- 1Department of Nephrology, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China
- 2School of Nursing, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 3Department of Nursing, Guizhou Nursing Vocational College, Guiyang, Guizhou, China
- 4Hospital infection Management Department, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China
- 5School of Nursing, Zunyi Medical University, Zunyi, Guizhou, China
- 6Department of Nursing, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
Objective: To explore the impact of various exercise doses on depressive symptoms among hemodialysis patients and offer valuable guidance for the selection of optimal exercise doses in clinical practice settings.
Methods: A comprehensive systematic review was conducted across four major databases, namely, PubMed, Embase, Web of Science, and Cochrane Library, covering the period from their inception until August 2024. Exercise interventions were classified based on adherence to American College of Sports Medicine (ACSM) recommendations, dividing studies into groups with high and low/uncertain ACSM adherence. A meta-analysis was performed utilising Review Manager5.4.1 to assess the effects of ACSM adherence on depression in hemodialysis patients.
Results: This meta-analysis incorporated a total of 19 randomized controlled trials, involving 1,285 patients. The mean age of the patients ranged from 33.2 to 70 years, and the average body mass index (BMI) fluctuated between 23.3 and 28.81 kg/m2. Males accounted for a relatively larger proportion of the participants. Among these trials, 14 were classified as having high ACSM adherence, while 5 were categorized as having low or uncertain adherence. Overall, exercise markedly improved depression in hemodialysis patients (SMD: −0.63, 95% CI: −0.87, −0.39; p < 0.05). The high ACSM adherence group showed greater improvement relative to the low/uncertain adherence group (SMD: −0.66 vs. −0.56). No notable disparities were noted in the effects of exercise duration or patient age on depression outcomes between the subgroups (p = 0.86, p = 0.48).
Conclusion: Exercise interventions that exhibit high adherence to the ACSM guidelines prove to be more efficacious in alleviating depression among hemodialysis patients as compared to those with low or uncertain adherence levels.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#myprospero
1 Introduction
The latest global burden of kidney disease report reveals that the international incidence of chronic kidney disease (CKD) attained 9.5% in 2022, which has become one of the most significant burdens on the global public health system (Bello et al., 2024; Jha et al., 2023). The terminal phase of CKD, namely, end-stage renal disease (ESRD), exhibits a global prevalence ranging from 8% to 16%, and this prevalence is on an upward trajectory over time (Kramer et al., 2018). Hemodialysis (HD) is one of the principal treatments for patients with ESRD, and it can enhance their prognosis and quality of life. However, depression is one of the most prevalent complications among HD patients and can trigger increased mortality and hospitalisation rates (Iida et al., 2020; Alshelleh et al., 2023). Research has demonstrated that more than a quarter of HD patients is diagnosed of major depression (Kalra et al., 2024). A systematic review and meta-analysis involving 80,932 CKD patients from 27 countries indicated a 30.6% incidence rate of depression in HD patients (Adejumo et al., 2024). The study results revealed that the occurrence of depression might lead patients who had previously undergone HD to discontinue dialysis (Silva et al., 2022; Virani et al., 2021), and treatment adherence would decrease (Cogley et al., 2022). Simultaneously, the probability of losing severe function would increase by 46% (Virani et al., 2021). Generally speaking, the incidence rate of depression among HD patients is relatively high, and the occurrence of depression will have numerous adverse effects on the health of HD patients. Therefore, the early and efficient management of depressive symptoms in HD patients are of paramount importance.
Current evidence supporting the role of exercise in mental health among clinical populations appears to be popular in the exercise community (Newsome et al., 2024). The treatment modalities for depression in HD patients incorporate both pharmacological and non-pharmacological approaches (Kubanek et al., 2021; Grigoriou et al., 2015; Nadort et al., 2020). Prolonged use of antidepressants in patients with CKD may exacerbate renal impairment, lead to adverse drug reactions and lead to poor treatment adherence (Menezes et al., 2021). Therefore, exercise, as a non-drug treatment approach,has become an essential means to improve the psychological and physiological wellbeing of the HD population (Gerogianni et al., 2019; Sovatzidis et al., 2020). Numerous studies indicate that exercise significantly relieves fatigue among HD patients (Lu et al., 2024) and promotes cardiovascular health (Davies et al., 2023), as well as ameliorates sarcopenia and physical function. Physical inactivity among HD patients also serves as a robust predictor of mortality (Huang et al., 2019). Moreover, exercise has been demonstrated to markedly alleviate depression symptoms and improve the overall wellbeing of individuals with HD (Greenwood et al., 2021; Yu et al., 2023; Bernier-Jean et al., 2022; Hargrove et al., 2021). The Centers for Disease Control and Prevention (CDC) advocates that physical activity serves as a preventive against conditions like depression, anxiety, cognitive decline and dementia (Halle et al., 2024). The American Kidney Foundation also underscores the importance of exercise in managing complications in HD patients (Huang et al., 2019). The 2021 guidelines for CKD by the British Society of Nephrology suggest that HD patients without contraindications should engage in at least 30 min of exercise 3–5 times weekly (Baker et al., 2022; Lambert et al., 2022). The effect of exercise seems to be dose-dependent. A recent consensus statement document from the Italian Society of Nephrology highlights that physical activity and exercise prescriptions should be tailored to each patient, considering factors such as physical function, comorbidities, space availability, and time to ensure adequacy, safety, and feasibility (Battaglia et al., 2024). In summary, lth/fitness-related outcomes in this cohort should be highlighted, focusing on the popularity. Newsome et al. (2024), safety, and effectiveness (Sovatzidis et al., 2020; Yu et al., 2023; Hargrove et al., 2021) of this particular exercise modality among people with or without health issues. However, there are few studies on exercise dose for depression in HD patients, and standardising exercise interventions remains a challenge. Therefore, further studies are required to investigate the optimal exercise dose for HD patients.
In 2014, the American College of Sports Medicine (ACSM) issued guidelines for the prescription exercise, emphasizing aerobic exercise, resistance training and flexibility exercises (Ferguson, 2014). Nevertheless, it remains unclear whether exercise programs that adhere to ACSM recommendations have a more significant influence on depression in HD patients. This systematic review seeks to evaluate the comparative outcomes of exercise interventions with high and low/uncertain adherence to ACSM suggestions on depression in HD patients.
2 Materials and methods
This systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Page et al., 2021) guidelines and has been registered with PROSPERO (CRD42024579620).
2.1 Search strategy
A comprehensive systematic review was conducted across four major databases, namely, PubMed, Embase, Web of Science and Cochrane Library, covering the period from their inception until August 2024. Additionally, manual searches were performed for relevant studies not retrieved from the databases. If needed, the corresponding authors were contacted for further information.The detailed search strategy is detailed in Supplementary Appendix S1.
2.2 Eligibility criteria
Eligibility criteria were established using the PICOS framework: (1) Participants: patients aged 18 years or older undergoing HD as renal replacement therapy, excluding those who received HD due to acute renal failure. (2) Interventions:any form of exercise, including aerobic exercise, resistance training, flexibility exercise, or exercise combined with video technology. (3) Comparisons: the control group either received no treatment or a treatment unrelated to exercise. (4) Outcomes:the primary endpoint was depression, assessed using tools such as the Beck Depression Inventory, The Center for Epidemiological Scale-Depression, or Zung Self-Rating Depression Scale. The Beck Depression Inventory is mainly used in clinical assessment and research to accurately assess the severity of depression. The Center for Epidemiological Scale-Depression focuses on large-scale epidemiological studies that screen populations for depressive symptoms. Zung Self-Rating Depression Scale is used for preliminary clinical judgment and mental health investigation, and also has a certain degree of severity. (5) Study design: randomised controlled trial (RCT).
The following studies were excluded: (1) Animal studies. (2) Reviews, case reports and conference abstracts. (3) Studies with missing data that could not be obtained by contacting the authors. (4) Duplicate publications, or studies without full-text access. (5) Case-control studies, cross-sectional studies, and longitudinal studies were excluded.
2.3 Data synthesis and analysis
Data were autonomously screened by two researchers (FY and HL) according to the established inclusion and exclusion criteria. When discrepancies arose, a third investigator (XB) adjudicated. Relevant data were extracted and recorded in Excel, encompassing the title, first author, year of publication, country, sample size, intervention details, age, intervention frequency, exercise intensity, duration and type of exercise. For investigations with several follow-up points, only the data immediately post-intervention were gathered. When multiple assessment tools were used for the same outcome, the most appropriate tool was selected based on a predetermined hierarchy.
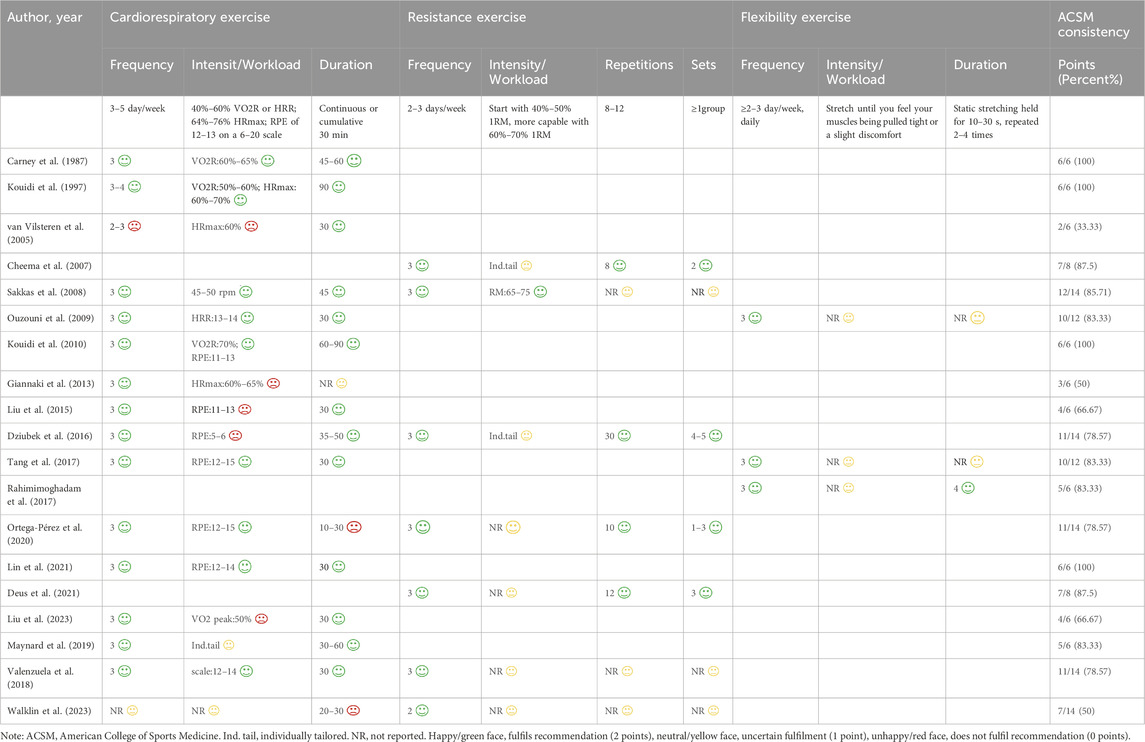
Following data extraction, both authors independently assessed the exercise intervention’s dosage (encompassing frequency, intensity, workload and duration) and compliance in HD patients, as per ACSM guidelines (Table 1) (Garber et al., 2011).
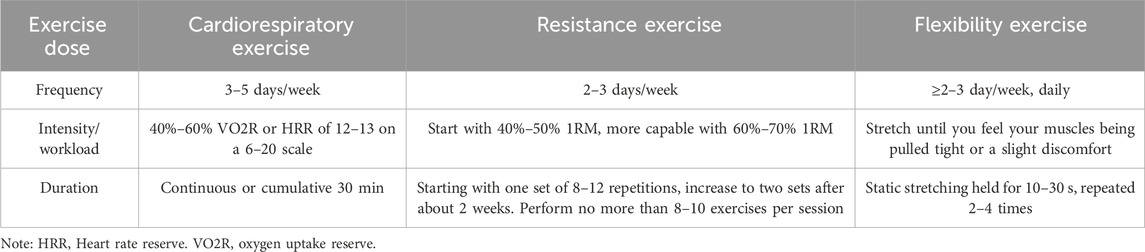
Table 1. The ACSM’s prescriptions for cardiorespiratory fitness, muscular strength, and flexibility in adults seemingly in good health.
2.4 Statistical analyses
Each physical activity metric was evaluated using a 0–2 point scale: 2 points were awarded for fulfilling the requirements, 1 point for ambiguity, and 0 points for failing to meet the criteria. When discrepancies arose in the evaluation process, the researchers consulted with an additional author to achieve agreement. Utilising this assessment method, the percentage of exercise regimens conforming to ACSM-suggested guidelines was determined for every investigation. A percentage of ≥75% signified strong compliance with ACSM recommendations, whereas <75% indicated low or questionable adherence.
Meta-analysis was executed utilising Review Manager 5.4.1 software. For data characterised by non-normal distribution and reported as medians (M) with interquartile ranges (P25, P75) in the original studies, the methods described by McGrath et al. (2020) were employed to calculate the mean value ±standard deviation (SD). The standardised mean difference (SMD) was used as the effect measure, and the Higgins I2 statistic assessed statistical variability across investigations. A fixed-effect model was deemed appropriate when treatment effects were homogeneous and heterogeneity was low (I2 < 50%). Conversely, a random-effects model was employed when heterogeneity was significant (I2 > 50%), with effect sizes denoted as SMD and 95% confidence intervals (95%CI). The investigations were categorised into two groups grounded in adherence to ACSM recommendations: high adherence and low or uncertain adherence. Publication bias was elevated by generating a funnel plot to evaluate the effect size against the standard deviation for each investigation, with a significance threshold established at p < 0.05. Sensitivity analysis was meticulously carried out by systematically omitting each of the included studies one by one with the application of Stata 17.0.
2.5 Quality appraisal
The methodological rigour of the incorporated investigations was autonomously evaluated by two separate teams of researchers employing the Cochrane (Higgins et al., 2011) risk of a bias assessment tool for RCTs. This appraisal encompassed six domains: random sequence generation (selection bias), allocation concealment (selection bias), participant and personnel blinding (performance bias), outcome data (attrition bias), reporting bias and other biases. The risk of bias was categorised into three categories: low risk, high risk and unclear risk.
3 Results
3.1 Study selection
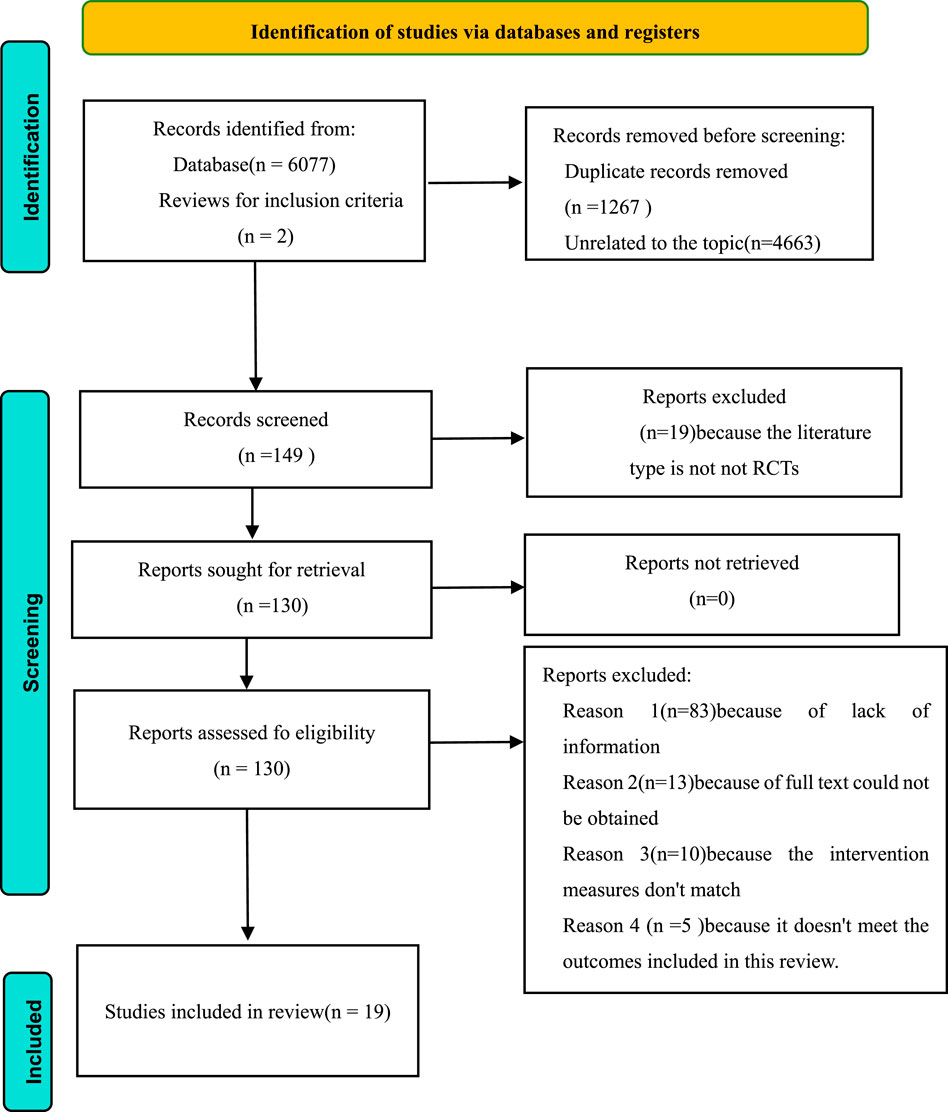
Altogether 6,077 publications were procured from four databases (PubMed: 2,065; Embase: 1,853; Web of Science: 1,027; Cochrane Library: 1,132), with 2 additional documents manually identified from alternative sources. After examining titles and abstracts, 149 articles were chosen for comprehensive evaluation. Ultimately, 19 investigations fulfilled the eligibility requirements and were incorporated into this analysis (Carney et al., 1987; Kouidi et al., 1997; van Vilsteren et al., 2005; Cheema et al., 2007; Sakkas et al., 2008; Ouzouni et al., 2009; Kouidi et al., 2010; Giannaki et al., 2013; Liu et al., 2015; Dziubek et al., 2016; Tang et al., 2017; Rahimimoghadam et al., 2017; Ortega-Pérez et al., 2020; Lin et al., 2021; Deus et al., 2021; Liu et al., 2023; Maynard et al., 2019; Valenzuela et al., 2018; Walklin et al., 2023) (Figure 1).
3.2 Study characteristics
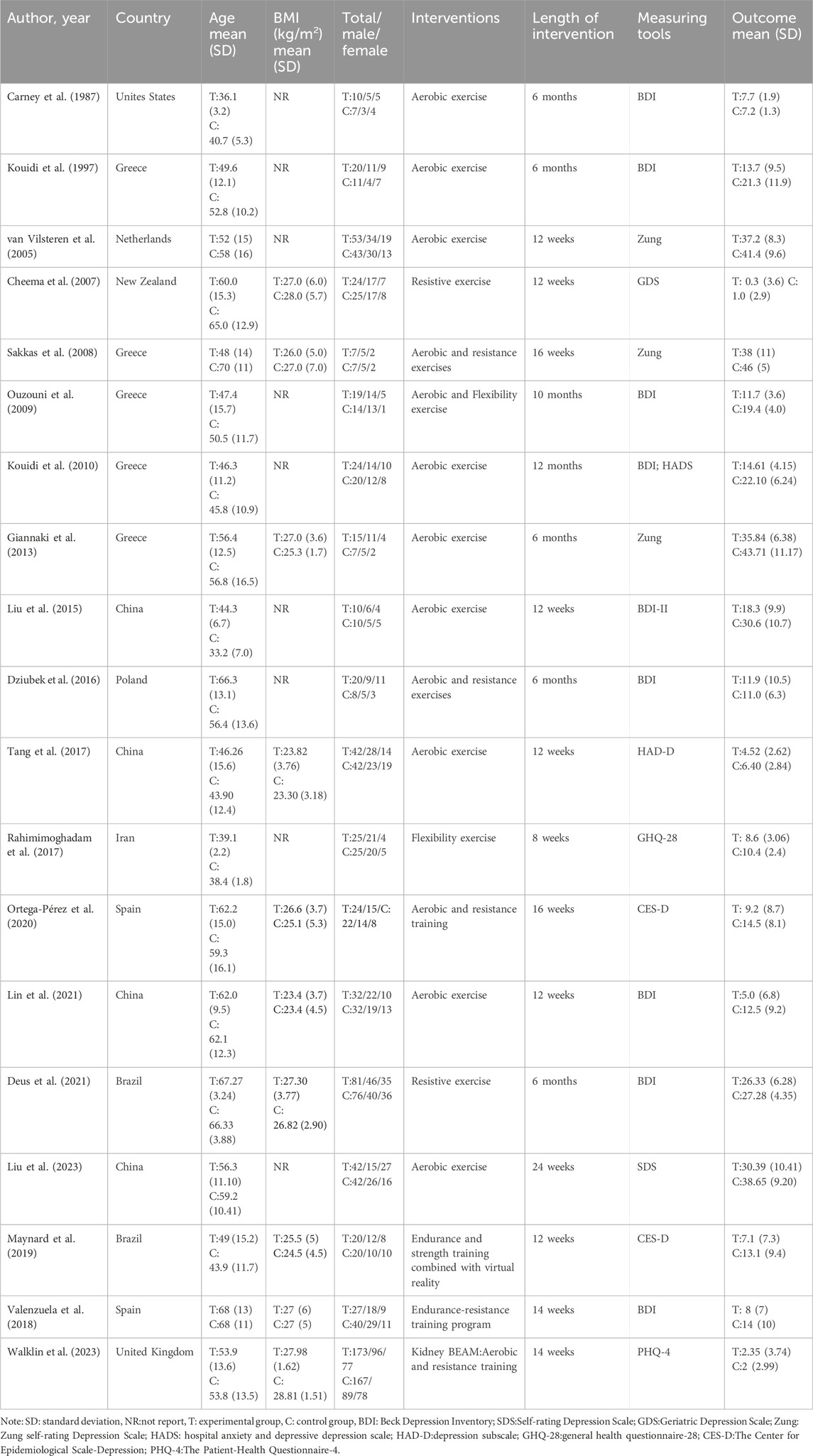
Table 2 depicts an overview of the primary attributes of the incorporated studies. In total, 19 investigations involved 1,285 participants, ranging in age from 33.2 to 70 years and the average body mass index (BMI) fluctuated between 23.3 and 28.81 kg/m2. Males accounted for a relatively larger proportion of the participants. The experimental groups included 667 participants, while the control groups comprised 618 participants. Intervention periods varied from 8 weeks to 12 months. Nine investigations focused on aerobic exercise, four on resistance exercise, one on flexibility exercises, four on a blend of aerobic and resistance exercises and one on a blend of aerobic and flexibility exercises. The depressive function was assessed using the Beck Depression Inventory (BDI) in nine studies, the Zung Self-Rating Depression Scale in three studies and Center for Epidemiological Scale-Depression (CES-D) in two studies. Additional tools used in a few studies included the Geriatric Depression Scale, the Hospital Anxiety and Depression Scale (HADS), Self-Rating Depression Scale (SDS), the General Health Questionnaire-28 (GHQ-28) and the Patient Health Questionnaire-4 (PHQ-4). Geographically, the studies were conducted in Greece (5), China (4), Spain (2), Brazil (2), New Zealand (2), and one study each in the United Kingdom, the Netherlands, Poland, Iran and the United States (Table 2).
3.3 Risk of bias
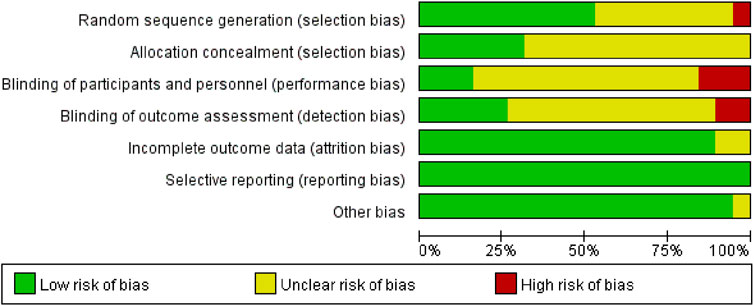
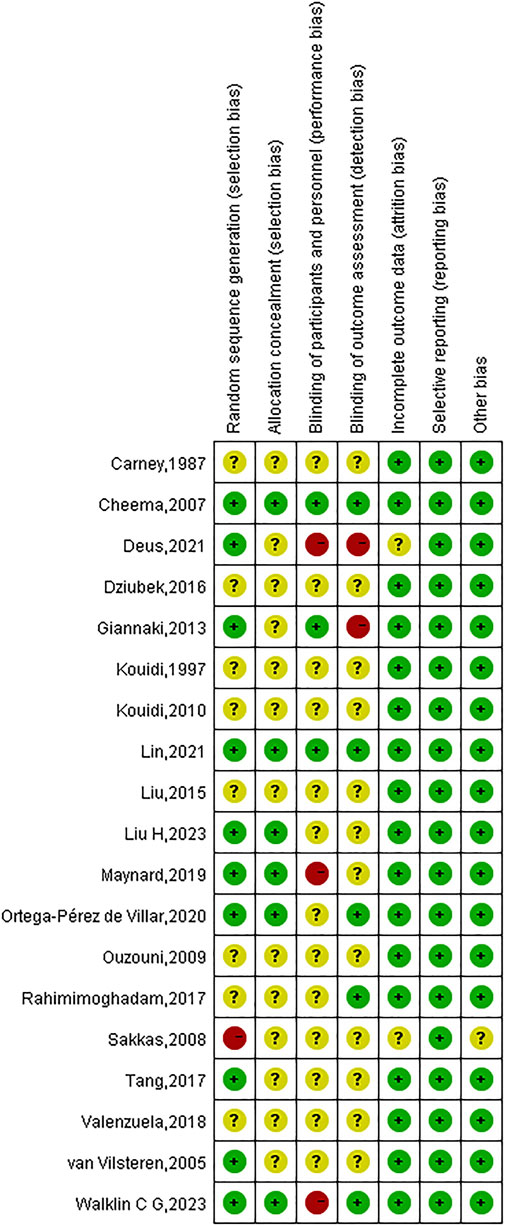
Due to incomplete methodological descriptions, the risk of bias in the examined research was largely categorised as high or uncertain. All 19 studies reported using randomisation, but only 10 (52.63%) provided detailed information on random sequence generation, and 6 studies described appropriate allocation concealment. Given the inherent challenges in blinding both participants and investigators in exercise interventions, only three studies blinded participants and five studies used blinded outcome assessments. Attrition rates were reported in most trials (89.47%), and complete data were available for all 19 studies. An in-depth evaluation of the calibre of the incorporated studies is depicted in Figures 2, 3.
3.4 Adherence to the ACSM guidelines
Following ACSM guidelines, the experimental groups were divided into two categories: high adherence and low or uncertain adherence. In 14 studies, physical activity achieved a compliance rate of ≥75%, meeting ACSM recommendations. In contrast, five studies had adherence rates of <75%, primarily due to experimental designs that did not incorporate all the recommended parameters. Additionally, a lack of sufficient detail on exercise prescription hindered accurate assessment of adherence (Table 3).
3.5 Meta-analysis
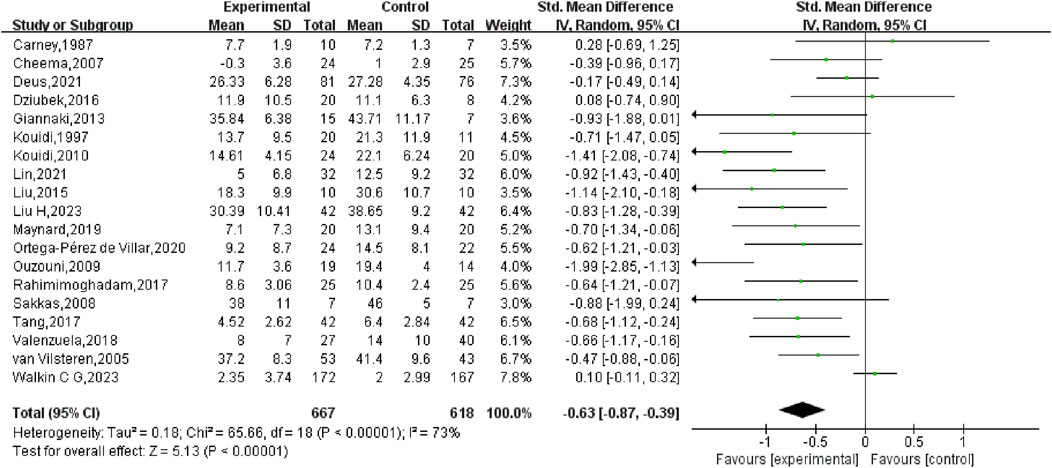
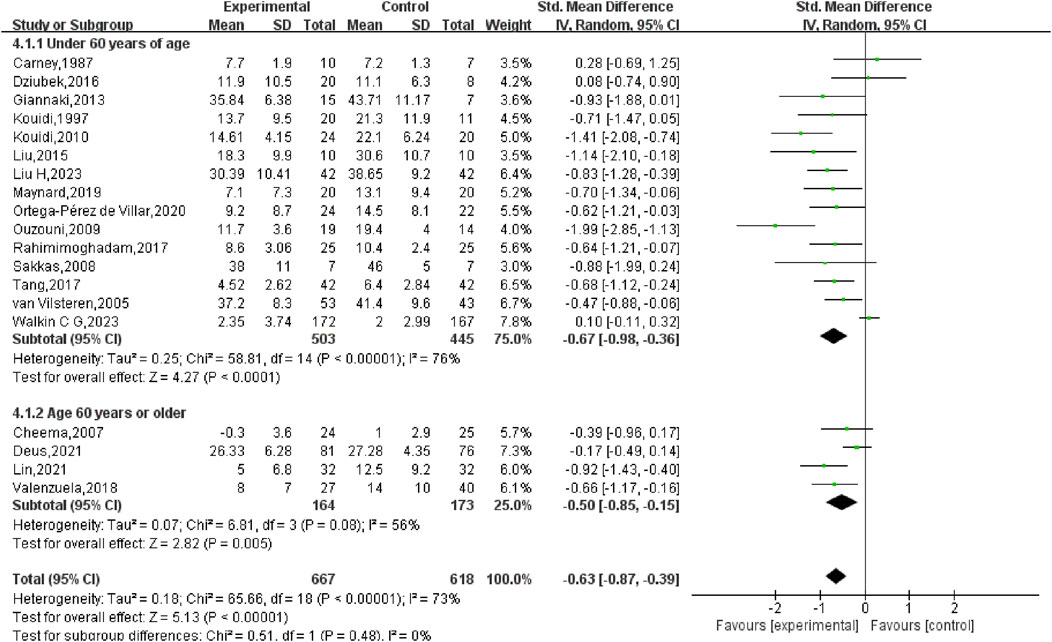
First, a comprehensive heterogeneity assessment of the incorporated studies was conducted, indicating substantial heterogeneity (I2 = 73%, p < 0.01). Consequently, a random-effects model was employed for statistical analysis. The outcomes suggested that exercise interventions in the experimental group had a greater positive effect on depression relative to the control group. The overall SMD was −0.63 (95% CI: −0.87, −0.39), with statistically significant results (p < 0.01), indicating that exercise exerted a considerable effect in alleviating depression in HD patients (Figure 4).
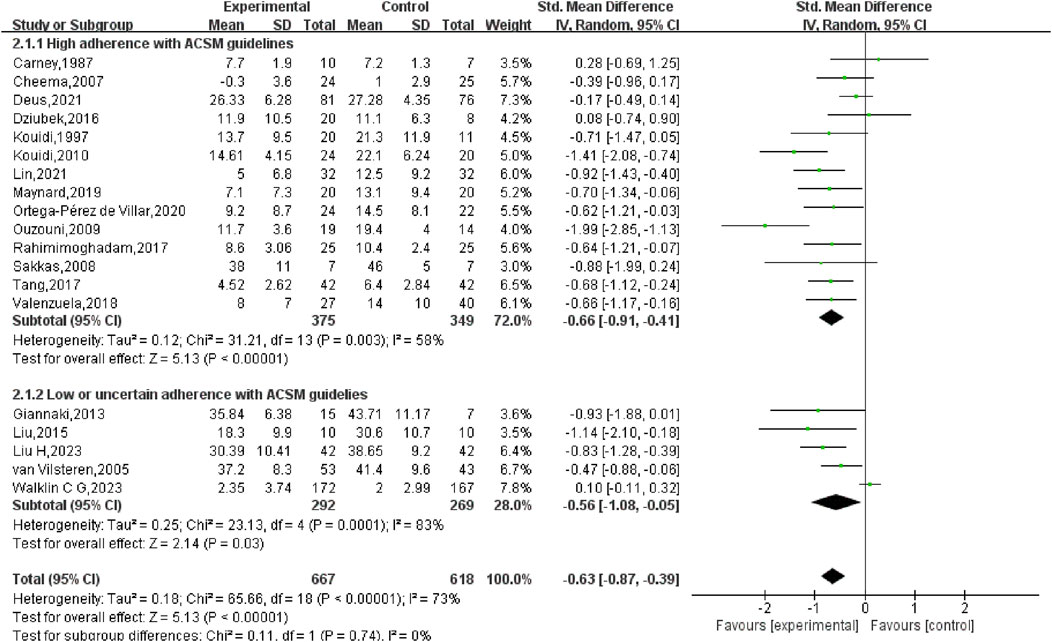
Subgroup analysis indicated that in the high adherence group, the SMD was −0.66 (95% CI: −0.91, −0.41), with moderate heterogeneity (I2 = 58%) and a statistically notable difference (p < 0.01). This suggests that exercise prescriptions closely aligned with ACSM recommendations had a considerable effect on reducing depression in HD patients. In the low or uncertain adherence group, the SMD was −0.56 (95% CI: −1.08, −0.05), with high heterogeneity (I2 = 83%) and a statistically notable difference (p = 0.03). This indicates that even exercise interventions with lower or uncertain adherence to ACSM guidelines had a significant effect on depression in HD patients. When comparing the two groups, exercise interventions highly adherent to ACSM guidelines demonstrated a slightly stronger effect on depression reduction (SMD: high adherence −0.66 vs low/uncertain adherence −0.56) (p < 0.05) (Figure 5).
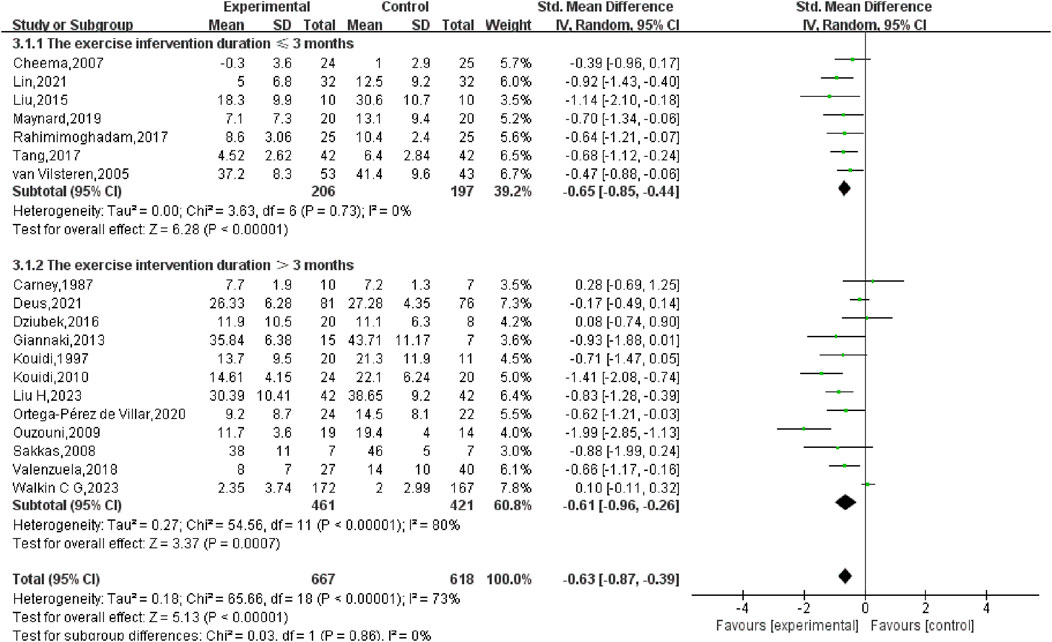
A subgroup analysis grounded in the length of the exercise intervention (ranging from 8 weeks to 12 months) was also performed. The results showed that for interventions lasting ≤3 months, the SMD was −0.65 (95% CI: −0.85, −0.44) (p < 0.01), while for interventions lasting >3 months, the SMD was −0.61 (95% CI: −0.96, −0.26) (p < 0.01). The comparison between the two groups yielded no statistically significant disparity (p = 0.86), suggesting that the intervention’s length did not markedly affect the overall results (Figure 6).
Given the wide age range of participants (33.2–70 years), a subgroup analysis based on age was also conducted. For participants younger than 60 years, the SMD was −0.67 (95% CI: −0.98, −0.36) (p < 0.01), while for participants aged 60 years or older, the SMD was −0.50 (95% CI: −0.85, −0.15) (p < 0.01). The analysis revealed no statistically significant disparity between the two age cohorts (p = 0.48), indicating that age did not markedly influence the overall results (Figure 7).
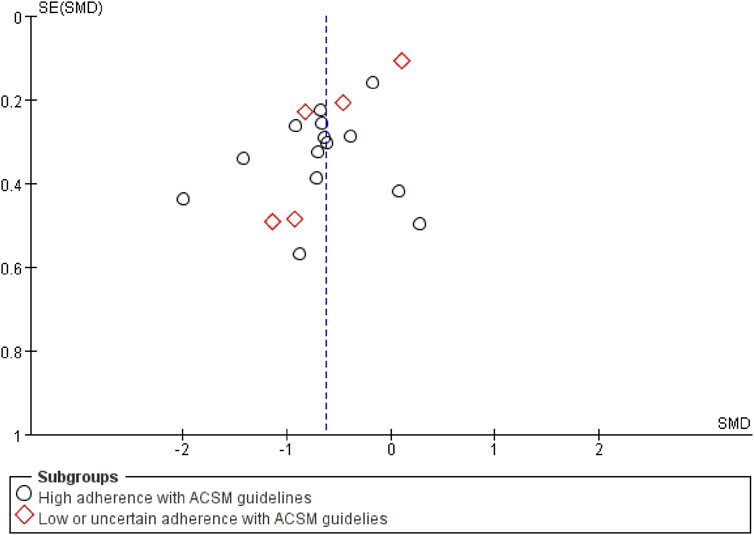
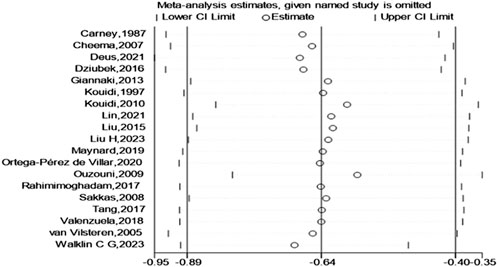
Finally, a test for publication bias was carried out by means of a funnel plot (Figure 8). The distribution of studies on both sides of the funnel plot seemed fairly balanced, implying the absence of significant publication bias. According to the sensitivity analysis (Figure 9), we found that no single study had a significant impact on the overall results, indicating the robustness of our research findings.
4 Discussion
This investigation compared the effects of exercise prescriptions with high adherence to the ACSM recommendations against those with low or uncertain adherence on depression in HD patients. The analysis encompassed 19 investigations involving 1,285 subjects.
The results confirmed that exercise remains a highly effective non-pharmacological intervention for alleviating depressive symptoms in HD patients (SMD: −0.63, 95% CI: −0.87, −0.39, p < 0.05), consistent with previous findings (Yu et al., 2023; Chung et al., 2017; Rezaei et al., 2015; Song et al., 2018; Hargrove et al., 2021; Wen et al., 2020). The mechanisms through which exercise reduces depressive symptoms are diverse and include the regulation of serotonin and norepinephrine levels, brain-derived neurotrophic factor and various immune-inflammatory pathways (Ross et al., 2023). However, prior investigations have emphasised the effectiveness of different exercise interventions varies. Consequently, investigating the impact of exercise doses on depressive symptoms in this patient population. Meta-analyses by Yu et al. (2023) and Hargrove et al. (2021) indicated that aerobic exercise is more effective than combined or resistance exercise in reducing depression levels. Chung et al. (2017) and Rezaei et al. (2015) found that exercise during dialysis markedly improved depressive symptoms when compared to no exercise, no resistance exercise, or walking at home. Similarly, Gomes et al. showed that a combination of aerobic and resistance exercises markedly improved depressive symptoms in HD patients. Gomes et al. (2018) the intensity and frequency of exercise are also critical factors. Current evidence suggests that any exercise intervention lasting 2–12 months may improve depressive symptoms, with sustained exercise beyond 4 months potentially leading to even greater improvements (Bernier-Jean et al., 2022). However, Hargrove et al. (2021) suggested that it may take 6 months or more for exercise programmes to maximise the relief of depressive symptoms in this population. Interestingly, Singh et al. (2023) found that the effectiveness of exercise interventions tends to decline with longer durations. Thus, while exercise is beneficial, the specific dose, including type, intensity, frequency and duration, requires further investigation to optimise the improvement of depressive symptoms in HD patients. This study highlights the need for more research into the precise parameters of exercise interventions tailored to this population.
In this systematic review, we integrated data from diverse research on multiple forms of exercise, varying intensities, frequency and duration to elevate the influence of exercise on depressive symptoms in patients with HD. We calculated adherence scores grounded in the ACSM guidelines, categorising subjects into high adherence and low/uncertain adherence groups. Subgroup analyses were subsequently executed to assess the effects of exercise dose on improving depressive symptoms in these individuals.
The subgroup analysis indicated that exercise exhibited a notable beneficial effect on diminishing depressive symptoms in both the high adherence and low/uncertain adherence groups [high adherence SMD = −0.66, 95% CI (−0.91, −0.41); low/uncertain adherence SMD = −0.56, 95% CI (−1.08, −0.05)], with statistical significance (p < 0.05). This implies that exercise, regardless of adherence to ACSM guidelines, is beneficial for improving depressive symptoms in HD patients. This aligns with previous studies highlighting the potential of exercise to alleviate depression (Singh et al., 2023). However, our study found that higher adherence to exercise prescriptions was more effective in reducing depressive symptoms than low or uncertain adherence (high SMD = −0.66 > low or uncertain SMD = −0.56).
The meta-analysis underscores the significant benefits of following exercise doses that align closely with ACSM guidelines relative to those with lower or uncertain compliance. These findings have strong clinical relevance and can provide a basis for developing standardised and methodical exercise intervention programmes for HD patients. Therefore, we recommend that healthcare professionals develop tailored exercise plans for HD patients with HD-related depression as early as possible. During exercise therapy, it is crucial to adjust the exercise dose based on individual patient characteristics and gradually increase it to achieve high adherence to ACSM recommendations, while prioritising patient safety. In clinical practice, customising exercise interventions per ACSM recommendations and providing personalised exercise prescriptions for HD patients with depression is essential. Future research should focus on conducting more RCTs that adhere to ACSM guidelines, with larger sample sizes, multicenter involvement and more rigorous study designs. This will further validate our findings and contribute to the development of systematic, standardised and repeatable exercise intervention protocols.
However, this study has several limitations. First, comprehensive outlines of exercise regimens in the interventions are essential for establishing a sensible spectrum of ACSM adherence scores. Unfortunately, the included studies exhibited disparities in the frequency, intensity and duration of exercise, making it difficult to develop common criteria for determining the most effective exercise interventions. Second, some investigations failed to report or inadequately documented exercise intervention doses. Thus, even with strict adherence to ACSM recommendations, exercise doses might be vaguely categorised as belonging to low or uncertain adherence groups. Lastly, the number of qualified RCTs examining the impact of exercise on depression in HD patients remains restricted. This paucity highlights the necessity for additional relevant investigations in this area to enhance the existing knowledge foundation. Overall, while the findings are promising, they warrant careful consideration, given the substantial variability noted across the examined research.
Despite its inherent limitations, this systematic review nonetheless presents valuable practical implications for clinical application. In exploring the optimal exercise dose for depression in HD patients, the exercise dose with high adherence to the ACSM guidelines is associated with better physical health outcomes, a classification that helps improve adherence to tailored exercise prescriptions. The findings support strict adherence to the ACSM recommended dose of exercise as one of the therapeutic strategies to improve depression in HD patients, while emphasizing personalized physical activity prescriptions to maximize the health benefits of exercise.
5 Conclusion
In this investigation, we observed that exercise exerts a notable beneficial influence on depressive symptoms in HD patients. Notably, our results suggest that strict adherence to the ACSM’s exercise guidelines has a greater impact on depressive symptoms than non-strict or uncertain adherence. This highlights the potential advantages of adhering to recommended physical activity protocols for HD patients. However, it is essential to recognise that the data derived from the meta-analysis is influenced by the limited number of studies, the unclear extent of participants’ adherence to exercise in specific investigations, and the varying composition of cases across the studies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YF: Writing–original draft, Writing–review and editing, Conceptualization. BX: Writing–original draft, Writing–review and editing, Conceptualization, Funding acquisition, Supervision. LH: Data curation, Investigation, Methodology, Writing–original draft. GY: Methodology, Resources, Data curation, Writing–review and editing. ZB: Formal Analysis, Software, Data curation, Methodology, Writing–review and editing. WM: Software, Validation, Visualization, Writing–review and editing. WJ: Validation, Visualization, Software, Writing–review and editing. LX: Resources, Validation, Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and publication of this article. This research has been funded by the Guizhou Nursing Vocational College Key Fund Project (gzhlyj2023-02) and the scientific research project of Guizhou Nursing Association in 2024 (GZHLKY202405).
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1513746/full#supplementary-material
References
Adejumo O. A., Edeki I. R., Sunday O. D., Falade J., Yisau O. E., Ige O. O., et al. (2024). Global prevalence of depression in chronic kidney disease: a systematic review and meta-analysis. J. Nephrol. 37, 2455–2472. doi:10.1007/s40620-024-01998-5
Alshelleh S., Alhawari H., Alhouri A., Abu-Hussein B., Oweis A. (2023). Level of depression and anxiety on quality of life among patients undergoing hemodialysis. Int. J. Gen. Med. 16, 161783–161795. doi:10.2147/ijgm.s406535
Baker L. A., March D. S., Wilkinson T. J., Billany R. E., Bishop N. C., Castle E. M., et al. (2022). Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 23 (1), 75. doi:10.1186/s12882-021-02618-1
Battaglia Y., Baciga F., Bulighin F., Amicone M., Mosconi G., Storari A., et al. (2024). Physical activity and exercise in chronic kidney disease: consensus statements from the Physical Exercise Working Group of the Italian Society of Nephrology. J. Nephrol. 37 (7), 1735–1765. doi:10.1007/s40620-024-02049-9
Bello A. K., Okpechi I. G., Levin A., Ye F., Damster S., Arruebo S., et al. (2024). An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob. Health 12 (3), e382–e395. doi:10.1016/S2214-109X(23)00570-3
Bernier-Jean A., Beruni N. A., Bondonno N. P., Williams G., Teixeira-Pinto A., Craig J. C., et al. (2022). Exercise training for adults undergoing maintenance dialysis. Cochrane Database Syst. Rev. 1 (1), CD014653. doi:10.1002/14651858.CD014653
Carney R. M., Templeton B., Hong B. A., Harter H. R., Hagberg J. M., Schechtman K. B., et al. (1987). Exercise training reduces depression and increases the performance of pleasant activities in hemodialysis patients. Nephron 47 (3), 194–198. doi:10.1159/000184490
Cheema B., Abas H., Smith B., O'Sullivan A., Chan M., Patwardhan A., et al. (2007). Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J. Am. Soc. Nephrol. 18 (5), 1594–1601. doi:10.1681/ASN.2006121329
Chung Y. C., Yeh M. L., Liu Y. M. (2017). Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J. Clin. Nurs. 26 (13-14), 1801–1813. doi:10.1111/jocn.13514
Cogley C., Carswell C., Bramham K., Chilcot J. (2022). Chronic kidney disease and severe mental illness: addressing disparities in access to health care and health outcomes. Clin. J. Am. Soc. Nephrol. 17 (9), 1413–1417. doi:10.2215/CJN.15691221
Davies M. D., Hughes F., Sandoo A., Alejmi A., Macdonald J. H. (2023). The effect of exercise on vascular health in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Am. J. Physiol. Ren. Physiol. 325 (5), F638–F655. doi:10.1152/ajprenal.00152.2023
Deus L. A., Corrêa H. L., Neves R., Reis A. L., Honorato F. S., Silva V. L., et al. (2021). Are resistance training-induced BDNF in hemodialysis patients associated with depressive symptoms, quality of life, antioxidant capacity, and muscle strength? An insight for the muscle-brain-renal Axis. Int. J. Environ. Res. Public Health 18 (21), 11299. doi:10.3390/ijerph182111299
Dziubek W., Kowalska J., Kusztal M., Rogowski Ł., Gołębiowski T., Nikifur M., et al. (2016). The level of anxiety and depression in dialysis patients undertaking regular physical exercise training--a preliminary study. Kidney Blood Press Res. 41 (1), 86–98. doi:10.1159/000368548
Ferguson B. (2014). ACSM's guidelines for exercise testing and prescription 9th ed. 2014. J. Can. Chiropr. Assoc. 58 (3), 328.
Garber C. E., Blissmer B., Deschenes M. R., Franklin B. A., Lamonte M. J., Lee I. M., et al. (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc 43 (7), 1334–1359. doi:10.1249/MSS.0b013e318213fefb
Gerogianni G., Babatsikou F., Polikandrioti M., Grapsa E. (2019). Management of anxiety and depression in haemodialysis patients: the role of non-pharmacological methods. Int. Urol. Nephrol. 51 (1), 113–118. doi:10.1007/s11255-018-2022-7
Giannaki C. D., Sakkas G. K., Karatzaferi C., Hadjigeorgiou G. M., Lavdas E., Kyriakides T., et al. (2013). Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 14194, 194. doi:10.1186/1471-2369-14-194
Gomes N. M., de Lacerda F., Lopes A. A., Martinez B. P., Saquetto M. B. (2018). Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin. Rehabil. 32 (9), 1189–1202. doi:10.1177/0269215518760380
Greenwood S. A., Koufaki P., Macdonald J. H., Bulley C., Bhandari S., Burton J. O., et al. (2021). Exercise programme to improve quality of life for patients with end-stage kidney disease receiving haemodialysis: the PEDAL RCT. Health Technol. Assess. 25 (40), 1–52. doi:10.3310/hta25400
Grigoriou S. S., Karatzaferi C., Sakkas G. K. (2015). Pharmacological and non-pharmacological treatment options for depression and depressive symptoms in hemodialysis patients. Health Psychol. Res. 3 (1), 1811. doi:10.4081/hpr.2015.1811
Halle M., Manfredini F., Floege J., Zoccali C. (2024). Physical exercise in haemodialysis patients: which type of exercise is more convenient? Clin. Kidney J. 17 (7), sfae165. doi:10.1093/ckj/sfae165
Hargrove N., EL T. N., Zhou O., Pinder M., Plant B., Askin N., et al. (2021). Effect of aerobic exercise on dialysis-related symptoms in individuals undergoing maintenance hemodialysis: a systematic review and meta-analysis of clinical trials. Clin. J. Am. Soc. Nephrol. 16 (4), 560–574. doi:10.2215/CJN.15080920
Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343d5928.
Huang M., Lv A., Wang J., Xu N., Ma G., Zhai Z., et al. (2019). Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am. J. Nephrol. 50 (4), 240–254. doi:10.1159/000502447
Iida H., Fujimoto S., Wakita T., Yanagi M., Suzuki T., Koitabashi K., et al. (2020). Psychological flexibility and depression in advanced CKD and dialysis. Kidney Med. 2 (6), 684–691.e1. doi:10.1016/j.xkme.2020.07.004
Jha V., AL-Ghamdi S., Li G., Wu M. S., Stafylas P., Retat L., et al. (2023). Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the inside CKD research programme. Adv. Ther. 40 (10), 4405–4420. doi:10.1007/s12325-023-02608-9
Kalra S., Sharma S., Sahay M. (2024). Dialysis distress. J. Pak Med. Assoc. 74 (5), 1000–1002. doi:10.47391/JPMA.24-37
Kouidi E., Iacovides A., Iordanidis P., Vassiliou S., Deligiannis A., Ierodiakonou C., et al. (1997). Exercise renal rehabilitation program: psychosocial effects. Nephron 77 (2), 152–158. doi:10.1159/000190266
Kouidi E., Karagiannis V., Grekas D., Iakovides A., Kaprinis G., Tourkantonis A., et al. (2010). Depression, heart rate variability, and exercise training in dialysis patients. Eur. J. Cardiovasc Prev. Rehabil. 17 (2), 160–167. doi:10.1097/HJR.0b013e32833188c4
Kramer A., Pippias M., Noordzij M., Stel V. S., Afentakis N., Ambühl P. M., et al. (2018). The European renal association - European dialysis and transplant association (ERA-EDTA) registry annual report 2015: a summary. Clin. Kidney J. 11 (1), 108–122. doi:10.1093/ckj/sfx149
Kubanek A., Paul P., Przybylak M., Kanclerz K., Rojek J. J., Renke M., et al. (2021). Use of sertraline in hemodialysis patients. Med. Kaunas. 57 (9), 949. doi:10.3390/medicina57090949
Lambert K., Lightfoot C. J., Jegatheesan D. K., Gabrys I., Bennett P. N. (2022). Physical activity and exercise recommendations for people receiving dialysis: a scoping review. PLoS One 17 (4), e0267290. doi:10.1371/journal.pone.0267290
Lin C. H., Hsu Y. J., Hsu P. H., Lee Y. L., Lin C. H., Lee M. S., et al. (2021). Effects of intradialytic exercise on dialytic parameters, health-related quality of life, and depression status in hemodialysis patients: a randomized controlled trial. Int. J. Environ. Res. Public Health 18 (17), 9205. doi:10.3390/ijerph18179205
Liu Y. M., Chung Y. C., Chang J. S., Yeh M. L. (2015). Effects of aerobic exercise during hemodialysis on physical functional performance and depression. Biol. Res. Nurs. 17 (2), 214–221. doi:10.1177/1099800414539548
Liu H., Zheng F., Yao W., Zhu J., Du X., Shi H., et al. (2023). The impact of aerobic exercise on health-related quality of life among patients undergoing maintenance hemodialysis. Med. Baltim. 102 (45), e35990. doi:10.1097/MD.0000000000035990
Lu X., Yang J., Bai D., Wu C., Cai M., Wang W., et al. (2024). Effect of exercise on fatigue in patients receiving maintenance hemodialysis treatment: a systematic review and network meta-analysis. Am. J. Phys. Med. Rehabil. 103 (4), 293–301. doi:10.1097/PHM.0000000000002348
Maynard L. G., de Menezes D. L., Lião N. S., de Jesus E. M., Andrade N., Santos J., et al. (2019). Effects of exercise training combined with virtual reality in functionality and health-related quality of life of patients on hemodialysis. Games Health J. 8 (5), 339–348. doi:10.1089/g4h.2018.0066
Mcgrath S., Zhao X., Steele R., Thombs B. D., Benedetti A.DEPRESsion Screening Data DEPRESSD Collaboration (2020). Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 29 (9), 2520–2537. doi:10.1177/0962280219889080
Menezes R., Sampaio T. L., Martins A., de Sousa A. R., Magalhães E. P. (2021). Prescription drug overdose, depression, and other mental disorders in the context of kidney disease. Contrib. Nephrol. 199, 199155–199161. doi:10.1159/000517700
Nadort E., Schouten R. W., Witte S., Broekman B., Honig A., Siegert C., et al. (2020). Treatment of current depressive symptoms in dialysis patients: a systematic review and meta-analysis. Gen. Hosp. Psychiatry 67, 6726–6734. doi:10.1016/j.genhosppsych.2020.07.012
Newsome A. M., Batrakoulis A., Camhi S. M., Mcavoy C., Sansone J. S., Reed R. (2024). 2024 ACSM worldwide fitness trends: future directions of the health and fitness industry. ACSM's Health & Fit. J. 28 (6), 14–26. doi:10.1249/fit.0000000000000933
Ortega-Pérez D. V. L., Martínez-Olmos F. J., Pérez-Domínguez F. B., Benavent-Caballer V., Montañez-Aguilera F. J., Mercer T., et al. (2020). Comparison of intradialytic versus home-based exercise programs on physical functioning, physical activity level, adherence, and health-related quality of life: pilot study. Sci. Rep. 10 (1), 8302. doi:10.1038/s41598-020-64372-y
Ouzouni S., Kouidi E., Sioulis A., Grekas D., Deligiannis A. (2009). Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin. Rehabil. 23 (1), 53–63. doi:10.1177/0269215508096760
Page M. J., Mckenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372n71, n71. doi:10.1136/bmj.n71
Rahimimoghadam Z., Rahemi Z., Mirbagher A. N., Sadat Z. (2017). Effects of Pilates exercise on general health of hemodialysis patients. J. Bodyw. Mov. Ther. 21 (1), 86–92. doi:10.1016/j.jbmt.2016.05.012
Rezaei J., Abdi A., Rezaei M., Heydarnezhadian J., Jalali R. (2015). Effect of regular exercise program on depression in hemodialysis patients. Int. Sch. Res. Not. 2015182030, 182030. doi:10.1155/2015/182030
Ross R. E., Vanderwerker C. J., Saladin M. E., Gregory C. M. (2023). The role of exercise in the treatment of depression: biological underpinnings and clinical outcomes. Mol. Psychiatry 28 (1), 298–328. doi:10.1038/s41380-022-01819-w
Sakkas G. K., Hadjigeorgiou G. M., Karatzaferi C., Maridaki M. D., Giannaki C. D., Mertens P. R., et al. (2008). Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J. 54 (2), 185–190. doi:10.1097/MAT.0b013e3181641b07
Silva O., Jaber D., Chiu A., Adams-Mardi C., Wicht E. (2022). Depression and capacity to withdraw from dialysis. J. Clin. Ethics 33 (3), 240–244. doi:10.1086/jce2022333240
Singh B., Olds T., Curtis R., Dumuid D., Virgara R., Watson A., et al. (2023). Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br. J. Sports Med. 57 (18), 1203–1209. doi:10.1136/bjsports-2022-106195
Song Y. Y., Hu R. J., Diao Y. S., Chen L., Jiang X. L. (2018). Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. J. Pain Symptom Manage 55 (4), 1184–1195. doi:10.1016/j.jpainsymman.2017.12.472
Sovatzidis A., Chatzinikolaou A., Fatouros I. G., Panagoutsos S., Draganidis D., Nikolaidou E., et al. (2020). Intradialytic cardiovascular exercise training alters redox status, reduces inflammation and improves physical performance in patients with chronic kidney disease. Antioxidants (Basel) 9 (9), 868. doi:10.3390/antiox9090868
Tang Q., Yang B., Fan F., Li P., Yang L., Guo Y. (2017). Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: a randomized controlled trial in China. Int. J. Nurs. Pract. 23 (2). doi:10.1111/ijn.12519
Valenzuela P. L., de Alba A., Pedrero-Chamizo R., Morales J. S., Cobo F., Botella A., et al. (2018). Intradialytic exercise: one size doesn't fit all. Front. Physiol. 9844, 844. doi:10.3389/fphys.2018.00844
van Vilsteren M. C., de Greef M. H., Huisman R. M. (2005). The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol. Dial. Transpl. 20 (1), 141–146. doi:10.1093/ndt/gfh560
Virani A., Shah R. P., Haneef G., Khan A. T., Dias C. C., Pereira K. N., et al. (2021). Depression impairs level of functioning in chronic kidney disease inpatients: a case-control study. Cureus 13 (6), e16017. doi:10.7759/cureus.16017
Walklin C. G., Young H., Asghari E., Bhandari S., Billany R. E., Bishop N., et al. (2023). The effect of a novel, digital physical activity and emotional well-being intervention on health-related quality of life in people with chronic kidney disease: trial design and baseline data from a multicentre prospective, wait-list randomised controlled trial (kidney BEAM). BMC Nephrol. 24 (1), 122. doi:10.1186/s12882-023-03173-7
Wen X., Wang Y., Zhao Q., Zhang H., Shi H., Wang M., et al. (2020). Nonpharmacological interventions for depressive symptoms in end-stage renal disease: a systematic review. West J. Nurs. Res. 42 (6), 462–473. doi:10.1177/0193945919857540
Keywords: hemodialysis, exercise, ACSM, depression, systematic review
Citation: Fang Y, Xiaoling B, Huan L, Yaping G, Binying Z, Man W, Juan W and Xinyu L (2025) Effects of exercise dose based on the ACSM recommendations on depression in hemodialysis patients: a systematic review and meta-analysis of randomized controlled trials. Front. Physiol. 15:1513746. doi: 10.3389/fphys.2024.1513746
Received: 19 October 2024; Accepted: 23 December 2024;
Published: 31 January 2025.
Edited by:
Ida Cariati, University of Rome Tor Vergata, ItalyReviewed by:
Alexios Batrakoulis, University of Thessaly, GreeceYuri Battaglia, University of Verona, Italy
Copyright © 2025 Fang, Xiaoling, Huan, Yaping, Binying, Man, Juan and Xinyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bai Xiaoling, YmFpeGlhb2xpbmcyMDAzQDE2My5jb20=
 Yang Fang
Yang Fang Bai Xiaoling
Bai Xiaoling Li Huan2,4
Li Huan2,4 Zhang Binying
Zhang Binying