
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Physiol. , 10 September 2024
Sec. Red Blood Cell Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fphys.2024.1426505
This article is a commentary on:
COVID-19 impairs oxygen delivery by altering red blood cell hematological, hemorheological, and oxygen transport properties
A Commentary on
Covid-19 impairs oxygen delivery by altering red blood cell hematological, hemorheological, and oxygen transport properties
by Rogers SC, Brummet M, Safari Z, Wang Q, Rowden T, Boyer T and Doctor A (2024). Front. Physiol. 14:1320697. doi: 10.3389/fphys.2023.1320697
Recently, Rogers and colleagues (Rogers et al., 2023) published an article on the effects of COVID-19 on “Oxygen Delivery by Altering Red Blood Cell Hematological, Hemorheological, and Oxygen Transport Properties“ in this journal. Venous samples from hospitalized patients were compared to blood of healthy controls.
One part of their study was the measurement of oxygen affinity, characterized by the half saturation pressure (P50) and the slope n in the logarithmic Hill plot at standard conditions (pH 7.2, 7.4, 7.6, 37°C). These measurement revealed a tendency for slightly increased P50 and decreased n values in 11 COVID-19 patients relative to 14 controls at all pH values, yet apparently without reaching the level of significance (P50 at pH 7.2: 28.77 ± 1.87 vs. 29.83 ± 2.31 mmHg, p = 0.2198; pH 7.4: 23.06 ± 1.69 vs. 24.3 ± 2.25 mmHg, p = 0.124; pH 7.6: 18.69 ± 1.67 vs. 19.59 ± 1.72 mmHg, p = 0.1929, healthy control vs COVID-19, respectively). The authors therefore concluded that COVID-19 exerts no effect on hemoglobin oxygen affinity.
We have some concerns with this interpretation, which can be summarized as follows:
Statistics: First, the statistical analysis only applied individual t-tests for each pH value, rather than analyzing variance for the complete data set. Our analysis (data supplied by Rogers et al., method “GraphPad Prism 9.3.1. (built 471) for Windows 64 bit, GraphPad Software, San Diego, California, USA”) revealed a significant group-effect between healthy subjects and COVID-19 patients (p = 0.0114) leading to greater values of standard-P50 at all investigated pH-values among COVID-19 patients (See Table 1).
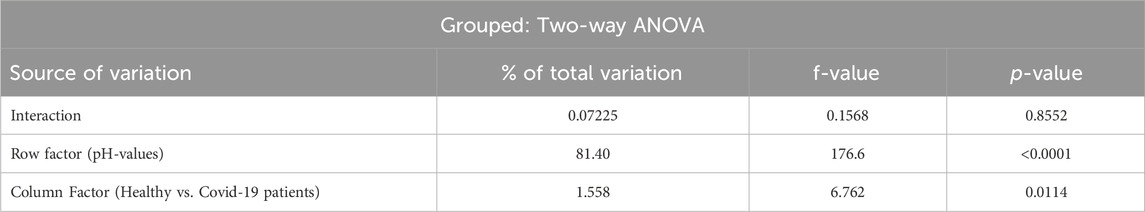
Table 1. Statistical analysis performed with the whole data set supplied by Rogers et al. through Grouped Two-way ANOVA.
As venous blood was investigated, this corresponds to the right shift in such samples described in our recent review in this journal (Böning et al., 2023) supporting oxygen delivery to tissue. The corresponding small decrease of Hill`s n is negligible.
Method: Second, the authors used the Hemox-Analyzer (Asakura, 1979; Guarnone et al., 1995) for registration of oxygen dissociation curves (ODC). In this apparatus venous blood was extremely diluted (120-fold) and analyzed under artificial conditions (no CO2, diluted in 3 mL of 50 mM BIS-Tris, and 100 mM NaCl, buffered to either pH 7.2, 7.4 or 7.6). Important extrinsic effects by substances like CO2, bicarbonate, or lactic acid directly affecting hemoglobin oxygen affinity, or ions that affect membrane potential like potassium or calcium and thus change intraerythrocytic pH are lacking in this assay, or are only present in subphysiological concentrations which will inherently affect the ODC. Consistently, the results correspond in part to former publications applying the same methodology who also failed to detect marked changes of the ODC in COVID-19 (Daniel et al., 2020; Renoux et al., 2021). Notably, however, these studies reported higher standard P50 values in both patients and healthy control subjects (>27 mmHg).
Comparison to publications applying different methods: Third, the reported effects of COVID-19 on the OCD were, however, variable in other investigations with different methodological approaches. Most investigators calculated P50 from single measurements in native blood with blood gas analyzers applying correction factors for pH and PCO2. In several articles a shift of the ODC (mainly leftward in arterial and rightward in venous blood) was observed. We have reviewed these studies last year in a publication in this journal (Böning et al., 2023); they are, however, not considered, cited or discussed in the recent work by Rogers and colleagues. The authors also fail to explain the rather low standard P50 values of, e.g., 23.1 mmHg for the control group at pH 7.4. Generally, P50 values amount to approximately 26–28 mmHg, with slightly higher values for females before menopause than for males (Humpeler et al., 1989).
2,3-Biphosphoglycerate: Fourth, as in virtually all studies on the ODC in COVID-19 so far, the concentration of 2,3-bisphosphoglycerate (2,3-BPG) as a critical determinant of the P50 was not measured. Until recently only Thomas and colleagues (Thomas et al., 2020) had communicated slighty elevated 2,3-BPG concentrations in moderately ill COVID-19 patients, but only arbitrary units were used. The reason for the relative lack of reported 2,3-BPG levels seems to be that most major companies (e.g., Roche, Sigma) had ended the production of the kits for photometric measurements prior to the pandemic. Yet, a new study on 2,3-BPG concentration was recently published as preproof by Bertilacchi and coworkers (Bertilacchi et al., 2024). Here, the authors applied a competitive enzyme-linked immunosorbent assay (ELISA, COD. MBS288269-96, MyBioSource) to measure 2,3-BPG levels in sixty eight patients on the intensive care unit. Patients were stratified into two subgroups based on a median arterial oxygen pressure of 67 mmHg. Interestingly, the mean value for 2,3-BPG (corrected by us for a calculation error) was 4.9 ± 1.4 mmol/L erythrocytes for patients with PaO2 > 67 mmHg, but 5.7 ± 1.5 mmol/L for patients with PaO2 < 67 mmHg. Both values are higher than in healthy subjects at sea level (approximately 4 mmol/L according to (Duhm and Gerlach, 1974)), and are consistent with the typical response to hypoxia (reviewed in West et al., 2007).
Based on these considerations, we suggest that the measurement of P50 with the Hemox-Analyzer in the study by Rogers and colleagues likely yields lower than actual half saturation pressures, and potential effects of COVID-19 such as a moderate decrease in oxygen affinity as formerly observed in venous blood failed to reach significance based on the applied statistical evaluation. Direct measurements of 2,3-BPG by Bertilacchi and colleagues (Bertilacchi et al., 2024) suggest increased concentrations in COVID-19 patients. Unfortunately these authors did not calculate half saturation pressures, or report 2,3-BPG values in healthy controls. As such, the ultimate word on the effects of COVID-19 on ODC, and the potential role of altered 2,3-BPG levels in this scenario still await to be elucidated.
DB: Conceptualization, Supervision, Writing–original draft, Writing–review and editing, Validation. WB: Formal Analysis, Validation, Writing–review and editing. DV: Formal Analysis, Validation, Writing–review and editing. MS: Formal Analysis, Writing original draft, Writing–review and editing. WK: Writing–original draft, Writing–review and editing, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Asakura T. (1979). Automated method for determination of oxygen equilibrium curves of red cell suspensions under controlled buffer conditions and its clinical applications. Crit. Care Med. 7, 391–395. doi:10.1097/00003246-197909000-00008
Bertilacchi M. S., Piccarducci R., Celi A., Germelli L., Romei C., Bartholmai B., et al. (2024). Blood oxygenation state in covid-19 patients: unexplored role of 2,3-bisphosphoglycerate. Biomed. J., 100723. Epub 20240405. doi:10.1016/j.bj.2024.100723
Böning D., Kuebler W. M., Vogel D., Bloch W. (2023). The oxygen dissociation curve of blood in covid-19-an update. Front. Med. (Lausanne) 10, 1098547. Epub 20230227. doi:10.3389/fmed.2023.1098547
Daniel Y., Hunt B. J., Retter A., Henderson K., Wilson S., Sharpe C. C., et al. (2020). Haemoglobin oxygen affinity in patients with severe covid-19 infection. Br. J. Haematol. 190 (3), e126–e127. Epub 20200628. doi:10.1111/bjh.16888
Duhm J., Gerlach E. (1974). Metabolism and function of 2,3-diphosphoglycerate in red blood cells. Hum. Red Cell Vitro, 111–152.
Guarnone R., Centenara E., Barosi G. (1995). Performance characteristics of hemox-analyzer for assessment of the hemoglobin dissociation curve. Haematologica 80 (5), 426–430.
Humpeler E., Vogel S., Schobersberger W., Mairbaurl H. (1989). Red cell oxygen transport in man in relation to gender and age. Mech. Ageing Dev. 47 (3), 229–239. doi:10.1016/0047-6374(89)90035-3
Renoux C., Fort R., Nader E., Boisson C., Joly P., Stauffer E., et al. (2021). Impact of covid-19 on red blood cell rheology. Br. J. Haematol. 192 (4), e108–e111. Epub 20210107. doi:10.1111/bjh.17306
Rogers S. C., Brummet M., Safari Z., Wang Q., Rowden T., Boyer T., et al. (2023). Covid-19 impairs oxygen delivery by altering red blood cell hematological, hemorheological, and oxygen transport properties. Front. Physiol. 14, 1320697. Epub 20240103. doi:10.3389/fphys.2023.1320697
Thomas T., Stefanoni D., Dzieciatkowska M., Issaian A., Nemkov T., Hill R. C., et al. (2020). Evidence of structural protein damage and membrane lipid remodeling in red blood cells from covid-19 patients. J. Proteome Res. 19 (11), 4455–4469. doi:10.1021/acs.jproteome.0c00606
Keywords: hemoglobin oxygen affinity, in vivo oxygen dissociation curve, hemox-analyzer, 2,3-bisphosphoglycerate, erythrocytes
Citation: Böning D, Bloch W, Vogel D, Steinach M and Kuebler WM (2024) Commentary: COVID-19 impairs oxygen delivery by altering red blood cell hematological, hemorheological, and oxygen transport properties. Front. Physiol. 15:1426505. doi: 10.3389/fphys.2024.1426505
Received: 01 May 2024; Accepted: 22 August 2024;
Published: 10 September 2024.
Edited by:
Anna Bogdanova, University of Zurich, SwitzerlandCopyright © 2024 Böning, Bloch, Vogel, Steinach and Kuebler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dieter Böning, ZGlldGVyLmJvZW5pbmdAY2hhcml0ZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.