
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Physiol. , 07 March 2025
Sec. Clinical and Translational Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1546307
This article is part of the Research Topic Physiological and Pathological Responses to Hypoxia and High Altitude, Volume III View all 8 articles
Acute mountain sickness (AMS) is a common condition following rapid exposure to high altitude, though severe complications such as acute gastrointestinal bleeding, systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) are rare. Herein, we report a case of SIRS and MODS in a young traveler who visited Lhasa, Tibet (elevation 3,650 m). Three days after arrival, the patient developed headache, abdominal pain, significant hematemesis, and persistent hypotension. Gastroscopy revealed diffuse bleeding of the gastric mucosa. Laboratory tests indicated multi-organ dysfunction involving the lungs, liver, and kidneys. The patient responded well to conservative treatment of continuous oxygen supplementation. This case represents one of the first reported instances of acute gastric mucosal injury and MODS induced by AMS, underscoring the significant medical risks associated with high-altitude environments.
Acute Mountain Sickness (AMS) is a common condition affecting individuals ascending to high altitudes, typically manifesting as headache, nausea, and dizziness (Gatterer et al., 2024). While AMS is generally self-limiting, severe cases can lead to life-threatening complications such as High Altitude Pulmonary Edema (HAPE) and High Altitude Cerebral Edema (HACE) (Pena et al., 2022; Miglani et al., 2020). However, critical illnesses stemming from AMS, particularly those involving multiple organ dysfunction syndrome (MODS), are relatively rare and may often be misdiagnosed in clinical settings (Richalet et al., 2024; Meier et al., 2017). Here, we present a case of a young patient who developed SIRS and MODS following rapid ascent to Lhasa, Tibet, at an elevation of 3,650 m. Despite the rarity of such severe manifestations, our patient exhibited significant clinical symptoms including persistent hypotension, diffuse gastric mucosal hemorrhage. Meanwhile, we conducted a literature review about the MODS in the context of AMS.
A 26-year-old male patient with normal body type presented to the emergency department of the General Hospital of Tibet Military Command (Lhasa, Tibet, China) on 5 August 2024, with a primary complaint of shortness of breath, headache and abdominal pain accompanied by hematemesis for 1 day. The patient arrived in Lhasa (average altitude 3,650 m) by airplane from Shanghai (average altitude 3 m) 3 days prior. One day before admission, he developed difficulty breathing, epigastric pain, and massive hematemesis. The abdominal pain was described as intermittent distension, and hematemesis occurred six times with large volumes each time. The patient denied any history of gastric disorders, acetaminophen and dexamethasone administration or alcohol consumption. Upon examination, vital signs revealed a body temperature of 36.8°C, pulse 141 beats per minute, respiratory rate 31 breaths per minute, and blood pressure 86/42 mmHg. Without supplemental oxygen, the patient’s blood oxygen saturation was 28% by blood gas analysis. Distinct cyanosis of the lips was observed. Auscultation of the lungs revealed coarse breath sounds with diffuse wet rales. Mild tenderness in the epigastric region was noted without rebound tenderness or muscle guarding.
An urgent gastroscopy was performed, revealing acute diffuse hemorrhagic gastritis (Figure 1). A chest X-ray suggested possible high-altitude pulmonary edema (Figure 2A). Laboratory tests indicated elevated white blood cell count (WBC) at 17.1 × 109/L (normal range: 3.5–9.5 × 109/L), decreased platelets (PLT) at 45 × 109/L (normal range: 125–350 × 109/L), increased C-reactive protein (CRP) at 38.1 mg/L (normal range: 0–10 mg/L), significantly elevated alanine aminotransferase (ALT) 3978 U/L (normal range: 9–50 U/L), aspartate aminotransferase (AST) 5687 U/L, normal range: 15–40 U/L), lactate dehydrogenase (LDH) 7825 U/L (normal range: 120–250 U/L), creatinine (Crea) at 348.7 μmol/L (normal range: 57–97 μmol/L), and Urea nitrogen (Urea) at 26.01 mmol/L (normal range: 3.1–8.0 mmol/L). Arterial blood gas analysis showed a partial pressure of oxygen (PaO2) of 55 mmHg (normal range: 83–108 mmHg). These laboratory findings suggested the presence of MODS, including impairment of pulmonary, hepatic, and renal functions.
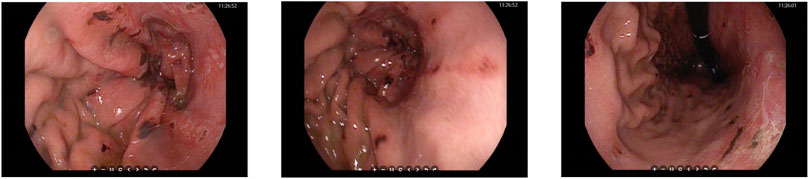
Figure 1. Gastroscopy images showing diffuse bleeding points on the gastric mucosa in the body of the stomach.
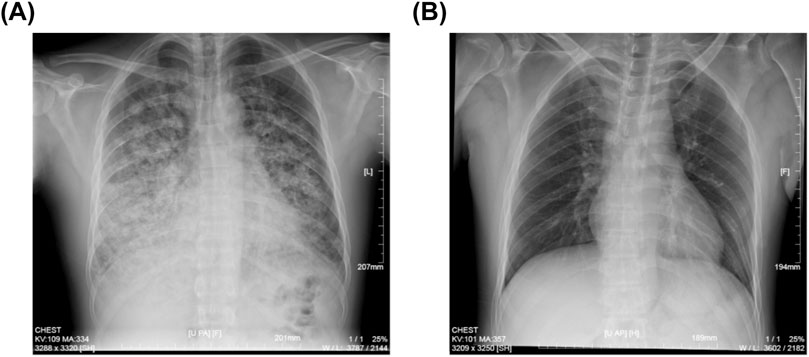
Figure 2. Chest X-ray images of the patient. (A) Spine position of chest X-ray image showing the HAPE at the patient’s admission to our hospital. (B) Upright position of chest X-ray image after continuous oxygen supplementation of 3 days.
The patient was immediately placed on continuous low-flow oxygen therapy (2–3 L/min). Following the initiation of oxygen therapy, the patient ceased hematemesis and the abdominal pain resolved. By the third day of hospitalization, fecal occult blood testing was negative. The patient’s respiratory distress improved significantly, and a follow-up chest X-ray on the third day demonstrated complete resolution of the pulmonary infiltrates (Figure 2B). Subsequent laboratory tests showed significant recovery in PaO2, PaCO2, pH, liver function tests, and renal function indicators (Figure 3).
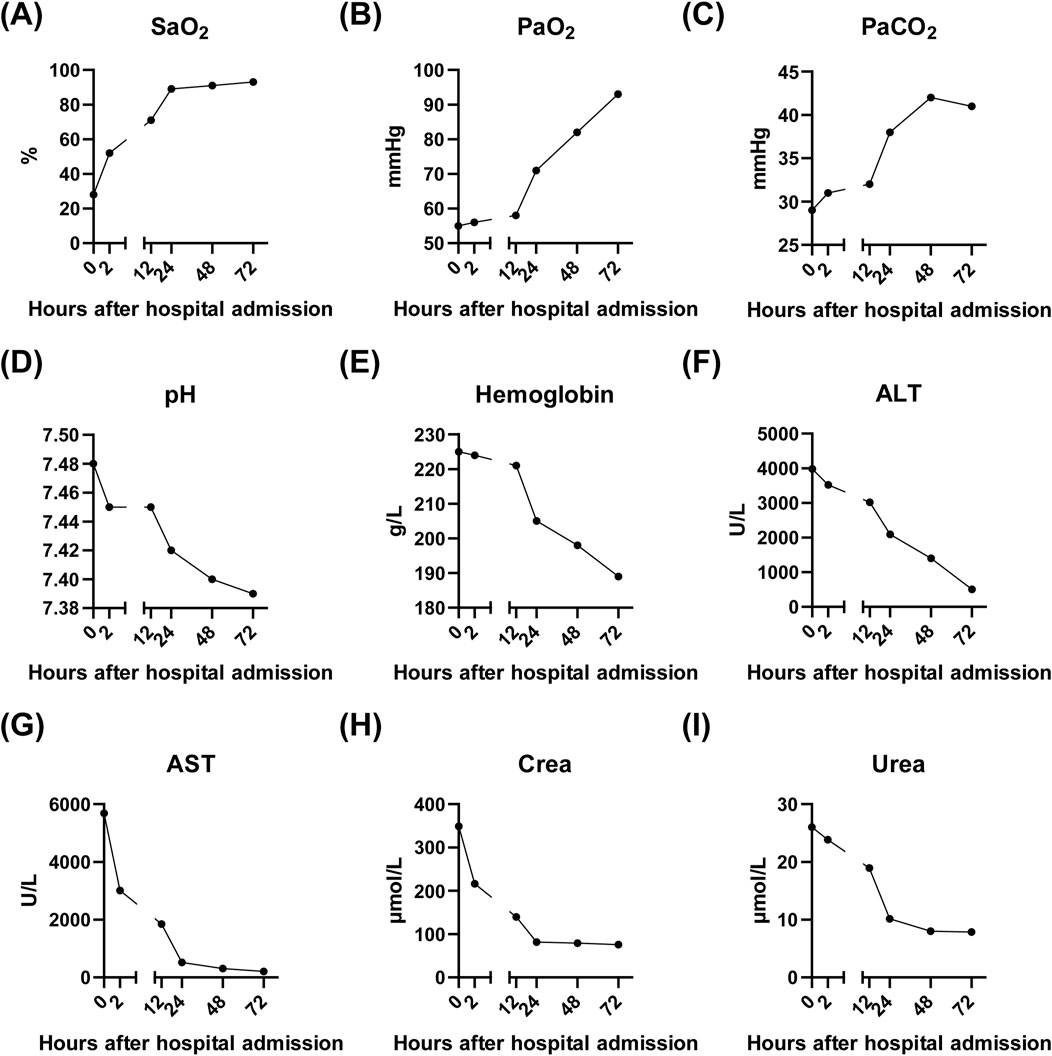
Figure 3. Laboratory results of the patient after admission to our hospital. (A–E) showing the recovery of lung function after continuous oxygen supplementation of 3 days. (F–G) showing the recovery of liver function after continuous oxygen supplementation of 3 days. (H–I) showing the recovery of kidney function after continuous oxygen supplementation of 3 days. SaO2, Arterial oxygen saturation; PaO2, Partial pressure of oxygen in arterial blood; PaCO2, Partial pressure of carbon dioxide in arterial blood; pH, Potential of hydrogen in arterial blood; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Crea, Creatinine; Urea, Urea nitrogen.
AMS symptoms vary in severity and onset time, typically appearing within 6–24 h after reaching high altitudes above 2,500 m (Gatterer et al., 2024). Severe forms of AMS can progress to HACE, HAPE, and even MODS, all of which are medical emergencies requiring prompt descent and medical intervention (Simancas-Racines et al., 2018). Early recognition and appropriate management are crucial to prevent life-threatening complications (Luks et al., 2017).
Table 1 summarizes the key features of nine patients reported in the literature who developed MODS following AMS. Notably, these patients were predominantly young or even children. They presented with initial symptoms of AMS, which were often dismissed or underestimated. Over subsequent days, their condition deteriorated significantly, necessitating intensive care unit (ICU) admission. A striking observation was the frequent occurrence of pulmonary dysfunction among these patients, suggesting that hypoxia post-ascent plays a pivotal role in triggering MODS. Fortunately, these patients responded well to oxygen therapy, and their condition was rapidly brought under control.
While AMS typically presents with headache, nausea, dizziness, fatigue, and sleep disturbances, severe cases can lead to complications such as gastrointestinal bleeding, SIRS and MODS. However, considering that AMS induced gastrointestinal ulcers and MODS are very rare, most clinics may focus more on the patient’s clinical symptoms (hematemesis, liver and kidney function damage, etc.) and overlook the patient’s medical history (Gatterer et al., 2024, Luks et al., 2017). Since oxygen therapy represents the cornerstone of AMS management, misdiagnosis and subsequent inappropriate treatment strategies may significantly compromise therapeutic efficacy.
The pathogenesis of AMS-induced gastrointestinal bleeding, SIRS and subsequent MODS involves a complex interplay of hypoxia, systemic inflammation, and oxidative stress (Pena et al., 2022, Wu et al., 2007a). Rapid ascent to high altitudes leads to reduced barometric pressure and oxygen availability, causing tissue hypoxia (Titz et al., 2024). This triggers compensatory mechanisms such as increased ventilation and heart rate, which, while attempting to enhance oxygen delivery, can exacerbate tissue hypoxia and contribute to organ dysfunction (Wilkins et al., 2015; Wu et al., 2007b; Wu and Liu, 2006). Concurrently, individuals ascending to elevated altitudes may encounter acid-base disturbances, notably respiratory alkalosis resulting in decreased PaCO2 levels. This physiological response could potentially serve as a pathogenic mechanism underlying acute gastric mucosal injury (Krishnan and Lotfollahzadeh, 2025). Furthermore, the possible influence of heightened adrenaline concentrations during stressful conditions must be considered, as it may also play a role in the development of gastric mucosal lesions (Guzman and Kruse, 1985).
Oxygen therapy is a cornerstone in the management of AMS and its complications, including gastrointestinal bleeding, SIRS and MODS (Schneider et al., 2024). By correcting hypoxia, oxygen therapy improves tissue oxygenation, reduces edema, and mitigates systemic inflammation (West, 2015). In patients with AMS-induced gastrointestinal bleeding, oxygen therapy can stabilize hemodynamic parameters, improve mucosal healing, and reduce the risk of further bleeding. For those progressing to MODS, oxygen therapy is still crucial in preventing organ failure and improving overall outcomes.
In conclusion, AMS-induced gastrointestinal bleeding and multiple organ dysfunction syndrome represent severe complications of high-altitude exposure that demand prompt recognition and aggressive management. This discussion underscores the importance of early diagnosis through meticulous clinical evaluation and emphasizes the lifesaving potential of timely oxygen therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethical Committee of General Hospital, Lhasa, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
BW: Formal Analysis, Funding acquisition, Writing–original draft. MP: Data curation, Writing–original draft. GK: Data curation, Writing–original draft. FF: Data curation, Writing–original draft. JG: Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Fund of Tibet Autonomous Region (ZRKX2024000383).
The authors are grateful to the many medical staff for their dedication of the patient.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Asseri A. A., Asiri I. A., Alwabel H. H., Asiri A. M., Asiri W. I. (2022). Severe acute reentry high altitude pulmonary edema in pediatric patients: report of three cases and literature review. Turk J. Pediatr. 64, 400–407. doi:10.24953/turkjped.2021.611
Bhandari S. S., Koirala P., Regmi N., Pant S. (2017). Retinal hemorrhage in a high-altitude aid post volunteer doctor: a case report. High. Alt. Med. Biol. 18, 285–287. doi:10.1089/ham.2017.0003
Brar K. S., Garg M. K. (2012). High altitude-induced pituitary apoplexy. Singap. Med. J. 53, e117–e119.
Gatterer H., Villafuerte F. C., Ulrich S., Bhandari S. S., Keyes L. E., Burtscher M. (2024). Altitude illnesses. Nat. Rev. Dis. Prim. 10, 43. doi:10.1038/s41572-024-00526-w
Gilbert-Kawai E., Martin D., Grocott M., Levett D. (2016). High altitude-related hypertensive crisis and acute kidney injury in an asymptomatic healthy individual. Extrem Physiol. Med. 5, 10. doi:10.1186/s13728-016-0051-3
Guzman J. A., Kruse J. A. (1985). Splanchnic hemodynamics and gut mucosal-arterial PCO(2) gradient during systemic hypocapnia. J. Appl. Physiol. 87, 1102–1106. doi:10.1152/jappl.1999.87.3.1102
Krishnan P., Lotfollahzadeh S. (2025). “Spontaneous intestinal perforation of the newborn,” in StatPearls (Treasure Island (FL): StatPearls Publishing).
Luks A. M., Swenson E. R., Bartsch P. (2017). Acute high-altitude sickness. Eur. Respir. Rev., 26. doi:10.1183/16000617.0096-2016
Meier D., Collet T. H., Locatelli I., Cornuz J., Kayser B., Simel D. L., et al. (2017). Does this patient have acute Mountain Sickness? The rational clinical examination systematic review. JAMA 318, 1810–1819. doi:10.1001/jama.2017.16192
Miglani M., Rain M., Pasha Q., Raj V. S., Thinlas T., Mohammad G., et al. (2020). Shorter telomere length, higher telomerase activity in association with tankyrase gene polymorphism contribute to high-altitude pulmonary edema. Hum. Mol. Genet. 29, 3094–3106. doi:10.1093/hmg/ddaa205
Pena E., El A. S., Siques P., Brito J. (2022). Oxidative stress and diseases associated with high-altitude exposure. Antioxidants (Basel) 11, 267. doi:10.3390/antiox11020267
Richalet J. P., Hermand E., Lhuissier F. J. (2024). Cardiovascular physiology and pathophysiology at high altitude. Nat. Rev. Cardiol. 21, 75–88. doi:10.1038/s41569-023-00924-9
Schneider S. R., Muller J., Bauer M., Mayer L., Luond L., Ulrich T., et al. (2024). Overnight exposure to high altitude in pulmonary hypertension: adverse events and effect of oxygen therapy. Eur. HEART J. 45, 309–311. doi:10.1093/eurheartj/ehad789
Simancas-Racines D., Arevalo-Rodriguez I., Osorio D., Franco J. V., Xu Y., Hidalgo R. (2018). Interventions for treating acute high altitude illness. Cochrane Database Syst. Rev. 6, CD009567. doi:10.1002/14651858.CD009567.pub2
Titz A., Schneider S., Mueller J., Mayer L., Lichtblau M., Ulrich S. (2024). Symposium review: high altitude travel with pulmonary vascular disease. J. Physiol. 602, 5505–5513. doi:10.1113/JP284585
Wang S. Y., Liang J., Zhao J. H. (2024). A case of high-altitude renal syndrome. High. Alt. Med. Biol. 25, 149–151. doi:10.1089/ham.2023.0077
Wei Q., Yu T. C., Hua M. X., Xue H. Z., Zi L., Ping F. (2009). Continuous renal replacement therapy in the treatment of severe acute mountain sickness. Ren. Fail 31, 175–177. doi:10.1080/08860220802598256
West J. B. (2015). High-altitude medicine. Lancet Respir. Med. 3, 12–13. doi:10.1016/S2213-2600(14)70238-3
Wilkins M. R., Ghofrani H. A., Weissmann N., Aldashev A., Zhao L. (2015). Pathophysiology and treatment of high-altitude pulmonary vascular disease. CIRCULATION 131, 582–590. doi:10.1161/CIRCULATIONAHA.114.006977
Wu T., Liu J. (2006). Alcohol and aspirin in combination with dexamethasone causes gastrointestinal bleeding at high altitude. Wilderness Environ. Med. 17, 69–71. doi:10.1580/1080-6032(2006)17[69:aaaicw]2.0.co;2
Wu T. Y., Ding S. Q., Liu J. L., Jia J. H., Dai R. C., Zhu D. C., et al. (2007b). High-altitude gastrointestinal bleeding: an observation in Qinghai-Tibetan railroad construction workers on Mountain Tanggula. World J. Gastroenterol. 13, 774–780. doi:10.3748/wjg.v13.i5.774
Wu T. Y., Ding S. Q., Liu J. L., Yu M. T., Jia J. H., Chai Z. C., et al. (2007a). Who should not go high: chronic disease and work at altitude during construction of the Qinghai-Tibet railroad. High. Alt. Med. Biol. 8, 88–107. doi:10.1089/ham.2007.1015
Keywords: acute mountain sickness, acute gastrointestinal bleeding, systemic inflammatory response syndrome, multiple organ dysfunction syndrome, oxygen therapy
Citation: Wang B, Peng M, Kou G, Fang F and Gao J (2025) Systemic inflammatory response syndrome and multiple organ dysfunction syndrome caused by acute mountain sickness: a case report and literature review. Front. Physiol. 16:1546307. doi: 10.3389/fphys.2025.1546307
Received: 31 December 2024; Accepted: 14 February 2025;
Published: 07 March 2025.
Edited by:
Tatum S. Simonson, University of California, San Diego, United StatesReviewed by:
Melissa L. Bates, The University of Iowa, United StatesCopyright © 2025 Wang, Peng, Kou, Fang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bowen Wang, MTQ5MTg2NzY0MkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.