- 1Sports Rehabilitation Research Center, China Institute of Sport Science, Beijing, China
- 2School of Exercise Medicine and Health, Chengdu Sport University, Chengdu, China
- 3School of Physical Education and Sport Science, Fujian Normal University, Fujian, China
- 4School of Sport Science, Beijing Sport University, Beijing, China
Objective: This systematic review aims to comprehensively analyze the efficacy and underlying mechanisms of vagus nerve stimulation (VNS) in enhancing cognitive functions and its therapeutic potential for various cognitive impairments. The review focuses on the impact of VNS on emotional processing, executive functions, learning, memory, and its clinical applications in conditions such as epilepsy, depression, Alzheimer’s disease, and other neurological disorders.
Methods: A systematic search of electronic databases (PubMed, Scopus, Web of Science) was conducted using the keywords “vagus nerve stimulation,” “cognitive enhancement,” “emotional processing,” “executive function,” “learning and memory,” “epilepsy,” “depression,” “Alzheimer’s disease,” “neurological disorders,” “attention-deficit/hyperactivity disorder,” “sleep disorders,” and “long COVID.” The inclusion criteria encompassed controlled trials, longitudinal studies, and meta-analyses published in English between 2000 and July 2024.
Results: A comprehensive review of 100 articles highlighted the cognitive effects of Vagus Nerve Stimulation (VNS). Studies show that VNS, especially through transcutaneous auricular VNS (taVNS), enhances emotional recognition, particularly for facial expressions, and improves selective attention under high cognitive demands. Additionally, VNS enhances learning and memory, including associative memory and spatial working memory tasks. In clinical applications, VNS exhibits promising benefits for improving cognitive functions in treatment-resistant epilepsy, depression, and Alzheimer’s disease.
Conclusion: VNS represents a promising therapeutic approach for enhancing cognitive function across diverse patient populations. The reviewed evidence highlights its efficacy in modulating cognitive domains in healthy individuals and improving cognition in neurological conditions. However, the comparative effectiveness of different VNS modalities and the differential effects of online versus offline VNS on cognitive psychology require further investigation. Future research should focus on optimizing VNS protocols and elucidating specific cognitive domains that benefit most from VNS interventions. This ongoing exploration is essential for maximizing the therapeutic potential of VNS in clinical practice.
1 Introduction
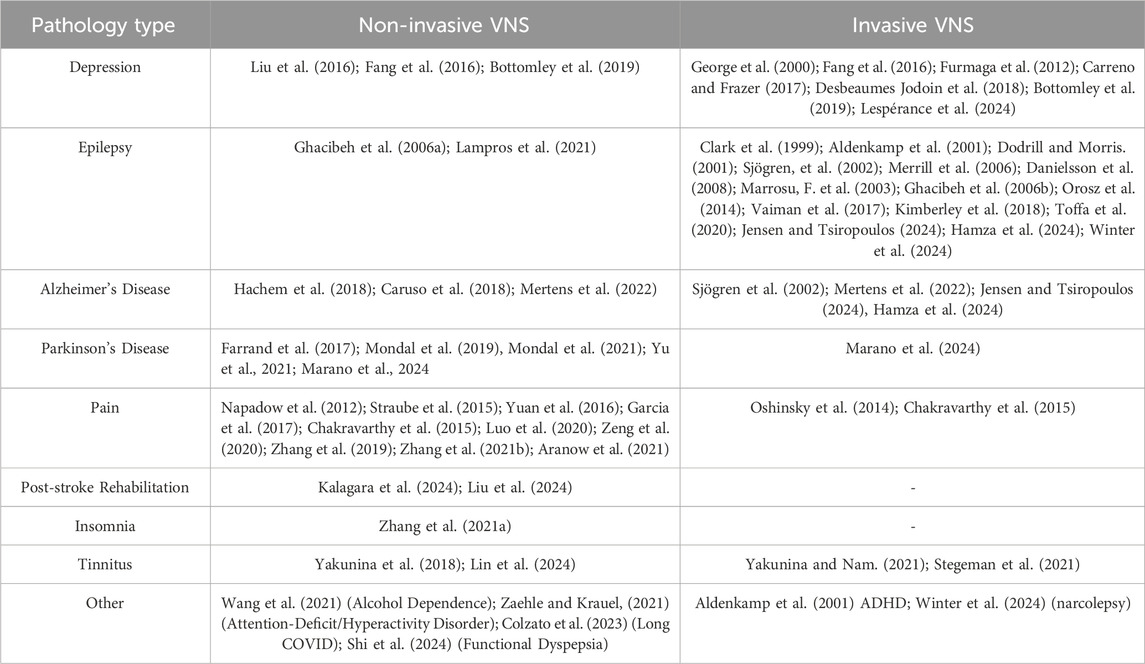
Vagus nerve stimulation (VNS) is a type of neuromodulation therapy that targets excitability and changes the balance of autonomic nervous function (Ma et al., 2019; Jung et al., 2024). It does this by electrically stimulating the vagus nerve network. In 1985, Zabara 1985 first reported that VNS could suppress epileptic seizures in dogs. Since then, research on VNS for epilepsy has increased, revealing its positive effects on various diseases. In 1997 and 2005, the U.S. Food and Drug Administration (FDA) approved implantable VNS (iVNS) for the clinical treatment of drug-resistant epilepsy and depression. In 2017, the FDA also approved transcutaneous cervical VNS (taVNS) for migraines and cluster headaches. Currently, VNS can be classified into two main categories: invasive and non-invasive. Hese modalities have been studied in a range of neuropsychiatric disorders beyond epilepsy and depression, including Alzheimer’s disease, chronic pain, tinnitus, Parkinson’s disease, and post-stroke rehabilitation (detailed in Table 1). These applications highlight the versatility and potential of VNS as a therapeutic intervention across various conditions. For instance, studies have indicated its efficacy in reducing symptoms and improving the quality of life for patients suffering from these disorders. With the advancement of research, recent years have seen the emergence of new VNS stimulation patterns and protocols both domestically and internationally, with significant progress in the field of cognitive function regulation (Kalagara et al., 2024). This article aims to review the stimulation patterns, mechanisms, and effects of VNS on cognitive function, providing guidance and reference for clinical treatment and scientific research.
2 Modes
2.1 Classification of VNS
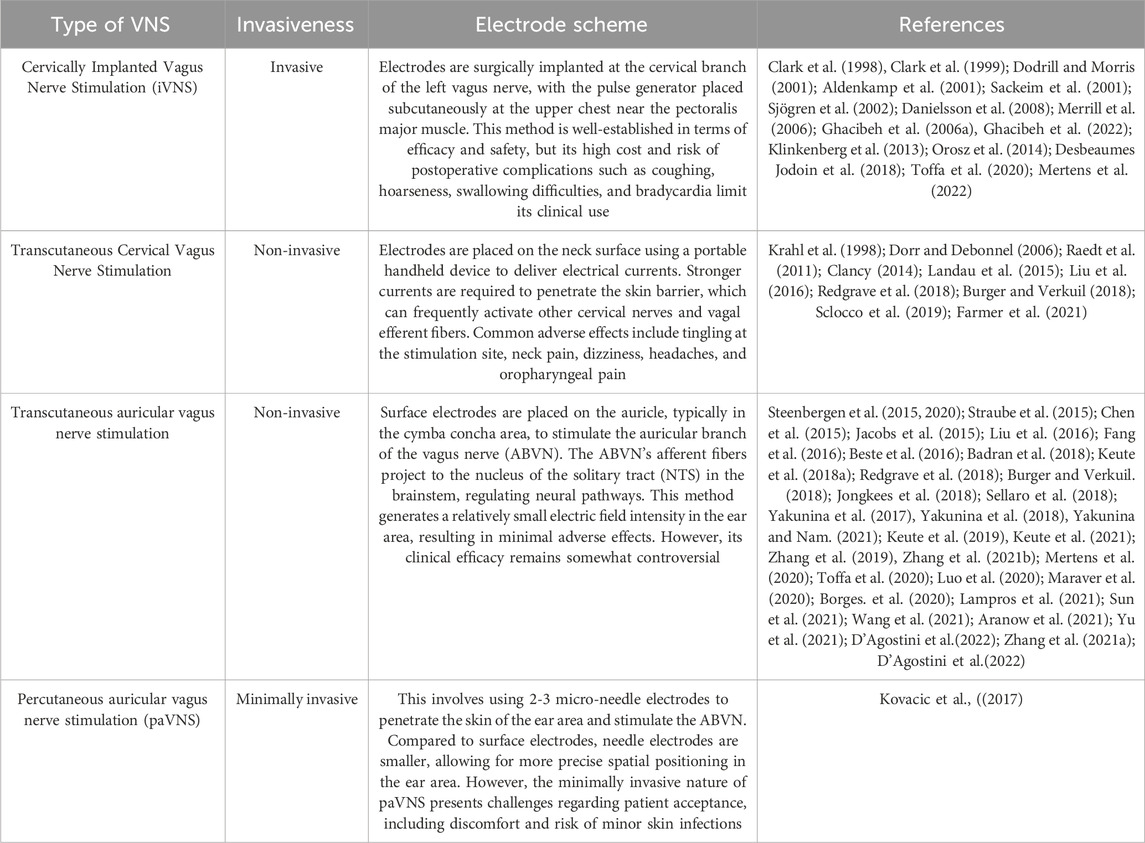
VNS is generally divided into four clinical forms. See Table 2 for details.
2.2 Methods of VNS
Conventional VNS typically follows an open-loop stimulation model, where the stimulation parameters are pre-set before treatment and do not change in response to neural activity during treatment. Clark et al. (1998), Clark et al. (1999) discovered that cervical iVNS in rats and epilepsy patients follow an intensity-dependent inverted U-shaped dose-effect relationship, where moderate current intensity yields better memory performance than lower or higher current intensities. Buell et al. (2018) recorded auditory cortex activity in rats via EEG and verified that VNS-induced cortical plasticity follows an inverted U-shaped function of VNS pulse frequency.
This phenomenon can be explained by the Yerkes-Dodson law: for simpler tasks, individual responses increase linearly with motivational stimuli; however, as task difficulty increases, the optimal response threshold for stimuli decreases (Yerkes and Dodson, 1908; Broadhurst, 1957; Calabrese, 2008a; Calabrese, 2008b). In tcVNS studies, reported stimulation parameters vary widely, and subject responses lack consistency across studies. This inconsistency may result from individual differences and methodological heterogeneity, such as the innervation patterns of the ABVN in the auricle (Yakunina et al., 2017; Burger and Verkuil, 2018), pre-stimulation vagal tone (Clancy, 2014), respiratory cycles (Sclocco et al., 2019), and stimulation sites. Some studies suggest that taVNS has consistent effects regardless of the stimulation site (left or right ear) or ear area (cymba concha or lobule) (Chen et al., 2015; Keute et al., 2021). Despite the lack of a standardized percutaneous VNS protocol, the “International Consensus on Minimum Reporting Standards for Transcutaneous Vagus Nerve Stimulation Studies (2020)" (Farmer et al., 2021) recommends standardizing commonly used stimulation parameters in tcVNS studies, including stimulation site, electrodes, duty cycle, frequency (Hz), intensity (mA), pulse width (μs), and waveform.
Closed-loop VNS involves continuously measuring subjects’ behavioral performance, brain activity, and peripheral physiological indicators during stimulation, dynamically adjusting stimulation parameters based on the subjects’ state. A team from Harvard Medical School developed the Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS) method, which uses a pressure sensor placed on the subject’s chest to deliver taVNS stimulation during the expiratory/inspiratory phase based on chest expansion. Results show that stimulation during exhalation (eRAVANS) enhances treatment efficacy, potentially because the ventral respiratory group (VRG) in the medulla sends excitatory signals to the NTS during exhalation, optimizing VNS stimulation (Napadow et al., 2012; Garcia et al., 2017; Sclocco et al., 2019). Besides RAVANS, Cook et al. designed a myoelectric-triggered auricular VNS system (MAAVNS) based on orofacial muscle movement to improve patients’ swallowing function (Cook et al., 2020). Thanks to advancements in wireless technology, wireless EEG, ECG, and subcutaneous fluid signal devices (SHS) are increasingly used in closed-loop taVNS systems (Yu et al., 2021). However, eliminating motion artifacts in closed-loop VNS and ensuring its sustainability and stimulation efficacy remain research priorities for the future.
3 Mechanisms of central regulation by VNS
3.1 Afferent network of the vagus nerve
The afferent branches of the vagus nerve can be functionally divided into: 1) General somatic afferent fibers (GSA) originating from the superior ganglion, transmitting general sensations from the posterior wall of the external auditory canal and the outer surface of the tympanic membrane, terminating in the spinal trigeminal nucleus;2) General visceral afferent fibers (GVA) originating from the inferior ganglion, distributed to the pharynx, larynx, trachea, lungs, esophagus, abdominal viscera, aortic pressure, chemoreceptors, and the dura mater of the posterior cranial fossa, terminating in the caudal part of the nucleus tractus solitarius (NTS); 3) Special visceral afferent fibers (SVA) originating from the inferior ganglion, receiving taste information from the epiglottis area, terminating in the rostral part of the NTS (Ruffoli et al., 2011). Approximately 80% of vagus nerve fibers are sensory afferent fibers (George et al., 2000), primarily transmitting general somatic and visceral sensations. The NTS is the main hub of the vagus nerve’s afferent network, where most afferent fibers terminate. For example, the NTS sends these fibers to the locus coeruleus (LC), the dorsal raphe nucleus (DRN), and the parabrachial nucleus (PBN). There are close neural connections between brainstem nuclei and the thalamus, hippocampus, and amygdala, regulating perception, learning, memory, and emotional functions. Furthermore, connections are established through the nucleus basalis and cingulate cortex with the prefrontal cortex (PFC), orbital frontal cortex (OFC), and sensorimotor cortex, which participate in the regulation of cortical and subcortical circuit excitability (Hachem et al., 2018).
3.2 Central mechanisms of VNS
3.2.1 Inhibition of neuroinflammatory response
Neuroinflammation, linked to neurovascular unit (NVU) damage, microglia and astrocyte activation, and increased blood-brain barrier (BBB) permeability, can be mitigated by VNS, which helps maintain BBB integrity (Chen et al., 2018). This has a “neuroprotective” effect and is used to treat neurological and psychiatric disorders (Borovikova et al., 2000; Shytle et al., 2004; Varatharaj and Galea, 2017; Fonseca et al., 2019). The classic anti-inflammatory mechanism of VNS is the “cholinergic anti-inflammatory pathway” (CAP). A study by Borovikova et al. (2000) found that acetylcholine (ACh) released after VNS can lower the levels of cytokines that cause inflammation (TNF-α, IL-1β, IL-6, and IL-8) that are caused by lipopolysaccharide (LPS) without changing the levels of cytokines that stop inflammatory responses (IL-10, IL-4, and TGF-β). In 2012, Olofsson et al., 2012 studied the CAP pathway in more detail and discovered that norepinephrine (NE), which is released when the vagus nerve stimulates the celiac ganglion and splenic nerve, can increase the number of T lymphocytes and ACh release through β2-adrenergic receptors (β2-AR). ACh can connect to α7-nicotinic acetylcholine receptors (α7nAChR), which turns on macrophages in the spleen and lowers the production of TNF-α. In the same way, Shytle et al. (2004) discovered that VNS can turn on microglial α7nAChR, which stops the CAP pathway from making pro-inflammatory cytokines. Kaczmarczyk et al. (2018) said that VNS can change the microglial phenotype from neurodestructive to neuroprotective. This is done by increasing the release of BDNF, bFGF, and anti-inflammatory factors while decreasing the release of pro-inflammatory factors. This protects the neurons. Besides that, VNS can turn on specific mAChR central muscarinic acetylcholine receptors. These can then activate vagal efferent fibers and stop the growth of inflammation (Pavlov et al., 2009). Additionally, mAChR is involved in hippocampal theta rhythm modulation, which is crucial for learning, memory, and anxiety regulation (Broncel et al., 2018). Increased BBB permeability is another critical factor in neuroinflammation, with NVU damage being the core mechanism. The NVU is made up of vascular endothelial cells, smooth muscle cells, cholinergic and adrenergic nerve terminals, astrocytes, and perivascular cells (microglia, macrophages, and mast cells). It is very important for keeping the brain’s microenvironment stable and regulating blood flow, BBB substance exchange, immune surveillance, nutritional support, and coagulation balance (Iadecola, 2010; Kalaria, 2010). Researchers have discovered that increasing the release of LC and NE can turn on endothelial cell α7nAChR, which makes them better at pinocytosis (Kimura et al., 2019). Excitation of the vagus nerve can stop microglia and reactive astrocyte activation (Chen et al., 2018; Yang et al., 2018), stop aquaporin-4 (AQP-4), lower TNF-α levels, and improve tight junction protein protection of the BBB (Lopez et al., 2012), which stops neurodegenerative changes caused by inflammation (Varatharaj and Galea, 2017). Also, dorsal motor nuclei of the vagus (DMV) and paraventricular nuclei of the hypothalamus (PVN) that are activated by the vagus nerve help release growth hormone-releasing peptides (Ghrelin) and oxytocin, which help control inflammation and protect the brain-blood barrier (Bansal et al., 2012; Yuan et al., 2016; Collden et al., 2017; Panaro et al., 2020).
Along with its impact on immune cytokines and BBB permeability, VNS may also help reduce inflammation by controlling gut microbiota and improving cerebrospinal fluid (CSF) exchange in the glymphatic system (Bohórquez et al., 2015; Bonaz et al., 2019; Cheng et al., 2020; Zhang et al., 2020), though the exact ways it does this need more research.
3.2.2 Promoting neurogenic signaling pathways
VNS promotes the release of neurotransmitters and chemical molecules in the brain. These are the main ones: the NE pathway, the 5-HT pathway, the dopamine (DA) pathway, the brain-derived neurotrophic factor-tyrosine kinase B (TrkB) pathway, and the -aminobutyric acid (GABA) pathway.
The neurotransmitter GABA is mostly found in the medial septum (MS), the nucleus ambiguus (NA), the dorsal motor nucleus of the vagus (DMV), and the nucleus tractus solitarius (NTS) (Bennett et al., 1987; Helm et al., 2005; Herman et al., 2009; Pelkey et al., 2017). It is an inhibitory neurotransmitter. Herman et al. (2009) discovered that stimulating NTS can increase GABA signaling and send it to DMN and NA, making epileptic seizures less severe. Marrosu et al. (2003) found that GABA can control cortical excitability through GABAA receptors on the cerebral cortex to help people with epilepsy. And Keute et al. (2018a), Keute et al. (2018b) discovered that VNS can also act on GABAergic neurons in the motor cortex to control autonomic behavior inhibition. Furthermore, it has been discovered that GABAA and GABAB receptors in MS help control changes in the hippocampus after VNS stimulation. This can impact how people deal with anxiety and their ability to learn new things (Broncel et al., 2019). It can be seen that VNS stimulation can promote GABAergic pathways to regulate abnormal brain excitation and achieve comprehensive protective effects.
Noradrenaline (NE) is an excitatory neurotransmitter, and the locus coeruleus (LC) is the most abundant region of noradrenergic neurons in the brain. Short-term and long-term VNS can raise the firing rate of LC neurons and keep them active for a long time, causing NE to build up in the prefrontal lobe, amygdala, and hippocampus (Groves, Bowman and Brown, 2005; Hulsey et al., 2017). The rise in NE is strongly connected to cognitive function and memory performance (Ciampa et al., 2022). Some scholars have observed that after VNS, there is also an increase in NE levels in the medial and thalamic areas, as well as other cerebral cortex areas (Landau et al., 2015). The LC, the core node of the noradrenergic pathway, can sustain damage in experiments. VNS-mediated antiepileptic effects are blocked by LC (Krahl et al., 1998; Raedt et al., 2011; Liu et al., 2016); additionally, LC mediates the release of 5-HT and dopamine (DA) (Ruffoli et al., 2011).
The dorsal raphe nucleus (DRN) is where the VNS controls the 5-HT pathway. Stimulating the VNS can raise the level of 5-HT in the DRN and hippocampus, which can help people with depression (Furmaga et al., 2011). At the moment, there is disagreement about whether NTS and DRN connect directly through fibers. However, electrophysiological studies have shown that NTS can be sent through LC to indirectly control DRN (Dorr and Debonnel, 2006). They found that the discharge rate of LC increased after long-term and short-term VNS, and the discharge rate of DRN increased significantly only after long-term VNS. A study by Manta et al. (2009) found that the increasing DRN discharge frequency stopped happening after selective inhibition of LC-mediated norepinephrine neurons. This means that 5-hydroxytryptamine and norepinephrine work together and have a purpose in the brain. Manta et al. (2013) and Farrand et al. (2017) also revealed dopamine (DA) in the midbrain ventral tegmentum, substantia nigra, striatum, subventricular nucleus, and frontal lobe.
Brain-derived neurotrophic factor (BDNF) can prevent neuronal death and is an important hippocampal plasticity neurotrophic factor (Hofer and Barde, 1988; Zhao et al., 2007). A study by Furmaga et al. (2012) and Carreno and Frazer (2014) shows that long-term VNS can change hippocampal neurons through the aTnAChR mechanism, raise BDNF levels, improve mouse memory, and boost hippocampal tyrosine kinase B receptor B (AAkt) and cellular external signals controlled by S6K and CAMP response element binding protein (CREP), a group of cytokines.
4 VNS and its effects on cognitive function
4.1 VNS’s role in cognitive processing
VNS is most commonly studied in the cognitive domain of emotional functioning. VNS can enhance subjects’ ability to recognize emotions in others’ facial expressions. Ventura-Bort et al. (2021) required subjects to complete memory tasks while receiving taVNS stimulation and conducted a recognition test 1 week later. The results showed that the taVNS group performed better on emotional images compared to the sham group, with no significant difference in performance on neutral images between the two groups. Colzato et al. (2017) found that taVNS only improved performance on simple tasks of the Reading the Mind in the Eyes Test (RMET), with no significant effect on complex RMET tasks. Sellaro et al. (2018), based on the above studies, further explored the differential effects of taVNS on facial and body emotion recognition, showing that subjects’ scores in facial emotion recognition improved without affecting body emotion recognition. Sellaro therefore suggests that taVNS can enhance the brain’s ability to recognize emotions but is sensitive only to prominent stimuli that enhance attention, such as faces and eyes. Maraver et al. (2020) discovered that people who received taVNS were more accurate in tasks that came after direct gaze stimuli. This improvement happened regardless of the emotion (anger, fear) or the time between tasks, which supports Sellaro’s findings even more. Additionally, VNS can regulate participants’ inner motivation and evaluation before and after task completion, with delayed satisfaction being an important characteristic of self-emotional control.
Researchers have found that the impact of taVNS on delayed reward discount rates is dependent on the individual’s positive emotional level, reflecting the effectiveness of taVNS in emotional control (Steenbergen et al., 2020). De Smet et al. (2021) found that after subjects receiving taVNS were required to re-evaluate pictures previously rated negatively, their intense emotions were significantly reduced compared to the control group. Neuser et al. (2020), Ferstl et al. (2021) did one of the most important studies on emotional control. They found that taVNS significantly increased participants’ positive emotions after they worked hard to complete low-reward tasks. They also found that lower baseline positive emotions were significantly related to better taVNS effects. This study suggests that taVNS can encourage participants to work harder under low-value rewards to enhance their behavioral motivation, which is highly significant for improving patient behavioral motivation in clinical rehabilitation settings.
VNS also has a significant impact on executive attention functions, particularly selective attention and cognitive control. Steenbergen et al. (2015) used the stop-change paradigm to test people’s selective attention function. They discovered that taVNS improved people’s ability to choose their responses during action cascade processes, which means they had faster reactions when doing two behaviors right after each other. In more research on inhibitory control, Beste et al. (2016) discovered that taVNS did not improve performance in the reverse inhibition paradigm but did significantly improve performance in response inhibition paradigms involving task loads. Jongkees et al. (2018) also discovered that subjects receiving taVNS did not show any return inhibition phenomenon in continuous response tasks with high cognitive loads, suggesting that taVNS can improve cognitive selection processes when high-selectivity requirements are present. However, its effect on cognitive control is limited under conditions of lower loads or involvement of working memory. Borges et al. (2020) partly corroborated these findings, minimizing working memory load and using the Flanker test and Stroop questionnaire to test subjects’ inhibitory control capabilities. The results showed that under taVNS stimulation, there was no improvement in subjects’ inhibitory control performance, but there was a significant improvement in their performance in tasks involving task switching. Colzato looks at the vagus nerve network’s GABAergic pathway and the locus coeruleus-norepinephrine (LC-NE) pathway and says that taVNS can stimulate the GABAergic pathway in the cortex to promote inhibition. This weakens competitive selection in tasks that need high selectivity and makes it easier for people to choose between competing options. Network reset theory says that taVNS turns on the LC-NE after a global attention reset to change people’s behavior and stop them from investing too much time in tasks (Colzato et al., 2018a; Colzato et al., 2018b).
Learning and memory are core functions in cognitive processing and are closely related to emotional and attentional domains. In 2015, Jacobs et al. found that VNS can enhance elderly associative memory for faces, while some studies have shown that taVNS has no significant effect on vocabulary recognition memory (Mertens et al., 2020). One possible explanation for this phenomenon is that taVNS drives subjects to allocate more attentional resources to targets related to faces and emotional cues through its enhanced emotional effects, thereby reinforcing the encoding and consolidation stages of memory. Research from the past has shown that the amygdala controls explicit emotion and declarative memory in the hippocampus. VNS can stimulate these areas (Badran et al., 2018; Singh et al., 2022). It is worth noting that Sun et al. (2021) found that taVNS significantly improved subjects’ performance on spatial working memory tasks using the n-Back paradigm, possibly related to taVNS activation of the prefrontal lobe, but the neuroimaging mechanisms require further study.
4.2 The effects of VNS on patients with cognitive impairments
4.2.1 VNS and Epilepsy
The cognitive improvement effects of VNS in patients were first reported by Clark et al. (1999). They found that moderate current intensity (0.5 mA) VNS had the best effect on language memory improvement in epilepsy patients, whereas higher intensities (0.75–1.5 mA) led to reduced memory performance. Dodrill and Morris (2001) divided epilepsy patients into high and low stimulation groups and found no significant improvement in cognitive function after 12–16 weeks of follow-up with iVNS, although the high stimulation group experienced fewer emotional and physiological problems compared to the low stimulation group. Ghacibeh et al. (2006a), Ghacibeh et al. (2006b) used the Hopkins Verbal Learning Test (HVLT) to study 10 epilepsy patients implanted with iVNS. The results showed that there was no significant improvement in the learning part of the HVLT. However, the recognition and recall scores were significantly higher than those in the sham group. This suggests that VNS improves the encoding-consolidation phase of short-term memory in people with epilepsy. However, Ghacibeh also found a certain degree of decline in creativity and cognitive flexibility in epilepsy patients after receiving iVNS. A study by Mertens et al. (2022) compared the effectiveness of iVNS and taVNS in epilepsy patients. They discovered that acute iVNS and taVNS had no effect on language memory, but chronic iVNS over 6 weeks improved patients’ immediate recall and delayed recognition. This suggests that cumulative effects are one potential mechanism of VNS for cognitive impairment therapy.
In addition to adult epilepsy, many scholars have studied the effects of VNS on cognitive improvement in pediatric epilepsy patients, but the results are still controversial (Aldenkamp et al., 2001; Danielsson et al., 2008; Klinkenberg et al., 2013; Orosz et al., 2014). The cognitive-functional efficacy of VNS in patients with depression has also been studied to some extent. Unlike epilepsy patients, Sackeim et al. (2001) found no significant correlation between cognitive improvement in patients with major depressive disorder and VNS current intensity. Fourteen depressed patients who were implanted with VNS were studied for 2 years. It was found that improvements in their depressive symptoms were strongly linked to their attention and visuospatial working memory (Desbeaumes Jodoin et al., 2018). It is inferred that VNS may indirectly improve cognitive function by alleviating patients’ depressive symptoms.
4.2.2 VNS and Alzheimer’s disease
Two-thirds of dementia cases are diagnosed with Alzheimer’s disease (AD), characterized by neuronal deposition of amyloid-β plaques and neurofibrillary tau tangles, inflammatory activation of glia, reduced synaptic capacity, and neuronal loss. The Sjogren team conducted a series of studies on whether iVNS could improve cognitive status in patients with AD. In 2002, Sjogren et al. reported that 6 months after VNS implantation, seven out of 10 AD patients showed improvement in ADAS-cog scores, while nine out of 10 patients showed improvement in MMSE scores. A subsequent extended study of 17 AD patients after iVNS surgery found a median decrease of 4.8% in tau protein in cerebrospinal fluid within 1 year, while phosphorylated tau protein increased by 5%, providing new evidence for the physiological mechanisms of VNS in cognitive improvement (Merrill et al., 2006).
Aside from tau protein levels, stress appears to be another significant factor influencing AD (Caruso et al., 2018). Research has found elevated cortisol levels in biological fluids such as plasma, saliva, and cerebrospinal fluid in AD patients, which can exacerbate disease progression. Chronic VNS has been shown to effectively reduce serum cortisol levels, potentially improving the prognosis for AD patients by mitigating stress severity (O’Keane et al., 2005). While VNS can reduce cortisol and thereby delay the progression of AD, research on the regulation of the hypothalamic-pituitary-adrenal (HPA) axis in AD patients remains limited. Understanding how VNS regulates cortisol and its mechanisms to limit the negative impact of stress on individuals with brain diseases is crucial.
Epel et al. (2004) found that psychological stress is associated with telomere shortening. Currently, there is significant research discussing the relationship between telomere length and AD. Most studies suggest that shorter telomeres are associated with an increased risk of AD (Hackenhaar et al., 2021). Previous case-control studies and meta-analyses have shown the presence of short leukocyte telomere length (LTL) in individuals diagnosed with AD (Boccardi et al., 2020). Short baseline LTL is associated with a higher risk of developing AD (Koh et al., 2020) and all-cause dementia (Honig et al., 2012), although some studies have found no association between LTL and AD (Hinterberger et al., 2017). Notably, another longitudinal time-to-event analysis study found a nonlinear relationship between LTL and AD, with both short and long LTL associated with an increased risk of AD (Fani et al., 2020). Similar short and long-term LTL risk correlations have been observed for amnestic mild cognitive impairment (aMCI), considered a prodromal stage of AD (Roberts et al., 2014).
Telomere dysfunction induced by damage can occur regardless of telomere length (Brandr, 2019). According to the neuro-immune-senescence integrative model (NISIM; Ask and Sütterlin, 2022), reduced vagal regulation capacity represents decreased splenic vagal input and increased inflammation. NISIM posits that the prefrontal cortex (PFC) influences splenic inflammation levels when exerting regulatory effects on arousal through the vagus nerve. Research by Torvald et al. suggests that the activity of the HPA axis is related to telomere length, possibly indicating that HPA axis dysregulation leads to increased inflammation levels and subsequent ROS-induced telomere damage. This provides preliminary evidence for the potential clinical intervention of vagus nerve stimulation in improving telomere dysfunction and reducing peripheral inflammation levels.
Overall, VNS emerges as a promising intervention for AD, targeting cognitive impairments through modulation of tau proteins and addressing inflammatory and stress-related pathways through cortisol and telomere regulation. Further research is necessary to elucidate the precise mechanisms and optimize VNS protocols for maximum therapeutic benefit.
4.2.3 VNS and Sleep
VNS has become an intervention for sleep disorders (Zhang S. et al., 2021). Srinivasan et al. (2023) investigated the effects of taVNS on sleep disorders exacerbated in elderly healthcare workers. The results showed that taVNS significantly improved sleep quality and reduced anxiety. taVNS may improve sleep quality by modulating the brain’s default mode network (DMN) and salience network (SN). The DMN, active during rest, is involved in self-relevant thoughts and introspection, with its dysfunction associated with mental health issues such as anxiety and depression, which in turn affect sleep quality. The SN is responsible for processing significant sensory information and helps shift attention from internal thoughts to external stimuli, maintaining good mood and sleep. Additionally, brain-derived neurotrophic factor (BDNF) plays a crucial role in promoting neuroplasticity and recovery. Increased levels of BDNF are associated with improved mood, cognitive function, and sleep quality. taVNS may support brain health and enhance sleep quality by increasing plasma BDNF levels.
Meanwhile, Werner et al. (2015) examined the relationship between cardiac vagal control (CVC) and sleep quality in healthy women, finding that higher levels of CVC, measured by high-frequency heart rate variability (HF-HRV), were associated with better sleep quality. This suggests that good autonomic regulation and higher CVC can improve sleep quarters.
The arousal and wake-promoting effects of VNS have been demonstrated in animal studies and are well-known side effects of VNS treatment for epilepsy and depression. Winter et al. (2024) suggested that VNS may be a promising non-drug treatment for narcolepsy. Moreover, VNS may further stabilize neural networks and improve sleep quality during sleep, reducing the risk of seizures (Vespa et al., 2021). However, research on VNS for sleep disorders is still limited, and further high-quality randomized controlled trials are needed to verify its efficacy.
4.2.4 VNS and other diseases
Furthermore, studies have found that VNS has certain therapeutic effects on alcohol dependence (Wang et al., 2021) and COVID-19-related symptoms. Recent studies, including those by Azabou et al. (2021), have highlighted the potential of VNS to modulate the immune response in COVID-19 patients. This modulation is believed to occur via CAP activation, leading to reduced levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. They found that VNS significantly reduced pro-inflammatory cytokine levels, suggesting a potential mechanism for alleviating the hyperinflammatory state often seen in severe COVID-19 cases.
In another study, Colzato et al. (2023) demonstrated that long COVID, characterized by persistent symptoms such as “brain fog,” anxiety, depression, and cognitive deficits, is believed to be associated with brainstem dysfunction and disrupted vagal signaling. Studies suggest that taVNS may help ameliorate these symptoms by enhancing vagal activity and directly activating brainstem nuclei involved in cognitive and affective regulation. Also, taVNS is a non-pharmacological intervention that can be self-administered, making it a practical and accessible treatment option. Its safety profile is favorable, with minimal adverse effects reported compared to other invasive vagus nerve stimulation methods.
Expanding on these findings, Zheng et al. (2024) conducted a pilot study on the efficacy of taVNS in a female cohort with Long COVID. The study included 24 female patients who underwent a 10-day t-VNS intervention. Results demonstrated significant improvements in cognitive functions, anxiety, depression, and sleep post-intervention, with sustained benefits observed at a 1-month follow-up. However, olfactory performance did not show significant improvement, indicating the need for further investigation into this specific symptom.
Despite of novel findings in researches, several limitations need to be addressed. First, the studies reviewed often have small sample sizes and lack diversity in patient demographics, particularly the study by Zheng et al. (2024), which focused exclusively on female patients. This limits the generalizability of the results to the broader population. Also, the variability in VNS protocols, such as stimulation parameters and duration, complicates the comparison of outcomes across studies. Moreover, the pilot nature of these studies means that long-term safety and efficacy data are limited. The studies primarily report short-term benefits, and there is a need for longitudinal research to determine the sustained impact of VNS on COVID-19-related symptoms. At the same time, potential side effects should not be ignored (Mastitskaya et al., 2021), such as voice alteration, cough, dyspnea, dysphagia, etc.
5 Conclusion
VNS as a neuroregulatory technique holds promising applications in both clinical practice and research. As more clinical trials are done, more expert opinions are published, and non-invasive stimulation devices and closed-loop feedback stimulation technology get better, the use of VNS is moving toward more standardized and varied growth. VNS has been proven effective in modulating various cognitive domains in healthy individuals, and it shows potential benefits in improving cognition in treatment-resistant epilepsy, depression, Alzheimer’s disease, addictive disorders, and sleep disorders. However, there is currently a lack of comparative studies between different VNS stimulation modalities. Moreover, the differential effects of online VNS versus offline VNS on cognitive psychology remain unclear. Existing clinical trials often provide broad assessments of cognitive functions in patients without detailed scrutiny of various cognitive processing stages. To address these gaps, future research should delve deeper into these areas.
Overall, VNS represents a promising avenue for enhancing cognitive function across different patient populations. Continued research efforts are crucial to elucidating the optimal protocols, mechanisms of action, and specific cognitive domains that can benefit most from VNS interventions.
Author contributions
WW: Conceptualization, Funding Acquisition, Project Administration, Writing–Original Draft. RL: Data Curation, Formal Analysis, Writing–Original Draft. CL: Investigation, Writing–Original Draft. QL: Methodology, Writing–Review and Editing. XG: Supervision, Writing–Review and Editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Project 24-52 supported by the Fundamental Research Funds for the China Institute of Sport Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldenkamp A. P., Van de Veerdonk S. H., Majoie H. J., Berfelo M. W., Evers S. M., Kessels A. G., et al. (2001). Effects of 6 months of treatment with vagus nerve stimulation on behavior in children with Lennox-Gastaut syndrome in an open clinical and nonrandomized study. Epilepsy and Behav. 2 (4), 343–350. doi:10.1006/ebeh.2001.0218
Aranow C., Atish-Fregoso Y., Lesser M., Mackay M., Anderson E., Chavan S., et al. (2021). Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann. Rheumatic Dis. 80 (2), 203–208. doi:10.1136/annrheumdis-2020-217872
Ask T. F., Sütterlin S. (2022). Prefrontally modulated vagal neuroimmunomodulation is associated with telomere length. Front. Neurosci. 16, 1063162. doi:10.3389/fnins.2022.1063162
Azabou E., Bao G., Bounab R., Heming N. (2021). Vagus nerve stimulation: a potential adjunct therapy for COVID-19. Front. Med. 8, 625836. doi:10.3389/fmed.2021.625836
Badran B. W., Dowdle L. T., Mithoefer O. J., LaBate N. T., Coatsworth J., Brown J. C., et al. (2018). Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 11 (3), 492–500. doi:10.1016/j.brs.2017.12.009
Bansal V., et al. (2012). Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation 35 (1), 214–220. doi:10.1007/s10753-011-9307-7
Bennett J. A., McWilliam P. N., Shepheard S. L. (1987). A gamma-aminobutyric-acid-mediated inhibition of neurones in the nucleus tractus solitarius of the cat. J. Physiology 392 (1), 417–430. doi:10.1113/physiol.1987.sp016788
Beste C., Steenbergen L., Sellaro R., Grigoriadou S., Zhang R., Chmielewski W., et al. (2016). Effects of concomitant stimulation of the GABAergic and norepinephrine system on inhibitory control - a study using transcutaneous vagus nerve stimulation. Brain Stimul. 9 (6), 811–818. doi:10.1016/j.brs.2016.07.004
Boccardi V., Arosio B., Cari L., Bastiani P., Scamosci M., Casati M., et al. (2020). Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 59 (1), 119–126. doi:10.1007/s00394-019-01892-y
Bohórquez D. V., Shahid R. A., Erdmann A., Kreger A. M., Wang Y., Calakos N., et al. (2015). Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investigation 125 (2), 782–786. doi:10.1172/JCI78361
Bonaz B., Sinniger V., Pellissier s. (2019). Vagus nerve stimulation at the interface of brain-gut interactions. Cold Spring Harb. Perspect. Med. 9 (8), a034199. do. doi:10.1101/cshperspect.a034199
Borges U., Knops L., Laborde S., Klatt S., Raab M. (2020). Transcutaneous vagus nerve stimulation may enhance only specific aspects of the core executive functions. A randomized crossover trial. Front. Neurosci. 14, 523. doi:10.3389/fnins.2020.00523
Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405 (6785), 458–462. doi:10.1038/35013070
Bottomley J. M., LeReun C., Diamantopoulos A., Mitchell S., Gaynes B. N. (2019). Vagus nerve stimulation (VNS) therapy in patients withtreatment resistant depression: a systematic review and meta-analysis. Compr. Psychiatry 98, 152156. doi:10.1016/j.comppsych.2019.152156
Brand T. (2019). Length doesn’t matter—telomere damage triggers cellular senescence in the ageing heart. EMBO J. 38, e101571. doi:10.15252/embj.2019101571
Broadhurst P. L. (1957). Emotionality and the yerkes-dodson law. J. Exp. Psychol. 54 (5), 345–352. doi:10.1037/h0049114
Broncel A., Bocian R., Kłos-Wojtczak P., Konopacki J. (2018). Medial septal cholinergic mediation of hippocampal theta rhythm induced by vagal nerve stimulation. PLOS ONE 13 (11), e0206532. doi:10.1371/journal.pone.0206532
Broncel A., Bocian R., Kłos-Wojtczak P., Konopacki J. (2019). GABAergic mediation of hippocampal theta rhythm induced by stimulation of the vagal nerve. Brain Res. Bull. 147, 110–123. doi:10.1016/j.brainresbull.2019.02.010
Buell E. P., Loerwald K. W., Engineer C. T., Borland M. S., Buell J. M., Kelly C. A., et al. (2018). Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul. 11 (6), 1218–1224. doi:10.1016/j.brs.2018.07.045
Burger A. M., Verkuil B. (2018). Transcutaneous nerve stimulation via the tragus: are we really stimulating the vagus nerve? Brain Stimul. 11 (4), 945–946. doi:10.1016/j.brs.2018.03.018
Calabrese E. J. (2008a). Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res. Rev. 7 (1), 8–20. doi:10.1016/j.arr.2007.07.001
Calabrese E. J. (2008b). Stress biology and hormesis: the Yerkes-Dodson law in psychology--a special case of the hormesis dose response. Crit. Rev. Toxicol. 38 (5), 453–462. doi:10.1080/10408440802004007
Carreno F. R., Frazer A. (2014). Activation of signaling pathways downstream of the brain-derived neurotrophic factor receptor, TrkB, in the rat brain by vagal nerve stimulation and antidepressant drugs. Int. J. Neuropsychopharmacol. 17 (2), 247–258. doi:10.1017/S1461145713000977
Carreno F. R., Frazer A. (2017). Vagal nerve stimulation for treatment-resistant depression. Neurother. J. Am. Soc. Exp. Neuro Ther. 14 (3), 716–727. doi:10.1007/s13311-017-0537-8
Caruso A., Nicoletti F., Mango D., Saidi A., Orlando R., Scaccianoce S. (2018). Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 132, 130–134. doi:10.1016/j.phrs.2018.04.017
Chakravarthy K., Chaudhry H., Williams K., Christo P. J. (2015). Review of the uses of vagal nerve stimulation in chronic pain management. Curr. Pain Headache Rep. 19 (12), 54. doi:10.1007/s11916-015-0528-6
Chen M., Yu L., Ouyang F., Liu Q., Wang Z., Wang S., et al. (2015). The right side or left side of noninvasive transcutaneous vagus nerve stimulation: based on conventional wisdom or scientific evidence? Int. J. Cardiol. 187, 44–45. doi:10.1016/j.ijcard.2015.03.351
Chen X., He X., Luo S., Feng Y., Liang F., Shi T., et al. (2018). Vagus nerve stimulation attenuates cerebral microinfarct and colitis-induced cerebral microinfarct aggravation in mice. Front. Neurology 9. doi:10.3389/fneur.2018.00798
Cheng K. P., Brodnick S. K., Blanz S. L., Zeng W., Kegel J., Pisaniello J. A., et al. (2020). Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul. Basic, Transl. Clin. Res. Neuromodulation 13 (4), 1024–1030. doi:10.1016/j.brs.2020.03.012
Ciampa C. J., Parent J. H., Harrison T. M., Fain R. M., Betts M. J., Maass A., et al. (2022). Associations among locus coeruleus catecholamines, tau pathology, and memory in aging. Neuropsychopharmacol. Official Publ. Am. Coll. Neuropsychopharmacol. 47 (5), 1106–1113. doi:10.1038/s41386-022-01269-6
Clancy J. A., Mary D. A., Witte K. K., Greenwood J. P., Deuchars S. A., Deuchars J. (2014). Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 7 (6), 871–877. doi:10.1016/j.brs.2014.07.031
Clark K. B., Smith D. C., Hassert D. L., Browning R. A., Naritoku D. K., Jensen R. A. (1998). Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol. Learn. Mem. 70 (3), 364–373. doi:10.1006/nlme.1998.3863
Clark K. B., Naritoku D. K., Smith D. C., Browning R. A., Jensen R. A. (1999). Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 2 (1), 94–98. doi:10.1038/4600
Collidén G., Tschöp M. H., Müller T. D. (2017). Therapeutic potential of targeting the ghrelin pathway. Int. J. Mol. Sci. 18 (4), 798. doi:10.3390/ijms18040798
Colzato L. S., Elmers J., Beste C., et al. (2023). A prospect to ameliorate affective symptoms and to enhance cognition in long COVID using auricular transcutaneous vagus nerve stimulation. *Journal of Clinical Medicine* 12 (3). doi:10.3390/jcm12031198
Colzato L. S., Ritter S. M., Steenbergen L. (2018a). Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia 111, 72–76. doi:10.1016/j.neuropsychologia.2018.01.003
Colzato L. S., Sellaro R., Beste C. (2017). Darwin revisited: the vagus nerve is a causal element in controlling recognition of other's emotions. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 92, 95–102. doi:10.1016/j.cortex.2017.03.017
Colzato L. S., Wolters G., Peifer C. (2018b). Transcutaneous vagus nerve stimulation(AVNS) modulates flow experience. Experimental Brain Research 236 (1), 253–257. doi:10.1007/s00221-017-5123-0
Cook D. N., Thompson S., Stomberg-Firestein S., Bikson M., George M. S., Jenkins D. D., et al. (2020). Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation. Brain Stimulation Basic, Translational, and Clinical Research in Neuromodulation 13 (3), 800–803. doi:10.1016/j.brs.2020.02.028
D'Agostini M., Burger A. M., Villca Ponce G., Claes S., von Leupoldt A., Van Diest I. (2022). No evidence for a modulating effect of continuous transcutaneous auricular vagus nerve stimulation on markers of noradrenergic activity. Psychophysiology Preprint. doi:10.1111/psyp.13984
Danielsson S., Viggedal G., Gillberg C., Olsson I. (2008). Lack of effects of vagus nerve stimulation on drug-resistant epilepsy in eight pediatric patients with autism spectrum disorders: a prospective 2-year follow-up study. Epilepsy and Behavior 12 (2), 298–304. doi:10.1016/j.yebeh.2007.10.007
Dawson J., Liu C. Y., Francisco G. E., Cramer S. C., Wolf S. L., Dixit A., et al. (2021). Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. TheLancet 397 (10284), 1545–1553. doi:10.1016/S0140-6736(21)00475-X
Desbeaumes Jodoin V., Richer F., Miron J. P., Fournier-Gosselin M. P., Lespérance P. (2018). Long-term sustained cognitive benefits of vagus nerve stimulation in refractory depression. The Journal of ECT 34 (4), 283–290. doi:10.1097/YCT.0000000000000502
De Smet S., Baeken C., Seminck N., Tilleman J., Carrette E., Vonck K., Vanderhasselt M. A. (2021). Non-invasive vagal nerve stimulation enhances cognitive emotion regulation. Behaviour Research and Therapy 145, 103933. doi:10.1016/.brat.2021.103933
Dodrill C. B., Morris G. L. (2001). Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy and Behavior 2 (1), 46–53. doi:10.1006/ebeh.2000.0148
Dorr A. E., Debonnel G. (2006). Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. The Journal of Pharmacology and Experimental Therapeutics 318 (2), 890–898. doi:10.1124/jpet.106.104166
Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., et al. (2004). Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 101, 17312–17315. doi:10.1073/pnas.0407162101
O'Keane V., Dinan T. G., Scott L., Corcoran C. (2005). Changes in hypothalamopituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry 58, 963–968.
Fang J., Rong P., Hong Y., Fan Y., Liu J., Wang H., et al. (2016). Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biological Psychiatry 79 (4), 266–273. doi:10.1016/j.biopsych.2015.03.025
Fani L., Hilal S., Sedaghat S., Broer L., Licher S., Arp P. P., et al. (2020). Telomere length and the risk of Alzheimer’s disease: the Rotterdam study. J Alzheimers Dis 73 (2), 707–714. doi:10.3233/JAD-190759
Farmer A. D., Strzelczyk A., Finisguerra A., Gourine A. V., Gharabaghi A., Hasan A., et al. (2021). International Consensus based review and recommendations for Minimum reporting Standards in research on transcutaneous vagus nerve stimulation (version 2020). Frontiers in Human Neuroscience 14, 568051. doi:10.3389/fnhum.2020.568051
Farrand A. Q., Helke K. L., Gregory R. A., Gooz M., Hinson V. K., Boger H. A. (2017). Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson's disease. Brain Stimulation Basic, Translational, and Clinical Research in Neuromodulation 10 (6), 1045–1054. doi:10.1016/j.brs.2017.08.008
Ferstl M., Teckentrup V., Lin W. M., Kräutlein F., Kühnel A., Klaus J., Walter M., Kroemer N. B. (2021). Non-invasive vagus nerve stimulation boosts mood recovery after effort exertion. Psychological Medicine, 1–11. doi:10.1017/S0033291720005073
Fonseca R. C., Bassi G. S., Brito C. C., Rosa L. B., David B. A., Araújo A. M., et al. (2019). Vagus nerve regulates the phagocytic and secretory activity of resident macrophages in the liver. Brain, Behavior, and Immunity 81, 414–454. doi:10.1016/j.bbi.2019.06.041
Furmaga H., Carreno F. R., Frazer A. (2012). Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLOS ONE 7 (5), e34844. doi:10.1371/journal.pone.0034844
Furmaga H., Shah A., Frazer A. (2011). Serotonergic and noradrenergic pathways are required for the anxiolytic-like and antidepressant-like behavioral effects of repeated vagal nerve stimulation in rats. Biological Psychiatry 70 (10), 937–945. doi:10.1016/j.biopsych.2011.07.020
Garcia R. G., Lin R. L., Lee J., Kim J., Barbieri R., Sclocco R., Wasan A. D., et al. (2017). Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain 158 (8), 1461–1472. doi:10.1097/j.pain.0000000000000930
George M. S., Sackeim H. A., Rush A. J., Marangell L. B., Nahas Z., Husain M. M., et al. (2000). Vagus nerve stimulation: a new tool for brain research and therapy. Biological Psychiatry 47 (4), 287–295. doi:10.1016/0006-3223(99)00308-x
Ghacibeh G. A., Shenker J. I., Shenal B., Uthman B. M., Heilman K. M. (2006a). Effect of vagus nerve stimulation on creativity and cognitive flexibility. EpilepsyBehavior 8 (4), 720–725. doi:10.1016/j.yebeh.2006.03.008
Ghacibeh G. A., Shenker J. I., Shenal B., Uthman B. M., Heilman K. M. (2006b). The influence of vagus nerve stimulation on memory. Cognitive and Behavioral Neurology 19 (3), 119–122. doi:10.1097/01.wnn.0000213908.34278.7d
Groves D. A., Bowman E. M., Brown V. J. (2005). Recordings from the rat locus coerules during acute vagal nerve stimulation in the anaesthetised rat. Neuroscience Letters 379 (3), 174–179. doi:10.1016/j.neulet.2004.12.055
Hachem L. D., Wong S. M., Ibrahim G. M. (2018). The vagus afferent network: emerging role in translational connectomics. Neurosurgical Focus 45 (3), E2.do. doi:10.3171/2018.6.FOCUS18216
Hackenhaar F. S., Josefsson M., Adolfsson A. N., Landfors M., Kauppi K., Hultdin M., et al. (2021). Short leukocyte telomeres predict 25-year Alzheimer’s disease incidence in non-APOE ε4-carriers. Alzheimer’s Res. Ther. 13, 130. doi:10.1186/s13195-021-00871-y
Hamza M., Carron R., Dibué M., et al. (2024). Right-sided vagus nerve stimulation for drug-resistant epilepsy: a systematic review of the literature and perspectives. Seizure 117, 298–304. doi:10.1016/j.seizure.2024.02.011
Helm K. A., Haberman R. P., Dean S. L., Hoyt E. C., Melcher T., Lund P. K., Gallagher M. (2005). GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element. CRE in the hippocampus Neuropharmacology 48 (7), 956–964. doi:10.1016/j.neuropharm.2005.01.019
Herman M. A., Cruz M. T., Sahibzada N., Verbalis J., Gillis R. A. (2009) GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am. J. Physiol. Gastrointest. Liver Physiol. 296 (1), G101–G111. doi:10.1152/ajpgi.90504.2008
Hinterberger M., Fischer P., Huber K., Krugluger W., Zehetmayer S. (2017). Leukocyte telomere length is linked to vascular risk factors not to Alzheimer’s disease in the VITA study. J Neural Transm (Vienna) 124 (7), 809–819. doi:10.1007/s00702-017-1721-z
Hofer M. M., Barde Y.-A. (1988). Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature 331 (6153), 261–262. doi:10.1038/331261a0
Honig L. S., Kang M. S., Schupf N., Lee J. H., Mayeux R. (2012). Association of shorterleukocyte telomere repeat length with dementia and mortality. Arch Neurol 69 (10), 1332–1339. doi:10.1001/archneurol.2012.1541
Hulsey D. R., Riley J. R., Loerwald K. W., Rennaker R. L., Kilgard M. P., Hays S. A. (2017). Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Experimental Neurology 289, 21–30. doi:10.1016/j.expneurol.2016.12.005
Jacobs H. I., Riphagen J. M., Razat C. M., Wiese S., Sack A. T. (2015). Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiology of Aging 36 (5), 1860–1867. doi:10.1016/j.neurobiolaging.2015.02.023
Jensen P., Tsiropoulos I. (2024). Vagus nerve stimulation for the treatment of treatment-refractory epilepsy. Ugeskr Laeger 186 (23), V10230638. Published 2024 Jun 3. doi:10.61409/V10230638
Jongkees B. J., et al. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during sequential action. Frontiers in Psychology 9, 1159. doi:10.3389/fpsyg.2018.01159
Jung B., Yang C., Lee S. H. (2024). Vagus nerves stimulation: clinical implication and practical issue as a neuropsychiatric treatment. Clin Psychopharmacol Neurosci 22 (1), 13–22. doi:10.9758/cpn.23.1101
Kaczmarczyk R., Tejera D., Simon B. J., Heneka M. T. (2018). Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer's disease. Journal of Neurochemistry 146 (1), 76–85. doi:10.1111/jnc.14284
Kalagara R., Chennareddy S., Reford E., Bhimani A. D., Cummins D. D., Downes M. H., et al. (2024). Complications of implanted vagus nerve stimulation: a systematic review and meta-analysis. Cerebrovasc Dis. doi:10.1159/000536362
Kalaria R. N. (2010). Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutrition Reviews 68 (Suppl. l), S74–S87. doi:10.1111/j.1753-4887.2010.00352.x
Keute M., Demirezen M., Graf A., Mueller N. G., Zaehle T. (2019). No modulation of pupil size and event-related pupil response by transcutaneous auricular vagus nerve stimulation (taVNS). Scientific Reports 9 (1), 11452. doi:10.1038/s41598-019-47961-4
Keute M., Machetanz K., Berelidze L., Guggenberger R., Gharabaghi A. (2021). Neuro-cardiac coupling predicts transcutaneous auricular vagus nerve stimulation effects. Brain Stimulation 14 (2), 209–216. doi:10.1016/j.brs.2021.01.001
Keute M., Krauel K., Heinze H. J., Stenner M. P. (2018a). Intact automatic motor inhibition in attention deficit hyperactivity disorder. Cortex 109, 215–225. doi:10.1016/j.cortex.2018.09.018
Keute M., Ruhnau P., Heinze H. J., Zaehle T. (2018b). Behavioral and electrophysiological evidence for GABAergic modulation through transcutaneous vagus nerve stimulation. Clinical Neurophysiology 129 (9), 1789–1795. doi:10.1016/j.clinph.2018.05.026
Kimberley T. J., Pierce D., Prudente C. N., Francisco G. E., Yozbatiran N., Smith P., et al. (2018). Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke 49 (11), 2789–2792. doi:10.1161/STROKEAHA.118.022279
Kimura I., Dohgu S., Takata F., Matsumoto J., Kawahara Y., Nishihira M., et al. (2019). Activation of the a7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in ratbrain endothelial cells. NeuroscienceLetters 694 (op), 9–13. doi:10.1016/i.neulet.2018.11.022
Klinkenberg S., van den Bosch C. N., Majoie H. J., Aalbers M. W., Leenen L., Hendriksen J., et al. (2013). Behavioural and cognitive effects during vagus nerve stimulation in children with intractable epilepsy - a randomized controlled trial. European journal of paediatric neurology EJPN official journal of the European Paediatric Neurology Society 17 (1), 82–90. doi:10.1016/j.ejpn.2012.07.003
Koh S. H., Choi S. H., Jeong J. H., Jang J. W., Park K. W., Kim E. J., et al. (2020). Telomereshortening reflecting physical aging is associated with cognitive declineand dementia conversion in mild cognitive impairment due to Alzheimer'sdisease. Aging (Albany NY). doi:10.18632/aging.102893
Kovacic K., Hainsworth K., Sood M., Chelimsky G., Unteutsch R., Nugent M., et al. (2017). Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. The Lancet Gastroenterology and Hepatology 2 (10), 727–737. doi:10.1016/S2468-1253(17)30253-4
Krahl S. E., Clark K. B., Smith D. C., Browning R. A. (1998). Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39 (7), 709–714. doi:10.1111/j.1528-1157.1998.tb01155.x
ladecola C. (2010). The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathologica 120 (3), 287–296. doi:10.1007/s00401-010-0718-6
Lampros M., Vlachos N., Zigouris A., Voulgaris S., Alexiou G. A. (2021). Transcutaneous Vagus Nerve Stimulation (t-VNS) and epilepsy: a systematic review of the literature. Seizure 91, 40–48. doi:10.1016/j.seizure.2021.05.017
Landau A. M., Dyve S., Jakobsen S., Alstrup A. K., Gjedde A., Doudet D. J. (2015). Acute vagal nerve stimulation lowers a2 adrenoceptor availability: possible mechanism of therapeutic action. Brain Stimulation Basic, Translational, and Clinical Research in Neuromodulation 8 (4), 702–707. doi:10.1016/j.brs.2015.02.003
Lespérance P., Desbeaumes Jodoin V., Drouin D., et al. (2024). Vagus nerve stimulation modulates inflammation in treatment-resistant depression patients: a pilot study. Int J Mol Sci 25 (5), 2679. Published 2024 Feb 26. doi:10.3390/ijms25052679
Lin X., Fang Y., Hu H., Ye Z. (2024). Efficacy and safety of transcutaneous auricular vagus nerve stimulation (ta-VNS) in the treatment of tinnitus: protocol for an updated systematic review and meta-analysis. BMJ Open 14 (5), e082906. Published 2024 May 21. doi:10.1136/bmjopen-2023-082906
Liu C., Tang H., Liu C., Ma J., Liu G., Niu L., et al. (2024). Transcutaneous auricular vagus nerve stimulation for post-stroke depression: a double-blind, randomized, placebo-controlled trial. J Affect Disord 354, 82–88. doi:10.1016/j.jad.2024.03.005
Liu J., Fang J., Wang Z., Rong P., Hong Y., Fan Y., et al. (2016). Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. Journal of Affective Disorders 205, 319–326. doi:10.1016/j.jad.2016.08.003
Lopez N. E., Krzyzaniak M. J., Costantini T. W., Putnam J., Hageny A. M., Eliceiri B., et al. (2012). Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. Journal of Trauma and Acute Care Surgery 72 (6), 1562–1566. doi:10.1097/TA.0b013e3182569875
Luo W., Zhang Y., Yan Z., Liu X., Hou X., Chen W., et al. (2020). The instant effects of continuous transcutaneous auricular vagus nerve stimulation at acupoints on the functional connectivity of amygdala in migraine without aura: a preliminary study. Neural Plasticity 2020, e8870589. doi:10.1155/2020/8870589
Ma J., Qiao P., Li Q., Wang Y., Zhang L., Yan L. J., Cai Z. (2019). Vagus nerve stimulation as a promising adjunctive treatment for ischemic stroke. Neurochemistry International 131, 104539. doi:10.1016/j.neuint.2019.104539
Manta S., Dong J., Debonnel G., Blier P. (2009). Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. Journal of psychiatry and neuroscience 34 (4), 272–280.
Manta S., El Mansari M., Debonnel G., Blier P. (2013). Electrophysiological and neurochemical effects of long-terr vagus nerve stimulation on the rat monoaminergic systems. The International Journal of Neuropsychopharmacology 162, 459–470. doi:10.1017/S1461145712000387
Marano M., Anzini G., Saltarocchi L., Ricciuti R., Capone F., Tan H., et al. (2024). Left vagus stimulation modulates contralateral subthalamic β power improving the gait in Parkinson's disease. Mov Disord 39 (2), 424–428. doi:10.1002/mds.29690
Maraver M. J., Steenbergen L., Hossein R., Actis-Grosso R., Ricciardelli P., Hommel B., Colzato L. S. (2020). Transcutaneous vagus nerve stimulation modulates attentional resource deployment towards social cues. Neuropsychologia 143, 107465. doi:10.1016/j.neuropsychologia.2020.107465
Marrosu F., Serra A., Maleci A., Puligheddu M., Biggio G., Piga M. (2003). Correlation between GABA receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Research 55 (1), 59–70. doi:10.1016/S0920-1211(03)00107-4
Mastitskaya S., Thompson N., Holder D. (2021). Selective vagus nerve stimulation as a therapeutic approach for the treatment of ards: a rationale for neuro-immunomodulation in COVID-19 disease. *Frontiers in Neuroscience* 15, 667036. doi:10.3389/fnins.2021.667036
Merrill C. A., Jonsson M. A., Minthon L., Ejnell H., C-son Silander H., Blennow K., et al. (2006). Vagus nerve stimulation in patients with Alzheimer's disease: additional follow-up results of a pilot study through 1 year. J Clin Psychiatry. 67 (8): 1171–8. doi:10.4088/jcp.v67n0801
Mertens A., Naert L., Miatton M., Poppa T., Carrette E., Gadeyne S., et al. (2020). Transcutaneous vagus nerve stimulation does not affect verbal memory performance in healthy volunteers. Frontiers in Psychology 11, 551. doi:10.3389/fpsyg.2020.00551
Mertens A., Gadeyne S., Lescrauwaet E., Carrette E., Meurs A., De Herdt V., et al. (2022). The potential of invasive and non-invasive vagus nerve stimulation to improve verbal memory performance in epilepsy patients. Scientific Reports 12 (1), 1984. doi:10.1038/s41598-022-05842-3
Mondal B., Choudhury S., Simon B., Baker M. R., Kumar H. (2019). Noninvasive vagus nerve stimulation improves gait and reduces freezing of gait in Parkinson's disease. Movement Disorders Official Journal of the Movement Disorder Society 34 (6), 917–918. doi:10.1002/mds.27662
Mondal B., Choudhury S., Banerjee R., Roy A., Chatterjee K., Basu P., et al. (2021). Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson's disease in patients with freezing of gait. NPJ Parkinson's disease 7 (1), 46. doi:10.1038/s41531-021-00190-x
Napadow V., Edwards R. R., Cahalan C. M., Mensing G., Greenbaum S., Valovska A., et al. (2012). Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Medicine (Malden, Mass.) 13 (6), 777–789. doi:10.1111/j.1526-4637.2012.01385.x
Neuser M. P., Teckentrup V., Kühnel A., Hallschmid M., Walter M., Kroemer N. B. (2020). Vagus nerve stimulation boosts the drive to work for rewards. Nature Communications 11 (1), 3555. doi:10.1038/s41467-020-17344-9
Olofsson P. S., Katz D. A., Rosas-Ballina M., Levine Y. A., Ochani M., Valdés-Ferrer S. I., et al. (2012). a7 nicotinic acetylcholine receptor (a7nACh) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Molecular Medicine 18 (3), 539–543. doi:10.2119/molmed.2011.00405
Orosz I., McCormick D., Zamponi N., Varadkar S., Feucht M., Parain D., et al. (2014). Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia 55 (10), 1576–1584. doi:10.1111/epi.12762
Oshinsky M. L., Murphy A. L., Hekierski H. Jr, Cooper M., Simon B. J. (2014). Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 155 (5), 1037–1042. doi:10.1016/j.pain.2014.02.009
Panaro M. A., Benameur T., Porro C. (2020). Hypothalamic neuropeptide brain protection: focus on oxytocin. Journal of Clinical Medicine 9 (5), 1534. doi:10.3390/jcm9051534
Pavlov V. A., Parrish W. R., Rosas-Ballina M., Ochani M., Puerta M., Ochani K., et al. (2009). Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity 23 (1), 41–45. doi:10.1016/j.bbi.2008.06.011
Pelkey K. A., Chittajallu R., Craig M. T., Tricoire L., Wester J. C., McBain C. J. (2017). Hippocampal GABAergic inhibitory interneurons. Physiological Reviews 97 (4), 1619–1747. doi:10.1152/physrev.00007.2017
Raedt R., Clinckers R., Mollet L., Vonck K., El Tahry R., Wyckhuys T., et al. (2011). Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. Journal of Neurochemistry 117 (3), 461–469. doi:10.1111/j.1471-4159.2011.07214.x
Redgrave J., Day D., Leung H., Laud P. J., Ali A., Lindert R., et al. (2018). Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans; a systematic review. Brain Stimulation 11 (6), 1225–1238. doi:10.1016/j.brs.2018.08.010
Roberts RO, Boardman LA, Cha RH, Pankratz VS, Johnson RA, Druliner BR, et al. (2014). Short and long telomeres increase risk of amnestic mild cognitive impairment. Mech Ageing Dev 141-142, 64–9. doi:10.1016/j.mad.2014.10.002
Ruffoli R., et al. (2011). The chemical neuroanatomy of vagus nerve stimulation. Journal of Chemical Neuroanatomy 42 (4), 288–296. doi:10.1016/j.jchemneu.2010.12.002
Sackeim H. A., Keilp J. G., Rush A. J., George M. S., Marangell L. B., Dormer J. S., et al. (2001). The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry, neuropsychology, and behavioral neurology 14 (1), 53–62.
Sclocco R., Garcia R. G., Kettner N. W., Isenburg K., Fisher H. P., Hubbard C. S., et al. (2019). The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: a multimodal ultrahigh-field (7T) MRI study. Brain Stimulation 12 (4), 911–921. doi:10.1016/j.brs.2019.02.003
Sellaro R., de Gelder B., Finisguerra A., Colzato L. S. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex 99, 213–223. doi:10.1016/j.cortex.2017.11.007
Shi X., Zhao L., Luo H., Deng H., Wang X., Ren G., et al. (2024). Transcutaneous auricular vagal nerve stimulation is effective for the treatment of functional dyspepsia: a multicenter, randomized controlled study. Am J Gastroenterol 119 (3), 521–531. doi:10.14309/ajg.0000000000002548
Shytle R. D., Mori T., Townsend K., Vendrame M., Sun N., Zeng J., et al. (2004). Cholinergic modulation of microglial activation by a7 nicotinic receptors. Journal of Neurochemistry 89 (2), 337–343. doi:10.1046/j.1471-4159.2004.02347.x
Singh K., García-Gomar M. G., Cauzzo S., Staab J. P., Indovina I., Bianciardi M. (2022). Structural connectivity of autonomic, pain, limbic, and sensory brainstem nuclei in living humans based on 7 Tesla and 3 Tesla MRI. Human Brain Mapping Preprint. doi:10.1002/hbm.25836
Sjögren M. J., Hellström P. T., Jonsson M. A., Runnerstam M., Silander H. C., Ben-Menachem E. (2002). Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: a pilot study. The Journal of Clinical Psychiatry 63 (11), 972–980. doi:10.4088/jcp.v63n1103
Srinivasan V., Abathsagayam K., Suganthirababu P., Alagesan J., Vishnuram V., Vasanthi R. K. (2023). Effect of vagus nerve stimulation (taVNS) on anxiety and sleep disturbances among elderly health care workers in the post COVID-19 pandemic. Work 78 (4), 1149–1156. doi:10.3233/WOR-231362
Steenbergen L., Colzato L. S., Maraver M. J. (2020). Vagal signaling and the somatic marker hypothesis: the effect of transcutaneous vagal nerve stimulation on delay discounting is modulated by positive mood. International Journal of PsychophysiologyOfficial Journal of the International Organization of Psychophysiology 148, 84–92. doi:10.1016/j.ijpsycho.2019.10.010
Steenbergen L., Sellaro R., Stock A. K., Verkuil B., Beste C., Colzato L. S. (2015). Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. European Neuropsychopharmacology 25 (6), 773–778. doi:10.1016/j.euroneuro.2015.03.015
Stegeman I., Velde H. M., Robe P. A. J. T., Stokroos R. J., Smit A. L. (2021). Tinnitus treatment by vagus nerve stimulation: a systematic review. PloS One 16 (3), e0247221. doi:10.1371/journal.pone.0247221
Straube A., Ellrich J., Eren O., Blum B., Ruscheweyh R. (2015). Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. The Journal of Headache and Pain 16, 543. doi:10.1186/s10194-015-0543-3
Sun J. B., Cheng C., Tian Q. Q., Yuan H., Yang X. J., Deng H., et al. (2021). Transcutaneous auricular vagus nerve stimulation improves spatial working memory in healthy young adults. Frontiers in Neuroscience 15, 790793. doi:10.3389/fins.2021.790793
Toffa D. H., Touma L., El Meskine T., Bouthillier A., Nguyen D. K. (2020). Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review. Seizure 83, 104–123. doi:10.1016/j.seizure.2020.09.027
Vaiman M., Heyman E., Lotan G. (2017). Neurological results of the modified treatment of epilepsy by stimulation of the vagus nerve. Child's nervous systemChNS official journal of the International Society for Pediatric Neurosurgery 33 (11), 2017–2022. doi:10.1007/00381-017-3490-2
Varatharaj A., Galea I. (2017). The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity 60, 1–12. doi:10.1016/j.bbi.2016.03.010
Ventura-Bort C., Wirkner J., Wendt J., Hamm A. O., Weymar M. (2021). Establishment of emotional memories is mediated by vagal nerve activation: evidence from noninvasive taVNS. Journal of Neuroscience 41 (36), 7636–7648. doi:10.1523/JNEUROSCI.2329-20.2021
Vespa S., Heyse J., Stumpp L., Liberati G., Ferrao Santos S., Rooijakkers H. (2021). Vagus nerve stimulation elicits sleep EEG desynchronization and network changes in responder patients in epilepsy. Neurotherapeutics 18 (4), 2623–2638. doi:10.1007/s13311-021-01124-4
Wang Y. M., Xu Y. Y., Zhai Y., Wu Q. Q., Huang W., Liang Y., et al. (2021). Effect of transcutaneous auricular vagus nerve stimulation on protracted alcohol withdrawal symptoms in male alcohol-dependent patients. Frontiers in Psychiatry 12, 678594. doi:10.3389/fpsyt.2021.678594
Werner G., Ford B. Q., Mauss I. B., Schabus M., Blechert J., Wilhelm F. H. (2015). High cardiac vagal control is related to better subjective and objective sleep quality. Biological Psychology 106, 79–85. doi:10.1016/j.biopsycho.2015.02.004
Winter Y., Sandner K., Bassetti C. L. A., Glaser M., Ciolac D., Ziebart A., et al. (2024). Vagus nerve stimulation for the treatment of narcolepsy. Brain Stimul 17 (1), 83–88. doi:10.1016/j.brs.2024.01.002
Yakunina N., Kim S. S., Nam E. C. (2017). Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation Technology A. T. the Neural Interface 20 (3), 290–300. doi:10.1111/ner.12541
Yakunina N., Kim S. S., Nam E.-C. (2018). BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PloS One 13 (11), e0207281. doi:10.1371/journal.pone.0207281
Yakunina N., Nam E.-C. (2021). Direct and transcutaneous vagus nerve stimulation for treatment of tinnitus: a scoping review. Frontiers in Neuroscience 15. doi:10.3389/fins.2021.680590Accessed March 29, 2022)
Yang Y., Yang L. Y., Orban L., Cuylear D., Thompson J., Simon B., Yang Y. (2018). Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimulation Basic, Translational, and Clinical Research in Neuromodulation 11 (4), 689–698. doi:10.1016/j.brs.2018.01.034
Yerkes R. M., Dodson J. D. (1908). The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 18, 459–482.
Yu Y., Ling J., Yu L., Liu P., Jiang M. (2021). Closed-loop transcutaneous auricular vagal nerve stimulation:current situation and future possibilities. Frontiers in Human Neuroscience 15, 785620. doi:10.3389/fnhum.2021.785620
Yuan L., Liu S., Bai X., Gao Y., Liu G., Wang X., et al. (2016). Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of Neuroinflammation 13 (1), 77. doi:10.1186/s12974-016-0541-7
Zabara J. (1985). Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalography and Clinical Neurophysiology 61 (3), S162. doi:10.1016/0013-4694(85)90626-1
Zaehle T., Krauel K. (2021). Transcutaneous vagus nerve stimulation in patients with attention-deficit/hyperactivity disorder: a viable option?. Prog Brain Res 264, 171–190. doi:10.1016/bs.pbr.2021.03.001
Zeng H., Pacheco-Barrios K., Cao Y., Li Y., Zhang J., Yang C., Fregni F. (2020). Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Scientific Reports 10 (1), 19184. doi:10.1038/s41598-020-75922-9
Zhang J., Ma L., Chang L., Pu Y., Qu Y., Hashimoto K. (2020). A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Translational Psychiatry 10 (1), 1–13. doi:10.1038/s41398-020-00878-3
Zhang S., He J. K., Meng H., Zhao B., Zhao Y. N., Wang Y., et al. (2021a). Effects of transcutaneous auricular vagus nerve stimulation on brain functional connectivity of medial prefrontal cortex in patients with primary insomnia. Anatomical Record (Hoboken, N.J.) 304 (11), 2426–2435. doi:10.1002/ar.24785
Zhang Y., Liu J., Li H., Yan Z., Liu X., Cao J., et al. (2019). 'Transcutaneous auricular vagus nerve stimulation at 1Hz modulates locus coeruleus activity and resting state functional connectivity in patients with migraine: an fMRI study. Neurolmage Clinical 24, 101971. doi:10.1016/j.nicl.2019.101971
Zhang Y., Huang Y., Li H., Yan Z., Zhang Y., Liu X., et al. (2021b). Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: an fMRI study. Regional Anesthesia and Pain Medicine 46 (2), 145–150. doi:10.1136/rapm-2020-102088
Zhao M., Li D., Shimazu K., Zhou Y. X., Lu B., Deng C. X. (2007). Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biological Psychiatry 62 (5), 381–390. doi:10.1016/j.biopsych.2006.10.019
Keywords: vagus nerve stimulation, cognitive enhancement, emotional processing, epilepsy, depression, Alzheimer’s disease, ADHD (attention deficit and hyperactivity disorder), long covid
Citation: Wang W, Li R, Li C, Liang Q and Gao X (2024) Advances in VNS efficiency and mechanisms of action on cognitive functions. Front. Physiol. 15:1452490. doi: 10.3389/fphys.2024.1452490
Received: 21 June 2024; Accepted: 08 August 2024;
Published: 09 October 2024.
Edited by:
Luiz Carlos Marques Vanderlei, São Paulo State University, BrazilReviewed by:
Claire Marie Rangon, Independent Researcher, Montmorency, FranceElias Manjarrez, Meritorious Autonomous University of Puebla, Mexico
Copyright © 2024 Wang, Li, Li, Liang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Gao, Z2FveGlhb2xpbkBjaXNzLmNu
†These authors have contributed equally to this work and share first authorship
 Wendi Wang
Wendi Wang Rui Li
Rui Li Chuangtao Li
Chuangtao Li Qimin Liang
Qimin Liang Xiaolin Gao
Xiaolin Gao