- 1School of Exercise Science and Health, Capital University of Physical Education and Sports, Beijing, China
- 2School of Arts and Sports, Dong-A University, Busan, Republic of Korea
Objective: Research evidence suggests that exercise is a potent therapeutic strategy for non-alcoholic fatty liver disease (NAFLD). Many investigations have delved into the curative potential of diverse exercise regimens on NAFLD. This investigation synthesizes findings from randomized controlled trials via a network meta-analysis to evaluate the efficacy of exercise-based interventions on NAFLD.
Methods: We conducted a search across five electronic databases (Web of Science, EMBASE, PubMed, SCOPUS, and CNKI)to identify randomized controlled trials (RCTs) comparing the effects of different exercise modalities on metabolic profiles and liver functions in patients with NAFLD. The literature search was comprehensive up to 15, December 2023. The selected studies were subjected to a rigorous quality appraisal and risk of bias analysis in accordance with the Cochrane Handbook’s guidelines, version 5.1.0. We employed Stata/MP 17 for the network meta-analysis, presenting effect sizes as standardized mean differences (SMD).
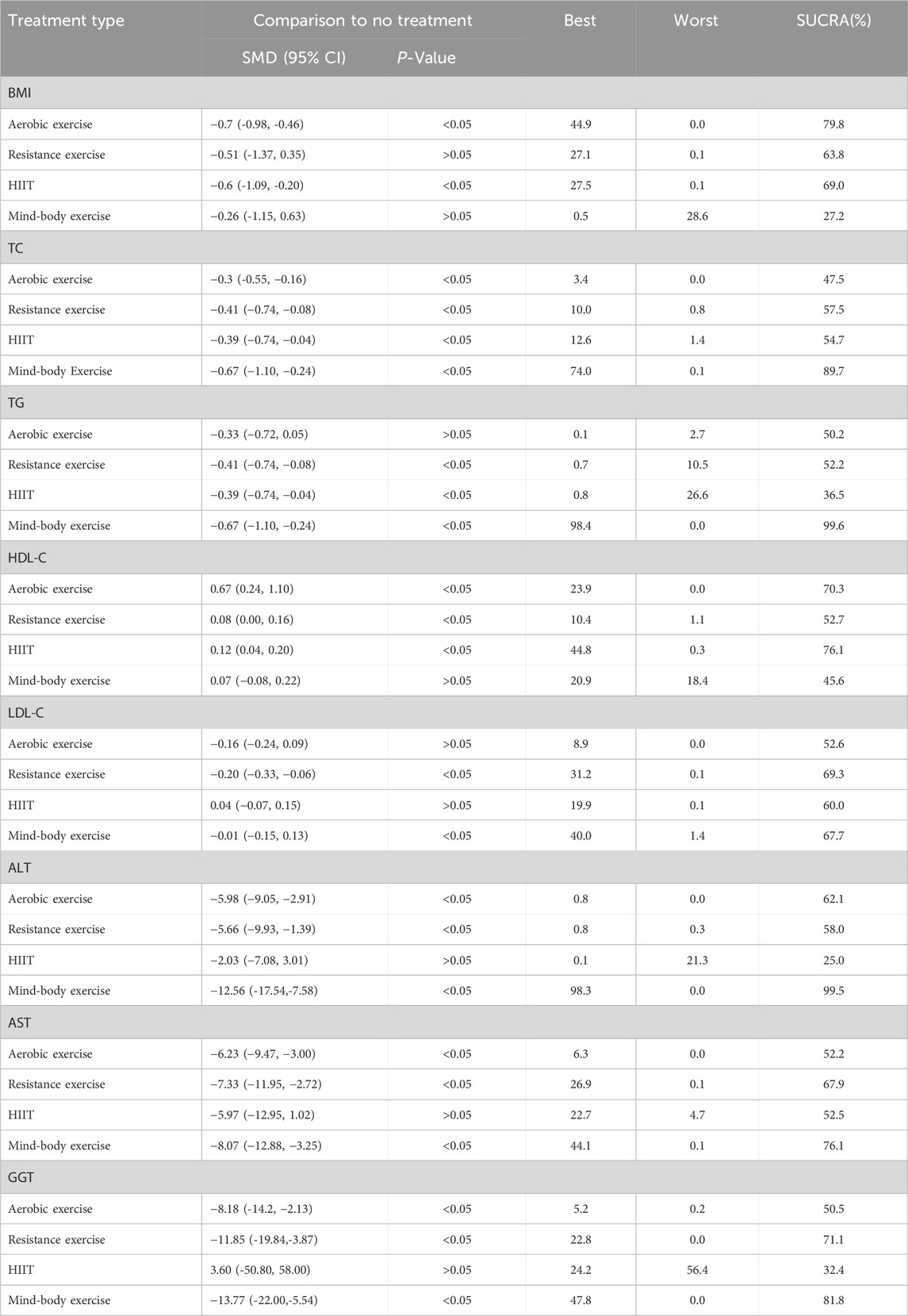
Results: This study aggregated results from 28 studies, involving a total of 1,606 participants. The network meta-analysis revealed that aerobic exercise was the most effective intervention for improving BMI in patients with NAFLD, demonstrating a significant decrease in BMI (−0.72, 95%CI: −0.98 to −0.46; p < 0.05; Surface Under the Cumulative Ranking (SUCRA) = 79.8%). HIIT was the top intervention for enhancing HDL-C (0.12, 95% CI: 0.04 to 0.20; p < 0.05; SUCRA = 76.1%). Resistance exercise was the most effective for reducing LDL-C (−0.20, 95% CI: −0.33 to −0.06; p < 0.05; SUCRA = 69.7%). Mind-body exercise showed superior effectiveness in improving TC (−0.67, 95% CI: −1.10 to −0.24; p < 0.05; SUCRA = 89.7%), TG = −0.67, 95% CI: −1.10 to −0.24; p < 0.05; SUCRA = 99.6%), AST (−8.07, 95% CI: −12.88 to −3.25; p < 0.05; SUCRA = 76.1%), ALT (−12.56, 95% CI: −17.54 to −7.58; p < 0.05; SUCRA = 99.5%), and GGT (−13.77, 95% CI: −22.00 to −5.54; p < 0.05; SUCRA = 81.8%).
Conclusion: This network meta-analysis demonstrates that exercise interventions positively affect various metabolic profiles and liver functions in NAFLD patients. Mind-body exercises are particularly effective, surpassing other exercise forms in improving metabolic profiles and liver functions.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier registration number CRD42024526332.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of hepatic dysfunctions primarily driven by metabolic dysregulation (Powell et al., 2021). Intrinsically linked to obesity, metabolic syndromes, type 2 diabetes, and insulin resistance, NAFLD’s prevalence is notably increasing in parallel with obesity rates, currently estimated at 25% among adults (Younossi et al., 2018). Notably, a study projected an increase in NAFLD incidence by 13.5%–29.5% by 2030 across diverse national contexts including the United States, the United Kingdom, China, and Italy (Estes et al., 2018).
Sedentary lifestyles and poor dietary habits augment the potential risk of NAFLD, prompting researchers to explore interventions targeting modifiable factors. Lifestyle modifications have emerged as efficacious strategies in early NAFLD management (Ding et al., 2017), and adopting a Mediterranean dietary pattern shown to significantly reduce hepatic fat in overweight NAFLD patients (Trovato et al., 2015). Furthermore, prospective research indicates associations between sedentary behavior changes and alterations in waist circumference and cardiovascular metabolic risk scores (Healy et al., 2008), thus underscoring the importance of considering sedentary status alteration and increased physical activity as avenues of investigation. Exercise has been found to mitigate insulin resistance, type 2 diabetes, and obesity (Snowling and Hopkins, 2006; Romero-Gómez et al., 2017), exhibiting favorable effects on transaminase levels and dyslipidemia (Li and Huang, 2021bib_li_and_huang_2021). Even without weight loss, consistent exercise regimens have been shown to reduce hepatic fat by 20%–30% (Hashida et al., 2017). However, despite numerous clinical interventions and randomized controlled trials targeting NAFLD, the differential impacts of various exercise modalities (aerobic, resistance, high-intensity interval training (HIIT), and mind-body exercise) remain inconclusive.
In recent years, numerous studies have focused on the impact of various forms of exercise on NAFLD (Nam et al., 2023; Hejazi and Hackett, 2023; Chun, 2023). Research has highlighted the effects of aerobic exercise and resistance training in improving liver health and overall metabolic function in NAFLD patients. Nam et al. (2023), in a systematic review of 11 studies, explored the effects of different exercise modalities on intrahepatic lipid (IHL), alanine aminotransferase (ALT), body mass index (BMI), and insulin resistance (IR) in patients with NAFLD. Their findings indicate that aerobic exercise can significantly improve IHL and ALT levels. However, a critical review of these studies reveals a predominant focus on aerobic and resistance exercises, with a noticeable lack of comprehensive examination of other exercise modalities. Furthermore, these studies often combined the effects of different exercise interventions without directly comparing their efficacy. This indicates a gap in the literature, underscoring the need for more detailed comparative studies on various exercise forms in the management of NAFLD.
Liver function and lipid biomarkers serve as common indicators for evaluating the severity of NAFLD and associated metabolic abnormalities (Wong et al., 2018). Within lipid metabolism indicators, total cholesterol (TC), triacylglycerol (TG), and low-density lipoprotein cholesterol (LDL-C) are commonly utilized to assess the severity of NAFLD and its close association with metabolic abnormalities (Wong et al., 2018; Vilar-Gomez and Chalasani, 2018). Low levels of high-density lipoprotein cholesterol (HDL-C) are frequently observed in lipid metabolism disorders (Fan et al., 2011) and are closely linked to the progression of NAFLD (Feng et al., 2020; Mato et al., 2019). Aspartate aminotransferase (AST) and ALT serve as sensitive indicators of hepatic cell damage (Senior, 2012), while sustained elevation of gamma-glutamyl transferase (GGT) may indicate progression to chronic hepatitis and is an important marker for identifying NAFLD (Kunutsor, 2016; Ha et al., 2022). BMI is considered the most commonly used measure for defining obesity and overweight populations across various genders and age groups (Daniels, 2009). Due to the close relationship between obesity and NAFLD, there are studies suggesting that BMI might be an important method for identifying NAFLD (Camhi et al., 2011). In summary, in our study, we selected eight outcome indicators closely related to NAFLD and aimed to provide relatively effective exercise modalities for adult NAFLD patients through network meta-analysis.
2 Methods
This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2010), with the research protocol prospectively registered in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/, CRD42024526332).
2.1 Search strategy
The literature search was conducted using Web of Science, EMBASE, PubMed, SCOPUS, and China National Knowledge Infrastructure (CNKI) databases up to December 15, 2023, to identify randomized controlled trials. Keywords such as “exercise,” “obesity,” “lifestyle,” “physical activity,” “aerobic,” “liver,” and “NAFLD” were combined using the “OR” operator. Specific search strategies included.
• Web of Science: TS=(“exercise” OR “physical activity” OR “aerobic” OR “lifestyle”) AND TS=(“obesity” OR “NAFLD” OR “liver”)
• EMBASE: “exercise”/exp OR “physical activity”/exp OR “aerobic exercise”/exp OR “lifestyle”/exp AND “obesity”/exp OR “nonalcoholic fatty liver disease”/exp OR “liver disease”/exp
• PubMed: (“exercise” [MeSH Terms] OR “physical activity” [MeSH Terms] OR “aerobic exercise” [MeSH Terms] OR “lifestyle” [MeSH Terms]) AND (“obesity” [MeSH Terms] OR “NAFLD” [MeSH Terms] OR “liver” [MeSH Terms])
• SCOPUS: TITLE-ABS-KEY (“exercise” OR “physical activity” OR “aerobic” OR “lifestyle”) AND TITLE-ABS-KEY (“obesity” OR “NAFLD” OR “liver”)
• CNKI: TS=(“exercise” OR “physical activity” OR “aerobic” OR “lifestyle”) AND TS=(“obesity” OR “NAFLD” OR “liver”)
Detailed search terms are documented in Supplementary Material S1.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (1) Randomized controlled trials (RCTs) that investigate the use of exercise therapy in patients with NAFLD; (2) Subjects diagnosed with NAFLD via histopathological or imaging examinations; (3) No significant differences in baseline values of outcome indicators before intervention in patients; (4) Outcome indicators include BMI, TG, TC, LDL-C, HDL-C, ALT, AST, and GGT; (5) The type of intervention must be one of the following: aerobic exercise, resistance exercise, HIIT, or mind-body exercise; (6) The exercise intervention period must last at least 4 weeks.
Exclusion criteria: (1) Specific data on outcome indicators (such as AST, ALT, TC, TG, BMI, etc.) are unavailable. (2) Excluded studies include animal experiments, abstracts, case reports, reviews, systematic evaluations, and duplicate publications. (3) Studies where the type, duration, and frequency of exercise are unclear. (4) Studies focusing only on lifestyle changes without specific exercise regimens.
2.3 Data extraction and quality assessment
The data extraction process included relevant information from the literature, such as the title, authors, publication year, journal, number of patients, age, gender, intervention measures, and outcome indicators. The intervention measures encompassed four types of exercise modalities: aerobic, resistance, HIIT, and mind-body exercise. The aerobic exercise included brisk walking, cycling, jogging, and swimming, while mind-body exercise involved yoga, tai chi, baduanjin, and Wushu qigong. Definitions for each intervention modality are provided in Supplementary Material S2. Outcome indicators included BMI, lipid metabolism indicators, and liver function markers. Lipid metabolism indicators comprised TC, HDL-C, TG, and LDL-C. Liver function markers included GGT, AST, and ALT. Extracted data were presented as mean ± standard deviation (SD) before and after the intervention. Data presented as median and quartiles were converted to mean ± SD.
We conducted a comprehensive assessment of potential biases in the included studies using the risk of bias assessment tool recommended in the Cochrane Handbook 5.1.0 (Higgins et al., 2011). Data extraction and risk assessment were independently performed by two individuals. To maintain objectivity, any discrepancies during the screening process were resolved through discussion with a third researcher.
2.4 Statistical analysis
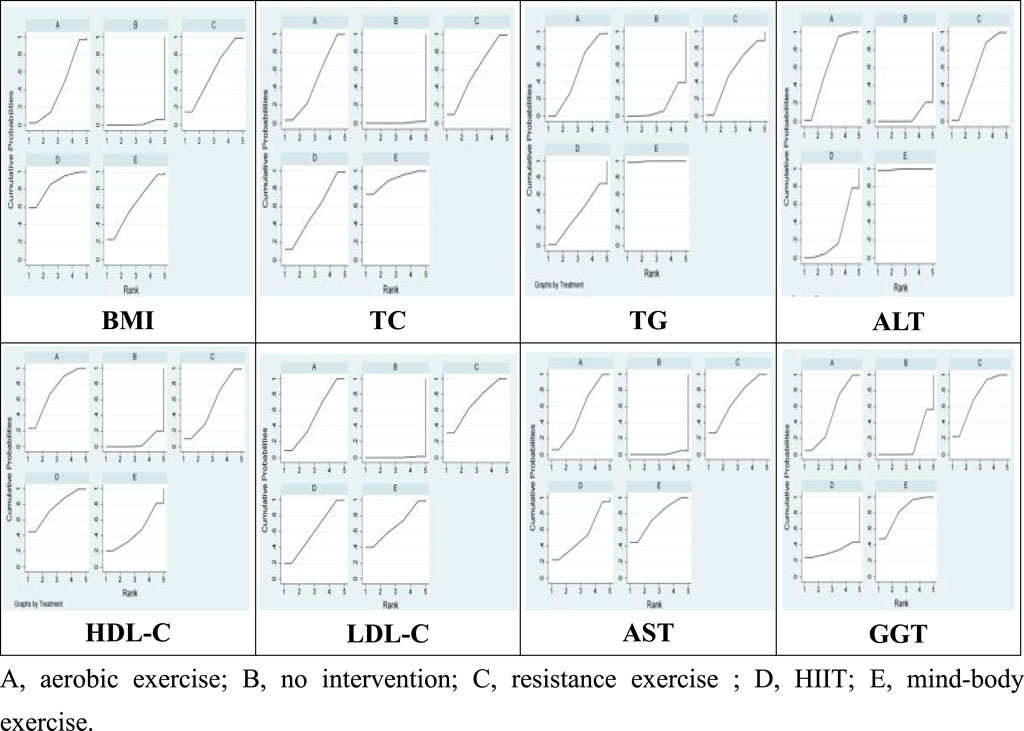
A frequency-based network meta-analysis of outcome indicators was conducted using Stata 17.0 software. For continuous variables, the mean difference (MD) was estimated via network meta-analysis. Given the consistency of outcome indicator units, MD and SD were used to calculate indicators. All meta-analysis results are thoroughly detailed in the results section. Network plots for various outcome indicators were constructed using the “network” command, where nodes represent different intervention methods. Node size correlates with the sample size of each treatment method, with larger nodes denoting greater sample sizes. The thickness of the lines between nodes indicates the number of studies, whereas thicker lines signify more studies. The SUCRA was used to determine the probability ranking of different exercise patterns as the optimal intervention method. A higher SUCRA value indicates a higher probability that a specific intervention will be the most effective (Rücker and Schwarzer, 2015).
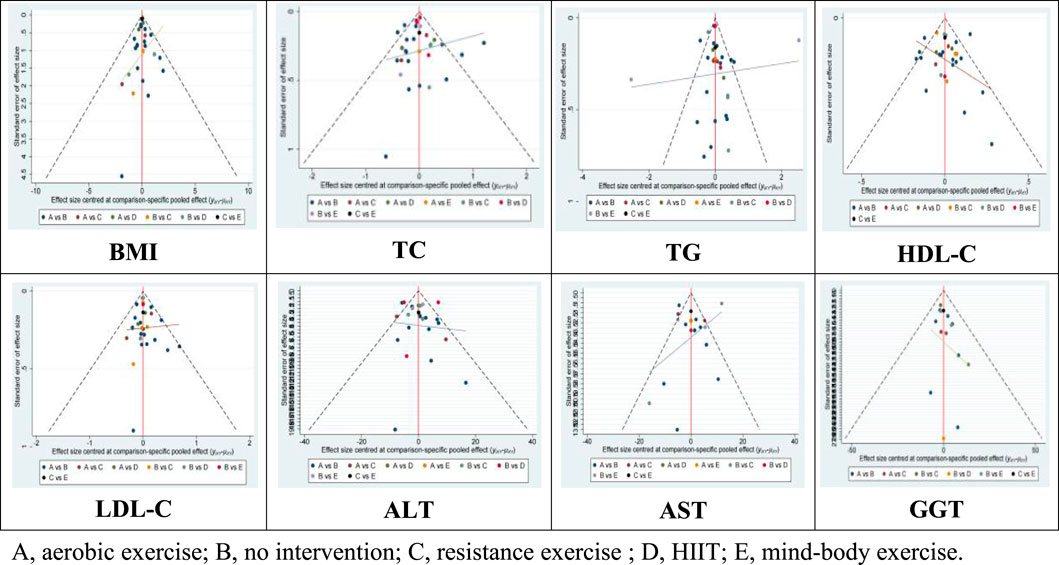
Comparative funnel plots were used to assess publication bias. In cases where closed loops were present, inconsistency tests were conducted. When the inconsistency tests yielded p < 0.05, significant inconsistencies between direct and indirect comparison results were noted. Two levels of inconsistency assessment were conducted: global and local. Overall inconsistency was evaluated first, followed by node-splitting methods to assess local inconsistencies. The global I2 statistic was used to evaluate heterogeneity; values exceeding 75% indicated substantial heterogeneity. A random-effects network meta-analysis model was used in cases of high heterogeneity, while a fixed-effect model was employed for lower heterogeneity.
3 Results
3.1 Search results
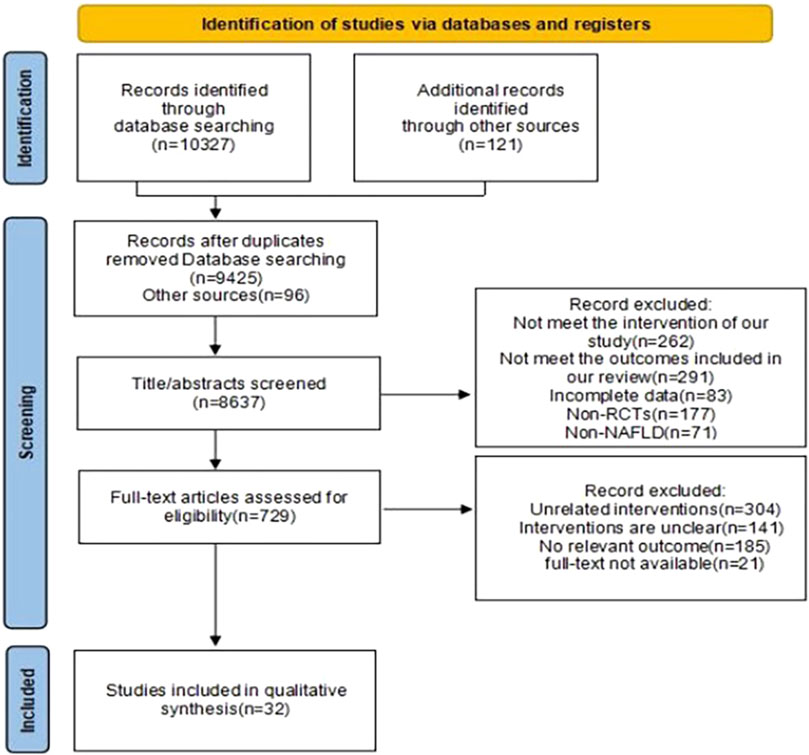
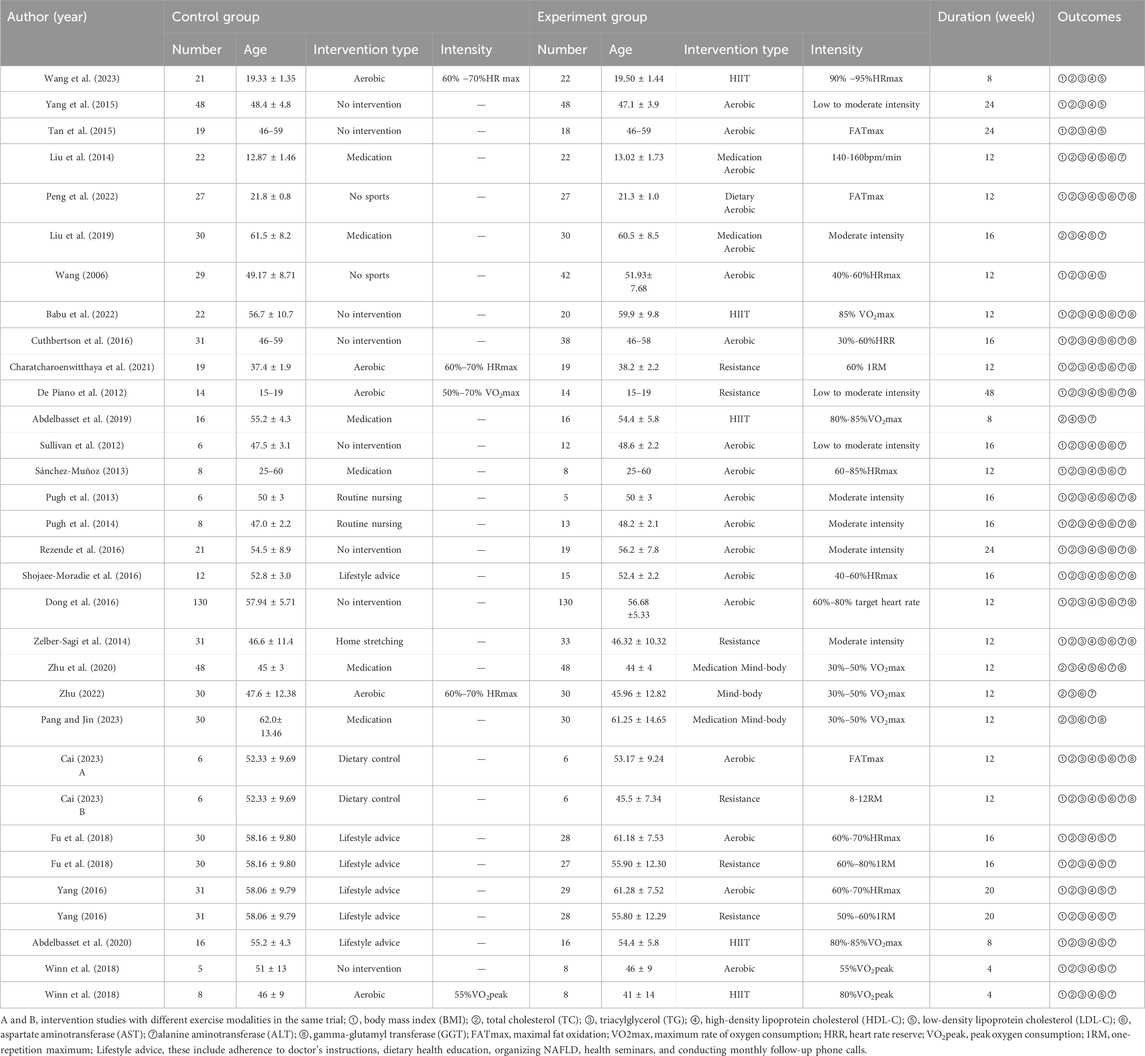
Our search identified 10,448 articles. Through processes including duplicate removal, abstract reviews, and full-text examinations, we excluded studies that failed to meet our inclusion criteria. Ultimately, 28 studies fulfilling the eligibility requirements were selected for analysis, The specific retrieval process is depicted in Figure 1. Of these, four studies compared two distinct exercise interventions (Cai, 2023; Fu et al., 2018; Yang, 2016; Winn et al., 2018). These studies were grouped into two separate groups, A and B, resulting in a total of 32 interventions, though the number of articles analyzed remained at 28. The included studies comprised 23 on aerobic exercise, six on resistance exercise, five on HIIT, and three on mind-body exercises. This collection included two studies comparing aerobic to resistance exercises, two comparing aerobic to HIIT, and one comparing aerobic to mind-body exercises. In total, 1,606 participants were involved in the included trials, with only two studies having a duration of less than 8 weeks. The basic characteristics of the included studies are presented in Table 1.
3.2 Risk of bias
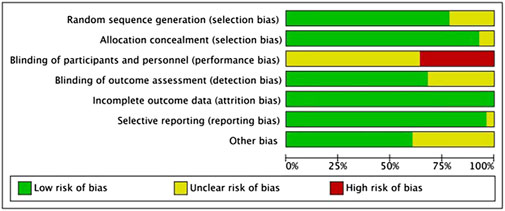
This investigation incorporated 28 RCTs. Among these, 22 studies clearly described the methods used for generating random sequences, and two studies implemented appropriate allocation concealment. On participants and personnel blinding, considering the nature and type of interventions, blinding of participants and controllers was challenging; however, an overall assessment indicated a generally low risk of bias (refer to Figure 2). Risk of bias in individual studies in Supplementary Material S3.
3.3 Network meta-analysis
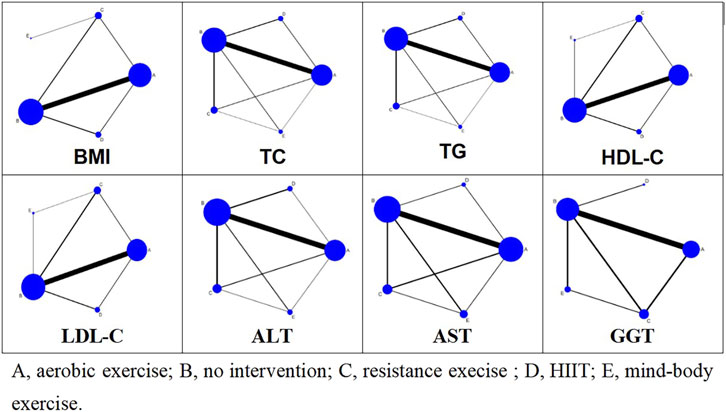
Figure 3 illustrates the network relationships among four types of exercise interventions and a control intervention. In the diagram, “A” represents aerobic exercise, “B” denotes no intervention, “C” signifies resistance exercise, “D” stands for HIIT, and “E” indicates mind-body exercise. Each intervention type is represented by a node, with lines connecting these nodes to depict direct comparisons made in studies. The absence of a line between any two nodes suggests no direct comparative research exists for these specific interventions within the reviewed literature. The size of each node reflects the number of studies associated with each intervention type, with larger nodes indicating a greater number of studies. Similarly, the thickness of the lines between two nodes correlates with the number of studies directly comparing these interventions.
3.3.1 Effects of exercise interventions on BMI
28 studies were included in this meta-analysis to investigate the effects of various interventions on the BMI of patients diagnosed with NAFLD. The findings from pairwise meta-analyses indicated that, compared to the control group, aerobic exercise (−0.72, 95% CI: −0.98 to −0.46; p < 0.05), HIIT (−0.65, 95% CI: −1.09 to −0.20; p < 0.05) were associated with significant improvements in BMI among NAFLD patients’ post-intervention. However, the effect of mind-body exercise (−0.26, 95% CI: −1.15 to 0.63; p > 0.05), and resistance exercise (−0.51, 95% CI: −1.37 to 0.35; p > 0.05) on BMI was not statistically significant. Furthermore, the SUCRA analysis, based on the consistency model, revealed that aerobic exercise SUCRA scores of 79.8, and HIIT SUCRA scores of 69.0 exhibited superior efficacy in improving BMI among NAFLD patients, while mind-body exercise SUCRA scores of 27.2, and resistance exercise SUCRA scores of 63.8 ranked the low in terms of effectiveness Table 2 and Figure 4). These results suggest that aerobic exercise may be more beneficial for managing BMI in NAFLD patients compared to mind-body exercise.
Moreover, the Wald test for inconsistency in the network did not yield significant results, indicating the absence of inconsistency among the included studies. Additionally, funnel plots did not reveal any evident bias (Figure 5).
3.3.2 The intervention effect of exercise lipid metabolism in NAFLD
Lipid metabolism markers, including TC, TG, HDL-C, and LDL-in patients with NAFLD are important for assessing treatment outcomes. There are 32 studies assessing the impact of various exercise modalities on TC levels in NAFLD patients, 31 studies on TG, 30 on HDL-C, and 30 on LDL-C. Compared to the control, aerobic exercise, HIIT, resistance, and mind-body exercises significantly improve TC, TG, HDL-C, and LDL-C levels. Notably, mind-body exercises demonstrate the most substantial effect on TC (−0.67; 95% CI: −1.10 to −0.24; p < 0.05) and TG (−0.67; 95% CI: −1.10 to −0.24; p < 0.05), achieving the highest SUCRA scores of 89.7 for TC and 99.6 for TG. HIIT is most effective in enhancing HDL-C levels (0.12; 95% CI: 0.04 to 0.20; p < 0.05), with a SUCRA score of 76.1. Resistance training shows significant improvements in LDL-C (−0.20; 95% CI: −0.33 to −0.06; p < 0.05), with a SUCRA score of 69.3 (Table 2 and Figure 4). The Wald test for network inconsistency yielded no significant results, and funnel plots exhibited no discernible biases (Figure 5).
3.3.3 Effects of exercise modalities on liver function in NAFLD
AST, ALT, and GGT are important biochemical markers for liver function tests and also serve as physiological bases for diagnosing NAFLD. Our review encompassed 20 studies on AST, 28 on ALT, and 15 on GGT. Among the different exercise modalities, mind-body exercises were found to significantly reduce AST (−8.07; 95% CI: −12.88 to −3.25; p < 0.05), ALT (−12.56; 95% CI: −17.54 to −7.58; p < 0.05), and GGT levels (−13.77; 95% CI: −22.00 to −5.54; p < 0.05). These interventions scored SUCRA values of 76.1 for AST, 99.5 for ALT, and 81.8 for GGT (Table 2 and Figure 4). Similar to the lipid metabolism studies, the Wald tests for inconsistency across the network of liver enzyme studies were not significant, and the funnel plots exhibited no discernible biases (Figure 5).
4 Discussion
This network meta-analysis assessed the efficacy of four distinct exercise modalities—aerobic, resistance, mind-body, and HIIT—on BMI in patients with NAFLD, alongside four lipid metabolism indicators (TC, TG, HDL-C, and LDL-C) and three liver function parameters (AST, ALT, and GGT). The comprehensive analysis substantiated the beneficial outcomes of these exercise forms in managing NAFLD, highlighting aerobic exercise as the most effective for BMI reduction. Resistance training showed superior results in LDL-C improvement, HIIT was most beneficial for HDL-C enhancement, and mind-body exercises showed significant efficacy in improving TC, TG, and liver function (AST, ALT, GGT). Existing literature has a varied evidence base, thereby advocating a cautious treatment of differing study designs and results in meta-analyses.
BMI, a crucial indicator associated with obesity and NAFLD (Camhi et al., 2011), was most effectively reduced through aerobic exercise in this analysis. The prolonged duration and moderate intensity of aerobic exercise might explain its efficacy in BMI reduction among NAFLD patients (Gao et al., 2021). Although high-intensity regimens have also been shown to decrease BMI (Zhu et al., 2020), aerobic exercise remains the focus of most research concerning NAFLD interventions. Moreover, aerobic exercise has demonstrated superior effects in ameliorating BMI and other NAFLD-related metrics such as glycated hemoglobin, resting blood pressure, and serum cholesterol levels (Kelley and Kelley, 2008). It also increases serum high-molecular-weight adiponectin, which influences insulin resistance and subsequently NAFLD (Haus et al., 2013). Moreover, compared to resistance exercise, aerobic exercise has a greater effect on reducing intrahepatic lipid content in patients with NAFLD (Orci et al., 2016; Slentz et al., 2011). Therefore, this study suggests that patients with NAFLD use aerobic exercise as a means of reducing BMI.
Our research indicates that resistance exercise is particularly effective in improving LDL-C levels. The likely mechanism underlying this benefit involves the enhancement of LDL receptor activity on hepatocyte membranes. This improvement in receptor activity increases the blood’s capacity to transport LDL-C, thereby lowering its concentration in the bloodstream (Chen et al., 2005). Furthermore, resistance exercise may facilitate the secretion of cytokines, known as myokines, from skeletal muscles. These myokines interact with various organs to mediate metabolic processes (Benatti and Pedersen, 2015; Karstoft and Pedersen, 2016). Notably, the secretion of myokine Irisin during resistance exercise is associated with the induction of browning in subcutaneous fat cells, which enhances thermogenesis and energy expenditure (Boström et al., 2012). Kim et al. (2016) observed a significant increase in circulating Irisin levels during resistance exercises, with negligible changes in aerobic exercises, highlighting Irisin’s potential role in improving NAFLD outcomes. Additionally, exercise may enhance insulin sensitivity and upregulate LDL-C mRNA expression, thereby accelerating LDL-C metabolism (Young and Stout, 1987). In line with previous findings, regular exercise has been shown to improve LDL-C markers in patients with NAFLD (Fu et al., 2018).
HIIT, which alternates between high-intensity exercise and lower-intensity recovery periods. Our findings indicate that HIIT is most effective in improving HDL-C levels. Previous studies have identified exercise intensity as a significant factor in the variations observed in HDL-C levels (Gao et al., 2021). Notably, studies reporting significant differences in HDL-C levels between control and experimental groups also observed higher exercise intensities (Wang et al., 2020). Despite these benefits, we recommend that individuals select an exercise regimen that is manageable over the long term. HIIT, while beneficial, may not be suitable for all populations, particularly the elderly, due to its demanding nature. This study has yielded a novel finding: mind-body exercises demonstrate superior efficacy in improving liver function markers (AST, ALT, GGT) and certain lipid metabolism indicators (TC, TG) compared to three other forms of exercise.
Most of the Mind-body exercises belong to low-intensity aerobic exercises such as yoga, tai chi, and baduanjin. In addition to its ability to increase energy expenditure and promote fat metabolism, a unique aspect of mind-body exercise is that it focuses on the unity of mind and body integrating physical movement with psychological processes. Exercise has been shown to reduce levels of TC and TG (Yu et al., 2017; Costa et al., 2020), yet the underlying mechanisms remain unclear. Some studies suggest that exercise increases the activity of lipolytic enzymes and accelerates the rate of breakdown into mitochondrial energy supply (Bianchi et al., 2021), while others associate it with adipocyte factors such as leptin and adiponectin (Izadi et al., 2013). However, the means by which exercise improves adipose factors are not yet clear, necessitating further research for confirmation. AST, ALT, and GGT are critical markers for identifying liver function and are closely related to NAFLD. Studies reporting improvements in ALT, AST, and GGT through exercise align with our findings (Mascaró et al., 2022; Gao et al., 2021).
In addition to exercise interventions, other methods such as dietary management and pharmacological treatments are effective strategies for managing NAFLD. Controlling intake is a crucial aspect of dietary intervention. When the body consumes excessive amounts of saturated and unsaturated fatty acids, there is a significant impact on hepatic fat accumulation (Rosqvist et al., 2014). However, there remains ongoing debate regarding optimal dietary composition and patterns for NAFLD management (El-agroudy et al., 2019; Moore et al., 2020). Pharmacological treatments predominantly target disease-related pathways, including pathogenic factors and associated metabolic disorders (Rong et al., 2023). Due to the complex pathogenesis of NAFLD, specific pharmacological treatments are still lacking (Rong et al., 2023; Romero-Gómez et al., 2017). Compared to these interventions, exercise appears more favorable, as most RCTs have demonstrated that exercise confers significant benefits for NAFLD (Nassir, 2022). Studies have shown that exercise reduces hepatic steatosis, improves metabolic function, and ameliorates fibrosis (Huber et al., 2019; O’Gorman et al., 2020). Various studies have explored the underlying mechanisms of exercise in NAFLD management. Some research suggests that exercise regulates hepatic fatty acid synthesis and oxidation, as well as mitochondrial structure, thereby preventing liver damage (Oh et al., 2021; Rector et al., 2011; Thyfault and Rector, 2020). Additionally, exercise has been found to improve hepatic inflammation, partly by increasing sirtuin (SIRT) activity (Bianchi et al., 2021). Furthermore, exercise enhances peripheral insulin sensitivity, which in turn reduces hepatic lipogenesis (Cuthbertson et al., 2016). Beyond these physiological mechanisms, the psychological benefits of exercise have also been explored. Physical and mental exercises, such as tai chi and Yoga, can reduce anxiety, depression, and stress (Dong et al., 2024). Consequently, some studies suggest that such exercises may improve NAFLD progression by alleviating stress and anxiety, improving insulin sensitivity and hepatic fat accumulation (Shomaker et al., 2010), and regulating hormone levels, including reducing cortisol (Targher et al., 2006). However, in clinical practice, NAFLD patients often demonstrate low readiness for behavioral change and may lack motivation to adopt healthier lifestyles (Centis et al., 2013). Moreover, long and demanding exercise regimens may pose adherence challenges for these patients.
In summary, the reason mind-body exercises excel in improving liver function and lipid profile markers in NAFLD may be due to their comprehensive action on both the physical and psychological levels, promoting health through various mechanisms including but not limited to stress management, psychological health improvement, endocrine and metabolic function regulation, as well as enhanced antioxidative and anti-inflammatory effects. This suggests that a holistic treatment approach may be more effective than singular exercise modalities in managing metabolic diseases like NAFLD. Therefore, we recommend incorporating mind-body exercise as a viable exercise modality in the treatment of NAFLD, and can also be combined with diet as an adjunct to the treatment of NAFLD.
5 Limitations and future direction
There are several noteworthy limitations in this study. Firstly, the study compared different types of exercise without strict regulations regarding exercise volume, intensity, duration, and age. The inconsistency in the methods of measuring exercise intensity across the studies and the lack of standardization may introduce a certain degree of bias to the results. Furthermore, the study encompassed research that investigated the combined effects of medication and exercise interventions. This aspect raises the possibility of synergistic effects influencing the outcomes, thereby introducing an additional layer of complexity and potential bias in interpreting the results. The final limitation arises from the specific geographic region; for instance, the mind-body exercises included in this study all originate from Asia. This is mainly due to the fact that mind-body exercises originated in Asia. However, this inadvertently increases the potential influence of cultural, genetic, and environmental factors on exercise interventions. It is important to note that the prevalence of NAFLD also varies significantly across different geographic regions. In light of these considerations, it is essential to approach the study’s conclusions with a degree of caution. This is because they may pose challenges to the overall validity and reliability of the study’s results.
To mitigate biases, future investigations should establish more stringent requirements for various exercise types. Further research is also needed to determine whether these findings can be replicated in different racial populations to understand potential race-specific responses to different exercise modalities. Additionally, combining different exercise modalities or integrating them with pharmacological interventions could provide deeper insights into their effects on NAFLD patients. Finally, there is a need for more in-depth research and exploration of the potential mechanisms underlying the therapeutic effects of different exercise modalities on NAFLD.
6 Conclusion
Our network meta-analysis revealed that aerobic exercise significantly enhances BMI in individuals with NAFLD, resistance exercise was found to be particularly effective in improving LDL-C, whereas HIIT markedly boosts HDL-C. Additionally, mind-body exercises were superior in enhancing TC, TG, and key liver function parameters (AST, ALT, GGT). Based on these findings, it is recommended that NAFLD patients incorporate mind-body exercises into their treatment regimen to optimize health outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MH: Writing–review and editing, Writing–original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization. JY: Writing–review and editing, Writing–original draft, Software, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. YW: Writing–original draft, Visualization, Software, Project administration, Methodology, Investigation, Data curation. JW: Writing–review and editing, Writing–original draft, Validation, Supervision, Resources, Project administration, Investigation, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1428723/full#supplementary-material
References
Abdelbasset W. K., Tantawy S. A., Kamel D. M., Alqahtani B. A., Elnegamy T. E., Soliman G. S., et al. (2020). Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: a comparative randomized controlled trial. Medicine 99 (10), e19471. doi:10.1097/MD.0000000000019471
Abdelbasset W. K., Tantawy S. A., Kamel D. M., Alqahtani B. A., Soliman G. S. (2019). A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine 98 (12), e14918. doi:10.1097/MD.0000000000014918
Babu A. F., Csader S., Männistö V., Tauriainen M. M., Pentikäinen H., Savonen K., et al. (2022). Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci. Rep. 12 (1), 6485. doi:10.1038/s41598-022-10481-9
Benatti F. B., Pedersen B. K. (2015). Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat. Rev. Rheumatol. 11 (2), 86–97. doi:10.1038/nrrheum.2014.193
Bianchi A., Marchetti L., Hall Z., Lemos H., Vacca M., Paish H., et al. (2021). Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 206 (4), 904–916. doi:10.4049/jimmunol.2001022
Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., et al. (2012). A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481 (7382), 463–468. doi:10.1038/nature10777
Cai Y. L. (2023). A 12-week study of the effects of different modalities ofexercise intervention on patients with non-alcoholic fatty liver disease. Capital University of Physical Education and Sports. doi:10.27340/d.cnki.gstxy.2023.000157
Camhi S. M., Bray G. A., Bouchard C., Greenway F. L., Johnson W. D., Newton R. L., et al. (2011). The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity 19 (2), 402–408. doi:10.1038/oby.2010.248
Centis E., Moscatiello S., Bugianesi E., Bellentani S., Fracanzani A. L., Calugi S., et al. (2013). Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J. hepatology 58 (4), 771–777. doi:10.1016/j.jhep.2012.11.031
Charatcharoenwitthaya P., Kuljiratitikal K., Aksornchanya O., Chaiyasoot K., Bandidniyamanon W., Charatcharoenwitthaya N. (2021). Moderate-intensity aerobic vs resistance exercise and dietary modification in patients with nonalcoholic fatty liver disease: a randomized clinical trial. Clin. Transl. Gastroenterology 12 (3), e00316. doi:10.14309/ctg.0000000000000316
Chen W., Penumetcha M., Santanam N., Liu Y. G., Parthasarathy S. (2005). Exercise might favor reverse cholesterol transport and lipoprotein clearance: potential mechanism for its anti-atherosclerotic effects. Biochim. Biophys. Acta. 1723, 124–127. doi:10.1016/j.bbagen.2005.03.005
Chun H. S. (2023). Aerobic and resistance exercise: synergistic influence for nonalcoholic fatty liver disease. Gut Liver 17 (4), 485–486. doi:10.5009/gnl230236
Costa R. R., Barroso B. M., Reichert T., Vieira A. F., Kruel L. F. M. (2020). Effects of supervised exercise training on lipid profile of children and adolescents: systematic review, meta-analysis and meta-regression. Sci. and Sports 35 (6), 321–329. doi:10.1016/j.scispo.2020.02.007
Cuthbertson D. J., Shojaee-Moradie F., Sprung V. S., Jones H., Pugh C. J., Richardson P., et al. (2016). Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 130 (2), 93–104. doi:10.1042/CS20150447
Daniels S. R. (2009). The use of BMI in the clinical setting. Pediatrics 124 (Suppl. ment_1), S35–S41. doi:10.1542/peds.2008-3586F
De Piano A., de Mello M. T., Sanches P. D. L., da Silva P. L., Campos R. M., Carnier J., et al. (2012). Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur. J. gastroenterology and hepatology 24 (11), 1313–1324. doi:10.1097/MEG.0b013e32835793ac
Ding Z., Bu L., Lu H., et al. (2017). Correlation of liver fat content with serum vitamin A level and insulin resistance in patients with nonalcoholic fatty liver disease. J. Clin. Hepatology 33 (12), 2361–2365.
Dong F., Zhang Y., Huang Y., Wang Y., Zhang G., Hu X., Bao Z. (2016). Long-term lifestyle interventions in middle-aged and elderly men with nonalcoholic fatty liver disease: a randomized controlled trial. Scientific reports, 6(1), 36783.
Dong Y., Zhang X., Zhao R., Cao L., Kuang X., Yao J. (2024). The effects of mind-body exercise on anxiety and depression in older adults: a systematic review and network meta-analysis. Frontiers in psychiatry. 15, 1305295.
El-agroudy N. N., Kurzbach A., Rodionov R. N., O’Sullivan J., Roden M., Birkenfeld A. L., et al. (2019). Are lifestyle therapies effective for NAFLD treatment? Trends Endocrinol. Metab. 30, 701–709. doi:10.1016/j.tem.2019.07.013
Estes C., Anstee Q. M., Arias-Loste M. T., Bantel H., Bellentani S., Caballeria J., et al. (2018). Modeling nafld disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. hepatology 69 (4), 896–904. doi:10.1016/j.jhep.2018.05.036
Fan J. G., Jia J. D., Li Y. M., Wang B. Y., Lu L. G., Shi J. P.Chinese Association for the Study of Liver Disease, (2011). Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18:163-166). J. Dig. Dis. 12 (1), 38–44. (published in Chinese on Chinese Journal of Hepatology 2010; 18. doi:10.1111/j.1751-2980.2010.00476.x
Feng G., Feng L., Zhao Y. (2020). Association between ratio of γ-glutamyl transpeptidase to high-density lipoprotein cholesterol and prevalence of nonalcoholic fatty liver disease and metabolic syndrome: a cross-sectional study. Ann. Transl. Med. 8 (10), 634. doi:10.21037/atm-19-4516
Fu Y. Y., Meng M. M., Rong N., Liu L., Zhang J., Chen S. H., et al. (2018). Study on the effect of aerobic exercise and resistance exercise on patients with non-alcoholic fatty liver disease. J. Nanjing Med. Univ. Nat. Sci. Ed. (04), 528–531. doi:10.7655/NYDXBNS20180422
Gao Y., Lu J., Liu X., Liu J., Ma Q., Shi Y., et al. (2021). Effect of long-term exercise on liver lipid metabolism in Chinese patients with NAFLD: a systematic review and meta-analysis. Front. physiology 12, 748517. doi:10.3389/fphys.2021.748517
Ha Y., Chon Y. E., Kim M. N., Lee J. H., Hwang S. G. (2022). Gamma-glutamyl transpeptidase dynamics as a biomarker for advanced fibrosis in non-alcoholic fatty liver disease. J. Gastroenterology Hepatology 37 (8), 1624–1632. doi:10.1111/jgh.15871
Hashida R., Kawaguchi T., Bekki M., Omoto M., Matsuse H., Nago T., et al. (2017). Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J. hepatology 66 (1), 142–152. doi:10.1016/j.jhep.2016.08.023
Haus J. M., Solomon T. P., Kelly K. R., Fealy C. E., Kullman E. L., Scelsi A. R., et al. (2013). Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J. Clin. Endocrinol. and Metabolism 98 (7), E1181–E1188. doi:10.1210/jc.2013-1229
Healy G. N., Dunstan D. W., Salmon J., Cerin E., Shaw J. E., Zimmet P. Z., et al. (2008). Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes care 31 (4), 661–666. doi:10.2337/dc07-2046
Hejazi K., Hackett D. (2023). Effect of exercise on liver function and insulin resistance markers in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 12 (8), 3011. doi:10.3390/jcm12083011
Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Huber Y., Pfirrmann D., Gebhardt I., Labenz C., Gehrke N., Straub B. K., et al. (2019). Improvement of non-invasive markers of nafld from an individualised, web-based exercise program. Aliment. Pharmacol. Ther. 50 (8), 930–939. doi:10.1111/apt.15427
Izadi V., Farabad E., Azadbakht L. (2013). Epidemiologic evidence on serum adiponectin level and lipid profile. Int. J. Prev. Med. 4 (2), 133–140.
Karstoft K., Pedersen B. K. (2016). Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. and Metabolic Care 19 (4), 270–275. doi:10.1097/MCO.0000000000000283
Kelley G. A., Kelley K. S. (2008). Efficacy of aerobic exercise on coronary heart disease risk factors. Prev. Cardiol. 11 (2), 71–75. doi:10.1111/j.1751-7141.2008.08037.x
Kim H. J., Lee H. J., So B., Son J. S., Yoon D., Song W. (2016). Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiological Res. 65 (2), 271–279. doi:10.33549/physiolres.932997
Kunutsor S. K. (2016). Gamma-glutamyl transferase—friend or foe within? Liver Int. 36 (12), 1723–1734. doi:10.1111/liv.13221
Li Y., Huang X. (2021). Effects of exercise prescription combined with dietary intervention on body morphology and blood biochemical indexes in patients with nonalcoholic fatty liver disease. China Chronic Dis. Prev. Control 29 (2), 115–118. doi:10.16386/j.cjpccd.issn.1004-6194.2021.02.008
Liu D. Y., Zhang X. H., Liang G. Q., Wang J., Wang G. C. (2014). Experimental study on the intervention effect of traditional Chinese medicine combined with exercise therapy on non-alcoholic fatty liver in children. Chin. J. Sports Med. (05), 426–430. doi:10.16038/j.1000-6710.2014.05.004
Liu F., Chen L., Jiang Z., Shang J. (2019). Cognitive reappraisal in children: neuropsychological evidence of up-regulating positive emotion from an ERP study. Chin. Foreign Med. Res. 10 (10), 147–148. doi:10.3389/fpsyg.2019.00147
Mascaró C. M., Bouzas C., Montemayor S., Casares M., Gómez C., Ugarriza L., et al. (2022). Association between physical activity and non-alcoholic fatty liver disease in adults with metabolic syndrome: the FLIPAN study. Nutrients 14 (5), 1063. doi:10.3390/nu14051063
Mato J. M., Alonso C., Noureddin M., Lu S. C. (2019). Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease. World J. gastroenterology 25 (24), 3009–3020. doi:10.3748/wjg.v25.i24.3009
Moher D., Liberati A., Tetzlaff J., Altman D. G.Prisma Group (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8 (5), 336–341. doi:10.1016/j.ijsu.2010.02.007
Moore M. P., Cunningham R. P., Dashek R. J., Mucinski J. M., Rector R. S. (2020). A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity 28, 1843–1852. doi:10.1002/oby.22964
Nam H., Yoo J. J., Cho Y., Kang S. H., Ahn S. B., Lee H. W., et al. (2023). Effect of exercise-based interventions in nonalcoholic fatty liver disease: a systematic review with meta-analysis. Dig. Liver Dis. 55 (9), 1178–1186. doi:10.1016/j.dld.2022.12.013
Nassir F. (2022). NAFLD: mechanisms, treatments, and biomarkers. Biomolecules 12 (6), 824. doi:10.3390/biom12060824
O'Gorman P., Naimimohasses S., Monaghan A., Kennedy M., Melo A. M., Nf D., et al. (2020). Improvement in histological endpoints of mafld following a 12-week aerobic exercise intervention. Aliment. Pharmacol. Ther. 52 (8), 1387–1398. doi:10.1111/apt.15989
Oh S., Tsujimoto T., Kim B., Uchida F., Suzuki H., Iizumi S., et al. (2021). Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD. JHEP Rep. 3, 100253. doi:10.1016/j.jhepr.2021.100253
Orci L. A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. (2016). Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin. gastroenterology hepatology 14 (10), 1398–1411. doi:10.1016/j.cgh.2016.04.036
Pang L., Jin W. (2023). Clinical observation on baduanjin in the treatment of non-alcoholic fatty liver disease. Bright Chin. Med. (04), 673–676. doi:10.3969/j.issn.1003-8914.2023.04.022
Peng Y., Zhu H., Yang M., Zhou H., Liu X. L., Wang C. K. (2022). Effects of 12 weeks of FATmax intensity exercise on glycemic lipids and liver function in obese patients with nonalcoholic fatty liver disease. Genomics Appl. Biol. (03), 648–658. doi:10.13417/j.gab.041.000648
Powell E. E., Wong V. W. S., Rinella M. (2021). Non-alcoholic fatty liver disease. Lancet 397 (10290), 2212–2224. doi:10.1016/S0140-6736(20)32511-3
Pugh C. J., Cuthbertson D. J., Sprung V. S., Kemp G. J., Richardson P., Margot Umpleby A., et al. (2013). Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am. J. Physiology-Endocrinology Metabolism 305 (1), E50–E58. doi:10.1152/ajpendo.00055.2013
Pugh C. J., Sprung V. S., Kemp G. J., Richardson P., Shojaee-Moradie F., Umpleby A. M., et al. (2014). Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am. J. Physiology-Heart Circulatory Physiology 307 (9), H1298–H1306. doi:10.1152/ajpheart.00306.2014
Rector R. S., Uptergrove G. M., Morris E. M., Borengasser S. J., Laughlin M. H., Booth F. W., et al. (2011). Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G874–G883. doi:10.1152/ajpgi.00510.2010
Rezende R. E., Duarte S. M., Stefano J. T., Roschel H., Gualano B., de Sá Pinto A. L., et al. (2016). Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause 23 (8), 876–883. doi:10.1097/GME.0000000000000647
Romero-Gómez M., Zelber-Sagi S., Trenell M. (2017). Treatment of NAFLD with diet, physical activity and exercise. J. hepatology 67 (4), 829–846. doi:10.1016/j.jhep.2017.05.016
Rong L., Zou J., Ran W., Qi X., Chen Y., Cui H., et al. (2023). Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 13, 1087260. doi:10.3389/fendo.2022.1087260
Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H. E., Larsson A., et al. (2014). Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 63 (7), 2356–2368. doi:10.2337/db13-1622
Rücker G., Schwarzer G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15 (1), 58–59. doi:10.1186/s12874-015-0060-8
Sánchez-Muñoz V., Salas-Romero R., Del Villar-Morales A., Martínez-Coria E., Pegueros-Pérez A., Franco-Sánchez J. G. (2013). Decrease of liver fat content by aerobic exercise of metformin therapy in overweight or obese women. Rev. Investig. Clínica 65 (4), 307–317.
Senior J. R. (2012). Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin. Pharmacol. and Ther. 92 (3), 332–339. doi:10.1038/clpt.2012.108
Shojaee-Moradie F., Cuthbertson D. J., Barrett M., Jackson N. C., Herring R., Thomas E. L., et al. (2016). Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J. Clin. Endocrinol. and Metabolism 101 (11), 4219–4228. doi:10.1210/jc.2016-2353
Shomaker L. B., Tanofsky-Kraff M., Young-Hyman D., Han J. C., Yanoff L. B., Brady S. M., et al. (2010). Psychological symptoms and insulin sensitivity in adolescents. Pediatr. diabetes 11 (6), 417–423. doi:10.1111/j.1399-5448.2009.00606.x
Slentz C. A., Bateman L. A., Willis L. H., Shields A. T., Tanner C. J., Piner L. W., et al. (2011). Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am. J. Physiology-Endocrinology Metabolism 301 (5), E1033–E1039. doi:10.1152/ajpendo.00291.2011
Snowling N. J., Hopkins W. G. (2006). Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes care 29 (11), 2518–2527. doi:10.2337/dc06-1317
Sullivan S., Kirk E. P., Mittendorfer B., Patterson B. W., Klein S. (2012). Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 55 (6), 1738–1745. doi:10.1002/hep.25548
Tan S. J., Xu D. Q., Cao L. Q., Guo Z. (2015). FATmax exercise intervention for non-alcoholic fatty liver disease in middle-aged women. J. Tianjin Sports Inst. (03), 185–189. doi:10.13297/j.cnki.issn1005-0000.2015.03.001
Targher G., Bertolini L., Rodella S., Zoppini G., Zenari L., Falezza G. (2006). Associations between liver histology and cortisol secretion in subjects with nonalcoholic fatty liver disease. Clin. Endocrinol. 64 (3), 337–341. doi:10.1111/j.1365-2265.2006.02466.x
Thyfault J. P., Rector R. S. (2020). Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes 69, 517–524. doi:10.2337/dbi18-0043
Trovato F. M., Catalano D., Martines G. F., Pace P., Trovato G. M. (2015). Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin. Nutr. 34 (1), 86–88. doi:10.1016/j.clnu.2014.01.018
Vilar-Gomez E., Chalasani N. (2018). Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J. hepatology 68 (2), 305–315. doi:10.1016/j.jhep.2017.11.013
Wang P., Liu B. L., Liu Y., Jiang D. (2023). Effects of exercise combined with dietary intervention on body composition and lipid metabolism and intestinal flora in obese female college students with nonalcoholic fatty liver disease. Chin. Sch. Health (08), 1169–1173. doi:10.16835/j.cnki.1000-9817.2023.08.011
Wang S. T., Zheng J., Peng H. W., Cai X. L., Pan X. T., Li H. Q., et al. (2020). Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 20, 66–12. doi:10.1186/s12876-020-01204-3
Wang Y. (2006) “Research on the effect of aerobic exercise on the intervention of non-alcoholic fatty liver disease,” in Master's thesis. Beijing Sport University. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD2008&filename=2007225785.nh.
Winn N. C., Liu Y., Rector R. S., Parks E. J., Ibdah J. A., Kanaley J. A. (2018). Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—a randomized trial. Metabolism 78, 128–140. doi:10.1016/j.metabol.2017.08.012
Wong V. W. S., Adams L. A., de Lédinghen V., Wong G. L. H., Sookoian S. (2018). Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat. Rev. Gastroenterology and hepatology 15 (8), 461–478. doi:10.1038/s41575-018-0014-9
Yang S. N. (2016). Effect of different modalities of exercise on patients with non-alcoholic fatty liver. Nanjing University of Traditional Chinese Medicine. Available at: https://kns.cnki.net/kcms2/article/abstract?v=WSSiAUnZHh0FC7q8iz2D0J5DmTgAIX4gCbDKhcgVZ0R9kyskKNXixSW91vkhwjcsPpa_6LHkZDhu7RDpv_y2s2VZtgCygo-yVYtsvtflyFcNdoPF8xSUCE0vkJFdi7rummKHWNDAamogf1TD4s45NQ==uniplatform=NZKPTlanguage=CHS=.
Yang Y. H., Wang F. L., Mao J. J. (2015). A comprehensive investigation of the effectiveness of aerobic exercise in patients with non-alcoholic fatty liver disease. Mod. Pract. Med. (08), 1055–1057. doi:10.3969/j.issn.1671-0800.2015.08.045
Young I. R., Stout R. W. (1987). Effects of insulin and glucose on the cells of the arterial wall: interaction of insulin with dibutyryl cyclic AMP and low-density lipoprotein in arterial cells. Diabete and Metabolisme 13 (3 Pt 2), 301–306.
Younossi Z., Anstee Q. M., Marietti M., Hardy T., Henry L., Eslam M., et al. (2018). Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterology and hepatology 15 (1), 11–20. doi:10.1038/nrgastro.2017.109
Yu N., Ruan Y., Gao X., Sun J. (2017). Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Hormone Metabolic Res. 49 (03), 164–173. doi:10.1055/s-0042-121605
Zelber-Sagi S., Buch A., Yeshua H., Vaisman N., Webb M., Harari G., Shibolet O., et al. (2014). Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World journal of gastroenterology: WJG 20 (15), 4382–4392. doi:10.3748/wjg.v20.i15.4382
Zhu L. (2022). Effect of abdominal massage combined with wuqinxi exercise on Nonalcoholic fatty liver (Master's thesis, Guangxi University of Traditional Chinese Medicine). Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202301&filename=1022081578.nh.
Keywords: nonalcoholic fatty liver disease, exercise, network meta-analysis, aerobic, physical activity
Citation: Huang M, Yang J, Wang Y and Wu J (2024) Comparative efficacy of different exercise modalities on metabolic profiles and liver functions in non-alcoholic fatty liver disease: a network meta-analysis. Front. Physiol. 15:1428723. doi: 10.3389/fphys.2024.1428723
Received: 06 May 2024; Accepted: 28 August 2024;
Published: 11 September 2024.
Edited by:
Mallikarjuna Korivi, Zhejiang Normal University, ChinaReviewed by:
Chang-Mo Oh, Kyung Hee University, Republic of KoreaZbigniew Ossowski, Gdańsk University of Physical Education and Sport, Poland
Bing Bo, Henan University, China
Copyright © 2024 Huang, Yang, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, d3VqaWFuY3VwZXNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Mingming Huang
Mingming Huang Jiafa Yang
Jiafa Yang Yihao Wang1
Yihao Wang1