- 1Department of Burn and Plastic Surgery, Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 2Department of Dermatology, Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 3Department of Medical Cosmetology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China
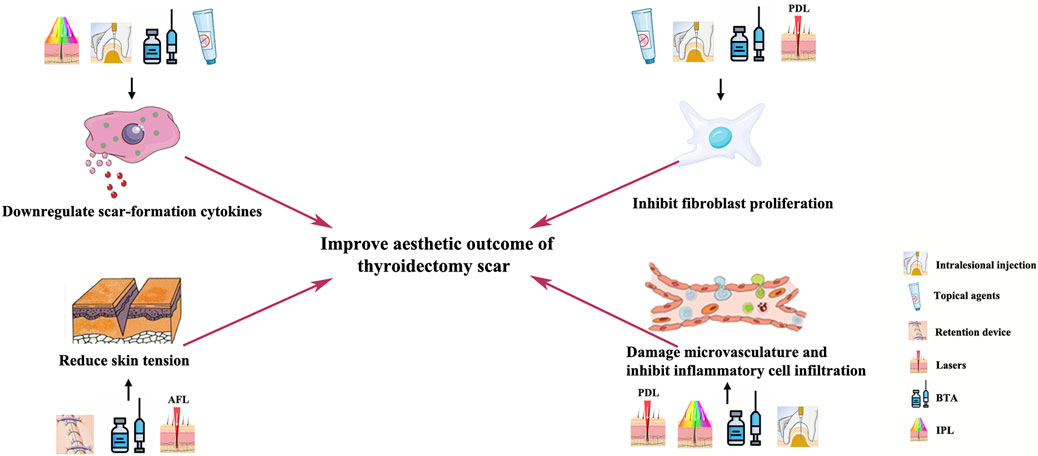
Thyroidectomy scars, located on the exposed site, can cause distress in patients. Owing to the cosmetic importance of thyroidectomy scars, many studies have been conducted on its prevention and treatment. Scar formation factors mainly include inflammatory cell infiltration, angiogenesis, fibroblast proliferation, secretion of cytokines such as transforming growth factor (TGF)-β1, and mechanical tension on the wound edges. Anti-scar methods including topical anti-scar agents, skin tension-bearing devices, and local injections of botulinum toxin, as well as lasers and phototherapies, that target these scar formation factors have been developed. However, current studies remain fragmented, and there is a lack of a comprehensive evaluation of the impacts of these anti-scar methods on treating thyroidectomy scars. Early intervention is a crucial but often neglected key to control hyperplastic thyroidectomy scars. Therefore, we review the currently adopted early postoperative strategies for thyroidectomy scar reduction, aiming to illustrate the mechanism of these anti-scar methods and provide flexible and comprehensive treatment selections for clinical physicians to deal with thyroidectomy scars.
Introduction
Thyroidectomy is often conducted on the anterior neck, which is a highly sensitive and visible anatomic location. Thus, postoperative scarring after thyroidectomy can make patients feel distressed (Bayat et al., 2003). The cosmetic outcome of the scar after thyroidectomy has raised wide interest in thyroid surgeons (Consorti et al., 2013), and many attempts to minimize thyroidectomy scars have been performed. The thyroidectomy incision length has been shortened from a 10-cm-long Kocher’s incision to a 15-mm-long access achieved by video-assisted thyroidectomy (Chung et al., 2021). In addition, thyroidectomy conducted by robotic surgery via an intraoral approach or endoscopic surgery via a postauricular approach leaves no visible scar on the neck (Teoh et al., 2019). However, robotic thyroidectomy and endoscopic thyroidectomy are conducted by few top facilities, and the traditional 10-cm-long Kocher’s incision remained the most adopted surgical approach. Therefore, early-stage interventions to improve the cosmetic outcome of thyroidectomy incision are of critical importance. Several surgical and nonsurgical approaches have been proposed for the prevention and treatment of thyroidectomy scars (Jung et al., 2011; Ha et al., 2014; Shin et al., 2014; An et al., 2019), and these interventions have displayed varying degrees of success.
Certain body sites, such as the neck area, shoulder area, anterior chest, lower abdomen, and earlobe, which have an overly bony prominence or bear greater skin tension, are more prone to an exaggerated scarring response (Aarabi et al., 2007; Ogawa, 2008). Patients of Afro-Caribbean descent and those with a personal or family history of hypertrophic scars or keloids are more likely to suffer from an exaggerated scarring response. Young age (less than 30 years) is reported to be another risk factor, especially for keloids (Berman et al., 2007; Profyris et al., 2012). However, little can be done to change the innate tendency of certain individuals or body sites, and further research aiming to reduce this scar formation risk is necessary.
Recent fundamental research also identified the major factors considered to affect scar formation and prevention, which are inflammatory cell infiltration, angiogenesis, fibroblast proliferation, secretion of cytokines such as transforming growth factor (TGF)-β1, and mechanical tension on wound edges (Bayat et al., 2003; Korntner et al., 2019; Zhang et al., 2020). Many anti-scar methods have emerged that target these scar formation factors, including topical drugs, tension-bearing devices, local injections, and lasers. However, so far, studies on anti-scar interventions concerning thyroidectomy scars remain fragmented. Therefore, in this review, we illustrate the mechanism and evaluate the clinical impacts of the current anti-scar methods for thyroidectomy scars.
Topical anti-scarring drugs
Topical anti-scar ingredient, mainly including silicone, asiaticoside, and onion extracts, lends itself as the most common and convenient postoperative scar prevention method. Silicone products could increase hydration in scars and local skin temperature under the occlusive membrane, leading to a decrease in scar size (Chang et al., 1995; Borgognoni, 2002; Chan et al., 2005; Berman et al., 2007). Silicone gel sheeting is a self-adhesive and semi-occlusive dressing for anti-scar purposes. Silicone gel sheeting is beneficial for creating a closed-wound environment, which will increase the hydration of the cuticle, contributing to the stability of mast cells, and inhibit the release of inflammatory cytokines (Trace et al., 2016). In addition, the hydrated occlusive wound environment, which was created by the silicone gel cream, can reduce capillary permeability and subsequently reduce the release of regeneration cytokines (Quinn et al., 1985; Sawada and Sone, 1990). The use of silicone gel sheeting alleviates symptoms like pain and itching associated with scarring (Trace et al., 2016). Previous studies proved silicone gel sheets or silicone oil-based cream to be effective in limiting hypertrophic growth of postoperative scars (Juckett and Hartman-Adams, 2009). In addition, small molecule types of silicone in silicone oil cream can penetrate the skin and inhibit the proliferation of fibroblasts, resulting in reduced collagen deposition (Kuhn et al., 2001). A randomized controlled trial of patients undergoing skin surgery showed that patients who used silicone cream after removal had a significantly reduced formation of hyperplastic scars and keloids (de Giorgi et al., 2009).
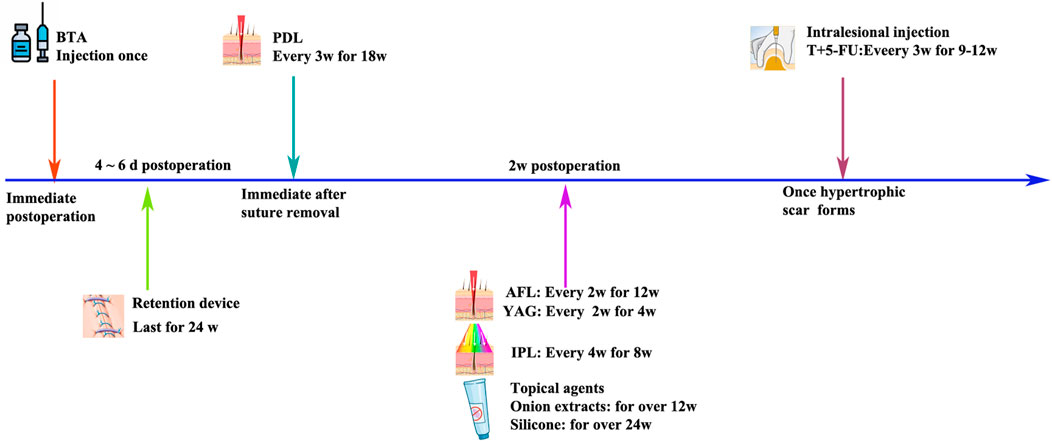
It is suggested that silicone gel sheets should be applied as early as 2 weeks post-operation (Son and Harijan, 2014), especially for patients with predisposing factors for hypertrophic scars. The gel sheet should be trimmed slightly larger than the scar and applied continually for up to 6 months after the operation. Meanwhile, the silicone oil-based cream could be used alternatively in locations where sheet attachment is difficult (Sawada and Sone, 1990). The silicone cream is recommended to be topically applied 3–4 times a day and massaged for 5–10 min with each application (Son and Harijan, 2014).
Asiaticoside is a white needle-like crystal extracted from the traditional herbal medicinal plant, Centella asiatica, a plant of the umbelliferone family, which has been used for many years for the treatment of dermal disorders, such as venous insufficiency and microangiopathy (Shukla et al., 1999). Asiaticoside can promote collagen crosslinking and re-epithelialization, leading to faster wound maturation and contraction, thus reducing erythema and pigmentation of hypertrophic scars. An in vitro experiment also demonstrated that the addition of asiaticoside can inhibit the proliferation of fibroblasts and downregulate the expression of TGF-β, leading to the prevention of excessive scarring (Cheng et al., 2004; Ju-Lin et al., 2009; Zoumalan, 2018).
Onion extract gel, with a much lower price, was proved by a recent clinical trial to have compliance, side effects, and efficacy similar to those of silicone gel in making surgical scars less distinct (Song et al., 2018). Onion extract possesses anti-inflammatory, antibiotic, and collagen-degradation properties (Augusti, 1996), which is suitable for scar management. A recent clinical trial proved that onion extract gel can help restore the stratum corneum barrier, reduce transdermal dehydration, and inhibit the keratinocytes related to scar formation. Topical onion extract combined with silicone derivate gel is proven to achieve safe and effective results in the prevention of hypertrophic surgical scarring (Jenwitheesuk et al., 2012). However, the anti-scar effect of onion extract is still controversial, and many researchers consider onion extract to have a limited effect in relieving redness and itching symptoms associated with surgical scarring. With few existing studies, most scholars agreed that the therapeutic mechanisms of onion extract gel on hypertrophic scars mainly depend on reducing inflammation and fibrotic cell proliferation, inhibition of connective tissue components (proteoglycans and collagen), moisturizing scar tissue, and antimicrobial capacity (Willital and Heine, 1994; Maragakis et al., 1995; Jackson and Shelton, 1999; Hosnuter et al., 2007).
Other related anti-scar drugs are also listed in few studies, such as hyaluronic acid, which supplements the extracellular matrix (ECM) and prevents scar formation (Bullard et al., 2003). Another anti-scar component worth mentioning here is curcumin, which regulates the inflammatory response during wound healing by inhibiting the production of IL-1 and TNF-α that activate monocytes and macrophages. Curcumin can also accelerate wound re-epithelialization, therefore yielding a better scar outcome (Tejada et al., 2016). A 16-week-long comparative study indicated that vitamin E lotion could achieve significant improvements in volume, length, induration, erythema, and pigmentation alteration of hypertrophic scars (Perez et al., 2010), while topical calcipotriol cream showed no statistically significant improvement in the prevention of hypertrophic scars (van der Veer et al., 2009).
Skin tension-reduction methods
The linear incisional scar response, which is often generated by thyroidectomy, is determined by modifiable factors including incision design, aseptic techniques, complete hemostasis, atraumatic handling of soft tissue, and skin tension (Larson et al., 2010). High skin tension is well known to be a critical causative factor for the development of wide and hypertrophic scars in humans (Wong et al., 2011; Suarez et al., 2013; Suarez et al., 2014). A cutaneous tension-reducing approach is mandatory in early-stage postoperative scar prevention and management. During surgeries, clinicians strive to make skin incisions that follow the relaxed tension lines on the body, the so-called Langer lines (Wilhelmi et al., 1999), aiming to minimalize incision tension.
In the case of sutured wounds, the epidermis can regenerate within 7–10 days, allowing both the patient and the doctor to believe that the wound has fully healed. In fact, it takes up to 3 months for the dermis to return to 90% of its normal strength. This long-term vulnerability to mechanical forces triggered by inflammation means that immature scars must be provided with long-term external mechanical support until maturity, which indicates the necessity of skin tension-reduction devices (Akaishi et al., 2008; Ogawa et al., 2011; Ogawa et al., 2016; Ogawa et al., 2021).
The skin tension-bearing tape or device is designed to shield the healing incision from the natural tension that is inherent in any break in skin that must be pulled together to close a wound (Atkinson et al., 2005; Longaker et al., 2014). It is estimated that tensile strength across an incision is only 3% of that of uninterrupted skin at 1 week after the surgery and increases to 20% by the third week when scar remodeling begins and to 80% after 12 weeks. The skin load-bearing tape or device is supposed to be applied across the incision for at least 12 weeks to reduce the tension through the whole wound remodeling phase. In addition, the load-bearing tape or device is suggested to be applied on the incision of convex skin surfaces rather than that of flexor crease locations (Atkinson et al., 2005; Son and Harijan, 2014).
The simple total surgical excision of keloids and hypertrophic scars with high recurrence rates associated is often disappointing for patients with cutaneous scars. Novel flap surgery and skin graft methods have been developed to release wound tension and change the orientation of the scar. The subtotal excision with a rim of keloids left behind was reported to achieve a better outcome owing to low wound tension and decreased collagen synthesis (Engrav et al., 1988). “Z” and “W” plastic surgery combined with postoperative nonsurgical adjuvant therapy are classic flap surgeries for successful management of small hypertrophic scars (English and Shenefelt, 1999). However, the evidence base of surgical strategies for scar treatment is, overall, inadequate and should be cautiously adopted in clinical practice.
Cryotherapy is another method for volume and tension reduction of hypertrophic scar tissues. A cryotherapy agent (most commonly liquid nitrogen) can induce scar tissue destruction by direct cell-freezing effects and cause vascular stasis during the thawing phase (Zouboulis, 1998). It is reported that the use of the intralesional cryo-needle, which is superior to the conventional open-spray method, can achieve approximately 60% volume reduction in hypertrophic scars and keloids with fewer adverse reactions after a single intralesional cryogenic treatment (Har-Shai et al., 2003; Har-Shai et al., 2006; Har-Shai et al., 2008).
Local injections of botulinum toxin, steroids, and chemotherapy agents
Botulinum toxin type A (BTA) is a potent neurotoxin that indirectly blocks neuromuscular transmission with inhibition of exocytosis of acetylcholine, leading to functional denervation of striated muscles and glands for 3–6 months (Noland et al., 2016; Sundaram et al., 2016). A major factor determining the final cosmetic appearance of a cutaneous scar is the tension acting on wound edges during the healing phase (Huang et al., 2013). Dermal injections of botulinum toxin type A and their diffusion into the surrounding muscles could reduce the mechanical tension on the wound edges (Hsu et al., 2004; Zhibo and Miaobo, 2008; Kim et al., 2014). A randomized, double-blind, placebo-controlled primate study indicated that BTA injections would lead to scar improvement by causing paralysis of surrounding muscles, thereby reducing continual tension on the wound (Larrabee, 2000). Another clinical retrospective analysis based on the medical records of 96 thyroidectomy patients also demonstrated that BTA injections act on the muscles surrounding the scar to reduce the pathologic role of mechanical stress (Kim J. H. et al., 2012).
In vitro and animal experiments have demonstrated that the mechanism of scar management by botulinum toxin injections involves suppressing infiltration by inflammatory cells and delaying the fibroblast cell cycle (Lee et al., 2009; Prodromidou et al., 2015). TGF-β-secreting macrophages are the most important inflammatory cells during the acute wound healing phase (Mahdavian Delavary et al., 2011). However, studies proved that BTA plays an important role in the inhibition of capsule formation through the TGF-β/Smad signaling pathway (Kim et al., 2016). In addition, BTA decreases the expression of connective tissue growth factors, which is a downstream regulator of TGF-β1 secreted by macrophages, thereby suppressing scar formation by fibroblasts (Xiao et al., 2011). However, it remains controversial whether BTA increases or decreases the vascular endothelial growth factor (VEGF) (Kim et al., 2009; Arnold et al., 2014). A few studies have shown changes in the melanin index after BTA injection, but there was no significant difference in melanin index between patients injected with BTA and the control groups in both studies (Zhu et al., 2016; Zhu et al., 2017). The presence of botulinum toxin type A reduces inflammation in the wound healing process, which leads to a significant difference in scar erythema, as reported by clinical research (Arnold et al., 2014).
Therefore, botulinum toxin type A has been applied clinically to prevent postoperative linear scars at multiple sites, including thyroidectomy scars (Kim et al., 2014; An et al., 2019), cleft lip scars (Chang et al., 2014), and maxillofacial and neck scars (Zhang et al., 2016). Three types of BTA-delivering methods have been reported: 1) for thyroidectomy scars: BTA injections were administered into the dermal layer 0.5 cm from the incision line in 2 rows (cephalad and caudad to the incision), 5 U at a time, at 1.5-cm intervals, and the total dose never exceeded 60 U (Kim et al., 2014); 2) for small-scale linear scars, injections were administered into the dermis 0.2 cm away from the wound edge with 5 U each site (Chang et al., 2014); 3) for normal-scale linear scars: BTA injections were administered into the dermal layer 0.5 cm away from the incision scar with 10 U each site at 1-cm intervals (Zhang et al., 2016). In addition, no obvious side effects of BTA treatment of linear scars have been reported by previous literature studies. The dose and site of BTA injections could be reduced according to the scale and the treatment response of the scar.
The time of injection in these studies varied from the day of surgery to within 3 days, 5 days, and 10 days after surgery (Ziade et al., 2013; Kim et al., 2014; Zhang et al., 2016; Hu et al., 2018; Lee et al., 2018). A clinical research based on 30 adult patients indicated that the operation-day BTA injection showed better outcomes with respect to the erythema index and skin elasticity (An et al., 2019). However, early BTA injections did not cause significant differences in the scar width and height.
Meanwhile, intralesional steroid injection has been widely used as a mainstay for scar management because it inhibits fibroblast growth and promotes collagen degeneration (Kelly, 2004; Zhang et al., 2016). Chemotherapy agents, such as 5-fluorouracil and bleomycin, which have been used in keloids (Nanda and Reddy, 2004; Tziotzios et al., 2012), could be carefully used in hypertrophic linear scars when satisfying results cannot be reached by a single steroid injection. The intralesional injection of steroids combined with 5-fluorouracil is suggested to be conducted at an early stage for patients with scarring predispositions (Srivastava et al., 2018). Therefore, when the scar appears to harden and swell after thyroidectomy, the above treatment methods can be used (Figure 1).
Lasers and phototherapy
Various types of lasers and phototherapy methods, mainly including pulsed dye laser (PDL), intense pulsed light (IPL), ablative fractional laser (AFL), and non-ablative fractional laser (NAFL), have been used to improve the cosmetic outcome of the scar after thyroidectomy (Jung et al., 2011; Chung et al., 2021).
IPL: IPL (400–1,200 nm; 500–600 nm) selectively targets hemoglobin in intravascular red blood cells to close local blood vessels and reduce blood supply for scar tissue growth (Fu et al., 2019). In a prospective study, the authors evaluated the safety and effectiveness of IPL in the treatment of burns, trauma, surgery, and acne scars with an initial treatment duration of 3 months and a treatment interval of 2–4 weeks, with patients receiving an average of eight treatments. IPL can not only effectively improve the appearance of hypertrophic scars and keloids but also reduce the height, redness, and hardness of scars (Erol et al., 2008). Some scholars also started IPL treatment immediately after removal, at 4 weeks and 8 weeks, and evaluated the therapeutic effect by using the Patient and Observer Scar Assessment Scale (POSAS) and Vancouver Scar Scale (VSS) scores, suggesting that IPL combined with erbium-doped yttrium aluminum garnet (Er:YAG) laser has a better preventive effect on scars than Er:YAG alone or no treatment (Kim et al., 2021). Therefore, IPL treatment of post-thyroidectomy scars should be carried out as early as possible, and effective treatment can be carried out within 6 months after surgery (Figure 2).
Pulsed dye laser, targeting the vasculature, has become a commonly used laser treatment for postoperative scarring. In 1993, the pulsed dye laser at 585 nm was reported to significantly improve erythematous and hypertrophic scars (Alster, 1993). In 2012, a randomized controlled trial with a follow-up time of 28 weeks proved that PDL treatment can improve the outcome of surgical scars in aspects of erythema, pigmentation, elasticity, and thickness (Davari et al., 2012). Although the exact anti-scarring mechanism remains unclear, previous literature proved that PDL emits light energy that is absorbed by hemoglobin, generating heat and resulting in damage to the microvasculature in the early scarring phase (Bouzari et al., 2007). Furthermore, 585-nm PDL is can decrease fibroblast proliferation and collagen type III deposition (Kuo et al., 2005). Several previous studies suggested PDL treatment should be conducted immediately after the removal of surgical stitches (Nouri et al., 2003; Alam et al., 2006; Conologue and Norwood, 2006; Nouri et al., 2009). However, for prevention of thyroidectomy scars, PDL treatment is suggested to be conducted 2–3 weeks after the removal of surgical stitches (Ha et al., 2014).
Ablative fractional laser based on the fractional approach, such as the 10,600-nm carbon dioxide (CO2) and 2,940-nm Er:YAG fractional laser system, is another common strategy for the early treatment of postoperative scars (Alster, 1999; Alster and Zaulyanov, 2007; Yun et al., 2011). AFL produces arrays of microscopic thermal wounds called microscopic treatment zones (MTZs) at specific depths. The intact epidermal architecture surrounding each MTZ rapidly heals, which in turn stimulates progressive collagen remodeling in scars (Manstein et al., 2004; Geronemus, 2006; Laubach et al., 2006). Several studies have proven the ablative CO2 fractional laser (CO2 AFL) system to be effective and safe in early postoperative interventions of thyroidectomy scars (Jung et al., 2011) and other surgical linear scars (Lee et al., 2013; Shin et al., 2014; Sobanko et al., 2015). Consensus has not been reached on the intervention time of CO2 AFL, with literature studies suggesting the time as on the day of suture removal (Sobanko et al., 2015), 2–3 weeks after stitch removal (Lee et al., 2013), and 2–3 months after surgery (Shin et al., 2014). However, one study suggested that 2–3 weeks after surgery could be an appropriate window for AFL treatment of thyroidectomy scars because re-epithelialization would be complete at this time point (Jung et al., 2011). The treatment energy of AFL should be carefully reduced to make the heat damage depth reach the dermis but without total penetration of the scar (Anderson et al., 2014) since another study found aggravation of hypertrophic scars when treating post-thyroidectomy scars 2–3 months after surgery, which is due to excessive AFL energy and density (Shin et al., 2014).
The development of non-ablative fractionated laser technology provided a new tool for the successful treatment of linear scars, offering the fractional thermolysis technique with minimal sequelae and a short downtime (Behroozan et al., 2006; Niwa et al., 2009). NAFL produces arrays of MTZs to stimulate collagen remodeling in scars, although sparing the epidermis while reaching a depth of 300–400 um in the dermis. Compared to NAFL, AFL caused more aggressive damage with the epidermis included in the MTZs, leading to slightly more side effects and a longer downtime, but ultimately has greater and more prolonged effects (Hantash et al., 2007a; Hantash et al., 2007b; Avram et al., 2009). Multiple studies have suggested that use of an erbium–glass (Er:Glass) NAFL for linear scar treatment might decrease the incidence of hypertrophic scarring and accelerate the improvement of postoperative scars (Choe et al., 2009; Ha et al., 2014; Karmisholt et al., 2018). The appropriate window for the NAFL treatment of the postoperative linear scar is unknown, with limited literature suggesting 2–3 weeks (Kim H. S. et al., 2012) or 2 months (Shin et al., 2014) after surgery. Of note, combination treatment, such as NAFL plus IPL (Kim et al., 2021) or NAFL combined with intralesional triamcinolone injection (Chung et al., 2021), achieves greater improvement in post-thyroidectomy scar prevention and management (Table 1).
Conclusion
Early clinical management of thyroidectomy scars is a comprehensive procedure with a duration of 3–6 months. The most modifiable factor of scar prevention is the design of the skin incision and suture material, which leads to the least amount of wound tension in the postoperative period. Local injection of BTA, tension-bearing devices, and AFL might contribute to reduction in skin tension during the early post-thyroidectomy stage. In addition, local injection of steroids and chemotherapy agents, topical anti-scar agents, and PDL might contribute to the damage of the microvasculature and the decrease in fibroblast proliferation during the early thyroidectomy scar stage. A flexible combination and selection of these early postoperative interventions might help thyroidectomy patients achieve an optimal esthetic outcome.
Author contributions
NH: conceptualization, data curation, funding acquisition, and writing–original draft. BS: data curation, formal analysis, and writing–review and editing. PY: supervision, writing–review and editing, conceptualization, and funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The Scientific Research Project (Nos YYMS2021022, 22LCZLXJS39, YYQN2021078, 22HLZX2 and K2023064) supported this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarabi S., Bhatt K. A., Shi Y., Paterno J., Chang E. I., Loh S. A., et al. (2007). Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21 (12), 3250–3261. doi:10.1096/fj.07-8218com
Akaishi S., Akimoto M., Ogawa R., Hyakusoku H. (2008). The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann. Plast. Surg. 60 (4), 445–451. doi:10.1097/SAP.0b013e3181238dd7
Alam M., Pon K., Van Laborde S., Kaminer M. S., Arndt K. A., Dover J. S. (2006). Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 32 (1), 21–25. doi:10.1111/1524-4725.2006.32029
Alster T., Zaulyanov L., Zaulyanov-Scanlon L. (2007). Laser scar revision: a review. Dermatol Surg. 33 (2), 131–140. doi:10.1111/j.1524-4725.2006.33030.x
Alster T. S. (1999). Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast. Reconstr. Surg. 103 (2), 619–632. doi:10.1097/00006534-199902000-00040
An M. K., Cho E. B., Park E. J., Kim K. H., Kim L. S., Kim K. J. (2019). Appropriate timing of early postoperative botulinum toxin type A injection for thyroidectomy scar management: a split-scar study. Plast. Reconstr. Surg. 144 (4), 659e–668e. doi:10.1097/PRS.0000000000006064
Anderson R. R., Donelan M. B., Hivnor C., Greeson E., Ross E. V., Shumaker P. R., et al. (2014). Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol 150 (2), 187–193. doi:10.1001/jamadermatol.2013.7761
Arnold P. B., Fang T., Songcharoen S. J., Ziakas G., Zhang F. (2014). Inflammatory response and survival of pedicled abdominal flaps in a rat model after perivascular application of botulinum toxin type A. Plast. Reconstr. Surg. 133 (4), 491e–498e. doi:10.1097/PRS.0000000000000030
Atkinson J. A., McKenna K. T., Barnett A. G., McGrath D. J., Rudd M. (2005). A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast. Reconstr. Surg. 116 (6), 1648–1656. doi:10.1097/01.prs.0000187147.73963.a5
Augusti K. T. (1996). Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J. Exp. Biol. 34 (7), 634–640.
Avram M. M., Tope W. D., Yu T., Szachowicz E., Nelson J. S. (2009). Hypertrophic scarring of the neck following ablative fractional carbon dioxide laser resurfacing. Lasers Surg. Med. 41 (3), 185–188. doi:10.1002/lsm.20755
Bayat A., McGrouther D. A., Ferguson M. W. (2003). Skin scarring. BMJ 326 (7380), 88–92. doi:10.1136/bmj.326.7380.88
Behroozan D. S., Goldberg L. H., Dai T., Geronemus R. G., Friedman P. M. (2006). Fractional photothermolysis for the treatment of surgical scars: a case report. J. Cosmet. Laser Ther. 8 (1), 35–38. doi:10.1080/14764170600607251
Berman B., Perez O. A., Konda S., Kohut B. E., Viera M. H., Delgado S., et al. (2007). A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg. 33 (11), 1291–1302. doi:10.1111/j.1524-4725.2007.33280.x
Borgognoni L. (2002). Biological effects of silicone gel sheeting. Wound Repair Regen. 10 (2), 118–121. doi:10.1046/j.1524-475x.2002.00205.x
Bouzari N., Davis S. C., Nouri K. (2007). Laser treatment of keloids and hypertrophic scars. Int. J. Dermatol 46 (1), 80–88. doi:10.1111/j.1365-4632.2007.03104.x
Bullard K. M., Longaker M. T., Lorenz H. P. (2003). Fetal wound healing: current biology. World J. Surg. 27 (1), 54–61. doi:10.1007/s00268-002-6737-2
Chan K. Y., Lau C. L., Adeeb S. M., Somasundaram S., Nasir-Zahari M. (2005). A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast. Reconstr. Surg. 116 (4), 1013–1020. doi:10.1097/01.prs.0000178397.05852.ce
Chang C. C., Kuo Y. F., Chiu H. C., Lee J. L., Wong T. W., Jee S. H. (1995). Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J. Surg. Res. 59 (6), 705–711. doi:10.1006/jsre.1995.1227
Chang C. S., Wallace C. G., Hsiao Y. C., Chang C. J., Chen P. K. (2014). Botulinum toxin to improve results in cleft lip repair. Plast. Reconstr. Surg. 134 (3), 511–516. doi:10.1097/PRS.0000000000000416
Cheng C. L., Guo J. S., Luk J., Koo M. W. (2004). The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci. 74 (18), 2237–2249. doi:10.1016/j.lfs.2003.09.055
Choe J. H., Park Y. L., Kim B. J., Kim M. N., Rho N. K., Park B. S., et al. (2009). Prevention of thyroidectomy scar using a new 1,550-nm fractional erbium-glass laser. Dermatol Surg. 35 (8), 1199–1205. doi:10.1111/j.1524-4725.2008.34428.x
Chung J. H., Kim D. S., Cheon J. H., Yoon J. M., Baek S. K., Jung K. Y., et al. (2021). Current protocol for aesthetic scar management in thyroid surgery. Laryngoscope 131 (7), E2188–E2195. doi:10.1002/lary.29441
Conologue T. D., Norwood C. (2006). Treatment of surgical scars with the cryogen-cooled 595 nm pulsed dye laser starting on the day of suture removal. Dermatol Surg. 32 (1), 13–20. doi:10.1111/1524-4725.2006.32002
Consorti F., Mancuso R., Piccolo A., Pretore E., Antonaci A. (2013). Quality of scar after total thyroidectomy: a single blinded randomized trial comparing octyl-cyanoacrylate and subcuticular absorbable suture. ISRN Surg. 2013, 270953. doi:10.1155/2013/270953
Davari P., Gorouhi F., Hashemi P., Behnia F., Ghassemi A., Nasiri-Kashani M., et al. (2012). Pulsed dye laser treatment with different onset times for new surgical scars: a single-blind randomized controlled trial. Lasers Med. Sci. 27 (5), 1095–1098. doi:10.1007/s10103-011-1044-5
de Giorgi V., Sestini S., Mannone F., Papi F., Alfaioli B., Gori A., et al. (2009). The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clin. Exp. Dermatol 34 (6), 688–693. doi:10.1111/j.1365-2230.2008.03096.x
English R. S., Shenefelt P. D. (1999). Keloids and hypertrophic scars. Dermatol Surg. 25 (8), 631–638. doi:10.1046/j.1524-4725.1999.98257.x
Engrav L. H., Gottlieb J. R., Millard S. P., Walkinshaw M. D., Heimbach D. M., Marvin J. A. (1988). A comparison of intramarginal and extramarginal excision of hypertrophic burn scars. Plast. Reconstr. Surg. 81 (1), 40–45. doi:10.1097/00006534-198801000-00007
Erol O. O., Gurlek A., Agaoglu G., Topcuoglu E., Oz H. (2008). Treatment of hypertrophic scars and keloids using intense pulsed light (IPL). Aesthetic Plast. Surg. 32 (6), 902–909. doi:10.1007/s00266-008-9161-7
Fu X., Dong J., Wang S., Yan M., Yao M. (2019). Advances in the treatment of traumatic scars with laser, intense pulsed light, radiofrequency, and ultrasound. Burns Trauma 7, 1. doi:10.1186/s41038-018-0141-0
Geronemus R. G. (2006). Fractional photothermolysis: current and future applications. Lasers Surg. Med. 38 (3), 169–176. doi:10.1002/lsm.20310
Ha J. M., Kim H. S., Cho E. B., Park G. H., Park E. J., Kim K. H., et al. (2014). Comparison of the effectiveness of nonablative fractional laser versus pulsed-dye laser in thyroidectomy scar prevention. Ann. Dermatol 26 (5), 615–620. doi:10.5021/ad.2014.26.5.615
Hantash B. M., Bedi V. P., Chan K. F., Zachary C. B. (2007a). Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg. Med. 39 (2), 87–95. doi:10.1002/lsm.20405
Hantash B. M., Bedi V. P., Kapadia B., Rahman Z., Jiang K., Tanner H., et al. (2007b). In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg. Med. 39 (2), 96–107. doi:10.1002/lsm.20468
Har-Shai Y., Amar M., Sabo E. (2003). Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast. Reconstr. Surg. 111 (6), 1841–1852. doi:10.1097/01.PRS.0000056868.42679.05
Har-Shai Y., Brown W., Labbe D., Dompmartin A., Goldine I., Gil T., et al. (2008). Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery: the results of a prospective observational study. Int. J. Low. Extrem Wounds 7 (3), 169–175. doi:10.1177/1534734608322813
Har-Shai Y., Sabo E., Rohde E., Hyams M., Assaf C., Zouboulis C. C. (2006). Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies. Wound Repair Regen. 14 (1), 18–27. doi:10.1111/j.1743-6109.2005.00084.x
Hosnuter M., Payasli C., Isikdemir A., Tekerekoglu B. (2007). The effects of onion extract on hypertrophic and keloid scars. J. Wound Care 16 (6), 251–254. doi:10.12968/jowc.2007.16.6.27070
Hsu T. S., Dover J. S., Arndt K. A. (2004). Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch. Dermatol 140 (11), 1351–1354. doi:10.1001/archderm.140.11.1351
Hu L., Zou Y., Chang S. J., Qiu Y., Chen H., Gang M., et al. (2018). Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast. Reconstr. Surg. 141 (3), 646–650. doi:10.1097/PRS.0000000000004110
Huang C., Miyazaki K., Akaishi S., Watanabe A., Hyakusoku H., Ogawa R. (2013). Biological effects of cellular stretch on human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 66 (12), e351–e361. doi:10.1016/j.bjps.2013.08.002
Jackson B. A., Shelton A. J. (1999). Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg. 25 (4), 267–269. doi:10.1046/j.1524-4725.1999.08240.x
Jenwitheesuk K., Surakunprapha P., Jenwitheesuk K., Kuptarnond C., Prathanee S., Intanoo W. (2012). Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int. Wound J. 9 (4), 397–402. doi:10.1111/j.1742-481X.2011.00898.x
Juckett G., Hartman-Adams H. (2009). Management of keloids and hypertrophic scars. Am. Fam. Physician 80 (3), 253–260.
Ju-Lin X., Shao-Hai Q., Tian-Zeng L., Bin H., Jing-Ming T., Ying-Bin X., et al. (2009). Effect of asiaticoside on hypertrophic scar in the rabbit ear model. J. Cutan. Pathol. 36 (2), 234–239. doi:10.1111/j.1600-0560.2008.01015.x
Jung J. Y., Jeong J. J., Roh H. J., Cho S. H., Chung K. Y., Lee W. J., et al. (2011). Early postoperative treatment of thyroidectomy scars using a fractional carbon dioxide laser. Dermatol Surg. 37 (2), 217–223. doi:10.1111/j.1524-4725.2010.01853.x
Karmisholt K. E., Banzhaf C. A., Glud M., Yeung K., Paasch U., Nast A., et al. (2018). Laser treatments in early wound healing improve scar appearance: a randomized split-wound trial with nonablative fractional laser exposures vs untreated controls. Br. J. Dermatol 179 (6), 1307–1314. doi:10.1111/bjd.17076
Kelly A. P. (2004). Medical and surgical therapies for keloids. Dermatol Ther. 17 (2), 212–218. doi:10.1111/j.1396-0296.2004.04022.x
Kim H. S., Lee J. H., Park Y. M., Lee J. Y. (2012a). Comparison of the effectiveness of nonablative fractional laser versus ablative fractional laser in thyroidectomy scar prevention: a pilot study. J. Cosmet. Laser Ther. 14 (2), 89–93. doi:10.3109/14764172.2012.672746
Kim J. C., Kang S. Y., Kim H. O., Park C. W., Kwon O., Chung B. Y. (2021). Efficacy of combined treatment with intense pulsed light and fractional erbium:YAG Laser in scar prevention: a randomized split wound trial. Dermatol Ther. 34 (5), e15061. doi:10.1111/dth.15061
Kim J. H., Sung J. Y., Kim Y. H., Lee Y. S., Chang H. S., Park C. S., et al. (2012b). Risk factors for hypertrophic surgical scar development after thyroidectomy. Wound Repair Regen. 20 (3), 304–310. doi:10.1111/j.1524-475X.2012.00784.x
Kim S., Ahn M., Piao Y., Ha Y., Choi D. K., Yi M. H., et al. (2016). Effect of botulinum toxin type A on TGF-β/smad pathway signaling: implications for silicone-induced capsule formation. Plast. Reconstr. Surg. 138 (5), 821e–829e. doi:10.1097/PRS.0000000000002625
Kim T. K., Oh E. J., Chung J. Y., Park J. W., Cho B. C., Chung H. Y. (2009). The effects of botulinum toxin A on the survival of a random cutaneous flap. J. Plast. Reconstr. Aesthet. Surg. 62 (7), 906–913. doi:10.1016/j.bjps.2007.12.034
Kim Y. S., Lee H. J., Cho S. H., Lee J. D., Kim H. S. (2014). Early postoperative treatment of thyroidectomy scars using botulinum toxin: a split-scar, double-blind randomized controlled trial. Wound Repair Regen. 22 (5), 605–612. doi:10.1111/wrr.12204
Korntner S., Lehner C., Gehwolf R., Wagner A., Grutz M., Kunkel N., et al. (2019). Limiting angiogenesis to modulate scar formation. Adv. Drug Deliv. Rev. 146, 170–189. doi:10.1016/j.addr.2018.02.010
Kuhn M. A., Moffit M. R., Smith P. D., Lyle W. G., Ko F., Meltzer D. D., et al. (2001). Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int. J. Surg. Investig. 2 (6), 467–474.
Kuo Y. R., Wu W. S., Jeng S. F., Huang H. C., Yang K. D., Sacks J. M., et al. (2005). Activation of ERK and p38 kinase mediated keloid fibroblast apoptosis after flashlamp pulsed-dye laser treatment. Lasers Surg. Med. 36 (1), 31–37. doi:10.1002/lsm.20129
Larrabee W. F. (2000). Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast. Reconstr. Surg. 105 (6), 1954–1955. doi:10.1097/00006534-200005000-00006
Larson B. J., Longaker M. T., Lorenz H. P. (2010). Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 126 (4), 1172–1180. doi:10.1097/PRS.0b013e3181eae781
Laubach H. J., Tannous Z., Anderson R. R., Manstein D. (2006). Skin responses to fractional photothermolysis. Lasers Surg. Med. 38 (2), 142–149. doi:10.1002/lsm.20254
Lee B. J., Jeong J. H., Wang S. G., Lee J. C., Goh E. K., Kim H. W. (2009). Effect of botulinum toxin type a on a rat surgical wound model. Clin. Exp. Otorhinolaryngol. 2 (1), 20–27. doi:10.3342/ceo.2009.2.1.20
Lee S. H., Min H. J., Kim Y. W., Cheon Y. W. (2018). The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthetic Plast. Surg. 42 (2), 530–537. doi:10.1007/s00266-017-1008-7
Lee S. H., Zheng Z., Roh M. R. (2013). Early postoperative treatment of surgical scars using a fractional carbon dioxide laser: a split-scar, evaluator-blinded study. Dermatol Surg. 39 (8), 1190–1196. doi:10.1111/dsu.12228
Longaker M. T., Rohrich R. J., Greenberg L., Furnas H., Wald R., Bansal V., et al. (2014). A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast. Reconstr. Surg. 134 (3), 536–546. doi:10.1097/PRS.0000000000000417
Mahdavian Delavary B., van der Veer W. M., van Egmond M., Niessen F. B., Beelen R. H. (2011). Macrophages in skin injury and repair. Immunobiology 216 (7), 753–762. doi:10.1016/j.imbio.2011.01.001
Manstein D., Herron G. S., Sink R. K., Tanner H., Anderson R. R. (2004). Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 34 (5), 426–438. doi:10.1002/lsm.20048
Maragakis M., Willital G. H., Michel G., Gortelmeyer R. (1995). Possibilities of scar treatment after thoracic surgery. Drugs Exp. Clin. Res. 21 (5), 199–206.
Nanda S., Reddy B. S. (2004). Intralesional 5-fluorouracil as a treatment modality of keloids. Dermatol Surg. 30 (1), 54–56. doi:10.1111/j.1524-4725.2004.29382.x
Niwa A. B., Mello A. P., Torezan L. A., Osorio N. (2009). Fractional photothermolysis for the treatment of hypertrophic scars: clinical experience of eight cases. Dermatol Surg. 35 (5), 773–777. doi:10.1111/j.1524-4725.2009.01127.x
Noland M. E., Lalonde D. H., Yee G. J., Rohrich R. J. (2016). Current uses of botulinum neurotoxins in plastic surgery. Plast. Reconstr. Surg. 138 (3), 519e–530e. doi:10.1097/PRS.0000000000002480
Nouri K., Jimenez G. P., Harrison-Balestra C., Elgart G. W. (2003). 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg. 29 (1), 65–73. doi:10.1046/j.1524-4725.2003.29014.x
Nouri K., Rivas M. P., Stevens M., Ballard C. J., Singer L., Ma F., et al. (2009). Comparison of the effectiveness of the pulsed dye laser 585 nm versus 595 nm in the treatment of new surgical scars. Lasers Med. Sci. 24 (5), 801–810. doi:10.1007/s10103-009-0698-8
Ogawa R. (2008). Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med. Hypotheses 71 (4), 493–500. doi:10.1016/j.mehy.2008.05.020
Ogawa R., Akaishi S., Huang C., Dohi T., Aoki M., Omori Y., et al. (2011). Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J. Nippon. Med. Sch. 78 (2), 68–76. doi:10.1272/jnms.78.68
Ogawa R., Akaishi S., Kuribayashi S., Miyashita T. (2016). Keloids and hypertrophic scars can now Be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J. Nippon. Med. Sch. 83 (2), 46–53. doi:10.1272/jnms.83.46
Ogawa R., Dohi T., Tosa M., Aoki M., Akaishi S. (2021). The latest strategy for keloid and hypertrophic scar prevention and treatment: the nippon medical school (NMS) protocol. J. Nippon. Med. Sch. 88 (1), 2–9. doi:10.1272/jnms.JNMS.2021_88-106
Perez O. A., Viera M. H., Patel J. K., Konda S., Amini S., Huo R., et al. (2010). A comparative study evaluating the tolerability and efficacy of two topical therapies for the treatment of keloids and hypertrophic scars. J. Drugs Dermatol 9 (5), 514–518.
Prodromidou A., Frountzas M., Vlachos D. E., Vlachos G. D., Bakoyiannis I., Perrea D., et al. (2015). Botulinum toxin for the prevention and healing of wound scars: a systematic review of the literature. Plast. Surg. (Oakv) 23 (4), 260–264. doi:10.4172/plastic-surgery.1000934
Profyris C., Tziotzios C., Do Vale I. (2012). Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol 66 (1), 1–10. doi:10.1016/j.jaad.2011.05.055
Quinn K. J., Evans J. H., Courtney J. M., Gaylor J. D., Reid W. H. (1985). Non-pressure treatment of hypertrophic scars. Burns Incl. Therm. Inj. 12 (2), 102–108. doi:10.1016/0305-4179(85)90035-x
Sawada Y., Sone K. (1990). Treatment of scars and keloids with a cream containing silicone oil. Br. J. Plast. Surg. 43 (6), 683–688. doi:10.1016/0007-1226(90)90189-7
Shin J. U., Gantsetseg D., Jung J. Y., Jung I., Shin S., Lee J. H. (2014). Comparison of non-ablative and ablative fractional laser treatments in a postoperative scar study. Lasers Surg. Med. 46 (10), 741–749. doi:10.1002/lsm.22297
Shukla A., Rasik A. M., Jain G. K., Shankar R., Kulshrestha D. K., Dhawan B. N. (1999). In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 65 (1), 1–11. doi:10.1016/s0378-8741(98)00141-x
Sobanko J. F., Vachiramon V., Rattanaumpawan P., Miller C. J. (2015). Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: a split-scar, evaluator-blinded study. Lasers Surg. Med. 47 (1), 1–5. doi:10.1002/lsm.22314
Son D., Harijan A. (2014). Overview of surgical scar prevention and management. J. Korean Med. Sci. 29 (6), 751–757. doi:10.3346/jkms.2014.29.6.751
Song T., Kim K. H., Lee K. W. (2018). Randomised comparison of silicone gel and onion extract gel for post-surgical scars. J. Obstet. Gynaecol. 38 (5), 702–707. doi:10.1080/01443615.2017.1400524
Srivastava S., Patil A., Prakash C., Kumari H. (2018). Comparison of intralesional triamcinolone acetonide, 5-fluorouracil, and their combination in treatment of keloids. World J. Plast. Surg. 7 (2), 212–219.
Suarez E., Syed F., Alonso-Rasgado T., Mandal P., Bayat A. (2013). Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, α2β1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast. Reconstr. Surg. 131 (2), 158e–173e. doi:10.1097/PRS.0b013e3182789b2b
Suarez E., Syed F., Rasgado T. A., Walmsley A., Mandal P., Bayat A. (2014). Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair Regen. 22 (5), 557–568. doi:10.1111/wrr.12215
Sundaram H., Signorini M., Liew S., Trindade de Almeida A. R., Wu Y., Vieira Braz A., et al. (2016). Global aesthetics consensus: botulinum toxin type A--evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast. Reconstr. Surg. 137 (3), 518e–529e. doi:10.1097/01.prs.0000475758.63709.23
Tejada S., Manayi A., Daglia M., Nabavi S. F., Sureda A., Hajheydari Z., et al. (2016). Wound healing effects of curcumin: a short review. Curr. Pharm. Biotechnol. 17, 1002–1007. doi:10.2174/1389201017666160721123109
Teoh L. Y., Chong S. S., Hoh S. Y., Teoh M. S., Ng K. L. (2019). A comparison of aesthetic outcome between tissue adhesive and subcuticular suture in thyroidectomy wound closure in a multiracial country: a randomized controlled trial. Asian J. Surg. 42 (5), 634–640. doi:10.1016/j.asjsur.2018.09.014
Trace A. P., Enos C. W., Mantel A., Harvey V. M. (2016). Keloids and hypertrophic scars: a spectrum of clinical challenges. Am. J. Clin. Dermatol 17 (3), 201–223. doi:10.1007/s40257-016-0175-7
Tziotzios C., Profyris C., Sterling J. (2012). Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part II. Strategies to reduce scar formation after dermatologic procedures. J. Am. Acad. Dermatol 66 (1), 13–24. doi:10.1016/j.jaad.2011.08.035
van der Veer W. M., Jacobs X. E., Waardenburg I. E., Ulrich M. M., Niessen F. B. (2009). Topical calcipotriol for preventive treatment of hypertrophic scars: a randomized, double-blind, placebo-controlled trial. Arch. Dermatol 145 (11), 1269–1275. doi:10.1001/archdermatol.2009.237
Wilhelmi B. J., Blackwell S. J., Phillips L. G. (1999). Langer's lines: to use or not to use. Plast. Reconstr. Surg. 104 (1), 208–214. doi:10.1097/00006534-199907000-00033
Willital G. H., Heine H. (1994). Efficacy of Contractubex gel in the treatment of fresh scars after thoracic surgery in children and adolescents. Int. J. Clin. Pharmacol. Res. 14 (5-6), 193–202.
Wong V. W., Rustad K. C., Akaishi S., Sorkin M., Glotzbach J. P., Januszyk M., et al. (2011). Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 18 (1), 148–152. doi:10.1038/nm.2574
Xiao Z., Zhang M., Liu Y., Ren L. (2011). Botulinum toxin type a inhibits connective tissue growth factor expression in fibroblasts derived from hypertrophic scar. Aesthetic Plast. Surg. 35 (5), 802–807. doi:10.1007/s00266-011-9690-3
Yun J. S., Choi Y. J., Kim W. S., Lee G. Y. (2011). Prevention of thyroidectomy scars in Asian adults using a 532-nm potassium titanyl phosphate laser. Dermatol Surg. 37 (12), 1747–1753. doi:10.1111/j.1524-4725.2011.02128.x
Zhang D. Z., Liu X. Y., Xiao W. L., Xu Y. X. (2016). Botulinum toxin type A and the prevention of hypertrophic scars on the maxillofacial area and neck: a meta-analysis of randomized controlled trials. PLoS One 11 (3), e0151627. doi:10.1371/journal.pone.0151627
Zhang T., Wang X. F., Wang Z. C., Lou D., Fang Q. Q., Hu Y. Y., et al. (2020). Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 129, 110287. doi:10.1016/j.biopha.2020.110287
Zhibo X., Miaobo Z. (2008). Potential therapeutical effects of botulinum toxin type A in keloid management. Med. Hypotheses 71 (4), 623. doi:10.1016/j.mehy.2008.04.018
Zhu J., Ji X., Li M., Chen X. E., Liu J., Zhang J. A., et al. (2016). The efficacy and safety of fractional CO₂ laser combined with topical type A botulinum toxin for facial rejuvenation: a randomized controlled split-face study. Biomed. Res. Int. 2016, 3853754. doi:10.1155/2016/3853754
Zhu J., Ji X., Xu Y., Liu J., Miao Y. Y., Zhang J. A., et al. (2017). The efficacy of intradermal injection of type A botulinum toxin for facial rejuvenation. Dermatol Ther. 30 (1), e12433. doi:10.1111/dth.12433
Ziade M., Domergue S., Batifol D., Jreige R., Sebbane M., Goudot P., et al. (2013). Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J. Plast. Reconstr. Aesthet. Surg. 66 (2), 209–214. doi:10.1016/j.bjps.2012.09.012
Keywords: thyroidectomy, linear scars, postoperative interventions, early-stage treatment, hypertrophic scars
Citation: Hong N, Sheng B and Yu P (2024) Early postoperative interventions in the prevention and management of thyroidectomy scars. Front. Physiol. 15:1341287. doi: 10.3389/fphys.2024.1341287
Received: 20 November 2023; Accepted: 26 February 2024;
Published: 06 March 2024.
Edited by:
Yiming Zhang, Xinqiao Hospital, ChinaReviewed by:
Jeremie Oliver Piña, National Institutes of Health (NIH), United StatesHulin Chen, Guangdong Province Women and Children Hospital, China
Diego Velasco, Universidad Carlos III de Madrid de Madrid, Spain
Copyright © 2024 Hong, Sheng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Yu, eXA1MkAxNjMuY29t
†These authors contributed equally to this work and share the first co-authorship.
 Nan Hong
Nan Hong Bin Sheng
Bin Sheng Pan Yu
Pan Yu