- 1Centre for Rehabilitation and Sports Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Department for Pulmonary Medicine, Allergology and Clinical Immunology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Objectives: To assess whether nasal breathing improves exercise ventilatory efficiency in patients with heart failure (HF) or chronic coronary syndromes (CCS).
Background: Exercise inefficient ventilation predicts disease progression and mortality in patients with cardiovascular diseases. In healthy people, improved ventilatory efficiency with nasal compared to oral breathing was found.
Methods: Four study groups were recruited: Patients with HF, patients with CCS, old (age≥45 years) and young (age 20–40 years) healthy control subjects. After a 3-min warm-up, measurements of 5 min with once nasal and once oral breathing were performed in randomized order at 50% peak power on cycle ergometer. Ventilation and gas exchange parameters measured with spiroergometry were analysed by Wilcoxon paired-sample tests and linear mixed models adjusted for sex, height, weight and test order.
Results: Groups comprised 15 HF, CCS, and young control and 12 old control. Ventilation/carbon dioxide production (
Conclusion: Nasal breathing during submaximal exercise significantly improved ventilatory efficiency and abnormal breathing patterns (rapid shallow breathing and EOV) in 80% of our patients with HF and CCS.
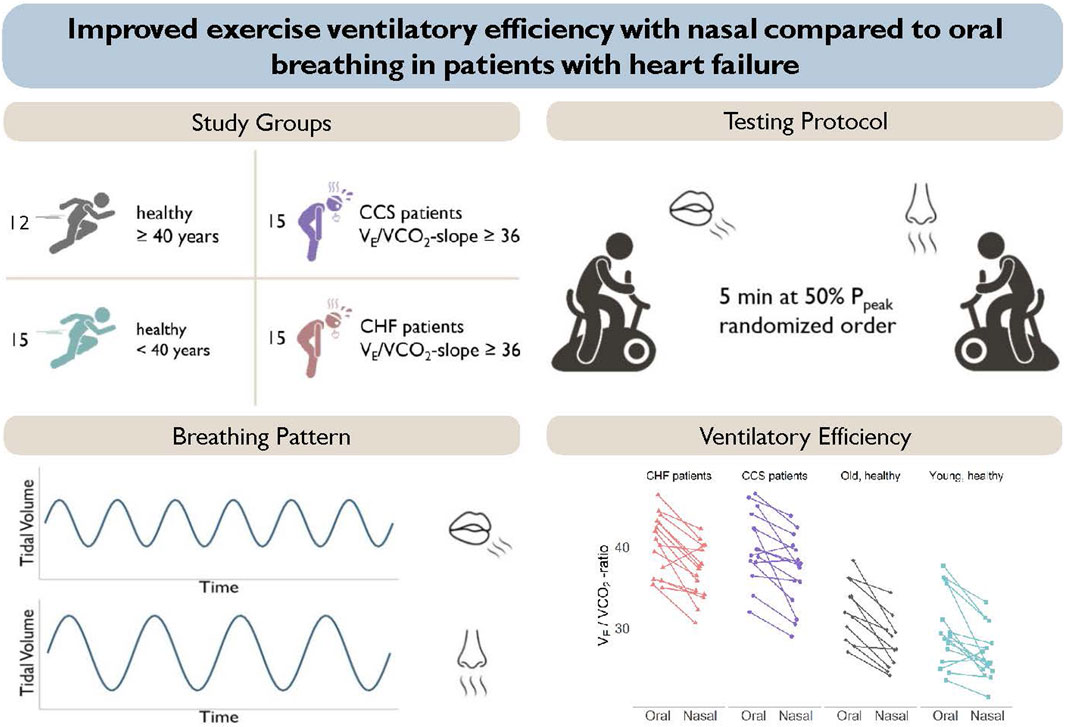
GRAPHICAL ABSTRACT | Presentation of the study groups and study protocol (top panels). Nasal breathing resulted in a pattern of slower and deeper breathing and a reduced ventilation to carbon dioxide production in all groups (lower panels).
1 Introduction
An exaggerated ventilatory response to exercise, often accompanied by early exertional dyspnea, is a hallmark in patients with chronic heart failure (HF) (Chua et al., 1996; Tomita et al., 2003). It has also been reported in patients with chronic coronary syndromes (CCS) and left ventricular dysfunction (Eser et al., 2023). Ventilatory inefficiency has not only been associated with reduced exercise capacity and quality of life but also with poorer prognosis (Ponikowski et al., 2001; Arena et al., 2004; Nadruz et al., 2017). It is quantified by an increased
Physiological dead space refers to the ventilated air that does not participate in gas exchange and is comprised of the anatomical dead space (i.e., the conducting airways) and the alveolar dead space (i.e., lung regions which are poorly perfused).
In patients with HF, impaired cardiac function may result in lung areas which are ventilated but poorly perfused (i.e., ventilation-perfusion mismatch) with
Pharmaceutical as well as exercise therapies have been shown to reduce the exaggerated ventilatory response to exercise in patients with HF (Hambrecht et al., 1995). However, adherence to exercise recommendations may be poor in patients suffering from dyspnea, as exercise tolerance may be low (Cooper et al., 2015). There is an unmet need for further therapies to improve ventilatory efficiency and exercise tolerance. Slow breathing training has been shown to have positive effects on cardiorespiratory function, (Bernardi et al., 2002; Parati et al., 2008; Lachowska et al., 2019), and ventilatory efficiency (Parati et al., 2008) in patients with HF. Furthermore, in healthy volunteers it has been shown that nasal breathing can reduce the
To date, no study has investigated whether nasal breathing is feasible in patients with HF or CCS and whether it is accompanied by a lower
The aims of the current study were to 1) Compare ventilatory efficiency and parameters of breathing pattern between oral and nasal breathing during submaximal exercise in patients with HF or CCS and inefficient ventilation; and 2) assess whether there is an age-related difference between oral and nasal breathing with regard to ventilatory efficiency by comparing healthy old volunteers (age-matched to the HF and CCS patients), and young healthy volunteers.
2 Methods
2.1 Study participants
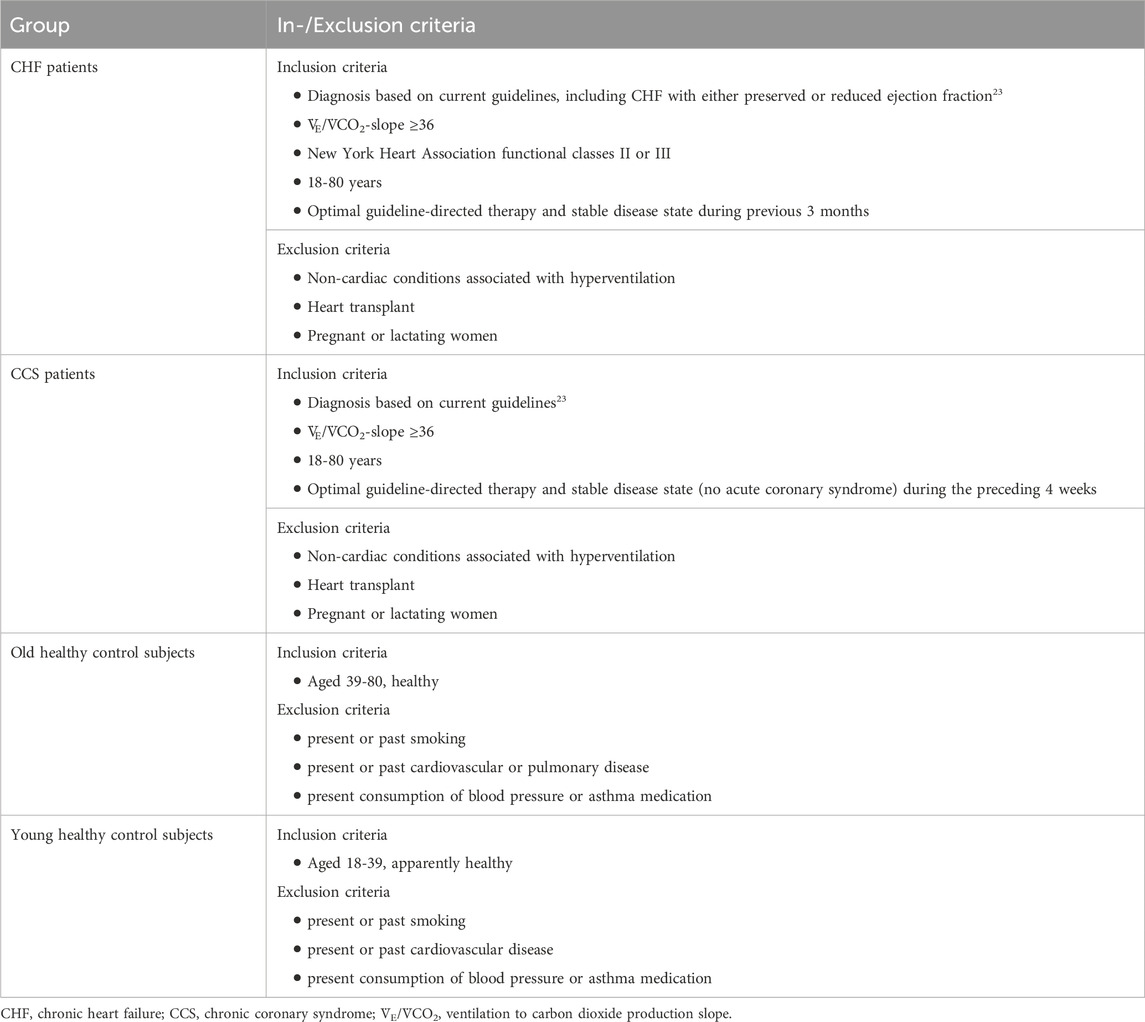
This study was conducted as a sub-study of the Breathe-HF trial (NCT05057884). The sub-study included four different groups of participants, two cardiac patient groups and two healthy control groups. The inclusion and exclusion criteria are listed in Table 1. The rational for only including patients with
2.2 Study procedures
Eligible patients with HF and CCS were identified and recruited during their yearly check-up visit at a tertiary university referral centre. Healthy young and old volunteers were recruited by word of mouth. If they met the inclusion criteria and consented in writing, they were included in the study and measurements were performed as summarized in Supplementary Figure S1. The study was approved by the ethics committee of the Canton of Berne.
Body composition was assessed by bioelectrical impedance with a body composition analyzer (inbody 770, best4health gmbh, Bassersdorf, Switzerland). Weight, lean muscle mass, and body fat percentage were measured and reported for comparison of anthropometric characteristics between groups. Moreover, body mass index (BMI) was calculated.
2.3 Cardiopulmonary exercise testing
Exercise capacity was assessed with a CPET on a cycle ergometer. Prior to the test, a vital capacity (FCV, l) and forced expiratory volume in one second (FEV1, l*min-1) was assessed by spirometry. Then, after sitting on the ergometer quietly for 3 min, blood pressure was measured two times and the lowest measurement was recorded. A 3 min warm-up was followed by an individually set ramp as previously described (Eser et al., 2022). Volumes, flows and gases were sampled continuously in an open spirometric system (Quark, Cosmed, Rome, Italy) and averaged over 8 breaths, as recommended (Glaab and Taube, 2022). Measured variables included oxygen uptake (
2.4 Oral and nasal submaximal tests
After the CPET following a 15-min resting period (or on a separate day if the CPET was performed as part of clinical routine testing), all subjects completed a submaximal constant load cycling protocol with exclusively oral and nasal breathing in randomized order. The protocol consisted of a 3 min warm-up phase followed by 5 min of constant load cycling at 50% of peak power output. This intensity was chosen based on a study by LaComb (LaComb et al., 2017) and the fact that some people have difficulties breathing through their nose at high intensities. During the oral breathing subjects were required to wear a nose clip under the mask, whereas during the nasal breathing their mouth was covered with tape.
Participants were instructed to always maintain a cadence of 60–70 min−1. The two trials were separated by a 10-min break to allow for some recovery. Rating of perceived exertion (RPE) was noted upon completion of each trial in the patient groups. All parameters of breathing patterns and gas exchange were calculated as averages of the fifth minute. The rapid shallow breathing index (RSBI, m2*min−1* l−1) was calculated by dividing fR by VT. Exercise ventilatory oscillation (EOV) was defined according to guidelines (Guazzi et al., 2012).
2.5 Statistical analysis
All analyses were performed by R (R Core Team, 2021; Version 4.1.0). Primary outcome was the within-subject difference in
Baseline characteristics were tested between groups by Kruskal-Wallis tests followed by post hoc testing (only patient groups and young control subjects were tested against old control subjects) adjusted for multiple testing by Benjamini-Hochberg correction. Categorical variables were tested by Fisher’s exact tests. Statistical significance for all tests was set at a p-value <0.05.
3 Results
3.1 Study population
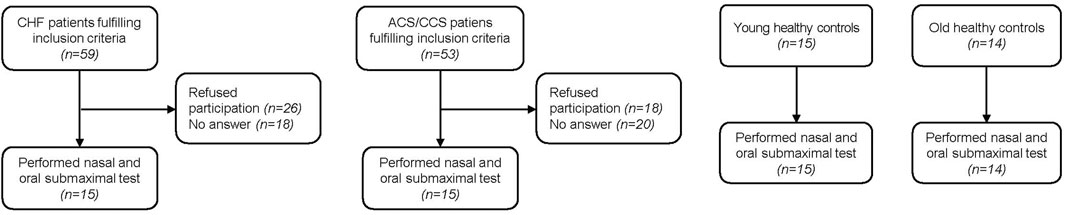
Fifteen young and 14 old healthy control subjects were recruited for the present study (Figure 1). Of patients with HF performing CPETs for yearly clinical visits, 59 qualified for inclusion. Eighteen could not be reached by phone and 26 declined participation in the study. Within the HF group, eleven patients were classified as having reduced (HFrEF), three as having mildly reduced (HFmrEF) and one as having preserved ejection fraction (HFpEF) (McDonagh et al., 2021). Of 53 patients with CCS qualifying for the study, 20 could not be contacted and 18 declined participation, leaving 15 who participated in the study. Two old healthy controls had a blocked nose and had to stop the nasal trial after 3 min, leaving data from 12 old healthy controls in the analyses. Otherwise the exercise bouts with the different breathing modes were tolerated well. There were no significant differences between old healthy control subjects and the two patient groups with regard to baseline characteristics (Table 2). The only significantly different baseline characteristics were age and percent body fat between old and young healthy control subjects.
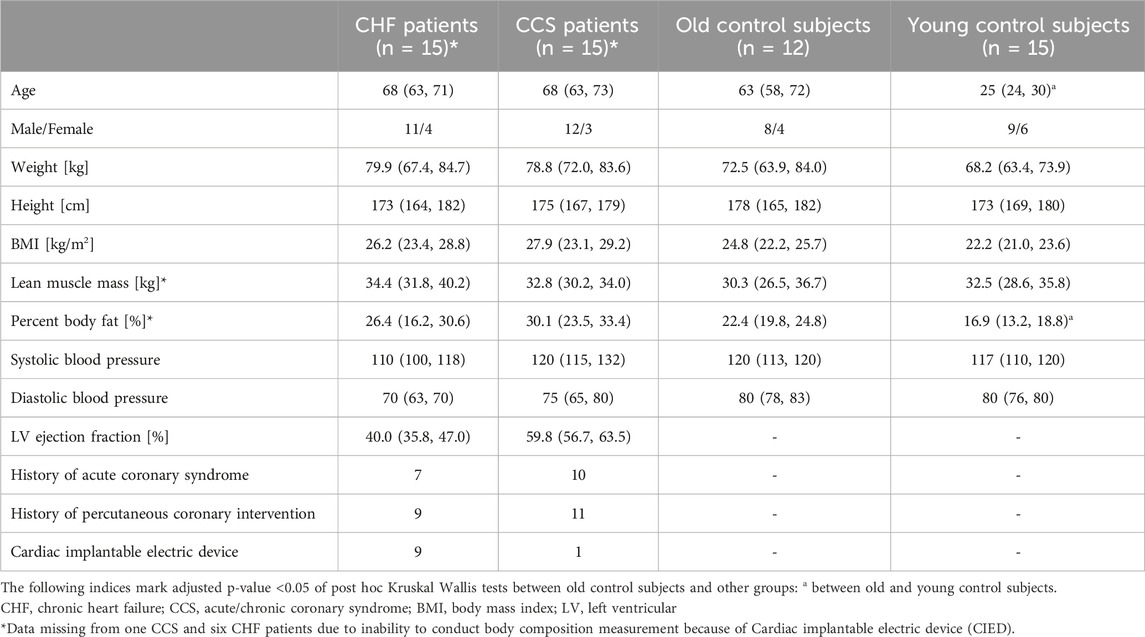
Table 2. Baseline characteristics of included subjects. Shown are medians and first and third quartiles in round brackets for each group.
3.2 Results of cardiopulmonary exercise tests
Resting parameters of the two patient groups were comparable to old control subjects except for PETCO2 that was lower in patient with CCS (Supplementary Table S1). The first ventilatory threshold occurred at lower power, lower VO2, lower
3.3 Nasal compared to oral breathing
Non-parametric data of ventilatory and circulatory parameters during the submaximal cycling with nasal and oral breathing modes of the four different groups are shown in Table 3. Power output was constant between nasal and oral breathing and was comparable to power at VT1 (range −14% to 8%), indicating that the tests were completed during aerobic metabolism. Subjectively perceived exertion did not differ between breathing modes.
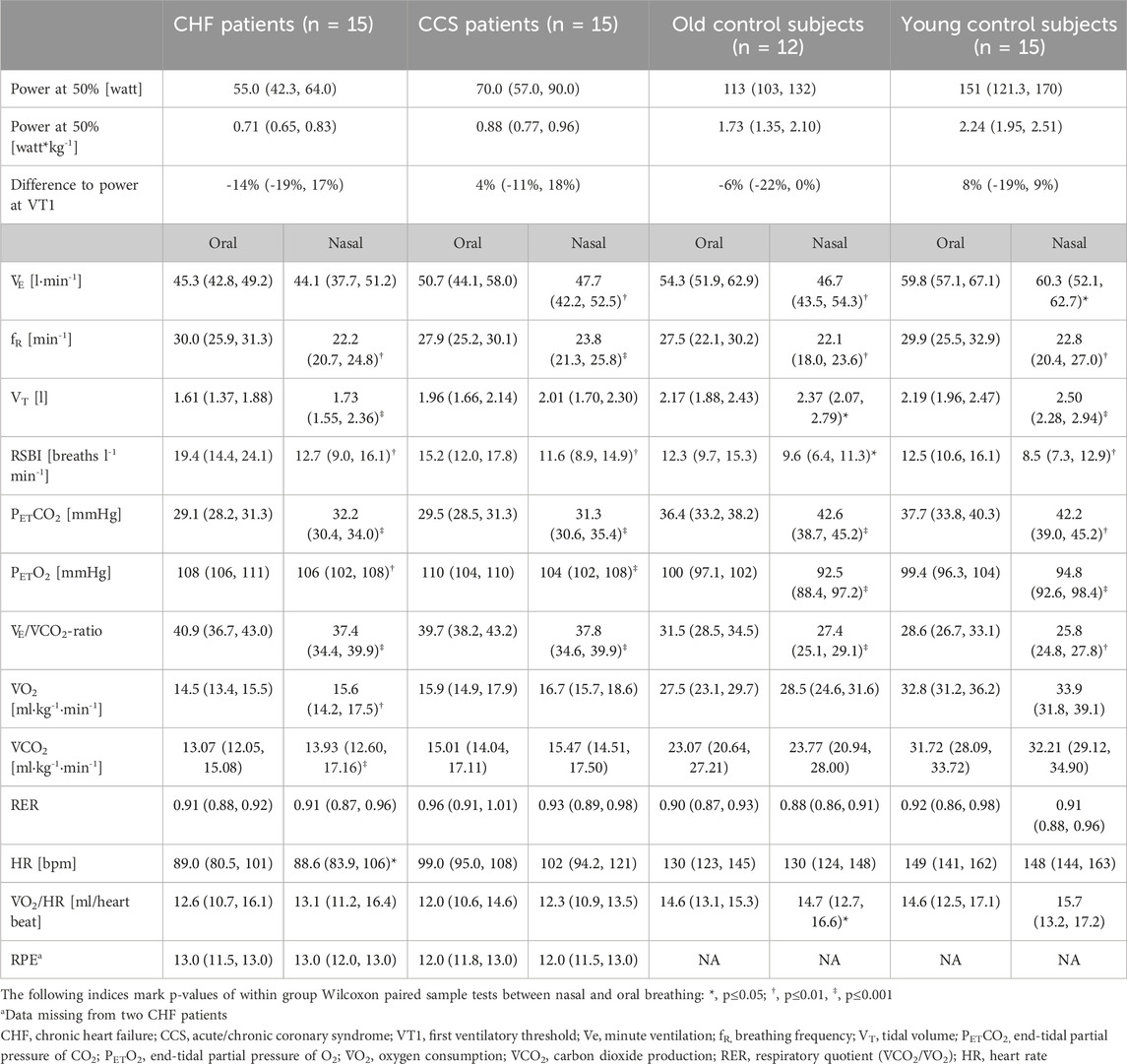
Table 3. Ventilatory and circulatory parameters during 5 minutes of submaximal cycling with exclusively nasal or oral breathing. The intensity was set at 50% of their peak power achieved during the CPET. For each participant values were averaged over the 5th minute. Shown are medians and first and third quartiles in round brackets for each group and each condition in randomized order.
Mean values during oral and nasal breathing of all primary and secondary outcome variables (

Figure 2. Interaction plots of effects of breathing modes and groups on
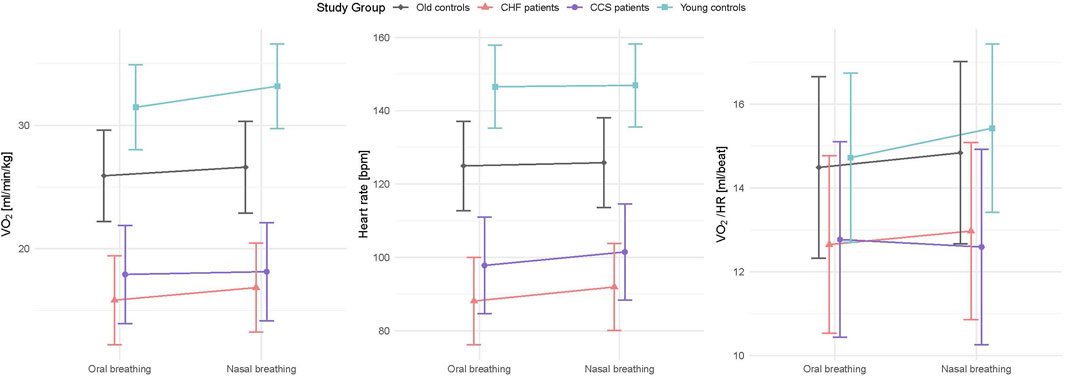
Figure 3. Interaction plots of effects of breathing modes and groups on
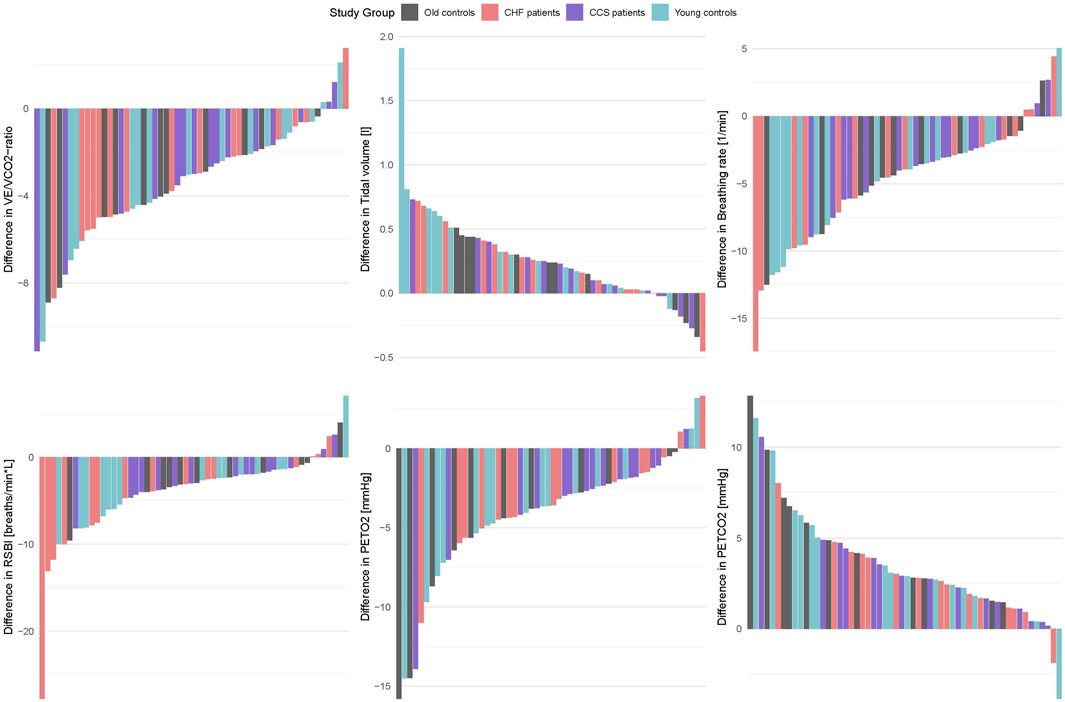
Figure 4. Barplots of within-subject differences between oral and nasal breathing (oral value–nasal value) for the same parameters as shown in Figure 2.
Patients with CCS had similar values to patients with HF for all measured parameters and similar improvements with nasal breathing (Figures 2, 3). Five patients with HF and one patient with CCS had EOV during oral breathing which in all these patients was markedly dampened with nasal breathing (Figure 5).
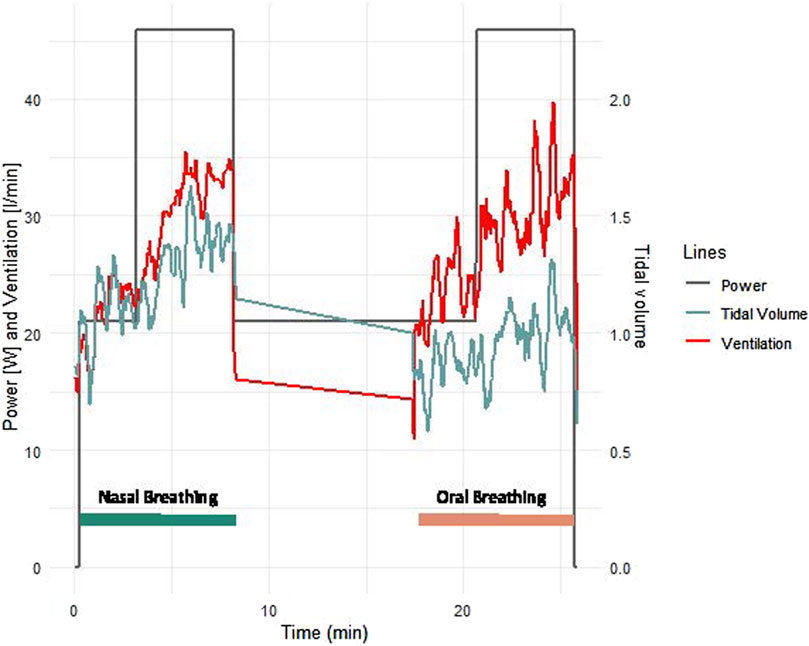
Figure 5. Example of ventilation and tidal volume of a typical female 63 years old patient with heart failure with reduced ejection fraction. The mask was not worn during the 10 min rest phase between exercise bouts.
Based on selection criteria, the young and old healthy group had lower
4 Discussion
Our study is the first to demonstrate that nasal breathing could reduce the excessive ventilatory response to exercise (represented by a lower
Nasal breathing compared to oral breathing lowered
In our CHF group, median
Our results are consistent with findings of previous studies in healthy individuals, where acute nasal breathing was found to reduce
Potential mechanisms underlying the improvement of ventilatory efficiency with nasal breathing may be the alteration of breathing pattern with lower fR and increased VT, with the latter having been shown to reduce muscle sympathetic neural activation in some (Hering et al., 2013; Oneda et al., 2010) but not all studies (Limberg et al., 2013). Another potential mechanism reducing
The increase in PETCO2 and decrease in PETO2 with nasal breathing indicates either a more efficient oxygen extraction or a better reflection of alveolar partial pressures of CO2 and O2, based on the fact that breathing at higher fR increases the ratio of air that goes to anatomical dead space. Further, nasal breathing has been suggested to lead to higher nitric oxide (NO) concentrations in the inhaled air than oral breathing as the main production site of NO, the paranasal sinuses, are circumvented by oral breathing (Lundberg et al., 1996; Lundberg et al., 1995). Nasal breathing has been shown to reduce pulmonary vascular resistance compared to oral breathing in an invasive study in patients with HF (Settergren et al., 1998). Inhaled NO, which is recommended intraoperatively in patients with pulmonary hypertension for selective pulmonary vasodilation, (Rajagopal et al., 2023), and during cardiopulmonary bypass, (Abouzid et al., 2023), has been shown to improve ventilation-perfusion matching (Dembinski et al., 2000; Hoffman and Nelin, 2005; Hajian et al., 2016).
It has been suggested that the rapid shallow breathing pattern may be adapted by patients with HF to avoid large intrathoracic pressure swings to preserve cardiac output (Lalande and Johnson, 2010). In our study, the O2 pulse, an accepted surrogate parameter for stroke volume, was not found to be different between nasal and oral breathing in neither of our patient groups, so we cannot confirm that a greater VT leads to a reduction of stroke volume in these patients.
Strengths of the present study were the inclusion of a representative cohort of well phenotyped patients with CHF and CCS and a rigorous within-subject study protocol, CPET based measurements, and random assignment of the order of the intervention to each study participant. Further, the inclusion of both sexes as well as a young healthy control group showed that the found effects of nasal breathing were independent of sex and age and similarly applied to all groups.
In the present study, we set the intensity at 50% of the maximal power despite results by LaComb and colleagues in healthy people suggesting that the effects of nasal breathing on reducing
The main limitation of our study was the lack of blood gas analyses due to logistic reasons, which prevented calculation of dead space. A further limitation was that no dyspnea perception rating was included, so we can only assume from the RPE that patients subjectively felt the same amount of dyspnea during both trials. Also, the chosen bout duration was 5 min in this study for logistic reasons so that all patients managed to perform two bouts during one visit to the lab. Whether the same differences between breathing modes would result from longer bouts would have to be tested in a more involving study protocol with bouts on separate days. Last but not least, since we only included patients with
We conclude that in healthy subjects and patients with HF or CCS alike, nasal breathing led to a reduced
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethikkommission des Kantons Bern. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. PC: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing–review and editing. AK: Investigation, Methodology, Project administration, Visualization, Writing–review and editing. LS: Project administration, Visualization, Writing–review and editing. SH: Investigation, Methodology, Project administration, Writing–review and editing. DK: Investigation, Methodology, Project administration, Writing–review and editing. SG: Writing–review and editing. MW: Conceptualization, Investigation, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1380562/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Protocol of study measurements comparing oral and nasal breathing at 50% or peak power output that was determined during a preceding maximal ramp test. *order of oral and nasal breathing was set randomly.
Abbreviations
BMI, body mass index; BSA, body surface area; CCS, chronic coronary syndrome; CO2, carbon dioxide; CPET, cardiopulmonary exercise test; EOV, exercise oscillatory ventilation; FCV, forced vital capacity; FEV1, forced expiratory volume in the first second; fR, respiratory frequency; HF, heart failure; HFpEF; heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; O2, oxygen; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PETCO2, end-tidal partial pressure of carbon dioxide; PETO2, end-tidal partial pressure of oxygen; RER,
References
Abouzid M., Roshdy Y., Daniel J. M., Rzk F. M., Ismeal A. A. A., Hendawy M., et al. (2023). The beneficial use of nitric oxide during cardiopulmonary bypass on postoperative outcomes in children and adult patients: a systematic review and meta-analysis of 2897 patients. Eur. J. Clin. Pharmacol. 79, 1425–1442. doi:10.1007/s00228-023-03554-9
Agostoni P., Guazzi M. (2017). Exercise ventilatory inefficiency in heart failure: some fresh news into the roadmap of heart failure with preserved ejection fraction phenotyping. Eur. J. heart Fail. 19 (12), 1686–1689. doi:10.1002/ejhf.940
Arena R., Myers J., Aslam S. S., Varughese E. B., Peberdy M. A. (2004). Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am. heart J. 147 (2), 354–360. doi:10.1016/j.ahj.2003.07.014
Bernardi L., Porta C., Spicuzza L., Bellwon J., Spadacini G., Frey A. W., et al. (2002). Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105 (2), 143–145. doi:10.1161/hc0202.103311
Chua T. P., Clark A. L., Amadi A. A., Coats A. J. (1996). Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J. Am. Coll. Cardiol. 27 (3), 650–657. doi:10.1016/0735-1097(95)00523-4
Cooper L. B., Mentz R. J., Sun J. L., Schulte P. J., Fleg J. L., Cooper L. S., et al. (2015). Psychosocial factors, exercise adherence, and outcomes in heart failure patients: insights from heart failure: a controlled trial investigating outcomes of exercise training (HF-action). Circ. Heart Fail. 8 (6), 1044–1051. doi:10.1161/CIRCHEARTFAILURE.115.002327
Cross T. J., Kim C. H., Johnson B. D., Lalande S. (2020). The interactions between respiratory and cardiovascular systems in systolic heart failure. J. Appl. Physiol. (1985) 128 (1), 214–224. doi:10.1152/japplphysiol.00113.2019
Dallam G., Kies B. (2020). The effect of nasal breathing versus oral and oronasal breathing during exercise: a review. J. Sports Res. 7 (1), 1–10. doi:10.18488/journal.90.2020.71.1.10
Dallam G., McClaran S., Cox D., P. Foust C. (2018). Effect of nasal versus oral breathing on Vo2max and physiological economy in recreational runners following an extended period spent using nasally restricted breathing. Int. J. Kinesiol. Sports Sci. 6 (2), 22–29. doi:10.7575/aiac.ijkss.v.6n.2p.22
Dembinski R., Max M., Lopez F., Kuhlen R., Sünner M., Rossaint R. (2000). Effect of inhaled nitric oxide in combination with almitrine on ventilation-perfusion distributions in experimental lung injury. Intensive Care Med. 26 (2), 221–228. doi:10.1007/s001340050051
Donelli da Silveira A., Beust de Lima J., da Silva Piardi D., Dos Santos Macedo D., Zanini M., Nery R., et al. (2020). High-intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: a randomized clinical trial. Eur. J. Prev. Cardiol. 27 (16), 1733–1743. doi:10.1177/2047487319901206
Douglas N. J., White D. P., Weil J. V., Zwillich C. W. (1983). Effect of breathing route on ventilation and ventilatory drive. Respir. Physiol. 51 (2), 209–218. doi:10.1016/0034-5687(83)90041-5
Eser P., Marcin T., Prescott E., Prins L. F., Kolkman E., Bruins W., et al. (2023). Breathing pattern and pulmonary gas exchange in elderly patients with and without left ventricular dysfunction—modification with exercise-based cardiac rehabilitation and prognostic value. Front. Cardiovasc. Med. 10, 1219589. doi:10.3389/fcvm.2023.1219589
Eser P., Trachsel L. D., Marcin T., Herzig D., Freiburghaus I., De Marchi S., et al. (2022). Short- and long-term effects of high-intensity interval training vs. Moderate-intensity continuous training on left ventricular remodeling in patients early after ST-segment elevation myocardial infarction-the HIIT-EARLY randomized controlled trial. Front. Cardiovasc Med. 9, 869501. doi:10.3389/fcvm.2022.869501
Glaab T., Taube C. (2022). Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 23 (1), 9. doi:10.1186/s12931-021-01895-6
Guazzi M., Adams V., Conraads V., Halle M., Mezzani A., Vanhees L., et al. (2012). EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 126 (18), 2261–2274. doi:10.1161/CIR.0b013e31826fb946
Guazzi M., Melzi G., Marenzi G. C., Agostoni P. (1999). Angiotensin-converting enzyme inhibition facilitates alveolar-capillary gas transfer and improves ventilation-perfusion coupling in patients with left ventricular dysfunction. Clin. Pharmacol. Ther. 65 (3), 319–327. doi:10.1016/S0009-9236(99)70111-6
Hajian B., De Backer J., Vos W., Van Holsbeke C., Ferreira F., Quinn D. A., et al. (2016). Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int. J. Chron. Obstruct Pulmon Dis. 11, 1533–1541. doi:10.2147/COPD.S106480
Hambrecht R., Niebauer J., Fiehn E., Kälberer B., Offner B., Hauer K., et al. (1995). Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J. Am. Coll. Cardiol. 25 (6), 1239–1249. doi:10.1016/0735-1097(94)00568-B
Hering D., Kucharska W., Kara T., Somers V. K., Parati G., Narkiewicz K. (2013). Effects of acute and long-term slow breathing exercise on muscle sympathetic nerve activity in untreated male patients with hypertension. J. Hypertens. 31 (4), 739–746. doi:10.1097/HJH.0b013e32835eb2cf
Hoffman G. M., Nelin L. D. (2005). Mean airway pressure and response to inhaled nitric oxide in neonatal and pediatric patients. Lung 183 (6), 441–453. doi:10.1007/s00408-005-2555-2
Iellamo F., Manzi V., Caminiti G., Vitale C., Castagna C., Massaro M., et al. (2013). Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int. J. Cardiol. 167 (6), 2561–2565. doi:10.1016/j.ijcard.2012.06.057
Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. (2020). 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. heart J. 41 (3), 407–477. doi:10.1093/eurheartj/ehz425
Lachowska K., Bellwon J., Narkiewicz K., Gruchała M., Hering D. (2019). Long-term effects of device-guided slow breathing in stable heart failure patients with reduced ejection fraction. Clin. Res. Cardiol. official J. Ger. Cardiac Soc. 108 (1), 48–60. doi:10.1007/s00392-018-1310-7
LaComb C. O., Tandy R. D., Lee S.-P., Young J. C., Navalta J. W. (2017). Oral versus nasal breathing during moderate to high intensity submaximal aerobic exercise. Int. J. Kinesiol. Sports Sci. 5, 8–16. doi:10.7575//aiac.ijkss.v.5n.1p.8
Lalande S., Johnson B. D. (2010). Breathing strategy to preserve exercising cardiac function in patients with heart failure. Med. Hypotheses 74 (3), 416–421. doi:10.1016/j.mehy.2009.09.030
Limberg J. K., Morgan B. J., Schrage W. G., Dempsey J. A. (2013). Respiratory influences on muscle sympathetic nerve activity and vascular conductance in the steady state. Am. J. Physiol. Heart Circ. Physiol. 304 (12), H1615–H1623. doi:10.1152/ajpheart.00112.2013
Lundberg J. O., Lundberg J. M., Settergren G., Alving K., Weitzberg E. (1995). Nitric oxide, produced in the upper airways, may act in an 'aerocrine' fashion to enhance pulmonary oxygen uptake in humans. Acta physiol. Scand. 155 (4), 467–468. doi:10.1111/j.1748-1716.1995.tb09998.x
Lundberg J. O., Settergren G., Gelinder S., Alving K., Weitzberg E. (1996). Inhalation of nasally derived nitric oxide modulates pulmonary function in humans. Acta physiol. Scand. 158 (4), 343–347. doi:10.1046/j.1365-201X.1996.557321000.x
Marcin T., Eser P., Prescott E., Prins L. F., Kolkman E., Bruins W., et al. (2020). Training intensity and improvements in exercise capacity in elderly patients undergoing European cardiac rehabilitation - the EU-CaRE multicenter cohort study. PloS one 15 (11), e0242503. doi:10.1371/journal.pone.0242503
McBride B., Whitelaw W. A. (1981). A physiological stimulus to upper airway receptors in humans. J. Appl. Physiol. Respir. Environ. Exerc Physiol. 51 (5), 1189–1197. doi:10.1152/jappl.1981.51.5.1189
McDonagh T. A., Metra M., Adamo M., Gardner R. S., Baumbach A., Böhm M., et al. (2021). 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. heart J. 42 (36), 3599–3726. doi:10.1093/eurheartj/ehab368
McSwain S. D., Hamel D. S., Smith P. B., Gentile M. A., Srinivasan S., Meliones J. N., et al. (2010). End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir. Care 55 (3), 288–293.
Nadruz W., West E., Sengelov M., Santos M., Groarke J. D., Forman D. E., et al. (2017). Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J. Am. Heart Assoc. 6 (11), e006000. doi:10.1161/JAHA.117.006000
Oneda B., Ortega K. C., Gusmão J. L., Araújo T. G., Mion D. (2010). Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens. Res. 33 (7), 708–712. doi:10.1038/hr.2010.74
Parati G., Malfatto G., Boarin S., Branzi G., Caldara G., Giglio A., et al. (2008). Device-guided paced breathing in the home setting: effects on exercise capacity, pulmonary and ventricular function in patients with chronic heart failure: a pilot study. Circ. Heart Fail. 1 (3), 178–183. doi:10.1161/CIRCHEARTFAILURE.108.772640
Piepoli M., Clark A. L., Volterrani M., Adamopoulos S., Sleight P., Coats A. J. (1996). Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93 (5), 940–952. doi:10.1161/01.cir.93.5.940
Ponikowski P., Chua T. P., Piepoli M., Ondusova D., Webb-Peploe K., Harrington D., et al. (1997). Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation 96 (8), 2586–2594. doi:10.1161/01.cir.96.8.2586
Ponikowski P. P., Chua T. P., Francis D. P., Capucci A., Coats A. J., Piepoli M. F. (2001). Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104 (19), 2324–2330. doi:10.1161/hc4401.098491
Rajagopal S., Ruetzler K., Ghadimi K., Horn E. M., Kelava M., Kudelko K. T., et al. (2023). Evaluation and management of pulmonary hypertension in noncardiac surgery: a scientific statement from the American heart association. Circulation 147 (17), 1317–1343. doi:10.1161/CIR.0000000000001136
Rappelt L., Held S., Wiedenmann T., Deutsch J. P., Hochstrate J., Wicker P., et al. (2023). Restricted nasal-only breathing during self-selected low intensity training does not affect training intensity distribution. Front. physiology 14, 1134778. doi:10.3389/fphys.2023.1134778
Settergren G., Angdin M., Astudillo R., Gelinder S., Liska J., Lundberg J. O., et al. (1998). Decreased pulmonary vascular resistance during nasal breathing: modulation by endogenous nitric oxide from the paranasal sinuses. Acta physiol. Scand. 163 (3), 235–239. doi:10.1046/j.1365-201x.1998.00352.x
Shi Y. X., Seto-Poon M., Wheatley J. R. (1999). The breathing route dependence of ventilatory responses to hypercapnia and exercise is modulated by upper airway resistance. Respirology 4 (4), 331–338. doi:10.1046/j.1440-1843.1999.00201.x
Smart N. A., Steele M. (2012). A comparison of 16 weeks of continuous vs intermittent exercise training in chronic heart failure patients. Congest. Heart Fail 18 (4), 205–211. doi:10.1111/j.1751-7133.2011.00274.x
Tanaka Y., Morikawa T., Honda Y. (1988). An assessment of nasal functions in control of breathing. J. Appl. Physiol.(1985) 65 (4), 1520–1524. doi:10.1152/jappl.1988.65.4.1520
Tomita T., Takaki H., Hara Y., Sakamaki F., Satoh T., Takagi S., et al. (2003). Attenuation of hypercapnic carbon dioxide chemosensitivity after postinfarction exercise training: possible contribution to the improvement in exercise hyperventilation. Heart 89 (4), 404–410. doi:10.1136/heart.89.4.404
Wang M. C., Corbridge T. C., McCrimmon D. R., Walter J. M. (2020). Teaching an intuitive derivation of the clinical alveolar equations: mass balance as a fundamental physiological principle. Adv. Physiol. Educ. 44 (2), 145–152. doi:10.1152/advan.00064.2019
Ward S. A., Whipp B. J. (1980). Ventilatory control during exercise with increased external dead space. J. Appl. Physiol. Respir. Environ. Exerc Physiol. 48 (2), 225–231. doi:10.1152/jappl.1980.48.2.225
Keywords: VE/VCO2 ratio, rapid shallow breathing index, exercise oscillatory ventilation, heart failure, nasal breathing
Citation: Eser P, Calamai P, Kalberer A, Stuetz L, Huber S, Kaesermann D, Guler S and Wilhelm M (2024) Improved exercise ventilatory efficiency with nasal compared to oral breathing in cardiac patients. Front. Physiol. 15:1380562. doi: 10.3389/fphys.2024.1380562
Received: 01 February 2024; Accepted: 02 July 2024;
Published: 06 August 2024.
Edited by:
David Cristóbal Andrade, University of Antofagasta, ChileReviewed by:
Stéphanie Saxer, OST Eastern Swiss University of Applied Sciences, SwitzerlandDaniella Cunha, Federal University of Pernambuco, Brazil
Copyright © 2024 Eser, Calamai, Kalberer, Stuetz, Huber, Kaesermann, Guler and Wilhelm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prisca Eser, cHJpc2NhLmVzZXJAaW5zZWwuY2g=
 Prisca Eser
Prisca Eser Pietro Calamai
Pietro Calamai Anja Kalberer1
Anja Kalberer1 Laura Stuetz
Laura Stuetz Dominic Kaesermann
Dominic Kaesermann Matthias Wilhelm
Matthias Wilhelm