
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 21 February 2025
Sec. Metabolic Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1537231
The circadian clock, an innate timing mechanism, governs a variety of physiological activities by producing near-24-h cycles in gene expression. These cycles are reflected in patterns of metabolism and behavior. This system consists of two parts: one is the central clock located in the suprachiasmatic nucleus of the hypothalamus, and the other is the peripheral clock located in tissues throughout the body. Glucokinase, also termed hexokinase 4, is a member of the hexokinase family. It acts as a glucose sensor, plays a pivotal role in glucose homeostasis. Here, we review the role of circadian rhythm in glucose metabolism across various tissues, look into the molecular mechanism of circadian disruption involvement in glucose metabolism and diabetic complications, with a particular focus on the role of glucokinase. Finally, we propose potential strategies for effectively treating metabolic disorders and diabetic complications by modulating circadian rhythm glucokinase.
Disruption of circadian rhythms is a frequently overlooked risk factor for diabetes (Stenvers et al., 2019). The circadian system, often referred to as the biological clock. It consists of two parts: one is the central clock located in the suprachiasmatic nucleus of the hypothalamus, and the other is the peripheral clock located in tissues throughout the body. These clocks are synchronized by neuronal and hormonal signals, body temperature, light, and feeding cues (Reppert and Weaver, 2002). Increasing evidence suggests that various clock genes play roles in lipid regulation, glucose balance and overall health (Manoogian and Panda, 2017; Poggiogalle et al., 2018). Recent research indicates a strong link between circadian disruption and the development of diabetes and its complications (Potter et al., 2016; Mason et al., 2020; Nakazawa et al., 2022).
Glucokinase (GCK), also known as hexokinase 4, belongs to the hexokinase family and acts as a glucose sensor pivotal for glucose homeostasis. While other hexokinases (HK1–3) exhibit high affinity for glucose (saturated at fasting levels of ∼5 mM) and are inhibited by glucose-6-phosphate (G6P), GCK has a low affinity for glucose (EC50 ∼8–10 mM), is not inhibited by G6P, and phosphorylates glucose proportionally across a broader physiological range (3–15 mM) (Ashcroft et al., 2023). These unique characteristics enable GCK to dynamically regulate glucose utilization and storage in response to postprandial glycemic fluctuations, making it a critical player in metabolic regulation and a key target for studying circadian rhythms in glucose metabolism.
The expression and activity of glucokinase are influenced by circadian rhythms, linking it directly to the metabolic disturbances observed in diabetes Studies have shown that the expression and activity of GCK exhibit circadian rhythmic fluctuations. Typically, GCK activity is increased after feeding but decreased during fasting, which helps process glucose absorbed from food. This is closely linked to the feeding-fasting cycle in animals. The circadian expression of GCK is directly controlled by the circadian locomotor output cycles kaput (CLOCK) and aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1), which bind to the E-box at the promoter region of GCK, making it a clock-controlled gene (CCG). Disruptions in these circadian genes may lead to abnormal GCK function, thereby affecting glucose metabolism (Llanos et al., 2023b). In addition to circadian regulation, GCK expression is also modulated by dietary signals. GCK is regulated by dietary signals primarily through the insulin-mediated sterol regulatory element-binding protein 1c (SREBP1c) pathway and direct glucose regulation. Postprandial insulin activates SREBP1c, enhancing GCK transcription, while elevated glucose levels directly upregulate GCK expression (Kim et al., 2004). Additionally, pathways such as Glucagon-like peptide-1 (GLP-1) and Liver X Receptor (LXR) further modulate GCK, enabling it to dynamically respond to dietary changes and maintain glucose homeostasis (Kim et al., 2009; Ding et al., 2011b).
In recent years, GCK activators have been considered an effective antidiabetic drug. However, the impact of GCK on lipid metabolism should not be overlooked. Activation of GCK leads to hepatic lipid accumulation and inflammation by altering the expression of liver genes involved in lipogenesis, lipolysis, and β-oxidation (Xie et al., 2023). Therefore, GCK activators can contradictorily affect both glucose and lipid metabolism, sometimes improving glucose control while potentially disrupting lipid balance (Jiang et al., 2024). This paradox underscores the complexity of targeting GCK for therapeutic purposes and highlights the importance of considering circadian rhythms in the development of treatments targeting GCK and metabolic disorders.
In this review, we will introduce the concept of circadian rhythms, analyze the impact of circadian rhythm disruption on diabetes and its complication, with a particular focus on the involvement of glucokinase. We will look into the molecular mechanism of circadian rhythm affecting these diseases, and explore the promising treatment strategies for diabetes and its complication.
The circadian clock is an internal system that predicts daily environmental changes (Young and Kay, 2001). In mammals, a central clock is located in the hypothalamic suprachiasmatic nuclei (SCN) and primarily responds to the light-dark cycle (Hastings et al., 2018). Peripheral clocks, similar to the SCN, exist in tissues such as the liver and pancreas (Mohawk et al., 2012). The circadian clock in every cell relies on a transcriptional–translational feedback loop composed of clock genes. Core mammalian clock genes include CLOCK and BMAL1, which form BMAL1/CLOCK heterodimers. The first loop involves cryptochrome (CRY) and period (PER) proteins. During the rest phase, the BMAL1/CLOCK heterodimer binds to E-Box DNA binding sequences (DBS), promoting the expression of CRY and PER. These proteins then form dimers, move into the nucleus, and inhibit BMAL1/CLOCK activity during the active phase. Post-translational modifications lead to the degradation of PER and CRY, initiating the next circadian cycle. In the rest phase, REV-ERB represses BMAL1 and CLOCK, while during the active phase, decreasing REV-ERB and increasing ROR levels activate their expression, maintaining the circadian rhythm (Asher and Schibler, 2011; Bass, 2012; Takahashi, 2017). Overall, circadian clock possesses intricate molecular mechanism and is present in various tissues and organs throughout the body (Ardlie et al., 2015; Mure et al., 2018).
Circadian rhythms regulate numerous physiological and metabolic functions, notably influencing glucose metabolism across all levels of mammalian organization. In healthy humans, plasma glucose concentrations are controlled at stable levels (Aparicio et al., 1974). Numerous human studies have documented a circadian rhythm in oral glucose tolerance, which usually peaks in the morning and declines in the afternoon and evening (Carroll and Nestel, 1973; Mayer et al., 1976; Wojtczak-Jaroszowa, 1977). As mentioned above, GCK activity is higher during feeding periods (typically during the day) and lower during fasting periods (typically at night). Therefore, the circadian rhythm of glucose tolerance may result from the combined actions of various peripheral tissues and GCK (Figure 1).

Figure 1. Circadian clocks regulate glucose metabolism. Circadian rhythms govern a large array of physiological and metabolic functions and permeate all levels of mammalian organization, especially in glucose metabolism. The main involvement of GCK is in the regulation of food intake and the control of peripheral tissue clocks, including the liver, pancreas, and gut. BMAL1, aryl hydrocarbon receptor nuclear translocator-like protein 1; CRY, cryptochrome; SCN, suprachiasmatic nuclei; ARC, arcuate nucleus.
Insulin secretion exhibits a circadian rhythm. Insulin secretion rate peaks in the mid-afternoon and is lowest at night while sleeping. The rhythm may be associated with habitual feeding times, consistent with GCK (Boden et al., 1996; Saad et al., 2012). Circadian clock genes are involved in various pathways for insulin secretion. GLP-1, an incretin hormone primarily produced by intestinal L-cells, is also synthesized by pancreatic α-cells. It plays a vital role in insulin secretion (Seino et al., 2016). Research indicates that GLP-1 stimulates insulin secretion via a mechanism involving glucokinase (GCK) and is regulated by the clock gene BMAL1 (Ding et al., 2011a; Biancolin et al., 2020). Therefore, in β-cells, clock genes may affect the rhythm of insulin secretion through the GLP-1-GCK pathway.
The liver’s circadian clocks employ various mechanisms to produce antiphasic rhythms in glucose metabolism. During habitual feeding periods, the liver clock gene CRY plays an important role in metabolic regulation by modulating the expression of Glucose transporter type 2 (GLUT2). CRY achieves this by inhibiting G protein-coupled receptor (GPCR) signaling pathways, which otherwise would downregulate GLUT2 expression. The upregulation of GLUT2 enhances the activity of Glucokinase (GCK), a key enzyme in glucose metabolism. This activation of GCK facilitates hepatic glucose uptake, thereby reducing blood glucose levels, and concurrently suppresses hepatic gluconeogenesis, the process by which the liver produces glucose (Kinsella et al., 2021). The overall result produces nearly constant blood levels of glucose throughout the day (Zhang et al., 2010; Lamia et al., 2011).
The circadian rhythm of glucose metabolism involves not only the pancreas and liver mentioned above, but also other peripheral tissues suck as the adipose tissue, kidneys, and muscles. These tissues are regulated by BMAL1, PER1, and CRY. This regulation causes a circadian rhythm in insulin sensitivity in muscle and adipose tissue, and glucose reabsorption in the kidney, thereby influencing the overall circadian rhythm of glucose metabolism (Verrillo et al., 1989; Iwashina et al., 2011; Barclay et al., 2013; Solocinski et al., 2015; Nikolaeva et al., 2016; Gliniak et al., 2017; Ansermet et al., 2022). However, the expression of GCK in these tissues is minimal. Hexokinase 2 (HK2) is another isoform of HKs, present in almost all tissues. Circadian rhythms may influence insulin sensitivity in muscle and adipose tissue through the BMAL1-HK2-glucose transporter (Glut4) pathway (Harfmann et al., 2016; Shimobayashi et al., 2023). HK2 is also associated with glycogen deposition in the kidneys. Therefore, the regulation of renal glucose reabsorption by circadian rhythms may also involve HK2 (Rabbani et al., 2022).
Researches find that the occurrence of glucose metabolic rhythm may be related to food intake (Zhao et al., 2021). This impact is mainly manifested in two aspects (Figure 1). On the one hand, there is a direct neuroanatomical connection between the SCN and the hypothalamic arcuate nucleus (ARC), the center for regulating food intake. The neuronal activity of ARC increases during hypoglycemia. SCN inhibits the activity of ARC neurons, avoiding the occurrence of more food intake and higher blood sugar levels after hypoglycemia (Herrera-Moro Chao et al., 2016). On the other hand, feeding-related neuropeptides expressed in the ARC are also affected by circadian clock genes, including the potent orexigenic hypothalamic neuropeptides, neuropeptide Y (NPY) and agouti-related peptide (AgRP), and the anorexigenic peptide α-melanocyte-stimulating hormone (POMC). The expression of these neuropeptides peak during eating. BMAL1 can inhibit the high expression of AgRP, NPY, and POMC, thereby avoiding overeating during both day and night (Clemenzi et al., 2020). Interestingly, GCK is widely expressed in the central nervous system, particularly in ARC of the hypothalamus. Previous studies have confirmed that GCK regulates AgRP and NPY in the hypothalamus. Since GCK is also influenced by circadian clock genes, the circadian rhythm of food intake might be related to GCK (Maria Uranga et al., 2017).
Circadian disruption will increase the susceptibility to diabetes complications. A case-control study shows that service workers, who have sometimes to work in shifts and eat at irregular times, are more likely to have complications of diabetes (Nakazawa et al., 2022).
In the following discussion we emphasize the epidemiological and background evidence linking circadian disruption to diabetic complications, and then explore examples of targetable mechanisms involving circadian disruption and GCK in these conditions (Figure 2).
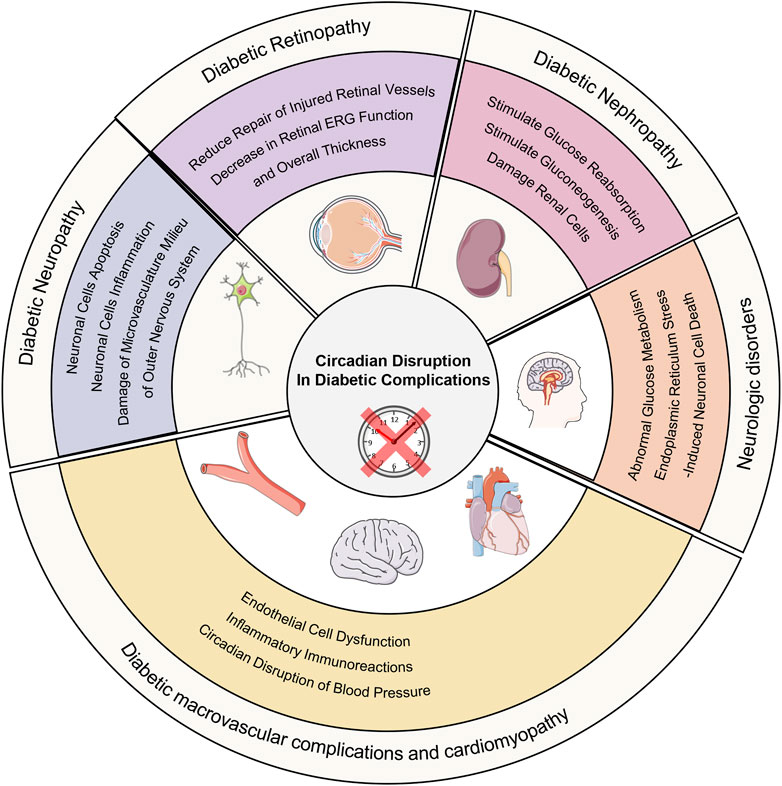
Figure 2. Circadian disruption in diabetic complications. Circadian disruption increases the occurrence of diabetic complications and its possible molecular mechanism.
Diabetic neuropathy, affecting over 50% of those with diabetes, involves peripheral and autonomic nervous system damage, resulting in pain, increased fall risk, and reduced quality of life (Feldman et al., 2019). Circadian disruption may cause imbalance between free radicals’ production and clearance, hyperactivation of ERK-MAPK signaling pathway and abnormal expression of proinflammatory factors leading to damage of microvasculature milieu of outer nervous system, neuronal cells inflammation and neuronal cells apoptosis. They aggravate the occurrence and development of diabetic neuropathy (Daulhac et al., 2006; Patel et al., 2009; Prather et al., 2009; Zielinski et al., 2016; Griffin et al., 2019; Fang et al., 2021; Budkowska et al., 2022). TNF-α is an inflammatory protein upregulated by various mediators. Research has shown that activation of GCK activity can result in elevated TNF-α levels. TNF-α may induce inflammation and neuronal damage in the stellate ganglion, impairing its ability to regulate the heart (Xu et al., 2020). The expression level of GCK is influenced by BMAL1 (Llanos et al., 2023a). Therefore, circadian disruption may lead to the development of diabetic autonomic neuropathy through this inflammatory mechanism.
Macrovascular complications of diabetes mellitus, including cardiovascular diseases, peripheral artery disease and cerebrovascular disease, and cardiomyopathy are the primary causes of mortality in diabetic patients. Evidence is mounting which links circadian rhythm and its effects to macrovascular complications and cardiomyopathy of diabetes. Humans those who suffer from sleep disorders and animal with environmental circadian disruption may have an increased chance of developing macrovascular complications and cardiomyopathy (Baguet et al., 2012; Earnest et al., 2016; Kecklund and Axelsson, 2016; Pan et al., 2016; Qiao et al., 2017; Yang et al., 2023). The influence of circadian rhythm on those may be multifaceted including circadian disruption of blood pressure, inflammation, vascular endothelial dysfunction, and so on (Stamler et al., 1993; Gibbs et al., 2014; Kokubo and Iwashima, 2015; Frati et al., 2017; Nishida and Otsu, 2017; Gonzalez-Guerra et al., 2021; Kelly et al., 2021; Kong et al., 2022). Current research has found that the lack of GCK accelerates the development of atherosclerosis and cardiomyopathy (Li et al., 2014; Adingupu et al., 2016). Activation of GCK can reduce the risk of cardiovascular disease in diabetes (Wang et al., 2022). GCK may be involved in circadian disruption leading to diabetic macrovascular diseases, but its specific mechanisms require further clarification.
Recent research has suggested that diabetic nephropathy and retinopathy will occur and progress more quickly due to circadian disruption (Firsov and Bonny, 2018). Disturbances of the kidney clock may affect the progression of diabetic nephropathy by stimulating renal tubular gluconeogenesis and glucose reabsorption or by damaging renal cells (Solocinski et al., 2015; Nikolaeva et al., 2016; Ansermet et al., 2022). In the progression of DR, multiple abnormal circadian rhythms including circadian disruption of systemic blood pressure (impaired nocturnal blood pressure), decreased melatonin levels (peak levels and amplitude), weakened daily cycling of enzymes for fatty acid β-oxidation, increased amplitude of inflammatory markers and loss of autophagic protein circadian rhythm all play important roles (Wang et al., 2014; Mateo-Gavira et al., 2016; Vancura et al., 2016; Coughlin et al., 2017; Hassan et al., 2017; Lemmer and Oster, 2018; Qi et al., 2020). However, the role of GCK in this process remains unclear. Current research has found that diabetic nephropathy is associated with the rs780094 polymorphism in the glucokinase regulatory protein (GCKR) gene, but the specific molecular mechanisms remain to be elucidated (Liu and Wan, 2023).
Currently, there are various treatments for the circadian rhythm of diseases. The main treatment methods can be divided into pharmacological treatment and non-pharmacological treatment (Figure 3). The first is pharmacological interventions for feeding-fasting and sleep-wake disorders. Besides, drugs that directly manipulate the core oscillator is a new perspective to circadian medicine. Non-pharmacological treatment of environmental and lifestyle regimens can also restore circadian rhythm, including light therapy, time-restricted feeding, sleep intervention, scheduled activity and combination.
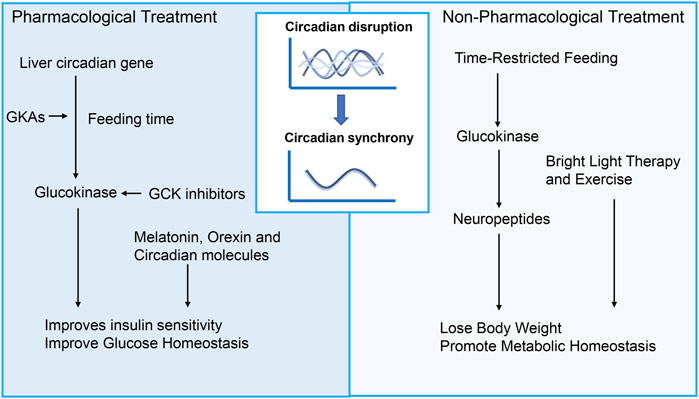
Figure 3. Treatment for diabetic complications targeting circadian rhythms. Promising therapeutic strategies targeting circadian disruption for diabetes and its complications can be divided into pharmacological treatment and non-pharmacological treatment. Pharmacological interventions targeting feeding-fasting and sleep-wake disorders encompass drugs that act upon GCK signaling, melatonin, orexin, and circadian molecules. In addition to these approaches, non-pharmacological treatments involving environmental and lifestyle adjustments hold promise for managing diabetic complications. Such treatments may include light therapy, time-restricted feeding, sleep intervention, scheduled activity, and combinations thereof. GCK, Glucokinase; GKAs, Glucokinase activators.
Various lifestyle changes, such as timed meals, sleep, and physical activity, have been studied for their effectiveness in restoring circadian rhythms and treating glucose disorders. Recent years have seen remarkable progress when it comes to feeding habits, such as Time-restricted feeding (TRF), which involves granting access to food for 8–9 h during the active phase, and has been demonstrated to be very effective in preventing and treating metabolic disorders, particularly diabetes. Studies have found that TRF improves glucose tolerance and insulin resistance, reduces lipid accumulation, and influences the SCN by regulating the expression of GCK and neuropeptides such as NPY and AgRP in hypothalamus and hippocampus (Chaix et al., 2014; Tacad et al., 2022). Furthermore, TRF has significant effects on peripheral organs such as the liver and pancreas. In the pancreas, improves β-cell function through the autophagy-lysosomal pathway, reducing β-cell apoptosis, and increasing insulin sensitivity (Liu et al., 2017; Marinho et al., 2020). In the liver, TRF improves glucose and lipid metabolism by restoring circadian rhythms of core clock gene, reducing gluconeogenesis via CREB phosphorylation, and enhancing glucose utilization through glycogen synthesis and the pentose phosphate pathway (Chaix et al., 2019). However, the involvement of GCK in the effects of TRF on peripheral tissues remains to be elucidated. In addition, TRF has shown promising protective effects on various diabetic complications, such as nephropathy, retinopathy, neuropathy and cardiovascular diseases (Beli et al., 2018; Malinowski et al., 2019; Yang et al., 2022). Other circadian rhythm-related therapies, including light therapy and exercise, have also been shown to effectively improve sleep, reduce insulin resistance, and lower glycated hemoglobin levels (Boulé et al., 2001; Colberg et al., 2016; Wang et al., 2024).
Activating GCK is a promising strategy for reducing glucose levels. Under hyperglycemia, GKAs promote insulin secretion and glycogen synthesis, while during fasting, GCK supports glycogenolysis and gluconeogenesis for glucose supply. However, GKAs can cause side effects like hyperlipidemia and hepatic steatosis. Early studies indicated that while GKAs lower glucose, they may increase liver lipids and inflammation. Tobias Kroon et al. found that timing GCK activation to feeding time improves insulin sensitivity, reverses liver steatosis, and reduces fibrosis markers (Kroon et al., 2022). This may be related to the circadian rhythm of hepatic glucose metabolism. As previously mentioned, under physiological conditions, during feeding time, the circadian rhythm genes in the liver regulate GCK to lower blood glucose levels. Taking GKAs during feeding aligns with this rhythm, which may help minimize side effects. The novel GKA, dorzagliatin, taken before meals, effectively lowers glucose without severe hypoglycemia, though slight increases in TG and TC were observed, posing potential risks (Jiang et al., 2024).
Recent study suggests that hyperactivation of glucose metabolism, leads to β cell function decline in diabetes (Brock et al., 2002). GCK plays an important role in glucose metabolism. Therefore, reducing GCK levels to lower glucose metabolism to normal levels may be a useful alternative strategy for protecting β-cells function. Existing study has found that inhibiting GCK levels in vivo or in vitro can maintain β-cell function and quality. However, the inhibition of GCK must consider whether it may lead to the occurrence of hyperglycemia. A special subtype of diabetes, glucokinase-maturity onset diabetes of the young (GCK-MODY), is characterized by patients who may have mild hyperglycemia, but their blood sugar levels do not further increase without medication, and the incidence of diabetes complications does not increase. In terms of side effects, GCK inhibition does not lead to hypoglycemia or abnormal blood lipid levels (Ashcroft et al., 2023). However, as previously mentioned, GCK inhibition seems to increase the risk of diabetic neuropathy and atherosclerosis. Further research is needed to determine whether combining GCK inhibitors with circadian rhythm can bring better therapeutic outcomes.
Other circadian rhythm-related medications include melatonin, orexin, and circadian molecules. Melatonin is a natural neurohormone synthesized from tryptophan, produced by the pineal gland and keeps the circadian rhythm (Arendt and Skene, 2005). Melatonin has been demonstrated to entrain the circadian system, and has been found to possess sleep-promoting properties, improve glucose homeostasis, and reduce insulin resistance (Gooley et al., 2011; Forrestel et al., 2017; Vasey et al., 2021). Orexin is a neuropeptide from the lateral hypothalamus (LH) crucial for regulating sleep and feeding behaviors (Tsujino and Sakurai, 2009). Furthermore, it has been reported that CRY stabilizers, REV-ERB agonists, and ROR agonists - which target the molecular clock directly - show promise in improving obesity and glucose metabolism in diabetic animal models (Solt et al., 2012; He et al., 2016; Humphries et al., 2016). Research on the relationship between these drugs and GCK remains limited. In terms of complications of diabetes, these medications help improve diabetic neuropathy, retinopathy, and macrovascular complications (Tsuneki et al., 2015; Niknia et al., 2019; Patel et al., 2022).
Diabetes mellitus is a globally prevalent metabolic disorder with increasing incidence and chronic complications due to long-term hyperglycemia. Disruptions in the body’s 24-h circadian rhythm are increasingly linked to severe mental and physical health impacts. GCK, which is expressed in multiple organs in the human, may play an indispensable role in these processes. In this review, we analyzed the effect of circadian rhythm disorder on diabetic complications, explored the potential molecular mechanism of circadian rhythm involvement in diabetic complications, with a particular focus on the role of GCK, and discussed the research regarding the treatment of diabetic complications through circadian rhythm.
GCK, as a key target for circadian rhythm intervention, has inconsistent effects on glucose or lipid metabolism in different tissues and under different conditions. Therefore, it is important to develop appropriate GCK-targeted intervention strategies based on the specific circadian disruptions in different tissues of diabetic patients. Most current research on circadian rhythms and pharmacological effects relies on in vitro or animal models, lacking substantial clinical data. These relationships highlight the need for further research to understand how circadian rhythms interact with external factors and disease processes for better prevention and treatment of diabetes and its complications.
ZZ: Conceptualization, Writing–original draft, Writing–review and editing. SW: Writing–review and editing. LG: Funding acquisition, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Science Foundation of China (Project # 82270861 to LG), the Fundamental Research Funds for the Central Universities (Project # 2042020kf1079 to LG), and the Planned international development Project of Wuhan University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adingupu D. D., Heinonen S. E., Andreasson A.-C., Brusberg M., Ahnmark A., Behrendt M., et al. (2016). Hyperglycemia induced by glucokinase deficiency accelerates atherosclerosis development and impairs lesion regression in combined heterozygous glucokinase and the apolipoprotein E-knockout mice. J. Diabetes Res. 2016, 8630961. doi:10.1155/2016/8630961
Ansermet C., Centeno G., Bignon Y., Ortiz D., Pradervand S., Garcia A., et al. (2022). Dysfunction of the circadian clock in the kidney tubule leads to enhanced kidney gluconeogenesis and exacerbated hyperglycemia in diabetes. Kidney Int. 101 (3), 563–573. doi:10.1016/j.kint.2021.11.016
Aparicio N. J., Puchulu F. E., Gagliardino J. J., Ruiz M., Llorens J. M., Ruiz J., et al. (1974). Circadian variation of the blood glucose, plasma insulin and human growth hormone levels in response to an oral glucose load in normal subjects. Diabetes 23 (2), 132–137. doi:10.2337/diab.23.2.132
Ardlie K. G., Deluca D. S., Segre A. V., Sullivan T. J., Young T. R., Gelfand E. T., et al. (2015). Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348 (6235), 648–660. doi:10.1126/science.1262110
Arendt J., Skene D. J. J. S. M. R. (2005). Melatonin as a chronobiotic. Sleep. Med. Rev. 9 (1), 25–39. doi:10.1016/j.smrv.2004.05.002
Ashcroft F. M., Lloyd M., Haythorne E. A. (2023). Glucokinase activity in diabetes: too much of a good thing? Trends Endocrinol. Metabolism 34 (2), 119–130. doi:10.1016/j.tem.2022.12.007
Asher G., Schibler U. J. C. M. (2011). Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13 (2), 125–137. doi:10.1016/j.cmet.2011.01.006
Baguet J.-P., Barone-Rochette G., Tamisier R., Levy P., Pepin J.-L. (2012). Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat. Rev. Cardiol. 9 (12), 679–688. doi:10.1038/nrcardio.2012.141
Barclay J. L., Shostak A., Leliavski A., Tsang A. H., Joehren O., Mueller-Fielitz H., et al. (2013). High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiology-Endocrinology Metabolism 304 (10), E1053–E1063. doi:10.1152/ajpendo.00512.2012
Bass J. J. N. (2012). Circadian topology of metabolism. Nature 491 (7424), 348–356. doi:10.1038/nature11704
Beli E., Yan Y., Moldovan L., Vieira C. P., Gao R., Duan Y., et al. (2018). Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes 67 (9), 1867–1879. doi:10.2337/db18-0158
Biancolin A. D., Martchenko A., Mitova E., Gurges P., Michalchyshyn E., Chalmers J. A., et al. (2020). The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab. 31, 124–137. doi:10.1016/j.molmet.2019.11.004
Boden G., Ruiz J., Urbain J., Chen X. J. a. J. O. P.-E. (1996). Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. 271 (2), E246–E252. doi:10.1152/ajpendo.1996.271.2.E246
Boulé N. G., Haddad E., Kenny G. P., Wells G. A., Sigal R. J. (2001). Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus -: a meta-analysis of controlled clinical trials. Jama-Journal Am. Med. Assoc. 286 (10), 1218–1227. doi:10.1001/jama.286.10.1218
Brock B., Mogensen J. H., Gregersen S., Hermansen K. (2002). Glucose desensitization in INS-1 cells: evidence of impaired function caused by glucose metabolite(s) rather than by the glucose molecule per se. Metabolism 51 (6), 671–677. doi:10.1053/meta.2002.32722
Budkowska M., Cecerska-Heryc E., Marcinowska Z., Siennicka A., Dolegowska B. (2022). The influence of circadian rhythm on the activity of oxidative stress enzymes. Int. J. Mol. Sci. 23 (22), 14275. doi:10.3390/ijms232214275
Carroll K. F., Nestel P. J. J. D. (1973). Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 22 (5), 333–348. doi:10.2337/diab.22.5.333
Chaix A., Manoogian E. N. C., Melkani G. C., Panda S. (2019). Time-restricted eating to prevent and manage chronic metabolic diseases. Diabetes 22 (5), 333–348. doi:10.1146/annurev-nutr-082018-124320
Chaix A., Zarrinpar A., Phuong M., Panda S. (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20 (6), 991–1005. doi:10.1016/j.cmet.2014.11.001
Clemenzi M. N., Martchenko A., Loganathan N., Erika K. T., Brubaker P. L., Belsham D. D. J. M., et al. (2020). Analysis of Western diet, palmitate and BMAL1 regulation of neuropeptide Y expression in the murine hypothalamus and BMAL1 knockout cell models. Mol. Cell Endocrinol. 507, 110773. doi:10.1016/j.mce.2020.110773
Colberg S. R., Sigal R. J., Yardley J. E., Riddell M. C., Dunstan D. W., Dempsey P. C., et al. (2016). Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 39 (11), 2065–2079. doi:10.2337/dc16-1728
Coughlin B. A., Feenstra D. J., Mohr S. (2017). Muller cells and diabetic retinopathy. Vis. Res. 139, 93–100. doi:10.1016/j.visres.2017.03.013
Daulhac L., Mallet C., Courteix C., Etienne M., Duroux E., Privat A.-M., et al. (2006). Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol. Pharmacol. 70 (4), 1246–1254. doi:10.1124/mol.106.025478
Ding S.-Y., Nkobena A., Kraft C. A., Markwardt M. L., Rizzo M. A. (2011a). Glucagon-like peptide 1 stimulates post-translational activation of glucokinase in pancreatic beta cells. J. Biol. Chem. 286 (19), 16768–16774. doi:10.1074/jbc.M110.192799
Ding S. Y., Nkobena A., Kraft C. A., Markwardt M. L., Rizzo M. A. (2011b). Glucagon-like peptide 1 stimulates post-translational activation of glucokinase in pancreatic beta cells. J. Biol. Chem. 286 (19), 16768–16774. doi:10.1074/jbc.M110.192799
Earnest D. J., Neuendorff N., Coffman J., Selvamani A., Sohrabji F. J. E. (2016). Sex differences in the impact of shift work schedules on pathological outcomes in an animal model of ischemic stroke. Endocrinology 157 (7), 2836–2843. doi:10.1210/en.2016-1130
Fang K., Liu D., Pathak S. S., Yang B., Li J., Karthikeyan R., et al. (2021). Disruption of circadian rhythms by ambient light during neurodevelopment leads to autistic-like molecular and behavioral alterations in adult mice. Cells 10 (12), 3314. doi:10.3390/cells10123314
Feldman E. L., Callaghan B. C., Pop-Busui R., Zochodne D. W., Wright D. E., Bennett D. L., et al. (2019). Diabetic neuropathy. Nat. Rev. Dis. Prim. 5, 41. doi:10.1038/s41572-019-0092-1
Firsov D., Bonny O. (2018). Circadian rhythms and the kidney. Nat. Rev. Nephrol. 14 (10), 626–635. doi:10.1038/s41581-018-0048-9
Forrestel A. C., Miedlich S. U., Yurcheshen M., Wittlin S. D., Sellix M. T. (2017). Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia 60 (5), 808–822. doi:10.1007/s00125-016-4175-1
Frati G., Schirone L., Chimenti I., Yee D., Biondi-Zoccai G., Volpe M., et al. (2017). An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc. Res. 113 (4), 378–388. doi:10.1093/cvr/cvx011
Gibbs J., Ince L., Matthews L., Mei J. J., Bell T., Yang N., et al. (2014). An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20 (8), 919–926. doi:10.1038/nm.3599
Gliniak C. M., Brown J. M., Noy N. (2017). The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J. Biol. Chem. 292 (36), 15080–15093. doi:10.1074/jbc.M117.782334
Gonzalez-Guerra A., Roche-Molina M., García-Quintáns N., Sánchez-Ramos C., Martín-Pérez D., Lytvyn M., et al. (2021). Sustained elevated blood pressure accelerates atherosclerosis development in a preclinical model of disease. Int. J. Mol. Sci. 22 (16), 8448. doi:10.3390/ijms22168448
Gooley J. J., Chamberlain K., Smith K. A., Khalsa S. B. S., Rajaratnam S. M., Van Reen E., et al. (2011). Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. and Metabolism 96 (3), E463–E472. doi:10.1210/jc.2010-2098
Griffin P., Dimitry J. M., Sheehan P. W., Lananna B. V., Guo C., Robinette M. L., et al. (2019). Circadian clock protein Rev-erba regulates neuroinflammation. Proc. Natl. Acad. Sci. U. S. A. 116 (11), 5102–5107. doi:10.1073/pnas.1812405116
Harfmann B. D., Schroder E. A., Kachman M. T., Hodge B. A., Zhang X., Esser K. A. (2016). Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 6, 12. doi:10.1186/s13395-016-0082-x
Hassan I., Luo Q. Y., Majumdar S., Dominguez J. M., Busik J. V., Bhatwadekar A. D. (2017). Tumor necrosis factor alpha (TNF-α) disrupts Kir4.1 channel expression resulting in muller cell dysfunction in the retina. Invest. Ophthalmol. and Vis. Sci. 58 (5), 2473–2482. doi:10.1167/iovs.16-20712
Hastings M. H., Maywood E. S., Brancaccio M. (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 19 (8), 453–469. doi:10.1038/s41583-018-0026-z
He B., Nohara K., Park N., Park Y.-S., Guillory B., Zhao Z., et al. (2016). The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23 (4), 610–621. doi:10.1016/j.cmet.2016.03.007
Herrera-Moro Chao D., León-Mercado L., Foppen E., Guzmán-Ruiz M., Basualdo M., Escobar C., et al. (2016). The suprachiasmatic nucleus modulates the sensitivity of arcuate nucleus to hypoglycemia in the male rat. Endocrinology 157 (9), 3439–3451. doi:10.1210/en.2015-1751
Humphries P. S., Bersot R., Kincaid J., Mabery E., Mccluskie K., Park T., et al. (2016). Carbazole-containing sulfonamides and sulfamides: discovery of cryptochrome modulators as antidiabetic agents. Bioorg. Med. Chem. Lett. 26 (3), 757–760. doi:10.1016/j.bmcl.2015.12.102
Iwashina I., Mochizuki K., Inamochi Y., Goda T. (2011). Clock genes regulate the feeding schedule-dependent diurnal rhythm changes in hexose transporter gene expressions through the binding of BMAL1 to the promoter/enhancer and transcribed regions. J. Nutr. Biochem. 22 (4), 334–343. doi:10.1016/j.jnutbio.2010.02.012
Jiang Y., Wang L., Dong Z., Xia B., Pang S. (2024). Recent drug development of dorzagliatin, a new glucokinase activator, with the potential to treat type 2 diabetes: a review study. J. Diabetes 16 (6), e13563. doi:10.1111/1753-0407.13563
Kecklund G., Axelsson J. J. B. (2016). Health consequences of shift work and insufficient sleep. BMJ 355, i5210. doi:10.1136/bmj.i5210
Kelly P. J., Lemmens R., Tsivgoulis G. (2021). Inflammation and stroke risk: a new target for prevention. Stroke 52 (8), 2697–2706. doi:10.1161/strokeaha.121.034388
Kim S. Y., Kim H. I., Kim T. H., Im S. S., Park S. K., Lee I. K., et al. (2004). SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J. Biol. Chem. 279 (29), 30823–30829. doi:10.1074/jbc.M313223200
Kim T. H., Kim H., Park J. M., Im S. S., Bae J. S., Kim M. Y., et al. (2009). Interrelationship between liver X receptor alpha, sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma, and small heterodimer partner in the transcriptional regulation of glucokinase gene expression in liver. J. Biol. Chem. 284 (22), 15071–15083. doi:10.1074/jbc.M109.006742
Kinsella G. K., Cannito S., Bordano V., Stephens J. C., Rosa A. C., Miglio G., et al. (2021). GPR21 inhibition increases glucose-uptake in HepG2 cells. Int. J. Mol. Sci. 22 (19), 10784. doi:10.3390/ijms221910784
Kokubo Y., Iwashima Y. (2015). Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension 66 (2), 254–259. doi:10.1161/hypertensionaha.115.03480
Kong P., Cui Z. Y., Huang X. F., Zhang D. D., Guo R. J., Han M. (2022). Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 7 (1), 131. doi:10.1038/s41392-022-00955-7
Kroon T., Hagstedt T., Alexandersson I., Ferm A., Petersson M., Maurer S., et al. (2022). Chronotherapy with a glucokinase activator profoundly improves metabolism in obese Zucker rats. Sci. Transl. Med. 14 (668), eabh1316. doi:10.1126/scitranslmed.abh1316
Lamia K. A., Papp S. J., Yu R. T., Barish G. D., Uhlenhaut N. H., Jonker J. W., et al. (2011). Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480 (7378), 552–556. doi:10.1038/nature10700
Lemmer B., Oster H. (2018). The role of circadian rhythms in the hypertension of diabetes mellitus and the metabolic syndrome. Curr. Hypertens. Rep. 20 (5), 43. doi:10.1007/s11906-018-0843-5
Li H., Wang X., Mao Y., Hu R., Xu W., Lei Z., et al. (2014). Long term liver specific glucokinase gene defect induced diabetic cardiomyopathy by up regulating NADPH oxidase and down regulating insulin receptor and p-AMPK. Cardiovasc. Diabetol. 13, 24. doi:10.1186/1475-2840-13-24
Liu H. Y., Javaheri A., Godar R. J., Murphy J., Ma X. C., Rohatgi N., et al. (2017). Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 13 (11), 1952–1968. doi:10.1080/15548627.2017.1368596
Liu Y.-Y., Wan Q. (2023). Relationship between GCKR gene rs780094 polymorphism and type 2 diabetes with albuminuria. World J. Diabetes 14 (12), 1803–1812. doi:10.4239/wjd.v14.i12.1803
Llanos P., Ordenes P., Rhoads D. B., Santibanez J. F., Garcia-Robles M., Millan C. (2023a). BMAL1 regulates glucokinase expression through E-box elements in vitro. Adv. Exp. Med. Biol. 1408, 235–249. doi:10.1007/978-3-031-26163-3_13
Llanos P., Ordenes P., Rhoads D. B., Santibanez J. F., García-Robles M., Millán C. (2023b). Advances in molecular pathology. Berlin, Germany: Springer, 235–249.
Malinowski B., Zalewska K., Wesierska A., Sokolowska M. M., Socha M., Liczner G., et al. (2019). Intermittent fasting in cardiovascular disorders-an overview. Nutrients 11 (3), 673. doi:10.3390/nu11030673
Manoogian E. N., Panda S. (2017). Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 39, 59–67. doi:10.1016/j.arr.2016.12.006
Maria Uranga R., Millan C., Jose Barahona M., Recabal A., Salgado M., Martinez F., et al. (2017). Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior. Sci. Rep. 7, 3697. doi:10.1038/s41598-017-03928-x
Marinho T. D., Borges C. C., Aguila M. B., Mandarim-De-Lacerda C. A. (2020). Intermittent fasting benefits on alpha- and beta-cell arrangement in diet-induced obese mice pancreatic islet. J. Diabetes Its Complicat. 34 (3), 107497. doi:10.1016/j.jdiacomp.2019.107497
Mason I. C., Qian J., Adler G. K., Scheer F. A. J. (2020). Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63 (3), 462–472. doi:10.1007/s00125-019-05059-6
Mateo-Gavira I., Vílchez-López F. J., García-Palacios M. V., Laureano F. C. S., Jiménez-Carmona S., Aguilar-Diosdado M. (2016). Nocturnal blood pressure is associated with the progression of microvascular complications and hypertension in patients with type 1 diabetes mellitus. J. Diabetes Complicat. 30 (7), 1326–1332. doi:10.1016/j.jdiacomp.2016.05.021
Mayer K. H., Stamler J., Dyer A., Freinkel N., Stamler R., Berkson D. M., et al. (1976). Epidemiologic findings on the relationship of time of day and time since last meal to glucose tolerance. Diabetes 25 (10), 936–943. doi:10.2337/diab.25.10.936
Mohawk J. A., Green C. B., Takahashi J. S. (2012). Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35 (35), 445–462. doi:10.1146/annurev-neuro-060909-153128
Mure L. S., Le H. D., Benegiamo G., Chang M. W., Rios L., Jillani N., et al. (2018). Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359 (6381), eaao0318. doi:10.1126/science.aao0318
Nakazawa S., Fukai K., Furuya Y., Kojimahara N., Hoshi K., Toyota A., et al. (2022). Occupations associated with diabetes complications: a nationwide-multicenter hospital-based case-control study. Diabetes Res. Clin. Pract. 186, 109809. doi:10.1016/j.diabres.2022.109809
Niknia S., Kaeidi A., Hajizadeh M. R., Mirzaei M. R., Khoshdel A., Hajializadeh Z., et al. (2019). Neuroprotective and antihyperalgesic effects of orexin-A in rats with painful diabetic neuropathy. Neuropeptides 73, 34–40. doi:10.1016/j.npep.2018.11.001
Nikolaeva S., Ansermet C., Centeno G., Pradervand S., Bize V., Mordasini D., et al. (2016). Nephron-specific deletion of circadian clock gene Bmal1 alters the plasma and renal metabolome and impairs drug disposition. J. Am. Soc. Nephrol. 27 (10), 2997–3004. doi:10.1681/asn.2015091055
Nishida K., Otsu K. (2017). Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 113 (4), 389–398. doi:10.1093/cvr/cvx012
Pan X., Bradfield C. A., Hussain M. M. (2016). Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 7, 13011. doi:10.1038/ncomms13011
Patel R., Parmar N., Palit S. P., Rathwa N., Ramachandran A. V., Begum R. (2022). Diabetes mellitus and melatonin: where are we? Biochimie 202, 2–14. doi:10.1016/j.biochi.2022.01.001
Patel S. R., Zhu X., Storfer-Isser A., Mehra R., Jenny N. S., Tracy R., et al. (2009). Sleep duration and biomarkers of inflammation. Sleep 32 (2), 200–204. doi:10.1093/sleep/32.2.200
Poggiogalle E., Jamshed H., Peterson C. M. (2018). Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 84, 11–27. doi:10.1016/j.metabol.2017.11.017
Potter G. D., Skene D. J., Arendt J., Cade J. E., Grant P. J., Hardie L. J. (2016). Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr. Rev. 37 (6), 584–608. doi:10.1210/er.2016-1083
Prather A. A., Marsland A. L., Hall M., Neumann S. A., Muldoon M. F., Manuck S. B. (2009). Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol. Psychol. 82 (1), 12–17. doi:10.1016/j.biopsycho.2009.04.008
Qi X. P., Mitter S. K., Yan Y. Q., Busik J. V., Grant M. B., Boulton M. E. (2020). Diurnal rhythmicity of autophagy is impaired in the diabetic retina. Cells 9 (4), 905. doi:10.3390/cells9040905
Qiao L., Guo B., Zhang H., Yang R., Chang L., Wang Y., et al. (2017). The clock gene, brain and muscle Arnt-like 1, regulates autophagy in high glucose-induced cardiomyocyte injury. Oncotarget 8 (46), 80612–80624. doi:10.18632/oncotarget.20811
Rabbani N., Xue M., Thornalley P. J. (2022). Hexokinase-2-linked glycolytic overload and unscheduled glycolysis-driver of insulin resistance and development of vascular complications of diabetes. Int. J. Mol. Sci. 23 (4), 2165. doi:10.3390/ijms23042165
Reppert S. M., Weaver D. R. J. N. (2002). Coordination of circadian timing in mammals. Nature 418 (6901), 935–941. doi:10.1038/nature00965
Saad A., Dalla Man C., Nandy D. K., Levine J. A., Bharucha A. E., Rizza R. A., et al. (2012). Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61 (11), 2691–2700. doi:10.2337/db11-1478
Seino Y., Maekawa R., Ogata H., Hayashi Y. (2016). Carbohydrate-induced secretion of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1. J. Diabetes Investig 7, 27–32. doi:10.1111/jdi.12449
Shimobayashi M., Thomas A., Shetty S., Frei I. C., Wolnerhanssen B. K., Weissenberger D., et al. (2023). Diet-induced loss of adipose hexokinase 2 correlates with hyperglycemia. Elife 12, e85103. doi:10.7554/eLife.85103
Solocinski K., Richards J., All S., Cheng K. Y., Khundmiri S. J., Gumz M. L. (2015). Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am. J. Physiol. Renal Physiol. 309 (11), F933–F942. doi:10.1152/ajprenal.00197.2014
Solt L. A., Wang Y., Banerjee S., Hughes T., Kojetin D. J., Lundasen T., et al. (2012). Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485 (7396), 62–68. doi:10.1038/nature11030
Stamler J., Stamler R., Neaton J. D. (1993). Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Archives Intern. Med. 153 (5), 598–615. doi:10.1001/archinte.153.5.598
Stenvers D. J., Scheer F. A., Schrauwen P., La Fleur S. E., Kalsbeek A. (2019). Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15 (2), 75–89. doi:10.1038/s41574-018-0122-1
Tacad D. K. M., Tovar A. P., Richardson C. E., Horn W. F., Keim N. L., Krishnan G. P., et al. (2022). Satiety associated with calorie restriction and time-restricted feeding: central neuroendocrine integration. Adv. Nutr. 13 (3), 758–791. doi:10.1093/advances/nmac011
Takahashi J. S. (2017). Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18 (3), 164–179. doi:10.1038/nrg.2016.150
Tsujino N., Sakurai T. (2009). Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol. Rev. 61 (2), 162–176. doi:10.1124/pr.109.001321
Tsuneki H., Tokai E., Nakamura Y., Takahashi K., Fujita M., Asaoka T., et al. (2015). Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64 (2), 459–470. doi:10.2337/db14-0695
Vancura P., Wolloscheck T., Baba K., Tosini G., Luvone P. M., Spessert R. (2016). Circadian and dopaminergic regulation of fatty acid oxidation pathway genes in retina and photoreceptor cells. PLoS One 11 (10), e0164665. doi:10.1371/journal.pone.0164665
Vasey C., Mcbride J., Penta K. J. N. (2021). Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13 (10), 3480. doi:10.3390/nu13103480
Verrillo A., Deteresa A., Martino C., Dichiara G., Pinto M., Verrillo L., et al. (1989). Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am. J. Physiol. 257 (4), E459–E465. doi:10.1152/ajpendo.1989.257.4.E459
Wang K., Shi M., Huang C., Fan B., Luk A. O. Y., Kong A. P. S., et al. (2022). Evaluating the impact of glucokinase activation on risk of cardiovascular disease: a Mendelian randomisation analysis. Cardiovasc. Diabetol. 21 (1), 192. doi:10.1186/s12933-022-01613-6
Wang Q., Bozack S. N., Yan Y. Q., Boulton M. E., Grant M. B., Busik J. V. (2014). Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Invest. Ophthalmol. Vis. Sci. 55 (6), 3986–3994. doi:10.1167/iovs.13-13076
Wang Q., Wu S., Luo Z., Pu L., Wang X., Guo M., et al. (2024). Effects of light therapy on sleep and circadian rhythm in older type 2 diabetics living in long-term care facilities: a randomized controlled trial. Front. Endocrinol. 15, 1307537. doi:10.3389/fendo.2024.1307537
Wojtczak-Jaroszowa J. J. C. (1977). Physiological and clinical aspects of circadian variations in glucose tolerance. Chronobiologia 4 (4), 363–384.
Xie Z., Xie T., Liu J., Zhang Q., Xiao X. (2023). Glucokinase inactivation ameliorates lipid accumulation and exerts favorable effects on lipid metabolism in hepatocytes. Int. J. Mol. Sci. 24 (5), 4315. doi:10.3390/ijms24054315
Xu X., Liu B., Yang J., Zou Y., Sun M., Li Z., et al. (2020). Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res. Bull. 165, 290–297. doi:10.1016/j.brainresbull.2020.10.004
Yang L., Feng H., Chen J., Wing Y. K., Benedict C., Tan X., et al. (2023). Association of circadian rest-activity rhythms with cardiovascular disease and mortality in type 2 diabetes. Diabetes Res. Clin. Pract. 197, 110262. doi:10.1016/j.diabres.2023.110262
Yang M., Chen W., He L., Liu D., Zhao L., Wang X. (2022). Intermittent fasting-A healthy dietary pattern for diabetic nephropathy. Nutrients 14 (19), 3995. doi:10.3390/nu14193995
Young M. W., Kay S. A. (2001). Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2 (9), 702–715. doi:10.1038/35088576
Zhang E. E., Liu Y., Dentin R., Pongsawakul P. Y., Liu A. C., Hirota T., et al. (2010). Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16 (10), 1152–1156. doi:10.1038/nm.2214
Zhao L., Hutchison A. T., Heilbronn L. K. J. C. O. I. C. N., Care M. (2021). Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 24 (4), 342–348. doi:10.1097/MCO.0000000000000756
Keywords: circadian rhythm, disruption, glucose metabolism, diabetes, diabetic complications, glucokinase
Citation: Zhang Z, Wang S and Gao L (2025) Circadian rhythm, glucose metabolism and diabetic complications: the role of glucokinase and the enlightenment on future treatment. Front. Physiol. 16:1537231. doi: 10.3389/fphys.2025.1537231
Received: 30 November 2024; Accepted: 04 February 2025;
Published: 21 February 2025.
Edited by:
Ganesh Kolumam, Calico Life Sciences LLC, United StatesReviewed by:
Masaaki Ikeda, Saitama Medical University, JapanCopyright © 2025 Zhang, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Gao, bGluZy5nYW9Ad2h1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.