
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 12 September 2011
Sec. Plant Proteomics and Protein Structural Biology
Volume 2 - 2011 | https://doi.org/10.3389/fpls.2011.00044
Using a series of multiplexed experiments we studied the quantitative changes in protein abundance of three Australian bread wheat cultivars (Triticum aestivum L.) in response to a drought stress. Three cultivars differing in their ability to maintain grain yield during drought, Kukri (intolerant), Excalibur (tolerant), and RAC875 (tolerant), were grown in the glasshouse with cyclic drought treatment that mimicked conditions in the field. Proteins were isolated from leaves of mature plants and isobaric tags were used to follow changes in the relative protein abundance of 159 proteins. This is the first shotgun proteomics study in wheat, providing important insights into protein responses to drought as well as identifying the largest number of wheat proteins (1,299) in a single study. The changes in the three cultivars at the different time points reflected their differing physiological responses to drought, with the two drought tolerant varieties (Excalibur and RAC875) differing in their protein responses. Excalibur lacked significant changes in proteins during the initial onset of the water deficit in contrast to RAC875 that had a large number of significant changes. All three cultivars had changes consistent with an increase in oxidative stress metabolism and reactive O2 species (ROS) scavenging capacity seen through increases in superoxide dismutases and catalases as well as ROS avoidance through the decreases in proteins involved in photosynthesis and the Calvin cycle.
Abiotic stresses (e.g., drought, heat, and salinity) are the major cause of grain yield loss, upward of 50% (Boyer, 1982), and hence have significant impact on our capacity to meet the food demands of an ever increasing global population (Tester and Langridge, 2010). A common feature of these abiotic stresses is a water deficit that results in a complex cellular and physiological response in plants. Generally photosynthesis is reduced under a water deficit either through stomatal closure or metabolic impairment (Reddy et al., 2004) and changes in mitochondrial respiration and the photosynthetic electron transport lead to the generation of highly toxic reactive O2 species (ROS) such as superoxide and peroxides, that cause chemical damage to DNA and proteins leading to serious effects on cellular metabolism (Mittler, 2002). Plants have evolved several strategies to deal with ROS, including avoidance such as anatomical adaption, suppression of photosynthesis and photosystem (PS), and antenna modulations, ROS scavenging through the production of chemical antioxidants such as ascorbate and glutathione, and enzymes such as peroxidases and superoxide dismutases (SODs; Chaves et al., 2003; Mittler, 2006). Another adaptive mechanism to deal with drought is to maintain turgor pressure by the production of osmolytes, such as proline, glycine betaine, and trehalose that can also provide secondary protective effects such as protecting proteins from unfolding (Hare et al., 1998). Additionally, drought responsive proteins such as dehydrins and heat shock proteins are produced to protect the intracellular metabolic machinery (Wang et al., 2003).
The advent of genomic technologies has now also defined a complex series of interacting pathways/networks controlled by transcription factors (e.g., DREB1, AREB, and NF-YB; Yang et al., 2010). Many of the signaling pathways are known to be regulated through post-translational modifications of proteins, primarily phosphorylation, and have therefore lead to other “omics” (proteomics and metabolomics) being applied to attempt to understand the complex cellular responses.
Southern Australia has a Mediterranean-type climate, with sufficient rainfall in winter for plant growth and intermittent rainfall in spring during the growing season. Izanloo et al. (2008) designed a glasshouse pot experiment with cyclic drought treatment to mimic these field conditions. The experiments analyzed the morphological and physiological responses of three wheat cultivars; Kukri (intolerant), Excalibur and RAC875 (both tolerant), that differ in their ability to maintain grain yield under drought conditions. The two tolerant cultivars differ in their drought tolerance mechanisms, with Excalibur showing a higher osmotic adjustment (OA) potential, low ABA content, higher stomatal conductance, and rapid recovery after stress compared to RAC875. In contrast, cultivar RAC875 stores more water-soluble carbohydrates in the stem and the leaf morphology is better adapted to minimizing water loss due to waxier and thicker leaves. To build on the Izanloo et al. (2008) study we used a similar cyclic drought treatment with the same three cultivars, adopting a proteomics profiling approach to monitor protein changes.
There have been several proteomic studies in wheat during drought stress (Hajheidari et al., 2007; Caruso et al., 2009; Peng et al., 2009; Kamal et al., 2010; Bazargani et al., 2011; Yang et al., 2011), all using 2D gel-based methods. These studies have identified a small sub-set of proteins that are drought responsive, including 77 (Yang et al., 2011), 33 (Kamal et al., 2010), and 57 (Hajheidari et al., 2007) in grains, 82 in stem (Bazargani et al., 2011), 21 in the first leaf (Caruso et al., 2009), and 49 and 30 in root and leaf, respectively (Peng et al., 2009). Recent advances in proteomics have allowed for large numbers of proteins to be identified using shotgun style approaches in plants (Oeljeklaus et al., 2009). These have not been applied to wheat due to the lack of genomic sequence information and the daunting task of protein identification from peptides in a hexaploid organism. We present to our knowledge the first shotgun proteomics study undertaken in wheat, resulting in some important insights into the protein response of wheat plants to drought stress as well as identifying the largest number of wheat proteins in a single proteomics experiment.
Cyclic drought experiments were conducted in growth room with a refrigerated cooling system at the Australian Centre for Plant Functional Genomics (ACPFG), the University of Adelaide, Australia, essentially as described by Izanloo et al. (2008). Three Australian wheat cultivars (Triticum aestivum L. cv Kukri, Excalibur, and RAC875) were grown in watertight bags containing 6 kg of dried soil collected from the field at Roseworthy Agricultural campus, University of Adelaide and mixed with Waikerie sand (50/50), basal nutrients were added at the start of the experiment as described by Izanloo et al. (2008). Plants were grown at 16°C day/4°C night for the first 4 weeks, 17°C day/6°C night for the next 4 weeks then 23°C day/10°C night for the remainder of the experiment [see Table 1 from Izanloo et al. (2008) for a detailed description]. Relative humidity was maintained at 40–50% during the day and at approximately 80% during the night cycle. There were two watering regimes, control plants were watered to field capacity daily (determined by weighing bags) and drought-treated plants were watered to field capacity daily until emergence of the first flag leaf and a drought treatment was applied by gradually reducing the quantity of water added each day until the least tolerant cultivar (Kukri) displayed wilting symptoms. Plants were then re-watered to field capacity and left to dry (without daily watering) until the least tolerant cultivar (Kukri) displayed wilting symptoms when they were again re-watered (Figure 1). Plant water status was monitored by measuring leaf relative water content (RWC) of all cultivars over the course of the experiment. RWC was measured on the flag leaf by the standard method (Barrs and Weatherley, 1962) as described in Izanloo et al. (2008). Mature leaves were collected from five plants for both control and drought-treated plants at 2 pm on days 5, 14, 24, and 25 after the beginning of the drought treatment. The day 5 samples are designated as water stressed (WS), day 14 is the first wilting point (WP1), day 24 is the second wilting point (WP2), and day 25 is after re-watering (RW). The leaves from the five plants were pooled for protein analysis.
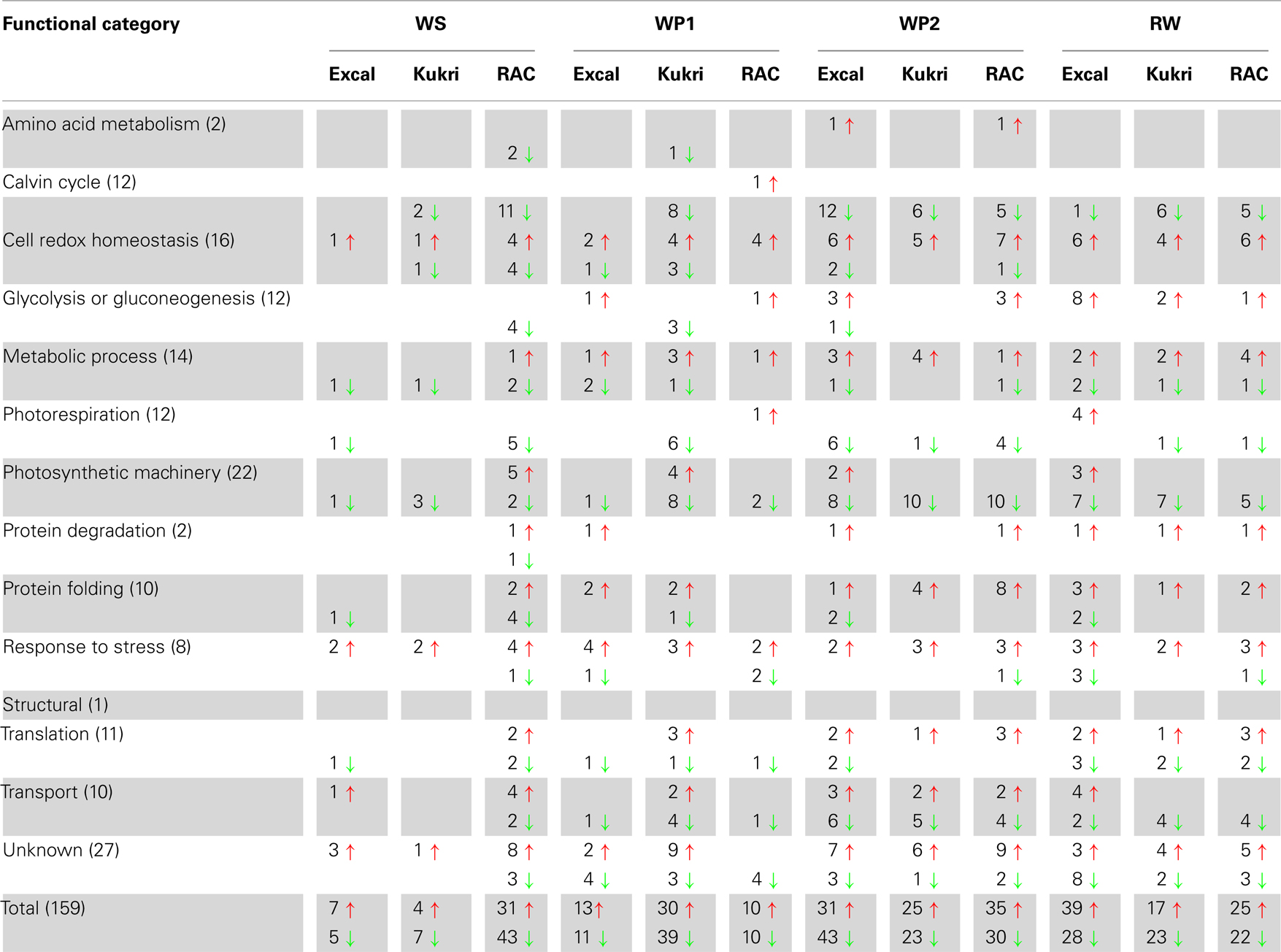
Table 1. The data from Table S2 in Supplementary Material of the 159 proteins identified by iTRAQ as changing during drought stress are summarized according to functional categories, increases indicated by ↑ in red, decreases indicated by ↓ in green.
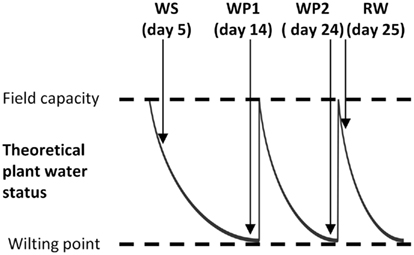
Figure 1. Schematic diagram of watering regime for wheat cyclic drought experiment. Abbreviations: WS, water stressed at day 5; WP1, first wilting point of intolerant cultivar at day 14; WP2, second wilting point of intolerant cultivar at day 24; and RW, re-watered at day 25.
Samples were ground in liquid nitrogen and TCA precipitated according to Damerval et al. (1986). Protein pellets were re-suspended in 8 M urea, an aliquot of each was diluted to 1 M urea and protein concentrations were estimated using the Bradford assay (Bio-Rad, Hercules, CA, USA) with BSA as a standard. 100 μg of protein from each sample was used for digestion and labeled in four batches (Figure 2) with the iTRAQ 8plex reagents (AB Sciex, Foster City, CA, USA) following the manufacturers protocol. A reference sample, containing an aliquot of each of the 24 samples was included in each batch to enable cross batch comparison. After pooling labeled samples into batches, removing isopropanol under vacuum, and adjusting pH to <3 with neat formic acid, peptides were passed over a C18 SepPak® Plus cartridge (Waters, Milford, MA, USA). Cartridges were initially washed with 10 mL of 0.1% formic acid in 60% acetonitrile, equilibrated with 10 mL of 0.1% formic acid, peptides were bound, then washed with 10 mL 0.1% formic acid and eluted with 2 mL of 0.1% formic acid in 60% acetonitrile. Peptides were then concentrated under vacuum to a volume of approximately 100 μl. The concentrated tryptic peptides were separated on a PolySULFOETHYL Aspartamide SCX column (4.6 mm × 200 mm, 5 μm, 300 Å, PolyLC Inc., Columbia, MD, USA) attached to an Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA USA) with the following separation gradient: buffer A (25% (v/v) acetonitrile in 5 mM phosphate buffer, pH 3) for 10 min, then up to 100% buffer B (300 mM potassium chloride, 25% (v/v) acetonitrile in 5 mM phosphate buffer, pH 5) over 30 min at a flow rate of 0.7 mL/min with 0.5 min fractions being collected in a 96-well plate.
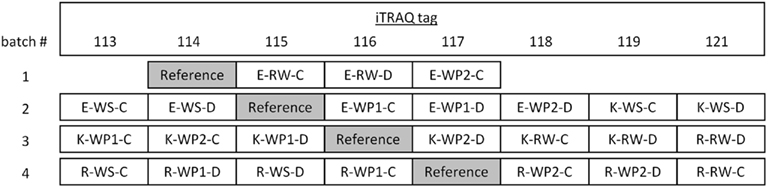
Figure 2. Schematic diagram of iTRAQ labeling for wheat cyclic drought experiment. Abbreviations: E, Excalibur; K, Kukri; R, RAC875; C, well-watered controls; D, cyclic drought-treated samples.
Fractions collected from 16 to 45 min from SCX-HPLC were reduced under vacuum and re-suspended in 0.1% formic acid (60 μL), filtered through a minisart membrane (0.2 μm; Sartorius Stedim Biotech, Aubagne, France) and one-tenth of each fraction was loaded onto a reversed-phase pre-column (300 μm × 5 mm Zorbax 300SB-C18; Agilent Technologies, Palo Alto, CA, USA) attached to a Shimadzu Prominence nano LC system (Shimadzu Corporation, Kyoto, Japan). The pre-column was washed with 0.1% formic acid in 5% acetonitrile for 15 min before placing in-line with a 75-μm i.d. × 150-mm Zorbax 300SB-C18 (Agilent Technologies, Palo Alto, CA, USA) reversed-phase column. Peptides were eluted using a gradient of 5–65% (v/v) acetonitrile in 0.1% formic acid over 60 min, at a flow rate of 0.25 μL min−1. Peptides were analyzed via electrospray ionization (ESI) on a QSTAR Elite hybrid quadrupole time-of-flight mass spectrometer (AB Sciex, Foster City, CA, USA). Each SCX-HPLC fraction was chromatographed and analyzed five times.
The MS was operated in the positive ion mode, ion source voltage of 2,200 V, using 10 μm uncoated SilicaTips™ (New Objective, Woburn, MA, USA). Analyst® QS 2.0 software (AB Sciex, Foster City, CA, USA) was used to collect data in a data-dependent acquisition mode for the three most intense ions fulfilling the following criteria: m/z between 450 and 2,000; ion intensity 40 counts; and charge state between +2 and +5. After MS/MS analysis, these ions were dynamically excluded for 18 s, using a mass tolerance of 50 mDa. MS scans were accumulated for 0.5 s, and MS/MS scans were collected in automatic accumulation mode for a maximum of 2 s. Mass and charge state-dependent rolling collision energy was used and the MS instrument was calibrated daily with [Glu]- fibrinopeptide B (Sigma-Aldrich, St. Louis, MO, USA).
Peak lists from the MS/MS spectra were made using ProteinPilot software version 2.0.1 (AB Sciex, Foster City, CA, USA). The peak lists were searched against a six-frame translation of the July 2008 release of the Wheat Gene Index (V11.0, DFCI) using MASCOT 2.06 (Perkins et al., 1999) and the Paragon™ Algorithm contained within ProteinPilot software version 2.0.1 (AB Sciex, Foster City, CA, USA). The MASCOT parameters were: enzyme: trypsin, fixed modifications: iTRAQ8plex (N-term); iTRAQ8plex (K); Methylthiol (C), variable modifications: iTRAQ8plex (Y), MS peptide tolerance: 0.25 Da, MS/MS tolerance: 0.15 Da, number of missed cleavages: up to 1. The Paragon™ Algorithm parameters were: sample type: iTRAQ 8plex (peptide labeled); Cys Alkylation: MMTS; Digestion: Trypsin; Instrument type: QSTAR ESI; Search effort: Thorough ID. The outputs from both search algorithms were combined and only peptides with a P < 0.05 in both search algorithms were reported, this corresponded to a minimum mascot ion score of 42. Peptides were then used to research the database using KNIME (Berthold et al., 2008) to find all proteins containing any of the peptides, with the constraint that they must be fully tryptic. The list was then reduced to a minimum list of proteins by selecting only those that contained two or more peptides and only keeping one of the proteins where more than one protein matched the same set of peptides; I and L were treated as the same amino acid. The false discovery rate based on a randomized version of the six-frame translation of the July 2008 release of the Wheat Gene Index (V11.0, DFCI) database searched with batch 1 data is <1%.
Protein function was determined by performing a BLAST search of the SwissProt database (August 2010) using the EBI WU-Blast 2.0 web service1 with an e-value cutoff of 1e-5. Sequences which satisfy this constraint were then subject to QuickGO analysis2 to identify available function for the reported BLAST hits. The 159 proteins with iTRAQ quantitation were checked manually using the NCBI Blast server3, ExPASy Proteomics Server4 and available literature.
It is not possible with the current publicly available sequence information to be certain of the uniqueness of a peptide, therefore the requirement that a peptide be unique to be used for protein quantification was relaxed. Peptides that are shared between proteins were only included in the quantitative information for the protein that had the most number of assigned peptides, insuring that a peptide was then only used once for quantification. Although proteins have been assigned quantitative information, those data could also have resulted from a change in a group of proteins with similar sequences.
The reporter ion peak areas generated in ProteinPilot were exported to KNIME and used for quantification, following similar calculations used in the Libra module in the trans-proteomic pipeline (Pedrioli et al., 2004) and previously applied in Rao et al. (2010), with some changes. Briefly, any peptide with a reporter ion peak area of less than 20 was removed from quantification. When a peptide was detected more than once, the peak area for each reporter ion was summed, each peptide was then normalized by the sum of its channel intensities (113, 114, 115, 116, 117, 118, 119, and 121). Peptides were ignored when the normalized peptide value was more than 2 SDs from the calculated mean of the protein the peptide matched to. The mean was then calculated for proteins with three or more peptides that fulfilled the above criteria. The time points were normalized to the reference and again by the average protein ratio for each time point. A change was considered significant if it was greater than 1.3-fold with less than 15% SE.
Under well-watered conditions all three wheat cultivars maintained leaf RWC of greater than 94% and showed little variation either between cultivars or during the growth cycle of the experiment. In contrast, the RWC of the three cultivars varied during the cyclic drought experiment (Figure 3). The tolerant variety, RAC875, maintained the highest RWC and had the least significant water loss. At the WP1 RAC875 had a considerably higher RWC (68%) compared to both Excalibur and Kukri that are similar with 50 and 54%, respectively. However, at the WP2, Excalibur retains considerably more water than Kukri and is now more similar to RAC875. Unlike the two tolerant cultivars the RWC of Kukri at WP2 is not significantly different from WP1. After re-watering (RW) the RWC of all cultivars have returned to control (well-watered) levels.
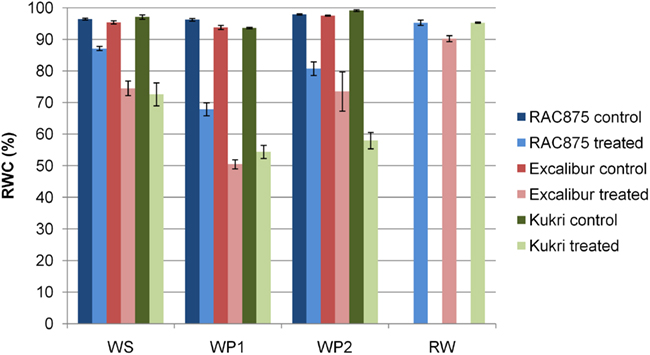
Figure 3. Relative water content (RWC) of flag leaf. Data represents the mean of 5 biological replicates, plus/minus the SD. WS, water stressed at 5 days; WP1, wilting point 1 for intolerant cultivar at day 14; WP2, wilting point 2 for intolerant cultivar at day 24; and RW, re-watered at day 25. Control plants were not measured at day 25 but are assumed to mirror results of controls from other sampling time points where values remained constant and greater than 90%.
Proteomic profiling was used to monitor changes in response to cyclic drought conditions in three wheat cultivars; RAC875 (drought tolerant), Excalibur (drought tolerant), and Kukri (drought intolerant), with differing tolerance based on grain yield (Izanloo et al., 2008). Using a 2D-LC-MS/MS approach 5,125 peptides were identified, matching 22,812 accessions in the Wheat Gene Index (V11.0, DFCI), this list was reduced to a minimum set of 1,299 proteins (Table S1 in Supplementary Material) with two or more fully tryptic peptide matches to be described. The lack of a genome sequence for wheat and the ploidy (hexaploid) of these cultivars created a significant challenge for protein identification from a shotgun proteomic approach, with only 10% of the spectra matched to a peptide. Previous studies performed in rice using the same conditions and instrument, matched approximately 30% of the spectra (Rao et al., 2010). The minimum set of 1,299 proteins that were described by the set of peptides identified is likely an underestimation of the total number of proteins due to the inability to separate all homologs and homeologs using peptide sequences and the fact that a minimum of two peptide matches was specified to ensure a positive identification. These proteins were assigned to different functional categories (Figure 4A) and included, in descending order: metabolic process (12%); cell redox homeostasis, translation, and transport (7% each); photosynthetic machinery (6%); response to stress and protein folding (4% each); Calvin cycle, glycolysis or gluconeogenesis, and protein degradation (3% each); amino acid biosynthesis, transcription, and photorespiration (2% each); structural (<1%); with the largest category of unknowns (38%).
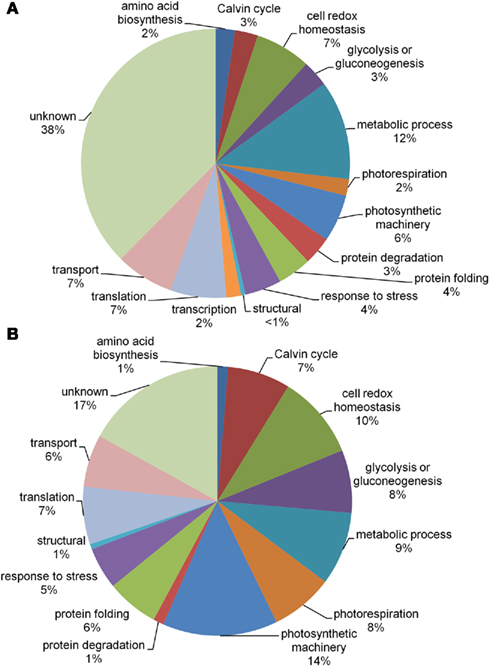
Figure 4. Pie charts of (A) all identified proteins (1,299) and (B) proteins with iTRAQ data (159) divided into functional categories.
The iTRAQ tagging system was used for relative quantification, with 159 proteins having relative quantification information across all time points (Table S2 in Supplementary Material). The 159 proteins could be classified into 14 functional categories (Figure 4B): photosynthetic machinery (14%); cell redox homeostasis (10%); metabolic process (9%); photorespiration (8%); glycolysis or gluconeogenesis (8%); translation and Calvin cycle (7% each); transport and protein folding (6% each); response to stress (5%); protein degradation, amino acid biosynthesis and structural (1% each), and unknowns (17%). Unsurprisingly, the quantitative experimental approach ensured an under-representation of the low abundance (e. g., transcription factors) and membrane proteins. Although the extraction method is biased toward hydrophilic proteins some hydrophobic proteins were detected in the iTRAQ results; thus the biased nature of the extraction method could have added some variation to the iTRAQ data. The iTRAQ results in Table S2 in Supplementary Material are summarized in Table 1; Figure 5. The initial response of the three wheat cultivars to drought is distinctly different (Figure 5).
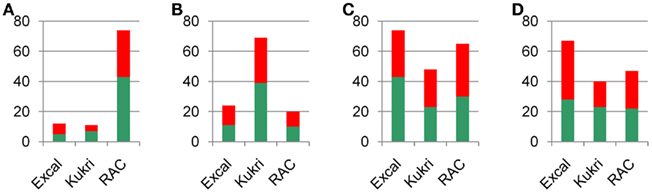
Figure 5. Summary of iTRAQ identified protein changes from Table S2 in Supplementary Material. The number of protein changes at (A) WS, (B) WP1, (C) WP2, and (D) RW. Red indicates an increase and green indicates a decrease. The scale indicates the number of proteins changing.
RAC875 responded early to a moderate water stress (RWC 87%) with 74 significant protein changes compared to Excalibur and Kukri with 12 and 11 significant changes, respectively, at WS. The early response in RAC875 resulted in down regulation of the Calvin cycle proteins under water stress as 11 out of the 12 proteins in that functional category significantly decreased compared to the well-watered controls, with the largest protein (Rubisco large subunit) decrease at this time point (2.3-fold) also occurring within this category. Decreases were also seen in glycolysis or gluconeogenesis, amino acid metabolism, and photorespiration. There were no changes in the structural group and the other functional categories had mixed responses. The largest increase in RAC875 in response to the cyclic drought treatment occurred at this early time with a 3.3-fold increase in catalase from the cell redox homeostasis category. At WP1 there were only 20 significant changes in the RAC875 treated samples compared to the well-watered controls. Although there were a lot fewer changes and the amplitude of the changes were not as large, the changes are almost the opposite of WS. There were significant increases in protein levels in the Calvin cycle, glycolysis or gluconeogenesis, photorespiration, metabolic processes, and cell redox homeostasis, decreases in photosynthetic machinery, transport, translation, and the unknown proteins and a mixed response in the response to stress protein category.
At WP2, RAC875 once again had a large number (65) of significant changes and the largest protein decrease (5.4-fold) in this cultivar in the photosystem I subunit VII protein from the photosynthetic machinery category. The protein responses include significant decreases in the Calvin cycle, photorespiration, and the photosynthetic machinery, and increases in amino acid metabolism, glycolysis or gluconeogenesis, protein degradation, protein folding, and translation. Both increases and decreases were seen in cell redox homeostasis, metabolic processes, response to stress, transport, and unknowns, although the majority of the changes in cell redox homeostasis, response to stress, and the unknown proteins were increases. After re-watering, there were fewer changes with 47 significant changes, however, the trends are the same, apart from no longer seeing any changes in amino acid metabolism.
Excalibur is much slower to respond to water stress at the protein level than RAC875. At WS, Excalibur had very few (12) significant changes in treated samples compared to the well-watered controls. The significant increases were in cell redox homeostasis, response to stress, transport, and the unknown proteins. The significant decreases in response to drought were one in each of the metabolic processes, photorespiration, photosynthetic machinery, protein folding, and translation. The largest protein change occurred in the dehydrin COR410 with a 2.1-fold increase in the response to stress category. At WP1, Excalibur has 24 significant changes in response to drought and larger protein fold changes. The largest changes were a 4.3-fold decrease in the photosystem I subunit VII and a 3.3-fold increase in the dehydrin COR410. At this time point there are mixed responses to drought in cell redox homeostasis, metabolic processes, response to stress proteins, and the unknowns. Significant increases are seen in glycolysis or gluconeogenesis, protein degradation, and protein folding and decreases were seen in transport, translation, and photosynthetic machinery.
At WP2 Excalibur had a larger number of proteins responding (74) to drought than at WP1. At this time point (WP2) there is a large coordinated decrease in the Calvin cycle proteins with all of the observed proteins decreasing, five of which, decreased by more than two-fold. Decreases also occurred in photorespiration and mixed responses were seen for the other categories apart from protein degradation and amino acid metabolism where an increase occurred in both and two response to stress proteins. The majority of responses in the photosynthetic machinery and transport were decreases and the majority of the increases were in cell redox homeostasis, glycolysis or gluconeogenesis, metabolic processes, and the unknown proteins. Within the cell redox homeostasis category the Cu/Zn superoxide dismutase and the catalase were both more than 2.5-fold higher than the controls. As with the other tolerant cultivar RAC875, Excalibur’s largest decrease (5.4-fold) occurred in the photosystem I subunit VII protein in the photosynthetic machinery category.
At RW, Excalibur had 67 significant changes, slightly less than it had at the WP2 (74). Unlike the other tolerant cultivar RAC875, the changes at RW in Excalibur are different to WP2. There is no longer a large decrease in the Calvin cycle proteins; there is only one decrease. There is a large increase in glycolysis or gluconeogenesis with 8 out of 11 proteins significantly increasing. There are increases in photorespiration, protein degradation, and cell redox homeostasis. A mixed response is seen in metabolic processes, photosynthetic machinery, response to stress, translation, transport, and the unknown proteins, the majority of proteins in photosynthetic machinery, and the unknown proteins decrease.
Kukri had a more intermediate number of proteins responding in comparison to the two tolerant cultivars. At WS it had 11 significant changes, peaking at 69 significant changes in response to drought at WP1 and then decreasing to 48 and 40 at WP2 and RW, respectively. At WS there are decreases in the Calvin cycle, metabolic processes, and photosynthetic machinery. Significant increases were in the response to stress proteins and the unknown proteins. There was a mixed response in cell redox homeostasis with one increase and one decrease. The largest changes occurred in the catalase (2.1-fold increase) and the dehydrin COR410 (2.5-fold increase). At WP1 there is a large decrease in the Calvin cycle with 8 out of the 12 proteins decreased, the largest decrease being a 2.3-fold in the Rubisco large subunit. A decrease was also seen in glycolysis or gluconeogenesis, photorespiration, and amino acid metabolism and increases in the response to stress proteins, where the largest increase in the entire dataset occurred, with a 4.3-fold increase in the dehydrin COR410. Mixed responses were seen in all other categories apart from amino acid metabolism, protein degradation, and the structural proteins where there were no changes. The majority of proteins in the metabolic process, translation, and the unknown proteins increased and the majority of proteins in photosynthetic machinery and transport decreased.
At WP2, Kukri has 48 significant changes compared to the controls representing a substantial decrease from WP1 (69). At WP2 there is a decrease in the Calvin cycle proteins (6), although not as many as at WP1 (8), decreases also occurred in the photosynthetic machinery proteins and one decrease in photorespiration. There was an increase in proteins in cell redox homeostasis, metabolic processes, protein folding, response to stress proteins, and translation. There were mixed responses to drought treatment in transport and unknown proteins, although the majority are increases in the unknowns and decreases in the transport category. At RW the number of significant changes in Kukri is similar to WP2 with 40 significant changes and the trends are very similar.
By the completion of the drought regime RAC 875 (tolerant) had the most number of protein changes (206) with Excalibur (tolerant) intermediate (177) and Kukri (intolerant; 168) the least. These are surprisingly similar total numbers (18% variation between the largest and smallest) given the morphological and physiological differences between these cultivars (Izanloo et al., 2008). Unsurprisingly, the proteins and their direction (up-/down-regulated) and the amplitude of change differed significantly between varieties. Excalibur is the slowest cultivar to respond in terms of the number of significant protein changes, increasing from 12 to 24 to 74 over the first three time points (see Table 1; Figure 5). At WS, RAC875 had the largest number of significant protein changes, followed by Kukri at WP1 and finally Excalibur at WP2. One possible explanation is that the cultivar Excalibur, known to have a higher initial OA potential than the other cultivars (Izanloo et al., 2008), allows this cultivar to maintain cellular function for longer under drought conditions before needing to respond (Morgan, 1980). Although at this stage it cannot be fully ruled out that the maturity differences between Excalibur and the other two cultivars also influenced differences for OA (Izanloo et al., 2008). However, this would not explain the large number of significant protein changes initially observed in RAC875 that is known to have a higher OA potential than Kukri (Izanloo et al., 2008). It does, however, suggest that the cultivar RAC875 has the highest capacity of the three cultivars for a cellular protein response to drought. In general we observed an increase in proteins involved in ROS scavenging and a down regulation of proteins involved in photosynthesis and the Calvin cycle, consistent with avoidance of ROS generation in all three cultivars. Known drought responsive proteins, including dehydrins, were also significantly up-regulated. The changes observed are discussed under the major functional categories reflecting metabolism.
During photosynthesis, light energy absorbed by the photosynthetic pigments in the chloroplasts is converted to chemical energy through the photosynthesis machinery with this chemical energy used for CO2 fixation in the Calvin cycle. Under a water deficit, the CO2 concentration in leaves decreases due to stomatal closure (Chaves, 1991; Kaiser and Kappen, 1997) leading to a corresponding decrease in the activities of enzymes involved in Calvin cycle (Chaves et al., 2002; Maroco et al., 2002). There were 12 proteins involved in the Calvin cycle responding to a water stress (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). These protein changes are consistent with those observed in the previous physiological studies (Chaves et al., 2002; Maroco et al., 2002), with decreases in this category of proteins in RAC875 (11 out of the 12 proteins) at WS, in Kukri at WP1 (9) and Excalibur (12) at WP2. Despite the coordinated down regulation of proteins involved in the Calvin cycle at WS in RAC875, at WP1 there is only one significant change in the drought-treated plants compared to the controls and this is an increase in the Rubisco large subunit, perhaps indicating that at this time point there is no reduction in the Calvin cycle compared to the controls. This could be either due the oxygenation of the Rubisco, feeding through photorespiration, where there was also one increase and then back to the Calvin cycle or as with many of the other observed changes, suggests an over-compensation of this cultivar to drought stress. At WP2, five of these proteins in RAC875 are significantly decreased again. After re-watering (RW), Excalibur had only one significant decrease in protein levels, RAC875 had five, and Kukri six, perhaps indicating faster recovery in Excalibur; it also has quicker stomatal conductance recovery after re-watering (Izanloo et al., 2008).
As plants are exposed to a water deficit the absorbed light energy through the photosynthetic pigments exceeds its rate of consumption through the Calvin cycle (as seen in this data set through decreases in proteins involved in the Calvin cycle), leading to photo-damage to the photosynthetic machinery, particularly the photosystem II (PSII) reaction center core proteins D1 and D2 (Aro et al., 1993). After the unknown proteins (discussed in section Unknown Proteins below) the photosynthetic machinery with 22 proteins was the largest functional category to show changes. There were 78 significant protein changes in response to cyclic drought stress in three cultivars and four time points with a large proportion (62) of these protein changes being significant decreases. There are more decreases in proteins within the photosynthesis machinery category in Kukri (28) compared to the two tolerant cultivars, Excalibur (17) and RAC875 (19); furthermore, the largest decrease (∼5.4-fold) in a protein was observed in this category in both tolerant cultivars in the PS I subunit VII protein. Plants have evolved several mechanisms to avoid damage to the photosynthetic machinery such as antenna modulations; decreasing the size of antennae to reduce the amount of absorbed light (Eberhard et al., 2008) is one mechanism. Proteins in the antennae of the photosystems are the light-harvesting complex proteins (LHC) and in our dataset there are four LHC proteins, three of which are significantly decreased in Kukri at the first three time points (WS, WP1, WP2) and only two at RW, whereas in RAC875 one is decreased at WS and three at WP2 and in Excalibur all four decreased at WP2. Other proteins changing within the photosynthesis machinery category are the extrinsic subunits of the PSII complex, known as oxygen-evolving complex (OEC) proteins, that are involved in the stabilization of the PSII complex (Ifuku et al., 2008) and its impairment is proposed to be the rate limiting step in the photo-damage process to the PSII (Takahashi and Murata, 2008). Four OEC proteins were observed to change in this dataset (PsbP, PsbQ, PsbO, and PPL a PsbP homolog), all of which increased significantly at the WS in RAC875, at WP1 the PsbQ and PPL increased significantly in Kukri and also WP2 in Excalibur. Significant decreases occurred in RAC875 at WP1 in PsbO and at RW in Kukri and the other OEC proteins, apart from PPL, decrease significantly in Excalibur and RAC875. HCF136, a protein that is essential for the repair and assembly of the PSII complex (Plucken et al., 2002), decreased at WS in RAC875 and in Kukri at WP1 whereas at RW in Excalibur it increased significantly consistent with the other observations that repair of the PSII complex occurred more rapidly in Excalibur after re-watering than in the other two cultivars.
There were 16 proteins with quantitative information involved in cell redox homeostasis (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). Cell redox homeostasis is important under water deficit as the use of the absorbed light decreases and the energy dissipation is insufficient resulting in the formation of ROS’ (Foyer et al., 1994). The largest change in protein levels in this category was catalase (CAT) with an increase in response to drought in all cultivars at all time points. CAT is essential during stress and is thought to be responsible for the removal of excess ROS (Willekens et al., 1997). Luna et al. (2005) showed increasing CAT activity following a water deficit in wheat. We also found three SODs, chloroplastic and cytosolic Cu/Zn-SOD, and a mitochondrial Mn-SOD, changing in response to drought stress in all three cultivars. The catalytic activities of these same four enzymes (CAT and SODs) were shown by Simova-Stoilova et al. (2009) to remain high in wheat cultivars of differing drought tolerance (tolerant – Yantar and Zlatitsa; sensitive – Miziya and Dobrudjanka) upon exposure to drought but with Mn-SOD and CAT increasing toward grain filling, especially in the drought-sensitive varieties (Miziya and Dobrudjanka). As enzyme activities were not measured in our study it is difficult to make direct comparisons between the two studies other than to note that these are critical drought response enzymes.
RAC875 (drought tolerant) had significant increases in response to drought in three enzymes involved in detoxification and anti-oxidant synthesis. Aldehyde dehydrogenase (ALDH) is proposed to have a role in detoxification of toxic aldehydes from lipid peroxidation due to the formation of ROS during drought stress. ALDH increased significantly in both drought tolerant cultivars (RAC875, at WP1 through to RW and Excalibur only at RW) but not in the intolerant cultivar. Guo et al. (2009) showed an increase in ALDH transcript in two drought tolerant barley cultivars compared to a drought insensitive cultivar and transgenic Arabidopsis plants over-expressing ALDH had improved tolerance to oxidative stress (Sunkar et al., 2003). Glyoxalase I, involved in the detoxification of methylglyoxyl, has been shown to increase under abiotic stress in plants (Yadav et al., 2005). Hajheidari et al. (2007) showed a decrease in glyoxalase I protein in two drought-sensitive wheat cultivars (Afghani and Arwand) and no change in a drought tolerant cultivar (Khazar). Our data shows a decrease in glyoxalase I levels at WP1 in the drought-sensitive cultivar (Kukri) and also an initial decrease in RAC875 at WS but it significantly increased in this drought tolerant cultivar at WP1. Geranylgeranyl reductase also increased significantly in both drought tolerant cultivars and in the same pattern as the ALDH. Geranylgeranyl reductase reduces free geranylgeranyl diphosphate to phytyl diphosphate that provides the side chain to chlorophylls, tocopherols, and plastoquinones. In transgenic tobacco plants transformed with the antisense geranylgeranyl reductase there was an increased sensitivity to photo-oxidative stress (Tanaka et al., 1999).
Twelve proteins from the glycolysis and gluconeogenesis functional category were shown to be changing in response to cyclic drought stress (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). Only in one cultivar, Excalibur, and at one time point (RW) was there a clear coordinated response of proteins in this category. Eight of the 12 proteins were significantly increased in response to drought compared to the control, reflecting an increased energy requirement for the quicker recovery of Excalibur after re-watering consistent with the stomatal conductance and photosynthesis responses (Izanloo et al., 2008). There were significant changes at other time points and cultivars, although not in a coordinated fashion.
There were 10 proteins in the protein folding category and eight of these increased significantly in RAC875 at WP2, compared to four in Kukri and only one in Excalibur (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). These proteins included peptidyl-prolyl cis-trans isomerases and chaperonins. Peptidyl-prolyl cis-trans isomerase activity was also found to increase in drought tolerant sorghum cultivars compared to drought-sensitive cultivars (Sharma and Singh, 2003). Cyclophilin 38 ensures correct assembly and stability of PSII in Arabidopsis (Sirpio et al., 2008) and a protein with homology to the Arabidopsis cyclophilin 38 changed significantly in RAC875 with a decrease at the WS and an increase at WP2.
There were 10 proteins that changed in response to cyclic drought stress (Figures 4B and 5; Table 1; Table S2 in Supplementary Material) in the transport category and over half of these were subunits of ATPases/synthases, although not all belonging to the same complex. All six subunits decreased in Excalibur at WP2, five in Kukri and four in RAC875, three of these increased in Excalibur RW and no increases were seen in the other two cultivars, consistent with Excalibur’s ability to recover quicker after drought. Three voltage-dependant anion channel proteins (VDAC) were detected and these increased significantly initially in RAC875 at WS, Kukri at WP1 and all three cultivars at WP2. VDAC’s are major transport proteins located on the outer membrane of mitochondria and are thought to regulate metabolite transport between the mitochondria and the cytoplasm and have been shown to be up-regulated in a variety of abiotic and biotic stresses (Kusano et al., 2009).
Eight known stress responsive proteins were found to change including the drought-induced protein SDi-6 and dehydrin COR410 (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). Dehydrin COR410 is proposed to stabilize the plasma membrane during freezing and dehydration stress (Danyluk et al., 1998). This protein showed the most significant quantitative change in response to drought stress in our experiment and it was significantly increased in all cultivars at all time points in response to drought with the most significant change observed in Kukri at WS (4.3-fold). The wheat dehydrin COR410 protein when transgenically introduced into strawberry resulted in improved chilling tolerance at 5°C after previous acclimation treatment (Houde et al., 2004). A protein similar to a drought-induced protein SDi-6 from sunflower, increased in response to drought in RAC875 (at WS, WP2, and RW) and in Kukri it increased significantly at all time points and in Excalibur at all time points except it significantly decreased at RW. The transcript level of the gene encoding the SDi-6 protein increased in sunflower (Helianthus annuus) leaves in response to drought in both drought tolerant and intolerant cultivars (Ouvrard et al., 1996).
There were two proteins involved in protein degradation, a type II metacaspase and a leucine aminopeptidase, that showed increases in response to drought especially at the later time points (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). Proteases are thought to play an important role in metabolism under a water deficit (Wisniewski and Zagdanska, 2001) as they are involved in the release of amino acids for metabolism, protein activation, and degradation of damaged proteins. Type II metacaspases are arginine/lysine-specific cysteine-dependant proteases (Vercammen et al., 2004), possibly involved in programmed cell death. Surprisingly there is a significant increase of this protease in RAC875 at WS, Excalibur at WP1 and both of these drought tolerant cultivars at WP2. At RW the two tolerant cultivars are back to control levels and Kukri is significantly increased. The early increases in a protease thought to be involved in programmed cell death in the two drought tolerant cultivars prior to the drought intolerant cultivar could be a protective mechanism of rapidly sacrificing some cells to ensure plant survival (Williams and Dickman, 2008). The other protease was a leucine aminopeptidase that decreased in RAC875 at WS and increased in both tolerant cultivars, RAC875, and Excalibur at RW. Aminopeptidases catalyze the hydrolysis of amino acids from the N-terminus of proteins and have been proposed by Miazek and Zagdanska (2008) to play an essential role in plant stress response through the activation of regulatory proteins and the turnover of damaged proteins. Simova-Stoilova et al. (2010) showed an increase in aminopeptidase activity in wheat leaves in response to a drought stress but no cultivar (cv. Pobeda, Katya, and Sadova) differences were observed.
Two proteins involved in amino acid metabolism, both cysteine synthases (a cytoplasmic and a plastidic form) changed in response to drought. There was only one significant change in the plastidic form at WS in RAC875. The cytoplasmic cysteine synthase decreased significantly in RAC875 at WS and Kukri at WP1 and then it increased significantly in the two tolerant cultivars at WP2 but not in Kukri, the intolerant cultivar. This wheat protein, when transformed into tobacco, made plants resistant to toxic levels of hydrogen sulfide gas (Youssefian et al., 1993). In Arabidopsis the knockout of the cytoplasmic cysteine synthase compromised the antioxidant capacity of the cytosol (Lopez-Martin et al., 2008) suggesting a function in maintaining the redox state of the cell, a key mechanism for drought stress tolerance.
This was the largest category with 27 proteins changing in abundance in response to cyclic drought stress (Figures 4B and 5; Table 1; Table S2 in Supplementary Material). Although there are many changes within the unknowns it is difficult to draw any conclusions. There are two proteins that stand out, the TC292219 as it only changed in the two tolerant cultivars and has homology to remorin, a protein that has been implicated in biotic stress and is thought to play a role in signal transduction processes (Lefebvre et al., 2010). The other protein, TC280943, has some similarity to 4-alpha-hydroxytetrahydrobiopterin dehydratase increased in RAC875 (drought tolerant) at WP2. This enzyme is thought to be involved in the recycling of oxidized tetrahydropterins, the cofactors of aromatic amino hydroxylases (AAH; Thony et al., 2000). In plants it seems that it may have an additional metabolic role in recycling another type of pterin, molybdenum cofactor (Naponelli et al., 2008). Naponelli et al. (2008) showed that the activity of the xanthine dehydrogenase, a molybdoenzyme, was significantly reduced in Arabidopsis 4-alpha-hydroxytetrahydrobiopterin dehydratase knockouts. Xanthine dehydrogenase is thought to be important in the drought response, as xanthine dehydrogenase-suppressed Arabidopsis plants exposed to drought have reduced growth and enhanced cell death (Watanabe et al., 2010).
Three wheat cultivars differing in their ability to maintain grain yield under drought conditions were studied. All three cultivars had changes consistent with an increase in ROS scavenging capacity seen through increases in SODs and CAT as well as ROS avoidance through the decreases in proteins involved in photosynthesis and the Calvin cycle. From a previous physiological study performed on these cultivars it was concluded that two were more drought tolerant and that their mechanisms of drought tolerance are different (Izanloo et al., 2008). Excalibur (tolerant variety) has a high OA allowing normal cellular function during dehydration and this was reflected in this proteomics study through the lack of significant changes at the protein level during the initial onset of water deficit. RAC875 (tolerant variety) has more water-soluble carbohydrates in the stem and the leaves are thicker and more waxed. While the anatomical adaption in RAC875 is unlikely to be observed in protein changes a larger number of significant protein changes at the beginning of the water stress were observed, indicating it has the highest capacity for cellular protein response as well as more increases in proteins involved in cell detoxification than the other cultivars. The findings from this proteomic study support the physiological and yield data (Izanloo et al., 2008) previously reported between the three wheat cultivars (Kukri, Excalibur, RAC875) in response to cyclic drought stress. This highlights the importance of proteomics as a complementary tool for identifying candidate genes in abiotic stress tolerance in cereals. This study has provided potential candidates for genetic manipulation of wheat cultivars to enhance drought tolerance. Any such studies require validation of the protein changes by transcriptomic and biochemical (enzyme assays) studies before the transgenic experiments are initiated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by grants to the Australian Centre for Plant Functional Genomics (ACPFG) from the Australian Research Council (ARC) and the Grains Research & Development Corporation (GRDC), the South Australian Government, the University of Adelaide, the University of Queensland and the University of Melbourne. The authors also wish to thank Dr. Siria Natera for protein extraction and Dr. Juan Juttner for the collection of plant material.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Plant_Proteomics/10.3389/fpls.2011.00044/abstract
Table S1. Complete list of proteins and corresponding peptides within functional categories.
Table S2. iTRAQ results, fold change and SE of cyclic drought-treated samples compared to well-watered controls. Significant changes indicated in bold, increases in red, and decreases in green.
Aro, E. M., Virgin, I., and Andersson, B. (1993). Photoinhibition of photosystem-II – inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–134.
Barrs, H. D., and Weatherley, P. E. (1962). A re-examination of relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 15, 413–428.
Bazargani, M. M., Sarhadi, E., Bushehri, A.-A. S., Matros, A., Mock, H.-P., Naghavi, M.-R., Hajihoseini, V., Mardi, M., Hajirezaei, M.-R., Moradi, F., Ehdaie, B., and Salekdeh, G. H. (2011). A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J. Prot. doi: 10.1016/j.jprot.2011.05.015. [Epub ahead of print].
Berthold, M. R., Cebron, N., Dill, F., Gabriel, T. R., Kotter, T., Meinl, T., Ohl, P., Sieb, C., Thiel, K., and Wiswedel, B. (2008). “KNIME: the Konstanz information miner,” in Data Analysis, Machine Learning and Applications, eds C. Preisach, H. Burkhardt, L. Schmidtthieme, and R. Decker (Berlin: Springer-Verlag), 319–326.
Caruso, G., Cavaliere, C., Foglia, P., Gubbiotti, R., Samperi, R., and Lagana, A. (2009). Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGE and MALDI-TOF mass spectrometry. Plant Sci. 177, 570–576.
Chaves, M. M., Maroco, J. P., and Pereira, J. S. (2003). Understanding plant responses to drought – from genes to the whole plant. Funct. Plant Biol. 30, 239–264.
Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P. P., Osorio, M. L., Carvalho, I., Faria, T., and Pinheiro, C. (2002). How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot. 89, 907–916.
Damerval, C., Devienne, D., Zivy, M., and Thiellement, H. (1986). Technical improvements in two-dimensional electrophoresis increase the level of genetic-variation detected in wheat-seedling proteins. Electrophoresis 7, 52–54.
Danyluk, J., Perron, A., Houde, M., Limin, A., Fowler, B., Benhamou, N., and Sarhan, F. (1998). Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell 10, 623–638.
Eberhard, S., Finazzi, G., and Wollman, F. A. (2008). The dynamics of photosynthesis. Annu. Rev. Genet. 42, 463–515.
Foyer, C. H., Lelandais, M., and Kunert, K. J. (1994). Photooxidative stress in plants. Physiol. Plant 92, 696–717.
Guo, P. G., Baum, M., Grando, S., Ceccarelli, S., Bai, G. H., Li, R. H., Von Korff, M., Varshney, R. K., Graner, A., and Valkoun, J. (2009). Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J. Exp. Bot. 60, 3531–3544.
Hajheidari, M., Eivazi, A., Buchanan, B. B., Wong, J. H., Majidi, I., and Salekdeh, G. H. (2007). Proteomics uncovers a role for redox in drought tolerance in wheat. J. Proteome Res. 6, 1451–1460.
Hare, P. D., Cress, W. A., and Van Staden, J. (1998). Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 21, 535–553.
Houde, M., Dallaire, S., N’dong, D., and Sarhan, F. (2004). Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol. J. 2, 381–387.
Ifuku, K., Ishihara, S., Shimamoto, R., Ido, K., and Sato, F. (2008). Structure, function, and evolution of the PsbP protein family in higher plants. Photosyn. Res. 98, 427–437.
Izanloo, A., Condon, A. G., Langridge, P., Tester, M., and Schnurbusch, T. (2008). Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 59, 3327–3346.
Kaiser, H., and Kappen, L. (1997). In situ observations of stomatal movements in different light-dark regimes: the influence of endogenous rhythmicity and long-term adjustments. J. Exp. Bot. 48, 1583–1589.
Kamal, A. H. M., Kim, K. H., Shin, K. H., Choi, J. S., Baik, B. K., Tsujimoto, H., Heo, H. Y., Park, C. S., and Woo, S. H. (2010). Abiotic stress responsive proteins of wheat grain determined using proteomics technique. Aust. J. Crop. Sci. 4, 196–208.
Kusano, T., Tateda, C., Berberich, T., and Takahashi, Y. (2009). Voltage-dependent anion channels: their roles in plant defense and cell death. Plant Cell Rep. 28, 1301–1308.
Lefebvre, B., Timmers, T., Mbengue, M., Moreau, S., Herve, C., Toth, K., Bittencourt-Silvestre, J., Klaus, D., Deslandes, L., Godiard, L., Murray, J. D., Udvardi, M. K., Raffaele, S., Mongrand, S., Cullimore, J., Gamas, P., Niebel, A., and Ott, T. (2010). A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. U.S.A. 107, 2343–2348.
Lopez-Martin, M. C., Becana, M., Romero, L. C., and Gotor, C. (2008). Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant Physiol. 147, 562–572.
Luna, C. M., Pastori, G. M., Driscoll, S., Groten, K., Bernard, S., and Foyer, C. H. (2005). Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 56, 417–423.
Maroco, J. P., Rodrigues, M. L., Lopes, C., and Chaves, M. M. (2002). Limitations to leaf photosynthesis in field-grown grapevine under drought – metabolic and modelling approaches. Funct. Plant Biol. 29, 451–459.
Miazek, A., and Zagdanska, B. (2008). Involvement of exopeptidases in dehydration tolerance of spring wheat seedlings. Biol. Plant. 52, 687–694.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410.
Mittler, R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11, 15–19.
Morgan, J. M. (1980). Osmotic adjustment in the spikelets and leaves of wheat. J. Exp. Bot. 31, 655–665.
Naponelli, V., Noiriel, A., Ziemak, M. J., Beverley, S. M., Lye, L. F., Plume, A. M., Botella, J. R., Loizeau, K., Ravanel, S., Rebeille, F., De Crecy-Lagard, V., and Hanson, A. D. (2008). Phylogenomic and functional analysis of pterin-4a-carbinolamine dehydratase family (COG2154) proteins in plants and microorganisms. Plant Physiol. 146, 1515–1527.
Oeljeklaus, S., Meyer, H. E., and Warscheid, B. (2009). Advancements in plant proteomics using quantitative mass spectrometry. J. Proteomics 72, 545–554.
Ouvrard, O., Cellier, F., Ferrare, K., Tousch, D., Lamaze, T., Dupuis, J. M., and Cassedelbart, F. (1996). Identification and expression of water stress- and abscisic acid-regulated genes in a drought-tolerant sunflower genotype. Plant Mol. Biol. 31, 819–829.
Pedrioli, P. G. A., Eng, J. K., Hubley, R., Vogelzang, M., Deutsch, E. W., Raught, B., Pratt, B., Nilsson, E., Angeletti, R. H., Apweiler, R., Cheung, K., Costello, C. E., Hermjakob, H., Huang, S., Julian, R. K., Kapp, E., Mccomb, M. E., Oliver, S. G., Omenn, G., Paton, N. W., Simpson, R., Smith, R., Taylor, C. F., Zhu, W. M., and Aebersold, R. (2004). A common open representation of mass spectrometry data and its application to proteomics research. Nat. Biotechnol. 22, 1459–1466.
Peng, Z. Y., Wang, M. C., Li, F., Lv, H. J., Li, C. L., and Xia, G. M. (2009). A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol. Cell. Proteomics 8, 2676–2686.
Perkins, D. N., Pappin, D. J. C., Creasy, D. M., and Cottrell, J. S. (1999). Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567.
Plucken, H., Muller, B., Grohmann, D., Westhoff, P., and Eichacker, L. A. (2002). The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 532, 85–90.
Rao, S. R., Ford, K. L., Cassin, A. M., Roessner, U., Patterson, J. H., and Bacic, A. (2010). Proteomic and metabolic profiling of rice suspension culture cells as a model to study abscisic acid signaling response pathways in plants. J. Proteome Res. 9, 6623–6634.
Reddy, A. R., Chaitanya, K. V., and Vivekanandan, M. (2004). Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 161, 1189–1202.
Sharma, A. D., and Singh, P. (2003). Comparative studies on drought-induced changes in peptidyl prolyl cis-trans isomerase activity in drought-tolerant and susceptible cultivars of Sorghum bicolor. Curr. Sci. 84, 911–918.
Simova-Stoilova, L., Demirevska, K., Petrova, T., Tsenov, N., and Feller, U. (2009). Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties subjected to long-term field drought. Plant Growth Regul. 58, 107–117.
Simova-Stoilova, L., Vaseva, I., Grigorova, B., Demirevska, K., and Feller, U. (2010). Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol. Biochem. 48, 200–206.
Sirpio, S., Khrouchtchova, A., Allahverdiyeva, Y., Hansson, M., Fristedt, R., Vener, A. V., Scheller, H. V., Jensen, P. E., Haldrup, A., and Aro, E. M. (2008). AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II. Plant J. 55, 639–651.
Sunkar, R., Bartels, D., and Kirch, H. H. (2003). Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 35, 452–464.
Takahashi, S., and Murata, N. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13, 178–182.
Tanaka, R., Oster, U., Kruse, E., Rudiger, W., and Grimm, B. (1999). Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol. 120, 695–704.
Tester, M., and Langridge, P. (2010). Breeding technologies to increase crop production in a changing world. Science 327, 818–822.
Thony, B., Auerbach, G., and Blau, N. (2000). Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347, 1–16.
Vercammen, D., Van De Cotte, B., De Jaeger, G., Eeckhout, D., Casteels, P., Vandepoele, K., Vandenberghe, I., Van Beeumen, J., Inze, D., and Van Breusegem, F. (2004). Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. J. Biol. Chem. 279, 45329–45336.
Wang, W. X., Vinocur, B., and Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14.
Watanabe, S., Nakagawa, A., Izumi, S., Shimada, H., and Sakamoto, A. (2010). RNA interference-mediated suppression of xanthine dehydrogenase reveals the role of purine metabolism in drought tolerance in Arabidopsis. FEBS Lett. 584, 1181–1186.
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Vanmontagu, M., Inze, D., and Vancamp, W. (1997). Catalase is a sink for H2O2 and is indispensable for stress defence in C-3 plants. EMBO J. 16, 4806–4816.
Williams, B., and Dickman, M. (2008). Plant programmed cell death: can’t live with it; can’t live without it. Mol. Plant Pathol. 9, 531–544.
Wisniewski, K., and Zagdanska, B. (2001). Genotype-dependent proteolytic response of spring wheat to water deficiency. J. Exp. Bot. 52, 1455–1463.
Yadav, S. K., Singla-Pareek, S. L., Ray, M., Reddy, M. K., and Sopory, S. K. (2005). Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 337, 61–67.
Yang, F., Jorgensen, A. D., Li, H. W., Sondergaard, I., Finnie, C., Svensson, B., Jiang, D., Wollenweber, B., and Jacobsen, S. (2011). Implications of high-temperature events and water deficits on protein profiles in wheat (Triticum aestivum L. cv. Vinjett) grain. Proteomics 11, 1684–1695.
Yang, S. J., Vanderbeld, B., Wan, J. X., and Huang, Y. F. (2010). Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol. Plant 3, 469–490.
Keywords: Triticum aestivum, bread wheat, drought, quantitative proteomics, iTRAQ
Citation: Ford KL, Cassin A and Bacic A (2011) Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance. Front. Plant Sci. 2:44. doi: 10.3389/fpls.2011.00044
Received: 21 June 2011;
Accepted: 13 August 2011;
Published online: 12 September 2011.
Edited by:
Harvey Millar, The University of Western Australia, AustraliaReviewed by:
Nicolas L. Taylor, The University of Western Australia, AustraliaCopyright: © 2011 Ford, Cassin and Bacic. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Antony Bacic, The Australian Research Council Centre of Excellence in Plant Cell Walls, School of Botany, University of Melbourne, Parkville, VIC 3010, Australia. e-mail:YWJhY2ljQHVuaW1lbGIuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.