
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 25 January 2013
Sec. Plant Proteomics and Protein Structural Biology
Volume 3 - 2012 | https://doi.org/10.3389/fpls.2012.00310
This article is part of the Research Topic Application of Proteomics for Improvement in Crop Protection/Artificial Regulation View all 19 articles
Modulation of plant proteome composition is an inevitable process to cope with the environmental challenges including heavy metal (HM) stress. Soil and water contaminated with hazardous metals not only cause permanent and irreversible health problems, but also result substantial reduction in crop yields. In course of time, plants have evolved complex mechanisms to regulate the uptake, mobilization, and intracellular concentration of metal ions to alleviate the stress damages. Since, the functional translated portion of the genome plays an essential role in plant stress response, proteomic studies provide us a finer picture of protein networks and metabolic pathways primarily involved in cellular detoxification and tolerance mechanism. In the present review, an attempt is made to present the state of the art of recent development in proteomic techniques and significant contributions made so far for better understanding the complex mechanism of plant metal stress acclimation. Role of metal stress-related proteins involved in antioxidant defense system and primary metabolism is critically reviewed to get a bird’s-eye view on the different strategies of plants to detoxify HMs. In addition to the advantages and disadvantages of different proteomic methodologies, future applications of proteome study of subcellular organelles are also discussed to get the new insights into the plant cell response to HMs.
High-throughput OMICS techniques are extensively being exploited in recent times to dissect plants molecular strategies of heavy metals (HMs) stress tolerance. Plants growing in HMs contaminated environment have developed coordinated homeostatic mechanisms to regulate the uptake, mobilization, and intracellular concentration of toxic metal ions to alleviate stress damages. As the functional translated portion of the genome play a key role in plant stress response, proteomic studies provide us a finer picture of protein networks and metabolic pathways primarily involved in cellular detoxification and tolerance mechanism against HM toxicity.
By definition, elements having specific gravity above five are considered as HMs. Nevertheless, the term HM commonly refers to toxic metals, e.g., cadmium (Cd), copper (Cu), chromium (Cr), lead (Pb), zinc (Zn) as well as hazardous metalloids viz., arsenic (As), boron (B), which exert negative effects on plant growth and development (Hossain et al., 2012a).
Most of the HMs get their entry into plant root system via specific/generic ion carriers or channels (Bubb and Lester, 1991). The lack of specificity of transporters that are primarily involved in uptake of essential elements such as Zn2+, Fe2+, and Ca2+ allow the entry of Cd2+, Pb2+ (Welch and Norvell, 1999; Perfus-Barbeoch et al., 2002). Once HM ions enter the cell, cellular functions are affected by a wide range of actions. The negative impact of HM includes binding of HM ions to sulfhydryl groups of proteins, replacement of essential cations from specific binding sites, leading to enzyme inactivation and production of reactive oxygen species (ROS), resulting in oxidative damages to lipids, proteins and nucleic acids (Sharma and Dietz, 2009).
Over the last decade, extensive research on plants response to HM stress has been conducted to unravel the tolerance mechanism. Genomics technologies have been useful in addressing plant abiotic stress responses including HM toxicity (Bohnert et al., 2006). However, changes in gene expression at transcript level have not always been reflected at protein level (Gygi et al., 1999). An in-depth proteomic analysis is thus of great importance to identify target proteins that actively take part in HM detoxification mechanism.
Plant response to HM stress has been reviewed extensively over the past decade (Sanita Di Toppi and Gabbrielli, 1999; Cobbett, 2000; Ma et al., 2001; Cobbett and Goldsbrough, 2002; Hall, 2002; Maksymiec, 2007; Sharma and Dietz, 2009; Verbruggen et al., 2009; Yang and Chu, 2011; Hossain et al., 2012a). However, review articles on application of proteomics in analyzing cellular mechanism for HM tolerance are limited (Ahsan et al., 2009; Luque-Garcia et al., 2011; Villiers et al., 2011).
Current review represents the state of art of recent developments in proteomic techniques and significant contributions made so far to strengthen our knowledge about plants HM-stress response cascade at protein level. Special emphasis is given to highlight the role of metal stress-related proteins engage in HM ions sequestration, antioxidant defense system, and primary metabolism for deeper understanding of coordinated pathways involve in detoxification of HM ions within plant cells. Furthermore, future applications of proteome study of subcellular organelles are discussed to get the new insights into the plant cell response to HMs.
Conventional two-dimensional gel electrophoresis (2-DE) approach coupled with protein identification by mass spectrometry (MS) has been the most widely used proteomic technique for investigation of HM-induced alteration of plant proteome composition (Table 1). Protein extraction and purification from the HM-stressed tissue is the most crucial step in 2-DE approach, as the amount and quality of the extracted proteins ultimately determine the protein spot number, resolution, and intensity. Phenolic compounds, proteolytic and oxidative enzymes, terpenes, pigments, organic acids, inhibitory ions, and carbohydrates are some common interfering substances present in recalcitrant plant tissues. Inferior 2-D separation results due to proteolytic breakdown, streaking, and charge heterogeneity. Proteomic studies on plant response against HM stress have revealed that trichloroacetic acid/acetone precipitation (Patterson et al., 2007; Zhen et al., 2007; Kieffer et al., 2008; Alves et al., 2011; Hossain et al., 2012b,2012c) and phenol-based (Bona et al., 2007; Alvarez et al., 2009; Vannini et al., 2009; Lee et al., 2010; Ritter et al., 2010; Rodríguez-Celma et al., 2010; Ahsan et al., 2012; Sharmin et al., 2012) protocols are the effective protein extraction methods for obtaining high quality proteome map. Nevertheless, phenol-based method is the most appropriate in extracting glycoproteins, and produce high-resolution proteome map for recalcitrant plant tissues (Saravanan and Rose, 2004; Komatsu and Ahsan, 2009).
As compared to classical staining procedure of 2-DE gel using CBB or silver staining, advanced fluorescence two-dimensional difference gel electrophoresis (2-D DIGE) proteomic approach is now being used which allows comparison of the differentially expressed proteins of control and HM-stressed tissue on one single gel (Kieffer et al., 2008; Alvarez et al., 2009). DIGE is basically a gel-based method where proteins were labeled with fluorescent dyes (CyDyes – Cy2, Cy3, and Cy5) prior to electrophoresis. With the advancement of technology multiplexed isobaric tagging (iTRAQ) of peptides has allowed comparative, quantitative analysis of multiple samples. This second generation gel free proteomic approach has been well exploited for gaining comprehensive understanding of plants response to Cd and B (Patterson et al., 2007; Alvarez et al., 2009; Schneider et al., 2009).
In course of time, higher plants have evolved sophisticated mechanisms to regulate the uptake, mobilization, and intracellular concentration of HM ions (Figure 1). Apart from the plasma membrane exclusion method, the most common way to protect the cell from the adverse effects of HMs includes synthesis of membrane transporters and thiol-containing chelating compounds for vacuolar sequestration. Furthermore, increased abundance of defense proteins for effective ROS scavenging and molecular chaperones for re-establishing normal protein conformation help HM-stressed plants to maintain redox homeostasis. Modulations of vital metabolic pathways – photosynthesis and mitochondrial respiration – further help the stressed plant to produce more reducing power to compensate high-energy demand of HM challenged cells.
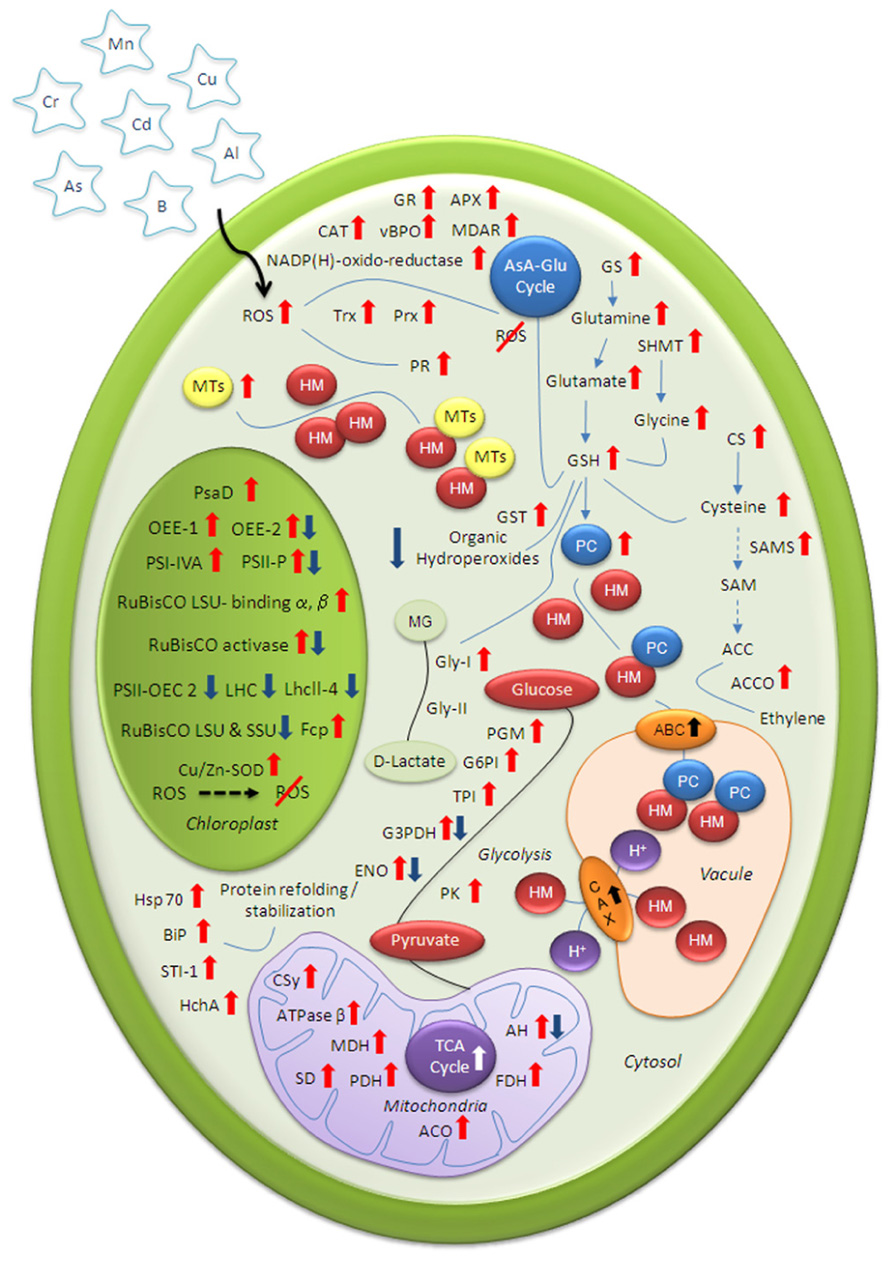
FIGURE 1. Schematic illustration of various cellular mechanisms for mitigating heavy metal (HM) stress. Information about highlighted proteins gathered from published proteomic articles related to plant HM-toxicity. Up and down arrows indicate HM-induced increase and decrease protein abundance respectively. ATPase β, ATP synthase subunit beta; AH, aconitate hydratase; AsA-Glu, ascorbate glutathione; APX, ascorbate peroxidase; ACC, 1-aminocyclopropane-1-carboxylic acid; ACO, aconitase; CAT, catalase; CAX, cation/proton exchanger; CS, cysteine synthase; CSy, citrate synthase; ENO, enolase; FDH, formate dehydrogenase; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; GR, glutathione reductase; Gly-I, glyoxalase I; GS, glutamine synthetase; GSH, reduced glutathione; LHC, light harvesting complex; LhcII-4, light-harvesting chlorophyll-a/b binding protein; LSU, large subunit; MTs, metallothioneins; MG, methylglyoxal; MDAR, monodehydroascorbate reductase; MDH, malate dehydrogenase; OEE, oxygen-evolving enhancer protein; PC, Phytochelatin; Prx, peroxidoxin; PR, pathogenesis-related; PDH, pyruvate dehydrogenase; PSII-OEC 2, photosystem II oxygen-evolving complex protein 2; PS, photosystem; ROS, reactive oxygen species; RuBisCO, ribulose-1,5-bisphosphate carboxylase oxygenase; SD, succinate dehydrogenase; SAM, S-adenosylmethionine; SSU, small subunit; Trx, thioredoxin; TPI, triosephosphate isomerase; TCA, tricarboxylic acid.
One of the important plant strategies of detoxifying HMs within cell is to synthesize low molecular weight chelators to minimize the binding of metal ions to functionally important proteins (Verbruggen et al., 2009). The thiol-containing chelating compounds strongly interact with HM, thus reducing free HM ions from cytosol and hence limiting HM toxicity (Cobbett and Goldsbrough, 2002). The phytochelatins (PCs) and metallothioneins (MTs), the two best characterized cysteine-rich HM binding protein molecules, play crucial roles in HM tolerance mechanism (Cobbett and Goldsbrough, 2002).
Phytochelatins synthesized from glutathione (GSH) by the enzyme PC synthase readily form complexes with HM in the cytosol and to facilitate their transport into vacuoles (Grill et al., 1989; Figure 1). Although PCs synthesis found to be induced in presence of most of the studied HMs, modulation of proteins, amino acids involved in PC biosynthesis have been the most widely studied in response to Cd. Our recent comparative proteome analysis of high and low Cd accumulating soybeans has revealed enhanced expression of glutamine synthetase (GS) under Cd stress. The enzyme GS is involved in the synthesis of GSH through glutamate biosynthesis pathway (Sarry et al., 2006; Semane et al., 2010). The enhanced expression of GS leads to more GSH formation (Hossain et al., 2012b). Induction of GSH synthesis implies higher metal binding capacity as well as enhanced cellular defense mechanism against oxidative stress (Verbruggen et al., 2009). Since GSH is the precursor of PC, enhanced expression of GS helps the cell to synthesize and accumulate more PC to detoxify cytosolic Cd2+. Our finding is in agreement with previous reports of up-regulation of GS in response to Cd (Kieffer et al., 2008; Hradilova et al., 2010; Semane et al., 2010; Ahsan et al., 2012). In contrast, sharp decline in GS abundance has been reported in Cd-stressed rice roots (Lee et al., 2010).
Ahsan et al. (2012) exploited proteomic technique in combination of metabolomics for deeper understanding of PC-mediated detoxification of Cd2+ in soybean roots. Comparative analysis revealed that proteins (GS beta 1) and amino acids (glycine, serine, glutamic acid) associated with Cd chelating pathways are highly active in low root-to-shoot Cd translocating cultivar. In addition, proteins involved in lignin biosynthesis were shown to be increased under stress. Proteomic findings suggest that translocation of Cd ions from the root to the aerial parts might be prevented by the increased xylem lignifications.
The PC biosynthetic pathway has been finely dissected in Cd-exposed Arabidopsis thaliana cells using protein and metabolite profiling (Sarry et al., 2006). At high Cd concentration global pool of GSH decreased dramatically with the increase in dipeptide γGlu-Cys, suggesting high cellular demand of GSH for sustaining PC [(γGlu-Cys)n-Gly] synthesis.
Alvarez et al. (2009) implemented two quantitative proteomics approaches – 2-D DIGE and iTRAQ – to find out the relation between Cd2+ sequestration and thiol metabolism. Both techniques identified an increased abundance of proteins involved in sulfur metabolism. Sulfite reductase and O-acetylserine sulfhydrylase, involved in reduction of sulfate to cysteine, were found to be overexpressed in Cd-treated Brassica juncea roots. Authors suggested that under Cd-stress, sulfate availability for synthesis of PCs and GSH may limit Cd tolerance. Significant inductions of GSH and PCs (PC3) in Cd-stressed rice roots further confirm the role of thiol-peptides in HM tolerance mechanism (Aina et al., 2007). Another proteomic study by Pandey et al. (2012) revealed higher abundance of cysteine synthase (CS) with higher contents of PCs and higher transcript of PC synthase in arsenic stressed Anabaena indicating their positive roles in As sequestration. Arsenic induced increases in GSH and PCs were also recorded in fronds of arsenic hyperaccumulator Pteris vittata (Bona et al., 2011). Interestingly, no such increase was evident in roots under As treatment. Proteomic results indicate that PCs could play role in As detoxification in P. vittata fronds only, but overall PC mediated detoxification is not the primary mechanism of As-tolerance in As hyperaccumulator, but to other adaptive mechanism. Up-regulation of proteins (CS and GSTs) and GSH pool involved in As detoxification has also been documented in proteomic study of As-stressed rice roots (Ahsan et al., 2008). Apart from Cd and As stress, CS and GSH also play essential role in Al adaptation for rice (Yang et al., 2007) and soybean (Zhen et al., 2007).
Unlike PCs, proteomics-based report on HM-induced alterations of MTs is very limited. Zhang et al. (2009) for the first time identified MT-like proteins from Cu-stressed germinating rice seed embryo. A number of gene expression studies have shown that MT genes are involved in Cu homeostasis and tolerance in Arabidopsis (Murphy and Taiz, 1995) and Silene vulgaris (van Hoof et al., 2001). Plant MTs not only play vital role in chelating Cu through the Cys thiol groups but are also considered as a potent scavenger of ROS (Cobbett and Goldsbrough, 2002; Wang et al., 2004).
The final step of HM detoxification involves sequestering of either free HMs or PCs-HMs complexes into cell vacuoles (Hall, 2002). This accumulation is mediated by tonoplast-bound cation/proton exchanger, P-type ATPase and ATP-dependent ABC transporter (Salt and Rauser, 1995; Hall, 2002). Transporters are also situated in plasma membrane and facilitate transport of HMs into apoplast. As the vacuoles or apoplasts have limited metabolic activity, accumulations of HMs in these compartments reduce the toxic effects of HMs (Schneider et al., 2009). The iTRAQ analysis of Cd-exposed barley leaf mesophyll tonoplast proteome led to the identification of ~50 vacuolar transporters, that include vacuolar ATPase subunits, MRP-like ABC transporter and two novel CAX transporters (CAX1a and CAX5) and one Al-activated malate transporter protein (Schneider et al., 2009). Induction of these transporters especially cation/proton exchanger 1a and ABC transporter assure Cd2+ transport into vacuole (Aina et al., 2007). Further proteomic study by Lee et al. (2010) revealed induction of vacuolar proton-ATPase in rice roots and leaves indicating their positive role in Cd detoxification through vacuolarisation.
Cellular ROS generation gets accelerated upon exposure to HM stress. HMs (Cu, Fe, Cr) that are directly involved in cellular redox reaction lead to ROS generation known as redox active, while redox inactive HMs (Cd, Al, As, Ni) trigger oxidative stress by depleting cells major thiol-containing antioxidants and enzymes, disrupting electron transport chain or by inducing lipid peroxidation (Ercal et al., 2001; Hossain et al., 2012a). The excess intracellular ROS level alters protein structure by inducing oxidation of both protein backbone and amino acid side chain residues (Villiers et al., 2011). To counter stress, plants have evolved robust antioxidant defense mechanism comprised of both enzymatic and non-enzymatic components (Hossain et al., 2012d).
Most of the proteomic research done so far on HM-related toxicity revealed positive correlation between tolerance and increased abundance of scavenger proteins. Within plant cells, SOD constitutes the first line of defense against ROS. It plays pivotal role in cellular defense against oxidative stress, as its activity directly modulates the amount of and H2O2, the two important Haber–Weiss reaction substrates. The excess generated under HM-stress usually disproportionate into H2O2 by the action of SOD, which is then metabolized by the components of the ascorbate–GSH cycle. Higher expressions of SOD isoforms (Cu/Zn-SOD, Fe-SOD) have been documented in plants exposed to excess Cd (Kieffer et al., 2008,2009; Alvarez et al., 2009; Farinati et al., 2009; Semane et al., 2010; Hossain et al., 2012b) and Al (Yang et al., 2007). Interestingly, root proteome analysis of Cd-exposed B. juncea revealed up-regulation of Fe-SOD while down regulation of Cu/Zn SOD (Alvarez et al., 2009). Ascorbate peroxidase (APX), peroxidase (POD), and catalase (CAT) are involved in scavenging H2O2, hence protecting cell membrane from hydroxyl radical-induced lipid peroxidation (Barber and Thomas, 1978). The scavenging roles of APX, POD, and CAT have been documented in several proteomic studies related to Cd stress (Sarry et al., 2006; Aina et al., 2007; Kieffer et al., 2008; Lee et al., 2010; Hossain et al., 2012b) and As (Pandey et al., 2012) toxicity. Interestingly, excessive Cu (Bona et al., 2007), Cr (Sharmin et al., 2012) treatments or B deficiency (Alves et al., 2011) lead to decreased abundance of APX and POD. The detected suppression of POD is in accordance with the decrease in POD reported in maize roots treated with Al (Wang et al., 2011).
The abundance of another antioxidant enzyme of ascorbate–GSH cycle, monodehydroascorbate reductase (MDAR) was found to be increased in response to Cd (Sarry et al., 2006; Alvarez et al., 2009). MDAR helps to scavenge monodehydroascorbate radical and by doing this it generates dehydroascorbate (DHA), the oxidized form of ascorbate. Up-regulation of MDAR assures production of DHA, the substrate of dehydroascorbate reductase (DHAR) enzyme that catalyzes reduction of DHA to AsA (reduced ascorbate). In contrary, shoot proteome analysis of Arabidopsis halleri has shown decreased expression of MDAR in response to Cd, Zn, and rhizosphere microorganisms (Farinati et al., 2009). This down-regulation is also evident in roots of Lupinus albus undergoing long-term B deficiency (Alves et al., 2011). Decreased MDAR abundance in HM-stressed plants might indicate non-enzymatic disproportionation of monodehydroascorbate into AsA, essential for maintenance of balanced redox status (Hossain et al., 2009). Yet another well documented antioxidant found to be up-regulated under HM stress is peroxiredoxin (Prx). The Prx is basically a thiol peroxidase with multiple functions. It (a) detoxifies hydroperoxides; (b) plays essential role in enzyme activation and redox sensoring; (c) acts as molecular chaperone similar to HSPs; (d) induces cell signaling (; Jang et al., 2004; Barranco-Medina et al., 2009). Prx was found to be induced under Cd (Sarry et al., 2006; Ahsan et al., 2007a; Hossain et al., 2012b) and As (Requejo and Tena, 2006; Pandey et al., 2012) stress.
Plants are also equipped with some additional defense proteins, shown to be up-regulated by HM stress. This group includes thioredoxin (Trx), Trx-dependent peroxidase, NADP(H)-oxido-reductase and glyoxylase I (Gly I). Trx is known to suppress apoptosis as well as supplies reducing equivalents to antioxidants (Hishiya et al., 2008). Excess Cu treatment seems to down-regulate the abundance of Trx and Trx-POD in germinating rice embryo (Zhang et al., 2009) and Cannabis sativa roots (Bona et al., 2007) respectively. However, enhanced expression of Trx was found to be helpful in mitigating oxidative stress in As-treated Anabaena (Pandey et al., 2012).
Methylglyoxal (MG), a cytotoxic by-product of glycolysis generally accumulated in cell in response to environmental stresses including HM (Espartero et al., 1995). MG readily interacts with nucleic acids and proteins causing alteration of function (Yadav et al., 2005). Detoxification of MG through glyoxalase pathway involves active participation of GSH and Gly I and Gly II enzymes. Up-regulation of Gly I was found to help the germinating rice seedlings in detoxifying MG under Cd (Ahsan et al., 2007a) and Cu (Ahsan et al., 2007b). Higher Gly I abundance was also reported in Cd + Zn + microorganisms treated A. halleri shoots (Farinati et al., 2009). Proteomic study also highlighted enhanced expression of NADP(H)-oxido-reductase by Cd (Sarry et al., 2006; Lee et al., 2010) and As (Pandey et al., 2012). This protein is a vital component of plants second line of defense, protecting cells from HM-induced oxidative damages.
Plants tolerance against HMs is often attributed to steady state of GSH pool for its multifunctional activities in PC synthesis, MG detoxification, ROS scavenging through ascorbate–GSH cycle, GSTs mediated decomposition of toxic compounds as well as stress signaling (Figure 1). Within GSH cycle, glutathione reductase (GR) acts as a rate limiting enzyme that catalyzes reduction of oxidized glutathione (GSSG) to GSH (reduced glutathione) and with the help of DHAR it maintains high AsA/DHA ratio necessary for tight control of HM-induced ROS scavenging. The delicate balance between GSH and GSSG is critical for keeping a favorable redox status for the detoxification of H2O2. Higher abundance of GSTs has been observed in response to Cd (Alvarez et al., 2009; Lee et al., 2010), As (Ahsan et al., 2008; Pandey et al., 2012), Cu (Zhang et al., 2009). Findings of Ahsan et al. (2008) revealed increased activity of GST-omega in rice roots following exposure of AsV, indicating the probable role of GST-omega in inorganic arsenic biotransformation and metabolism. The authors also suggested that depletion in GSH content may be associated with high rate of PCs synthesis thus detoxification of As through compartmentalization or due to down-regulation of enzymes of GSH biosynthetic pathways such as GR and CS. The HM-induced PCs synthesis coupled with GSH depletion is in agreement with earlier studies by Sarry et al. (2006) and Di Baccio et al. (2005).
Proteomic analyses strongly indicate that accumulation of defense proteins chiefly enzymatic components of ascorbate–GSH cycle, POD, CAT, GSH, GSTs, Gly I, Prx, Trx help cells to mitigate HM-induced oxidative stress by scavenging ROS.
Protein dysfunction is an inevitable consequence of a wide range of adverse environmental conditions including HM toxicity. Molecular chaperones/heat-shock proteins (HSPs) are responsible for protein stabilization, proper folding, assembly, and translocation under both optimum and adverse growth conditions (Wang et al., 2004). In our study, enhanced abundance (>2-fold) of HSP70 protein was detected in leaves of high Cd-accumulating soybean cultivar Harosoy while low Cd-accumulating cv. Fukuyutaka exhibited decreased expression (Hossain et al., 2012b). Cd-induced up-regulation of HSP70 is also evident in response to various HMs including Cd (Kieffer et al., 2009; Hradilova et al., 2010; Rodríguez-Celma et al., 2010), Cr (Sharmin et al., 2012), and B deficiency (Alves et al., 2011). Ahsan et al. (2007a) reported increase of DnaK-type molecular chaperone BiP and chaperone protein HchA in germinating rice seedlings exposed to acute Cd toxicity. Al-stress also is known to induce one LMW-HSP and three DnaJ-like proteins in Al-stressed soybean (Zhen et al., 2007). To sum up, HSPs/chaperones play pivotal role in combating HM stress by re-establishing normal protein conformation and hence, cellular homeostasis.
Down-regulation of photosynthetic machinery is a known phenomenon of Cd stress. Low abundance of proteins involved in photosynthetic electron transport chain and Calvin cycle has been reported in Cd-exposed Populus (Kieffer et al., 2008,2009; Durand et al., 2010) and Thlaspi (Tuomainen et al., 2006). Pioneer proteomic work by Hajduch et al. (2001) of rice leaves exposed to HMs revealed drastic reduction in abundance/fragmentation of large and small subunits of RuBisCO (LSU and SSU), suggesting complete disruption of photosynthetic machinery by HM stress. This decrease in RuBisCO has also been documented in other HMs toxicity like As (Duquesnoy et al., 2009) and Cd (Kieffer et al., 2008). Proteomic analysis for other toxic HMs like As-exposed leaf proteome of Agrostis tenuis has shown total disruption of RuBisCO LSU and SSU along with oxygen-evolving enhancer protein 1 and oxygen evolving protein 2 in response to 134 μM As(V) treatment (Duquesnoy et al., 2009). Potassium dichromate treatment had similar effects on algal RuBisCO LSU and some antenna proteins namely light harvesting Chl a/b protein complex. However, Vannini et al. (2009) reported higher abundance of RuBisCO activase in Pseudokirchneriella subcapitata under chromate treatment. Interestingly, in our proteomic experiment with Cd-exposed soybean, increased abundance of RuBisCO LSU-binding protein subunits alpha and beta, RuBisCO activase, oxygen-evolving enhancer protein 1 and 2, NAD(P)H-dependent oxido-reductase, photosystem I and II-related proteins were evident (Hossain et al., 2012b). Enhanced expressions of proteins involved in photosystem I, II, and Calvin cycle might be an adaptive feature to overcome the Cd injury in soybean. This increased abundance is in accordance with the findings of Semane et al. (2010), who also reported increase of photosynthetic protein abundance in leaves of Arabidopsis treated with mild Cd stress. In our opinion, contribution of high photosynthetic assimilates into respiration would help plants to yield more energy needed to combat the Cd2+ stress.
To maintain the normal growth and development under stressed environment, plants need to up regulate metabolic pathways such as glycolysis and tricarboxylic acid (TCA) cycle. Detailed analysis of HM toxicity-related proteomic works has shown higher abundance of glycolytic enzymes phosphoglycerate mutase (PGM), glucose-6-phosphate isomerase (G6PI), triose phosphate isomerase (TPI), glyceraldehyde-3-phosphate dehydrogenase (G3PDH), enolase (ENO), and pyruvate kinase (PK) in response to Cd (Sarry et al., 2006; Kieffer et al., 2008; Rodríguez-Celma et al., 2010; Hossain et al., 2012b), Cr (Labra et al., 2006). However, down-regulation of G3PDH was reported in As-treated rice roots (Ahsan et al., 2008) and roots of Lupinus albus under B deficiency (Alves et al., 2011). Similarly, Cu-treated Cannabis roots exhibited down-regulation of another glycolytic enzyme ENO, the metalloenzyme that catalyzes penultimate step of glycolysis – conversion of 2-phosphoglycerate to phosphoenolpyruvate (Bona et al., 2007).
Like glycolysis, enzymes of TCA cycle citrate synthase (CS), succinate dehydrogenase (SD), malate dehydrogenase (MDH), aconitase (ACO), aconitate hydratase (AH) were found to be up-regulated under Cd stress (Sarry et al., 2006; Kieffer et al., 2009; Rodríguez-Celma et al., 2010; Semane et al., 2010; Hossain et al., 2012b; Figure 1). In contrast, suppressions of several AH isoforms were evident in long-term B deficiency (Alves et al., 2011). Overall, up-regulation of glycolysis and TCA cycle might help the stressed plant to produce more reducing power to compensate high-energy demand of HM challenged cell.
Plant cells trigger some common defense machineries whenever they encounter a biotic or abiotic stress. Accumulation of PR proteins is one of such plant defense strategies and often associated with systemic acquired resistance (SAR) against a wide range of pathogens (Van Loon, 1997; Durrant and Dong, 2004). Using the 2-DE approach, Elvira et al. (2008) successfully identified different PR protein isoforms (viz. PR-1, β-1,3-glucanases PR-2, chitinases PR-3, osmotin-like protein PR-5, peroxidases PR-9, germin-like protein PR-16, and NtPRp27-like protein PR-17) in Capsicum chinense leaves and additionally resolved their specific accumulation pattern in both the compatible and incompatible PMMoV–C. chinense interactions. Apart from the assigned role in plant defense against pathogenic constraints, PR proteins also play key role in adaptation to stressful environments including HM toxicity (Hensel et al., 1999; Rakwal et al., 1999; Van Loon and Van Strien, 1999; Hajduch et al., 2001; Akiyama et al., 2004; Edreva, 2005). Kieffer et al. (2008) documented marked increase in abundance of PR proteins class I chitinases (PR-3 family), several β-1,3-glucanases (PR-2 family), and thaumatin-like protein (PR-5 family) in Cd-exposed poplar leaves. Endo-1,3-beta-glucanase, a class 2 PR protein, also found to be induced in rice roots under short-term Cd stress (Lee et al., 2010). Higher abundance of PR proteins under HM as documented in many proteomic studies is in accordance with previous transcriptomic analysis of mercuric chloride-treated Zea mays leaves (Didierjean et al., 1996). Like Cd stress, PR-10 and LIR18B protein (both belong to PR-10 family), and an acidic chitinase (PR-8 family) were de novo expressed under B deficiency (Alves et al., 2011). Stress-induced increase in ROS level has been shown to induce PR protein accumulation (Jwa et al., 2006). Treatment with excess Cu increased abundance of two PR proteins (PR-10a and putative PR proteins) in germinating rice embryos (Zhang et al., 2009). Analysis of the Vigna unguiculata leaf apoplast proteome using 2-DE and LC-MS/MS also revealed accumulation of several PR-like proteins glucanase, chitinase, and thaumatin-like proteins in response to excess Mn supply (Fecht-Christoffers et al., 2003). Transgenic tobacco overexpressing pepper gene CABPR1 encoding basic PR-1 protein showed enhanced resistance against HMs as well as pathogen stresses (Sarowar et al., 2005). These transgenic lines exhibited significant decline in total POD activity, suggesting that overexpression of CABPR1 in tobacco cells altered redox balance. Although, the precise role of PR proteins in combating HM stress is not yet clearly understood, the authors suggested that the induced redox imbalance might lead to H2O2 accumulation, triggering stress tolerance cascade. Several in vitro experiments have demonstrated that PR proteins display additional functions related to growth and development by modulating signal molecules (Kasprzewska, 2003; Liu and Ekramoddoullah, 2006). However, further proteomic investigations need to be undertaken to resolve the underlying molecular mechanism of PR proteins mediated plants HM tolerance.
The present review outlines the impact of HMs stresses on plant proteome constituents. Most of the investigations done so far primarily highlighted the differential expression of proteins involved in plant defense and detoxification pathways, namely ROS scavenging, chelation, compartmentalization. In addition, accumulation of PR proteins and modulation of plants vital metabolic pathways CO2 assimilation, mitochondrial respiration in maintaining steady state of reducing power and energy required for combating HM-induced stress has been discussed in detail. Careful analysis of published proteomic works on HM toxicity has revealed that classical 2-DE coupled with MS-based protein identification has been the most widely used proteomic technique in investigating plant HM tolerance at organ/whole plant level. These proteomic findings have enriched us for deeper understanding plants HM tolerance mechanism.
The cellular mechanism of sensing stress and transduction of stress signals into the cell organelle represent the initial reaction of plant cells toward any kind of stress including HM. Communication through intracellular compartments plays a significant role in stress signal transduction process that finally activates defense gene cascade (Hossain et al., 2012d). To dissect the underlying molecular mechanism of how a plant cell modulates its protein signature to cope with stress, in depth study on organelle proteome would be of great contribution toward development of HM-tolerant crops.
As the PCs mediated HM-ion detoxification pathway ends in sequestration of PC-HM complexes into vacuole through various transporter proteins present in tonoplast membrane, more research on vacuole proteome needs to be undertaken for identification and characterization of novel metal transporter proteins responsible for cytoplasmic efflux of transition metal cations into vacuole. Legendary work by Schneider et al. (2009) on quantitative detection of changes in barley leaf mesophyll tonoplast proteome using advanced gel free iTRAQ method has enriched our knowledge about contribution of vacuolar transporters to Cd2+ detoxification. Plasma membrane proteome should be another target of future proteomic research on HM stress, as it acts as a primary interface between the cellular cytoplasm and the extracellular environment, thus playing a vital role in stress signal perception and transduction. Furthermore, transporter proteins present in cell membrane have importance in up-taking HM-ions into the cell. As most of the organelle membrane proteins are hydrophobic in nature, MS-based gel free system would be the most promising technique for identification of such proteins.
Plants response to multiple HMs would be another interesting area of future proteomic research (Sharma and Dietz, 2009). This could shed some light on cross talk between different HM stress signal pathways.
Heavy metal-induced protein oxidation study through redox proteomic approach has been the focus of much interest. More initiatives in this topic need to be taken as PTM/redox modification of proteins provides fundamental information about HM toxicity mechanism and biomarker discovery (Dowling and Sheehan, 2006; Braconi et al., 2011).
In summary, we believe that more research on sub-proteome-based HM approach would provide new insights into plants HM-stress response mechanism. HM-induced novel marker proteins would further enable us to design HM-tolerant transgenic crops.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thankfully acknowledge support from the Department of Science and Technology, Government of India, through DST-BOYSCAST Fellowship Programme and National Agriculture and Food Research Organization, Japan.
CBB, coomassie brilliant blue; 2-DE, two-dimensional polyacrylamide gel electrophoresis; GS, glutamine synthetase; GSH, glutathione; GST, glutathione S-transferase; IEF, isoelectric focusing; LC, liquid chromatography; MS, mass spectrometry; MTs, metallothioneins; PCs, phytochelatins; pI, isoelectric point; PR, pathogenesis related; ROS, reactive oxygen species; SOD, superoxide dismutase.
Ahsan, N., Nakamura, T., and Komatsu, S. (2012). Differential responses of microsomal proteins and metabolites in two contrasting cadmium (Cd)-accumulating soybean cultivars under Cd stress. Amino Acids 42, 317–327.
Ahsan, N., Lee, D. G., Alam, I., Kim, P. J., Lee, J. J., Ahn, Y. O., et al. (2008). Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 8, 3561–3576.
Ahsan, N., Lee, D. G., Kim, K. H., Alam, I., Lee, S. H., Lee, K. W., et al. (2010). Analysis of arsenic stress-induced differentially expressed proteins in rice leaves by two-dimensional gel electrophoresis coupled with mass spectrometry. Chemosphere 78, 224–231.
Ahsan, N., Lee, D. G., Lee, S. H., Kang, K. Y., Lee, J. J., Kim, P. J., et al. (2007b). Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67, 1182–1193.
Ahsan, N., Lee, S. H., Lee, D. G., Lee, H., Lee, S. W., Bahk, J. D., et al. (2007a). Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. C. R. Biol. 330, 735–746.
Ahsan, N., Renaut, J., and Komatsu, S. (2009). Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 9, 2602–2621.
Aina, R., Labra, M., Fumagalli, P., Vannini, C., Marsoni, M., Cucchi, U., et al. (2007). Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ. Exp. Bot. 59, 381–392.
Akiyama, T., Pillai, M. A., and Sentoku N. (2004). Cloning, characterization and expression of OsGLN2, a rice endo-1,3-beta-glucanase gene regulated developmentally in flowers and hormonally in germinating seeds. Planta 220, 129–139.
Alvarez, S., Berla, B. M., Sheffield, J., Cahoon, R. E., Jez, J. M., and Hicks, L. M. (2009). Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. Proteomics 9, 2419–2431.
Alves, M., Moes, S., Jenö, P., Pinheiro, C., Passarinho, J., and Ricardo, C. P. (2011). The analysis of Lupinus albus root proteome revealed cytoskeleton altered features due to long-term boron deficiency. J. Proteomics 74, 1351–1363.
Barber, D. J. W., and Thomas, J. K. (1978). Reactions of radicals with lecithin bilayers. Radiat. Res. 74, 51–65.
Barranco-Medina, S., Lázaro, J. J., and Dietz, K. J. (2009). The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 583, 1809–1816.
Bohnert, H. J., Gong, Q., Li, P., and Ma, S. (2006). Unraveling abiotic stress tolerance mechanisms – getting genomics going. Curr. Opin. Plant Biol. 9, 180–188.
Bona, E., Marsano, F., Massa, N., Cattaneo, C., Cesaro, P., Argese, E., et al. (2011). Proteomic analysis as a tool for investigating arsenic stress in Pteris vittata roots colonized or not by arbuscular mycorrhizal symbiosis. J. Proteomics 74, 1338–1350.
Bona, E., Marsano, F., Cavaletto, M., and Berta, G. (2007). Proteomic characterization of copper stress response in Cannabis sativa roots. Proteomics 7, 1121–1130.
Braconi, D., Bernardini, G., and Santucci, A. (2011). Linking protein oxidation to environmental pollutants: redox proteomic approaches. J. Proteomics 74, 2324–2337.
Bubb, J. M., and Lester, J. N. (1991). The impact of heavy metals on lowland rivers and the implications for man and the environment. Sci. Total Environ. 100, 207–233.
Cobbett, C. S. (2000). Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123, 825–832.
Cobbett, C., and Goldsbrough, P. (2002). Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53, 159–182.
Di Baccio, D., Kopriva, S., Sebastiani, L., and Rennenberg, H. (2005). Does glutathione metabolism have a role in the defence of poplar against zinc excess? New Phytol. 167, 73–80.
Didierjean, L., Frendo, P., Nasser, W., Genot, G., Marivet, J., and Burkard, G. (1996). Heavy-metal-responsive genes in maize: identification and comparison of their expression upon various forms of abiotic stress. Planta 199, 1–8.
Dietz, K. J., Jacob, S., Oelze, M. L., Laxa, M., Tognetti, V., de Miranda, S. M., et al. (2006). The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 57, 1697–1709.
Dowling, V. A., and Sheehan, D. (2006). Proteomics as a route to identification of toxicity targets in environmental toxicology. Proteomics 6, 5597–5604.
Durand, T. C., Sergeant, K., Planchon, S., Carpin, S., Label, P., Morabito, D., et al. (2010). Acute metal stress in Populus tremula × P. alba (717-1B4 genotype): leaf and cambial proteome changes induced by cadmium(2+). Proteomics 10, 349–368.
Durrant, W. E., and Dong, X. (2004). Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209.
Duquesnoy, I., Goupil, P., Nadaud, I., Branlard, G., Piquet-Pissaloux, A., and Ledoigt, G. (2009). Identification of Agrostis tenuis leaf proteins in response to As(V) and As(III) induced stress using a proteomics approach. Plant Sci. 176, 206–213.
Edreva, A. (2005). Pathogenesis-related proteins. Research progress in the last 15 years. Gen. Appl. Plant Physiol. 31, 105–124.
Elvira, M. I., Galdeano, M. M., Gilardi, P., García-Luque, I., and Serra, M. T. (2008). Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. J. Exp. Bot. 59, 1253–1265.
Ercal, N., Gurer-Orhan, H., and Aykin-Burns, N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 1, 529–539.
Espartero, J., Sanchez-Aguayo, I., and Pardo, J. M. (1995). Molecular characterization of glyoxalase-I from a higher plant; upregulation by stress. Plant Mol. Biol. 29, 1223–1233.
Farinati, S., DalCorso, G., Bona, E., Corbella, M., Lampis, S., Cecconi, D., et al. (2009). Proteomic analysis of Arabidopsis halleri shoots in response to the heavy metals cadmium and zinc and rhizosphere microorganisms. Proteomics 9, 4837–4850.
Fecht-Christoffers, M. M., Braun, H. P., Lemaitre-Guillier, C., VanDorsselaer, A., and Horst, W. J. (2003). Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol. 133, 1935–1946.
Führs, H., Hartwig, M., Molina, L. E., Heintz, D., Van Dorsselaer, A., Braun, H. P., et al. (2008). Early manganese-toxicity response in Vigna unguiculata L. – a proteomic and transcriptomic study. Proteomics 8, 149–159.
Grill, E., Loffler, S., Winnacker, E. L., and Zenk, M. H. (1989). Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. U.S.A. 86, 6838–6842.
Gygi, S. P., Rochon, Y., Franza, B. R., and Aebersold, R. (1999). Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19, 1720–1730.
Hajduch, M., Rakwal, R., Agrawal, G. K., Yonekura, M., and Pretova, A. (2001). High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electro-phoresis 22, 2824–2831.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53, 1–11.
Hensel, G., Kunze, G., and Kunze, I. (1999). Expression of the tobacco gene CBP20 in response to developmental stage, wounding, salicylic acid and heavy metals. Plant Sci. 148, 165–174.
Hishiya, S., Hatakeyama, W., Mizota, Y., Hosoya-Matsuda, N., Motohashi, K., Ikeuchi, M., et al. (2008). Binary reducing equivalent pathways using NADPH-thioredoxin reductase and ferredoxin-thioredoxin reductase in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 49, 11–18.
Hossain, M. A., Piyatida, P., Teixeira da Silva, J. A., and Fujita, M. (2012a). Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 1–37.
Hossain, Z., Hajika, M., and Komatsu, S. (2012b). Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids 43, 2393–2416.
Hossain, Z., Lopez-Climent, M. F., Arbona, V., Perez-Clemente, R. M., and Gomez-Cadenas, A. (2009). Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 166, 1391–1404.
Hossain, Z., Makino, T., and Komatsu, S. (2012c). Proteomic study of β -aminobutyric acid-mediated cadmium stress alleviation in soybean. J. Proteomics 75, 4151–4164.
Hossain, Z., Nouri, M. Z., and Komatsu, S. (2012d). Plant cell organelle proteomics in response to abiotic stress. J. Proteome Res. 11, 37–48.
Hradilova, J., Rehulka, P., Rehulkova, H., Vrbova, M., Griga, M., and Brzobohaty, B. (2010). Comparative analysis of proteomic changes in contrasting flax cultivars upon cadmium exposure. Electrophoresis 31, 421–431.
Jang, H. H., Lee, K. O., Chi, Y. H., Jung, B. G., Park, S. K., Park, J. H., et al. (2004). Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635.
Jwa, N. S., Agrawal, G. K., Tamogami, S., Yonekura, M., Han, O., Iwahashi, H., et al. (2006). Role of defense/stress related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 44, 261–273.
Kieffer, P., Dommes, J., Hoffmann, L., Hausman, J. F., and Renaut, J. (2008). Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics 8, 2514–2530.
Kieffer, P., Planchon, S., Oufir, M., Ziebel, J., Dommes, J., Hoffmann, L., et al. (2009). Combining proteomics and metabolite analyses to unravel cadmium stress-response in poplar leaves. J. Proteome Res. 8, 400–417.
Komatsu, S., and Ahsan, N. (2009). Soybean proteomics and its application to functional analysis. J. Proteomics 72, 325–336.
Kasprzewska, A. (2003). Plant chitinases – regulation and function. Cell. Mol. Biol. Lett. 8, 809–824.
Labra, M., Gianazza, E., Waitt, R., Eberini, I., Sozzi, A., Regondi, S., et al. (2006). Zea mays L. protein changes in response to potassium dichromate treatments. Chemosphere 62, 1234–1244.
Lee, K., Bae, D. W., Kim, S. H., Han, H. J., Liu, X., Park, H. C., et al. (2010). Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J. Plant Physiol. 167, 161–168.
Liu, J. J., and Ekramoddoullah, A. (2006). The family 10 of plant pathogenesis-related proteins: their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 68, 3–13.
Luque-Garcia, J. L., Cabezas-Sanchez, P., and Camara, C. (2011). Proteomics as a tool for examining the toxicity of heavy metals. Trends Anal. Chem. 30, 703–716.
Ma, J. F., Ryan, P. R., and Delhaize, E. (2001). Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 6, 273–278.
Maksymiec, W. (2007). Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 29, 177–187.
Murphy, A., and Taiz, L. (1995). Comparison of metallothionein gene expression and nonprotein thiols in ten Arabidopsis ecotypes. Correlation with copper tolerance. Plant Physiol. 109, 945–954.
Pandey, S., Rai, R., and Rai, L. C. (2012). Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J. Proteomics 75, 921–937.
Patterson, J., Ford, K., Cassin, A., Natera, S., and Bacic, A. (2007). Increased abundance of proteins involved in phytosiderophore production in boron-tolerant barley. Plant Physiol. 144, 1612–1631.
Perfus-Barbeoch, L., Leonhardt, N., Vavasseur, A., and Forestier, C. (2002). Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 32, 539–548.
Rakwal, R., Agrawal, G. K., and Yonekura, M. (1999). Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 20, 3472–3478.
Requejo, R., and Tena, M. (2006). Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 6, S156–S162.
Ritter, A., Ubertini, M., Romac, S., Gaillard, F., Delage, L., Mann, A., et al. (2010). Copper stress proteomics highlights local adaptation of two strains of the model brown alga Ectocarpus siliculosus. Proteomics 10, 2074–2088.
Rodríguez-Celma, J., Rellan-Alvarez, R., Abadia, A., Abadia, J., and Lopez-Millan, A. F. (2010). Changes induced by two levels of cadmium toxicity in the 2-DE protein profile of tomato roots. J. Proteomics 73, 1694–1706.
Salt, D. E., and Rauser, W. E. (1995). MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 107, 1293–1301.
Sanita Di Toppi, L., and Gabbrielli, R. (1999). Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105–130.
Saravanan, R. S., and Rose, J. K. (2004). A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4, 2522–2532.
Sarowar, S., Kim, Y. J., Kim, E. N., Kim, K. D., Hwang, B. K., Islam, R., et al. (2005). Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 24, 216–224.
Sarry, J. E., Kuhn, L., Ducruix, C., Lafaye, A., Junot, C., Hugouvieux, V., et al. (2006). The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 6, 2180–2198.
Schneider, T., Schellenberg, M., Meyer, S., Keller, F., Gehrig, P., Riedel, K., et al. (2009). Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics 9, 2668–2677.
Semane, B., Dupae, J., Cuypers, A., Noben, J. P., Tuomainen, M., Tervahauta, A., et al. (2010). Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J. Plant Physiol. 167, 247–254.
Sharma, S. S., and Dietz, K. J. (2009). The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 14, 43–50.
Sharmin, S. A., Alam, I., Kim, K. H., Kim, Y. G., Kim, P. J., Bahk, J. D., et al. (2012). Chromium-induced physiological and proteomic alterations in roots of Miscanthus sinensis. Plant Sci. 187, 113–126.
Tuomainen, M. H., Nunan, N., Lehesranta, S. J., Tervahauta, A. I., Hassinen, V. H., Schat, H., et al. (2006). Multivariate analysis of protein profiles of metal hyperaccumulator Thlaspi caerulescens accessions. Proteomics 6, 3696–3706.
van Hoof, N. A., Hassinen, V. H., Hakvoort, H. W., Ballintijn, K. F., Schat, H., Verkleij, J. A., et al. (2001). Enhanced copper tolerance in Silene vulgaris (Moench) Garcke populations from copper mines is associated with increased transcript levels of a 2b-type metallothionein gene. Plant Physiol. 126, 1519–1526.
Van Loon, L., and Van Strien, E. (1999). The families of pathogenesis related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 55, 85–97.
Van Loon, L. C. (1997). Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 103, 753–765.
Vannini, C., Marsoni, M., Domingo, G., Antognoni, F., Biondi, S., and Bracale, M. (2009). Proteomic analysis of chromate-induced modifications in Pseudokirchneriella subcapitata. Chemosphere 76, 1372–1379.
Verbruggen, N., Hermans, C., and Schat, H. (2009). Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 12, 364–372.
Villiers, F., Ducruix, C., Hugouvieux, V., Jarno, N., Ezan, E., Garin, J., et al. (2011). Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 11, 1650–1663.
Wang, L., Zhao, J.-Y., Wu, S.-M., Pan, J.-L., Huang, Z.-B., Wu, Z.-K., et al. (2011). No statistic correlation between superoxide dismutase and peroxidase activities and aluminum-induced lipid peroxidation in maize, implying limited roles of both enzymes in prevention against aluminum-induced lipid peroxidation. Am. J. Plant Sci. 2, 156–164.
Wang, W., Vinocur, B., Shoseyov, O., and Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252.
Welch, R. M., and Norvell, W. A. (1999) “Mechanisms of cadmium uptake, translocation and deposition in plants,” in Cadmium in Soils and Plants, eds M. J. McLaughlin and B. R. Singh (Dordrecht: The Kluwer Academic Publishers), 125–150.
Yadav, S. K., Singla-Pareek, S. L., Ray, M., Reddy, M. K., and Sopory, S. K. (2005). Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 337, 61–67.
Yang, Z., and Chu, C. (2011). “Towards understanding plant response to heavy metal stress,” in Abiotic Stress in Plants – Mechanisms and Adaptations, eds A. Shanker and B. Venkateswarlu (Shanghai: InTech). Available at: http://www.intechopen.com/books/abiotic-stress-in-plants-mechanisms-and-adaptations
Yang, Q., Wang, Y., Zhang, J., Shi, W., Qian, C., and Peng, X. (2007). Identification of aluminum-responsive proteins in rice roots by a proteomic approach: cysteine synthase as a key player in Al response. Proteomics 7, 737–749.
Zhang, H., Lian, C., and Shen, Z. (2009). Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 103, 923–930.
Keywords: antioxidant, heavy metal, HSPs, phytochelatins, proteomics, PR protein
Citation: Hossain Z and Komatsu S (2013) Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant Sci. 3:310. doi: 10.3389/fpls.2012.00310
Received: 29 November 2012; Paper pending published: 13 December 2012;
Accepted: 24 December 2012; Published online: 25 January 2013.
Edited by:
Pingfang Yang, Wuhan Botanical Garden, Chinese Academy of Sciences, ChinaReviewed by:
Hans-Peter Mock, Institute of Plant Genetics and Crop Plant Research, GermanyCopyright: © 2013 Hossain and Komatsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Setsuko Komatsu, National Institute of Crop Science, Kannondai 2-1-18, Tsukuba 305-8518, Japan. e-mail:c2tvbWF0c3VAYWZmcmMuZ28uanA=; Zahed Hossain, Department of Botany, West Bengal State University, Kolkata 700126, West Bengal, India. e-mail:emFoZWRfa2x5QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.