- 1Molecular Biology, Institute for Biochemistry and Biology, University of Potsdam, Potsdam, Germany
- 2IMPRS-PMPG, Max-Planck Institute of Molecular Plant Physiology, Potsdam, Germany
- 3Centro de Biotecnologia y Genomica de Plantas, Universidad Politécnica de Madrid, Madrid, Spain
Potassium (K+) is inevitable for plant growth and development. It plays a crucial role in the regulation of enzyme activities, in adjusting the electrical membrane potential and the cellular turgor, in regulating cellular homeostasis and in the stabilization of protein synthesis. Uptake of K+ from the soil and its transport to growing organs is essential for a healthy plant development. Uptake and allocation of K+ are performed by K+ channels and transporters belonging to different protein families. In this review we summarize the knowledge on the versatile physiological roles of plant K+ channels and their behavior under stress conditions in the model plant Arabidopsis thaliana.
Introduction
Potassium (K+) is essential for growth and development of an organism. It is involved in various important cellular processes, like stabilization of protein synthesis, activation of enzymes, neutralization of negative charges on proteins and many more. In addition to the above mentioned tasks, in plants it is a key player in osmotic processes contributing to cellular turgor, cell elongation, translocation of photosynthates, maintenance of cytosolic pH homeostasis, and the setting of the membrane potential along with the proton motive force (Maathuis, 2009; Marschner, 2012). All these functions justify it being the most abundant inorganic cation in plants, contributing to up to 10% of their dry mass (Leigh and Wyn Jones, 1984).
Potassium is a major factor in resistance to drought, salinity, and fungal diseases (Amtmann et al., 2008). This explains why it is of crucial importance in agriculture affecting crop yield. For performing the tasks explained above, plants require potassium concentrations ranging between 100–200 mM in the cytoplasm (Wyn Jones and Pollard, 1983). In contrast, concentration of potassium in soil (10–100 μM) is 3–4 orders of magnitude lower (Schroeder et al., 1994). Therefore, a plant has to invest energy for the uptake of K+ and its distribution throughout the plant.
The transport of potassium is accomplished by a variety of transporter proteins. In the plant model organism Arabidopsis thaliana a total of 71 K+ channels and transporters have already been identified (Mäser et al., 2001; Véry and Sentenac, 2003; Amtmann et al., 2004; Wang and Wu, 2010). They have been categorized into six different gene families, comprising of three channel families and three transporter families (KUP/HAK/KT, HKT, and CPA families; Gierth and Mäser, 2007; Chanroj et al., 2012; Gomez-Porras et al., 2012).
The three identified families of K+ channels are Shaker, Tandem-Pore K+ (TPK) and Kir-like channels. Recent phylogenetic data, however, evidenced that Kir-like channels in fact belong to the TPK family and originated by evolutionarily recent gene duplication and partial deletion events (Marcel et al., 2010; Voelker et al., 2010; Gomez-Porras et al., 2012). We therefore do not consider Kir-like channels as a separate family anymore. K+ channels are active as multimeric proteins composed of two or four α-subunits, which are characterized by the presence of either one or two pore (P) domains. In the functional multimeric protein, four P domains are associated to form part of the conduction pathway, including its selectivity filter. K+ selective channels have the hallmark motif TXGYGD/E in their P domains (Lebaudy et al., 2007; Table 1, Figure 1).
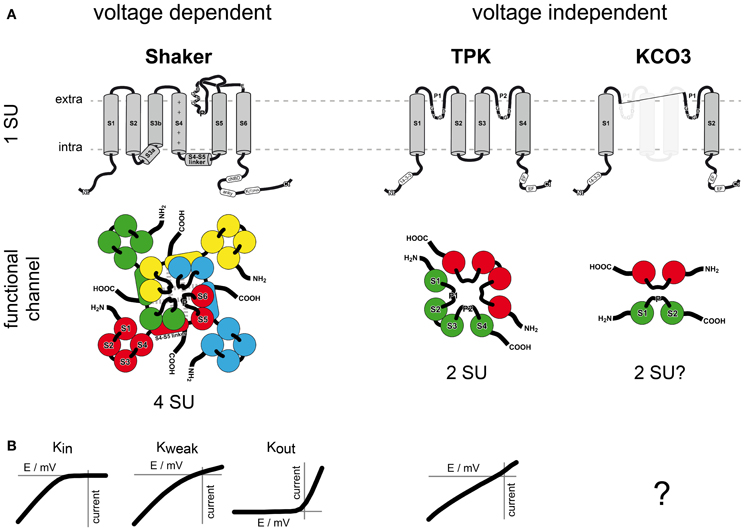
Figure 1. Structure and function of K+ channel families in plants. The two plant K+ channel families vary in (A) structure and (B) function. Shaker channels form the most versatile family among plant K+ channels. Nine members segregate into inwardly, outwardly and weakly rectifying channels. Functional channels are tetramers and operate in a voltage dependent manner. One subunit consists of six transmembrane domains (S1–S6) and one pore domain (P). The fourth transmembrane region S4 is rich in positively charged amino acids and acts together with S1, S2, and S3 as voltage sensor. Five TPK channels have been identified. One subunit contains two pore domains (P1 and P2) and two subunits are sufficient to form a functional channel. TPKs act in a largely voltage independent manner and exhibit leak like currents. KCO3 was initially classified as a Kir-like channel showing two transmembrane regions and one pore domain. In fact, “plant Kir-like channels” originate from TPKs by partial deletion of one selectivity-filter and two transmembrane domains. In line with this notion, only stable dimers have been detected. A K+ transport function has not been shown for these truncated channels. Abbreviations: extra, extracellular side; intra, intracellular side; SU, subunit; +, positively charged amino acids; cNBD, cyclic nucleotide binding domain; anky, ankyrin repeat domain; K(T)/HA, acidic domain; EF, EF hand domain.
In 1992, AKT1 and KAT1, two inward rectifying channels from Arabidopsis were identified by functional complementation of yeast strains defective in potassium uptake. These two members of the Shaker-like channel family were the first cloned plant potassium channels (Anderson et al., 1992; Schachtman et al., 1992; Sentenac et al., 1992).
In 1997, a first member of the TPK channel family was identified by in silico approaches, utilizing the Arabidopsis gene sequencing program. TPK channels are the plant counterparts of animal Tandem Pore (TWIK-like) channels (Czempinski et al., 1997). While searching for TPK1-related sequences in genome sequence database, KCO3 was identified and was thought to be structurally similar to animal potassium inward rectifying channels (Czempinski et al., 1999) leading to its initial classification into a separate family of plant Kir-like channels.
Voltage Independent K+ Channels in Arabidopsis
Tandem Pore Potassium Channels
The Tandem-Pore K+ (TPK) channel family comprises six members (TPK1-TPK5 and KCO3, see also below for this special case) in the model plant Arabidopsis thaliana. TPK homologues were identified in higher plants and green algae (Voelker et al., 2010; Gomez-Porras et al., 2012). A phylogenetic analysis has shown that plant TPK channels are divided into two subfamilies: TPK1 belongs to one and TPK2, TPK3, TPK4, and TPK5 to the second subfamily. This sub division in families indicates a common ancestral origin of the channels TPK2, TPK3, TPK4, and TPK5; a hypothesis that was further supported by the analysis of chromosome segment duplication in the Arabidopsis genome (Marcel et al., 2010; Voelker et al., 2010; Gomez-Porras et al., 2012).
The first TPK channel (AtTPK1) was cloned via an A. thaliana EST database search for the conserved K+ channel pore domain motif TXGYGD (Czempinski et al., 1997). TPKs show a TM-P-TM-TM-P-TM structure with a duplicated transmembrane-pore-transmembrane module (Figure 1A). In general, these channels contain one or two Ca2+-binding EF hands in the cytosolic C-terminal part and binding sites for 14-3-3 proteins in the cytosolic N-terminal part, as well as a putative N-glycosylation site in the luminal loop between the pore domain and the second transmembrane domain.
Functional TPK channels are built of two of such subunits and exist as dimers (Maitrejean et al., 2011). They show a high Ca2+ dependency, which might be important for channel regulation (Latz et al., 2007a). TPK channels have been localized in the vacuolar membrane (Czempinski et al., 2002; Schönknecht et al., 2002). One exception is TPK4, which has been reported to localize in the plasma membrane (Becker et al., 2004; Dunkel et al., 2008). TPK4 shares 85% similarity (53% identity) with TPK5 but lacks the regulatory domains and the 14-3-3 protein interaction motif. It might thus be speculated that TPK4 evolved from TPK5 and subsequently underwent truncation events. Another exception from exclusive vacuolar localization might be TPK3. In Western blots TPK3 was also identified in thylakoid membranes (Zanetti et al., 2010) raising the question whether TPKs may have multiple subcellular locations.
Expression analysis of TPKs through quantitative real-time PCR experiments evidenced their presence in different plant tissues like roots, leaves and flowers (Deeken et al., 2003; Voelker et al., 2010). Among all TPKs, TPK1 showed the highest expression levels in all tissues analysed, followed by TPK3 and TPK5. Expression levels of TPK2 and TPK4 were very low. Elevated levels of TPK2 transcripts were detected in stamen and pollen. TPK3 transcript levels were more abundant in petals, stamen, seeds and senescent leaves.
Assembly status of tandem-pore K+ channels
Promoter-reporter gene studies and qRT-PCR experiments revealed overlapping expression patterns for members of the TPK/KCO3 channel family (Czempinski et al., 2002; Deeken et al., 2003; Philippar et al., 2003; Becker et al., 2004; Voelker et al., 2006). Expression of TPK1 overlaps with that of TPK3 in root tips and with that of TPK5 and KCO3 in vascular tissues. Additionally, TPK1, TPK2, TPK3, and TPK4 express in pollen. The overlapping expression patterns and their common localization in the tonoplast propose that heteromeric channel subunit combination might occur under different developmental stages or physiological conditions (Latz et al., 2007a).
Dimerization of TPK channels has been shown experimentally by using velocity sucrose gradient centrifugation of leaf homogenates expressing TPK1-GFP. This confirms the contribution of four pore domains to the K+ selectivity filter of the TPK1 channel (Maitrejean et al., 2011). Using the same technique, AtKCO3 and AtKCO3-GFP have been observed to exist as dimers, too. These channels would thus have only two pore domains in a dimerized state (Figure 1A), which is not considered to be sufficient for an active, K+-selective channel (Rocchetti et al., 2012).
With the aim of studying the assembly status of TPK/KCO family members, various experiments have been performed employing techniques like FRET and BiFC (split-YFP). Results from these approaches indicated the existence of homomeric TPK/KCO3 channels, as e.g., in the case of TPK1 or TPK5 (Voelker et al., 2006). However, so far no evidence for heteromeric channel formation could be provided. Nevertheless, there are neither convincing data ruling out this possibility. Thus it cannot be excluded that in vivo heteromeric channel formation might occur under different developmental and physiological conditions.
Localization of tandem-pore K+ channels
In a first approach to detect the subcellular localization, the TPK1 channel has been stably over-expressed in tobacco BY-2 cells. After protein fractionation with a sucrose gradient, this K+ channel was found to co-fractionate with tonoplast markers, giving a first clue of its localization on the vacuolar membrane (Czempinski et al., 2002). Further localization studies were performed by creating GFP fusion constructs followed by their transient expression in A. thaliana protoplasts. Such experiments demonstrated vacuolar localization of TPK1, TPK2, TPK3, and TPK5 (Voelker et al., 2006). In contrast, when a TPK4:GFP fusion construct was expressed in onion epidermal cells, it was found to localize partially in the plasma membrane. A major fraction, however, was detected in the ER (Becker et al., 2004; Dunkel et al., 2008). This might be either due to ER-retention or may indicate that besides TPK3, been found in the tonoplast and in the thylakoid membrane, also TPK4 may exhibit at least a dual localization profile.
Unfortunately till now, no general targeting sequence is known that “guides” TPK channels to the appropriate membrane (Vitale and Hinz, 2005; Dunkel et al., 2008). With the purpose of identifying the sorting signal of vacuolar TPK channels, various chimeras were generated between TPK4 (plasma membrane protein) and TPK1 (tonoplast protein). It is not handed down why this particular pair has been chosen and not the “twins” TPK5 and TPK4; TPK4 sharing 85% of similar amino acids with TPK5. Nevertheless, the chimeras showed that complete replacement of the cytosolic C-terminus of TPK1 results in ER retention. Further detailed analysis indicated that the terminal 25 amino acids are not important for the trafficking process. An analysis of amino acids 292–308 in the C-terminus of TPK1 could identify three diacidic motifs. Out of these three motifs, mutations in (D296G/E298G) resulted in ER-stuck TPK1 proteins, suggesting that this diacidic motif is crucial for the export of TPK1 from the ER (Dunkel et al., 2008; Voelker et al., 2010). A related study on rice TPKs identified amino acids in the cytosolic C-terminal domain that determine differential targeting of TPKs to the endomembranes of the large central lytic vacuole or of protein storage vacuoles (Isayenkov et al., 2011a) indicating a general role of certain regions in the cytosolic C-terminus for channel targeting.
Retention of TPK1 channel protein in the ER also occurred when plant leaves were treated with Brefeldin A, a fungal toxin which causes redistribution of Golgi membranes. From this observation it was inferred that the transport of TPK channel proteins to the vacuolar membrane is through a Golgi-dependent pathway and that the Golgi apparatus is the first compartment crossed by the protein after it leaves the ER (Dunkel et al., 2008). Experiments in rice indicated a more complex situation of TPK targeting. TPKs targeted to the lytic vacuole indeed cross the Golgi apparatus. However, TPKs targeted to protein storage vacuoles apparently reach the endomembrane in a Golgi-independent way (Isayenkov et al., 2011a).
Regulation and function of tandem-pore K+ channels
At present the knowledge on function and regulation of plant TPKs is limited. Research is often fuelled by comparison with related channels from other kingdoms. Animal two-pore channel activity has been shown to be regulated by interacting 14-3-3 proteins (Rajan et al., 2002). Also in plants down-regulation of K+ channel activity in the tonoplast has been observed to be caused by interaction with 14-3-3 proteins. In TPKs, the cytosolic N-terminus comprises a classical binding motif for 14-3-3 proteins (RSXpS/pTXP)1. Phosphorylation of these serine or threonine residues is crucial for the interaction with 14-3-3 proteins (Latz et al., 2007a).
TPK channels are proposed to be involved in the K+ homeostasis of plant cells by allowing the controlled intracellular K+ transport from and into organelles. Recent experiments employing the patch clamp technique have demonstrated a mechano-sensitive nature of TPK channels suggesting especially a role in osmoregulation. This concept was further supported by protoplast disruption assays (Maathuis, 2011) and seedling germination tests (Gobert et al., 2007).
AtTPK1 is ubiquitously expressed in A. thaliana. Using promoter-reporter gene (GUS) fusion, TPK1 promoter activity was observed in root cortex, vascular tissue, mesophyll cells, guard cells and pollen grains (Czempinski et al., 2002). When expressed in yeast, TPK1 has characteristics of K+-selective channels from Vicia faba (VK channels) previously characterized in vivo with strong selectivity for K+ over Na+ (Bihler et al., 2005; Gobert et al., 2007; Latz et al., 2007b). The activity of TPK1 is independent of the membrane voltage but was shown to be dependent on the cytosolic pH with a maximum open probability at pH 6.7, decreasing 20–30% at physiological pH 7.5–7.8. It is activated by cytosolic Ca2+, remarkably exhibiting the highest affinity for calcium ions among the proteins tested including calmodulin. Interaction of TPK1 with the 14-3-3 protein GRF6 (General Release Factor 6) increases the channel activity in a dose dependent manner. This interaction does not play any role in targeting of the protein to the tonoplast (Latz et al., 2007a). All these data indicate that TPK1 is tightly controlled by cellular signals. TPK1 has been reported to participate in vacuolar K+ release during stomatal closure and also during seed germination and radicle growth (Gobert et al., 2007).
AtTPK4 is an instantaneously activating, K+ selective channel that is also found in the plasma membrane when expressed in Xenopus oocytes and yeast. In planta, TPK4 exhibits low transcript abundance. It is predominantly expressed in pollen, as observed by promoter-GUS fusion analysis. TPK4 is blocked by extracellular Ca2+ and is insensitive toward changes in extracellular pH, but it is efficiently blocked by cytosolic acidification. Activation of TPK4 by heat has also been reported (Becker et al., 2004). TPK4 is proposed to contribute to the K+ conductance of the pollen tube plasma membrane, where it operates as a so called “open rectifier” with saturating current at depolarizing membrane potentials.
AtTPK5 is targeted to the tonoplast. At the mRNA level, TPK5 shows higher abundance in senescent leaves and petals (Voelker et al., 2010). Promoter GUS studies of TPK5 have shown expression in the vascular tissues of leaves, roots, hydathodes, floral tissues and stems. TPK5 transcript level is increased or decreased in response to external factors.
Recently AtTPK1, AtTPK2, and AtTPK5 were functionally characterized in Escherichia coli. The three isoforms were able to complement the K+ uptake deficient E. coli mutant LB2003 on low K+ medium (Isayenkov and Maathuis, 2013). Interestingly, in the same experiments AtTPK3 could not complement LB2003. This may indicate that this channel might be active in a different membrane environment, as for instance the thylakoid membrane (Zanetti et al., 2010).
Different isoforms of tandem-pore K+ channels
Tandem-pore K+ channels have also been identified and characterized in plant species other than A. thaliana, for example Hordeum vulgare, Nicotiana tabacum, Solanum tuberosum, Oryza sativa (Czempinski et al., 1999; Hamamoto et al., 2008a,b; Isayenkov et al., 2011a,b). It is fascinating to see that NtTPK1 from tobacco exhibits properties different from other plant TPK channels, since it is active even in the absence of Ca2+. Nevertheless, increase in cytosolic Ca2+ resulted in an up to two fold increase in the K+ current amplitude (Hamamoto et al., 2008a). Its current profile shows an instantaneous and a time-dependent component (Hamamoto et al., 2008b). The most interesting distinguishing feature is that two of the four identified isoforms in N. tabacum do not contain the conserved TXGYGD motif in the second pore domain. Instead, NtTPKb and NtTPKc possess VHG or GHG, respectively.
Plant Kir-Like Channels
Plant Kir-like channels were initially classified as an own group although they are similar to TPK channels. To date, they have been found only in the genus Arabidopsis, (A. thaliana and A. lyrata; Gomez-Porras et al., 2012). Thus, they apparently emerged just recently in evolution. Phylogenetic analyses indicated them to have originated from gene duplication of an TPK channel gene followed by a partial deletion event that resulted in the loss of one pore domain (Figure 1A; Marcel et al., 2010; Voelker et al., 2010). As a consequence, a plant Kir-like channel subunit contains only two TM and one P region. Based on that structural feature it was speculated that plant Kir-like channels are tetramers. This concept, however, is rather questionable. The genome of A. thaliana contains only one gene (called KCO3) coding for a Kir-like subunit. Recently, KCO3 could be detected only as stable dimer at the biochemical level (Rocchetti et al., 2012) pointing further to its origin from TPK channels. Very low transcript abundance has been observed for KCO3. Promoter-GUS fusion constructs for KCO3 show expression in vascular tissue of leaves, roots, flower tissue and stem and also in hydathodes as seen also for TPK5. KCO3 might play a role in osmoregulation, as the knock-out plant for the KCO3 gene shows reduced growth under osmotic stress condition. However, this change in the plant phenotype can be complemented by expressing a mutant KCO3 gene with an inactive pore region. These results indicate that the function of KCO3 under osmotic stress conditions is independent of its ability to transport potassium ions (Rocchetti et al., 2012). In conclusion, based on the current knowledge, plant Kir-like channels should be re-integrated into the TPK family, instead of being considered as a separate channel family. Their occurrence in Arabidopsis, only, may suggest that they just are “a freak of nature” without fundamental physiological importance outside this genus.
Voltage Dependent K+ Channels in Arabidopsis
The so-called plant Shaker-family is a group of voltage gated K+ channels. In A. thaliana it comprises nine members. This group can be divided into three subfamilies regarding their response to the membrane voltage (Lebaudy et al., 2007; Dreyer and Blatt, 2009). Six members activate upon membrane hyperpolarization and are closed when the driving force for potassium is outwardly directed. As a consequence they elicit only inward K+ currents (Kin). Two members activate upon membrane depolarization. They are closed when the driving force for potassium is inwardly directed. Thus, they elicit only outward K+ currents (Kout). And one member exhibits weak voltage dependence and can mediate both, K+ efflux and K+ influx (Kweak; Figure 1B).
Functional plant Shaker channels are built of four α-subunits. Each α-subunit contains six transmembrane domains and one pore domain between the fifth and the sixth transmembrane domain. The C-terminus contains various regulatory elements, like the cyclic nucleotide binding domain, an ankyrin repeat domain, the acidic domain KHA and in Kin channels the KT domain (Sentenac et al., 1992; Ehrhardt et al., 1997; Gaymard et al., 1998; Dreyer et al., 2004). Besides being functional as homotetramers, the formation of heterotetramers is common and proven to occur in plants (Dreyer et al., 1997; Lebaudy et al., 2008a).
Versatile physiological roles of plant Shaker channels were identified in numerous experiments. Knock-out and overexpressing mutant plants, as well as heterologous expression systems like Saccharomyces cerevisiae, Xenopus laevis oocytes, HEK293, COS, or Sf9 cells were used to study the functionality of K+ channels (Dreyer et al., 1999). The physiological roles and impacts of plant Shaker channels on the plant are described in the following sections.
K+ Uptake into Roots via AKT1
Various conditions necessitate different uptake systems
K+ uptake from soil is performed by a well-organized system of transport proteins each contributing in its own manner (Alemán et al., 2011). All uptake systems together operate on a broad range of K+ concentrations and are part of an extensive regulatory network (Figure 2). As main K+ uptake systems in Arabidopsis roots the Shaker-like K+ channel AKT1 and the K+ transporter AtHAK5 have been identified (Hirsch et al., 1998; Gierth et al., 2005; Rubio et al., 2008). At external K+ concentrations below 0.01 mM the proton-driven H+/K+ co-transporter AtHAK5 is the only system responsible for K+ uptake from the soil. At K+ concentrations between 0.01 mM and 0.05 mM AtHAK5 and AKT1 together contribute to K+ uptake. At higher external K+ concentrations, AKT1 together with other unknown low affinity K+ uptake systems are responsible for K+ uptake from the soil (Rubio et al., 2010; Pyo et al., 2010; Caballero et al., 2012).
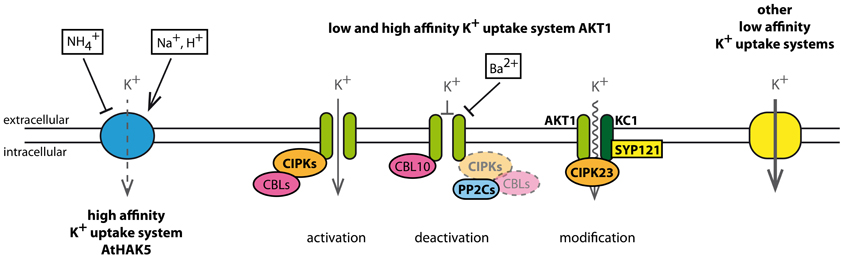
Figure 2. K+ uptake into Arabidopsis roots and its regulation. Depending on the actual K+ concentration in the soil different low or high affinity K+ uptake systems are active. At K+ concentrations below 0.01 mM only the high affinity transporter AtHAK5 is active. It is blocked by extracellular NH+4 and stimulated by extracellular Na+ and H+. The Shaker-like K+ channel AKT1 is involved in high and low affinity K+ uptake. It is a target of an extensive regulatory network that includes calcium sensors (CBLs), kinases (CIPKs), phosphatases (PP2Cs), and the ability to form heterotetramers with AtKC1. In the presence of CBL1 or CBL9, CIPK23 phosphorylates and activates AKT1. The interaction of CIPK 6, 16, and 23 each with CBL1, 2, 3, and 9 and its effect on AKT1 were shown in yeast two-hybrid assays and Xenopus laevis oocytes (Lee et al., 2007). AKT1 is deactivated by a direct interaction with CBL10, external Ba2+, or dephosphorylation via PP2C phosphatases. Phosphatases act directly on AKT1 or on the CIPK-CBL machinery to inactivate AKT1 (Lan et al., 2011). Furthermore, AKT1 is able to form heterotetramers with AtKC1. The heteromeric channel exhibits changed gating and permeation properties that block efficiently potential K+ release under low external K+ concentrations (Geiger et al., 2009). In addition, an interaction of CIPK23 with the heteromeric AKT1-AtKC1 was suggested and the contribution of SYP121 to the native characteristics of AKT1-AtKC1 was described (Honsbein et al., 2009).
AKT1 and AtHAK5 are affected by different environmental conditions. Both transport proteins work at different K+ concentration spectra and exhibit individual sensitivity toward other ions. For instance, AtHAK5 is sensitive to ammonium (NH+4) whereas AKT1 remains unaffected in the presence of NH+4. On the contrary, Ba2+ blocks AKT1 while AtHAK5 remains unaffected, and Na+ and H+ stimulate activity of AtHAK5 (Hirsch et al., 1998; Spalding et al., 1999; Rubio et al., 2008). Therefore, the different K+ uptake systems complement one another and even permit K+ uptake when one uptake system is disabled. AKT1 provides hence an alternative K+ uptake system to the NH+4 sensitive AtHAK5 under low K+ conditions.
Regulation of AKT1
AKT1 itself contributes to high and low affinity K+ uptake and is target of a regulatory network. Xu et al. and Li et al. showed in 2006 that CIPK23 and CBL1 or CBL9 are required to activate AKT1. The two calcineurin B-like calcium sensors CBL1 and CBL9 bind to the CBL-interacting protein kinase CIPK23, which then in turn phosphorylates AKT1. All three components (AKT1-CIPK23-CBL1/9) are essential for a functional expression of AKT1 in oocytes of X. laevis.
Shortly after, further components of this highly complex and flexible regulatory network were discovered. Besides several CIP kinases a 2C-type protein phosphatase (PP2C), AIP1, was shown to bind and inactivate AKT1 (Lee et al., 2007). Subsequent studies detected more interrelations between several CBLs and CIPKs with AKT1 (Lee et al., 2007; Lan et al., 2011; Ren et al., 2013). Lan et al. suggested that PP2C phosphatases also interact with the CIPK-CBL complex to inhibit the phosphorylation activity of the kinase and to dephosphorylate AKT1. And Ren et al. (2013) demonstrated that CBL10 directly binds AKT1 and diminishes its activity in a concentration-dependent and CIPK-independent manner.
Many different associations of AKT1 with CBLs, CIPKs, and PP2Cs have been reported. Grefen and Blatt (2012) argue that the method used to investigate interactions between proteins, positioning of tags and the way of analysis have a decisive impact on detectable interactions. Nevertheless, CBLs, CIPKs and PP2Cs provide a comprehensive system to regulate the K+ uptake mediated by AKT1. Especially, as different CBLs are involved in different signaling pathways this phosphorylation-dephosphorylation system provides a powerful regulatory network for the plant to respond to a broad range of environmental changes (for review see Kudla et al., 2010).
Internal regulation via heteromerization
Besides the regulation by kinases and phosphatases another member of the Shaker-like family alters the functionality of AKT1: AtKC1. AtKC1 is known as regulatory or silent α-subunit of Kin Shaker-like channels as it shows no currents in Xenopus oocytes when expressed alone and affects only Kin channels (Dreyer et al., 1997; Jeanguenin et al., 2011). Nevertheless, its participation in K+ uptake and its connection to AKT1 has been recognized since long (Reintanz et al., 2002; Pilot et al., 2003). Duby et al. (2008) demonstrated AtKC1′s impact on AKT1. They described that AtKC1 shifts the activation threshold of AKT1 toward more negative values. This in turn would avert K+ efflux through AKT1 under unfavorable conditions. The reduction of potential outward currents prevents the plant from K+ loss under low K+ concentrations. However, the cost of such “a valve” is a reduced channel activity that in turn implies decreased K+ influx under more favourable conditions. Geiger et al. (2009) supported, further broadened and fine-tuned this “valve” hypothesis. They showed in electrophysiological experiments the effect of AtKC1 on AKT1 inward and outward currents under varying K+ concentrations. Besides affecting the activation threshold, also the K+ dependent stability of the pore has been altered in AKT1-AtKC1 heteromers. When the external K+ concentration drops, the permeation pathway of K+ channels gets instable and collapses (Zhou et al., 2001). The threshold concentration, below which this happens, appears to be a characteristic feature of each channel. Geiger et al. (2009) found that the pore of AKT1-AtKC1 heteromers collapses at higher K+ concentrations than that of AKT1 homomers. Thus, heteromers comprise a more efficient block of the K+ passage in the unfavorable outward direction.
On top of that, the association of CIPK23 with the heteromeric AKT1-AtKC1 channel has been suggested from interaction analyses in yeast (Grefen and Blatt, 2012) along with an impact of the membrane vesicle trafficking SNARE protein SYP121 (Honsbein et al., 2009). In contrast to CIPK23, SYP121 binds only to the AtKC1 α-subunit but not to the AKT1 α-subunit. SNARE proteins are involved in vesicle targeting and fusion. Thus, K+ transport is not only regulated via the channel activity but also by membrane trafficking processes. Interestingly, the transcript level of AKT1 is constant under different environmental conditions (Lagarde et al., 1996; Pilot et al., 2003). But, the expression levels of its regulators change according to environmental stimuli (Pilot et al., 2003, review: Batistic and Kudla, 2004; Tripathi et al., 2009).
Outward Rectifiers in Roots
GORK in root hairs
Alongside the inward rectifying K+ channel AKT1, the outward rectifying K+ channel GORK is expressed in root epidermal cells (Ivashikina et al., 2001). GORK activates upon membrane depolarization and its gating depends on the extracellular K+ concentration. Environmental changes in the surrounding of root hairs can appear rapidly and in response the membrane depolarizes (Càrdenas et al., 2000). GORK activates under these conditions and is considered to initiate the repolarization of the membrane. By controlling the membrane potential and the turgor in root hairs, the plant can react on environmental changes, like absence or abundance of water that cause changes in solute concentrations and affect the mechanical stability and the hydration status of the root. Furthermore, the ability of GORK to sense the extracellular K+ concentration is supposed to enable the root hair to sense and flexibly react on the K+ content in the soil.
Root to shoot communication via SKOR
K+ is transported from roots to the upper parts of the plant via the xylem. The outward rectifying Shaker-like channel SKOR is expressed in the pericycle and the xylem parenchyma in roots. SKOR was identified as transport protein responsible for loading K+ to the xylem based on the finding that its disruption strongly reduced the K+ content in the shoot while the K+ content in roots remained unaffected (Gaymard et al., 1998).
In addition to the membrane voltage, SKOR is modulated by the external K+ concentration. In the presence of ample external K+, the channel needs a higher membrane voltage to open and thus minimizes the risk to serve as an undesirable K+-influx pathway. Such behavior is achieved by a complex interplay between the pore region and the last transmembrane domain of the channel that is responsible for final channel opening and closure. When the external K+ concentration is high, the pore region is quite rigid and strongly interacts with the last transmembrane domain of the channel. As a consequence the channel is stabilized in a closed state. Under low external K+ conditions the pore region is less occupied by K+ ions. As a consequence, the pore is more flexible and does not interact with the surrounding transmembrane domains anymore. Opening of the channel is possible with less energy input, i.e., at less positive membrane voltages. If the last transmembrane domains rearrange and unclench the conduction pathway, intracellular K+ ions can re-enter the pore, stabilize it in a permeable conformation and thus enable a K+ outward current (the K+-sensing mechanisms has been animated in the supplementary material of Johansson et al., 2006).
K+ distribution is also influenced by factors that are involved in stress signaling. SKOR expression is inhibited by abscisic acid (ABA). It was proposed that the reduced K+ release to the xylem in response to ABA could be a possibility to adjust osmotic conditions by roots in stress situations (Gaymard et al., 1998). Besides, intra- and extracellular acidification negatively affects the SKOR currents. As the regulation via ABA appears on the transcriptional level, the pH sensitivity might be a complementary process to prevent K+ loss from roots toward the shoot tissue (Lacombe et al., 2000a).
Hydrogen peroxide (H2O2) exhibits a contrary effect on SKOR currents. Reactive oxygen species function as signal and regulator in plant development and in responses to environmental stress situations (Torres and Dangl, 2005; Gapper and Dolan, 2006). Treatment with H2O2 leads to an increase in SKOR outward currents and a decrease in its half activation time (Garcia-Mata et al., 2010). This finding points to a relation between reactive oxygen species and K+ partitioning during developmental processes and stress responses.
Phloem-Allocation and Retrieval
Once loaded into the xylem, K+ circulates within the whole plant. There, other K+ channels contribute to the further distribution. The Shaker-like potassium channel AKT22 is mainly expressed in the vascular tissue of aerial parts and in guard cells of plants. However, it is not expressed until the plant is widely independent of carbohydrates provided by the seed (Marten et al., 1999; Deeken et al., 2000; Lacombe et al., 2000b; Szyroki et al., 2001; Ivashikina et al., 2005).
Charging and using the potassium battery
As the only member of the Shaker-like channels in plants, AKT2 features a unique channel property and can mediate both, inward and outward K+ currents. AKT2 is in fact a specialized inward rectifying channel that can be changed into a non-rectifying channel. It exhibits two phosphorylation status-dependent gating modes that are inter-convertible (Dreyer et al., 2001; Chèrel et al., 2002; Michard et al., 2005a,b). The non-phosphorylated AKT2 (mode 1) is lacking its outward component and behaves like an inward rectifying channel. In contrast, the phosphorylated AKT2 (mode 2) is permanently open and able to conduct K+ in the inward and in the outward direction. Two serine residues located near the intracellular side of the channel are identified as targets for phosphorylation (Michard et al., 2005a). Nevertheless, it is proposed that the two phospho-serine residues alone are not sufficient to completely convert AKT2 between its modes. Sandmann et al. (2011) proposed rather a transition via a cascade of posttranslational (so far unknown) modifications. This hypothesis is fuelled by experimental observations. A lysine within the voltage sensor enables AKT2 to sense its phosphorylation status and to change between the two modes. Replacement of the lysine by serine or arginine keeps AKT2 in its inward rectifying mode 1 (Michard et al., 2005b; Sandmann et al., 2011).
Summing up, AKT2 can modulate the membrane voltage by switching between its modes of an inward or a non-rectifying channel, respectively, and phosphorylation acts as a tool for fine tuning (Deeken et al., 2002; Michard et al., 2005a,b). Gajdanowicz et al. (2011) embedded AKT2 as a central player in a “potassium battery” model in which K+ serves as mobile energy source in vascular tissues. In source tissues, the plant invests energy to load K+ into the phloem sieve element companion cell complexes. The loaded potassium is then circulating with the phloem stream. Under energy limiting conditions, the AKT2 channel can be switched from its inward-rectifying to its non-rectifying mode and thus enables a passage for K+ efflux. This in turn enables the use of the K+ gradient between the phloem and the apoplast for the reloading of photoassimilates into the phloem. This “potassium battery” concept is illustrated in the supplementary material of Gajdanowicz et al. (2011). Limiting conditions occur for example under ATP shortage or when the H+-ATPase is down-regulated by cellular signals. The normally used H+ gradient is then complemented by the K+ gradient. Besides tapping the “battery,” the AKT2 channel is also proposed to charge it depending on its actual gating mode (Michard et al., 2005b).
Further effects on AKT2
In addition to the gating mode modulations, AKT2 was also demonstrated to act on diverse signals involved in stress responses. The expression level of AKT2 increases in the presence of ABA, light and CO2 assimilates (Deeken et al., 2000; Lacombe et al., 2000b). Primarily, the influences of the last two factors led to the view that AKT2 plays a role in phloem transport.
Macroscopic K+currents mediated by AKT2 are modulated by changes in internal and external pH and external Ca2+ (Marten et al., 1999; Lacombe et al., 2000b). While external Ca2+ blocks inward currents at negative voltages in a voltage-dependent manner, acidification on both sides of the membrane diminishes AKT2 currents in the whole voltage range. Changes in pH and Ca2+ do not affect the gating mode of the channel indicating that H+ and Ca2+ affect only the permeation pathway of AKT2. The sensitivity of AKT2 toward Ca2+ was investigated in guard cells. Ivashikina et al. (2005) showed in experiments on guard cell protoplasts that the Ca2+ sensitivity of K+ uptake channels correlates with the presence of AKT2 subunits.
Recently, Held et al. (2011) demonstrated the association of AKT2 with CIPK6 and CBL4 and the effect of this assembly on macroscopic AKT2 currents. In contrast to the AKT1-CIPK-CBL complexes, no phosphorylation events could be detected in vitro so far. Held et al. therefore proposed for these findings a Ca2+ dependent targeting of AKT2 to the plasma membrane that depends solely on the physical interaction of AKT2 with CIPK6/CBL4 rather than a regulation of the channel via phosphorylation.
Guard Cells and its K+ Channel Population
Two third of the Shaker channel family members are expressed in guard cells. Besides AKT2, also KAT1, KAT2, AKT1, AtKC1, and GORK are detectable there and have important impacts on stomatal opening and closure (Szyroki et al., 2001; Ivashikina et al., 2005; Lebaudy et al., 2008b). Vast signal transduction pathways coordinate stomatal movement. In case of stomatal opening, they result in the activation of Kin channels and the uptake of K+and anions, which finally leads to an increase of guard cell turgor. In case of stomatal closure, Kout channels are activated, K+ is released together with anions, water is passively flowing out and guard cell turgor decreases. Recent comprehensive reviews of signal transduction pathways that affect stomatal opening and closure were published by Pandey et al. (2007) or Kim et al. (2010). Figure 3 shows an overview of the regulation of K+ channels involved in stomatal movements.

Figure 3. K+ channels in Arabidopsis guard cells and their effectors. Changes in membrane potential lead to stomatal opening or closure, respectively. Membrane hyperpolarisation in response to H+-ATPase activity activates inward-rectifying K+ channels. Transcripts for KAT1, KAT2, AKT1, AKT2, and AtKC1 are detectable in guard cells (Szyroki et al., 2001). All these K+ channel subunits form heterotetrameric channels like KAT1-KAT2, AKT2-KAT2, and AKT1-AtKC1. The impact of AKT1 and Kin-AtKC1 on stomatal movement has not been investigated in detail. Acidification affects directly the currents through K+ channels in guard cells. Kin channels are activated upon extracellular acidification, while currents through Kweak channels decrease. Besides, the Kweak channel AKT2 is negatively affected by extracellular Ca2+. KAT1 currents are furthermore modulated by intracellular pH, ATP, and cGMP. ATP and cGMP have antagonistic effects. Moreover, stomatal K+ channels are affected by signals via signal transduction cascades. Effects of 14-3-3 proteins, Ca2+ and kinases have been reported. Membrane depolarization, on the other hand, caused by the inactivation of H+-ATPases and activation of anion channels activates the Kout channel GORK. GORK currents are positively influenced by extra- and intra-cellular alkalinisation. Furthermore, the current enhancing effect of H2O2 is under investigation. Both, stomatal opening and closure are affected by phytohormones. While Auxin evokes stomatal opening, ABA inhibits its opening but evokes closure of stomata. Abbreviations: pHac, acidification; pHba, alkalinisation.
Channel variability in guard cells
Although KAT1 represents the dominant Kin channel in guard cells, it is not essential for stomatal opening (Ichida et al., 1997; Kwak et al., 2001; Szyroki et al., 2001). The coevally expressed Kin channel subunits AKT1, AKT2, and KAT2 are able to compensate for the loss of KAT1. The expression pattern of all guard cell Kin channel subunits is not exactly identical as exemplified by KAT1 and KAT2. Both subunits are expressed in guard cells. But, KAT1 is only expressed in guard cells of leaves and petioles, while KAT2 is additionally expressed in guard cells of the stem (Nakamura et al., 1995; Pilot et al., 2001). That points to different available sets of K+ channels dependent on the guard cell location. Furthermore, K+ channel subunits are able to form heteromeric channels in plants (Dreyer et al., 1997; Lebaudy et al., 2008a). For KAT1-KAT2 heterotetramers it has been shown that their basic properties are similar to properties observed for the homotetrameric KAT1 and KAT2 channels (Pilot et al., 2001; Lebaudy et al., 2010). In contrast, the AKT2-KAT2 heterotetramer combines different properties of its parental channels and forms a new functional type of a K+ channel (Xicluna et al., 2007). The gating properties of the heterotetramer are inherited from AKT2, a weak-rectifying K+ channel described above. The sensitivities to Ca2+ and H+ are inherited from KAT2. Thus, K+ channel heteromers notably contribute to an increase in channel variability and enhance the regulatory possibilities of K+ channels.
Kin channels contribute to stomatal opening
For activation of Kin channels the membrane potential needs to be hyperpolarized. Hyperpolarization is achieved through the activity of H+-ATPases that transport protons under ATP consumption out of the cell. The membrane voltage is sensed by the intrinsic voltage sensor that is formed by the transmembrane regions S1–S4. An important role is played especially by the positive charges in S4 (Figure 1A). The four voltage sensors of the channel induce conformational changes in the protein that then result in an opening of the permeation pathway. This voltage-sensitivity is modulated by other factors that interact with the channel protein. Indeed, many experiments show the sensitivity of guard cell Kin channels to changes in pH (Hedrich et al., 1995; Hoshi, 1995; Marten et al., 1999; Pilot et al., 2001; Xicluna et al., 2007). KAT1, KAT2 and the heteromeric KAT1-KAT2 are activated by extracellular and intracellular acidification due to a shift of the voltage dependence of the channels to more positive values. A histidine residue conserved among plant Kin channels that is located in the pore was suggested to sense pH changes in Kin channels (Hoth et al., 1997; Hoth and Hedrich, 1999). For KST1, a Kin channel from potato guard cells, it has been shown that this histidine is part of the pH sensor. Surprisingly, mutations of this histidine in KAT1 did not affect its pH dependence. Further investigation revealed that KAT1 senses pH changes via a sensory cloud rather than a single residue (Gonzalez et al., 2012). Besides, KAT1 is also modulated by ATP and cGMP. While cGMP reduces KAT1 currents, ATP affects KAT1 positively. Thus, ATP and cGMP show antagonistic effects (Hoshi, 1995).
Another regulator of guard cell Kin currents might be extracellular Ca2+. Here, AKT2 is the only channel affected directly by external Ca2+ (Marten et al., 1999; Latz et al., 2007b). While AKT2 is blocked by Ca2+, KAT1, KAT2 and AKT1 do not show any response (Szyroki et al., 2001; Ivashikina et al., 2005; Brüggemann et al., 1999). It is therefore proposed that experimentally observed sensitivity of guard cell Kin channels to extracellular Ca2+ is conferred by AKT2 subunits (Ivashikina et al., 2005).
Furthermore, effects of regulatory proteins on KAT1 have been shown. For instance, KAT1 is phosphorylated in a Ca2+-dependent manner in the presence of CDPK-a Ca2+-dependent protein kinase with a calmodulin-like domain (Li et al., 1998). This study used a recombinant CDPK from the bean Vicia faba and did not show whether KAT1 is phosphorylated directly by CDPK or rather other proteins are affected by the kinase. Berkowitz et al. (2000) showed in electrophysiological experiments that the recombinant CDPK has a negative effect on KAT1 currents. Ca2+-dependent phosphorylation of KAT1 is further supported by a study that manipulated a protein kinase C (PKC) present in X. laevis oocytes (Sato et al., 2010). Upon activation of PKC that has similar target sites as plant Ca2+-dependent kinases, KAT1 currents decline. In addition, recombinant 14-3-3 proteins from maize stimulated KAT1 currents (Sottocornola et al., 2006, 2008). These studies provide a first glimpse on the broad range of feasible effectors of K+ channels in guard cells.
Additionally it was found that the channel population within the membrane undergoes regulation as well (Mikosch et al., 2006, 2009; Sutter et al., 2006, 2007; Sieben et al., 2008; Reuff et al., 2010). It has been shown that KAT1 interacts with SNARE proteins (see above), and ABA triggers endocytosis of KAT1 from the plasma membrane. Furthermore, the ER export motif of KAT1 subunits is important for proper channel trafficking. It has been shown that efficient ER export of KAT1 depends on an acidic motif in the C-terminus. Therefore, endo- and exo-cytosis, as well as the ER export of K+ channels might be another level for regulating channel densities and K+ currents across the membrane.
Kout channels during stomatal closure
Kout channels are activated upon depolarization. Such a membrane voltage change is achieved by inhibition of the H+-ATPase and activation of anion channels. GORK is the only Kout channel identified in guard cells and is responsible for stomatal closure (Szyroki et al., 2001; Hosy et al., 2003). In contrast to inward rectifying channels, GORK currents are reduced with decreasing internal and external pH (Blatt, 1992; Ache et al., 2000). GORK also senses the external K+ concentration, so that at higher external K+ it requires more positive voltage for its activation (Ache et al., 2000). A similar analogy to SKOR might also hold for the direct interaction of GORK with the stress signaling molecule H2O2. GORK and SKOR share the cysteine residue that has been shown to be responsible for the activation effect in SKOR (Garcia-Mata et al., 2010). Nevertheless, the impact of H2O2 on GORK and the role of this presumed regulation in guard cell physiology still need to be investigated. Earlier reports have shown that H2O2 is an important player in stomatal signaling (reviewed by Wang and Song, 2008).
Alongside the activation of Kout channels during stomatal closure, Kin channels are deactivated (Blatt, 1990; Thiel et al., 1992). A knock-out mutant of the Kin channel AKT1 has been shown to be more resistant toward water stress than wild type plants (Nieves-Cordones et al., 2012). Transpiration was reduced and stomata closure was more efficient in knock-out plants treated with ABA. Thus, the inactivation of Kin channels favors stomatal closure but it is not essential for the process of closure itself (MacRobbie, 1998). Furthermore, very similar phenotypes of akt1 and cipk23 knock-out plants could be observed suggesting a regulation of AKT1 by CIPK23 also in guard cells as it has been shown already for AKT1 in roots.
Influences of phytohormones
The phytohormones auxin and ABA cause opposing effects on stomata. Auxin is involved in plant developmental processes and promotes stomatal opening, whereas, ABA is involved in various stress responses. It prevents the opening and promotes the closure of stomata (Gehring et al., 1990). The direct influence of ABA on guard cell K+ currents was shown by Blatt and Armstrong (1993). ABA treatment leads to inactivation of Kin and activation of Kout channels in guard cells. Although the phytohormon affects the transcription level of the Kout channel GORK in roots and shoots, the transcript level in guard cells remains unaffected (Becker et al., 2003). Besides, electrophysiological analyses exclude the direct effect of ABA on outward currents in guard cells. Therefore, ABA seems to affect guard cell Kout currents indirectly. As ABA signals from roots come along with alkalinisation of the guard cell cytoplasm (Blatt and Armstrong, 1993), the pH sensitive GORK can be activated and affected by ABA by this long distance signaling pathway (Blatt, 1992; Ache et al., 2000; Becker et al., 2003).
Auxin, on the other hand, stimulates the transcription of KAT1 and KAT2 (Philippar et al., 2004). It is not clear, however, whether this stimulation is tissue specific as in the case of GORK or whether it is a general feature of these Kin channel genes in different parts of the plant.
Influence on Pollen Tube Development
The Shaker channel SPIK is the main Kin channel in pollen and exclusively expressed there (Mouline et al., 2002; Zhao et al., 2013). Its disruption affects negatively pollen tube growth. The activity of SPIK is enhanced by decreasing external pH and negatively affected by the Ca2+-dependent protein kinases CDPK11 and CDPK24. Ca2+ affects Kin currents only in the pollen tube but not in pollen grain protoplasts. It has been shown that the effect of Ca2+ is dependent on the presence of both kinases. In the absence of one of the kinases Ca2+ cannot block pollen Kin currents. Zhao and colleagues propose that Ca2+ acts negatively on SPIK via a kinase cascade, in which CDPK11 phosphorylates CDPK24.
Conclusions
K+ channels are important for K+ uptake from the soil, its distribution within the plant and processes to maintain and support plant growth. The past two decades revealed crucial information especially for plant Shaker like channels regarding the structure, physiological role and-to a minor extent-regarding their regulation. In contrast, our knowledge on TPK channels is far more rudimentary. The challenge of the future of plant K+ channel research will be to identify the complex regulatory networks that regulate their activity and to understand the dynamics of these networks.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Spanish Ministerio de Economía y Competitividad (BFU2011-28815), a Marie-Curie Career Integration Grant (FP7-PEOPLE-2011-CIG No. 303674—Regopoc), and by grants of the Deutsche Forschungsgemeinschaft to Ingo Dreyer (DFG grants DR430/8-1 and DR430/9-1). Tripti Sharma and Janin Riedelsberger were recipients of doctoral fellowships from the International Max-Planck Research School “Primary Metabolism and Plant Growth”. The authors are grateful to the reviewers for their helpful comments.
Abbreviations
ABA, abscisic acid; ATP, adenosine triphosphate; CBL, calcineurin B-like calcium sensor; CDPK, Ca2+ dependent protein kinase; cGMP, cyclic guanosine monophosphate; CIPK, CBL-interacting protein kinase; ER, endoplasmic reticulum; GFP, green fluorescent protein; P, pore; PKC, protein kinase C; PP2C, 2C-type protein phosphatase; SNARE, soluble N-ethylmaleimide–sensitive factor protein attachment protein receptor; TM, transmembrane; YFP, yellow fluorescent protein.
Footnotes
- ^pS/pT indicate the potential phosphorylation of the serine or threonine residue, respectively.
- ^In literature, the gene encoded by the locus At4g22200 has been named AKT2, AKT3 and AKT2/3. To avoid confusions, we will summarize the data under the name AKT2 irrespective of the alternative names used in the original publications.
References
Ache, P., Becker, D., Ivashikina, N., Dietrich, P., Roelfsema, M., and Hedrich, R. (2000). GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 486, 93–98. doi: 10.1016/S0014-5793(00)02248-1
Alemán, F., Nieves-Cordones, M., Martínez, V., and Rubio, F. (2011). Root K+ acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol. 52, 1603–1612. doi: 10.1093/pcp/pcr096
Amtmann, A., Armengaud, P., and Volkov, V. (2004). “Potassium nutrition and salt stress,” in Membrane Transport in Plants, ed M. R. Blatt (Oxford: Blackwell), 316–348.
Amtmann, A., Troufflard, S., and Armengaud, P. (2008). The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133, 682–691. doi: 10.1111/j.1399-3054.2008.01075.x
Anderson, J. A., Huprikar, S. S., Kochian, L. V., Lucas, W. J., and Gaber, R. F. (1992). Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 89, 3736–3740.
Basset, M., Conejero, G., Lepetit, M., Fourcroy, P., and Sentenac, H. (1995). Organization and expression of the gene coding for the potassium transport system AKT1 of Arabidopsis thaliana. Plant Mol. Biol. 29, 947–958.
Batistic, O., and Kudla, J. (2004). Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 219, 915–924. doi: 10.1007/s00425-004-1333-3
Becker, D., Geiger, D., Dunkel, M., and Roller, A. (2004). AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 101, 15621–15626.
Becker, D., Hoth, S., Ache, P., Wenkel, S., Roelfsema, M. R. G., Meyerhoff, O., et al. (2003). Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 554, 119–126. doi: 10.1016/S0014-5793(03)01118-9
Berkowitz, G., Zhang, X., Mercier, R., Leng, Q., and Lawton, M. (2000). Co- expression of calcium-dependent protein kinase with the inward rectified guard cell K+ channel KAT1 alters current parameters in Xenopus laevis oocytes. Plant Cell Physiol. 41, 785–790. doi: 10.1093/pcp/41.6.785
Bihler, H., Eing, C., Hebeisen, S., Roller, A., Czempinski, K., and Bertl, A. (2005). TPK1 is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol. 139, 417–424. doi: 10.1104/pp.105.065599
Blatt, M. R. (1990). Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta 180, 445–455. doi: 10.1007/BF01160403
Blatt, M. R. (1992). K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J. Gen. Physiol. 99, 615–644.
Blatt, M. R., and Armstrong, F. (1993). K+ channels of stomatal guard cells: abscisic- acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191, 330–341. doi: 10.1007/BF00195690
Brüggemann, L., Dietrich, P., Dreyer, I., and Hedrich, R. (1999). Pronounced differences between the native K+ channels and KAT1 and KST1 alpha-subunit homomers of guard cells. Planta 207, 370–376. doi: 10.1007/s004250050494
Caballero, F., Botella, M. A., Rubio, L., Fernández, J. A., Martínez, V., and Rubio, F. (2012). A Ca2+-sensitive system mediates low-affinity K+ uptake in the absence of AKT1 in Arabidopsis plants. Plant Cell Physiol. 53, 2047–2059. doi: 10.1093/pcp/pcs140
Càrdenas, L., Holdaway-Clarke, T. L., Saìnchez, F., Quinto, C., Feijoì, J. A., Kunkel, J. G., et al. (2000). Ion changes in legume root hairs responding to Nod factors. Plant Physiol. 123, 443–452. doi: 10.1104/pp.123.2.443
Chèrel, I., Michard, E., Platet, N., Mouline, K., Alcon, C., Sentenac, H., et al. (2002). Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell 14, 1133–1146. doi: 10.1105/tpc.000943
Chanroj, S., Wang, G., Venema, K., Zhang, M. W., Delwiche, C. F., and Sze, H. (2012). Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front. Plant Sci. 3:25. doi: 10.3389/fpls.2012.00025
Czempinski, K., Frachisse, J.-M., Maurel, C., Barbier-Brygoo, H., and Mueller-Roeber, B. (2002). Vacuolar membrane localization of the Arabidopsis 'two- pore' K+ channel KCO1. Plant J. 29, 809–820. doi: 10.1046/j.1365-313X.2002.01260.x
Czempinski, K., Gaedeke, N., Zimmermann, S., and Muĺller-Roĺber, B. (1999). Molecular mechanisms and regulation of plant ion channels. J. Exp. Bot. 50, 955–966.
Czempinski, K., Zimmermann, S., Ehrhardt, T., and Muĺller-Roĺber, B. (1997). New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J. 16, 2565–2575. doi: 10.1093/emboj/16.10.2565
Deeken, R., Geiger, D., Fromm, J., Koroleva, O., Ache, P., Langenfeld-Heyser, R., et al. (2002). Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 216, 334–344. doi: 10.1007/s00425-002-0895-1
Deeken, R., Ivashikina, N., Czirjak, T., Philippar, K., Becker, D., Ache, P., et al. (2003). Tumour development in Arabidopsis thaliana involves the Shaker-like K+ channels AKT1 and AKT2/3. Plant J. 34, 778–787. doi: 10.1046/j.1365-313X.2003.01766.x
Deeken, R., Sanders, C., Ache, P., and Hedrich, R. (2000). Developmental and light-dependent regulation of a phloem-localised K+ channel of Arabidopsis thaliana. Plant J. 23, 285–290. doi: 10.1046/j.1365-313x.2000.00791.x
Dennison, K. L., Robertson, W. R., Lewis, B. D., Hirsch, R. E., Sussman, M. R., and Spalding, E. P. (2001). Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 127, 1012–1019. doi: 10.1104/pp.010193
Dreyer, I., Antunes, S., Hoshi, T., Muĺller-Roĺber, B., Palme, K., Pongs, O., et al. (1997). Plant K+ channel alpha-subunits assemble indiscriminately. Biophys. J. 72, 2143–2150.
Dreyer, I., and Blatt, M. R. (2009). What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 14, 383–390. doi: 10.1016/j.tplants.2009.04.001
Dreyer, I., Horeau, C., Lemaillet, G., Zimmermann, S., Bush, D. R., Rodríguez-Navarro, A., et al. (1999). Identification and characterization of plant transporters. J. Exp. Bot. 50, 1073–1087.
Dreyer, I., Michard, E., Lacombe, B., and Thibaud, J. B. (2001). A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 505, 233–239. doi: 10.1016/S0014-5793(01)02832-0
Dreyer, I., Poree, F., Schneider, A., Mittelstadt, J., Bertl, A., Sentenac, H., et al. (2004). Assembly of plant Shaker-like Kout channels requires two distinct sites of the channel alpha-subunit. Biophys. J. 87, 858–872.
Duby, G., Hosy, E., Fizames, C., Alcon, C., Costa, A., Sentenac, H., et al. (2008). AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J. 53, 115–123. doi: 10.1111/j.1365-313X.2007.03324.x
Dunkel, M., Latz, A., Schumacher, K., Muĺller, T., Becker, D., and Hedrich, R. (2008). Targeting of vacuolar membrane localized members of the TPK channel family. Mol. Plant 1, 938–949.
Ehrhardt, T., Zimmermann, S., and Müller-Röber, B. (1997). Association of plant K+in channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett. 409, 166–170. doi: 10.1016/S0014-5793(97)00502-4
Gajdanowicz, P., Michard, E., Sandmann, M., Rocha, M., Corre^a, L. G. G., Ramiìrez-Aguilar, S. J., et al. (2011). Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. U.S.A. 108, 864–869.
Gapper, C., and Dolan, L. (2006). Control of plant development by reactive oxygen species. Plant Physiol. 141, 341–345. doi: 10.1104/pp.106.079079
Garcia-Mata, C., Wang, J., Gajdanowicz, P., Gonzalez, W., Hills, A., Donald, N., et al. (2010). A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 285, 29286–29294.
Gaymard, F., Pilot, G., Lacombe, B., Bouchez, D., Bruneau, D., Boucherez, J., et al. (1998). Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. doi: 10.1016/S0092-8674(00)81606-2
Gehring, C. A., Irving, H. R., and Parish, R. W. (1990). Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc. Natl. Acad. Sci. U.S.A. 87, 9645–9649.
Geiger, D., Becker, D., Vosloh, D., Gambale, F., Palme, K., Rehers, M., et al. (2009). Heteromeric AtKC1-AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 284, 21288–21295.
Gierth, M., Maser, P., and Schroeder, J. I. (2005). The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 137, 1105–1114. doi: 10.1104/pp.104.057216
Gierth, M., and Mäser, P. (2007). Potassium transporters in plants–involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 581, 2348–2356. doi: 10.1016/j.febslet.2007.03.035
Gobert, A., Isayenkov, S., Voelker, C., Czempinski, K., and Maathuis, F. J. M. (2007). The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. U.S.A. 104, 10726–10731.
Gomez-Porras, J. L., Riaño-Pachón, D. M., Benito, B., Haro, R., Sklodowski, K., Rodriguez-Navarro, A., et al. (2012). Phylogenetic analysis of K+ transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Front. Plant Sci. 3:167. doi: 10.3389/fpls.2012.00167
Gonzalez, W., Riedelsberger, J., Morales-Navarro, S. E., Caballero, J., Alzate-Morales, J. H., Gonzaìlez-Nilo, F. D., et al. (2012). The pH-sensor of the plant K+ uptake channel KAT1 is built of a sensory cloud rather than of single key amino acids. Biochem. J. 442, 57–63.
Grefen, C., and Blatt, M. R. (2012). Do calcineurin B-like proteins interact independently of the serine threonine kinase CIPK23 with the K+ channel AKT1? Lessons learned from a meìnage aÌ trois. Plant Physiol. 159, 915–919. doi: 10.1104/pp.112.198051
Grefen, C., Chen, Z., Honsbein, A., Donald, N., Hills, A., and Blatt, M. R. (2010). A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 22, 3076–3092. doi: 10.1105/tpc.110.077768
Hamamoto, S., Marui, J., Matsuoka, K., Higashi, K., Igarashi, K., Nakagawa, T., et al. (2008a). Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J. Biol. Chem. 283, 1911–1920.
Hamamoto, S., Yabe, I., and Uozumi, N. (2008b). Electrophysiological properties of NtTPK1 expressed in yeast tonoplast. Biosci. Biotechnol. Biochem. 72, 2785–2787.
Hedrich, R., Moran, O., Conti, F., Busch, H., Becker, D., Gambale, F., et al. (1995). Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators. Eur. Biophys. J. 24, 107–115.
Held, K., Pascaud, F., Eckert, C., Gajdanowicz, P., Hashimoto, K., Corratgeì-Faillie, C., et al. (2011). Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 21, 1116–1130. doi: 10.1038/cr.2011.50
Hirsch, R. E., Lewis, B. D., Spalding, E. P., and Sussman, M. R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. doi: 10.1126/science.280.5365.918
Honsbein, A., Sokolovski, S., Grefen, C., Campanoni, P., Pratelli, R., Paneque, M., Chen, Z., et al. (2009). A tripartite SNARE- K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21, 2859–2877. doi: 10.1105/tpc.109.066118
Hoshi, T. (1995). Regulation of voltage dependence of the KAT1 channel by intracellular factors. J. Gen. Physiol. 105, 309–328.
Hosy, E., Vavasseur, A., Mouline, K., Dreyer, I., Gaymard, F., Poree, F., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. U.S.A. 100, 5549–5554.
Hoth, S., Dreyer, I., Dietrich, P., Becker, D., Müller-Röber, B., and Hedrich, R. (1997). Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. U.S.A. 94, 4806–4810.
Hoth, S., and Hedrich, R. (1999). Distinct molecular bases for pH sensitivity of the guard cell K+ channels KST1 and KAT1. J. Biol. Chem. 274, 11599–11603.
Ichida, A. M., Pei, Z. M., Baizabal-Aguirre, V. M., Turner, K. J., and Schroeder, J. I. (1997). Expression of a Cs+-resistant guard cell K+ channel confers Cs+- resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell 9, 1843–1857.
Isayenkov, S., Isner, J. C., and Maathuis, F. J. (2011a). Rice two-pore K+ channels are expressed in different types of vacuoles. Plant Cell 23, 756–768.
Isayenkov, S., Isner, J. C., and Maathuis, F. J. (2011b). Membrane localisation diversity of TPK channels and their physiological role. Plant Signal Behav. 6, 1201–1204.
Isayenkov, S., and Maathuis, F. J. (2013). Arabidopsis thaliana vacuolar TPK channels form functional K+ uptake pathways in Escherichia coli. Plant Signal. Behav. 8, e24665.
Ivashikina, N., Becker, D., Ache, P., Meyerhoff, O., Felle, H. H., and Hedrich, R. (2001). K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 508, 463–469. doi: 10.1016/S0014-5793(01)03114-3
Ivashikina, N., Deeken, R., Fischer, S., Ache, P., and Hedrich, R. (2005). AKT2/3 subunits render guard cell K+ channels Ca2+ sensitive. J. Gen. Physiol. 125, 483–492.
Jeanguenin, L., Alcon, C., Duby, G., Boeglin, M., Chèrel, I., Gaillard, I., et al. (2011). AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 67, 570–582. doi: 10.1111/j.1365-313X.2011.04617.x
Johansson, I., Wulfetange, K., Poree, F., Michard, E., Gajdanowicz, P., Lacombe, B., et al. (2006). External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 46, 269–281. doi: 10.1111/j.1365-313X.2006.02690.x
Kim, T. H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Kudla, J., Batistic, O., and Hashimoto, K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell 22, 541–563. doi: 10.1105/tpc.109.072686
Kwak, J. M., Murata, Y., Baizabal-Aguirre, V. M., Merrill, J., Wang, M., Kemper, A., et al. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127, 473–485. doi: 10.1104/pp.010428
Lacombe, B., Pilot, G., Gaymard, F., Sentenac, H., and Thibaud, J. B. (2000a). PH control of the plant outwardly-rectifying potassium channel SKOR. FEBS Lett. 466, 351–354.
Lacombe, B., Pilot, G., Michard, E., Gaymard, F., Sentenac, H., and Thibaud, J. B. (2000b). A Shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12, 837–851.
Lagarde, D., Basset, M., Lepetit, M., Conejero, G., Gaymard, F., Astruc, S., et al. (1996). Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 9, 195–203. doi: 10.1046/j.1365-313X.1996.09020195.x
Lan, W.-Z., Lee, S.-C., Che, Y.-F., Jiang, Y.-Q., and Luan, S. (2011). Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol. Plant 4, 527–536.
Latz, A., Becker, D., Hekman, M., Muller, T., Beyhl, D., Marten, I., et al. (2007a). TPK1, a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14–3-3 proteins. Plant J. 52, 449–459.
Latz, A., Ivashikina, N., Fischer, S., Ache, P., Sano, T., Becker, D., et al. (2007b). In planta AKT2 subunits constitute a pH- and Ca2+- sensitive inward rectifying K+ channel. Planta 225, 1179–1191.
Lebaudy, A., Hosy, E., Simonneau, T., Sentenac, H., Thibaud, J., and Dreyer, I. (2008a). Heteromeric K+ channels in plants. Plant J. 54, 1076–1082.
Lebaudy, A., Vavasseur, A., Hosy, E., Dreyer, I., Leonhardt, N., Thibaud, J.-B., et al. (2008b). Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. U.S.A. 105, 5271–5276.
Lebaudy, A., Pascaud, F., Veìry, A.-A., Alcon, C., Dreyer, I., Thibaud, J.-B., et al. (2010). Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 285, 6265–6274.
Lebaudy, A., Véry, A., and Sentenac, H. (2007). K+ channel activity in plants: genes, regulations and functions. FEBS Lett. 581, 2357–2366. doi: 10.1016/j.febslet.2007.03.058
Lee, S. C., Lan, W.-Z., Kim, B.-G., Li, L., Cheong, Y. H., Pandey, G. K., et al. (2007). A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. U.S.A. 104, 15959–15964.
Leigh, R. A., and Wyn Jones, R. G. (1984). A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97, 1–13. doi: 10.1111/j.1469-8137.1984.tb04103.x
Li, J., Lee, Y. J., and Assmann, S. M. (1998). Guard cells possess a calcium- dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 116, 785–795. doi: 10.1104/pp.116.2.785
Li, L., Kim, B.-G., Cheong, Y. H., Pandey, G. K., and Luan, S. (2006). A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 12625–12630.
Maathuis, F. J. M. (2009). Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258.
Maathuis, F. J. M. (2011). Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 191, 84–91. doi: 10.1111/j.1469-8137.2011.03664.x
MacRobbie, E. A. C. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. B. 353, 1475–1488.
Maitrejean, M., Wudick, M. M., Voelker, C., Prinsi, B., Mueller-Roeber, B., Czempinski, K., et al. (2011). Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol. 156, 1783–1796. doi: 10.1104/pp.111.177816
Marcel, D., Müller, T., Hedrich, R., and Geiger, D. (2010). K+ transport characteristics of the plasma membrane tandem-pore channel TPK4 and pore chimeras with its vacuolar homologs. FEBS letters 584, 2433–2439. doi: 10.1016/j.febslet.2010.04.038
Marten, I., Hoth, S., Deeken, R., Ache, P., Ketchum, K. A., Hoshi, T., et al. (1999). AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. U.S.A. 96, 7581–7586.
Mäser, P., Thomine, S., Schroeder, J., Ward, J., Hirschi, K., Sze, H., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. doi: 10.1104/pp.126.4.1646
Michard, E., Dreyer, I., Lacombe, B., Sentenac, H., and Thibaud, J.-B. (2005a). Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 44, 783–797.
Michard, E., Lacombe, B., Poreìe, F., Mueller-Roeber, B., Sentenac, H., Thibaud, J.-B., et al. (2005b). A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J. Gen. Physiol. 126, 605–617.
Mikosch, M., Hurst, A. C., Hertel, B., and Homann, U. (2006). Diacidic motif is required for efficient transport of the K+ channel KAT1 to the plasma membrane. Plant Physiol. 142, 923–930. doi: 10.1104/pp.106.087064
Mikosch, M., Käberich, K., and Homann, U. (2009). ER export of KAT1 is correlated to the number of acidic residues within a triacidic motif. Traffic 10, 1481–1487. doi: 10.1111/j.1600-0854.2009.00962.x
Mouline, K., Veìry, A.-A., Gaymard, F., Boucherez, J., Pilot, G., Devic, M., et al. (2002). Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 16, 339–350. doi: 10.1101/gad.213902
Nakamura, R. L., McKendree, W. L., Hirsch, R. E., Sedbrook, J. C., Gaber, R. F., and Sussman, M. R. (1995). Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374. doi: 10.1104/pp.109.2.371
Nieves-Cordones, M., Caballero, F., Martínez, V., and Rubio, F. (2012). Disruption of the Arabidopsis thaliana inward-rectifier K+ channel AKT1 improves plant responses to water stress. Plant Cell Physiol. 53, 423–432. doi: 10.1093/pcp/pcr194
Pandey, S., Zhang, W., and Assmann, S. M. (2007). Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 581, 2325–2336. doi: 10.1016/j.febslet.2007.04.008
Philippar, K., Büchsenschutz, K., Abshagen, M., Fuchs, I., Geiger, D., Lacombe, B., et al. (2003). The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays. J. Biol. Chem. 278, 16973–16981.
Philippar, K., Ivashikina, N., Ache, P., Christian, M., Luĺthen, H., Palme, K., et al. (2004). Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana. Plant J. 37, 815–827. doi: 10.1111/j.1365-313X.2003.02006.x
Pilot, G., Gaymard, F., Mouline, K., Chèrel, I., and Sentenac, H. (2003). Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 51, 773–787.
Pilot, G., Lacombe, B., Gaymard, F., Cherel, I., Boucherez, J., Thibaud, J. B., et al. (2001). Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221.
Pyo, Y. J., Gierth, M., Schroeder, J. I., and Cho, M. H. (2010). High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 153, 863–875. doi: 10.1104/pp.110.154369
Rajan, S., Preisig-Muller, R., Wischmeyer, E., Nehring, R., Hanley, P. J., Renigunta, V., et al. (2002). Interaction with 14-3-3 proteins promotes functional expression of the potassium channels TASK-1 and TASK-3. J. Physiol. 545, 13–26. doi: 10.1113/jphysiol.2002.027052
Reintanz, B., Szyroki, A., Ivashikina, N., Ache, P., Godde, M., Becker, D., et al. (2002). AtKC1, a silent Arabidopsis potassium channelalpha-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. U.S.A. 99, 4079–4084.
Ren, X.-L., Qi, G.-N., Feng, H.-Q., Zhao, S., Zhao, S.-S., Wang, Y., et al. (2013). Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 74, 258–266. doi: 10.1111/tpj.12123
Reuff, M., Mikosch, M., and Homann, U. (2010). Trafficking, lateral mobility and segregation of the plant K+ channel KAT1*. Plant Biol. 12, 99–104. doi: 10.1111/j.1438-8677.2010.00355.x
Rocchetti, A., Sharma, T., Wulfetange, C., Scholz-Starke, J., Grippa, A., Carpaneto, A., et al. (2012). The putative K+ channel subunit AtKCO3 forms stable dimers in Arabidopsis. Front. Plant Sci. 3:251. doi: 10.3389/fpls.2012.00251.
Rubio, F., Alemaìn, F., Nieves-Cordones, M., and Martiìnez, V. (2010). Studies on ArabidopsisAtHAK5, AtAKT1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K uptake. Physiol. Plant. 139, 220–228. doi: 10.1111/j.1399-3054.2010.01354.x
Rubio, F., Nieves-Cordones, M., Alemaìn, F., and Martiìnez, V. (2008). Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant 134, 598–608. doi: 10.1111/j.1399-3054.2008.01168.x
Sandmann, M., Skłodowski, K., Gajdanowicz, P., Michard, E., Rocha, M., Gomez-Porras, J. L., et al. (2011). The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal. Behav. 6, 558–562.
Sato, A., Gambale, F., Dreyer, I., and Uozumi, N. (2010). Modulation of the Arabidopsis KAT1 channel by an activator of protein kinase C in Xenopus laevis oocytes. FEBS J. 277, 2318–2328. doi: 10.1111/j.1742-4658.2010.07647.x
Schachtman, D. P., Schroeder, J. I., Lucas, W. J., Anderson, J. A., and Gaber, R. F. (1992). Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258, 1654–1658. doi: 10.1126/science.8966547
Schönknecht, G., Spoormaker, P., Steinmeyer, R., Brüggemann, L., Ache, P., Dutta, R., et al. (2002). KCO1 is a component of the slow-vacuolar (SV) ion channel. FEBS Lett. 511, 28–32. doi: 10.1016/S0014-5793(01)03273-2
Schroeder, J. I., Ward, J. M., and Gassmann, W. (1994). Perspectives on the physiology and structure of inward-rectifying K+channels in higher plants: biophysical implications for K+ uptake. Annu. Rev. Biophys. Biomol. Struc. 23, 441–471.
Sentenac, H., Bonneaud, N., Minet, M., Lacroute, F., Salmon, J. M., Gaymard, F., et al. (1992). Cloning and expression in yeast of a plant potassium ion transport system. Science 256, 663–665. doi: 10.1126/science.1585180
Sieben, C., Mikosch, M., Brandizzi, F., and Homann, U. (2008). Interaction of the K+-channel KAT1 with the coat protein complex II coat component Sec24 depends on a di-acidic endoplasmic reticulum export motif. Plant J. 56, 997–1006. doi: 10.1111/j.1365-313X.2008.03658.x
Sottocornola, B., Gazzarrini, S., Olivari, C., Romani, G., Valbuzzi, P., Thiel, G., et al. (2008). 14-3-3 proteins regulate the potassium channel KAT1 by dual modes. Plant Biol. 10, 231–236. doi: 10.1111/j.1438-8677.2007.00028.x
Sottocornola, B., Visconti, S., Orsi, S., Gazzarrini, S., Giacometti, S., Olivari, C., et al. (2006). The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J. Biol. Chem. 281, 35735–35741.
Spalding, E. P., Hirsch, R. E., Lewis, D. R., Qi, Z., Sussman, M. R., and Lewis, B. D. (1999). Potassium uptake supporting plant growth in the absence of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. J. Gen. Physiol. 113, 909–918.
Sutter, J.-U., Campanoni, P., Tyrrell, M., and Blatt, M. R. (2006). Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18, 935–954. doi: 10.1105/tpc.105.038950
Sutter, J.-U., Sieben, C., Hartel, A., Eisenach, C., Thiel, G., and Blatt, M. R. (2007). Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17, 1396–1402.
Szyroki, A., Ivashikina, N., Dietrich, P., Roelfsema, M. R., Ache, P., Reintanz, B., et al. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. U.S.A. 98, 2917–2921.
Thiel, G., MacRobbie, E. A. C., and Blatt, M. B. (1992). Membrane transport in stomatal guard cells: the importance of voltage control. J. Membr. Biol. 126, 1–18.
Torres, M. A., and Dangl, J. L. (2005). Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8, 397–403.
Tripathi, V., Parasuraman, B., Laxmi, A., and Chattopadhyay, D. (2009). CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. Plant J. 58, 778–790. doi: 10.1111/j.1365-313X.2009.03812.x
Véry, A., Gaymard, F., Bosseux, C., Sentenac, H., and Thibaud, J. (1995). Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 7, 321–332. doi: 10.1046/j.1365-313X.1995.7020321.x
Véry, A., and Sentenac, H. (2003). Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54, 575–603. doi: 10.1146/annurev.arplant.54.031902.134831
Vitale, A., and Hinz, G. (2005). Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 10, 316–323.
Voelker, C., Gomez-Porras, J. L., Becker, D., Hamamoto, S., Uozumi, N., Gambale, F., et al. (2010). Roles of tandem-pore K+ channels in plants - a puzzle still to be solved. Plant Biol. 12(Suppl. 1), 56–63. doi: 10.1111/j.1438-8677.2010.00353.x
Voelker, C., Schmidt, D., Mueller-Roeber, B., and Czempinski, K. (2006). Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 48, 296–306. doi: 10.1111/j.1365-313X.2006.02868.x
Wang, P., and Song, C. P. (2008). Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 178, 703–718. doi: 10.1111/j.1469-8137.2008.02431.x
Wang, Y., and Wu, W.-H. (2010). Plant sensing and signaling in response to K+- deficiency. Mol. Plant 3, 280–287.
Wyn Jones, R. G., and Pollard, A. (1983). “Proteins, enzymes and inorganic ions,” in Encyclopedia of Plant Physiology, eds A. Lauchli and A. Pirson (Berlin: Springer), 528–562.
Xicluna, J., Lacombe, B., Dreyer, I., Alcon, C., Jeanguenin, L., Sentenac, H., et al. (2007). Increased functional diversity of plant K+ channels by preferential heteromerization of the Shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 282, 486–494.
Xu, J., Li, H.-D., Chen, L.-Q., Wang, Y., Liu, L.-L., He, L., et al. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125, 1347–1360. doi: 10.1016/j.cell.2006.06.011
Zanetti, M., Teardo, E., La Rocca, N., Zulkifli, L., Checchetto, V., Shijuku, T., et al. (2010). A novel potassium channel in photosynthetic cyanobacteria. PLoS ONE 5:e10118. doi: 10.1371/journal.pone.0010118
Zhao, L.-N., Shen, L.-K., Zhang, W.-Z., Zhang, W., Wang, Y., and Wu, W.-H. (2013). Ca2+-Dependent Protein Kinase11 and 24 Modulate the Activity of the Inward Rectifying K+ Channels in Arabidopsis Pollen Tubes. Plant Cell 25, 649–661. doi: 10.1105/tpc.112.103184
Keywords: plant potassium channel, Shaker, TPK, Kir-like, Arabidopsis thaliana, voltage-dependent, voltage-independent
Citation: Sharma T, Dreyer I and Riedelsberger J (2013) The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 4:224. doi: 10.3389/fpls.2013.00224
Received: 13 March 2013; Accepted: 09 June 2013;
Published online: 27 June 2013.
Edited by:
Sergey Shabala, University of Tasmania, AustraliaReviewed by:
Nava Moran, The Hebrew University of Jerusalem, IsraelIgor Pottosin, Universidad de Colima, Mexico
Copyright © 2013 Sharma, Dreyer and Riedelsberger. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Ingo Dreyer, Plant Biophysics, Centro de Biotecnología y Genómica de Plantas, Universidad Politécnica de Madrid, Campus de Montegancedo, Carretera M-40, km 37.7, Pozuelo de Alarcón, Madrid E-28223, Spain e-mail:aW5nby5kcmV5ZXJAdXBtLmVz;
Janin Riedelsberger, Molecular Biology, Institute for Biochemistry and Biology, University of Potsdam, Karl-Liebknecht-Str. 24/25, House 20, D-14476 Potsdam, Germany e-mail:amFuaW4ucmllZGVsc2JlcmdlckB1bmktcG90c2RhbS5kZQ==