
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 29 October 2024
Sec. Integrative Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1440307
This article is part of the Research Topic Crosstalk between lung and brain, heart, kidney and vascular system in critical illness View all 6 articles
 Ah Young Leem1†§
Ah Young Leem1†§ Hee Tae Yu2†
Hee Tae Yu2† MinDong Sung1§
MinDong Sung1§ Kyung Soo Chung1§
Kyung Soo Chung1§ Yeonkyeong Kim1
Yeonkyeong Kim1 Ala Woo1§
Ala Woo1§ Song Yee Kim1§
Song Yee Kim1§ Moo Suk Park1§
Moo Suk Park1§ Young Sam Kim1§
Young Sam Kim1§ Young Ho Yang3§
Young Ho Yang3§ Ha Eun Kim3
Ha Eun Kim3 Jin Gu Lee3
Jin Gu Lee3 Kyuseok Kim4
Kyuseok Kim4 Kyu Bom Kim5
Kyu Bom Kim5 Boyoung Joung2
Boyoung Joung2 Junbeom Park6,7*‡
Junbeom Park6,7*‡ Su Hwan Lee1*‡§
Su Hwan Lee1*‡§Introduction: End-stage lung disease causes cardiac remodeling and induces electrocardiogram (ECG) changes. On the other way, whether lung transplantation (LTx) in end-stage lung disease patients are associated with ECG change is unknown. The object of this study was to investigate ECG changes before and after LTx in end-stage lung disease patients and whether these changes had clinical significance.
Method: This was a single-center retrospective cohort study of 280 end-stage lung disease patients who consecutively underwent LTx at a tertiary referral hospital. ECG findings before LTx and within 1 week and 1, 3, and 6 months after LTx were obtained and analyzed. To find clinical meaning, the ECG at 1 month after LTx was analyzed according to 1-year survival (survivor vs non-survivor groups). Survival data were estimated using the Kaplan–Meier method.
Results: Significant differences were observed in the PR interval, QRS duration, QT interval, QTc interval, and heart rate before LTx and 1 month after LTx; the PR interval, QRS duration, QTc interval, and heart rate were decreased. Particularly, the QTc interval was significantly decreased 1 month after LTx, whereas there was no significant change in the QTc interval from 1 to 6 months thereafter. The PR interval, QT interval, QTc interval, and heart rate were significantly different between the survivor and non-survivor groups. The serial changes in QTc interval before LTx and 1 and 3 months after LTx were also significantly different between the survivor and non-survivor groups (p = 0.040 after adjusting for age and body mass index). Upon dividing the patients based on the range of QTc interval change ≤ -8 ms, >-8–10 ms, >10–35 ms, >35 ms), the survival rate was significantly lower in the group whose QTc interval at 1 month after LTx decreased by > 35 m (p = 0.019).
Conclusion: LTx in patients with end-stage lung disease may induce ECG changes. Patients whose QTc interval at 1 month after LTx decreased by > 35 ms have a significantly higher 1-year mortality rate. Hence, these ECG changes may have clinical and prognostic significance.
End-stage lung disease is a severe disease that causes cardiac remodeling, and lung transplantation (LTx) can also induce marked changes to the heart (Panagiotou et al., 2017). The heart is connected to pulmonary vessels within the thorax, and the condition of the lung and pulmonary vessels can affect the heart. Hyperexpanded lungs compress the heart and diaphragm, which may cause clockwise rotation of the heart location. Chronic hypoxemia due to chronic lung disease also causes pulmonary vasoconstriction with elevation of pulmonary artery pressure. Destruction of lung tissues causes pulmonary capillary injury and increases the resistance of pulmonary vasculature, and increased pulmonary arterial pressures cause right atrial and ventricular afterload (Pinsky, 2018; Harrigan and Jones, 2002). Furthermore, changes in intrathoracic pressure affect the systemic venous return and left ventricular systolic pressure (Lalande et al., 2012).
The 12-lead electrocardiogram (ECG) provides several information regarding cardiac electric activity (Stracina et al., 2022). Abnormalities in heart function affect ECG findings, and thus, the ECG plays an important role in the diagnosis of arrhythmias and several cardiovascular diseases such as myocardial infarction, myocarditis, pericarditis, myocardial fibrosis, and inherited defects (Yu et al., 2021; Van Mieghem et al., 2004). Hence, cardiac changes related to lung disease can affect ECG findings. Some patients with chronic lung disease or pulmonary thromboembolism show abnormal ECG (Pinsky, 2018; Van Mieghem et al., 2004). Several studies have shown ECG abnormalities in end-stage heart failure and confirmed ECG changes after heart transplantation, and these ECG changes are clinically helpful (Hashim et al., 2022; Babuty et al., 1993). Moreover, electrolyte imbalances also affect ECG findings. In patients with end-stage kidney disease with electrolyte abnormalities, ECG changes are observed after kidney transplantation (Johri et al., 2009; Lee et al., 2021).
The ECG may also change in patients with end-stage lung disease who undergo LTx. Some studies reported arrhythmias after LTx (Orrego et al., 2014; Kim et al., 2020), and one study reported that bilateral LTx reduced heart rate variability and complexity of cardiac autonomic modulation (Tobaldini et al., 2021). However, data on changes in individual ECG parameters such as PR interval, QT interval, and QTc interval after LTx are scarce. Therefore, in this study, we aimed to investigate ECG changes before and after LTx in end-stage lung disease patients and whether these changes had clinical significance. We hypothesized that there are cardiac changes after LTx, and these changes can be reflected in the ECG.
This was a single-center retrospective cohort study of patients with end-stage lung disease who underwent LTx at Severance Hospital in South Korea between October 2012 and August 2020. Among the 294 patients identified, 14 were excluded due to young age (<19 years, n = 4), re-transplantation (n = 5), and multi-organ transplantation (n = 5). Finally, 280 patients were evaluated (Figure 1). To find clinical meaning, the ECG findings at 1 month after LTx was analyzed according to 1-year survival; the patients were accordingly divided into the survivor and non-survivor groups.
All LTx procedures were performed under intraoperative extracorporeal membrane oxygenation or cardiopulmonary bypass, and all patients received induction immunosuppression with methylprednisolone 250 mg intraoperatively and 0.5 mg/kg/day for 3 days postoperatively. After LTx, most patients received a triple immunosuppression regimen comprising prednisolone, tacrolimus, and mycophenolate mofetil. Prophylaxis against cytomegalovirus and invasive aspergillosis was provided until 6 months after LTx using valganciclovir and voriconazole or itraconazole. Life-long prophylaxis for Pneumocystis jirovecii pneumonia was provided using trimethoprim/sulfamethoxazole.
Patient data were obtained from the electronic medical records of the hospital. Clinicodemographic data including age, sex, cause of LTx, presence of hypertension and diabetes mellitus, and survival or death were evaluated.
The LTx protocol includes hospitalization for routine check-ups at 1, 3, 6, and 12 months after surgery, and ECGs were taken at these times. Therefore, ECG findings before LTx and within 1 week after LTx and at 1, 3, and 6 months after LTx were obtained.
All patients underwent a regular 12-lead ECG check-up and 2-dimensional transthoracic echocardiography (TTE) before and after LTx. Data on the following ECG parameters were obtained: PR interval; QRS duration; QT interval; QTc interval; and P, R, and T axes. The PR interval extended from the beginning of the P wave until the beginning of the QRS complex. The QRS duration was defined as the time from the first downward deflection after the P wave to the second downward deflection after the R wave. The QT interval was measured from the onset of the QRS complex to end of the T wave that returned to the TP baseline. Every QT interval was corrected for the patient heart rate using Bazett’s formula: QTc = QT√RR (in ms) (Page et al., 2016). The normal range of the QTc interval was as 350–450 m for males and 360–460 m for females (Rezuş et al., 2015). On TTE, we acquired the ejection fraction (measured by M-mode), right ventricular systolic pressure (RVSP), tissue Doppler imaging (TDI), peak systolic velocities of tricuspid annulus derived from TDI (TDIS), left ventricle end diastolic dimension (LVEDD), left ventricle end-systolic dimension (LVESD), (measured by Modified Simpson’s method), and E/E′ ratio (E: mitral peak velocity of early filling, E’: early diastolic mitral annular velocity).
This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4–2022–0122) and was conducted according to the principles set forth in the Declaration of Helsinki. The requirement for obtaining informed consent was waived owing to the retrospective nature of the study.
Continuous variables did not satisfy normality, so they were analyzed using the Mann-Whitney U test and expressed as medians with interquartile ranges (IQRs). Meanwhile, categorical variables are expressed as numbers with corresponding percentages and were analyzed using chi-squared or Fisher’s exact tests. Owing to non-normal distribution of the data, ECG findings before and after LTx were analyzed using Wilcoxon signed rank test. Serial ECG findings at different time points were compared between the two groups using generalized estimating equation (GEE) to estimate the parameters of a generalized linear model with a possible unmeasured correlation. Survival data were estimated using the Kaplan–Meier method, and significant differences between the two groups were determined using the log-rank test. All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY), and p < 0.05 was set to indicate statistical significance.
The median patient age was 57 (IQR: 48.3–63) years, and 64.3% (n = 180) of the patients were male. Idiopathic pulmonary fibrosis (n = 151, 53.9%) was a major cause of LTx, and almost all patients (n = 269, 96.1%) underwent bilateral LTx. In the preoperative TTE, the mean right ventricular systolic pressure was 48.5 (IQR, 38–79.9) mmHg. For the preoperative ECG, 37.5% (n = 105) of the patients showed abnormal QTc interval. Table 1 shows the other baseline patient characteristics.
Table 2 shows the ECG findings before LTx and at 1, 3, and 6 months after LTx. The PR interval, QRS duration, QT interval, and QTc interval were significantly different before LTx and 1 month after LTx (all p < 0.001). The PR interval, QRS duration, QTc interval, and heart rate were all decreased after LTx than before LTx. The QTc interval was significantly decreased 1 month after LTx, although no significant change was observed from 1 month to 6 months thereafter. Figure 2 shows the trend in QTc interval after LTx; the QTc interval was consistently reduced after LTx (p < 0.001). Additionally, we conducted further analysis to explore potential differences based on the type of terminal lung disease and the type of lung transplantation (bilateral vs single). There were no significant differences in the trend of QTc interval changes after LTx between idiopathic pulmonary fibrosis and other lung diseases (p = 0.514). Furthermore, we found no differences in the trend of QTc changes after LTx between bilateral and single lung transplantation (p = 0.445).
Among the 259 patients who survived at 1 month and underwent ECG, 166 (64.1%) showed decreased QTc interval. Although the QTc interval of the 93 patients (35.9%) was increased, the interval was within the normal range in 47 patients. At 1 month after LTx, 71 patients (27.4% 71/259) showed abnormal QTc interval. Although the P- and R-axes were not significantly different before and after LTx, the T-axis was significantly increased after LTx (p < 0.001). There was no increase in the prevalence of atrial fibrillation during the follow-up period. In the TTE findings before and after LTx, there were no significant differences in EF, TDIS, LVEDD, or LVESD. However, RVSP significantly decreased from a median of 54 (40–66) before LTx to a median of 31.5 (26–43) after LTx (p < 0.001).
The survivor group was significantly younger (55 years vs. 62 years, p < 0.001) and had significantly lower BMI (20.7 kg/m2 vs. 21.3 kg/m2, p = 0.045) than the non-survivor group. For ECG findings, the PR interval, QT interval, QTc interval, and heart rate were significantly different between the two groups; the QTc interval was significantly higher in the survivor group (437 m vs 421 m, p = 0.005). However, the proportion of patients with abnormal QTc interval was not significantly different between the two groups (29.2% vs 23.5%, p = 0.209). There were also no significant differences in TTE parameters at the preoperative period and 1 month after LTx between survivor and non survivors (Table 3). Additionally, Cox proportional hazards regression analysis, including variables such as age, sex, BMI and ECG parameters, was conducted to gain a deeper understanding of the associations between ECG changes and outcomes. In the multivariate analysis, age, QT interval, and QTc interval from the 1-month ECG after LTx were significantly associated with 1-year mortality (Table 4).
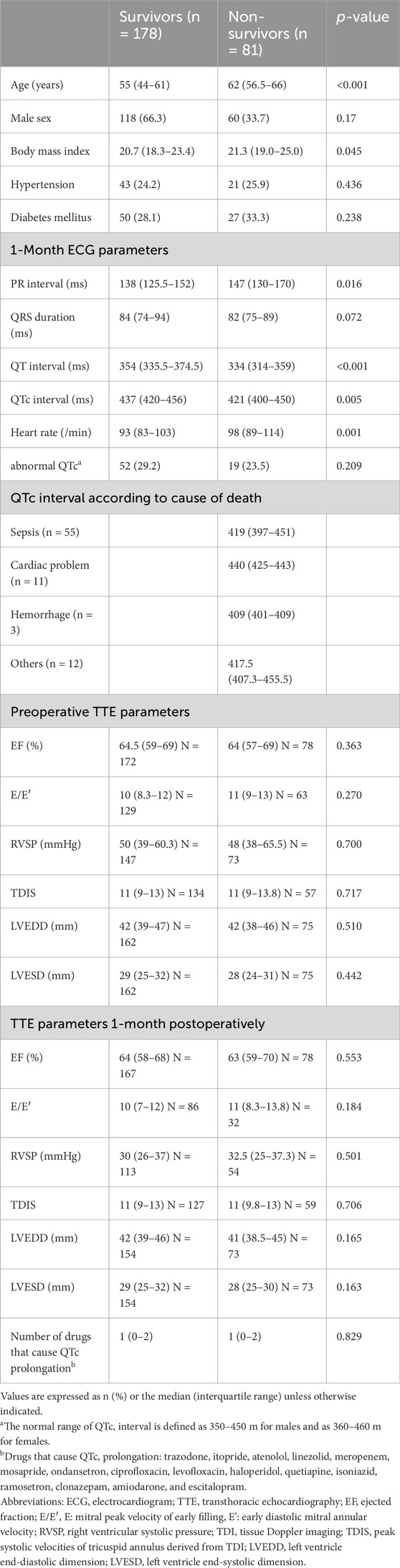
Table 3. Comparison of patient characteristics according to survival at 1 year after lung transplantation.
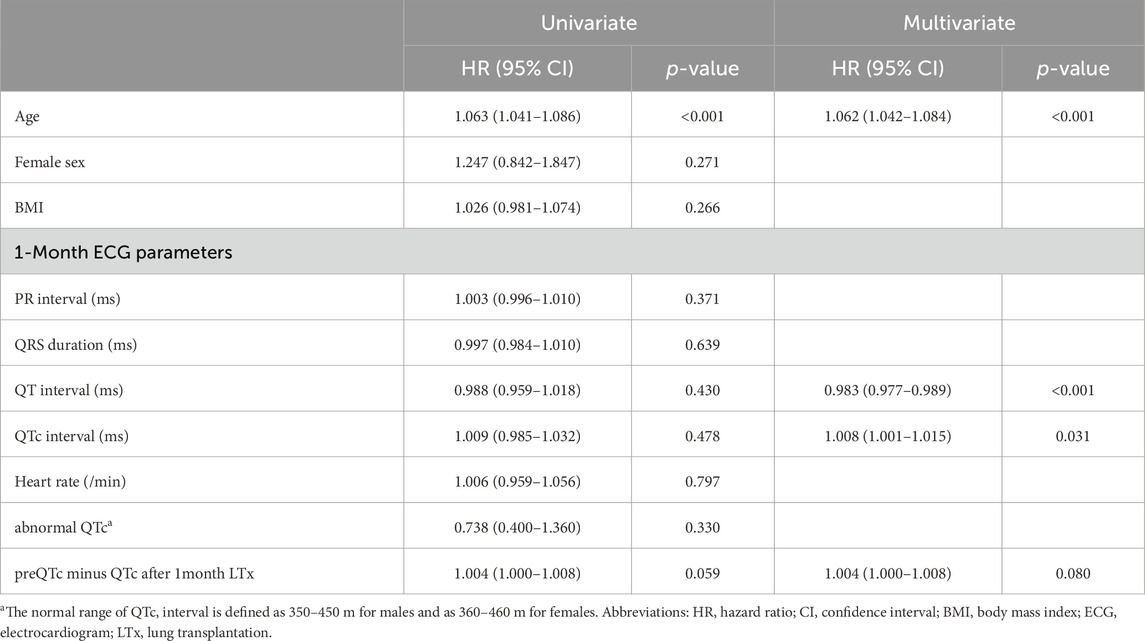
Table 4. Cox proportional hazards model of the 1-month ECG after lung transplantation and 1-year mortality.
As shown in Figure 3A, the serial change in QTc interval before LTx and 1 month after LTx was significantly different between the survivor and non-survivor groups after adjusting for age and BMI (p = 0.033). The serial changes in QTc interval before LTx and at 1 and 3 months after LTx was also significantly different between the two groups after adjusting for age and BMI (p = 0.040, Figure 3B). The non-survivor group showed a more dynamic change in QTc than the survivor group. The number of drugs causing QTc prolongation such as trazodone, itopride, atenolol, linezolid, meropenem, mosapride, ondansetron, ciprofloxacin, levofloxacin, haloperidol, quetiapine, isoniazid, ramosetron, clonazepam, amiodarone, and escitalopram at 1 month after LTx were not significantly different between the two groups.
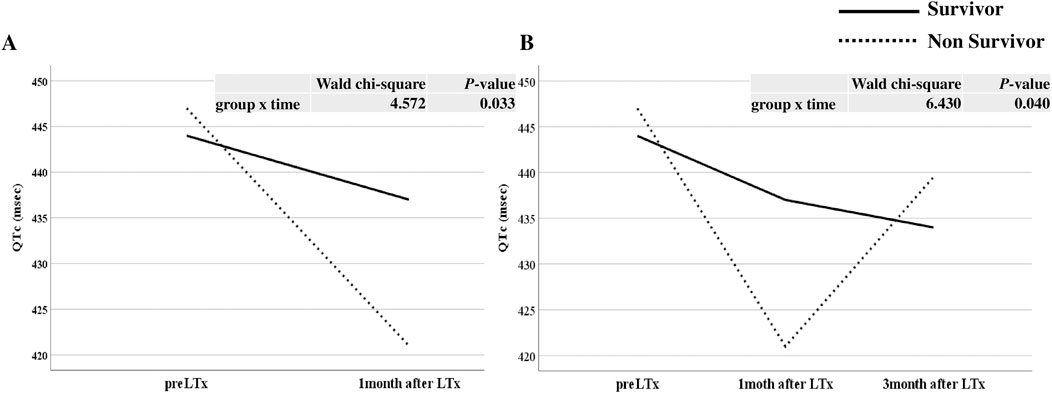
Figure 3. The serial change in QTc interval before and after lung transplantation (LTx) between survivor and non survivor. (A) Comparison of changes before LTx and one month after LTx, (B) Comparison of changes before LTx, one month after LTx, and three months after LTx.
Given the findings mentioned earlier, additional analysis was conducted to determine whether the change in the range of QTc interval after LTx was associated with prognosis. The median difference in QTc interval before LTx and 1 month after LTx was 10 (IQR, -8–35 m). There was a significant difference in the change of QTc interval before LTx and 1 month after LTx between survivors and non-survivors (6.5 [-11–29]ms vs 18 [0.5–46]ms, p = 0.022, Figure 4). The patients were then divided into four groups according to the interquartile range of QTc interval change (≤-8 ms, -8–10 m, 10–35 m, >35 m). Kaplan–Meier survival analysis showed that the survival rate was significantly lower in the group whose QTc interval at 1 month after LTx was decreased by > 35 m (p = 0.019, Figure 5). Table 5 shows the comparison of characteristics between patients whose QTc interval before LTx was changed by ≤ 35 m and >35 m 1 month after LTx. The group with a >35 m change had a significantly higher 1-year mortality rate (27.3% vs 43.1%, p = 0.021). Regarding the cause of death, the rate of sepsis was also significantly higher in this group (17.5% vs 32.3%, p = 0.022). There was no significant difference in other variables such as age, sex, and comorbidities.
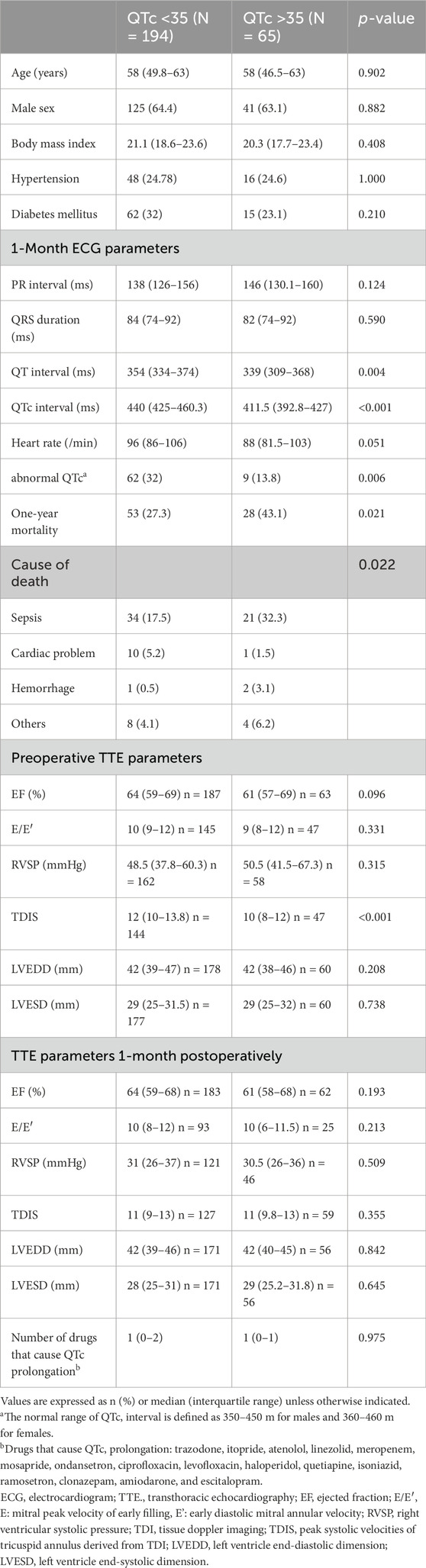
Table 5. Comparison of patient characteristics according to change in QTc interval from pre-to post-lung transplantation.
In this study, we investigated ECG changes before and after LTx in end-stage lung disease patients and whether these changes had clinical significance. The current study found that ECG parameters were changed after LTx in end-stage lung disease patients. The QTc interval decreased 1 month after LTx and remained lower than that before LTx. Furthermore, the change in QTc interval was associated with prognosis after LTx. Patients whose QTc interval was decreased by > 35 m at 1 month after LTx showed poorer prognosis than those whose QTc interval decreased by ≤ 35 m at 1 month.
The heart and lungs are closely connected; therefore, in patients with chronic lung disease, anatomical remodeling in the heart occurs (Panagiotou et al., 2017). This remodeling can be diagnosed through anatomical alterations that may present as hypertrophy or enlargement of the right ventricle or can be confirmed through hypertrophy of the left ventricle or enlargement of the atrium (Azevedo et al., 2016). However, our data showed that RVSP was significantly reduced but there were no differences in cardiac ejection fraction, left ventricular size, or right ventricular function.
The occurrence of these anatomical changes in the heart depends on the type of lung diseases that cause hemodynamic changes and varies depending on the duration of the disease (Han et al., 2007). Therefore, predicting the severity or duration of lung disease based on these anatomical changes of the heart is challenging. Our analysis showed that electrophysiological changes on the ECG might precede these anatomical changes. The T axis is related to ventricular repolarization (Kors et al., 1998), and the current study reports significant changes in the T axis on ECG 1 month after LTx. The QTc interval is related to repolarization of the cardiac muscle cells (Sarazan, 2014). In the current study, all QRS durations on the ECG were within the normal range of ≤120 m. While the QTc interval was maintained within the normal range, the change was sensitive to changes before and after LTx. The QTc interval tended to temporarily increase immediately after LTx but then decrease 1 month after LTx and remained stable thereafter. However, patients with large changes before and after surgery (>35 m) had poor prognosis after LTx.
There are two crucial points to consider regarding our results. The first pertains to the electrical remodeling of the heart, indicated by the QTc interval, which sensitively mirrors hemodynamic changes due to chronic lung disease and transplantation. As previously mentioned, anatomical changes in the heart resulting from chronic lung disease necessitate considerable time and energy; thus, these changes are not as responsive as electrical remodeling. Conversely, notable alterations in the electrical remodeling of the heart signify a substantial impact of transplantation on chronic lung disease. The patients in this study showed a decreased in QTc interval after LTx, which may reflect the cardiac remodeling caused by the lung disease prior to transplantation, potentially influencing the postoperative prognosis. The second point of consideration is that the primary cause of patient mortality was sepsis rather than deteriorating cardiovascular disease. Sepsis-induced alterations in blood oxygen saturation and ion concentrations (Na, K, Ca, and Mg) may have impacted the electrical remodeling, including repolarization of the heart muscle cells. In general, the QTc interval exhibits wide variations not only because of changes in blood ion concentration but also owing to the administration of antibiotics and other medications. Consequently, we conducted an analysis of the use of antibiotics and psychiatric drugs known to influence the QTc interval, and confirmed no significant difference between the survivor and non-survivor groups (Table 3). Electrical remodeling of the heart, represented by the QTc interval, sensitively reflects the hemodynamic changes resulting from chronic lung disease. In the current study, no association between change of QTc interval and cardiac disease-related mortality was observed. The cardiac-related mortality rate was 18%, and over 50% of the patients died during the early period after LTx. This result supports that a large change in QTc interval after LTx may indicate vulnerability to sepsis through an indirect correlation with electrical remodeling of the heart rather than direct cardiac problems. Significant QTc interval changes after LTx may be indicative of the disease severity before transplantation. Furthermore, by representing sepsis-induced alterations in ion concentration after transplantation, electrical alteration is presumably a more rapid marker. Although, the observed increase in mortality does not necessarily indicate that patients with prolonged QTc interval are inherently more susceptible to sepsis. However, changes in QTc on an ECG may serve as subtle markers of early septic conditions. Therefore, further research with stronger controls and prospective study designs is needed to clarify the underlying mechanisms.
To our best knowledge, this is the first study to analyze changes in individual ECG parameters including PR interval, QRS duration, QT interval, QTc interval, and axis after LTx. However, this study is limited by its retrospective single-center design and the insufficient follow-up period, which do not allow for the examination of long-term changes. The results may not be generalizable to other countries due to the study being conducted at a single center and because all enrolled patients are Asian. Furthermore, other factors that may influence ECG changes including post-operative course and several drugs that were not adjusted for, may have been overlooked. Prospective studies in which several factors that can affect the ECG are well controlled are needed to validate our findings. In addition, future studies with larger and more diverse encompassing a wide range of lung diseases, will build upon this knowledge.
This study found that ECG findings may change after LTx in patients with end-stage lung disease. Particularly, a QTc interval decreased by > 35 m at 1 month after LTx is associated with a significantly higher 1-year mortality rate. Thus, these ECG changes may have clinical and prognostic significance.
The datasets presented in this article are not readily available because Limited due to IRB policy. Data requests require re-review by the IRB. Requests to access the datasets should be directed to SL, aGlob2dvZ29AeXVocy5hYw==.
The studies involving humans were approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2022-0122). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participant’s legal guardians/next of kin because to the retrospective nature of the study.
AL: Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. HY: Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. MS: Data curation, Formal Analysis, Investigation, Software, Writing–review and editing. KC: Data curation, Formal Analysis, Investigation, Supervision, Writing–review and editing. YeK: Formal Analysis, Investigation, Methodology, Software, Writing–review and editing. AW: Data curation, Formal Analysis, Investigation, Writing–review and editing. SK: Data curation, Formal Analysis, Investigation, Writing–review and editing. MP: Data curation, Formal Analysis, Investigation, Writing–review and editing. YoK: Data curation, Formal Analysis, Investigation, Visualization, Writing–review and editing. YY: Data curation, Formal Analysis, Investigation, Writing–review and editing. HK: Data curation, Formal Analysis, Investigation, Writing–review and editing. JL: Data curation, Formal Analysis, Investigation, Writing–review and editing. KsK: Formal Analysis, Investigation, Software, Writing–review and editing. KbK: Formal Analysis, Investigation, Resources, Software, Writing–review and editing. BJ: Formal Analysis, Supervision, Writing–review and editing. JP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing. SL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Korea Medical Device Development Fund awarded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety) (Project Number: RS-2020-KD000032), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00252863), the Ministry of Science, ICT and Future Planning (NRF-2022R1A2C1093352) and Institute of Information and communications Technology Planning and Evaluation (IITP) grant funded by the Korea government (MSIT) (No. RS-2022-00155966, Artificial Intelligence Convergence Innovation Human Resources Development (Ewha Womans University).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ECG, electrocardiogram; IQRs, interquartile ranges; LTx, lung transplantation; TTE, transthoracic echocardiography.
Azevedo P. S., Polegato B. F., Minicucci M. F., Paiva S. A., Zornoff L. A. (2016). Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 106 (1), 62–69. doi:10.5935/abc.20160005
Babuty D., Neville P., Aupart M., Rouchet S., Marchand M., Fauchier J. P., et al. (1993). Electrophysiological properties of the transplanted heart. Clinical applications. Arch. Mal. Coeur Vaiss. 86 (7), 1053–1060.
Han M. K., McLaughlin V. V., Criner G. J., Martinez F. J. (2007). Pulmonary diseases and the heart. Circulation 116 (25), 2992–3005. doi:10.1161/circulationaha.106.685206
Harrigan R. A., Jones K. (2002). ABC of clinical electrocardiography. Conditions affecting the right side of the heart. Bmj 324 (7347), 1201–1204. doi:10.1136/bmj.324.7347.1201
Hashim H. T., Ramadhan M. A., Ahmad S., Shah J., Varney J., Motawea K. R., et al. (2022). The role of the electrocardiogram in the recognition of cardiac transplant rejection: a systematic review and meta-analysis. Clin. Cardiol. 45 (3), 258–264. doi:10.1002/clc.23783
Johri A. M., Baranchuk A., Simpson C. S., Abdollah H., Redfearn D. P. (2009). ECG manifestations of multiple electrolyte imbalance: peaked T wave to P wave (“tee-pee sign”). Ann. Noninvasive Electrocardiol. 14 (2), 211–214. doi:10.1111/j.1542-474X.2009.00283.x
Kim B. G., Uhm J. S., Yang P. S., Yu H. T., Kim T. H., Joung B., et al. (2020). Clinical significance of postoperative atrial arrhythmias in patients who underwent lung transplantation. Korean J. Intern Med. 35 (4), 897–905. doi:10.3904/kjim.2018.326
Kors J. A., de Bruyne M. C., Hoes A. W., van Herpen G., Hofman A., van Bemmel J. H., et al. (1998). T axis as an indicator of risk of cardiac events in elderly people. Lancet 352 (9128), 601–605. doi:10.1016/s0140-6736(97)10190-8
Lalande S., Luoma C. E., Miller A. D., Johnson B. D. (2012). Effect of changes in intrathoracic pressure on cardiac function at rest and during moderate exercise in health and heart failure. Exp. Physiol. 97 (2), 248–256. doi:10.1113/expphysiol.2011.061945
Lee H. J., Choe A. R., Lee H., Ryu D. R., Kang E. W., Park J. T., et al. (2021). Clinical associations between serial electrocardiography measurements and sudden cardiac death in patients with end-stage renal disease undergoing hemodialysis. J. Clin. Med. 10 (9), 1933. doi:10.3390/jcm10091933
Orrego C. M., Cordero-Reyes A. M., Estep J. D., Seethamraju H., Scheinin S., Loebe M., et al. (2014). Atrial arrhythmias after lung transplant: underlying mechanisms, risk factors, and prognosis. J. Heart Lung Transpl. 33 (7), 734–740. doi:10.1016/j.healun.2014.02.032
Page R. L., Joglar J. A., Caldwell M. A., Calkins H., Conti J. B., Deal B. J., et al. (2016). 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 67 (13), e27–e115. doi:10.1016/j.jacc.2015.08.856
Panagiotou M., Church A. C., Johnson M. R., Peacock A. J. (2017). Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur. Respir. Rev. 26 (143), 160053. doi:10.1183/16000617.0053-2016
Pinsky M. R. (2018). Cardiopulmonary interactions: physiologic basis and clinical applications. Ann. Am. Thorac. Soc. 15 (Suppl. 1), S45-S48–s8. doi:10.1513/AnnalsATS.201704-339FR
Rezuş C., Moga V. D., Ouatu A., Floria M. (2015). QT interval variations and mortality risk: is there any relationship? Anatol. J. Cardiol. 15 (3), 255–258. doi:10.5152/akd.2015.5875
Sarazan R. D. (2014). “The QT interval of the electrocardiogram,” in Encyclopedia of toxicology. Third Edition (Oxford: Academic Press), 10–15.
Stracina T., Ronzhina M., Redina R., Novakova M. (2022). Golden standard or obsolete method? Review of ECG applications in clinical and experimental context. Front. Physiol. 13, 867033. doi:10.3389/fphys.2022.867033
Tobaldini E., Rodrigues G. D., Mantoan G., Monti A., Zelati G. C., Furlan L., et al. (2021). Effects of bilateral lung transplantation on cardiac autonomic modulation and cardiorespiratory coupling: a prospective study. Respir. Res. 22 (1), 156. doi:10.1186/s12931-021-01752-6
Van Mieghem C., Sabbe M., Knockaert D. (2004). The clinical value of the ECG in noncardiac conditions. Chest 125 (4), 1561–1576. doi:10.1378/chest.125.4.1561
Keywords: electrocardiogram, end-stage lung disease, lung transplantation, risk factor, prognosis
Citation: Leem AY, Yu HT, Sung M, Chung KS, Kim Y, Woo A, Kim SY, Park MS, Kim YS, Yang YH, Kim HE, Lee JG, Kim K, Kim KB, Joung B, Park J and Lee SH (2024) Clinical implication of electrocardiogram change in patients experiencing lung transplantation with end stage lung disease. Front. Physiol. 15:1440307. doi: 10.3389/fphys.2024.1440307
Received: 08 June 2024; Accepted: 14 October 2024;
Published: 29 October 2024.
Edited by:
Patricia R. M. Rocco, Federal University of Rio de Janeiro, BrazilReviewed by:
Eduardo Butturini De Carvalho, University of Vassouras, BrazilCopyright © 2024 Leem, Yu, Sung, Chung, Kim, Woo, Kim, Park, Kim, Yang, Kim, Lee, Kim, Kim, Joung, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junbeom Park, cGFya2piQGV3aGEuYWMua3I=; Su Hwan Lee, aGlob2dvZ29AeXVocy5hYw==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
§ORCID: Ah Young Leem, orcid.org/0000-0001-5165-3704; MinDong Sung, orcid.org/0000-0002-5217-8877; Kyung Soo Chung, orcid.org/0000-0003-1604-8730; Ala Woo, orcid.org/0000-0002-8818-6115; Song Yee Kim, orcid.org/0000-0001-8627-486X; Moo Suk Park, orcid.org/0000-0003-0820-7615; Young Sam Kim, orcid.org/0000-0001-9656-8482; Young Ho Yang, orcid.org/0000-0002-0977-0525; Su Hwan Lee, orcid.org/0000-0002-3487-2574
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.