
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol. , 13 February 2025
Sec. Exercise Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1542322
Knee pain, affecting an estimated 654 million people worldwide, so blood flow restriction training (BFRT) is catching the spotlight as an effective intervention. Evidence continues to demonstrate the effectiveness of BFRT in managing knee pain. However, the mechanism by which BFRT alleviates knee pain remains unclear, thereby limiting its application in clinical pain management. This study aims to elucidate the underlying mechanisms of BFRT to better understand its efficacy in treating knee pain. This review will discuss the influence of muscle hypertrophy, endogenous opioid system, endocannabinoids, inflammation regulation, and conditional pain regulation on BFRT treatment of knee pain. Current studies on BFRT have limitations, such as small sample sizes, relatively low-quality evidence, and lack of mechanistic studies. Therefore, further research on BFRT is needed, particularly high-quality and large-sized randomized controlled trials.
Knee pain affects millions worldwide, diminishing quality of life and mobility. As conventional treatments demonstrate limitations, blood flow restriction training (BFRT) emerges as a promising alternative. Approximately 5% of all primary care visits in adults are related to knee pain, which reduces function and mobility (Duong, et al., 2023). The prevalence of knee pain and symptomatic knee osteoarthritis (KOA) has increased over the course of 20 years, approximately doubling in women and tripling in men, and they now account for almost 4 million primary care visits per year (Nguyen et al., 2011). Patellar Tendinopathy (PT), anterior cruciate ligament (ACL) injury, and KOA are some of the factors that contribute to knee pain. The most typical reason for knee pain in persons 45 years and older is KOA (Duong, et al., 2023). More than half of the elderly (Kim et al., 2011) and 19%–31% of adolescents (Smith et al., 2018) currently have knee pain, and long-term knee pain may lead to a decline in quality of life (Crossley et al., 2016; Deshpande et al., 2016).
For various diseases that cause knee pain, exercise is recommended for pain management (Kolasinski et al., 2020; Uthman et al., 2013). Different types of exercise have been shown to have a positive effect on chronic pain (Fransen et al., 2015; Goh et al., 2019; Hussain et al., 2016; Imoto et al., 2019; Kan et al., 2019; van den Ende et al., 2000). Several authoritative organizations, including the American Academy of Orthopedic Surgeons (Jevsevar, 2013), the Osteoarthritis Research Society International (Bannuru et al., 2019), and the American College of Rheumatology (Hochberg et al., 2012), have noted that the enhancement of lower limb muscle strength could effectively reduce pain. BFRT was defined as a method of blood flow restriction combined with resistance training (Barber-Westin and Noyes, 2019). The benefit of BFRT is that loads around 30% of maximum force provide outcomes that are comparable to or greater than 80% of maximum force (Jack et al., 2022; Minniti, et al., 2020). BFRT has been demonstrated to be a secure and efficient training technique that builds muscular growth and strength (Loenneke et al., 2012b; Minniti et al., 2020).
To date, several reviews and meta-analyses have investigated the clinical efficacy of BFRT in the treatment of knee disease. A systematic review showed evidence of a potential benefit of perioperative BFRT for muscular mass in those undergoing ACL reconstruction (Lu et al., 2020). High-load BFRT could considerably enhance muscular strength in individuals with knee injuries according to the meta-analysis of Li et al., and low-load BFRT could remarkably reduce pain intensity (Li et al., 2021). Recent literature has demonstrated the efficacy of BFRT in alleviating pain associated with PT (Burton and McCormack, 2022). Besides, a systematic review of KOA showed that BFRT was effective in increasing muscle strength and reducing pain (Pitsillides et al., 2021). For both patients with KOA and those with ACL injuries, BFRT can provide more effective pain relief. Although the current review supports the role of BFRT, less clinical evidence exists and more studies are needed to draw more definitive conclusions. However, all of these reviews only described the role of BFRT and not the underlying mechanisms that BFRT is used for knee pain. To the authors’ knowledge, only one review has mentioned the mechanism of pain reduction after BFRT (Song et al., 2021), and the study mentioned that pain reduction after BFRT may be related to activation of endogenous opioid and cannabinoid system, high threshold motor unit recruitment, cardiovascular system, and conditioned pain modulation. However, no studies have reviewed the underlying mechanisms of BFRT in the treatment of knee pain. This review explores the underlying mechanisms of BFRT to provide a comprehensive understanding of its role in knee pain management, thereby providing a theoretical foundation for future research on the impact of BFRT on patients with knee pain.
BFRT have been increasingly used to treat knee pain. Mahmoud et al. recruited 35 subjects and observed that a combination of 70% of total occlusion pressure with 30% 1-RM training was effective in relieving pain (Mahmoud et al., 2021). Giles et al. recruited 79 people to perform BFRT or resistance training three times a week for 8 weeks. The results showed that the BFRT group experienced a 93% reduction in daily life pain over 8 weeks, but the worst pain was not statistically different between the two groups (Giles et al., 2017). Knee pain was also lower with BFRT during and at 24 h post-training after 8 weeks of twice-weekly BFRT than with high-load resistance training (HL-RT) (Hughes et al., 2019). Another study compared the clinical effects of 3-week conventional resistance training and twice-daily BFRT. The results showed that the pain scores during training were considerably lower over time in the BFRT group (Ladlow et al., 2018). Ferraz et al. also supported that BFRT could relieve knee pain and reduce joint stress compared with HL-RT (Ferraz et al., 2018). In addition, Tennent et al. recruited 17 people and assigned them to either the BFRT group or the standard physical therapy group for 12 sessions. The Knee Injury and Osteoarthritis Outcome Scores improved obviously on several subscales in both groups, with the BFRT group showing 1.5–2 times improvement in all subscales (Tennent et al., 2017). However, research by Segal et al. showed that low-load BFRT (LL-BFRT) did not considerably improve knee pain (Segal et al., 2015). In this study, quadriceps strength exhibited a significant increase; however, no substantial improvements were observed in quadriceps volume or pain levels. This may potentially be attributed to insufficient training frequency or intensity (Segal et al., 2015). Despite the varying results observed, integrating these findings with an exploration of the underlying mechanisms will lead to more robust and substantiated conclusions. Table 1 summarizes the study characteristics of BFRT for knee pain. BFRT was effective in reducing knee pain in the majority of cases.
A strong quadriceps muscle is generally believed to provide structural stability and assistance to damaged and degenerated knees and thus effectively relieve knee pain (Lee et al., 2018). Meanwhile, muscle atrophy is also a serious complication of various knee joint diseases (Bannuru et al., 2019; Hochberg et al., 2012; Jevsevar, 2013). BFRT could induce muscle hypertrophic adaptation and increase muscle strength at the same time (Patterson et al., 2019). At present, muscle hypertrophy is considered to be mainly caused by mechanical tension and metabolic stress, and a large number of studies have shown that mechanical tension is the main mechanism of muscle growth. However, most BFRTs only use low-load resistance (30%–50% 1-RM), Therefore, BFRT generally could not induce mechanisms related to mechanical tension (Pearson and Hussain, 2015). Goto et al. (2005) conducted two groups of exercise programs (3–5 groups of 10 repetitions; the intensity was only 10-RM; the rest time between groups was 1 min; and the movements used pull-ups, shoulder presses, and bilateral knee extensions) for a completely consistent experimental comparison. The only difference between the acute and chronic effects was that one of the sets included a 30-s break at the midpoint of each set to alter exercise-induced metabolic stress. Acute hormonal responses were measured for both regimens followed by 12 weeks of resistance training. The results showed that the no-rest regimen induced stronger blood lactate concentration, growth hormone, epinephrine, and norepinephrine responses than the rest regimen. After 12 weeks of training, the no-rest program group had considerably increased maximal strength, maximal isometric strength, muscular endurance, and muscle cross-sectional area (CSA) in knee extension compared with the rest program group, revealing a link between metabolic stress and muscle hypertrophy.
Numerous studies have also shown that metabolic stress linked to BFRT may have hypertrophic effects, in which low-load resistance training (LL-RT, 30%–50% 1-RM) resulted in a remarkable increase in muscle CSA. In addition, literature has reported a direct relationship between other indicators of exercise metabolic stress and muscle hypertrophy after LL-BFRT (20% 1-RM). This finding may highlight a major role for metabolic stress causing muscle hypertrophic adaptations after BFRT. Exercise-induced metabolic stress has been hypothesized to mediate muscle hypertrophy through various mechanisms, including increased systemic hormone production, increased recruitment of fast-twitch fibers, cellular swelling, muscle damage, and increased reactive oxygen species (ROS) production. All of these factors are hypothesized to mediate muscle protein signaling and/or satellite cell proliferation to induce muscle growth.
This level of metabolic stress is also amplified under BFRT conditions. Blood lactate concentrations (Kon et al., 2012; Takarada et al., 2000) and Growth hormone increases were stronger and greater following LL-RT than after the same workout program without BFRT (Takarada et al., 2000). A number of studies have demonstrated the potential hypertrophic effects of metabolic stress associated with BFRT, in which LL-RT (30%–50% 1-RM) resulted in a evident increase in muscle CSA (Takarada et al., 2000; Takarada et al., 2002; Takarada et al., 2004). In addition, the literature has reported a direct relationship between other indicators of exercise metabolic stress and muscle hypertrophy after LL-BFRT (20% 1RM) (Takada et al., 2012). This may highlight a major role for metabolic stress causing muscle hypertrophic adaptations after BFRT. Exercise-induced metabolic stress is thought to mediate muscle hypertrophy via a variety of mechanisms, including increased systemic hormone production (Reeves et al., 2006), increased recruitment of fast-twitch fibers (Takarada et al., 2002), cellular swelling (Berneis, et al., 1999; Loenneke, et al., 2012a), muscle damage (Schoenfeld, 2013), and increased ROS production (Pope et al., 2013; Schiaffino et al., 2013), all of these are hypothesized to play a role in satellite cell proliferation and/or muscle protein signaling to promote muscular growth. It is also noteworthy that a recent review has highlighted the varying effects of BFRT on muscle hypertrophy across different training states. Highly trained individuals may achieve superior strength and hypertrophy gains through BFRT compared to their untrained counterparts (Geng, et al., 2024). This represents a significant advancement for individuals suffering from PT. PT is a degenerative condition that affects the patellar tendon, typically resulting from prolonged overstretching or repetitive overuse of the tendon (Burton and McCormack, 2022). This suggests that BFRT may yield superior outcomes for the treatment of PT.
Beta-endorphin (BE) is an opioid neuropeptide that is considered one of the important substances affecting exercise-induced hypoalgesia (EIH) (Rice et al., 2019; Vaegter and Jones, 2020). The circulating concentration of BE increases after human exercise, which results from the activation of the opioid system after stimulating afferent fibers in groups III and IV in contractile muscles (Thorén et al., 1990). This phenomenon triggers the release of BE by the pituitary gland and peripheral neurons functioning as an agonist for opioid receptors, which are widely distributed in the central and peripheral nervous systems’ descending pain control circuits. BEs provide analgesic effects by attaching to opioid receptors in presynaptic and postsynaptic nerve endings in the peripheral nervous system, thereby inhibiting the tachykinin substance P, which is known to play a major role in pain transmission. Considering that the central nervous system also contains opioid receptors, BEs could act as an analgesic through this system. Instead of blocking the tachykinin substance P in the central nervous system, BEs provide analgesia by boosting dopamine release (Sprouse-Blum et al., 2010). Studies have shown that taking opioid agonists, such as naloxone, may reduce hypoalgesia, affirming the part the opioid system plays in hypoalgesia (Haier et al., 1981). Hughes and Patterson (2020) compared the acute effects of LL-RT and BFRT on pain sensitivity. The pain threshold of the moving limb was found to be higher after low- and high-pressure BFRT and HL-RT than after LL-RT (26%–48% vs. 10%), and after BFRT40 (p < 0.01, d = 0.47) and BFRT80 (p > 0.01, d > 0.49) than after HL-RT, indicating that BFRT was involved in the opioid-mediated mechanisms in EIH. However, after 24 h of exercise, the BE levels of all four groups and the pressure pain thresholds (PPTs) after LLRE and HLRE returned to baseline levels. Moreover, the PPT of motor limbs after low- and high-pressure BFRT remained above baseline (15% and 24%, respectively). Therefore, EIH caused by BFRT may be associated with more than just endogenous opioid production.
The endogenous cannabinoid (ECB) system belongs to the non-opioid mechanism (Koltyn, et al., 2014), which is situated in the central nervous system’s region responsible for processing pain (Hohmann and Suplita, 2006). Some studies suggest that the non-opioid mechanism may also be related to EIH (Vaegter et al., 2019). The ECB system is a neuromodulatory system that is composed of cannabinoid receptors and endogenous ligand agonists (including anandamide and 2-indoloylglycerol [2-AG]) found at pain-treating sites on the peripheral and spinal cord and in proteins that regulate their metabolism (Starowicz et al., 2013; Vaegter et al., 2019). Under stress conditions such as exercise, cells synthesize and rapidly release ECB, and ECB produces analgesic effects by binding to cannabinoid receptors (Feuerecker et al., 2012). Studies have shown elevated ECB concentrations following exercise, pointing to a potential role for the ECB system in EIH. This hypothesis are supports by the ECB receptors that activated during muscle contraction, which dense exist on Aδ and Cδ primary afferent fibers (Feuerecker et al., 2012). The receptor activated during muscle contraction, resulting in changes in circulating concentrations of ECB (Koltyn et al., 2014).
Current animal studies have shown that opioid or non-opioid hypoalgesia mechanisms could be elicited by adjusting the duration and intensity of exercise stressors (Starowicz et al., 2013). However, studies on BFRT have only measured the concentration of 2-AG and discovered that it remained constant over time (pre-exercise, 5 min post-workout, and 24 h post-workout) (Hughes and Patterson, 2020). Previous research has demonstrated that intense exercise does not alter circulating 2-AG concentrations, whereas another ECB (called anandamide) has been found to increase (Heyman, et al., 2012). Which could be based on their various metabolic pathways. As a result, how BFRT affects other ECB substances is unclear.
Under normal physiological conditions, the inflammatory response is the response of the immune system when harmful stimulus causes damage to the body (Weiss, 2008). In recent years, evidence showed that the inflammatory response has a remarkable effect on knee pain, such as increased inflammation, increased interleukin (IL)-1β, and increased macrophages in the subpatellar fat pad (Bastiaansen-Jenniskens et al., 2012), which could speed up the deterioration of the cartilage by allowing the deep-lying nerve fibers in the subchondral bone and cartilage to send pain signals to the brain (Adatia et al., 2012). Studies have revealed that elevated inflammatory molecules, such as IL-6, tumor necrosis factor-α (TNF-α), and MMP-13, were present in the knee synovial fluid of patients with KOA who are in pain (Runhaar et al., 2019). After treatment, the inflammatory molecules were remarkably reduced (Schell et al., 2017). Thus, synovial fluid inflammation is a major contributor to the emergence of KOA-related pain. These results suggested that reducing knee pain by suppressing inflammation may be a viable mechanism.
At present, a large number of animal experiments could prove that exercise training could reduce the expression of inflammatory molecules, such as IL-1β, caspase-3, and MMP-13, to effectively prevent inflammation (Adatia et al., 2012). However, the effect of BFRT on inflammatory biomarkers is minimal. BFRT only resulted in a 163% increase in the anti-inflammatory macrophage phenotype after 3 weeks as opposed to LL-RT (20% 1-RM) or HL-RT (70% 1-RM). In addition, the pro-inflammatory macrophage levels were higher in the LL-RT group than in the BFRT group 3 days after the end of the training period. An 18% decrease in plasma IL-6 before BFRT treatment to 180 min was also recorded, whereas no change was observed in the high-load control group (Nielsen et al., 2017). Biweekly BFRT after 8 weeks tended to lower the levels of inflammatory biomarkers after training (Kambič et al., 2019). Some evidence showed that BFRT may have anti-inflammatory properties. However, few research has been conducted in this area, and additional investigation is required to understand the precise mechanistic connection between BFRT and pro-inflammatory factors.
CPM is a phenomenon known as “pain suppression” that is often used to assess the body’s ability to regulate pain (Kennedy et al., 2016). In human studies, CPM is usually triggered by the application of a nourishing harmful cold stimulus, which could lead to an acute ectopic decrease in pain sensitivity (Pud et al., 2005). Therefore, it is occasionally utilized as an experimental measurement of human endogenous pain suppression pathways in humans (Pud et al., 2009; van Wijk and Veldhuijzen, 2010). In brief, by comparing the pain sensitivity in different parts of the body, CPM measures the conditioned pain conditioning during or after painful stimuli (i.e., conditioned stimuli and test stimulus) and in the absence of conditioned stimuli (Yarnitsky et al., 2010). Opioid decreasing the inhibition of pathway activation has been suggested as an underlying mechanism for CPM, as opioid-treated subjects experienced reduced CPM (Ram et al., 2008). Therefore, some studies suggested that CPM and EIH may have a common mechanism. For example, in populations with and without chronic pain, a considerable correlation was found between the magnitude of CPM and EIH, indicating that CPM and EIH may activate the same downward inhibitory pathway (Fingleton et al., 2017; Lemley et al., 2015). Meanwhile, studies advocating the opposing viewpoint contended that the time course and spatial distribution of CPM and EIH are different (Rice et al., 2019). When a rise in pain threshold was observed only during and immediately after conditioned stimulation, EIH lasted longer (about 15 min) than CPM (Vaegter et al., 2014). In addition to differences in time course, their analgesic effects present a different spatial distribution. For example, CPM has greater analgesia effects in non-localized sites than in localized sites. Conversely, EIH has a greater effect of analgesia in local sites than in non-localized sites (Vaegter et al., 2014). In conclusion, the research highlighted the possibility that the mechanisms underlying conditioned pain regulation and EIH are distinct.
The discomfort in BFRT is similar to that in HL-RT (Martín-Hernández et al., 2017; Mattocks et al., 2019). The increase in discomfort during BFRT may act as a conditioned stimulus and trigger CPM (Hughes and Patterson, 2019). In comparison to LL-RT and HL-RT without BFRT, BFRT caused an increase in PPTs and muscle discomfort (Hughes and Patterson, 2020). In a recent study, there was a significant association between muscle discomfort and pain. This suggesting that CPM may be triggered after BFRT. However, the PPT in this study remained higher than baseline 24 h after exercise under BFRT, which could not be explained by CPM because muscle soreness due to exercise is unlikely to affect outcomes 24 h after exercise (Hughes and Patterson, 2020). This result led to the conclusion that local mechanisms, as opposed to CPM, are primarily responsible for prolonged hypoalgesia. However, CPM remains one of the underlying mechanisms of BFRT in the treatment of knee pain.
This review delves into the underlying mechanisms of BFRT (Figure 1). BFRT may hold promise for patients with KOA and ACL injuries; however, additional research is warranted, particularly high-quality, large-sample randomized controlled trials. A thorough understanding of the mechanism of BFRT is essential for developing personalized rehabilitation programs tailored to patients with various conditions.
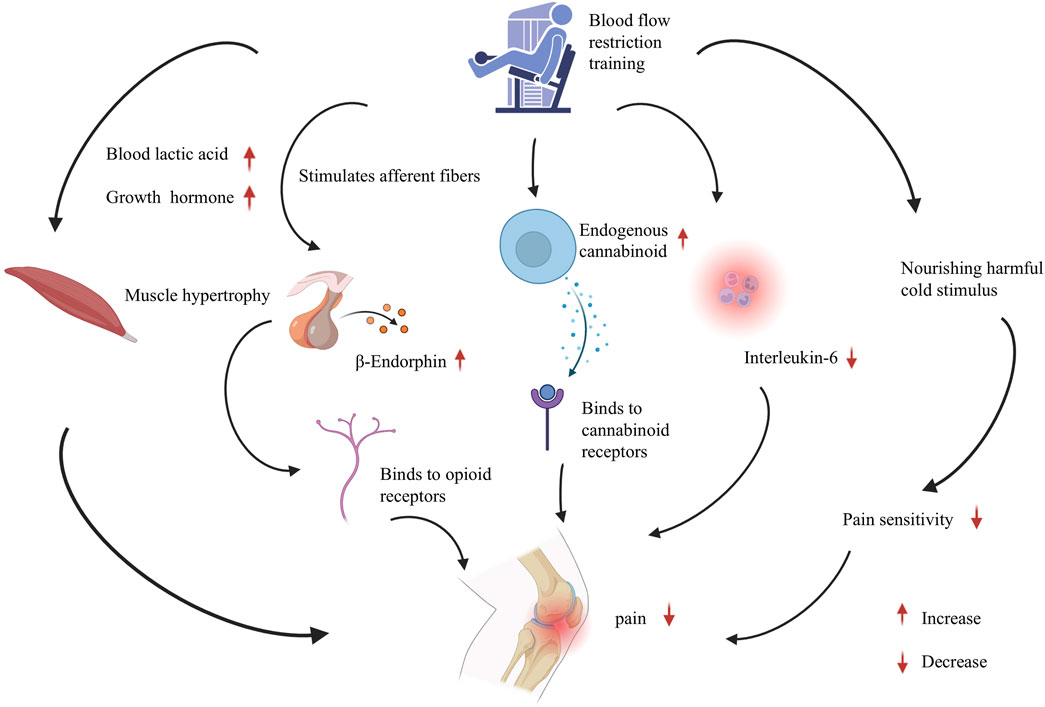
Figure 1. The involved mechanisms in blood flow restriction training on knee pain. The analgesic mechanism of blood flow restriction training for knee pain, involving muscle hypertrophy, endogenous opioid system, endocannabinoids, inflammation regulation, and conditional pain regulation. Abbreviations: BFRT, Blood flow restriction training; BE, Beta-endorphin; ECB, Endogenous cannabinoid; IL-6, nterleukin-6.
At present, only one study has examined the changes of BE and 2-AG in patients after BFRT, without valuable findings. The limited studies could not fully explain that EIH and ECB are the potential mechanisms of BFRT in the treatment of knee pain. The relationship between another endogenous ligand agonist (anandamide) and BFRT has yet to be studied to fill the gap between BFRT and ECB. A substantial body of high-quality randomized controlled trials is warranted in future research to investigate underexplored areas, particularly focusing on anandamide levels and CPM mechanisms. Additionally, further studies should examine the long-term outcomes of BFRT and its effects across various age groups.
S-YX: Writing–original draft, Writing–review and editing. XJ: Writing–review and editing, Writing–original draft. J-BY: Writing–review and editing. JL: Writing–original draft, Data curation. SS: Writing–review and editing. H-YH: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. The authors disclosed receipt of financial support from the following for the research, authorship and/or publication of this article: Shanghai Commission of Science and Technology, Research on Healthy Aging and Smart Exercise Programs for the Elderly, 23DZ1204203, 23DZ1204200.
The authors thank all the participants and clinical researchers involved in the publications cited in this review and peer reviewers who contributed to the continuous improvement of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adatia A., Rainsford K. D., Kean W. F. (2012). Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J. Pharm. Pharmacol. 64 (5), 617–625. doi:10.1111/j.2042-7158.2012.01458.x
Bannuru R. R., Osani M. C., Vaysbrot E. E., Arden N. K., Bennell K., Bierma-Zeinstra S. M. A., et al. (2019). OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27 (11), 1578–1589. doi:10.1016/j.joca.2019.06.011
Barber-Westin S., Noyes F. R. (2019). Blood flow-restricted training for lower extremity muscle weakness due to knee pathology: a systematic review. Sports Health 11 (1), 69–83. doi:10.1177/1941738118811337
Bastiaansen-Jenniskens Y. M., Clockaerts S., Feijt C., Zuurmond A. M., Stojanovic-Susulic V., Bridts C., et al. (2012). Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann. Rheum. Dis. 71 (2), 288–294. doi:10.1136/ard.2011.153858
Berneis K., Ninnis R., Häussinger D., Keller U. (1999). Effects of hyper- and hypoosmolality on whole body protein and glucose kinetics in humans. Am. J. Physiol. 276 (1), E188–E195. doi:10.1152/ajpendo.1999.276.1.E188
Burton I., McCormack A. (2022). Blood flow restriction resistance training in tendon rehabilitation: a scoping review on intervention parameters, physiological effects, and outcomes. Front. Sports Act. Living 4 (879860), 879860. doi:10.3389/fspor.2022.879860
Crossley K. M., Stefanik J. J., Selfe J., Collins N. J., Davis I. S., Powers C. M., et al. (2016). 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br. J. Sports Med. 50 (14), 839–843. doi:10.1136/bjsports-2016-096384
Deshpande B. R., Katz J. N., Solomon D. H., Yelin E. H., Hunter D. J., Messier S. P., et al. (2016). Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. Hob. 68 (12), 1743–1750. doi:10.1002/acr.22897
Duong V., Oo W. M., Ding C., Culvenor A. G., Hunter D. J. (2023). Evaluation and treatment of knee pain: a review. Jama 330 (16), 1568–1580. doi:10.1001/jama.2023.19675
Ferraz R. B., Gualano B., Rodrigues R., Kurimori C. O., Fuller R., Lima F. R., et al. (2018). Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med. Sci. Sports Exerc 50 (5), 897–905. doi:10.1249/mss.0000000000001530
Feuerecker M., Hauer D., Toth R., Demetz F., Hölzl J., Thiel M., et al. (2012). Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur. J. Appl. Physiol. 112 (7), 2777–2781. doi:10.1007/s00421-011-2237-0
Fingleton C., Smart K. M., Doody C. M. (2017). Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin. J. Pain 33 (5), 395–404. doi:10.1097/ajp.0000000000000418
Fransen M., McConnell S., Harmer A. R., Van der Esch M., Simic M., Bennell K. L. (2015). Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br. J. Sports Med. 49 (24), 1554–1557. doi:10.1136/bjsports-2015-095424
Geng Y., Wu X., Zhang Y., Zhang M. (2024). Potential moderators of the effects of blood flow restriction training on muscle strength and hypertrophy: a meta-analysis based on a comparison with high-load resistance training. Sports Med. Open 10 (1), 58. doi:10.1186/s40798-024-00719-3
Giles L., Webster K. E., McClelland J., Cook J. L. (2017). Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br. J. Sports Med. 51 (23), 1688–1694. doi:10.1136/bjsports-2016-096329
Goh S. L., Persson M. S. M., Stocks J., Hou Y., Welton N. J., Lin J., et al. (2019). Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: systematic review and network meta-analysis. Sports Med. 49 (5), 743–761. doi:10.1007/s40279-019-01082-0
Goto K., Ishii N., Kizuka T., Takamatsu K. (2005). The impact of metabolic stress on hormonal responses and muscular adaptations. Med. Sci. Sports Exerc 37 (6), 955–963. doi:10.1249/01.mss.0000170470.98084.39
Haier R. J., Quaid K., Mills J. C. (1981). Naloxone alters pain perception after jogging. Psychiatry Res. 5 (2), 231–232. doi:10.1016/0165-1781(81)90052-4
Heyman E., Gamelin F. X., Goekint M., Piscitelli F., Roelands B., Leclair E., et al. (2012). Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology 37 (6), 844–851. doi:10.1016/j.psyneuen.2011.09.017
Hochberg M. C., Altman R. D., April K. T., Benkhalti M., Guyatt G., McGowan J., et al. (2012). American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. Hob. 64 (4), 465–474. doi:10.1002/acr.21596
Hohmann A. G., Suplita R. L. (2006). Endocannabinoid mechanisms of pain modulation. Aaps J. 8 (4), E693–E708. doi:10.1208/aapsj080479
Hughes L., Patterson S. D. (2019). Low intensity blood flow restriction exercise: rationale for a hypoalgesia effect. Med. Hypotheses 132, 109370. doi:10.1016/j.mehy.2019.109370
Hughes L., Patterson S. D. (2020). The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J. Appl. Physiol. (1985) 128 (4), 914–924. doi:10.1152/japplphysiol.00768.2019
Hughes L., Patterson S. D., Haddad F., Rosenblatt B., Gissane C., McCarthy D., et al. (2019). Examination of the comfort and pain experienced with blood flow restriction training during post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: a UK National Health Service trial. Phys. Ther. Sport 39, 90–98. doi:10.1016/j.ptsp.2019.06.014
Hussain S. M., Neilly D. W., Baliga S., Patil S., Meek R. (2016). Knee osteoarthritis: a review of management options. Scott Med. J. 61 (1), 7–16. doi:10.1177/0036933015619588
Imoto A. M., Pardo J. P., Brosseau L., Taki J., Desjardins B., Thevenot O., et al. (2019). Evidence synthesis of types and intensity of therapeutic land-based exercises to reduce pain in individuals with knee osteoarthritis. Rheumatol. Int. 39 (7), 1159–1179. doi:10.1007/s00296-019-04289-6
Jack R. A., Lambert B. S., Hedt C. A., Delgado D., Goble H., McCulloch P. C. (2022). Blood flow restriction therapy preserves lower extremity bone and muscle mass after ACL reconstruction. Sports Health 15, 361–371. doi:10.1177/19417381221101006
Jevsevar D. S. (2013). Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 21 (9), 571–576. doi:10.5435/jaaos-21-09-571
Kambič T., Novaković M., Tomažin K., Strojnik V., Jug B. (2019). Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front. Physiol. 10, 656. doi:10.3389/fphys.2019.00656
Kan H. S., Chan P. K., Chiu K. Y., Yan C. H., Yeung S. S., Ng Y. L., et al. (2019). Non-surgical treatment of knee osteoarthritis. Hong Kong Med. J. 25 (2), 127–133. doi:10.12809/hkmj187600
Kennedy D. L., Kemp H. I., Ridout D., Yarnitsky D., Rice A. S. C. (2016). Reliability of conditioned pain modulation: a systematic review. Pain 157 (11), 2410–2419. doi:10.1097/j.pain.0000000000000689
Kim I. J., Kim H. A., Seo Y. I., Jung Y. O., Song Y. W., Jeong J. Y., et al. (2011). Prevalence of knee pain and its influence on quality of life and physical function in the Korean elderly population: a community based cross-sectional study. J. Korean Med. Sci. 26 (9), 1140–1146. doi:10.3346/jkms.2011.26.9.1140
Kolasinski S. L., Neogi T., Hochberg M. C., Oatis C., Guyatt G., Block J., et al. (2020). 2019 American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. Hob. 72 (2), 149–162. doi:10.1002/acr.24131
Koltyn K. F., Brellenthin A. G., Cook D. B., Sehgal N., Hillard C. (2014). Mechanisms of exercise-induced hypoalgesia. J. Pain 15 (12), 1294–1304. doi:10.1016/j.jpain.2014.09.006
Kon M., Ikeda T., Homma T., Suzuki Y. (2012). Effects of low-intensity resistance exercise under acute systemic hypoxia on hormonal responses. J. Strength Cond. Res. 26 (3), 611–617. doi:10.1519/JSC.0b013e3182281c69
Ladlow P., Coppack R. J., Dharm-Datta S., Conway D., Sellon E., Patterson S. D., et al. (2018). Low-load resistance training with blood flow restriction improves clinical outcomes in musculoskeletal rehabilitation: a single-blind randomized controlled trial. Front. Physiol. 9, 1269. doi:10.3389/fphys.2018.01269
Lee J. Y., Han K., McAlindon T. E., Park Y. G., Park S. H. (2018). Lower leg muscle mass relates to knee pain in patients with knee osteoarthritis. Int. J. Rheum. Dis. 21 (1), 126–133. doi:10.1111/1756-185x.12896
Lemley K. J., Hunter S. K., Bement M. K. (2015). Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med. Sci. Sports Exerc 47 (1), 176–184. doi:10.1249/mss.0000000000000381
Li S., Shaharudin S., Abdul Kadir M. R. (2021). Effects of blood flow restriction training on muscle strength and pain in patients with knee injuries: a meta-analysis. Am. J. Phys. Med. Rehabil. 100 (4), 337–344. doi:10.1097/phm.0000000000001567
Loenneke J. P., Fahs C. A., Rossow L. M., Abe T., Bemben M. G. (2012a). The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 78 (1), 151–154. doi:10.1016/j.mehy.2011.10.014
Loenneke J. P., Wilson J. M., Marín P. J., Zourdos M. C., Bemben M. G. (2012b). Low intensity blood flow restriction training: a meta-analysis. Eur. J. Appl. Physiol. 112 (5), 1849–1859. doi:10.1007/s00421-011-2167-x
Lu Y., Patel B. H., Kym C., Nwachukwu B. U., Beletksy A., Forsythe B., et al. (2020). Perioperative blood flow restriction rehabilitation in patients undergoing ACL reconstruction: a systematic review. Orthop. J. Sports Med. 8 (3), 2325967120906822. doi:10.1177/2325967120906822
Mahmoud W. S., Osailan A., Ahmed S. A., Elnaggar R. K., Radwan N. L. (2021). Optimal parameters of blood flow restriction and resistance training on quadriceps strength and cross-sectional area and pain in knee osteoarthritis. Isokinet. Exerc. Sci. 1 (2021), 393–402. doi:10.3233/IES-200235
Martín-Hernández J., Ruiz-Aguado J., Herrero A. J., Loenneke J. P., Aagaard P., Cristi-Montero C., et al. (2017). Adaptation of perceptual responses to low-load blood flow restriction training. J. Strength Cond. Res. 31 (3), 765–772. doi:10.1519/jsc.0000000000001478
Mattocks K. T., Mouser J. G., Jessee M. B., Buckner S. L., Dankel S. J., Bell Z. W., et al. (2019). Perceptual changes to progressive resistance training with and without blood flow restriction. J. Sports Sci. 37 (16), 1857–1864. doi:10.1080/02640414.2019.1599315
Minniti M. C., Statkevich A. P., Kelly R. L., Rigsby V. P., Exline M. M., Rhon D. I., et al. (2020). The safety of blood flow restriction training as a therapeutic intervention for patients with musculoskeletal disorders: a systematic review. Am. J. Sports Med. 48 (7), 1773–1785. doi:10.1177/0363546519882652
Nguyen U. S., Zhang Y., Zhu Y., Niu J., Zhang B., Felson D. T. (2011). Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann. Intern Med. 155 (11), 725–732. doi:10.7326/0003-4819-155-11-201112060-00004
Nielsen J. L., Aagaard P., Prokhorova T. A., Nygaard T., Bech R. D., Suetta C., et al. (2017). Blood flow restricted training leads to myocellular macrophage infiltration and upregulation of heat shock proteins, but no apparent muscle damage. J. Physiol. 595 (14), 4857–4873. doi:10.1113/jp273907
Patterson S. D., Hughes L., Warmington S., Burr J., Scott B. R., Owens J., et al. (2019). Blood flow restriction exercise: considerations of methodology, application, and safety. Front. Physiol. 10, 533. doi:10.3389/fphys.2019.00533
Pearson S. J., Hussain S. R. (2015). A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 45 (2), 187–200. doi:10.1007/s40279-014-0264-9
Pitsillides A., Stasinopoulos D., Mamais I. (2021). Blood flow restriction training in patients with knee osteoarthritis: systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 27, 477–486. doi:10.1016/j.jbmt.2021.04.015
Pope Z. K., Willardson J. M., Schoenfeld B. J. (2013). Exercise and blood flow restriction. J. Strength Cond. Res. 27 (10), 2914–2926. doi:10.1519/JSC.0b013e3182874721
Pud D., Granovsky Y., Yarnitsky D. (2009). The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144 (1-2), 16–19. doi:10.1016/j.pain.2009.02.015
Pud D., Sprecher E., Yarnitsky D. (2005). Homotopic and heterotopic effects of endogenous analgesia in healthy volunteers. Neurosci. Lett. 380 (3), 209–213. doi:10.1016/j.neulet.2005.01.037
Ram K. C., Eisenberg E., Haddad M., Pud D. (2008). Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 139 (2), 431–438. doi:10.1016/j.pain.2008.05.015
Reeves G. V., Kraemer R. R., Hollander D. B., Clavier J., Thomas C., Francois M., et al. (2006). Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J. Appl. Physiol. (1985) 101 (6), 1616–1622. doi:10.1152/japplphysiol.00440.2006
Rice D., Nijs J., Kosek E., Wideman T., Hasenbring M. I., Koltyn K., et al. (2019). Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J. Pain 20 (11), 1249–1266. doi:10.1016/j.jpain.2019.03.005
Runhaar J., Beavers D. P., Miller G. D., Nicklas B. J., Loeser R. F., Bierma-Zeinstra S., et al. (2019). Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthr. Cartil. 27 (8), 1118–1123. doi:10.1016/j.joca.2019.04.009
Schell J., Scofield R. H., Barrett J. R., Kurien B. T., Betts N., Lyons T. J., et al. (2017). Strawberries improve pain and inflammation in obese adults with radiographic evidence of knee osteoarthritis. Nutrients 9 (9), 949. doi:10.3390/nu9090949
Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. Febs J. 280 (17), 4294–4314. doi:10.1111/febs.12253
Schoenfeld B. J. (2013). Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 43 (3), 179–194. doi:10.1007/s40279-013-0017-1
Segal N. A., Williams G. N., Davis M. C., Wallace R. B., Mikesky A. E. (2015). Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. Pm R. 7 (4), 376–384. doi:10.1016/j.pmrj.2014.09.014
Smith B. E., Selfe J., Thacker D., Hendrick P., Bateman M., Moffatt F., et al. (2018). Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS One 13 (1), e0190892. doi:10.1371/journal.pone.0190892
Song J. S., Spitz R. W., Yamada Y., Bell Z. W., Wong V., Abe T., et al. (2021). Exercise-induced hypoalgesia and pain reduction following blood flow restriction: a brief review. Phys. Ther. Sport 50, 89–96. doi:10.1016/j.ptsp.2021.04.005
Sprouse-Blum A. S., Smith G., Sugai D., Parsa F. D. (2010). Understanding endorphins and their importance in pain management. Hawaii Med. J. 69 (3), 70–71.
Starowicz K., Makuch W., Korostynski M., Malek N., Slezak M., Zychowska M., et al. (2013). Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One 8 (4), e60040. doi:10.1371/journal.pone.0060040
Takada S., Okita K., Suga T., Omokawa M., Kadoguchi T., Sato T., et al. (2012). Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J. Appl. Physiol. (1985) 113 (2), 199–205. doi:10.1152/japplphysiol.00149.2012
Takarada Y., Nakamura Y., Aruga S., Onda T., Miyazaki S., Ishii N. (2000). Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. (1985) 88 (1), 61–65. doi:10.1152/jappl.2000.88.1.61
Takarada Y., Sato Y., Ishii N. (2002). Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 86 (4), 308–314. doi:10.1007/s00421-001-0561-5
Takarada Y., Tsuruta T., Ishii N. (2004). Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn. J. Physiol. 54 (6), 585–592. doi:10.2170/jjphysiol.54.585
Tennent D. J., Hylden C. M., Johnson A. E., Burns T. C., Wilken J. M., Owens J. G. (2017). Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin. J. Sport Med. 27 (3), 245–252. doi:10.1097/jsm.0000000000000377
Thorén P., Floras J. S., Hoffmann P., Seals D. R. (1990). Endorphins and exercise: physiological mechanisms and clinical implications. Med. Sci. Sports Exerc 22 (4), 417–428. doi:10.1249/00005768-199008000-00001
Uthman O. A., van der Windt D. A., Jordan J. L., Dziedzic K. S., Healey E. L., Peat G. M., et al. (2013). Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. Bmj 347 (f5555), f5555. doi:10.1136/bmj.f5555
Vaegter H. B., Handberg G., Graven-Nielsen T. (2014). Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 155 (1), 158–167. doi:10.1016/j.pain.2013.09.023
Vaegter H. B., Jones M. D. (2020). Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain Rep. 5 (5), e823. doi:10.1097/pr9.0000000000000823
Vaegter H. B., Lyng K. D., Yttereng F. W., Christensen M. H., Sørensen M. B., Graven-Nielsen T. (2019). Exercise-induced hypoalgesia after isometric wall squat exercise: a test-retest reliabilty study. Pain Med. 20 (1), 129–137. doi:10.1093/pm/pny087
van den Ende C. H., Breedveld F. C., le Cessie S., Dijkmans B. A., de Mug A. W., Hazes J. M. (2000). Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann. Rheum. Dis. 59 (8), 615–621. doi:10.1136/ard.59.8.615
van Wijk G., Veldhuijzen D. S. (2010). Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J. Pain 11 (5), 408–419. doi:10.1016/j.jpain.2009.10.009
Keywords: BFRT, arthralgia, patellar, injuries, inflammation
Citation: Xie S-Y, Jiang X, Yuan J-B, Luo J, Song S and Hu H-Y (2025) Mechanisms of blood flow restriction training for knee pain: a mini review. Front. Physiol. 16:1542322. doi: 10.3389/fphys.2025.1542322
Received: 09 December 2024; Accepted: 21 January 2025;
Published: 13 February 2025.
Edited by:
Alejandro Santos-Lozano, Miguel de Cervantes European University, SpainReviewed by:
António Miguel Monteiro, Instituto Politécnico de Bragança, PortugalCopyright © 2025 Xie, Jiang, Yuan, Luo, Song and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shun Song, c29uZ3NodW5Ac2p0dS5lZHUuY24=; Hao-Yu Hu, aGh5MTIzempsQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.