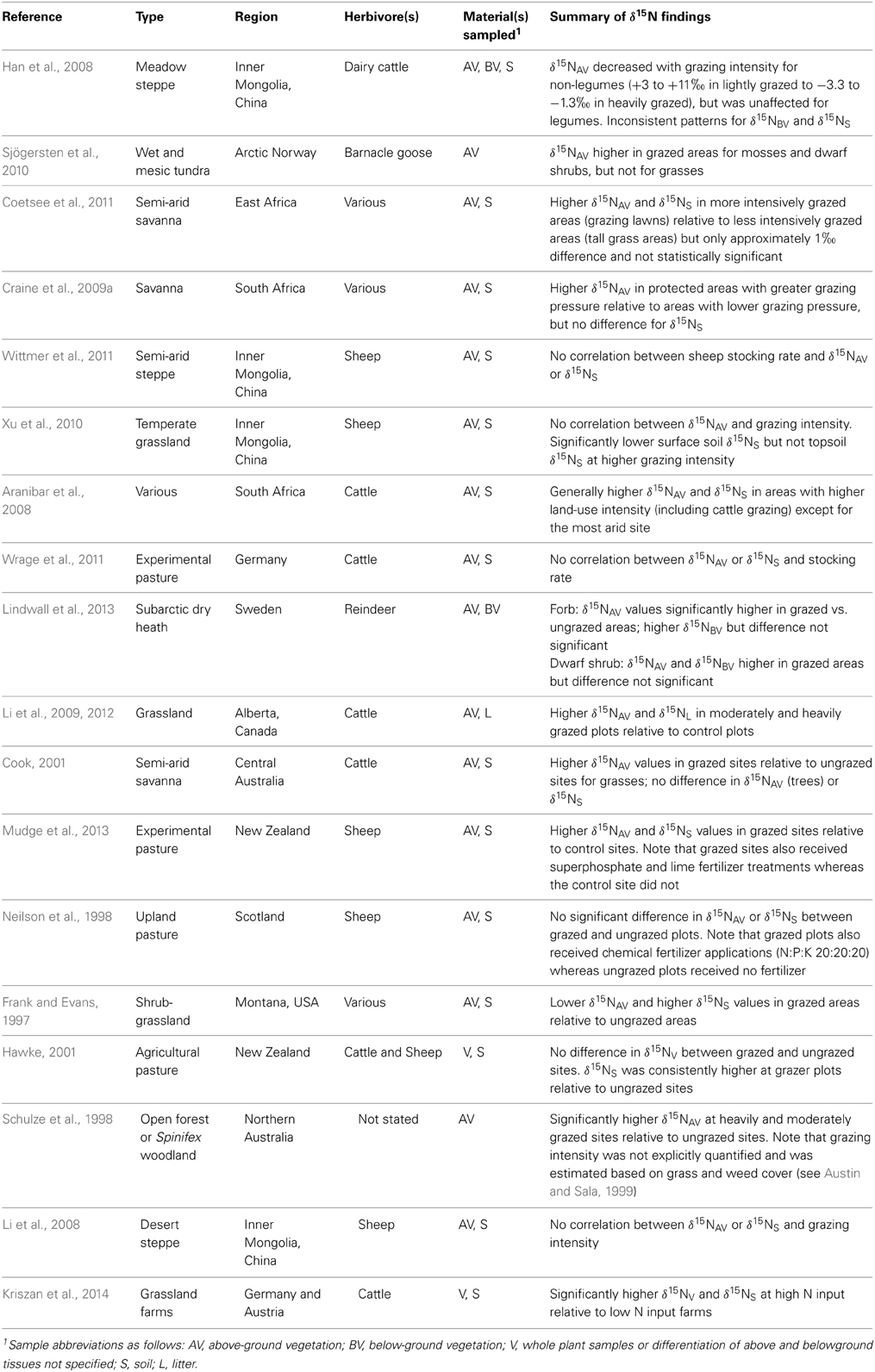
Table 2. Summary of studies examining the effects of grazing intensity or stocking rate on plant and soil N isotopic compositions.
While a trend toward higher plant and soil δ15N with higher grazing intensity exists, several studies have demonstrated significantly lower δ15N values in plant shoots relative to roots and/or soil in areas of high NH3 volatilization (Frank and Evans, 1997; Erskine et al., 1998; Frank et al., 2004; Han et al., 2008), suggesting that leaves actively absorb 15N-depleted NH3 and assimilate it into organic N (Vallano and Sparks, 2013). In agricultural contexts the emission of NH3 and N2O can be very high in areas with large amounts of manure, such as occurs with intense grazing (Amon et al., 2001). The uptake of these isotopically light gaseous N compounds by plant leaves may have a mediating effect against the uptake of 15N-enriched soil compounds that occur with heavy grazing, and cause significant spatial variability in foliar δ15N values within and between fields if grazing intensity and the distribution of animal waste is uneven (see Erskine et al., 1998).
Because archaeological studies overwhelmingly analyze animal skeletal or dental tissues for their N isotopic compositions (rather than plant materials), an important consideration is whether or not the elevated δ15N values with higher grazing intensity/stocking rate are also reflected in animal tissues. From a strictly theoretical perspective, if the soil and plant δ15N values increase with increased stocking rate, so too should the δ15N values of herbivores grazing in these ecosystems. Wittmer et al. (2011) found no relationship between stocking rate and sheep tissue δ15N, but they also did not detect any significant differences in vegetation or soil δ15N with variable stocking rate; similar results were obtained by Wrage et al. (2011) with cattle. Schwertl et al. (2005) and Kriszan et al. (2014) found that higher grazing intensities were correlated with higher animal tissue δ15N values, with differences between stocking rates as high as 4‰ in both studies. This is strongly suggestive that such a pattern may also be present in the tissues of archaeological animals, although one wonders to what extent results derived from modern confinement dairy farms (with very high stocking rates) are in any way a realistic analog for ancient animal management systems. A more conservative and appropriate strategy for ancient contexts might be to compare data derived from small-scale organic farms with variable stocking rates.
The type of foods that animals consume is one of the many behavioral changes that may differentiate wild and domestic species. This control exerted over the diet of animals through spatial and behavioral restrictions is an important, and perhaps a defining, aspect of relations between humans and livestock. Indeed, in some areas of the world there may have been some degree of symbiotic development of animal and crop husbandry, with animal excreta being instrumental in maintaining soil fertility and cultivated plants (or byproducts) providing valuable fodder for animals (Charles et al., 1996). Many studies have interpreted isotopic data from prehistoric animals within the context of foddering strategies (Finucane et al., 2006; Madgwick et al., 2012; Makarewicz, 2014), although most have focused on variable C3 and C4 plant consumption on the basis of carbon isotopic data. There are, however, some important considerations with respect to N isotopic compositions of agricultural plants within the context of animal foddering and some processes that would be expected to significantly influence δ15N values in plants and animals.
The types of plants used as animal fodder are highly variable. In prehistoric Europe, when forests were much more widespread than in the present day, tree leaves were an important source of animal fodder (Regnell, 2002). While some crops may have been grown specifically to be fed to animals (Ross and Zutter, 2007), the most common source of animal fodder is agricultural byproducts, typically the stems and leaves remaining after the harvest of grains (Jones, 1996). In these instances where animals and humans consume different parts of the same plant, there are important isotopic consequences driven by N isotopic variability within plants. This is especially important because animal N isotopic data are often used for comparative or baseline purposes to assess the importance of plant- and animal-derived protein in human diets (Privat et al., 2002; Müldner and Richards, 2005).
Most of the preceding discussion has focused on factors influencing the N isotopic composition of N species taken up by plants, but there are also processes that occur within plants that have the potential to alter their N isotopic composition. Even plants grown under controlled conditions with a single N source have displayed considerable (up to 7‰) within-plant variation in δ15N (Yoneyama et al., 1986; Yoneyama and Kaneko, 1989; Evans et al., 1996; Robinson et al., 2000; Kolb and Evans, 2002; Szpak et al., 2012a). One reason for this within-plant variation is the assimilation of NH+4 into organic N occurs only in the roots, but the assimilation of NO−3 occurs both in the roots and the shoots. This is significant because the NO−3 that is moved to the shoot and assimilated there has already undergone some fractionation in the roots (due to the assimilation of NO−3 in the root) and is enriched in 15N relative to the NO−3 pool that was assimilated in the root (Evans et al., 1996). Thus, where NO−3 is the predominant N source, there exists a possibility of shoots being enriched in 15N relative to roots (Yoneyama and Kaneko, 1989; Evans et al., 1996; Evans, 2001).
More pertinent to the foddering of animals, however, is that when N that has been previously acquired is remobilized to areas of new growth, this may result in fractionation (Gebauer et al., 1994; Näsholm, 1994; Szpak et al., 2012a; Kalcsits and Guy, 2013). There tends to be a difference in δ15N between tissues that act as nitrogen sinks (e.g., grains that form during reproductive growth when vegetative growth has slowed or ceased) and nitrogen sources (e.g., leaves and stems that may reallocate much of their N to reproductive tissues such as flowers and fruits). There are two reasons that this variation occurs. First, because metabolic pathways leading to the synthesis of different amino acids are characterized by differing levels of fractionation against 15N (Werner and Schmidt, 2002), the selective import or export of particular amino acids between plant tissues may contribute to this intraplant variation (Tcherkez, 2011; Gauthier et al., 2013). Similarly, alterations in the distribution of proteins, free amino acids, amino sugars, and alkaloids between different tissues or organs may drive this variability because proteins tend to be 15N enriched relative to bulk cell N, while the other compounds listed tend to be relatively depleted in 15N (Werner and Schmidt, 2002). Second, the fractionations associated with the catabolism and eventual reassimilation of various N compounds (deamination and transamination) could also influence the δ15N values of source and sink tissues (Macko et al., 1986, 1994; Yoneyama et al., 2003) as N is remobilized during reproductive growth.
Grains or fruits tend to act as strong N sinks. The amount of N that is remobilized to the grain from previously absorbed N is substantial, up to 85% in maize (Ta and Weiland, 1992), 100% in wheat (Martre et al., 2003; Tahir and Nakata, 2005), and 65% in rice (Mae and Ohira, 1981). Thus, fruits and grains should be systematically depleted in 15N relative to whole plants, leaves, and stems. In support of this notion, several studies have found lower δ15N values in grains relative to the leaves, stems, or shoots (Figure 8), the magnitude of which varies strongly with growing conditions, but is typically on the order of 1–4‰. Therefore, if animals were foddered to a significant extent on agricultural byproducts and humans consumed variable proportions of grains and those animals, there should be a convergence in the nitrogen isotopic compositions of the human and animal tissues relative to humans consuming variable proportions of grains and animals grazing on open pastures. This must be kept in mind when contemporaneous human and animal δ15N data are directly compared for paleodietary reconstructions, and considered as a possibility if higher than expected δ15N values are recorded in domestic animal tissues. Finally, agricultural systems tend to be characterized by higher N inputs (and in turn higher N losses) than non-agricultural systems, and are thus expected to be more prone to the loss of 14N (Kriszan et al., 2014). Therefore, animals foddered to a large extent on agricultural products or byproducts should be characterized by higher tissue δ15N values than animals grazing on wild pastures.
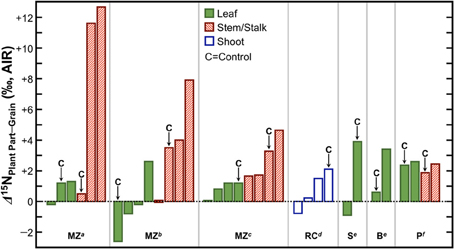
Figure 8. Differences in δ15N between grains/fruits and above-ground plant parts. The grain or fruit tends to be depleted in 15N relative to the leaves and stems/stalks. Data are from studies examining effects of fertilizers on plant δ15N values and a “C” above a particular bar denotes that these data are from a control treatment in which no fertilizer was applied. Abbreviations at the bottom of the figure denote species represented and source for data. MZ, maize; RC, rice; S, squash; B, bean; P, pepper. Data obtained from: a Szpak et al. (2012a), b Szpak et al. (2012b), c Choi et al. (2002), d Yun et al. (2011), e Szpak et al. (2014), fDel Amor et al. (2008).
The potential influence of both stocking rate and foddering require much more research to resolve what influence they may actually have on animal tissue N isotopic compositions. As suggested previously for agricultural practices, considerable potential lies in the isotopic analysis of animal tissues derived from small-scale or traditional herders. In many regions, particularly in Europe, where the production of particular kinds of meats, cheeses, and other animal products is regulated and occurs on relatively small or at least non-industrial scales, there is potential to investigate a wide-variety of animal management strategies that have been maintained over long periods of time. Certainly isotopic analysis have already been used extensively in attempts to verify the geographic origin of particular animal products (reviewed by Gonzalvez et al., 2009), but more directed efforts should also be made to assess the consequences of different management techniques on animal tissue (and potentially plant tissue) isotopic compositions (Von Holstein et al., 2013).
A large number of environmental and cultural variables may strongly influence the N isotopic compositions of plant-soil systems. With respect to agricultural systems, the following generalizations can be made about the three areas focused on in this paper:
1. Animal Fertilizers. The use of animal fertilizers will increase plant δ15N values by a variable amount depending on the type of fertilizer applied, the amount applied, and the duration of application. For some fertilizers (such as cattle manure) the effect is relatively small, on the order of +2 to +8‰, while the effect is substantial for others such as pig manure (+15 to +20‰) or seabird guano (+10 to +40‰).
2. Burning/Shifting Cultivation. Soil and vegetation δ15N values will increase in the years immediately after burning, and subsequently return to pre-fire levels. The magnitude of the difference in plant δ15N between recently burned and unburned vegetation may be between 2 and 8‰, although there is considerable variation.
3. Tillage. Ploughing or tillage is unlikely to influence plant δ15N in agricultural fields because these soils are typically not characterized by the large depth-related variation in δ15N values observed in forests.
The impact of animal fertilizers on plant N isotopic compositions has been relatively well investigated insomuch as it is now firmly established that plant N isotopic compositions consistently increase with manuring. While additional studies in this area would certainly be useful, more attention must be paid to other aspects of prehistoric cultivation practices (e.g., tillage, crop rotations, irrigation and floodplain agriculture, intercropping), and how they might affect soil and plant δ15N values. The end product will likely be a more complicated pattern of N isotopic variation with relatively few consistent and predictable effects for individual processes. At the very least, this will result in the ability to better and more accurately convey these complexities and incorporate various levels of uncertainty into reconstructions of ancient diet and agricultural practices.
With respect to animal husbandry, the following generalizations can be made about the two areas focused on in this paper:
1. Grazing Intensity/Stocking Rate. Generally, with increased grazing intensity, plant and soil δ15N values tend to increase. Some experimental work with modern animals has also shown that their tissues are positively correlated with stocking rate, although it is not clear whether or not these rates are reasonable proxies for ancient animal management regimes.
2. Foddering. The foddering of domestic animals with agricultural byproducts may increase animal tissue δ15N values because plant parts (leaves and stems) that supply N to reproductive structures are typically enriched in 15N relative to grains. Currently there is no supporting evidence for this notion from studies of modern animals foddered on agricultural byproducts.
Additional research focusing on the isotopic consequences of different animal management strategies in modern contexts would be useful for the interpretation of isotopic data derived from ancient animal populations.
Given the extreme complexities of N isotopic biogeochemistry in plant-soil systems and the multitude of factors that may influence plant δ15N values, how do we move forward in dealing with N isotopic data from ancient contexts? While early efforts in the field established general patterns that were useful for the qualitative interpretation of isotopic data (DeNiro and Epstein, 1981; Schoeninger et al., 1983; Schoeninger and DeNiro, 1984), much of the work discussed in this paper has focused on how the complexities of the N cycle lead to large variation and uncertainty in plant N isotopic compositions. First and foremost, the complexities of these systems must be acknowledged and effectively communicated in archaeological literature. A simplistic treatment of N isotopic data wherein there is a trophic level effect and a distinction between marine and terrestrial environments inadequately captures the nature of this variation. With respect to data treatment, recent mixing models that utilize a Bayesian framework have the capacity to incorporate uncertainty in source parameters (Moore and Semmens, 2008; Parnell et al., 2010) and because of this, these models have the potential to more realistically convey these baseline complexities and uncertainties, although quantitative mixing models have not been embraced by archaeologists to date (but see Kellner and Schoeninger, 2007). These models certainly have limitations and any dietary reconstruction of ancient populations is necessarily fraught with considerable uncertainty, but there is greater potential to more honestly communicate this uncertainty to the non-specialist via the Bayesian approaches. Methodologically, additional work focused on the N isotopic analysis of individual amino acids isolated from bone collagen (Naito et al., 2010) or plant remains (Styring et al., 2014) has considerable potential with respect to elucidating the relative importance of different biogeochemical processes in determining N isotopic compositions, but this field is still in its infancy.
A diverse array of factors can influence plant and animal δ15N values. Because of this, we need to explicitly consider N isotopic measurements as integrators of the complexities of the N cycle (Robinson, 2001) rather than tracers of individual processes. With respect to archaeological samples, in the absence of abundant supporting evidence, it is rare that one process can be singled out as causative for any pattern. Generally speaking, N isotopic compositions are rarely well positioned to directly answer specific questions about many processes that may be of interest to archaeologists, but are most effective when integrated with other lines of evidence.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The quality of this manuscript was significantly improved by the comments of Mike Richards, Joan Coltrain, Erik Hobbie, Laurie Reitsema, as well as discussions with Eric Guiry, Jessica Metcalfe, Farnoush Tahmasebi, Christine White, Terry Hnatyshyn, Megan Wong, Christina Cheung, Joe Hepburn, Catherine Cooper, and Rachel-Schwartz Narbonne.
Amon, B., Amon, T., Boxberger, J., and Alt, C. (2001). Emissions of NH3, N2O and CH4 from dairy cows housed in a farmyard manure tying stall (housing, manure storage, manure spreading). Nutr. Cycl. Agroecosys. 60, 103–113. doi: 10.1023/A:1012649028772
Amundson, R., Austin, A. T., Schuur, E. A. G., Yoo, K., Matzek, V., Kendall, C., et al. (2003). Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem. Cycl. 17, 1031. doi: 10.1029/2002gb001903
Aranibar, J. N., Anderson, I. C., Epstein, H. E., Feral, C. J. W., Swap, R. J., Ramontsho, J., et al. (2008). Nitrogen isotope composition of soils, C3 and C4 plants along land use gradients in southern Africa. J. Arid Environ. 72, 326–337. doi: 10.1016/j.jaridenv.2007.06.007
Austin, A. T., and Sala, O. E. (1999). Foliar δ15N is negatively correlated with rainfall along the IGBP transect in Australia. Aust. J. Plant Physiol. 26, 293–298. doi: 10.1071/PP98144
Austin, A. T., and Vitousek, P. M. (1998). Nutrient dynamics on a precipitation gradient in Hawai'i. Oecologia 113, 519–529. doi: 10.1007/s004420050405
Bardgett, R. D., and Wardle, D. A. (2003). Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84, 2258–2268. doi: 10.1890/02-0274
Bateman, A. S., and Kelly, S. D. (2007). Fertilizer nitrogen isotope signatures. Isot. Environ. and Health Stud. 43, 237–247. doi: 10.1080/10256010701550732
Bateman, A. S., Kelly, S. D., and Jickells, T. D. (2005). Nitrogen isotope relationships between crops and fertilizer: implications for using nitrogen isotope analysis as an indicator of agricultural regime. J. Agric. Food Chem. 53, 5760–5765. doi: 10.1021/jf050374h
Beghin, R., Cherubini, P., Battipaglia, G., Siegwolf, R., Saurer, M., and Bovio, G. (2011). Tree-ring growth and stable isotopes (13C and 15N) detect effects of wildfires on tree physiological processes in Pinus sylvestris L. Trees 25, 627–636. doi: 10.1007/s00468-011-0539-9
Bocherens, H., Polet, C., and Toussaint, M. (2007). Palaeodiet of Mesolithic and Neolithic populations of Meuse Basin (Belgium): evidence from stable isotopes. J. Archaeol. Sci. 34, 10–27. doi: 10.1016/j.jas.2006.03.009
Bogaard, A. (2002). Questioning the relevance of shifting cultivation to Neolithic farming in the loess belt of Europe: evidence from the Hambach Forest experiment. Veg. Hist. Archaeobot. 11, 155–168. doi: 10.1007/s003340200017
Bogaard, A., Fraser, R., Heaton, T. H. E., Wallace, M., Vaiglova, P., Charles, M., et al. (2013). Crop manuring and intensive land management by Europe's first farmers. Proc. Natl. Acad. Sci. U.S.A. 110, 12589–12594. doi: 10.1073/pnas.1305918110
Bogaard, A., Heaton, T. H. E., Poulton, P., and Merbach, I. (2007). The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 34, 335–343. doi: 10.1016/j.jas.2006.04.009
Borić, D., and Price, T. D. (2013). Strontium isotopes document greater human mobility at the start of the Balkan Neolithic. Proc. Natl. Acad. Sci. U.S.A. 110, 3298–3303. doi: 10.1073/pnas.1211474110
Britton, K., Müldner, G., and Bell, M. (2008). Stable isotope evidence for salt-marsh grazing in the Bronze Age Severn Estuary, UK: implications for palaeodietary analysis at coastal sites. J. Archaeol. Sci. 35, 2111–2118. doi: 10.1016/j.jas.2008.01.012
Broadbent, F. E., Rauschkolb, R. S., Lewis, K. A., and Chang, G. Y. (1980). Spatial variability of nitrogen-15 and total nitrogen in some virgin and cultivated soils. Soil Sci. Soc. Am. J. 44, 524–527. doi: 10.2136/sssaj1980.03615995004400030017x
Brundrett, M. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320, 37–77. doi: 10.1007/s11104-008-9877-9
Caut, S., Angulo, E., and Courchamp, F. (2009). Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. doi: 10.1111/j.1365-2664.2009.01620.x
Charles, M., Halstead, P., and Jones, G. (1996). The archaeology of fodder: introduction. Environ. Archaeol. 1, i–ii. doi: 10.1179/env.1996.1.1.i
Choi, W., Arshad, M., Chang, S., and Kim, T. (2006). Grain 15N of crops applied with organic and chemical fertilizers in a four-year rotation. Plant Soil 284, 165–174. doi: 10.1007/s11104-006-0038-8
Choi, W.-J., Lee, S.-M., Ro, H.-M., Kim, K.-C., and Yoo, S.-H. (2002). Natural 15N abundances of maize and soil amended with urea and composted pig manure. Plant Soil 245, 223–232. doi: 10.1023/A:1020475017254
Choi, W.-J., Ro, H.-M., and Hobbie, E. A. (2003). Patterns of natural 15N in soils and plants from chemically and organically fertilized uplands. Soil Biol. Biochem. 35, 1493–1500. doi: 10.1016/S0038-0717(03)00246-3
Choi, W. J., Chang, S. X., Kwak, J. H., Jung, J. W., Lim, S. S., Yoon, K. S., et al. (2007). Nitrogen transformations and ammonia volatilization losses from 15N-urea as affected by the co-application of composted pig manure. Can. J. Soil Sci. 87, 485–493. doi: 10.4141/CJSS07002
Clementz, M. T. (2012). New insight from old bones: stable isotope analysis of fossil mammals. J. Mammal. 93, 368–380. doi: 10.1644/11-MAMM-S-179.1
Codron, D., Sponheimer, M., Codron, J., Hammer, S., Tschuor, A., Braun, U., et al. (2012). Tracking the fate of digesta 13C and 15N compositions along the ruminant gastrointestinal tract: does digestion influence the relationship between diet and faeces? Eur. J. Wildlife Res. 58, 303–313. doi: 10.1007/s10344-011-0581-3
Coetsee, C., Stock, W. D., and Craine, J. M. (2011). Do grazers alter nitrogen dynamics on grazing lawns in a South African savannah? Afr. J. Ecol. 49, 62–69. doi: 10.1111/j.1365-2028.2010.01236.x
Commisso, R. G., and Nelson, D. E. (2006). Modern plant δ15N values reflect ancient human activity. J. Archaeol. Sci. 33, 1167–1176. doi: 10.1016/j.jas.2005.12.005
Cook, G. D. (2001). Effects of frequent fires and grazing on stable nitrogen isotope ratios of vegetation in northern Australia. Austral Ecol. 26, 630–636. doi: 10.1046/j.1442-9993.2001.01150.x
Craine, J. M., Ballantyne, F., Peel, M., Zambatis, N., Morrow, C., and Stock, W. D. (2009a). Grazing and landscape controls on nitrogen availability across 330 South African savanna sites. Austral Ecol. 34, 731–740. doi: 10.1111/j.1442-9993.2009.01978.x
Craine, J. M., Elmore, A. J., Aidar, M. P. M., Bustamante, M., Dawson, T. E., Hobbie, E. A., et al. (2009b). Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 183, 980–992. doi: 10.1111/j.1469-8137.2009.02917.x
Del Amor, F. M., Navarro, J., and Aparicio, P. M. (2008). Isotopic discrimination as a tool for organic farming certification in sweet pepper. J. Environ. Qual. 37, 182–185. doi: 10.2134/jeq2007.0329
DeNiro, M. J., and Epstein, S. (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. doi: 10.1016/0016-7037(78)90199-0
DeNiro, M. J., and Epstein, S. (1981). Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351. doi: 10.1016/0016-7037(81)90244-1
DeNiro, M. J., and Hastorf, C. A. (1985). Alteration of 15N/14N and 13C/12C ratios of plant matter during the initial stages of diagenesis: studies utilizing archaeological specimens from Peru. Geochim. Cosmochim. Acta 49, 97–115. doi: 10.1016/0016-7037(85)90194-2
Eghball, B., Wienhold, B. J., Gilley, J. E., and Eigenberg, R. A. (2002). Mineralization of manure nutrients. J. Soil Water Conserv. 57, 470–473.
Emmett, B. A., Kjønaas, O. J., Gundersen, P., Koopmans, C., Tietema, A., and Sleep, D. (1998). Natural abundance of 15N in forests across a nitrogen deposition gradient. Forest Ecol. Manag. 101, 9–18. doi: 10.1016/s0378-1127(97)00121-7
Erickson, A. E. (1982). “Tillage effects on soil aeration,” in Predicting Tillage Effects on Soil Physical Properties and Processes. ASA Special Publication 44, eds P. W. Unger and D. M. Van Doren Jr. (Madison, WI: American Society of Agronomy and Soil Science Society of America), 91–104.
Erskine, P. D., Bergstrom, D. M., Schmidt, S., Stewart, G. R., Tweedie, C. E., and Shaw, J. D. (1998). Subantarctic Macquarie Island – a model ecosystem for studying animal-derived nitrogen sources using 15N natural abundance. Oecologia 117, 187–193. doi: 10.1007/s004420050647
Evans, R. D. (2001). Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 6, 121–126. doi: 10.1016/S1360-1385(01)01889-1
Evans, R. D., Bloom, A. J., Sukrapanna, S. S., and Ehleringer, J. R. (1996). Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ. 19, 1317–1323. doi: 10.1111/j.1365-3040.1996.tb00010.x
Finlay, R. D. (2008). Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 59, 1115–1126. doi: 10.1093/jxb/ern059
Finucane, B., Agurto, P. M., and Isbell, W. H. (2006). Human and animal diet at Conchopata, Peru: stable isotope evidence for maize agriculture and animal management practices during the Middle Horizon. J. Archaeol. Sci. 33, 1766–1776. doi: 10.1016/j.jas.2006.03.012
Fogel, M. L., Wooller, M. J., Cheeseman, J., Smallwood, B. J., Roberts, Q., Romero, I., et al. (2008). Unusually negative nitrogen isotopic compositions (δ15N) of mangroves and lichens in an oligotrophic, microbially-influenced ecosystem. Biogeosciences 5, 1693–1704. doi: 10.5194/bg-5-1693-2008
Frank, D., Evans, R. D., and Tracy, B. (2004). The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland. Biogeochemistry 68, 169–178. doi: 10.1023/b:biog.0000025736.19381.91
Frank, D., and Zhang, Y. (1997). Ammonia volatilization from a seasonally and spatially variable grazed grassland: Yellowstone National Park. Biogeochemistry 36, 189–203. doi: 10.1023/A:1005705121160
Frank, D. A., and Evans, R. D. (1997). Effects of native grazers on grassland in cycling in Yellowstone National Park. Ecology 78, 2238–2248. doi: 10.1890/0012-9658(1997)078[2238:eongog]2.0.co;2
Fraser, R. A., Bogaard, A., Charles, M., Styring, A. K., Wallace, M., Jones, G., et al. (2013). Assessing natural variation and the effects of charring, burial and pre-treatment on the stable carbon and nitrogen isotope values of archaeobotanical cereals and pulses. J. Archaeol. Sci. 40, 4754–4766. doi: 10.1016/j.jas.2013.01.032
Fraser, R. A., Bogaard, A., Heaton, T., Charles, M., Jones, G., Christensen, B. T., et al. (2011). Manuring and stable nitrogen isotope ratios in cereals and pulses: towards a new archaeobotanical approach to the inference of land use and dietary practices. J. Archaeol. Sci. 38, 2790–2804. doi: 10.1016/j.jas.2011.06.024
Gauthier, P. P. G., Lamothe, M., Mahé, A., Molero, G., Nogués, S., Hodges, M., et al. (2013). Metabolic origin of δ15N values in nitrogenous compounds from Brassica napus L. leaves. Plant Cell Environ. 36, 128–137. doi: 10.1111/j.1365-3040.2012.02561.x
Gebauer, G., Giesemann, A., Schulze, E., and Jäger, H. (1994). Isotope ratios and concentrations of sulfur and nitrogen in needles and soils of Picea abies stands as influenced by atmospheric deposition of sulfur and nitrogen compounds. Plant Soil 164, 267–281. doi: 10.1007/bf00010079
Gonzalvez, A., Armenta, S., and De La Guardia, M. (2009). Trace-element composition and stable-isotope ratio for discrimination of foods with protected designation of origin. Trends Anal. Chem. 28, 1295–1311. doi: 10.1016/j.trac.2009.08.001
Gosling, P., Hodge, A., Goodlass, G., and Bending, G. D. (2006). Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 113, 17–35. doi: 10.1016/j.agee.2005.09.009
Grogan, P., Burns, T. D., and Chapin, F. S. 3rd. (2000). Fire effects on ecosystem nitrogen cycling in a Californian bishop pine forest. Oecologia 122, 537–544. doi: 10.1007/s004420050977
Gustine, D. D., Barboza, P. S., Adams, L. G., Farnell, R. G., and Parker, K. L. (2011). An isotopic approach to measuring nitrogen balance in caribou. J. Wildlife Manag. 75, 178–188. doi: 10.1002/jwmg.11
Gustine, D. D., Barboza, P. S., and Lawler, J. P. (2010). Dynamics of body protein and the implications for reproduction in captive muskoxen (Ovibos moschatus) during winter. Physiol. Biochem. Zool. 83, 687–697. doi: 10.1086/652729
Han, G., Hao, X., Zhao, M., Wang, M., Ellert, B. H., Willms, W., et al. (2008). Effect of grazing intensity on carbon and nitrogen in soil and vegetation in a meadow steppe in Inner Mongolia. Agric. Ecosyst. Environ. 125, 21–32. doi: 10.1016/j.agee.2007.11.009
Handley, L. L., Austin, A. T., Stewart, G. R., Robinson, D., Scrimgeour, C. M., Raven, J. A., et al. (1999). The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Aust. J. Plant Physiol. 26, 185–199. doi: 10.1071/PP98146
Handley, L. L., and Scrimgeour, C. M. (1997). Terrestrial plant ecology and 15N natural abundance: the present limits to interpretation for uncultivated systems with original data from a Scottish old field. Adv. Ecol. Res. 27, 133–212.
Hawke, D. J. (2001). Variability of δ15N in soil and plants at a New Zealand hill country site: correlations with soil chemistry and nutrient inputs. Aust. J. Soil Res. 39, 373–383. doi: 10.1071/SR99094
Herman, D. J., and Rundel, P. W. (1989). Nitrogen isotope fractionation in burned and unburned chaparral soils. Soil Sci. Soc. Am. J. 53, 1229–1236. doi: 10.2136/sssaj1989.03615995005300040040x
Hobbie, E., and Ouimette, A. (2009). Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95, 355–371. doi: 10.1007/s10533-009-9328-6
Hobbie, E. A., and Agerer, R. (2010). Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327, 71–83. doi: 10.1007/s11104-009-0032-z
Hobbie, E. A., and Högberg, P. (2012). Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 196, 367–382. doi: 10.1111/j.1469-8137.2012.04300.x
Hobbie, E. A., Macko, S. A., and Shugart, H. H. (1999). Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118, 353–360. doi: 10.1007/s004420050736
Hobbs, N. T. (1996). Modification of ecosystems by Ungulates. J. Wildlife Manag. 60, 695–713. doi: 10.2307/3802368
Högberg, P. (1997). Tansley Review No. 95. 15N natural abundance in soil-plant systems. New Phytol. 137, 179–203. doi: 10.1046/j.1469-8137.1997.00808.x
Högberg, P., Högbom, L., Schinkel, H., Högberg, M., Johannisson, C., and Wallmark, H. (1996). 15N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 108, 207–214. doi: 10.1007/bf00334643
Högberg, P., and Johannisson, C. (1993). 15N Abundance of forests is correlated with losses of nitrogen. Plant Soil 157, 147–150. doi: 10.1007/BF02390237
Högberg, P., Johannisson, C., and Högberg, M. N. (2014). Is the high 15N natural abundance of trees in N-loaded forests caused by an internal ecosystem N isotope redistribution or a change in the ecosystem N isotope mass balance? Biogeochemistry 117, 351–358. doi: 10.1007/s10533-013-9873-x
Högberg, P., Johannisson, C., Yarwood, S., Callesen, I., Näsholm, T., Myrold, D. D., et al. (2011). Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest. New Phytol. 189, 515–525. doi: 10.1111/j.1469-8137.2010.03485.x
Huber, E., Bell, T., and Adams, M. (2013). Combustion influences on natural abundance nitrogen isotope ratio in soil and plants following a wildfire in a sub-alpine ecosystem. Oecologia 173, 1063–1074. doi: 10.1007/s00442-013-2665-0
Hwang, Y. T., Millar, J. S., and Longstaffe, F. J. (2007). Do δ15N and δ13C values of feces reflect the isotopic composition of diets in small mammals? Can. J. Zool. 85, 388–396. doi: 10.1139/z07-019
Jensen, A., and Jakobsen, I. (1980). The occrrence of vesicular-arbuscular mycorrhiza in barley and wheat grown in some Danish soils with different fertilizer treatments. Plant Soil 55, 403–414. doi: 10.1007/BF02182701
Johnson, B. G., Johnson, D. W., Chambers, J. C., and Blank, R. R. (2011). Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant Soil 341, 437–445. doi: 10.1007/s11104-010-0656-z
Jones, G. (1996). Distinguishing food from fodder in the archaeobotanical record. Environ. Archaeol. 1, 95–98.
Jones, R., (ed.). (2012). Manure Matters: Historical, Archaeological and Ethnographic Perspectives. Surrey: Ashgate.
Juo, A. S. R., and Manu, A. (1996). Chemical dynamics in slash-and-burn agriculture. Agric. Ecosyst. Environ. 58, 49–60. doi: 10.1016/0167-8809(95)00656-7
Kabir, Z., O'Halloran, I. P., Fyles, J. W., and Hamel, C. (1997). Seasonal changes of arbuscular mycorrhizal fungi as affected by tillage practices and fertilization: hyphal density and mycorrhizal root colonization. Plant Soil 192, 285–293. doi: 10.1023/A:1004205828485
Kahiluoto, H., Ketoja, E., Vestberg, M., and Saarela, I. (2001). Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231, 65–79. doi: 10.1023/A:1010366400009
Kalcsits, L. A., and Guy, R. D. (2013). Quantifying remobilization of pre-existing nitrogen from cuttings to new growth of woody plants using 15N at natural abundance. Plant Methods 9, 27. doi: 10.1186/1746-4811-9-27
Kanstrup, M., Holst, M. K., Jensen, P. M., Thomsen, I. K., and Christensen, B. T. (in press). Searching for long-term trends in prehistoric manuring practice. δ15N analyses of charred cereal grains from the 4th to the 1st millennium BC. J. Archaeol. Sci. doi: 10.1016/j.jas.2013.04.018
Kanstrup, M., Thomsen, I. K., Mikkelsen, P. H., and Christensen, B. T. (2012). Impact of charring on cereal grain characteristics: linking prehistoric manuring practice to δ15N signatures in archaeobotanical material. J. Archaeol. Sci. 39, 2533–2540. doi: 10.1016/j.jas.2012.03.007
Karamanos, R. E., Voroney, R. P., and Rennie, D. A. (1981). Variation in natural N-15 abundance of central Saskatchewan soils. Soil Sci. Soc. Am. J. 45, 826–828. doi: 10.2136/sssaj1981.03615995004500040031x
Keegan, W. F., and DeNiro, M. J. (1988). Stable carbon- and nitrogen-isotope ratios of bone collagen used to study coral-reef and terrestrial components of prehistoric Bahamian diet. Am. Antiq. 53, 320–336. doi: 10.2307/281022
Kellner, C. M., and Schoeninger, M. J. (2007). A simple carbon isotope model for reconstructing prehistoric human diet. Am. J. Phys. Anthropol. 133, 1112–1127. doi: 10.1002/ajpa.20618
Kim, Y.-J., Choi, W.-J., Lim, S.-S., Kwak, J.-H., Chang, S. X., Kim, H.-Y., et al. (2008). Changes in nitrogen isotopic compositions during composting of cattle feedlot manure: effects of bedding material type. Bioresour. Technol. 99, 5452–5458. doi: 10.1016/j.biortech.2007.11.012
Kirchmann, H. (1985). Losses, plant uptake and utilisation of manure nitrogen during a production cycle. Acta Agric. Scand. Suppl. 24, 5–77.
Klopatek, J. M., Klopatek, C. C., and DeBano, L. F. (1990). Potential variation of nitrogen transformations in pinyon-juniper ecosystems resulting from burning. Biol. Fertil. Soils 10, 35–44. doi: 10.1007/BF00336122
Knobbe, N., Vogl, J., Pritzkow, W., Panne, U., Fry, H., Lochotzke, H., et al. (2006). C and N stable isotope variation in urine and milk of cattle depending on the diet. Anal. Bioanal. Chem. 386, 104–108. doi: 10.1007/s00216-006-0644-6
Kolb, K. J., and Evans, R. D. (2002). Implications of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol. 156, 57–64. doi: 10.1046/j.1469-8137.2002.00490.x
Koopmans, C. J., Dam, D. V., Tietema, A., and Verstraten, J. M. (1997). Natural 15N abundance in two nitrogen saturated forest ecosystems. Oecologia 111, 470–480. doi: 10.1007/s004420050260
Kreitler, C. W., and Jones, D. C. (1975). Natural soil nitrate: the cause of the nitrate contamination of ground water in Runnels County, Texasa. Ground Water 13, 53–62. doi: 10.1111/j.1745-6584.1975.tb03065.x
Kriszan, M., Schellberg, J., Amelung, W., Gebbing, T., Pötsch, E. M., and Kühbauch, W. (2014). Revealing N management intensity on grassland farms based on natural δ15N abundance. Agric. Ecosyst. Environ. 184, 158–167. doi: 10.1016/j.agee.2013.11.028
Laird, D., Fleming, P., Wang, B., Horton, R., and Karlen, D. (2010). Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158, 436–442. doi: 10.1016/j.geoderma.2010.05.012
Ledgard, S. F., Freney, J. R., and Simpson, J. R. (1984). Variations in natural enrichment of 15N in the profiles of some Australian pasture soils. Aust. J. Soil Res. 22, 155–164. doi: 10.1071/sr9840155
Leduc, S. D., Rothstein, D. E., Yermakov, Z., and Spaulding, S. E. (2013). Jack pine foliar δ15N indicates shifts in plant nitrogen acquisition after severe wildfire and through forest stand development. Plant Soil 373, 955–965. doi: 10.1007/s11104-013-1856-0
Lehmann, J., Pereira Da Silva, J. Jr., Steiner, C., Nehls, T., Zech, W., and Glaser, B. (2003). Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249, 343–357. doi: 10.1023/A:1022833116184
Li, C., Hao, X., Willms, W. D., and McAllister, T. A. (2012). Responses of herbage and cattle tail switch hair δ15N value to long-term stocking rates on a rough fescue grassland. Soil Sci. Plant Nutr. 58, 326–333. doi: 10.1080/00380768.2012.682283
Li, C., Hao, X., Willms, W. D., Zhao, M., and Han, G. (2009). Seasonal response of herbage production and its nutrient and mineral contents to long-term cattle grazing on a Rough Fescue grassland. Agric. Ecosyst. Environ. 132, 32–38. doi: 10.1016/j.agee.2009.02.010
Li, C., Hao, X., Zhao, M., Han, G., and Willms, W. D. (2008). Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agric. Ecosyst. Environ. 128, 109–116. doi: 10.1016/j.agee.2008.05.008
Lightfoot, E., and Stevens, R. E. (2012). Stable isotope investigations of charred barley (Hordeum vulgare) and wheat (Triticum spelta) grains from Danebury Hillfort: implications for palaeodietary reconstructions. J. Archaeol. Sci. 39, 656–662. doi: 10.1016/j.jas.2011.10.026
Lim, S.-S., Choi, W.-J., Kwak, J.-H., Jung, J.-W., Chang, S., Kim, H.-Y., et al. (2007). Nitrogen and carbon isotope responses of Chinese cabbage and chrysanthemum to the application of liquid pig manure. Plant Soil 295, 67–77. doi: 10.1007/s11104-007-9262-0
Lindwall, F., Vowles, T., Ekblad, A., and Björk, R. G. (2013). Reindeer grazing has contrasting effect on species traits in Vaccinium vitis-idaea L. and Bistorta vivipara (L.) Gray. Acta Oecologica 53, 33–37. doi: 10.1016/j.actao.2013.08.006
Macko, S. A., Engel, M. H., and Qian, Y. (1994). Early diagenesis and organic matter preservation – a molecular stable carbon isotope perspective. Chem. Geol. 114, 365–379. doi: 10.1016/0009-2541(94)90064-7
Macko, S. A., Estep, M. L. F., Engel, M. H., and Hare, P. E. (1986). Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim. Cosmochim. Acta 50, 2143–2146. doi: 10.1016/0016-7037(86)90068-2
Madgwick, R., Mulville, J., and Stevens, R. E. (2012). Diversity in foddering strategy and herd management in late Bronze Age Britain: an isotopic investigation of pigs and other fauna from two midden sites. Environm. Archaeol. 17, 126–140. doi: 10.1179/1461410312Z.00000000011
Mae, T., and Ohira, K. (1981). The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol. 22, 1067–1074.
Makarewicz, C. A. (2014). Winter pasturing practices and variable fodder provisioning detected in nitrogen (δ15N) and carbon (δ13C) isotopes in sheep dentinal collagen. J. Archaeol. Sci. 41, 502–510. doi: 10.1016/j.jas.2013.09.016
Martinelli, L. A., Piccolo, M. C., Townsend, A. R., Vitousek, P. M., Cuevas, E., McDowell, W., et al. (1999). Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46, 45–65. doi: 10.1023/a:1006100128782
Martre, P., Porter, J. R., Jamieson, P. D., and Triboï, E. (2003). Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiol. 133, 1959–1967. doi: 10.1104/pp.103.030585
Matson, P. A., Parton, W. J., Power, A. G., and Swift, M. J. (1997). Agricultural intensification and ecosystem properties. Science 277, 504–509. doi: 10.1126/science.277.5325.504
McLauchlan, K. K., Craine, J. M., Oswald, W. W., Leavitt, P. R., and Likens, G. E. (2007). Changes in nitrogen cycling during the past century in a northern hardwood forest. Proc. Natl. Acad. Sci. U.S.A. 104, 7466–7470. doi: 10.1073/pnas.0701779104
McNaughton, S. J., Ruess, R. W., and Seagle, S. W. (1988). Large mammals and process dynamics in African ecosystems. Bioscience 38, 794–800. doi: 10.2307/1310789
Moore, J. W., and Semmens, B. X. (2008). Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480. doi: 10.1111/j.1461-0248.2008.01163.x
Mordelet, P., Cook, G., Abbadie, L. U. C., Grably, M., and Mariotti, A. (1996). Natural 15N abundance of vegetation and soil in the Kapalga savanna, Australia. Aust. J. Ecol. 21, 336–340. doi: 10.1111/j.1442-9993.1996.tb00617.x
Mozafar, A., Anken, T., Ruh, R., and Frossard, E. (2000). Tillage intensity, mycorrhizal and nonmycorrhizal fungi, and nutrient concentrations in maize, wheat, and canola. Agron. J. 92, 1117–1124. doi: 10.2134/agronj2000.9261117x
Mudge, P. L., Schipper, L. A., Ghani, A., Upsdell, M., and Baisden, W. T. (2013). Changes in natural 15N abundance in pastoral soils receiving differing amounts of superphosphate fertilizer and irrigation for 50 years. Soil Sci. Soc. Am. J. 77, 830–841. doi: 10.2136/sssaj2012.0333
Müldner, G., Britton, K., and Ervynck, A. (2014). Inferring animal husbandry strategies in coastal zones through stable isotope analysis: new evidence from the Flemish coastal plain (Belgium, 1st–15th century AD). J. Archaeol. Sci. 41, 322–332. doi: 10.1016/j.jas.2013.08.010
Müldner, G., and Richards, M. P. (2005). Fast or feast: reconstructing diet in later Medieval England by stable isotope analysis. J. Archaeol. Sci. 32, 39–48. doi: 10.1016/j.jas.2004.05.007
Murphy, B. P., and Bowman, D. M. J. S. (2006). Kangaroo metabolism does not cause the relationship between bone collagen δ15N and water availability. Funct. Ecol. 20, 1062–1069. doi: 10.1111/j.1365-2435.2006.01186.x
Nadelhoffer, K., Shaver, G., Fry, B., Giblin, A., Johnson, L., and McKane, R. (1996). 15N natural abundances and N use by tundra plants. Oecologia 107, 386–394. doi: 10.1007/bf00328456
Nadelhoffer, K. J., and Fry, B. (1994). “Nitrogen isotope studies in forest ecosystems,” in Stable Isotopes in Ecology and Environmental Science, eds K. Lathja and R. H. Michener (Oxford: Blackwell Scientific), 22–44.
Naito, Y. I., Honch, N. V., Chikaraishi, Y., Ohkouchi, N., and Yoneda, M. (2010). Quantitative evaluation of marine protein contribution in ancient diets based on nitrogen isotope ratios of individual amino acids in bone collagen: an investigation at the Kitakogane Jomon site. Am. J. Phys. Anthropol. 143, 31–40. doi: 10.1002/ajpa.21287
Nájera-Hillman, E., and Mandujano, S. (2013). Faecal stable isotope analysis in white-tailed deer (Odocoileus virginianus), an alternative method for alimentary ecology studies. Wildlife Biol. Pract. 9, 63–75. doi: 10.2461/wbp.2013.9.8
Nakano, A., and Uehara, Y. (2007). Effects of different kinds of fertilizer and application methods on δ15N values of tomato. Jpn. Agric. Res. Q. 41, 219–226. doi: 10.6090/jarq.41.219
Näsholm, T. (1994). Removal of nitrogen during needle senescence in Scots pine (Pinus sylvestris L.). Oecologia 99, 290–296. doi: 10.1007/bf00627741
Neilson, R., Hamilton, D., Wishart, J., Marriott, C. A., Boag, B., Handley, L. L., et al. (1998). Stable isotope natural abundances of soil, plants and soil invertebrates in an upland pasture. Soil Biol. Biochem. 30, 1773–1782. doi: 10.1016/s0038-0717(98)00038-8
Newman, E. I., and Reddell, P. (1987). The distribution of mycorrhizas among families of vascular plants. New Phytol. 106, 745–751. doi: 10.1111/j.1469-8137.1987.tb00175.x
Oelze, V. M., Siebert, A., Nicklisch, N., Meller, H., Dresely, V., and Alt, K. W. (2011). Early Neolithic diet and animal husbandry: stable isotope evidence from three Linearbandkeramik (LBK) sites in Central Germany. J. Archaeol. Sci. 38, 270–279. doi: 10.1016/j.jas.2010.08.027
Pardo, L. H., Templer, P. H., Goodale, C. L., Duke, S., Groffman, P. M., Adams, M. B., et al. (2006). Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80, 143–171. doi: 10.1007/s10533-006-9015-9
Parnell, A. C., Inger, R., Bearhop, S., and Jackson, A. L. (2010). Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672. doi: 10.1371/journal.pone.0009672
Petersen, S. O., Lind, A.-M., and Sommer, S. G. (1998). Nitrogen and organic matter losses during storage of cattle and pig manure. J. Agric. Sci. 130, 69–79. doi: 10.1017/S002185969700508X
Petzke, K. J., Boeing, H., Klaus, S., and Metges, C. C. (2005). Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J. Nutr. 135, 1515–1520.
Piccolo, M., Neill, C., Melillo, J., Cerri, C., and Steudler, P. (1996). 15N natural abundance in forest and pasture soils of the Brazilian Amazon Basin. Plant Soil 182, 249–258. doi: 10.1007/BF00029056
Privat, K. L., O'Connell, T. C., and Richards, M. P. (2002). Stable isotope analysis of human and faunal remains from the Anglo-Saxon cemetery at Berinsfield, Oxfordshire: dietary and social implications. J. Archaeol. Sci. 29, 779–790. doi: 10.1006/jasc.2001.0785
Rapisarda, P., Camin, F., Fabroni, S., Perini, M., Torrisi, B., and Intrigliolo, F. (2010). Influence of different organic fertilizers on quality parameters and the δ15N, δ13C, δ2H, δ34S, and δ18O values of orange fruit (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 58, 3502–3506. doi: 10.1021/jf903952v
Regnell, M. (2002). “Charcoals from Uppåkra as indicators of leaf fodder,” in Centrality – Regionality. The Social Structure of Southern Sweden during the Iron Age, eds L. Larsson and B. Hårdh (Stockholm: Almqvist & Wiksell International), 105–115.
Riga, A., Van Praag, H. J., and Brigode, N. (1971). Rapport isotopique naturel de l'azote dans quelques sols forestiers et agricoles de Belgique soumis à divers traitements culturaux. Geoderma 6, 213–222. doi: 10.1016/0016-7061(71)90064-4
Robinson, D. (2001). δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 16, 153–162. doi: 10.1016/S0169-5347(00)02098-X
Robinson, D., Handley, L. L., Scrimgeour, C. M., Gordon, D. C., Forster, B. P., and Ellis, R. P. (2000). Using stable isotope natural abundances (δ15N and δ13C) to integrate the stress responses of wild barley (Hordeum spontaneum C. Koch.) genotypes. J. Exp. Bot. 51, 41–50. doi: 10.1093/jexbot/51.342.41
Ross, J. M., and Zutter, C. (2007). Comparing Norse animal husbandry practices: paleoethnobotanical analyses from Iceland and Greenland. Arctic Anthropol. 44, 62–85. doi: 10.3368/aa.44.1.62
Rowley-Conwy, P., and Layton, R. (2011). Foraging and farming as niche construction: stable and unstable adaptations. Philos. Trans. R. Soc. B Biol. Sci. 366, 849–862. doi: 10.1098/rstb.2010.0307
Ruess, R. W., and McNaughton, S. J. (1987). Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands. Oikos 49, 101–110. doi: 10.2307/3565559
Ruess, R. W., and McNaughton, S. J. (1988). Ammonia volatilization and the effects of large grazing mammals on nutrient loss from East African grasslands. Oecologia 77, 382–386. doi: 10.1007/BF00378047
Saito, L., Miller, W. W., Johnson, D. W., Qualls, R. G., Provencher, L., Carroll, E., et al. (2007). Fire effects on stable isotopes in a sierran forested watershed. J. Environ. Qual. 36, 91–100. doi: 10.2134/jeq2006.0233
Salvarina, I., Yohannes, E., Siemers, B. M., and Koselj, K. (2013). Advantages of using fecal samples for stable isotope analysis in bats: evidence from a triple isotopic experiment. Rapid Commun. Mass Spectrom. 27, 1945–1953. doi: 10.1002/rcm.6649
Schafer, J. L., and Mack, M. C. (2010). Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 334, 433–447. doi: 10.1007/s11104-010-0394-2
Schoeninger, M. J., and DeNiro, M. J. (1984). Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim. Cosmochim. Acta 48, 625–639. doi: 10.1016/0016-7037(84)90091-7
Schoeninger, M. J., DeNiro, M. J., and Tauber, H. (1983). Stable nitrogen isotope ratios of bone collagen reflect marine and terrestrial components of prehistoric human diet. Science 220, 1381–1383. doi: 10.1126/science.6344217
Schulze, E. D., Chapin, F. S., and Gebauer, G. (1994). Nitrogen nutrition and isotope differences among life forms at the northern treeline of Alaska. Oecologia 100, 406–412. doi: 10.1007/BF00317862
Schulze, E. D., Williams, R. J., Farquhar, G. D., Schulze, W., Langridge, J., Miller, J. M., et al. (1998). Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern Australia. Aust. J. Plant Physiol. 25, 413–425. doi: 10.1071/PP97113
Schwarcz, H. P., White, C. D., and Longstaffe, F. J. (2010). “Stable and radiogenic isotopes in biological archaeology: some applications,” in Isoscapes: Understanding Movement, Pattern, and Process on Earth through Stable Isotope Mapping, eds J. B. West, G. J. Bowen, T. E. Dawson, and K. P. Tu (Heidelberg: Springer-Verlag), 335–356.
Schwertl, M., Auerswald, K., Schäufele, R., and Schnyder, H. (2005). Carbon and nitrogen stable isotope composition of cattle hair: ecological fingerprints of production systems? Agric. Ecosyst. Environ. 109, 153–165. doi: 10.1016/j.agee.2005.01.015
Selles, F., Karamanos, R. E., and Bowren, K. E. (1984). Changes in natural 15N abundance of soils associated with tillage practices. Can. J. Soil Sci. 64, 345–354. doi: 10.4141/cjss84-036
Shearer, G., Kohl, D. H., and Chien, S.-H. (1978). The nitrogen-15 abundance in a wide variety of soils. Soil Sci. Soc. Am. J. 42, 899–902. doi: 10.2136/sssaj1978.03615995004200060013x
Sherratt, A. (1980). Water, soil and seasonality in early cereal cultivation. World Archaeol. 11, 313–330. doi: 10.2307/124253
Silgram, M., and Shepherd, M. A. (1999). The effects of cultivation on soil nitrogen mineralization. Adv. Agron. 65, 267–311. doi: 10.1016/s0065-2113(08)60915-3
Singer, F. J., and Schoenecker, K. A. (2003). Do ungulates accelerate or decelerate nitrogen cycling? Forest Ecol. Manag. 181, 189–204. doi: 10.1016/s0378-1127(03)00133-6
Sjögersten, S., Kuijper, D. P. J., Wal, R., Loonen, M. J. J. E., Huiskes, A. H. L., and Woodin, S. J. (2010). Nitrogen transfer between herbivores and their forage species. Polar Biol. 33, 1195–1203. doi: 10.1007/s00300-010-0809-9
Sponheimer, M., Robinson, T. F., Roeder, B. L., Passey, B. H., Ayliffe, L. K., Cerling, T. E., et al. (2003). An experimental study of nitrogen flux in llamas: is 14N preferentially excreted? J. Archaeol. Sci. 30, 1649–1655. doi: 10.1016/s0305-4403(03)00066-9
Steele, K. W., and Daniel, R. M. J. (1978). Fractionation of nitrogen isotopes by animals: a further complication to the use of variations in the natural abundance of 15N for tracer studies. J. Agric. Sci. 90, 7–9. doi: 10.1017/S002185960004853X
Steele, K. W., Wilson, A. T., and Saunders, W. M. H. (1981). Nitrogen isotope ratios in surface and sub-surface horizons of New Zealand improved grassland soils. N.Z. J. Agric. Res. 24, 167–170. doi: 10.1080/00288233.1981.10420885
Stock, W. D., Wienand, K. T., and Baker, A. C. (1995). Impacts of invading N2-fixing Acacia species on patterns of nutrient cycling in two Cape ecosystems: evidence from soil incubation studies and 15N natural abundance values. Oecologia 101, 375–382. doi: 10.1007/BF00328825
Styring, A. K., Fraser, R. A., Bogaard, A., and Evershed, R. P. (2014). Cereal grain, rachis and pulse seed amino acid δ15N values as indicators of plant nitrogen metabolism. Phytochemistry 97, 20–29. doi: 10.1016/j.phytochem.2013.05.009
Styring, A. K., Manning, H., Fraser, R. A., Wallace, M., Jones, G., Charles, M., et al. (2013). The effect of charring and burial on the biochemical composition of cereal grains: investigating the integrity of archaeological plant material. J. Archaeol. Sci. 40, 4767–4779. doi: 10.1016/j.jas.2013.03.024
Sutoh, M., Koyama, T., and Yoneyama, T. (1987). Variations of natural 15N abundances in the tissues and digesta of domestic animals. Radioisotopes 36, 74–77.
Sutoh, M., Obara, Y., and Yoneyama, T. (1993). The effects of feeding regimen and dietary sucrose supplementation on natural abundance of 15N in some components of ruminal fluid and plasma of sheep. J. Anim. Sci. 71, 226–231.
Szpak, P., Longstaffe, F. J., Millaire, J.-F., and White, C. D. (2012a). Stable isotope biogeochemistry of seabird guano fertilization: results from growth chamber studies with Maize (Zea mays). PLoS ONE 7:e33741. doi: 10.1371/journal.pone.0033741
Szpak, P., Longstaffe, F. J., Millaire, J.-F., and White, C. D. (2014). Large variation in nitrogen isotopic composition of a fertilized legume. J. Archaeol. Sci. 45, 72–79. doi: 10.1016/j.jas.2014.02.007
Szpak, P., Millaire, J.-F., White, C. D., and Longstaffe, F. J. (2012b). Influence of seabird guano and camelid dung fertilization on the nitrogen isotopic composition of field-grown maize (Zea mays). J. Archaeol. Sci. 39, 3721–3740. doi: 10.1016/j.jas.2012.06.035
Szpak, P., Orchard, T. J., Mckechnie, I., and Gröcke, D. R. (2012c). Historical ecology of late Holocene sea otters (Enhydra lutris) from northern British Columbia: isotopic and zooarchaeological perspectives. J. Archaeol. Sci. 39, 1553–1571. doi: 10.1016/j.jas.2011.12.006
Szpak, P., White, C. D., Longstaffe, F. J., Millaire, J.-F., and Vásquez Sánchez, V. F. (2013). Carbon and nitrogen isotopic survey of northern Peruvian plants: baselines for paleodietary and paleoecological studies. PLoS ONE 8:e53763. doi: 10.1371/journal.pone.0053763
Ta, C. T., and Weiland, R. T. (1992). Nitrogen partitioning in maize during ear development. Crop Sci. 32, 443–451. doi: 10.2135/cropsci1992.0011183X003200020032x
Tahir, I. S. A., and Nakata, N. (2005). Remobilization of nitrogen and carbohydrate from stems of bread wheat in response to heat stress during grain filling. J. Agron. Crop Sci. 191, 106–115. doi: 10.1111/j.1439-037X.2004.00127.x
Tcherkez, G. (2011). Natural 15N/14N isotope composition in C3 leaves: are enzymatic isotope effects informative for predicting the 15N-abundance in key metabolites? Funct. Plant Biol. 38, 1–12. doi: 10.1071/FP10091
Tiessen, H., Karamanos, R. E., Stewart, J. W. B., and Selles, F. (1984). Natural nitrogen-15 abundance as an indicator of soil organic matter transformations in native and cultivated soils. Soil Sci. Soc. Am. J. 48, 312–315. doi: 10.2136/sssaj1984.03615995004800020017x
Vaiglova, P., Bogaard, A., Collins, M., Cavanagh, W., Mee, C., Renard, J., et al. (2014). An integrated stable isotope study of plants and animals from Kouphovouno, southern Greece: a new look at Neolithic farming. J. Archaeol. Sci. 42, 201–215. doi: 10.1016/j.jas.2013.10.023
Vallano, D. M., and Sparks, J. P. (2013). Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 172, 47–58. doi: 10.1007/s00442-012-2489-3
Van Groenigen, K.-J., Bloem, J., Bååth, E., Boeckx, P., Rousk, J., Bodé, S., et al. (2010). Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 42, 48–55. doi: 10.1016/j.soilbio.2009.09.023
Von Holstein, I. C. C., Hamilton, J., Craig, O. E., Newton, J., and Collins, M. J. (2013). Comparison of isotopic variability in proteinaceous tissues of a domesticated herbivore: a baseline for zooarchaeological investigation. Rapid Commun. Mass Spectrom. 27, 2601–2615. doi: 10.1002/rcm.6725
Warinner, C., Garcia, N. R., and Tuross, N. (2013). Maize, beans and the floral isotopic diversity of highland Oaxaca, Mexico. J. Archaeol. Sci. 40, 868–873. doi: 10.1016/j.jas.2012.07.003
Watzka, M., Buchgraber, K., and Wanek, W. (2006). Natural 15N abundance of plants and soils under different management practices in a montane grassland. Soil Biol. Biochem. 38, 1564–1576. doi: 10.1016/j.soilbio.2005.11.007
Werner, R. A., and Schmidt, H.-L. (2002). The in vivo nitrogen isotope discrimination among organic plant compounds. Phytochemistry 61, 465–484. doi: 10.1016/s0031-9422(02)00204-2
Wittmer, M., Auerswald, K., Schönbach, P., Bai, Y., and Schnyder, H. (2011). 15N fractionation between vegetation, soil, faeces and wool is not influenced by stocking rate. Plant Soil 340, 25–33. doi: 10.1007/s11104-010-0411-5
Wrage, N., Küchenmeister, F., and Isselstein, J. (2011). Isotopic composition of soil, vegetation or cattle hair no suitable indicator of nitrogen balances in permanent pasture. Nutr. Cycl. Agroecosyst. 90, 189–199. doi: 10.1007/s10705-011-9421-9
Xu, Y., He, J., Cheng, W., Xing, X., and Li, L. (2010). Natural 15N abundance in soils and plants in relation to N cycling in a rangeland in Inner Mongolia. J. Plant Ecol. 3, 201–207. doi: 10.1093/jpe/rtq023
Yoneyama, T., Fujita, K., Yoshida, T., Matsumoto, T., Kambayashi, I., and Yazaki, J. (1986). Variation in natural abundance of 15N among plant parts and in 15N/14N fractionation during N2 fixation in the legume-rhizobia symbiotic system. Plant Cell Physiol. 27, 791–799.
Yoneyama, T., Ito, O., and Engelaar, W. M. H. G. (2003). Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: progress over the last 30 years. Phytochem. Rev. 2, 121–132. doi: 10.1023/B:PHYT.0000004198.95836.ad
Yoneyama, T., and Kaneko, A. (1989). Variations in the natural abundance of 15N in nitrogenous fractions of komatsuna plants supplied with nitrate. Plant Cell Physiol. 30, 957–962.
Yuan, Y., Zhao, M., Zhang, Z., Chen, T., Yang, G., and Wang, Q. (2012). Effect of different fertilizers on nitrogen isotope composition and nitrate content of Brassica campestris. J. Agric. Food Chem. 60, 1456–1460. doi: 10.1021/jf203105t
Yun, S.-I., Lim, S.-S., Lee, G.-S., Lee, S.-M., Kim, H.-Y., Ro, H.-M., et al. (2011). Natural 15N abundance of paddy rice (Oryza sativa L.) grown with synthetic fertilizer, livestock manure compost, and hairy vetch. Biol. Fertil. Soils 47, 607–617. doi: 10.1007/s00374-011-0571-3
Yun, S.-I., and Ro, H.-M. (2009). Natural 15N abundance of plant and soil inorganic-N as evidence for over-fertilization with compost. Soil Biol. Biochem. 41, 1541–1547. doi: 10.1016/j.soilbio.2009.04.014
Yun, S.-I., Ro, H.-M., Choi, W.-J., and Chang, S. X. (2006). Interactive effects of N fertilizer source and timing of fertilization leave specific N isotopic signatures in Chinese cabbage and soil. Soil Biol. Biochem. 38, 1682–1689. doi: 10.1016/j.soilbio.2005.11.022
Keywords: stable isotopes, nitrogen, archaeology, agriculture, animal management
Citation: Szpak P (2014) Complexities of nitrogen isotope biogeochemistry in plant-soil systems: implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 5:288. doi: 10.3389/fpls.2014.00288
Received: 01 April 2014; Accepted: 02 June 2014;
Published online: 23 June 2014.
Edited by:
Raymond Dave Evans, Washington State University, USAReviewed by:
Erik Alan Hobbie, Earth Systems Research Center, USACopyright © 2014 Szpak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Szpak, Department of Anthropology, University of British Columbia, 6303 NW Marine Drive, Vancouver, BC V6T 1Z1, Canada e-mail:cGF1bC5zenBha0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.