
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol., 30 October 2024
Sec. Red Blood Cell Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1499308
The development of red blood cell (RBC) storage lesion during hypothermic storage has long posed challenges for blood transfusion efficacy. These alterations are primarily driven by oxidative stress, concern both structural and biochemical aspects of RBCs, and affect their interactions with the recipient’s tissues post-transfusion. Efforts to counteract these effects focus on improving the antioxidant capacity within stored RBCs, reducing oxygen exposure, and scavenging harmful molecules that accumulate during storage. Various supplements, such as ascorbic acid, N-acetylcysteine, polyphenolic compounds, and specific metabolites have shown the potential to improve RBC quality by reducing oxidative lesions and lysis phenomena, and enhancing antioxidant, energy, or proteostasis networks. Accordingly, anaerobic storage has emerged as a promising strategy, demonstrating improved RBC storability and recovery in both animal models and preliminary human studies. Finally, targeted scavenging of harmful storage-related phenotypes and molecules, like removal signals, oxidized proteins, and extracellular hemoglobin, while not so studied, also has the potential to benefit both the unit and the patient in need. Omics technologies have aided a lot in these endeavors by revealing biomarkers of superior storability and, thus, potential novel supplementation strategies. Nonetheless, while the so far examined storage modifications show significant promise, there are not many post-transfusion studies (either in vitro, in animal models, or humans) to evaluate RBC efficacy in the transfusion setting. Looking ahead, the future of blood storage and transfusion will likely depend on the optimization of these interventions to extend the shelf-life and quality of stored RBCs, as well as their therapeutic outcome.
During their hypothermic storage, red blood cells (RBCs) accumulate a wide array of defects, collectively known as storage lesion. The primary culprit is oxidative stress, where hemoglobin (Hb) oxidation (Yoshida et al., 2019) leads to the formation of methemoglobin (metHb) and superoxide anions (O2•−). Low temperatures accelerate metHb denaturation, releasing free hemin or heme and forming hemichromes (Samuel et al., 2020), which tightly bind to the membrane, increasing rigidity, promoting removal signals, like band three aggregates (Badior and Casey, 2018), and disrupting phosphorylation events (Pantaleo et al., 2016). At the same time, Fenton and Haber-Weiss reactions can produce hydroxyl radicals (HO•), one of the most potent oxidizing radicals in biology (Moller et al., 2023). While superoxide anions are not toxic per se, they can react with nitric oxide (NO•) to produce peroxynitrite (ONOO−) (Cortese-Krott, 2023), which further reacts with CO2 to generate powerful oxidants like nitrogen dioxide (NO2•) and carbonate radical (CO3•−) (Denicola et al., 1996). All these radicals attack cellular targets, leading to lipid peroxidation and protein carbonylation, initiating a cascade of cellular and biochemical alterations. Hb is one of the main targets of oxidative stress, further propagating the production of radical species. Additionally, ferrylHb is formed and exhibits extremely high redox potential, rendering it highly toxic to cellular molecules (Alayash, 2022). In terms of RBC integrity, structural proteins also undergo oxidation (D'Alessandro et al., 2012), and many oxidized molecules migrate to the membrane, disrupting its connections with the cytoskeleton and reducing RBC deformability (Barshtein et al., 2021). These changes affect phospholipid distribution in the membrane, exposing phosphatidylserine (PS), a marker of cell removal (Koshkaryev et al., 2020). In parallel, the “do-not-eat-me” signal of CD47 is also converted to an “eat-me” signal through oxidatively induced conformational changes (Burger et al., 2012). Moreover, several bioactive products of oxidation or glycoxidation processes, including oxylipins and advanced glycation end products (AGEs), accumulate during storage (Lysenko et al., 2006; Fu et al., 2016). The oxidation of metabolic enzymes, combined with acidic conditions, reduces key metabolites like 2,3-diphosphoglycerate (2,3-DPG), ATP, and NADPH. At the same time, systems that redirect glucose to the pentose phosphate pathway to enhance reducing power in stored RBCs (Rinalducci et al., 2015) become less effective due to fragmentation of the N-terminus of band 3, which regulates metabolic fluxes (Rinalducci et al., 2012; D'Alessandro et al., 2023). Finally, ATP depletion and low temperatures restrict Na+-K+ ATPase functionality, altering the cation gradient across the membrane (Flatt et al., 2014), and affecting RBC volume and shape (Zimna et al., 2021).
Despite being equipped with an antioxidant defense system, including enzymes like superoxide dismutase, peroxiredoxin 2, and catalase, and non-enzymatic scavengers like glutathione (GSH) and ascorbate (Moller et al., 2023; Chatzinikolaou et al., 2024), stored RBCs are characterized by redox imbalance. Many antioxidant enzymes become oxidized (Delobel et al., 2016), as in the case of peroxiredoxin 2; its oxidized form is accumulated during storage due to its decreased reduction (Sadowska-Bartosz and Bartosz, 2023). Accordingly, GSH gets depleted, especially since its de novo synthesis is minimal (Whillier et al., 2011; D'Alessandro et al., 2017). Additionally, the oxidation of membrane-related molecules and translocation of oxidized proteins to the membrane creates a source of reactive species that is less accessible to the cytosolic antioxidant system (Mohanty et al., 2014). Proteasomal degradation of carbonylated proteins can help alleviate cellular stress (Delobel et al., 2012), but proteasomal activity decreases in the cytosol during storage (Anastasiadi et al., 2021; Peltier et al., 2024). Although proteasome’s subunits translocate to the membrane around the middle of the storage period, their activity declines later, likely due to accumulated defects (Tzounakas et al., 2022c) or inability to process overoxidized aggregates (Delobel et al., 2012). Another way of dealing with damaged molecules is their compartmentalization and release through extracellular vesicles (EVs). While this ostracization of potentially toxic molecules is helpful, it exacerbates membrane integrity issues, altering RBC morphology and leading to the ultimate storage lesion phenotype: hemolysis (Figure 1) (Melzak et al., 2021; Tzounakas et al., 2022b).

Figure 1. Hallmarks of stored red blood cell oxidative lesions. Oxyhemoglobin (OxyHb) oxidation leads to the generation of methemoglobin (metHb) and reactive oxygen species (ROS). MetHb can form hemichromes that get attached to band 3 (1) and aggravate red blood cell (RBC) deformability. ROS can fuel the production of reactive nitrogen species and altogether oxidize lipid (2) and protein (3) molecules. All these phenomena aid in the emergence of removal signals, by modifying self-antigens, like CD47 (4), or exposing phosphatidylserine on the RBC surface (5). The fragmentation of the cytosolic N-terminus of band 3 (6) alters metabolic fluxes, while ATP depletion (7) affects the normal function of ion channels, and thus, ion homeostasis, ultimately leading to shape modifications (8). At the same time, both enzymatic and non-enzymatic antioxidant mechanisms are compromised (9), the proteasome machinery’s activity is reduced (10), and the cell sacrifices part of its membrane through vesiculation to remove oxidized molecules (11). Altogether, these lesions lead to the occurrence of hemolysis (12), the gold standard of blood storage. CAT: catalase; DAMP: damage-associated molecular pattern; GSH: glutathione; NO: nitric oxide; prdx2: peroxiredoxin 2; SOD: superoxide dismutase; TSP-1: thrombospondin 1. Created in BioRender.com/f93h148.
The extensive antioxidant system of RBCs can benefit every plasma-accessible part of the organism since redox buffering is one of the many altruistic functions of these cells (Anastasiadi et al., 2024a). This is possible through transmembrane electron transport, which allows the reduction of extracellular oxidants. Potential cytosolic sources of reducing equivalents include NADH, ascorbate, and flavonoids, while the plasma membrane redox system of RBCs seems to be comprised of distinct oxidoreductases, with the involvement of cytochromes and sulfhydryl groups (Matteucci and Giampietro, 2007). A notable example concerns the recycling of ascorbic acid. RBCs can retrieve dehydroascorbate from their environment and convert it to ascorbic acid. This boosts the plasma membrane electron transport through the duodenal isoform of cytochrome b561, the main substrate of which is the extracellular ascorbate free radical, reducing it back to ascorbic acid (Eigenschink et al., 2021). The disturbance of RBC redox potential during storage, along with the elevated lysis and subsequent release of prooxidant molecules in the circulation post-transfusion can have detrimental consequences on systemic redox signaling, especially in the vasculature. Reactive oxygen species (ROS) regulate vascular function, but they must be within specific limits to remain harmless (Obradovic et al., 2020). Redox disturbance in the endothelium can also be caused by AGEs that accumulate on the surface of stored RBCs, since they can interact with their receptor in the microvasculature, induce ROS production, and deplete antioxidant powers (Mangalmurti et al., 2010).
It should not be omitted that the interaction of RBCs with vascular function is predominantly through nitric oxide (NO) metabolism. NO is a powerful vasodilator that initiates a cascade that relaxes smooth muscle by lowering intracellular calcium (Zhao et al., 2015). RBC membrane is believed to possess functional domains, one of which is related to NO production via the interaction of deoxyHb with nitrite (Gladwin et al., 2005). Additionally, RBCs are equipped with an active endothelial NO synthase that converts L-arginine to citrulline, releasing NO in the process (Cortese-Krott and Kelm, 2014), a reaction that is affected by elevated oxidative stress (Cortese-Krott and Shiva, 2019). Yet, both free and vesicular Hb can capture NO in the circulation upon transfusion, limiting its bioavailability (Buehler and Alayash, 2004) and potentially decreasing blood flow in the microcirculation, ultimately leading to organ compromise (Roback, 2011; Weinberg and Patel, 2016). In the same context, AGEs have been also shown to quench NO and at the same time induce endothelin-1 (a powerful vasoconstrictor), further tipping the scales in favor of vasoconstriction (Basta et al., 2004). In addition, in vitro experiments support that PS-exposing RBCs can be targeted by secretory phospholipase A2, releasing lysophosphatidic acid, a potent lipid mediator that can induce endothelial dysfunction and even vascular leak (Neidlinger et al., 2006). Finally, oxylipins are also linked to hemodynamic properties post-transfusion, probably explaining the increase in blood flow even when NO synthesis is compromised (Figure 1) (D'Alessandro et al., 2019).
Upon recognizing the relation between oxidative stress and storage lesions and by utilizing elegant omics technologies (Nemkov et al., 2024b; Thomas et al., 2024) and advanced platforms for the evaluation of multiple storage strategies (Nemkov et al., 2022), blood transfusion research has increasingly focused on discovering biomarkers of superior storability and refining storage conditions to mitigate oxidative events. Addressing such lesions holds the promise of not only extending the quality and shelf-life of blood units, but also of reducing adverse post-transfusion reactions to improve patient outcomes, paving the way for precision blood transfusion, where blood units and distinct storage strategies can be selected to match specific patient cohorts. In this context, three main routes have been explored: the enhancement of extracellular and intracellular antioxidant defenses, the reduction of oxygen, and the targeted scavenging of harmful biological molecules (Figure 2).
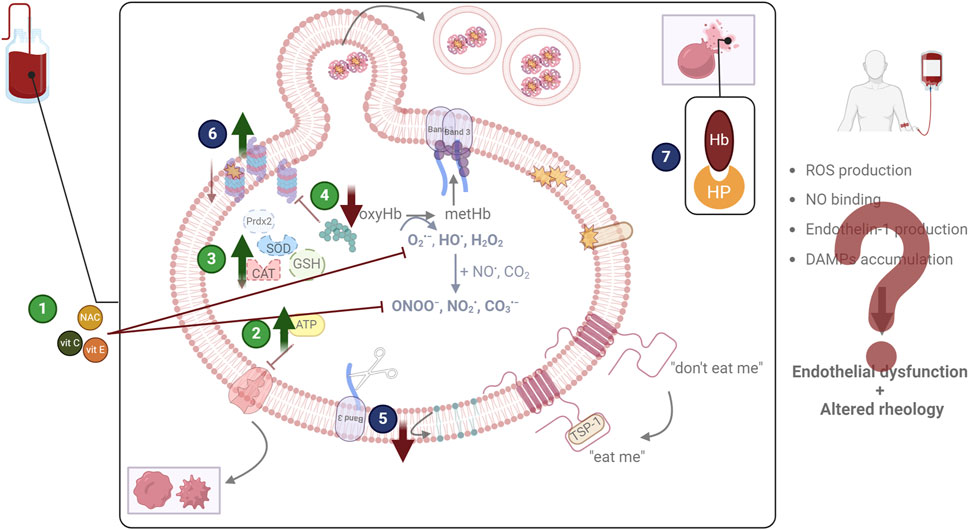
Figure 2. Examined and proposed interventions to address oxidative lesions. The most widely tested storage modification regards the addition of antioxidant molecules (1) to scavenge reactive species and inhibit their detrimental effects. Other approaches focus on the enhancement of metabolism (either energy or redox) (2), or of antioxidant enzymes (3), again to counteract oxidative stress. Finally, the reduction of oxygen (4) by storing red blood cells in anaerobic conditions is also being examined since it targets the root of the problem. Apart from addressing oxidative stress (green circles), other efforts aim at diminishing the lesions that emerge as its consequence (blue circles). The decrease of removal signals (5), the enhancement of proteostasis (6), and the scavenging of damage-associated molecular patterns (DAMPs), including hemoglobin (Hb) through haptoglobin (HP) addition (7) have been discussed. CAT: catalase; GSH: glutathione; NAC: N-acetyl cysteine; NO: nitric oxide; prdx2: peroxiredoxin 2; ROS: reactive oxygen species; SOD: superoxide dismutase; TSP-1: thrombospondin 1; vit: vitamin. Created in BioRender.com/q76t533.
The most studied antioxidant supplement in stored RBCs is ascorbic acid, with human studies indicating that it attenuates oxidative stress and lesions (Bardyn et al., 2021; Tzounakas et al., 2022a) and boosts antioxidant defenses (Anastasiadi et al., 2023), as also supported by the elevated protein sulfhydryls in stored RBCs from rats (Ravikumar and Rajashekaraiah, 2017). However, results regarding hemolysis are contradictory (Raval et al., 2013; Czubak et al., 2017). Notably, supplemented murine RBCs present better recovery (Stowell et al., 2013), something not evident upon transfusion in xenobiotic models (Tzounakas et al., 2022a). When in combination with uric acid there is an improvement in terms of redox equilibrium, metabolic lesions (Bardyn et al., 2020), and proteostasis (Tzounakas et al., 2022a). Another molecule that has been examined in combination with ascorbic acid is N-acetylcysteine (NAC), a precursor of GSH, leading to reduced hemolysis and ROS accumulation, and improved antioxidant metabolism (Pallotta et al., 2014). Similar results also emerge upon the sole addition of NAC (Amen et al., 2017). Combining vitamins C and E in stored rat RBCs does not seem to alter their storability features (Pallavi and Rajashekaraiah, 2023); yet, vitamin E alone efficiently reduces ROS and hemolysis in stored RBCs from healthy humans (Silva et al., 2017; Nemkov et al., 2022). While most studies focus on the addition of antioxidants in the stored units, the antioxidant “treatment” of donors some days before blood donation, also has the potential to provide RBCs with improved storability (Addis and St Cyr, 2017; Kim et al., 2022). Such is the case of taurine, which boosts the antioxidant metabolism of stored RBCs both after supplementation of the donors and of the units, and results in higher post-transfusion survival rates in mouse models (Bertolone et al., 2020).
Other potentially beneficial supplements are derived from plant extracts. For instance, resveratrol can induce the activity of antioxidant enzymes and the levels of GSH in stored RBCs, protecting them from oxidative insults (Huyut et al., 2018). The fact that the same compound upregulates the plasma membrane redox system of RBCs (Pandey and Rizvi, 2013) could also benefit the patients post-transfusion. Similar improvements are seen when caffeic acid (Huyut et al., 2016) or astaxanthin are added in packed RBCs, but in the second case, there is an additional reduction in hemolysis and ROS accumulation (Wang et al., 2015; Wang and Wang, 2021), as also seen when naringin is used (She et al., 2022). Recently, the supplementation of stored RBCs with curcumin improved ATP preservation and slowed oxidative lesion emergence. However, the most promising outcomes concern post-transfusion aspects: a drop was observed in fibrinogen subunit levels that could lead to minor aggregation, while recovery was increased, satisfying the gold standard of transfusion therapy (Hicks et al., 2024). Antioxidant supplementation can also be used in distinct settings. In the context of γ-irradiated RBCs, the presence of quercetin seems to partly improve some redox parameters, but at the same time exacerbates hemolysis phenomena (Zbikowska et al., 2014). Such interventions could be useful for targeted supplementation of units from donor cohorts with compromised redox metabolism (Tzounakas et al., 2016; Hazegh et al., 2021).
Metabolites could not be absent from such a list. Since GSH rapidly decreases during storage, its direct addition has a protective effect (Dumaswala et al., 2000), whereas the addition of its precursors only slightly elevates its levels (Whillier et al., 2011; D'Alessandro et al., 2017). Based on the ability of RBCs to synthesize NAD+ from nicotinic acid, supplementing them with the latter protects them from oxidation and lysis by increasing intracellular antioxidants (Arun et al., 1999). Similar interventions have been made to maintain adequate levels of 2,3-DPG and ATP, as the ones using phosphate or pyruvate (Oski et al., 1971; Dawson et al., 1981). In the same context, since sphingosine-1-phosphate promotes glycolysis, units supplemented with it better maintain their energy, but at the cost of reducing agents, rendering them inferior regarding post-transfusion recovery (Hay et al., 2023b). Supporting energy metabolism also affects redox dynamics, as shown by the improvement of antioxidant defenses and the minor post-transfusion liver oxidation and inflammation after supplementation of stored murine RBCs with sodium pyruvate (Xia et al., 2016). Moving on to lipid metabolism, L-carnitine is one of the molecules that stand out due to its role in repairing oxidized membrane lipids. Indeed, its addition to stored RBCs protects them from oxidative lesions (Ravikumar et al., 2020), enhances their resilience to lysis (Arduini et al., 1997), and leads to superior recovery and Hb increment post-transfusion (Nemkov et al., 2024a). Again, the consumption of such metabolites by the donor could also benefit their stored RBCs. To support this, stored RBCs from mice fed with diets enriched in long-chain polyunsaturated fatty acids −molecules that can support RBC membrane properties− are characterized by improved deformability and lipid peroxidation, and a boosted post-transfusion survival (Kim et al., 2022; Kim et al., 2023).
Finally, the addition of antioxidant enzymes, albeit understudied, is also interesting. Barzegar et al., developed nanoparticles containing two of the main RBC antioxidant enzymes, superoxide dismutase, and catalase, to counteract the drop in activity that is observed during storage. Their presence in the blood bag attenuates the observed increase in oxidative stress and morphology deterioration (Barzegar et al., 2021; Barzegar et al., 2022). The use of small molecules that mimic the activity of enzymes has been also examined in vitro. More specifically, selenium-based peroxiredoxin mimetics have been suggested to protect RBCs from oxidative insults and the emergence of eryptotic phenotypes (Chakrabarty et al., 2020). Another promising approach concerns the modulation of the expression of antioxidant enzymes in RBC precursors. Recently, reticulocytes with enhanced peroxiredoxin and glutathione peroxidase proteins were successfully engineered, giving hope for the potential to improve RBC storability (Langlands et al., 2024).
Anaerobic storage has emerged as an alternative to normoxic conditions that could potentially enhance RBC quality by removing oxygen at the beginning of storage and maintaining the hypoxic state throughout its duration. The outcomes so far are promising, showing improved storability and beneficial post-transfusion outcomes. For instance, under hypoxic storage RBCs are rendered metabolically superior, since they better maintain their 2,3-DPG levels, are characterized by improved GSH homeostasis, and present minor purine oxidation (Meng et al., 2019; D'Alessandro et al., 2020). This metabolic rewiring is also linked to better oxygen-unloading kinetics (Rabcuka et al., 2022). Oxidative stress is indeed mitigated since anaerobic RBCs accumulate less ROS during their storage (Bencheikh et al., 2022), and at the same time, they better retain their morphology and integrity (Meng et al., 2019). All these findings corroborate the potential of storage extension by using this strategy (Yoshida et al., 2007). Investigations in mice report similar storability findings and take it a step further by stating the superior post-transfusion survival (Hay et al., 2023a) and efficacy (Williams et al., 2020) of hypoxic RBCs. Notably, in vitro models of transfusion hint at the possibility of better performance in sickle cell transfusion recipients (Karafin et al., 2023), while autologous transfusion events are linked to improved RBC recovery when hypoxic storage is used (D'Alessandro et al., 2020). Recently an interim report, regarding the outcomes of anaerobically stored RBC administration to patients with hematologic malignancies, demonstrated the tolerance of these cells by the patients and gave hope for increasing the window between consecutive transfusions (Reikvam et al., 2023).
Instead of targeting oxidative stress, one could focus on its outcomes. Such an example is the addition of erythropoietin (Epo) in RBC units. Epo interacts with erythroid precursors and promotes their viability and differentiation. There are indications for mature RBCs to retain some Epo receptors, and therefore Epo supplementation could better retain RBC viability in the unit; indeed, the emergence of removal signals was mitigated (Penuela et al., 2016). Similar was the aim of the study of Hoehn et al., in which acid sphingomyelinase, an enzyme that aids in the emergence of removal signals on RBCs, was inhibited during storage (Hoehn et al., 2016). With this approach, the integrity of RBCs was improved, and less PS was exposed on their surface, while at the same time, the release of Hb in the circulation of the recipient was minor. Another potential molecular target is the proteasome machinery. Based on its role in decongesting stored RBCs from accumulating oxidized proteins, and the decline in its activity during storage, its enhancement has been suggested as a promising approach to counteract storage lesion (Delobel et al., 2016; Anastasiadi et al., 2023). Preliminary observations of our team, indeed support that upregulated proteasome can benefit stored RBCs, since donor cohorts of high proteasomal activity are characterized by an improved RBC storability profile in terms of lysis and oxidation parameters (Anastasiadi et al., 2024b). Other approaches focus on the extracellular compartment to reduce the accumulating damage-associated molecular patterns (DAMPs), since they are harmful both to stored RBCs, as well as to the recipient. A delicate attempt was recently made with the use of nanofibrous sheets that were able to scavenge DAMPs and slow the integrity lesions of stored RBCs. Importantly, this intervention also positively affected the post-transfusion survival of RBCs in mice models (Pandey et al., 2022). Since free Hb is one of the most abundant and potent DAMPs, the addition of haptoglobin in the units has been discussed (Wang et al., 2014). If effective, such supplementation could protect the patient from unwanted free Hb-related outcomes, as in the case of NO binding and ROS production, but its applicability and success remain to be determined.
Oxidative stress is a pivotal factor in the development of RBC storage lesions, leading to a cascade of cellular and biochemical alterations that compromise quality and function. Addressing these lesions by enhancing antioxidant defenses, reducing oxygen levels, and scavenging harmful molecules shows promise in improving the storability and efficacy of stored blood. Omics technologies and biomarker identification offer a pathway to optimizing storage conditions, ultimately leading to better patient outcomes. However, while the potential for these strategies is promising, the field still lacks comprehensive studies on post-transfusion efficacy and adverse effects. Current data are limited to in vitro models and animal studies, with few clinical trials providing conclusive results. To bridge this gap, more studies should focus on pre-clinical evaluations, moving towards clinical trials, and eventually integrating these findings into clinical practice. Like a story yet to be written, the future of blood storage and transfusion will require a deeper understanding of the complex interplay of oxidative stress, storability, and transfusion outcomes.
AA: Visualization, Writing–original draft. KS: Writing–review and editing. AK: Writing–review and editing. VT: Funding acquisition, Supervision, Visualization, Writing–original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This paper has been financed by the funding programme “MEDICUS”, of the University of Patras.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Addis P. B., St Cyr J. A. (2017). Nutritional supplementation of donors may improve outcomes following the transfusion of stored red blood cells. J. Diet. Suppl. 14, 485–488. doi:10.1080/19390211.2016.1272660
Alayash A. I. (2022). Hemoglobin oxidation reactions in stored blood. Antioxidants (Basel) 11, 747. doi:10.3390/antiox11040747
Amen F., Machin A., Tourino C., Rodriguez I., Denicola A., Thomson L. (2017). N-acetylcysteine improves the quality of red blood cells stored for transfusion. Arch. Biochem. Biophys. 621, 31–37. doi:10.1016/j.abb.2017.02.012
Anastasiadi A. T., Arvaniti V. Z., Hudson K. E., Kriebardis A. G., Stathopoulos C., D'alessandro A., et al. (2024a). Exploring unconventional attributes of red blood cells and their potential applications in biomedicine. Protein Cell 15, 315–330. doi:10.1093/procel/pwae001
Anastasiadi A. T., Fortis S., Solomou E., Stamoulis K., Kriebardis A. G., Tzounakas V. L. (2024b). “Effect of proteasome activity on red blood cell phenotypes linked to storage lesion,” in 38th international Congress of the ISBT: vox sanguinis), 55.
Anastasiadi A. T., Stamoulis K., Papageorgiou E. G., Lelli V., Rinalducci S., Papassideri I. S., et al. (2023). The time-course linkage between hemolysis, redox, and metabolic parameters during red blood cell storage with or without uric acid and ascorbic acid supplementation. Front. Aging 4, 1161565. doi:10.3389/fragi.2023.1161565
Anastasiadi A. T., Tzounakas V. L., Arvaniti V. Z., Dzieciatkowska M., Stamoulis K., Lekka M. E., et al. (2021). Red blood cell proteasome in beta-thalassemia trait: topology of activity and networking in blood bank conditions. Membr. (Basel) 11, 716. doi:10.3390/membranes11090716
Arduini A., Holme S., Sweeney J. D., Dottori S., Sciarroni A. F., Calvani M. (1997). Addition of L-carnitine to additive solution-suspended red cells stored at 4 degrees C reduces in vitro hemolysis and improves in vivo viability. Transfusion 37, 166–174. doi:10.1046/j.1537-2995.1997.37297203519.x
Arun P., Padmakumaran Nair K. G., Manojkumar V., Deepadevi K. V., Lakshmi L. R., Kurup P. A. (1999). Decreased hemolysis and lipid peroxidation in blood during storage in the presence of nicotinic acid. Vox Sang. 76, 220–225. doi:10.1159/000031055
Badior K. E., Casey J. R. (2018). Molecular mechanism for the red blood cell senescence clock. IUBMB Life 70, 32–40. doi:10.1002/iub.1703
Bardyn M., Allard J., Crettaz D., Rappaz B., Turcatti G., Tissot J. D., et al. (2021). Image- and fluorescence-based test shows oxidant-dependent damages in red blood cells and enables screening of potential protective molecules. Int. J. Mol. Sci. 22, 4293. doi:10.3390/ijms22084293
Bardyn M., Chen J., Dussiot M., Crettaz D., Schmid L., Langst E., et al. (2020). Restoration of physiological levels of uric acid and ascorbic acid reroutes the metabolism of stored red blood cells. Metabolites 10, 226. doi:10.3390/metabo10060226
Barshtein G., Pajic-Lijakovic I., Gural A. (2021). Deformability of stored red blood cells. Front. Physiol. 12, 722896. doi:10.3389/fphys.2021.722896
Barzegar S., Asri Kojabad A., Manafi Shabestari R., Barati M., Rezvany M. R., Safa M., et al. (2022). Use of antioxidant nanoparticles to reduce oxidative stress in blood storage. Biotechnol. Appl. Biochem. 69, 1712–1722. doi:10.1002/bab.2240
Barzegar S., Rezvani M. R., Safa M., Amani A., Abbaspour A., Pourfathollah A., et al. (2021). Dose-dependent efficacy of antioxidant nanoparticles on red blood cells storage. J. Educ. Health Promot 10, 256. doi:10.4103/jehp.jehp_1638_20
Basta G., Schmidt A. M., De Caterina R. (2004). Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 63, 582–592. doi:10.1016/j.cardiores.2004.05.001
Bencheikh L., Nguyen K. A., Chadebech P., Kiger L., Bodivit G., Jouard A., et al. (2022). Preclinical evaluation of the preservation of red blood cell concentrates by hypoxic storage technology for transfusion in sickle cell disease. Haematologica 107, 1944–1949. doi:10.3324/haematol.2021.279721
Bertolone L., Roy M. K., Hay A. M., Morrison E. J., Stefanoni D., Fu X., et al. (2020). Impact of taurine on red blood cell metabolism and implications for blood storage. Transfusion 60, 1212–1226. doi:10.1111/trf.15810
Buehler P. W., Alayash A. I. (2004). Oxygen sensing in the circulation: cross talk between red blood cells and the vasculature. Antioxid. Redox Signal 6, 1000–1010. doi:10.1089/ars.2004.6.1000
Burger P., Hilarius-Stokman P., De Korte D., Van Den Berg T. K., Van Bruggen R. (2012). CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119, 5512–5521. doi:10.1182/blood-2011-10-386805
Chakrabarty G., Naveenkumar S. K., Kumar S., Mugesh G. (2020). Modulation of redox signaling and thiol homeostasis in red blood cells by peroxiredoxin mimetics. ACS Chem. Biol. 15, 2673–2682. doi:10.1021/acschembio.0c00309
Chatzinikolaou P. N., Margaritelis N. V., Paschalis V., Theodorou A. A., Vrabas I. S., Kyparos A., et al. (2024). Erythrocyte metabolism. Acta Physiol. (Oxf) 240, e14081. doi:10.1111/apha.14081
Cortese-Krott M. M. (2023). The reactive species interactome in red blood cells: oxidants, antioxidants, and molecular targets. Antioxidants (Basel) 12, 1736. doi:10.3390/antiox12091736
Cortese-Krott M. M., Kelm M. (2014). Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2, 251–258. doi:10.1016/j.redox.2013.12.027
Cortese-Krott M. M., Shiva S. (2019). The redox physiology of red blood cells and platelets: implications for their interactions and potential use as systemic biomarkers. Curr. Opin. Physiology 9, 56–66. doi:10.1016/j.cophys.2019.04.016
Czubak K., Antosik A., Cichon N., Zbikowska H. M. (2017). Vitamin C and Trolox decrease oxidative stress and hemolysis in cold-stored human red blood cells. Redox Rep. 22, 445–450. doi:10.1080/13510002.2017.1289314
D'alessandro A., Anastasiadi A. T., Tzounakas V. L., Nemkov T., Reisz J. A., Kriebardis A. G., et al. (2023). Red blood cell metabolism in vivo and in vitro. Metabolites 13, 793. doi:10.3390/metabo13070793
D'alessandro A., D'amici G. M., Vaglio S., Zolla L. (2012). Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica 97, 107–115. doi:10.3324/haematol.2011.051789
D'alessandro A., Nemkov T., Yoshida T., Bordbar A., Palsson B. O., Hansen K. C. (2017). Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 57, 325–336. doi:10.1111/trf.13892
D'alessandro A., Reisz J. A., Zhang Y., Gehrke S., Alexander K., Kanias T., et al. (2019). Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 3, 884–896. doi:10.1182/bloodadvances.2018029629
D'alessandro A., Yoshida T., Nestheide S., Nemkov T., Stocker S., Stefanoni D., et al. (2020). Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion 60, 786–798. doi:10.1111/trf.15730
Dawson R. B., Sisk L. D., Meyer D. R., Hershey R. T., Myers-Hilbert C. S. (1981). Blood preservation 33. Phosphate enhancement of ribose maintenance of 2,3-DPG and ATP. Transfusion 21, 215–218. doi:10.1046/j.1537-2995.1981.21281178160.x
Delobel J., Prudent M., Rubin O., Crettaz D., Tissot J. D., Lion N. (2012). Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J. Proteomics 76 (Spec No.), 181–193. doi:10.1016/j.jprot.2012.05.004
Delobel J., Prudent M., Tissot J. D., Lion N. (2016). Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteomics Clin. Appl. 10, 257–266. doi:10.1002/prca.201500074
Denicola A., Freeman B. A., Trujillo M., Radi R. (1996). Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys. 333, 49–58. doi:10.1006/abbi.1996.0363
Dumaswala U. J., Wilson M. J., Wu Y. L., Wykle J., Zhuo L., Douglass L. M., et al. (2000). Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic. Res. 33, 517–529. doi:10.1080/10715760000301061
Eigenschink M., Savran D., Zitterer C. P., Granitzer S., Fritz M., Baron D. M., et al. (2021). Redox properties of human erythrocytes are adapted for vitamin C recycling. Front. Physiol. 12, 767439. doi:10.3389/fphys.2021.767439
Flatt J. F., Bawazir W. M., Bruce L. J. (2014). The involvement of cation leaks in the storage lesion of red blood cells. Front. Physiol. 5, 214. doi:10.3389/fphys.2014.00214
Fu X., Felcyn J. R., Odem-Davis K., Zimring J. C. (2016). Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion 56, 2560–2570. doi:10.1111/trf.13748
Gladwin M. T., Schechter A. N., Kim-Shapiro D. B., Patel R. P., Hogg N., Shiva S., et al. (2005). The emerging biology of the nitrite anion. Nat. Chem. Biol. 1, 308–314. doi:10.1038/nchembio1105-308
Hay A., Dziewulska K., Gamboni F., Nerguizian D., Dzieciatkowska M., Zimring J. C., et al. (2023a). Hypoxic storage of murine red blood cells improves energy metabolism and post-transfusion recoveries. Blood Transfus. 21, 50–61. doi:10.2450/2022.0172-22
Hay A., Nemkov T., Gamboni F., Dzieciatkowska M., Key A., Galbraith M., et al. (2023b). Sphingosine 1-phosphate has a negative effect on RBC storage quality. Blood Adv. 7, 1379–1393. doi:10.1182/bloodadvances.2022008936
Hazegh K., Fang F., Bravo M. D., Tran J. Q., Muench M. O., Jackman R. P., et al. (2021). Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 61, 435–448. doi:10.1111/trf.16168
Hicks W., Jana S., Kassa T., Prince R., Cabrales P., Friedman J., et al. (2024). Biopreservation and reversal of oxidative injury during blood storage by a novel curcumin-based gel formulation. Res. Sq. doi:10.21203/rs.3.rs-4277591/v1
Hoehn R. S., Jernigan P. L., Chang A. L., Edwards M. J., Caldwell C. C., Gulbins E., et al. (2016). Acid sphingomyelinase inhibition prevents hemolysis during erythrocyte storage. Cell Physiol. Biochem. 39, 331–340. doi:10.1159/000445627
Huyut Z., Sekeroglu M. R., Balahoroglu R., Huyut M. T. (2018). Characteristics of resveratrol and serotonin on antioxidant capacity and susceptibility to oxidation of red blood cells in stored human blood in a time-dependent manner. J. Int. Med. Res. 46, 272–283. doi:10.1177/0300060517725450
Huyut Z., Sekeroglu M. R., Balahoroglu R., Karakoyun T., Cokluk E. (2016). The relationship of oxidation sensitivity of red blood cells and carbonic anhydrase activity in stored human blood: effect of certain phenolic compounds. Biomed. Res. Int. 2016, 3057384. doi:10.1155/2016/3057384
Karafin M. S., Field J. J., Ilich A., Li L., Qaquish B. F., Shevkoplyas S. S., et al. (2023). Hypoxic storage of donor red cells preserves deformability after exposure to plasma from adults with sickle cell disease. Transfusion 63, 193–202. doi:10.1111/trf.17163
Kim C. Y., Johnson H., Peltier S., Spitalnik S. L., Hod E. A., Francis R. O., et al. (2022). Deuterated linoleic acid attenuates the RBC storage lesion in a mouse model of poor RBC storage. Front. Physiol. 13, 868578. doi:10.3389/fphys.2022.868578
Kim C. Y., Larsen H. J., Spitalnik S. L., Hod E. A., Francis R. O., Hudson K. E., et al. (2023). Low-dose dietary fish oil improves RBC deformability without improving post-transfusion recovery in mice. Nutrients 15, 4456. doi:10.3390/nu15204456
Koshkaryev A., Livshits L., Pajic-Lijakovic I., Gural A., Barshtein G., Yedgar S. (2020). Non-oxidative band-3 clustering agents cause the externalization of phosphatidylserine on erythrocyte surfaces by a calcium-independent mechanism. Biochim. Biophys. Acta Biomembr. 1862, 183231. doi:10.1016/j.bbamem.2020.183231
Langlands H. D., Shoemark D. K., Toye A. M. (2024). Modulation of antioxidant enzyme expression of in vitro culture-derived reticulocytes. Antioxidants (Basel) 13, 1070. doi:10.3390/antiox13091070
Lysenko L., Mierzchala M., Gamian A., Durek G., Kubler A., Kozlowski R., et al. (2006). The effect of packed red blood cell storage on arachidonic acid and advanced glycation end-product formation. Arch. Immunol. Ther. Exp. Warsz. 54, 357–362. doi:10.1007/s00005-006-0042-y
Mangalmurti N. S., Chatterjee S., Cheng G., Andersen E., Mohammed A., Siegel D. L., et al. (2010). Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion 50, 2353–2361. doi:10.1111/j.1537-2995.2010.02689.x
Matteucci E., Giampietro O. (2007). Electron pathways through erythrocyte plasma membrane in human physiology and pathology: potential redox biomarker? Biomark. Insights 2, 117727190700200–117727190700329. doi:10.1177/117727190700200026
Melzak K. A., Spouge J. L., Boecker C., Kirschhofer F., Brenner-Weiss G., Bieback K. (2021). Hemolysis pathways during storage of erythrocytes and inter-donor variability in erythrocyte morphology. Transfus. Med. Hemother 48, 39–47. doi:10.1159/000508711
Meng Q., Peng X., Zhao S., Xu T., Wang S., Liu Q., et al. (2019). Hypoxic storage of erythrocytes slows down storage lesions and prolongs shelf-life. J. Cell Physiol. 234, 22833–22844. doi:10.1002/jcp.28847
Mohanty J. G., Nagababu E., Rifkind J. M. (2014). Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 5, 84. doi:10.3389/fphys.2014.00084
Moller M. N., Orrico F., Villar S. F., Lopez A. C., Silva N., Donze M., et al. (2023). Oxidants and antioxidants in the redox biochemistry of human red blood cells. ACS Omega 8, 147–168. doi:10.1021/acsomega.2c06768
Neidlinger N. A., Larkin S. K., Bhagat A., Victorino G. P., Kuypers F. A. (2006). Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J. Biol. Chem. 281, 775–781. doi:10.1074/jbc.M505790200
Nemkov T., Key A., Stephenson D., Earley E. J., Keele G. R., Hay A., et al. (2024a). Genetic regulation of carnitine metabolism controls lipid damage repair and aging RBC hemolysis in vivo and in vitro. Blood 143, 2517–2533. doi:10.1182/blood.2024023983
Nemkov T., Stephenson D., Earley E. J., Keele G. R., Hay A., Key A., et al. (2024b). Biological and genetic determinants of glycolysis: phosphofructokinase isoforms boost energy status of stored red blood cells and transfusion outcomes. Cell Metab. 36, 1979–1997.e13. doi:10.1016/j.cmet.2024.06.007
Nemkov T., Yoshida T., Nikulina M., D'alessandro A. (2022). High-throughput metabolomics platform for the rapid data-driven development of novel additive solutions for blood storage. Front. Physiol. 13, 833242. doi:10.3389/fphys.2022.833242
Obradovic M., Essack M., Zafirovic S., Sudar-Milovanovic E., Bajic V. P., Van Neste C., et al. (2020). Redox control of vascular biology. Biofactors 46, 246–262. doi:10.1002/biof.1559
Oski F. A., Travis S. F., Miller L. D., Delivoria-Papadopoulos M., Cannon E. (1971). The in vitro restoration of red cell 2,3-diphosphoglycerate levels in banked blood. Blood 37, 52–58. doi:10.1182/blood.v37.1.52.52
Pallavi M., Rajashekaraiah V. (2023). Synergistic activity of vitamin-C and vitamin-E to ameliorate the efficacy of stored erythrocytes. Transfus. Clin. Biol. 30, 87–95. doi:10.1016/j.tracli.2022.09.002
Pallotta V., Gevi F., D'alessandro A., Zolla L. (2014). Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 12, 376–387. doi:10.2450/2014.0266-13
Pandey K. B., Rizvi S. I. (2013). Resveratrol up-regulates the erythrocyte plasma membrane redox system and mitigates oxidation-induced alterations in erythrocytes during aging in humans. Rejuvenation Res. 16, 232–240. doi:10.1089/rej.2013.1419
Pandey S., Mahato M., Srinath P., Bhutani U., Goap T. J., Ravipati P., et al. (2022). Intermittent scavenging of storage lesion from stored red blood cells by electrospun nanofibrous sheets enhances their quality and shelf-life. Nat. Commun. 13, 7394. doi:10.1038/s41467-022-35269-3
Pantaleo A., Ferru E., Pau M. C., Khadjavi A., Mandili G., Matte A., et al. (2016). Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p (72) syk. Oxid. Med. Cell Longev. 2016, 6051093. doi:10.1155/2016/6051093
Peltier S., Marin M., Dzieciatkowska M., Dussiot M., Roy M. K., Bruce J., et al. (2024). Proteostasis and metabolic dysfunction in a distinct subset of storage-induced senescent erythrocytes targeted for clearance. Biorxiv, 612195. doi:10.1101/2024.09.11.612195
Penuela O. A., Palomino F., Gomez L. A. (2016). Erythropoietin reduces storage lesions and decreases apoptosis indices in blood bank red blood cells. Rev. Bras. Hematol. Hemoter. 38, 15–20. doi:10.1016/j.bjhh.2015.10.003
Rabcuka J., Blonski S., Meli A., Sowemimo-Coker S., Zaremba D., Stephenson D., et al. (2022). Metabolic reprogramming under hypoxic storage preserves faster oxygen unloading from stored red blood cells. Blood Adv. 6, 5415–5428. doi:10.1182/bloodadvances.2022007774
Raval J. S., Fontes J., Banerjee U., Yazer M. H., Mank E., Palmer A. F. (2013). Ascorbic acid improves membrane fragility and decreases haemolysis during red blood cell storage. Transfus. Med. 23, 87–93. doi:10.1111/tme.12013
Ravikumar S., Prabhu S., Vani R. (2020). Effects of L-carnitine on the erythrocytes of stored human blood. Transfus. Med. 30, 215–225. doi:10.1111/tme.12645
Ravikumar S., Rajashekaraiah V. (2017). Vitamin C as a modulator of oxidative stress in erythrocytes of stored blood. Acta Haematol. Pol. 48, 350–356. doi:10.1016/j.achaem.2017.08.005
Reikvam H., Hetland G., Ezligini F., Dorsch K., Omert L., Dunham A., et al. (2023). Safety of hypoxic red blood cell administration in patients with transfusion-dependent hematological malignancies: an interim analysis. Transfus. Apher. Sci. 62, 103755. doi:10.1016/j.transci.2023.103755
Rinalducci S., Ferru E., Blasi B., Turrini F., Zolla L. (2012). Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 10 (Suppl. 2), s55–s62. doi:10.2450/2012.009S
Rinalducci S., Marrocco C., Zolla L. (2015). Thiol-based regulation of glyceraldehyde-3-phosphate dehydrogenase in blood bank-stored red blood cells: a strategy to counteract oxidative stress. Transfusion 55, 499–506. doi:10.1111/trf.12855
Roback J. D. (2011). Vascular effects of the red blood cell storage lesion. Hematol. Am. Soc. Hematol. Educ. Program 2011, 475–479. doi:10.1182/asheducation-2011.1.475
Sadowska-Bartosz I., Bartosz G. (2023). Peroxiredoxin 2: an important element of the antioxidant defense of the erythrocyte. Antioxidants (Basel) 12, 1012. doi:10.3390/antiox12051012
Samuel P. P., White M. A., Ou W. C., Case D. A., Phillips G. N., Olson J. S. (2020). The interplay between molten globules and heme disassociation defines human hemoglobin disassembly. Biophys. J. 118, 1381–1400. doi:10.1016/j.bpj.2020.01.031
She Y., Liu Q., Xiong X., Li N., Zhang J. (2022). Erythrocyte storage lesion improvements mediated by naringin screened from vegetable/fruit juice using cell extract and HPLC-MS. J. Anal. Methods Chem. 2022, 7556219. doi:10.1155/2022/7556219
Silva C. a.L., Azevedo Filho C. A., Pereira G., Silva D. C. N., Castro M., Almeida A. F., et al. (2017). Vitamin E nanoemulsion activity on stored red blood cells. Transfus. Med. 27, 213–217. doi:10.1111/tme.12394
Stowell S. R., Smith N. H., Zimring J. C., Fu X., Palmer A. F., Fontes J., et al. (2013). Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion 53, 2248–2257. doi:10.1111/trf.12106
Thomas T. A., Francis R. O., Zimring J. C., Kao J. P., Nemkov T., Spitalnik S. L. (2024). The role of ergothioneine in red blood cell biology: a review and perspective. Antioxidants (Basel) 13, 717. doi:10.3390/antiox13060717
Tzounakas V. L., Anastasiadi A. T., Arvaniti V. Z., Lelli V., Fanelli G., Paronis E. C., et al. (2022a). Supplementation with uric and ascorbic acid protects stored red blood cells through enhancement of non-enzymatic antioxidant activity and metabolic rewiring. Redox Biol. 57, 102477. doi:10.1016/j.redox.2022.102477
Tzounakas V. L., Anastasiadi A. T., Lekka M. E., Papageorgiou E. G., Stamoulis K., Papassideri I. S., et al. (2022b). Deciphering the relationship between free and vesicular hemoglobin in stored red blood cell units. Front. Physiol. 13, 840995. doi:10.3389/fphys.2022.840995
Tzounakas V. L., Dzieciatkowska M., Anastasiadi A. T., Karadimas D. G., Vergaki A., Siourounis P., et al. (2022c). Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. 20, 27–39. doi:10.2450/2020.0179-20
Tzounakas V. L., Kriebardis A. G., Georgatzakou H. T., Foudoulaki-Paparizos L. E., Dzieciatkowska M., Wither M. J., et al. (2016). Glucose 6-phosphate dehydrogenase deficient subjects may be better storers than donors of red blood cells. Free Radic. Biol. Med. 96, 152–165. doi:10.1016/j.freeradbiomed.2016.04.005
Wang S. L., He L. J., He T. B., Han W., Wang Q. (2015). Effect of astaxanthin on oxidative stress of red blood cells and peroxidation damage of membrane. Zhongguo Shi Yan Xue Ye Xue Za Zhi 23, 552–556. doi:10.7534/j.issn.1009-2137.2015.02.050
Wang S. L., Wang Q. (2021). Effect of astaxanthin on antioxidant enzyme activities in suspended leukocyte-depleted red blood cells stored for transfusion. Zhongguo Shi Yan Xue Ye Xue Za Zhi 29, 1312–1317. doi:10.19746/j.cnki.issn.1009-2137.2021.04.047
Wang Y., Zhang Y., Zhao L., Yin Y., Wang Q., Zhou H. (2014). Addition of haptoglobin to RBCs storage, a new strategy to improve quality of stored RBCs and transfusion. Med. Hypotheses 82, 125–128. doi:10.1016/j.mehy.2013.09.020
Weinberg J. A., Patel R. P. (2016). Red blood cell transfusion and its effect on microvascular dysfunction in shock states. Best. Pract. Res. Clin. Anaesthesiol. 30, 491–498. doi:10.1016/j.bpa.2016.10.005
Whillier S., Raftos J. E., Sparrow R. L., Kuchel P. W. (2011). The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion 51, 1450–1459. doi:10.1111/j.1537-2995.2010.03026.x
Williams A. T., Jani V. P., Nemkov T., Lucas A., Yoshida T., Dunham A., et al. (2020). Transfusion of anaerobically or conventionally stored blood after hemorrhagic shock. Shock 53, 352–362. doi:10.1097/SHK.0000000000001386
Xia S., Chen G., Wang B., Yin Y., Sun Z., Zhao J., et al. (2016). Addition of sodium pyruvate to stored red blood cells attenuates liver injury in a murine transfusion model. Mediat. Inflamm. 2016, 3549207. doi:10.1155/2016/3549207
Yoshida T., Aubuchon J. P., Tryzelaar L., Foster K. Y., Bitensky M. W. (2007). Extended storage of red blood cells under anaerobic conditions. Vox Sang. 92, 22–31. doi:10.1111/j.1423-0410.2006.00860.x
Yoshida T., Prudent M., D'alessandro A. (2019). Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 17, 27–52. doi:10.2450/2019.0217-18
Zbikowska H. M., Antosik A., Szejk M., Bijak M., Nowak P. (2014). A moderate protective effect of quercetin against gamma-irradiation- and storage-induced oxidative damage in red blood cells for transfusion. Int. J. Radiat. Biol. 90, 1201–1210. doi:10.3109/09553002.2013.877173
Zhao Y., Vanhoutte P. M., Leung S. W. (2015). Vascular nitric oxide: beyond eNOS. J. Pharmacol. Sci. 129, 83–94. doi:10.1016/j.jphs.2015.09.002
Keywords: red blood cells, storage lesion, oxidative stress, blood transfusion, storage improvement, antioxidant enhancement, hypoxia, proteasome
Citation: Anastasiadi AT, Stamoulis K, Kriebardis AG and Tzounakas VL (2024) Molecular modifications to mitigate oxidative stress and improve red blood cell storability. Front. Physiol. 15:1499308. doi: 10.3389/fphys.2024.1499308
Received: 20 September 2024; Accepted: 22 October 2024;
Published: 30 October 2024.
Edited by:
Stephen C. Rogers, University of Maryland, United StatesReviewed by:
Leonor Thomson, University of the Republic, UruguayCopyright © 2024 Anastasiadi, Stamoulis, Kriebardis and Tzounakas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vassilis L. Tzounakas, dnR6b3VuYWthc0B1cGF0cmFzLmdy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.